Chamazulene Induces Metabolic Reprogramming and Mitigates Inflammation in Photoaged Skin: PPARα/γ as Potential Regulators
Abstract
1. Introduction
2. Materials and Methods
2.1. A 0.4% CHA Formulation Preparation
2.2. Animal Experiment and Treatment
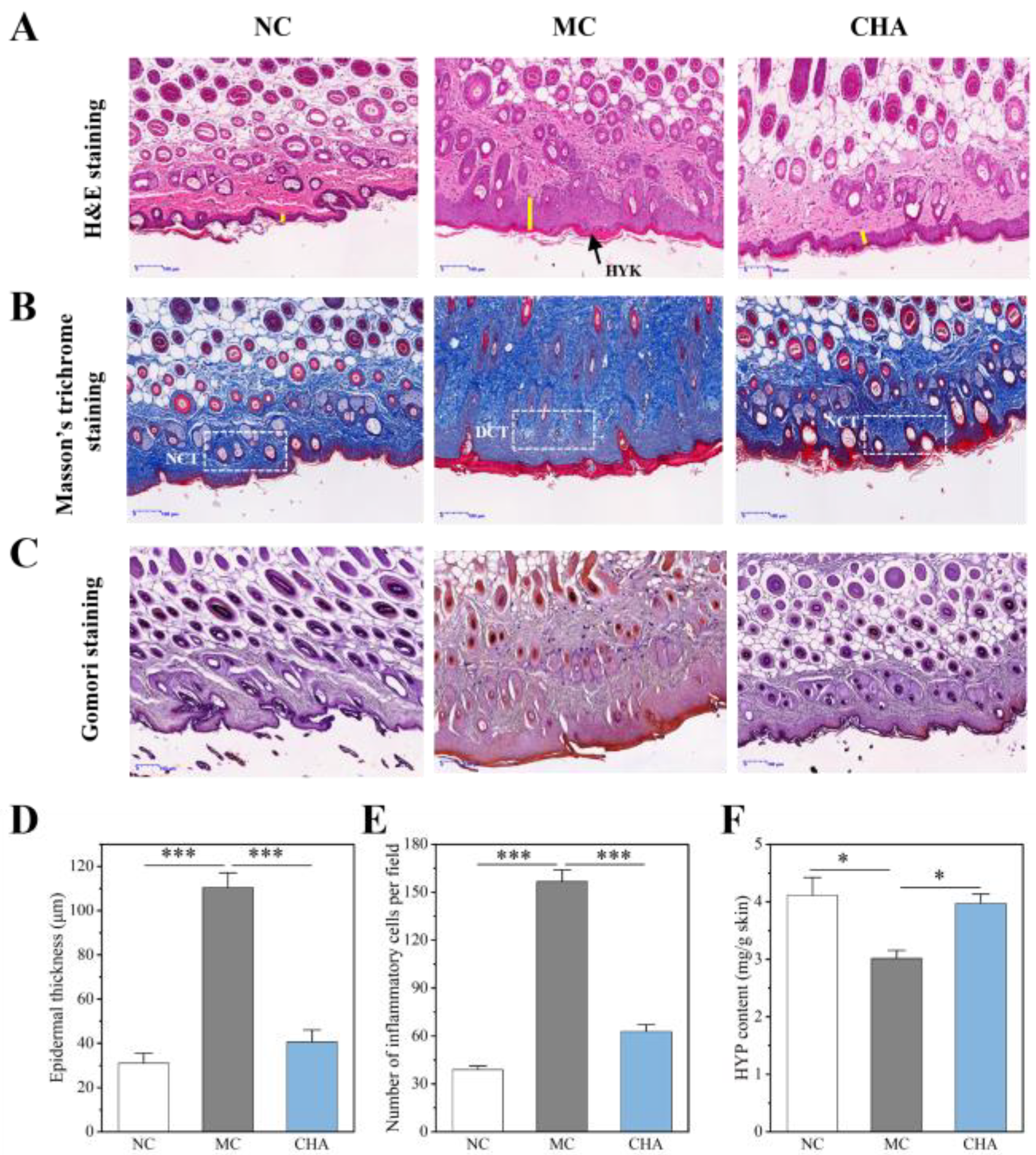
2.3. Histopathological Analysis
2.4. Determination of Hydroxyproline
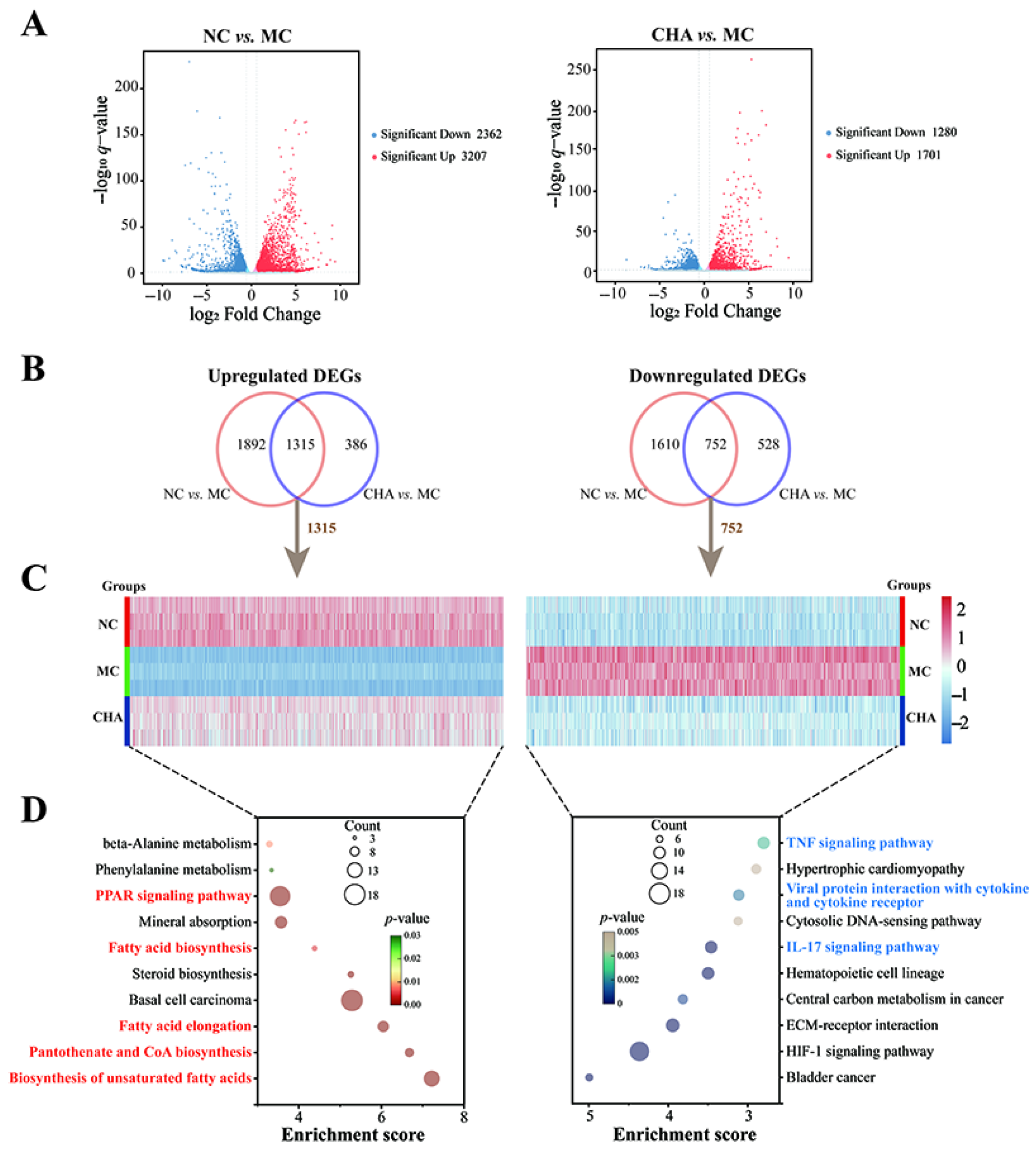
2.5. RNA Sequencing Analysis
2.6. Untargeted Metabolomic Analysis
2.6.1. Sample Preparation and Ultra-Performance Liquid Chromatography–Tandem Mass Spectrometric (UPLC-MS/MS) Analysis
2.6.2. Data Processing and Multivariate Analysis
2.7. Determination of Fatty Acid Composition by Gas Chromatography–Mass Spectrometry (GC-MS)
2.8. Quantitative Real-Time PCR (qRT-PCR) Analysis
2.9. Western Blotting
2.10. Statistical Analysis
3. Results
3.1. CHA Exhibited Anti-Photoaging Effect by Mitigating UVB-Induced Damage in Mouse Skin
3.2. CHA Induced Transcriptomic Changes in the Skin of Photoaged Mouse Model
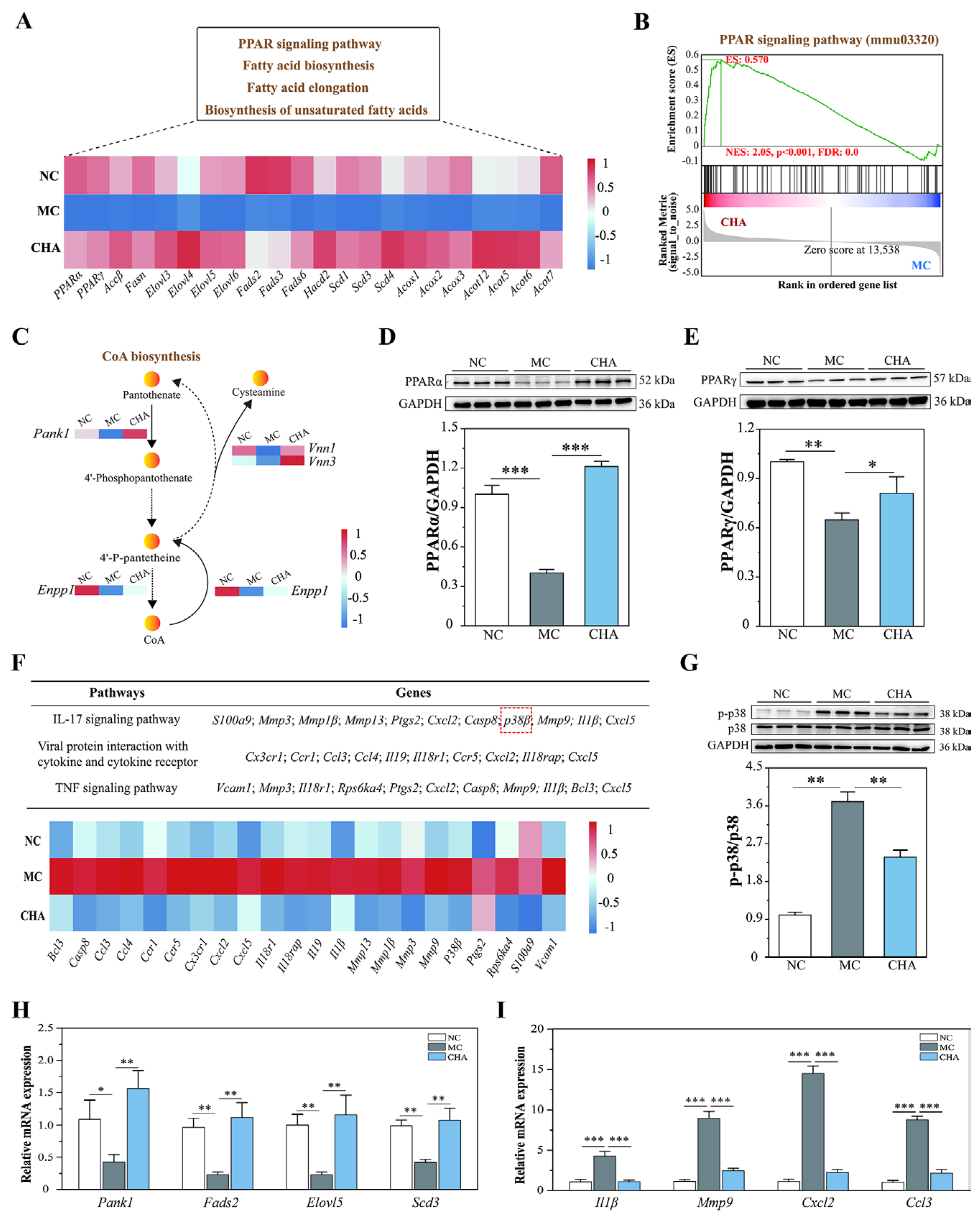
3.3. CHA Treatment Enhanced Fatty Acid Metabolism in the Skin of Photoaged Mouse Model, Potentially Involving PPARα/γ Activation
3.4. CHA Treatment Blocked p38 MAPK Signaling in the Skin of Photoaged Mouse Model
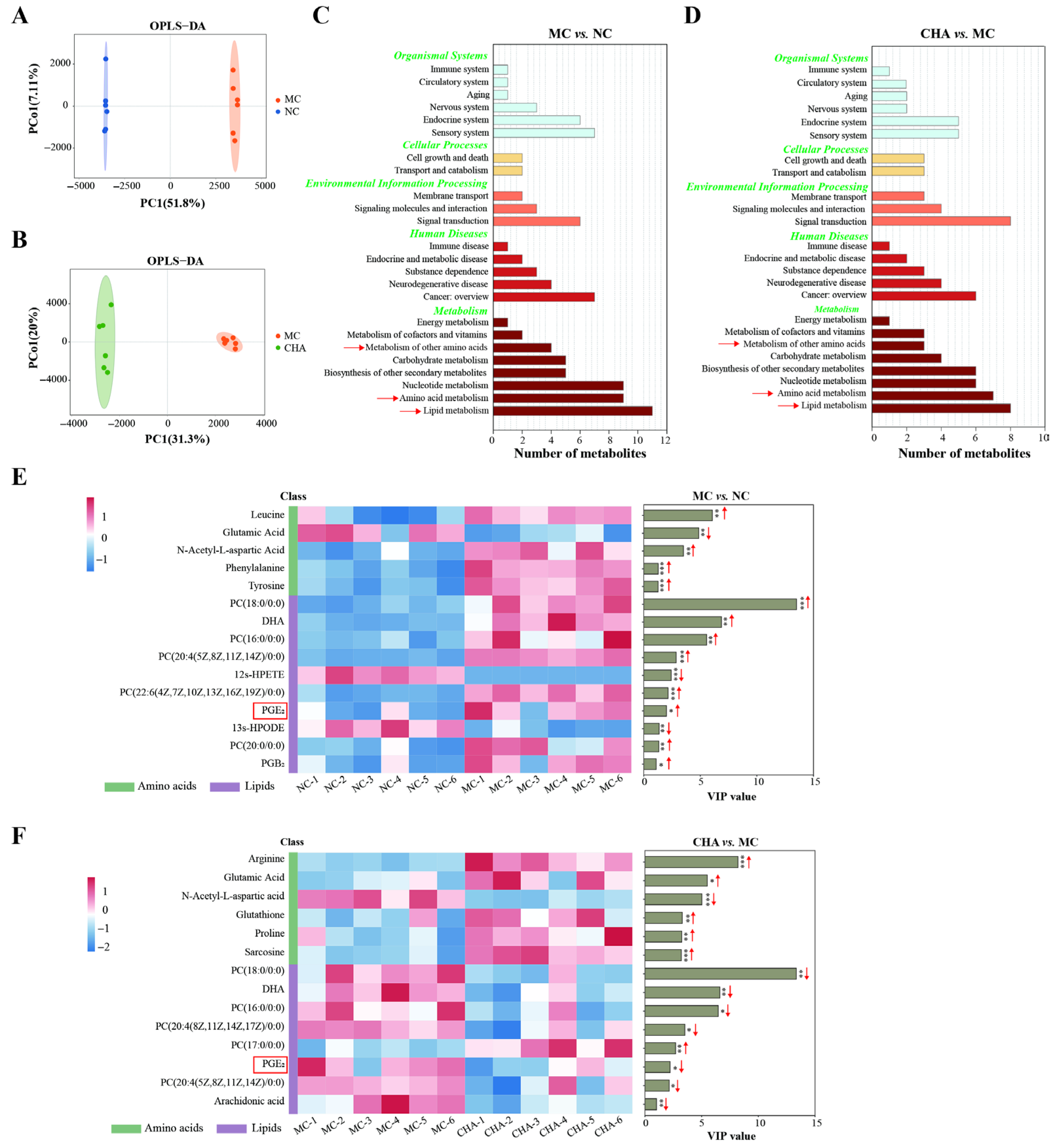
3.5. CHA Treatment Stimulated Metabolic Reprogramming in the Skin of Photoaged Mouse Model
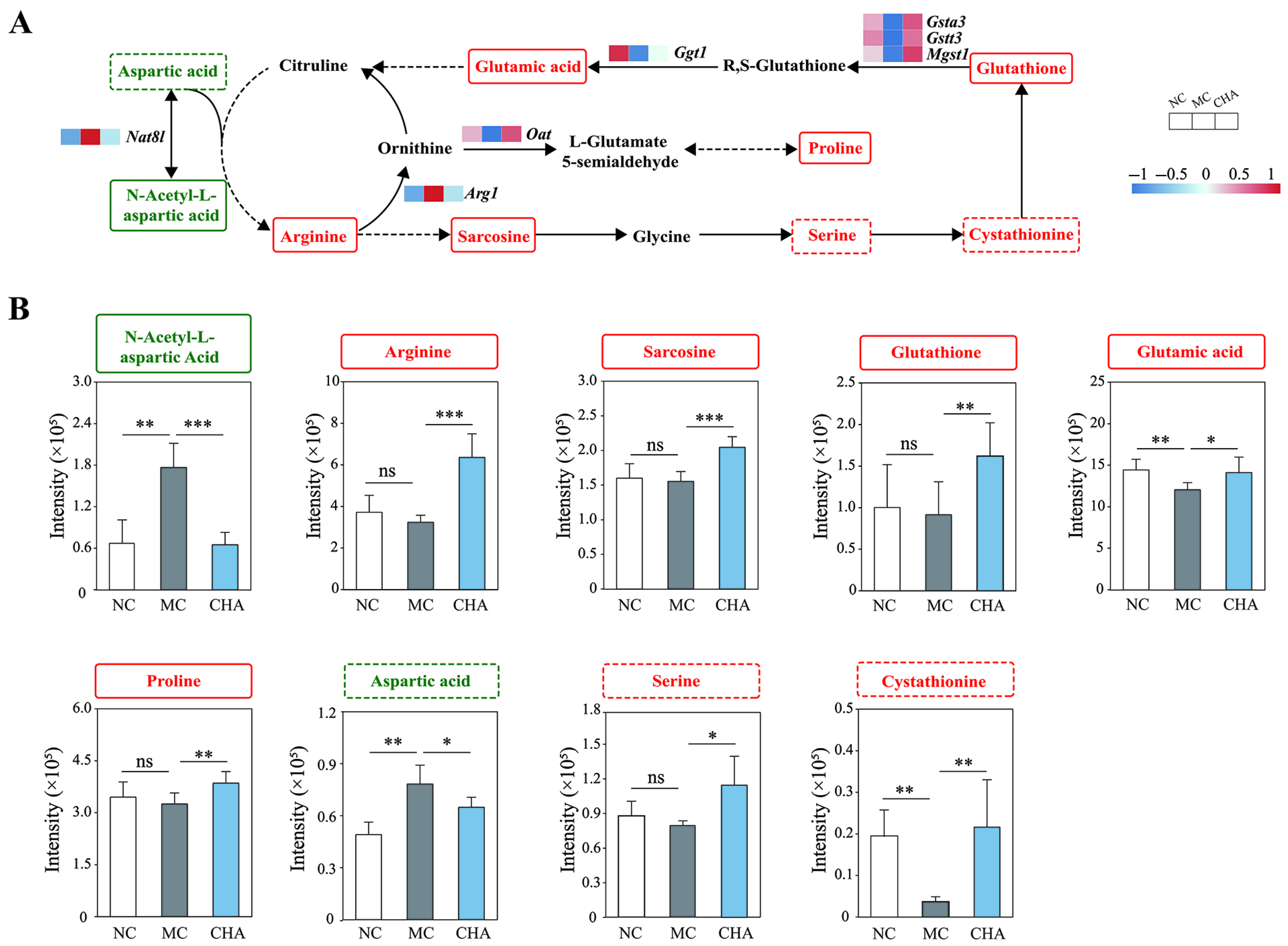
3.6. CHA Treatment Upregulated Non-Essential Amino Acid Metabolism in the Skin of Photoaged Mouse Model
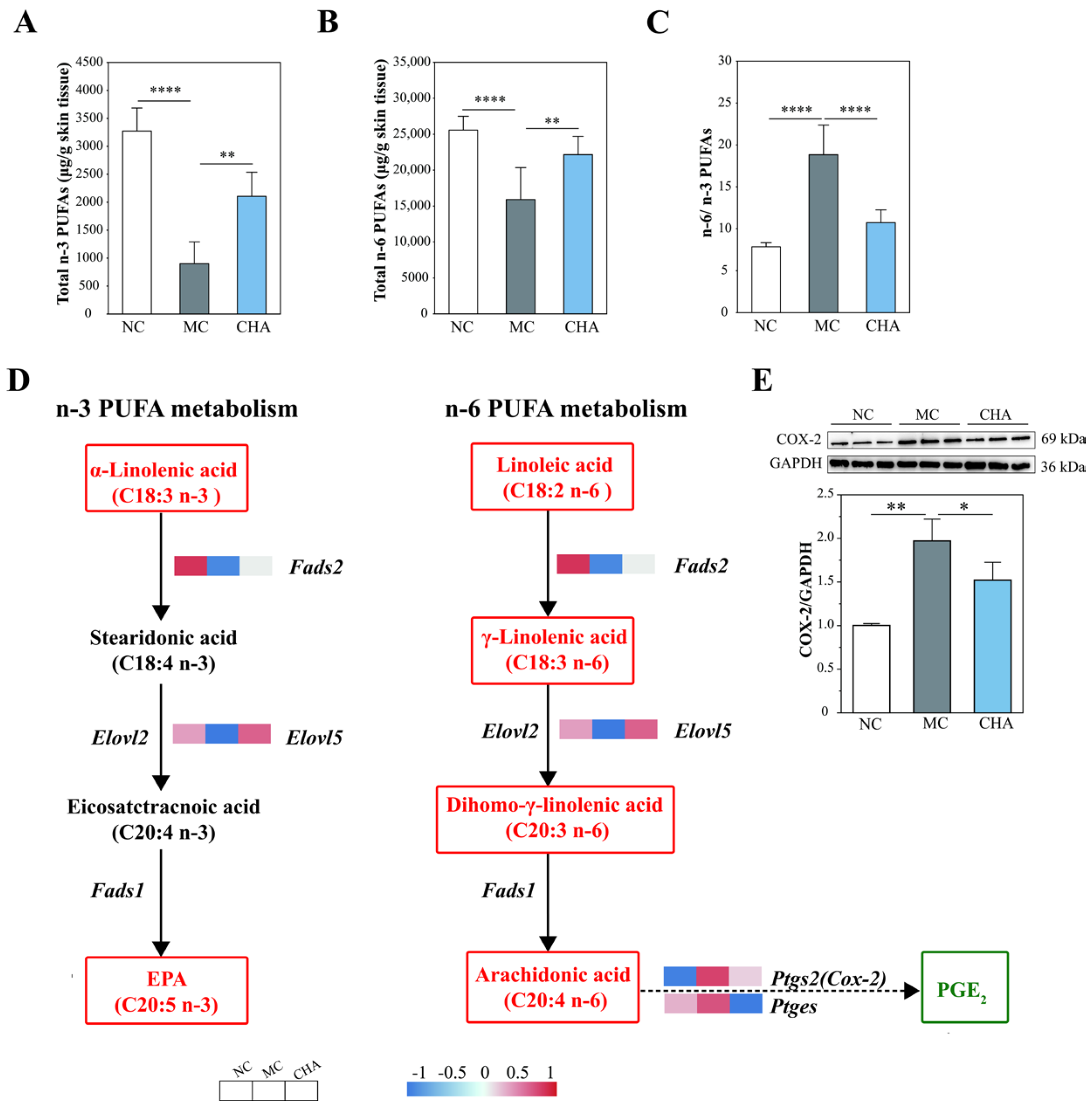
3.7. CHA Treatment Modulated Polyunsaturated Fatty Acid Metabolism in the Skin of Photoaged Mouse Model
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AEO | Artemisia sieversiana Ehrhart ex Willd. Essential oil |
| AA | Arachidonic acid |
| CHA | Chamazulene |
| CCL | CC ligand |
| CXCL | CXC chemokine ligand |
| COX-2 | Cyclooxygenase-2 |
| DEGs | Differentially expressed genes |
| ECM | Extracellular matrix |
| FAME | Fatty acid methyl ester |
| GC-MS | Gas chromatography–mass spectrometry |
| GSEA | Gene set enrichment analysis |
| HYP | Hydroxyproline |
| IL | Interleukin |
| MAPK | Mitogen-activated protein kinase |
| MMP | Matrix metalloproteinase |
| NEAA | Non-essential amino acid |
| OPLS-DA | Orthogonal partial least squares discriminant analysis |
| PCA | Principal component analysis |
| PUFA | Polyunsaturated fatty acid |
| PPAR | Peroxisome proliferator-activated receptor |
| UPLC-MS/MS | Ultra-performance liquid chromatography–tandem mass spectrometry |
References
- Wong, Q.Y.A.; Chew, F.T. Defining skin aging and its risk factors: A systematic review and meta-analysis. Sci Rep. 2021, 11, 22075. [Google Scholar] [CrossRef] [PubMed]
- Hussein, R.S.; Bin Dayel, S.; Abahussein, O.; El-Sherbiny, A.A. Influences on skin and intrinsic aging: Biological, environmental, and therapeutic insights. J. Cosmet. Dermatol. 2024, 24, e16688. [Google Scholar] [CrossRef] [PubMed]
- Krutmann, J.; Schikowski, T.; Morita, A.; Berneburg, M. Environmentally-induced (extrinsic) skin aging: Exposomal factors and underlying mechanisms. J. Investig. Dermatol. 2021, 141, 1096–1103. [Google Scholar] [CrossRef] [PubMed]
- Huang, A.H.; Chien, A.L. Photoaging: A review of current literature. Curr. Dermatol. Rep. 2020, 9, 22–29. [Google Scholar] [CrossRef]
- Poon, F.; Kang, S.; Chien, A.L. Mechanisms and treatments of photoaging. Photodermatol. Photoimmunol. Photomed. 2015, 31, 65–74. [Google Scholar] [CrossRef]
- Salminen, A.; Kaarniranta, K.; Kauppinen, A. Photoaging: UV radiation-induced inflammation and immunosuppression accelerate the aging process in the skin. Inflamm. Res. 2022, 71, 817–831. [Google Scholar] [CrossRef]
- Randhawa, M.; Sangar, V.; Tucker-Samaras, S.; Southall, M. Metabolic signature of sun exposed skin suggests catabolic pathway overweighs anabolic pathway. PLoS ONE 2014, 9, e90367. [Google Scholar] [CrossRef]
- Kim, E.J.; Jin, X.J.; Kim, Y.K.; Oh, I.K.; Kim, J.E.; Park, C.H.; Chung, J.H. UV decreases the synthesis of free fatty acids and triglycerides in the epidermis of human skin in vivo, contributing to development of skin photoaging. J. Dermatol. Sci. 2010, 57, 19–26. [Google Scholar] [CrossRef]
- Randhawa, M.; Southall, M.; Samaras, S.T. Metabolomic analysis of sun exposed skin. Mol. Biosyst. 2013, 9, 2045–2050. [Google Scholar] [CrossRef]
- Nicolaou, A.; Pilkington, S.M.; Rhodes, L.E. Ultraviolet-radiation induced skin inflammation: Dissecting the role of bioactive lipids. Chem. Phys. Lipids 2011, 164, 535–543. [Google Scholar] [CrossRef]
- Calder, P.C. Functional roles of fatty acids and their effects on human health. J. Parenter. Enter. Nutr. 2015, 39, 18s–32s. [Google Scholar] [CrossRef]
- Kiezel-Tsugunova, M.; Kendall, A.C.; Nicolaou, A. Fatty acids and related lipid mediators in the regulation of cutaneous inflammation. Biochem. Soc. Trans. 2018, 46, 119–129. [Google Scholar] [CrossRef]
- Cho, B.A.; Yoo, S.K.; Seo, J.S. Signatures of photo-aging and intrinsic aging in skin were revealed by transcriptome network analysis. Aging 2018, 10, 1609–1626. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.W.; Evans, R. PPARs and ERRs: Molecular mediators of mitochondrial metabolism. Curr. Opin. Cell Biol. 2015, 33, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Sertznig, P.; Seifert, M.; Tilgen, W.; Reichrath, J. Peroxisome proliferator-activated receptors (PPARs) and the human skin-Importance of PPARs in skin physiology and dermatologic diseases. Am. J. Clin. Dermatol. 2008, 9, 15–31. [Google Scholar] [CrossRef] [PubMed]
- Kippenberger, S.; Loitsch, S.M.; Grundmann-Kollmann, M.; Simon, S.; Dang, T.A.; Hardt-Weinelt, K.; Kaufmann, R.; Bernd, A. Activators of peroxisome proliferator-activated receptors protect human skin from ultraviolet-B-light-induced inflammation. J. Investig. Dermatol. 2001, 117, 1430–1436. [Google Scholar] [CrossRef]
- Sahu, R.P.; DaSilva, S.C.; Rashid, B.; Martel, K.C.; Jernigan, D.; Mehta, S.R.; Mohamed, D.R.; Rezania, S.; Bradish, J.R.; Armstrong, A.B.; et al. Mice lacking epidermal PPARγ exhibit a marked augmentation in photocarcinogenesis associated with increased UVB-induced apoptosis, inflammation and barrier dysfunction. Int. J. Cancer 2012, 131, E1055–E1066. [Google Scholar] [CrossRef]
- Shin, M.H.; Lee, S.R.; Kim, M.K.; Shin, C.Y.; Lee, D.H.; Chung, J.H. Activation of peroxisome proliferator-activated receptor alpha improves aged and UV-irradiated skin by catalase induction. PLoS ONE 2016, 11, e0162628. [Google Scholar] [CrossRef]
- Jung, Y.R.; Lee, E.K.; Kim, D.H.; Park, C.H.; Park, M.H.; Jeong, H.O.; Yokozawa, T.; Tanaka, T.; Im, D.S.; Kim, N.D.; et al. Upregulation of collagen expression via PPARβ/δ activation in aged skin by magnesium lithospermate B from Salvia miltiorrhiza. J. Nat. Prod. 2015, 78, 2110–2115. [Google Scholar] [CrossRef]
- Chen, L.; Bi, B.; Zeng, J.P.; Zhou, Y.; Yang, P.; Guo, Y.; Zhu, J.; Yang, Q.; Zhu, N.; Liu, T.; et al. Rosiglitazone ameliorates senescence-like phenotypes in a cellular photoaging model. J. Dermatol. Sci. 2015, 77, 173–181. [Google Scholar] [CrossRef]
- Briganti, S.; Flori, E.; Mastrofrancesco, A.; Kovacs, D.; Camera, E.; Ludovici, M.; Cardinali, G.; Picardo, M. Azelaic acid reduced senescence-like phenotype in photo-irradiated human dermal fibroblasts: Possible implication of PPAR? Exp. Dermatol. 2013, 22, 41–47. [Google Scholar] [CrossRef]
- Park, M.H.; Park, J.Y.; Lee, H.J.; Kim, D.H.; Chung, K.W.; Park, D.; Jeong, H.O.; Kim, H.R.; Park, C.H.; Kim, S.R.; et al. The novel PPAR α/γ dual agonist MHY 966 modulates UVB-induced skin inflammation by inhibiting NF-κB activity. PLoS ONE 2013, 8, e76820. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.K.; Mun, S.; Kim, M.S.; Kim, M.B.; Sa, B.K.; Hwang, J.K. 5,7-Dimethoxyflavone, an activator of PPARα/γ, inhibits UVB-induced MMP expression in human skin fibroblast cells. Exp. Dermatol. 2012, 21, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, Z.; Zufall, A.; Nash, M.; Rao, D.; Hirani, R.; Russo, M. Comparing tretinoin to other topical therapies in the treatment of skin photoaging: A systematic review. Am. J. Clin. Dermatol. 2024, 25, 873–890. [Google Scholar] [CrossRef] [PubMed]
- Olivero-Verbel, J.; Quintero-Rincón, P.; Caballero-Gallardo, K. Aromatic plants as cosmeceuticals: Benefits and applications for skin health. Planta 2024, 260, 132. [Google Scholar] [CrossRef]
- Gabbanini, S.; Neba, J.N.; Matera, R.; Valgimigli, L. Photochemical and oxidative degradation of chamazulene contained in, and essential oils and setup of protection strategies. Molecules 2024, 29, 2604. [Google Scholar] [CrossRef]
- Bakun, P.; Czarczynska-Goslinska, B.; Goslinski, T.; Lijewski, S. In vitro and in vivo biological activities of azulene derivatives with potential applications in medicine. Med. Chem. Res. 2021, 30, 834–846. [Google Scholar] [CrossRef]
- Zhou, Y.; He, L.; Wang, W.; Wei, G.; Ma, L.; Liu, H.; Yao, L. Artemisia sieversiana Ehrhart ex Willd. essential oil and its main component, chamazulene: Their photoprotective effect against UVB-induced cellular damage and potential as novel natural sunscreen additives. ACS Sustain. Chem. Eng. 2023, 11, 17675–17686. [Google Scholar] [CrossRef]
- Zhou, Y.; He, L.; Zhang, N.; Ma, L.; Yao, L. Photoprotective effect of Artemisia sieversiana Ehrhart essential oil against UVB-induced photoaging in mice. Photochem. Photobiol. 2022, 98, 958–968. [Google Scholar] [CrossRef]
- Watanabe, S.; Hiraoka, Y.; Endo, S.; Tanimoto, Y.; Tozawa, Y.; Watanabe, Y. An enzymatic method to estimate the content of L-hydroxyproline. J. Biotechnol. 2015, 199, 9–16. [Google Scholar] [CrossRef]
- Zhou, C.H.; Chen, Y.H.; Xue, S.S.; Shi, Q.Q.; Guo, L.; Yu, H.; Xue, F.; Cai, M.; Wang, H.N.; Peng, Z.W. rTMS ameliorates depressive-like behaviors and regulates the gut microbiome and medium- and long-chain fatty acids in mice exposed to chronic unpredictable mild stress. CNS Neurosci. Ther. 2023, 29, 3549–3566. [Google Scholar] [CrossRef] [PubMed]
- Tahri-Joutey, M.; Andreoletti, P.; Surapureddi, S.; Nasser, B.; Cherkaoui-Malki, M.; Latruffe, N. Mechanisms mediating the regulation of peroxisomal fatty acid beta-oxidation by PPARα. Int. J. Mol. Sci. 2021, 22, 8969. [Google Scholar] [CrossRef] [PubMed]
- Dongol, B.; Shah, Y.; Kim, I.; Gonzalez, F.J.; Hunt, M.C. The acyl-CoA thioesterase I is regulated by PPARα and HNF4α via a distal response element in the promoter. J. Lipid. Res. 2007, 48, 1781–1791. [Google Scholar] [CrossRef] [PubMed]
- Varga, T.; Czimmerer, Z.; Nagy, L. PPARs are a unique set of fatty acid regulated transcription factors controlling both lipid metabolism and inflammation. Biochim. Biophys. Acta-Mol. Basis Dis. 2011, 1812, 1007–1022. [Google Scholar] [CrossRef]
- Lee, Y.K.; Park, J.E.; Lee, M.; Hardwick, J.P. Hepatic lipid homeostasis by peroxisome proliferator-activated receptor gamma 2. Liver. Res. 2018, 2, 209–215. [Google Scholar] [CrossRef]
- Kobayashi, T.; Fujimori, K. Very long-chain-fatty acids enhance adipogenesis through coregulation of Elovl3 and PPARγ in 3T3-L1 cells. Am. J. Physiol.-Endocrinol. Metab. 2012, 302, E1461–E1471. [Google Scholar] [CrossRef]
- Yao-Borengasser, A.; Rassouli, N.; Varma, V.; Bodles, A.M.; Rasouli, N.; Unal, R.; Phanavanh, B.; Ranganathan, G.; McGehee, R.E.J.; Kern, P.A. Stearoyl-coenzyme a desaturase 1 gene expression increases after pioglitazone treatment and is associated with peroxisomal proliferator-activated receptor-γ responsiveness. J. Clin. Endocrinol. Metab. 2008, 93, 4431–4439. [Google Scholar] [CrossRef]
- Czumaj, A.; Szrok-Jurga, S.; Hebanowska, A.; Turyn, J.; Swierczynski, J.; Sledzinski, T.; Stelmanska, E. The pathophysiological role of CoA. Int. J. Mol. Sci. 2020, 21, 9057. [Google Scholar] [CrossRef]
- Naquet, P.; Kerr, E.W.; Vickers, S.D.; Leonardi, R. Regulation of coenzyme A levels by degradation: The ‘Ins and Outs’. Prog. Lipid Res. 2020, 78, 101028. [Google Scholar] [CrossRef]
- Sheikh, K.; Camejo, G.; Lanne, B.; Halvarsson, T.; Landergren, M.R.; Oakes, N.D. Beyond lipids, pharmacological PPARα activation has important effects on amino acid metabolism as studied in the rat. Am. J. Physiol.-Endocrinol. Metab. 2007, 292, E1157–E1165. [Google Scholar] [CrossRef]
- Ericsson, A.; Turner, N.; Hansson, G.I.; Wallenius, K.; Oakes, N.D. Pharmacological PPARα activation markedly alters plasma turnover of the amino acids glycine, serine and arginine in the rat. PLoS ONE 2014, 9, e113328. [Google Scholar] [CrossRef] [PubMed]
- Contreras, A.V.; Rangel-Escareño, C.; Torres, N.; Alemán-Escondrillas, G.; Ortiz, V.; Noriega, L.G.; Torre-Villalvazo, I.; Granados, O.; Velázquez-Villegas, L.A.; Tobon-Cornejo, S.; et al. PPARα via HNF4α regulates the expression of genes encoding hepatic amino acid catabolizing enzymes to maintain metabolic homeostasis. Genes Nutr. 2015, 10, 452. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.Y.; Wu, Z.L.; Dai, Z.L.; Yang, Y.; Wang, W.; Liu, C.; Wang, B.; Wang, J.; Yin, Y. Dietary requirements of “nutritionally non-essential amino acids” by animals and humans. Amino Acids 2013, 44, 1107–1113. [Google Scholar] [CrossRef] [PubMed]
- Choi, B.H.; Coloff, J.L. The diverse functions of non-essential amino acids in cancer. Cancers 2019, 11, 675. [Google Scholar] [CrossRef]
- Safayhi, H.; Sabieraj, J.; Sailer, E.R.; Ammon, H.P. Chamazulene: An antioxidant-type inhibitor of leukotriene B4 formation. Planta. Med. 1994, 60, 410–413. [Google Scholar] [CrossRef]
- Capuzzo, A.; Occhipinti, A.; Maffei, M.E. Antioxidant and radical scavenging activities of chamazulene. Nat. Prod. Res. 2014, 28, 2321–2323. [Google Scholar] [CrossRef]
- Maoqiang, M.; Elias, P.M.; Feingold, K.R. Fatty-acids are required for epidermal permeability barrier function. J. Clin. Invest. 1993, 92, 791–798. [Google Scholar] [CrossRef]
- Sassa, T.; Ohno, Y.; Suzuki, S.; Nomura, T.; Nishioka, C.; Kashiwagi, T.; Hirayama, T.; Akiyama, M.; Taguchi, R.; Shimizu, H.; et al. Impaired epidermal permeability barrier in mice lacking, the gene responsible for very-long-chain fatty acid production. Mol. Cell. Biol. 2013, 33, 2787–2796. [Google Scholar] [CrossRef]
- Binczek, E.; Jenke, B.; Holz, B.; Günter, R.H.; Thevis, M.; Stoffel, W. Obesity resistance of the stearoyl-CoA desaturase-deficient (scd1-/-) mouse results from disruption of the epidermal lipid barrier and adaptive thermoregulation. Biol. Chem. 2007, 388, 405–418. [Google Scholar] [CrossRef]
- Velarde, M.C. Epidermal barrier protects against age-associated systemic inflammation. J. Investig. Dermatol. 2017, 137, 1206–1208. [Google Scholar] [CrossRef]
- Liu, B.; Meng, Q.F.; Gao, X.; Sun, H.; Xu, Z.; Wang, Y.; Zhou, H. Lipid and glucose metabolism in senescence. Front. Nutr. 2023, 10, 1157352. [Google Scholar] [CrossRef]
- Vamecq, J.; Andreoletti, P.; El Kebbaj, R.; Saih, F.E.; Latruffe, N.; El Kebbaj, M.H.S.; Lizard, G.; Nasser, B.; Cherkaoui-Malki, M. Peroxisomal acyl-CoA oxidase type 1: Anti-inflammatory and anti-aging properties with a special emphasis on studies with LPS and argan oil as a model transposable to aging. Oxidative Med. Cell. Longev. 2018, 2018, 6986984. [Google Scholar] [CrossRef]
- Marion-Letellier, R.; Savoye, G.; Ghosh, S. Fatty acids, eicosanoids and PPAR gamma. Eur. J. Pharmacol. 2016, 785, 44–49. [Google Scholar] [CrossRef]
- Muthusamy, V.; Piva, T.J. The UV response of the skin: A review of the MAPK, NFκB and TNFα signal transduction pathways. Arch. Dermatol. Res. 2010, 302, 5–17. [Google Scholar] [CrossRef] [PubMed]
- Saika, A.; Tiwari, P.; Nagatake, T.; Node, E.; Hosomi, K.; Honda, T.; Kabashima, K.; Kunisawa, J. Mead acid inhibits retinol-induced irritant contact dermatitis via peroxisome proliferator-activated receptor alpha. Front. Mol. Biosci. 2023, 10, 1097955. [Google Scholar] [CrossRef] [PubMed]
- Briganti, S.; Flori, E.; Bellei, B.; Picardo, M. Modulation of PPARγ provides new insights in a stress induced premature senescence model. PLoS ONE 2014, 9, e104045. [Google Scholar] [CrossRef] [PubMed]
- Balić, A.; Vlašić, D.; Žužul, K.; Marinović, B.; Bukvić Mokos, Z. Omega-3 versus omega-6 polyunsaturated fatty acids in the prevention and treatment of inflammatory skin diseases. Int. J. Mol. Sci. 2020, 21, 741. [Google Scholar] [CrossRef]
- Barman, M.; Stråvik, M.; Broberg, K.; Sandin, A.; Wold, A.E.; Sandberg, A.S. Proportions of polyunsaturated fatty acids in umbilical cord blood at birth are related to atopic eczema development in the first year of life. Nutrients 2021, 13, 3779. [Google Scholar] [CrossRef]
- Morin, S.; Tremblay, A.; Dumais, E.; Julien, P.; Flamand, N.; Pouliot, R. Eicosapentaenoic acid influences the lipid profile of an in vitro psoriatic skin model produced with T cells. Biomolecules 2023, 13, 1413. [Google Scholar] [CrossRef]
- Pilkington, S.M.; Watson, R.E.B.; Nicolaou, A.; Rhodes, L.E. Omega-3 polyunsaturated fatty acids: Photoprotective macronutrients. Exp. Dermatol. 2011, 20, 537–543. [Google Scholar] [CrossRef]
- Biernacki, M.; Skrzydlewska, E. Metabolic pathways of eicosanoids-derivatives of arachidonic acid and their significance in skin. Cell. Mol. Biol. Lett. 2025, 30, 7. [Google Scholar] [CrossRef]
- Hanson, D.; Deleo, V. Long-wave ultraviolet-light induces phospholipase activation in cultured human epidermal-keratinocytes. J. Investig. Dermatol. 1990, 95, 158–163. [Google Scholar] [CrossRef] [PubMed]
- Kangrotondo, C.H.; Miller, C.C.; Morrison, A.R.; Pentland, A.P. Enhanced keratinocyte prostaglandin synthesis after UV injury is due to increased phospholipase-activity. Am. J. Physiol. 1993, 264, C396–C401. [Google Scholar] [CrossRef] [PubMed]
- Tripp, C.S.; Blomme, E.A.G.; Chinn, K.S.; Hardy, M.M.; LaCelle, P.; Pentland, A.P. Epidermal COX-2 induction following ultraviolet irradiation: Suggested mechanism for the role of COX-2 inhibition in photoprotection. J. Investig. Dermatol. 2003, 121, 853–861. [Google Scholar] [CrossRef] [PubMed]
- Seo, J.Y.; Kim, E.K.; Lee, S.H.; Park, K.C.; Kim, K.H.; Eun, H.C.; Chung, J.H. Enhanced expression of cylooxygenase-2 by UV in aged human skin in vivo. Mech. Ageing Dev. 2003, 124, 903–910. [Google Scholar] [CrossRef]
- Chen, W.X.; Tang, Q.B.; Gonzales, M.S.; Bowden, G.T. Role of p38 MAP kinases and ERK in mediating ultraviolet-B induced cyclooxygenase-2 gene expression in human keratinocytes. Oncogene 2001, 20, 3921–3926. [Google Scholar] [CrossRef]
- Cho, J.W.; Park, K.; Kweon, G.R.; Jang, B.C.; Baek, W.K.; Suh, M.H.; Kim, C.W.; Lee, K.S.; Suh, S.I. Curcumin inhibits the expression of COX-2 in UVB-irradiated human keratinocytes (HaCaT) by inhibiting activation of AP-1: p38 MAP kinase and JNK as potential upstream targets. Exp. Mol. Med. 2005, 37, 186–192. [Google Scholar] [CrossRef]
- Kuehne, A.; Hildebrand, J.; Soehle, J.; Wenck, H.; Terstegen, L.; Gallinat, S.; Knott, A.; Winnefeld, M.; Zamboni, N. An integrative metabolomics and transcriptomics study to identify metabolic alterations in aged skin of humans. BMC Genom. 2017, 18, 169. [Google Scholar] [CrossRef]
- Solano, F. Photoprotection versus photodamage: Updating an old but still unsolved controversy about melanin. Polym. Int. 2016, 65, 1276–1287. [Google Scholar] [CrossRef]
- Wu, G.Y. Amino acids in nutrition, health, and disease. Front. Biosci. 2021, 26, 1386–1392. [Google Scholar] [CrossRef]
- Debats, I.B.J.G.; Wolfs, T.G.A.M.; Gotoh, T.; Cleutjens, J.P.; Peutz-Kootstra, C.J.; van der Hulst, R.R. Role of arginine in superficial wound healing in man. Nitric. Oxide-Biol. Chem. 2009, 21, 175–183. [Google Scholar] [CrossRef]
- Karna, E.; Szoka, L.; Huynh, T.Y.L.; Palka, J.A. Proline-dependent regulation of collagen metabolism. Cell. Mol. Life. Sci. 2020, 77, 1911–1918. [Google Scholar] [CrossRef]
- Oh, Y.; Lim, H.W.; Huang, Y.H.; Kwon, H.S.; Jin, C.D.; Kim, K.; Lim, C.J. Attenuating properties of Agastache rugosa leaf extract against ultraviolet-B-induced photoaging via up regulating glutathione and superoxide dismutase in a human keratinocyte cell line. J. Photochem. Photobiol. B Biol. 2016, 163, 170–176. [Google Scholar] [CrossRef]
- Cappello, A.; Mancini, M.; Madonna, S.; Rinaldo, S.; Paone, A.; Scarponi, C.; Belardo, A.; Zolla, L.; Zuccotti, A.; Panatta, E.; et al. Extracellular serine empowers epidermal proliferation and psoriasis-like symptoms. Sci. Adv. 2022, 8, eabm7902. [Google Scholar] [CrossRef]
| Metabolites | Abbreviation | RT (min) | Concentration (μg/g Skin Tissue) | ||
|---|---|---|---|---|---|
| NC | MC | CHA | |||
| Linolelaidic acid | C18:2n-6 | 14.56 | 20.04 ± 3.96 a | 8.47 ± 1.50 b | 15.39 ± 1.70 a |
| Linoleic acid | C18:2n-6 (LA) | 14.90 | 24,308.99 ± 1867.58 a | 15,052.33 ± 4389.97 b | 21,228.26 ± 2533.12 a |
| γ-Linolenic acid | C18:3n-6 | 15.23 | 113.00 ± 16.37 a | 36.90 ± 6.70 b | 83.13 ± 27.85 a |
| α-Linolenic acid | C18:3n-3 (ALA) | 15.62 | 3026.82 ± 410.89 a | 663.88 ± 162.19 c | 1899. 28 ± 180.36 b |
| 11Z,14Z-Eicosadienoic acid | C20:2n-6 | 17.38 | 225.62 ± 36.75 a | 111.60 ± 13.30 c | 174.74 ± 16.03 b |
| Dihomo-γ-linolenic acid | C20:3n-6 (DGLA) | 17.77 | 119.20 ± 17.33 a | 45.29 ± 12.27 c | 71.58 ± 5.56 b |
| 11Z,14Z,17Z-Eicosatrienoic acid | C20:3n-3 | 18.21 | 14.00 ± 1.96 a | 5.10 ± 0.72 c | 9.48 ± 0.61 b |
| Arachidonic acid | C20:4n-6 (AA) | 18.02 | 714.07 ± 100.62 a | 573.43 ± 72.77 b | 532.03 ± 63.57 b |
| 5Z,8Z,11Z,14Z,17Z-Eicosapentaenoic acid | C20:5n-3 (EPA) | 18.876 | 16.54 ± 3.21 a | 5.86 ± 2.10 b | 9.43 ± 2.36 b |
| 13Z,16Z-Docosadienoic acid | C22:2n-6 | 20.06 | 3.30 ± 1.06 a | 2.70 ± 0.59 b | 6.04 ± 0.93 b |
| Adrenic acid | C22:4n-6 | 20.87 | 43.71 ± 3.28 a | 47.45 ± 9.67 a | 39.69 ± 5.01 a |
| 7Z,10Z,13Z,16Z,19Z-Docosapentaenoic acid | C22:5n-3 (DPA) | 21.88 | 47.03 ± 4.33 a | 42.76 ± 6.76 a | 39.23 ± 9.04 a |
| 4Z,7Z,10Z,13Z,16Z,19Z-Docosahexaenoic acid | C22:6n-3 (DHA) | 22.13 | 186.56 ± 23.03 a | 197.88 ± 32.88 a | 162.90 ± 40.55 a |
| Total | 28,838.89 ± 2315.94 a | 16,973.63 ± 4806.88 b | 24,271.17 ± 2908.76 a | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Y.; Wang, W.; He, L.; Zhang, N.; Zhou, B.; Chen, Z.; Ma, L.; Yao, L. Chamazulene Induces Metabolic Reprogramming and Mitigates Inflammation in Photoaged Skin: PPARα/γ as Potential Regulators. Antioxidants 2025, 14, 1320. https://doi.org/10.3390/antiox14111320
Zhou Y, Wang W, He L, Zhang N, Zhou B, Chen Z, Ma L, Yao L. Chamazulene Induces Metabolic Reprogramming and Mitigates Inflammation in Photoaged Skin: PPARα/γ as Potential Regulators. Antioxidants. 2025; 14(11):1320. https://doi.org/10.3390/antiox14111320
Chicago/Turabian StyleZhou, Ying, Wencui Wang, Lei He, Nan Zhang, Bowen Zhou, Zimeng Chen, Li Ma, and Lei Yao. 2025. "Chamazulene Induces Metabolic Reprogramming and Mitigates Inflammation in Photoaged Skin: PPARα/γ as Potential Regulators" Antioxidants 14, no. 11: 1320. https://doi.org/10.3390/antiox14111320
APA StyleZhou, Y., Wang, W., He, L., Zhang, N., Zhou, B., Chen, Z., Ma, L., & Yao, L. (2025). Chamazulene Induces Metabolic Reprogramming and Mitigates Inflammation in Photoaged Skin: PPARα/γ as Potential Regulators. Antioxidants, 14(11), 1320. https://doi.org/10.3390/antiox14111320






