Identifying Baicalein as a Key Bioactive Compound in XueBiJing Targeting KEAP1: Implications for Antioxidant Effects
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. In Vivo Evaluation
2.2.1. Zebrafish Models
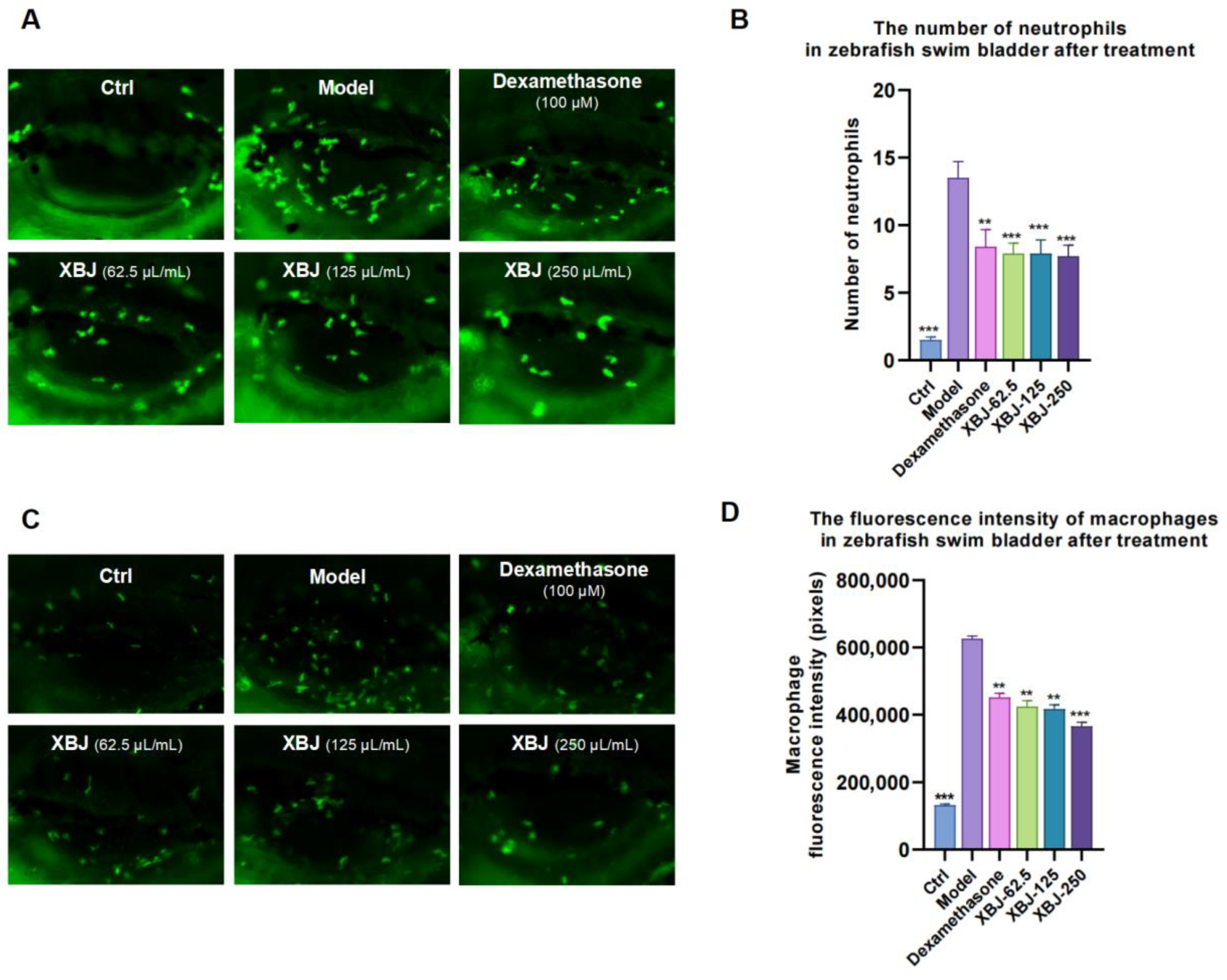
2.2.2. Poly (I:C)-Induced Viral Infection Model (Neutrophils)
2.2.3. Poly (I:C)-Induced Viral Infection Model (Macrophage)
2.3. Cell Culture
2.4. CCK-8 Assay and Drug Treatment
2.5. Transcriptomic and Enrichment Analysis
2.6. Network Construction and Analysis
2.7. Real-Time qPCR
2.8. Western Blotting
2.9. Molecular Docking, Dynamics Simulations, and Trajectory Data Analysis
2.10. Cellular Thermal Shift Assay—Western Blotting (CETSA-WB)
2.11. Surface Plasmon Resonance (SPR) Analysis
2.12. siRNA Transfection and ROS Measurement
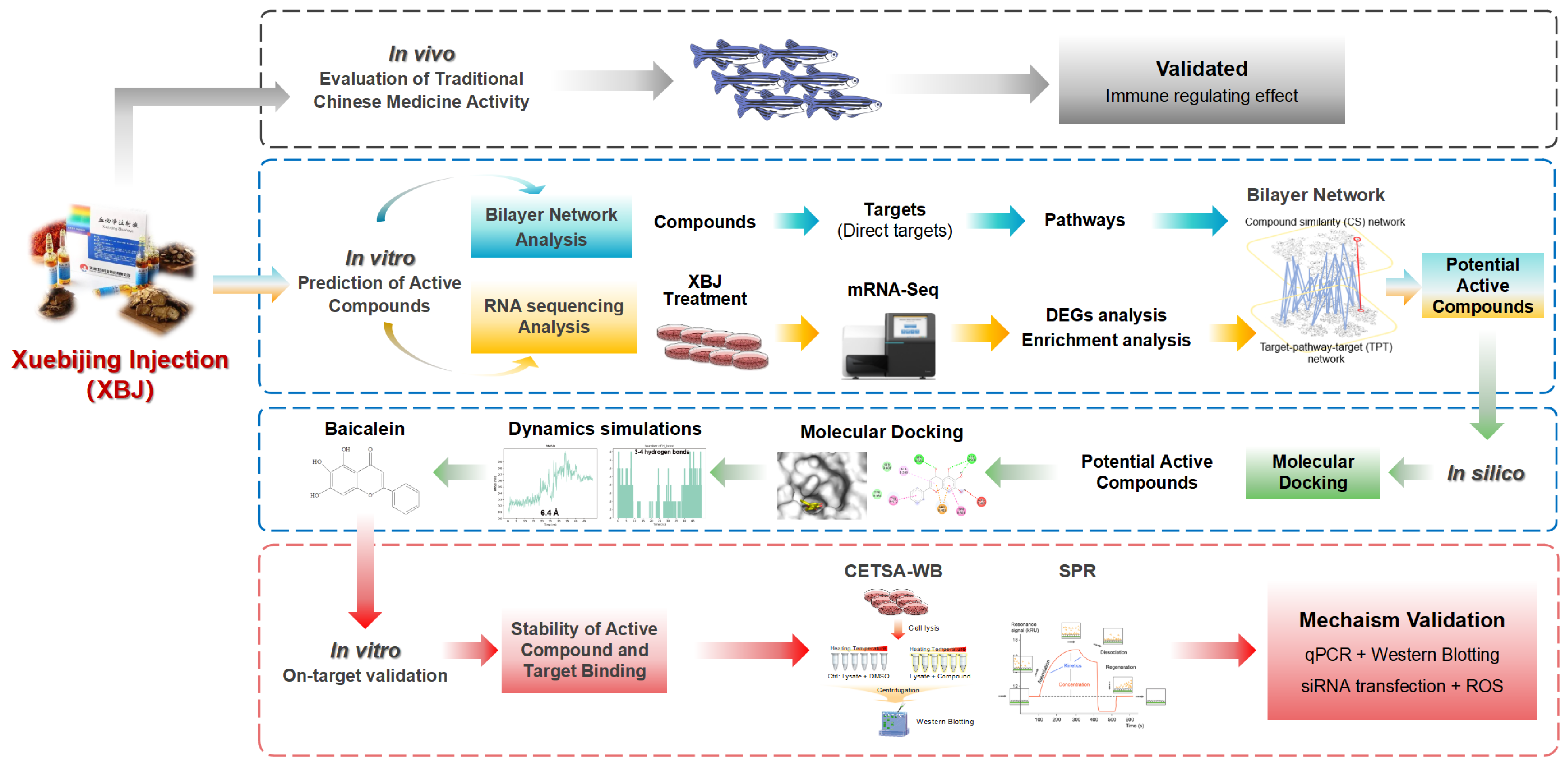
3. Results
3.1. Anti-Inflammatory Effect of XBJ on Poly (I:C)-Induced Infection
3.2. Genes Involved in NRF2-Regulated Oxidative Stress Response Were Inferred by XBJ
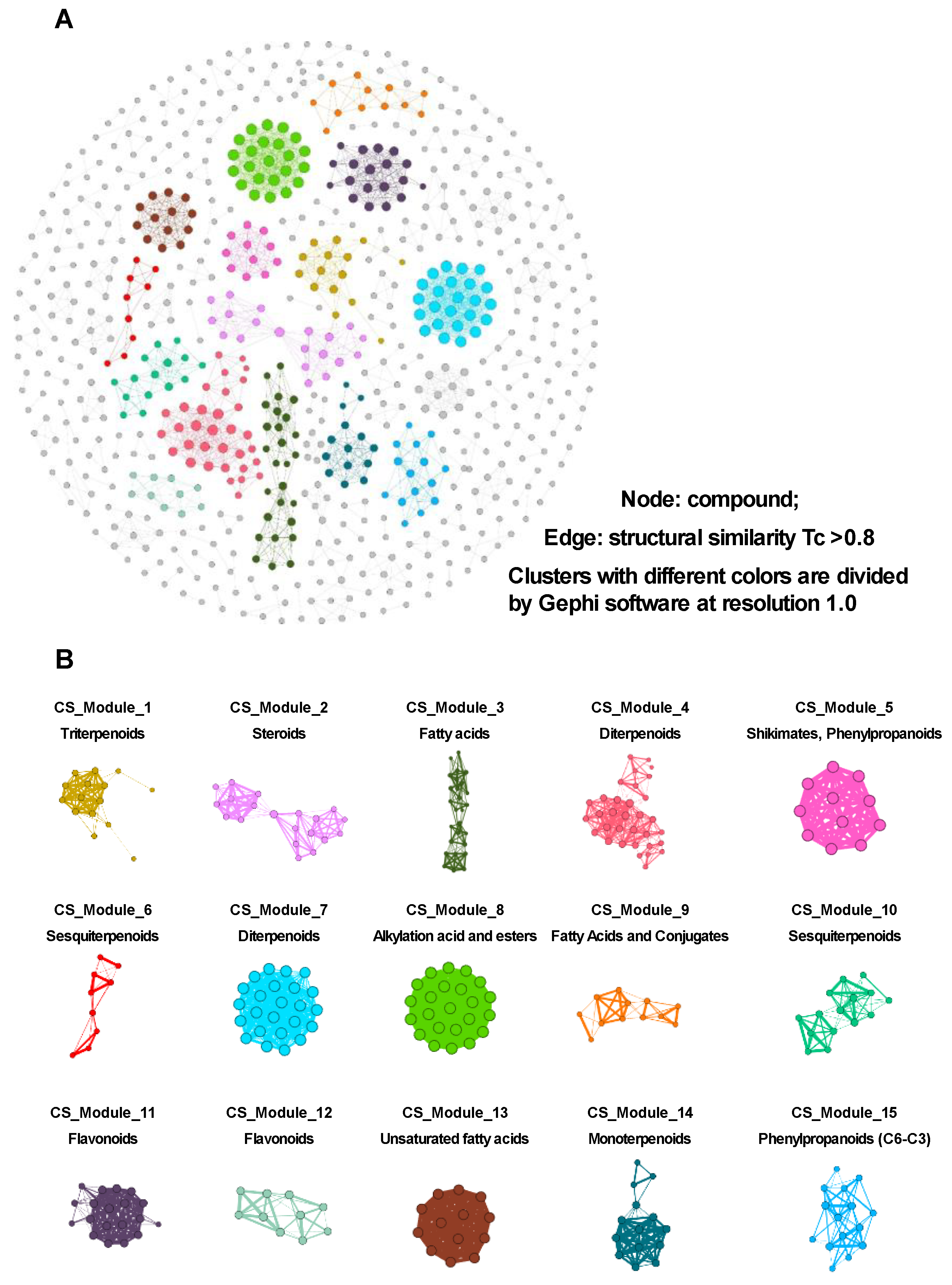
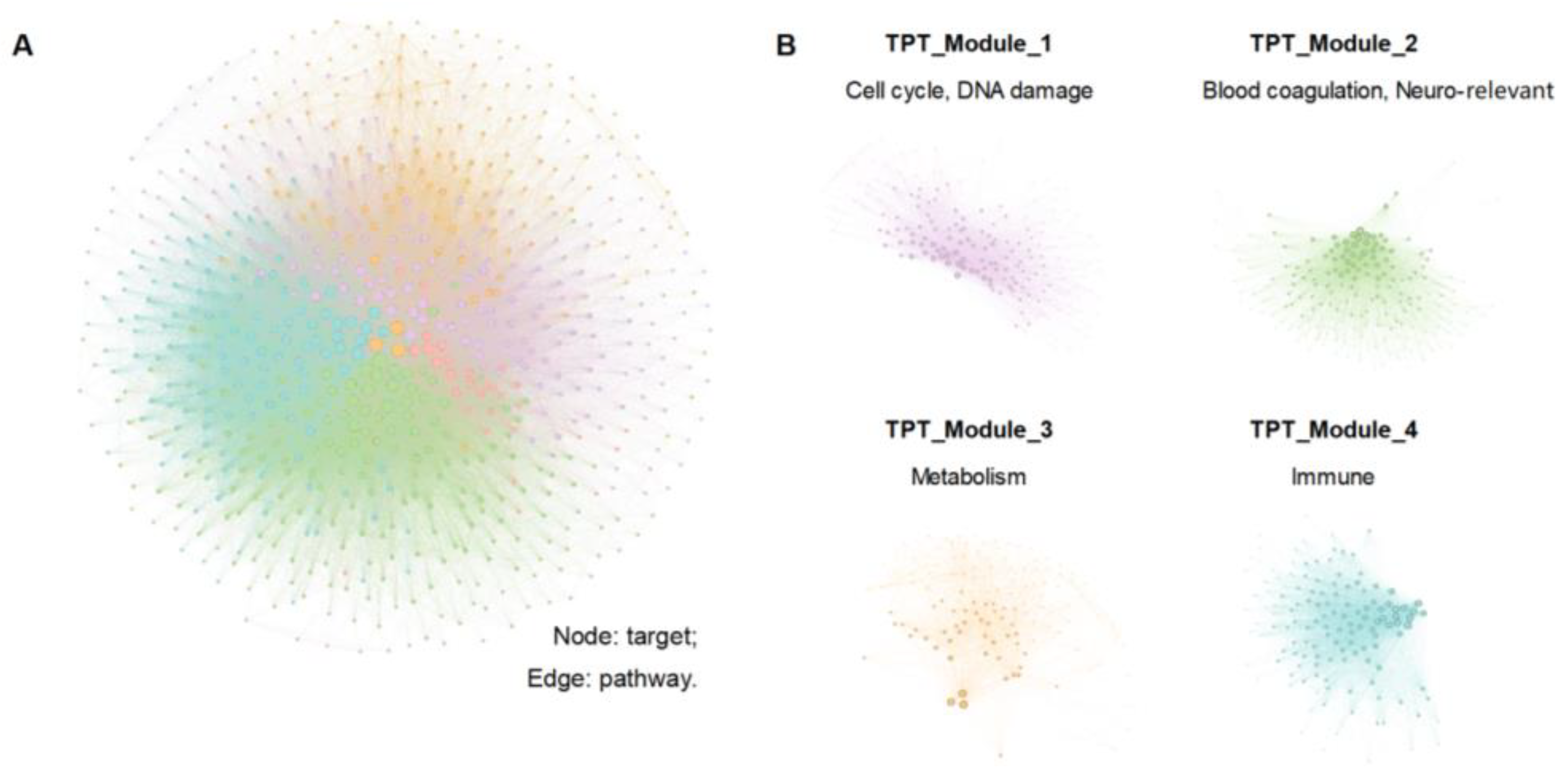
3.3. Conducting a Network Analysis to Identify the Potential Active Ingredients
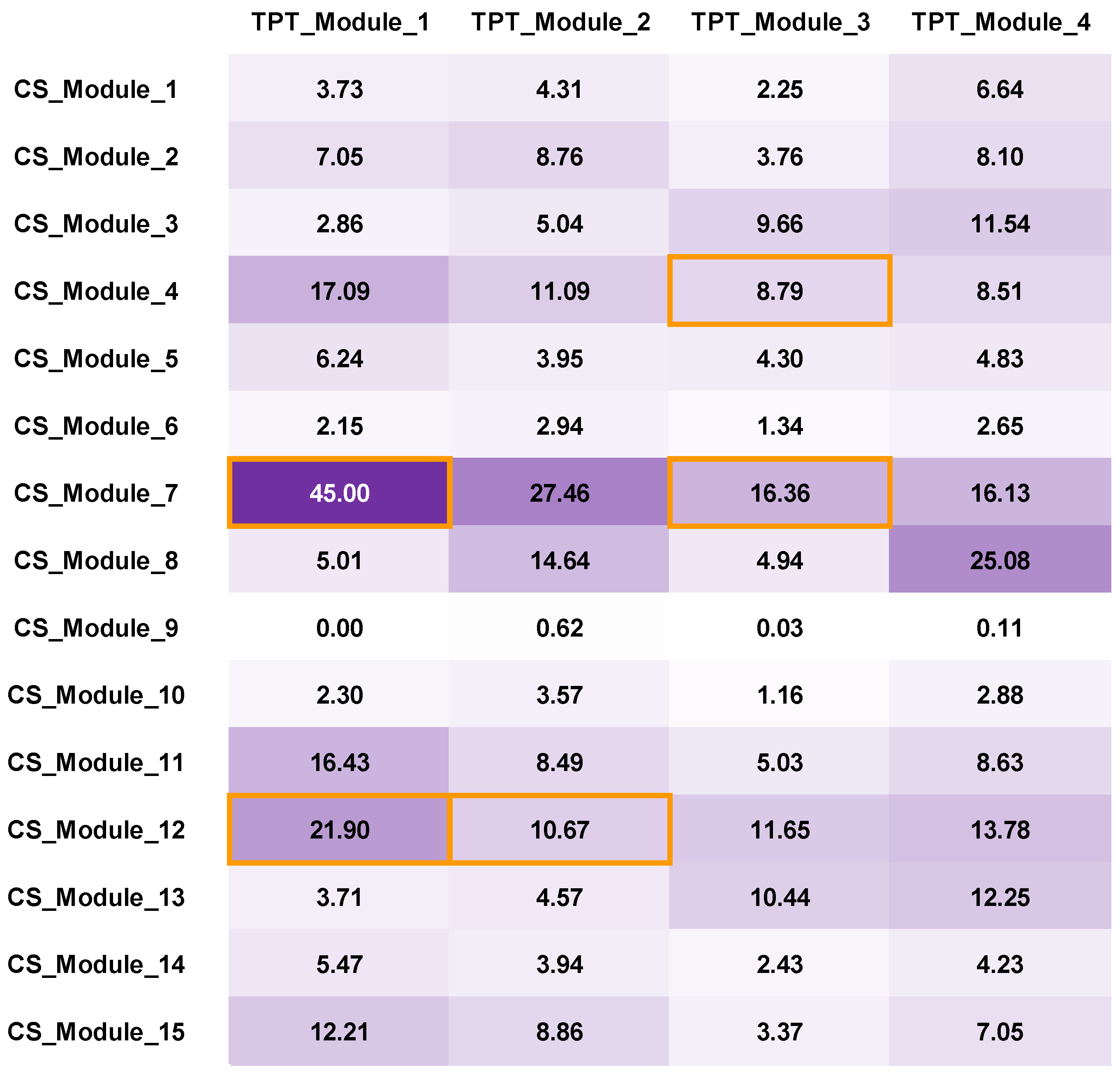
3.3.1. CS Network Construction and Network Analysis
3.3.2. TPT Network Construction and Network Analysis
3.3.3. Bilayer Network Analysis and Selected Important Compounds
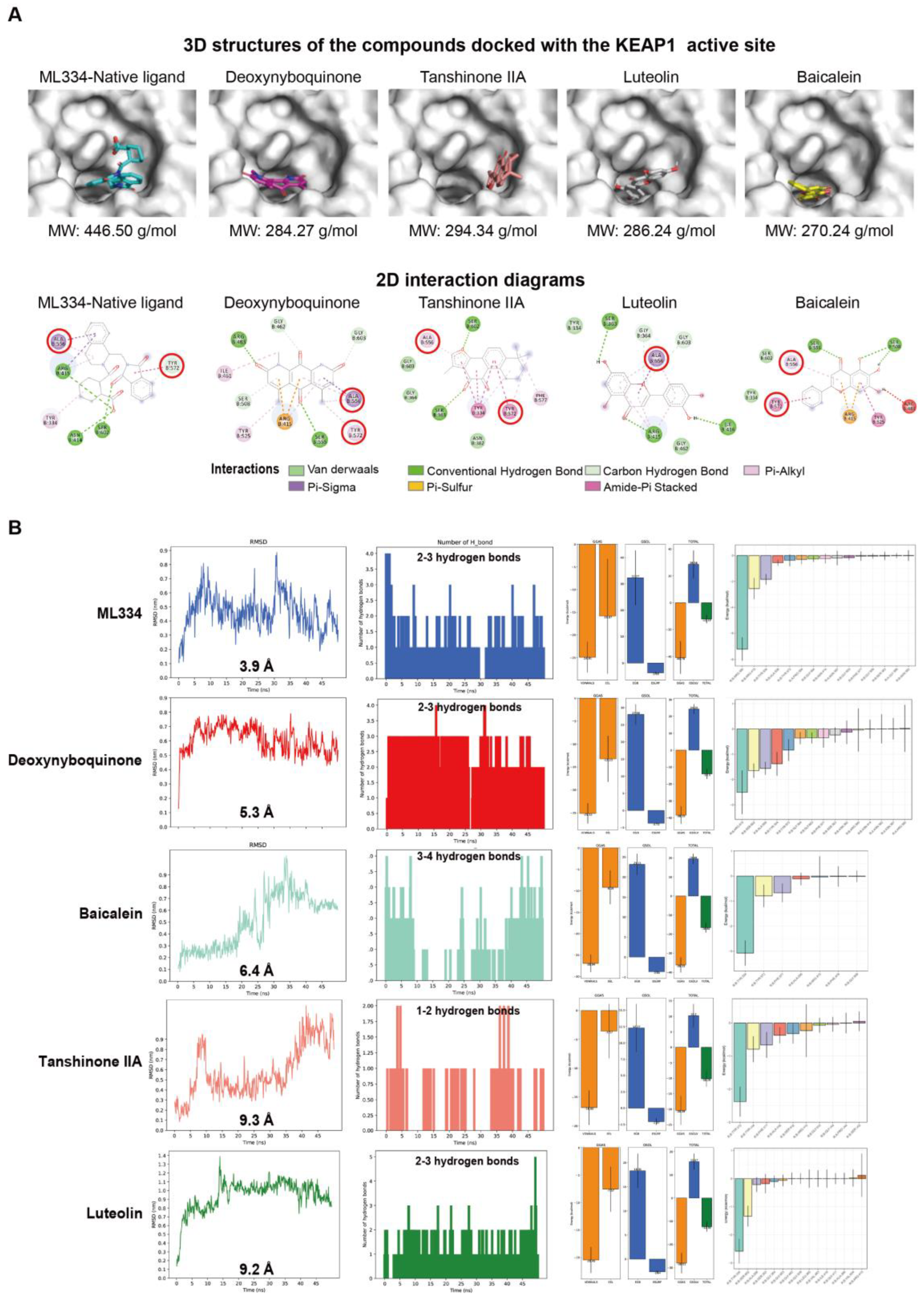
3.4. Molecular Docking and Dynamic Simulation Insights into the Binding Efficacy of Predicted Compounds with KEAP1
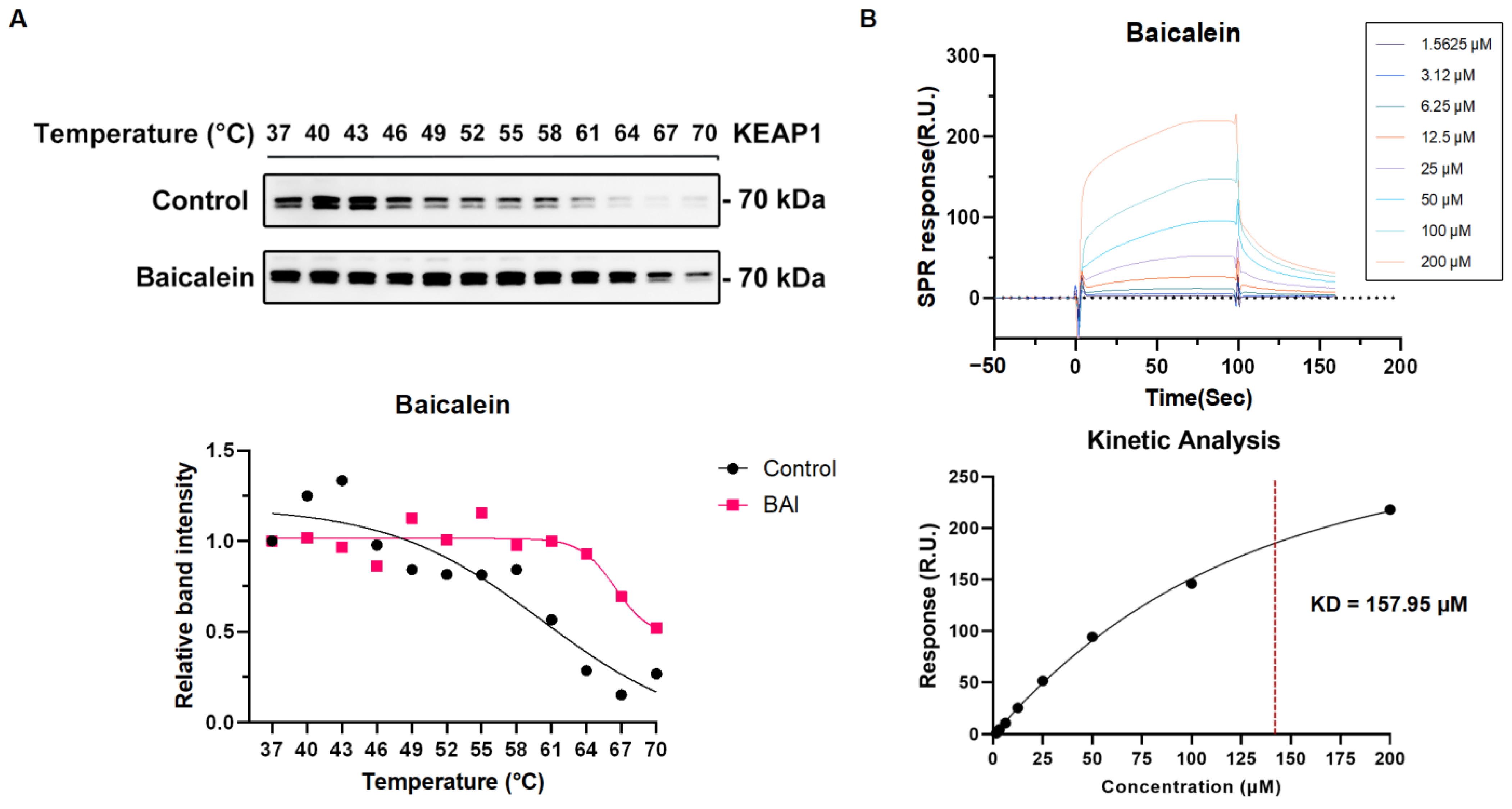
3.5. The Active Compound Baicalein of XBJ Directly Target KEAP1 Protein
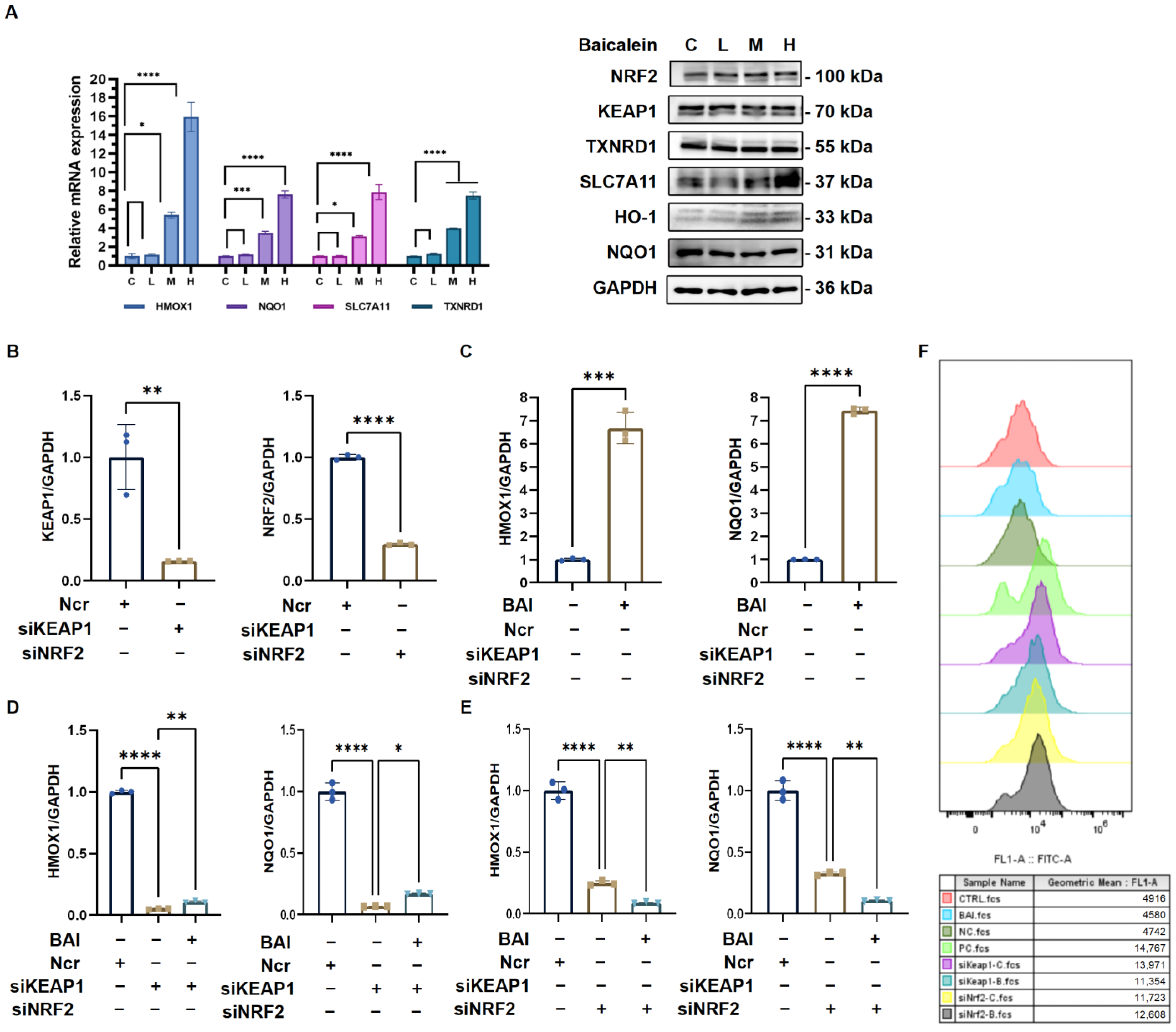
3.6. Baicalein Activates Antioxidative Response via KEAP1/NRF2 Pathway
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| XBJ | XueBiJing injection |
| ROS | Reactive oxygen species |
| KEAP1 | Kelch-like ECH associating protein 1 |
| NRF2 | Nuclear factor erythroid 2-related factor 2 |
| HO-1 | Heme-induced heme oxygenase-1 |
| DEGs | Differentially expressed genes |
| MPX | Myeloperoxidase |
| CS network | Compound similarity network |
| TPT network | Target–pathway–target network |
| MD | Molecular dynamics |
| RMSD | Root mean square deviation |
| SPR | Surface plasmon resonance |
| WB | Western blotting |
| CETSA | Cellular thermal shift assay |
| NCR | Non-coding RNA |
| siNRF2 | siRNA against Nrf2 |
| siKEAP1 | siRNA against KEAP1 |
| BAI | Baicalein |
References
- Guo, H.; Zheng, J.; Huang, G.; Xiang, Y.; Lang, C.; Li, B.; Huang, D.; Sun, Q.; Luo, Y.; Zhang, Y.; et al. Xuebijing injection in the treatment of COVID-19: A retrospective case-control study. Ann. Palliat. Med. 2020, 9, 3235–3248. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.; Ren, C.; Li, M.Z.; Liu, Y.H.; Yao, R.Q.; Yu, Y.; Yu, X.; Wang, J.L.; Wang, L.X.; Leng, Y.C.; et al. Pharmacologically significant constituents collectively responsible for anti-sepsis action of XueBiJing, a Chinese herb-based intravenous formulation. Acta Pharmacol. Sin. 2024, 45, 1077–1092. [Google Scholar] [CrossRef]
- Li, C.; Wang, P.; Zhang, L.; Li, M.; Lei, X.; Liu, S.; Feng, Z.; Yao, Y.; Chang, B.; Liu, B.; et al. Efficacy and safety of Xuebijing injection (a Chinese patent) for sepsis: A meta-analysis of randomized controlled trials. J. Ethnopharmacol. 2018, 224, 512–521. [Google Scholar] [CrossRef]
- Huang, H.; Ji, L.; Song, S.; Wang, J.; Wei, N.; Jiang, M.; Bai, G.; Luo, G. Identification of the major constituents in Xuebijing injection by HPLC-ESI-MS. Phytochem. Anal. 2011, 22, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.-J.; Yan, Q.; Ni, Y.-S.; Zhan, S.-F.; Yang, L.-L.; Zhuang, H.-F.; Liu, X.-H.; Jiang, Y. Examining the effector mechanisms of Xuebijing injection on COVID-19 based on network pharmacology. BioData Min. 2020, 13, 17. [Google Scholar] [CrossRef] [PubMed]
- Tianyu, Z.; Liying, G. Identifying the molecular targets and mechanisms of xuebijing injection for the treatment of COVID-19 via network pharmacology and molecular docking. Bioengineered 2021, 12, 2274–2287. [Google Scholar] [CrossRef]
- Elebeedy, D.; Elkhatib, W.F.; Kandeil, A.; Ghanem, A.; Kutkat, O.; Alnajjar, R.; Saleh, M.A.; Abd El Maksoud, A.I.; Badawy, I.; Al-Karmalawy, A.A. Anti-SARS-CoV-2 activities of tanshinone IIA, carnosic acid, rosmarinic acid, salvianolic acid, baicalein, and glycyrrhetinic acid between computational and in vitro insights. RSC Adv. 2021, 11, 29267–29286. [Google Scholar] [CrossRef]
- Shen, Y.-C.; Chiou, W.-F.; Chou, Y.-C.; Chen, C.-F. Mechanisms in mediating the anti-inflammatory effects of baicalin and baicalein in human leukocytes. Eur. J. Pharmacol. 2003, 465, 171–181. [Google Scholar] [CrossRef]
- Song, J.; Zhang, L.; Xu, Y.; Yang, D.; Zhang, L.; Yang, S.; Zhang, W.; Wang, J.; Tian, S.; Yang, S.; et al. The comprehensive study on the therapeutic effects of baicalein for the treatment of COVID-19 in vivo and in vitro. Biochem. Pharmacol. 2021, 183, 114302. [Google Scholar] [CrossRef] [PubMed]
- Zou, F.; Zou, J.; Du, Q.; Liu, L.; Li, D.; Zhao, L.; Tang, M.; Zuo, L.; Sun, Z. XueBiJing injection improves the symptoms of sepsis-induced acute lung injury by mitigating oxidative stress and ferroptosis. J. Ethnopharmacol. 2025, 337, 118732. [Google Scholar] [CrossRef] [PubMed]
- Guan, T.; Li, N.; Xu, X.; Xiong, D.; Wang, B.; Xiao, L.; Yang, W.; Chu, G.; Yusuf, A.; Zhang, J.; et al. Involvement of the Keap1-Nrf2-ARE pathway in the antioxidant activity of sinomenine. Arch. Biochem. Biophys. 2024, 753, 109928. [Google Scholar] [CrossRef]
- Khan, S.; Wang, T.; Cobo, E.R.; Liang, B.; Khan, M.A.; Xu, M.; Qu, W.; Gao, J.; Barkema, H.W.; Kastelic, J.P.; et al. Antioxidative Sirt1 and the Keap1-Nrf2 Signaling Pathway Impair Inflammation and Positively Regulate Autophagy in Murine Mammary Epithelial Cells or Mammary Glands Infected with Streptococcus uberis. Antioxid. 2024, 13, 171. [Google Scholar] [CrossRef]
- Liu, J.; Li, Y.; Wu, S.; Zhang, Z.; Li, D. Hyperbaric Oxygen Upregulates Mst1 to Activate Keap1/Nrf2/HO-1 Pathway Resisting Oxidative Stress in a Rat Model of Acute Myocardial Infarction. Mol. Biotechnol. 2024, 67, 284–293. [Google Scholar] [CrossRef]
- Park, J.S.; Kang, D.H.; Lee, D.H.; Bae, S.H. Fenofibrate activates Nrf2 through p62-dependent Keap1 degradation. Biochem. Biophys Res. Commun. 2015, 465, 542–547. [Google Scholar] [CrossRef] [PubMed]
- Zakkar, M.; Van der Heiden, K.; Luong, L.A.; Chaudhury, H.; Cuhlmann, S.; Hamdulay, S.S.; Krams, R.; Edirisinghe, I.; Rahman, I.; Carlsen, H. Activation of Nrf2 in endothelial cells protects arteries from exhibiting a proinflammatory state. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 1851–1857. [Google Scholar] [CrossRef] [PubMed]
- Dong, W.; Li, Q.; Lu, X.; Lan, J.; Qiu, Z.; Wang, X.; Wang, J.; Zheng, X.; Chen, S.; Zhang, C.; et al. Ceramide kinase-mediated C1P metabolism attenuates acute liver injury by inhibiting the interaction between KEAP1 and NRF2. Exp. Mol. Med. 2024, 56, 946–958. [Google Scholar] [CrossRef] [PubMed]
- Song, D.; Niu, Y.; Wang, J.; Xue, J.; Lv, X.; Yang, J.; Wang, B. Anti-allergic effects of xuebijing and potential role of heme oxygenase-1 against ovalbumin-induced murine allergic rhinitis model. Lin. Chuang Er Bi Yan Hou Tou Jing Wai Ke Za Zhi = J. Clin. Otorhinolaryngol. Head. Neck Surg. 2013, 27, 899–904. [Google Scholar]
- Espinoza, J.A.; León, M.A.; Céspedes, P.F.; Gómez, R.S.; Canedo-Marroquín, G.; Riquelme, S.A.; Salazar-Echegarai, F.J.; Blancou, P.; Simon, T.; Anegon, I.; et al. Heme Oxygenase-1 Modulates Human Respiratory Syncytial Virus Replication and Lung Pathogenesis during Infection. J. Immunol. 2017, 199, 212–223. [Google Scholar] [CrossRef] [PubMed]
- Batra, N.; De Souza, C.; Batra, J.; Raetz, A.G.; Yu, A.-M. The HMOX1 Pathway as a Promising Target for the Treatment and Prevention of SARS-CoV-2 of 2019 (COVID-19). Int. J. Mol. Sci. 2020, 21, 6412. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.-L.; Zhang, P.; Wang, H.-Q.; Li, Y.-F.; Hu, J.; Jiang, J.-D.; Li, Y.-H. heme oxygenase-1 agonist CoPP suppresses influenza virus replication through IRF3-mediated generation of IFN-α/β. Virology 2019, 528, 80–88. [Google Scholar] [CrossRef]
- Zhou, W.; Lai, X.; Wang, X.; Yao, X.; Wang, W.; Li, S. Network pharmacology to explore the anti-inflammatory mechanism of Xuebijing in the treatment of sepsis. Phytomedicine 2021, 85, 153543. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, Y.; Cao, C.; Zhu, Y.; Huang, W.; Yang, Y.; Qiu, H.; Liu, S.; Wang, D. Beneficial effect of Xuebijing against Pseudomonas aeruginosa infection in Caenorhabditis elegans. Front. Pharmacol. 2022, 13, 949608. [Google Scholar] [CrossRef] [PubMed]
- Ewels, P.A.; Peltzer, A.; Fillinger, S.; Patel, H.; Alneberg, J.; Wilm, A.; Garcia, M.U.; Di Tommaso, P.; Nahnsen, S. The nf-core framework for community-curated bioinformatics pipelines. Nat. Biotechnol. 2020, 38, 276–278. [Google Scholar] [CrossRef]
- Ge, S.X.; Son, E.W.; Yao, R. iDEP: An integrated web application for differential expression and pathway analysis of RNA-Seq data. BMC Bioinform. 2018, 19, 534. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.Y.; Peng, R.Q.; Wang, X.; Zuo, H.L.; Lyu, L.Y.; Yang, F.Q.; Hu, Y.J. A network pharmacology-based study on the quality control markers of antithrombotic herbs: Using Salvia miltiorrhiza—Ligusticum chuanxiong as an example. J. Ethnopharmacol. 2022, 292, 115197. [Google Scholar] [CrossRef]
- Liu, Z.; Shang, Q.; Zuo, H.; Li, H.; Fang, D.; Zhang, J.; Huang, H.D.; Granato, D.; Chen, J.; Chen, J. Cynomorium songaricum: UHPLC/ESI-LTQ-Orbitrap-MS analysis and mechanistic study on insulin sensitivity of a flavonoid-enriched fraction. Phytomedicine 2024, 132, 155862. [Google Scholar] [CrossRef]
- Kurien, B.T.; Scofield, R.H. Western blotting: An introduction. Methods Mol. Biol. 2015, 1312, 17–30. [Google Scholar] [CrossRef] [PubMed]
- Rose, P.W.; Prlic, A.; Altunkaya, A.; Bi, C.; Bradley, A.R.; Christie, C.H.; Costanzo, L.D.; Duarte, J.M.; Dutta, S.; Feng, Z.; et al. The RCSB protein data bank: Integrative view of protein, gene and 3D structural information. Nucleic Acids Res. 2017, 45, D271–D281. [Google Scholar] [CrossRef] [PubMed]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Jnoff, E.; Albrecht, C.; Barker, J.J.; Barker, O.; Beaumont, E.; Bromidge, S.; Brookfield, F.; Brooks, M.; Bubert, C.; Ceska, T.; et al. Binding mode and structure-activity relationships around direct inhibitors of the Nrf2-Keap1 complex. ChemMedChem 2014, 9, 699–705. [Google Scholar] [CrossRef]
- Linghu, K.-G.; Zhang, T.; Zhang, G.-T.; Lv, P.; Zhang, W.-J.; Zhao, G.-D.; Xiong, S.-H.; Ma, Q.-S.; Zhao, M.-M.; Chen, M.; et al. Small molecule deoxynyboquinone triggers alkylation and ubiquitination of Keap1 at Cys489 on Kelch domain for Nrf2 activation and inflammatory therapy. J. Pharm. Anal. 2023, 14, 401–415. [Google Scholar] [CrossRef]
- Abraham, M.J.; Murtola, T.; Schulz, R.; Páll, S.; Smith, J.C.; Hess, B.; Lindahl, E. GROMACS: High performance molecular simulations through multi-level parallelism from laptops to supercomputers. SoftwareX 2015, 1–2, 19–25. [Google Scholar] [CrossRef]
- Lindahl, M.A.; Andrey, A.; Cathrine, B.; Christian, B.; Eliane, B.; Mahesh, D.; Stefan, F.; Vytautas, G.; Gaurav, G.; Sergey, G.; et al. GROMACS 2023.2 Source Code, Version 2023.2; Zenodo: Garching, Germany, 2023. [Google Scholar] [CrossRef]
- Jo, S.; Kim, T.; Iyer, V.G.; Im, W. CHARMM-GUI: A web-based graphical user interface for CHARMM. J. Comput. Chem. 2008, 29, 1859–1865. [Google Scholar] [CrossRef]
- Lee, J.; Cheng, X.; Swails, J.M.; Yeom, M.S.; Eastman, P.K.; Lemkul, J.A.; Wei, S.; Buckner, J.; Jeong, J.C.; Qi, Y.; et al. CHARMM-GUI Input Generator for NAMD, GROMACS, AMBER, OpenMM, and CHARMM/OpenMM Simulations Using the CHARMM36 Additive Force Field. J. Chem. Theory Comput. 2016, 12, 405–413. [Google Scholar] [CrossRef] [PubMed]
- Leontyev, I.; Stuchebrukhov, A. Accounting for electronic polarization in non-polarizable force fields. Phys. Chem. Chem. Phys. 2011, 13, 2613–2626. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; MacKerell, A.D. CHARMM36 all-atom additive protein force field: Validation based on comparison to NMR data. J. Comput. Chem. 2013, 34, 2135–2145. [Google Scholar] [CrossRef]
- Parrinello, M.; Rahman, A. Polymorphic transitions in single crystals: A new molecular dynamics method. J. Appl. Phys. 1981, 52, 7182–7190. [Google Scholar] [CrossRef]
- Evans, D.J.; Holian, B.L. The Nose–Hoover thermostat. J. Chem. Phys. 1985, 83, 4069–4074. [Google Scholar] [CrossRef]
- Hess, B.; Bekker, H.; Berendsen, H.J.C.; Fraaije, J.G.E.M. LINCS: A linear constraint solver for molecular simulations. J. Comput. Chem. 1997, 18, 1463–1472. [Google Scholar] [CrossRef]
- Li, Y.; Xu, X.L.; Zhao, D.; Pan, L.N.; Huang, C.W.; Guo, L.J.; Lu, Q.; Wang, J. TLR3 ligand Poly IC Attenuates Reactive Astrogliosis and Improves Recovery of Rats after Focal Cerebral Ischemia. CNS Neurosci. Ther. 2015, 21, 905–913. [Google Scholar] [CrossRef] [PubMed]
- Hancock, L.A.; Hennessy, C.E.; Solomon, G.M.; Dobrinskikh, E.; Estrella, A.; Hara, N.; Hill, D.B.; Kissner, W.J.; Markovetz, M.R.; Grove Villalon, D.E.; et al. Muc5b overexpression causes mucociliary dysfunction and enhances lung fibrosis in mice. Nat. Commun. 2018, 9, 5363. [Google Scholar] [CrossRef]
- Seibold, M.A.; Wise, A.L.; Speer, M.C.; Steele, M.P.; Brown, K.K.; Loyd, J.E.; Fingerlin, T.E.; Zhang, W.; Gudmundsson, G.; Groshong, S.D.; et al. A common MUC5B promoter polymorphism and pulmonary fibrosis. N. Engl. J. Med. 2011, 364, 1503–1512. [Google Scholar] [CrossRef] [PubMed]
- Niemi, M.E.K.; Daly, M.J.; Ganna, A. The human genetic epidemiology of COVID-19. Nat. Rev. Genet. 2022, 23, 533–546. [Google Scholar] [CrossRef]
- Silvin, A.; Chapuis, N.; Dunsmore, G.; Goubet, A.G.; Dubuisson, A.; Derosa, L.; Almire, C.; Henon, C.; Kosmider, O.; Droin, N.; et al. Elevated Calprotectin and Abnormal Myeloid Cell Subsets Discriminate Severe from Mild COVID-19. Cell 2020, 182, 1401–1418.e1418. [Google Scholar] [CrossRef]
- Battino, M.; Giampieri, F.; Pistollato, F.; Sureda, A.; De Oliveira, M.R.; Pittala, V.; Fallarino, F.; Nabavi, S.F.; Atanasov, A.G.; Nabavi, S.M. Nrf2 as regulator of innate immunity: A molecular Swiss army knife! Biotechnol. Adv. 2018, 36, 358–370. [Google Scholar] [CrossRef]
- Kim, J.; Surh, Y.J. The Role of Nrf2 in Cellular Innate Immune Response to Inflammatory Injury. Toxicol. Res. 2009, 25, 159–173. [Google Scholar] [CrossRef] [PubMed]
- Olagnier, D.; Farahani, E.; Thyrsted, J.; Blay-Cadanet, J.; Herengt, A.; Idorn, M.; Hait, A.; Hernaez, B.; Knudsen, A.; Iversen, M.B.; et al. SARS-CoV2-mediated suppression of NRF2-signaling reveals potent antiviral and anti-inflammatory activity of 4-octyl-itaconate and dimethyl fumarate. Nat. Commun. 2020, 11, 4938. [Google Scholar] [CrossRef]
- Zuo, H.; Zhang, Q.; Su, S.; Chen, Q.; Yang, F.; Hu, Y. A network pharmacology-based approach to analyse potential targets of traditional herbal formulas: An example of Yu Ping Feng decoction. Sci. Rep. 2018, 8, 11418. [Google Scholar] [CrossRef]
- Zuo, H.L.; Zhang, Q.R.; Chen, C.; Yang, F.Q.; Yu, H.; Hu, Y.J. Molecular evidence of herbal formula: A network-based analysis of Si-Wu decoction. Phytochem. Anal. 2021, 32, 198–205. [Google Scholar] [CrossRef] [PubMed]
- Zuo, H.L.; Linghu, K.G.; Wang, Y.L.; Liu, K.M.; Gao, Y.; Yu, H.; Yang, F.Q.; Hu, Y.J. Interactions of antithrombotic herbal medicines with Western cardiovascular drugs. Pharmacol. Res. 2020, 159, 104963. [Google Scholar] [CrossRef] [PubMed]
- Ge, W.; Zhao, K.; Wang, X.; Li, H.; Yu, M.; He, M.; Xue, X.; Zhu, Y.; Zhang, C.; Cheng, Y.; et al. iASPP Is an Antioxidative Factor and Drives Cancer Growth and Drug Resistance by Competing with Nrf2 for Keap1 Binding. Cancer Cell 2017, 32, 561–573.e566. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Pi, J.; Zhang, Q. Signal amplification in the KEAP1-NRF2-ARE antioxidant response pathway. Redox Biol. 2022, 54, 102389. [Google Scholar] [CrossRef]
- Laforge, M.; Elbim, C.; Frère, C.; Hémadi, M.; Massaad, C.; Nuss, P.; Benoliel, J.J.; Becker, C. Tissue damage from neutrophil-induced oxidative stress in COVID-19. Nat. Rev. Immunol. 2020, 20, 515–516. [Google Scholar] [CrossRef] [PubMed]
- Yachie, A. Heme Oxygenase-1 Deficiency and Oxidative Stress: A Review of 9 Independent Human Cases and Animal Models. Int. J. Mol. Sci. 2021, 22, 1514. [Google Scholar] [CrossRef]
- Toro, A.; Ruiz, M.S.; Lage-Vickers, S.; Sanchis, P.; Sabater, A.; Pascual, G.; Seniuk, R.; Cascardo, F.; Ledesma-Bazan, S.; Vilicich, F.; et al. A Journey into the Clinical Relevance of Heme Oxygenase 1 for Human Inflammatory Disease and Viral Clearance: Why Does It Matter on the COVID-19 Scene? Antioxidants 2022, 11, 276. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, J.; Wang, L.; Aliyari, S.; Cheng, G. SARS-CoV-2 virus NSP14 Impairs NRF2/HMOX1 activation by targeting Sirtuin 1. Cell Mol. Immunol. 2022, 19, 872–882. [Google Scholar] [CrossRef] [PubMed]
- Itoh, K.; Wakabayashi, N.; Katoh, Y.; Ishii, T.; Igarashi, K.; Engel, J.D.; Yamamoto, M. Keap1 represses nuclear activation of antioxidant responsive elements by Nrf2 through binding to the amino-terminal Neh2 domain. Genes. Dev. 1999, 13, 76–86. [Google Scholar] [CrossRef] [PubMed]
- Xue, F.; Cooley, L. Kelch encodes a component of intercellular bridges in Drosophila egg chambers. Cell 1993, 72, 681–693. [Google Scholar] [CrossRef]
- Wilson, A.J.; Kerns, J.K.; Callahan, J.F.; Moody, C.J. Keap Calm, and Carry on Covalently. J. Med. Chem. 2013, 56, 7463–7476. [Google Scholar] [CrossRef]
- Liu, M.W.; Liu, R.; Wu, H.Y.; Zhang, W.; Xia, J.; Dong, M.N.; Yu, W.; Wang, Q.; Xie, F.M.; Wang, R.; et al. Protective effect of Xuebijing injection on D-galactosamine- and lipopolysaccharide-induced acute liver injury in rats through the regulation of p38 MAPK, MMP-9 and HO-1 expression by increasing TIPE2 expression. Int. J. Mol. Med. 2016, 38, 1419–1432. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Buttar, H.S.; Kaur, G.; Tuli, H.S. Baicalein: Promising therapeutic applications with special reference to published patents. Pharm. Pat. Anal. 2022, 11, 23–32. [Google Scholar] [CrossRef]
- Moghaddam, E.; Teoh, B.T.; Sam, S.S.; Lani, R.; Hassandarvish, P.; Chik, Z.; Yueh, A.; Abubakar, S.; Zandi, K. Baicalin, a metabolite of baicalein with antiviral activity against dengue virus. Sci. Rep. 2014, 4, 5452. [Google Scholar] [CrossRef]
- Qin, S.; Deng, F.; Wu, W.; Jiang, L.; Yamashiro, T.; Yano, S.; Hou, D.X. Baicalein modulates Nrf2/Keap1 system in both Keap1-dependent and Keap1-independent mechanisms. Arch. Biochem. Biophys. 2014, 559, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Jo, S.; Kim, S.; Shin, D.H.; Kim, M.S. Inhibition of SARS-CoV 3CL protease by flavonoids. J. Enzym. Inhib. Med. Chem. 2020, 35, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Wan, L.; Li, Y.; Liao, W.; Lei, L.; Zhao, M.; Zeng, J.; Zhao, Z.; Tang, J. Synergistic inhibition effects of andrographolide and baicalin on coronavirus mechanisms by downregulation of ACE2 protein level. Sci. Rep. 2024, 14, 4287. [Google Scholar] [CrossRef] [PubMed]
- Liao, H.; Ye, J.; Gao, Y.; Lian, C.; Liu, L.; Xu, X.; Feng, Y.; Yang, Y.; Yang, Y.; Shen, Q.; et al. Baicalein self-microemulsion based on drug-phospholipid complex for the alleviation of cytokine storm. Bioeng. Transl. Med. 2023, 8, e10357. [Google Scholar] [CrossRef]
- Gao, Y.; Snyder, S.A.; Smith, J.N.; Chen, Y.C. Anticancer properties of baicalein: A review. Med. Chem. Res. 2016, 25, 1515–1523. [Google Scholar] [CrossRef] [PubMed]
- Fang, Z.Y.; Zhang, M.; Liu, J.N.; Zhao, X.; Zhang, Y.Q.; Fang, L. Tanshinone IIA: A Review of its Anticancer Effects. Front. Pharmacol. 2020, 11, 611087. [Google Scholar] [CrossRef]
- Ru, J.; Li, P.; Wang, J.; Zhou, W.; Li, B.; Huang, C.; Li, P.; Guo, Z.; Tao, W.; Yang, Y.; et al. TCMSP: A database of systems pharmacology for drug discovery from herbal medicines. J. Cheminform 2014, 6, 13. [Google Scholar] [CrossRef]
- Fang, S.; Dong, L.; Liu, L.; Guo, J.; Zhao, L.; Zhang, J.; Bu, D.; Liu, X.; Huo, P.; Cao, W.; et al. HERB: A high-throughput experiment- and reference-guided database of traditional Chinese medicine. Nucleic Acids Res. 2021, 49, D1197–D1206. [Google Scholar] [CrossRef]
- Nickel, J.; Gohlke, B.O.; Erehman, J.; Banerjee, P.; Rong, W.W.; Goede, A.; Dunkel, M.; Preissner, R. SuperPred: Update on drug classification and target prediction. Nucleic Acids Res. 2014, 42, W26–W31. [Google Scholar] [CrossRef] [PubMed]
- Keiser, M.J.; Roth, B.L.; Armbruster, B.N.; Ernsberger, P.; Irwin, J.J.; Shoichet, B.K. Relating protein pharmacology by ligand chemistry. Nat. Biotechnol. 2007, 25, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Zuo, H.L. An Analytical Method of Pathway-Based Target Networks and Its Application in Modern Research of Herbal Formulae. Ph.D. Thesis, University of Macau, Macau, China, 2020. [Google Scholar]
- Hassan, M.; Elzallat, M.; Aboushousha, T.; Elhusseny, Y.; El-Ahwany, E. MicroRNA-122 mimic/microRNA-221 inhibitor combination as a novel therapeutic tool against hepatocellular carcinoma. Noncoding RNA Res. 2023, 8, 126–134. [Google Scholar] [CrossRef]
- Subelj, L.; Bajec, M. Unfolding communities in large complex networks: Combining defensive and offensive label propagation for core extraction. Phys. Rev. E Stat. Nonlin Soft Matter Phys. 2011, 83, 036103. [Google Scholar] [CrossRef] [PubMed]


| Forward Primer (5′–3′) | Reverse Primer (5′–3′) | |
|---|---|---|
| GAPDH | ACCCACTCCTCCACCTTTGAC | TGTTGCTGTAGCCAAATTCGTT |
| HMOX1 | AAGACTGCGTTCCTGCTCAAC | AAAGCCCTACAGCAACTGTCG |
| SLC7A11 | TCTCCAAAGGAGGTTACCTGC | AGACTCCCCTCAGTAAAGTGAC |
| NQO1 | GAAGAGCACTGATCGTACTGGC | GGATACTGAAAGTTCGCAGGG |
| TXNRD1 | ATATGGCAAGAAGGTGATGGTCC | GGGCTTGTCCTAACAAAGCTG |
| NRF2 | TCAGCGACGGAAAGAGTATGA | CCACTGGTTTCTGACTGGATGT |
| KEAP1 | CTGGAGGATCATACCAAGCAGG | GGATACCCTCAATGGACACCAC |
| Target Protein | Compounds | PubChem CID | Molecular Weight (g/mol) | Structure | Source | Binding Energy | Ligand–Protein RMSD (Å) | MMPB (GB)SA ΔGTotal (kcal/mol) | Number of Hydrogen Bonds |
|---|---|---|---|---|---|---|---|---|---|
| KEAP1 (PDB ID: 4L7B) | ML 334 | 56840728 | 446.5 | 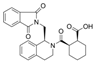 | Native ligand | −10.8 | 3.9 | −12.19 | 2–3 |
| Deoxynyboquinone | 295934 | 284.27 | 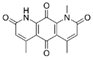 | NRF2 activator | −8.7 | 5.3 | −14.1 | 2–3 | |
| Tanshinone IIA | 164676 | 294.3 | 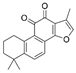 | Danshen | −8.6 | 9.3 | −10.19 | 1–2 | |
| Luteolin | 5280445 | 286.24 | 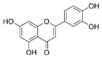 | Honghua | −8.6 | 9.2 | −12.35 | 2–3 | |
| Baicalein | 5281605 | 270.24 |  | Honghua/Chishao | −8.2 | 6.4 | −16.65 | 3–4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, T.-S.; Cai, X.-X.; Wang, Y.-B.; Xu, J.-T.; Xiao, J.-H.; Huang, H.-Y.; Li, S.-F.; Liu, K.-M.; Chen, J.-H.; Li, L.-P.; et al. Identifying Baicalein as a Key Bioactive Compound in XueBiJing Targeting KEAP1: Implications for Antioxidant Effects. Antioxidants 2025, 14, 248. https://doi.org/10.3390/antiox14030248
Lin T-S, Cai X-X, Wang Y-B, Xu J-T, Xiao J-H, Huang H-Y, Li S-F, Liu K-M, Chen J-H, Li L-P, et al. Identifying Baicalein as a Key Bioactive Compound in XueBiJing Targeting KEAP1: Implications for Antioxidant Effects. Antioxidants. 2025; 14(3):248. https://doi.org/10.3390/antiox14030248
Chicago/Turabian StyleLin, Ting-Syuan, Xiao-Xuan Cai, Yi-Bing Wang, Jia-Tong Xu, Ji-Han Xiao, Hsi-Yuan Huang, Shang-Fu Li, Kun-Meng Liu, Ji-Hang Chen, Li-Ping Li, and et al. 2025. "Identifying Baicalein as a Key Bioactive Compound in XueBiJing Targeting KEAP1: Implications for Antioxidant Effects" Antioxidants 14, no. 3: 248. https://doi.org/10.3390/antiox14030248
APA StyleLin, T.-S., Cai, X.-X., Wang, Y.-B., Xu, J.-T., Xiao, J.-H., Huang, H.-Y., Li, S.-F., Liu, K.-M., Chen, J.-H., Li, L.-P., Ni, J., Chen, Y.-G., Zhu, Z.-H., Li, J., Hu, Y.-J., Huang, H.-D., Zuo, H.-L., & Lin, Y.-C.-D. (2025). Identifying Baicalein as a Key Bioactive Compound in XueBiJing Targeting KEAP1: Implications for Antioxidant Effects. Antioxidants, 14(3), 248. https://doi.org/10.3390/antiox14030248








