Phenolic Fingerprints of Spanish Olive Mill Wastewaters (Alpechin): A Step Toward Regional Valorization Through Antioxidant Recovery
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples
2.2. Experimental Design for the Valorization of OMW
2.2.1. OMW Defatting
2.2.2. Evaluating the Influence of pH
2.2.3. Solid/Liquid Extraction
2.2.4. Liquid/Liquid Extraction
2.3. Chemical Analysis of the Extracts
2.3.1. Total and LC/MS/MS Determination of Phenolic Profile
2.3.2. ICP-MS Evaluation of Mineral and Heavy Metal Contents
2.3.3. Statistical Analysis
3. Results and Discussion
3.1. Characterization and Pretreatment of OMW
3.2. Hexane Defatting Treatment
3.3. Effect of pH on TPC Extraction
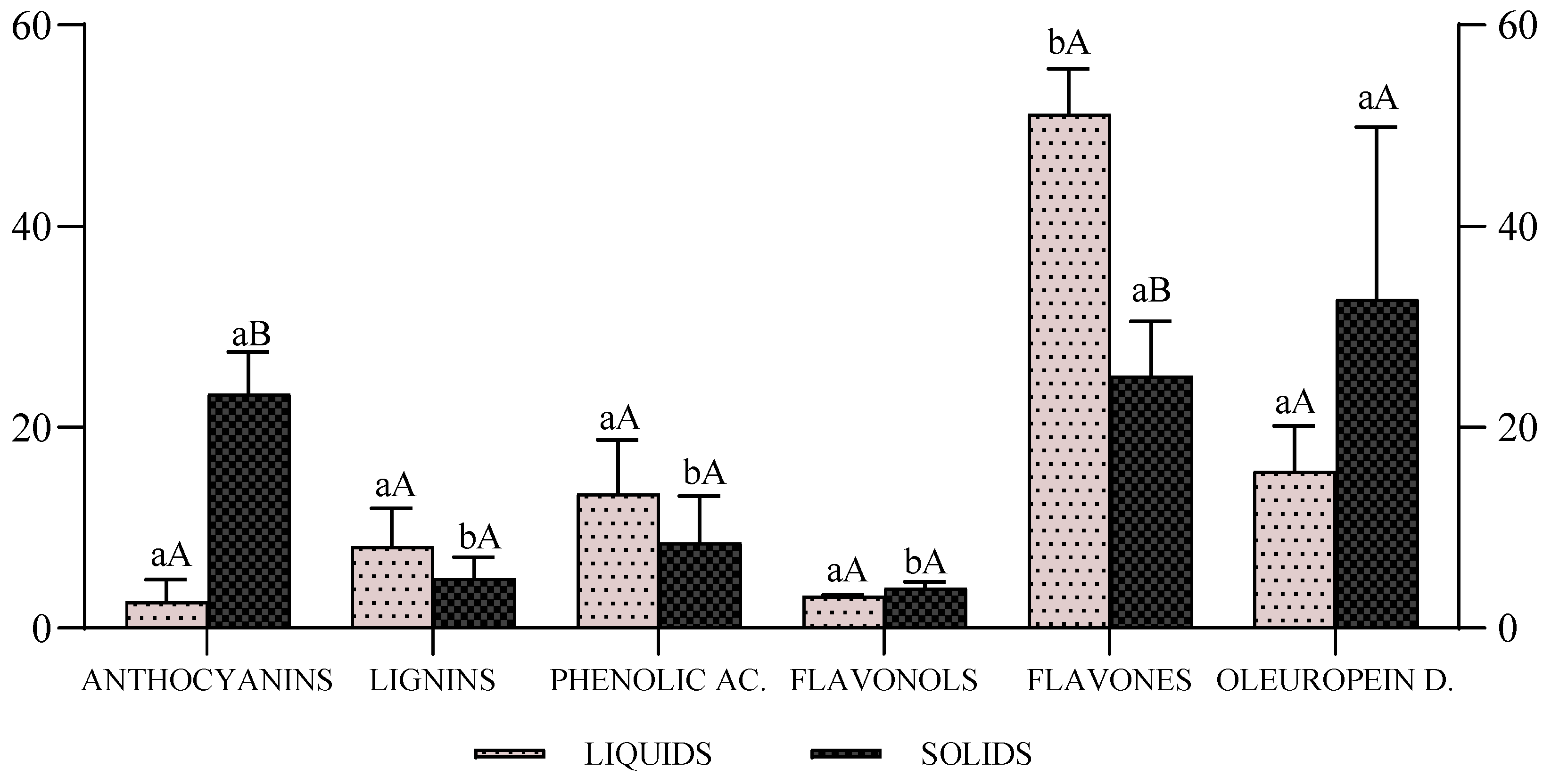
3.4. LC/MS/MS Analysis and Characterization of Phenolic Profile
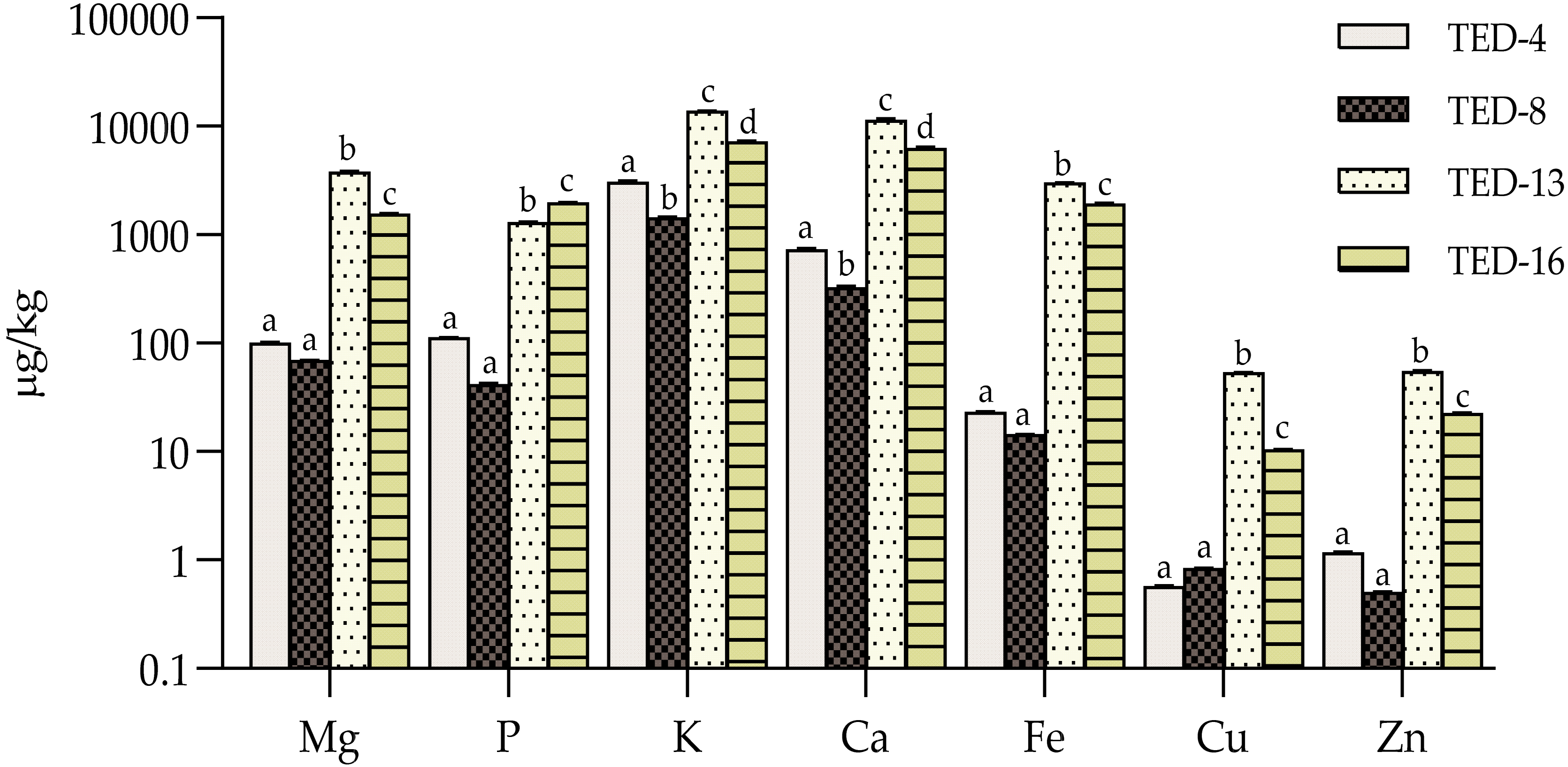
3.5. Mineral Profiles and Contents
3.6. Heavy Metal Profiles and Contents
4. Conclusions
5. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
References
- Galliou, F.; Markakis, N.; Fountoulakis, M.S.; Nikolaidis, N.; Manios, T. Production of Organic Fertilizer from Olive Mill Wastewater by Combining Solar Greenhouse Drying and Composting. Waste Manag. 2018, 75, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Huertas-Alonso, A.J.; Gonzalez-Serrano, D.J.; Hadidi, M.; Salgado-Ramos, M.; Orellana-Palacios, J.C.; Sánchez-Verdú, M.P.; Xia, Q.; Simirgiotis, M.J.; Barba, F.J.; Dar, B.N.; et al. Table Olive Wastewater as a Potential Source of Biophenols for Valorization: A Mini Review. Fermentation 2022, 8, 215. [Google Scholar] [CrossRef]
- Barbera, A.C.; Maucieri, C.; Cavallaro, V.; Ioppolo, A.; Spagna, G. Effects of Spreading Olive Mill Wastewater on Soil Properties and Crops, a Review. Agric. Water Manag. 2013, 119, 43–53. [Google Scholar] [CrossRef]
- Souilem, S.; El-Abbassi, A.; Kiai, H.; Hafidi, A.; Sayadi, S.; Galanakis, C.M. Olive Oil Production Sector: Environmental Effects and Sustainability Challenges. In Olive Mill Waste: Recent Advances for Sustainable Management; Academic Press: San Diego, CA, USA, 2017; pp. 1–28. [Google Scholar] [CrossRef]
- Cabrera, F. Características y Tratamiento de Las Aguas Residuales Industriales Por Sectores: Molturado de Aceituna Para La Obtención de Aceite de Oliva Virgen. In Spanish Inland Water Quality Current State and Research; Consejo Superior de Investigaciones Científicas: Madrid, Spain, 1995; pp. 141–154. Available online: https://digital.csic.es/handle/10261/81064 (accessed on 4 May 2024).
- Caporaso, N.; Formisano, D.; Genovese, A. Use of Phenolic Compounds from Olive Mill Wastewater as Valuable Ingredients for Functional Foods. Crit. Rev. Food Sic. Nutr. 2017, 58, 2829–2841. [Google Scholar] [CrossRef]
- Martínez-Gallardo, M.R.; López, M.J.; López-González, J.A.; Jurado, M.M.; Suárez-Estrella, F.; Pérez-Murcia, M.D.; Sáez, J.A.; Moral, R.; Moreno, J. Microbial Communities of the Olive Mill Wastewater Sludge Stored in Evaporation Ponds: The Resource for Sustainable Bioremediation. J. Environ. Manag. 2021, 279, 111810. [Google Scholar] [CrossRef]
- Kavvadias, V.; Elaiopoulos, K.; Theocharopoulos, S.; Soupios, P. Fate of Potential Contaminants Due to Disposal of Olive Mill Wastewaters in Unprotected Evaporation Ponds. Bull. Environ. Contam. Toxicol. 2017, 98, 323–330. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Gallardo, M.R. Estudio y Manejo de Comunidades Microbianas de Lodos de Alpechín En Balsas de Evaporación Para Su Biorremediación y Aprovechamiento. Ph.D. Thesis, Universidad de Almería, Almeria, Spain, 2021. [Google Scholar]
- Shabir, S.; Ilyas, N.; Saeed, M.; Bibi, F.; Sayyed, R.Z.; Almalki, W.H. Treatment Technologies for Olive Mill Wastewater with Impacts on Plants. Environ. Res. 2023, 216, 114399. [Google Scholar] [CrossRef]
- Doula, M.K.; Moreno-Ortego, J.L.; Tinivella, F.; Inglezakis, V.J.; Sarris, A.; Komnitsas, K. Olive Mill Waste: Recent Advances for the Sustainable Development of Olive Oil Industry. In Olive Mill Waste: Recent Advances for Sustainable Management; Academic Press: San Diego, CA, USA, 2017; pp. 29–56. [Google Scholar] [CrossRef]
- Paredes, C.; Cegarra, J.; Roig, A.; Sánchez-Monedero, M.A.; Bernal, M.P. Characterization of Olive Mill Wastewater (Alpechin) and Its Sludge for Agricultural Purposes. Bioresour. Technol. 1999, 67, 111–115. [Google Scholar] [CrossRef]
- Dermeche, S.; Nadour, M.; Larroche, C.; Moulti-Mati, F.; Michaud, P. Olive Mill Wastes: Biochemical Characterizations and Valorization Strategies. Process Biochem. 2013, 48, 1532–1552. [Google Scholar] [CrossRef]
- Velasco-Muñoz, J.F.; Mendoza, J.M.F.; Aznar-Sánchez, J.A.; Gallego-Schmid, A. Circular Economy Implementation in the Agricultural Sector: Definition, Strategies and Indicators. Resour. Conserv. Recycl. 2021, 170, 105618. [Google Scholar] [CrossRef]
- Donner, M.; Verniquet, A.; Broeze, J.; Kayser, K.; De Vries, H. Critical Success and Risk Factors for Circular Business Models Valorising Agricultural Waste and By-Products. Resour. Conserv. Recycl. 2021, 165, 105236. [Google Scholar] [CrossRef]
- Roselló-Soto, E.; Koubaa, M.; Moubarik, A.; Lopes, R.P.; Saraiva, J.A.; Boussetta, N.; Grimi, N.; Barba, F.J. Emerging Opportunities for the Effective Valorization of Wastes and By-Products Generated during Olive Oil Production Process: Non-Conventional Methods for the Recovery of High-Added Value Compounds. Trends Food Sci. Technol. 2015, 45, 296–310. [Google Scholar] [CrossRef]
- De Marco, E.; Savarese, M.; Paduano, A.; Sacchi, R. Characterization and Fractionation of Phenolic Compounds Extracted from Olive Oil Mill Wastewaters. Food Chem. 2007, 104, 858–867. [Google Scholar] [CrossRef]
- Martínez-Gallardo, M.R.; Jiménez, R.; Suárez-Estrella, F.; Toribio, A.; Estrella-González, M.J.; Mira-Urios, M.A.; Jurado, M.M.; López-González, J.A.; Martínez-Culebras, P.V.; López, M.J. Cataloging Olive Oil Mill Wastewater Sludge Based on Toxicological Profiles and Functional Microbial Diversity. Sci. Total Environ. 2025, 976, 179348. [Google Scholar] [CrossRef]
- Hernández-Fernández, A.; Garrido, Y.; Iniesta-López, E.; Pérez de los Ríos, A.; Quesada-Medina, J.; Hernández-Fernández, F.J. Recovering Polyphenols in Aqueous Solutions from Olive Mill Wastewater and Olive Leaf for Biological Applications. Processes 2023, 11, 2668. [Google Scholar] [CrossRef]
- Kalogerakis, N.; Politi, M.; Foteinis, S.; Chatzisymeon, E.; Mantzavinos, D. Recovery of Antioxidants from Olive Mill Wastewaters: A Viable Solution That Promotes Their Overall Sustainable Management. J. Environ. Manag. 2013, 128, 749–758. [Google Scholar] [CrossRef]
- Carrara, M.; Kelly, M.T.; Roso, F.; Larroque, M.; Margout, D. Potential of Olive Oil Mill Wastewater as a Source of Polyphenols for the Treatment of Skin Disorders: A Review. J. Agric. Food Chem. 2021, 69, 7268–7284. [Google Scholar] [CrossRef]
- Cuffaro, D.; Bertolini, A.; Bertini, S.; Ricci, C.; Cascone, M.G.; Danti, S.; Saba, A.; Macchia, M.; Digiacomo, M. Olive Mill Wastewater as Source of Polyphenols with Nutraceutical Properties. Nutrients 2023, 15, 3746. [Google Scholar] [CrossRef]
- Rahmanian, N.; Jafari, S.M.; Galanakis, C.M. Recovery and Removal of Phenolic Compounds from Olive Mill Wastewater. J. Am. Oil Chem. Soc. 2014, 91, 1–18. [Google Scholar] [CrossRef]
- Sicari, V.; Custureri, I.M.G.; Tundis, R.; Loizzo, M.R. Comparison of Physicochemical Characteristics and Bioactivity of Olive Oil Mill Wastewaters from Traditional and Water-Saving ARA-Controlled Three-Phase Decanter. Sustainability 2023, 15, 3890. [Google Scholar] [CrossRef]
- Silvan, J.M.; Pinto-Bustillos, M.A.; Vásquez-Ponce, P.; Prodanov, M.; Martinez-Rodriguez, A.J. Olive Mill Wastewater as a Potential Source of Antibacterial and Anti-Inflammatory Compounds against the Food-Borne Pathogen Campylobacter. Innov. Food Sci. Emerg. Technol. 2019, 51, 177–185. [Google Scholar] [CrossRef]
- Borzì, A.M.; Biondi, A.; Basile, F.; Luca, S.; Vicari, E.S.D.; Vacante, M. Olive Oil Effects on Colorectal Cancer. Nutrients 2018, 11, 32. [Google Scholar] [CrossRef]
- Barbara Bassani, T.R. Effect of a Purified Extract of Olive Mill Waste Water on Endothelial Cell Proliferation, Apoptosis, Migration and Capillary-Like Structure in Vitro and in Vivo. J. Bioanal. Biomed. 2015, S12, 006. [Google Scholar] [CrossRef]
- Rodrigues, F.; Pimentel, F.B.; Oliveira, M.B.P.P. Olive By-Products: Challenge Application in Cosmetic Industry. Ind. Crops Prod. 2015, 70, 116–124. [Google Scholar] [CrossRef]
- Nunes, M.A.; Pimentel, F.B.; Costa, A.S.G.; Alves, R.C.; Oliveira, M.B.P.P. Olive By-Products for Functional and Food Applications: Challenging Opportunities to Face Environmental Constraints. Innov. Food Sci. Emerg. Technol. 2016, 35, 139–148. [Google Scholar] [CrossRef]
- Sciubba, F.; Chronopoulou, L.; Pizzichini, D.; Lionetti, V.; Fontana, C.; Aromolo, R.; Socciarelli, S.; Gambelli, L.; Bartolacci, B.; Finotti, E.; et al. Olive Mill Wastes: A Source of Bioactive Molecules for Plant Growth and Protection against Pathogens. Biology 2020, 9, 450. [Google Scholar] [CrossRef] [PubMed]
- Food and Drug Administration (FDA). Summary of Information Supporting the Generally Recognized As Safe (GRAS) Status of ElaVidaTM (A Polyphenol Preparation From Olive Fruits) for Use as an Ingredient in Selected Foods. Available online: https://www.floridaolive.org/wp-content/uploads/2021/09/FDA-Olive-Leaf-Extract-GRAS-Notice.pdf (accessed on 4 May 2024).
- Veciana Galindo, C.; Cortés Castell, E.; Torro Montell, L.; Sirvent Segura, E.; Rizo Baeza, M.M.; Gil Guillén, V. Evaluación de La Citotoxicidad y Bioseguridad de Un Extracto de Polifenoles de Huesos de Aceitunas. Nutr. Hosp. 2014, 29, 1388–1393. [Google Scholar] [CrossRef]
- Ministerio de Agricultura, Pesca y Alimentación. Spain. Aforo Nacional de Cosecha de Aceite de Oliva 2023/2024. Available online: https://launio.org/download-doc/451324 (accessed on 4 May 2024).
- De Leonardis, A.; Macciola, V.; Lembo, G.; Aretini, A.; Nag, A. Studies on Oxidative Stabilisation of Lard by Natural Antioxidants Recovered from Olive-Oil Mill Wastewater. Food Chem. 2007, 100, 998–1004. [Google Scholar] [CrossRef]
- El-Abbassi, A.; Kiai, H.; Hafidi, A. Phenolic Profile and Antioxidant Activities of Olive Mill Wastewater. Food Chem. 2012, 132, 406–412. [Google Scholar] [CrossRef] [PubMed]
- El-Abbassi, A.; Kiai, H.; Raiti, J.; Hafidi, A. Application of Ultrafiltration for Olive Processing Wastewaters Treatment. J. Clean. Prod. 2014, 65, 432–438. [Google Scholar] [CrossRef]
- Suárez, M.; Romero, M.P.; Ramo, T.; Macià, A.; Motilva, M.J. Methods for Preparing Phenolic Extracts from Olive Cake for Potential Application as Food Antioxidants. J. Agric. Food Chem. 2009, 57, 1463–1472. [Google Scholar] [CrossRef]
- Galanakis, C.M.; Goulas, V.; Tsakona, S.; Manganaris, G.A.; Gekas, V. A Knowledge Base for The Recovery of Natural Phenols with Different Solvents. Int. J. Food Prop. 2012, 16, 382–396. [Google Scholar] [CrossRef]
- Allouche, N.; Fki, I.; Sayadi, S. Toward a High Yield Recovery of Antioxidants and Purified Hydroxytyrosol from Olive Mill Wastewaters. J. Agric. Food Chem. 2004, 52, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Huertas-alonso, A.J.; Gavahian, M.; González-serrano, D.J.; Hadidi, M.; Salgado-ramos, M.; Sanchez-Verdu, M.P.; Simirgiotis, M.J.; Barba, F.J.; Franco, D.; Lorenzo, J.M.; et al. Valorization of Wastewater from Table Olives: NMR Identification of Antioxidant Phenolic Fraction and Microwave Single-Phase Reaction of Sugary Fraction. Antioxidants 2021, 10, 1652. [Google Scholar] [CrossRef] [PubMed]
- Tsao, R. Chemistry and Biochemistry of Dietary Polyphenols. Nutrients 2010, 2, 1231–1246. [Google Scholar] [CrossRef] [PubMed]
- Azzam, M.O.J.; Hazaimeh, S.A. Olive Mill Wastewater Treatment and Valorization by Extraction/Concentration of Hydroxytyrosol and Other Natural Phenols. Process Saf. Environ. Prot. 2021, 148, 495–523. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of Total Phenols and Other Oxidation Substrates and Antioxidants by Means of Folin-Ciocalteu Reagent. In Methods in Enzymology; Packer, L., Ed.; Academic Press: San Diego, CA, USA, 1999; Volume 299, pp. 152–178. [Google Scholar]
- Martí-Quijal, F.J.; Ramon-Mascarell, F.; Pallarés, N.; Ferrer, E.; Berrada, H.; Phimolsiripol, Y.; Barba, F.J. Extraction of Antioxidant Compounds and Pigments from Spirulina (Arthrospira Platensis) Assisted by Pulsed Electric Fields and the Binary Mixture of Organic Solvents and Water. Appl. Sci. 2021, 11, 7629. [Google Scholar] [CrossRef]
- Zhou, J.; Wang, M.; Berrada, H.; Zhu, Z.; Grimi, N.; Barba, F.J. Pulsed Electric Fields (PEF), Pressurized Liquid Extraction (PLE) and Combined PEF + PLE Process Evaluation: Effects on Spirulina Microstructure, Biomolecules Recovery and Triple TOF-LC-MS-MS Polyphenol Composition. Innov. Food Sci. Emerg. Technol. 2022, 77, 102989. [Google Scholar] [CrossRef]
- Martí-Quijal, F.J.; Pallarés, N.; Dawidowicz, K.; Ruiz, M.-J.; Barba, F.J. Enhancing Nutrient Recovery and Bioactive Compound Extraction from Spirulina through Supercritical Fluid Extraction: Implications for SH-SY5Y Cell Viability. Foods 2023, 12, 2509. [Google Scholar] [CrossRef]
- Schymanski, E.L.; Jeon, J.; Gulde, R.; Fenner, K.; Ruff, M.; Singer, H.P.; Hollender, J. Identifying Small Molecules via High Resolution Mass Spectrometry: Communicating Confidence. Environ. Sci. Technol. 2014, 48, 2097–2098. [Google Scholar] [CrossRef]
- Alburquerque, J.A.; Gonzálvez, J.; García, D.; Cegarra, J. Agrochemical Characterisation of “Alperujo”, a Solid by-Product of the Two-Phase Centrifugation Method for Olive Oil Extraction. Bioresour. Technol. 2004, 91, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Hernández, D.; Astudillo, C.A.; Fernández-Palacios, E.; Cataldo, F.; Tenreiro, C.; Gabriel, D. Evolution of Physical-Chemical Parameters, Microbial Diversity and VOC Emissions of Olive Oil Mill Waste Exposed to Ambient Conditions in Open Reservoirs. Waste Manag. 2018, 79, 501–509. [Google Scholar] [CrossRef]
- Andreozzi, R.; Longo, G.; Majone, M.; Modesti, G. Integrated Treatment of Olive Oil Mill Effluents (OME): Study of Ozonation Coupled with Anaerobic Digestion. Water Res. 1998, 32, 2357–2364. [Google Scholar] [CrossRef]
- Kissi, M.; Mountadar, M.; Assobhei, O.; Gargiulo, E.; Palmieri, G.; Giardina, P.; Sannia, G. Roles of Two White-Rot Basidiomycete Fungi in Decolorisation and Detoxification of Olive Mill Waste Water. Appl. Microbiol. Biotechnol. 2001, 57, 221–226. [Google Scholar] [CrossRef]
- Hung, Y.-T.; Salman, H.; Awad, A. Olive Oil Waste Treatment. In Waste Treatment in the Food Processing Industry; CRC Press: Boca Raton, FL, USA, 2005; pp. 119–192. [Google Scholar] [CrossRef]
- Lawag, I.L.; Nolden, E.S.; Schaper, A.A.M.; Lim, L.Y.; Locher, C. A Modified Folin-Ciocalteu Assay for the Determination of Total Phenolics Content in Honey. Appl. Sci. 2023, 13, 2135. [Google Scholar] [CrossRef]
- Seçmeler, Ö.; Güçlü Üstündağ, Ö. Behavior of Lipophilic Bioactives during Olive Oil Processing. Eur. J. Lipid Sci. Technol. 2017, 119, 1600404. [Google Scholar] [CrossRef]
- Lefebvre, T.; Destandau, E.; Lesellier, E. Selective Extraction of Bioactive Compounds from Plants Using Recent Extraction Techniques: A Review. J. Chromatogr. A 2021, 1635, 461770. [Google Scholar] [CrossRef]
- Domínguez-Rodríguez, G.; Marina, M.L.; Plaza, M. Strategies for the Extraction and Analysis of Non-Extractable Polyphenols from Plants. J. Chromatogr. A 2017, 1514, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Rico, A.; Inarejos-García, A.M.; Salvador, M.D.; Fregapane, G. Effect of Malaxation Conditions on Phenol and Volatile Profiles in Olive Paste and the Corresponding Virgin Olive Oils (Olea Europaea L. Cv. Cornicabra). J. Agric. Food Chem. 2009, 57, 3587–3595. [Google Scholar] [CrossRef]
- Alcántara, C.; Žugčić, T.; Abdelkebir, R.; García-Pérez, J.V.; Jambrak, A.R.; Lorenzo, J.M.; Collado, M.C.; Granato, D.; Barba, F.J. Effects of Ultrasound-Assisted Extraction and Solvent on the Phenolic Profile, Bacterial Growth, and Anti-Inflammatory/Antioxidant Activities of Mediterranean Olive and Fig Leaves Extracts. Molecules 2020, 25, 1718. [Google Scholar] [CrossRef] [PubMed]
- Şahin, S.; Samli, R.; Tan, A.S.B.; Barba, F.J.; Chemat, F.; Cravotto, G.; Lorenzo, J.M. Solvent-Free Microwave-Assisted Extraction of Polyphenols from Olive Tree Leaves: Antioxidant and Antimicrobial Properties. Molecules 2017, 22, 1056. [Google Scholar] [CrossRef] [PubMed]
- Abbattista, R.; Losito, I.; Castellaneta, A.; De Ceglie, C.; Calvano, C.D.; Cataldi, T.R.I. Insight into the Storage-Related Oxidative/Hydrolytic Degradation of Olive Oil Secoiridoids by Liquid Chromatography and High-Resolution Fourier Transform Mass Spectrometry. J. Agric. Food Chem. 2020, 68, 12310–12325. [Google Scholar] [CrossRef] [PubMed]
- Peralbo-Molina, Á.; Priego-Capote, F.; Luque de Castro, M.D. Tentative Identification of Phenolic Compounds in Olive Pomace Extracts Using Liquid Chromatography–Tandem Mass Spectrometry with a Quadrupole–Quadrupole-Time-of-Flight Mass Detector. J. Agric. Food Chem. 2012, 60, 11542–11550. [Google Scholar] [CrossRef] [PubMed]
- Vilaplana-Pérez, C.; Auñón, D.; García-Flores, L.A.; Gil-Izquierdo, A. Hydroxytyrosol and Potential Uses in Cardiovascular Diseases, Cancer, and AIDS. Front. Nutr. 2014, 1, 27. [Google Scholar] [CrossRef]
- European Commission. Commission Regulation (EU) 2023/915 of 25 April 2023 on Maximum Levels for Certain Contaminants in Food and Repealing Regulation (EC) No 1881/2006. Off. J. Eur. Union 2023, 119, 103–157. [Google Scholar]
- Ministerio de la Presidencia, Relaciones con las Cortes y Memoria Democrática (Spain). Real Decreto 1051/2022, de 27 de diciembre, por el que se establecen normas para la nutrición sostenible en los suelos agrarios. Boletín Of. Del Estado 2022, 312, BOE-A-2022-23052. [Google Scholar]
- European Commission. European Commission Directive 86/278/EEC of 12 June 1986 on the Protection of the Environment, and in Particular of the Soil, When Sewage Sludge Is Used in Agriculture. Off. J. Eur. Union 1986, L 181, 6–12. Available online: http://data.europa.eu/eli/dir/1986/278/oj (accessed on 11 November 2025).
- Khalil, J.; Jaafar, A.A.K.; Habib, H.; Bouguerra, S.; Nogueira, V.; Rodríguez-Seijo, A. The Impact of Olive Mill Wastewater on Soil Properties, Nutrient and Heavy Metal Availability—A Study Case from Syrian Vertisols. J. Environ. Manag. 2024, 351, 119861. [Google Scholar] [CrossRef]
- Kabata-Pendias, A. The Anthroposphere. In Trace Elements in Soils and Plants; CRC Press: Boca Raton, FL, USA, 2010; pp. 33–64. [Google Scholar]


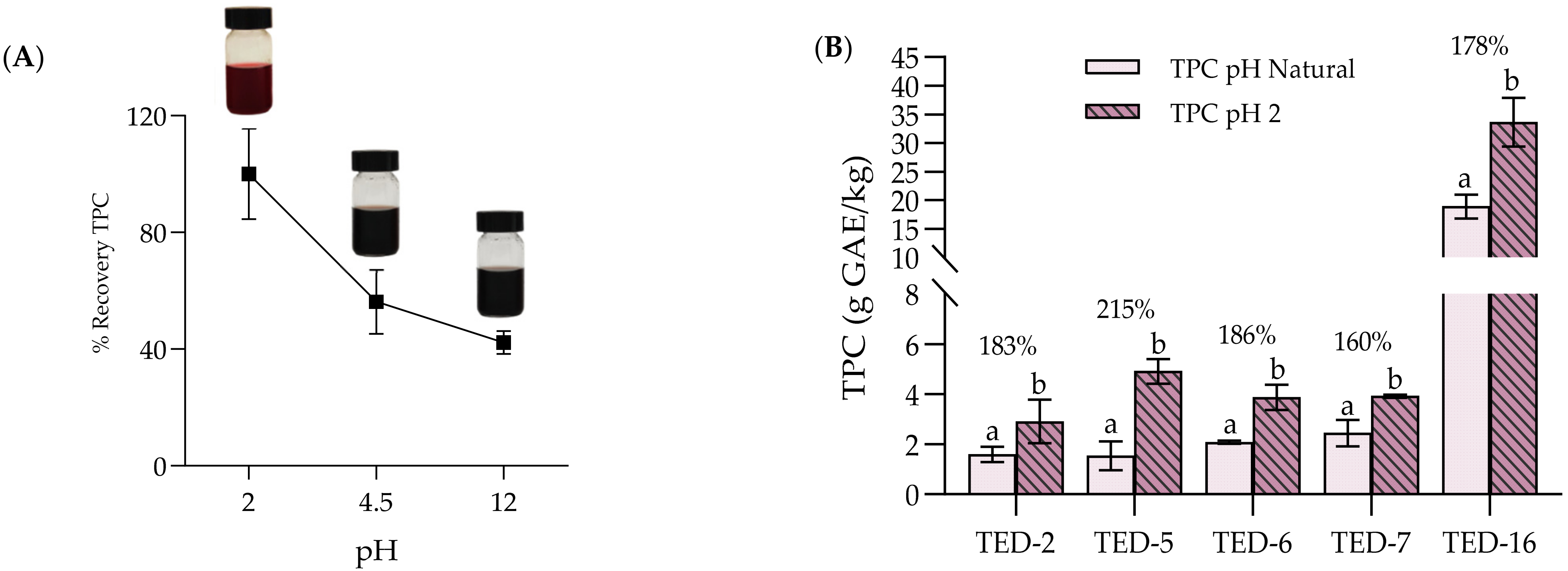

| TPC 1 (g GAE/kg) | Fats and Oils (g/100 g) | Moisture (g/100 g) | pH | State of Matrix | Colour | Area | Reference |
|---|---|---|---|---|---|---|---|
| 3.5 ± 1.9 a | 0.2 ± 0.1 | 96.9 ± 3.9 | 4.6 ± 0.2 | Liquid | Dark Brown to Dark Red | Spain | This study |
| 13.7 ± 9.8 b | 5.7 ± 3.3 | 41.1 ± 5.7 | 6.1 ± 0.3 | Solid | Dark Brown | Spain | |
| 8.65 * (3.8–14.6) | 7.4 (4.6–11.9) | 64.0 (55.6–74.5) | 5.32 (4.86–6.45) | “Alperujo” Dry weight | - | Spain | [48] |
| 5 | - | 70 | 4.8 | OMW | Black Brown | Greece | [20] |
| 13.2 | 8.7 | - | 5.7 | “Alperujo” | - | Chile | [49] |
| 6.2 | 9.8 | - | 5.1 | OMW | - | Italy | [50] |
| 12 ** | - | - | 4.2 | OMW | - | Morocco | [51] |
| 0.002–80 ** | 0.1–2.3 | - | 3.0–5.9 | OMW | Dark | Mediterranean Area | [52] |
| Compound | Formula | RT | Liquid | Solid | Group |
|---|---|---|---|---|---|
| Malvidin 3-O-rutinoside | C29H35O16 | 10.36 | TED-4, 6 | nd | Anthocyanins |
| Petunidin 3-O-rutinoside | C28H33O16 | 9.95 | TED-4 | TED-13 | |
| Cyanidin 3,5-O-diglucoside | C27H31O16 | 9.14 | TED-4, 8 | TED-13, 16 | |
| Cyanidin 3-O-(2-xylosyl-galactoside) | C26H29O15 | 9.11 | TED-4 | TED-16 | |
| Malvidin 3-O-glucoside | C23H25O12 | 9.23 | TED-4 | TED-13, 16 | |
| Petunidin 3-O-galactoside | C22H23O12 | 8.25 | TED-4 | TED-13, 16 | |
| Delphinidin 3-O-glucoside | C21H21O12 | 8.51 | TED-4, 6 | TED-13, 16 | |
| 7-Hydroxysecoisolariciresinol | C20H26O7 | 6.66 | TED-6, 8 | TED-13, 15, 16 | Lignins |
| Pinoresinol | C20H22O6 | 7.02 | TED-4 | nd | |
| Sinapic acid | C11H12O5 | 5.04 | TED-4, 6, 8 | nd | Phenolic acids |
| p-Coumaric acid | C9H8O3 | 4.98 | TED-4, 6 | TED-13, 15, 16 | |
| Cinnamic acid | C9H8O2 | 5.18 | TED-4,8 | TED-16 | |
| Vanilic acid | C8H8O4 | 1.29 | TED-4, 6, 8 | TED-13, 15, 16 | |
| Vanillin | C8H8O3 | 0.64 | TED-4, 6, 8 | TED-13, 15, 16 | |
| Hydroxybenzoic acid | C7H6O3 | 2.02 | TED-4, 6, 8 | TED-13, 15, 16 | |
| Quercetin | C15H10O7 | 7.68 | TED-4, 6, 8 | TED-13, 16 | Flavonol |
| Catechin | C15H14O6 | 4.18 | TED-4, 6, 8 | TED-13, 15, 16 | |
| Diosmetin | C16H12O6 | 8.73 | TED-4, 8 | nd | Flavones and Flavanol |
| Luteolin | C15H10O6 | 7.98 | TED-4, 6, 8 | TED-13, 15, 16 | |
| Apigenin | C15H10O5 | 8.66 | TED-4, 6, 8 | TED-15, 16 | |
| Verbascoside | C29H36O15 | 6.26 | TED-4, 8 | TED-13, 16 | Oleuropein derivatives |
| Oleuropein | C25H32O13 | 7.15 | TED-4, 6, 8 | TED-13, 15, 16 | |
| Ligustroside | C25H32O12 | 8.63 | TED-4, 8 | TED-13 | |
| 3,4-DHPEA-EA | C19H22O8 | 5.62 | TED-4, 6 | nd | |
| p-HPEA-EA | C19H22O7 | 6.92 | TED-6 | TED-13, 16 | |
| Oleoside 11-methylester | C17H24O11 | 5.69 | TED-6, 8 | TED-16 | |
| 3,4-DHPEA-EDA | C17H20O6 | 5.37 | TED-6, 8 | TED-13, 15 | |
| 3,4-DHPEA-AC | C10H12O4 | 6.32 | TED-4, 6, 8 | TED-13, 15 | |
| p-HPEA-AC | C10H12O3 | 7.07 | TED-4, 6, 8 | TED-15, 16 | |
| Tyrosol | C8H10O2 | 6.30 | TED-4, 6, 8 | TED-13, 15, 16 | |
| Hydroxytyrosol | C8H10O3 | 1.28 | TED-4, 6, 8 | TED-13, 15, 16 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez-Terol, S.; Ferrer, E.; Martínez-Culebras, P.V.; Berrada, H.; Pallarés, N.; Saez-Tovar, J.; Orden, L.; Martínez-Gallardo, M.R.; Toribio, A.J.; Barba, F.J. Phenolic Fingerprints of Spanish Olive Mill Wastewaters (Alpechin): A Step Toward Regional Valorization Through Antioxidant Recovery. Antioxidants 2025, 14, 1371. https://doi.org/10.3390/antiox14111371
Martínez-Terol S, Ferrer E, Martínez-Culebras PV, Berrada H, Pallarés N, Saez-Tovar J, Orden L, Martínez-Gallardo MR, Toribio AJ, Barba FJ. Phenolic Fingerprints of Spanish Olive Mill Wastewaters (Alpechin): A Step Toward Regional Valorization Through Antioxidant Recovery. Antioxidants. 2025; 14(11):1371. https://doi.org/10.3390/antiox14111371
Chicago/Turabian StyleMartínez-Terol, Sergio, Emilia Ferrer, Pedro V. Martínez-Culebras, Houda Berrada, Noelia Pallarés, Jose Saez-Tovar, Luciano Orden, María R. Martínez-Gallardo, Ana J. Toribio, and Francisco J. Barba. 2025. "Phenolic Fingerprints of Spanish Olive Mill Wastewaters (Alpechin): A Step Toward Regional Valorization Through Antioxidant Recovery" Antioxidants 14, no. 11: 1371. https://doi.org/10.3390/antiox14111371
APA StyleMartínez-Terol, S., Ferrer, E., Martínez-Culebras, P. V., Berrada, H., Pallarés, N., Saez-Tovar, J., Orden, L., Martínez-Gallardo, M. R., Toribio, A. J., & Barba, F. J. (2025). Phenolic Fingerprints of Spanish Olive Mill Wastewaters (Alpechin): A Step Toward Regional Valorization Through Antioxidant Recovery. Antioxidants, 14(11), 1371. https://doi.org/10.3390/antiox14111371










