Superoxide Dismutase 3 Deficiency Disrupts the Regulation of Oxidative Stress Caused by Polystyrene Nanoplastics
Abstract
1. Introduction
2. Materials and Methods
2.1. Zebrafish Husbandry
2.2. Generation of a Zebrafish sod3a Mutant Line
2.3. In Situ Hybridization and Gene Expression Analysis
2.4. Fluorescent Quantification of PSNP Accumulation
2.5. ROS Detection (CM-H2DCFDA Assay)
2.6. Hydrogen Peroxide Detection (Amplex Red Assay)
2.7. Acridine Orange Staining
2.8. Zebrafish Intestinal Motility
2.9. Statistical Analysis
3. Results
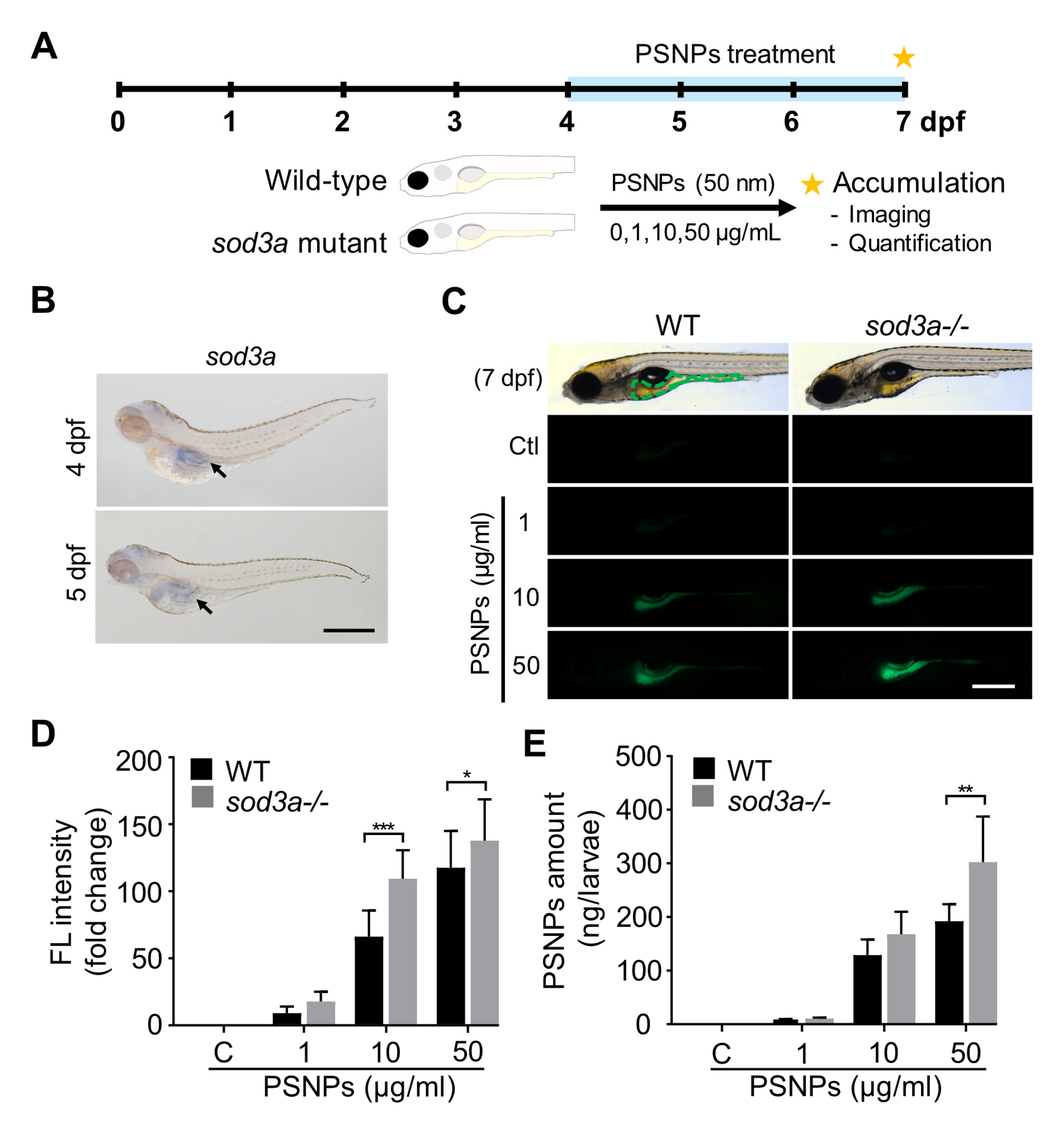
3.1. Spatial Expression of sod3a and Its Association with PSNP Accumulation
3.2. Assessment of PSNP-Induced Oxidative Stress
3.3. Cell Death Comparison PSNP Accumulation
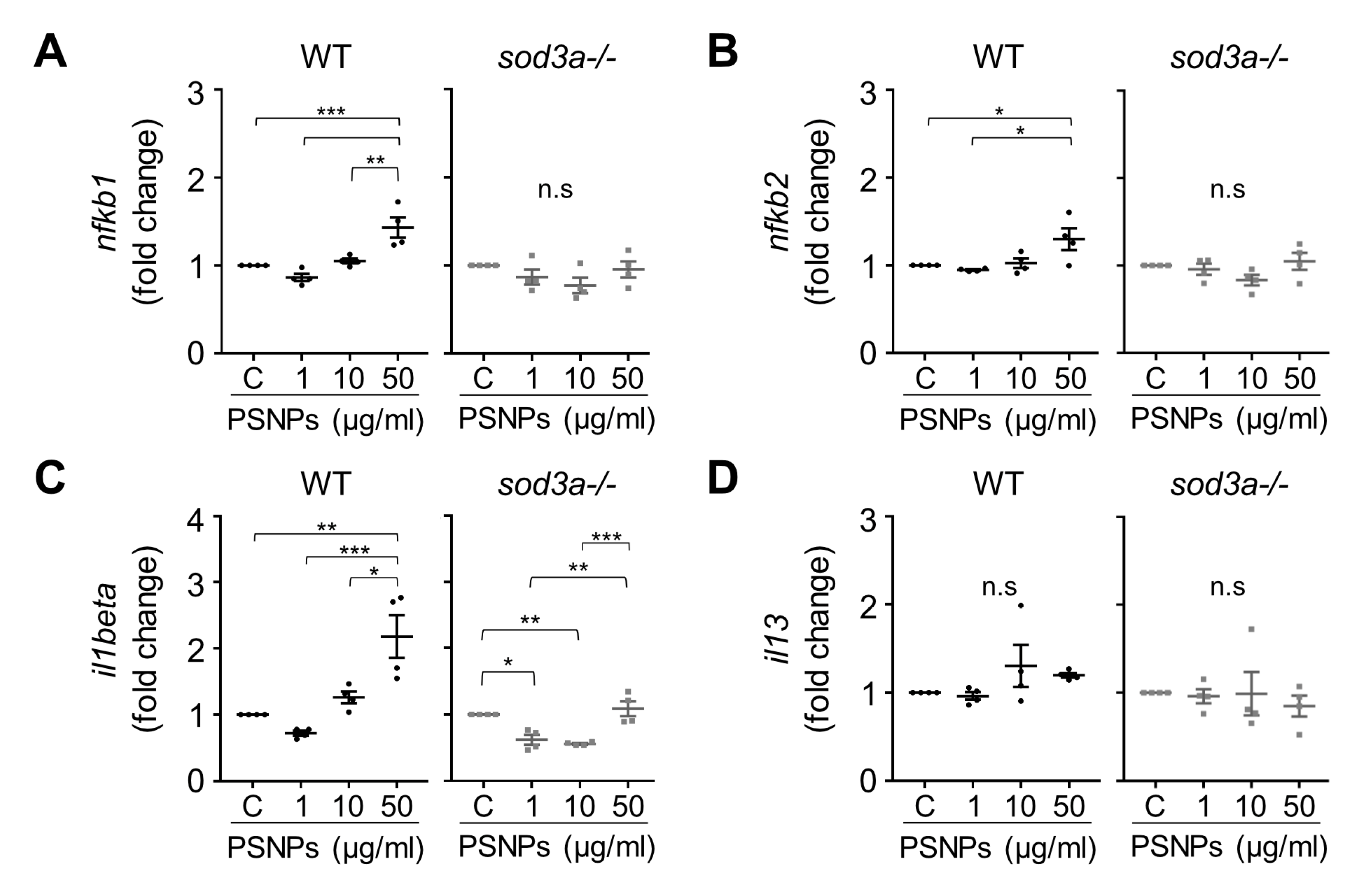
3.4. Differential Immune Responses to PSNPs
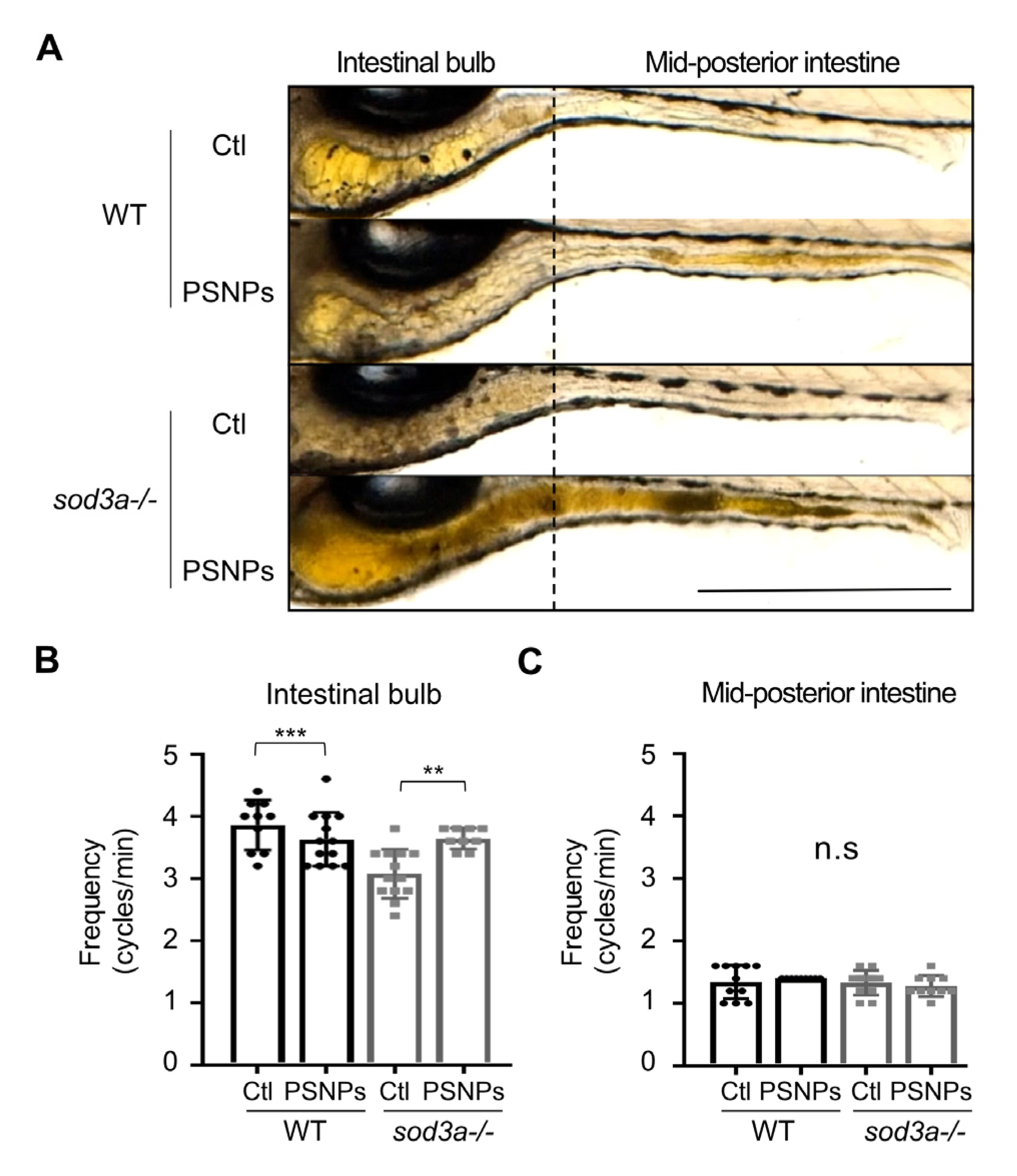
3.5. Differential Intestinal Motility Responses to PSNP Exposure
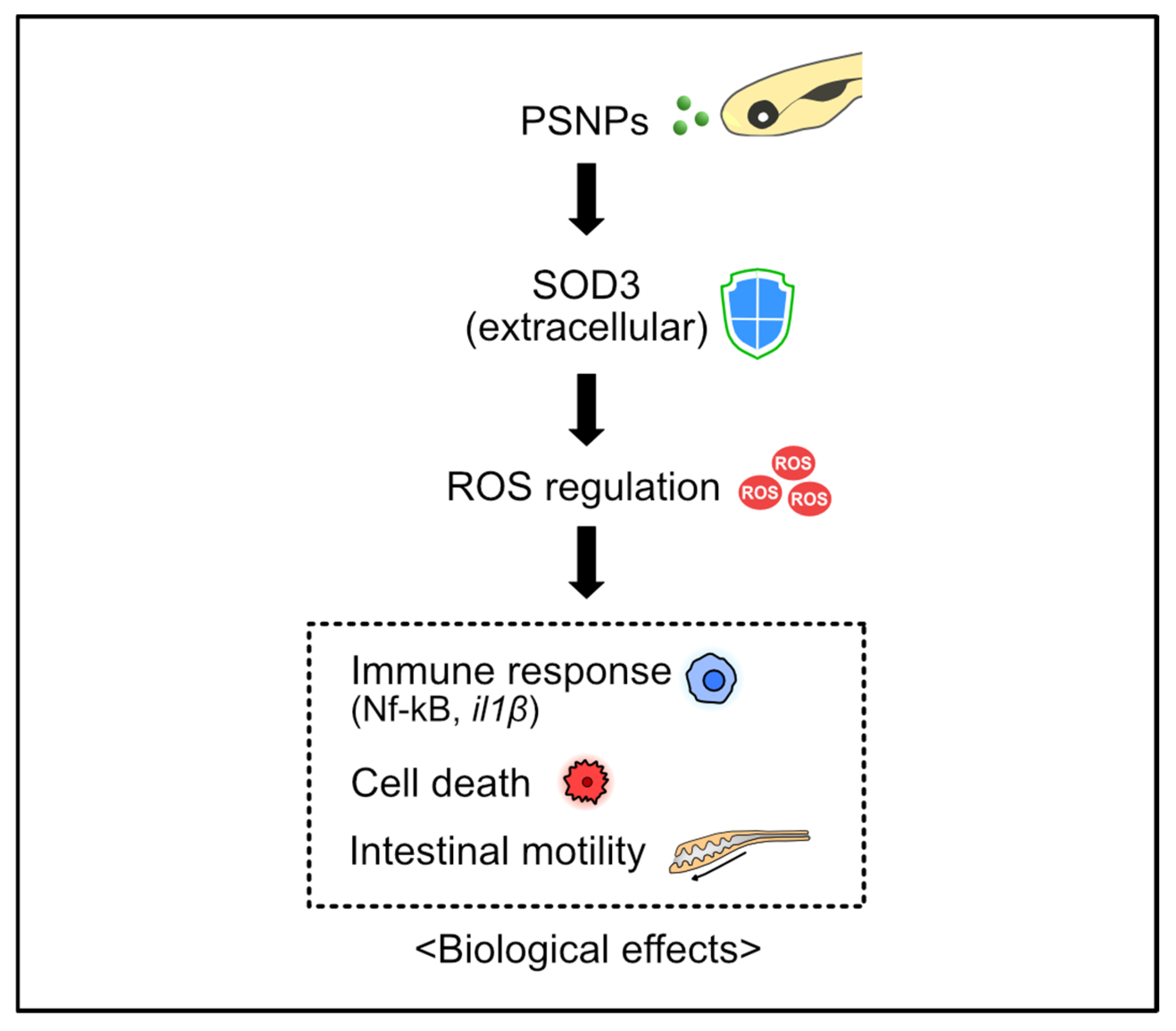
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ANOVA | One-way analysis of variance |
| AO | Acridine orange |
| Cu | Copper |
| ECM | Extracellular matrix |
| dpf | days of post fertilization |
| H2O2 | Hydrogen peroxide |
| hpi | hours of post incubation |
| HRP | Horseradish peroxidase |
| MD | Molecular dynamics |
| MNPs | Microplastics and nanoplastics |
| O2− | Superoxide anions |
| PSNPs | Polystyrene nanoplastics |
| ROS | Reactive oxygen species |
| sgRNA | single-guide RNA |
| SOD3 | Superoxide dismutase 3 |
| WT | Wild-type |
| WISH | Whole-mount in situ hybridization |
| Zn | Zinc |
References
- Alimi, O.S.; Farner Budarz, J.; Hernandez, L.M.; Tufenkji, N. Microplastics and Nanoplastics in Aquatic Environments: Aggregation, Deposition, and Enhanced Contaminant Transport. Environ. Sci. Technol. 2018, 52, 1704–1724. [Google Scholar] [CrossRef] [PubMed]
- Pfohl, P.; Wagner, M.; Meyer, L.; Domercq, P.; Praetorius, A.; Hüffer, T.; Hofmann, T.; Wohlleben, W. Environmental Degradation of Microplastics: How to Measure Fragmentation Rates to Secondary Micro- and Nanoplastic Fragments and Dissociation into Dissolved Organics. Environ. Sci. Technol. 2022, 56, 11323–11334. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Tao, L.; Wang, Q.; Wang, F.; Li, G.; Song, M. Potential Health Impact of Microplastics: A Review of Environmental Distribution, Human Exposure, and Toxic Effects. Environ. Health 2023, 1, 249–257. [Google Scholar] [CrossRef]
- Yang, H.; Niu, S.; Guo, M.; Xue, Y. A Critical Review of the Ecotoxic Effects of Microplastics on Aquatic, Soil and Atmospheric Ecosystems and Current Research Challenges. Environ. Res. 2025, 274, 121361. [Google Scholar] [CrossRef]
- Raffaele, M.; Francesco, P.; Celestino, S.; Gianluca, F.; Laura, G.; Tatiana, S.; Nunzia, D.; Lucia, S.; Rosalba, L.G.; Chiara, F.; et al. Microplastics and Nanoplastics in Atheromas and Cardiovascular Events. N. Engl. J. Med. 2024, 390, 900–910. [Google Scholar] [CrossRef]
- Nihart, A.J.; Garcia, M.A.; El Hayek, E.; Liu, R.; Olewine, M.; Kingston, J.D.; Castillo, E.F.; Gullapalli, R.R.; Howard, T.; Bleske, B.; et al. Bioaccumulation of Microplastics in Decedent Human Brains. Nat. Med. 2025, 31, 1114–1119. [Google Scholar] [CrossRef]
- Gecegelen, E.; Ucdal, M.; Dogu, B.B. A Novel Risk Factor for Dementia: Chronic Microplastic Exposure. Front. Neurol. 2025, 16, 1581109. [Google Scholar] [CrossRef]
- Gopinath, P.M.; Saranya, V.; Vijayakumar, S.; Mythili Meera, M.; Ruprekha, S.; Kunal, R.; Pranay, A.; Thomas, J.; Mukherjee, A.; Chandrasekaran, N. Assessment on Interactive Prospectives of Nanoplastics with Plasma Proteins and the Toxicological Impacts of Virgin, Coronated and Environmentally Released-Nanoplastics. Sci. Rep. 2019, 9, 8860. [Google Scholar] [CrossRef]
- Khan, A.; Jia, Z. Recent Insights into Uptake, Toxicity, and Molecular Targets of Microplastics and Nanoplastics Relevant to Human Health Impacts. iScience 2023, 26, 106061. [Google Scholar] [CrossRef] [PubMed]
- Casella, C.; Ballaz, S.J. Genotoxic and Neurotoxic Potential of Intracellular Nanoplastics: A Review. J. Appl. Toxicol. 2024, 44, 1657–1678. [Google Scholar] [CrossRef]
- Engin, A.B.; Nikitovic, D.; Neagu, M.; Henrich-Noack, P.; Docea, A.O.; Shtilman, M.I.; Golokhvast, K.; Tsatsakis, A.M. Mechanistic Understanding of Nanoparticles’ Interactions with Extracellular Matrix: The Cell and Immune System. Part. Fibre Toxicol. 2017, 14, 22. [Google Scholar] [CrossRef]
- Zhou, Z.; Zhang, Y.; Zeng, Y.; Yang, D.; Mo, J.; Zheng, Z.; Zhang, Y.; Xiao, P.; Zhong, X.; Yan, W. Effects of Nanomaterials on Synthesis and Degradation of the Extracellular Matrix. ACS Nano 2024, 18, 7688–7710. [Google Scholar] [CrossRef]
- Huang, H.; Hou, J.; Liao, Y.; Wei, F.; Xing, B. Polyethylene Microplastics Impede the Innate Immune Response by Disrupting the Extracellular Matrix and Signaling Transduction. iScience 2023, 26, 107390. [Google Scholar] [CrossRef]
- Bhagat, J.; Zang, L.; Nishimura, N.; Shimada, Y. Zebrafish: An Emerging Model to Study Microplastic and Nanoplastic Toxicity. Sci. Total Environ. 2020, 728, 138707. [Google Scholar] [CrossRef]
- Feng, M.; Luo, J.; Wan, Y.; Zhang, J.; Lu, C.; Wang, M.; Dai, L.; Cao, X.; Yang, X.; Wang, Y. Polystyrene Nanoplastic Exposure Induces Developmental Toxicity by Activating the Oxidative Stress Response and Base Excision Repair Pathway in Zebrafish (Danio rerio). ACS Omega 2022, 7, 32153–32163. [Google Scholar] [CrossRef] [PubMed]
- Saputra, F.; Pramata, A.D.; Soegianto, A.; Hu, S.-Y. Polystyrene Nanoplastics Cause Developmental Abnormalities, Oxidative Damage and Immune Toxicity in Early Zebrafish Development. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2025, 295, 110216. [Google Scholar] [CrossRef]
- Hu, M.; Palić, D. Micro- and Nano-Plastics Activation of Oxidative and Inflammatory Adverse Outcome Pathways. Redox Biol. 2020, 37, 101620. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.J.; Lee, W.S.; Jeong, J.; Lee, J.S. A review on the impacts of nanomaterials on neuromodulation and neurological dysfunction using a zebrafish animal model. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2022, 261, 109428. [Google Scholar] [CrossRef]
- Kadac-Czapska, K.; Ośko, J.; Knez, E.; Grembecka, M. Microplastics and Oxidative Stress—Current Problems and Prospects. Antioxidants 2024, 13, 579. [Google Scholar] [CrossRef]
- Mahmud, F.; Sarker, D.B.; Jocelyn, J.A.; Sang, Q.-X.A. Molecular and Cellular Effects of Microplastics and Nanoplastics: Focus on Inflammation and Senescence. Cells 2024, 13, 1788. [Google Scholar] [CrossRef] [PubMed]
- Matthiesen, C.L.; Hu, L.; Torslev, A.S.; Poulsen, E.T.; Larsen, U.G.; Kjaer-Sorensen, K.; Thomsen, J.S.; Brüel, A.; Enghild, J.J.; Oxvig, C.; et al. Superoxide Dismutase 3 Is Expressed in Bone Tissue and Required for Normal Bone Homeostasis and Mineralization. Free Radic. Biol. Med. 2021, 164, 399–409. [Google Scholar] [CrossRef]
- Thisse, C.; Thisse, B. High-Resolution in Situ Hybridization to Whole-Mount Zebrafish Embryos. Nat. Protoc. 2008, 3, 59–69. [Google Scholar] [CrossRef]
- Karakuzu, O.; Cruz, M.R.; Liu, Y.; Garsin, D.A. Amplex Red Assay for Measuring Hydrogen Peroxide Production from Caenorhabditis elegans. Bio-Protocol 2019, 9, e3409. [Google Scholar] [CrossRef]
- Veal, E.A.; Day, A.M.; Morgan, B.A. Hydrogen Peroxide Sensing and Signaling. Mol. Cell 2007, 26, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Sies, H. Hydrogen Peroxide as a Central Redox Signaling Molecule in Physiological Oxidative Stress: Oxidative Eustress. Redox Biol. 2017, 11, 613–619. [Google Scholar] [CrossRef] [PubMed]
- Karra, R.; Knecht, A.K.; Kikuchi, K.; Poss, K.D. Myocardial NF-ΚB Activation Is Essential for Zebrafish Heart Regeneration. Proc. Natl. Acad. Sci. USA 2015, 112, 13255–13260. [Google Scholar] [CrossRef]
- Hall, J.E.; Hall, M.E. Guyton and Hall Textbook of Medical Physiology E-Book: Guyton Physiology; Elsevier: Amsterdam, The Netherlands, 2020; ISBN 9780323640039. [Google Scholar]
- Shepherd, I.; Eisen, J. Chapter 6—Development of the Zebrafish Enteric Nervous System. In Methods in Cell Biology; Detrich, H.W., Westerfield, M., Zon, L.I., Eds.; Academic Press: New York, NY, USA, 2011; Volume 101, pp. 143–160. ISBN 0091-679X. [Google Scholar]
- Kuil, L.E.; Chauhan, R.K.; Cheng, W.W.; Hofstra, R.M.W.; Alves, M.M. Zebrafish: A Model Organism for Studying Enteric Nervous System Development and Disease. Front. Cell Dev. Biol. 2021, 8, 629073. [Google Scholar] [CrossRef]
- Kim, B.-M.; Amores, A.; Kang, S.; Ahn, D.-H.; Kim, J.-H.; Kim, I.-C.; Lee, J.H.; Lee, S.G.; Lee, H.; Lee, J.; et al. Antarctic Blackfin Icefish Genome Reveals Adaptations to Extreme Environments. Nat. Ecol. Evol. 2019, 3, 469–478. [Google Scholar] [CrossRef] [PubMed]
- Zou, H.; Qu, H.; Bian, Y.; Sun, J.; Wang, T.; Ma, Y.; Yuan, Y.; Gu, J.; Bian, J.; Liu, Z. Polystyrene Microplastics Induce Oxidative Stress in Mouse Hepatocytes in Relation to Their Size. Int. J. Mol. Sci. 2023, 24, 7382. [Google Scholar] [CrossRef]
- Milillo, C.; Aruffo, E.; Di Carlo, P.; Patruno, A.; Gatta, M.; Bruno, A.; Dovizio, M.; Marinelli, L.; Dimmito, M.P.; Di Giacomo, V.; et al. Polystyrene Nanoplastics Mediate Oxidative Stress, Senescence, and Apoptosis in a Human Alveolar Epithelial Cell Line. Front. Public Health 2024, 12, 1385387. [Google Scholar] [CrossRef]
- Wu, Y.; Tan, X.; Shi, X.; Han, P.; Liu, H. Combined Effects of Micro- and Nanoplastics at the Predicted Envi-ronmental Concentration on Functional State of Intestinal Barrier in Caenorhabditis elegans. Toxics 2023, 11, 653. [Google Scholar] [CrossRef] [PubMed]
- Karami, A.; Groman, D.B.; Wilson, S.P.; Ismail, P.; Neela, V.K. Biomarker Responses in Zebrafish (Danio rerio) Larvae Exposed to Pristine Low-Density Polyethylene Fragments. Environ. Pollut. 2017, 223, 466–475. [Google Scholar] [CrossRef] [PubMed]
- Bhagat, J.; Zang, L.; Nakayama, H.; Nishimura, N.; Shimada, Y. Effects of Nanoplastic on Toxicity of Azole Fungicides (Ketoconazole and Fluconazole) in Zebrafish Embryos. Sci. Total Environ. 2021, 800, 149463. [Google Scholar] [CrossRef] [PubMed]
- Martin-Folgar, R.; Torres-Ruiz, M.; de Alba, M.; Cañas-Portilla, A.I.; González, M.C.; Morales, M. Molecular Effects of Polystyrene Nanoplastics Toxicity in Zebrafish Embryos (Danio rerio). Chemosphere 2023, 312, 137077. [Google Scholar] [CrossRef]
- Ding, P.; Xiang, C.; Li, X.; Chen, H.; Shi, X.; Li, X.; Huang, C.; Yu, Y.; Qi, J.; Li, A.J.; et al. Photoaged Microplastics Induce Neurotoxicity via Oxidative Stress and Abnormal Neurotransmission in Zebrafish Larvae (Danio rerio). Sci. Total Environ. 2023, 881, 163480. [Google Scholar] [CrossRef]
- Labuschagne, C.F.; Brenkman, A.B. Current Methods in Quantifying ROS and Oxidative Damage in Caenorhabditis elegans and Other Model Organism of Aging. Ageing Res. Rev. 2013, 12, 918–930. [Google Scholar] [CrossRef]
- Chang, J.; Zhu, Y.; Yang, Z.; Wang, Z.; Wang, M.; Chen, L. Airborne Polystyrene Nanoplastics Exposure Leads to Heart Failure via ECM-Receptor Interaction and PI3K/AKT/BCL-2 Pathways. Sci. Total Environ. 2024, 954, 176469. [Google Scholar] [CrossRef]
- Bojic, S.; Falco, M.M.; Stojkovic, P.; Ljujic, B.; Gazdic Jankovic, M.; Armstrong, L.; Markovic, N.; Dopazo, J.; Lako, M.; Bauer, R.; et al. Platform to Study Intracellular Polystyrene Nanoplastic Pollution and Clinical Outcomes. Stem Cells 2020, 38, 1321–1325. [Google Scholar] [CrossRef]
- Łazarski, G.; Rajtar, N.; Romek, M.; Jamróz, D.; Rawski, M.; Kepczynski, M. Interaction of Polystyrene Nano-plastic with Lipid Membranes. J. Phys. Chem. B 2025, 129, 4110–4122. [Google Scholar] [CrossRef]
- Dahl, M.; Bowler, R.P.; Juul, K.; Crapo, J.D.; Levy, S.; Nordestgaard, B.G. Superoxide dismutase 3 polymorphism associated with reduced lung function in two large populations. Am. J. Respir. Crit. Care Med. 2008, 178, 906–912. [Google Scholar] [CrossRef]
- Yao, H.; Arunachalam, G.; Hwang, J.W.; Chung, S.; Sundar, I.K.; Kinnula, V.L.; James, D.C.; Rahman, I. Extracellular superoxide dismutase protects against pulmonary emphysema by attenuating oxidative fragmentation of ECM. Proc. Natl. Acad. Sci. USA 2010, 107, 15571–15576. [Google Scholar] [CrossRef]
- Lee, M.J.; Agrahari, G.; Kim, H.Y.; An, E.J.; Chun, K.H.; Kang, H.; Kim, Y.S.; Bang, C.W.; Tak, L.J.; Kim, T.Y. Extracellular superoxide dismutase prevents skin aging by promoting collagen production through the activation of AMPK and Nrf2/HO-1 cascades. J. Investig. Dermatol. 2021, 141, 2344–2353. [Google Scholar] [CrossRef] [PubMed]
- Sah, S.K.; Agrahari, G.; Kim, T.Y. Insights into superoxide dismutase 3 in regulating biological and functional properties of mesenchymal stem cells. Cell Biosci. 2020, 10, 22. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.; Deng, S.; Wang, B.; Zhang, F.; Luo, T.; Kuang, H.; Kuang, X.; Yuan, Y.; Huang, J.; Zhang, D. Exposure to Polystyrene Nanoplastics Induces Hepatotoxicity Involving NRF2-NLRP3 Signaling Pathway in Mice. Ecotoxicol. Environ. Saf. 2024, 278, 116439. [Google Scholar] [CrossRef]
- Meng, X.; Yin, K.; Zhang, Y.; Wang, D.; Lu, H.; Hou, L.; Zhao, H.; Xing, M. Polystyrene Microplastics Induced Oxidative Stress, Inflammation and Necroptosis via NF-ΚB and RIP1/RIP3/MLKL Pathway in Chicken Kidney. Toxicology 2022, 478, 153296. [Google Scholar] [CrossRef]
- Cao, J.; Xu, R.; Geng, Y.; Xu, S.; Guo, M. Exposure to Polystyrene Microplastics Triggers Lung Injury via Tar-geting Toll-like Receptor 2 and Activation of the NF-ΚB Signal in Mice. Environ. Pollut. 2023, 320, 121068. [Google Scholar] [CrossRef]
- Zeng, G.; Li, J.; Wang, Y.; Su, J.; Lu, Z.; Zhang, F.; Ding, W. Polystyrene Microplastic-Induced Oxidative Stress Triggers Intestinal Barrier Dysfunction via the NF-ΚB/NLRP3/IL-1β/MCLK Pathway. Environ. Pollut. 2024, 345, 123473. [Google Scholar] [CrossRef] [PubMed]
- Lingappan, K. NF-κB in oxidative stress. Curr. Opin. Toxicol. 2018, 7, 81–86. [Google Scholar] [CrossRef]
- Ashrafi, S.; Zahmatkesh, N.; Tehrani, M.K.; Hadizadeh, N.; Rahimzadeh, S.; Mahdavi, M.; Abdollahi, M.; Yazdi, M.H. How Oxidative Stress Modifies the Immune Response Landscape in Cancer. Biomed. Res. Bull. 2024, 2, 140–161. [Google Scholar] [CrossRef]
- Liu, J.; Han, X.; Zhang, T.; Tian, K.; Li, Z.; Luo, F. Reactive oxygen species (ROS) scavenging biomaterials for anti-inflammatory diseases: From mechanism to therapy. J. Hematol. Oncol. 2023, 16, 116. [Google Scholar] [CrossRef]

| Species | MNPs Type | MNPs Size | MNPs Concentration | Exposure Time | Oxidative Unbalance | Reference |
|---|---|---|---|---|---|---|
| Mouse hepatocytes | PS | 0.5, 5 μm | 10 mg/L | 90 d | SOD2↓ | [31] |
| A549 cell line | PS | 0.8 μm | 10~500 μg/mL | 24 h | SOD1↓, SOD2↓ | [32] |
| Caenorhabditis elegans | PS | 50, 500 nm | 1, 10, 15 μg/L | L1 to adult | sod-2↑, sod-3↑ * | [33] |
| Zebrafish larvae (Danio rerio) | LDPE | <17.6 μm | 5, 50, 500 mg/L | 10, 20 d | No difference in sod1 | [34] |
| PS | 50 nm | 1 mg/L | 96 h | sod1↓, sod2↓ | [35] | |
| PS | 30 nm | 0.1, 0.5, 3 μg/mL | 120 h | SOD1↑, SOD2↑ | [36] | |
| Photoaged PS | 10 μm | 0, 0.1, 1, 10, 100 μg/L | 120 h | sod1↓ | [37] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sim, Y.; Kim, J.-H.; Lee, J.-S.; Jeong, J.; Cho, H.-J. Superoxide Dismutase 3 Deficiency Disrupts the Regulation of Oxidative Stress Caused by Polystyrene Nanoplastics. Antioxidants 2025, 14, 1378. https://doi.org/10.3390/antiox14111378
Sim Y, Kim J-H, Lee J-S, Jeong J, Cho H-J. Superoxide Dismutase 3 Deficiency Disrupts the Regulation of Oxidative Stress Caused by Polystyrene Nanoplastics. Antioxidants. 2025; 14(11):1378. https://doi.org/10.3390/antiox14111378
Chicago/Turabian StyleSim, Yugyeong, Jin-Hyoung Kim, Jeong-Soo Lee, Jinyoung Jeong, and Hyun-Ju Cho. 2025. "Superoxide Dismutase 3 Deficiency Disrupts the Regulation of Oxidative Stress Caused by Polystyrene Nanoplastics" Antioxidants 14, no. 11: 1378. https://doi.org/10.3390/antiox14111378
APA StyleSim, Y., Kim, J.-H., Lee, J.-S., Jeong, J., & Cho, H.-J. (2025). Superoxide Dismutase 3 Deficiency Disrupts the Regulation of Oxidative Stress Caused by Polystyrene Nanoplastics. Antioxidants, 14(11), 1378. https://doi.org/10.3390/antiox14111378









