Role of Matricellular Proteins in Endothelial Cell Inflammation and Atherosclerosis
Abstract
1. Introduction
2. Role of Vascular Endothelium in Atherogenesis
2.1. Endothelial Activation
| Name of the Marker | Function | Refs. |
|---|---|---|
| C-reactive protein (CRP) | CRP is produced by the liver in response to inflammation. CRP binds to damaged ECs and exacerbates inflammation and oxidative stress. In addition, it reduces NO production, increases endothelin-1 synthesis, and suppresses endothelium-dependent arterial relaxation. | [35,36] |
| IL-6 | IL-6 is a pro-inflammatory cytokine secreted by ECs and other cells during inflammation. It plays an important role in upregulating the production of CRP and other inflammatory mediators. It also promotes the expression of chemokines and adhesion molecules on ECs, which aids in leukocyte recruitment. | [35,37] |
| VCAM-1 | VCAM-1, an adhesion molecule, binds to integrins found on leukocytes, facilitating their attachment and transmigration across the endothelium. VCAM-1 expression on ECs increases in response to various inflammatory stimuli. | [38,39] |
| E-selectin | It interacts with sialylated glycoproteins on leukocytes, enabling leukocyte rolling and initial attachment to the endothelium. | [35,40] |
| ICAM-1 | Similar to VCAM-1, it helps in leukocyte attachment and transmigration across the endothelium. | [35,41] |
| Endothelin-1 | It is a potent vasoconstrictor and pro-inflammatory agent synthesized by ECs. It can stimulate the expression of adhesion molecules and chemokines on ECs. Additionally, it activates the NF-κB pathway, a key regulator of inflammation. | [35,42] |
| MCP-1/CCL2 | ECs and VSMCs secrete MCP-1, which plays a crucial role in attracting monocytes and macrophages to the subendothelial cell layer. This recruitment process facilitates the accumulation of lipids within these immune cells, ultimately contributing to the formation of atherosclerotic lesions. | [35,43,44] |
| TNF-α | TNF-α, originally identified for its anti-tumor properties, is a key pro-inflammatory cytokine associated with various CVDs. TNF-α contributes to endothelial dysfunction via promoting oxidative stress and reducing NO production, thus impairing endothelium-dependent vasodilation across different vascular beds. | [35,45] |
| Interleukin-1 β (IL-1β) | IL-1β promotes the development of early atherosclerotic lesions by enhancing the adherence of monocytes to ECs through the elevation of adhesion molecules. | [35,46] |
| Transforming growth factor β (TGF-β) | TGF-β induces the expression of several pro-inflammatory chemokines, cytokines, their receptors, and adhesion molecules on ECs. Moreover, it stimulates the expression of matrix metalloproteinases and fibronectin (FN), which are closely associated with inflammation. | [47] |
| Interleukin-18 (IL-18) | IL-18 belongs to the IL-1 cytokine family and was originally found in macrophages and Kupffer cells. IL-18 triggers IFN-γ production by T cells. | [35] |
| CD40/CD40L | CD40L, a TNF family member, and its receptor CD40 are co-expressed in activated T lymphocytes, ECs, SMCs, and macrophages in atherosclerotic lesions. | [35] |
| Interleukin-8 (IL-8) | IL-8 is a pro-inflammatory cytokine that plays a significant role in EC inflammation, particularly in the context of CVDs. IL-8 functions through its receptors, CXCR1 and CXCR2, present on circulating immune cells, particularly neutrophils, leading to their chemotaxis and adhesion to the EC surface. IL-8 also plays a role in promoting ICAM-1 and VCAM-1 expression on ECs. | [48,49] |
| CXCL12 | CXCL12, also known as stromal cell-derived factor 1 (SDF-1), is a chemokine that plays a crucial role in vascular development, tissue repair, and inflammation. In ECs, CXCL12 is involved in various processes: EC migration, inflammation, and angiogenesis. | [50] |
2.2. Endothelial Dysfunction
2.3. Endothelial Senescence
2.4. LDL Transcytosis Across the Arterial Endothelium
2.5. Endothelial-to-Mesenchymal Transition
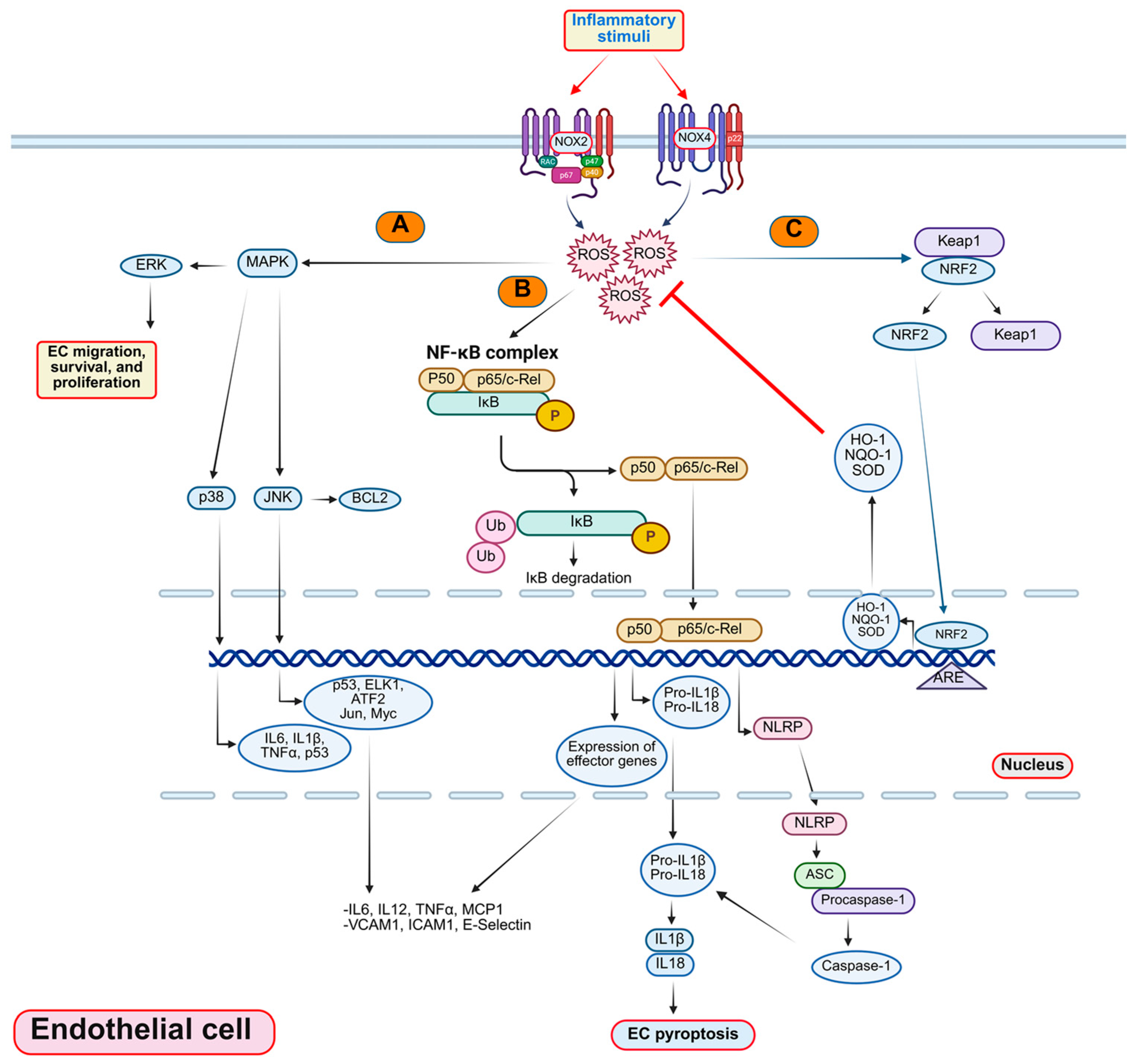
3. Mechanisms of EC Inflammation
3.1. Oxidative Stress in EC Inflammation
3.1.1. Reactive Oxygen Species and Their Role in EC Inflammation
| Molecule | Function | Refs. |
|---|---|---|
| Superoxide anion (O2•−) | It is produced by the one-electron reduction of molecular oxygen (O2). Superoxide can activate various signaling pathways in ECs, such as MAPK, NF-κB, and nucleotide-binding domain, leucine-rich-containing family, pyrin domain–containing-3 (NLRP3), and induce the expression of pro-inflammatory cytokines, chemokines, and adhesion molecules. | [90,91] |
| Hydrogen peroxide (H2O2) | It is produced by the two-electron reduction of O2 or by the dismutation of superoxide. H2O2 can modulate the activity of various transcription factors in ECs, such as NF-κB, Nrf2, and AP-1, and regulate the expression of genes involved in inflammation, antioxidant defense, and cell death. | [90,92,93] |
| Hydroxyl radical (•OH) | It is the most reactive and damaging ROS molecule, which is produced by the one-electron reduction of H2O2 or Fenton reaction. Hydroxyl radical can cause oxidative damage to various biomolecules in ECs, such as lipids, proteins, and DNA, and trigger inflammation, apoptosis, and senescence. | [90,92] |
| NO radical (•NO) | NO is a free radical, which readily reacts with various molecules, particularly iron centers and oxygen. It plays critical roles in vasodilation, neuronal signaling, and microbial defense. | [94] |
| Peroxynitrite (ONOO−) | It is reactive nitrogen species, produced by the reaction of superoxide with NO. Peroxynitrite can impair the function of various enzymes and proteins in ECs, such as nitric oxide synthase (NOS), cyclooxygenase (COX), and SOD, and induce inflammation, nitrosative stress, and endothelial dysfunction. | [90,95] |
3.1.2. Sources of NO and Endothelial Cell Biology
3.1.3. Effects of Various Circulating Risk Factors of Atherosclerosis on ROS Generation
3.2. Disturbed Flow in EC Inflammation and Atherosclerosis
3.3. ER Stress in EC Inflammation and Atherosclerosis
3.4. Autophagy in EC Inflammation and Atherosclerosis
4. Matricellular Proteins in EC Inflammation and Atherosclerosis
| Protein | Primary Receptors | Signaling Pathway and Phenotype | Cell/Animal/Human Model Type | Role | Refs. |
|---|---|---|---|---|---|
| TSP1 | CD47 CD36 | Decreases cAMP/cGMP levels by inhibiting EC NO production | Bovine aortic ECs Human umbilical vein ECs Thbs1−/− and Cd47−/− mice arteries | EC dysfunction and pro-hypertensive | [214] |
| Thbs1 deletion prevents leptin-induced atherosclerosis Deletion blocks leptin-induced vascular inflammation Deletion inhibits SMC dedifferentiation | Apoe−/− and Apoe−/−/Thbs1−/− mice | Pro-atherogenic Pro-inflammatory Pro-atherogenic | [215] | ||
| In the early stage, a deficiency of Thbs1 reduces plaque area In the advanced stage, Thbs1 loss promotes plaque necrosis | Apoe−/− and Apoe−/−/Thbs1−/− mice | Pro-atherogenic Anti-plaque vulnerability | [216] | ||
| Thbs1 deletion in mice promotes maladaptive remodeling in response to pressure overload via inhibiting Thbs1/integrin β1/YAP signaling Thbs1 deletion inhibits neointima formation upon carotid artery ligation | Thbs1−/− mice | Promotes intimal hyperplasia | [217] | ||
| COMP | α5β1 α7β1 | Lack of Comp induces aging-related vascular dysfunction, stiffness, and senescence | Comp−/− mice | Promotes vascular function | [218] |
| Comp deletion augments atherosclerosis | Apoe−/− and Comp−/−/Apoe−/− mice | Anti-atherogenic | [219] | ||
| RSPO2 | LGR4 | Suppresses lymphangiogenesis via PI3K-AKT-eNOS signaling and inhibits Wnt-β-catenin pathway in lymphatic ECs | Human dermal lymphatic ECs | Anti-lymphangiogenic Pro-atherogenic | [220] |
| Perivascular application of LGR4-ECD promotes arterial lymphangiogenesis and reduces atherosclerosis | Apoe−/− mice | ||||
| RSPO1 | LGR4-5 | Wnt/β-catenin/VEGFaa-induced abnormal angiogenesis | Zebrafish | Pro-angiogenic | [221] |
| RSPO3 | LGR4-5 | Non-canonical WNT/Ca2+/NFAT signaling and vascular defects | EC-specific Rspo3-deficient mice | Pro-angiogenic | [222] |
| Tenascin-C | Integrins | TN-C polymorphisms correlate with atherosclerosis/CAD | Human aorta samples and CATHGEN cardiovascular study | Three SNPs correlate with atherosclerosis | [223] |
| Osteopontin (OPN/SSP1) | Integrins CD44 | Deletion reduces atherosclerosis Deletion stimulates vascular calcification Deficiency reduces atherogenesis | Apo−/−/Spp1−/− mice Apoe−/−/Ldlr−/− /Spp1−/− triple knockout mice | Pro-atherogenic Pro-atherogenic | [224] |
| Expression levels associate with plaque severity | Human aorta samples | Pro-atherogenic | [225] | ||
| CCN1 | Integrins | Upregulated levels in atherosclerotic aortas of Apoe−/− mice Promotes atherosclerosis | Apoe−/− mice | Pro-atherogenic | [226] |
| Elevated Ccn1 expression in atherosclerotic arteries | Apoe−/− mice and human ECs | Mediates TNFα-induced EC apoptosis | [227] | ||
| Promotes neovascularization | C57BL/6 wild-type mice and human venous ECs | Pro-angiogenic | [228] |
4.1. Thrombospondins
4.1.1. Thrombospondin1 (TSP1)
4.1.2. Thrombospondin 5/Cartilage Oligomeric Matrix Protein (COMP)
4.2. Osteopontin (OPN)
4.3. Roof Plate-Specific Spondins (RSPOs)
4.4. Tenascins
4.5. CCN Proteins
4.6. Secreted Protein Acidic and Rich in Cysteine
5. Therapeutic Potential of Targeting Different Matricellular Proteins
6. Conclusions and Future Perspectives
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ECM | Extracellular Matrix |
| EC/ECs | Endothelial Cells |
| CVDs | Cardiovascular Diseases |
| LDL | Low-Density Lipoprotein |
| VSMCs | Vascular Smooth Muscle Cells |
| CAD | Coronary Artery Disease |
| NO | Nitric Oxide |
| TNF-α | Tumor Necrosis Factor-alpha |
| IL-1 | Interleukin-1 |
| ICAM-1 | Intercellular Adhesion Molecule-1 |
| VCAM-1 | Vascular Cell Adhesion Molecule-1 |
| NF-κB | Nuclear Factor-Kappa B |
| MAPK | Mitogen-Activated Protein Kinase |
| MCP-1 | Monocyte Chemoattractant Protein-1 |
| EndMT | Endothelial-to-Mesenchymal Transition |
| ROS | Reactive oxygen species |
| NOXs | NADPH Oxidases |
| O2•− | Superoxide Ion |
| SOD | Superoxide Dismutase |
| TSPs | Thrombospondins |
| OPN | Osteopontin |
| RSPOs | Roof Plate-Specific Spondins |
| TN-C | Tenascin-C |
| SPARC | Secreted Protein Acidic and Rich in Cysteine |
| HUVECs | Human Umbilical Vein ECs |
| AML | Acute Myeloid Leukemia |
References
- Piepoli, M.F.; Hoes, A.W.; Agewall, S.; Albus, C.; Brotons, C.; Catapano, A.L.; Cooney, M.T.; Corra, U.; Cosyns, B.; Deaton, C.; et al. 2016 European Guidelines on cardiovascular disease prevention in clinical practice: The Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts)Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur. Heart J. 2016, 37, 2315–2381. [Google Scholar] [CrossRef] [PubMed]
- Martin, S.S.; Aday, A.W.; Allen, N.B.; Almarzooq, Z.I.; Anderson, C.A.M.; Arora, P.; Avery, C.L.; Baker-Smith, C.M.; Bansal, N.; Beaton, A.Z.; et al. 2025 Heart Disease and Stroke Statistics: A Report of US and Global Data From the American Heart Association. Circulation 2025, 151, e41–e660. [Google Scholar] [CrossRef] [PubMed]
- Stary, H.C. The sequence of cell and matrix changes in atherosclerotic lesions of coronary arteries in the first forty years of life. Eur. Heart J. 1990, 11 (Suppl. E), 3–19. [Google Scholar] [CrossRef]
- Wang, Y.; Nanda, V.; Direnzo, D.; Ye, J.; Xiao, S.; Kojima, Y.; Howe, K.L.; Jarr, K.U.; Flores, A.M.; Tsantilas, P.; et al. Clonally expanding smooth muscle cells promote atherosclerosis by escaping efferocytosis and activating the complement cascade. Proc. Natl. Acad. Sci. USA 2020, 117, 15818–15826. [Google Scholar] [CrossRef]
- Depuydt, M.A.C.; Prange, K.H.M.; Slenders, L.; Ord, T.; Elbersen, D.; Boltjes, A.; de Jager, S.C.A.; Asselbergs, F.W.; de Borst, G.J.; Aavik, E.; et al. Microanatomy of the Human Atherosclerotic Plaque by Single-Cell Transcriptomics. Circ. Res. 2020, 127, 1437–1455. [Google Scholar] [CrossRef]
- Virmani, R.; Kolodgie, F.D.; Burke, A.P.; Farb, A.; Schwartz, S.M. Lessons from sudden coronary death: A comprehensive morphological classification scheme for atherosclerotic lesions. Arterioscler. Thromb. Vasc. Biol. 2000, 20, 1262–1275. [Google Scholar] [CrossRef] [PubMed]
- Davignon, J.; Ganz, P. Role of endothelial dysfunction in atherosclerosis. Circulation 2004, 109, III27–III32. [Google Scholar] [CrossRef]
- Cahill, P.A.; Redmond, E.M. Vascular endothelium—Gatekeeper of vessel health. Atherosclerosis 2016, 248, 97–109. [Google Scholar] [CrossRef]
- Ricard, N.; Bailly, S.; Guignabert, C.; Simons, M. The quiescent endothelium: Signalling pathways regulating organ-specific endothelial normalcy. Nat. Rev. Cardiol. 2021, 18, 565–580. [Google Scholar] [CrossRef]
- Medina-Leyte, D.J.; Zepeda-Garcia, O.; Dominguez-Perez, M.; Gonzalez-Garrido, A.; Villarreal-Molina, T.; Jacobo-Albavera, L. Endothelial Dysfunction, Inflammation and Coronary Artery Disease: Potential Biomarkers and Promising Therapeutical Approaches. Int. J. Mol. Sci. 2021, 22, 3850. [Google Scholar] [CrossRef]
- Pervaiz, N.; Kathuria, I.; Aithabathula, R.V.; Singla, B. Matricellular proteins in atherosclerosis development. Matrix Biol. 2023, 120, 1–23. [Google Scholar] [CrossRef]
- Bornstein, P. Diversity of function is inherent in matricellular proteins: An appraisal of thrombospondin 1. J. Cell Biol. 1995, 130, 503–506. [Google Scholar] [CrossRef]
- Kong, P.; Cui, Z.Y.; Huang, X.F.; Zhang, D.D.; Guo, R.J.; Han, M. Inflammation and atherosclerosis: Signaling pathways and therapeutic intervention. Signal Transduct. Target. Ther. 2022, 7, 131. [Google Scholar] [CrossRef]
- Ivanova, E.A.; Myasoedova, V.A.; Melnichenko, A.A.; Grechko, A.V.; Orekhov, A.N. Small Dense Low-Density Lipoprotein as Biomarker for Atherosclerotic Diseases. Oxid. Med. Cell Longev. 2017, 2017, 1273042. [Google Scholar] [CrossRef]
- Mauersberger, C.; Hinterdobler, J.; Schunkert, H.; Kessler, T.; Sager, H.B. Where the Action Is-Leukocyte Recruitment in Atherosclerosis. Front. Cardiovasc. Med. 2021, 8, 813984. [Google Scholar] [CrossRef]
- Rafieian-Kopaei, M.; Setorki, M.; Doudi, M.; Baradaran, A.; Nasri, H. Atherosclerosis: Process, indicators, risk factors and new hopes. Int. J. Prev. Med. 2014, 5, 927–946. [Google Scholar] [PubMed]
- Libby, P. Inflammation in atherosclerosis. Nature 2002, 420, 868–874. [Google Scholar] [CrossRef] [PubMed]
- Marchio, P.; Guerra-Ojeda, S.; Vila, J.M.; Aldasoro, M.; Victor, V.M.; Mauricio, M.D. Targeting Early Atherosclerosis: A Focus on Oxidative Stress and Inflammation. Oxid. Med. Cell Longev. 2019, 2019, 8563845. [Google Scholar] [CrossRef]
- Forstermann, U.; Xia, N.; Li, H. Roles of Vascular Oxidative Stress and Nitric Oxide in the Pathogenesis of Atherosclerosis. Circ. Res. 2017, 120, 713–735. [Google Scholar] [CrossRef] [PubMed]
- Loeffen, R.; Spronk, H.M.; ten Cate, H. The impact of blood coagulability on atherosclerosis and cardiovascular disease. J. Thromb. Haemost. 2012, 10, 1207–1216. [Google Scholar] [CrossRef]
- Girolami, A.; Sambado, L.; Lombardi, A.M. The impact of blood coagulability on atherosclerosis and cardiovascular disease: A rebuttal. J. Thromb. Haemost. 2013, 11, 213–214, discussion 215–216. [Google Scholar] [CrossRef] [PubMed]
- Botts, S.R.; Fish, J.E.; Howe, K.L. Dysfunctional Vascular Endothelium as a Driver of Atherosclerosis: Emerging Insights Into Pathogenesis and Treatment. Front. Pharmacol. 2021, 12, 787541. [Google Scholar] [CrossRef]
- Zhu, Y.; Liao, H.; Wang, N.; Ma, K.S.; Verna, L.K.; Shyy, J.Y.; Chien, S.; Stemerman, M.B. LDL-activated p38 in endothelial cells is mediated by Ras. Arterioscler. Thromb. Vasc. Biol. 2001, 21, 1159–1164. [Google Scholar] [CrossRef]
- Pietersma, A.; Tilly, B.C.; Gaestel, M.; de Jong, N.; Lee, J.C.; Koster, J.F.; Sluiter, W. p38 mitogen activated protein kinase regulates endothelial VCAM-1 expression at the post-transcriptional level. Biochem. Biophys. Res. Commun. 1997, 230, 44–48. [Google Scholar] [CrossRef] [PubMed]
- Reustle, A.; Torzewski, M. Role of p38 MAPK in Atherosclerosis and Aortic Valve Sclerosis. Int. J. Mol. Sci. 2018, 19, 3761. [Google Scholar] [CrossRef]
- Gan, J.; Guo, L.; Zhang, X.; Yu, Q.; Yang, Q.; Zhang, Y.; Zeng, W.; Jiang, X.; Guo, M. Anti-inflammatory therapy of atherosclerosis: Focusing on IKKbeta. J. Inflamm. 2023, 20, 8. [Google Scholar] [CrossRef]
- Hajra, L.; Evans, A.I.; Chen, M.; Hyduk, S.J.; Collins, T.; Cybulsky, M.I. The NF-kappa B signal transduction pathway in aortic endothelial cells is primed for activation in regions predisposed to atherosclerotic lesion formation. Proc. Natl. Acad. Sci. USA 2000, 97, 9052–9057. [Google Scholar] [CrossRef]
- Chen, Y.; Ye, X.; Escames, G.; Lei, W.; Zhang, X.; Li, M.; Jing, T.; Yao, Y.; Qiu, Z.; Wang, Z.; et al. The NLRP3 inflammasome: Contributions to inflammation-related diseases. Cell Mol. Biol. Lett. 2023, 28, 51. [Google Scholar] [CrossRef]
- Blevins, H.M.; Xu, Y.; Biby, S.; Zhang, S. The NLRP3 Inflammasome Pathway: A Review of Mechanisms and Inhibitors for the Treatment of Inflammatory Diseases. Front. Aging Neurosci. 2022, 14, 879021. [Google Scholar] [CrossRef]
- Chen, M.Y.; Ye, X.J.; He, X.H.; Ouyang, D.Y. The Signaling Pathways Regulating NLRP3 Inflammasome Activation. Inflammation 2021, 44, 1229–1245. [Google Scholar] [CrossRef]
- Saha, S.; Buttari, B.; Panieri, E.; Profumo, E.; Saso, L. An Overview of Nrf2 Signaling Pathway and Its Role in Inflammation. Molecules 2020, 25, 5474. [Google Scholar] [CrossRef]
- Zgorzynska, E.; Dziedzic, B.; Walczewska, A. An Overview of the Nrf2/ARE Pathway and Its Role in Neurodegenerative Diseases. Int. J. Mol. Sci. 2021, 22, 9592. [Google Scholar] [CrossRef]
- Mimura, J.; Itoh, K. Role of Nrf2 in the pathogenesis of atherosclerosis. Free Radic. Biol. Med. 2015, 88, 221–232. [Google Scholar] [CrossRef]
- Braile, M.; Marcella, S.; Cristinziano, L.; Galdiero, M.R.; Modestino, L.; Ferrara, A.L.; Varricchi, G.; Marone, G.; Loffredo, S. VEGF-A in Cardiomyocytes and Heart Diseases. Int. J. Mol. Sci. 2020, 21, 5294. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, A.; Wakabayashi, Y.; Mori, T. Cellular and molecular basis for the regulation of inflammation by TGF-beta. J. Biochem. 2010, 147, 781–792. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Kishimoto, T. Interplay between interleukin-6 signaling and the vascular endothelium in cytokine storms. Exp. Mol. Med. 2021, 53, 1116–1123. [Google Scholar] [CrossRef]
- Immanuel, J.; Yun, S. Vascular Inflammatory Diseases and Endothelial Phenotypes. Cells 2023, 12, 1640. [Google Scholar] [CrossRef] [PubMed]
- Hu, D.; Yin, C.; Luo, S.; Habenicht, A.J.R.; Mohanta, S.K. Vascular Smooth Muscle Cells Contribute to Atherosclerosis Immunity. Front. Immunol. 2019, 10, 1101. [Google Scholar] [CrossRef]
- Kwaifa, I.K.; Bahari, H.; Yong, Y.K.; Noor, S.M. Endothelial Dysfunction in Obesity-Induced Inflammation: Molecular Mechanisms and Clinical Implications. Biomolecules 2020, 10, 291. [Google Scholar] [CrossRef]
- Dhananjayan, R.; Koundinya, K.S.; Malati, T.; Kutala, V.K. Endothelial Dysfunction in Type 2 Diabetes Mellitus. Indian. J. Clin. Biochem. 2016, 31, 372–379. [Google Scholar] [CrossRef]
- Gimbrone, M.A., Jr.; Garcia-Cardena, G. Endothelial Cell Dysfunction and the Pathobiology of Atherosclerosis. Circ. Res. 2016, 118, 620–636. [Google Scholar] [CrossRef]
- Sitia, S.; Tomasoni, L.; Atzeni, F.; Ambrosio, G.; Cordiano, C.; Catapano, A.; Tramontana, S.; Perticone, F.; Naccarato, P.; Camici, P.; et al. From endothelial dysfunction to atherosclerosis. Autoimmun. Rev. 2010, 9, 830–834. [Google Scholar] [CrossRef]
- Hunt, B.J.; Jurd, K.M. Endothelial cell activation. A central pathophysiological process. BMJ 1998, 316, 1328–1329. [Google Scholar] [CrossRef]
- Yau, J.W.; Teoh, H.; Verma, S. Endothelial cell control of thrombosis. BMC Cardiovasc. Disord. 2015, 15, 130. [Google Scholar] [CrossRef]
- Yang, X.; Chang, Y.; Wei, W. Endothelial Dysfunction and Inflammation: Immunity in Rheumatoid Arthritis. Mediat. Inflamm. 2016, 2016, 6813016. [Google Scholar] [CrossRef]
- Dolmatova, E.V.; Wang, K.; Mandavilli, R.; Griendling, K.K. The effects of sepsis on endothelium and clinical implications. Cardiovasc. Res. 2021, 117, 60–73. [Google Scholar] [CrossRef]
- Chen, J.; Somanath, P.R.; Razorenova, O.; Chen, W.S.; Hay, N.; Bornstein, P.; Byzova, T.V. Akt1 regulates pathological angiogenesis, vascular maturation and permeability in vivo. Nat. Med. 2005, 11, 1188–1196. [Google Scholar] [CrossRef]
- Lee, M.Y.; Luciano, A.K.; Ackah, E.; Rodriguez-Vita, J.; Bancroft, T.A.; Eichmann, A.; Simons, M.; Kyriakides, T.R.; Morales-Ruiz, M.; Sessa, W.C. Endothelial Akt1 mediates angiogenesis by phosphorylating multiple angiogenic substrates. Proc. Natl. Acad. Sci. USA 2014, 111, 12865–12870. [Google Scholar] [CrossRef]
- Ackah, E.; Yu, J.; Zoellner, S.; Iwakiri, Y.; Skurk, C.; Shibata, R.; Ouchi, N.; Easton, R.M.; Galasso, G.; Birnbaum, M.J.; et al. Akt1/protein kinase Balpha is critical for ischemic and VEGF-mediated angiogenesis. J. Clin. Investig. 2005, 115, 2119–2127. [Google Scholar] [CrossRef]
- Szmitko, P.E.; Wang, C.H.; Weisel, R.D.; de Almeida, J.R.; Anderson, T.J.; Verma, S. New markers of inflammation and endothelial cell activation: Part I. Circulation 2003, 108, 1917–1923. [Google Scholar] [CrossRef]
- Badimon, L.; Pena, E.; Arderiu, G.; Padro, T.; Slevin, M.; Vilahur, G.; Chiva-Blanch, G. C-Reactive Protein in Atherothrombosis and Angiogenesis. Front. Immunol. 2018, 9, 430. [Google Scholar] [CrossRef]
- Barthel, S.R.; Gavino, J.D.; Descheny, L.; Dimitroff, C.J. Targeting selectins and selectin ligands in inflammation and cancer. Expert Opin. Ther. Targets 2007, 11, 1473–1491. [Google Scholar] [CrossRef]
- Kaur, R.; Singh, V.; Kumari, P.; Singh, R.; Chopra, H.; Emran, T.B. Novel insights on the role of VCAM-1 and ICAM-1: Potential biomarkers for cardiovascular diseases. Ann. Med. Surg. 2022, 84, 104802. [Google Scholar] [CrossRef]
- Sutton, G.; Pugh, D.; Dhaun, N. Developments in the Role of Endothelin-1 in Atherosclerosis: A Potential Therapeutic Target? Am. J. Hypertens. 2019, 32, 813–815. [Google Scholar] [CrossRef]
- Harrington, J.R. The role of MCP-1 in atherosclerosis. Stem Cells 2000, 18, 65–66. [Google Scholar] [CrossRef]
- Lin, J.; Kakkar, V.; Lu, X. Impact of MCP-1 in atherosclerosis. Curr. Pharm. Des. 2014, 20, 4580–4588. [Google Scholar] [CrossRef]
- Picchi, A.; Gao, X.; Belmadani, S.; Potter, B.J.; Focardi, M.; Chilian, W.M.; Zhang, C. Tumor necrosis factor-alpha induces endothelial dysfunction in the prediabetic metabolic syndrome. Circ. Res. 2006, 99, 69–77. [Google Scholar] [CrossRef]
- Li, X.; Zhao, W.; Li, X.; Chen, X.; Li, Y.; He, J.; Qin, Y.; Li, L.; Zhang, H. The association of SPARC with hypertension and its function in endothelial-dependent relaxation. Atherosclerosis 2024, 388, 117390. [Google Scholar] [CrossRef]
- Chen, P.Y.; Qin, L.; Li, G.; Wang, Z.; Dahlman, J.E.; Malagon-Lopez, J.; Gujja, S.; Cilfone, N.A.; Kauffman, K.J.; Sun, L.; et al. Endothelial TGF-beta signalling drives vascular inflammation and atherosclerosis. Nat. Metab. 2019, 1, 912–926. [Google Scholar] [CrossRef]
- Li, A.; Dubey, S.; Varney, M.L.; Dave, B.J.; Singh, R.K. IL-8 directly enhanced endothelial cell survival, proliferation, and matrix metalloproteinases production and regulated angiogenesis. J. Immunol. 2003, 170, 3369–3376. [Google Scholar] [CrossRef]
- Yu, H.; Huang, X.; Ma, Y.; Gao, M.; Wang, O.; Gao, T.; Shen, Y.; Liu, X. Interleukin-8 regulates endothelial permeability by down-regulation of tight junction but not dependent on integrins induced focal adhesions. Int. J. Biol. Sci. 2013, 9, 966–979. [Google Scholar] [CrossRef] [PubMed]
- Doring, Y.; van der Vorst, E.P.C.; Duchene, J.; Jansen, Y.; Gencer, S.; Bidzhekov, K.; Atzler, D.; Santovito, D.; Rader, D.J.; Saleheen, D.; et al. CXCL12 Derived From Endothelial Cells Promotes Atherosclerosis to Drive Coronary Artery Disease. Circulation 2019, 139, 1338–1340. [Google Scholar] [CrossRef]
- Theofilis, P.; Sagris, M.; Oikonomou, E.; Antonopoulos, A.S.; Siasos, G.; Tsioufis, C.; Tousoulis, D. Inflammatory Mechanisms Contributing to Endothelial Dysfunction. Biomedicines 2021, 9, 781. [Google Scholar] [CrossRef]
- Moore, K.J.; Sheedy, F.J.; Fisher, E.A. Macrophages in atherosclerosis: A dynamic balance. Nat. Rev. Immunol. 2013, 13, 709–721. [Google Scholar] [CrossRef]
- Alfaddagh, A.; Martin, S.S.; Leucker, T.M.; Michos, E.D.; Blaha, M.J.; Lowenstein, C.J.; Jones, S.R.; Toth, P.P. Inflammation and cardiovascular disease: From mechanisms to therapeutics. Am. J. Prev. Cardiol. 2020, 4, 100130. [Google Scholar] [CrossRef]
- Pober, J.S.; Sessa, W.C. Evolving functions of endothelial cells in inflammation. Nat. Rev. Immunol. 2007, 7, 803–815. [Google Scholar] [CrossRef]
- Bloom, S.I.; Islam, M.T.; Lesniewski, L.A.; Donato, A.J. Mechanisms and consequences of endothelial cell senescence. Nat. Rev. Cardiol. 2023, 20, 38–51. [Google Scholar] [CrossRef]
- Han, Y.; Kim, S.Y. Endothelial senescence in vascular diseases: Current understanding and future opportunities in senotherapeutics. Exp. Mol. Med. 2023, 55, 1–12. [Google Scholar] [CrossRef]
- Yang, Z.; Li, H.; Luo, P.; Yan, D.; Yang, N.; Zhang, Y.; Huang, Y.; Liu, Y.; Zhang, L.; Yan, J.; et al. UNC5B Promotes Vascular Endothelial Cell Senescence via the ROS-Mediated P53 Pathway. Oxid. Med. Cell Longev. 2021, 2021, 5546711. [Google Scholar] [CrossRef]
- Davalli, P.; Mitic, T.; Caporali, A.; Lauriola, A.; D’Arca, D. ROS, Cell Senescence, and Novel Molecular Mechanisms in Aging and Age-Related Diseases. Oxid. Med. Cell Longev. 2016, 2016, 3565127. [Google Scholar] [CrossRef]
- Donato, A.J.; Machin, D.R.; Lesniewski, L.A. Mechanisms of Dysfunction in the Aging Vasculature and Role in Age-Related Disease. Circ. Res. 2018, 123, 825–848. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Sessa, W.C.; Fernandez-Hernando, C. Endothelial Transcytosis of Lipoproteins in Atherosclerosis. Front. Cardiovasc. Med. 2018, 5, 130. [Google Scholar] [CrossRef]
- Ramirez, C.M.; Zhang, X.; Bandyopadhyay, C.; Rotllan, N.; Sugiyama, M.G.; Aryal, B.; Liu, X.; He, S.; Kraehling, J.R.; Ulrich, V.; et al. Caveolin-1 Regulates Atherogenesis by Attenuating Low-Density Lipoprotein Transcytosis and Vascular Inflammation Independently of Endothelial Nitric Oxide Synthase Activation. Circulation 2019, 140, 225–239. [Google Scholar] [CrossRef]
- Huang, L.; Chambliss, K.L.; Gao, X.; Yuhanna, I.S.; Behling-Kelly, E.; Bergaya, S.; Ahmed, M.; Michaely, P.; Luby-Phelps, K.; Darehshouri, A.; et al. SR-B1 drives endothelial cell LDL transcytosis via DOCK4 to promote atherosclerosis. Nature 2019, 569, 565–569. [Google Scholar] [CrossRef]
- Kraehling, J.R.; Chidlow, J.H.; Rajagopal, C.; Sugiyama, M.G.; Fowler, J.W.; Lee, M.Y.; Zhang, X.; Ramirez, C.M.; Park, E.J.; Tao, B.; et al. Genome-wide RNAi screen reveals ALK1 mediates LDL uptake and transcytosis in endothelial cells. Nat. Commun. 2016, 7, 13516. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Fernandez-Hernando, C. Transport of LDLs into the arterial wall: Impact in atherosclerosis. Curr. Opin. Lipidol. 2020, 31, 279–285. [Google Scholar] [CrossRef]
- Bolanle, I.O.; de Liedekerke Beaufort, G.C.; Weinberg, P.D. Transcytosis of LDL Across Arterial Endothelium: Mechanisms and Therapeutic Targets. Arterioscler. Thromb. Vasc. Biol. 2025, 45, 468–480. [Google Scholar] [CrossRef] [PubMed]
- Tamargo, I.A.; Baek, K.I.; Kim, Y.; Park, C.; Jo, H. Flow-induced reprogramming of endothelial cells in atherosclerosis. Nat. Rev. Cardiol. 2023, 20, 738–753. [Google Scholar] [CrossRef]
- Gorelova, A.; Berman, M.; Al Ghouleh, I. Endothelial-to-Mesenchymal Transition in Pulmonary Arterial Hypertension. Antioxid. Redox Signal. 2021, 34, 891–914. [Google Scholar] [CrossRef]
- Zhang, Z.; Fang, Z.; Ge, J.; Li, H. Endothelial-to-mesenchymal transition in cardiovascular diseases. Trends Mol. Med. 2025, 25, S1471–S1486. [Google Scholar] [CrossRef]
- Chen, P.Y.; Schwartz, M.A.; Simons, M. Endothelial-to-Mesenchymal Transition, Vascular Inflammation, and Atherosclerosis. Front. Cardiovasc. Med. 2020, 7, 53. [Google Scholar] [CrossRef]
- Xiong, J.; Kawagishi, H.; Yan, Y.; Liu, J.; Wells, Q.S.; Edmunds, L.R.; Fergusson, M.M.; Yu, Z.X.; Rovira, I.I.; Brittain, E.L.; et al. A Metabolic Basis for Endothelial-to-Mesenchymal Transition. Mol. Cell 2018, 69, 689–698.e7. [Google Scholar] [CrossRef] [PubMed]
- Alvandi, Z.; Bischoff, J. Endothelial-Mesenchymal Transition in Cardiovascular Disease. Arterioscler. Thromb. Vasc. Biol. 2021, 41, 2357–2369. [Google Scholar] [CrossRef]
- Evrard, S.M.; Lecce, L.; Michelis, K.C.; Nomura-Kitabayashi, A.; Pandey, G.; Purushothaman, K.R.; d’Escamard, V.; Li, J.R.; Hadri, L.; Fujitani, K.; et al. Endothelial to mesenchymal transition is common in atherosclerotic lesions and is associated with plaque instability. Nat. Commun. 2016, 7, 11853. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.G.; Lee, A.; Chang, W.; Lee, M.S.; Kim, J. Endothelial to Mesenchymal Transition Represents a Key Link in the Interaction between Inflammation and Endothelial Dysfunction. Front. Immunol. 2018, 9, 294. [Google Scholar] [CrossRef] [PubMed]
- Hall, I.F.; Kishta, F.; Xu, Y.; Baker, A.H.; Kovacic, J.C. Endothelial to mesenchymal transition: At the axis of cardiovascular health and disease. Cardiovasc. Res. 2024, 120, 223–236. [Google Scholar] [CrossRef]
- Kim, M.; Choi, S.H.; Jin, Y.B.; Lee, H.J.; Ji, Y.H.; Kim, J.; Lee, Y.S.; Lee, Y.J. The effect of oxidized low-density lipoprotein (ox-LDL) on radiation-induced endothelial-to-mesenchymal transition. Int. J. Radiat. Biol. 2013, 89, 356–363. [Google Scholar] [CrossRef]
- Kovacic, J.C.; Dimmeler, S.; Harvey, R.P.; Finkel, T.; Aikawa, E.; Krenning, G.; Baker, A.H. Endothelial to Mesenchymal Transition in Cardiovascular Disease: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2019, 73, 190–209. [Google Scholar] [CrossRef]
- Shi, X.; Li, P.; Liu, H.; Prokosch, V. Oxidative Stress, Vascular Endothelium, and the Pathology of Neurodegeneration in Retina. Antioxidants 2022, 11, 543. [Google Scholar] [CrossRef]
- Singla, B.; Aithabathula, R.V.; Kiran, S.; Kapil, S.; Kumar, S.; Singh, U.P. Reactive Oxygen Species in Regulating Lymphangiogenesis and Lymphatic Function. Cells 2022, 11, 1750. [Google Scholar] [CrossRef]
- Meyer, J.W.; Holland, J.A.; Ziegler, L.M.; Chang, M.M.; Beebe, G.; Schmitt, M.E. Identification of a functional leukocyte-type NADPH oxidase in human endothelial cells: A potential atherogenic source of reactive oxygen species. Endothelium 1999, 7, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.; Zhang, X.; Zheng, H.; Hu, D.; Zhang, Y.; Guan, Q.; Liu, L.; Ding, Q.; Li, Y. Clematichinenoside inhibits VCAM-1 and ICAM-1 expression in TNF-alpha-treated endothelial cells via NADPH oxidase-dependent IkappaB kinase/NF-kappaB pathway. Free Radic. Biol. Med. 2015, 78, 190–201. [Google Scholar] [CrossRef]
- Kim, H.J.; Park, K.G.; Yoo, E.K.; Kim, Y.H.; Kim, Y.N.; Kim, H.S.; Kim, H.T.; Park, J.Y.; Lee, K.U.; Jang, W.G.; et al. Effects of PGC-1alpha on TNF-alpha-induced MCP-1 and VCAM-1 expression and NF-kappaB activation in human aortic smooth muscle and endothelial cells. Antioxid. Redox Signal. 2007, 9, 301–307. [Google Scholar] [CrossRef]
- Bloodsworth, A.; O’Donnell, V.B.; Freeman, B.A. Nitric oxide regulation of free radical- and enzyme-mediated lipid and lipoprotein oxidation. Arterioscler. Thromb. Vasc. Biol. 2000, 20, 1707–1715. [Google Scholar] [CrossRef] [PubMed]
- Beckman, J.S.; Koppenol, W.H. Nitric oxide, superoxide, and peroxynitrite: The good, the bad, and ugly. Am. J. Physiol. 1996, 271, C1424–C1437. [Google Scholar] [CrossRef] [PubMed]
- Brandes, R.P.; Weissmann, N.; Schroder, K. Nox family NADPH oxidases: Molecular mechanisms of activation. Free Radic. Biol. Med. 2014, 76, 208–226. [Google Scholar] [CrossRef]
- Ushio-Fukai, M.; Ash, D.; Nagarkoti, S.; Belin de Chantemele, E.J.; Fulton, D.J.R.; Fukai, T. Interplay Between Reactive Oxygen/Reactive Nitrogen Species and Metabolism in Vascular Biology and Disease. Antioxid. Redox Signal. 2021, 34, 1319–1354. [Google Scholar] [CrossRef] [PubMed]
- Van Buul, J.D.; Fernandez-Borja, M.; Anthony, E.C.; Hordijk, P.L. Expression and localization of NOX2 and NOX4 in primary human endothelial cells. Antioxid. Redox Signal. 2005, 7, 308–317. [Google Scholar] [CrossRef]
- Bayraktutan, U.; Blayney, L.; Shah, A.M. Molecular characterization and localization of the NAD(P)H oxidase components gp91-phox and p22-phox in endothelial cells. Arterioscler. Thromb. Vasc. Biol. 2000, 20, 1903–1911. [Google Scholar] [CrossRef] [PubMed]
- Aldosari, S.; Awad, M.; Harrington, E.O.; Sellke, F.W.; Abid, M.R. Subcellular Reactive Oxygen Species (ROS) in Cardiovascular Pathophysiology. Antioxidants 2018, 7, 14. [Google Scholar] [CrossRef]
- Shafique, E.; Torina, A.; Reichert, K.; Colantuono, B.; Nur, N.; Zeeshan, K.; Ravichandran, V.; Liu, Y.; Feng, J.; Zeeshan, K.; et al. Mitochondrial redox plays a critical role in the paradoxical effects of NAPDH oxidase-derived ROS on coronary endothelium. Cardiovasc. Res. 2017, 113, 234–246. [Google Scholar] [CrossRef]
- Craige, S.M.; Chen, K.; Pei, Y.; Li, C.; Huang, X.; Chen, C.; Shibata, R.; Sato, K.; Walsh, K.; Keaney, J.F., Jr. NADPH oxidase 4 promotes endothelial angiogenesis through endothelial nitric oxide synthase activation. Circulation 2011, 124, 731–740. [Google Scholar] [CrossRef]
- Schroder, K.; Zhang, M.; Benkhoff, S.; Mieth, A.; Pliquett, R.; Kosowski, J.; Kruse, C.; Luedike, P.; Michaelis, U.R.; Weissmann, N.; et al. Nox4 is a protective reactive oxygen species generating vascular NADPH oxidase. Circ. Res. 2012, 110, 1217–1225. [Google Scholar] [CrossRef]
- Shafique, E.; Choy, W.C.; Liu, Y.; Feng, J.; Cordeiro, B.; Lyra, A.; Arafah, M.; Yassin-Kassab, A.; Zanetti, A.V.; Clements, R.T.; et al. Oxidative stress improves coronary endothelial function through activation of the pro-survival kinase AMPK. Aging 2013, 5, 515–530. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.M.; Kim, S.J.; Tatsunami, R.; Yamamura, H.; Fukai, T.; Ushio-Fukai, M. ROS-induced ROS release orchestrated by Nox4, Nox2, and mitochondria in VEGF signaling and angiogenesis. Am. J. Physiol. Cell Physiol. 2017, 312, C749–C764. [Google Scholar] [CrossRef]
- Schilder, Y.D.; Heiss, E.H.; Schachner, D.; Ziegler, J.; Reznicek, G.; Sorescu, D.; Dirsch, V.M. NADPH oxidases 1 and 4 mediate cellular senescence induced by resveratrol in human endothelial cells. Free Radic. Biol. Med. 2009, 46, 1598–1606. [Google Scholar] [CrossRef]
- Konior, A.; Schramm, A.; Czesnikiewicz-Guzik, M.; Guzik, T.J. NADPH oxidases in vascular pathology. Antioxid. Redox Signal. 2014, 20, 2794–2814. [Google Scholar] [CrossRef]
- Nazari, B.; Jaquet, V.; Krause, K.H. NOX family NADPH oxidases in mammals: Evolutionary conservation and isoform-defining sequences. Redox Biol. 2023, 66, 102851. [Google Scholar] [CrossRef] [PubMed]
- Szekeres, F.L.M.; Walum, E.; Wikstrom, P.; Arner, A. A small molecule inhibitor of Nox2 and Nox4 improves contractile function after ischemia-reperfusion in the mouse heart. Sci. Rep. 2021, 11, 11970. [Google Scholar] [CrossRef]
- Lassegue, B.; Griendling, K.K. NADPH oxidases: Functions and pathologies in the vasculature. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 653–661. [Google Scholar] [CrossRef] [PubMed]
- Alhayaza, R.; Haque, E.; Karbasiafshar, C.; Sellke, F.W.; Abid, M.R. The Relationship Between Reactive Oxygen Species and Endothelial Cell Metabolism. Front. Chem. 2020, 8, 592688. [Google Scholar] [CrossRef] [PubMed]
- Takenouchi, Y.; Kobayashi, T.; Matsumoto, T.; Kamata, K. Gender differences in age-related endothelial function in the murine aorta. Atherosclerosis 2009, 206, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Lassegue, B.; San Martin, A.; Griendling, K.K. Biochemistry, physiology, and pathophysiology of NADPH oxidases in the cardiovascular system. Circ. Res. 2012, 110, 1364–1390. [Google Scholar] [CrossRef]
- Taye, A.; Saad, A.H.; Kumar, A.H.; Morawietz, H. Effect of apocynin on NADPH oxidase-mediated oxidative stress-LOX-1-eNOS pathway in human endothelial cells exposed to high glucose. Eur. J. Pharmacol. 2010, 627, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Duerrschmidt, N.; Stielow, C.; Muller, G.; Pagano, P.J.; Morawietz, H. NO-mediated regulation of NAD(P)H oxidase by laminar shear stress in human endothelial cells. J. Physiol. 2006, 576, 557–567. [Google Scholar] [CrossRef]
- Hwang, J.; Saha, A.; Boo, Y.C.; Sorescu, G.P.; McNally, J.S.; Holland, S.M.; Dikalov, S.; Giddens, D.P.; Griendling, K.K.; Harrison, D.G.; et al. Oscillatory shear stress stimulates endothelial production of O2− from p47phox-dependent NAD(P)H oxidases, leading to monocyte adhesion. J. Biol. Chem. 2003, 278, 47291–47298. [Google Scholar] [CrossRef]
- Li, J.M.; Fan, L.M.; George, V.T.; Brooks, G. Nox2 regulates endothelial cell cycle arrest and apoptosis via p21cip1 and p53. Free Radic. Biol. Med. 2007, 43, 976–986. [Google Scholar] [CrossRef]
- Gray, S.P.; Shah, A.M.; Smyrnias, I. NADPH oxidase 4 and its role in the cardiovascular system. Vasc. Biol. 2019, 1, H59–H66. [Google Scholar] [CrossRef]
- Goettsch, C.; Goettsch, W.; Arsov, A.; Hofbauer, L.C.; Bornstein, S.R.; Morawietz, H. Long-term cyclic strain downregulates endothelial Nox4. Antioxid. Redox Signal. 2009, 11, 2385–2397. [Google Scholar] [CrossRef]
- Hwang, J.; Ing, M.H.; Salazar, A.; Lassegue, B.; Griendling, K.; Navab, M.; Sevanian, A.; Hsiai, T.K. Pulsatile versus oscillatory shear stress regulates NADPH oxidase subunit expression: Implication for native LDL oxidation. Circ. Res. 2003, 93, 1225–1232. [Google Scholar] [CrossRef]
- Datla, S.R.; Peshavariya, H.; Dusting, G.J.; Mahadev, K.; Goldstein, B.J.; Jiang, F. Important role of Nox4 type NADPH oxidase in angiogenic responses in human microvascular endothelial cells in vitro. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 2319–2324. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Kirber, M.T.; Xiao, H.; Yang, Y.; Keaney, J.F., Jr. Regulation of ROS signal transduction by NADPH oxidase 4 localization. J. Cell Biol. 2008, 181, 1129–1139. [Google Scholar] [CrossRef]
- Tang, X.; Wang, J.; Abboud, H.E.; Chen, Y.; Wang, J.J.; Zhang, S.X. Sustained Upregulation of Endothelial Nox4 Mediates Retinal Vascular Pathology in Type 1 Diabetes. Diabetes 2023, 72, 112–125. [Google Scholar] [CrossRef]
- Basuroy, S.; Bhattacharya, S.; Leffler, C.W.; Parfenova, H. Nox4 NADPH oxidase mediates oxidative stress and apoptosis caused by TNF-alpha in cerebral vascular endothelial cells. Am. J. Physiol. Cell Physiol. 2009, 296, C422–C432. [Google Scholar] [CrossRef] [PubMed]
- Salazar, G. NADPH Oxidases and Mitochondria in Vascular Senescence. Int. J. Mol. Sci. 2018, 19, 1327. [Google Scholar] [CrossRef] [PubMed]
- Przybylska, D.; Janiszewska, D.; Gozdzik, A.; Bielak-Zmijewska, A.; Sunderland, P.; Sikora, E.; Mosieniak, G. NOX4 downregulation leads to senescence of human vascular smooth muscle cells. Oncotarget 2016, 7, 66429–66443. [Google Scholar] [CrossRef]
- Munoz, M.; Lopez-Oliva, M.E.; Rodriguez, C.; Martinez, M.P.; Saenz-Medina, J.; Sanchez, A.; Climent, B.; Benedito, S.; Garcia-Sacristan, A.; Rivera, L.; et al. Differential contribution of Nox1, Nox2 and Nox4 to kidney vascular oxidative stress and endothelial dysfunction in obesity. Redox Biol. 2020, 28, 101330. [Google Scholar] [CrossRef]
- Canugovi, C.; Stevenson, M.D.; Vendrov, A.E.; Hayami, T.; Robidoux, J.; Xiao, H.; Zhang, Y.Y.; Eitzman, D.T.; Runge, M.S.; Madamanchi, N.R. Increased mitochondrial NADPH oxidase 4 (NOX4) expression in aging is a causative factor in aortic stiffening. Redox Biol. 2019, 26, 101288. [Google Scholar] [CrossRef]
- Cucoranu, I.; Clempus, R.; Dikalova, A.; Phelan, P.J.; Ariyan, S.; Dikalov, S.; Sorescu, D. NAD(P)H oxidase 4 mediates transforming growth factor-beta1-induced differentiation of cardiac fibroblasts into myofibroblasts. Circ. Res. 2005, 97, 900–907. [Google Scholar] [CrossRef]
- Hahner, F.; Moll, F.; Warwick, T.; Hebchen, D.M.; Buchmann, G.K.; Epah, J.; Abplanalp, W.; Schader, T.; Gunther, S.; Gilsbach, R.; et al. Nox4 promotes endothelial differentiation through chromatin remodeling. Redox Biol. 2022, 55, 102381. [Google Scholar] [CrossRef]
- Kim, J.; Yoo, J.Y.; Suh, J.M.; Park, S.; Kang, D.; Jo, H.; Bae, Y.S. The flagellin-TLR5-Nox4 axis promotes the migration of smooth muscle cells in atherosclerosis. Exp. Mol. Med. 2019, 51, 1–13. [Google Scholar] [CrossRef]
- Miyano, K.; Okamoto, S.; Yamauchi, A.; Kawai, C.; Kajikawa, M.; Kiyohara, T.; Tamura, M.; Taura, M.; Kuribayashi, F. The NADPH oxidase NOX4 promotes the directed migration of endothelial cells by stabilizing vascular endothelial growth factor receptor 2 protein. J. Biol. Chem. 2020, 295, 11877–11890. [Google Scholar] [CrossRef]
- Petry, A.; Djordjevic, T.; Weitnauer, M.; Kietzmann, T.; Hess, J.; Gorlach, A. NOX2 and NOX4 mediate proliferative response in endothelial cells. Antioxid. Redox Signal. 2006, 8, 1473–1484. [Google Scholar] [CrossRef]
- Yan, F.; Wang, Y.; Wu, X.; Peshavariya, H.M.; Dusting, G.J.; Zhang, M.; Jiang, F. Nox4 and redox signaling mediate TGF-beta-induced endothelial cell apoptosis and phenotypic switch. Cell Death Dis. 2014, 5, e1010. [Google Scholar] [CrossRef]
- Hendricks, K.S.; To, E.E.; Luong, R.; Liong, F.; Erlich, J.R.; Shah, A.M.; Liong, S.; O’Leary, J.J.; Brooks, D.A.; Vlahos, R.; et al. Endothelial NOX4 Oxidase Negatively Regulates Inflammation and Improves Morbidity During Influenza A Virus Lung Infection in Mice. Front. Cell. Infect. Microbiol. 2022, 12, 883448. [Google Scholar] [CrossRef]
- Yuan, S.; Hahn, S.A.; Miller, M.P.; Sanker, S.; Calderon, M.J.; Sullivan, M.; Dosunmu-Ogunbi, A.M.; Fazzari, M.; Li, Y.; Reynolds, M.; et al. Cooperation between CYB5R3 and NOX4 via coenzyme Q mitigates endothelial inflammation. Redox Biol. 2021, 47, 102166. [Google Scholar] [CrossRef]
- Cathcart, M.K. Regulation of superoxide anion production by NADPH oxidase in monocytes/macrophages: Contributions to atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 23–28. [Google Scholar] [CrossRef]
- Schurmann, C.; Rezende, F.; Kruse, C.; Yasar, Y.; Lowe, O.; Fork, C.; van de Sluis, B.; Bremer, R.; Weissmann, N.; Shah, A.M.; et al. The NADPH oxidase Nox4 has anti-atherosclerotic functions. Eur. Heart J. 2015, 36, 3447–3456. [Google Scholar] [CrossRef]
- Gray, S.P.; Di Marco, E.; Kennedy, K.; Chew, P.; Okabe, J.; El-Osta, A.; Calkin, A.C.; Biessen, E.A.; Touyz, R.M.; Cooper, M.E.; et al. Reactive Oxygen Species Can Provide Atheroprotection via NOX4-Dependent Inhibition of Inflammation and Vascular Remodeling. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 295–307. [Google Scholar] [CrossRef]
- Sheehan, A.L.; Carrell, S.; Johnson, B.; Stanic, B.; Banfi, B.; Miller, F.J., Jr. Role for Nox1 NADPH oxidase in atherosclerosis. Atherosclerosis 2011, 216, 321–326. [Google Scholar] [CrossRef]
- Gray, S.P.; Di Marco, E.; Okabe, J.; Szyndralewiez, C.; Heitz, F.; Montezano, A.C.; de Haan, J.B.; Koulis, C.; El-Osta, A.; Andrews, K.L.; et al. NADPH oxidase 1 plays a key role in diabetes mellitus-accelerated atherosclerosis. Circulation 2013, 127, 1888–1902. [Google Scholar] [CrossRef]
- Douglas, G.; Bendall, J.K.; Crabtree, M.J.; Tatham, A.L.; Carter, E.E.; Hale, A.B.; Channon, K.M. Endothelial-specific Nox2 overexpression increases vascular superoxide and macrophage recruitment in ApoE-/- mice. Cardiovasc. Res. 2012, 94, 20–29. [Google Scholar] [CrossRef]
- Batty, M.; Bennett, M.R.; Yu, E. The Role of Oxidative Stress in Atherosclerosis. Cells 2022, 11, 3843. [Google Scholar] [CrossRef]
- Hofmann, A.; Frank, F.; Wolk, S.; Busch, A.; Klimova, A.; Sabarstinski, P.; Gerlach, M.; Egorov, D.; Kopaliani, I.; Weinert, S.; et al. NOX4 mRNA correlates with plaque stability in patients with carotid artery stenosis. Redox Biol. 2022, 57, 102473. [Google Scholar] [CrossRef] [PubMed]
- Ho, F.; Watson, A.M.D.; Elbatreek, M.H.; Kleikers, P.W.M.; Khan, W.; Sourris, K.C.; Dai, A.; Jha, J.; Schmidt, H.; Jandeleit-Dahm, K.A.M. Endothelial reactive oxygen-forming NADPH oxidase 5 is a possible player in diabetic aortic aneurysm but not atherosclerosis. Sci. Rep. 2022, 12, 11570. [Google Scholar] [CrossRef] [PubMed]
- Mayer, B.; Hemmens, B. Biosynthesis and action of nitric oxide in mammalian cells. Trends Biochem. Sci. 1997, 22, 477–481. [Google Scholar] [CrossRef] [PubMed]
- Forstermann, U.; Sessa, W.C. Nitric oxide synthases: Regulation and function. Eur. Heart J. 2012, 33, 829–837. [Google Scholar] [CrossRef]
- Lundberg, J.O.; Weitzberg, E. Nitric oxide signaling in health and disease. Cell 2022, 185, 2853–2878. [Google Scholar] [CrossRef]
- Li, H.; Forstermann, U. Uncoupling of endothelial NO synthase in atherosclerosis and vascular disease. Curr. Opin. Pharmacol. 2013, 13, 161–167. [Google Scholar] [CrossRef]
- Zamora, R.; Vodovotz, Y.; Billiar, T.R. Inducible nitric oxide synthase and inflammatory diseases. Mol. Med. 2000, 6, 347–373. [Google Scholar] [CrossRef]
- Li, H.; Horke, S.; Forstermann, U. Vascular oxidative stress, nitric oxide and atherosclerosis. Atherosclerosis 2014, 237, 208–219. [Google Scholar] [CrossRef] [PubMed]
- Cominacini, L.; Rigoni, A.; Pasini, A.F.; Garbin, U.; Davoli, A.; Campagnola, M.; Pastorino, A.M.; Lo Cascio, V.; Sawamura, T. The binding of oxidized low density lipoprotein (ox-LDL) to ox-LDL receptor-1 reduces the intracellular concentration of nitric oxide in endothelial cells through an increased production of superoxide. J. Biol. Chem. 2001, 276, 13750–13755. [Google Scholar] [CrossRef] [PubMed]
- Chavakis, E.; Dernbach, E.; Hermann, C.; Mondorf, U.F.; Zeiher, A.M.; Dimmeler, S. Oxidized LDL inhibits vascular endothelial growth factor-induced endothelial cell migration by an inhibitory effect on the Akt/endothelial nitric oxide synthase pathway. Circulation 2001, 103, 2102–2107. [Google Scholar] [CrossRef]
- Mehta, J.L.; Chen, J.; Hermonat, P.L.; Romeo, F.; Novelli, G. Lectin-like, oxidized low-density lipoprotein receptor-1 (LOX-1): A critical player in the development of atherosclerosis and related disorders. Cardiovasc. Res. 2006, 69, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, T.; Edelstein, D.; Du, X.L.; Yamagishi, S.; Matsumura, T.; Kaneda, Y.; Yorek, M.A.; Beebe, D.; Oates, P.J.; Hammes, H.P.; et al. Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature 2000, 404, 787–790. [Google Scholar] [CrossRef]
- Dikalov, S.I.; Nazarewicz, R.R. Angiotensin II-induced production of mitochondrial reactive oxygen species: Potential mechanisms and relevance for cardiovascular disease. Antioxid. Redox Signal. 2013, 19, 1085–1094. [Google Scholar] [CrossRef] [PubMed]
- Khoi, C.S.; Lin, T.Y.; Chiang, C.K. Targeting Insulin Resistance, Reactive Oxygen Species, Inflammation, Programmed Cell Death, ER Stress, and Mitochondrial Dysfunction for the Therapeutic Prevention of Free Fatty Acid-Induced Vascular Endothelial Lipotoxicity. Antioxidants 2024, 13, 1486. [Google Scholar] [CrossRef]
- Schulz, E.; Anter, E.; Keaney, J.F., Jr. Oxidative stress, antioxidants, and endothelial function. Curr. Med. Chem. 2004, 11, 1093–1104. [Google Scholar] [CrossRef]
- Langille, B.L. Arterial remodeling: Relation to hemodynamics. Can. J. Physiol. Pharmacol. 1996, 74, 834–841. [Google Scholar] [CrossRef] [PubMed]
- Chiu, J.J.; Chien, S. Effects of disturbed flow on vascular endothelium: Pathophysiological basis and clinical perspectives. Physiol. Rev. 2011, 91, 327–387. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.Y.; Chiu, J.J. Atherosclerosis and flow: Roles of epigenetic modulation in vascular endothelium. J. Biomed. Sci. 2019, 26, 56. [Google Scholar] [CrossRef]
- Zhou, J.; Li, Y.S.; Chien, S. Shear stress-initiated signaling and its regulation of endothelial function. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 2191–2198. [Google Scholar] [CrossRef]
- Davies, P.F. Flow-mediated endothelial mechanotransduction. Physiol. Rev. 1995, 75, 519–560. [Google Scholar] [CrossRef]
- Tzima, E.; Irani-Tehrani, M.; Kiosses, W.B.; Dejana, E.; Schultz, D.A.; Engelhardt, B.; Cao, G.; DeLisser, H.; Schwartz, M.A. A mechanosensory complex that mediates the endothelial cell response to fluid shear stress. Nature 2005, 437, 426–431. [Google Scholar] [CrossRef]
- Jalali, S.; del Pozo, M.A.; Chen, K.; Miao, H.; Li, Y.; Schwartz, M.A.; Shyy, J.Y.; Chien, S. Integrin-mediated mechanotransduction requires its dynamic interaction with specific extracellular matrix (ECM) ligands. Proc. Natl. Acad. Sci. USA 2001, 98, 1042–1046. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Miao, H.; Li, S.; Chen, K.D.; Li, Y.S.; Yuan, S.; Shyy, J.Y.; Chien, S. Interplay between integrins and FLK-1 in shear stress-induced signaling. Am. J. Physiol. Cell Physiol. 2002, 283, C1540–C1547. [Google Scholar] [CrossRef] [PubMed]
- Dimmeler, S.; Fleming, I.; Fisslthaler, B.; Hermann, C.; Busse, R.; Zeiher, A.M. Activation of nitric oxide synthase in endothelial cells by Akt-dependent phosphorylation. Nature 1999, 399, 601–605. [Google Scholar] [CrossRef]
- Wu, W.; Xiao, H.; Laguna-Fernandez, A.; Villarreal, G., Jr.; Wang, K.C.; Geary, G.G.; Zhang, Y.; Wang, W.C.; Huang, H.D.; Zhou, J.; et al. Flow-Dependent Regulation of Kruppel-Like Factor 2 Is Mediated by MicroRNA-92a. Circulation 2011, 124, 633–641. [Google Scholar] [CrossRef] [PubMed]
- Boo, Y.C.; Hwang, J.; Sykes, M.; Michell, B.J.; Kemp, B.E.; Lum, H.; Jo, H. Shear stress stimulates phosphorylation of eNOS at Ser(635) by a protein kinase A-dependent mechanism. Am. J. Physiol. Heart Circ. Physiol. 2002, 283, H1819–H1828. [Google Scholar] [CrossRef]
- Takabe, W.; Warabi, E.; Noguchi, N. Anti-atherogenic effect of laminar shear stress via Nrf2 activation. Antioxid. Redox Signal. 2011, 15, 1415–1426. [Google Scholar] [CrossRef]
- Chen, Z.; Peng, I.C.; Cui, X.; Li, Y.S.; Chien, S.; Shyy, J.Y. Shear stress, SIRT1, and vascular homeostasis. Proc. Natl. Acad. Sci. USA 2010, 107, 10268–10273. [Google Scholar] [CrossRef]
- Wang, W.; Ha, C.H.; Jhun, B.S.; Wong, C.; Jain, M.K.; Jin, Z.G. Fluid shear stress stimulates phosphorylation-dependent nuclear export of HDAC5 and mediates expression of KLF2 and eNOS. Blood 2010, 115, 2971–2979. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.C.; Garmire, L.X.; Young, A.; Nguyen, P.; Trinh, A.; Subramaniam, S.; Wang, N.; Shyy, J.Y.; Li, Y.S.; Chien, S. Role of microRNA-23b in flow-regulation of Rb phosphorylation and endothelial cell growth. Proc. Natl. Acad. Sci. USA 2010, 107, 3234–3239. [Google Scholar] [CrossRef]
- Lin, K.; Hsu, P.P.; Chen, B.P.; Yuan, S.; Usami, S.; Shyy, J.Y.; Li, Y.S.; Chien, S. Molecular mechanism of endothelial growth arrest by laminar shear stress. Proc. Natl. Acad. Sci. USA 2000, 97, 9385–9389. [Google Scholar] [CrossRef]
- Abe, J.; Berk, B.C. Novel mechanisms of endothelial mechanotransduction. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 2378–2386. [Google Scholar] [CrossRef]
- Lin, J.H.; Walter, P.; Yen, T.S. Endoplasmic reticulum stress in disease pathogenesis. Annu. Rev. Pathol. 2008, 3, 399–425. [Google Scholar] [CrossRef]
- Han, J.; Kaufman, R.J. The role of ER stress in lipid metabolism and lipotoxicity. J. Lipid Res. 2016, 57, 1329–1338. [Google Scholar] [CrossRef] [PubMed]
- Hetz, C.; Zhang, K.; Kaufman, R.J. Mechanisms, regulation and functions of the unfolded protein response. Nat. Rev. Mol. Cell Biol. 2020, 21, 421–438. [Google Scholar] [CrossRef]
- Yang, S.; Wu, M.; Li, X.; Zhao, R.; Zhao, Y.; Liu, L.; Wang, S. Role of Endoplasmic Reticulum Stress in Atherosclerosis and Its Potential as a Therapeutic Target. Oxid. Med. Cell Longev. 2020, 2020, 9270107. [Google Scholar] [CrossRef]
- Darling, N.J.; Cook, S.J. The role of MAPK signalling pathways in the response to endoplasmic reticulum stress. Biochim. Biophys. Acta 2014, 1843, 2150–2163. [Google Scholar] [CrossRef] [PubMed]
- Lenna, S.; Han, R.; Trojanowska, M. Endoplasmic reticulum stress and endothelial dysfunction. IUBMB Life 2014, 66, 530–537. [Google Scholar] [CrossRef] [PubMed]
- Galan, M.; Kassan, M.; Kadowitz, P.J.; Trebak, M.; Belmadani, S.; Matrougui, K. Mechanism of endoplasmic reticulum stress-induced vascular endothelial dysfunction. Biochim. Biophys. Acta 2014, 1843, 1063–1075. [Google Scholar] [CrossRef]
- Sanson, M.; Auge, N.; Vindis, C.; Muller, C.; Bando, Y.; Thiers, J.C.; Marachet, M.A.; Zarkovic, K.; Sawa, Y.; Salvayre, R.; et al. Oxidized low-density lipoproteins trigger endoplasmic reticulum stress in vascular cells: Prevention by oxygen-regulated protein 150 expression. Circ. Res. 2009, 104, 328–336. [Google Scholar] [CrossRef]
- Civelek, M.; Manduchi, E.; Riley, R.J.; Stoeckert, C.J., Jr.; Davies, P.F. Chronic endoplasmic reticulum stress activates unfolded protein response in arterial endothelium in regions of susceptibility to atherosclerosis. Circ. Res. 2009, 105, 453–461. [Google Scholar] [CrossRef]
- Tabas, I. The role of endoplasmic reticulum stress in the progression of atherosclerosis. Circ. Res. 2010, 107, 839–850. [Google Scholar] [CrossRef]
- Jiang, C.; Xie, S.; Yang, G.; Wang, N. Spotlight on NLRP3 Inflammasome: Role in Pathogenesis and Therapies of Atherosclerosis. J. Inflamm. Res. 2021, 14, 7143–7172. [Google Scholar] [CrossRef] [PubMed]
- Gora, S.; Maouche, S.; Atout, R.; Wanherdrick, K.; Lambeau, G.; Cambien, F.; Ninio, E.; Karabina, S.A. Phospholipolyzed LDL induces an inflammatory response in endothelial cells through endoplasmic reticulum stress signaling. FASEB J. 2010, 24, 3284–3297. [Google Scholar] [CrossRef]
- Hang, L.; Peng, Y.; Xiang, R.; Li, X.; Li, Z. Ox-LDL Causes Endothelial Cell Injury Through ASK1/NLRP3-Mediated Inflammasome Activation via Endoplasmic Reticulum Stress. Drug Des. Devel Ther. 2020, 14, 731–744. [Google Scholar] [CrossRef]
- Wei, M.C.; Zong, W.X.; Cheng, E.H.; Lindsten, T.; Panoutsakopoulou, V.; Ross, A.J.; Roth, K.A.; MacGregor, G.R.; Thompson, C.B.; Korsmeyer, S.J. Proapoptotic BAX and BAK: A requisite gateway to mitochondrial dysfunction and death. Science 2001, 292, 727–730. [Google Scholar] [CrossRef]
- Li, G.; Scull, C.; Ozcan, L.; Tabas, I. NADPH oxidase links endoplasmic reticulum stress, oxidative stress, and PKR activation to induce apoptosis. J. Cell Biol. 2010, 191, 1113–1125. [Google Scholar] [CrossRef] [PubMed]
- Timmins, J.M.; Ozcan, L.; Seimon, T.A.; Li, G.; Malagelada, C.; Backs, J.; Backs, T.; Bassel-Duby, R.; Olson, E.N.; Anderson, M.E.; et al. Calcium/calmodulin-dependent protein kinase II links ER stress with Fas and mitochondrial apoptosis pathways. J. Clin. Investig. 2009, 119, 2925–2941. [Google Scholar] [CrossRef] [PubMed]
- Ryter, S.W.; Cloonan, S.M.; Choi, A.M. Autophagy: A critical regulator of cellular metabolism and homeostasis. Mol. Cells 2013, 36, 7–16. [Google Scholar] [CrossRef]
- Hua, Y.; Zhang, J.; Liu, Q.; Su, J.; Zhao, Y.; Zheng, G.; Yang, Z.; Zhuo, D.; Ma, C.; Fan, G. The Induction of Endothelial Autophagy and Its Role in the Development of Atherosclerosis. Front. Cardiovasc. Med. 2022, 9, 831847. [Google Scholar] [CrossRef]
- Lyu, Z.S.; Cao, X.N.; Wen, Q.; Mo, X.D.; Zhao, H.Y.; Chen, Y.H.; Wang, Y.; Chang, Y.J.; Xu, L.P.; Zhang, X.H.; et al. Autophagy in endothelial cells regulates their haematopoiesis-supporting ability. EBioMedicine 2020, 53, 102677. [Google Scholar] [CrossRef]
- Schaaf, M.B.; Houbaert, D.; Mece, O.; Agostinis, P. Autophagy in endothelial cells and tumor angiogenesis. Cell Death Differ. 2019, 26, 665–679. [Google Scholar] [CrossRef]
- Wu, Q.; Hu, Y.; Jiang, M.; Wang, F.; Gong, G. Effect of Autophagy Regulated by Sirt1/FoxO1 Pathway on the Release of Factors Promoting Thrombosis from Vascular Endothelial Cells. Int. J. Mol. Sci. 2019, 20, 4132. [Google Scholar] [CrossRef]
- Potente, M.; Ghaeni, L.; Baldessari, D.; Mostoslavsky, R.; Rossig, L.; Dequiedt, F.; Haendeler, J.; Mione, M.; Dejana, E.; Alt, F.W.; et al. SIRT1 controls endothelial angiogenic functions during vascular growth. Genes. Dev. 2007, 21, 2644–2658. [Google Scholar] [CrossRef]
- Dou, Y.Q.; Kong, P.; Li, C.L.; Sun, H.X.; Li, W.W.; Yu, Y.; Nie, L.; Zhao, L.L.; Miao, S.B.; Li, X.K.; et al. Smooth muscle SIRT1 reprograms endothelial cells to suppress angiogenesis after ischemia. Theranostics 2020, 10, 1197–1212. [Google Scholar] [CrossRef]
- Mattagajasingh, I.; Kim, C.S.; Naqvi, A.; Yamamori, T.; Hoffman, T.A.; Jung, S.B.; DeRicco, J.; Kasuno, K.; Irani, K. SIRT1 promotes endothelium-dependent vascular relaxation by activating endothelial nitric oxide synthase. Proc. Natl. Acad. Sci. USA 2007, 104, 14855–14860. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Bi, X.; Chen, T.; Zhang, Q.; Wang, S.X.; Chiu, J.J.; Liu, G.S.; Zhang, Y.; Bu, P.; Jiang, F. Shear stress regulates endothelial cell autophagy via redox regulation and Sirt1 expression. Cell Death Dis. 2015, 6, e1827. [Google Scholar] [CrossRef] [PubMed]
- Hirota, Y.; Yamashita, S.; Kurihara, Y.; Jin, X.; Aihara, M.; Saigusa, T.; Kang, D.; Kanki, T. Mitophagy is primarily due to alternative autophagy and requires the MAPK1 and MAPK14 signaling pathways. Autophagy 2015, 11, 332–343. [Google Scholar] [CrossRef]
- Lin, L.; Zhang, M.X.; Zhang, L.; Zhang, D.; Li, C.; Li, Y.L. Autophagy, Pyroptosis, and Ferroptosis: New Regulatory Mechanisms for Atherosclerosis. Front. Cell Dev. Biol. 2021, 9, 809955. [Google Scholar] [CrossRef]
- Shao, B.Z.; Han, B.Z.; Zeng, Y.X.; Su, D.F.; Liu, C. The roles of macrophage autophagy in atherosclerosis. Acta Pharmacol. Sin. 2016, 37, 150–156. [Google Scholar] [CrossRef]
- Zhu, L.; Wu, G.; Yang, X.; Jia, X.; Li, J.; Bai, X.; Li, W.; Zhao, Y.; Li, Y.; Cheng, W.; et al. Low density lipoprotein mimics insulin action on autophagy and glucose uptake in endothelial cells. Sci. Rep. 2019, 9, 3020. [Google Scholar] [CrossRef]
- Perrotta, P.; Van der Veken, B.; Van Der Veken, P.; Pintelon, I.; Roosens, L.; Adriaenssens, E.; Timmerman, V.; Guns, P.J.; De Meyer, G.R.Y.; Martinet, W. Partial Inhibition of Glycolysis Reduces Atherogenesis Independent of Intraplaque Neovascularization in Mice. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 1168–1181. [Google Scholar] [CrossRef]
- Ding, Z.; Liu, S.; Wang, X.; Khaidakov, M.; Dai, Y.; Mehta, J.L. Oxidant stress in mitochondrial DNA damage, autophagy and inflammation in atherosclerosis. Sci. Rep. 2013, 3, 1077. [Google Scholar] [CrossRef]
- Cho, K.; Choi, S.H. ASK1 Mediates Apoptosis and Autophagy during oxLDL-CD36 Signaling in Senescent Endothelial Cells. Oxid. Med. Cell Longev. 2019, 2019, 2840437. [Google Scholar] [CrossRef] [PubMed]
- Torisu, K.; Singh, K.K.; Torisu, T.; Lovren, F.; Liu, J.; Pan, Y.; Quan, A.; Ramadan, A.; Al-Omran, M.; Pankova, N.; et al. Intact endothelial autophagy is required to maintain vascular lipid homeostasis. Aging Cell 2016, 15, 187–191. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.L.; Cao, Y.J.; Zhang, X.; Liu, H.H.; Tong, T.; Xiao, G.D.; Yang, Y.P.; Liu, C.F. The autophagy-lysosome pathway: A novel mechanism involved in the processing of oxidized LDL in human vascular endothelial cells. Biochem. Biophys. Res. Commun. 2010, 394, 377–382. [Google Scholar] [CrossRef]
- Bornstein, P. Matricellular proteins: An overview. J. Cell Commun. Signal. 2009, 3, 163–165. [Google Scholar] [CrossRef] [PubMed]
- Frangogiannis, N.G. Matricellular proteins in cardiac adaptation and disease. Physiol. Rev. 2012, 92, 635–688. [Google Scholar] [CrossRef]
- Wong, G.S.; Rustgi, A.K. Matricellular proteins: Priming the tumour microenvironment for cancer development and metastasis. Br. J. Cancer 2013, 108, 755–761. [Google Scholar] [CrossRef]
- Vincent, K.M.; Postovit, L.M. Matricellular proteins in cancer: A focus on secreted Frizzled-related proteins. J. Cell Commun. Signal. 2018, 12, 103–112. [Google Scholar] [CrossRef]
- Bornstein, P. Thrombospondins as matricellular modulators of cell function. J. Clin. Investig. 2001, 107, 929–934. [Google Scholar] [CrossRef]
- Armstrong, L.C.; Bjorkblom, B.; Hankenson, K.D.; Siadak, A.W.; Stiles, C.E.; Bornstein, P. Thrombospondin 2 inhibits microvascular endothelial cell proliferation by a caspase-independent mechanism. Mol. Biol. Cell 2002, 13, 1893–1905. [Google Scholar] [CrossRef] [PubMed]
- Krady, M.M.; Zeng, J.; Yu, J.; MacLauchlan, S.; Skokos, E.A.; Tian, W.; Bornstein, P.; Sessa, W.C.; Kyriakides, T.R. Thrombospondin-2 modulates extracellular matrix remodeling during physiological angiogenesis. Am. J. Pathol. 2008, 173, 879–891. [Google Scholar] [CrossRef] [PubMed]
- Sadvakassova, G.; Dobocan, M.C.; Congote, L.F. Osteopontin and the C-terminal peptide of thrombospondin-4 compete for CD44 binding and have opposite effects on CD133+ cell colony formation. BMC Res. Notes 2009, 2, 215. [Google Scholar] [CrossRef] [PubMed]
- Frolova, E.G.; Pluskota, E.; Krukovets, I.; Burke, T.; Drumm, C.; Smith, J.D.; Blech, L.; Febbraio, M.; Bornstein, P.; Plow, E.F.; et al. Thrombospondin-4 regulates vascular inflammation and atherogenesis. Circ. Res. 2010, 107, 1313–1325. [Google Scholar] [CrossRef]
- Unger, S.; Hecht, J.T. Pseudoachondroplasia and multiple epiphyseal dysplasia: New etiologic developments. Am. J. Med. Genet. 2001, 106, 244–250. [Google Scholar] [CrossRef]
- Wang, L.; Zheng, J.; Du, Y.; Huang, Y.; Li, J.; Liu, B.; Liu, C.J.; Zhu, Y.; Gao, Y.; Xu, Q.; et al. Cartilage oligomeric matrix protein maintains the contractile phenotype of vascular smooth muscle cells by interacting with alpha(7)beta(1) integrin. Circ. Res. 2010, 106, 514–525. [Google Scholar] [CrossRef]
- Chen, F.H.; Thomas, A.O.; Hecht, J.T.; Goldring, M.B.; Lawler, J. Cartilage oligomeric matrix protein/thrombospondin 5 supports chondrocyte attachment through interaction with integrins. J. Biol. Chem. 2005, 280, 32655–32661. [Google Scholar] [CrossRef]
- Gao, Q.; Chen, K.; Gao, L.; Zheng, Y.; Yang, Y.G. Thrombospondin-1 signaling through CD47 inhibits cell cycle progression and induces senescence in endothelial cells. Cell Death Dis. 2016, 7, e2368. [Google Scholar] [CrossRef]
- Singla, B.; Aithbathula, R.V.; Pervaiz, N.; Kathuria, I.; Swanson, M.; Ekuban, F.A.; Ahn, W.; Park, F.; Gyamfi, M.; Cherian-Shaw, M.; et al. CD47 Activation by Thrombospondin-1 in Lymphatic Endothelial Cells Suppresses Lymphangiogenesis and Promotes Atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2023, 43, 1234–1250. [Google Scholar] [CrossRef]
- Stein, E.V.; Miller, T.W.; Ivins-O’Keefe, K.; Kaur, S.; Roberts, D.D. Secreted Thrombospondin-1 Regulates Macrophage Interleukin-1beta Production and Activation through CD47. Sci. Rep. 2016, 6, 19684. [Google Scholar] [CrossRef]
- Ganguly, R.; Khanal, S.; Mathias, A.; Gupta, S.; Lallo, J.; Sahu, S.; Ohanyan, V.; Patel, A.; Storm, K.; Datta, S.; et al. TSP-1 (Thrombospondin-1) Deficiency Protects ApoE(-/-) Mice Against Leptin-Induced Atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2021, 41, e112–e127. [Google Scholar] [CrossRef] [PubMed]
- Moura, R.; Tjwa, M.; Vandervoort, P.; Van Kerckhoven, S.; Holvoet, P.; Hoylaerts, M.F. Thrombospondin-1 deficiency accelerates atherosclerotic plaque maturation in ApoE-/- mice. Circ. Res. 2008, 103, 1181–1189. [Google Scholar] [CrossRef]
- Murphy-Ullrich, J.E.; Suto, M.J. Thrombospondin-1 regulation of latent TGF-beta activation: A therapeutic target for fibrotic disease. Matrix Biol. 2018, 68–69, 28–43. [Google Scholar] [CrossRef]
- Chen, D.; Asahara, T.; Krasinski, K.; Witzenbichler, B.; Yang, J.; Magner, M.; Kearney, M.; Frazier, W.A.; Isner, J.M.; Andres, V. Antibody blockade of thrombospondin accelerates reendothelialization and reduces neointima formation in balloon-injured rat carotid artery. Circulation 1999, 100, 849–854. [Google Scholar] [CrossRef] [PubMed]
- Isenberg, J.S.; Roberts, D.D. THBS1 (thrombospondin-1). Atlas Genet. Cytogenet. Oncol. Haematol. 2020, 24, 291–299. [Google Scholar] [CrossRef]
- Asch, A.S.; Silbiger, S.; Heimer, E.; Nachman, R.L. Thrombospondin sequence motif (CSVTCG) is responsible for CD36 binding. Biochem. Biophys. Res. Commun. 1992, 182, 1208–1217. [Google Scholar] [CrossRef] [PubMed]
- Gao, A.G.; Lindberg, F.P.; Finn, M.B.; Blystone, S.D.; Brown, E.J.; Frazier, W.A. Integrin-associated protein is a receptor for the C-terminal domain of thrombospondin. J. Biol. Chem. 1996, 271, 21–24. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Mosher, D.F.; Rapraeger, A. Heparan sulfate-mediated binding of epithelial cell surface proteoglycan to thrombospondin. J. Biol. Chem. 1989, 264, 2885–2889. [Google Scholar] [CrossRef]
- Calzada, M.J.; Annis, D.S.; Zeng, B.; Marcinkiewicz, C.; Banas, B.; Lawler, J.; Mosher, D.F.; Roberts, D.D. Identification of novel beta1 integrin binding sites in the type 1 and type 2 repeats of thrombospondin-1. J. Biol. Chem. 2004, 279, 41734–41743. [Google Scholar] [CrossRef]
- Calzada, M.J.; Sipes, J.M.; Krutzsch, H.C.; Yurchenco, P.D.; Annis, D.S.; Mosher, D.F.; Roberts, D.D. Recognition of the N-terminal modules of thrombospondin-1 and thrombospondin-2 by alpha6beta1 integrin. J. Biol. Chem. 2003, 278, 40679–40687. [Google Scholar] [CrossRef]
- Lawler, J.; Weinstein, R.; Hynes, R.O. Cell attachment to thrombospondin: The role of ARG-GLY-ASP, calcium, and integrin receptors. J. Cell Biol. 1988, 107, 2351–2361. [Google Scholar] [CrossRef]
- Lawler, J.; Hynes, R.O. An integrin receptor on normal and thrombasthenic platelets that binds thrombospondin. Blood 1989, 74, 2022–2027. [Google Scholar] [CrossRef] [PubMed]
- Mirochnik, Y.; Kwiatek, A.; Volpert, O.V. Thrombospondin and apoptosis: Molecular mechanisms and use for design of complementation treatments. Curr. Drug Targets 2008, 9, 851–862. [Google Scholar] [CrossRef] [PubMed]
- Jimenez, B.; Volpert, O.V.; Crawford, S.E.; Febbraio, M.; Silverstein, R.L.; Bouck, N. Signals leading to apoptosis-dependent inhibition of neovascularization by thrombospondin-1. Nat. Med. 2000, 6, 41–48. [Google Scholar] [CrossRef]
- Nor, J.E.; Mitra, R.S.; Sutorik, M.M.; Mooney, D.J.; Castle, V.P.; Polverini, P.J. Thrombospondin-1 induces endothelial cell apoptosis and inhibits angiogenesis by activating the caspase death pathway. J. Vasc. Res. 2000, 37, 209–218. [Google Scholar] [CrossRef]
- Dawson, D.W.; Pearce, S.F.; Zhong, R.; Silverstein, R.L.; Frazier, W.A.; Bouck, N.P. CD36 mediates the In vitro inhibitory effects of thrombospondin-1 on endothelial cells. J. Cell Biol. 1997, 138, 707–717. [Google Scholar] [CrossRef]
- Isenberg, J.S.; Ridnour, L.A.; Perruccio, E.M.; Espey, M.G.; Wink, D.A.; Roberts, D.D. Thrombospondin-1 inhibits endothelial cell responses to nitric oxide in a cGMP-dependent manner. Proc. Natl. Acad. Sci. USA 2005, 102, 13141–13146. [Google Scholar] [CrossRef]
- Chu, L.Y.; Ramakrishnan, D.P.; Silverstein, R.L. Thrombospondin-1 modulates VEGF signaling via CD36 by recruiting SHP-1 to VEGFR2 complex in microvascular endothelial cells. Blood 2013, 122, 1822–1832. [Google Scholar] [CrossRef]
- Isenberg, J.S.; Ridnour, L.A.; Dimitry, J.; Frazier, W.A.; Wink, D.A.; Roberts, D.D. CD47 is necessary for inhibition of nitric oxide-stimulated vascular cell responses by thrombospondin-1. J. Biol. Chem. 2006, 281, 26069–26080. [Google Scholar] [CrossRef]
- Rogers, N.M.; Sharifi-Sanjani, M.; Csanyi, G.; Pagano, P.J.; Isenberg, J.S. Thrombospondin-1 and CD47 regulation of cardiac, pulmonary and vascular responses in health and disease. Matrix Biol. 2014, 37, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Narizhneva, N.V.; Razorenova, O.V.; Podrez, E.A.; Chen, J.; Chandrasekharan, U.M.; DiCorleto, P.E.; Plow, E.F.; Topol, E.J.; Byzova, T.V. Thrombospondin-1 up-regulates expression of cell adhesion molecules and promotes monocyte binding to endothelium. FASEB J. 2005, 19, 1158–1160. [Google Scholar] [CrossRef] [PubMed]
- Peng, B.; Zhou, Y.; Fu, X.; Chen, L.; Pan, Z.; Yi, Q.; Zhao, T.; Fu, Z.; Wang, T. THBS1 mediates hypoxia driven EndMT in pulmonary hypertension. Pulm. Circ. 2024, 14, e70019. [Google Scholar] [CrossRef] [PubMed]
- Yamashiro, Y.; Thang, B.Q.; Shin, S.J.; Lino, C.A.; Nakamura, T.; Kim, J.; Sugiyama, K.; Tokunaga, C.; Sakamoto, H.; Osaka, M.; et al. Role of Thrombospondin-1 in Mechanotransduction and Development of Thoracic Aortic Aneurysm in Mouse and Humans. Circ. Res. 2018, 123, 660–672. [Google Scholar] [CrossRef]
- Aburima, A.; Berger, M.; Spurgeon, B.E.J.; Webb, B.A.; Wraith, K.S.; Febbraio, M.; Poole, A.W.; Naseem, K.M. Thrombospondin-1 promotes hemostasis through modulation of cAMP signaling in blood platelets. Blood 2021, 137, 678–689. [Google Scholar] [CrossRef]
- Stirling, E.R.; Terabe, M.; Wilson, A.S.; Kooshki, M.; Yamaleyeva, L.M.; Alexander-Miller, M.A.; Zhang, W.; Miller, L.D.; Triozzi, P.L.; Soto-Pantoja, D.R. Targeting the CD47/thrombospondin-1 signaling axis regulates immune cell bioenergetics in the tumor microenvironment to potentiate antitumor immune response. J. Immunother. Cancer 2022, 10, e004712. [Google Scholar] [CrossRef]
- Roberts, D.D.; Isenberg, J.S. CD47 and thrombospondin-1 regulation of mitochondria, metabolism, and diabetes. Am. J. Physiol. Cell Physiol. 2021, 321, C201–C213. [Google Scholar] [CrossRef]
- Zhao, W.; Shen, B.; Cheng, Q.; Zhou, Y.; Chen, K. Roles of TSP1-CD47 signaling pathway in senescence of endothelial cells: Cell cycle, inflammation and metabolism. Mol. Biol. Rep. 2023, 50, 4579–4585. [Google Scholar] [CrossRef]
- Gao, L.; Chen, K.; Gao, Q.; Wang, X.; Sun, J.; Yang, Y.G. CD47 deficiency in tumor stroma promotes tumor progression by enhancing angiogenesis. Oncotarget 2017, 8, 22406–22413. [Google Scholar] [CrossRef]
- Singh, B.; Cui, K.; Peng, Q.; Li, K.; Zhu, B.; Bhattacharjee, S.; Osorio, D.; Wang, B.; Dong, Y.; Wang, D. Novel Role Of Endothelial Cd47 In The Regulation Of Pathogenesis Of Atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2023, 43, A338. [Google Scholar] [CrossRef]
- Meijles, D.N.; Sahoo, S.; Al Ghouleh, I.; Amaral, J.H.; Bienes-Martinez, R.; Knupp, H.E.; Attaran, S.; Sembrat, J.C.; Nouraie, S.M.; Rojas, M.M.; et al. The matricellular protein TSP1 promotes human and mouse endothelial cell senescence through CD47 and Nox1. Sci. Signal. 2017, 10, eaaj1784. [Google Scholar] [CrossRef] [PubMed]
- LeBlanc, A.J.; Kelm, N.Q. Thrombospondin-1, Free Radicals, and the Coronary Microcirculation: The Aging Conundrum. Antioxid. Redox Signal. 2017, 27, 785–801. [Google Scholar] [CrossRef] [PubMed]
- Ridnour, L.A.; Isenberg, J.S.; Espey, M.G.; Thomas, D.D.; Roberts, D.D.; Wink, D.A. Nitric oxide regulates angiogenesis through a functional switch involving thrombospondin-1. Proc. Natl. Acad. Sci. USA 2005, 102, 13147–13152. [Google Scholar] [CrossRef]
- Novelli, E.M.; Little-Ihrig, L.; Knupp, H.E.; Rogers, N.M.; Yao, M.; Baust, J.J.; Meijles, D.; St Croix, C.M.; Ross, M.A.; Pagano, P.J.; et al. Vascular TSP1-CD47 signaling promotes sickle cell-associated arterial vasculopathy and pulmonary hypertension in mice. Am. J. Physiol. Lung Cell Mol. Physiol. 2019, 316, L1150–L1164. [Google Scholar] [CrossRef]
- Kale, A.; Rogers, N.M.; Ghimire, K. Thrombospondin-1 CD47 Signalling: From Mechanisms to Medicine. Int. J. Mol. Sci. 2021, 22, 4062. [Google Scholar] [CrossRef]
- Rogers, N.M.; Sharifi-Sanjani, M.; Yao, M.; Ghimire, K.; Bienes-Martinez, R.; Mutchler, S.M.; Knupp, H.E.; Baust, J.; Novelli, E.M.; Ross, M.; et al. TSP1-CD47 signaling is upregulated in clinical pulmonary hypertension and contributes to pulmonary arterial vasculopathy and dysfunction. Cardiovasc. Res. 2017, 113, 15–29. [Google Scholar] [CrossRef]
- Csanyi, G.; Yao, M.; Rodriguez, A.I.; Al Ghouleh, I.; Sharifi-Sanjani, M.; Frazziano, G.; Huang, X.; Kelley, E.E.; Isenberg, J.S.; Pagano, P.J. Thrombospondin-1 regulates blood flow via CD47 receptor-mediated activation of NADPH oxidase 1. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 2966–2973. [Google Scholar] [CrossRef]
- Csanyi, G.; Feck, D.M.; Ghoshal, P.; Singla, B.; Lin, H.; Nagarajan, S.; Meijles, D.N.; Al Ghouleh, I.; Cantu-Medellin, N.; Kelley, E.E.; et al. CD47 and Nox1 Mediate Dynamic Fluid-Phase Macropinocytosis of Native LDL. Antioxid. Redox Signal. 2017, 26, 886–901. [Google Scholar] [CrossRef]
- Lin, H.P.; Singla, B.; Ahn, W.; Ghoshal, P.; Blahove, M.; Cherian-Shaw, M.; Chen, A.; Haller, A.; Hui, D.Y.; Dong, K.; et al. Receptor-independent fluid-phase macropinocytosis promotes arterial foam cell formation and atherosclerosis. Sci. Transl. Med. 2022, 14, eadd2376. [Google Scholar] [CrossRef] [PubMed]
- Bond, A.R.; Hultgardh-Nilsson, A.; Knutsson, A.; Jackson, C.L.; Rauch, U. Cartilage oligomeric matrix protein (COMP) in murine brachiocephalic and carotid atherosclerotic lesions. Atherosclerosis 2014, 236, 366–372. [Google Scholar] [CrossRef] [PubMed]
- Hultman, K.; Edsfeldt, A.; Bjorkbacka, H.; Duner, P.; Sundius, L.; Nitulescu, M.; Persson, A.; Boyle, J.J.; Nilsson, J.; Hultgardh-Nilsson, A.; et al. Cartilage Oligomeric Matrix Protein Associates With a Vulnerable Plaque Phenotype in Human Atherosclerotic Plaques. Stroke 2019, 50, 3289–3292. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.F.; Ha, L.; Yu, H.Y.; Mi, L.; Han, J.L.; Gao, W. Altered serum level of cartilage oligomeric matrix protein and its association with coronary calcification in patients with coronary heart disease. J. Geriatr. Cardiol. 2017, 14, 87–92. [Google Scholar] [CrossRef]
- Fu, Y.; Gao, C.; Liang, Y.; Wang, M.; Huang, Y.; Ma, W.; Li, T.; Jia, Y.; Yu, F.; Zhu, W.; et al. Shift of Macrophage Phenotype Due to Cartilage Oligomeric Matrix Protein Deficiency Drives Atherosclerotic Calcification. Circ. Res. 2016, 119, 261–276. [Google Scholar] [CrossRef]
- Lv, H.; Wang, H.; Quan, M.; Zhang, C.; Fu, Y.; Zhang, L.; Lin, C.; Liu, X.; Yi, X.; Chen, J.; et al. Cartilage oligomeric matrix protein fine-tunes disturbed flow-induced endothelial activation and atherogenesis. Matrix Biol. 2021, 95, 32–51. [Google Scholar] [CrossRef]
- Riessen, R.; Fenchel, M.; Chen, H.; Axel, D.I.; Karsch, K.R.; Lawler, J. Cartilage oligomeric matrix protein (thrombospondin-5) is expressed by human vascular smooth muscle cells. Arterioscler. Thromb. Vasc. Biol. 2001, 21, 47–54. [Google Scholar] [CrossRef]
- Muqri, F.; Helkin, A.; Maier, K.G.; Gahtan, V. Thrombospondin-5 and fluvastatin promote angiogenesis and are protective against endothelial cell apoptosis. J. Cell. Biochem. 2020, 121, 4154–4165. [Google Scholar] [CrossRef]
- Wang, H.; Yuan, Z.; Wang, B.; Li, B.; Lv, H.; He, J.; Huang, Y.; Cui, Z.; Ma, Q.; Li, T.; et al. COMP (Cartilage Oligomeric Matrix Protein), a Novel PIEZO1 Regulator That Controls Blood Pressure. Hypertension 2022, 79, 549–561. [Google Scholar] [CrossRef]
- Agarwal, P.; Schulz, J.N.; Blumbach, K.; Andreasson, K.; Heinegard, D.; Paulsson, M.; Mauch, C.; Eming, S.A.; Eckes, B.; Krieg, T. Enhanced deposition of cartilage oligomeric matrix protein is a common feature in fibrotic skin pathologies. Matrix Biol. 2013, 32, 325–331. [Google Scholar] [CrossRef]
- Vuga, L.J.; Milosevic, J.; Pandit, K.; Ben-Yehudah, A.; Chu, Y.; Richards, T.; Sciurba, J.; Myerburg, M.; Zhang, Y.; Parwani, A.V.; et al. Cartilage oligomeric matrix protein in idiopathic pulmonary fibrosis. PLoS ONE 2013, 8, e83120. [Google Scholar] [CrossRef]
- Farina, G.; Lemaire, R.; Korn, J.H.; Widom, R.L. Cartilage oligomeric matrix protein is overexpressed by scleroderma dermal fibroblasts. Matrix Biol. 2006, 25, 213–222. [Google Scholar] [CrossRef]
- Farina, G.; Lemaire, R.; Pancari, P.; Bayle, J.; Widom, R.L.; Lafyatis, R. Cartilage oligomeric matrix protein expression in systemic sclerosis reveals heterogeneity of dermal fibroblast responses to transforming growth factor beta. Ann. Rheum. Dis. 2009, 68, 435–441. [Google Scholar] [CrossRef]
- Sanchez-Duffhues, G.; Garcia de Vinuesa, A.; Ten Dijke, P. Endothelial-to-mesenchymal transition in cardiovascular diseases: Developmental signaling pathways gone awry. Dev. Dyn. 2018, 247, 492–508. [Google Scholar] [CrossRef]
- Yao, J.; Guihard, P.J.; Blazquez-Medela, A.M.; Guo, Y.; Moon, J.H.; Jumabay, M.; Bostrom, K.I.; Yao, Y. Serine Protease Activation Essential for Endothelial-Mesenchymal Transition in Vascular Calcification. Circ. Res. 2015, 117, 758–769. [Google Scholar] [CrossRef]
- Bauer, E.M.; Qin, Y.; Miller, T.W.; Bandle, R.W.; Csanyi, G.; Pagano, P.J.; Bauer, P.M.; Schnermann, J.; Roberts, D.D.; Isenberg, J.S. Thrombospondin-1 supports blood pressure by limiting eNOS activation and endothelial-dependent vasorelaxation. Cardiovasc. Res. 2010, 88, 471–481. [Google Scholar] [CrossRef] [PubMed]
- Yamashiro, Y.; Thang, B.Q.; Ramirez, K.; Shin, S.J.; Kohata, T.; Ohata, S.; Nguyen, T.A.V.; Ohtsuki, S.; Nagayama, K.; Yanagisawa, H. Matrix mechanotransduction mediated by thrombospondin-1/integrin/YAP in the vascular remodeling. Proc. Natl. Acad. Sci. USA 2020, 117, 9896–9905. [Google Scholar] [CrossRef]
- Wang, M.; Fu, Y.; Gao, C.; Jia, Y.; Huang, Y.; Liu, L.; Wang, X.; Wang, W.; Kong, W. Cartilage oligomeric matrix protein prevents vascular aging and vascular smooth muscle cells senescence. Biochem. Biophys. Res. Commun. 2016, 478, 1006–1013. [Google Scholar] [CrossRef] [PubMed]
- Singla, B.; Lin, H.P.; Chen, A.; Ahn, W.; Ghoshal, P.; Cherian-Shaw, M.; White, J.; Stansfield, B.K.; Csanyi, G. Role of R-spondin 2 in arterial lymphangiogenesis and atherosclerosis. Cardiovasc. Res. 2021, 117, 1489–1509. [Google Scholar] [CrossRef] [PubMed]
- Fu, S.; Tan, R.; Feng, Y.; Yu, P.; Mo, Y.; Xiao, W.; Wang, S.; Zhang, J. N-methyl-N-nitrosourea induces zebrafish anomalous angiogenesis through Wnt/beta-catenin pathway. Ecotoxicol. Environ. Saf. 2022, 239, 113674. [Google Scholar] [CrossRef]
- Scholz, B.; Korn, C.; Wojtarowicz, J.; Mogler, C.; Augustin, I.; Boutros, M.; Niehrs, C.; Augustin, H.G. Endothelial RSPO3 Controls Vascular Stability and Pruning through Non-canonical WNT/Ca(2+)/NFAT Signaling. Dev. Cell 2016, 36, 79–93. [Google Scholar] [CrossRef] [PubMed]
- Minear, M.A.; Crosslin, D.R.; Sutton, B.S.; Connelly, J.J.; Nelson, S.C.; Gadson-Watson, S.; Wang, T.; Seo, D.; Vance, J.M.; Sketch, M.H., Jr.; et al. Polymorphic variants in tenascin-C (TNC) are associated with atherosclerosis and coronary artery disease. Hum. Genet. 2011, 129, 641–654. [Google Scholar] [CrossRef]
- Matsui, Y.; Rittling, S.R.; Okamoto, H.; Inobe, M.; Jia, N.; Shimizu, T.; Akino, M.; Sugawara, T.; Morimoto, J.; Kimura, C.; et al. Osteopontin deficiency attenuates atherosclerosis in female apolipoprotein E-deficient mice. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 1029–1034. [Google Scholar] [CrossRef]
- Hirota, S.; Imakita, M.; Kohri, K.; Ito, A.; Morii, E.; Adachi, S.; Kim, H.M.; Kitamura, Y.; Yutani, C.; Nomura, S. Expression of osteopontin messenger RNA by macrophages in atherosclerotic plaques. A possible association with calcification. Am. J. Pathol. 1993, 143, 1003–1008. [Google Scholar] [PubMed]
- Zhao, J.F.; Chen, H.Y.; Wei, J.; Jim Leu, S.J.; Lee, T.S. CCN family member 1 deregulates cholesterol metabolism and aggravates atherosclerosis. Acta Physiol. 2019, 225, e13209. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wu, G.; Dai, H. The matricellular protein CCN1 regulates TNF-alpha induced vascular endothelial cell apoptosis. Cell Biol. Int. 2016, 40, 1–6. [Google Scholar] [CrossRef]
- Grote, K.; Salguero, G.; Ballmaier, M.; Dangers, M.; Drexler, H.; Schieffer, B. The angiogenic factor CCN1 promotes adhesion and migration of circulating CD34+ progenitor cells: Potential role in angiogenesis and endothelial regeneration. Blood 2007, 110, 877–885. [Google Scholar] [CrossRef]
- Chen, J.; Singh, K.; Mukherjee, B.B.; Sodek, J. Developmental expression of osteopontin (OPN) mRNA in rat tissues: Evidence for a role for OPN in bone formation and resorption. Matrix 1993, 13, 113–123. [Google Scholar] [CrossRef]
- Brown, L.F.; Berse, B.; Van de Water, L.; Papadopoulos-Sergiou, A.; Perruzzi, C.A.; Manseau, E.J.; Dvorak, H.F.; Senger, D.R. Expression and distribution of osteopontin in human tissues: Widespread association with luminal epithelial surfaces. Mol. Biol. Cell 1992, 3, 1169–1180. [Google Scholar] [CrossRef]
- Xu, C.; Wu, Y.; Liu, N. Osteopontin in autoimmune disorders: Current knowledge and future perspective. Inflammopharmacology 2022, 30, 385–396. [Google Scholar] [CrossRef] [PubMed]
- Hu, D.D.; Lin, E.C.; Kovach, N.L.; Hoyer, J.R.; Smith, J.W. A biochemical characterization of the binding of osteopontin to integrins alpha v beta 1 and alpha v beta 5. J. Biol. Chem. 1995, 270, 26232–26238. [Google Scholar] [CrossRef]
- Yokosaki, Y.; Tanaka, K.; Higashikawa, F.; Yamashita, K.; Eboshida, A. Distinct structural requirements for binding of the integrins alphavbeta6, alphavbeta3, alphavbeta5, alpha5beta1 and alpha9beta1 to osteopontin. Matrix Biol. 2005, 24, 418–427. [Google Scholar] [CrossRef]
- Denda, S.; Reichardt, L.F.; Muller, U. Identification of osteopontin as a novel ligand for the integrin alpha8 beta1 and potential roles for this integrin-ligand interaction in kidney morphogenesis. Mol. Biol. Cell 1998, 9, 1425–1435. [Google Scholar] [CrossRef]
- Liaw, L.; Skinner, M.P.; Raines, E.W.; Ross, R.; Cheresh, D.A.; Schwartz, S.M.; Giachelli, C.M. The adhesive and migratory effects of osteopontin are mediated via distinct cell surface integrins. Role of alpha v beta 3 in smooth muscle cell migration to osteopontin in vitro. J. Clin. Investig. 1995, 95, 713–724. [Google Scholar] [CrossRef]
- O’Regan, A.; Berman, J.S. Osteopontin: A key cytokine in cell-mediated and granulomatous inflammation. Int. J. Exp. Pathol. 2000, 81, 373–390. [Google Scholar] [CrossRef] [PubMed]
- Liaw, L.; Lindner, V.; Schwartz, S.M.; Chambers, A.F.; Giachelli, C.M. Osteopontin and beta 3 integrin are coordinately expressed in regenerating endothelium in vivo and stimulate Arg-Gly-Asp-dependent endothelial migration in vitro. Circ. Res. 1995, 77, 665–672. [Google Scholar] [CrossRef]
- Qu, H.; Brown, L.F.; Senger, D.R.; Geng, L.L.; Dvorak, H.F.; Dvorak, A.M. Ultrastructural immunogold localization of osteopontin in human gallbladder epithelial cells. J. Histochem. Cytochem. 1994, 42, 351–361. [Google Scholar] [CrossRef]
- O’Brien, E.R.; Garvin, M.R.; Stewart, D.K.; Hinohara, T.; Simpson, J.B.; Schwartz, S.M.; Giachelli, C.M. Osteopontin is synthesized by macrophage, smooth muscle, and endothelial cells in primary and restenotic human coronary atherosclerotic plaques. Arterioscler. Thromb. 1994, 14, 1648–1656. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Pimental, D.R.; Lohan, S.; Vasertriger, A.; Pligavko, C.; Colucci, W.S.; Singh, K. Regulation of angiotensin II-stimulated osteopontin expression in cardiac microvascular endothelial cells: Role of p42/44 mitogen-activated protein kinase and reactive oxygen species. J. Cell. Physiol. 2001, 188, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Golledge, J.; McCann, M.; Mangan, S.; Lam, A.; Karan, M. Osteoprotegerin and osteopontin are expressed at high concentrations within symptomatic carotid atherosclerosis. Stroke 2004, 35, 1636–1641. [Google Scholar] [CrossRef]
- Gravallese, E.M. Osteopontin: A bridge between bone and the immune system. J. Clin. Investig. 2003, 112, 147–149. [Google Scholar] [CrossRef] [PubMed]
- Ashkar, S.; Weber, G.F.; Panoutsakopoulou, V.; Sanchirico, M.E.; Jansson, M.; Zawaideh, S.; Rittling, S.R.; Denhardt, D.T.; Glimcher, M.J.; Cantor, H. Eta-1 (osteopontin): An early component of type-1 (cell-mediated) immunity. Science 2000, 287, 860–864. [Google Scholar] [CrossRef]
- Kadoglou, N.P.E.; Khattab, E.; Velidakis, N.; Gkougkoudi, E. The Role of Osteopontin in Atherosclerosis and Its Clinical Manifestations (Atherosclerotic Cardiovascular Diseases)-A Narrative Review. Biomedicines 2023, 11, 3178. [Google Scholar] [CrossRef]
- Moschetta, D.; Di Minno, M.N.D.; Porro, B.; Perrucci, G.L.; Valerio, V.; Alfieri, V.; Massaiu, I.; Orekhov, A.N.; Di Minno, A.; Songia, P.; et al. Relationship Between Plasma Osteopontin and Arginine Pathway Metabolites in Patients With Overt Coronary Artery Disease. Front. Physiol. 2020, 11, 982. [Google Scholar] [CrossRef]
- Isoda, K.; Kamezawa, Y.; Ayaori, M.; Kusuhara, M.; Tada, N.; Ohsuzu, F. Osteopontin transgenic mice fed a high-cholesterol diet develop early fatty-streak lesions. Circulation 2003, 107, 679–681. [Google Scholar] [CrossRef]
- Chiba, S.; Okamoto, H.; Kon, S.; Kimura, C.; Murakami, M.; Inobe, M.; Matsui, Y.; Sugawara, T.; Shimizu, T.; Uede, T.; et al. Development of atherosclerosis in osteopontin transgenic mice. Heart Vessels 2002, 16, 111–117. [Google Scholar] [CrossRef]
- Kale, S.; Raja, R.; Thorat, D.; Soundararajan, G.; Patil, T.V.; Kundu, G.C. Osteopontin signaling upregulates cyclooxygenase-2 expression in tumor-associated macrophages leading to enhanced angiogenesis and melanoma growth via alpha9beta1 integrin. Oncogene 2014, 33, 2295–2306. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Peng, L.; Fan, K.; Wang, H.; Wei, R.; Ji, G.; Cai, J.; Lu, B.; Li, B.; Zhang, D.; et al. Osteopontin induces angiogenesis through activation of PI3K/AKT and ERK1/2 in endothelial cells. Oncogene 2009, 28, 3412–3422. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Suzuki, K.; Goldberg, H.A.; Rittling, S.R.; Denhardt, D.T.; McCulloch, C.A.; Sodek, J. Osteopontin modulates CD44-dependent chemotaxis of peritoneal macrophages through G-protein-coupled receptors: Evidence of a role for an intracellular form of osteopontin. J. Cell. Physiol. 2004, 198, 155–167. [Google Scholar] [CrossRef]
- Wang, Y.; Yan, W.; Lu, X.; Qian, C.; Zhang, J.; Li, P.; Shi, L.; Zhao, P.; Fu, Z.; Pu, P.; et al. Overexpression of osteopontin induces angiogenesis of endothelial progenitor cells via the avbeta3/PI3K/AKT/eNOS/NO signaling pathway in glioma cells. Eur. J. Cell Biol. 2011, 90, 642–648. [Google Scholar] [CrossRef]
- Scatena, M.; Almeida, M.; Chaisson, M.L.; Fausto, N.; Nicosia, R.F.; Giachelli, C.M. NF-kappaB mediates alphavbeta3 integrin-induced endothelial cell survival. J. Cell Biol. 1998, 141, 1083–1093. [Google Scholar] [CrossRef]
- Raja, R.; Kale, S.; Thorat, D.; Soundararajan, G.; Lohite, K.; Mane, A.; Karnik, S.; Kundu, G.C. Hypoxia-driven osteopontin contributes to breast tumor growth through modulation of HIF1alpha-mediated VEGF-dependent angiogenesis. Oncogene 2014, 33, 2053–2064. [Google Scholar] [CrossRef] [PubMed]
- Lyle, A.N.; Remus, E.W.; Fan, A.E.; Lassegue, B.; Walter, G.A.; Kiyosue, A.; Griendling, K.K.; Taylor, W.R. Hydrogen peroxide regulates osteopontin expression through activation of transcriptional and translational pathways. J. Biol. Chem. 2014, 289, 275–285. [Google Scholar] [CrossRef]
- Lyle, A.N.; Joseph, G.; Fan, A.E.; Weiss, D.; Landazuri, N.; Taylor, W.R. Reactive oxygen species regulate osteopontin expression in a murine model of postischemic neovascularization. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 1383–1391. [Google Scholar] [CrossRef]
- Okumura, N.; Nakamura, T.; Kay, E.P.; Nakahara, M.; Kinoshita, S.; Koizumi, N. R-spondin1 regulates cell proliferation of corneal endothelial cells via the Wnt3a/beta-catenin pathway. Invest. Ophthalmol. Vis. Sci. 2014, 55, 6861–6869. [Google Scholar] [CrossRef]
- Yoon, J.K.; Lee, J.S. Cellular signaling and biological functions of R-spondins. Cell Signal 2012, 24, 369–377. [Google Scholar] [CrossRef]
- Chen, P.H.; Chen, X.; Lin, Z.; Fang, D.; He, X. The structural basis of R-spondin recognition by LGR5 and RNF43. Genes. Dev. 2013, 27, 1345–1350. [Google Scholar] [CrossRef]
- Kim, K.A.; Wagle, M.; Tran, K.; Zhan, X.; Dixon, M.A.; Liu, S.; Gros, D.; Korver, W.; Yonkovich, S.; Tomasevic, N.; et al. R-Spondin family members regulate the Wnt pathway by a common mechanism. Mol. Biol. Cell 2008, 19, 2588–2596. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.R.; Yoon, J.K. The R-spondin family of proteins: Emerging regulators of WNT signaling. Int. J. Biochem. Cell Biol. 2012, 44, 2278–2287. [Google Scholar] [CrossRef] [PubMed]
- Nam, J.S.; Turcotte, T.J.; Smith, P.F.; Choi, S.; Yoon, J.K. Mouse cristin/R-spondin family proteins are novel ligands for the Frizzled 8 and LRP6 receptors and activate beta-catenin-dependent gene expression. J. Biol. Chem. 2006, 281, 13247–13257. [Google Scholar] [CrossRef]
- de Lau, W.B.; Snel, B.; Clevers, H.C. The R-spondin protein family. Genome Biol. 2012, 13, 242. [Google Scholar] [CrossRef]
- Zerlin, M.; Julius, M.A.; Kitajewski, J. Wnt/Frizzled signaling in angiogenesis. Angiogenesis 2008, 11, 63–69. [Google Scholar] [CrossRef]
- Breton-Romero, R.; Feng, B.; Holbrook, M.; Farb, M.G.; Fetterman, J.L.; Linder, E.A.; Berk, B.D.; Masaki, N.; Weisbrod, R.M.; Inagaki, E.; et al. Endothelial Dysfunction in Human Diabetes Is Mediated by Wnt5a-JNK Signaling. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 561–569. [Google Scholar] [CrossRef]
- Kim, J.; Kim, J.; Kim, D.W.; Ha, Y.; Ihm, M.H.; Kim, H.; Song, K.; Lee, I. Wnt5a induces endothelial inflammation via beta-catenin-independent signaling. J. Immunol. 2010, 185, 1274–1282. [Google Scholar] [CrossRef] [PubMed]
- Skaria, T.; Burgener, J.; Bachli, E.; Schoedon, G. IL-4 Causes Hyperpermeability of Vascular Endothelial Cells through Wnt5A Signaling. PLoS ONE 2016, 11, e0156002. [Google Scholar] [CrossRef]
- Wright, M.; Aikawa, M.; Szeto, W.; Papkoff, J. Identification of a Wnt-responsive signal transduction pathway in primary endothelial cells. Biochem. Biophys. Res. Commun. 1999, 263, 384–388. [Google Scholar] [CrossRef]
- Masckauchan, T.N.; Shawber, C.J.; Funahashi, Y.; Li, C.M.; Kitajewski, J. Wnt/beta-catenin signaling induces proliferation, survival and interleukin-8 in human endothelial cells. Angiogenesis 2005, 8, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Gore, A.V.; Swift, M.R.; Cha, Y.R.; Lo, B.; McKinney, M.C.; Li, W.; Castranova, D.; Davis, A.; Mukouyama, Y.S.; Weinstein, B.M. Rspo1/Wnt signaling promotes angiogenesis via Vegfc/Vegfr3. Development 2011, 138, 4875–4886. [Google Scholar] [CrossRef]
- Binnerts, M.E.; Kim, K.A.; Bright, J.M.; Patel, S.M.; Tran, K.; Zhou, M.; Leung, J.M.; Liu, Y.; Lomas, W.E., 3rd; Dixon, M.; et al. R-Spondin1 regulates Wnt signaling by inhibiting internalization of LRP6. Proc. Natl. Acad. Sci. USA 2007, 104, 14700–14705. [Google Scholar] [CrossRef]
- Caruso, M.; Ferranti, F.; Corano Scheri, K.; Dobrowolny, G.; Ciccarone, F.; Grammatico, P.; Catizone, A.; Ricci, G. R-spondin 1/dickkopf-1/beta-catenin machinery is involved in testicular embryonic angiogenesis. PLoS ONE 2015, 10, e0124213. [Google Scholar] [CrossRef]
- Jackson, S.R.; Costa, M.; Pastore, C.F.; Zhao, G.; Weiner, A.I.; Adams, S.; Palashikar, G.; Quansah, K.; Hankenson, K.; Herbert, D.R.; et al. R-spondin 2 mediates neutrophil egress into the alveolar space through increased lung permeability. BMC Res. Notes 2020, 13, 54. [Google Scholar] [CrossRef] [PubMed]
- Carmon, K.S.; Gong, X.; Yi, J.; Thomas, A.; Liu, Q. RSPO-LGR4 functions via IQGAP1 to potentiate Wnt signaling. Proc. Natl. Acad. Sci. USA 2014, 111, E1221–E1229. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Zhao, Y.; Pei, Y.; Lian, F.; Lin, H. Role of the NF-kB signalling pathway in heterotopic ossification: Biological and therapeutic significance. Cell Commun. Signal 2024, 22, 159. [Google Scholar] [CrossRef]
- Tachibana, N.; Chijimatsu, R.; Okada, H.; Oichi, T.; Taniguchi, Y.; Maenohara, Y.; Miyahara, J.; Ishikura, H.; Iwanaga, Y.; Arino, Y.; et al. RSPO2 defines a distinct undifferentiated progenitor in the tendon/ligament and suppresses ectopic ossification. Sci. Adv. 2022, 8, eabn2138. [Google Scholar] [CrossRef]
- Kempe, S.; Kestler, H.; Lasar, A.; Wirth, T. NF-kappaB controls the global pro-inflammatory response in endothelial cells: Evidence for the regulation of a pro-atherogenic program. Nucleic Acids Res. 2005, 33, 5308–5319. [Google Scholar] [CrossRef] [PubMed]
- Aithabathula, R.V.; Kathuria, I.; Pervaiz, N.; Sharma, B.K.; Samake, T.I.; Ofosu-Boateng, M.; Gebreyesus, L.H.; Gyamfi, M.A.; Sprague, C.; Stayton, A.; et al. R-spondin 2 suppresses hepatic steatosis via activation of AMPK-ACC signaling. JHEP Rep. 2025, 101551, in press. [Google Scholar] [CrossRef]
- Skaria, T.; Bachli, E.; Schoedon, G. RSPO3 impairs barrier function of human vascular endothelial monolayers and synergizes with pro-inflammatory IL-1. Mol. Med. 2018, 24, 45. [Google Scholar] [CrossRef]
- Matsumoto, K.I.; Aoki, H. The Roles of Tenascins in Cardiovascular, Inflammatory, and Heritable Connective Tissue Diseases. Front. Immunol. 2020, 11, 609752. [Google Scholar] [CrossRef]
- Hashimoto, K.; Kajitani, N.; Miyamoto, Y.; Matsumoto, K.I. Wound healing-related properties detected in an experimental model with a collagen gel contraction assay are affected in the absence of tenascin-X. Exp. Cell Res. 2018, 363, 102–113. [Google Scholar] [CrossRef]
- Egging, D.; van Vlijmen-Willems, I.; van Tongeren, T.; Schalkwijk, J.; Peeters, A. Wound healing in tenascin-X deficient mice suggests that tenascin-X is involved in matrix maturation rather than matrix deposition. Connect. Tissue Res. 2007, 48, 93–98. [Google Scholar] [CrossRef]
- Rathjen, F.G.; Hodge, R. Early Days of Tenascin-R Research: Two Approaches Discovered and Shed Light on Tenascin-R. Front. Immunol. 2020, 11, 612482. [Google Scholar] [CrossRef]
- David, L.S.; Schachner, M.; Saghatelyan, A. The extracellular matrix glycoprotein tenascin-R affects adult but not developmental neurogenesis in the olfactory bulb. J. Neurosci. 2013, 33, 10324–10339. [Google Scholar] [CrossRef] [PubMed]
- Saghatelyan, A.; de Chevigny, A.; Schachner, M.; Lledo, P.M. Tenascin-R mediates activity-dependent recruitment of neuroblasts in the adult mouse forebrain. Nat. Neurosci. 2004, 7, 347–356. [Google Scholar] [CrossRef]
- Xu, J.C.; Xiao, M.F.; Jakovcevski, I.; Sivukhina, E.; Hargus, G.; Cui, Y.F.; Irintchev, A.; Schachner, M.; Bernreuther, C. The extracellular matrix glycoprotein tenascin-R regulates neurogenesis during development and in the adult dentate gyrus of mice. J. Cell Sci. 2014, 127, 641–652. [Google Scholar] [CrossRef] [PubMed]
- Hargus, G.; Cui, Y.; Schmid, J.S.; Xu, J.; Glatzel, M.; Schachner, M.; Bernreuther, C. Tenascin-R promotes neuronal differentiation of embryonic stem cells and recruitment of host-derived neural precursor cells after excitotoxic lesion of the mouse striatum. Stem Cells 2008, 26, 1973–1984. [Google Scholar] [CrossRef] [PubMed]
- Chiovaro, F.; Chiquet-Ehrismann, R.; Chiquet, M. Transcriptional regulation of tenascin genes. Cell Adh Migr. 2015, 9, 34–47. [Google Scholar] [CrossRef]
- Alcaraz, L.B.; Exposito, J.Y.; Chuvin, N.; Pommier, R.M.; Cluzel, C.; Martel, S.; Sentis, S.; Bartholin, L.; Lethias, C.; Valcourt, U. Tenascin-X promotes epithelial-to-mesenchymal transition by activating latent TGF-beta. J. Cell Biol. 2014, 205, 409–428. [Google Scholar] [CrossRef]
- Martina, E.; Degen, M.; Ruegg, C.; Merlo, A.; Lino, M.M.; Chiquet-Ehrismann, R.; Brellier, F. Tenascin-W is a specific marker of glioma-associated blood vessels and stimulates angiogenesis in vitro. FASEB J. 2010, 24, 778–787. [Google Scholar] [CrossRef]
- Midwood, K.S.; Hussenet, T.; Langlois, B.; Orend, G. Advances in tenascin-C biology. Cell Mol. Life Sci. 2011, 68, 3175–3199. [Google Scholar] [CrossRef]
- Midwood, K.; Sacre, S.; Piccinini, A.M.; Inglis, J.; Trebaul, A.; Chan, E.; Drexler, S.; Sofat, N.; Kashiwagi, M.; Orend, G.; et al. Tenascin-C is an endogenous activator of Toll-like receptor 4 that is essential for maintaining inflammation in arthritic joint disease. Nat. Med. 2009, 15, 774–780. [Google Scholar] [CrossRef]
- Willems, I.E.; Arends, J.W.; Daemen, M.J. Tenascin and fibronectin expression in healing human myocardial scars. J. Pathol. 1996, 179, 321–325. [Google Scholar] [CrossRef]
- Imanaka-Yoshida, K.; Yoshida, T.; Miyagawa-Tomita, S. Tenascin-C in development and disease of blood vessels. Anat. Rec. 2014, 297, 1747–1757. [Google Scholar] [CrossRef]
- Mehri, H.; Aslanabadi, N.; Nourazarian, A.; Shademan, B.; Khaki-Khatibi, F. Evaluation of the serum levels of Mannose binding lectin-2, tenascin-C, and total antioxidant capacity in patients with coronary artery disease. J. Clin. Lab. Anal. 2021, 35, e23967. [Google Scholar] [CrossRef]
- Liabeuf, S.; Barreto, D.V.; Kretschmer, A.; Barreto, F.C.; Renard, C.; Andrejak, M.; Choukroun, G.; Massy, Z. High circulating levels of large splice variants of tenascin-C is associated with mortality and cardiovascular disease in chronic kidney disease patients. Atherosclerosis 2011, 215, 116–124. [Google Scholar] [CrossRef]
- Gao, W.; Li, J.; Ni, H.; Shi, H.; Qi, Z.; Zhu, S.; Hao, C.; Xie, Q.; Luo, X.; Xie, K. Tenascin C: A Potential Biomarker for Predicting the Severity of Coronary Atherosclerosis. J. Atheroscler. Thromb. 2019, 26, 31–38. [Google Scholar] [CrossRef]
- Gholipour, A.; Shakerian, F.; Zahedmehr, A.; Oveisee, M.; Maleki, M.; Mowla, S.J.; Malakootian, M. Tenascin-C as a noninvasive biomarker of coronary artery disease. Mol. Biol. Rep. 2022, 49, 9267–9273. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, J.; Huang, J.W.; Song, J.C.; Ma, Z.L.; Shi, H.B. In vivo MRI detection of atherosclerosis in ApoE-deficient mice by using tenascin-C-targeted USPIO. Acta Radiol. 2018, 59, 1431–1437. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wei, Q.; Han, L.; Cao, K.; Lan, T.; Xu, Z.; Wang, Y.; Gao, Y.; Xue, J.; Shan, F.; et al. Tenascin-c renders a proangiogenic phenotype in macrophage via annexin II. J. Cell. Mol. Med. 2018, 22, 429–438. [Google Scholar] [CrossRef]
- Hedin, U.; Holm, J.; Hansson, G.K. Induction of tenascin in rat arterial injury. Relationship to altered smooth muscle cell phenotype. Am. J. Pathol. 1991, 139, 649–656. [Google Scholar] [PubMed]
- Rupp, T.; Langlois, B.; Koczorowska, M.M.; Radwanska, A.; Sun, Z.; Hussenet, T.; Lefebvre, O.; Murdamoothoo, D.; Arnold, C.; Klein, A.; et al. Tenascin-C Orchestrates Glioblastoma Angiogenesis by Modulation of Pro- and Anti-angiogenic Signaling. Cell Rep. 2016, 17, 2607–2619. [Google Scholar] [CrossRef]
- Saupe, F.; Schwenzer, A.; Jia, Y.; Gasser, I.; Spenle, C.; Langlois, B.; Kammerer, M.; Lefebvre, O.; Hlushchuk, R.; Rupp, T.; et al. Tenascin-C downregulates wnt inhibitor dickkopf-1, promoting tumorigenesis in a neuroendocrine tumor model. Cell Rep. 2013, 5, 482–492. [Google Scholar] [CrossRef]
- Luo, H.; Wang, J.; Qiao, C.; Zhang, X.; Zhang, W.; Ma, N. ATF3 Inhibits Tenascin-C-induced Foam Cell Formation in LPS-Stimulated THP-1 Macrophages by Suppressing TLR-4. J. Atheroscler. Thromb. 2015, 22, 1214–1223. [Google Scholar] [CrossRef]
- Liu, R.; He, Y.; Li, B.; Liu, J.; Ren, Y.; Han, W.; Wang, X.; Zhang, L. Tenascin-C produced by oxidized LDL-stimulated macrophages increases foam cell formation through Toll-like receptor-4. Mol. Cells 2012, 34, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Hongu, T.; Pein, M.; Insua-Rodriguez, J.; Gutjahr, E.; Mattavelli, G.; Meier, J.; Decker, K.; Descot, A.; Bozza, M.; Harbottle, R.; et al. Perivascular tenascin C triggers sequential activation of macrophages and endothelial cells to generate a pro-metastatic vascular niche in the lungs. Nat. Cancer 2022, 3, 486–504. [Google Scholar] [CrossRef]
- Radwanska, A.; Grall, D.; Schaub, S.; Divonne, S.B.F.; Ciais, D.; Rekima, S.; Rupp, T.; Sudaka, A.; Orend, G.; Van Obberghen-Schilling, E. Counterbalancing anti-adhesive effects of Tenascin-C through fibronectin expression in endothelial cells. Sci. Rep. 2017, 7, 12762. [Google Scholar] [CrossRef]
- Matsui, K.; Torii, S.; Hara, S.; Maruyama, K.; Arai, T.; Imanaka-Yoshida, K. Tenascin-C in Tissue Repair after Myocardial Infarction in Humans. Int. J. Mol. Sci. 2023, 24, 10184. [Google Scholar] [CrossRef] [PubMed]
- Okada, T.; Suzuki, H. The Role of Tenascin-C in Tissue Injury and Repair After Stroke. Front. Immunol. 2020, 11, 607587. [Google Scholar] [CrossRef]
- Babic, A.M.; Chen, C.C.; Lau, L.F. Fisp12/mouse connective tissue growth factor mediates endothelial cell adhesion and migration through integrin alphavbeta3, promotes endothelial cell survival, and induces angiogenesis in vivo. Mol. Cell. Biol. 1999, 19, 2958–2966. [Google Scholar] [CrossRef] [PubMed]
- Zaykov, V.; Chaqour, B. The CCN2/CTGF interactome: An approach to understanding the versatility of CCN2/CTGF molecular activities. J. Cell Commun. Signal 2021, 15, 567–580. [Google Scholar] [CrossRef]
- Wang, Y.K.; Weng, H.K.; Mo, F.E. The regulation and functions of the matricellular CCN proteins induced by shear stress. J. Cell Commun. Signal 2023, 17, 361–370. [Google Scholar] [CrossRef]
- Luan, Y.; Zhang, H.; Ma, K.; Liu, Y.; Lu, H.; Chen, X.; Liu, Y.; Zhang, Z. CCN3/NOV Regulates Proliferation and Neuronal Differentiation in Mouse Hippocampal Neural Stem Cells via the Activation of the Notch/PTEN/AKT Pathway. Int. J. Mol. Sci. 2023, 24, 10324. [Google Scholar] [CrossRef]
- Jun, J.I.; Lau, L.F. Taking aim at the extracellular matrix: CCN proteins as emerging therapeutic targets. Nat. Rev. Drug Discov. 2011, 10, 945–963. [Google Scholar] [CrossRef] [PubMed]
- Naughton, M.; Moffat, J.; Eleftheriadis, G.; de la Vega Gallardo, N.; Young, A.; Falconer, J.; Hawkins, K.; Pearson, B.; Perbal, B.; Hogan, A.; et al. CCN3 is dynamically regulated by treatment and disease state in multiple sclerosis. J. Neuroinflammation 2020, 17, 349. [Google Scholar] [CrossRef]
- Russo, J.W.; Castellot, J.J. CCN5: Biology and pathophysiology. J. Cell Commun. Signal 2010, 4, 119–130. [Google Scholar] [CrossRef] [PubMed]
- Karagiannis, E.D.; Popel, A.S. Peptides derived from type I thrombospondin repeat-containing proteins of the CCN family inhibit proliferation and migration of endothelial cells. Int. J. Biochem. Cell Biol. 2007, 39, 2314–2323. [Google Scholar] [CrossRef]
- Tanaka, I.; Morikawa, M.; Okuse, T.; Shirakawa, M.; Imai, K. Expression and regulation of WISP2 in rheumatoid arthritic synovium. Biochem. Biophys. Res. Commun. 2005, 334, 973–978. [Google Scholar] [CrossRef] [PubMed]
- Delmolino, L.M.; Stearns, N.A.; Castellot, J.J., Jr. COP-1, a member of the CCN family, is a heparin-induced growth arrest specific gene in vascular smooth muscle cells. J. Cell. Physiol. 2001, 188, 45–55. [Google Scholar] [CrossRef]
- Hilfiker, A.; Hilfiker-Kleiner, D.; Fuchs, M.; Kaminski, K.; Lichtenberg, A.; Rothkotter, H.J.; Schieffer, B.; Drexler, H. Expression of CYR61, an angiogenic immediate early gene, in arteriosclerosis and its regulation by angiotensin II. Circulation 2002, 106, 254–260. [Google Scholar] [CrossRef]
- Malik, A.R.; Liszewska, E.; Jaworski, J. Matricellular proteins of the Cyr61/CTGF/NOV (CCN) family and the nervous system. Front. Cell. Neurosci. 2015, 9, 237. [Google Scholar] [CrossRef]
- Zuo, G.W.; Kohls, C.D.; He, B.C.; Chen, L.; Zhang, W.; Shi, Q.; Zhang, B.Q.; Kang, Q.; Luo, J.; Luo, X.; et al. The CCN proteins: Important signaling mediators in stem cell differentiation and tumorigenesis. Histol. Histopathol. 2010, 25, 795–806. [Google Scholar] [CrossRef]
- Perbal, B.V.; Takigawa, M. CCN Proteins: A New Family of Cell Growth and Differentiation Regulators; London Imperial College Press: London, UK, 2005. [Google Scholar]
- Parisi, M.S.; Gazzerro, E.; Rydziel, S.; Canalis, E. Expression and regulation of CCN genes in murine osteoblasts. Bone 2006, 38, 671–677. [Google Scholar] [CrossRef]
- Lau, L.F.; Lam, S.C. The CCN family of angiogenic regulators: The integrin connection. Exp. Cell Res. 1999, 248, 44–57. [Google Scholar] [CrossRef] [PubMed]
- Hsu, P.L.; Chen, J.S.; Wang, C.Y.; Wu, H.L.; Mo, F.E. Shear-Induced CCN1 Promotes Atheroprone Endothelial Phenotypes and Atherosclerosis. Circulation 2019, 139, 2877–2891. [Google Scholar] [CrossRef]
- Su, B.C.; Hsu, P.L.; Mo, F.E. CCN1 triggers adaptive autophagy in cardiomyocytes to curb its apoptotic activities. J. Cell Commun. Signal 2020, 14, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Kubota, S.; Takigawa, M. CCN family proteins and angiogenesis: From embryo to adulthood. Angiogenesis 2007, 10, 1–11. [Google Scholar] [CrossRef]
- Park, M.H.; Kim, A.K.; Manandhar, S.; Oh, S.Y.; Jang, G.H.; Kang, L.; Lee, D.W.; Hyeon, D.Y.; Lee, S.H.; Lee, H.E.; et al. CCN1 interlinks integrin and hippo pathway to autoregulate tip cell activity. Elife 2019, 8, e46012. [Google Scholar] [CrossRef]
- Niu, C.C.; Zhao, C.; Yang, Z.; Zhang, X.L.; Pan, J.; Zhao, C.; Si, W.K. Inhibiting CCN1 blocks AML cell growth by disrupting the MEK/ERK pathway. Cancer Cell Int. 2014, 14, 74. [Google Scholar] [CrossRef]
- Xie, D.; Yin, D.; Tong, X.; O’Kelly, J.; Mori, A.; Miller, C.; Black, K.; Gui, D.; Said, J.W.; Koeffler, H.P. Cyr61 is overexpressed in gliomas and involved in integrin-linked kinase-mediated Akt and beta-catenin-TCF/Lef signaling pathways. Cancer Res. 2004, 64, 1987–1996. [Google Scholar] [CrossRef] [PubMed]
- Yeger, H.; Perbal, B. The CCN axis in cancer development and progression. J. Cell Commun. Signal 2021, 15, 491–517. [Google Scholar] [CrossRef]
- Li, Z.Q.; Ding, W.; Sun, S.J.; Li, J.; Pan, J.; Zhao, C.; Wu, W.R.; Si, W.K. Cyr61/CCN1 is regulated by Wnt/beta-catenin signaling and plays an important role in the progression of hepatocellular carcinoma. PLoS ONE 2012, 7, e35754. [Google Scholar] [CrossRef]
- Li, H.; Li, T.; Wang, H.; He, X.; Li, Y.; Wen, S.; Peng, R.; Nie, Y.; Lu, Y.; Yang, H.; et al. Diabetes Promotes Retinal Vascular Endothelial Cell Injury by Inducing CCN1 Expression. Front. Cardiovasc. Med. 2021, 8, 689318. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Qian, Y.; Li, H.; Wang, T.; Jiang, Q.; Wang, Y.; Zhu, Y.; Li, S.; He, X.; Shi, G.; et al. Cellular communication network factor 1 promotes retinal leakage in diabetic retinopathy via inducing neutrophil stasis and neutrophil extracellular traps extrusion. Cell Commun. Signal 2024, 22, 275. [Google Scholar] [CrossRef] [PubMed]
- Bai, T.; Chen, C.C.; Lau, L.F. Matricellular protein CCN1 activates a proinflammatory genetic program in murine macrophages. J. Immunol. 2010, 184, 3223–3232. [Google Scholar] [CrossRef]
- Matsumae, H.; Yoshida, Y.; Ono, K.; Togi, K.; Inoue, K.; Furukawa, Y.; Nakashima, Y.; Kojima, Y.; Nobuyoshi, M.; Kita, T.; et al. CCN1 knockdown suppresses neointimal hyperplasia in a rat artery balloon injury model. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 1077–1083. [Google Scholar] [CrossRef]
- Lobel, M.; Bauer, S.; Meisel, C.; Eisenreich, A.; Kudernatsch, R.; Tank, J.; Rauch, U.; Kuhl, U.; Schultheiss, H.P.; Volk, H.D.; et al. CCN1: A novel inflammation-regulated biphasic immune cell migration modulator. Cell Mol. Life Sci. 2012, 69, 3101–3113. [Google Scholar] [CrossRef] [PubMed]
- Jorgensen, L.H.; Petersson, S.J.; Sellathurai, J.; Andersen, D.C.; Thayssen, S.; Sant, D.J.; Jensen, C.H.; Schroder, H.D. Secreted protein acidic and rich in cysteine (SPARC) in human skeletal muscle. J. Histochem. Cytochem. 2009, 57, 29–39. [Google Scholar] [CrossRef]
- Parfenova, O.K.; Kukes, V.G.; Grishin, D.V. Follistatin-Like Proteins: Structure, Functions and Biomedical Importance. Biomedicines 2021, 9, 999. [Google Scholar] [CrossRef]
- Sage, H.; Vernon, R.B.; Funk, S.E.; Everitt, E.A.; Angello, J. SPARC, a secreted protein associated with cellular proliferation, inhibits cell spreading in vitro and exhibits Ca+2-dependent binding to the extracellular matrix. J. Cell Biol. 1989, 109, 341–356. [Google Scholar] [CrossRef]
- Chandrasekhar, S.; Harvey, A.K.; Johnson, M.G.; Becker, G.W. Osteonectin/SPARC is a product of articular chondrocytes/cartilage and is regulated by cytokines and growth factors. Biochim. Biophys. Acta 1994, 1221, 7–14. [Google Scholar] [CrossRef]
- Ham, S.M.; Song, M.J.; Yoon, H.S.; Lee, D.H.; Chung, J.H.; Lee, S.T. SPARC Is Highly Expressed in Young Skin and Promotes Extracellular Matrix Integrity in Fibroblasts via the TGF-beta Signaling Pathway. Int. J. Mol. Sci. 2023, 24, 12179. [Google Scholar] [CrossRef]
- Hu, S.; Zhang, X.; Ding, Y.; Liu, X.; Xia, R.; Wang, X. Inhibition of SPARC signal by aerobic exercise to ameliorate atherosclerosis. Int. Immunopharmacol. 2024, 132, 111856. [Google Scholar] [CrossRef]
- Bradshaw, A.D.; Sage, E.H. SPARC, a matricellular protein that functions in cellular differentiation and tissue response to injury. J. Clin. Investig. 2001, 107, 1049–1054. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.L.; Sukkar, M.B. The SPARC protein: An overview of its role in lung cancer and pulmonary fibrosis and its potential role in chronic airways disease. Br. J. Pharmacol. 2017, 174, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Kelly, K.A.; Allport, J.R.; Yu, A.M.; Sinh, S.; Sage, E.H.; Gerszten, R.E.; Weissleder, R. SPARC is a VCAM-1 counter-ligand that mediates leukocyte transmigration. J. Leukoc. Biol. 2007, 81, 748–756. [Google Scholar] [CrossRef]
- Kupprion, C.; Motamed, K.; Sage, E.H. SPARC (BM-40, osteonectin) inhibits the mitogenic effect of vascular endothelial growth factor on microvascular endothelial cells. J. Biol. Chem. 1998, 273, 29635–29640. [Google Scholar] [CrossRef] [PubMed]
- Sage, H.; Decker, J.; Funk, S.; Chow, M. SPARC: A Ca2+-binding extracellular protein associated with endothelial cell injury and proliferation. J. Mol. Cell. Cardiol. 1989, 21 (Suppl. 1), 13–22. [Google Scholar] [CrossRef]
- Funk, S.E.; Sage, E.H. The Ca2(+)-binding glycoprotein SPARC modulates cell cycle progression in bovine aortic endothelial cells. Proc. Natl. Acad. Sci. USA 1991, 88, 2648–2652. [Google Scholar] [CrossRef]
- Raines, E.W.; Lane, T.F.; Iruela-Arispe, M.L.; Ross, R.; Sage, E.H. The extracellular glycoprotein SPARC interacts with platelet-derived growth factor (PDGF)-AB and -BB and inhibits the binding of PDGF to its receptors. Proc. Natl. Acad. Sci. USA 1992, 89, 1281–1285. [Google Scholar] [CrossRef]
- Goldblum, S.E.; Ding, X.; Funk, S.E.; Sage, E.H. SPARC (secreted protein acidic and rich in cysteine) regulates endothelial cell shape and barrier function. Proc. Natl. Acad. Sci. USA 1994, 91, 3448–3452. [Google Scholar] [CrossRef]
- Zhao, Y.; Yu, J.; Huang, A.; Yang, Q.; Li, G.; Yang, Y.; Chen, Y. ROS impairs tumor vasculature normalization through an endocytosis effect of caveolae on extracellular SPARC. Cancer Cell Int. 2023, 23, 152. [Google Scholar] [CrossRef]
- Alkabie, S.; Basivireddy, J.; Zhou, L.; Roskams, J.; Rieckmann, P.; Quandt, J.A. SPARC expression by cerebral microvascular endothelial cells in vitro and its influence on blood-brain barrier properties. J. Neuroinflammation 2016, 13, 225. [Google Scholar] [CrossRef]
- Rivera, L.B.; Brekken, R.A. SPARC promotes pericyte recruitment via inhibition of endoglin-dependent TGF-beta1 activity. J. Cell Biol. 2011, 193, 1305–1319. [Google Scholar] [CrossRef]
- Arnold, S.; Mira, E.; Muneer, S.; Korpanty, G.; Beck, A.W.; Holloway, S.E.; Manes, S.; Brekken, R.A. Forced expression of MMP9 rescues the loss of angiogenesis and abrogates metastasis of pancreatic tumors triggered by the absence of host SPARC. Exp. Biol. Med. 2008, 233, 860–873. [Google Scholar] [CrossRef]
- Francki, A.; Bradshaw, A.D.; Bassuk, J.A.; Howe, C.C.; Couser, W.G.; Sage, E.H. SPARC regulates the expression of collagen type I and transforming growth factor-beta1 in mesangial cells. J. Biol. Chem. 1999, 274, 32145–32152. [Google Scholar] [CrossRef] [PubMed]
- Jendraschak, E.; Sage, E.H. Regulation of angiogenesis by SPARC and angiostatin: Implications for tumor cell biology. Semin. Cancer Biol. 1996, 7, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Sage, E.H.; Reed, M.; Funk, S.E.; Truong, T.; Steadele, M.; Puolakkainen, P.; Maurice, D.H.; Bassuk, J.A. Cleavage of the matricellular protein SPARC by matrix metalloproteinase 3 produces polypeptides that influence angiogenesis. J. Biol. Chem. 2003, 278, 37849–37857. [Google Scholar] [CrossRef] [PubMed]
- Martel, C.; Li, W.; Fulp, B.; Platt, A.M.; Gautier, E.L.; Westerterp, M.; Bittman, R.; Tall, A.R.; Chen, S.H.; Thomas, M.J.; et al. Lymphatic vasculature mediates macrophage reverse cholesterol transport in mice. J. Clin. Investig. 2013, 123, 1571–1579. [Google Scholar] [CrossRef]
- Vuorio, T.; Nurmi, H.; Moulton, K.; Kurkipuro, J.; Robciuc, M.R.; Ohman, M.; Heinonen, S.E.; Samaranayake, H.; Heikura, T.; Alitalo, K.; et al. Lymphatic vessel insufficiency in hypercholesterolemic mice alters lipoprotein levels and promotes atherogenesis. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 1162–1170. [Google Scholar] [CrossRef]
- Kojima, Y.; Volkmer, J.P.; McKenna, K.; Civelek, M.; Lusis, A.J.; Miller, C.L.; Direnzo, D.; Nanda, V.; Ye, J.; Connolly, A.J.; et al. CD47-blocking antibodies restore phagocytosis and prevent atherosclerosis. Nature 2016, 536, 86–90. [Google Scholar] [CrossRef]
- Jarr, K.U.; Nakamoto, R.; Doan, B.H.; Kojima, Y.; Weissman, I.L.; Advani, R.H.; Iagaru, A.; Leeper, N.J. Effect of CD47 Blockade on Vascular Inflammation. N. Engl. J. Med. 2021, 384, 382–383. [Google Scholar] [CrossRef]
- Bouwstra, R.; van Meerten, T.; Bremer, E. CD47-SIRPalpha blocking-based immunotherapy: Current and prospective therapeutic strategies. Clin. Transl. Med. 2022, 12, e943. [Google Scholar] [CrossRef]
- Maute, R.; Xu, J.; Weissman, I.L. CD47-SIRPalpha-targeted therapeutics: Status and prospects. Immunooncol. Technol. 2022, 13, 100070. [Google Scholar] [CrossRef]
- Flores, A.M.; Hosseini-Nassab, N.; Jarr, K.U.; Ye, J.; Zhu, X.; Wirka, R.; Koh, A.L.; Tsantilas, P.; Wang, Y.; Nanda, V.; et al. Pro-efferocytic nanoparticles are specifically taken up by lesional macrophages and prevent atherosclerosis. Nat. Nanotechnol. 2020, 15, 154–161. [Google Scholar] [CrossRef]
- Bamezai, S.; Zhang, Y.; Kumari, M.; Lotfi, M.; Alsaigh, T.; Luo, L.; Kumar, G.S.; Wang, F.; Ye, J.; Puri, M.; et al. Pro-efferocytic nanotherapies reduce vascular inflammation without inducing anemia in a large animal model of atherosclerosis. Nat. Commun. 2024, 15, 8034. [Google Scholar] [CrossRef]
- Afroz, R.; Goodwin, J.E. Wnt Signaling in Atherosclerosis: Mechanisms to Therapeutic Implications. Biomedicines 2024, 12, 276. [Google Scholar] [CrossRef]
- Lang, F.; Li, Y.; Yao, R.; Jiang, M. Osteopontin in Chronic Inflammatory Diseases: Mechanisms, Biomarker Potential, and Therapeutic Strategies. Biology 2025, 14, 428. [Google Scholar] [CrossRef]
- Ge, Q.; Ruan, C.C.; Ma, Y.; Tang, X.F.; Wu, Q.H.; Wang, J.G.; Zhu, D.L.; Gao, P.J. Osteopontin regulates macrophage activation and osteoclast formation in hypertensive patients with vascular calcification. Sci. Rep. 2017, 7, 40253. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Zhang, Y.; Zhang, W.; Qin, C.; Zhu, Y.; Fang, Y.; Wang, Y.; Tang, C.; Cao, F. Osteopontin-Targeted and PPARdelta-Agonist-Loaded Nanoparticles Efficiently Reduce Atherosclerosis in Apolipoprotein E(-/-) Mice. ACS Omega 2022, 7, 28767–28778. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Wang, Y.; Ren, C.; Yu, S.; Wang, C.; Xing, J.; Xu, J.; Yan, S.; Zhang, T.; Li, Q.; et al. The role of TNC in atherosclerosis and drug development opportunities. Int. J. Biol. Sci. 2024, 20, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Kathuria, I.; Prasad, A.; Sharma, B.K.; Aithabathula, R.V.; Ofosu-Boateng, M.; Gyamfi, M.A.; Jiang, J.; Park, F.; Singh, U.P.; Singla, B. Nidogen 2 Overexpression Promotes Hepatosteatosis and Atherosclerosis. Int. J. Mol. Sci. 2024, 25, 12782. [Google Scholar] [CrossRef] [PubMed]
- Pervaiz, N.; Mehmood, R.; Aithabathula, R.V.; Kathuria, I.; Ahn, W.; Le, B.T.; Kim, K.S.; Singh, U.P.; Csanyi, G.; Singla, B. Smooth muscle cell-specific CD47 deletion suppresses atherosclerosis. Life Sci. 2025, 361, 123315. [Google Scholar] [CrossRef] [PubMed]
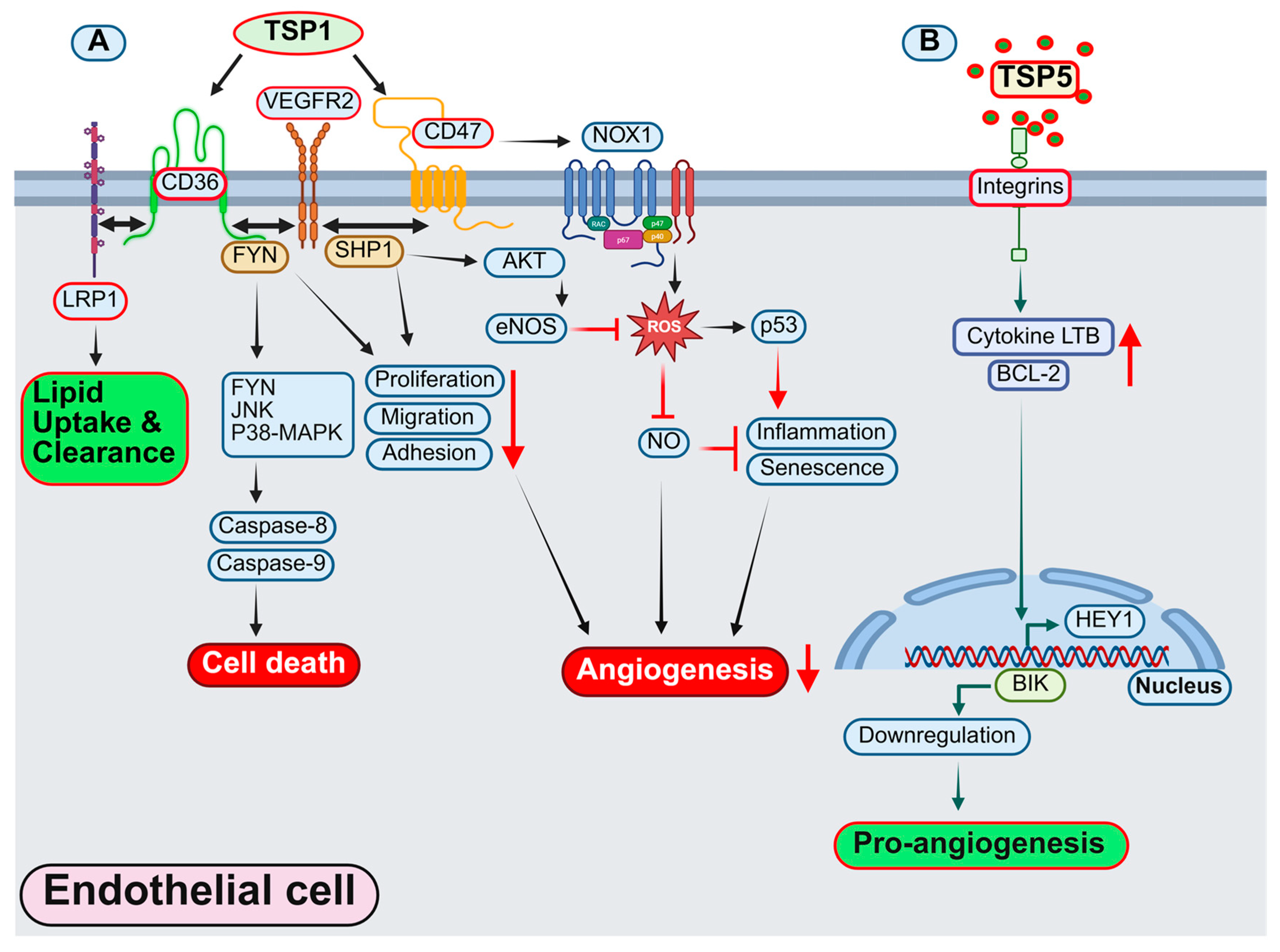

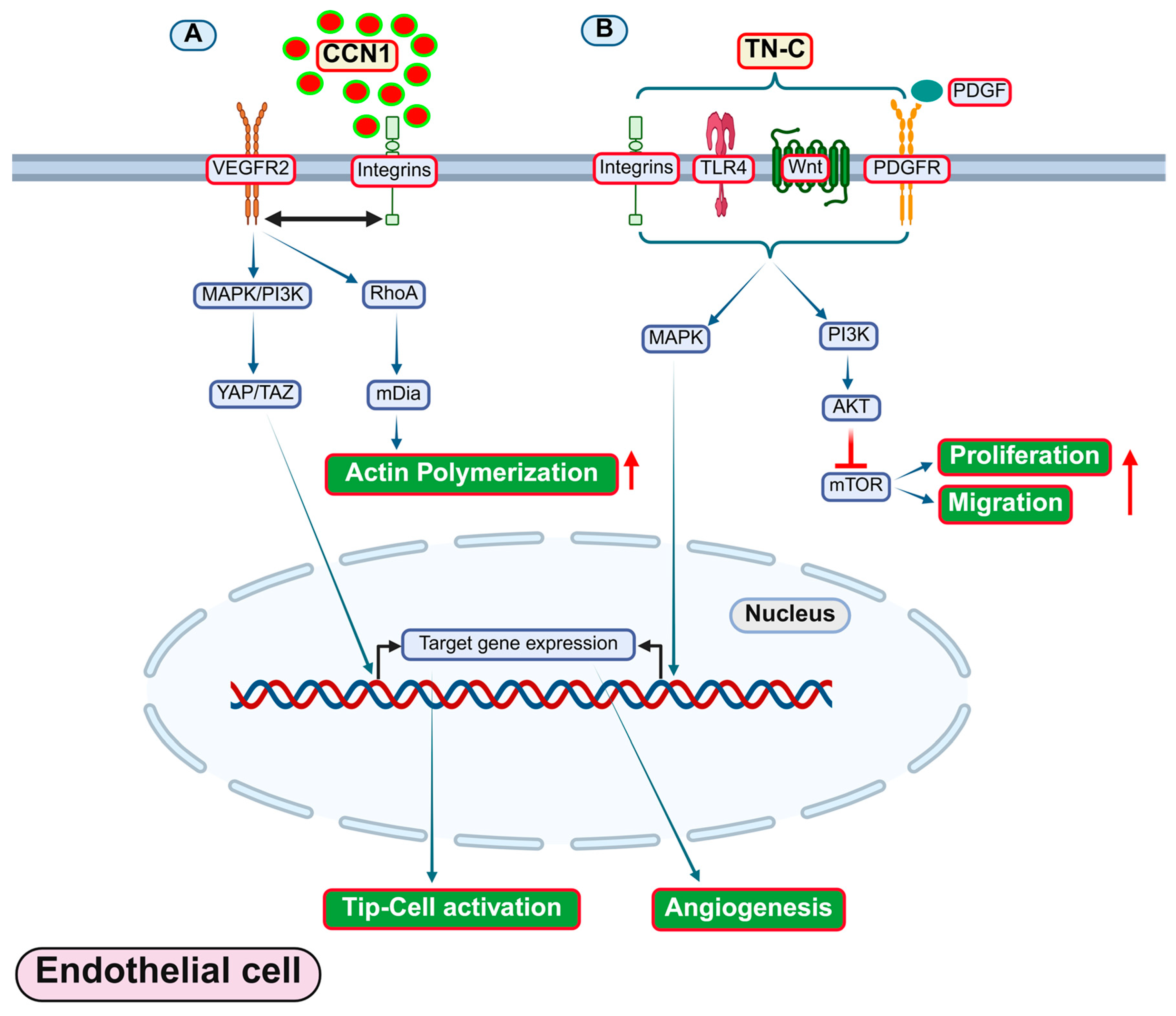
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aithabathula, R.V.; Kumar, S.; Singla, B. Role of Matricellular Proteins in Endothelial Cell Inflammation and Atherosclerosis. Antioxidants 2025, 14, 1338. https://doi.org/10.3390/antiox14111338
Aithabathula RV, Kumar S, Singla B. Role of Matricellular Proteins in Endothelial Cell Inflammation and Atherosclerosis. Antioxidants. 2025; 14(11):1338. https://doi.org/10.3390/antiox14111338
Chicago/Turabian StyleAithabathula, Ravi Varma, Santosh Kumar, and Bhupesh Singla. 2025. "Role of Matricellular Proteins in Endothelial Cell Inflammation and Atherosclerosis" Antioxidants 14, no. 11: 1338. https://doi.org/10.3390/antiox14111338
APA StyleAithabathula, R. V., Kumar, S., & Singla, B. (2025). Role of Matricellular Proteins in Endothelial Cell Inflammation and Atherosclerosis. Antioxidants, 14(11), 1338. https://doi.org/10.3390/antiox14111338








