Oxidative Stress and Postoperative Outcomes: An Umbrella Review of Systematic Reviews and Meta-Analyses
Abstract
1. Introduction
2. Materials and Methods
2.1. Methods Design
2.2. Selection Criteria
2.3. Information Sources and Search Strategy
2.4. Study Selection
2.5. Quality and Risk of Bias Assessment
2.6. Results Synthesis
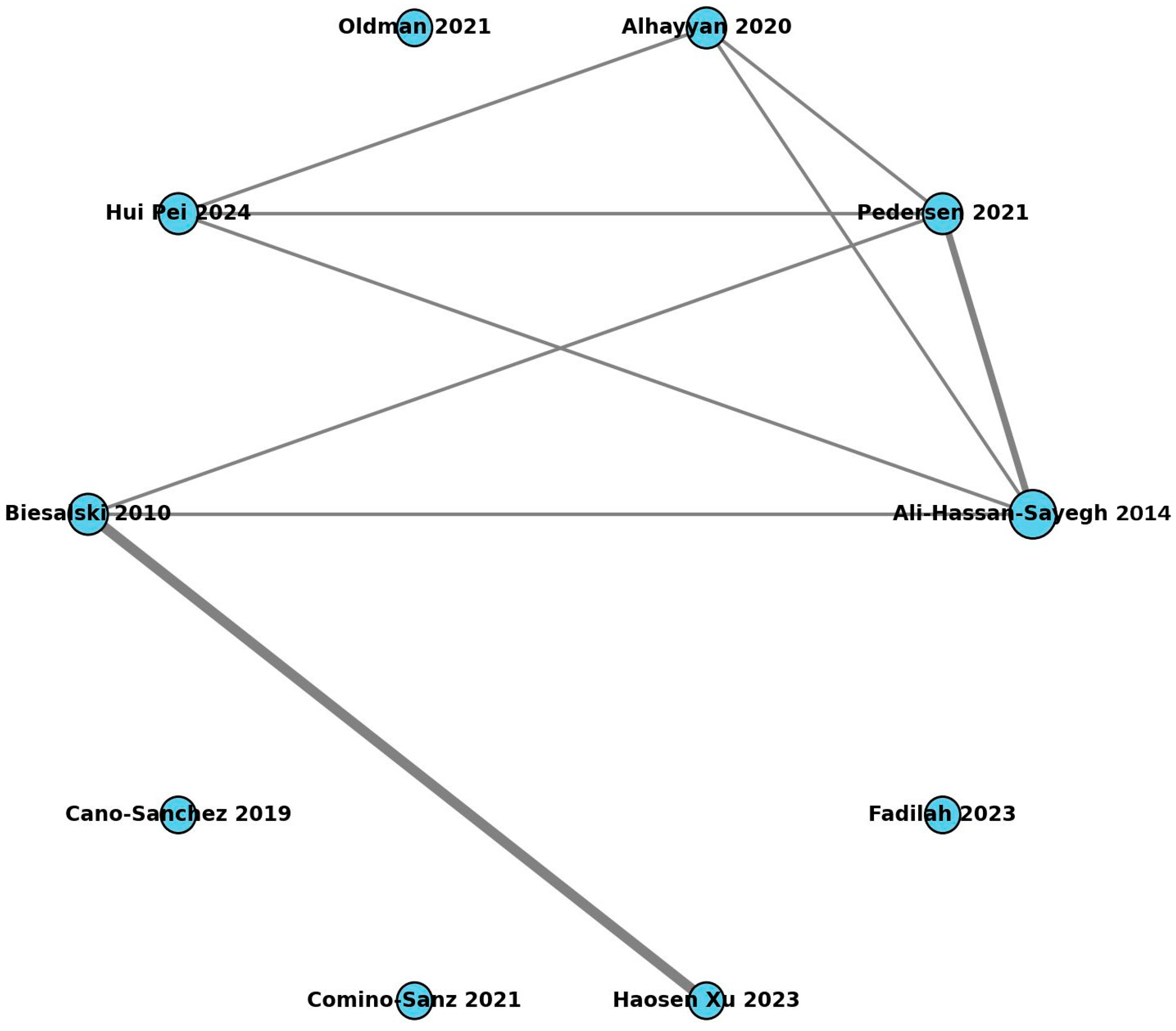
2.7. Assessment of Study Overlap (CCA)
2.8. Construction of the Evidence Map
2.9. Certainty of Evidence and Credibility Grading
3. Results
3.1. Methodological Quality and Risk of Bias
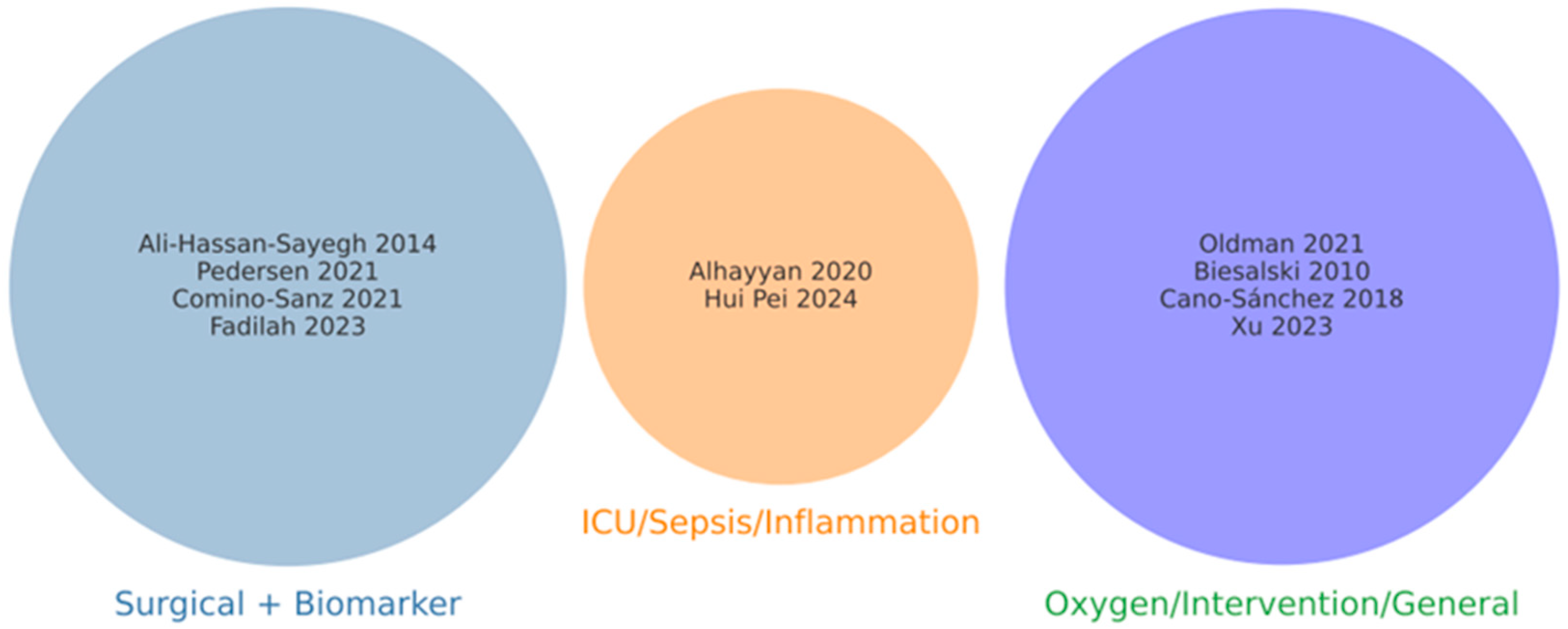
3.2. Conceptual Classification of Included Reviews
3.3. Study Overlap and Redundancy
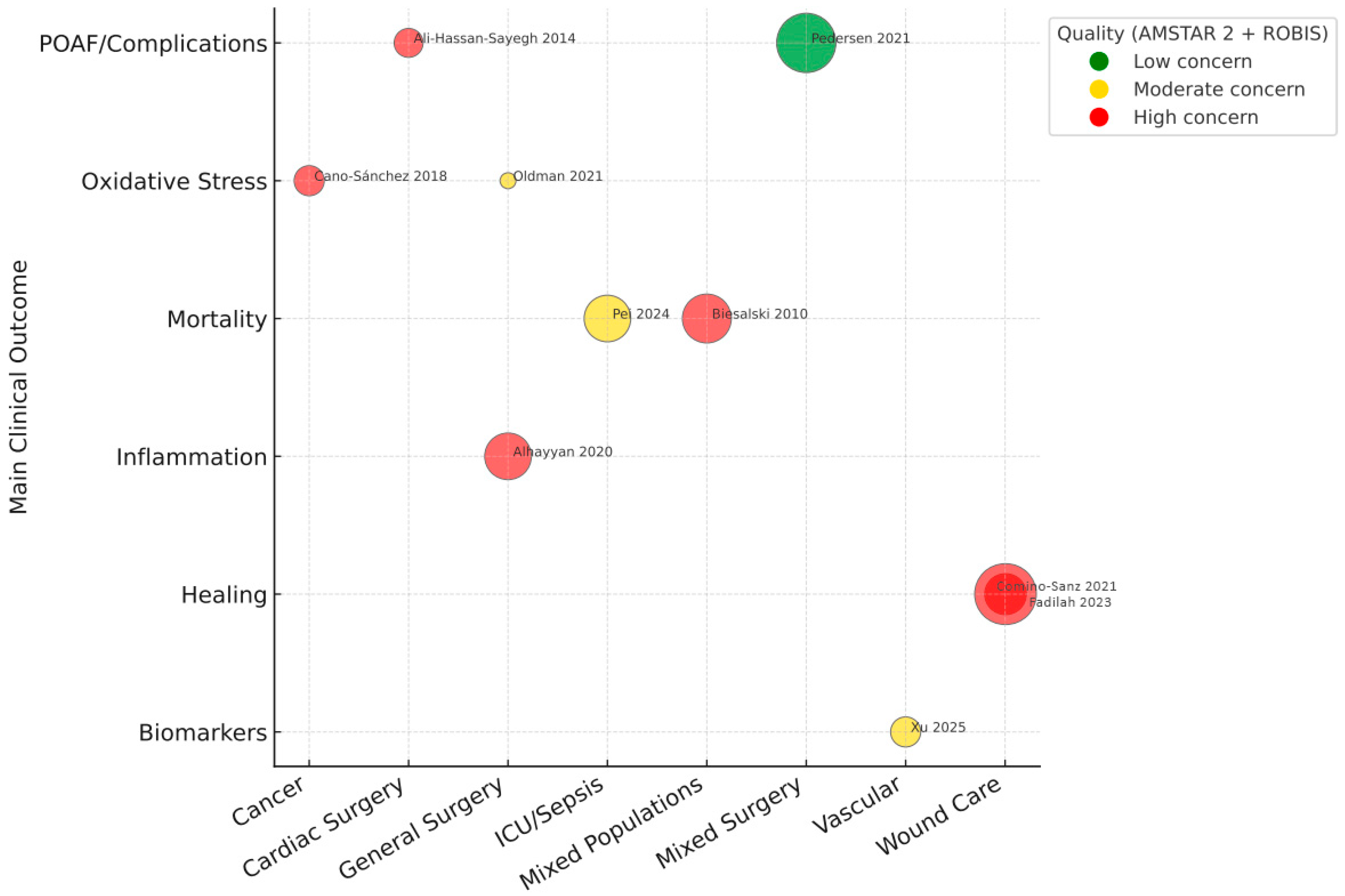
3.4. Evidence Mapping Results
3.5. Biomarkers and Clinical Outcomes
3.6. Graded Certainty by Outcome, Specialty, and Biomarker
3.7. Principal Findings by Thematic Areas
3.7.1. Infectious Complications and Delayed Healing
3.7.2. Cardiovascular and Systemic Complications
3.7.3. Risk Prediction and Personalized Perioperative Management
3.7.4. Methodological Assessment
4. Discussion
4.1. Clinical Implications
- (1)
- Incorporation of Biomarkers in Preoperative Assessment
- ○
- The measurement of TAC, GSH, SOD, MDA, and 8-OHdG constitutes a valuable tool for identifying patients with a higher probability of complications, allowing for a more preventive and individualized approach based on risk.
- (2)
- Personalized Antioxidant Interventions
- ○
- The use of vitamin C and N-acetylcysteine is supported by evidence in critical or septic patients. At the same time, intravenous anesthesia with propofol offers advantages over inhalational anesthesia in surgeries with a high inflammatory risk. Likewise, the careful titration of intraoperative oxygen therapy is essential to avoid hyperoxia and reduce lipid peroxidation.
- (3)
- Innovation in Antioxidant Biomaterials and Dressings
- ○
- New perspectives are opened in reconstructive surgery and in the management of complex wounds by the development of hydrogels and dressings enriched with antioxidants, particularly in patients with comorbidities such as diabetes or malnutrition.
- (4)
- Multidisciplinary Approach
- ○
- The successful implementation of these strategies relies on coordinated participation from surgeons, anesthesiologists, intensivists, and nursing professionals, guaranteeing an integral management of oxidative stress and favoring safer surgical outcomes.
4.2. Recommendations for Future Research
4.3. Study Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 8-OHdG | 8-hydroxy-2′-deoxyguanosine |
| AKI | Acute kidney injury |
| AMSTAR 2 | A Measurement Tool to Assess Systematic Reviews 2 |
| CCA | Corrected Covered Area |
| CRP | C-reactive protein |
| ELAM | Elamipretide |
| ERAS | Enhanced Recovery After Surgery |
| FiO2 | Fraction of inspired oxygen |
| GPx | Glutathione peroxidase |
| GSH | Glutathione |
| IL-6 | Interleukin-6 |
| MAPK | Mitogen-activated protein kinase |
| MDA | Malondialdehyde |
| MI | Myocardial infarction |
| NAC | N-acetylcysteine |
| NF-κB | Nuclear factor kappa B |
| NLRP3 | NOD-like receptor family pyrin domain containing 3 inflammasome |
| NO | Nitric oxide |
| Nrf2-ARE | Nuclear factor erythroid 2–related factor 2/antioxidant response element |
| OS | Oxidative stress |
| POAF | Postoperative atrial fibrillation |
| PUFA | Polyunsaturated fatty acids |
| RCTs | Randomized controlled trials |
| ROBIS | Risk of Bias in Systematic Reviews |
| ROS | Reactive oxygen species |
| SAE | Serious adverse events |
| SANRA | Scale for the Assessment of Narrative Review Articles |
| SkQ1 | Plastoquinone conjugated with decyl |
| SOD | Superoxide dismutase |
| TAC | Total antioxidant capacity |
| TAS | Total antioxidant status |
| TSA | Trial sequential analysis |
| WBC | White blood cells |
References
- Gómez-Serrano, M.; Camafeita, E.; López, J.A.; Rubio, M.A.; Bretón, I.; García-Consuegra, I.; García-Santos, E.; Lago, J.; Sánchez-Pernaute, A.; Torres, A.; et al. Differential proteomic and oxidative profiles unveil dysfunctional protein import to adipocyte mitochondria in obesity-associated aging and diabetes. Redox Biol. 2017, 11, 415–428. [Google Scholar] [CrossRef]
- Dhalla, N.S.; Temsah, R.M.; Netticadan, T. Role of oxidative stress in cardiovascular diseases. J. Hypertens. 2000, 18, 655–673. [Google Scholar] [CrossRef]
- Leimkühler, M.; Bourgonje, A.R.; van Goor, H.; Campmans-Kuijpers, M.J.E.; de Bock, G.H.; van Leeuwen, B.L. Oxidative Stress Predicts Post-Surgery Complications in Gastrointestinal Cancer Patients. Ann. Surg. Oncol. 2022, 29, 4540–4547. [Google Scholar] [CrossRef]
- Gu, W.J.; Wei, C.Y.; Yin, R.X. Antioxidant supplementation for the prevention of post-endoscopic retrograde cholangiopancreatography pancreatitis: A meta-analysis of randomized controlled trials. Nutr. J. 2013, 12, 23. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Li, Y.; Guo, L.; Zhang, C.; Tang, S. An umbrella review of systematic reviews and meta-analyses of observational investigations of obstructive sleep apnea and health outcomes. Sleep Breath. 2022, 26, 167–188. [Google Scholar] [CrossRef] [PubMed]
- Aromataris, E.; Fernandez, R.; Godfrey, C.M.; Holly, C.; Khalil, H.; Tungpunkom, P. Summarizing systematic reviews: Methodological development, conduct and reporting of an umbrella review approach. Int. J. Evid. Based. Healthc. 2015, 13, 132–140. [Google Scholar] [CrossRef]
- Rojas-Rueda, D.; Morales-Zamora, E.; Alsufyani, W.A.; Herbst, C.H.; AlBalawi, S.M.; Alsukait, R.; Alomran, M. Environmental risk factors and health: An umbrella review of meta-analyses. Int. J. Environ. Res. Public Health 2021, 18, 704. [Google Scholar] [CrossRef]
- D’Amato, A.; Cestiè, C.; Ferranti, F.; Segato, C.; Prosperi, S.; Germanò, R.; Myftari, V.; Bartimoccia, S.; Castellani, V.; Badagliacca, R.; et al. Implications of Oxidative Stress in the Pathophysiological Pathways of Heart Failure. Int. J. Mol. Sci. 2025, 26, 5165. [Google Scholar] [CrossRef]
- Yuba, T.; Koyama, Y.; Takahashi, A.; Fujino, Y.; Shimada, S. Association between oxidative stress and postoperative delirium in joint replacement using diacron-reactive oxygen metabolites and biological antioxidant potential tests. Sci. Rep. 2024, 14, 29854. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Ioannidis, J.P.A. Integration of evidence from multiple meta-analyses: A primer on umbrella reviews, treatment networks and multiple treatments meta-analyses. Can. Med. Assoc. J. 2009, 181, 488–493. [Google Scholar] [CrossRef] [PubMed]
- Tsagris, M.; Fragkos, K.C. Umbrella Reviews, Overviews of Reviews, and Meta-epidemiologic Studies: Similarities and Differences. In Umbrella Reviews: Evidence Synthesis with Overviews of Reviews and Meta-Epidemiologic Studies; Springer: Berlin/Heidelberg, Germany, 2016; pp. 43–54. [Google Scholar] [CrossRef]
- Shea, B.J.; Reeves, B.C.; Wells, G.; Thuku, M.; Hamel, C.; Moran, J.; Moher, D.; Tugwell, P.; Welch, V.; Kristjansson, E.; et al. AMSTAR 2: A critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ 2017, 358, 4008. [Google Scholar] [CrossRef]
- Whiting, P.; Savović, J.; Higgins, J.P.T.; Caldwell, D.M.; Reeves, B.C.; Shea, B.; Davies, P.; Kleijnen, J.; Churchill, R. ROBIS: A new tool to assess risk of bias in systematic reviews was developed. J. Clin. Epidemiol. 2016, 69, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Baethge, C.; Goldbeck-Wood, S.; Mertens, S. SANRA—A scale for the quality assessment of narrative review articles. Res. Integr. Peer Rev. 2019, 4, 5. [Google Scholar] [CrossRef]
- Ali-Hassan-Sayegh, S.; Mirhosseini, S.J.; Rezaeisadrabadi, M.; Dehghan, H.R.; Sedaghat-Hamedani, F.; Kayvanpour, E.; Popov, A.F.; Liakopoulos, O.J. Antioxidant supplementations for prevention of atrial fibrillation after cardiac surgery: An updated comprehensive systematic review and meta-analysis of 23 randomized controlled trials. Interact. Cardiovasc. Thorac. Surg. 2014, 18, 646–654. [Google Scholar] [CrossRef]
- Pedersen, S.S.; Fabritius, M.L.; Kongebro, E.K.; Meyhoff, C.S. Antioxidant treatment to reduce mortality and serious adverse events in adult surgical patients: A systematic review with meta-analysis and trial sequential analysis. Acta Anaesthesiol. Scand. 2021, 65, 438–450. [Google Scholar] [CrossRef]
- Alhayyan, A.; McSorley, S.; Roxburgh, C.; Kearns, R.; Horgan, P.; McMillan, D. The effect of anesthesia on the postoperative systemic inflammatory response in patients undergoing surgery: A systematic review and meta-analysis. Surg. Open Sci. 2020, 2, 1–21. [Google Scholar] [CrossRef]
- Oldman, A.H.; Martin, D.S.; Feelisch, M.; Grocott, M.P.W.; Cumpstey, A.F. Effects of perioperative oxygen concentration on oxidative stress in adult surgical patients: A systematic review. Br. J. Anaesth. 2021, 126, 622–632. [Google Scholar] [CrossRef]
- Pei, H.; Qu, J.; Chen, J.M.; Zhang, Y.L.; Zhang, M.; Zhao, G.J.; Lu, Z.Q. The effects of antioxidant supplementation on short-term mortality in sepsis patients. Heliyon 2024, 10, e29156. [Google Scholar] [CrossRef] [PubMed]
- Biesalski, H.K.; Grune, T.; Tinz, J.; Zöllner, I.; Blumberg, J.B. Reexamination of a meta-analysis of the effect of antioxidant supplementation on mortality and health in randomized trials. Nutrients 2010, 2, 929–949. [Google Scholar] [CrossRef]
- Sanchez, M.C.; Lancel, S.; Boulanger, E.; Neviere, R. Targeting oxidative stress and mitochondrial dysfunction in the treatment of impaired wound healing: A systematic review. Antioxidants 2018, 7, 98. [Google Scholar] [CrossRef] [PubMed]
- Comino-Sanz, I.M.; López-Franco, M.D.; Castro, B.; Pancorbo-Hidalgo, P.L. The role of antioxidants on wound healing: A review of the current evidence. J. Clin. Med. 2021, 10, 3558. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Yang, J.; Wei, Z.; Bao, S.; Liu, Z. Oxidative stress in vascular surgical diseases: Mechanisms, impacts and therapeutic perspectives. Front. Pharmacol. 2025, 16, 1527684. [Google Scholar] [CrossRef]
- Fadilah, N.I.M.; Phang, S.J.; Kamaruzaman, N.; Salleh, A.; Zawani, M.; Sanyal, A.; Maarof, M.; Fauzi, M.B. Antioxidant Biomaterials in Cutaneous Wound Healing and Tissue Regeneration: A Critical Review. Antioxidants 2023, 12, 787. [Google Scholar] [CrossRef]
- Beyaztas, H.; Ersoz, C.; Ozkan, B.N.; Olgun, I.; Polat, H.S.; Dastan, A.I.; Cetinkaya, E.; Guler, E.M. The role of oxidative stress and inflammation biomarkers in pre- and postoperative monitoring of prostate cancer patients. Free Radic. Res. 2024, 58, 98–106. [Google Scholar] [CrossRef] [PubMed]

| (a) | ||||||
|---|---|---|---|---|---|---|
| Year | Country | Authors | Type and Design of the Study | Main Results | Methodological Quality | ROBIS |
| 2014 | International | Ali-Hassan-Sayegh et al. [16]. | Meta-analysis of 23 RCTs (4278 patients) | NAC, PUFA, and vitamin C reduce POAF. PUFA and vitamin C reduce hospital stay. | High | Low |
| 2020 | Denmark | Pedersen et al. [17] | Systematic review + meta-analysis + TSA (RCTs) | 26% reduction in mortality (RR 0.74), not significant in low-bias analysis | High | Low |
| 2020 | United Kingdom | Alhayyan et al. [18] | Systematic review + meta-analysis (27 RCTs) | Propofol reduces postoperative oxidative stress compared with inhalational anesthesia (↓ MDA, IL-6, ↑ SOD) | High | Low |
| 2021 | United Kingdom | Oldman et al. [19] | Systematic review of RCTs | High FiO2 is associated with increased MDA and carbonyls, and it decreases antioxidant levels. | Moderate–High | Moderate |
| 2024 | China | Pei et al. [20] | Systematic review + meta-analysis (60 studies, 130,986 patients) | Significant reduction in hospital and 28-day mortality (highlighted ascorbic acid) | High | Low |
| 2010 | Germany/USA | Biesalski et al. [21] | Re-evaluation of meta-analyses (66 RCTs) | 36% showed benefit; only 5% showed harm. Greater benefits in malnourished populations. | Moderate–High | Moderate |
| (b) | ||||||
| Year | Country | Authors | Type and Design of the Study | Main Results | Methodological Quality | ROBIS |
| 2018 | France/Spain | Cano Sanchez et al. [22]. | Narrative systematic review | Mitochondrial antioxidants (SkQ1, elamipretide) improve wound healing in animal models. | Moderate–High | Low |
| 2021 | Spain | Comino-Sanz et al. [23]. | Narrative systematic review (46 in vitro, animal, and human studies) | Curcumin, NAC, chitosan, quercetin: Improves wound healing. Limited human evidence. | Moderate | Low |
| 2025 | China | Xu et al. [24]. | Narrative systematic review | Antioxidants reduce oxidative stress in vascular diseases. Limited clinical evidence. | Moderate–High | Moderate |
| 2023 | Malaysia/India | Fadilah et al. [25]. | Critical review (in vitro, in vivo, clinical) | Antioxidant biomaterials improve healing; solid clinical evidence is lacking. | Moderate–High | Moderate |
| Authors | Type of Study (Design) | Type of Surgery/Specialty | Associated Biomarkers | Associated Complications |
|---|---|---|---|---|
| Ali-Hassan-Sayegh et al. [16] | Meta-analysis (23 RCTs) | Cardiac surgery | NAC, PUFA, vitamin C | Reduced POAF and hospital stay |
| Biesalski et al. [21] | Re-evaluation of meta-analyses (66 RCTs) | Multisystem (primary/secondary prevention) | N/A (overall mortality analysis) | Mortality; contextual benefits depending on nutritional status |
| Pedersen et al. [17] | Systematic review + meta-analysis + TSA (RCTs) | General/Adult surgery | Non-specific (mortality, POAF, MI, stroke) | Mortality, POAF, MI, stroke, AKI, SAE |
| Alhayyan et al. [18] | Systematic review + meta-analysis (27 RCTs) | General surgery (multiple types) | IL-6, CRP, WBC | Inflammatory response intensity; risk of postoperative complications |
| Oldman et al. [19] | Systematic review (RCTs) | Non-cardiac surgery (cesarean, colorectal) | MDA, carbonyls, xanthine oxidase, SOD, TAS | Increased oxidative stress; potential adverse effects |
| Pei et al. [20] | Systematic review + meta-analysis (60 studies, 130,986 patients) | Sepsis (ICU, adults) | N/A (clinical variables: hospital and 28–30-day mortality) | Mortality, multiorgan dysfunction |
| Cano Sánchez et al. [22] | Narrative systematic review | Chronic wound healing (diabetes, hypoxia) | ROS, inflammation, NLRP3, Nrf2-ARE | Delayed healing, chronic inflammation, cellular senescence |
| Comino-Sanz et al. [23] | Narrative systematic review | Skin wounds (animal and human models) | SOD, lipid peroxidation, cell proliferation | Slow wound closure, low angiogenesis, inflammation |
| Xu et al. [24] | Systematic narrative review | Vascular surgery | ROS, NO, NF-κB, MAPK, apoptosis | Thrombosis, aneurysms, diabetic foot, vascular inflammation |
| Fadilah et al. [25] | Narrative and systematic review | Skin wounds and tissue regeneration | Collagen, angiogenesis, inflammation, oxidative stress | Ineffective healing, persistent inflammation |
| Outcome | Specialty | Biomarker (s) | No. SRs | Consistency | AMSTAR 2/ROBIS | Overlap (CCA) | Certainty (GRADE) | Notes |
|---|---|---|---|---|---|---|---|---|
| Infectious complications | General surgery | MDA ↑ | 2 SRs | Consistent ↑ risk | Moderate/Low | Low | Moderate | Heterogeneity in assays/timing |
| Organ dysfunction | Mixed | TAC ↓, GSH ↓, SOD ↓ | 3 SRs | Consistent | High/Low | Low | Moderate | Timing variability |
| Persistent pain | Orthopedic | 8-OHdG ↑ | 1 SR | Inconsistent | Moderate/Moderate | Low | Low | Imprecision, indirectness |
| POAF | Cardiac | Vit C, NAC, PUFA | 2 SRs | Moderate ↓ risk | High/Low | Low | Moderate | Small-study bias |
| Mortality | Sepsis/Cardiac | Multiple | 3 SRs | Variable | Mixed | Low | Low–Moderate | Fragile estimates |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cuevas-Budhart, M.A.; Sánchez-Garre, M.; Sánchez-Bermúdez, A.; Sobrino-Rodríguez, A.; Arniella-Blanco, M.M.; Renghea, A.; Crespo-Cañizares, A.; Cavero-Redondo, I.; Gallardo, J.M.; Gómez del Pulgar, M. Oxidative Stress and Postoperative Outcomes: An Umbrella Review of Systematic Reviews and Meta-Analyses. Antioxidants 2025, 14, 1349. https://doi.org/10.3390/antiox14111349
Cuevas-Budhart MA, Sánchez-Garre M, Sánchez-Bermúdez A, Sobrino-Rodríguez A, Arniella-Blanco MM, Renghea A, Crespo-Cañizares A, Cavero-Redondo I, Gallardo JM, Gómez del Pulgar M. Oxidative Stress and Postoperative Outcomes: An Umbrella Review of Systematic Reviews and Meta-Analyses. Antioxidants. 2025; 14(11):1349. https://doi.org/10.3390/antiox14111349
Chicago/Turabian StyleCuevas-Budhart, Miguel Angel, María Sánchez-Garre, Alba Sánchez-Bermúdez, Aurora Sobrino-Rodríguez, María Mastel Arniella-Blanco, Alina Renghea, Almudena Crespo-Cañizares, Iván Cavero-Redondo, Juan Manuel Gallardo, and Mercedes Gómez del Pulgar. 2025. "Oxidative Stress and Postoperative Outcomes: An Umbrella Review of Systematic Reviews and Meta-Analyses" Antioxidants 14, no. 11: 1349. https://doi.org/10.3390/antiox14111349
APA StyleCuevas-Budhart, M. A., Sánchez-Garre, M., Sánchez-Bermúdez, A., Sobrino-Rodríguez, A., Arniella-Blanco, M. M., Renghea, A., Crespo-Cañizares, A., Cavero-Redondo, I., Gallardo, J. M., & Gómez del Pulgar, M. (2025). Oxidative Stress and Postoperative Outcomes: An Umbrella Review of Systematic Reviews and Meta-Analyses. Antioxidants, 14(11), 1349. https://doi.org/10.3390/antiox14111349









