Tobacco Smoke Exposure and Oxidative Stress: The Role of Circulating Lipopolysaccharides in Heated and Conventional Products
Abstract
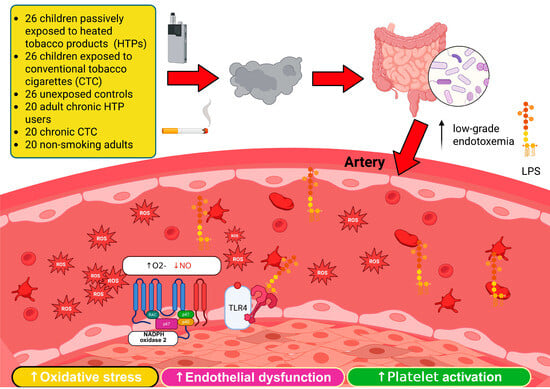
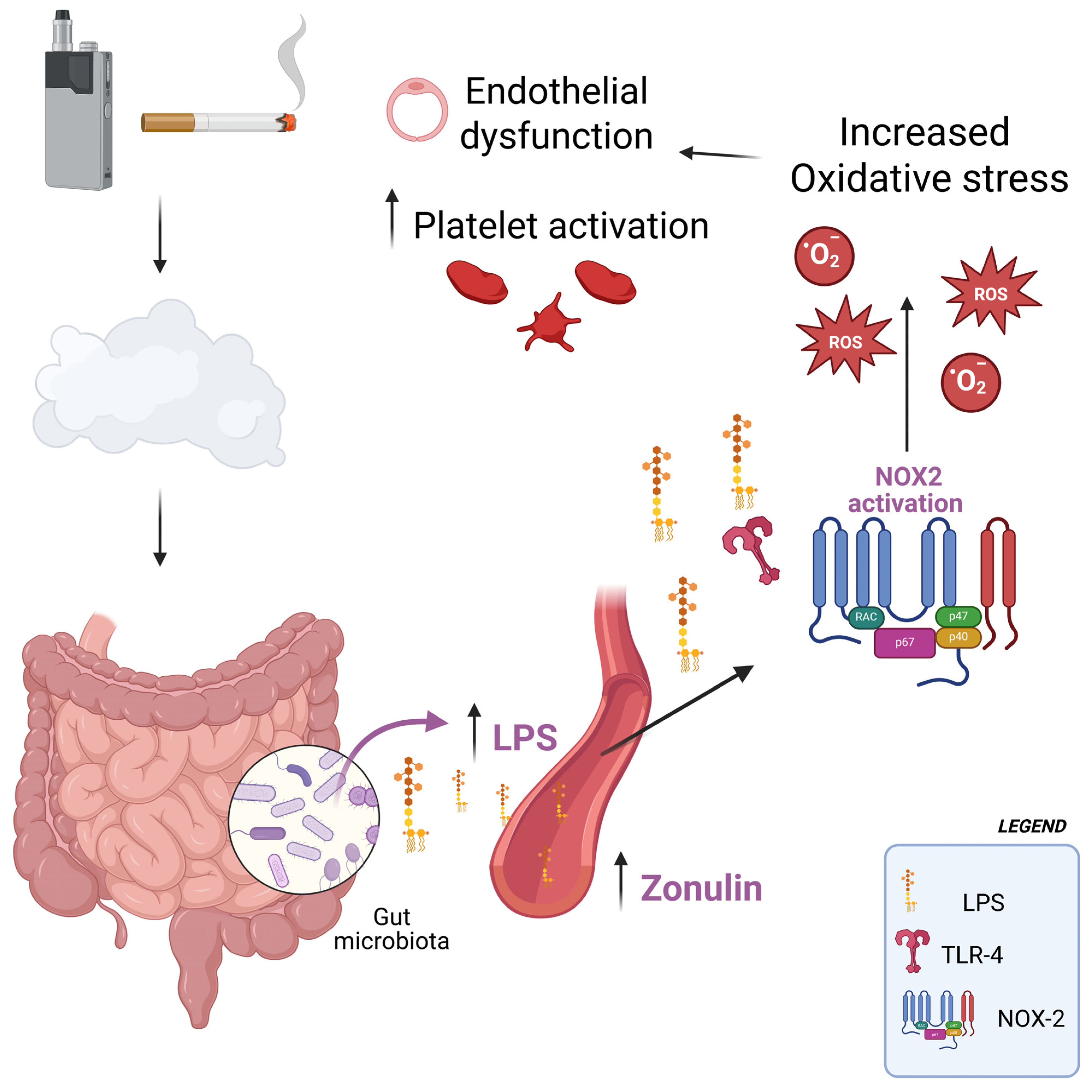
1. Introduction
2. Materials and Methods
2.1. Aim of the Study
2.2. Study Population: Passive Smoke Exposure and Controls
2.3. Study Population: Chronic Active Smokers and Controls
2.4. Sample Collection
2.4.1. Biochemical and Functional Assessments
NO2−/NO3− (Serum Nitric Oxide Metabolites)
2.4.2. 8-Isoprostane (8-Iso-PGF2α)
2.4.3. Soluble NOX2-Derived Peptide (sNOX2-dp)
2.4.4. Hydrogen Peroxide (H2O2)
2.4.5. Hydrogen Peroxide Breakdown Activity (HBA)
2.4.6. Soluble P-Selectin
2.4.7. Soluble CD40 Ligand (sCD40L)
2.4.8. Cotinine
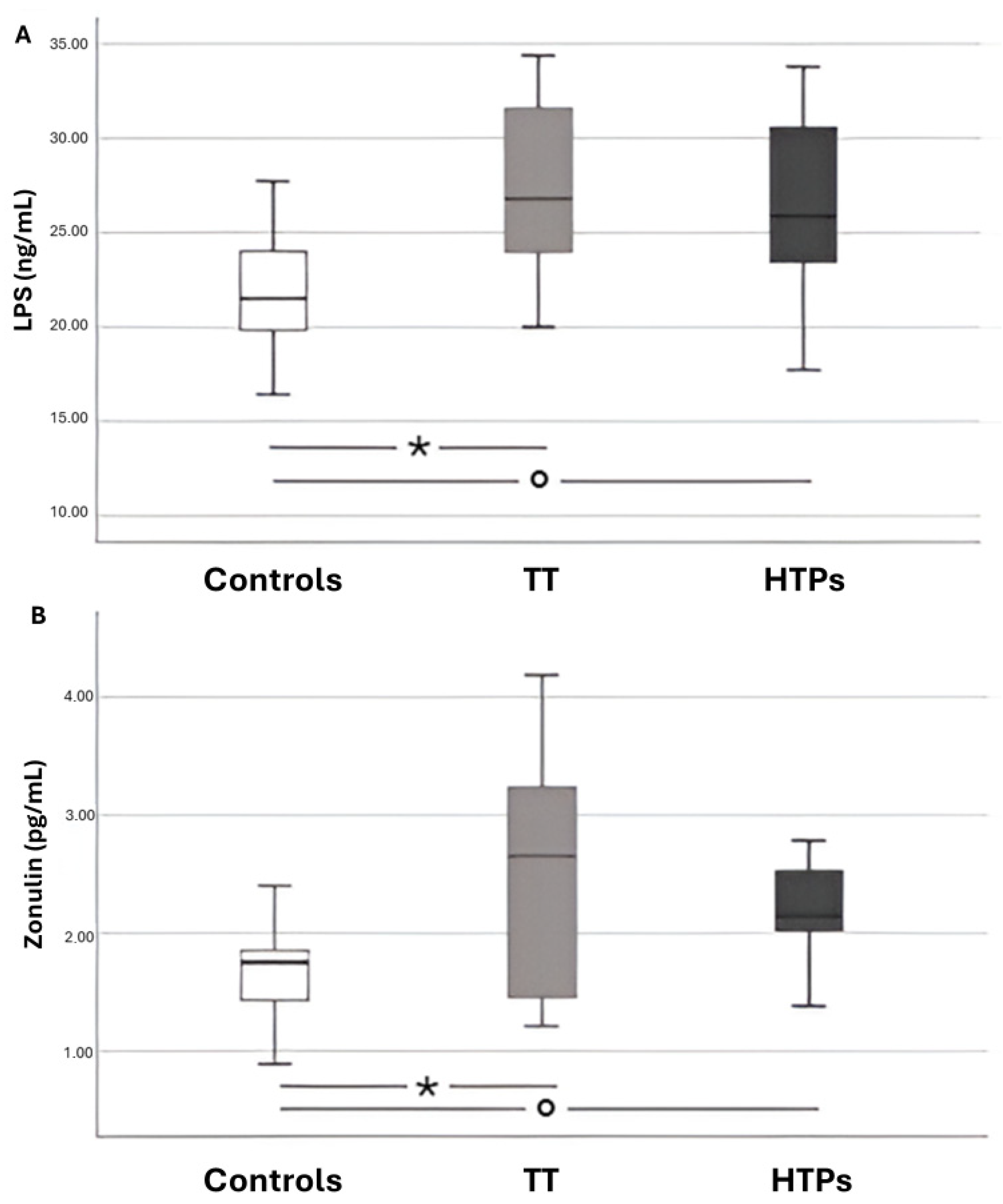
2.4.9. Lipopolysaccharide (LPS)
2.4.10. Zonulin
2.5. Flow-Mediated Dilation (FMD)
2.6. Statistical Analysis
- Passive smoke exposure group: Continuous variables were expressed as mean ± standard deviation; categorical variables as percentages. Normality was assessed with the Kolmogorov–Smirnov test. Between-group differences were analyzed using chi-square for categorical variables and ANOVA with Bonferroni correction for normally distributed variables. Bivariate correlations were evaluated by Spearman’s test. Variables with p < 0.10 were included in multivariate linear regression (stepwise procedure). Variables with p < 0.10 were included in multivariate linear regression (stepwise procedure). Multivariate linear regression models, adjusted for age, sex, BMI, mean arterial pressure and cotinine were used to compare non-smokers vs. conventional smokers, and non-smokers vs. HTP users. Statistical significance was set at p < 0.05. Analyses were performed using SPSS v25.0 (IBM, Armonk, NY, USA) and GraphPad Prism 7 (GraphPad Software, San Diego, CA, USA). Sample size was calculated based on a two-sided Student’s t-test: a difference (δ) of 2.5% in FMD between exposed and unexposed children, standard deviation of 2.5%, α = 0.05, and power (1 − β) = 0.95, yielding 26 patients per group.
- Active smokers group: Continuous variables were reported as median (IQR), categorical as counts (%). Group comparisons were performed using Kruskal–Wallis tests and Wilcoxon–Mann–Whitney tests. Correlations were analyzed by Spearman’s test and visualized with clustered heatmaps. Multivariate linear regression models, adjusted for age, sex, BMI, mean arterial pressure, total cholesterol, and cotinine, were used to compare non-smokers vs. conventional smokers, and non-smokers vs. HTP users. Comparisons between conventional smokers and HTP users were further adjusted for smoking duration and cigarettes/day. A two-tailed p < 0.05 was considered statistically significant. Analyses were conducted with SPSS v18.0 (SPSS, Chicago, IL, USA), Stata 13 (StataCorp, College Station, TX, USA), and R 3.5.3 (R Foundation for Statistical Computing, Vienna, Austria).
3. Results
3.1. Passive Smoking
3.2. Active Smokers
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Magna, A.; Polisena, N.; Polisena, L.; Bagnato, C.; Pacella, E.; Carnevale, R.; Nocella, C.; Loffredo, L. The Hidden Dangers: E-Cigarettes, Heated Tobacco, and Their Impact on Oxidative Stress and Atherosclerosis-A Systematic Review and Narrative Synthesis of the Evidence. Antioxidants 2024, 13, 1395. [Google Scholar] [CrossRef] [PubMed]
- Talhout, R.; Schulz, T.; Florek, E.; van Benthem, J.; Wester, P.; Opperhuizen, A. Hazardous compounds in tobacco smoke. Int. J. Environ. Res. Public Health 2011, 8, 613–628. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.G.; Noggle, B.; Vansickel, A.R.; Largo, E.G.; Magnani, P. Tobacco Use, Risk Perceptions, and Characteristics of Adults Who Used a Heated Tobacco Product (IQOS) in the United States: Cross-Sectional Survey Study. JMIR Form. Res. 2025, 9, e57398. [Google Scholar] [CrossRef]
- E-cigarettes, heat-not-burn and smokeless tobacco products. Breathe 2020, 16, 161ELF. [CrossRef]
- Violi, F.; Cammisotto, V.; Bartimoccia, S.; Pignatelli, P.; Carnevale, R.; Nocella, C. Gut-derived low-grade endotoxaemia, atherothrombosis and cardiovascular disease. Nat. Rev. Cardiol. 2023, 20, 24–37. [Google Scholar] [CrossRef]
- Capurso, G.; Lahner, E. The interaction between smoking, alcohol and the gut microbiome. Best Pract. Res. Clin. Gastroenterol. 2017, 31, 579–588. [Google Scholar] [CrossRef]
- Hasday, J.D.; Bascom, R.; Costa, J.J.; Fitzgerald, T.; Dubin, W. Bacterial endotoxin is an active component of cigarette smoke. Chest 1999, 115, 829–835. [Google Scholar] [CrossRef]
- Loffredo, L.; Soresina, A.; Cinicola, B.L.; Capponi, M.; Salvatori, F.; Bartimoccia, S.; Picchio, V.; Forte, M.; Caputi, C.; Poscia, R.; et al. Impaired arterial dilation and increased NOX2 generated oxidative stress in subjects with ataxia-telangiectasia mutated (ATM) kinase. Redox Biol. 2024, 77, 103347. [Google Scholar] [CrossRef]
- Violi, F.; Sanguigni, V.; Carnevale, R.; Plebani, A.; Rossi, P.; Finocchi, A.; Pignata, C.; De Mattia, D.; Martire, B.; Pietrogrande, M.C.; et al. Hereditary deficiency of gp91(phox) is associated with enhanced arterial dilatation: Results of a multicenter study. Circulation 2009, 120, 1616–1622. [Google Scholar] [CrossRef] [PubMed]
- Gui, X.; Yang, Z.; Li, M.D. Effect of Cigarette Smoke on Gut Microbiota: State of Knowledge. Front. Physiol. 2021, 12, 673341. [Google Scholar] [CrossRef]
- Loffredo, L.; Carnevale, R.; Battaglia, S.; Marti, R.; Pizzolo, S.; Bartimoccia, S.; Nocella, C.; Cammisotto, V.; Sciarretta, S.; Chimenti, I.; et al. Impact of chronic use of heat-not-burn cigarettes on oxidative stress, endothelial dysfunction and platelet activation: The SUR-VAPES Chronic Study. Thorax 2021, 76, 618–620. [Google Scholar] [CrossRef]
- Loffredo, L.; Carnevale, R.; Pannunzio, A.; Cinicola, B.L.; Palumbo, I.M.; Bartimoccia, S.; Nocella, C.; Cammisotto, V.; Violi, F.; Biondi-Zoccai, G.; et al. Impact of heat-not-burn cigarette passive smoking on children’s oxidative stress, endothelial and platelet function. Environ. Pollut. 2024, 345, 123304. [Google Scholar] [CrossRef]
- Loffredo, L.; Zicari, A.M.; Occasi, F.; Perri, L.; Carnevale, R.; Angelico, F.; Del Ben, M.; Martino, F.; Nocella, C.; De Castro, G.; et al. Role of NADPH oxidase-2 and oxidative stress in children exposed to passive smoking. Thorax 2018, 73, 986–988. [Google Scholar] [CrossRef] [PubMed]
- Emelyanov, A.; Fedoseev, G.; Abulimity, A.; Rudinski, K.; Fedoulov, A.; Karabanov, A.; Barnes, P.J. Elevated concentrations of exhaled hydrogen peroxide in asthmatic patients. Chest 2001, 120, 1136–1139. [Google Scholar] [CrossRef]
- Corretti, M.C.; Anderson, T.J.; Benjamin, E.J.; Celermajer, D.; Charbonneau, F.; Creager, M.A.; Deanfield, J.; Drexler, H.; Gerhard-Herman, M.; Herrington, D.; et al. Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: A report of the International Brachial Artery Reactivity Task Force. J. Am. Coll. Cardiol. 2002, 39, 257–265. [Google Scholar] [CrossRef]
- Thijssen, D.H.J.; Bruno, R.M.; van Mil, A.; Holder, S.M.; Faita, F.; Greyling, A.; Zock, P.L.; Taddei, S.; Deanfield, J.E.; Luscher, T.; et al. Expert consensus and evidence-based recommendations for the assessment of flow-mediated dilation in humans. Eur. Heart J. 2019, 40, 2534–2547. [Google Scholar] [CrossRef]
- Thijssen, D.H.; Black, M.A.; Pyke, K.E.; Padilla, J.; Atkinson, G.; Harris, R.A.; Parker, B.; Widlansky, M.E.; Tschakovsky, M.E.; Green, D.J. Assessment of flow-mediated dilation in humans: A methodological and physiological guideline. Am. J. Physiol.-Heart Circ. Physiol. 2011, 300, H2–H12. [Google Scholar] [CrossRef] [PubMed]
- Zuo, L.; Li, Y.; Wang, H.; Wu, R.; Zhu, W.; Zhang, W.; Cao, L.; Gu, L.; Gong, J.; Li, N.; et al. Cigarette smoking is associated with intestinal barrier dysfunction in the small intestine but not in the large intestine of mice. J. Crohn’s Colitis 2014, 8, 1710–1722. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Wei, H.; Liu, W.; Coker, O.O.; Gou, H.; Liu, C.; Zhao, L.; Li, C.; Zhou, Y.; Wang, G.; et al. Cigarette smoke promotes colorectal cancer through modulation of gut microbiota and related metabolites. Gut 2022, 71, 2439–2450. [Google Scholar] [CrossRef]
- Antinozzi, M.; Giffi, M.; Sini, N.; Galle, F.; Valeriani, F.; De Vito, C.; Liguori, G.; Romano Spica, V.; Cattaruzza, M.S. Cigarette Smoking and Human Gut Microbiota in Healthy Adults: A Systematic Review. Biomedicines 2022, 10, 510. [Google Scholar] [CrossRef]
- Sharma, A.; Lee, J.; Fonseca, A.G.; Moshensky, A.; Kothari, T.; Sayed, I.M.; Ibeawuchi, S.R.; Pranadinata, R.F.; Ear, J.; Sahoo, D.; et al. E-cigarettes compromise the gut barrier and trigger inflammation. iScience 2021, 24, 102035. [Google Scholar] [CrossRef]
- Wang, L.; Cai, Y.; Garssen, J.; Henricks, P.A.J.; Folkerts, G.; Braber, S. The Bidirectional Gut-Lung Axis in Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 2023, 207, 1145–1160. [Google Scholar] [CrossRef]
- Fricker, M.; Goggins, B.J.; Mateer, S.; Jones, B.; Kim, R.Y.; Gellatly, S.L.; Jarnicki, A.G.; Powell, N.; Oliver, B.G.; Radford-Smith, G.; et al. Chronic cigarette smoke exposure induces systemic hypoxia that drives intestinal dysfunction. JCI Insight 2018, 3, e94040. [Google Scholar] [CrossRef]
- Pral, L.P.; Fachi, J.L.; Correa, R.O.; Colonna, M.; Vinolo, M.A.R. Hypoxia and HIF-1 as key regulators of gut microbiota and host interactions. Trends Immunol. 2021, 42, 604–621. [Google Scholar] [CrossRef]
- Daijo, H.; Hoshino, Y.; Kai, S.; Suzuki, K.; Nishi, K.; Matsuo, Y.; Harada, H.; Hirota, K. Cigarette smoke reversibly activates hypoxia-inducible factor 1 in a reactive oxygen species-dependent manner. Sci. Rep. 2016, 6, 34424. [Google Scholar] [CrossRef] [PubMed]
- Larsson, L.; Pehrson, C.; Dechen, T.; Crane-Godreau, M. Microbiological components in mainstream and sidestream cigarette smoke. Tob. Induc. Dis. 2012, 10, 13. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Cheng, J.; Yang, Y.; Wang, H.; Fu, Y.; Li, X.; Wang, W.; Ma, S.; Xu, X.; Lu, F.; et al. A 90-Day Subchronic Exposure to Heated Tobacco Product Aerosol Caused Differences in Intestinal Inflammation and Microbiome Dysregulation in Rats. Nicotine Tob. Res. 2025, 27, 438–446. [Google Scholar] [CrossRef] [PubMed]
- Fuke, N.; Nagata, N.; Suganuma, H.; Ota, T. Regulation of Gut Microbiota and Metabolic Endotoxemia with Dietary Factors. Nutrients 2019, 11, 2277. [Google Scholar] [CrossRef]
- Mukohda, M.; Yano, T.; Matsui, T.; Nakamura, S.; Miyamae, J.; Toyama, K.; Mitsui, R.; Mizuno, R.; Ozaki, H. Treatment with Ligilactobacillus murinus lowers blood pressure and intestinal permeability in spontaneously hypertensive rats. Sci. Rep. 2023, 13, 15197. [Google Scholar] [CrossRef]
- Moludi, J.; Kafil, H.S.; Qaisar, S.A.; Gholizadeh, P.; Alizadeh, M.; Vayghyan, H.J. Effect of probiotic supplementation along with calorie restriction on metabolic endotoxemia, and inflammation markers in coronary artery disease patients: A double blind placebo controlled randomized clinical trial. Nutr. J. 2021, 20, 47. [Google Scholar] [CrossRef]
- Battey, J.N.D.; Szostak, J.; Phillips, B.; Teng, C.; Tung, C.K.; Lim, W.T.; Yeo, Y.S.; Ouadi, S.; Baumer, K.; Thomas, J.; et al. Impact of 6-Month Exposure to Aerosols From Potential Modified Risk Tobacco Products Relative to Cigarette Smoke on the Rodent Gastrointestinal Tract. Front. Microbiol. 2021, 12, 587745. [Google Scholar] [CrossRef]
- Fasano, A.; Not, T.; Wang, W.; Uzzau, S.; Berti, I.; Tommasini, A.; Goldblum, S.E. Zonulin, a newly discovered modulator of intestinal permeability, and its expression in coeliac disease. Lancet 2000, 355, 1518–1519. [Google Scholar] [CrossRef]
- Lu, Y.C.; Yeh, W.C.; Ohashi, P.S. LPS/TLR4 signal transduction pathway. Cytokine 2008, 42, 145–151. [Google Scholar] [CrossRef]
- Mignini, I.; Galasso, L.; Piccirilli, G.; Calvez, V.; Termite, F.; Esposto, G.; Borriello, R.; Miele, L.; Ainora, M.E.; Gasbarrini, A.; et al. Interplay of Oxidative Stress, Gut Microbiota, and Nicotine in Metabolic-Associated Steatotic Liver Disease (MASLD). Antioxidants 2024, 13, 1532. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhao, M.; Ji, K.; Li, J.; Wang, S.; Lu, L.; Chen, Z.; Zeng, J. Association of nicotine dependence and gut microbiota: A bidirectional two-sample Mendelian randomization study. Front. Immunol. 2023, 14, 1244272. [Google Scholar] [CrossRef] [PubMed]
- Salman, R.; Talih, S.; El-Hage, R.; Haddad, C.; Karaoghlanian, N.; El-Hellani, A.; Saliba, N.A.; Shihadeh, A. Free-Base and Total Nicotine, Reactive Oxygen Species, and Carbonyl Emissions From IQOS, a Heated Tobacco Product. Nicotine Tob. Res. 2019, 21, 1285–1288. [Google Scholar] [CrossRef]
- Yuri, K.; Higashiyama, A.; Takemura, S.; Suzuki, H.; Bassole Epse Brou, M.A.M.; Zhang, Y.; Aono, N.; Tabuchi, T.; Fujiyoshi, A. Do perceptions of the harm of heated tobacco products differ by smoking status? A cross-sectional analysis of the Japan Society and New Tobacco Internet Survey (JASTIS) 2020 in Japan. BMJ Public Health 2025, 3, e002516. [Google Scholar] [CrossRef]
- Loffredo, L.; Alfano, A.R.; Ettorre, E.; Desideri, G.; Carnevale, R.; Forte, M.; Maglione, V.; Bartimoccia, S.; Castellani, V.; Tote, C.M.; et al. Effect of the probiotic Escherichia coli Nissle 1917 on serum levels of NADPH oxidase-2 and lipopolysaccharide in patients with Alzheimer’s disease. J. Alzheimer’s Dis. 2025, 106, 13872877251345157. [Google Scholar] [CrossRef]
- Pannunzio, A.; Baratta, F.; Maggio, E.; Palumbo, I.M.; Magna, A.; Trivigno, C.; Carnevale, R.; Simona, B.; Cammisotto, V.; Vidili, G.; et al. Dark chocolate’s impact on low-grade endotoxemia in metabolic dysfunction-associated steatohepatitis. Nutrition 2025, 131, 112643. [Google Scholar] [CrossRef] [PubMed]

| Children | |||
| Variable | Controls (N = 26) | TT (N = 26) | HTPs (N = 26) |
| Age | 9 ± 3 | 9 ± 3 | 10 ± 3 |
| Female Gender | 19 (73%) | 16 (62%) | 17 (65%) |
| FMD (%) | 7.93 ± 2.30 | 5.78 ± 2.92 | 5.51 ± 3.00 |
| NO (μM) | 60.69 ± 11.44 | 48.12 ± 11.15 | 49.92 ± 9.01 |
| sNox2-dp (pg/mL) | 17.65 ± 7.92 | 25.96 ± 5.26 | 24.87 ± 7.64 |
| H2O2 (μM) | 23.19 ± 5.41 | 32.35 ± 7.61 | 29.04 ± 6.13 |
| sCD40L (ng/mL) | 1.83 ± 0.48 | 2.78 ± 0.81 | 2.44 ± 0.84 |
| sP-selectin (ng/mL) | 5.10 ± 1.74 | 6.77 ± 1.92 | 6.33 ± 1.20 |
| Platelet aggregation (%) | 25.60 ± 3.78 | 39.60 ± 5.90 | 37.00 ± 7.18 |
| Cotinine (ng/mL) | 1.45 ± 1.25 | 36.59 ± 9.42 | 33.46 ± 6.58 |
| LPS (pg/mL) | 21.95 ± 3.01 | 25.89 ± 3.95 | 26.88 ± 4.31 |
| Zonulin (ng/mL) | 1.94 ± 0.77 | 2.55 ± 0.86 | 2.71 ± 0.90 |
| 8-iso-PGF2α (pg/mL) | 142.50 ± 20.89 | 176.43 ± 43.75 | 178.5 ± 36.26 |
| HBA (%) | 53.37 ± 7.68 | 40.95 ± 6.37 | 45.11 ± 5.73 |
| Adults | |||
| Variable | Controls (N = 20) | TT (N = 20) | HTPs (N = 20) |
| Age | 28 (23–33) | 27 (24– 30) | 33 (28–44) |
| Female Gender | 11 (55%) | 10 (50%) | 12 (60%) |
| FMD (%) | 7.1 (2.8–11.5) | 1.6 (0–3.9) | 3.3 (2.4–6.0) |
| NO (μM) | 41 (38–49) | 10 (9–13) | 10 (8–13) |
| sNox2-dp (pg/mL) | 19 (15–23) | 46 (41–50) | 40 (34–41) |
| H2O2 (μM) | 8.8 (7.2–11.9) | 33.5 (19.5–52.7) | 26.7 (21.9–33.8) |
| sCD40L (ng/mL) | 1.6 (1.1–2.1) | 3.2 (2.5–4.4) | 3.0 (2.5–3.3) |
| sP-selectin (ng/mL) | 3.0 (2.0–3.9) | 9.2 (6.7–12.0) | 8.1 (5.5–9.2) |
| Platelet aggregation (%) | 62 (58–70) | 80 (77–80) | 76 (70–80) |
| Cotinine (ng/mL) | 2 (2–3) | 139 (130–148) | 137 (103–163) |
| LPS (pg/mL) | 21.68 ± 4.82 | 26.62 ± 4.58 | 27.43 ± 4.31 |
| Zonulin (ng/mL) | 1.68 ± 0.38 | 2.28 ± 0.53 | 2.55 ± 0.92 |
| β | SE | p | |
|---|---|---|---|
| Zonulin (ng/mL) | 0.441 | 0.452 | <0.001 |
| sCD40L (ng/mL) | 0.042 | 0.504 | 0.665 |
| sP-Selectin (ng/mL) | 0.017 | 0.262 | 0.861 |
| HBA (%) | −0.027 | 0.054 | 0.798 |
| Cotinin (ng/mL) | 0.343 | 0.029 | 0.005 |
| β | SE | p | |
|---|---|---|---|
| Zonulin (ng/mL) | 0.477 | 0.751 | <0.001 |
| NO (µM) | −0.307 | 0.033 | 0.007 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Loffredo, L.; Maggio, E.; Bartimoccia, S.; Magna, A.; Totè, C.M.; Bagnato, C.; Cinicola, B.L.; Armeli, F.; Leonardo, A.; D’Amico, A.; et al. Tobacco Smoke Exposure and Oxidative Stress: The Role of Circulating Lipopolysaccharides in Heated and Conventional Products. Antioxidants 2025, 14, 1316. https://doi.org/10.3390/antiox14111316
Loffredo L, Maggio E, Bartimoccia S, Magna A, Totè CM, Bagnato C, Cinicola BL, Armeli F, Leonardo A, D’Amico A, et al. Tobacco Smoke Exposure and Oxidative Stress: The Role of Circulating Lipopolysaccharides in Heated and Conventional Products. Antioxidants. 2025; 14(11):1316. https://doi.org/10.3390/antiox14111316
Chicago/Turabian StyleLoffredo, Lorenzo, Enrico Maggio, Simona Bartimoccia, Arianna Magna, Chiara Maria Totè, Chiara Bagnato, Bianca Laura Cinicola, Federica Armeli, Angela Leonardo, Alessandra D’Amico, and et al. 2025. "Tobacco Smoke Exposure and Oxidative Stress: The Role of Circulating Lipopolysaccharides in Heated and Conventional Products" Antioxidants 14, no. 11: 1316. https://doi.org/10.3390/antiox14111316
APA StyleLoffredo, L., Maggio, E., Bartimoccia, S., Magna, A., Totè, C. M., Bagnato, C., Cinicola, B. L., Armeli, F., Leonardo, A., D’Amico, A., Greco, E., Frati, G., Biondi-Zoccai, G., Spalice, A., Angeloni, A., Pignatelli, P., Violi, F., Zicari, A. M., Carnevale, R., & Smoking Prevention Study Group. (2025). Tobacco Smoke Exposure and Oxidative Stress: The Role of Circulating Lipopolysaccharides in Heated and Conventional Products. Antioxidants, 14(11), 1316. https://doi.org/10.3390/antiox14111316