Granulosa Cell-Secreted KITL Is Involved in Maintaining Zinc Homeostasis in the Oocytes of Neonatal Mouse Ovaries
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Chemicals
2.2. Neonatal Mouse Ovary Culture
2.3. Neonatal Mouse Injection Experiment
2.4. Isolation of Oocytes from Neonatal Mice
2.5. Histological and Morphological Analysis
2.6. Immunofluorescent Staining
2.7. Zinc Measurement and ROS Staining of Oocytes
2.8. In Situ Cell Death Detection
2.9. Immunohistochemical Staining
2.10. Western Blotting Analysis
2.11. RNA Isolation and Analysis
2.12. RNA-Sequencing Analysis
2.13. Statistical Analysis
3. Results
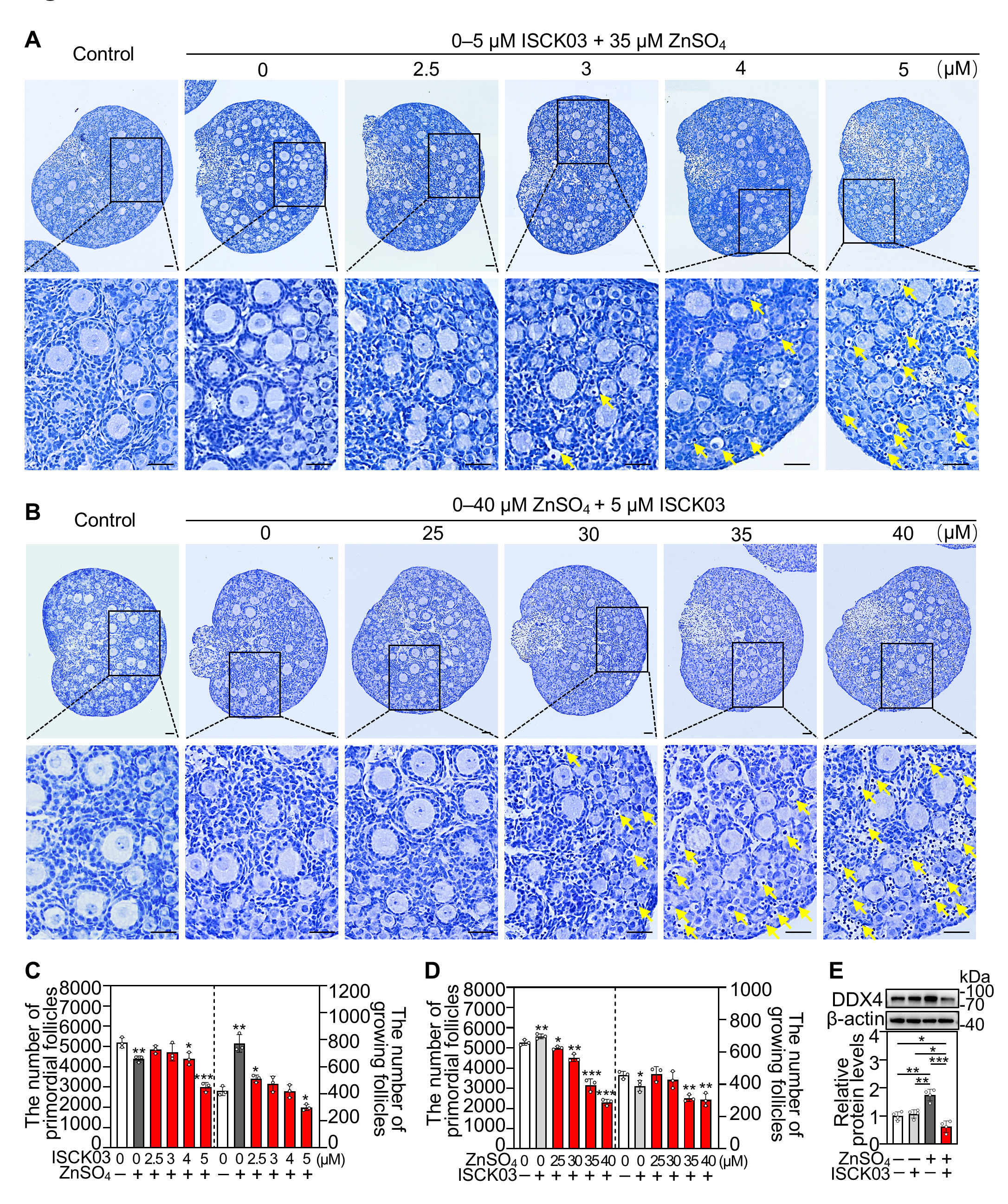
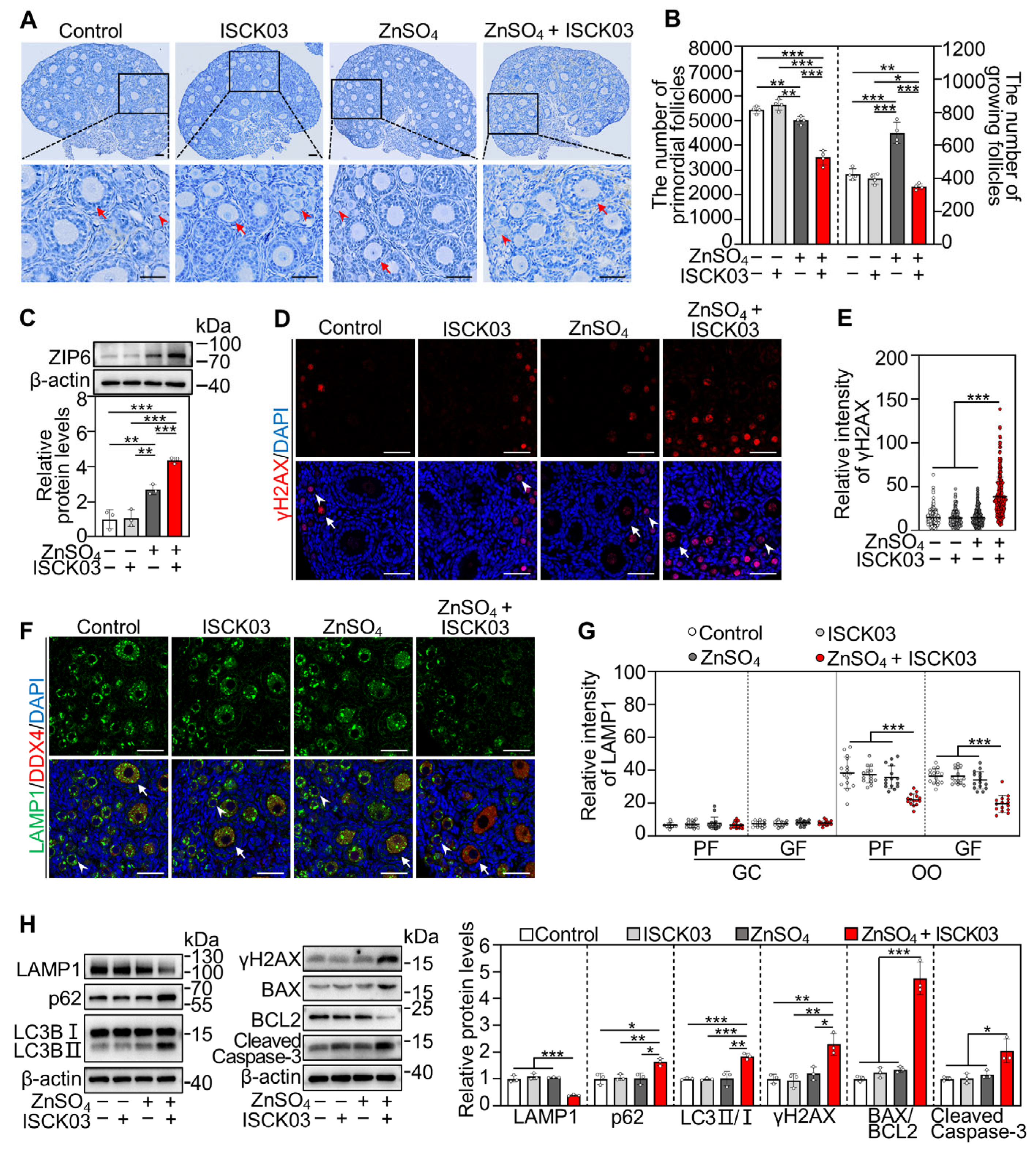
3.1. ZnSO4 Induces Follicular Atresia in Cultured Neonatal Mouse Ovaries in the Presence of ISCK03
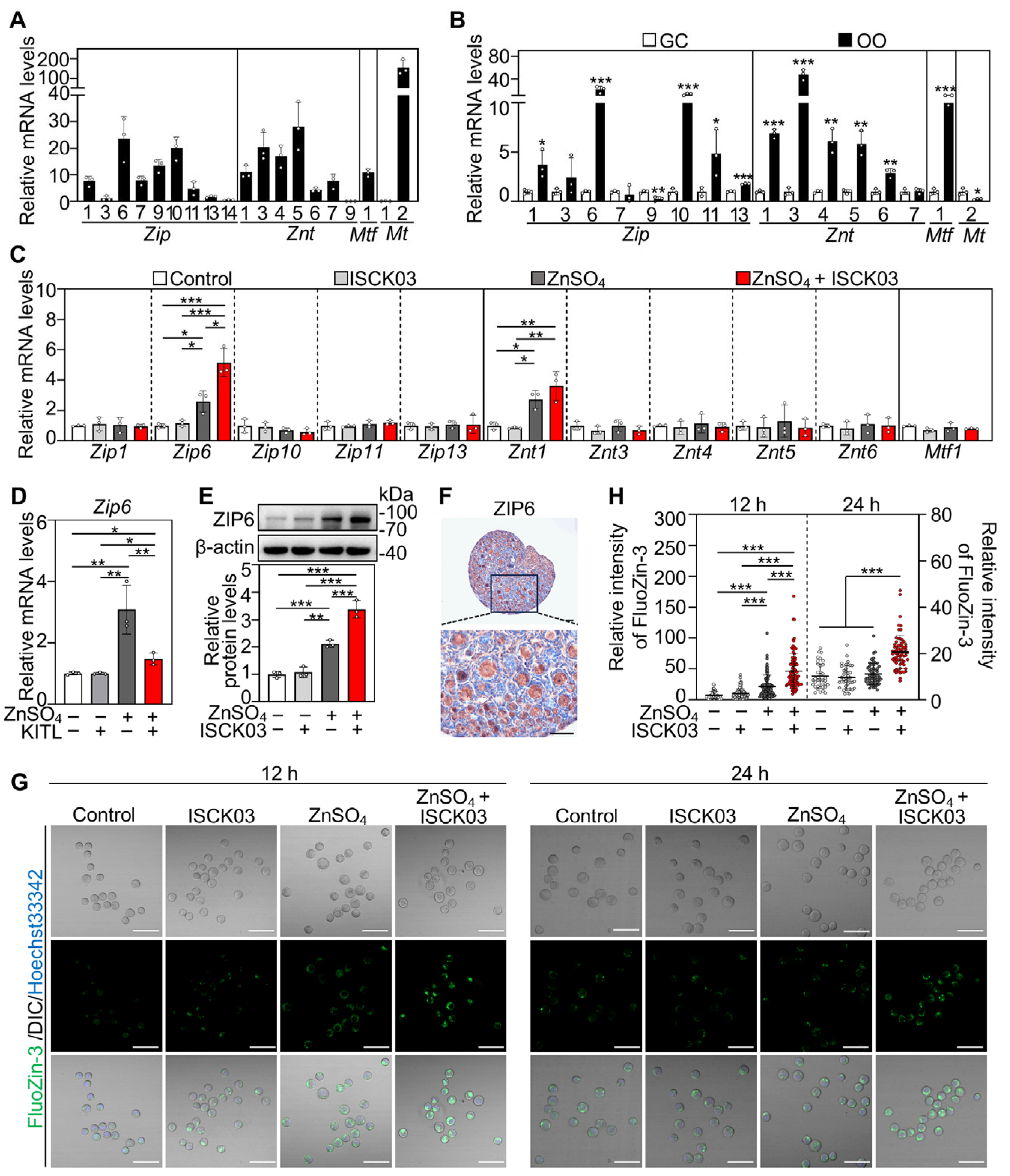
3.2. ZnSO4 Induces Zinc Overload in the Oocytes of Cultured Neonatal Mouse Ovaries in the Presence of ISCK03
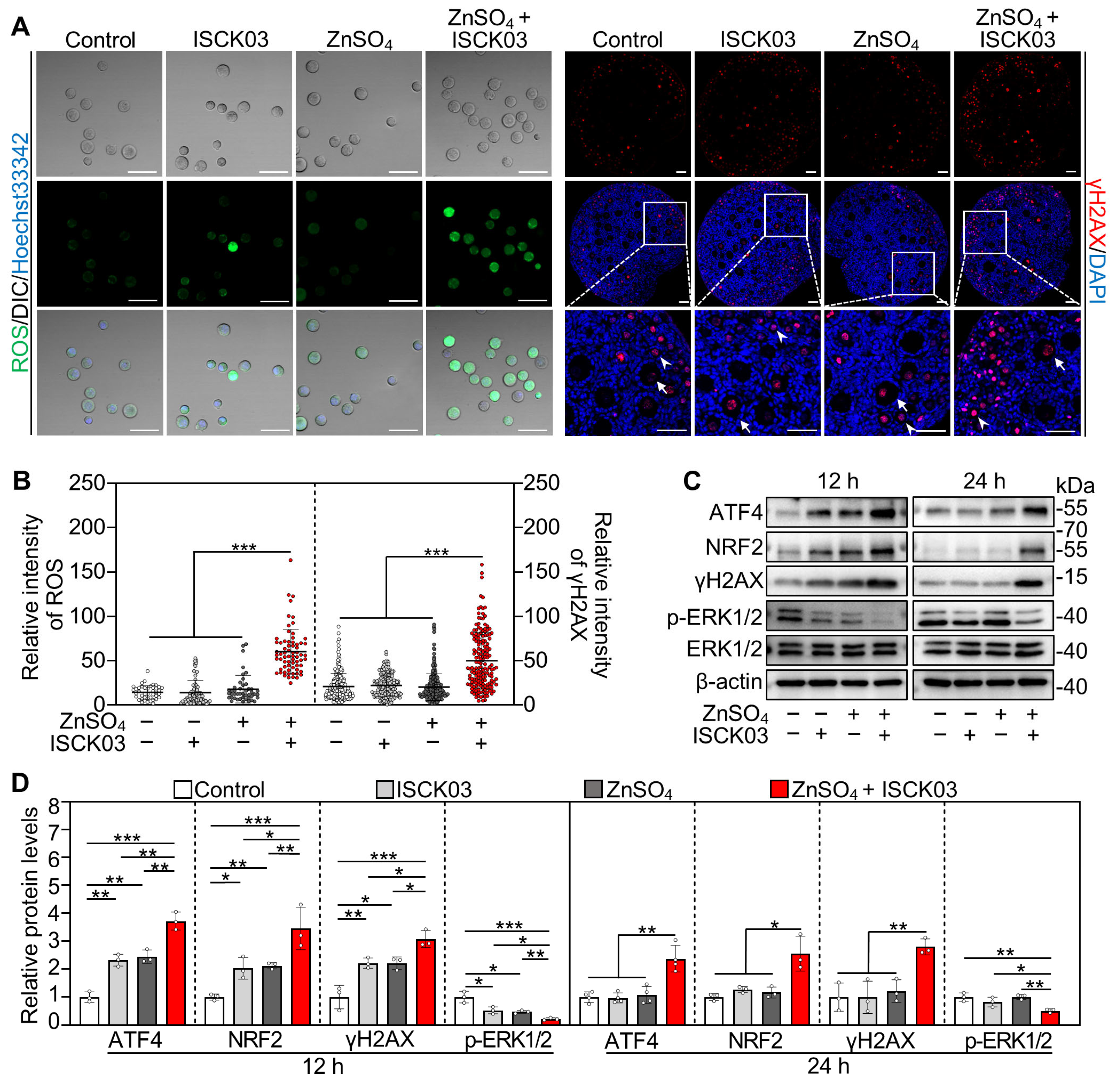
3.3. Zinc Overload Induces Oxidative Stress and DNA Damage in the Oocytes of Cultured Neonatal Mouse Ovaries
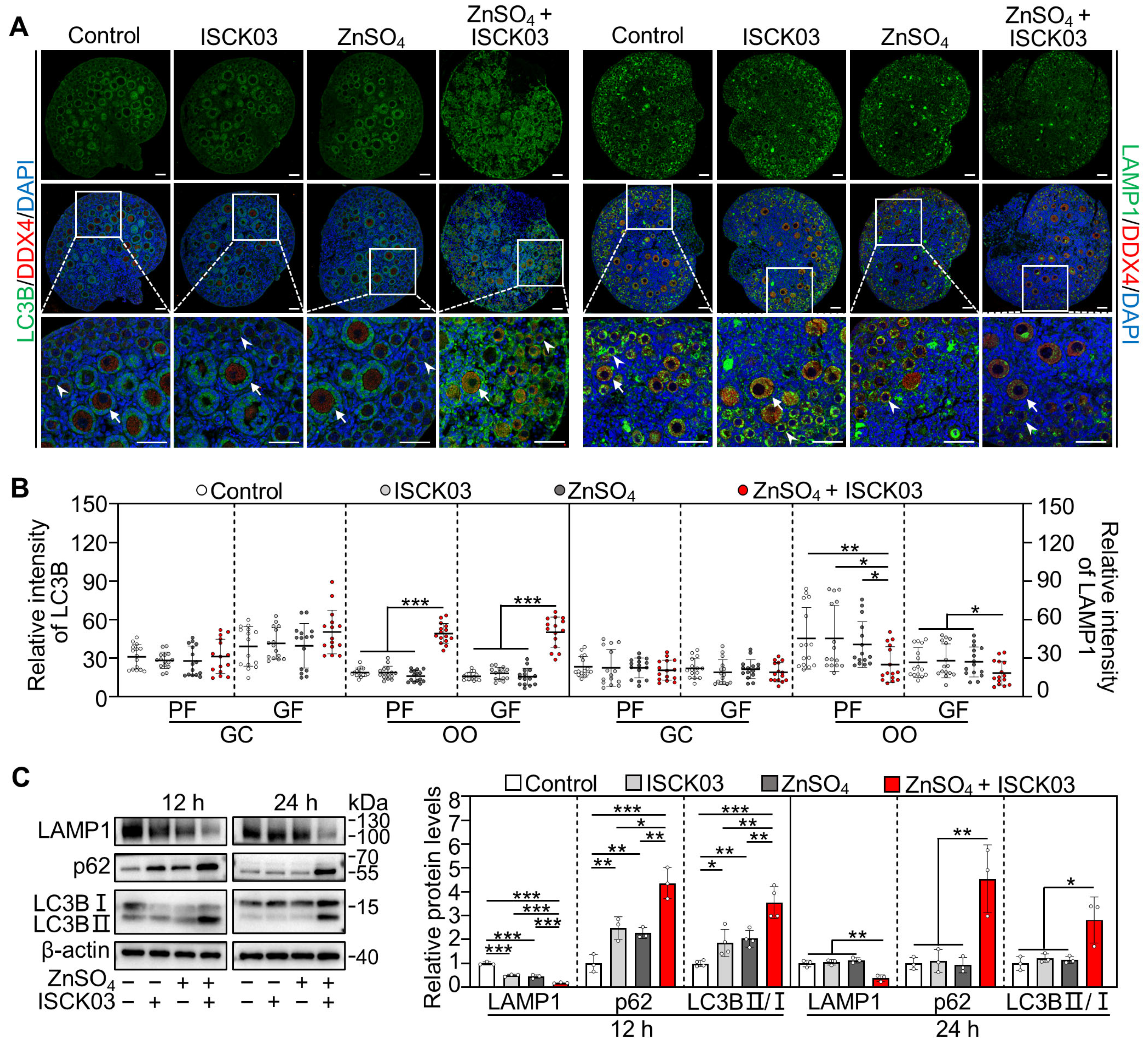
3.4. Zinc Overload Blocks the Autophagic Flux in the Oocytes of Cultured Neonatal Mouse Ovaries
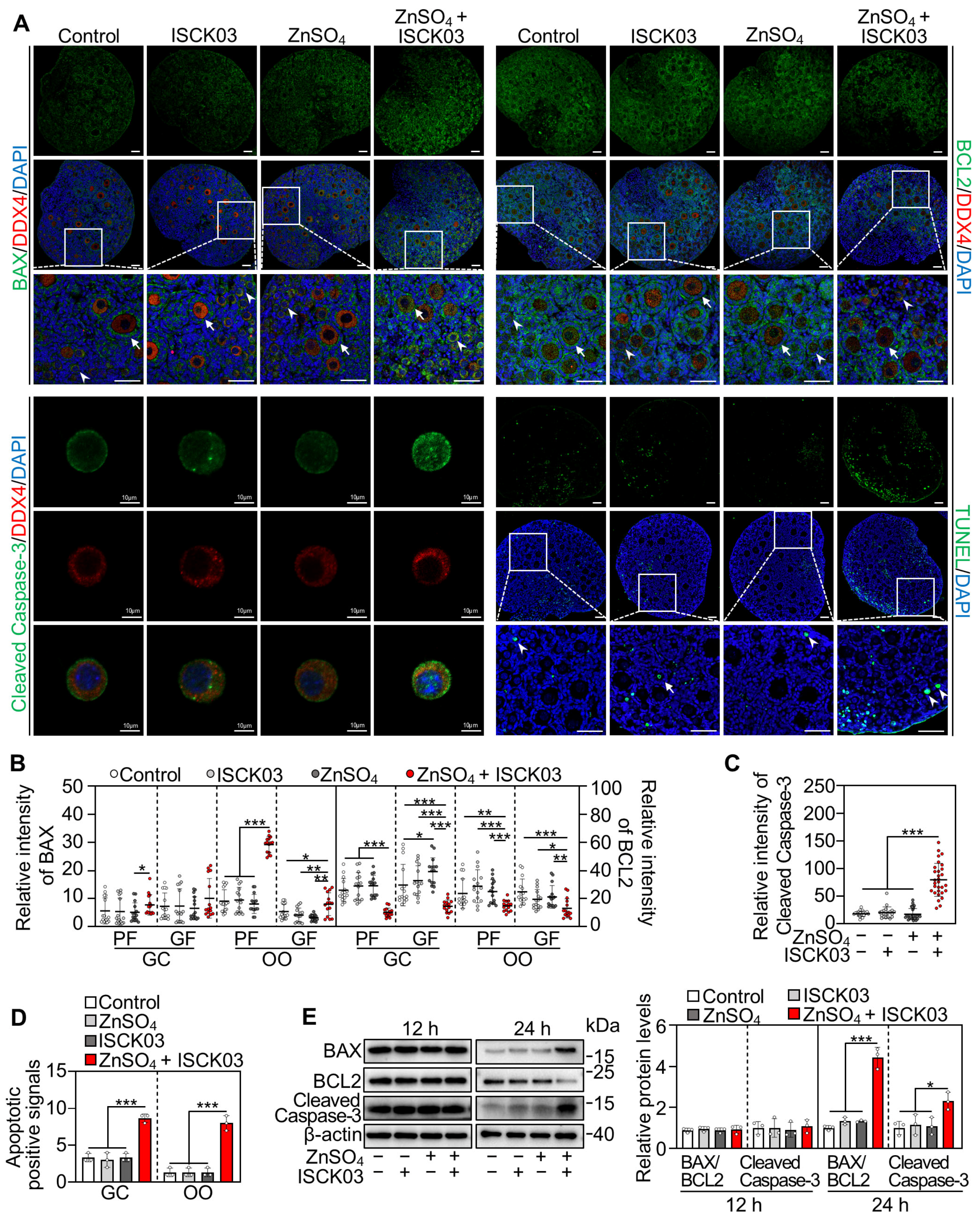
3.5. Zinc Overload Induces Oocyte Apoptosis in Cultured Neonatal Mouse Ovaries
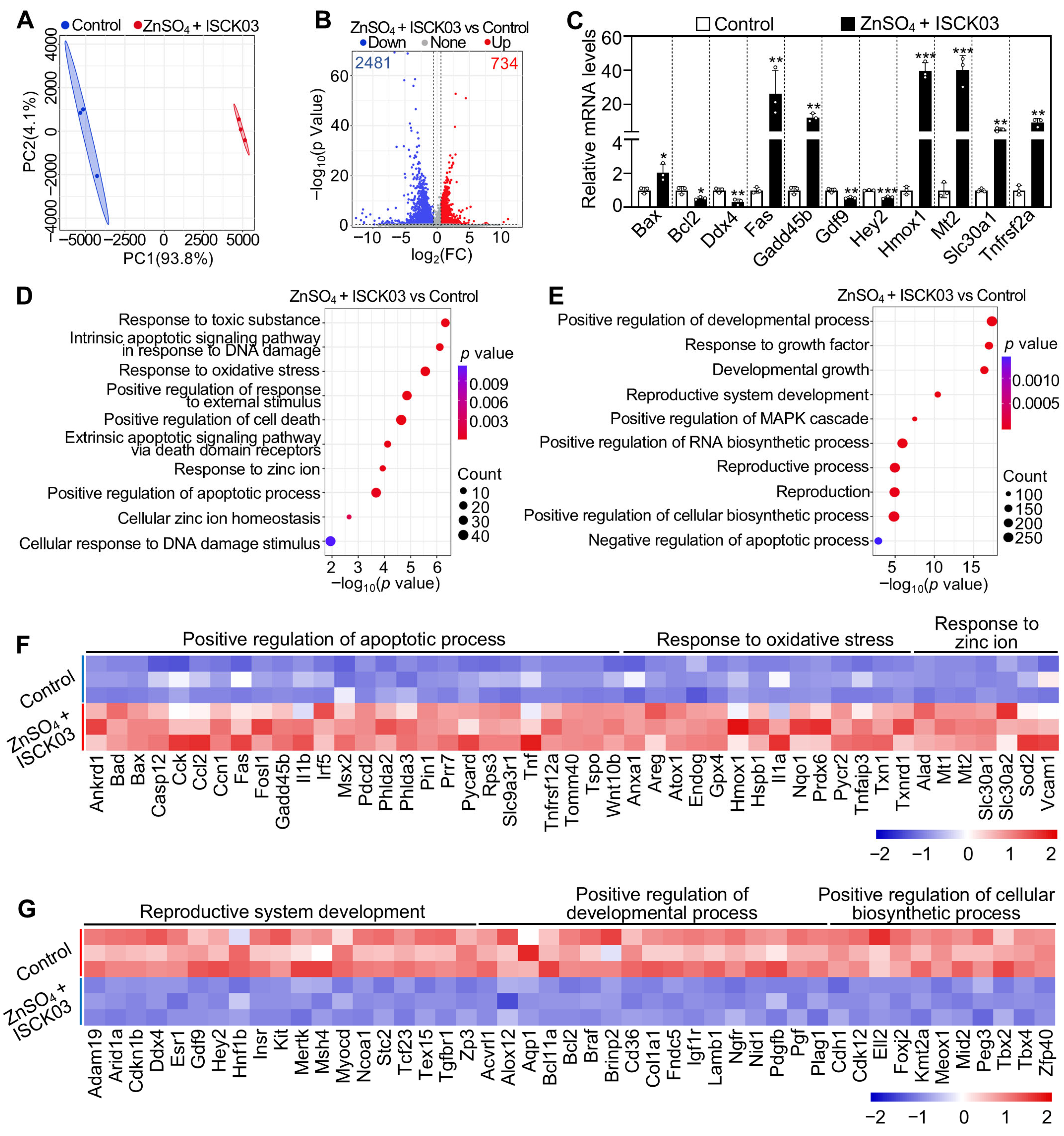
3.6. Effects of Zinc Overload on the Transcriptome of Cultured Neonatal Mouse Ovaries
3.7. ZnSO4 Induces Mouse Oocyte Apoptosis in the Presence of ISCK03 In Vivo
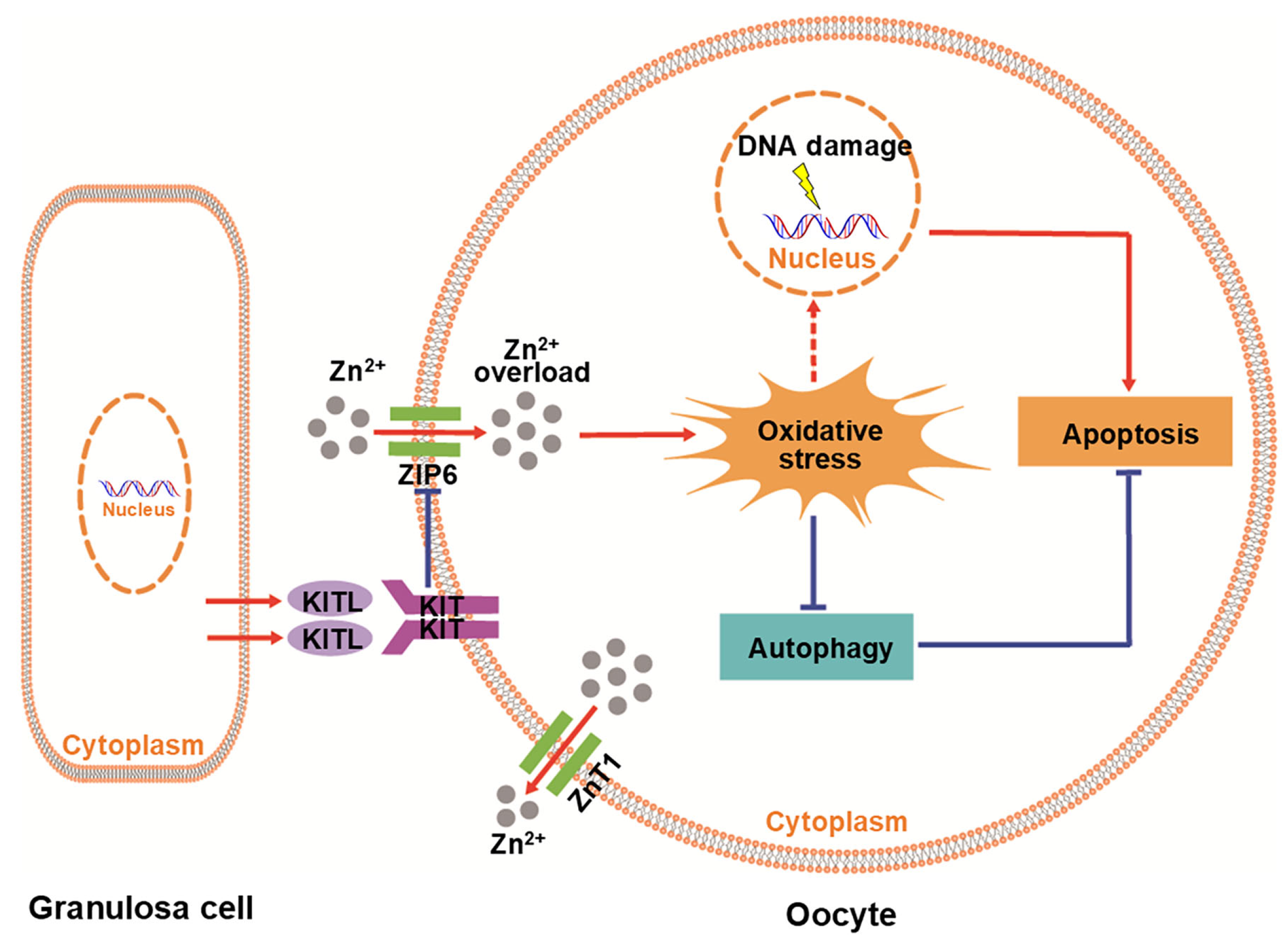
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Akt | protein kinase B |
| ATF4 | activating transcription factor 4 |
| ATM | ataxia telangiectasia mutated |
| BAX | BCL2-associated X protein |
| BCL2 | B-cell lymphoma 2 |
| BSA | bovine serum albumin |
| Caspase-3 | cysteine-dependent aspartate-specific protease-3 |
| DAPI | 4′,6-diamidino-2-phenylindole |
| DDX4 | DEAD-box helicase 4 |
| DMEM/F12 | Dulbecco’s modified Eagle’s medium/Ham’s F12 nutrient mixture |
| DMSO | dimethyl sulfoxide |
| dpp | days postpartum |
| FBS | fetal bovine serum |
| GAPDH | glyceraldehyde-3-phosphate dehydrogenase |
| GC | granulosa cell |
| GEO | Gene Expression Omnibus |
| GF | growing follicle |
| γH2AX | phosphorylated H2AX |
| HMOX1 | heme oxygenase 1 |
| JAK | Janus kinase |
| KIT | proto-oncogenic receptor tyrosine kinase |
| KITL | KIT ligand |
| LAMP1 | lysosomal-associated membrane protein 1 |
| LC3B | microtubule-associated protein 1 light chain 3 beta |
| MAPK | mitogen-activated protein kinase |
| mTOR | mammalian target of rapamycin |
| MTF1 | metal regulatory transcription factor 1 |
| MTs | metallothioneins |
| NRF2 | nuclear factor erythroid 2-related factor 2 |
| OO | oocyte |
| PBS | phosphate-buffered saline |
| PCA | principal-component analysis |
| PF | primordial follicle |
| PFA | paraformaldehyde |
| PI3K | phosphoinositide 3-kinase |
| p-ERK1/2 | phosphorylated extracellular signal-regulated kinases 1 and 2 |
| PVDF | polyvinylidene fluoride |
| qRT-PCR | quantitative real-time PCR |
| ROS | reactive oxygen species |
| SDS | sodium dodecyl sulfate |
| SMAD | Sma- and Mad-related protein |
| p62/SQSTM1 | sequestosome 1 |
| ZIPs | Zrt/Irt-like proteins |
| ZnTs | zinc transporters |
References
- Zhang, Y.; Wang, Y.; Feng, X.; Zhang, S.; Xu, X.; Li, L.; Niu, S.; Bo, Y.; Wang, C.; Li, Z.; et al. Oocyte-derived microvilli control female fertility by optimizing ovarian follicle selection in mice. Nat. Commun. 2021, 12, 2523. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yang, W.; Shi, X.; Zhang, C.; Song, G.; Huang, D. The factors and pathways regulating the activation of mammalian primordial follicles in vivo. Front. Cell Dev. Biol. 2020, 8, 575706. [Google Scholar] [CrossRef] [PubMed]
- Liao, B.; Qi, X.; Yun, C.; Qiao, J.; Pang, Y. Effects of androgen excess-related metabolic disturbances on granulosa cell function and follicular development. Front. Endocrinol. 2022, 13, 815968. [Google Scholar] [CrossRef] [PubMed]
- Takase, H.M.; Mishina, T.; Hayashi, T.; Yoshimura, M.; Kuse, M.; Nikaido, I.; Kitajima, T.S. Transcriptomic signatures of WNT-driven pathways and granulosa cell-oocyte interactions during primordial follicle activation. PLoS ONE 2024, 19, e0311978. [Google Scholar] [CrossRef]
- Kim, E.; Cai, L.; Hyun, S.H. Effects of stem cell factor/c-Kit signaling on in vitro maturation of porcine oocytes and subsequent developmental competence after fertilization. Front. Vet. Sci. 2021, 8, 745488. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, K. Cellular and molecular regulation of the activation of mammalian primordial follicles: Somatic cells initiate follicle activation in adulthood. Hum. Reprod. Update 2015, 21, 779–786. [Google Scholar] [CrossRef]
- Dai, Y.; Bo, Y.; Wang, P.; Xu, X.; Singh, M.; Jia, L.; Zhang, S.; Niu, S.; Cheng, K.; Liang, J.; et al. Asynchronous embryonic germ cell development leads to a heterogeneity of postnatal ovarian follicle activation and may influence the timing of puberty onset in mice. BMC Biol. 2022, 20, 109. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, W.; Wang, Z.; Zheng, N.; Yuan, F.; Li, B.; Li, X.; Deng, L.; Lin, M.; Chen, X.; et al. Enhanced glycolysis in granulosa cells promotes the activation of primordial follicles through mTOR signaling. Cell Death Dis. 2022, 13, 87. [Google Scholar] [CrossRef]
- Burton, J.J.N.; Luke, A.J.; Pepling, M.E. Regulation of mouse primordial follicle formation by signaling through the PI3K pathway. Biol. Reprod. 2022, 106, 515–525. [Google Scholar] [CrossRef]
- Luan, Y.; So, W.; Dong, R.; Abazarikia, A.; Kim, S.Y. KIT in oocytes: A key factor for oocyte survival and reproductive lifespan. eBioMedicine 2024, 106, 105263. [Google Scholar] [CrossRef]
- Doherty, C.A.; Amargant, F.; Shvartsman, S.Y.; Duncan, F.E.; Gavis, E.R. Bidirectional communication in oogenesis: A dynamic conversation in mice and Drosophila. Trends Cell. Biol. 2022, 32, 311–323. [Google Scholar] [CrossRef]
- Lim, J.M.; Sung, H.Y.; Park, S.W.; Hwang, J.S. IL-7 secreted by keratinocytes induces melanogenesis via c-kit/MAPK signaling pathway in Melan-a melanocytes. Arch. Dermatol. Res. 2025, 317, 275. [Google Scholar] [CrossRef]
- Chen, B.; He, H.; Sun, X.; Li, Q.; Yang, Q.; Zhao, L.; Li, X.; Zhou, G. Mechanism of Bushen Shengjing Decoction on the spermatogenic function of in rats with oligoasthenospermia: Focus on SCF/c-Kit signaling pathway-mediated apoptosis. Mol. Reprod. Dev. 2025, 92, e70044. [Google Scholar] [CrossRef]
- Shen, H.; Nie, J.; Li, G.; Tian, H.; Zhang, J.; Luo, X.; Xu, D.; Sun, J.; Zhang, D.; Zhang, H.; et al. Stem cell factor restrains endoplasmic reticulum stress-associated apoptosis through c-Kit receptor activation of JAK2/STAT3 axis in hippocampal neuronal cells. PLoS ONE 2024, 19, e0310872. [Google Scholar] [CrossRef]
- Chen, B.; Yu, P.; Chan, W.N.; Xie, F.; Zhang, Y.; Liang, L.; Leung, K.T.; Lo, K.W.; Yu, J.; Tse, G.M.K.; et al. Cellular zinc metabolism and zinc signaling: From biological functions to diseases and therapeutic targets. Signal Transduct. Target Ther. 2024, 9, 6. [Google Scholar] [CrossRef] [PubMed]
- Krall, R.F.; Tzounopoulos, T.; Aizenman, E. The function and regulation of zinc in the brain. Neuroscience 2021, 457, 235–258. [Google Scholar] [CrossRef] [PubMed]
- Omanakuttan, A.; Nambiar, J.; Harris, R.M.; Bose, C.; Pandurangan, N.; Varghese, R.K.; Kumar, G.B.; Tainer, J.A.; Banerji, A.; Perry, J.J.; et al. Anacardic acid inhibits the catalytic activity of matrix metalloproteinase-2 and matrix metalloproteinase-9. Mol. Pharmacol. 2012, 82, 614–622. [Google Scholar] [CrossRef] [PubMed]
- Chu, X.P.; Jiang, Q.; Chu, Y. Zinc in health and disease condition-2nd edition. Biomolecules 2025, 15, 609. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, H.; Xu, Z.; Cheng, X. Zinc dysregulation in cancers and its potential as a therapeutic target. Cancer Biol. Med. 2020, 17, 612–625. [Google Scholar] [CrossRef]
- Sugita, H.; Takarabe, S.; Kageyama, A.; Kawata, Y.; Ito, J. Molecular mechanism of oocyte activation in mammals: Past, present, and future directions. Biomolecules 2024, 14, 359. [Google Scholar] [CrossRef]
- Kong, B.Y.; Duncan, F.E.; Que, E.L.; Kim, A.M.; O’Halloran, T.V.; Woodruff, T.K. Maternally-derived zinc transporters ZIP6 and ZIP10 drive the mammalian oocyte-to-egg transition. Mol. Hum. Reprod. 2014, 20, 1077–1089. [Google Scholar] [CrossRef]
- Converse, A.; Thomas, P. The zinc transporter ZIP9 (Slc39a9) regulates zinc dynamics essential to egg activation in zebrafish. Sci. Rep. 2020, 10, 15673. [Google Scholar] [CrossRef] [PubMed]
- Kageyama, A.; Terakawa, J.; Takarabe, S.; Sugita, H.; Kawata, Y.; Ito, J.; Kashiwazaki, N. Zinc transporter ZnT3/Slc30a3 has a potential role in zinc ion influx in mouse oocytes. J. Reprod. Dev. 2024, 70, 338–342. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Wang, X.; Zhang, X.; Yu, W.; Guo, X.; Wang, R.; Xie, C.; Ma, J.; Wang, S. ZnT 9 involvement in estradiol-modulated zinc homeostasis of the human follicular microenvironment. Biol. Trace. Elem. Res. 2024, 202, 1901–1909. [Google Scholar] [CrossRef] [PubMed]
- Ogushi, S.; Kimura, T. The difference in zinc concentrations required for induction among metallothionein isoforms can be explained by the different MTF1 affinities to MREs in its promoter. Int. J. Mol. Sci. 2022, 24, 283. [Google Scholar] [CrossRef]
- Wang, Y.S.; Yang, S.J.; Ahmad, M.J.; Ding, Z.M.; Duan, Z.Q.; Chen, Y.W.; Liu, M.; Liang, A.X.; Hua, G.H.; Huo, L.J. Zinc pyrithione exposure compromises oocyte maturation through involving in spindle assembly and zinc accumulation. Ecotoxicol. Environ. Saf. 2022, 234, 113393. [Google Scholar] [CrossRef]
- Tang, W.; Zhu, X.; Chen, Y.; Yang, S.; Wu, C.; Chen, D.; Xue, L.; Guo, Y.; Dai, Y.; Wei, S.; et al. Towards prolonging ovarian reproductive life: Insights into trace elements homeostasis. Ageing Res. Rev. 2024, 97, 102311. [Google Scholar] [CrossRef]
- Han, L.; Huang, Y.; Li, B.; Wang, W.; Sun, Y.L.; Zhang, X.; Zhang, W.; Liu, S.; Zhou, W.; Xia, W.; et al. The metallic compound promotes primordial follicle activation and ameliorates fertility deficits in aged mice. Theranostics 2023, 13, 3131–3148. [Google Scholar] [CrossRef]
- Tanaka, K.I.; Shiota, S.; Sakakibara, O.; Shimoda, M.; Takafuji, A.; Takabatake, M.; Kadota, Y.; Kawakami, T.; Suzuki, S.; Kawahara, M. Exacerbation of elastase-induced emphysema via increased oxidative stress in metallothionein-knockout mice. Biomolecules 2022, 12, 583. [Google Scholar] [CrossRef]
- Lisle, R.S.; Anthony, K.; Randall, M.A.; Diaz, F.J. Oocyte-cumulus cell interactions regulate free intracellular zinc in mouse oocytes. Reproduction 2013, 145, 381–390. [Google Scholar] [CrossRef]
- Chen, Y.Y.; Chen, S.; Ok, K.; Duncan, F.E.; O’Halloran, T.V.; Woodruff, T.K. Zinc dynamics regulate early ovarian follicle development. J. Biol. Chem. 2023, 299, 102731. [Google Scholar] [CrossRef]
- Tian, K.; Wang, Y.X.; Li, L.X.; Liu, Y.Q. Neuronal death/apoptosis induced by intracellular zinc deficiency associated with changes in amino-acid neurotransmitters and glutamate receptor subtypes. J. Inorg. Biochem. 2018, 179, 54–59. [Google Scholar] [CrossRef]
- Sinan, M.; Yalcin, O.; Karakas, Z.; Goksel, E.; Ertan, N.Z. Zinc improved erythrocyte deformability and aggregation in patients with beta-thalassemia: An in vitro study. Clin. Hemorheol. Microcirc. 2023, 85, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Nishito, Y.; Kambe, T. Zinc transporter 1 (ZNT1) expression on the cell surface is elaborately controlled by cellular zinc levels. J. Biol. Chem. 2019, 294, 15686–15697. [Google Scholar] [CrossRef] [PubMed]
- Wedler, N.; Matthaus, T.; Strauch, B.; Dilger, E.; Waterstraat, M.; Mangerich, A.; Hartwig, A. Impact of the cellular zinc status on PARP-1 activity and genomic stability in HeLa S3 cells. Chem. Res. Toxicol. 2021, 34, 839–848. [Google Scholar] [CrossRef]
- Khalil, A.; Morgan, R.N.; Adams, B.R.; Golding, S.E.; Dever, S.M.; Rosenberg, E.; Povirk, L.F.; Valerie, K. ATM-dependent ERK signaling via AKT in response to DNA double-strand breaks. Cell Cycle 2011, 10, 481–491. [Google Scholar] [CrossRef]
- Zhou, Y.J.; Tang, Y.; Liu, S.J.; Zeng, P.H.; Qu, L.; Jing, Q.C.; Yin, W.J. Radiation-induced liver disease: Beyond DNA damage. Cell Cycle 2023, 22, 506–526. [Google Scholar] [CrossRef]
- Chang, R.; Sun, X.; Jia, H.; Xu, Q.; Dong, Z.; Tang, Y.; Luo, S.; Jiang, Q.; Loor, J.J.; Xu, C. Inhibiting nuclear factor erythroid 2 related factor 2-mediated autophagy in bovine mammary epithelial cells induces oxidative stress in response to exogenous fatty acids. J. Anim. Sci. Biotechnol. 2022, 13, 48. [Google Scholar] [CrossRef]
- Xing, Y.; Sui, Z.; Liu, Y.; Wang, M.M.; Wei, X.; Lu, Q.; Wang, X.; Liu, N.; Lu, C.; Chen, R.; et al. Blunting TRPML1 channels protects myocardial ischemia/reperfusion injury by restoring impaired cardiomyocyte autophagy. Basic Res. Cardiol. 2022, 117, 20. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, P.; Guo, J.; Ma, T.; Hu, Y.; Huang, L.; Xing, B.; He, Y.; Xi, J. Zinc overload induces damage to H9c2 cardiomyocyte through mitochondrial dysfunction and ROS-mediated mitophagy. Cardiovasc. Toxicol. 2023, 23, 388–405. [Google Scholar] [CrossRef]
- Lu, Z.; Ren, Y.; Yang, L.; Jia, A.; Hu, Y.; Zhao, Y.; Zhao, W.; Yu, B.; Zhao, W.; Zhang, J.; et al. Inhibiting autophagy enhances sulforaphane-induced apoptosis via targeting NRF2 in esophageal squamous cell carcinoma. Acta. Pharm. Sin. B 2021, 11, 1246–1260. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, Y.; Han, L.; Wei, H.; Zhang, X.; Zhang, W.; Weng, Y.; Wang, W.; Zhang, L.; He, S.; Zhang, M.; et al. Granulosa Cell-Secreted KITL Is Involved in Maintaining Zinc Homeostasis in the Oocytes of Neonatal Mouse Ovaries. Antioxidants 2025, 14, 1345. https://doi.org/10.3390/antiox14111345
Du Y, Han L, Wei H, Zhang X, Zhang W, Weng Y, Wang W, Zhang L, He S, Zhang M, et al. Granulosa Cell-Secreted KITL Is Involved in Maintaining Zinc Homeostasis in the Oocytes of Neonatal Mouse Ovaries. Antioxidants. 2025; 14(11):1345. https://doi.org/10.3390/antiox14111345
Chicago/Turabian StyleDu, Yan, Lincheng Han, Hongwei Wei, Xiaodan Zhang, Wenbo Zhang, Yashuang Weng, Weiyong Wang, Luchun Zhang, Sihui He, Meijia Zhang, and et al. 2025. "Granulosa Cell-Secreted KITL Is Involved in Maintaining Zinc Homeostasis in the Oocytes of Neonatal Mouse Ovaries" Antioxidants 14, no. 11: 1345. https://doi.org/10.3390/antiox14111345
APA StyleDu, Y., Han, L., Wei, H., Zhang, X., Zhang, W., Weng, Y., Wang, W., Zhang, L., He, S., Zhang, M., & Li, J. (2025). Granulosa Cell-Secreted KITL Is Involved in Maintaining Zinc Homeostasis in the Oocytes of Neonatal Mouse Ovaries. Antioxidants, 14(11), 1345. https://doi.org/10.3390/antiox14111345





