Untargeted UHPLC-HRMS Metabolomic Profiling of Cornus mas L. Fruits: Impact of Conventional and Emerging Extraction Methods on Phenolic Composition and Bioactivity
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Plant Material
2.3. Extraction of Bioactive Compounds
2.4. Total Phenolic Content (TPC) and Total Flavonoid Content (TFC)
2.5. UHPLC-HRMS Analysis of Phenolic Profile
2.6. In Vitro Assays of Antioxidant Capacity
2.7. Enzyme Inhibitory Activity
2.8. Statistical and Correlation Analysis
3. Results
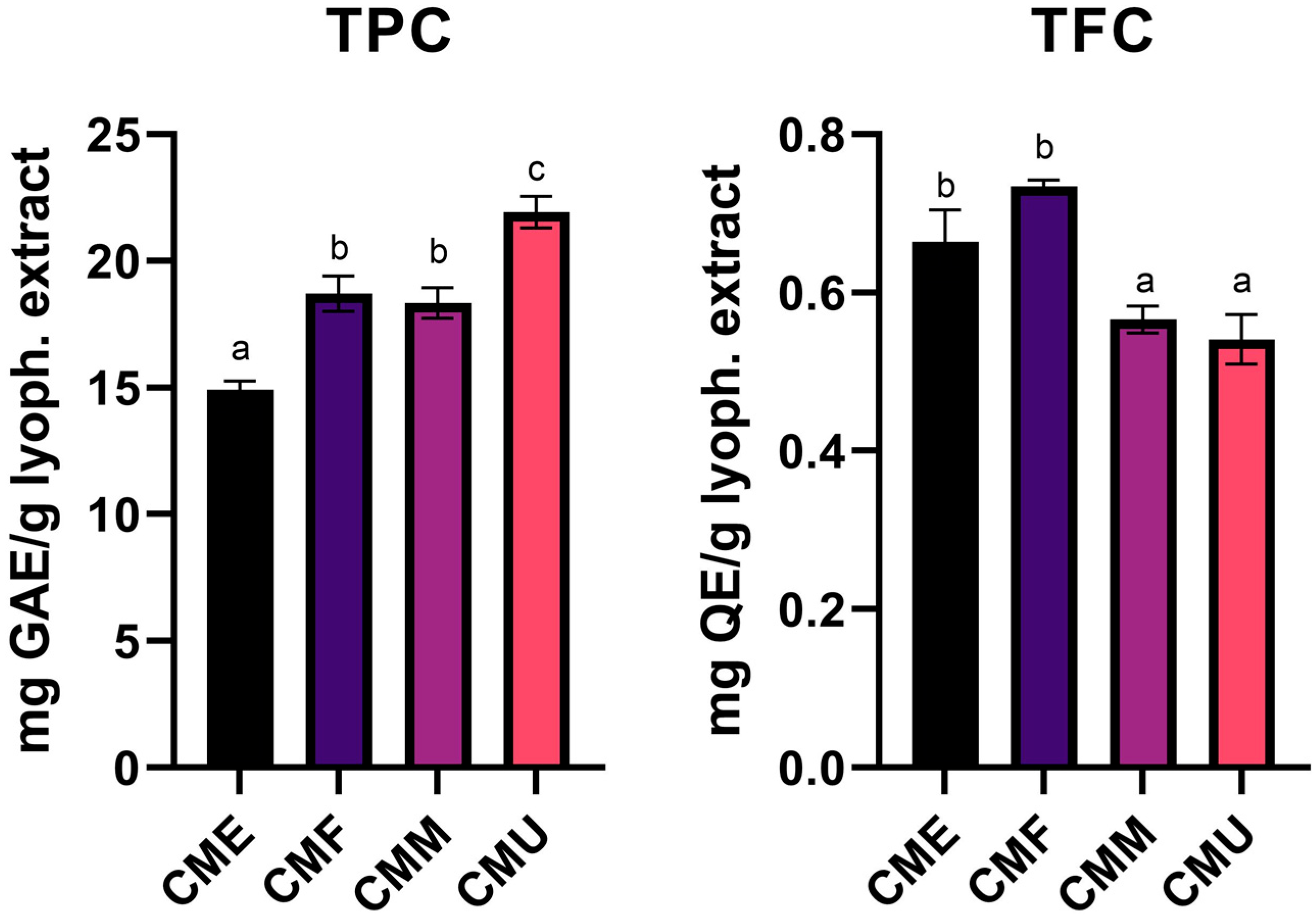
3.1. Cornelian Cherry Fruit Extracts Phytochemical Analysis
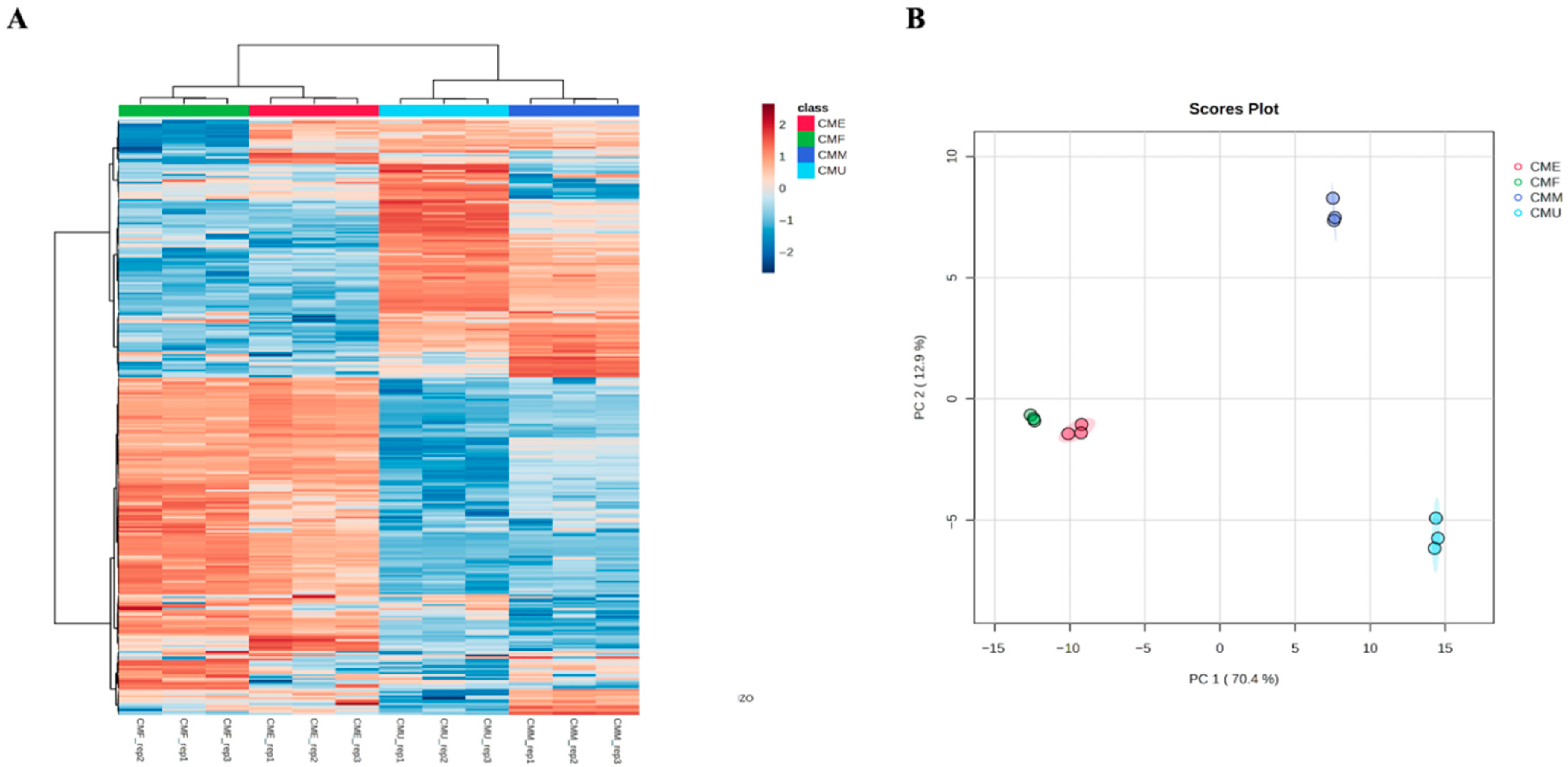
3.2. Multivariate Discrimination of Extraction Methods
3.3. Identification of Discriminant Markers by OPLS-DA
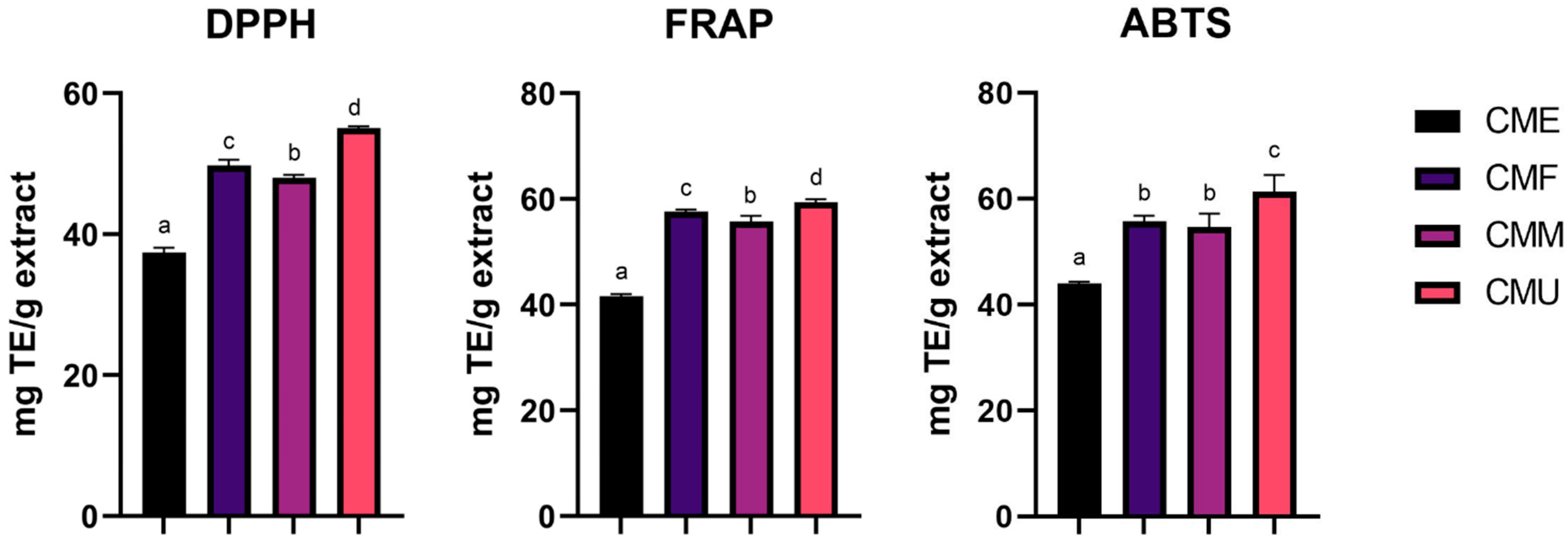
3.4. Antioxidant Activity of Cornelian Cherry Fruit Extracts
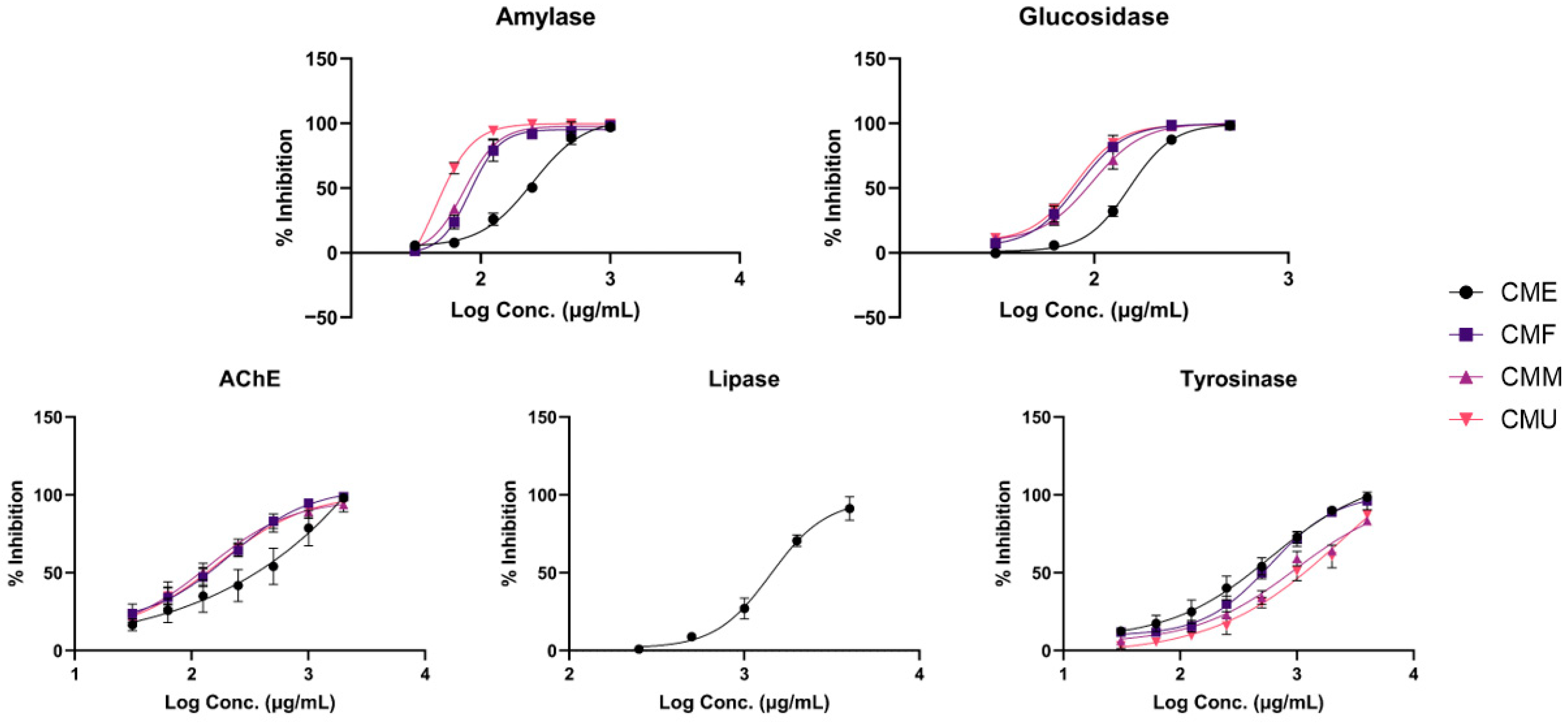
3.5. Assessment of the In Vitro Anti-Enzymatic Activity of Extracts from Cornelian Cherry Fruit Extracts
3.6. Correlation Coefficients Between Bioactivities and Phenolics
4. Discussion
4.1. Phytochemical Variability Observed in Cornelian Cherry Fruit Extracts
4.2. Antioxidant Potential of Cornelian Cherry Fruit Extracts and Influence of Extraction Technique
4.3. Comparative Interpretation of Extraction-Dependent Metabolite Patterns
4.4. Enzyme Inhibitory Activities of Cornelian Cherry Fruit Extracts and Their Potential Biological Implications
4.5. Correlation Between Phenolic Classes, Multifunctional Bioactivities, and Study Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Plant Finder. Available online: https://www.missouribotanicalgarden.org/PlantFinder/PlantFinderDetails.aspx?kempercode=c290 (accessed on 15 November 2024).
- Lidiková, J.; Čeryová, N.; Grygorieva, O.; Bobková, A.; Bobko, M.; Árvay, J.; Šnirc, M.; Brindza, J.; Ňorbová, M.; Harangozo, Ľ.; et al. Cornelian Cherry (Cornus mas L.) as a Promising Source of Antioxidant Phenolic Substances and Minerals. Eur. Food Res. Technol. 2024, 250, 1745–1754. [Google Scholar] [CrossRef]
- Cornus mas L. Plants of the World Online. Kew Science. Available online: https://powo.science.kew.org/taxon/urn:lsid:ipni.org:names:271612-1 (accessed on 26 November 2025).
- Butură, V. Corn (Cornus mas L.). In Enciclopedie de Etnobotanică Românească; Editura Științifică și Enciclopedică: Bucharest, Romania, 1979; pp. 78–79. [Google Scholar]
- Hosseinpour-Jaghdani, F.; Shomali, T.; Gholipour-Shahraki, S.; Rahimi-Madiseh, M.; Rafieian-Kopaei, M. Cornus Mas: A Review on Traditional Uses and Pharmacological Properties. J. Complement. Integr. Med. 2017, 14, 20160137. [Google Scholar] [CrossRef]
- Czerwińska, M.E.; Bobińska, A.; Cichocka, K.; Buchholz, T.; Woliński, K.; Melzig, M.F. Cornus mas and Cornus officinalis—A Comparison of Antioxidant and Immunomodulatory Activities of Standardized Fruit Extracts in Human Neutrophils and Caco-2 Models. Plants 2021, 10, 2347. [Google Scholar] [CrossRef]
- Brodyak, I.; Moroz, A.; Bernacka, K.; Kucharska, A.Z.; Sybirna, N. Alleviation of Hyperglycaemia and Oxidative Stress by Fruit Extracts of Different Cultivars of the Cornelian Cherry (Cornus mas L. and Cornus mas × Cornus Officinalis) in Rats with Diabetes Mellitus. Food Funct. 2025, 16, 2136–2155. [Google Scholar] [CrossRef]
- Brodyak, I.; Moroz, A.; Bernacka, K.; Kucharska, A.Z.; Sybirna, N. Erythrocyte-Related Indicators as Biomarkers in Diabetes: Improvement of Erythrocyte Function by Fruit Extracts from Different Cultivars of the Cornelian Cherry (Cornus mas L. and Cornus mas × Cornus officinalis Hybrids). Biomed. Pharmacother. 2025, 188, 118194. [Google Scholar] [CrossRef] [PubMed]
- Pomianek, T.; Zagórska-Dziok, M.; Skóra, B.; Ziemlewska, A.; Nizioł-Łukaszewska, Z.; Wójciak, M.; Sowa, I.; Szychowski, K.A. Comparison of the Antioxidant and Cytoprotective Properties of Extracts from Different Cultivars of Cornus mas L. Int. J. Mol. Sci. 2024, 25, 5495. [Google Scholar] [CrossRef] [PubMed]
- Wójciak, M.; Zagórska-Dziok, M.; Nizioł-Łukaszewska, Z.; Ziemlewska, A.; Furman-Toczek, D.; Szczepanek, D.; Sowa, I. In Vitro Evaluation of Anti-Inflammatory and Protective Potential of an Extract from Cornus mas L. Fruit against H2O2-Induced Oxidative Stress in Human Skin Keratinocytes and Fibroblasts. Int. J. Mol. Sci. 2022, 23, 13755. [Google Scholar] [CrossRef] [PubMed]
- Aryaeian, N.; Amiri, F.; Rahideh, S.T.; Abolghasemi, J.; Jazayeri, S.; Gholamrezayi, A.; Motevalian, M.; Solaymani-Dodaran, M.; Taghizadeh, M.; Heshmati, E.; et al. The Effect of Cornus Mas Extract Consumption on Bone Biomarkers and Inflammation in Postmenopausal Women: A Randomized Clinical Trial. Phyther. Res. 2021, 35, 4425–4432. [Google Scholar] [CrossRef]
- Yarhosseini, F.; Sangouni, A.A.; Sangsefidi, Z.S.; Hosseinzadeh, M.; Akhondi-Meybodi, M.; Ranjbar, A.; Fallahzadeh, H.; Mozaffari-Khosravi, H. Effect of Cornus mas L. Fruit Extract on Blood Pressure, Anthropometric and Body Composition Indices in Patients with Non-Alcoholic Fatty Liver Disease: A Double-Blind Randomized Controlled Trial. Clin. Nutr. ESPEN 2023, 56, 18–24. [Google Scholar] [CrossRef]
- Frumuzachi, O.; Kieserling, H.; Rohn, S.; Mocan, A.; Crișan, G. The Impact of Cornelian Cherry (Cornus mas L.) on Cardiometabolic Risk Factors: A Meta-Analysis of Randomised Controlled Trials. Nutrients 2024, 16, 2173. [Google Scholar] [CrossRef]
- Omrani, M.; Norouzzadeh, M.; Ahmadirad, H.; Abbasi, M.; Jahromi, M.K.; Teymoori, F.; Rashedi, M.H.; Rahideh, S.T.; Farhadnejad, H.; Mirmiran, P.; et al. Cornelian Cherry, Cardiometabolic Health, and Dietary Intake: A GRADE-Assessed Systematic Review and Meta-Analysis. Nutr. Metab. 2025, 22, 92. [Google Scholar] [CrossRef]
- Li, D.; Liu, Y.; Fu, Q.; Han, R.; Wu, J.; Zhang, Q.; Fang, F.; Zhu, D. Effects of Cornus mas L. Supplementation on Anthropometric and Metabolic Characteristics in Patients with Metabolic Syndrome and Related Disorders: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. Phyther. Res. 2025, 39, 1565–1577. [Google Scholar] [CrossRef]
- Tenuta, M.C.; Loizzo, M.R.; Tundis, R.; Dugay, A.; Bouzidi, C.; Marie, A.; Acquaviva, R.; Cappello, A.R.; Deguin, B. Iridoid- and Flavonoid-Enriched Fractions of Cornus sanguinea and Cornus mas Exert Antioxidant and Anti-Inflammatory Effects and Inhibit Key Enzymes in the Treatment of Metabolic Disorders. Food Funct. 2023, 14, 8838–8853. [Google Scholar] [CrossRef] [PubMed]
- Dzydzan, O.; Bila, I.; Kucharska, A.Z.; Brodyak, I.; Sybirna, N. Antidiabetic Effects of Extracts of Red and Yellow Fruits of Cornelian Cherries (Cornus mas L.) on Rats with Streptozotocin-Induced Diabetes Mellitus. Food Funct. 2019, 10, 6459–6472. [Google Scholar] [CrossRef] [PubMed]
- Deng, S.; West, B.J.; Jensen, C.J. UPLC-TOF-MS Characterization and Identification of Bioactive Iridoids in Cornus mas Fruit. J. Anal. Methods Chem. 2013, 2013, 710972. [Google Scholar] [CrossRef]
- Perova, I.B.; Zhogova, A.A.; Polyakova, A.V.; Eller, K.L.; Ramenskaya, G.V.; Samylina, I.A. Biologically Active Substances of Cornelian Cherry Fruits (Cornus mas L.). Vopr. Pitan. 2014, 83, 86–94. [Google Scholar]
- Zagórska-Dziok, M.; Ziemlewska, A.; Mokrzyńska, A.; Nizioł-Łukaszewska, Z.; Wójciak, M.; Sowa, I. Evaluation of the Biological Activity of Hydrogel with Cornus mas L. Extract and Its Potential Use in Dermatology and Cosmetology. Molecules 2023, 28, 7384. [Google Scholar] [CrossRef] [PubMed]
- Dinda, B.; Kyriakopoulos, A.M.; Dinda, S.; Zoumpourlis, V.; Thomaidis, N.S.; Velegraki, A.; Markopoulos, C.; Dinda, M. Cornus mas L. (Cornelian Cherry), an Important European and Asian Traditional Food and Medicine: Ethnomedicine, Phytochemistry and Pharmacology for Its Commercial Utilization in Drug Industry. J. Ethnopharmacol. 2016, 193, 670–690. [Google Scholar] [CrossRef]
- Huang, J.; Zhang, Y.; Dong, L.; Gao, Q.; Yin, L.; Quan, H.; Chen, R.; Fu, X.; Lin, D. Ethnopharmacology, Phytochemistry, and Pharmacology of Cornus officinalis Sieb. et Zucc. J. Ethnopharmacol. 2018, 213, 280–301. [Google Scholar] [CrossRef]
- He, K.; Song, S.; Zou, Z.; Feng, M.; Wang, D.; Wang, Y.; Li, X.; Ye, X. The Hypoglycemic and Synergistic Effect of Loganin, Morroniside, and Ursolic Acid Isolated from the Fruits of Cornus officinalis. Phyther. Res. 2016, 30, 283–291. [Google Scholar] [CrossRef]
- Bhadange, Y.A.; Carpenter, J.; Saharan, V.K. A Comprehensive Review on Advanced Extraction Techniques for Retrieving Bioactive Components from Natural Sources. ACS Omega 2024, 9, 31274–31297. [Google Scholar] [CrossRef]
- Sridhar, A.; Ponnuchamy, M.; Kumar, P.S.; Kapoor, A.; Vo, D.V.N.; Prabhakar, S. Techniques and Modeling of Polyphenol Extraction from Food: A Review. Environ. Chem. Lett. 2021, 19, 3409. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Pang, S.; Zhong, M.; Sun, Y.; Qayum, A.; Liu, Y.; Rashid, A.; Xu, B.; Liang, Q.; Ma, H.; et al. A Comprehensive Review of Ultrasonic Assisted Extraction (UAE) for Bioactive Components: Principles, Advantages, Equipment, and Combined Technologies. Ultrason. Sonochem. 2023, 101, 106646. [Google Scholar] [CrossRef]
- Streimikyte, P.; Viskelis, P.; Viskelis, J. Enzymes-Assisted Extraction of Plants for Sustainable and Functional Applications. Int. J. Mol. Sci. 2022, 23, 2359. [Google Scholar] [CrossRef]
- Yousefi, B.; Abasi, M.; Abbasi, M.M.; Jahanban-Esfahlan, R. Anti-Proliferative Properties of Cornus Mass Fruit in Different Human Cancer Cells. Asian Pacific J. Cancer Prev. 2015, 16, 5727–5731. [Google Scholar] [CrossRef]
- Klymenko, S.; Kucharska, A.Z.; Sokół-łętowska, A.; Piórecki, N.; Przybylska, D.; Grygorieva, O. Iridoids, Flavonoids, and Antioxidant Capacity of Cornus mas, C. officinalis, and C. mas × C. officinalis Fruits. Biomolecules 2021, 11, 776. [Google Scholar] [CrossRef] [PubMed]
- Sip, S.; Szymanowska, D.; Chanaj-Kaczmarek, J.; Skalicka-Woźniak, K.; Budzyńska, B.; Wronikowska-Denysiuk, O.; Słowik, T.; Szulc, P.; Cielecka-Piontek, J. Potential for Prebiotic Stabilized Cornus mas L. Lyophilized Extract in the Prophylaxis of Diabetes Mellitus in Streptozotocin Diabetic Rats. Antioxidants 2022, 11, 380. [Google Scholar] [CrossRef]
- Dumitraşcu, L.; Enachi, E.; Stănciuc, N.; Aprodu, I. Optimization of Ultrasound Assisted Extraction of Phenolic Compounds from Cornelian Cherry Fruits Using Response Surface Methodology. CYTA—J. Food 2019, 17, 814–823. [Google Scholar] [CrossRef]
- Sokoł-Łȩtowska, A.; Kucharska, A.Z.; Wińska, K.; Szumny, A.; Nawirska-Olszańska, A.; Mizgier, P.; Wyspiańska, D. Composition and Antioxidant Activity of Red Fruit Liqueurs. Food Chem. 2014, 157, 533–539. [Google Scholar] [CrossRef] [PubMed]
- You, Q.; Yin, X.; Zhao, Y. Enzyme Assisted Extraction of Polysaccharides from the Fruit of Cornus officinalis. Carbohydr. Polym. 2013, 98, 607–610. [Google Scholar] [CrossRef]
- You, Q.; Yin, X.; Xu, H. Ultrasonic-Enchanced Compound-Enzymes-Assisted Extraction of Polysaccharides from Cornus officinalis. Chem. Ind. Chem. Eng. Q. 2014, 21, 15. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, X.; Fan, E. Extraction, Structure, and Bioactivities of the Polysaccharides from Fructus. In Polysaccharides; Ramawat, K., Mérillon, J.M., Eds.; Springer: Cham, Switzerland, 2014; pp. 1–21. [Google Scholar] [CrossRef]
- Zhang, L.; Li, J.L.; Jiang, L.J. Studies on Process of Aqueous Enzymatic Extraction of Cornus wilsoniana Fruit Oil. In Proceedings of the 2012 International Conference on Biobase Material Science and Engineering, BMSE 2012, Changsha, China, 21–23 October 2012; pp. 83–86. [Google Scholar]
- Farmacopeea Română, 10th ed.; Editura Medicala: Bucharest, Romania, 1993.
- Gillani, F.; Raftani Amiri, Z.; Esmaeilzadeh Kenari, R. Assay of Antioxidant Activity and Bioactive Compounds of Cornelian Cherry (Cornus mas L.) Fruit Extracts Obtained by Green Extraction Methods: Ultrasound-Assisted, Supercritical Fluid, and Subcritical Water Extraction. Pharm. Chem. J. 2022, 56, 692–699. [Google Scholar] [CrossRef]
- Nicolescu, A.; Babotă, M.; Zhang, L.; Bunea, C.I.; Gavrilaș, L.; Vodnar, D.C.; Mocan, A.; Crișan, G.; Rocchetti, G. Optimized Ultrasound-Assisted Enzymatic Extraction of Phenolic Compounds from Rosa canina L. Pseudo-Fruits (Rosehip) and Their Biological Activity. Antioxidants 2022, 11, 1123. [Google Scholar] [CrossRef]
- Frumuzachi, O.; Nicolescu, A.; Martău, G.A.; Odocheanu, R.; Ranga, F.; Mocan, A.; Vodnar, D.C. Enhancing the Phenolic Profile and Antioxidant Activity in Corn Silk via Solid-State Fermentation with Aspergillus niger. Innov. Food Sci. Emerg. Technol. 2025, 105, 104239. [Google Scholar] [CrossRef]
- Babotă, M.; Frumuzachi, O.; Gâvan, A.; Iacoviță, C.; Pinela, J.; Barros, L.; Ferreira, I.C.F.R.; Zhang, L.; Lucini, L.; Rocchetti, G.; et al. Optimized Ultrasound-Assisted Extraction of Phenolic Compounds from Thymus comosus Heuff. Ex Griseb. et Schenk (Wild Thyme) and Their Bioactive Potential. Ultrason. Sonochem. 2022, 84, 105954. [Google Scholar] [CrossRef] [PubMed]
- García-Pérez, P.; Becchi, P.P.; Zhang, L.; Rocchetti, G.; Lucini, L. Metabolomics and Chemometrics: The next-Generation Analytical Toolkit for the Evaluation of Food Quality and Authenticity. Trends Food Sci. Technol. 2024, 147, 104481. [Google Scholar] [CrossRef]
- Frumuzachi, O.; Nicolescu, A.; Martău, G.-A.; Odocheanu, R.; Ranga, F.; Mocan, A.; Vodnar, D.C. Sustainable Valorization of Lignocellulosic Corn Husks via Solid-State Fermentation: Enhanced Recovery of Phenolic Compounds and Organic Acids. Bioresour. Technol. 2025, 436, 132959. [Google Scholar] [CrossRef]
- Babotă, M.; Frumuzachi, O.; Nicolescu, A.; Dias, M.I.; Pinela, J.; Barros, L.; Añibarro-Ortega, M.; Stojković, D.; Carević, T.; Mocan, A.; et al. Thymus Species from Romanian Spontaneous Flora as Promising Source of Phenolic Secondary Metabolites with Health-Related Benefits. Antioxidants 2023, 12, 390. [Google Scholar] [CrossRef] [PubMed]
- Babotă, M.; Frumuzachi, O.; Mocan, A.; Tămaș, M.; Dias, M.I.; Pinela, J.; Stojković, D.; Soković, M.; Bădărău, A.S.; Crișan, G.; et al. Unravelling Phytochemical and Bioactive Potential of Three Hypericum Species from Romanian Spontaneous Flora: H. alpigenum, H. perforatum, and H. rochelii. Plants 2022, 11, 2773. [Google Scholar] [CrossRef]
- Zengin, G.; Sarikurkcu, C.; Aktumsek, A.; Ceylan, R.; Ceylan, O. A Comprehensive Study on Phytochemical Characterization of Haplophyllum myrtifolium Boiss. Endemic to Turkey and Its Inhibitory Potential against Key Enzymes Involved in Alzheimer, Skin Diseases, and Type II Diabetes. Ind. Crops Prod. 2014, 53, 244–251. [Google Scholar] [CrossRef]
- Moldovan, C.; Frumuzachi, O.; Babotă, M.; Pinela, J.; Barros, L.; Rocchetti, G.; López, V.; Lucini, L.; Crișan, G.; Mocan, A. Untargeted Phytochemical Profiling and Biological Activity of Small Yellow Onion (Allium flavum L.) from Different Regions of Romania. Food Chem. 2023, 426, 136503. [Google Scholar] [CrossRef]
- Nicolescu, A.; Bunea, C.I.; Mocan, A. Total Flavonoid Content Revised: An Overview of Past, Present, and Future Determinations in Phytochemical Analysis. Anal. Biochem. 2025, 700, 115794. [Google Scholar] [CrossRef]
- Frumuzachi, O.; Nicolescu, A.; Babotă, M.; Mocan, A.; Sisea, C.R.; Hera, O.; Sturzeanu, M.; Rohn, S.; Lucini, L.; Crișan, G.; et al. D-Optimal Design-Based Ultrasound-Assisted Extraction Optimization and Extensive Bio-Structural Analysis of Phenolic Compounds from Romanian Cornelian Cherry (Cornus mas L.) Genotypes. Food Bioprocess Technol. 2025, 18, 7915–7932. [Google Scholar] [CrossRef]
- Aurori, M.; Niculae, M.; Hanganu, D.; Pall, E.; Cenariu, M.; Vodnar, D.C.; Bunea, A.; Fiţ, N.; Andrei, S. Phytochemical Profile, Antioxidant, Antimicrobial and Cytoprotective Effects of Cornelian Cherry (Cornus mas L.) Fruit Extracts. Pharmaceuticals 2023, 16, 420. [Google Scholar] [CrossRef]
- Spaggiari, C.; Othibeng, K.; Tugizimana, F.; Rocchetti, G.; Righetti, L. Towards a Greener Future: The Role of Sustainable Methodologies in Metabolomics Research. Adv. Sample Prep. 2025, 14, 100186. [Google Scholar] [CrossRef]
- Bayram, H.M.; Arda Ozturkcan, S. Bioactive Components and Biological Properties of Cornelian Cherry (Cornus mas L.): A Comprehensive Review. J. Funct. Foods 2020, 75, 104252. [Google Scholar] [CrossRef]
- Belarbi, S.; Vivier, M.; Zaghouani, W.; De Sloovere, A.; Agasse-Peulon, V.; Cardinael, P. Comparison of New Approach of GC-HRMS (Q-Orbitrap) to GC–MS/MS (Triple-Quadrupole) in Analyzing the Pesticide Residues and Contaminants in Complex Food Matrices. Food Chem. 2021, 359, 129932. [Google Scholar] [CrossRef]
- Gonçalves, L.M.; Valente, I.M.; Rodrigues, J.A. An Overview on Cardamonin. J. Med. Food 2014, 17, 633. [Google Scholar] [CrossRef] [PubMed]
- Sevindik, M.; Khassanov, V.T.; Sevindik, E.; Uysal, I.; Mohammed, F.S. Cornelian Cherry (Cornus mas L.): A Comprehensive Review on Its Usage Areas, Biological Activities, Mineral, Phenolic and Chemical Contents and Applications. Appl. Fruit Sci. 2024, 66, 2061–2071. [Google Scholar] [CrossRef]
- Drkenda, P.; Spahić, A.; Begić-Akagić, A.; Gaši, F.; Vranac, A.; Hudina, M.; Blanke, M. Pomological Characteristics of Some Autochthonous Genotypes of Cornelian Cherry (Cornus mas L.) in Bosnia and Herzegovina. Erwerbs-Obstbau 2014, 56, 59–66. [Google Scholar] [CrossRef]
- Sumner, L.W.; Amberg, A.; Barrett, D.; Beale, M.H.; Beger, R.; Daykin, C.A.; Fan, T.W.M.; Fiehn, O.; Goodacre, R.; Griffin, J.L.; et al. Proposed Minimum Reporting Standards for Chemical Analysis: Chemical Analysis Working Group (CAWG) Metabolomics Standards Initiative (MSI). Metabolomics 2007, 3, 211–221. [Google Scholar] [CrossRef]
- Mialon, N.; Roig, B.; Capodanno, E.; Cadiere, A. Untargeted Metabolomic Approaches in Food Authenticity: A Review That Showcases Biomarkers. Food Chem. 2023, 398, 133856. [Google Scholar] [CrossRef]
- Bedair, M.; Glenn, K.C. Evaluation of the Use of Untargeted Metabolomics in the Safety Assessment of Genetically Modified Crops. Metabolomics 2020, 16, 111. [Google Scholar] [CrossRef]
- Senoner, T.; Dichtl, W. Oxidative Stress in Cardiovascular Diseases: Still a Therapeutic Target? Nutrients 2019, 11, 2090. [Google Scholar] [CrossRef] [PubMed]
- Barnham, K.J.; Masters, C.L.; Bush, A.I. Neurodegenerative Diseases and Oxidative Stress. Nat. Rev. Drug Discov. 2004, 3, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Roberts, C.K.; Sindhu, K.K. Oxidative Stress and Metabolic Syndrome. Life Sci. 2009, 84, 705–712. [Google Scholar] [CrossRef] [PubMed]
- Behrangi, N.; Ghafoori, H.; Farahmand, Z.; Khani, E.M.; Sanati, M.H. Comparison among Cornelian Cherry and Prunus cerasus According to Phenolic Content and Antioxidant Capacity by Three Various Methods of Extraction. Food Nutr. Sci. 2015, 6, 1166–1173. [Google Scholar] [CrossRef]
- Enache, I.M.; Benito-Román, Ó.; Coman, G.; Vizireanu, C.; Stănciuc, N.; Andronoiu, D.G.; Mihalcea, L.; Sanz, M.T. Extraction Optimization and Valorization of the Cornelian Cherry Fruits Extracts: Evidence on Antioxidant Activity and Food Applications. Appl. Sci. 2021, 11, 10729. [Google Scholar] [CrossRef]
- Rocchetti, G.; Blasi, F.; Montesano, D.; Ghisoni, S.; Marcotullio, M.C.; Sabatini, S.; Cossignani, L.; Lucini, L. Impact of Conventional/Non-Conventional Extraction Methods on the Untargeted Phenolic Profile of Moringa Oleifera Leaves. Food Res. Int. 2019, 115, 319–327. [Google Scholar] [CrossRef]
- Granato, D.; Fidelis, M.; Haapakoski, M.; dos Santos Lima, A.; Viil, J.; Hellström, J.; Rätsep, R.; Kaldmäe, H.; Bleive, U.; Azevedo, L.; et al. Enzyme-Assisted Extraction of Anthocyanins and Other Phenolic Compounds from Blackcurrant (Ribes nigrum L.) Press Cake: From Processing to Bioactivities. Food Chem. 2022, 391, 133240. [Google Scholar] [CrossRef]
- Rocchetti, G.; Zhang, L.; Bocchi, S.; Giuberti, G.; Ak, G.; Elbasan, F.; Yıldıztugay, E.; Ceylan, R.; Picot-Allain, M.C.N.; Mahomoodally, M.F.; et al. The Functional Potential of Nine Allium Species Related to Their Untargeted Phytochemical Characterization, Antioxidant Capacity and Enzyme Inhibitory Ability. Food Chem. 2022, 368, 130782. [Google Scholar] [CrossRef]
- Alara, O.R.; Abdurahman, N.H.; Ukaegbu, C.I. Extraction of Phenolic Compounds: A Review. Curr. Res. Food Sci. 2021, 4, 200–214. [Google Scholar] [CrossRef] [PubMed]
- Tucci, S. The Role of Lipid and Carbohydrate Digestive Enzyme Inhibitors in the Management of Obesity: A Review of Current and Emerging Therapeutic Agents. Diabetes Metab. Syndr. Obes. Targets Ther. 2010, 3, 125. [Google Scholar] [CrossRef]
- Deri, B.; Kanteev, M.; Goldfeder, M.; Lecina, D.; Guallar, V.; Adir, N.; Fishman, A. The Unravelling of the Complex Pattern of Tyrosinase Inhibition. Sci. Rep. 2016, 6, 34993. [Google Scholar] [CrossRef]
- Marucci, G.; Buccioni, M.; Ben, D.D.; Lambertucci, C.; Volpini, R.; Amenta, F. Efficacy of Acetylcholinesterase Inhibitors in Alzheimer’s Disease. Neuropharmacology 2021, 190, 108352. [Google Scholar] [CrossRef]
- Świerczewska, A.; Buchholz, T.; Melzig, M.F.; Czerwińska, M.E. In Vitro α-Amylase and Pancreatic Lipase Inhibitory Activity of Cornus mas L. and Cornus alba L. Fruit Extracts. J. Food Drug Anal. 2019, 27, 249–258. [Google Scholar] [CrossRef] [PubMed]
- Blagojević, B.; Agić, D.; Serra, A.T.; Matić, S.; Matovina, M.; Bijelić, S.; Popović, B.M. An in Vitro and in Silico Evaluation of Bioactive Potential of Cornelian Cherry (Cornus mas L.) Extracts Rich in Polyphenols and Iridoids. Food Chem. 2021, 335, 127619. [Google Scholar] [CrossRef]
- Dzydzan, O.; Brodyak, I.; Strugała-Danak, P.; Strach, A.; Kucharska, A.Z.; Gabrielska, J.; Sybirna, N. Biological Activity of Extracts of Red and Yellow Fruits of Cornus mas L.—An in Vitro Evaluation of Antioxidant Activity, Inhibitory Activity against α-Glucosidase, Acetylcholinesterase, and Binding Capacity to Human Serum Albumin. Molecules 2022, 27, 2244. [Google Scholar] [CrossRef]
- Natić, M.; Pavlović, A.; Lo Bosco, F.; Stanisavljević, N.; Zagorac, D.D.; Akšić, M.F.; Papetti, A. Nutraceutical Properties and Phytochemical Characterization of Wild Serbian Fruits. Eur. Food Res. Technol. 2019, 245, 469–478. [Google Scholar] [CrossRef]
- Nizioł-Lukaszewska, Z.; Wasilewski, T.; Bujak, T.; Osika, P. Iridoids from Cornus mas L. and Their Potential as Innovative Ingredients in Cosmetics. Polish J. Chem. Technol. 2017, 19, 122–127. [Google Scholar] [CrossRef]
- Frumuzachi, O.; Flanagan, A.; Rohn, S.; Mocan, A. The Dichotomy between Functional and Functionalized Foods—A Critical Characterization of Concepts. Food Res. Int. 2025, 208, 116173. [Google Scholar] [CrossRef] [PubMed]
| Phenolic Equivalents (Eq.) | Tentatively Identified Compounds | CME (µg/g) | CMF (µg/g) | CMM (µg/g) | CMU (µg/g) |
|---|---|---|---|---|---|
| Cyanidin eq. | Cyanidin-3-glucoside; Pelargonidin 3-O-arabinoside; Delphinidin 3-O-galactoside; Malvidin 3-O-galactoside; Peonidin 3-O-diglucoside-5-O-glucoside | 48.6 ± 3.5 a | 49.7 ± 2.1 a | 63.6 ± 0.4 b | 62.8 ± 4.2 b |
| Catechin eq. | (+)-Catechin; (−)-Epigallocatechin 3-O-gallate; Epicatechin 3’-O-glucuronide; 3’-O-Methylepicatechin | 11.8 ± 2.6 a | 11.9 ± 2.2 a | 28.6 ± 0.9 b | 90.2 ± 1.4 c |
| Quercetin eq. | Quercetin; Kaempferol 3-O-rhamnoside; Myricetin 3-O-galactoside; Isorhamnetin 4’-O-glucuronide | 10.7 ± 0.2 a | 7.0 ± 0.1 a | 25.6 ± 1.5 b | 47.0 ± 5.4 c |
| Luteolin eq. | Luteolin 7-O-glucoside; Apigenin 7-O-glucuronide; Baicalein; Chrysoeriol 7-O-glucoside; Sinensetin | 222.9 ± 47.4 b | 196.3 ± 3.7 ab | 150.8 ± 27.9 a | 233.6 ± 1.6 b |
| Oleuropein eq. | Caftaric acid; Pyrocatechol; Hydroxytyrosol derivatives; Oleuropein-type phenolic fragments; Secoiridoid-linked phenolics | 1975.4 ± 35.1 b | 1506.5 ± 40.6 a | 1701.3 ± 94.9 ab | 1350.7 ± 30.3 a |
| Ferulic acid eq. | Ellagic acid; Syringic acid; Caffeic acid; p-Coumaric acid; Ferulic acid; 3-Caffeoylquinic acid | 104.4 ± 2.2 b | 46.0 ± 0.2 a | 263.1 ± 5.0 c | 420.2 ± 17.2 d |
| Resveratrol eq. | trans-Resveratrol; Piceatannol; Dihydroresveratrol | 107.9 ± 33.5 | 88.9 ± 1.7 | 107.6 ± 11.0 | 94.5 ± 8.0 |
| IC50 Value (μg/mL) | |||||
|---|---|---|---|---|---|
| Bioassay | CME | CMF | CMM | CMU | Reference Inhibitor |
| α-glucosidase Inhibition | 151.42 ± 4.36 c | 80.61 ± 4.61 ab | 92.38 ± 8.1 b | 77.17 ± 4.80 a | Acarbose: 48.15 ± 2.83 * |
| α-amylase Inhibition | 233.85 ± 14.29 c | 85.54 ± 5.46 b | 76.47 ± 4.54 b | 52.54 ± 1.58 a | Acarbose: 19.37 ± 1.12 * |
| Lipase Inhibition | 1434.68 ± 99.58 | 55.40 ± 0.86% | 52.24 ± 0.55% | 54.80 ± 0.32% | Orlistat: 68.47 ± 2.98 * |
| Acetylcholinesterase Inhibition | 280.81 ± 86.17 b | 140.07 ± 24.78 a | 118.43 ± 24.80 a | 134.76 ± 26.40 a | Galantamine: 0.12 ± 0.01 * |
| Tyrosinase Inhibition | 406.27 ± 91.26 a | 512.89 ± 71.52 a | 891.31 ± 128.11 b | 1184.17 ± 224.72 b | Kojic acid: 0.11 ± 0.01 * |
| Anthocyanins | Flavanols | Flavonols | Other Phenolics | Phenolic Acids | |
|---|---|---|---|---|---|
| AChE | −0.610 * | ns | ns | ns | ns |
| Amylase | −0.675 * | ns | ns | 0.695 * | ns |
| Glucosidase | ns | ns | ns | 0.738 ** | ns |
| Lipase | 0.597 * | ns | ns | −0.598 * | ns |
| Tyrosinase | 0.757 ** | 0.852 ** | 0.925 ** | ns | 0.890 ** |
| DPPH | ns | 0.880 ** | 0.743 ** | ns | 0.673 * |
| FRAP | ns | ns | ns | −0.771 ** | ns |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Frumuzachi, O.; Nicolescu, A.; Babotă, M.; Mocan, A.; Lucini, L.; Rohn, S.; Crișan, G.; Rocchetti, G. Untargeted UHPLC-HRMS Metabolomic Profiling of Cornus mas L. Fruits: Impact of Conventional and Emerging Extraction Methods on Phenolic Composition and Bioactivity. Antioxidants 2025, 14, 1419. https://doi.org/10.3390/antiox14121419
Frumuzachi O, Nicolescu A, Babotă M, Mocan A, Lucini L, Rohn S, Crișan G, Rocchetti G. Untargeted UHPLC-HRMS Metabolomic Profiling of Cornus mas L. Fruits: Impact of Conventional and Emerging Extraction Methods on Phenolic Composition and Bioactivity. Antioxidants. 2025; 14(12):1419. https://doi.org/10.3390/antiox14121419
Chicago/Turabian StyleFrumuzachi, Oleg, Alexandru Nicolescu, Mihai Babotă, Andrei Mocan, Luigi Lucini, Sascha Rohn, Gianina Crișan, and Gabriele Rocchetti. 2025. "Untargeted UHPLC-HRMS Metabolomic Profiling of Cornus mas L. Fruits: Impact of Conventional and Emerging Extraction Methods on Phenolic Composition and Bioactivity" Antioxidants 14, no. 12: 1419. https://doi.org/10.3390/antiox14121419
APA StyleFrumuzachi, O., Nicolescu, A., Babotă, M., Mocan, A., Lucini, L., Rohn, S., Crișan, G., & Rocchetti, G. (2025). Untargeted UHPLC-HRMS Metabolomic Profiling of Cornus mas L. Fruits: Impact of Conventional and Emerging Extraction Methods on Phenolic Composition and Bioactivity. Antioxidants, 14(12), 1419. https://doi.org/10.3390/antiox14121419












