The Regulation of γ-Aminobutyric Acid on Antioxidative Defense Response of Pacific Oyster upon High-Temperature Stress
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal and Exposure Experiment
2.2. RNA Extraction and cDNA Synthesis
2.3. RT-qPCR Analysis for mRNA Expression of Genes
2.4. Quantification of GABA Content
2.5. Measurement of Oxidative Stress Indexes
2.6. Statistical Analysis
3. Results
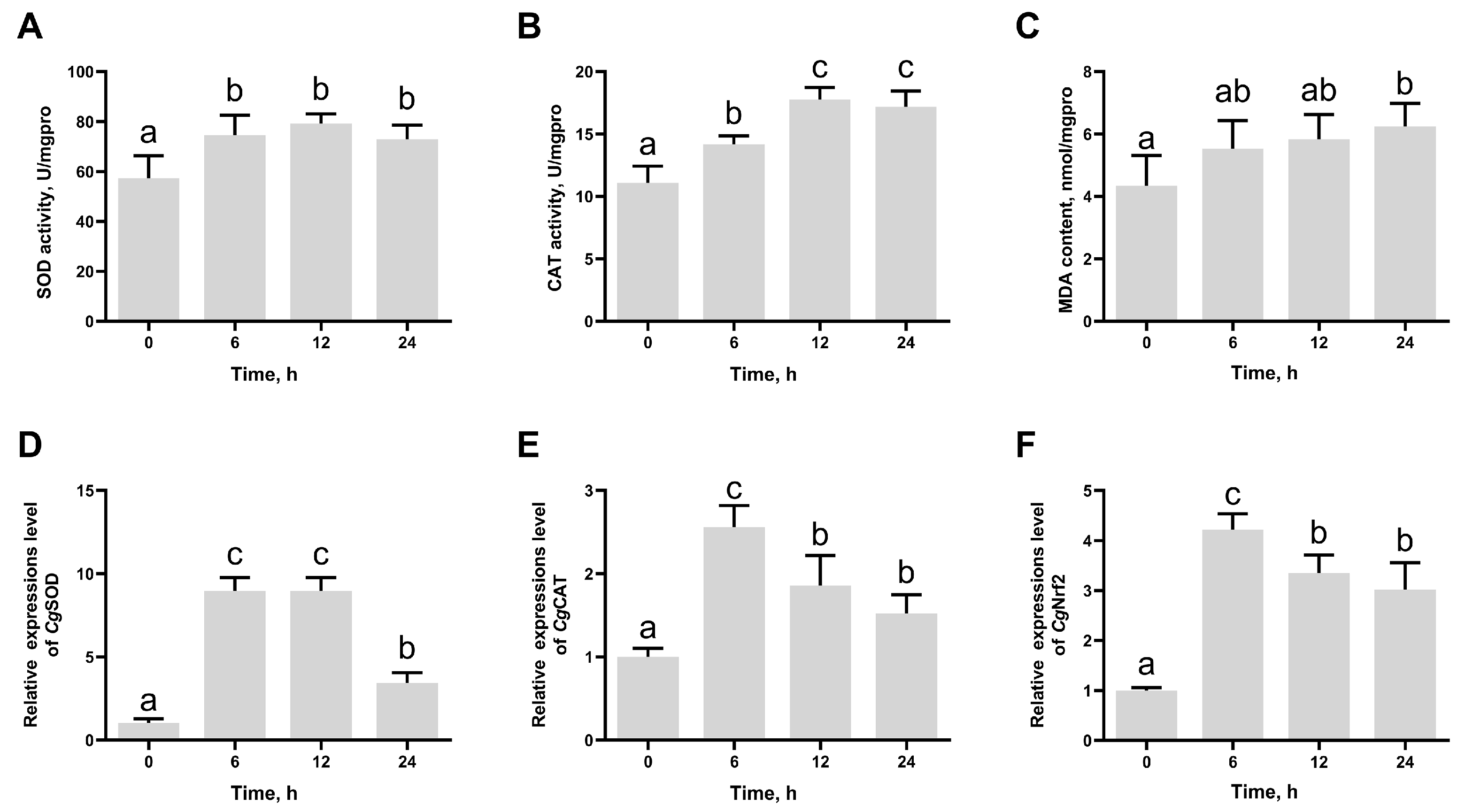
3.1. The SOD Activity, CAT Activity, MDA Content, and the mRNA Expression Levels of CgSOD, CgCAT, and CgNrf2 in Gill After High-Temperature Stress
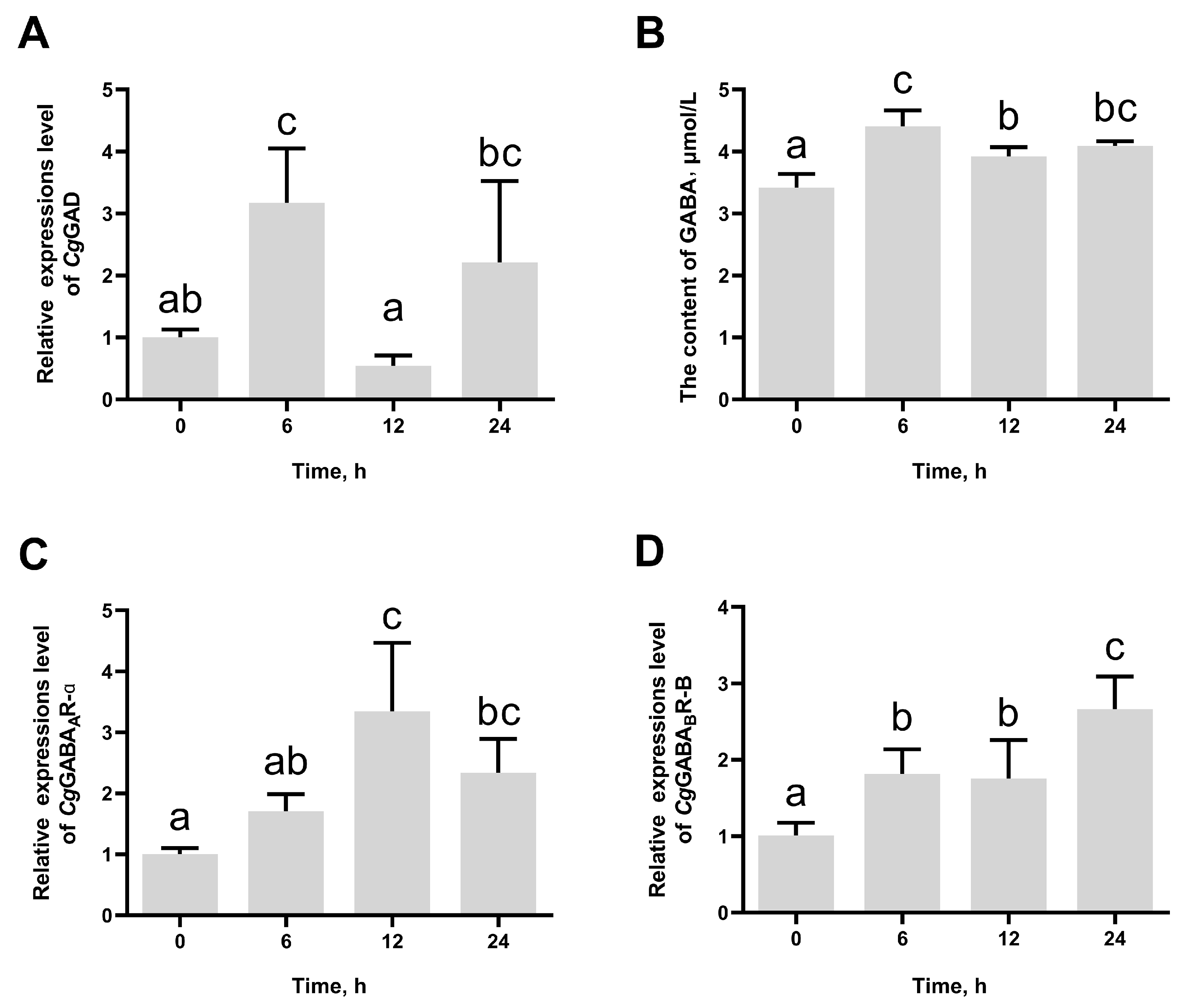
3.2. The Change in CgGAD, CgGABAAR-α, and CgGABABR-B Expression and GABA Content After High-Temperature Stress
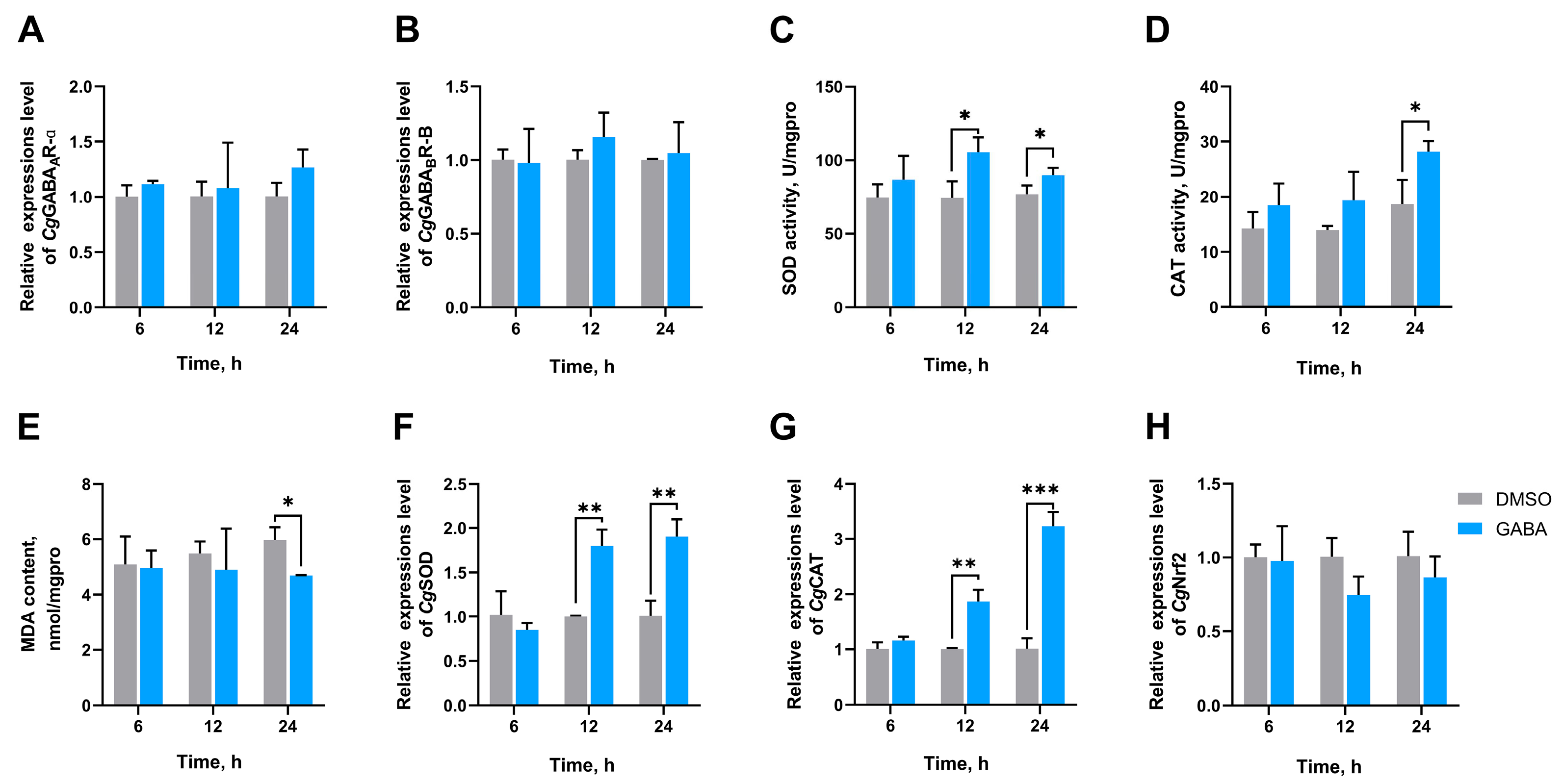
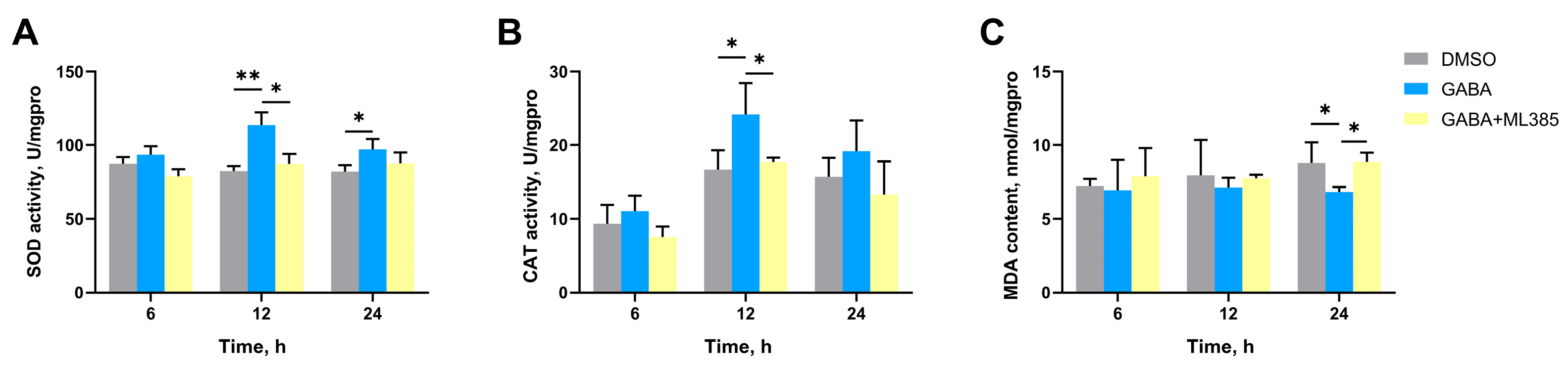
3.3. The SOD Activity, CAT Activity, MDA Content, and the mRNA Expression Levels of CgGABAAR-α, CgGABABR-B, CgSOD, CgCAT, and CgNrf2 in Gills After GABA Treatment
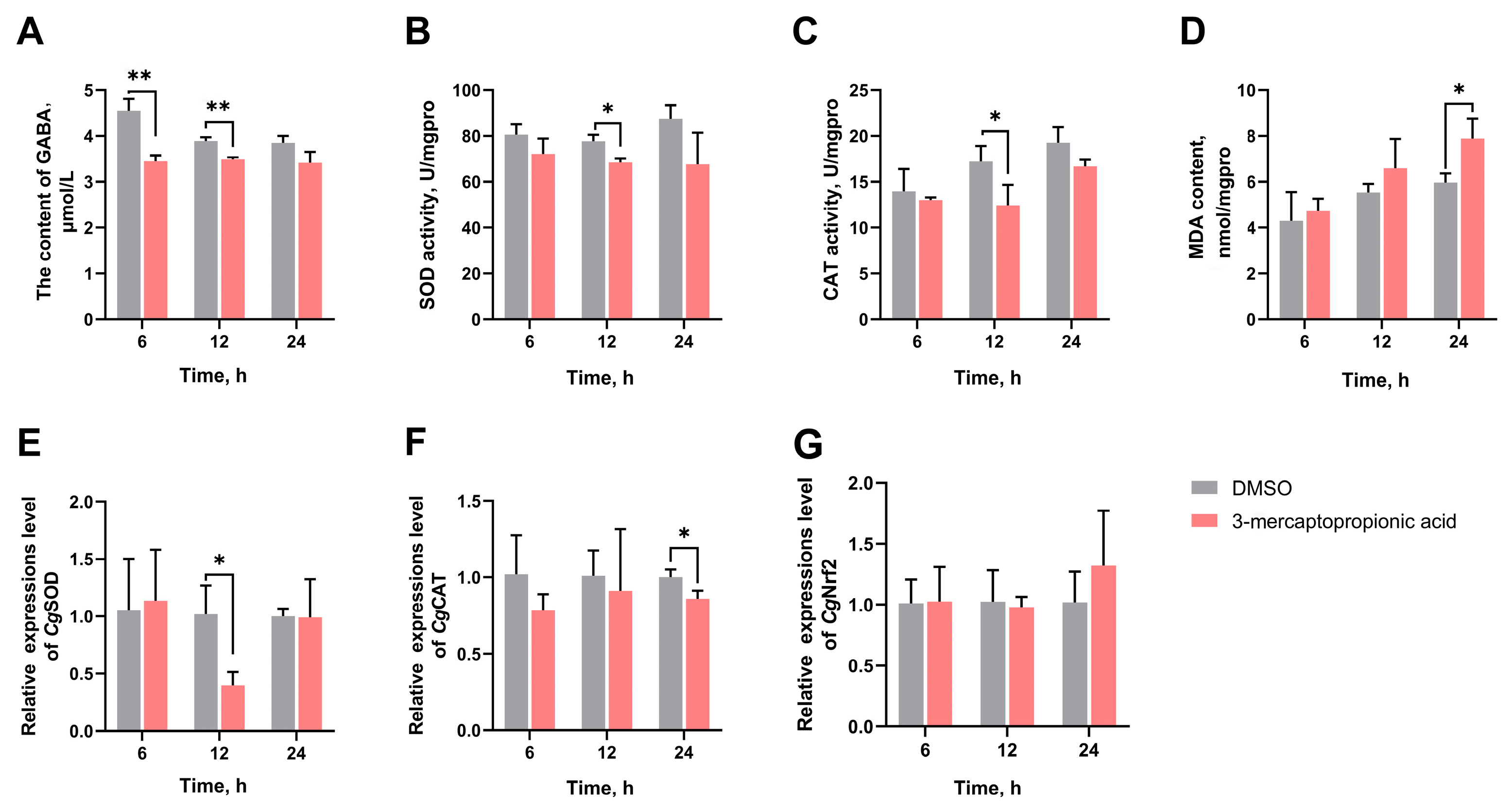
3.4. The GABA Content in Haemolymph Supernatant, SOD Activity, CAT Activity, MDA Content, and the mRNA Expression Levels of CgSOD, CgCAT, and CgNrf2 in Gills After GAD Inhibition
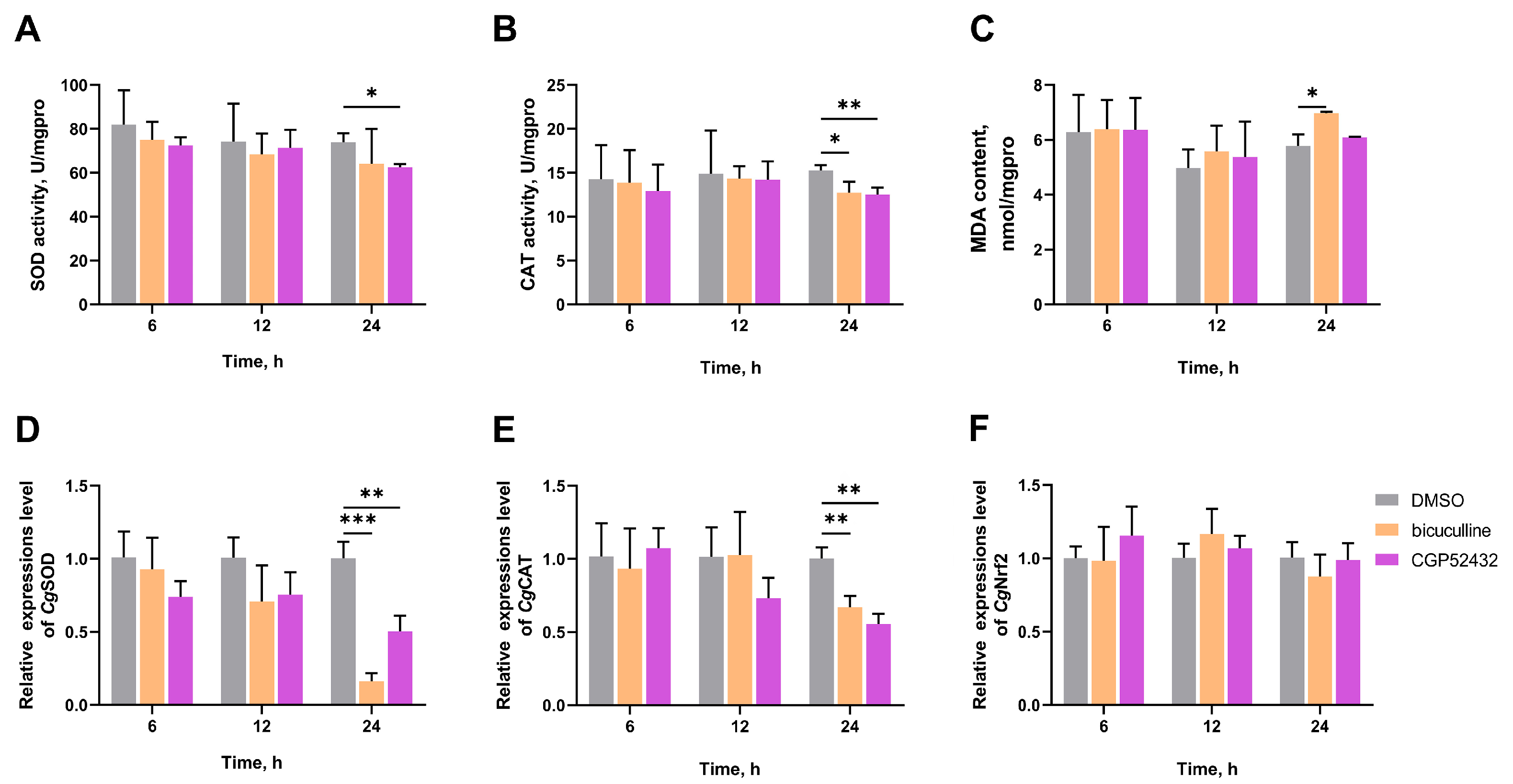
3.5. The SOD Activity, CAT Activity, MDA Content, and the mRNA Expression Levels of CgSOD, CgCAT, and CgNrf2 in Gills After Treatment with GABA Receptor Inhibitors
3.6. The SOD Activity, CAT Activity, and MDA Content in Gills After Nrf2 Inhibition
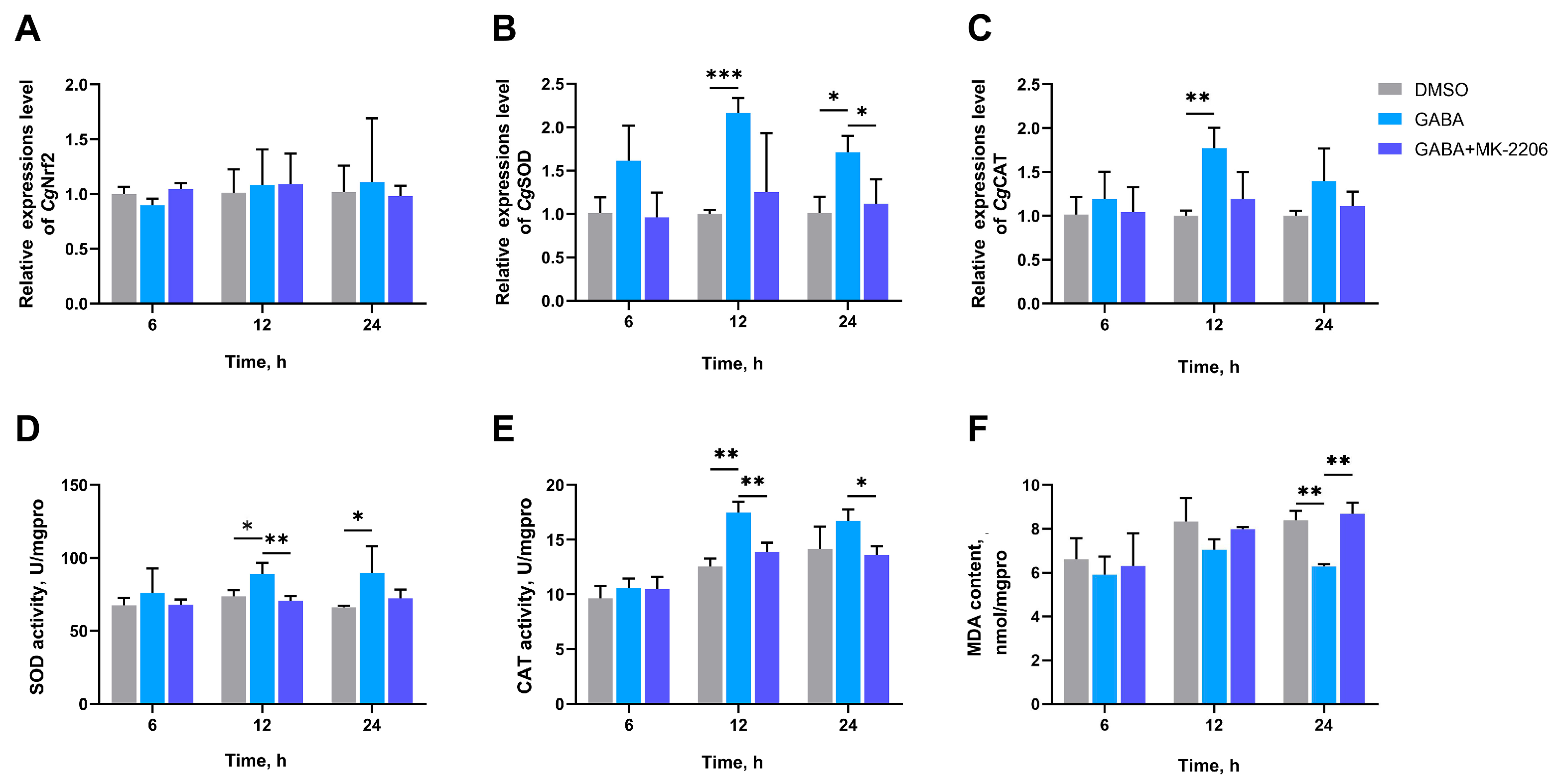
3.7. The SOD Activity, CAT Activity, MDA Content, and the mRNA Expression Levels of CgNrf2, CgSOD, and CgCAT in Gills After AKT Inhibition
3.8. The SOD Activity, CAT Activity, MDA Content, and the mRNA Expression Levels of CgNrf2, CgSOD, and CgCAT in Gills After GSK-3β Inhibition
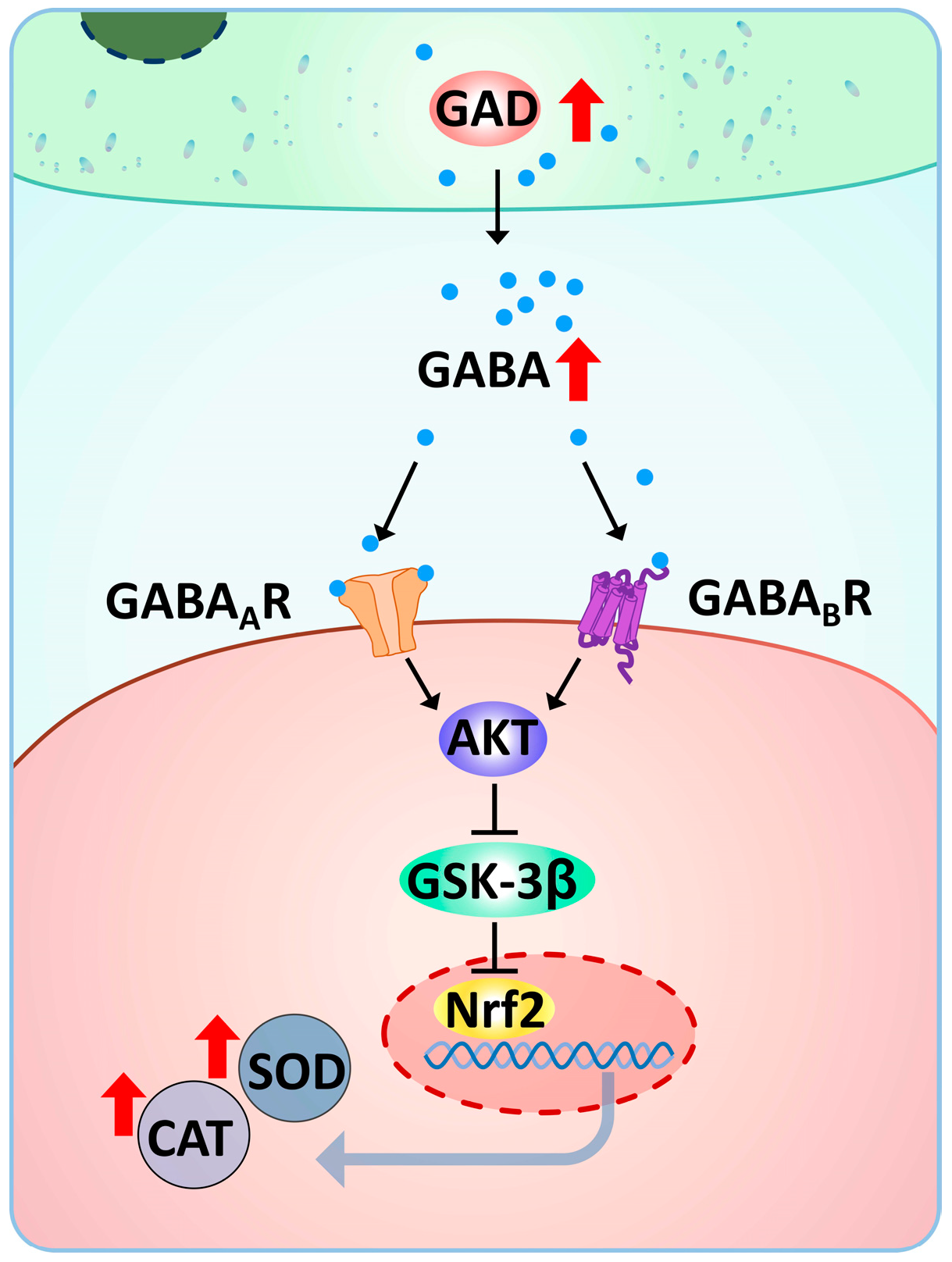
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gervais, C.R.; Huveneers, C.; Rummer, J.L.; Brown, C. Population variation in the thermal response to climate change reveals differing sensitivity in a benthic shark. Glob. Chang. Biol. 2021, 27, 108–120. [Google Scholar] [CrossRef] [PubMed]
- Schnytzer, Y.; Simon-Blecher, N.; Li, J.; Waldman Ben-Asher, H.; Salmon-Divon, M.; Achituv, Y.; Hughes, M.E.; Levy, O. Tidal and diel orchestration of behaviour and gene expression in an intertidal mollusc. Sci. Rep. 2018, 8, 4917. [Google Scholar] [CrossRef] [PubMed]
- Masanja, F.; Yang, K.; Xu, Y.; He, G.; Liu, X.; Xu, X.; Xiaoyan, J.; Xin, L.; Mkuye, R.; Deng, Y.; et al. Impacts of marine heat extremes on bivalves. Front. Mar. Sci. 2023, 10, 2296. [Google Scholar] [CrossRef]
- Kim, M.J.; Kim, J.A.; Lee, D.W.; Park, Y.S.; Kim, J.H.; Choi, C.Y. Oxidative stress and apoptosis in disk abalone (Haliotis discus hannai) caused by water temperature and pH changes. Antioxidants 2023, 12, 1003. [Google Scholar] [CrossRef]
- Birnie-Gauvin, K.; Costantini, D.; Cooke, S.J.; Willmore, W.G. A comparative and evolutionary approach to oxidative stress in fish: A review. Fish Fish. 2017, 18, 928–942. [Google Scholar] [CrossRef]
- Chainy, G.B.N.; Sahoo, D.K. Hormones and oxidative stress: An overview. Free Radic. Res. 2020, 54, 1–26. [Google Scholar] [CrossRef]
- Kloet, E.R.d.; Kloet, S.F.d.; Kloet, C.S.d.; Kloet, A.D.d. Top-down and bottom-up control of stress-coping. J. Neuroendocrinol. 2019, 31, e12675. [Google Scholar] [CrossRef]
- Danielli, N.M.; Trevisan, R.; Mello, D.F.; Fischer, K.; Deconto, V.S.; da Silva Acosta, D.; Bianchini, A.; Bainy, A.C.D.; Dafre, A.L. Upregulating Nrf2-dependent antioxidant defenses in Pacific oysters Crassostrea gigas: Investigating the Nrf2/Keap1 pathway in bivalves. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2017, 195, 16–26. [Google Scholar] [CrossRef]
- Qiu, L.; Chen, X.; Guo, B.; Liao, Z.; Buttino, I.; Yan, X.; Qi, P. Unraveling the protective role of Nrf2 in molluscs: Insights into mitochondrial and apoptosis pathways in the defense against Bap-induced oxidative stress. Aquat. Toxicol. 2023, 264, 106728. [Google Scholar] [CrossRef]
- Wang, H.; Pan, L.; Si, L.; Miao, J. The role of Nrf2-Keap1 signaling pathway in the antioxidant defense response induced by PAHs in the calm Ruditapes philippinarum. Fish Shellfish Immunol. 2018, 80, 325–334. [Google Scholar] [CrossRef]
- Feng, M.; Hu, Y.; Yang, L.; Wu, J.; Yang, G.; Jian, S.; Hu, B.; Wen, C. GST-Mu of Cristaria plicata is regulated by Nrf2/Keap1 pathway in detoxification microcystin and has antioxidant function. Aquat. Toxicol. 2023, 263, 106708. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.; Nioi, P.; Pickett, C.B. The Nrf2-Antioxidant Response Element Signaling Pathway and Its Activation by Oxidative Stress. J. Biol. Chem. 2009, 284, 13291–13295. [Google Scholar] [CrossRef] [PubMed]
- Abramov, T.; Suwansaard, S.; da Silva, P.M.; Wang, T.; Dove, M.; O’Connor, W.; Parker, L.; Lovejoy, D.A.; Cummins, S.F.; Elizur, A. Teneurin and TCAP phylogeny and physiology: Molecular analysis, immune activity, and transcriptomic analysis of the stress response in the Sydney rock oyster (Saccostrea glomerata) hemocytes. Front. Endocrinol. 2022, 13, 1664. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.A.; Henderson, S.; Miller-Ezzy, P.; Li, X.X.; Qin, J.G. Immune response to temperature stress in three bivalve species: Pacific oyster Crassostrea gigas, Mediterranean mussel Mytilus galloprovincialis and mud cockle Katelysia rhytiphora. Fish Shellfish Immunol. 2019, 86, 868–874. [Google Scholar] [CrossRef]
- Kopacz, A.; Kloska, D.; Forman, H.J.; Jozkowicz, A.; Grochot-Przeczek, A. Beyond repression of Nrf2: An update on Keap1. Free Radic. Biol. Med. 2020, 157, 63–74. [Google Scholar] [CrossRef]
- Glory, A.; Averill-Bates, D.A. The antioxidant transcription factor Nrf2 contributes to the protective effect of mild thermotolerance (40 °C) against heat shock-induced apoptosis. Free Radic. Biol. Med. 2016, 99, 485–497. [Google Scholar] [CrossRef]
- Yu, C.; Xiao, J.H. The Keap1-Nrf2 System: A Mediator between Oxidative Stress and Aging. Oxid. Med. Cell. Longev. 2021, 2021, 6635460. [Google Scholar] [CrossRef]
- Rada, P.; Rojo, A.I.; Evrard-Todeschi, N.; Innamorato, N.G.; Cotte, A.; Jaworski, T.; Tobón-Velasco, J.C.; Devijver, H.; García-Mayoral, M.F.; Van Leuven, F.; et al. Structural and functional characterization of Nrf2 degradation by the glycogen synthase kinase 3/β-TrCP axis. Mol. Cell. Biol. 2012, 32, 3486–3499. [Google Scholar] [CrossRef]
- Danielli, N.M.; Trevisan, R.; Mello, D.F.; Fischer, K.; Deconto, V.S.; Bianchini, A.; Bainy, A.C.D.; Dafre, A.L. Contrasting effects of a classic Nrf2 activator, tert-butylhydroquinone, on the glutathione-related antioxidant defenses in Pacific oysters, Crassostrea gigas. Mar. Environ. Res. 2017, 130, 142–149. [Google Scholar] [CrossRef]
- Regoli, F.; Giuliani, M.E. Oxidative pathways of chemical toxicity and oxidative stress biomarkers in marine organisms. Mar. Environ. Res. 2014, 93, 106–117. [Google Scholar] [CrossRef]
- Sasaki, S.; Yokozawa, T.; Cho, E.J.; Oowada, S.; Kim, M. Protective role of γ-aminobutyric acid against chronic renal failure in rats. J. Pharm. Pharmacol. 2006, 58, 1515–1525. [Google Scholar] [CrossRef] [PubMed]
- Fathi, M.; Saeedyan, S.; Kaoosi, M. Gamma-amino butyric acid (GABA) supplementation alleviates dexamethasone treatment-induced oxidative stress and inflammation response in broiler chickens. Stress 2023, 26, 2185861. [Google Scholar] [CrossRef] [PubMed]
- Hata, T.; Rehman, F.; Hori, T.; Nguyen, J.H. GABA, γ-Aminobutyric Acid, Protects Against Severe Liver Injury. J. Surg. Res. 2019, 236, 172–183. [Google Scholar] [CrossRef] [PubMed]
- Polenzani, L.; Woodward, R.; Miledi, R. Expression of mammalian gamma-aminobutyric acid receptors with distinct pharmacology in Xenopus oocytes. Proc. Natl. Acad. Sci. USA 1991, 88, 4318–4322. [Google Scholar] [CrossRef] [PubMed]
- Kochiyama, T.; Li, X.; Nakayama, H.; Kage, M.; Yamane, Y.; Takamori, K.; Iwabuchi, K.; Inada, E. Effect of propofol on the production of inflammatory cytokines by human polarized macrophages. Mediat. Inflamm. 2019, 2019, 1919538. [Google Scholar] [CrossRef]
- Tang, X.; Yu, R.; Zhou, Q.; Jiang, S.; Le, G. Protective effects of γ-aminobutyric acid against H2O2-induced oxidative stress in RIN-m5F pancreatic cells. Nutr. Metab. 2018, 15, 60. [Google Scholar] [CrossRef]
- Katila, N.; Bhurtel, S.; Park, P.; Choi, D. Metformin attenuates rotenone-induced oxidative stress and mitochondrial damage via the AKT/Nrf2 pathway. Neurochem. Int. 2021, 148, 105120. [Google Scholar] [CrossRef]
- Oliveira, M.R.d.; Ferreira, G.C.; Schuck, P.F.; Dal Bosco, S.M. Role for the PI3K/Akt/Nrf2 signaling pathway in the protective effects of carnosic acid against methylglyoxal-induced neurotoxicity in SH-SY5Y neuroblastoma cells. Chem.-Biol. Interact. 2015, 242, 396–406. [Google Scholar] [CrossRef]
- Purwana, I.; Zheng, J.; Li, X.; Deurloo, M.; Son, D.O.; Zhang, Z.; Liang, C.; Shen, E.; Tadkase, A.; Feng, Z.-P.; et al. GABA Promotes Human β-Cell Proliferation and Modulates Glucose Homeostasis. Diabetes 2014, 63, 4197–4205. [Google Scholar] [CrossRef]
- Zhang, C.; He, J.; Wang, X.; Su, R.; Huang, Q.; Qiao, F.; Qin, C.; Qin, J.; Chen, L. Dietary gamma-aminobutyric acid (GABA) improves non-specific immunity and alleviates lipopolysaccharide (LPS)-induced immune overresponse in juvenile Chinese mitten crab (Eriocheir sinensis). Fish Shellfish Immunol. 2022, 124, 480–489. [Google Scholar] [CrossRef]
- Bae, J.; Hamidoghli, A.; Farris, N.W.; Olowe, O.S.; Choi, W.; Lee, S.; Won, S.; Ohh, M.; Lee, S.; Bai, S.C. Dietary γ-Aminobutyric Acid (GABA) Promotes Growth and Resistance to Vibrio alginolyticus in Whiteleg Shrimp Litopenaeus vannamei. Aquac. Nutr. 2022, 2022, 9105068. [Google Scholar] [CrossRef]
- Li, J.; Jiang, M.; Han, Q.; Peng, R.; Jiang, X. Effects of γ-aminobutyric acid supplementation on the growth performance, serum biochemical indices and antioxidant status of pharaoh cuttlefish, Sepia pharaonis. Aquac. Nutr. 2020, 26, 1026–1034. [Google Scholar] [CrossRef]
- MartínezGarcía, M.F.; Ruesink, J.L.; GrijalvaChon, J.M.; Lodeiros, C.; ArreolaLizárraga, J.A.; de la ReVega, E.; VarelaRomero, A.; ChávezVillalba, J. Socioecological factors related to aquaculture introductions and production of Pacific oysters (Crassostrea gigas) worldwide. Rev. Aquac. 2022, 14, 613–629. [Google Scholar] [CrossRef]
- Siboni, N.; King, W.L.; Williams, N.L.R.; Scanes, E.; Giardina, M.; Green, T.J.; Ostrowski, M.; O’Connor, W.; Dove, M.; Labbate, M.; et al. Increased abundance of potentially pathogenic Vibrio and a marine heatwave co-occur with a Pacific oyster summer mortality event. Aquaculture 2024, 583, 740618. [Google Scholar] [CrossRef]
- Park, M.S.; Jo, P.G.; Choi, Y.K.; An, K.W.; Choi, C.Y. Characterization and mRNA expression of Mn-SOD and physiological responses to stresses in the Pacific oyster Crassostrea gigas. Mar. Biol. Res. 2009, 5, 451–461. [Google Scholar] [CrossRef]
- Elia, A.C.; Burioli, E.; Magara, G.; Pastorino, P.; Caldaroni, B.; Menconi, V.; Dörr, A.J.M.; Colombero, G.; Abete, M.C.; Prearo, M. Oxidative stress ecology on Pacific oyster Crassostrea gigas from lagoon and offshore Italian sites. Sci. Total Environ. 2020, 739, 139886. [Google Scholar] [CrossRef]
- Liu, Z.; Li, M.; Yi, Q.; Wang, L.; Song, L. The neuroendocrine-immune regulation in response to environmental stress in marine bivalves. Front. Physiol. 2018, 9, 1664. [Google Scholar] [CrossRef]
- Yu, Y.; Tian, D.; Ri, S.; Kim, T.; Ju, K.; Zhang, J.; Teng, S.; Zhang, W.; Shi, W.; Liu, G. Gamma-aminobutyric acid (GABA) suppresses hemocyte phagocytosis by binding to GABA receptors and modulating corresponding downstream pathways in blood clam, Tegillarca granosa. Fish Shellfish Immunol. 2023, 134, 108608. [Google Scholar] [CrossRef]
- Horton, R.W.; Meldrum, B.S. Seizures induced by allylglycine, 3-mercaptopropionic acid and 4-deoxypyridoxine in mice and photosensitive baboons, and different modes of inhibition of cerebral glutamic acid decarboxylase. Br. J. Pharmacol. 1973, 49, 52–63. [Google Scholar] [CrossRef]
- Hirai, H.; Sootome, H.; Nakatsuru, Y.; Miyama, K.; Taguchi, S.; Tsujioka, K.; Ueno, Y.; Hatch, H.; Majumder, P.K.; Pan, B.-S.; et al. MK-2206, an allosteric Akt inhibitor, enhances antitumor efficacy by standard chemotherapeutic agents or molecular targeted drugs in vitro and in vivo. Mol. Cancer Ther. 2010, 9, 1956–1967. [Google Scholar] [CrossRef]
- Jin, J.; Li, Y.; Liu, Y.; Jordan, A.A.; McIntosh, J.; Vargas, J.; Che, Y.; Yao, Y.; Wang, M. Bispecific CD19-CD20 and CD19-CD22 CAR-T Cells with Glycogen Synthase Kinase (GSK)-3β Inhibitor TWS119 Treatment Have Superior Therapeutic Effects on Mantle Cell Lymphoma. Blood 2021, 138, 1698. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, T.; Wang, L.; Xu, J.; Li, M.; Zhang, A.; Qiu, L.; Song, L. A new non-phagocytic TLR6 with broad recognition ligands from Pacific oyster Crassostrea gigas. Dev. Comp. Immunol. 2016, 65, 182–190. [Google Scholar] [CrossRef] [PubMed]
- Xing, Z.; Gao, L.; Liu, R.; Yang, Q.; Li, Q.; Wang, L.; Song, L. The oxidative stress of the Pacific oyster Crassostrea gigas under high-temperature stress. Aquaculture 2023, 577, 739998. [Google Scholar] [CrossRef]
- Lee, M.; Schwab, C.; McGeer, P.L. Astrocytes are GABAergic cells that modulate microglial activity. Glia 2011, 59, 152–165. [Google Scholar] [CrossRef]
- Dong, W.; Liu, Z.; Qiu, L.; Wang, W.; Song, X.; Wang, X.; Li, Y.; Xin, L.; Wang, L.; Song, L. The modulation role of serotonin in Pacific oyster Crassostrea gigas in response to air exposure. Fish Shellfish Immunol. 2017, 62, 341–348. [Google Scholar] [CrossRef]
- Rahman, M.S.; Rahman, M.S. Effects of elevated temperature on prooxidant-antioxidant homeostasis and redox status in the American oyster: Signaling pathways of cellular apoptosis during heat stress. Environ. Res. 2021, 196, 110428. [Google Scholar] [CrossRef]
- Ruenkoed, S.; Nontasan, S.; Phudkliang, J.; Phudinsai, P.; Pongtanalert, P.; Panprommin, D.; Mongkolwit, K.; Wangkahart, E. Effect of dietary gamma aminobutyric acid (GABA) modulated the growth performance, immune and antioxidant capacity, digestive enzymes, intestinal histology and gene expression of Nile tilapia (Oreochromis niloticus). Fish Shellfish Immunol. 2023, 141, 109056. [Google Scholar] [CrossRef]
- Menon, S.V.; Kumar, A.; Middha, S.K.; Paital, B.; Mathur, S.; Johnson, R.; Kademan, A.; Usha, T.; Hemavathi, K.N.; Dayal, S.; et al. Water physicochemical factors and oxidative stress physiology in fish, a review. Front. Environ. Sci. 2023, 11, 1240813. [Google Scholar] [CrossRef]
- Zhao, J.; Zhao, B.; Kong, N.; Li, F.; Liu, J.; Wang, L.; Song, L. Increased abundances of potential pathogenic bacteria and expressions of inflammatory cytokines in the intestine of oyster Crassostrea gigas after high temperature stress. Dev. Comp. Immunol. 2023, 141, 104630. [Google Scholar] [CrossRef]
- Meistertzheim, A.L.; Tanguy, A.; Moraga, D.; Thébault, M.T. Identification of differentially expressed genes of the Pacific oyster Crassostrea gigas exposed to prolonged thermal stress. Febs J. 2007, 274, 6392–6402. [Google Scholar] [CrossRef]
- Chen, C.; Li, P.; Wang, W.; Li, Z. Response of growth performance, serum biochemical parameters, antioxidant capacity, and digestive enzyme activity to different feeding strategies in common carp (Cyprinus carpio) under high-temperature stress. Aquaculture 2022, 548, 737636. [Google Scholar] [CrossRef]
- Li, A.; Li, L.; Song, K.; Wang, W.; Zhang, G. Temperature, energy metabolism, and adaptive divergence in two oyster subspecies. Ecol. Evol. 2017, 7, 6151–6162. [Google Scholar] [CrossRef] [PubMed]
- Ding, F.; Li, A.; Cong, R.; Wang, X.; Wang, W.; Que, H.; Zhang, G.; Li, L. The phenotypic and the genetic response to the extreme high temperature provides new insight into thermal tolerance for the pacific oyster Crassostrea gigas. Front. Mar. Sci. 2020, 7, 2296. [Google Scholar] [CrossRef]
- Lu, Y.; Wu, Z.; Song, Z.; Xiao, P.; Liu, Y.; Zhang, P.; You, F. Insight into the heat resistance of fish via blood: Effects of heat stress on metabolism, oxidative stress and antioxidant response of olive flounder Paralichthys olivaceus and turbot Scophthalmus maximus. Fish Shellfish Immunol. 2016, 58, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Zhao, L.; Liao, L.; Tang, X.; Cui, C.; Liu, Q.; He, K.; Ma, J.; Jin, L.; Yan, T.; et al. Interactive effect of thermal and hypoxia on largemouth bass (Micropterus salmoides) gill and liver: Aggravation of oxidative stress, inhibition of immunity and promotion of cell apoptosis. Fish Shellfish Immunol. 2020, 98, 923–936. [Google Scholar] [CrossRef]
- Hogg, D.W.; Hawrysh, P.J.; Buck, L.T. Environmental remodelling of GABAergic and glutamatergic neurotransmission: Rise of the anoxia-tolerant turtle brain. J. Therm. Biol. 2014, 44, 85–92. [Google Scholar] [CrossRef]
- Nilsson, G.E. Evidence for a role of gaba in metabolic depression during anoxia in crucian carp (Carassius carassius). J. Exp. Biol. 1992, 164, 243–259. [Google Scholar] [CrossRef]
- Li, M.; Wang, L.; Qiu, L.; Wang, W.; Xin, L.; Xu, J.; Wang, H.; Song, L. A glutamic acid decarboxylase (CgGAD) highly expressed in hemocytes of Pacific oyster Crassostrea gigas. Dev. Comp. Immunol. 2016, 63, 56–65. [Google Scholar] [CrossRef]
- Liang, Y.; Wu, Y.; Li, J.; Peng, R.; Jiang, M.; Jiang, X.; Chen, S.; Lin, J. Effects of ammonia toxicity on the histopathology, detoxification, oxidative stress, and immune response of the cuttlefish Sepia pharaonis and the mitigation of γ-aminobutyric acid. Ecotoxicol. Environ. Saf. 2022, 232, 113256. [Google Scholar] [CrossRef]
- Guan, X.; Shi, W.; Zha, S.; Rong, J.; Su, W.; Liu, G. Neurotoxic impact of acute TiO2 nanoparticle exposure on a benthic marine bivalve mollusk, Tegillarca granosa. Aquat. Toxicol. 2018, 200, 241–246. [Google Scholar] [CrossRef]
- Haider, F.; Falfushynska, H.I.; Timm, S.; Sokolova, I.M. Effects of hypoxia and reoxygenation on intermediary metabolite homeostasis of marine bivalves Mytilus edulis and Crassostrea gigas. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2020, 242, 110657. [Google Scholar] [CrossRef] [PubMed]
- Lauder, J.M. Neurotransmitters as growth regulatory signals: Role of receptors and second messengers. Trends Neurosci. 1993, 16, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Hori, T.; Uemoto, S.; Walden, L.B.; Chen, F.; Baine, A.T.; Hata, T.; Nguyen, J.H. Pretreatment of small-for-size grafts in vivo by γ-aminobutyric acid receptor regulation against oxidative stress-induced injury in rat split orthotopic liver transplantation. Int. J. Hepatol. 2013, 2013, 149123. [Google Scholar] [CrossRef] [PubMed]
- Richmond, J.E.; Murphy, A.D.; Lukowiak, K.; Bulloch, A.G.M. GABA regulates the buccal motor output of Helisoma by two pharmacologically distinct actions. J. Comp. Physiol. A 1994, 174, 593–600. [Google Scholar] [CrossRef]
- Hutton, M.L.; Harvey, R.J.; Earley, F.G.P.; Barnard, E.A.; Darlison, M.G. A novel invertebrate GABAA receptor-like polypeptide Sequence and pattern of gene expression. FEBS Lett. 1993, 326, 112–116. [Google Scholar] [CrossRef]
- Jiang, W.; Fang, J.; Rastrick, S.P.S.; Samuelsen, O.B.; Liang, B.; Mao, Y.; Strand, Ø.; Fang, J.; Jiang, Z. CO2-Induced ocean acidification alters the burrowing behavior of Manila clam Ruditapes philippinarum by Reversing GABAA receptor function. Environ. Sci. Technol. 2023, 57, 8921–8932. [Google Scholar] [CrossRef]
- Johnston, G.A.R. Advantages of an antagonist: Bicuculline and other GABA antagonists. Br. J. Pharmacol. 2013, 169, 328–336. [Google Scholar] [CrossRef]
- Deng, Z.; Li, D.; Wang, L.; Lan, J.; Wang, J.; Ma, Y. Activation of GABABR attenuates intestinal inflammation by reducing oxidative stress through modulating the TLR4/MyD88/NLRP3 pathway and gut microbiota abundance. Antioxidants 2024, 13, 1141. [Google Scholar] [CrossRef]
- Khan, M.Z.; Khan, A.; Chen, W.; Chai, W.; Wang, C. Advancements in genetic biomarkers and exogenous antioxidant supplementation for safeguarding mammalian cells against heat-induced oxidative stress and apoptosis. Antioxidants 2024, 13, 258. [Google Scholar] [CrossRef]
- Lin, Y.; Li, X.; Chen, X.; Chen, J.; Jin, X.; Sun, J.; Niu, X.; Kong, Y.; Li, M.; Wang, G. γ-aminobutyric acid effectively modulate growth performance, physiological response of Largemouth bass (Micropterus salmoides) under combined stress of flow velocity and density. Aquac. Nutr. 2024, 2024, 9180554. [Google Scholar] [CrossRef]
- Yan, Z.; Liu, B.; Liu, J.; Guo, Z.; Kou, Y.; Lu, W.; Sun, J.; Li, Y. Enhancing resilience to chronic ammonia stress in crucian carp (Carassius carassius) through dietary gamma-aminobutyric acid (GABA) supplementation: Effects on growth performance, immune function, hepatotoxicity, and apoptosis. Aquac. Rep. 2024, 37, 102259. [Google Scholar] [CrossRef]
- Saha, S.; Buttari, B.; Panieri, E.; Profumo, E.; Saso, L. An overview of Nrf2 signaling pathway and its role in inflammation. Molecules 2020, 25, 5474. [Google Scholar] [CrossRef] [PubMed]
- Soltani, N.; Qiu, H.; Aleksic, M.; Glinka, Y.; Zhao, F.; Liu, R.; Li, Y.; Zhang, N.; Chakrabarti, R.; Ng, T.; et al. GABA exerts protective and regenerative effects on islet beta cells and reverses diabetes. Proc. Natl. Acad. Sci. USA 2011, 108, 11692–11697. [Google Scholar] [CrossRef] [PubMed]
- Gardner, L.B.; Hori, T.; Chen, F.; Baine, A.T.; Hata, T.; Uemoto, S.; Nguyen, J.H. Effect of specific activation of γ-aminobutyric acid receptor in vivo on oxidative stress-induced damage after extended hepatectomy. Hepatol. Res. 2012, 42, 1131–1140. [Google Scholar] [CrossRef]
- Salazar, M.; Rojo, A.I.; Velasco, D.; de Sagarra, R.M.; Cuadrado, A. Glycogen synthase kinase-3beta inhibits the xenobiotic and antioxidant cell response by direct phosphorylation and nuclear exclusion of the transcription factor Nrf2. J. Biol. Chem. 2006, 281, 14841–14851. [Google Scholar] [CrossRef]
- Chowdhry, S.; Zhang, Y.; McMahon, M.; Sutherland, C.; Cuadrado, A.; Hayes, J.D. Nrf2 is controlled by two distinct β-TrCP recognition motifs in its Neh6 domain, one of which can be modulated by GSK-3 activity. Oncogene 2013, 32, 3765–3781. [Google Scholar] [CrossRef]
- Hou, L.; Qiao, X.; Li, Y.; Jin, Y.; Liu, R.; Wang, S.; Zhou, K.; Wang, L.; Song, L. A RAC-alpha serine/threonine-protein kinase (CgAKT1) involved in the synthesis of CgIFNLP in oyster Crassostrea gigas. Fish Shellfish Immunol. 2022, 127, 129–139. [Google Scholar] [CrossRef]
- Sun, J.; Gao, L.; Huang, S.; Wang, L.; Yang, W.; Zhang, T.; Jin, Y.; Song, L. CLec-TM1–ERK–GSK3β pathway regulates Vibrio splendidus–induced IL-17 production in oyster. J. Immunol. 2021, 207, 640–650. [Google Scholar] [CrossRef]


| Primer Name | Sequence (5′–3′) |
|---|---|
| CgEF-RT-F | AGTCACCAAGGCTGCACAGAAAG |
| CgEF-RT-R | TCCGACGTATTTCTTTGCGATGT |
| CgGAD-RT-F | GCTATGTGCGGATTACCTCTACCAG |
| CgGAD-RT-R | GATTCGCTAAGTCTTGGGTTGGATA |
| CgGABAAR-α-RT-F | GAGTTCTTTTAGCGGCCGTG |
| CgGABAAR-α-RT-R | TGCAGACGTTCAGGAAGACG |
| CgGABABR-B-RT-F | CACCTTCATGCTGACGTCCT |
| CgGABABR-B-RT-R | TCCACCAAAACCGCACCTTT |
| CgNrf2-RT-F | ACACAGCCTGTCAGACTTCAC |
| CgNrf2-RT-R | CACATCGAACATCTCCTTCCCT |
| CgSOD-RT-F | TGACAGAAGCGTCCGTTGGC |
| CgSOD-RT-R | CCGCCTTGAATCTTTCGTTG |
| CgCAT-RT-F | AACTACTTCGCTGAGGTG |
| CgCAT-RT-R | GGTCTTGGCTTTGTATGG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, R.; Gao, L.; Zhang, X.; Ge, P.; Wang, L.; Zhou, K.; Yang, C.; Wang, L.; Song, L. The Regulation of γ-Aminobutyric Acid on Antioxidative Defense Response of Pacific Oyster upon High-Temperature Stress. Antioxidants 2025, 14, 222. https://doi.org/10.3390/antiox14020222
Liu R, Gao L, Zhang X, Ge P, Wang L, Zhou K, Yang C, Wang L, Song L. The Regulation of γ-Aminobutyric Acid on Antioxidative Defense Response of Pacific Oyster upon High-Temperature Stress. Antioxidants. 2025; 14(2):222. https://doi.org/10.3390/antiox14020222
Chicago/Turabian StyleLiu, Ranyang, Lei Gao, Xueshu Zhang, Pingan Ge, Ling Wang, Keli Zhou, Chuanyan Yang, Lingling Wang, and Linsheng Song. 2025. "The Regulation of γ-Aminobutyric Acid on Antioxidative Defense Response of Pacific Oyster upon High-Temperature Stress" Antioxidants 14, no. 2: 222. https://doi.org/10.3390/antiox14020222
APA StyleLiu, R., Gao, L., Zhang, X., Ge, P., Wang, L., Zhou, K., Yang, C., Wang, L., & Song, L. (2025). The Regulation of γ-Aminobutyric Acid on Antioxidative Defense Response of Pacific Oyster upon High-Temperature Stress. Antioxidants, 14(2), 222. https://doi.org/10.3390/antiox14020222







