Antioxidant and Antidiabetic Potential of the Antarctic Lichen Gondwania regalis Ethanolic Extract: Metabolomic Profile and In Vitro and In Silico Evaluation
Abstract
1. Introduction
2. Materials and Methods
2.1. Lichen Material
2.2. Taxonomic Determination
2.3. Preparation of Ethanolic Extracts
2.4. LC Parameters and MS Parameters
2.5. Total Phenolic Content
2.6. Antioxidant Activity
2.6.1. Ferric-Reducing Antioxidant Power (FRAP) Assay
2.6.2. Oxygen Radical Absorbance Capacity (ORAC) Assay
2.6.3. DPPH Scavenging Activity
2.7. Enzymatic Inhibitory Activity
2.7.1. α-Glucosidase Inhibition Assay
2.7.2. α-Amylase Inhibition Assay
2.7.3. Lipase Pancreatic Inhibition Assay
2.8. Calculation of the Pharmacological Properties and Risk Toxicity
2.9. In Silico Analysis
2.10. Molecular Dynamic Simulations
2.11. Statistical Analysis
3. Results
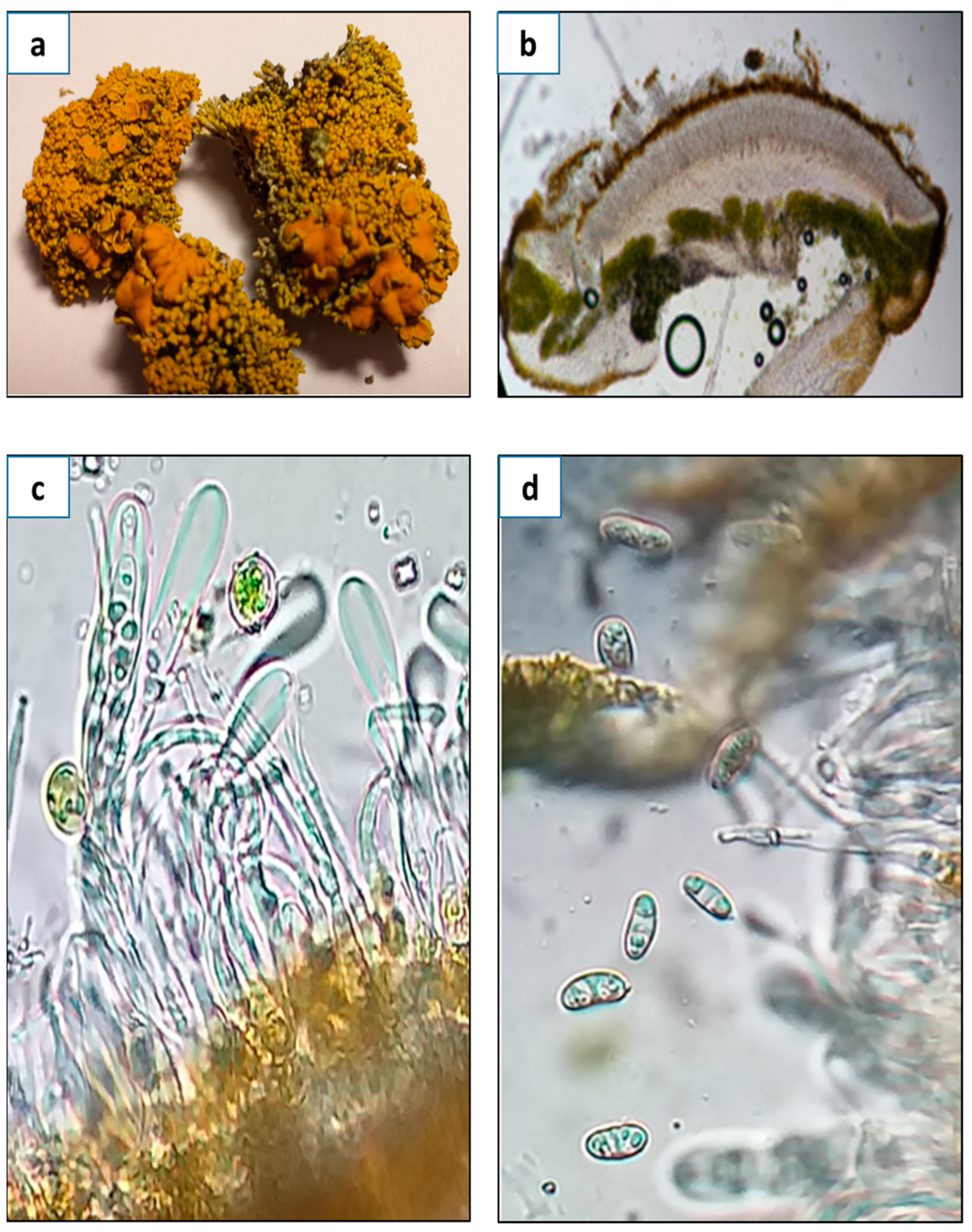
3.1. Morphological, Ecological, and Taxonomic Description of G. regalis
3.1.1. Morphological Description
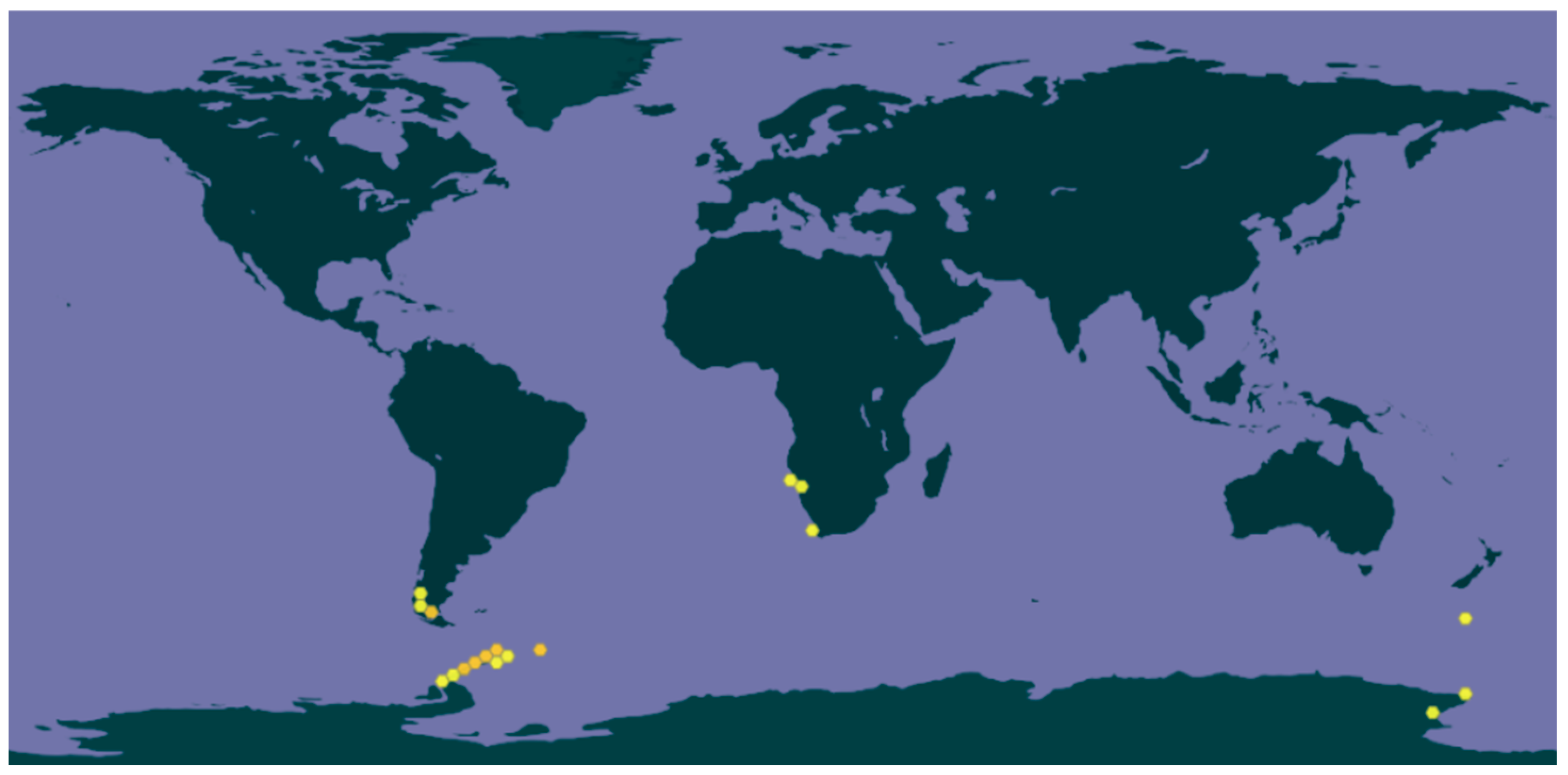
3.1.2. Ecology and Distribution
3.1.3. Taxonomic Notes
3.2. Chromatographic Analysis of G. regalis
3.3. Total Phenolic Content and Antioxidant Activity
3.4. Enzymatic Inhibitory Activity
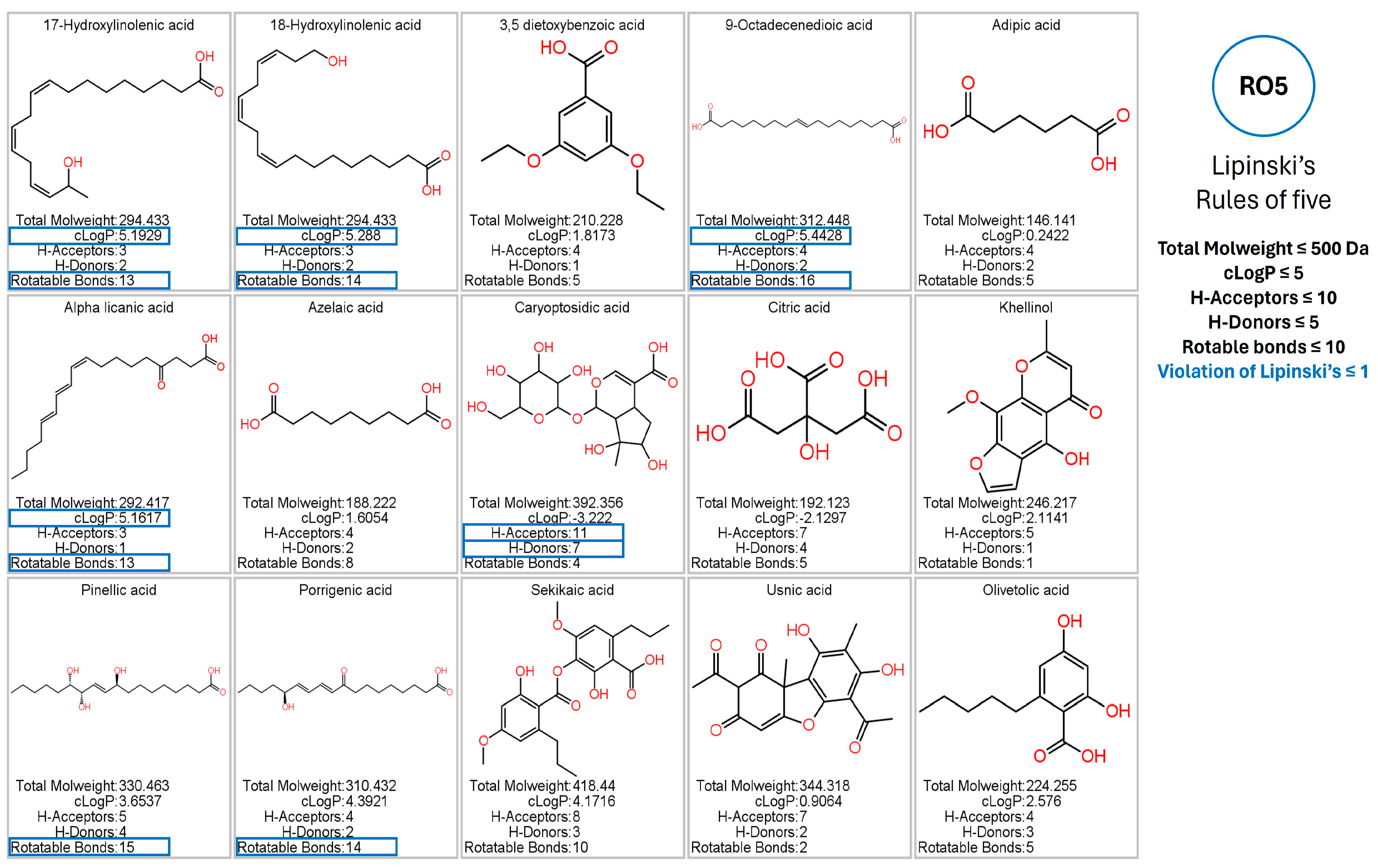
3.5. Prediction of Pharmacokinetic and Toxicological Properties
3.6. Docking Studies
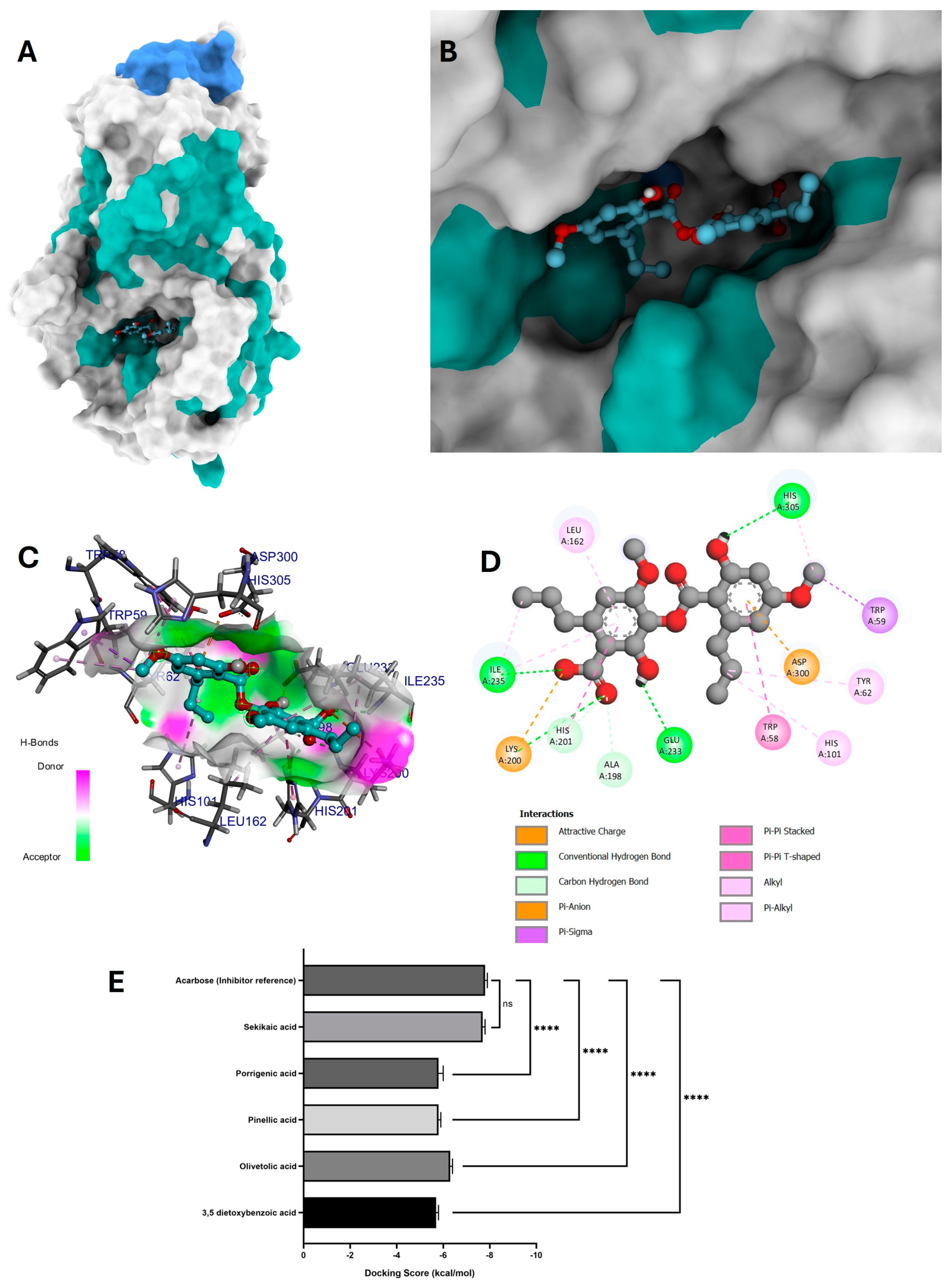
3.6.1. Molecular Docking of Phytochemicals for α-Amylase Inhibition
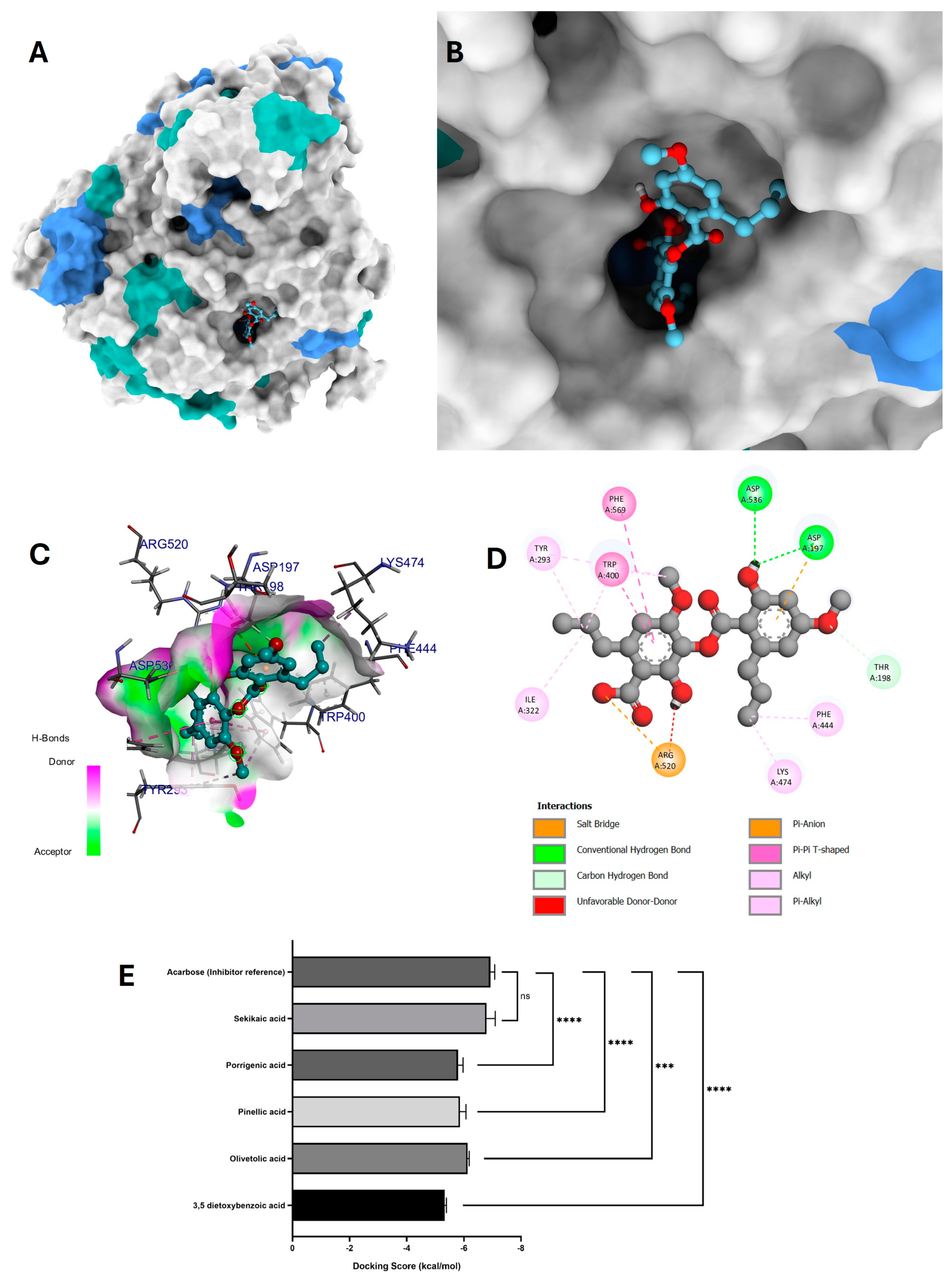
3.6.2. Molecular Docking of Phytochemicals for α-Glucosidase Inhibition
3.6.3. Molecular Docking of Phytochemicals for Lipase Pancreatic Inhibition
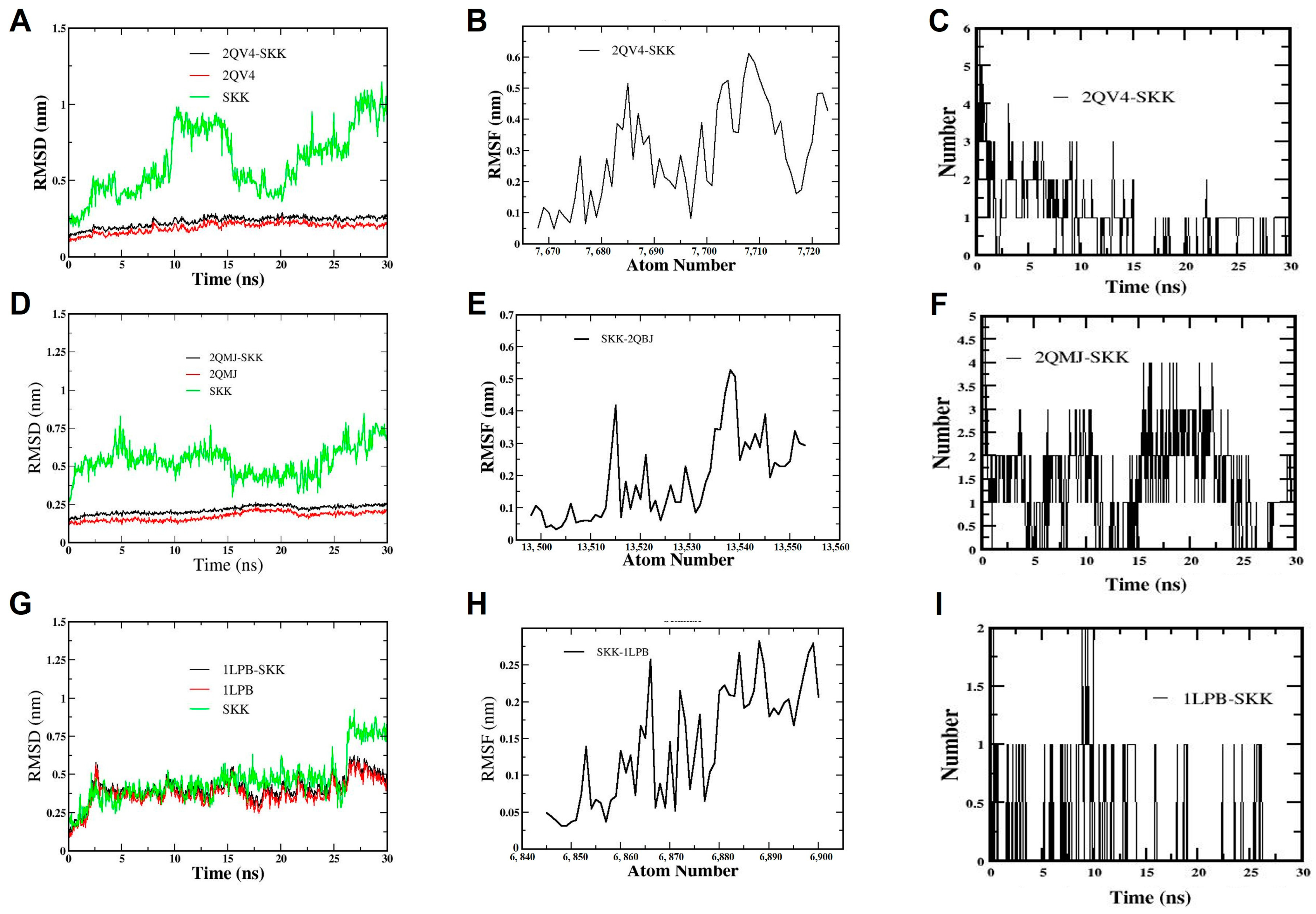
3.7. Molecular Dynamic Simulation
3.7.1. Molecular Dynamic Simulation of Sekikaic Acid for α-Amylase Inhibition
3.7.2. Molecular Dynamic Simulation of Sekikaic Acid for α-Glucosidase Inhibition
3.7.3. Molecular Dynamic Simulation of Sekikaic Acid for Human Pancreatic Lipase Inhibition
4. Discussion
4.1. Chemical Analysis
4.2. Antioxidant Activity
4.3. Inhibitory Activity
4.4. Molecular Docking
4.4.1. Molecular Docking of Phytochemicals for α-Amylase Inhibition
4.4.2. Molecular Docking of Phytochemicals for α-Glucosidase Inhibition
4.4.3. Molecular Docking of Phytochemicals for Human Pancreatic Lipase Inhibition
4.5. Molecular Dynamics (MD) Simulation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Spribille, T.; Tuovinen, V.; Resl, P.; Vanderpool, D.; Wolinski, H.; Aime, M.C.; Schneider, K.; Stabentheiner, E.; Toome-Heller, M.; Thor, G.; et al. Basidiomycete yeasts in the cortex of ascomycete macrolichens. Science 2016, 353, 488–492. [Google Scholar] [CrossRef] [PubMed]
- Spribille, T. Relative symbiont input and the lichen symbiotic outcome. Curr. Opin. Plant Biol. 2018, 44, 57–63. [Google Scholar] [CrossRef]
- Spribille, T.; Tagirdzhanova, G.; Goyette, S.; Tuovinen, V.; Case, R.; Zandberg, W.F. 3D biofilms: In search of the polysaccharides holding together lichen symbioses. FEMS Microbiol. Lett. 2020, 367, fnaa023. [Google Scholar] [CrossRef]
- Spribille, T.; Resl, P.; Stanton, D.E.; Tagirdzhanova, G. Evolutionary biology of lichen symbioses. New Phytol. 2022, 234, 1566–1582. [Google Scholar] [CrossRef] [PubMed]
- Naranjo-Ortiz, M.A.; Gabaldón, T. Fungal Evolution: Diversity, Taxonomy and Phylogeny of the Fungi. Biol. Rev. 2019, 94, 2101–2137. [Google Scholar] [CrossRef] [PubMed]
- Redón, J. Antarctic Lichens; Instituto Antártico Chileno: Santiago, Chile, 1985; 123p. [Google Scholar]
- Øvstedal, D.O.; Smith, R.L. Lichens of Antarctica and South Georgia: A Guide to Their Identification and Ecology; Cambridge University Press: Cambridge, UK, 2001; 411p. [Google Scholar]
- So, J.E.; Halda, J.P.; Hong, S.G.; Hur, J.S.; Kim, J.H. The Revision of Lichen Flora Around Maxwell Bay, King George Island, Maritime Antarctic. J. Microbiol. 2023, 61, 159–173. [Google Scholar] [CrossRef]
- Sancho, L.G.; Aramburu, A.; Etayo, J.; Beltrán-Sanz, N. Floristic Similarities between the Lichen Flora of Both Sides of the Drake Passage: A Biogeographical Approach. J. Fungi 2023, 10, 9. [Google Scholar] [CrossRef]
- Ureña-Vacas, I.; González-Burgos, E.; Divakar, P.K.; Gómez-Serranillos, M.P. Lichen depsides and tridepsides: Progress in pharmacological approaches. J. Fungi 2023, 9, 116. [Google Scholar] [CrossRef]
- Ureña-Vacas, I.; González-Burgos, E.; Divakar, P.K.; Gómez-Serranillos, M.P. Lichen depsidones with biological interest. Planta Med. 2022, 88, 855–880. [Google Scholar] [CrossRef]
- Ureña-Vacas, I.; Burgos, E.G.; Divakar, P.K.; Gómez-Serranillos, M.P. Dibenzofurans from lichens—A pharmacological overview. Curr. Top. Med. Chem. 2021, 21, 2397–2408. [Google Scholar] [CrossRef]
- White, P.A.; Oliveira, R.C.; Oliveira, A.P.; Serafini, M.R.; Araújo, A.A.; Gelain, D.P.; Moreira, J.C.; Almeida, J.R.; Quintans, J.S.; Quintans-Junior, L.J.; et al. Antioxidant activity and mechanisms of action of natural compounds isolated from lichens: A systematic review. Molecules 2014, 19, 14496–14527. [Google Scholar] [CrossRef] [PubMed]
- Kosanic, M.; Rankovic, B.; Stanojkovic, T.; Vasiljevic, P.; Manojlovic, N. Biological activities and chemical composition of lichens from Serbia. EXCLI J. 2014, 13, 1226–1238. [Google Scholar]
- Kosanić, M.; Ranković, B.; Stanojković, T. Antioxidant, antimicrobial, and anticancer activity of 3 Umbilicaria species. J. Food Sci. 2012, 77, T20–T25. [Google Scholar] [CrossRef]
- Singh, S.M.; Singh, P.; Ravindra, R. Screening of antioxidant potential of Arctic lichens. Polar Biol. 2011, 34, 1775–1782. [Google Scholar] [CrossRef]
- Devkota, S.; Chaudhary, R.P.; Werth, S.; Scheidegger, C. Indigenous knowledge and use of lichens by the lichenophilic communities of the Nepal Himalaya. J. Ethnobiol. Ethnomed. 2017, 13, 15. [Google Scholar] [CrossRef] [PubMed]
- Shukla, P.; Upreti, D.K.; Tiwari, P. Assessment of dye yielding potential of Indian lichens. Indian J. Plant Sci. 2014, 3, 57–63. [Google Scholar]
- Backor, M.; Loppi, S. Interactions of lichens with heavy metals. Biol. Plant. 2009, 53, 214–222. [Google Scholar] [CrossRef]
- Moreno-Palacios, M.; Torres-Benítez, A.; Soto-Medina, E.; Sánchez, M.; Divakar, P.K.; Pereira, I.; Gómez-Serranillos, M.P. Corticolous Lichen Communities and Their Bioindication Potential in an Urban and Peri-Urban Ecosystem in the Central Region of Colombia. Land 2024, 13, 932. [Google Scholar] [CrossRef]
- Adenubi, O.T.; Famuyide, I.M.; McGaw, L.J.; Eloff, J.N. Lichens: An update on their ethnopharmacological uses and potential as sources of drug leads. J. Ethnopharmacol. 2022, 298, 115657. [Google Scholar] [CrossRef]
- Aoussar, N.; Laasri, F.E.; Bourhia, M.; Manoljovic, N.; Mhand, R.A.; Rhallabi, N.; Ullah, R.; Shahat, A.A.; Noman, O.M.; Nasr, F.A.; et al. Phytochemical Analysis, Cytotoxic, Antioxidant, and Antibacterial Activities of Lichens. Evid. Based Complement. Alternat Med. 2020, 2020, 8104538. [Google Scholar] [CrossRef]
- Ranković, B.; Kosanić, M.; Stanojković, T.; Vasiljević, P.; Manojlović, N. Biological activities of Toninia candida and Usnea barbata together with their norstictic acid and usnic acid constituents. Int. J. Mol. Sci. 2012, 13, 14707–14722. [Google Scholar] [CrossRef] [PubMed]
- Žugić, A.; Tadić, V.; Kundaković, T.; Savić, S. Chimical composition and biological activities of the extracts and secondary metabolites of lichens belonging to the genus Usnea, Parmeliaceae. Lek. Sirovine 2018, 38, 68–80. [Google Scholar] [CrossRef]
- Thadhani, V.M.; Karunaratne, V. Potential of Lichen Compounds as Antidiabetic Agents with Antioxidative Properties: A Review. Oxid. Med. Cell Longev. 2017, 2017, 2079697. [Google Scholar] [CrossRef]
- Paukov, A.; Teptina, A.; Morozova, M.; Kruglova, E.; Favero-Longo, S.E.; Bishop, C.; Rajakaruna, N. The Effects of Edaphic and Climatic Factors on Secondary Lichen Chemistry: A Case Study Using Saxicolous Lichens. Diversity 2019, 11, 94. [Google Scholar] [CrossRef]
- Gaya, E.; Fernández-Brime, S.; Vargas, R.; Lachlan, R.F.; Gueidan, C.; Ramírez-Mejía, M.; Lutzoni, F. The adaptive radiation of lichen-forming Teloschistaceae is associated with sunscreening pigments and a bark-to-rock substrate shift. Proc. Natl. Acad. Sci. USA 2015, 112, 11600–11605. [Google Scholar] [CrossRef]
- Salgado, F.; Caballero, J.; Vargas, R.; Cornejo, A.; Areche, C. Continental and Antarctic Lichens: Isolation, identification and molecular modeling of the depside tenuiorin from the Antarctic lichen Umbilicaria antarctica as tau protein inhibitor. Nat. Prod. Res. 2020, 34, 646–650. [Google Scholar] [CrossRef] [PubMed]
- Kumar, J.; Dhar, P.; Tayade, A.B.; Gupta, D.; Chaurasia, O.P.; Upreti, D.K.; Arora, R.; Srivastava, R.B. Antioxidant capacities, phenolic profile and cytotoxic effects of saxicolous lichens from trans-Himalayan cold desert of Ladakh. PLoS ONE 2014, 9, e98696. [Google Scholar] [CrossRef]
- Leavitt, S.D.; Fankhauser, J.D.; Leavitt, D.H.; Porter, L.D.; Johnson, L.A.; St Clair, L.L. Complex patterns of speciation in cosmopolitan “rock posy” lichens—Discovering and delimiting cryptic fungal species in the lichen-forming Rhizoplaca melanophthalma species-complex (Lecanoraceae, Ascomycota). Mol. Phylogenet. Evol. 2011, 59, 587–602. [Google Scholar] [CrossRef]
- Blainski, A.; Lopes, G.C.; De Mello, J.C.P. Application and Analysis of the Folin Ciocalteu Method for the Determination of the Total Phenolic Content from Limonium brasiliense L. Molecules 2013, 18, 6852–6865. [Google Scholar] [CrossRef]
- Alreshidi, M.; Abdulhakeem, M.A.; Badraoui, R.; Amato, G.; Caputo, L.; De Martino, L.; Nazzaro, F.; Fratianni, F.; Formisano, C.; De Feo, V.; et al. Pulicaria incisa (Lam.) DC. as a Potential Source of Antioxidant, Antibacterial, and Anti-Enzymatic Bioactive Molecules: Phytochemical Constituents, In Vitro and In Silico Pharmacological Analysis. Molecules 2023, 28, 7439. [Google Scholar] [CrossRef]
- Huang, D.; Ou, B.; Hampsch-Woodill, M.; Flanagan, J.A.; Prior, R.L. High-throughput Assay of Oxygen Radical Absorbance Capacity (ORAC) Using a Multichannel Liquid Handling System Coupled with a Microplate Fluorescence Reader in 96-Well Format. J. Agric. Food Chem. 2002, 50, 4437–4444. [Google Scholar] [CrossRef] [PubMed]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Liu, L.; Deseo, M.A.; Morris, C.; Winter, K.M.; Leach, D.N. Investigation of α-glucosidase inhibitory activity of wheat bran and germ. Food Chem. 2011, 126, 553–561. [Google Scholar] [CrossRef]
- Yusuf, E.; Wojdyło, A.; Oszmiański, J.; Nowicka, P. Nutritional, Phytochemical Characteristics and In Vitro Effect on α-Amylase, α-Glucosidase, Lipase, and Cholinesterase Activities of 12 Coloured Carrot Varieties. Foods 2021, 10, 808. [Google Scholar] [CrossRef]
- Haguet, Q.A.-O.X.; Le Joubioux, F.A.-O.; Chavanelle, V.; Groult, H.A.-O.; Schoonjans, N.; Langhi, C.A.-O.; Michaux, A.; Otero, Y.F.; Boisseau, N.A.-O.; Peltier, S.L.; et al. Inhibitory Potential of α-Amylase, α-Glucosidase, and Pancreatic Lipase by a Formulation of Five Plant Extracts: TOTUM-63. Int. J. Mol. Sci. 2023, 24, 4. [Google Scholar] [CrossRef]
- Ley-Martínez, J.S.; Ortega-Valencia, J.E.; García-Barradas, O.; Jiménez-Fernández, M.; Uribe-Lam, E.; Vencedor-Meraz, C.I.; Oliva-Ramírez, J. Active Compounds in Zingiber officinale as Possible Redox Inhibitors of 5-Lipoxygenase Using an In Silico Approach. Int. J. Mol. Sci. 2022, 23, 6093. [Google Scholar] [CrossRef]
- Swargiary, A.; Daimari, M. Identification of bioactive compounds by GC-MS and α-amylase and α-glucosidase inhibitory activity of Rauvolfia tetraphylla L. and Oroxylum indicum (L.) Kurz: An in vitro and in silico approach. Clin. Phytosci. 2020, 6, 75. [Google Scholar] [CrossRef]
- Torres-Benítez, A.; Ortega-Valencia, J.E.; Sanchez, M.; Divakar, P.K.; Simirgiotis, M.J.; Gómez-Serranillos, M.P. Metabolomic Profiling, Antioxidant and Enzyme Inhibition Properties and Molecular Docking Analysis of Antarctic Lichens. Molecules 2022, 27, 8086. [Google Scholar] [CrossRef] [PubMed]
- Torres-Benítez, A.; Ortega-Valencia, J.E.; Sánchez, M.; Hillmann-Eggers, M.; Gómez-Serranillos, M.P.; Vargas-Arana, G.; Simirgiotis, M.J. UHPLC-MS Chemical Fingerprinting and Antioxidant, Enzyme Inhibition, Anti-Inflammatory In Silico and Cytoprotective Activities of Cladonia chlorophaea and C. gracilis (Cladoniaceae) from Antarctica. Antioxidants 2022, 12, 10. [Google Scholar] [CrossRef]
- Søchting, U.; Sancho, L.G.; Arup, U. The lichen genera Gondwania and Transdrakea gen. nov. (Teloschistaceae)—Speciation in three southern continents. Plant Fungal Syst. 2023, 68, 304–331. [Google Scholar] [CrossRef]
- Poelt, J.; Pelleter, U. Zwergstrauchige Arten der Flechtengattung Caloplaca. Plant Syst. Evol. 1984, 148, 51–88. [Google Scholar] [CrossRef]
- Arup, U.; Søchting, U.; Frödén, P. A new taxonomy of Teloschistaceae. Nord. J. Bot. 2013, 31, 16–83. [Google Scholar] [CrossRef]
- Torres-Benítez, A.; Ortega-Valencia, J.E.; Jara-Pinuer, N.; Sanchez, M.; Vargas-Arana, G.; Gómez-Serranillos, M.P.; Simirgiotis, M.J. Antioxidant and antidiabetic activity and phytoconstituents of lichen extracts with temperate and polar distribution. Front. Pharmacol. 2023, 14, 1251856. [Google Scholar] [CrossRef] [PubMed]
- Torres-Benítez, A.; Ortega-Valencia, J.E.; Hillmann-Eggers, M.; Sanchez, M.; Pereira, I.; Gómez-Serranillos, M.P.; Simirgiotis, M.J. Chemical composition and antioxidant, enzyme inhibition and cytoprotective activity of two Antarctic lichens of the genus Psoroma (Pannariaceae). Nat. Prod. Res. 2024, 1–14. [Google Scholar] [CrossRef]
- Areche, C.; Parra, J.R.; Sepulveda, B.; García-Beltrán, O.; Simirgiotis, M.J. UHPLC-MS Metabolomic Fingerprinting, Antioxidant, and Enzyme Inhibition Activities of Himantormia lugubris from Antarctica. Metabolites 2022, 12, 560. [Google Scholar] [CrossRef]
- Sepúlveda, B.; Cornejo, A.; Bárcenas-Pérez, D.; Cheel, J.; Areche, C. Two New Fumarprotocetraric Acid Lactones Identified and Characterized by UHPLC-PDA/ESI/ORBITRAP/MS/MS from the Antarctic Lichen Cladonia metacorallifera. Separations 2022, 9, 41. [Google Scholar] [CrossRef]
- Torres-Benítez, A.; Rivera-Montalvo, M.; Sepúlveda, B.; Castro, O.N.; Nagles, E.; Simirgiotis, M.J.; García-Beltrán, O.; Areche, C. Metabolomic Analysis of Two Parmotrema Lichens: P. robustum (Degel.) Hale and P. andinum (Mull. Arg.) Hale Using UHPLC-ESI-OT-MS-MS. Molecules 2017, 22, 1861. [Google Scholar] [CrossRef]
- Albornoz, L.; Torres-Benítez, A.; Moreno-Palacios, M.; Simirgiotis, M.J.; Montoya-Serrano, S.A.; Sepulveda, B.; Stashenko, E.; García-Beltrán, O.; Areche, C. Phylogenetic Studies and Metabolite Analysis of Sticta Species from Colombia and Chile by Ultra-High Performance Liquid Chromatography-High Resolution-Q-Orbitrap-Mass Spectrometry. Metabolites 2022, 12, 156. [Google Scholar] [CrossRef]
- Kerboua, M.; Monia, A.A.; Samba, N.; Silva, L.; Raposo, C.; Díez, D.; Rodilla, J.M. Phytochemical Composition of Lichen Parmotrema hypoleucinum (J. Steiner) Hale from Algeria. Molecules 2022, 27, 5229. [Google Scholar] [CrossRef]
- Vega-Bello, M.J.; Moreno, M.L.; Estellés-Leal, R.; Hernández-Andreu, J.M.; Prieto-Ruiz, J.A. Usnea aurantiaco-atra (Jacq) Bory: Metabolites and Biological Activities. Molecules 2023, 28, 7317. [Google Scholar] [CrossRef]
- Studzińska-Sroka, E.; Majchrzak-Celińska, A.; Zalewski, P.; Szwajgier, D.; Baranowska-Wójcik, E.; Kaproń, B.; Plech, T.; Żarowski, M.; Cielecka-Piontek, J. Lichen-Derived Compounds and Extracts as Biologically Active Substances with Anticancer and Neuroprotective Properties. Pharmaceuticals 2021, 14, 1293. [Google Scholar] [CrossRef] [PubMed]
- Kello, M.; Goga, M.; Kotorova, K.; Sebova, D.; Frenak, R.; Tkacikova, L.; Mojzis, J. Screening Evaluation of Antiproliferative, Antimicrobial and Antioxidant Activity of Lichen Extracts and Secondary Metabolites In Vitro. Plants 2023, 12, 611. [Google Scholar] [CrossRef]
- Legouin, B.; Lohézic-Le Dévéhat, F.; Ferron, S.; Rouaud, I.; Le Pogam, P.; Cornevin, L.; Bertrand, M.; Boustie, J. Specialized Metabolites of the Lichen Vulpicida pinastri Act as Photoprotective Agents. Molecules 2017, 22, 1162. [Google Scholar] [CrossRef] [PubMed]
- Marante, F.J.T.; Castellano, A.G.; Rosas, F.E.; Aguiar, J.Q.; Barrera, J.J.B. Identification and quantitation of allelochemicals from the lichen Lethariella canariensis: Phytotoxicity and antioxidative activity. Chem. Ecol. 2003, 29, 2049–2071. [Google Scholar] [CrossRef] [PubMed]
- Pavlovic, V.; Stojanovic, I.; Jadranin, M.; Vajs, V.; Djordjević, I.; Smelcerovic, A.; Stojanovic, G. Effect of four lichen acids isolated from Hypogymnia physodes on viability of rat thymocytes. Food Chem. Toxicol. 2013, 51, 160–164. [Google Scholar] [CrossRef]
- Paudel, B.; Bhattarai, H.D.; Koh, H.Y.; Lee, S.G.; Han, S.J.; Lee, H.K.; Oh, H.; Shin, H.W.; Yim, H. Ramalin, a novel nontoxic antioxidant compound from the Antarctic lichen Ramalina terebrata. Phytomedicine 2011, 18, 1285–1290. [Google Scholar] [CrossRef]
- Do, T.-H.; Duong, T.-H.; Nguyen, H.T.; Nguyen, T.-H.; Sichaem, J.; Nguyen, C.H.; Nguyen, H.-H.; Long, N.P. Biological Activities of Lichen-Derived Monoaromatic Compounds. Molecules 2022, 27, 2871. [Google Scholar] [CrossRef]
- Studzińska-Sroka, E.; Bulicz, M.; Henkel, M.; Rosiak, N.; Paczkowska-Walendowska, M.; Szwajgier, D.; Baranowska-Wójcik, E.; Korybalska, K.; Cielecka-Piontek, J. Pleiotropic Potential of Evernia prunastri Extracts and Their Main Compounds Evernic Acid and Atranorin: In Vitro and In Silico Studies. Molecules 2023, 29, 233. [Google Scholar] [CrossRef]
- Tatipamula, V.B.; Annam, S.S.P.; Nguyen, H.T.; Polimati, H.; Yejella, R.P. Sekikaic acid modulates pancreatic β-cells in streptozotocin-induced type 2 diabetic rats by inhibiting digestive enzymes. Nat. Prod. Res. 2021, 35, 5420–5424. [Google Scholar] [CrossRef]
- Kumar, T.K.; Siva, B.; Kiranmai, B.; Alli, V.J.; Jadav, S.S.; Reddy, A.M.; Boustie, J.; Le Devehat, F.; Tiwari, A.K.; Suresh Babu, K. Salazinic Acid and Norlobaridone from the Lichen Hypotrachyna cirrhata: Antioxidant Activity, α-Glucosidase Inhibitory and Molecular Docking Studies. Molecules 2023, 28, 7840. [Google Scholar] [CrossRef]
- Thuy Le, H.; Vu, Y.T.; Duong, G.H.; Le, T.K.; Dang, M.K.; Pham, D.D.; Pham, N.K.; Sichaem, J.; Nguyen, N.H.; Duong, T.H. Bio-Guided Isolation of Alpha-Glucosidase Inhibitory Compounds from Vietnamese Lichen Roccella Montagnei. Chem. Biodivers. 2024, 21, e202400438. [Google Scholar] [CrossRef] [PubMed]
- Do, T.H.; Duong, T.H.; Vu, Y.T.; Tran, H.P.; Nguyen, T.T.; Sichaem, J.; Nguyen, N.H.; Nguyen, H.T.; Pham, D.D. Alpha-glucosidase inhibitory compounds from Vietnamese lichen Usnea baileyi: In vitro and in silico aspects. RSC Adv. 2024, 14, 32624–32636. [Google Scholar] [CrossRef]
- Kumar, T.K.; Siva, B.; Anand, A.; Anusha, K.; Mohabe, S.; Reddy, A.M.; Le Devehat, F.; Tiwari, A.K.; Boustie, J.; Babu, K.S. Comprehensive Lichenometabolomic Exploration of Ramalina conduplicans Vain Using UPLC-Q-ToF-MS/MS: An Identification of Free Radical Scavenging and Anti-Hyperglycemic Constituents. Molecules 2022, 27, 6720. [Google Scholar] [CrossRef] [PubMed]
- Duong, T.H.; Hang, T.X.; Pogam, P.L.; Tran, T.N.; Mac, D.H.; Dinh, M.H.; Sichaem, J. α-Glucosidase Inhibitory Depsidones from the Lichen Parmotrema tsavoense. Planta Med. 2020, 86, 776–781. [Google Scholar] [CrossRef] [PubMed]
- Hoang, L.T.; Phan, H.V.; Nguyen-Si, H.V.; Tran, T.N.; Vo, T.P.; Le, H.T.T.; Dao, N.V.; Huynh, T.M.; Mai, D.T.; Dong, P.S.; et al. Tinctoric acid A-B, two new hopan-type triterpenoids from the Vietnamese lichen, Parmotrema tinctorum (Despr. ex Nyl.) hale with α-glucosidase inhibitory activity. Nat. Prod. Res. 2024, 23, 1–8. [Google Scholar] [CrossRef]
- Rao, M.M.V.; Hariprasad, T.P.N. In silico analysis of a potential antidiabetic phytochemical erythrin against therapeutic targets of diabetes. Silico Pharmacol. 2021, 9, 5. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, R.; Zhang, K.; Chen, X.; Zhang, Y. Antioxidant, Hypoglycemic and Molecular Docking Studies of Methanolic Extract, Fractions and Isolated Compounds from Aerial Parts of Cymbopogon citratus (DC.) Stapf. Molecules 2022, 27, 2858. [Google Scholar] [CrossRef]
- Chigurupati, S.; Al-Murikhy, A.; Almahmoud, S.A.; Almoshari, Y.; Saber Ahmed, A.; Vijayabalan, S.; Ghazi Felemban, S.; Raj Palanimuthu, V. Molecular docking of phenolic compounds and screening of antioxidant and antidiabetic potential of Moringa oleifera ethanolic leaves extract from Qassim region, Saudi Arabia. Saudi J. Biol. Sci. 2022, 29, 854–859. [Google Scholar] [CrossRef]
- AlMousa, L.A.; AlFaris, N.A.; Alshammari, G.M.; ALTamimi, J.Z.; Alsyadi, M.M.; Alagal, R.I.; Abdo Yahya, M. Antioxidant and antimicrobial potential of two extracts from Capparis spinosa L. and Rumex nervosus and molecular docking investigation of selected major compounds. Saudi J. Biol. Sci. 2022, 29, 103346. [Google Scholar] [CrossRef]
- Ismail, N.Z.; Md Toha, Z.; Muhamad, M.; Nik Mohamed Kamal, N.N.S.; Mohamad Zain, N.N.; Arsad, H. Antioxidant Effects, Antiproliferative Effects, and Molecular Docking of Clinacanthus nutans Leaf Extracts. Molecules 2020, 25, 2067. [Google Scholar] [CrossRef]
- Raj, P.S.; Prathapan, A.; Sebastian, J.; Antony, A.K.; Riya, M.P.; Rani, M.R.; Biju, H.; Priya, S.; Raghu, K.G. Parmotrema tinctorum exhibits antioxidant, antiglycation and inhibitory activities against aldose reductase and carbohydrate digestive enzymes: An in vitro study. Nat. Prod. Res. 2014, 28, 1480–1484. [Google Scholar] [PubMed]
- Karagöz, Y.; Öztürk Karagöz, B. Lichens in Pharmacological Action: What Happened in the Last Decade? Eurasian J. Med. 2022, 54, 195–208. [Google Scholar] [CrossRef] [PubMed]
- Piñeiro, M.; Manrique, S.; Gómez, J.; Rodriguez, J.M.; Barrera, P.; Caballero, D.; Sosa, M.A.; Vargas-Arana, G.; Tapia, A.; Lima, B.; et al. Biological activities of Usnea lethariiformis lichen extracts and UHPLC-ESI-QTOF-MS analysis of their secondary metabolites. Front. Pharmacol. 2025, 15, 1508835. [Google Scholar] [CrossRef]
- Sivakumar, S.; Girija, A.S.S.; Priyadharsini, J.V. Evaluation of the inhibitory effect of caffeic acid and gallic acid on tetR and tetM efflux pumps mediating tetracycline resistance in Streptococcus sp., using computational approach. J. King Saud. Univ. Sci. 2022, 32, 904–909. [Google Scholar] [CrossRef]
- Yu, Q.; Fan, L.; Duan, Z. Five individual polyphenols as tyrosinase inhibitors: Inhibitory activity, synergistic effect, action mechanism, and molecular docking. Food Chem. 2019, 297, 124910. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Sahu, N.; Singh, A.P.; Singh, V.K.; Singh, S.C.; Upadhye, V.J.; Mathew, A.T.; Kumar, R.; Sinha, R.P. Exploration of novel lichen compounds as inhibitors of SARS-CoV-2 Mpro: Ligand-based design, molecular dinamics, and ADMET analyses. Appl. Biochem. Biotechnol. 2022, 194, 6386–6406. [Google Scholar] [CrossRef]
- Kopeć, W.; Telenius, J.; Khandelia, H. Molecular dynamics simulations of the interactions of medicinal plant extracts and drugs with lipid bilayer membranes. FEBS J. 2013, 280, 2785–2805. [Google Scholar] [CrossRef]
- Ureña-Vacas, I.; González-Burgos, E.; De Vita, S.; Divakar, P.K.; Bifulco, G.; Gómez-Serranillos, M.P. Phytochemical Characterization and Pharmacological Properties of Lichen Extracts from Cetrarioid Clade by Multivariate Analysis and Molecular Docking. Evid. Based Complement. Altern. Med. 2022, 2022, 5218248. [Google Scholar] [CrossRef]
- Olaokun, O.O.; Zubair, M.S. Antidiabetic Activity, Molecular Docking, and ADMET Properties of Compounds Isolated from Bioactive Ethyl Acetate Fraction of Ficus lutea Leaf Extract. Molecules 2023, 28, 7717. [Google Scholar] [CrossRef]
- Ahmed, S.; Ali, M.C.; Ruma, R.A.; Mahmud, S.; Paul, G.K.; Saleh, M.A.; Alshahrani, M.M.; Obaidullah, A.J.; Biswas, S.K.; Rahman, M.M.; et al. Molecular Docking and Dynamics Simulation of Natural Compounds from Betel Leaves (Piper betle L.) for Investigating the Potential Inhibition of Alpha-Amylase and Alpha-Glucosidase of Type 2 Diabetes. Molecules 2022, 27, 4526. [Google Scholar] [CrossRef]
- Herrera, A.C.; Gonzalez de Mejia, E. Feasibility of commercial breadmaking using chickpea as an ingredient: Functional properties and potential health benefits. J Food Sci. 2021, 86, 2208–2224. [Google Scholar] [CrossRef] [PubMed]
- Sudhan; Janakiraman; Ahmad, S.F.; Wani, A.; Ahmed, S.S.S.J. Phytochemicals from Piper betle (L.) as Putative Modulators of a Novel Network-Derived Drug Target for Coronary Artery Disease: An In Silico Study. Processes 2023, 11, 3064. [Google Scholar] [CrossRef]
- Hajji, H.; Tabti, K.; En-nahli, F.; Bouamrane, S.; Lakhlifi, T.; Aziz Ajana, M.; Bouachrine, M. In silico investigation on the beneficial effects of medicinal plants on Diabetes and Obesity: Molecular docking, molecular dynamic simulations, and ADMET studies. Biointerface Res. Appl. Chem. 2022, 11, 6933–6949. [Google Scholar]
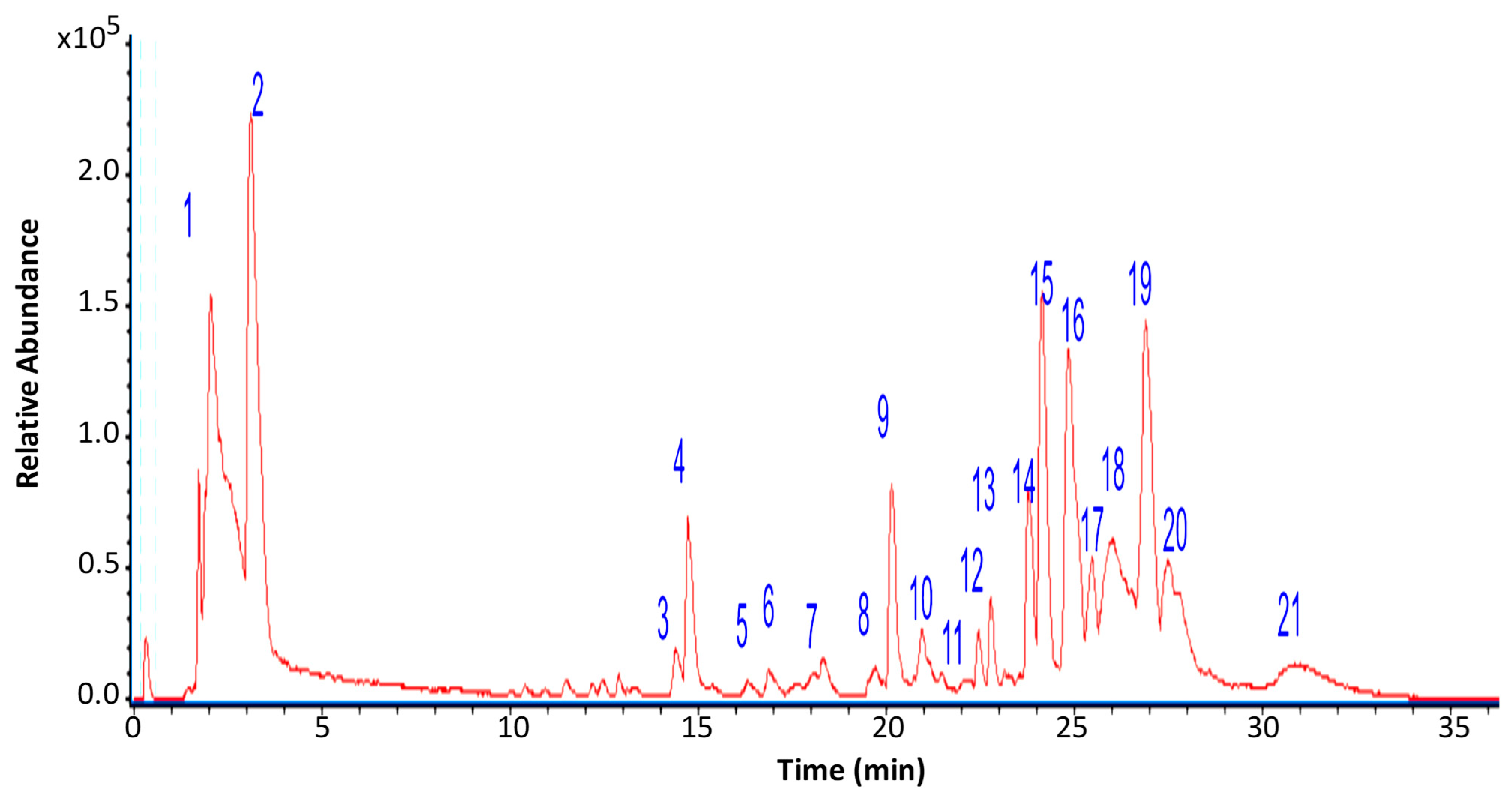
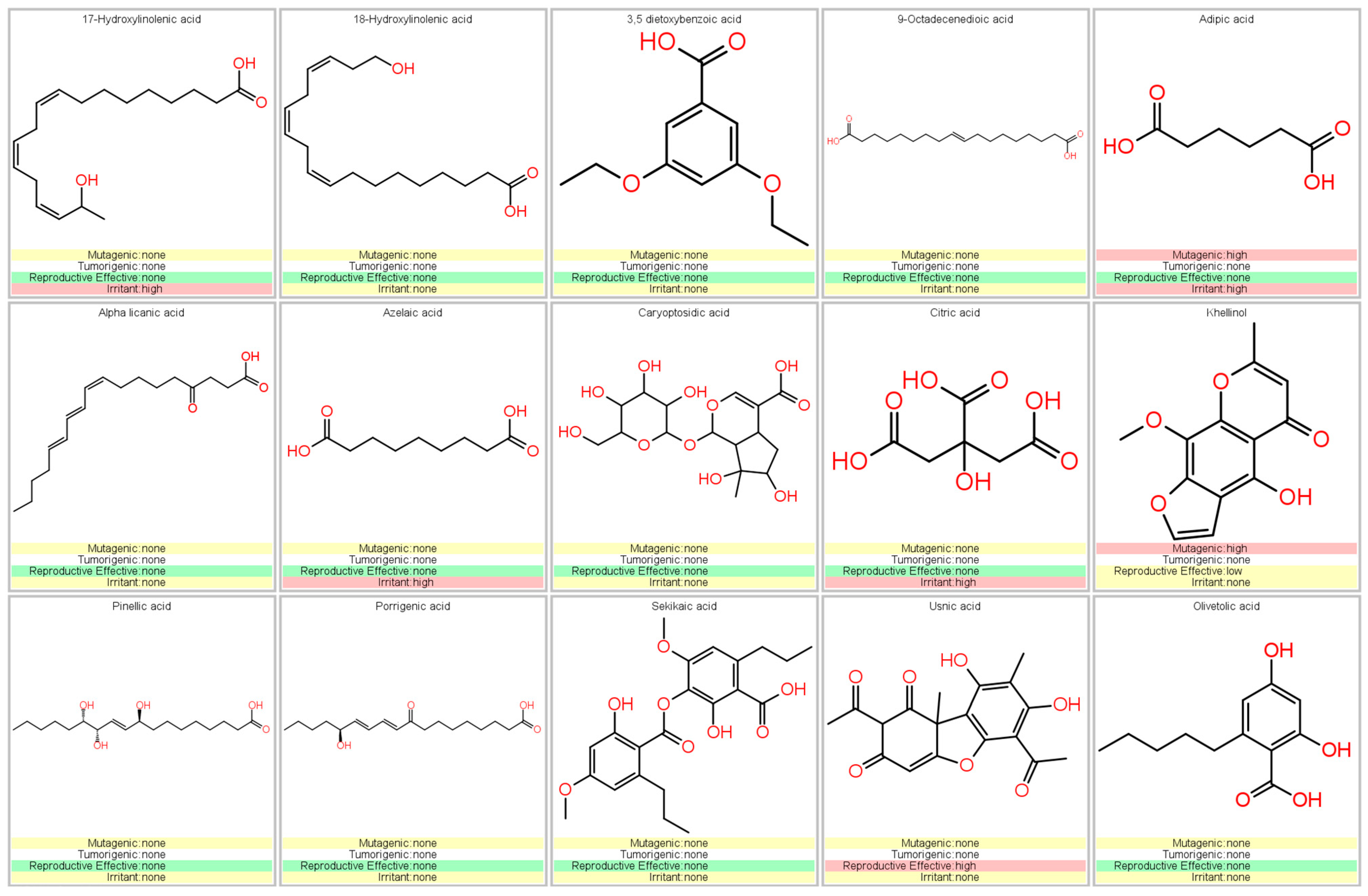

| Peak | Retention Time (min) | Tentative Identification | [M-H]− | Theoretical Mass (m/z) | Measured Mass (m/z) | Accuracy (ppm) | Metabolite Type | MS Ions (ppm) |
|---|---|---|---|---|---|---|---|---|
| 1 | 1.34 | Mannitol | C6H13O6 | 181.0712 | 181.0723 | 6.07 | C | 151.0598 |
| 2 | 3.21 | Citric acid | C6H7O7 | 191.0192 | 191.0184 | −4.19 | OA | 111.0074 |
| 3 | 14.46 | Azelaic acid | C9H15O4 | 187.0775 | 187.0769 | −3.21 | FA | - |
| 4 | 14.72 | Unknown | C25H27O | 179.0311 | 179.0321 | 5.58 | - | 165.0923 |
| 5 | 16.33 | Khellinol | C13H9O5 | 245.0489 | 245.0431 | −23.67 | C | 165.0914 |
| 6 | 17.56 | 9-Octadecenedioic acid | C18H31O4 | 311.2227 | 311.2228 | 0.32 | FA | - |
| 7 | 18.23 | Pinellic acid | C18H33O5 | 329.2333 | 329.2345 | 3.64 | FA | 251.0674, 215.1239 |
| 8 | 19.54 | Pinellic acid isomer | C18H33O5 | 329.2333 | 329.2345 | 3.64 | FA | 251.0674, 215.1239 |
| 9 | 20.22 | 2,4-Dihydroxy-6-pentylbenzoate(Olivetolic acid) | C12H15O4 | 223.0983 | 223.0981 | −0.89 | FA | 165.0923 |
| 10 | 21.01 | Unknown | C26H16O4 | 392.1075 | 392.1054 | −5.36 | - | 350.0945 |
| 11 | 22.03 | Lecanoric acid * | C16H13O7 | 317.0666 | 317.0653 | −4.10 | d | 167.034 |
| 12 | 22.50 | 3,5-Dietoxybenzoic acid | C11H13O4 | 209.0822 | 209.0823 | 0.48 | A | 163.0360 |
| 13 | 22.77 | Caryoptosidic acid | C16H23O11 | 391.1231 | 391.1245 | 3.58 | I | 311.2178, 263.1603 |
| 14 | 23.78 | Adipic acid | C16H27O4 | 283.1914 | 283.1869 | −15.89 | OA | 273.1797 |
| 15 | 24.37 | Sekikaic acid | C22H25O8 | 417.1553 | 417.1571 | 4.31 | d | 247.16944 |
| 16 | 25.19 | 17-Hydroxylinolenic acid | C18H29O3 | 293.2122 | 293.2136 | 4.77 | FA | 243.19740 |
| 17 | 25.31 | Porrigenic acid | C18H29O4 | 309.2070 | 309.2091 | 6.79 | FA | 291.19653 |
| 18 | 26.13 | Usnic acid * | C18H15O7 | 343.0823 | 343.0822 | −0.29 | DBF | 295.2291; 231.0647; 328.0570 |
| 19 | 26.93 | 18-Hydroxylinoleic acid | C18H31O3 | 295.2278 | 295.2279 | 0.34 | FA | 277.2133 |
| 20 | 27.7 | 18-Hydroxylinolenic acid | C18H29O3 | 293.2122 | 293.2071 | −17.39 | FA | 243.19740 |
| 21 | 31.2 | Alpha licanic acid | C18H27O3 | 291.1965 | 291.1907 | −19.92 | FA | 265.1444 |
| Assay | TPC (mg GAE/g) | FRAP (µmol Trolox/g) | ORAC (µmol Trolox/g) | DPPH IC50 (µg/mL) |
|---|---|---|---|---|
| G. regalis | 31.9 ± 0.016 * | 6.802 ± 0.062 * | 13.463 ± 0.15 * | 2246.149 ± 0.086 * |
| Gallic acid # | - | - | - | 2.24 ± 0.04 |
| Assay | α-Glucosidase IC50 (µg/mL) | α-Amylase IC50 (µg/mL) | Pancreatic Lipase IC50 (µg/mL) |
|---|---|---|---|
| G. regalis | 19.49 ± 0.027 * | 585.216 ± 0.026 * | 326.451 ± 0.066 * |
| Orlistat # | - | - | 2.149 ± 0.008 * |
| Acarbose # | 206.614 ± 0.008 * | 6.477 ± 0.003 * | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Torres-Benítez, A.; Ortega-Valencia, J.E.; Jara-Pinuer, N.; Ley-Martínez, J.S.; Velarde, S.H.; Pereira, I.; Sánchez, M.; Gómez-Serranillos, M.P.; Sasso, F.C.; Simirgiotis, M.; et al. Antioxidant and Antidiabetic Potential of the Antarctic Lichen Gondwania regalis Ethanolic Extract: Metabolomic Profile and In Vitro and In Silico Evaluation. Antioxidants 2025, 14, 298. https://doi.org/10.3390/antiox14030298
Torres-Benítez A, Ortega-Valencia JE, Jara-Pinuer N, Ley-Martínez JS, Velarde SH, Pereira I, Sánchez M, Gómez-Serranillos MP, Sasso FC, Simirgiotis M, et al. Antioxidant and Antidiabetic Potential of the Antarctic Lichen Gondwania regalis Ethanolic Extract: Metabolomic Profile and In Vitro and In Silico Evaluation. Antioxidants. 2025; 14(3):298. https://doi.org/10.3390/antiox14030298
Chicago/Turabian StyleTorres-Benítez, Alfredo, José Erick Ortega-Valencia, Nicolás Jara-Pinuer, Jaqueline Stephanie Ley-Martínez, Salvador Herrera Velarde, Iris Pereira, Marta Sánchez, María Pilar Gómez-Serranillos, Ferdinando Carlo Sasso, Mario Simirgiotis, and et al. 2025. "Antioxidant and Antidiabetic Potential of the Antarctic Lichen Gondwania regalis Ethanolic Extract: Metabolomic Profile and In Vitro and In Silico Evaluation" Antioxidants 14, no. 3: 298. https://doi.org/10.3390/antiox14030298
APA StyleTorres-Benítez, A., Ortega-Valencia, J. E., Jara-Pinuer, N., Ley-Martínez, J. S., Velarde, S. H., Pereira, I., Sánchez, M., Gómez-Serranillos, M. P., Sasso, F. C., Simirgiotis, M., & Caturano, A. (2025). Antioxidant and Antidiabetic Potential of the Antarctic Lichen Gondwania regalis Ethanolic Extract: Metabolomic Profile and In Vitro and In Silico Evaluation. Antioxidants, 14(3), 298. https://doi.org/10.3390/antiox14030298








