Neuron–Glia Crosstalk in the Regulation of Astrocytic Antioxidative Mechanisms Following CNS Injury
Abstract
1. Introduction
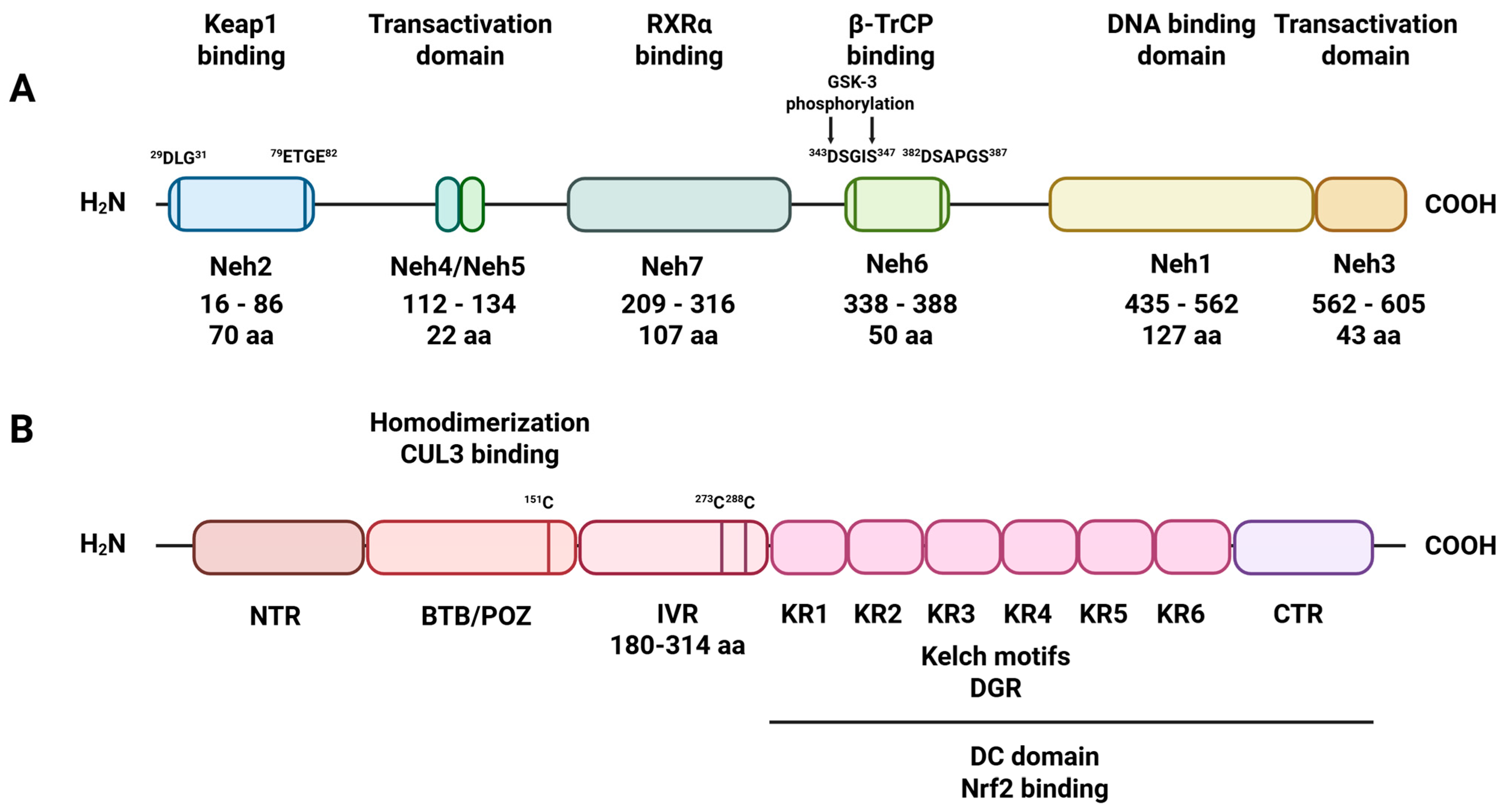
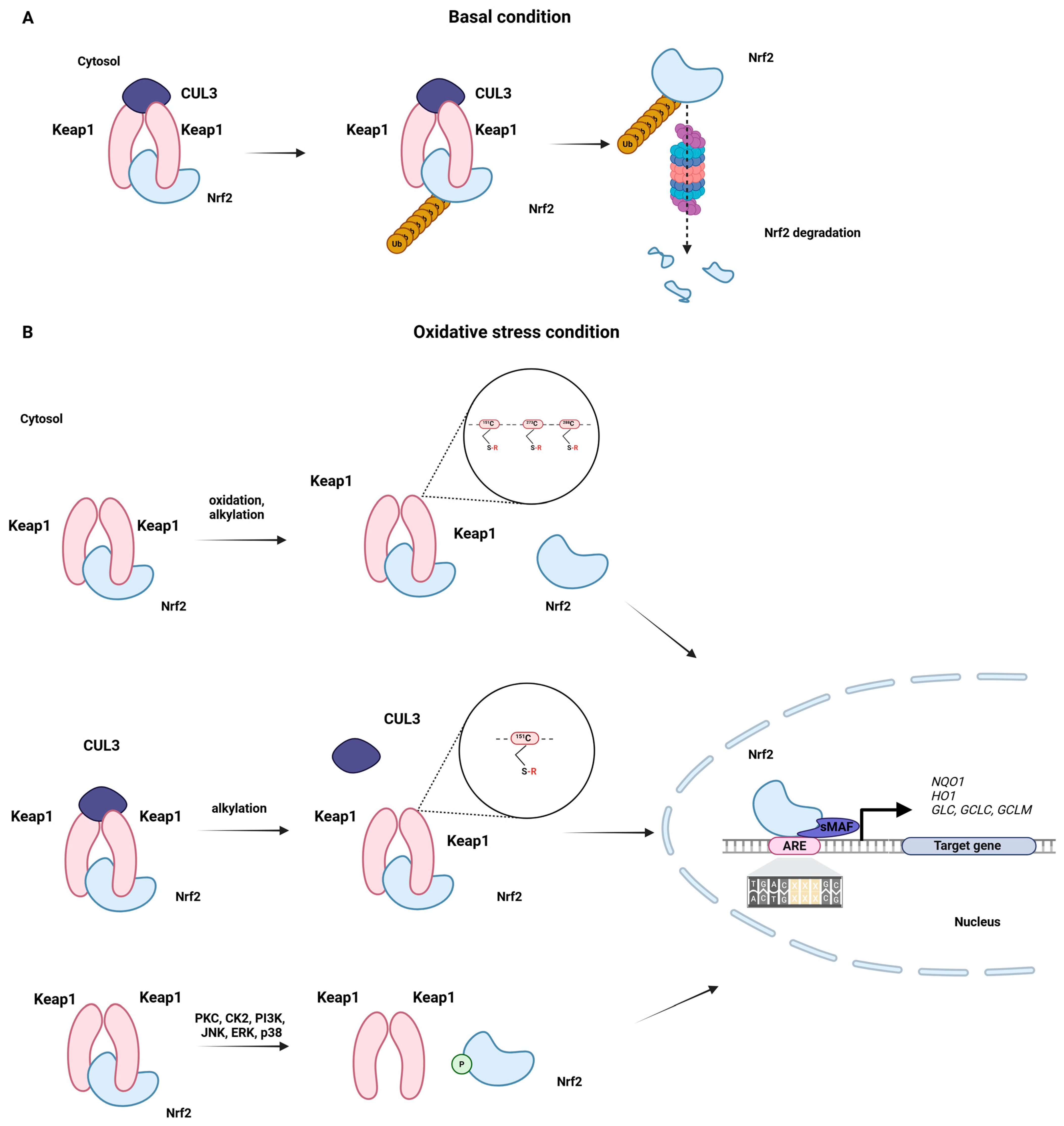
2. Astrocytic Antioxidant Machinery
3. Neuron–Astrocyte Crosstalk in Redox Regulation
4. Neuronal Signals That Boost Astrocytic Antioxidant Defences
5. Astrocytic Response Pathways to Neuronal Signals
6. Pathological Scenarios of Neuron–Glial Redox Signalling
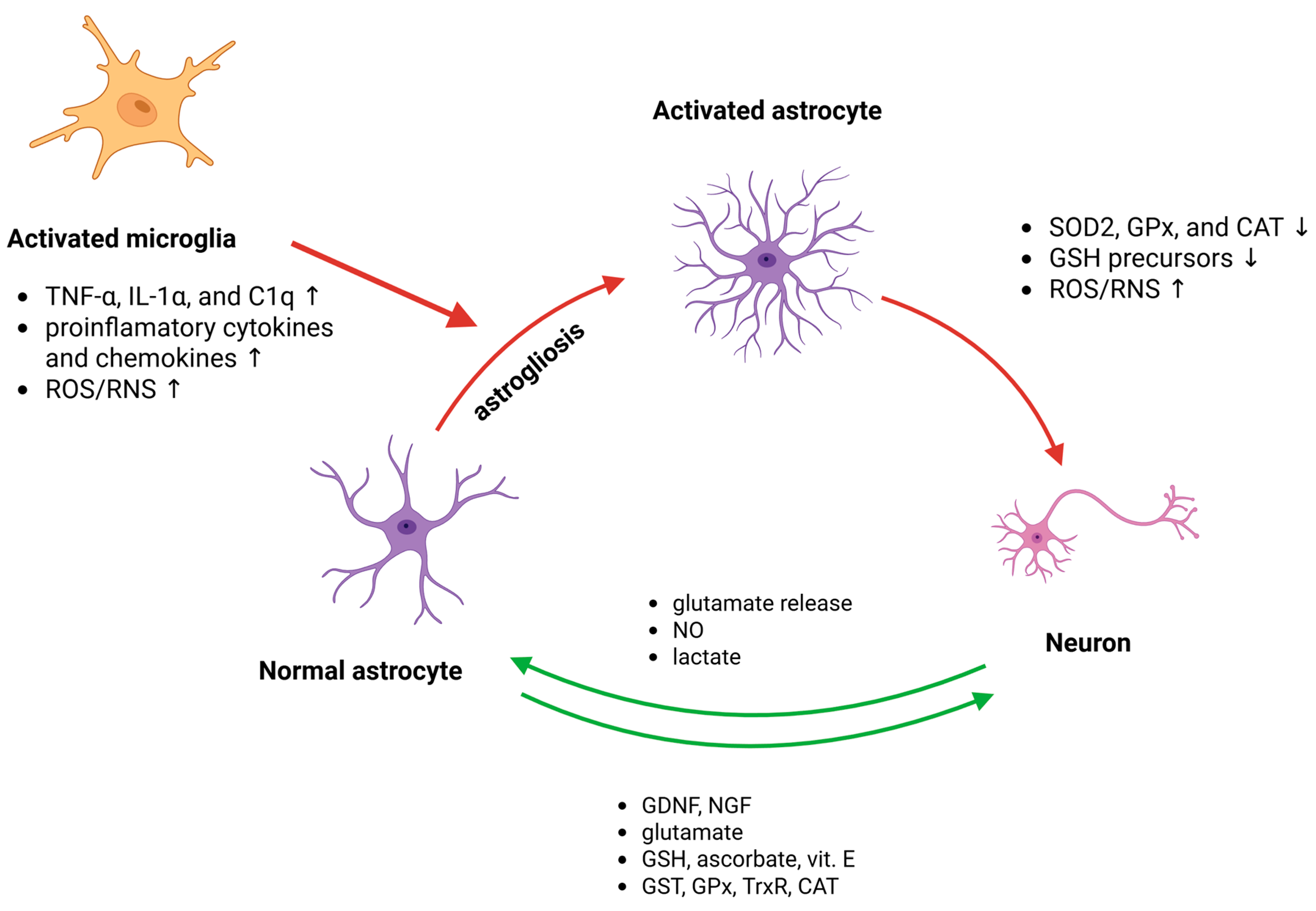
6.1. Neuron–Glial Redox Signalling Alteration
6.2. Neuron–Glia Redox Signalling in CNS Injuries
6.3. Neurodegenerative-Related Neuron–Glial Redox Signalling
7. Therapeutic Potential
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AAV | Adeno-Associated Virus |
| AD | Alzheimer’s Disease |
| ALS | Amyotrophic Lateral Sclerosis |
| AP-1 | Activator Protein 1 |
| ApoE | Apolipoprotein E |
| ARE | Antioxidant Response Element |
| ATF4 | Activating Transcription Factor 4 |
| ATP | Adenosine Triphosphate |
| Aβ | Amyloid Beta Peptide |
| BBB | Blood–Brain Barrier |
| BDNF | Brain-Derived Neurotrophic Factor |
| bFGF | Basic Fibroblast Growth Factor |
| C1q | Complement Component 1q |
| Ca2+ | Calcium Ion |
| CAT | Catalase |
| CBP | CREB-Binding Protein |
| CHD6 | Chromo-ATPase/Helicase DNA-Binding Protein 6 |
| CNS | Central Nervous System |
| CNTF | Ciliary Neurotrophic Factor |
| CREB | cAMP Response Element-Binding Protein |
| CUL3 | Cullin 3 |
| DAMPs | Damage-Associated Molecular Patterns |
| DT-13 | Saponin Compound from Liriope muscari |
| EAAT | Excitatory Amino Acid Transporter |
| ERK | Extracellular Signal-Regulated Kinase |
| GCL | γ-Glutamate-Cysteine Ligase |
| GCLC | γ-Glutamate-Cysteine Ligase Catalytic Subunit |
| GCLM | γ-Glutamate-Cysteine Ligase Modifier Subunit |
| GDNF | Glial Cell Line–Derived Neurotrophic Factor |
| GLT-1 | Glutamate Transporter-1 |
| GPx | Glutathione Peroxidase |
| GSH | Glutathione (reduced form) |
| GSK-3 | Glycogen Synthase Kinase 3 |
| GSSG | Glutathione Disulfide (oxidized form) |
| GST | Glutathione S-Transferase |
| HD | Huntington’s Disease |
| HO-1 | Heme Oxygenase-1 |
| IL-1β,3,4,10,33 | Interleukin-1β,3,4,10,33 |
| JAK/STAT | Janus Kinase/Signal Transducer and Activator of Transcription |
| JNK | c-Jun N-terminal Kinase |
| Keap1 | Kelch-Like ECH-Associated Protein 1 |
| MAF | Musculoaponeurotic Fibrosarcoma |
| MAPK | Mitogen-Activated Protein Kinase |
| mAβ | Monomeric Amyloid Beta |
| miRNA | MicroRNA |
| Mito-Q | Mitoquinone (ubiquinone derivative) |
| Mito-CP | Mito-Carboxy Proxyl |
| Mrp1, 5 | Multidrug Resistance Protein 1, 5 |
| MTA | Mitochondria-Targeted Antioxidant |
| NAC | N-Acetylcysteine |
| NADPH | Nicotinamide Adenine Dinucleotide Phosphate (reduced form) |
| NF-κB | Nuclear Factor Kappa B |
| NGF | Nerve Growth Factor |
| NOX2 | NADPH Oxidase 2 |
| NQO1 | NAD(P)H Quinone Oxidoreductase 1 |
| Nrf2 | Nuclear Factor Erythroid 2-Related Factor 2 |
| PD | Parkinson’s Disease |
| PDGF | Platelet-Derived Growth Factor |
| PKA | Protein Kinase A |
| PKC | Protein Kinase C |
| PPP | Pentose Phosphate Pathway |
| Prx | Peroxiredoxin |
| RAGE | Receptor for Advanced Glycation End-Products |
| Rg1/Rh1/Rb1 | Ginsenosides Rg1, Rh1, Rb1 (ginseng-derived saponins) |
| RIPK3 | Receptor-Interacting Serine/Threonine-Protein Kinase 3 |
| RNS | Reactive Nitrogen Species |
| ROS | Reactive Oxygen Species |
| RXRα | Retinoic X Receptor Alpha |
| SCI | Spinal Cord Injury |
| siRNA | Small Interfering RNA |
| SkQ1 | Plastoquinone-Derivative Antioxidant |
| sMAF | Small MAF Protein (MAFG/MAFK) |
| SOD | Superoxide Dismutase |
| SQSTM1/p62 | Sequestosome 1 Gene Encoding Protein p62 |
| SS-31 | Elamipretide (Szeto–Schiller peptide 31) |
| tBHQ | Tert-Butylhydroquinone |
| TBI | Traumatic Brain Injury |
| TGFβ | Transforming Growth Factor Beta |
| TNF-α | Tumor Necrosis Factor Alpha |
| Trx | Thioredoxin |
| TrxR | Thioredoxin Reductase |
| Wnt | Wingless/Integrated signalling patway |
| xc− | Cystine/Glutamate Antiporter |
| α-Syn | Alpha-Synuclein |
| β-TrCP | β-Transducing Repeat Containing Protein |
References
- Pathak, D.; Sriram, K. Neuron-Astrocyte Omnidirectional Signaling in Neurological Health and Disease. Front. Mol. Neurosci. 2023, 16, 1169320. [Google Scholar] [CrossRef]
- Huang, M.; Long, A.; Hao, L.; Shi, Z.; Zhang, M. Astrocyte in Neurological Disease: Pathogenesis and Therapy. MedComm 2025, 6, e70299. [Google Scholar] [CrossRef]
- Won, W.; Bhalla, M.; Lee, J.H.; Lee, C.J. Astrocytes as Key Regulators of Neural Signaling in Health and Disease. Annu. Rev. Neurosci. 2025, 48, 251–276. [Google Scholar] [CrossRef]
- Liu, T.; Rong, Z.; Li, J.; Wu, H.; Wei, J. Three-Dimensional Interactive Network: Mitochondrial-Metabolic-Calcium Homeostasis Driving Alzheimer’s Disease. Genes Dis. 2025, 101846. [Google Scholar] [CrossRef]
- Guo, C.Y.; Sun, L.; Chen, X.P.; Zhang, D.S. Oxidative Stress, Mitochondrial Damage and Neurodegenerative Diseases. Neural Regen. Res. 2013, 8, 2003–2014. [Google Scholar] [CrossRef]
- Beard, E.; Lengacher, S.; Dias, S.; Magistretti, P.J.; Finsterwald, C. Astrocytes as Key Regulators of Brain Energy Metabolism: New Therapeutic Perspectives. Front. Physiol. 2022, 12, 825816. [Google Scholar] [CrossRef]
- Zhang, Y.M.; Qi, Y.B.; Gao, Y.N.; Chen, W.G.; Zhou, T.; Zang, Y.; Li, J. Astrocyte Metabolism and Signaling Pathways in the CNS. Front. Neurosci. 2023, 17, 1217451. [Google Scholar] [CrossRef]
- Vargas, M.R.; Johnson, J.A. The Nrf2-ARE Cytoprotective Pathway in Astrocytes. Expert Rev. Mol. Med. 2009, 11, e17. [Google Scholar] [CrossRef]
- Todd, A.C.; Hardingham, G.E. The Regulation of Astrocytic Glutamate Transporters in Health and Neurodegenerative Diseases. Int. J. Mol. Sci. 2020, 21, 9607. [Google Scholar] [CrossRef]
- Achzet, L.M.; Davison, C.J.; Shea, M.; Sturgeon, I.; Jackson, D.A. Oxidative Stress Underlies the Ischemia/Reperfusion-Induced Internalization and Degradation of AMPA Receptors. Int. J. Mol. Sci. 2021, 22, 717. [Google Scholar] [CrossRef]
- Jurcau, A.; Ardelean, I.A. Molecular Pathophysiological Mechanisms of Ischemia/Reperfusion Injuries after Recanalization Therapy for Acute Ischemic Stroke. J. Integr. Neurosci. 2021, 20, 727–744. [Google Scholar] [CrossRef]
- Belov Kirdajova, D.; Kriska, J.; Tureckova, J.; Anderova, M. Ischemia-Triggered Glutamate Excitotoxicity from the Perspective of Glial Cells. Front. Cell. Neurosci. 2020, 14, 516047. [Google Scholar] [CrossRef]
- Hasan, A.R.; Tasnim, F.; Aktaruzzaman, M.; Islam, M.T.; Rayhan, R.; Brishti, A.; Hur, J.; Porter, J.E.; Raihan, M.O. The Alteration of Microglial Calcium Homeostasis in Central Nervous System Disorders: A Comprehensive Review. Neuroglia 2024, 5, 410–444. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, X.; Chai, Y.; Wang, Y.; Zhang, J.; Chen, X. Astrocyte-Mediated Inflammatory Responses in Traumatic Brain Injury: Mechanisms and Potential Interventions. Front. Immunol. 2025, 16, 1584577. [Google Scholar] [CrossRef]
- Wilson, D.M.; Cookson, M.R.; Van Den Bosch, L.; Zetterberg, H.; Holtzman, D.M.; Dewachter, I. Hallmarks of Neurodegenerative Diseases. Cell 2023, 186, 693–714. [Google Scholar] [CrossRef]
- Percário, S.; Da Silva Barbosa, A.; Varela, E.L.P.; Gomes, A.R.Q.; Ferreira, M.E.S.; De Nazaré Araújo Moreira, T.; Dolabela, M.F. Oxidative Stress in Parkinson’s Disease: Potential Benefits of Antioxidant Supplementation. Oxidative Med. Cell. Longev. 2020, 2020, 2360872. [Google Scholar] [CrossRef]
- Abramov, A.Y.; Canevari, L.; Duchen, M.R. β-Amyloid Peptides Induce Mitochondrial Dysfunction and Oxidative Stress in Astrocytes and Death of Neurons through Activation of NADPH Oxidase. J. Neurosci. 2004, 24, 565–575. [Google Scholar] [CrossRef]
- Sochocka, M.; Diniz, B.S.; Leszek, J. Inflammatory Response in the CNS: Friend or Foe? Mol. Neurobiol. 2017, 54, 8071–8089. [Google Scholar] [CrossRef]
- Fischer, R.; Maier, O. Interrelation of Oxidative Stress and Inflammation in Neurodegenerative Disease: Role of TNF. Oxidative Med. Cell. Longev. 2015, 2015, 610813. [Google Scholar] [CrossRef]
- Baxter, P.S.; Hardingham, G.E. Adaptive Regulation of the Brain’s Antioxidant Defences by Neurons and Astrocytes. Free Radic. Biol. Med. 2016, 100, 147–152. [Google Scholar] [CrossRef]
- Buendia, I.; Michalska, P.; Navarro, E.; Gameiro, I.; Egea, J.; León, R. Nrf2-ARE Pathway: An Emerging Target against Oxidative Stress and Neuroinflammation in Neurodegenerative Diseases. Pharmacol. Ther. 2016, 157, 84–104. [Google Scholar] [CrossRef]
- Gote, S.; Dubey, S.; Nargund, S.L.; Thapa, S. A Systematic Review of Natural Products Targeting Nrf2-Keap1-ARE Pathway and Their Influence on Neurodegenerative Disorders. Inflammopharmacology 2025, 33, 5097–5111. [Google Scholar] [CrossRef]
- Sengoku, T.; Shiina, M.; Suzuki, K.; Hamada, K.; Sato, K.; Uchiyama, A.; Kobayashi, S.; Oguni, A.; Itaya, H.; Kasahara, K.; et al. Structural Basis of Transcription Regulation by CNC Family Transcription Factor, Nrf2. Nucleic Acids Res. 2022, 50, 12543–12557. [Google Scholar] [CrossRef]
- Jaramillo, M.C.; Zhang, D.D. The Emerging Role of the Nrf2-Keap1 Signaling Pathway in Cancer. Genes Dev. 2013, 27, 2179–2191. [Google Scholar] [CrossRef]
- Suzuki, T.; Motohashi, H.; Yamamoto, M. Toward Clinical Application of the Keap1–Nrf2 Pathway. Trends Pharmacol. Sci. 2013, 34, 340–346. [Google Scholar] [CrossRef]
- Hayes, J.D.; McMahon, M.; Chowdhry, S.; Dinkova-Kostova, A.T. Cancer Chemoprevention Mechanisms Mediated Through the Keap1–Nrf2 Pathway. Antioxid. Redox Signal. 2010, 13, 1713–1748. [Google Scholar] [CrossRef]
- Zipper, L.M.; Timothy Mulcahy, R. The Keap1 BTB/POZ Dimerization Function Is Required to Sequester Nrf2 in Cytoplasm. J. Biol. Chem. 2002, 277, 36544–36552. [Google Scholar] [CrossRef]
- Lee, S.; Hu, L. Nrf2 Activation through the Inhibition of Keap1-Nrf2 Protein-Protein Interaction. Med. Chem. Res. 2020, 29, 846–867. [Google Scholar] [CrossRef]
- Li, X.; Zhang, D.; Hannink, M.; Beamer, L.J. Crystal Structure of the Kelch Domain of Human Keap1. J. Biol. Chem. 2004, 279, 54750–54758. [Google Scholar] [CrossRef]
- Dinkova-Kostova, A.T.; Kostov, R.V.; Canning, P. Keap1, the Cysteine-Based Mammalian Intracellular Sensor for Electrophiles and Oxidants. Arch. Biochem. Biophys. 2017, 617, 84–93. [Google Scholar] [CrossRef]
- Dayalan Naidu, S.; Dinkova-Kostova, A.T. KEAP1, a Cysteine-Based Sensor and a Drug Target for the Prevention and Treatment of Chronic Disease. Open Biol. 2020, 10, 200105. [Google Scholar] [CrossRef]
- Hayes, J.D.; Dinkova-Kostova, A.T. The Nrf2 Regulatory Network Provides an Interface between Redox and Intermediary Metabolism. Trends Biochem. Sci. 2014, 39, 199–218. [Google Scholar] [CrossRef]
- Barrera-Rodríguez, R. Importance of the Keap1-Nrf2 Pathway in NSCLC: Is it a Possible Biomarker? Biomed. Rep. 2018, 9, 375–382. [Google Scholar] [CrossRef]
- Li, W.; Yu, S.; Liu, T.; Kim, J.H.; Blank, V.; Li, H.; Kong, A.N.T. Heterodimerization with Small Maf Proteins Enhances Nuclear Retention of Nrf2 via Masking the NESzip Motif. Biochim. Biophys. Acta Mol. Cell Res. 2008, 1783, 1847–1856. [Google Scholar] [CrossRef]
- Baird, P.N.; Saw, S.M.; Lanca, C.; Guggenheim, J.A.; Smith, E.L.; Zhou, X.; Matsui, K.O.; Wu, P.C.; Sankaridurg, P.; Chia, A.; et al. Myopia. Nat. Rev. Dis. Primers 2020, 6, 99. [Google Scholar] [CrossRef]
- Kobayashi, M.; Yamamoto, M. Nrf2–Keap1 Regulation of Cellular Defense Mechanisms against Electrophiles and Reactive Oxygen Species. Adv. Enzym. Regul. 2006, 46, 113–140. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Lv, Y.F.; Zhao, J.L.; You, Q.D.; Jiang, Z.Y. Regulation of Nrf2 by Phosphorylation: Consequences for Biological Function and Therapeutic Implications. Free Radic. Biol. Med. 2021, 168, 129–141. [Google Scholar] [CrossRef] [PubMed]
- Cuadrado, A. Structural and Functional Characterization of Nrf2 Degradation by Glycogen Synthase Kinase 3/β-TrCP. Free Radic. Biol. Med. 2015, 88, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.M.; Calkins, M.J.; Chan, K.; Kan, Y.W.; Johnson, J.A. Identification of the NF-E2-Related Factor-2-Dependent Genes Conferring Protection against Oxidative Stress in Primary Cortical Astrocytes Using Oligonucleotide Microarray Analysis. J. Biol. Chem. 2003, 278, 12029–12038. [Google Scholar] [CrossRef]
- Pajares, M.; Jiménez-Moreno, N.; García-Yagüe, Á.J.; Escoll, M.; de Ceballos, M.L.; Van Leuven, F.; Rábano, A.; Yamamoto, M.; Rojo, A.I.; Cuadrado, A. Transcription Factor NFE2L2/NRF2 Is a Regulator of Macroautophagy Genes. Autophagy 2016, 12, 1902–1916. [Google Scholar] [CrossRef]
- Liberto, C.M.; Albrecht, P.J.; Herx, L.M.; Yong, V.W.; Levison, S.W. Pro-Regenerative Properties of Cytokine-Activated Astrocytes. J. Neurochem. 2004, 89, 1092–1100. [Google Scholar] [CrossRef] [PubMed]
- Cabezas, R.; Avila-Rodriguez, M.; Vega-Vela, N.E.; Echeverria, V.; González, J.; Hidalgo, O.A.; Santos, A.B.; Aliev, G.; Barreto, G.E. Growth Factors and Astrocytes Metabolism: Possible Roles for Platelet Derived Growth Factor. Med. Chem. 2016, 12, 204–210. [Google Scholar] [CrossRef]
- Liu, B.; Teschemacher, A.G.; Kasparov, S. Neuroprotective Potential of Astroglia. J. Neurosci. Res. 2017, 95, 2126–2139. [Google Scholar] [CrossRef]
- Bélanger, M.; Magistretti, P.J. The Role of Astroglia in Neuroprotection. Dialogues Clin. Neurosci. 2009, 11, 281–296. [Google Scholar] [CrossRef]
- Dringen, R.; Kussmaul, L.; Gutterer, J.M.; Hirrlinger, J.; Hamprecht, B. The Glutathione System of Peroxide Detoxification is Less Efficient in Neurons than in Astroglial Cells. J. Neurochem. 1999, 72, 2523–2530. [Google Scholar] [CrossRef]
- McBean, G.J. Cysteine, Glutathione, and Thiol Redox Balance in Astrocytes. Antioxidants 2017, 6, 62. [Google Scholar] [CrossRef]
- Chen, Y.; Vartiainen, N.E.; Ying, W.; Chan, P.H.; Koistinaho, J.; Swanson, R.A. Astrocytes Protect Neurons from Nitric Oxide Toxicity by a Glutathione-Dependent Mechanism. J. Neurochem. 2001, 77, 1601–1610. [Google Scholar] [CrossRef]
- Chen, Y.; Qin, C.; Huang, J.; Tang, X.; Liu, C.; Huang, K.; Xu, J.; Guo, G.; Tong, A.; Zhou, L. The Role of Astrocytes in Oxidative Stress of Central Nervous System: A Mixed Blessing. Cell Prolif. 2020, 53, e12781. [Google Scholar] [CrossRef]
- Lee, K.H.; Cha, M.; Lee, B.H. Neuroprotective Effect of Antioxidants in the Brain. Int. J. Mol. Sci. 2020, 21, 7152. [Google Scholar] [CrossRef] [PubMed]
- Castro, M.A.; Beltrán, F.A.; Brauchi, S.; Concha, I.I. A Metabolic Switch in Brain: Glucose and Lactate Metabolism Modulation by Ascorbic Acid. J. Neurochem. 2009, 110, 423–440. [Google Scholar] [CrossRef] [PubMed]
- Castro, M.A.; Pozo, M.; Cortés, C.; García, M.D.L.A.; Concha, I.I.; Nualart, F. Intracellular Ascorbic Acid Inhibits Transport of Glucose by Neurons, but Not by Astrocytes. J. Neurochem. 2007, 102, 773–782. [Google Scholar] [CrossRef]
- Salazar, K.; Espinoza, F.; Cerda-Gallardo, G.; Ferrada, L.; Magdalena, R.; Ramírez, E.; Ulloa, V.; Saldivia, N.; Troncoso, N.; Oviedo, M.J.; et al. SVCT2 Overexpression and Ascorbic Acid Uptake Increase Cortical Neuron Differentiation, Which is Dependent on Vitamin C Recycling between Neurons and Astrocytes. Antioxidants 2021, 10, 1413. [Google Scholar] [CrossRef]
- Miyazaki, I.; Asanuma, M. Multifunctional Metallothioneins as a Target for Neuroprotection in Parkinson’s Disease. Antioxidants 2023, 12, 894. [Google Scholar] [CrossRef]
- Li, Z.-D.; Kang, S.; Li, H.; Yu, P.; Xie, R.; Li, C.; Jing, Q.; Gong, Z.; Li, L.; Li, Z.; et al. Absence of Astrocytic Ceruloplasmin Reverses the Senescence Process with Aging of Learning and Memory Abilities. Redox Biol. 2025, 82, 103611. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Blasco, D.; Santofimia-Castanõ, P.; Gonzalez, A.; Almeida, A.; Bolanõs, J.P. Astrocyte NMDA Receptors’ Activity Sustains Neuronal Survival through a Cdk5-Nrf2 Pathway. Cell Death Differ. 2015, 22, 1877–1889. [Google Scholar] [CrossRef] [PubMed]
- Bell, K.F.S.; Al-Mubarak, B.; Martel, M.A.; McKay, S.; Wheelan, N.; Hasel, P.; Márkus, N.M.; Baxter, P.; Deighton, R.F.; Serio, A.; et al. Neuronal Development is Promoted by Weakened Intrinsic Antioxidant Defences Due to Epigenetic Repression of Nrf2. Nat. Commun. 2015, 6, 7066. [Google Scholar] [CrossRef] [PubMed]
- Baxter, P.S.; Márkus, N.M.; Dando, O.; He, X.; Al-Mubarak, B.R.; Qiu, J.; Hardingham, G.E. Targeted De-Repression of Neuronal Nrf2 Inhibits α-Synuclein Accumulation. Cell Death Dis. 2021, 12, 218. [Google Scholar] [CrossRef]
- Wilson, C.; Muñoz-Palma, E.; González-Billault, C. From Birth to Death: A Role for Reactive Oxygen Species in Neuronal Development. Semin. Cell Dev. Biol. 2018, 80, 43–49. [Google Scholar] [CrossRef]
- Bórquez, D.A.; Urrutia, P.J.; Wilson, C.; Van Zundert, B.; Núñez, M.T.; González-Billault, C. Dissecting the Role of Redox Signaling in Neuronal Development. J. Neurochem. 2016, 137, 506–517. [Google Scholar] [CrossRef]
- Funato, Y.; Michiue, T.; Asashima, M.; Miki, H. The Thioredoxin-Related Redox-Regulating Protein Nucleoredoxin Inhibits Wnt–β-Catenin Signalling through Dishevelled. Nat. Cell Biol. 2006, 8, 501–508. [Google Scholar] [CrossRef]
- Rharass, T.; Lemcke, H.; Lantow, M.; Kuznetsov, S.A.; Weiss, D.G.; Panáková, D. Ca2+-Mediated Mitochondrial Reactive Oxygen Species Metabolism Augments Wnt/β-Catenin Pathway Activation to Facilitate Cell Differentiation. J. Biol. Chem. 2014, 289, 27937–27951. [Google Scholar] [CrossRef]
- Yu, X.; Malenka, R.C. β-Catenin Is Critical for Dendritic Morphogenesis. Nat. Neurosci. 2003, 6, 1169–1177. [Google Scholar] [CrossRef]
- Rosso, S.B.; Sussman, D.; Wynshaw-Boris, A.; Salinas, P.C. Wnt Signaling through Dishevelled, Rac and JNK Regulates Dendritic Development. Nat. Neurosci. 2005, 8, 34–42. [Google Scholar] [CrossRef]
- Yang, Y.; Higashimori, H.; Morel, L. Developmental Maturation of Astrocytes and Pathogenesis of Neurodevelopmental Disorders. J. Neurodev. Disord. 2013, 5, 22. [Google Scholar] [CrossRef]
- Li, J.; Pan, L.; Pembroke, W.G.; Rexach, J.E.; Godoy, M.I.; Condro, M.C.; Alvarado, A.G.; Harteni, M.; Chen, Y.W.; Stiles, L.; et al. Conservation and Divergence of Vulnerability and Responses to Stressors between Human and Mouse Astrocytes. Nat. Commun. 2021, 12, 3958. [Google Scholar] [CrossRef]
- Aoyama, K. Glutathione in the Brain. Int. J. Mol. Sci. 2021, 22, 5010. [Google Scholar] [CrossRef] [PubMed]
- Almeida, A.; Jimenez-Blasco, D.; Bolaños, J.P. Cross-Talk between Energy and Redox Metabolism in Astrocyte-Neuron Functional Cooperation. Essays Biochem. 2023, 67, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Mattson, M.P.; Liu, D. Energetics and Oxidative Stress in Synaptic Plasticity and Neurodegenerative Disorders. Neuromolecular Med. 2002, 2, 215–231. [Google Scholar] [CrossRef] [PubMed]
- Cai, Q.; Sheng, Z.H. Molecular Motors and Synaptic Assembly. Neuroscientist 2009, 15, 78–89. [Google Scholar] [CrossRef]
- Almeida, A.; Bolaños, J.P. A Transient Inhibition of Mitochondrial ATP Synthesis by Nitric Oxide Synthase Activation Triggered Apoptosis in Primary Cortical Neurons. J. Neurochem. 2001, 77, 676–690. [Google Scholar] [CrossRef]
- Bolaños, J.P. Bioenergetics and Redox Adaptations of Astrocytes to Neuronal Activity. J. Neurochem. 2016, 139, 115–125. [Google Scholar] [CrossRef]
- Herrero-Mendez, A.; Almeida, A.; Fernández, E.; Maestre, C.; Moncada, S.; Bolaños, J.P. The Bioenergetic and Antioxidant Status of Neurons Is Controlled by Continuous Degradation of a Key Glycolytic Enzyme by APC/C-Cdh1. Nat. Cell Biol. 2009, 11, 747–752. [Google Scholar] [CrossRef]
- Bouzier-Sore, A.K.; Bolaños, J.P. Uncertainties in Pentose-Phosphate Pathway Flux Assessment Underestimate its Contribution to Neuronal Glucose Consumption: Relevance for Neurodegeneration and Aging. Front. Aging Neurosci. 2015, 7, 89. [Google Scholar] [CrossRef]
- Dringen, R.; Pfeiffer, B.; Hamprecht, B. Synthesis of the Antioxidant Glutathione in Neurons: Supply by Astrocytes of CysGly as Precursor for Neuronal Glutathione. J. Neurosci. 1999, 19, 562–569. [Google Scholar] [CrossRef]
- Quintana-Cabrera, R.; Bolaños, J.P. Glutathione and γ-Glutamylcysteine in Hydrogen Peroxide Detoxification. Methods Enzymol. 2013, 527, 129–144. [Google Scholar] [CrossRef]
- Fernandez-Fernandez, S.; Almeida, A.; Bolaños, J.P. Antioxidant and Bioenergetic Coupling between Neurons and Astrocytes. Biochem. J. 2012, 443, 3–12. [Google Scholar] [CrossRef]
- Minich, T.; Riemer, J.; Schulz, J.B.; Wielinga, P.; Wijnholds, J.; Dringen, R. The Multidrug Resistance Protein 1 (Mrp1), but Not Mrp5, Mediates Export of Glutathione and Glutathione Disulfide from Brain Astrocytes. J. Neurochem. 2006, 97, 373–384. [Google Scholar] [CrossRef]
- Bell, K.F.S.; Fowler, J.H.; Al-Mubarak, B.; Horsburgh, K.; Hardingham, G.E. Activation of Nrf2-Regulated Glutathione Pathway Genes by Ischemic Preconditioning. Oxidative Med. Cell. Longev. 2011, 2011, 689524. [Google Scholar] [CrossRef] [PubMed]
- Shih, A.Y.; Johnson, D.A.; Wong, G.; Kraft, A.D.; Jiang, L.; Erb, H.; Johnson, J.A.; Murphy, T.H. Coordinate Regulation of Glutathione Biosynthesis and Release by Nrf2-Expressing Glia Potently Protects Neurons from Oxidative Stress. J. Neurosci. 2003, 23, 3394–3406. [Google Scholar] [CrossRef] [PubMed]
- Davis, C.H.O.; Kim, K.Y.; Bushong, E.A.; Mills, E.A.; Boassa, D.; Shih, T.; Kinebuchi, M.; Phan, S.; Zhou, Y.; Bihlmeyer, N.A.; et al. Transcellular Degradation of Axonal Mitochondria. Proc. Natl. Acad. Sci. USA 2014, 111, 9633–9638. [Google Scholar] [CrossRef] [PubMed]
- Hayakawa, K.; Esposito, E.; Wang, X.; Terasaki, Y.; Liu, Y.; Xing, C.; Ji, X.; Lo, E.H. Transfer of Mitochondria from Astrocytes to Neurons after Stroke. Nature 2016, 535, 551–555. [Google Scholar] [CrossRef]
- Berridge, M.V.; Schneider, R.T.; McConnell, M.J. Mitochondrial Transfer from Astrocytes to Neurons Following Ischemic Insult: Guilt by Association? Cell Metab. 2016, 24, 376–378. [Google Scholar] [CrossRef]
- Ioannou, M.S.; Jackson, J.; Sheu, S.H.; Chang, C.L.; Weigel, A.V.; Liu, H.; Pasolli, H.A.; Xu, C.S.; Pang, S.; Matthies, D.; et al. Neuron-Astrocyte Metabolic Coupling Protects against Activity-Induced Fatty Acid Toxicity. Cell 2019, 177, 1522–1535.e14. [Google Scholar] [CrossRef]
- Qiu, J.; Dando, O.; Febery, J.A.; Fowler, J.H.; Chandran, S.; Hardingham, G.E. Neuronal Activity and Its Role in Controlling Antioxidant Genes. Int. J. Mol. Sci. 2020, 21, 1933. [Google Scholar] [CrossRef]
- Hasel, P.; Dando, O.; Jiwaji, Z.; Baxter, P.; Todd, A.C.; Heron, S.; Márkus, N.M.; McQueen, J.; Hampton, D.W.; Torvell, M.; et al. Neurons and Neuronal Activity Control Gene Expression in Astrocytes to Regulate Their Development and Metabolism. Nat. Commun. 2017, 8, 15132. [Google Scholar] [CrossRef]
- Habas, A.; Hahn, J.; Wang, X.; Margeta, M. Neuronal Activity Regulates Astrocytic Nrf2 Signaling. Proc. Natl. Acad. Sci. USA 2013, 110, 18291–18296. [Google Scholar] [CrossRef] [PubMed]
- Papadia, S.; Soriano, F.X.; Léveillé, F.; Martel, M.A.; Dakin, K.A.; Hansen, H.H.; Kaindl, A.; Sifringer, M.; Fowler, J.; Stefovska, V.; et al. Synaptic NMDA Receptor Activity Boosts Intrinsic Antioxidant Defenses. Nat. Neurosci. 2008, 11, 476–487. [Google Scholar] [CrossRef] [PubMed]
- Hardingham, G.E.; Lipton, S.A. Regulation of Neuronal Oxidative and Nitrosative Stress by Endogenous Protective Pathways and Disease Processes. Antioxid. Redox Signal. 2011, 14, 1421–1424. [Google Scholar] [CrossRef]
- Nguyen, T.; Yang, C.S.; Pickett, C.B. The Pathways and Molecular Mechanisms Regulating Nrf2 Activation in Response to Chemical Stress. Free Radic. Biol. Med. 2004, 37, 433–441. [Google Scholar] [CrossRef] [PubMed]
- Mayorquin, L.C.; Rodriguez, A.V.; Sutachan, J.J.; Albarracín, S.L. Connexin-Mediated Functional and Metabolic Coupling Between Astrocytes and Neurons. Front. Mol. Neurosci. 2018, 11, 118. [Google Scholar] [CrossRef]
- Jiwaji, Z.; Hardingham, G.E. The Consequences of Neurodegenerative Disease on Neuron-Astrocyte Metabolic and Redox Interactions. Neurobiol. Dis. 2023, 185, 106255. [Google Scholar] [CrossRef]
- Schipke, C.G.; Kettenmann, H. Astrocyte Responses to Neuronal Activity. Glia 2004, 47, 226–232. [Google Scholar] [CrossRef] [PubMed]
- Fellin, T.; Carmignoto, G. Neurone-to-Astrocyte Signalling in the Brain Represents a Distinct Multifunctional Unit. J. Physiol. 2004, 559, 3–15. [Google Scholar] [CrossRef]
- Parpura, V.; Basarsky, T.A.; Liu, F.; Jeftinija, K.; Jeftinija, S.; Haydon, P.G. Glutamate-Mediated Astrocyte–Neuron Signalling. Nature 1994, 369, 744–747. [Google Scholar] [CrossRef]
- Sofroniew, M.V. Molecular Dissection of Reactive Astrogliosis and Glial Scar Formation. Trends Neurosci. 2009, 32, 638–647. [Google Scholar] [CrossRef]
- Rosales-Corral, S.; Reiter, R.J.; Tan, D.X.; Ortiz, G.G.; Lopez-Armas, G. Functional Aspects of Redox Control during Neuroinflammation. Antioxid. Redox Signal. 2010, 13, 193–247. [Google Scholar] [CrossRef] [PubMed]
- Butterfield, D.A. Redox Signaling in Neurodegeneration. Neurobiol. Dis. 2015, 84, 1–3. [Google Scholar] [CrossRef]
- Aguilera, G.; Colín-González, A.L.; Rangel-López, E.; Chavarría, A.; Santamaría, A. Redox Signaling, Neuroinflammation, and Neurodegeneration. Antioxid. Redox Signal. 2018, 28, 1626–1651. [Google Scholar] [CrossRef]
- Franco, R.; Vargas, M.R. Redox Biology in Neurological Function, Dysfunction, and Aging. Antioxid. Redox Signal. 2018, 28, 1583–1586. [Google Scholar] [CrossRef]
- Spiers, J.G.; Chen, H.J.C.; Steinert, J.R. Redox Mechanisms and Their Pathological Role in Prion Diseases: The Road to Ruin. PLoS Pathog. 2023, 19, e1011309. [Google Scholar] [CrossRef] [PubMed]
- Raivich, G.; Bohatschek, M.; Kloss, C.U.A.; Werner, A.; Jones, L.L.; Kreutzberg, G.W. Neuroglial Activation Repertoire in the Injured Brain: Graded Response, Molecular Mechanisms and Cues to Physiological Function. Brain Res. Rev. 1999, 30, 77–105. [Google Scholar] [CrossRef]
- Guo, X.; Kang, J.; Wang, Z.; Wang, Y.; Liu, M.; Zhu, D.; Yang, F.; Kang, X. Nrf2 Signaling in the Oxidative Stress Response After Spinal Cord Injury. Neuroscience 2022, 498, 311–324. [Google Scholar] [CrossRef]
- Dugue, R.; Nath, M.; Dugue, A.; Barone, F.C. Roles of Pro- and Anti-Inflammatory Cytokines in Traumatic Brain Injury and Acute Ischemic Stroke. In Mechanisms of Neuroinflammation; IntechOpen: London, UK, 2017. [Google Scholar] [CrossRef]
- Jin, W.; Wang, H.; Yan, W.; Xu, L.; Wang, X.; Zhao, X.; Yang, X.; Chen, G.; Ji, Y. Disruption of Nrf2 Enhances Upregulation of Nuclear Factor-ΚB Activity, Proinflammatory Cytokines, and Intercellular Adhesion Molecule-1 in the Brain after Traumatic Brain Injury. Mediat. Inflamm. 2008, 2008, 725174. [Google Scholar] [CrossRef] [PubMed]
- Samarghandian, S.; Pourbagher-Shahri, A.M.; Ashrafizadeh, M.; Khan, H.; Forouzanfar, F.; Aramjoo, H.; Farkhondeh, T. A Pivotal Role of the Nrf2 Signaling Pathway in Spinal Cord Injury: A Prospective Therapeutics Study. CNS Neurol. Disord. Drug Targets 2020, 19, 207–219. [Google Scholar] [CrossRef] [PubMed]
- Silvestro, S.; Mazzon, E. Nrf2 Activation: Involvement in Central Nervous System Traumatic Injuries. A Promising Therapeutic Target of Natural Compounds. Int. J. Mol. Sci. 2022, 24, 199. [Google Scholar] [CrossRef] [PubMed]
- Heurtaux, T.; Bouvier, D.S.; Benani, A.; Helgueta Romero, S.; Frauenknecht, K.B.M.; Mittelbronn, M.; Sinkkonen, L. Normal and Pathological NRF2 Signalling in the Central Nervous System. Antioxidants 2022, 11, 1426. [Google Scholar] [CrossRef]
- Fadoul, G.; Ikonomovic, M.; Zhang, F.; Yang, T. The Cell-Specific Roles of Nrf2 in Acute and Chronic Phases of Ischemic Stroke. CNS Neurosci. Ther. 2024, 30, e14462. [Google Scholar] [CrossRef]
- Sivandzade, F.; Prasad, S.; Bhalerao, A.; Cucullo, L. NRF2 and NF-ҚB Interplay in Cerebrovascular and Neurodegenerative Disorders: Molecular Mechanisms and Possible Therapeutic Approaches. Redox Biol. 2019, 21, 101059. [Google Scholar] [CrossRef]
- Chandran, R.; Kim, T.H.; Mehta, S.L.; Udho, E.; Chanana, V.; Cengiz, P.; Kim, H.W.; Kim, C.; Vemuganti, R. A Combination Antioxidant Therapy to Inhibit NOX2 and Activate Nrf2 Decreases Secondary Brain Damage and Improves Functional Recovery after Traumatic Brain Injury. J. Cereb. Blood Flow Metab. 2018, 38, 1818–1827. [Google Scholar] [CrossRef]
- Muneer, P.M.A. Nrf2 as a Potential Therapeutic Target for Traumatic Brain Injury. J. Integr. Neurosci. 2023, 22, 81. [Google Scholar] [CrossRef]
- Liu, W.; Tang, Y.; Feng, J. Cross Talk between Activation of Microglia and Astrocytes in Pathological Conditions in the Central Nervous System. Life Sci. 2011, 89, 141–146. [Google Scholar] [CrossRef]
- Liddelow, S.A.; Guttenplan, K.A.; Clarke, L.E.; Bennett, F.C.; Bohlen, C.J.; Schirmer, L.; Bennett, M.L.; Münch, A.E.; Chung, W.S.; Peterson, T.C.; et al. Neurotoxic Reactive Astrocytes Are Induced by Activated Microglia. Nature 2017, 541, 481–487. [Google Scholar] [CrossRef]
- Li, J.; Wang, X.; Qin, S. Molecular Mechanisms and Signaling Pathways of Reactive Astrocytes Responding to Traumatic Brain Injury. Histol. Histopathol. 2021, 36, 921–929. [Google Scholar] [CrossRef]
- He, Y.; Liu, X.; Chen, Z. Glial Scar—A Promising Target for Improving Outcomes After CNS Injury. J. Mol. Neurosci. 2020, 70, 340–352. [Google Scholar] [CrossRef]
- Gao, Z.; Zhu, Q.; Zhang, Y.; Zhao, Y.; Cai, L.; Shields, C.B.; Cai, J. Reciprocal Modulation between Microglia and Astrocyte in Reactive Gliosis Following the CNS Injury. Mol. Neurobiol. 2013, 48, 690–701. [Google Scholar] [CrossRef] [PubMed]
- Kigerl, K.A.; Ankeny, D.P.; Garg, S.K.; Wei, P.; Guan, Z.; Lai, W.; Mctigue, D.M.; Banerjee, R.; Popovich, P.G. System Xc− Regulates Microglia and Macrophage Glutamate Excitotoxicity In Vivo. Exp. Neurol. 2011, 233, 333–341. [Google Scholar] [CrossRef]
- Guerriero, R.M.; Giza, C.C.; Rotenberg, A. Glutamate and GABA Imbalance Following Traumatic Brain Injury. Curr. Neurol. Neurosci. Rep. 2015, 15, 27. [Google Scholar] [CrossRef] [PubMed]
- Aizenman, E.; Loring, R.H.; Reynolds, I.J.; Rosenberg, P.A. The Redox Biology of Excitotoxic Processes: The NMDA Receptor, TOPA Quinone, and the Oxidative Liberation of Intracellular Zinc. Front. Neurosci. 2020, 14, 562891. [Google Scholar] [CrossRef]
- Ma, M.; Jiang, W.; Zhou, R. DAMPs and DAMP-Sensing Receptors in Inflammation and Diseases. Immunity 2024, 57, 752–771. [Google Scholar] [CrossRef] [PubMed]
- Chang, N.P.; DaPrano, E.M.; Lindman, M.; Estevez, I.; Chou, T.W.; Evans, W.R.; Nissenbaum, M.; McCourt, M.; Alzate, D.; Atkins, C.; et al. Neuronal DAMPs Exacerbate Neurodegeneration via Astrocytic RIPK3 Signaling. JCI Insight 2024, 9, e177002. [Google Scholar] [CrossRef]
- Reyes, R.C.; Brennan, A.M.; Shen, Y.; Baldwin, Y.; Swanson, R.A. Activation of Neuronal NMDA Receptors Induces Superoxide-Mediated Oxidative Stress in Neighboring Neurons and Astrocytes. J. Neurosci. 2012, 32, 12973–12978. [Google Scholar] [CrossRef] [PubMed]
- Haskew-Layton, R.E.; Payappilly, J.B.; Smirnova, N.A.; Ma, T.C.; Chan, K.K.; Murphy, T.H.; Guo, H.; Langley, B.; Sultana, R.; Butterfield, D.A.; et al. Controlled Enzymatic Production of Astrocytic Hydrogen Peroxide Protects Neurons from Oxidative Stress via an Nrf2-Independent Pathway. Proc. Natl. Acad. Sci. USA 2010, 107, 17385–17390. [Google Scholar] [CrossRef]
- Huang, Q.; Zhang, H.; Chen, S.; Wang, Y.; Zhou, J. Ferroptosis in Central Nervous System Injuries: Molecular Mechanisms, Diagnostic Approaches, and Therapeutic Strategies. Front. Cell. Neurosci. 2025, 19, 1593963. [Google Scholar] [CrossRef]
- Krajciova, G.; Filipcik, P.; Cente, M.; Skrabana, R.; Novak, M.; Shenk, J.C.; Castellani, R.J.; Moreira, P.; Aliev, G.; Siedlak, S.L.; et al. Iron-Induced Oxidative Stress in Primary Culture of Resting and Activated Astrocytes. Alzheimer’s Dement. 2009, 5, P314–P315. [Google Scholar] [CrossRef]
- Hoepken, H.H.; Korten, T.; Robinsont, S.R.; Dringen, R. Iron Accumulation, Iron-Mediated Toxicity and Altered Levels of Ferritin and Transferrin Receptor in Cultured Astrocytes during Incubation with Ferric Ammonium Citrate. J. Neurochem. 2004, 88, 1194–1202. [Google Scholar] [CrossRef] [PubMed]
- Geppert, M.; Hohnholt, M.C.; Nürnberger, S.; Dringen, R. Ferritin Up-Regulation and Transient ROS Production in Cultured Brain Astrocytes after Loading with Iron Oxide Nanoparticles. Acta Biomater. 2012, 8, 3832–3839. [Google Scholar] [CrossRef]
- Juurlink, B.H.J. Response of Glial Cells to Ischemia: Roles of Reactive Oxygen Species and Glutathione. Neurosci. Biobehav. Rev. 1997, 21, 151–166. [Google Scholar] [CrossRef]
- Hohnholt, M.C.; Dringen, R. Uptake and Metabolism of Iron and Iron Oxide Nanoparticles in Brain Astrocytes. Biochem. Soc. Trans. 2013, 41, 1588–1592. [Google Scholar] [CrossRef] [PubMed]
- Cheli, V.T.; Correale, J.; Paez, P.M.; Pasquini, J.M. Iron Metabolism in Oligodendrocytes and Astrocytes, Implications for Myelination and Remyelination. ASN Neuro 2020, 12, 1759091420962681. [Google Scholar] [CrossRef]
- Li, B.; Xia, M.; Zorec, R.; Parpura, V.; Verkhratsky, A. Astrocytes in Heavy Metal Neurotoxicity and Neurodegeneration. Brain Res. 2021, 1752, 147234. [Google Scholar] [CrossRef]
- David, S.; Jhelum, P.; Ryan, F.; Jeong, S.Y.; Kroner, A. Dysregulation of Iron Homeostasis in the CNS and the Role of Ferroptosis in Neurodegenerative Disorders. Antioxid. Redox Signal. 2021, 37, 150–170. [Google Scholar] [CrossRef]
- Xu, S.Y.; Ni, S.M.; Zeng, C.L.; Peng, Y.J. Role of Ferroptosis in Glial Cells after Ischemic Stroke. Front. Biosci. 2023, 28, 208. [Google Scholar] [CrossRef]
- Li, Y.; Xiao, D.; Wang, X. The Emerging Roles of Ferroptosis in Cells of the Central Nervous System. Front. Neurosci. 2022, 16, 1032140. [Google Scholar] [CrossRef]
- Ageeva, T.; Rizvanov, A.; Mukhamedshina, Y. NF-ΚB and JAK/STAT Signaling Pathways as Crucial Regulators of Neuroinflammation and Astrocyte Modulation in Spinal Cord Injury. Cells 2024, 13, 581. [Google Scholar] [CrossRef] [PubMed]
- Wardyn, J.D.; Ponsford, A.H.; Sanderson, C.M. Dissecting Molecular Cross-Talk between Nrf2 and NF-ΚB Response Pathways. Biochem. Soc. Trans. 2015, 43, 621–626. [Google Scholar] [CrossRef]
- Bonvento, G.; Bolaños, J.P. Astrocyte-Neuron Metabolic Cooperation Shapes Brain Activity. Cell Metab. 2021, 33, 1546–1564. [Google Scholar] [CrossRef]
- Bartnik-Olson, B.L.; Oyoyo, U.; Hovda, D.A.; Sutton, R.L. Astrocyte Oxidative Metabolism and Metabolite Trafficking after Fluid Percussion Brain Injury in Adult Rats. J. Neurotrauma 2010, 27, 2191–2202. [Google Scholar] [CrossRef] [PubMed]
- Burda, J.E.; Bernstein, A.M.; Sofroniew, M.V. Astrocyte Roles in Traumatic Brain Injury. Exp. Neurol. 2016, 275, 305–315. [Google Scholar] [CrossRef]
- Chen, Y.; Swanson, R.A. Astrocytes and Brain Injury. J. Cereb. Blood Flow Metab. 2003, 23, 137–149. [Google Scholar] [CrossRef] [PubMed]
- von Leden, R.E.; Parker, K.N.; Bates, A.A.; Noble-Haeusslein, L.J.; Donovan, M.H. The Emerging Role of Neutrophils as Modifiers of Recovery After Traumatic Injury to the Developing Brain. Exp. Neurol. 2019, 317, 144–154. [Google Scholar] [CrossRef]
- Morganti-Kossmann, M.C.; Semple, B.D.; Hellewell, S.C.; Bye, N.; Ziebell, J.M. The Complexity of Neuroinflammation Consequent to Traumatic Brain Injury: From Research Evidence to Potential Treatments. Acta Neuropathol. 2019, 137, 731–755. [Google Scholar] [CrossRef]
- Jantzie, L.; El Demerdash, N.; Newville, J.C.; Robinson, S. Time to Reconsider Extended Erythropoietin Treatment for Infantile Traumatic Brain Injury? Exp. Neurol. 2019, 318, 205–215. [Google Scholar] [CrossRef]
- Karve, I.P.; Taylor, J.M.; Crack, P.J. The Contribution of Astrocytes and Microglia to Traumatic Brain Injury. Br. J. Pharmacol. 2016, 173, 692–702. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.F.; Li, Y.; Song, J.N.; Pang, H.G. Role of Hydrogen Sulfide in Secondary Neuronal Injury. Neurochem. Int. 2014, 64, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.M.; Rzigalinski, B.A.; Willoughby, K.A.; Sitterding, H.A.; Ellis, E.F. Stretch-Induced Injury Alters Mitochondrial Membrane Potential and Cellular ATP in Cultured Astrocytes and Neurons. J. Neurochem. 2000, 74, 1951–1960. [Google Scholar] [CrossRef]
- Hilkens, N.A.; Casolla, B.; Leung, T.W.; de Leeuw, F.E. Stroke. Lancet 2024, 403, 2820–2836. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Emery, J.F.; Ouyang, Y.B.; Voloboueva, L.A.; Giffard, R.G. Astrocyte Targeted Overexpression of Hsp72 or SOD2 Reduces Neuronal Vulnerability to Forebrain Ischemia. Glia 2010, 58, 1042–1049. [Google Scholar] [CrossRef]
- Li, L.; Stary, C.M. Targeting Glial Mitochondrial Function for Protection from Cerebral Ischemia: Relevance, Mechanisms, and the Role of MicroRNAs. Oxidative Med. Cell. Longev. 2016, 2016, 6032306. [Google Scholar] [CrossRef]
- Choudhury, G.R.; Ding, S. Reactive Astrocytes and Therapeutic Potential in Focal Ischemic Stroke. Neurobiol. Dis. 2016, 85, 234–244. [Google Scholar] [CrossRef]
- Gouix, E.; Buisson, A.; Nieoullon, A.; Kerkerian-Le Goff, L.; Tauskela, J.S.; Blondeau, N.; Had-Aissouni, L. Oxygen Glucose Deprivation-Induced Astrocyte Dysfunction Provokes Neuronal Death through Oxidative Stress. Pharmacol. Res. 2014, 87, 8–17. [Google Scholar] [CrossRef]
- Kurkinen, M.; Fułek, M.; Fułek, K.; Beszłej, J.A.; Kurpas, D.; Leszek, J. The Amyloid Cascade Hypothesis in Alzheimer’s Disease: Should We Change Our Thinking? Biomolecules 2023, 13, 453. [Google Scholar] [CrossRef]
- Taniguchi, K.; Yamamoto, F.; Amano, A.; Tamaoka, A.; Sanjo, N.; Yokota, T.; Kametani, F.; Araki, W. Amyloid-β Oligomers Interact with NMDA Receptors Containing GluN2B Subunits and Metabotropic Glutamate Receptor 1 in Primary Cortical Neurons: Relevance to the Synapse Pathology of Alzheimer’s Disease. Neurosci. Res. 2022, 180, 90–98. [Google Scholar] [CrossRef]
- De Felice, F.G.; Velasco, P.T.; Lambert, M.P.; Viola, K.; Fernandez, S.J.; Ferreira, S.T.; Klein, W.L. Aβ Oligomers Induce Neuronal Oxidative Stress through an N-Methyl-D-Aspartate Receptor-Dependent Mechanism that is Blocked by the Alzheimer Drug Memantine. J. Biol. Chem. 2007, 282, 11590–11601. [Google Scholar] [CrossRef] [PubMed]
- Quintana, D.D.; Garcia, J.A.; Anantula, Y.; Rellick, S.L.; Engler-Chiurazzi, E.B.; Sarkar, S.N.; Brown, C.M.; Simpkins, J.W. Amyloid-β Causes Mitochondrial Dysfunction via a Ca2+-Driven Upregulation of Oxidative Phosphorylation and Superoxide Production in Cerebrovascular Endothelial Cells. J. Alzheimer’s Dis. 2020, 75, 119–138. [Google Scholar] [CrossRef]
- Cheignon, C.; Tomas, M.; Bonnefont-Rousselot, D.; Faller, P.; Hureau, C.; Collin, F. Oxidative Stress and the Amyloid Beta Peptide in Alzheimer’s Disease. Redox Biol. 2018, 14, 450–464. [Google Scholar] [CrossRef] [PubMed]
- Ramsey, C.P.; Glass, C.A.; Montgomery, M.B.; Lindl, K.A.; Ritson, G.P.; Chia, L.A.; Hamilton, R.L.; Chu, C.T.; Jordan-Sciutto, K.L. Expression of Nrf2 in Neurodegenerative Diseases. J. Neuropathol. Exp. Neurol. 2007, 66, 75–85. [Google Scholar] [CrossRef]
- Lastres-Becker, I.; Innamorato, N.G.; Jaworski, T.; Rábano, A.; Kügler, S.; Van Leuven, F.; Cuadrado, A. Fractalkine Activates NRF2/NFE2L2 and Heme Oxygenase 1 to Restrain Tauopathy-Induced Microgliosis. Brain 2014, 137, 78–91. [Google Scholar] [CrossRef] [PubMed]
- Tanji, K.; Maruyama, A.; Odagiri, S.; Mori, F.; Itoh, K.; Kakita, A.; Takahashi, H.; Wakabayashi, K. Keap1 Is Localized in Neuronal and Glial Cytoplasmic Inclusions in Various Neurodegenerative Diseases. J. Neuropathol. Exp. Neurol. 2013, 72, 18–28. [Google Scholar] [CrossRef]
- Raina, A.K.; Templeton, D.J.; Deak, J.C.; Perry, G.; Smith, M.A. Quinone Reductase (NQO1), a Sensitive Redox Indicator, Is Increased in Alzheimer’s Disease. Redox Rep. 1999, 4, 23–27. [Google Scholar] [CrossRef]
- Wang, Y.; Santa-Cruz, K.; Decarli, C.; Johnson, J.A. NAD(P)H:Quinone Oxidoreductase Activity Is Increased in Hippocampal Pyramidal Neurons of Patients with Alzheimer’s Disease. Neurobiol. Aging 2000, 21, 525–531. [Google Scholar] [CrossRef]
- SantaCruz, K.S.; Yazlovitskaya, E.; Collins, J.; Johnson, J.; DeCarli, C. Regional NAD(P)H:Quinone Oxidoreductase Activity in Alzheimer’s Disease. Neurobiol. Aging 2004, 25, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Schipper, H.M.; Bennett, D.A.; Liberman, A.; Bienias, J.L.; Schneider, J.A.; Kelly, J.; Arvanitakis, Z. Glial Heme Oxygenase-1 Expression in Alzheimer Disease and Mild Cognitive Impairment. Neurobiol. Aging 2006, 27, 252–261. [Google Scholar] [CrossRef]
- Schipper, H.M.; Cissé, S.; Stopa, E.G. Expression of Heme Oxygenase-1 in the Senescent and Alzheimer-Diseased Brain. Ann. Neurol. 1995, 37, 758–768. [Google Scholar] [CrossRef]
- Smith, M.A.; Kutty, R.K.; Richey, P.L.; Yan, S.D.; Stern, D.; Chader, G.J.; Wiggert, B.; Petersen, R.B.; Perry, G. Heme Oxygenase-1 is Associated with the Neurofibrillary Pathology of Alzheimer’s Disease. Am. J. Pathol. 1994, 145, 42–47. [Google Scholar]
- Fiebig, C.; Keiner, S.; Ebert, B.; Schäffner, I.; Jagasia, R.; Lie, D.C.; Beckervordersandforth, R. Mitochondrial Dysfunction in Astrocytes Impairs the Generation of Reactive Astrocytes and Enhances Neuronal Cell Death in the Cortex upon Photothrombotic Lesion. Front. Mol. Neurosci. 2019, 12, 421444. [Google Scholar] [CrossRef]
- Burda, J.E.; Sofroniew, M.V. Reactive Gliosis and the Multicellular Response to CNS Damage and Disease. Neuron 2014, 81, 229–248. [Google Scholar] [CrossRef]
- Lemoine, L.; Saint-Aubert, L.; Nennesmo, I.; Gillberg, P.G.; Nordberg, A. Cortical Laminar Tau Deposits and Activated Astrocytes in Alzheimer’s Disease Visualised by 3 H-THK5117 and 3 H-Deprenyl Autoradiography. Sci. Rep. 2017, 7, 45496. [Google Scholar] [CrossRef]
- Marutle, A.; Gillberg, P.G.; Bergfors, A.; Yu, W.; Ni, R.; Nennesmo, I.; Voytenko, L.; Nordberg, A. 3H-Deprenyl and 3H-PIB Autoradiography Show Different Laminar Distributions of Astroglia and Fibrillar β-Amyloid in Alzheimer Brain. J. Neuroinflamm. 2013, 10, 861. [Google Scholar] [CrossRef] [PubMed]
- Serrano-Pozo, A.; Mielke, M.L.; Gómez-Isla, T.; Betensky, R.A.; Growdon, J.H.; Frosch, M.P.; Hyman, B.T. Reactive Glia Not Only Associates with Plaques but Also Parallels Tangles in Alzheimer’s Disease. Am. J. Pathol. 2011, 179, 1373–1384. [Google Scholar] [CrossRef]
- Heneka, M.T.; Rodríguez, J.J.; Verkhratsky, A. Neuroglia in Neurodegeneration. Brain Res. Rev. 2010, 63, 189–211. [Google Scholar] [CrossRef] [PubMed]
- Ye, B.; Shen, H.; Zhang, J.; Zhu, Y.G.; Ransom, B.R.; Chen, X.C.; Ye, Z.C. Dual Pathways Mediate β-Amyloid Stimulated Glutathione Release from Astrocytes. Glia 2015, 63, 2208–2219. [Google Scholar] [CrossRef]
- Bantle, C.M.; Hirst, W.D.; Weihofen, A.; Shlevkov, E. Mitochondrial Dysfunction in Astrocytes: A Role in Parkinson’s Disease? Front. Cell Dev. Biol. 2021, 8, 608026. [Google Scholar] [CrossRef] [PubMed]
- Sznejder-Pachołek, A.; Joniec-Maciejak, I.; Wawer, A.; Ciesielska, A.; Mirowska-Guzel, D. The Effect of α-Synuclein on Gliosis and IL-1α, TNFα, IFNγ, TGFβ Expression in Murine Brain. Pharmacol. Rep. 2017, 69, 242–251. [Google Scholar] [CrossRef] [PubMed]
- Valdinocci, D.; Radford, R.A.W.; Siow, S.M.; Chung, R.S.; Pountney, D.L. Potential Modes of Intercellular α-Synuclein Transmission. Int. J. Mol. Sci. 2017, 18, 469. [Google Scholar] [CrossRef]
- Song, Y.J.C.; Halliday, G.M.; Holton, J.L.; Lashley, T.; Osullivan, S.S.; McCann, H.; Lees, A.J.; Ozawa, T.; Williams, D.R.; Lockhart, P.J.; et al. Degeneration in Different Parkinsonian Syndromes Relates to Astrocyte Type and Astrocyte Protein Expression. J. Neuropathol. Exp. Neurol. 2009, 68, 1073–1083. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Suk, J.E.; Patrick, C.; Bae, E.J.; Cho, J.H.; Rho, S.; Hwang, D.; Masliah, E.; Lee, S.J. Direct Transfer of α-Synuclein from Neuron to Astroglia Causes Inflammatory Responses in Synucleinopathies. J. Biol. Chem. 2010, 285, 9262–9272. [Google Scholar] [CrossRef]
- Fellner, L.; Irschick, R.; Schanda, K.; Reindl, M.; Klimaschewski, L.; Poewe, W.; Wenning, G.K.; Stefanova, N. Toll-like Receptor 4 is Required for α-Synuclein Dependent Activation of Microglia and Astroglia. Glia 2013, 61, 349–360. [Google Scholar] [CrossRef]
- Chavarría, C.; Rodríguez-Bottero, S.; Quijano, C.; Cassina, P.; Souza, J.M. Impact of Monomeric, Oligomeric and Fibrillar Alpha-Synuclein on Astrocyte Reactivity and Toxicity to Neurons. Biochem. J. 2018, 475, 3153–3169. [Google Scholar] [CrossRef]
- Angelova, P.R.; Horrocks, M.H.; Klenerman, D.; Gandhi, S.; Abramov, A.Y.; Shchepinov, M.S. Lipid Peroxidation is Essential for α-Synuclein-Induced Cell Death. J. Neurochem. 2015, 133, 582–589. [Google Scholar] [CrossRef]
- Lee, J.; Hyeon, S.J.; Im, H.; Ryu, H.; Kim, Y.; Ryu, H. Astrocytes and Microglia as Non-Cell Autonomous Players in the Pathogenesis of ALS. Exp. Neurobiol. 2016, 25, 233–240. [Google Scholar] [CrossRef]
- Ferraiuolo, L.; Higginbottom, A.; Heath, P.R.; Barber, S.; Greenald, D.; Kirby, J.; Shaw, P.J. Dysregulation of Astrocyte-Motoneuron Cross-Talk in Mutant Superoxide Dismutase 1-Related Amyotrophic Lateral Sclerosis. Brain 2011, 134, 2627–2641. [Google Scholar] [CrossRef] [PubMed]
- Bakshi, R.; Xu, Y.; Mueller, K.A.; Chen, X.; Granucci, E.; Paganoni, S.; Sadri-Vakili, G.; Schwarzschild, M.A. Urate Mitigates Oxidative Stress and Motor Neuron Toxicity of Astrocytes Derived from ALS-Linked SOD1 G93A Mutant Mice. Mol. Cell. Neurosci. 2018, 92, 12–16. [Google Scholar] [CrossRef]
- Kraft, A.D.; Johnson, D.A.; Johnson, J.A. Nuclear Factor E2-Related Factor 2-Dependent Antioxidant Response Element Activation by Tert-Butylhydroquinone and Sulforaphane Occurring Preferentially in Astrocytes Conditions Neurons against Oxidative Insult. J. Neurosci. 2004, 24, 1101–1112. [Google Scholar] [CrossRef]
- Zhang, Z.W.; Liang, J.; Yan, J.X.; Ye, Y.C.; Wang, J.J.; Chen, C.; Sun, H.T.; Chen, F.; Tu, Y.; Li, X.H. TBHQ Improved Neurological Recovery after Traumatic Brain Injury by Inhibiting the Overactivation of Astrocytes. Brain Res. 2020, 1739, 146818. [Google Scholar] [CrossRef]
- Lu, X.Y.; Wang, H.D.; Xu, J.G.; Ding, K.; Li, T. Pretreatment with Tert-Butylhydroquinone Attenuates Cerebral Oxidative Stress in Mice after Traumatic Brain Injury. J. Surg. Res. 2014, 188, 206–212. [Google Scholar] [CrossRef]
- Jin, W.; Kong, J.; Wang, H.; Wu, J.; Lu, T.; Jiang, J.; Ni, H.; Liang, W. Protective Effect of Tert-Butylhydroquinone on Cerebral Inflammatory Response Following Traumatic Brain Injury in Mice. Injury 2011, 42, 714–718. [Google Scholar] [CrossRef]
- Khezerlou, A.; Akhlaghi, A.p.; Alizadeh, A.M.; Dehghan, P.; Maleki, P. Alarming Impact of the Excessive Use of Tert-Butylhydroquinone in Food Products: A Narrative Review. Toxicol. Rep. 2022, 9, 1066–1075. [Google Scholar] [CrossRef] [PubMed]
- Danilov, C.A.; Chandrasekaran, K.; Racz, J.; Soane, L.; Zielke, C.; Fiskum, G. Sulforaphane Protects Astrocytes against Oxidative Stress and Delayed Death Caused by Oxygen and Glucose Deprivation. Glia 2009, 57, 645–656. [Google Scholar] [CrossRef]
- Bergström, P.; Andersson, H.C.; Gao, Y.; Karlsson, J.O.; Nodin, C.; Anderson, M.F.; Nilsson, M.; Hammarsten, O. Repeated Transient Sulforaphane Stimulation in Astrocytes Leads to Prolonged Nrf2-Mediated Gene Expression and Protection from Superoxide-Induced Damage. Neuropharmacology 2011, 60, 343–353. [Google Scholar] [CrossRef]
- Jazwa, A.; Rojo, A.I.; Innamorato, N.G.; Hesse, M.; Fernández-Ruiz, J.; Cuadrado, A. Pharmacological Targeting of the Transcription Factor Nrf2 at the Basal Ganglia Provides Disease Modifying Therapy for Experimental Parkinsonism. Antioxid. Redox Signal. 2011, 14, 2347–2360. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Bai, H.; Yang, D.; Wuhanqimuge; Bai, S.; Xiao, H.; Baigude, H.; Gao, N. Overexpression of BDNF by Astrocytes Targeted Delivery of MRNA Ameliorates Cognitive Impairment in Mouse Model of TBI. ACS Chem. Neurosci. 2025, 16, 3465–3471. [Google Scholar] [CrossRef]
- Sun, J.; Hu, H.; Ren, X.; Simpkins, J.W. Tert-Butylhydroquinone Compromises Survival in Murine Experimental Stroke. Neurotoxicology Teratol. 2016, 54, 15–21. [Google Scholar] [CrossRef]
- Klomparens, E.A.; Ding, Y. The Neuroprotective Mechanisms and Effects of Sulforaphane. Brain Circ. 2019, 5, 74–83. [Google Scholar] [CrossRef]
- Zheng, W.; Li, X.; Zhang, T.; Wang, J. Biological Mechanisms and Clinical Efficacy of Sulforaphane for Mental Disorders. Gen. Psychiatr. 2022, 35, e100700. [Google Scholar] [CrossRef]
- Hu, L.; Cao, Y.; Chen, H.; Xu, L.; Yang, Q.; Zhou, H.; Li, J.; Yu, Q.; Dou, Z.; Li, Y.; et al. The Novel Nrf2 Activator Omaveloxolone Regulates Microglia Phenotype and Ameliorates Secondary Brain Injury after Intracerebral Hemorrhage in Mice. Oxidative Med. Cell. Longev. 2022, 2022, 4564471. [Google Scholar] [CrossRef] [PubMed]
- Abeti, R.; Baccaro, A.; Esteras, N.; Giunti, P. Novel Nrf2-Inducer Prevents Mitochondrial Defects and Oxidative Stress in Friedreich’s Ataxia Models. Front. Cell. Neurosci. 2018, 12, 374847. [Google Scholar] [CrossRef]
- Reisman, S.A.; Gahir, S.S.; Lee, C.Y.I.; Proksch, J.W.; Sakamoto, M.; Ward, K.W. Pharmacokinetics and Pharmacodynamics of the Novel Nrf2 Activator Omaveloxolone in Primates. Drug Des. Dev. Ther. 2019, 13, 1259–1270. [Google Scholar] [CrossRef]
- Liu, S.; Chen, W.; Zhao, Y.; Zong, Y.; Li, J.; He, Z. Research Progress on Effects of Ginsenoside Rg2 and Rh1 on Nervous System and Related Mechanisms. Molecules 2023, 28, 7935. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Evankovich, J.W.; Lear, T.B.; Tuncer, F.; Kennerdell, J.R.; Camarco, D.P.; Shishido, M.S.; Liu, Y.; Chen, B.B. A Small Molecule NRF2 Activator BC-1901S Ameliorates Inflammation through DCAF1/NRF2 Axis. Redox Biol. 2020, 32, 101485. [Google Scholar] [CrossRef] [PubMed]
- Chu, S.-F.; Zhang, Z.; Zhou, X.; He, W.-B.; Chen, C.; Luo, P.; Liu, D.-D.; Ai, Q.-D.; Gong, H.-F.; Wang, Z.-Z.; et al. Ginsenoside Rg1 Protects against Ischemic/Reperfusion-Induced Neuronal Injury through MiR-144/Nrf2/ARE Pathway. Acta Pharmacol. Sin. 2019, 40, 13–25. [Google Scholar] [CrossRef]
- Li, F.; Lv, Y.N.; Tan, Y.S.; Shen, K.; Zhai, K.F.; Chen, H.L.; Kou, J.P.; Yu, B.Y. An Integrated Pathway Interaction Network for the Combination of Four Effective Compounds from ShengMai Preparations in the Treatment of Cardio-Cerebral Ischemic Diseases. Acta Pharmacol. Sin. 2015, 36, 1337–1348. [Google Scholar] [CrossRef]
- Zhao, Y.N.; Shao, X.; Ouyang, L.F.; Chen, L.; Gu, L. Qualitative Detection of Ginsenosides in Brain Tissues after Oral Administration of High-Purity Ginseng Total Saponins by Using Polyclonal Antibody against Ginsenosides. Chin. J. Nat. Med. 2018, 16, 175–183. [Google Scholar] [CrossRef]
- Wang, R.; Li, Y.N.; Wang, G.J.; Hao, H.P.; Wu, X.L.; Zhou, F. Neuroprotective Effects and Brain Transport of Ginsenoside Rg1. Chin. J. Nat. Med. 2009, 7, 315–320. [Google Scholar] [CrossRef]
- Park, J.D. Metabolism and Drug Interactions of Korean Ginseng Based on the Pharmacokinetic Properties of Ginsenosides: Current Status and Future Perspectives. J. Ginseng Res. 2024, 48, 253–265. [Google Scholar] [CrossRef]
- Shieh, P.; Jan, C.R.; Liang, W.Z. The Protective Effects of the Antioxidant N-Acetylcysteine (NAC) against Oxidative Stress-Associated Apoptosis Evoked by the Organophosphorus Insecticide Malathion in Normal Human Astrocytes. Toxicology 2019, 417, 1–14. [Google Scholar] [CrossRef]
- Ramaswamy, S.; Rodriguez, A.; Driscoll, D.; Rao, V. Nutraceuticals for Traumatic Brain Injury: Should You Recommend Their Use. Curr. Psychiatry 2017, 16, 34–38, 40, 41–45. [Google Scholar]
- Pandya, J.D.; Readnower, R.D.; Patel, S.P.; Yonutas, H.M.; Pauly, J.R.; Goldstein, G.A.; Rabchevsky, A.G.; Sullivan, P.G. N-Acetylcysteine Amide Confers Neuroprotection, Improves Bioenergetics and Behavioral Outcome Following TBI. Exp. Neurol. 2014, 257, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Kawoos, U.; McCarron, R.M.; Chavko, M. Protective Effect of N-Acetylcysteine Amide on Blast-Induced Increase in Intracranial Pressure in Rats. Front. Neurol. 2017, 8, 219. [Google Scholar] [CrossRef] [PubMed]
- Kawoos, U.; Abutarboush, R.; Zarriello, S.; Qadri, A.; Ahlers, S.T.; McCarron, R.M.; Chavko, M. N-Acetylcysteine Amide Ameliorates Blast-Induced Changes in Blood-Brain Barrier Integrity in Rats. Front. Neurol. 2019, 10, 650. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Wang, H.D.; Zhou, X.M.; Fang, J.; Zhu, L.; Ding, K. N-Acetylcysteine Amide Provides Neuroprotection via Nrf2-ARE Pathway in a Mouse Model of Traumatic Brain Injury. Drug Des. Dev. Ther. 2018, 12, 4117–4127. [Google Scholar] [CrossRef]
- Clark, R.S.B.; Empey, P.E.; Kochanek, P.M.; Bell, M.J. N-Acetylcysteine and Probenecid Adjuvant Therapy for Traumatic Brain Injury. Neurotherapeutics 2023, 20, 1529–1537. [Google Scholar] [CrossRef]
- Olsson, B.; Johansson, M.; Gabrielsson, J.; Bolme, P. Pharmacokinetics and Bioavailability of Reduced and Oxidized N-Acetylcysteine. Eur. J. Clin. Pharmacol. 1988, 34, 77–82. [Google Scholar] [CrossRef]
- Hara, Y.; McKeehan, N.; Dacks, P.A.; Fillit, H.M. Evaluation of the Neuroprotective Potential of N-Acetylcysteine for Prevention and Treatment of Cognitive Aging and Dementia. J. Prev. Alzheimer’s Dis. 2017, 4, 201–206. [Google Scholar] [CrossRef]
- Katz, M.; Won, S.J.; Park, Y.; Orr, A.; Jones, D.P.; Swanson, R.A.; Glass, G.A. Cerebrospinal Fluid Concentrations of N-Acetylcysteine after Oral Administration in Parkinson’s Disease. Park. Relat. Disord. 2015, 21, 500–503. [Google Scholar] [CrossRef]
- Dehkordi, H.T.; Ghasemi, S. Glutathione Therapy in Diseases: Challenges and Potential Solutions for Therapeutic Advancement. Curr. Mol. Med. 2023, 24, 1219–1230. [Google Scholar] [CrossRef]
- Cacciatore, I.; Baldassarre, L.; Fornasari, E.; Mollica, A.; Pinnen, F. Recent Advances in the Treatment of Neurodegenerative Diseases Based on GSH Delivery Systems. Oxidative Med. Cell. Longev. 2012, 2012, 240146. [Google Scholar] [CrossRef] [PubMed]
- Lewerenz, J.; Maher, P. Control of Redox State and Redox Signaling by Neural Antioxidant Systems. Antioxid. Redox Signal. 2011, 14, 1449–1465. [Google Scholar] [CrossRef]
- Bridges, R.J.; Natale, N.R.; Patel, S.A. System Xc− Cystine/Glutamate Antiporter: An Update on Molecular Pharmacology and Roles within the CNS. Br. J. Pharmacol. 2012, 165, 20–34. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; He, Y.; Hewett, S.J.; Hewett, J.A. Interleukin 1β Regulation of the System Xc− Substrate-Specific Subunit, XCT, in Primary Mouse Astrocytes Involves the RNA-Binding Protein HuR. J. Biol. Chem. 2016, 291, 1643–1651. [Google Scholar] [CrossRef]
- Dahlmanns, M.; Dahlmanns, J.K.; Savaskan, N.; Steiner, H.H.; Yakubov, E. Glial Glutamate Transporter-Mediated Plasticity: System Xc−/XCT/SLC7A11 and EAAT1/2 in Brain Diseases. Front. Biosci. 2023, 28, 57. [Google Scholar] [CrossRef] [PubMed]
- Sprimont, L.; Janssen, P.; De Swert, K.; Van Bulck, M.; Rooman, I.; Gilloteaux, J.; Massie, A.; Nicaise, C. Cystine–Glutamate Antiporter Deletion Accelerates Motor Recovery and Improves Histological Outcomes Following Spinal Cord Injury in Mice. Sci. Rep. 2021, 11, 12227. [Google Scholar] [CrossRef]
- Stelmashook, E.V.; Isaev, N.K.; Genrikhs, E.E.; Novikova, S.V. Mitochondria-Targeted Antioxidants as Potential Therapy for the Treatment of Traumatic Brain Injury. Antioxidants 2019, 8, 124. [Google Scholar] [CrossRef]
- Jou, M.J. Pathophysiological and Pharmacological Implications of Mitochondria-Targeted Reactive Oxygen Species Generation in Astrocytes. Adv. Drug Deliv. Rev. 2008, 60, 1512–1526. [Google Scholar] [CrossRef]
- Hayakawa, K. Commentary: Can Astrocytic Mitochondria Therapy Be Used as Antioxidant Conditioning to Protect Neurons? Cond. Med. 2022, 5, 192–195. [Google Scholar] [PubMed]
- Zhang, H.; Chen, Y.; Li, F.; Wu, C.; Cai, W.; Ye, H.; Su, H.; He, M.; Yang, L.; Wang, X.; et al. Elamipretide Alleviates Pyroptosis in Traumatically Injured Spinal Cord by Inhibiting CPLA2-Induced Lysosomal Membrane Permeabilization. J. Neuroinflamm. 2023, 20, 6. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Wang, H.; Fang, J.; Dai, W.; Zhou, J.; Wang, X.; Zhou, M. SS-31 Provides Neuroprotection by Reversing Mitochondrial Dysfunction after Traumatic Brain Injury. Oxidative Med. Cell. Longev. 2018, 2018, 4783602. [Google Scholar] [CrossRef]
- Zhou, J.; Wang, H.; Shen, R.; Fang, J.; Yang, Y.; Dai, W.; Zhu, Y.; Zhou, M. Mitochondrial-Targeted Antioxidant MitoQ Provides Neuroprotection and Reduces Neuronal Apoptosis in Experimental Traumatic Brain Injury Possibly via the Nrf2-ARE Pathway. Am. J. Transl. Res. 2018, 10, 1887–1899. [Google Scholar]
- Cen, J.; Zhang, R.; Zhao, T.; Zhang, X.; Zhang, C.; Cui, J.; Zhao, K.; Duan, S.; Guo, Y. A Water-Soluble Quercetin Conjugate with Triple Targeting Exerts Neuron-Protective Effect on Cerebral Ischemia by Mitophagy Activation. Adv. Healthc. Mater. 2022, 11, 2200817. [Google Scholar] [CrossRef] [PubMed]
- Hemachandra Reddy, P.; Manczak, M.; Kandimalla, R. Mitochondria-Targeted Small Molecule SS31: A Potential Candidate for the Treatment of Alzheimer’s Disease. Hum. Mol. Genet. 2017, 26, 1483–1496. [Google Scholar] [CrossRef]
- Murphy, M.P.; Smith, R.A.J. Targeting Antioxidants to Mitochondria by Conjugation to Lipophilic Cations. Annu. Rev. Pharmacol. Toxicol. 2007, 47, 629–656. [Google Scholar] [CrossRef]
- Tung, C.; Varzideh, F.; Farroni, E.; Mone, P.; Kansakar, U.; Jankauskas, S.S.; Santulli, G. Elamipretide: A Review of Its Structure, Mechanism of Action, and Therapeutic Potential. Int. J. Mol. Sci. 2025, 26, 944. [Google Scholar] [CrossRef]
- Liu, Z.Q.; Chan, K.; Zhou, H.; Jiang, Z.H.; Wong, Y.F.; Xu, H.X.; Liu, L. The Pharmacokinetics and Tissue Distribution of Sinomenine in Rats and Its Protein Binding Ability In Vitro. Life Sci. 2005, 77, 3197–3209. [Google Scholar] [CrossRef] [PubMed]
- Long, L.H.; Wu, P.F.; Chen, X.L.; Zhang, Z.; Chen, Y.; Li, Y.Y.; Jin, Y.; Chen, J.G.; Wang, F. HPLC and LC-MS Analysis of Sinomenine and Its Application in Pharmacokinetic Studies in Rats. Acta Pharmacol. Sin. 2010, 31, 1508–1514. [Google Scholar] [CrossRef]
- Sharma, R.; Kambhampati, S.P.; Zhang, Z.; Sharma, A.; Chen, S.; Duh, E.I.; Kannan, S.; Tso, M.O.M.; Kannan, R.M. Dendrimer Mediated Targeted Delivery of Sinomenine for the Treatment of Acute Neuroinflammation in Traumatic Brain Injury. J. Control. Release 2020, 323, 361–375. [Google Scholar] [CrossRef] [PubMed]
- Cassina, P.; Cassina, A.; Pehar, M.; Castellanos, R.; Gandelman, M.; De León, A.; Robinson, K.M.; Mason, R.P.; Beckman, J.S.; Barbeito, L.; et al. Mitochondrial Dysfunction in SOD1G93A-Bearing Astrocytes Promotes Motor Neuron Degeneration: Prevention by Mitochondrial-Targeted Antioxidants. J. Neurosci. 2008, 28, 4115–4122. [Google Scholar] [CrossRef]
- Rudnitskaya, E.A.; Burnyasheva, A.O.; Kozlova, T.A.; Peunov, D.A.; Kolosova, N.G.; Stefanova, N.A. Changes in Glial Support of the Hippocampus during the Development of an Alzheimer’s Disease-like Pathology and Their Correction by Mitochondria-Targeted Antioxidant SkQ1. Int. J. Mol. Sci. 2022, 23, 1134. [Google Scholar] [CrossRef]
- Muraleva, N.A.; Stefanova, N.A.; Kolosova, N.G. SkQ1 Suppresses the P38 MAPK Signaling Pathway Involved in Alzheimer’s Disease-Like Pathology in OXYS Rats. Antioxidants 2020, 9, 676. [Google Scholar] [CrossRef] [PubMed]
- Genrikhs, E.E.; Stelmashook, E.V.; Alexandrova, O.P.; Novikova, S.V.; Voronkov, D.N.; Glibka, Y.A.; Skulachev, V.P.; Isaev, N.K. The Single Intravenous Administration of Mitochondria-Targeted Antioxidant SkQR1 after Traumatic Brain Injury Attenuates Neurological Deficit in Rats. Brain Res. Bull. 2019, 148, 100–108. [Google Scholar] [CrossRef]
- Polyzos, A.; Holt, A.; Brown, C.; Cosme, C.; Wipf, P.; Gomez-Marin, A.; Castro, M.R.; Ayala-Peña, S.; McMurray, C.T. Mitochondrial Targeting of XJB-5-131 Attenuates or Improves Pathophysiology in HdhQ150 Animals with Well-Developed Disease Phenotypes. Hum. Mol. Genet. 2016, 25, 1792–1802. [Google Scholar] [CrossRef]
- Qin, R.; Lai, X.; Xu, W.; Qin, Q.; Liang, X.; Xie, M.; Chen, L. The Mechanisms and Application Prospects of Astrocyte Reprogramming into Neurons in Central Nervous System Diseases. Curr. Neuropharmacol. 2025, 23, 58–73. [Google Scholar] [CrossRef]
- Xia, S.; Xu, C.; Liu, F.; Chen, G. Development of MicroRNA-Based Therapeutics for Central Nervous System Diseases. Eur. J. Pharmacol. 2023, 956, 175956. [Google Scholar] [CrossRef] [PubMed]
- Sun, P.; Liu, D.Z.; Jickling, G.C.; Sharp, F.R.; Yin, K.J. MicroRNA-Based Therapeutics in Central Nervous System Injuries. J. Cereb. Blood Flow Metab. 2018, 38, 1125–1148. [Google Scholar] [CrossRef] [PubMed]
- Ishii, T.; Warabi, E.; Mann, G.E. Circadian Control of BDNF-Mediated Nrf2 Activation in Astrocytes Protects Dopaminergic Neurons from Ferroptosis. Free Radic. Biol. Med. 2019, 133, 169–178. [Google Scholar] [CrossRef]
- Saba, J.; Turati, J.; Ramírez, D.; Carniglia, L.; Durand, D.; Lasaga, M.; Caruso, C. Astrocyte Truncated Tropomyosin Receptor Kinase B Mediates Brain-Derived Neurotrophic Factor Anti-Apoptotic Effect Leading to Neuroprotection. J. Neurochem. 2018, 146, 686–702. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.A.; Braga, A.; Verheyen, J.; Basilico, S.; Bandiera, S.; Alfaro-Cervello, C.; Peruzzotti-Jametti, L.; Shu, D.; Haque, F.; Guo, P.; et al. RNA Nanotherapeutics for the Amelioration of Astroglial Reactivity. Mol. Ther. Nucleic Acids 2018, 10, 103–121. [Google Scholar] [CrossRef]
- Xu, T.; Chang, Y.; Wang, R.; Xu, J.; Qian, D.; Yao, H.; Gan, L.; Deng, S.; Lian, Q.; Ye, J.; et al. Lipid Nanoparticle-Mediated Targeted Delivery of MEGF10 SiRNA to Astrocytes Reduced Synaptic Phagocytosis and Promoted Stroke Recovery in Mice. ACS Appl. Mater. Interfaces 2025, 17, 57936–57952. [Google Scholar] [CrossRef]
- Guo, S.; Wei, F.; Sun, H.; Jin, H.; Cheng, W.; Fu, C.; Wang, H.; Yin, Y. Astrocyte-Specific Nrf2 Expression Transforms Neurotoxic Reactive Astrocytes to Neuroprotective Phenotype in 3xTg-AD Mice. Glia, 2025; early view. [Google Scholar] [CrossRef]
- Zhao, W.; Gasterich, N.; Clarner, T.; Voelz, C.; Behrens, V.; Beyer, C.; Fragoulis, A.; Zendedel, A. Astrocytic Nrf2 Expression Protects Spinal Cord from Oxidative Stress Following Spinal Cord Injury in a Male Mouse Model. J. Neuroinflamm. 2022, 19, 134. [Google Scholar] [CrossRef]
- Nanou, A.; Higginbottom, A.; Valori, C.F.; Wyles, M.; Ning, K.; Shaw, P.; Azzouz, M. Viral Delivery of Antioxidant Genes as a Therapeutic Strategy in Experimental Models of Amyotrophic Lateral Sclerosis. Mol. Ther. 2013, 21, 1486–1496. [Google Scholar] [CrossRef]
- Xiong, W.; Garfinkel, A.E.M.C.; Li, Y.; Benowitz, L.I.; Cepko, C.L. NRF2 Promotes Neuronal Survival in Neurodegeneration and Acute Nerve Damage. J. Clin. Investig. 2015, 125, 1433–1445. [Google Scholar] [CrossRef]
- Jiang, T.; Harder, B.; Rojo De La Vega, M.; Wong, P.K.; Chapman, E.; Zhang, D.D. P62 Links Autophagy and Nrf2 Signaling. Free Radic. Biol. Med. 2015, 88, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Ichimura, Y.; Komatsu, M. Activation of P62/SQSTM1-Keap1-Nuclear Factor Erythroid 2-Related Factor 2 Pathway in Cancer. Front. Oncol. 2018, 8, 377225. [Google Scholar] [CrossRef] [PubMed]
- Robertson, H.; Dinkova-Kostova, A.T.; Hayes, J.D. NRF2 and the Ambiguous Consequences of Its Activation during Initiation and the Subsequent Stages of Tumourigenesis. Cancers 2020, 12, 3609. [Google Scholar] [CrossRef]
- D’Souza, A.; Nozohouri, S.; Bleier, B.S.; Amiji, M.M. CNS Delivery of Nucleic Acid Therapeutics: Beyond the Blood–Brain Barrier and Towards Specific Cellular Targeting. Pharm. Res. 2022, 40, 77–105. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zakrzewski, P.K.; Boczek, T. Neuron–Glia Crosstalk in the Regulation of Astrocytic Antioxidative Mechanisms Following CNS Injury. Antioxidants 2025, 14, 1415. https://doi.org/10.3390/antiox14121415
Zakrzewski PK, Boczek T. Neuron–Glia Crosstalk in the Regulation of Astrocytic Antioxidative Mechanisms Following CNS Injury. Antioxidants. 2025; 14(12):1415. https://doi.org/10.3390/antiox14121415
Chicago/Turabian StyleZakrzewski, Piotr K., and Tomasz Boczek. 2025. "Neuron–Glia Crosstalk in the Regulation of Astrocytic Antioxidative Mechanisms Following CNS Injury" Antioxidants 14, no. 12: 1415. https://doi.org/10.3390/antiox14121415
APA StyleZakrzewski, P. K., & Boczek, T. (2025). Neuron–Glia Crosstalk in the Regulation of Astrocytic Antioxidative Mechanisms Following CNS Injury. Antioxidants, 14(12), 1415. https://doi.org/10.3390/antiox14121415






