Anti-Inflammatory and Antioxidant Mechanisms of Dendrobium moschatum Polysaccharide in Intestinal Epithelial Cells via TLR4-NF-κB and Nrf2 Signaling Pathways
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Isolation and Purification of D. Moschatum Polysaccharides
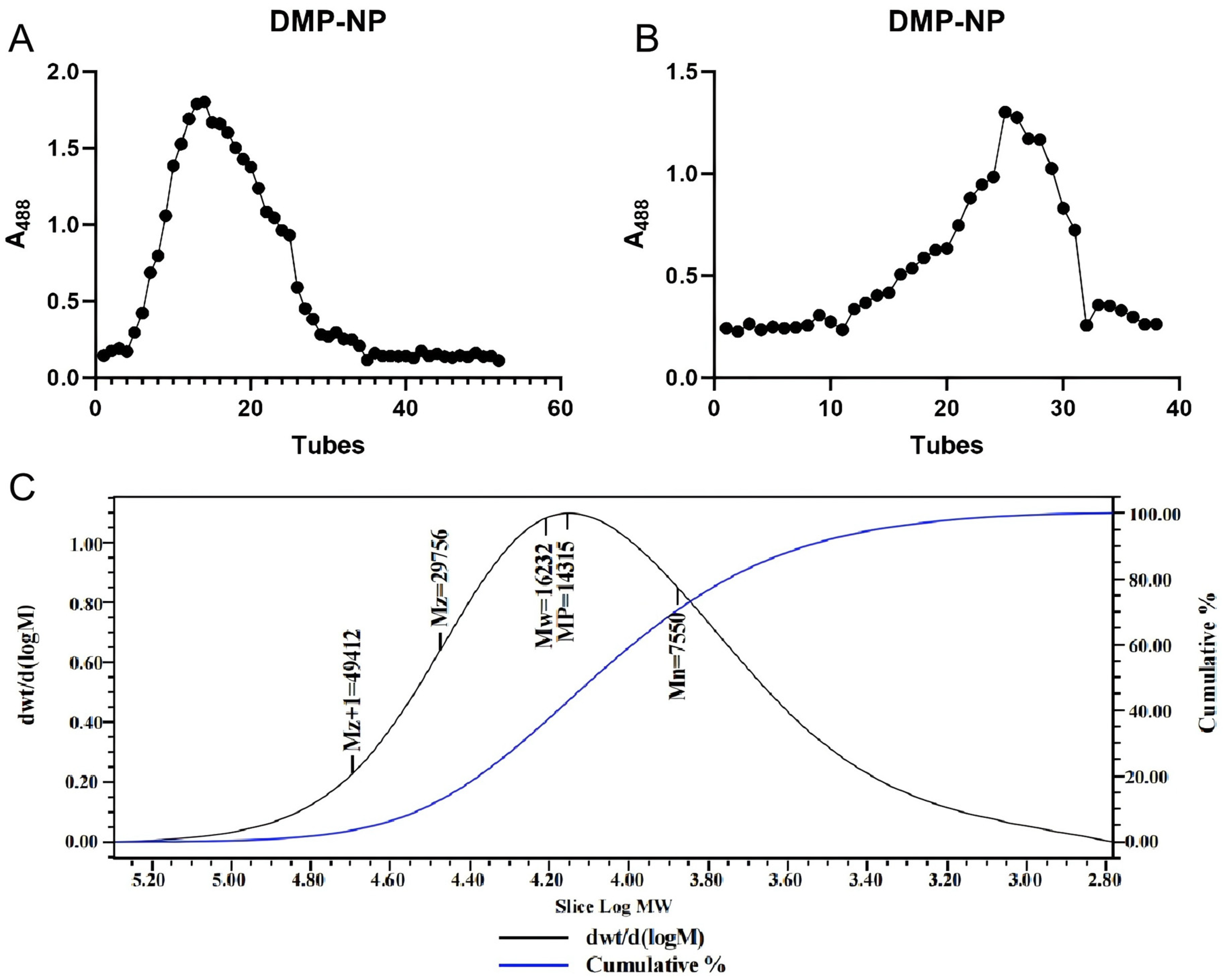
2.3. Molecular Weight Determination of DMP-NP
2.4. Chemical Composition and Monosaccharide Composition Analysis
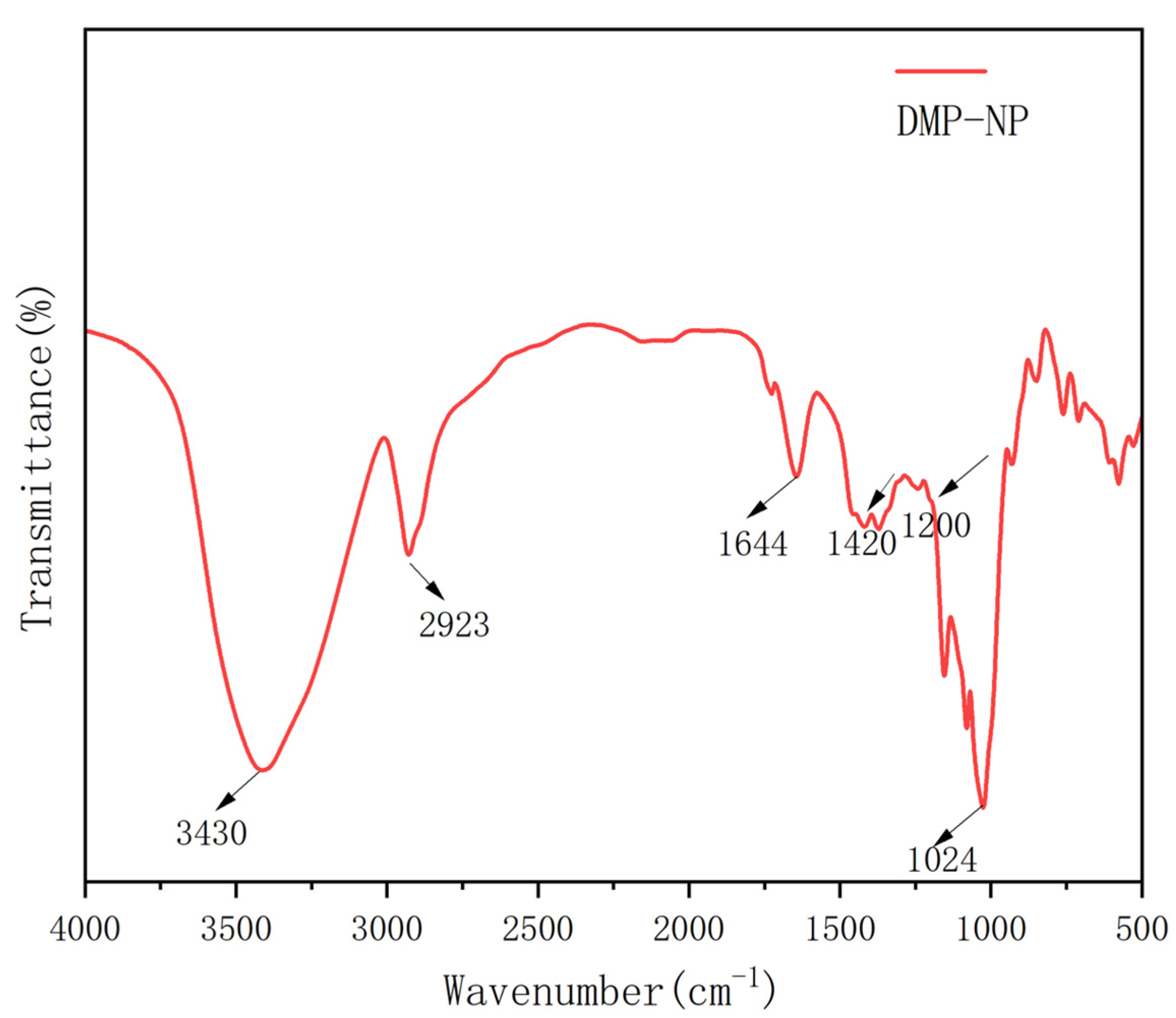
2.5. FT-IR and NMR Analyses
2.6. Anti-Inflammatory and Antioxidant Activity of D. Moschatum Polysaccharides on Intestinal Epithelial Cells (IPEC-J2)
2.6.1. Cell Culture
2.6.2. Prevention and Treatment of LPS-Induced Inflammation in IPEC-J2 Cells
2.7. Quantitative Real-Time PCR Analysis
2.8. Statistical Analysis
3. Results
3.1. Isolation of a Structurally Uniform Neutral Polysaccharide from D. moschatum
3.2. Purification Enhances Polysaccharide Purity and Yields a Novel Glucose-Rich Heteropolysaccharide from D. moschatum
3.3. Infrared Spectroscopy Reveals Novel Structural Features of Dendrobium Neutral Polysaccharides
3.4. Structural Characterization of D. moschatum Polysaccharides Using NMR Spectroscopy
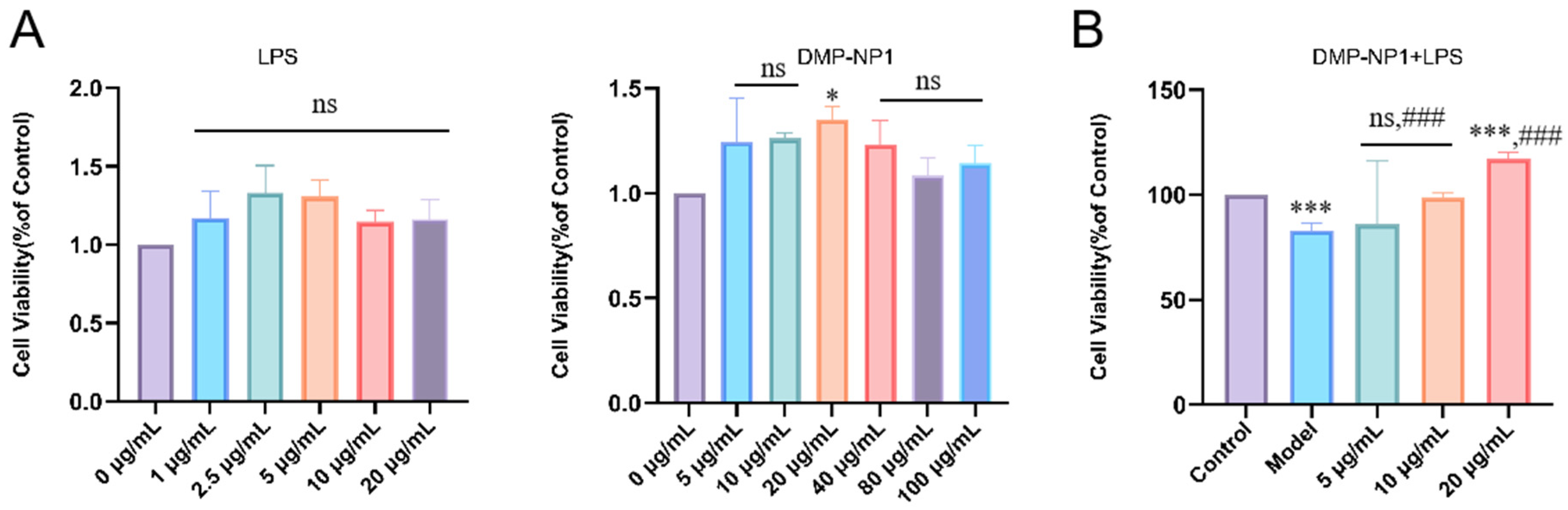
3.5. Anti-Inflammatory Activity of D. moschatum Polysaccharides
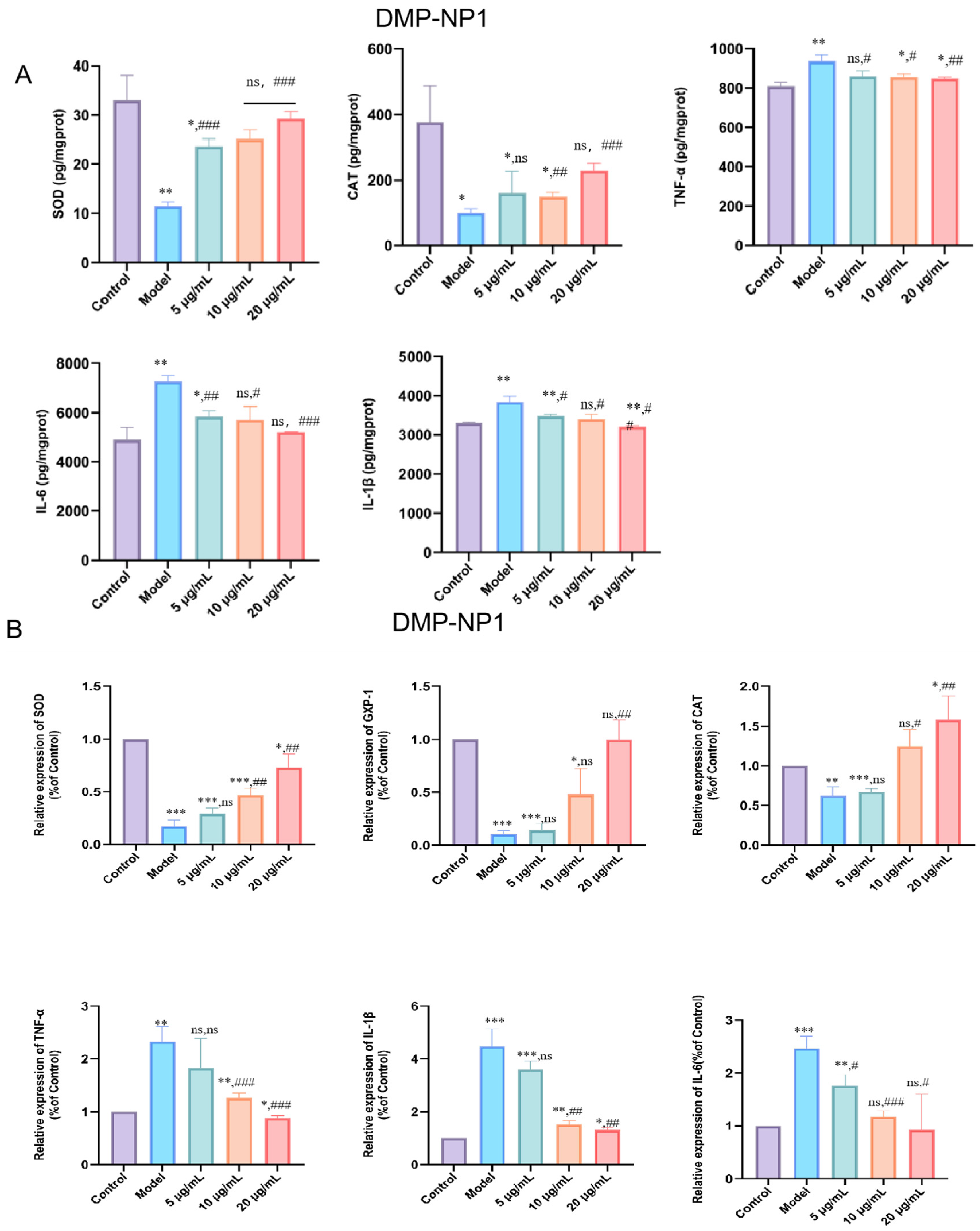
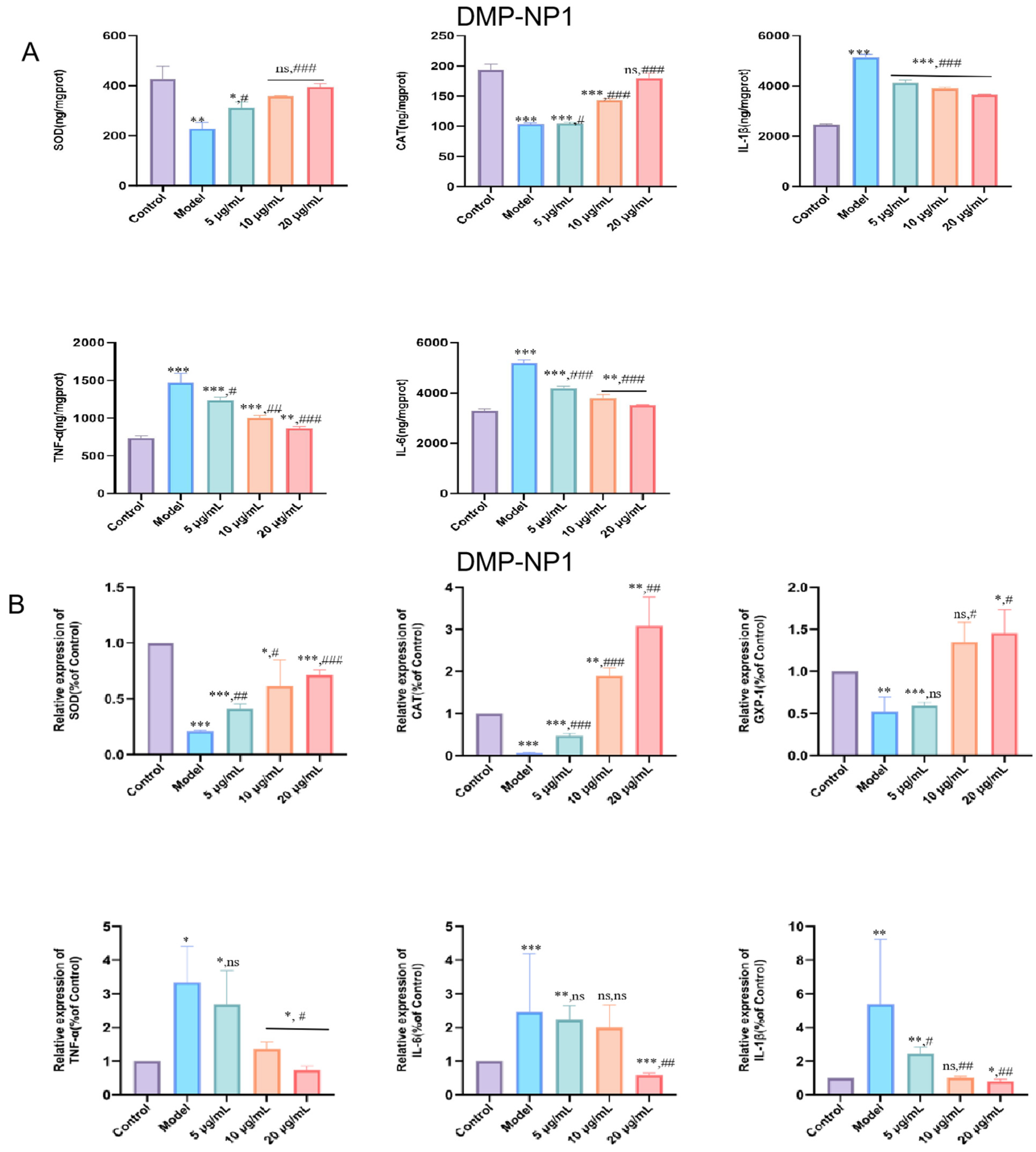
3.5.1. Preventive Effects of D. moschatum Polysaccharides on LPS-Induced Inflammation in IPEC-J2 Cells
3.5.2. Therapeutic Effect of D. moschatum Polysaccharides on LPS-Induced Inflammation in IPEC-J2 Cells
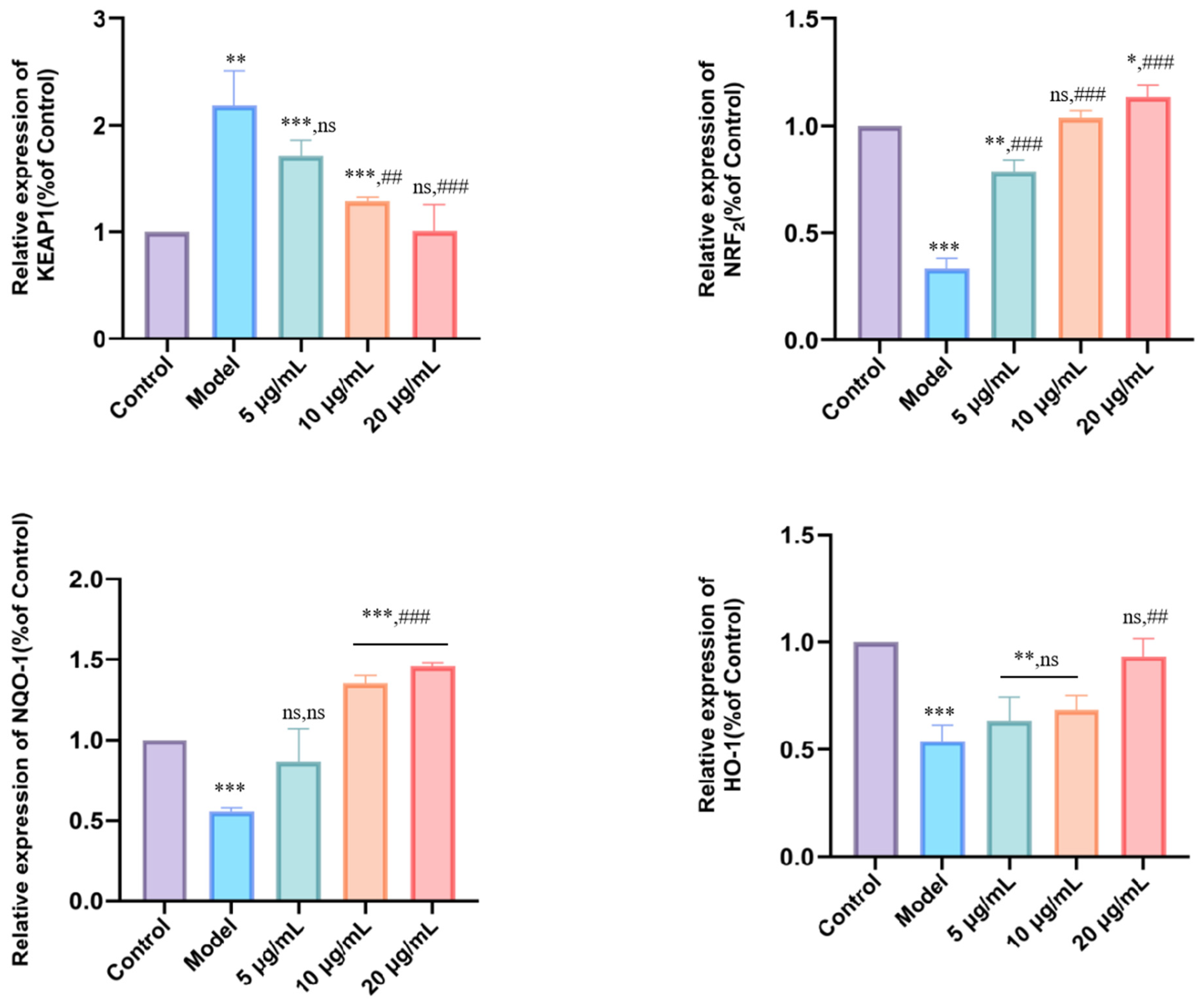
3.6. DMP-NP1 Enhances Antioxidant Defense via Modulation of the Keap1/Nrf2/HO-1/NQO1 Pathway in IPEC-J2 Cells
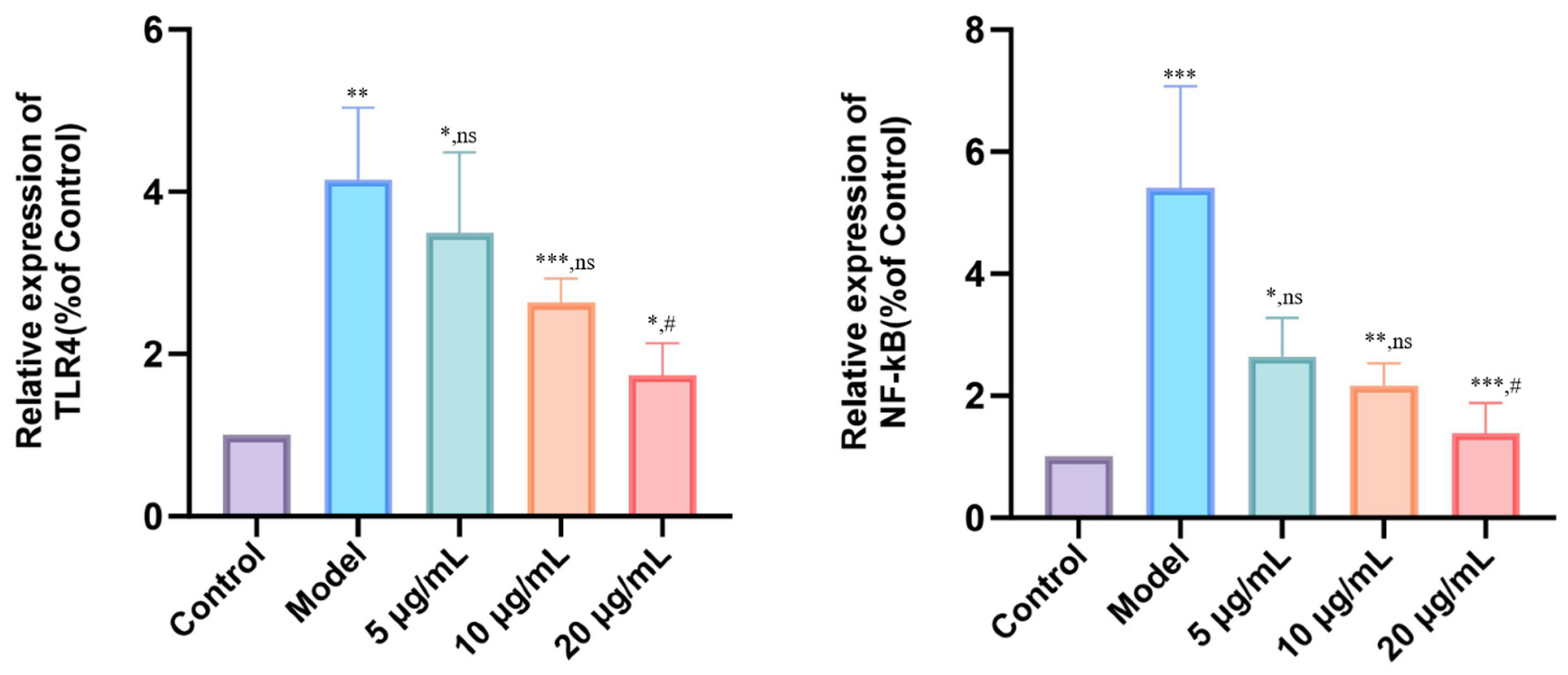
3.7. DMP-NP1 Effectively Suppresses TLR4/NF-κB Activation in IPEC-J2 Cells
3.8. Correlation Analysis Between Inflammatory and Oxidative Stress Signaling Pathways
4. Discussion
4.1. Monosaccharide Composition of Dendrobium Neutral Polysaccharides and Its Impact on Polysaccharide Bioactivity
4.2. D. moschatum Neutral Polysaccharides Exhibit Significant Anti-Inflammatory Activity
4.3. D. moschatum Neutral Polysaccharides Exert Significant Protective Effects Against Inflammation in Intestinal Epithelial Cells
4.4. Structural Features of DMP-NP1 Suggest Potential Mechanisms for Antioxidant and Anti-Inflammatory Functions
4.5. Structure–Activity Relationship and Mechanistic Insights
4.6. Limitations of the Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhou, H.L.; Zeng, X.J.; Sun, D.C.; Chen, Z.; Chen, W.X.; Fan, L.W.; Limpanont, Y.; Dekumyoy, P.; Maleewong, W.; Lv, Z.Y. Monosexual Cercariae of Schistosoma japonicum Infection Protects Against DSS-Induced Colitis by Shifting the Th1/Th2 Balance and Modulating the Gut Microbiota. Front. Microbiol. 2021, 11, 606605. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.X.; Dawulieti, J.; Shi, F.Y.; Yang, C.; Qin, Q.; Shi, T.F.; Wang, L.Z.; Hu, H.Z.; Sun, M.D.; Ren, L.; et al. A nanoparticulate dual scavenger for targeted therapy of inflammatory bowel disease. Sci. Adv. 2022, 8, eabj2372. [Google Scholar] [CrossRef]
- Li, X.L.; Hu, H.; Ren, Q.L.; Wang, M.; Du, Y.M.; He, Y.Q.; Wang, Q. Comparative analysis of endophyte diversity of Dendrobium officinale lived on rock and tree. Plant Biotechnol. 2023, 40, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.; Chen, Z.X.; Chen, X.Y.; Zhang, H.J.; Sun, Z.R.; Wang, Z.; Liu, M. Elucidating the anticancer potential of dendrobine in renal cell carcinoma treatment. Naunyn Schmiedebergs Arch. Pharmacol. 2025, 398, 7517–7528. [Google Scholar] [CrossRef]
- Luo, L.; Zhang, J.; Li, X.; Zhu, Y.; Wang, Y.; Liu, D. Sericic Acid Ameliorates DSS-induced Ulcerative Colitis in Mice by Modulating the NF-κappaB and Nrf2 Pathways. Curr. Mol. Pharmacol. 2023, 16, 759–770. [Google Scholar]
- Luo, A.X.; Ge, Z.F.; Fan, Y.J.; Luo, A.S.; Chun, Z.; He, X.J. In vitro and in vivo antioxidant activity of a water-soluble polysaccharide from Dendrobium denneanum. Molecules 2011, 16, 1579–1592. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, H.Y.; Yuan, Y.D. A Comparison of the Flavonoid Biosynthesis Mechanisms of Dendrobium Species by Analyzing the Transcriptome and Metabolome. Int. J. Mol. Sci. 2022, 23, 11980. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, T.T.; Wang, X.; Zhou, L.Y.; Qi, J.; An, S.Y. Structural characterization, antioxidant activity and anti-inflammatory of the phosphorylated polysaccharide from Pholiota nameko. Front. Nutr. 2022, 9, 976552. [Google Scholar] [CrossRef]
- Fincheira, P.; Espinoza, J.; Vera, J.; Berrios, D.; Nahuelcura, J.; Ruiz, A.; Quiroz, A.; Bustamante, L.; Cornejo, P.; Tortella, G.; et al. The Impact of 2-Ketones Released from Solid Lipid Nanoparticles on Growth Modulation and Antioxidant System of Lactuca sativa. Plants 2023, 12, 3094. [Google Scholar] [CrossRef]
- Li, Z.W.; Wei, Y.H.; Wang, Y.W.; Zhang, R.; Zhang, C.J.; Wang, C.X.; Yan, X.B. Preparation of Highly Substituted Sulfated Alfalfa Polysaccharides and Evaluation of Their Biological Activity. Foods 2022, 11, 737. [Google Scholar] [CrossRef] [PubMed]
- Ke, Y.F.; Wu, Y.X.; Cui, X.; Liu, X.; Yu, M.; Yang, C.B.; Li, X.R. Polysaccharide hydrogel combined with mesenchymal stem cells promotes the healing of corneal alkali burn in rats. PLoS ONE 2015, 10, e0119725. [Google Scholar] [CrossRef]
- Qi, X.D.; Yu, Y.; Wang, X.Y.; Xu, J.L.; Wang, X.; Feng, Z.K.; Zhou, Y.F.; Xiao, H.X.; Sun, L. Structural characterization and anti-oxidation activity evaluation of pectin from Lonicera japonica Thunb. Front. Nutr. 2022, 9, 998462. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.J.; Qiao, Y.J.; Wang, X.; Zhang, Y.; Fu, L.L. Extraction, Structural Characterization, Biological Functions, and Application of Rice Bran Polysaccharides: A Review. Foods 2023, 12, 639. [Google Scholar] [CrossRef] [PubMed]
- Habibi, S.C.; Nagy, G. Assessing the Use of Host-Guest Chemistry in Conjunction with Cyclic Ion Mobility Separations for the Linkage-Specific Characterization of Human Milk Oligosaccharides. Int. J. Mass Spectrom. 2023, 483, 116977. [Google Scholar] [CrossRef]
- Peng, X.; Wang, J.Y.; Gao, K.X.; Wang, Z.K.; Deng, Q.L.; Wang, Y.; Hu, M.B.; Liu, Y.J. Utilizing ultrasound for the extraction of polysaccharides from the tuber of Typhonium giganteum Engl.: Extraction conditions, structural characterization and bioactivities. Ultrason. Sonochem. 2025, 113, 107243. [Google Scholar] [CrossRef]
- Chen, X.L.; Sheng, Z.C.; Qiu, S.L.; Yang, H.F.; Jia, J.P.; Wang, J.; Jiang, C.M. Purification, characterization and in vitro and in vivo immune enhancement of polysaccharides from mulberry leaves. PLoS ONE 2019, 14, e0208611. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, T.; Hao, R.D.; Wang, Y.M. Construction Strategy and Mechanism of a Novel Wood Preservative with Excellent Antifungal Effects. Molecules 2024, 29, 1013. [Google Scholar] [CrossRef]
- Apriceno, A.; Silvestro, I.; Girelli, A.; Francolini, I.; Pietrelli, L.; Piozzi, A. Preparation and Characterization of Chitosan-Coated Manganese-Ferrite Nanoparticles Conjugated with Laccase for Environmental Bioremediation. Polymers 2021, 13, 1453. [Google Scholar] [CrossRef]
- Handoko, F.; Yusuf, Y. Synthesis and Physicochemical Properties of Poly(vinyl) Alcohol Nanocomposites Reinforced with Nanocrystalline Cellulose from Tea (Camellia sinensis) Waste. Materials 2021, 14, 7154. [Google Scholar] [CrossRef]
- Liang, H.; Tao, S.M.; Wang, Y.Y.; Zhao, J.; Yan, C.; Wu, Y.J.; Liu, N.; Qin, Y.H. Astragalus polysaccharide: Implication for intestinal barrier, anti-inflammation, and animal production. Front. Nutr. 2024, 11, 1364739. [Google Scholar] [CrossRef]
- Song, Z.Y.; Li, H.L.; Wen, J.; Zeng, Y.D.; Ye, X.Y.; Zhao, W.B.; Xu, T.T.; Xu, N.G.; Zhang, D.Y. Consumers’ attention on identification, nutritional compounds, and safety in heavy metals of Canadian sea cucumber in Chinese food market. Food Sci. Nutr. 2020, 8, 5962–5975. [Google Scholar] [CrossRef]
- Thorakkattu, P.; Khanashyam, A.C.; Shah, K.; Babu, K.S.; Mundanat, A.S.; Deliephan, A.; Deokar, G.S.; Santivarangkna, C.; Nirmal, N.P. Postbiotics: Current Trends in Food and Pharmaceutical Industry. Foods 2022, 11, 3094. [Google Scholar] [CrossRef]
- Ratto, M.; Suihko, M.; Siika-aho, M. Polysaccharide-producing bacteria isolated from paper machine slime deposits. J. Ind. Microbiol. Biotechnol. 2005, 32, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.K.; Geng, Y.W.; Lin, Q.Q. Adipose tissue aging as a risk factor for metabolic organ abnormalities: Mechanistic insights and the role of exercise interventions. Lipids Health Dis. 2025, 24, 274. [Google Scholar] [CrossRef]
- Shively, D.; Amin, N. Platelet-Rich Plasma for Rheumatoid Arthritis: A Case Series. Cureus 2021, 13, e19629. [Google Scholar] [CrossRef]
- Chen, T.T.; Li, Q.G.; Ai, G.X.; Huang, Z.W.; Liu, J.; Zeng, L.F.; Su, Z.R.; Dou, Y.X. Enhancing hepatoprotective action: Oxyberberine amorphous solid dispersion system targeting TLR4. Sci. Rep. 2024, 14, 14924. [Google Scholar] [CrossRef]
- Zerikiotis, S.; Efentakis, P.; Dapola, D.; Agapaki, A.; Seiradakis, G.; Kostomitsopoulos, N.; Skaltsounis, A.L.; Tseti, I.; Triposkiadis, F.; Andreadou, I. Synergistic Pulmonoprotective Effect of Natural Prolyl Oligopeptidase Inhibitors in In Vitro and In Vivo Models of Acute Respiratory Distress Syndrome. Int. J. Mol. Sci. 2023, 24, 14235. [Google Scholar] [CrossRef] [PubMed]
- Ganji, N.; Kalish, B.; Offringa, M.; Li, B.; Anderson, J.; Baruchel, S.; Blakely, M.; De Coppi, P.; Eaton, S.; Gauda, E.; et al. Translating regenerative medicine therapies in neonatal necrotizing enterocolitis. Pediatr. Res. 2024, 96, 1609–1615. [Google Scholar] [CrossRef] [PubMed]
- Song, X.L.; Liu, Z.H.; Zhang, J.J.; Zhang, C.; Dong, Y.H.; Ren, Z.Z.; Gao, Z.; Liu, M.; Zhao, H.J.; Jia, L. Antioxidative and hepatoprotective effects of enzymatic and acidic-hydrolysis of Pleurotus geesteranus mycelium polysaccharides on alcoholic liver diseases. Carbohydr. Polym. 2018, 201, 75–86. [Google Scholar] [CrossRef]
- Wang, Y.J.; Wang, H.Y.; Li, Q.M.; Zha, X.Q.; Luo, J.P. Dendrobium fimbriatum polysaccharide ameliorates DSS-induced intestinal mucosal injury by IL-22-regulated intestinal stem cell regeneration. Int. J. Biol. Macromol. 2023, 230, 123199. [Google Scholar] [CrossRef]
- Papatheodorou, E.M. Interventions Change Soil Functions and the Mechanisms Controlling the Structure of Soil Microbial Communities. Microorganisms 2023, 11, 1502. [Google Scholar] [CrossRef]
- Bhullar, K.A.; Horgan, M.I.M.; Le, A.; Fania, D.; Wuhrer, R.; Razmovski-Naumovski, V.; Chan, K.; Castignolles, P.; Gaborieau, M. Assessing the quantification of acetylation in konjac glucomannan via ATR-FTIR and solid-state NMR spectroscopy. Carbohydr. Polym. 2022, 291, 119659. [Google Scholar] [CrossRef] [PubMed]
- Casillo, A.; Fabozzi, A.; Russo Krauss, I.; Parrilli, E.; Biggs, C.I.; Gibson, M.I.; Lanzetta, R.; Appavou, M.S.; Radulescu, A.; Tutino, M.L.; et al. Physicochemical Approach to Understanding the Structure, Conformation, and Activity of Mannan Polysaccharides. Biomacromolecules 2021, 22, 1445–1457. [Google Scholar] [CrossRef]
- Chen, R.X.; Xu, J.X.; Wu, W.H.; Wen, Y.X.; Lu, S.Y.; El-Seedi, H.R.; Zhao, C. Structure-immunomodulatory activity relationships of dietary polysaccharides. Curr. Res. Food Sci. 2022, 5, 1330–1341. [Google Scholar] [CrossRef]
- Murphy, E.J.; Fehrenbach, G.W.; Abidin, I.Z.; Buckley, C.; Montgomery, T.; Pogue, R.; Murray, P.; Major, I.; Rezoagli, E. Polysaccharides-Naturally Occurring Immune Modulators. Polymers 2023, 15, 2373. [Google Scholar] [CrossRef] [PubMed]
- Bai, L.; Xu, D.; Zhou, Y.M.; Zhang, Y.B.; Zhang, H.; Chen, Y.B.; Cui, Y.L. Antioxidant Activities of Natural Polysaccharides and Their Derivatives for Biomedical and Medicinal Applications. Antioxidants 2022, 11, 2491. [Google Scholar] [CrossRef] [PubMed]
- Salazar, F.; Hall, L.; Negm, O.H.; Awuah, D.; Tighe, P.J.; Shakib, F.; Ghaemmaghami, A.M. The mannose receptor negatively modulates the Toll-like receptor 4-aryl hydrocarbon receptor-indoleamine 2,3-dioxygenase axis in dendritic cells affecting T helper cell polarization. J. Allergy Clin. Immunol. 2016, 137, 1841–1851.e2. [Google Scholar] [CrossRef]
- Villar, J.; Salazar, M.L.; Jiménez, J.M.; Del Campo, M.; Manubens, A.; Gleisner, M.A.; Ávalos, I.; Salazar-Onfray, F.; Salazar, F.; Mitchell, D.A.; et al. C-type lectin receptors MR and DC-SIGN are involved in recognition of hemocyanins, shaping their immunostimulatory effects on human dendritic cells. Eur. J. Immunol. 2021, 51, 1715–1731. [Google Scholar] [CrossRef]
- Deng, G.H.; Zhao, C.C.; Cai, X.; Zhang, X.Q.; Ma, M.Z.; Lv, J.H.; Jiang, W.L.; Peng, D.Y.; Wang, Y.Y.; Xing, L.H.; et al. Untargeted metabonomics and TLR4/NF-κappaB signaling pathway analysis reveals potential mechanism of action of Dendrobium huoshanense polysaccharide in nonalcoholic fatty liver disease. Front. Pharmacol. 2024, 15, 1374158. [Google Scholar] [CrossRef]
- Liu, C.; Hua, H.Y.; Zhu, H.K.; Cheng, Y.L.; Guo, Y.H.; Yao, W.R.; Qian, H. Aloe polysaccharides ameliorate acute colitis in mice via Nrf2/HO-1 signaling pathway and short-chain fatty acids metabolism. Int. J. Biol. Macromol. 2021, 185, 804–812. [Google Scholar] [CrossRef]
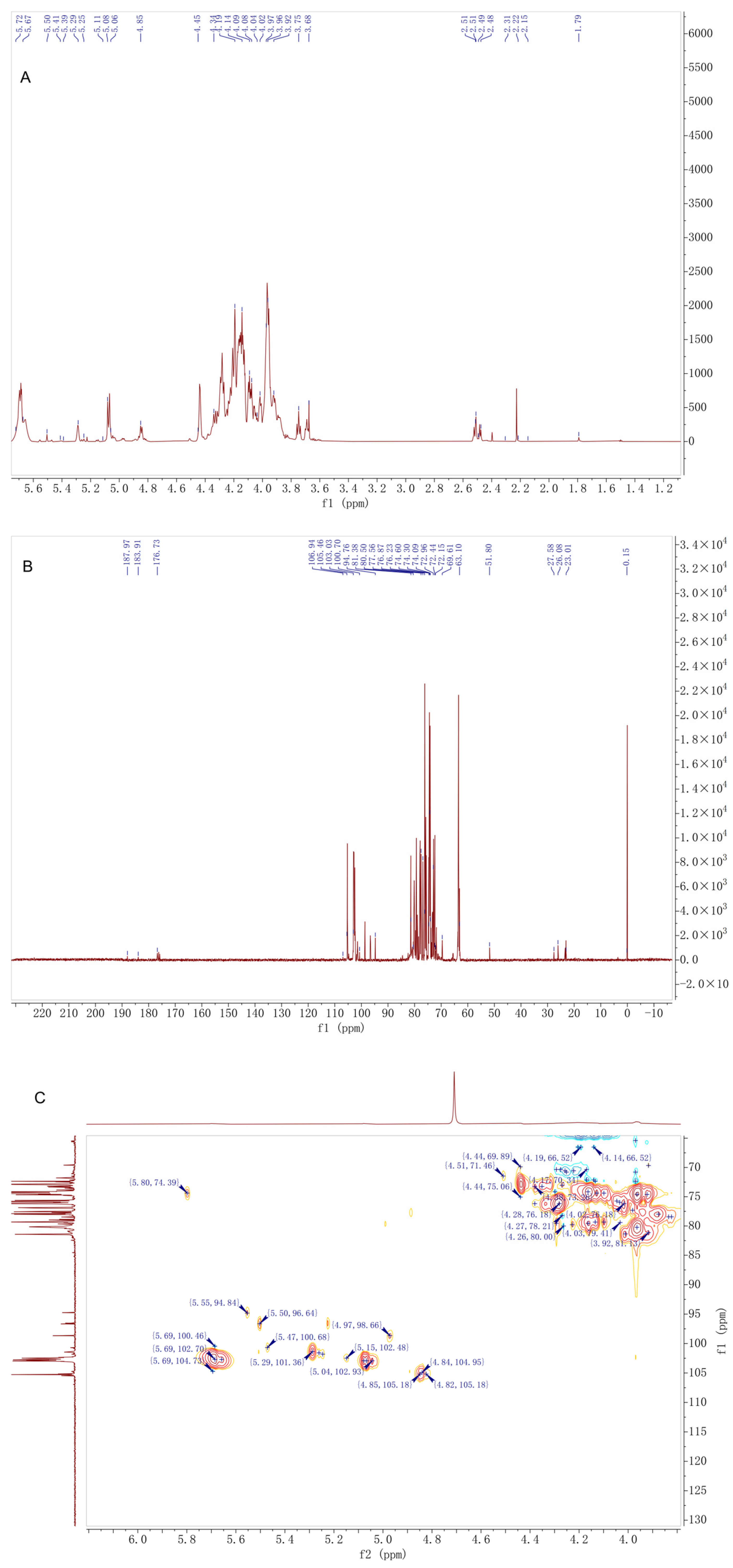

| Residues | H1/C1 | H2/C2 | H3/C3 | H4/C4 | H5/C5 | H6/C6 |
|---|---|---|---|---|---|---|
| →4)-α-Glcp-(1→ | 5.29/102.93 | 3.64/74.60 | 3.97/90.03 | 3.64/81.35 | 3.85/76.18 | 3.93,3.77/62.80 |
| Glcp-(1→) | 5.08/102.70 | 3.59/74.30 | 3.70/72.96 | 3.44/72.44 | 3.81/76.18 | 3.93,3.77/62.60 |
| →4,6)-α-Glcp-(1→) | 4.97/98.66 | 3.64/74.60 | 3.69/72.15 | 3.60/81.38 | 3.77/77.56 | 3.90,3.94/70.57 |
| →4)-β-Manp-(1→) | 4.71/103.03 | 4.11/73.04 | 3.94/79.33 | 3.84/80.50 | 3.59/76.87 | 3.92,3.77/62.40 |
| →4)-2-O-Ac-β-Manp-(1→) * | 4.85/105.18 | 5.48/74.39 | 4.02/79.56 | 3.84/80.50 | 3.61/77.56 | 3.92,3.77/62.20 |
| →4)-3-O-Ac-β-Manp-(1→) * | 4.84/101.36 | 4.18/70.57 | 5.09/76.23 | 3.81/80.50 | 3.59/76.87 | 3.92,3.77/62.00 |
| →4)-α-Manp | 5.06/96.64 | 3.58/74.09 | n.d./n.d. | n.d./n.d. | n.d./n.d. | n.d./n.d. |
| →4)-β-Glcp-(1→) | 4.45/105.46 | 3.37/72.44 | 3.63/71.60 | 3.81/79.33 | n.d./n.d. | 3.93,3.77/62.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, J.; Ma, C.; Mo, X.; Li, L.; Wu, L.; Zhang, C.; Li, R.; Zou, Y.; Liu, F.; Tian, M. Anti-Inflammatory and Antioxidant Mechanisms of Dendrobium moschatum Polysaccharide in Intestinal Epithelial Cells via TLR4-NF-κB and Nrf2 Signaling Pathways. Antioxidants 2025, 14, 1384. https://doi.org/10.3390/antiox14111384
Chen J, Ma C, Mo X, Li L, Wu L, Zhang C, Li R, Zou Y, Liu F, Tian M. Anti-Inflammatory and Antioxidant Mechanisms of Dendrobium moschatum Polysaccharide in Intestinal Epithelial Cells via TLR4-NF-κB and Nrf2 Signaling Pathways. Antioxidants. 2025; 14(11):1384. https://doi.org/10.3390/antiox14111384
Chicago/Turabian StyleChen, Ji, Chunyan Ma, Xu Mo, Linhong Li, Lijuan Wu, Chaowen Zhang, Rui Li, Yuanfeng Zou, Fan Liu, and Mengliang Tian. 2025. "Anti-Inflammatory and Antioxidant Mechanisms of Dendrobium moschatum Polysaccharide in Intestinal Epithelial Cells via TLR4-NF-κB and Nrf2 Signaling Pathways" Antioxidants 14, no. 11: 1384. https://doi.org/10.3390/antiox14111384
APA StyleChen, J., Ma, C., Mo, X., Li, L., Wu, L., Zhang, C., Li, R., Zou, Y., Liu, F., & Tian, M. (2025). Anti-Inflammatory and Antioxidant Mechanisms of Dendrobium moschatum Polysaccharide in Intestinal Epithelial Cells via TLR4-NF-κB and Nrf2 Signaling Pathways. Antioxidants, 14(11), 1384. https://doi.org/10.3390/antiox14111384







