Parthenolide Restores Testosterone Biosynthesis After Nanoplastic Exposure by Blocking ROS-Driven NF-κB Nuclear Translocation
Abstract
1. Introduction
2. Materials and Methods
2.1. PS-NPs Source
2.2. Animal
2.3. Experimental Protocol
2.4. Sample Collection
2.5. Hematoxylin and Eosin (H&E) Staining
2.6. TEM
2.7. RNA Sequencing and Bioinformatic Analysis
2.8. Cell Culture and Treatment
2.9. Ros
2.10. Western Blotting
2.11. Immunofluorescence (IF)
2.12. Reverse Transcription Quantitative PCR (RT–qPCR)
2.13. Determination of Testosterone, Malondialdehyde (MDA), and Glutathione (GSH) Levels
2.14. Co-Immunoprecipitation (Co-IP)
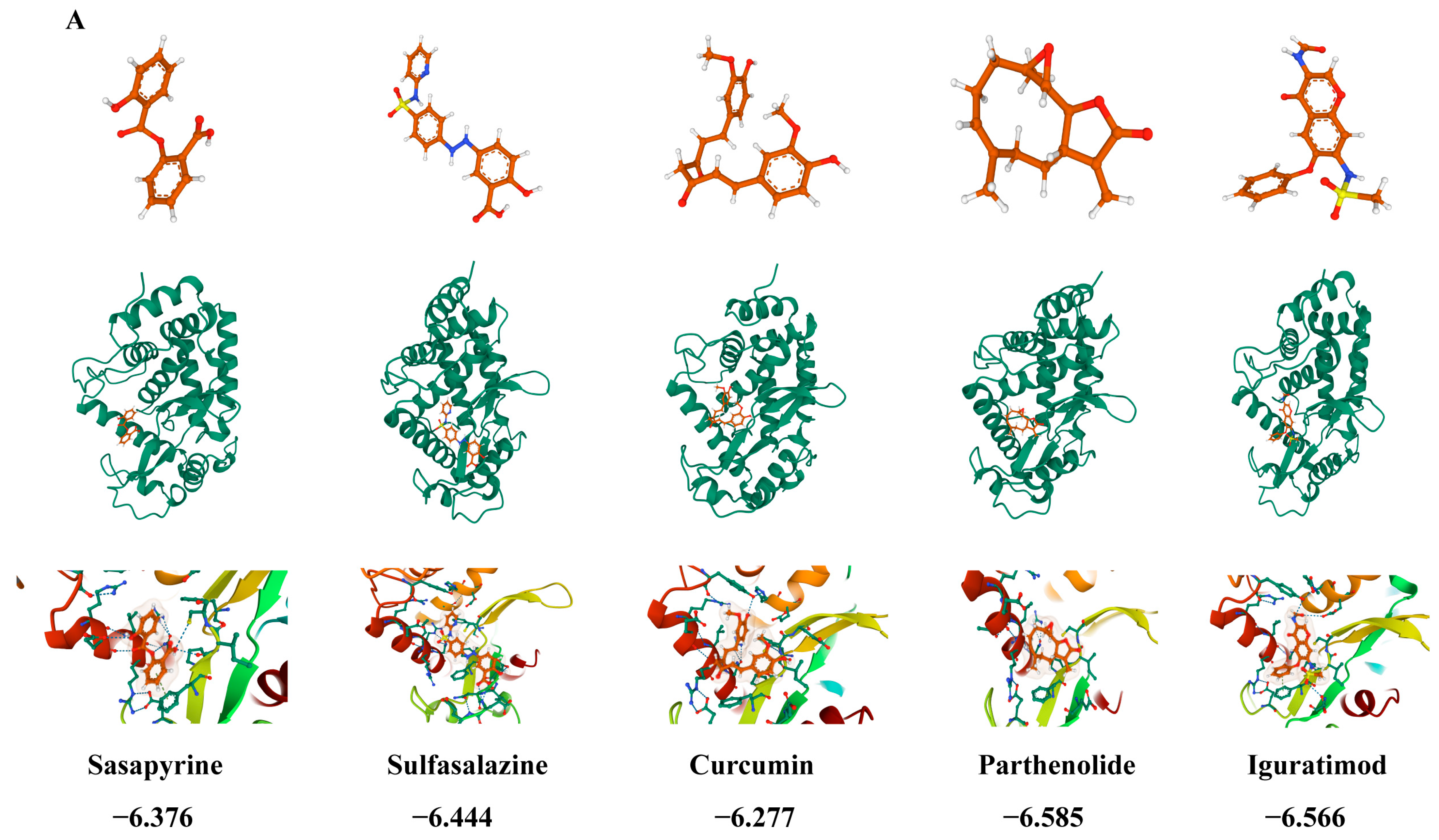
2.15. Drug Prediction and Molecular Docking
2.16. Statistical Analysis
3. Results
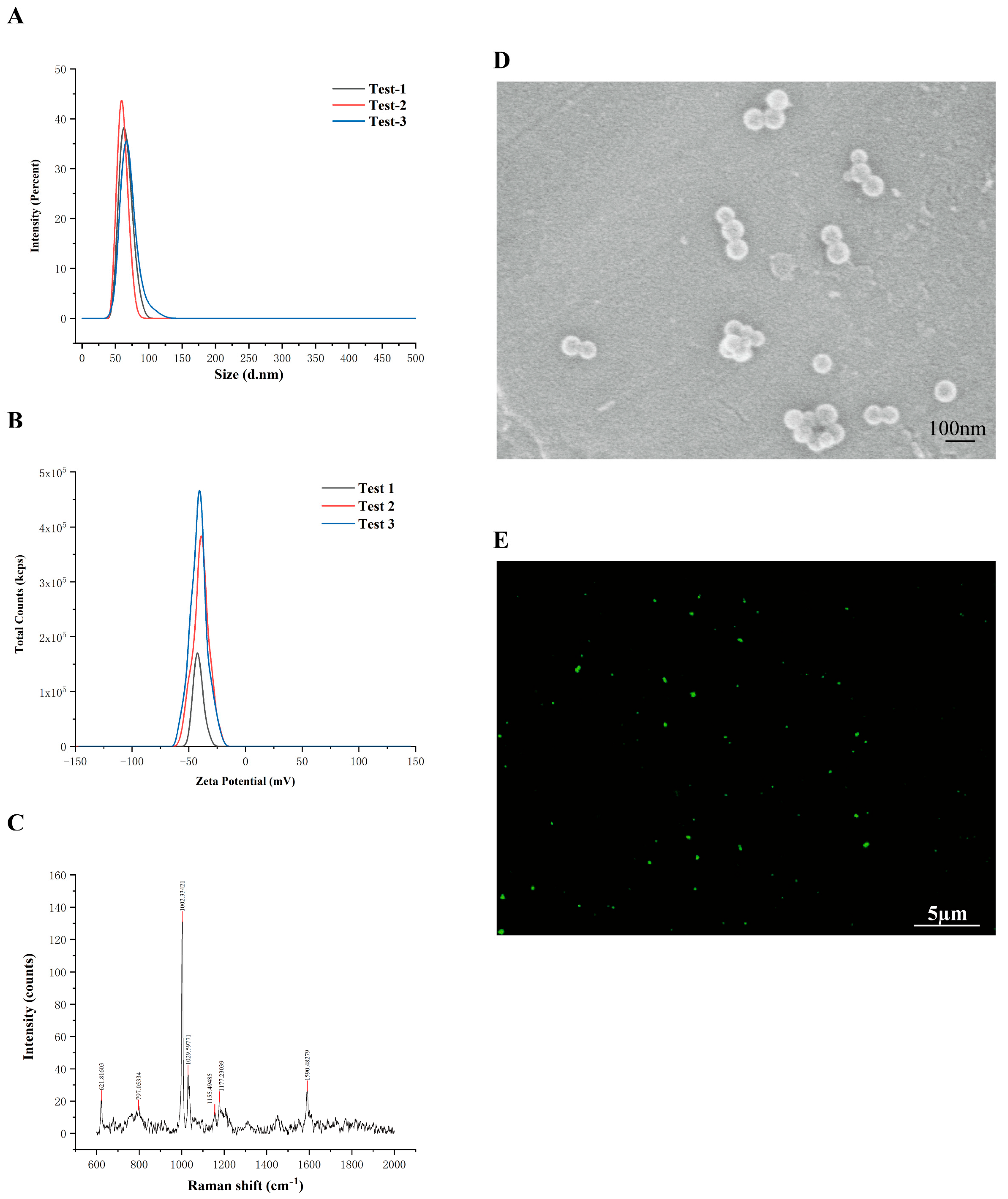
3.1. Phenotypic Characteristics of PS-NPs
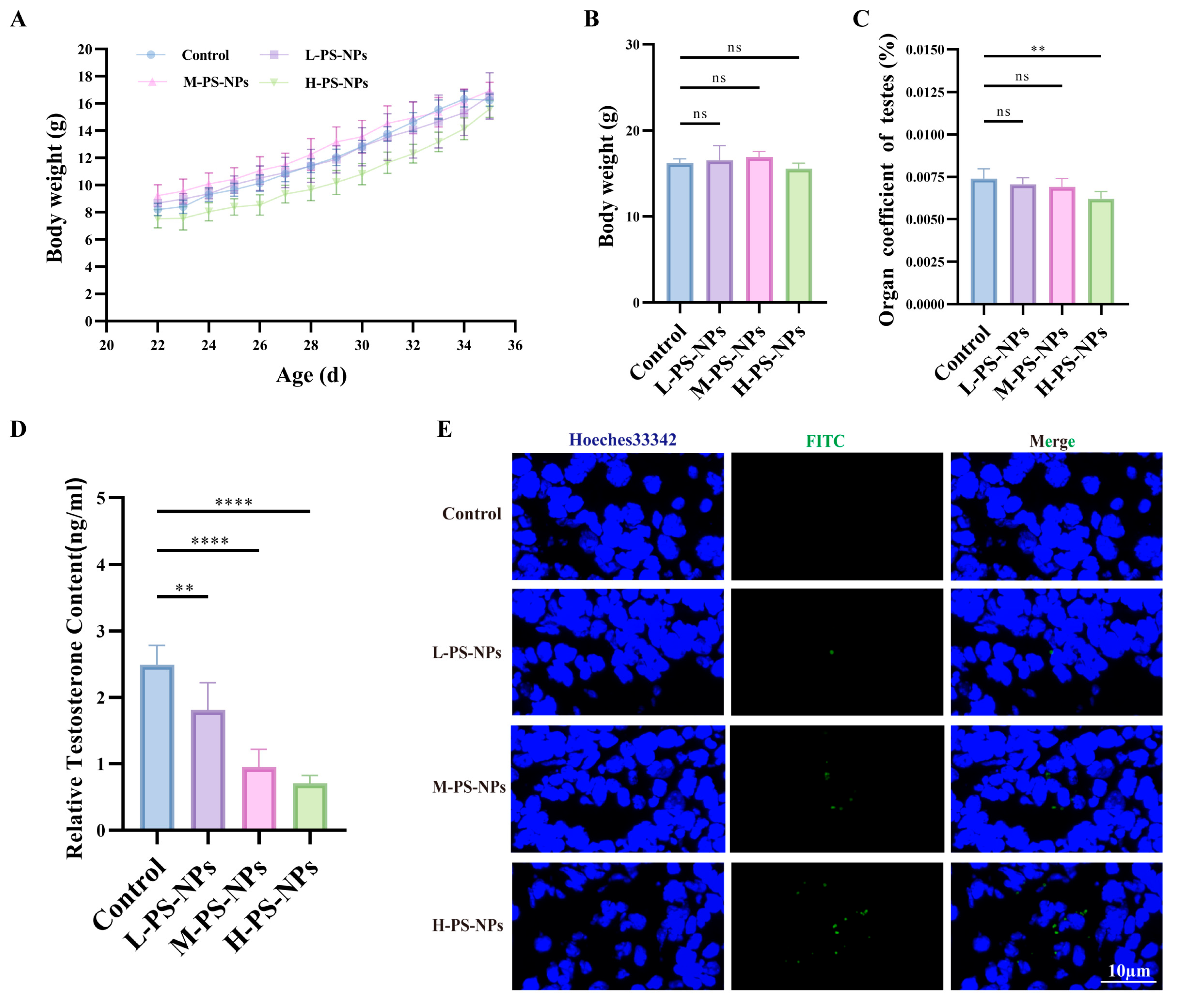
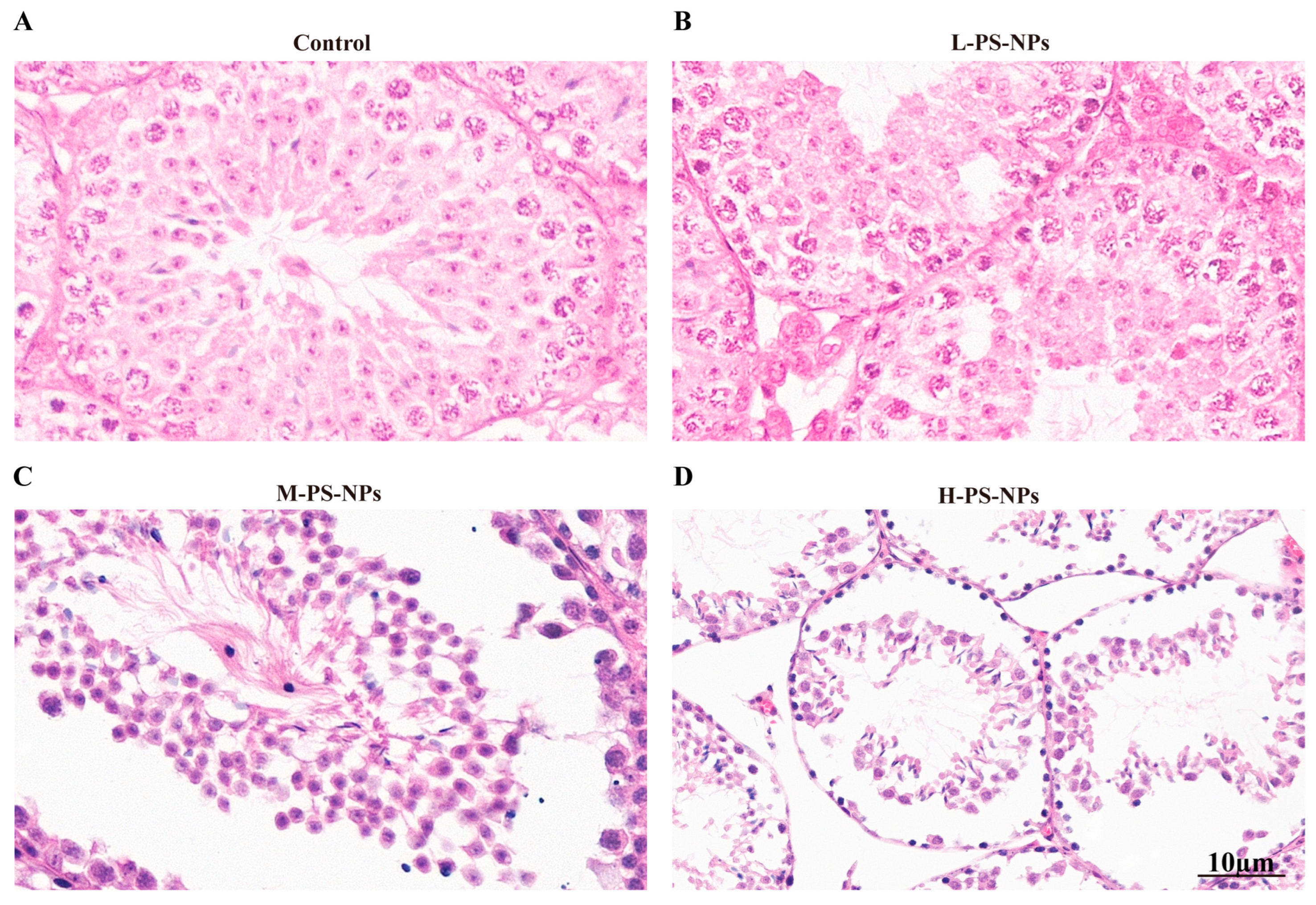
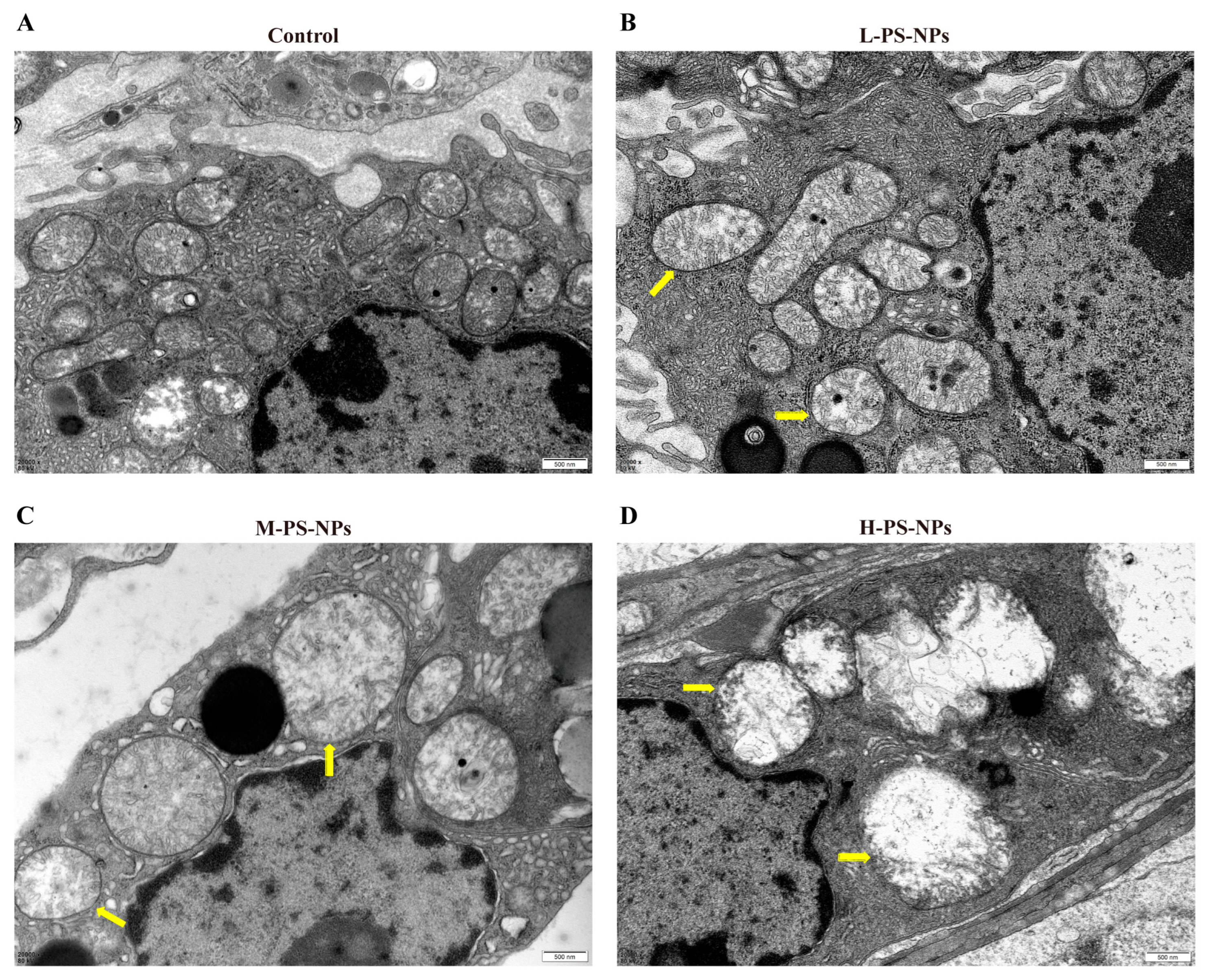
3.2. Exposure to PS-NPs Induces Structural Damage in Immature Mouse Testes
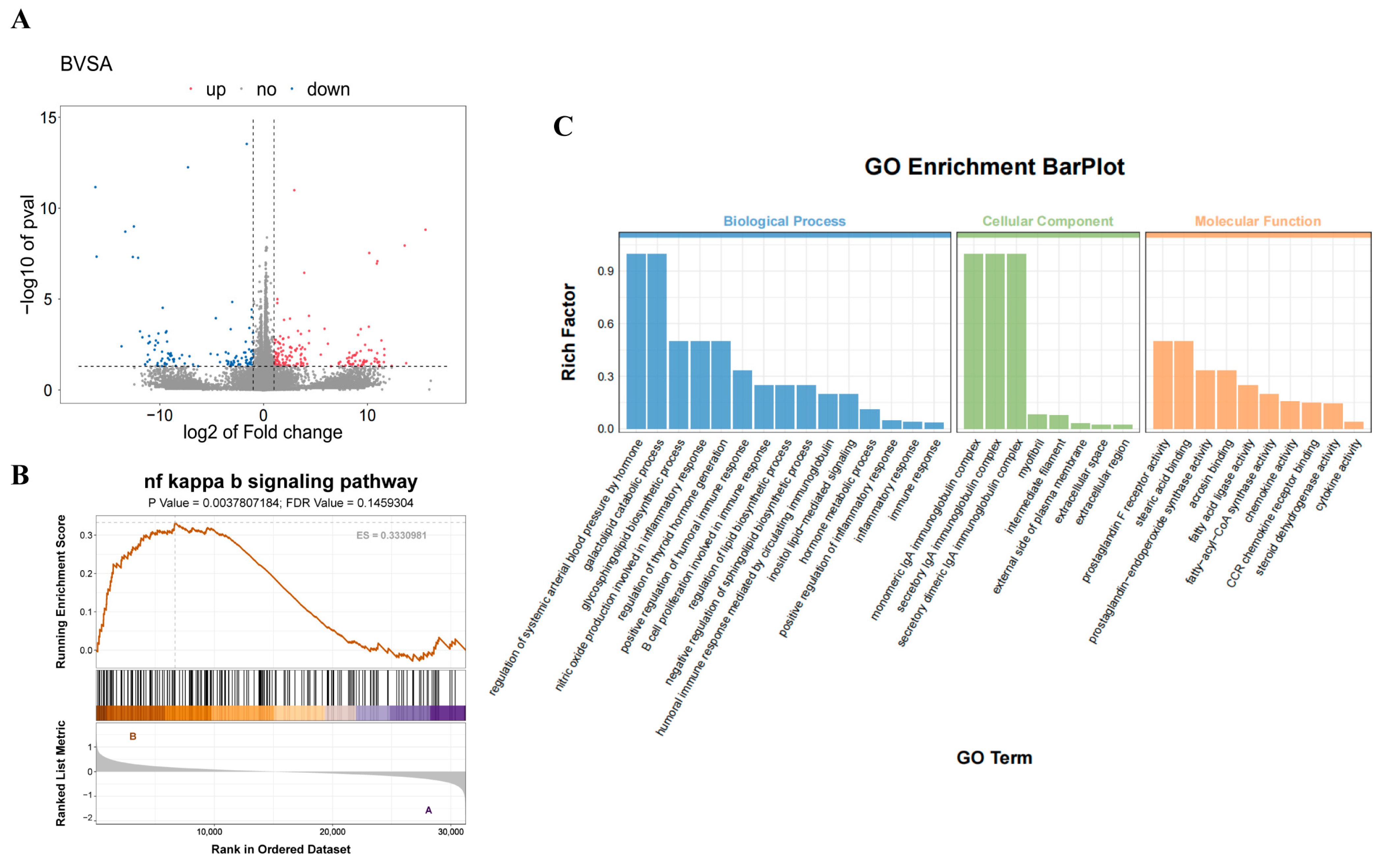
3.3. Transcriptomic Analysis Reveals PS-NPs-Induced Impairment of Testicular Testosterone Synthesis
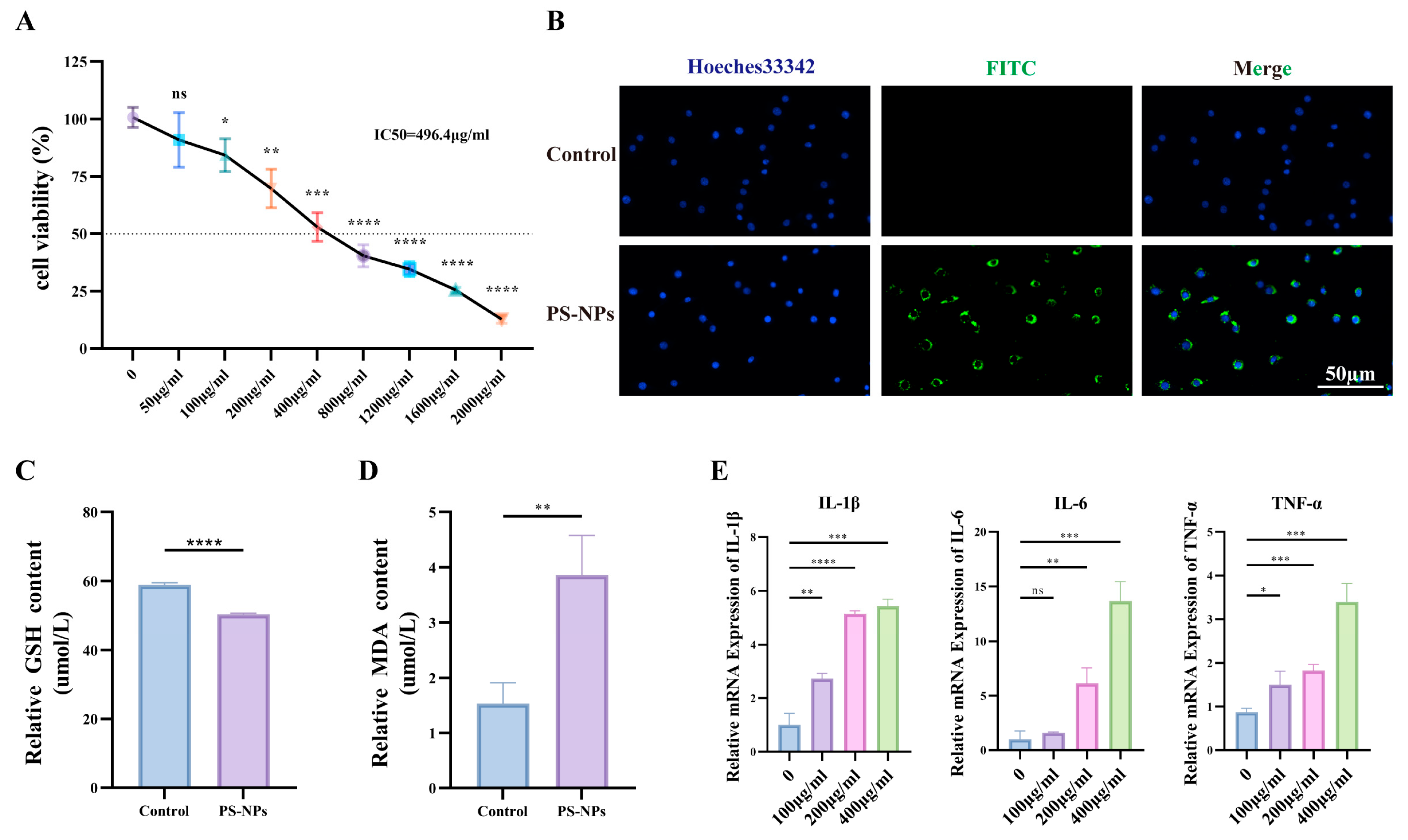
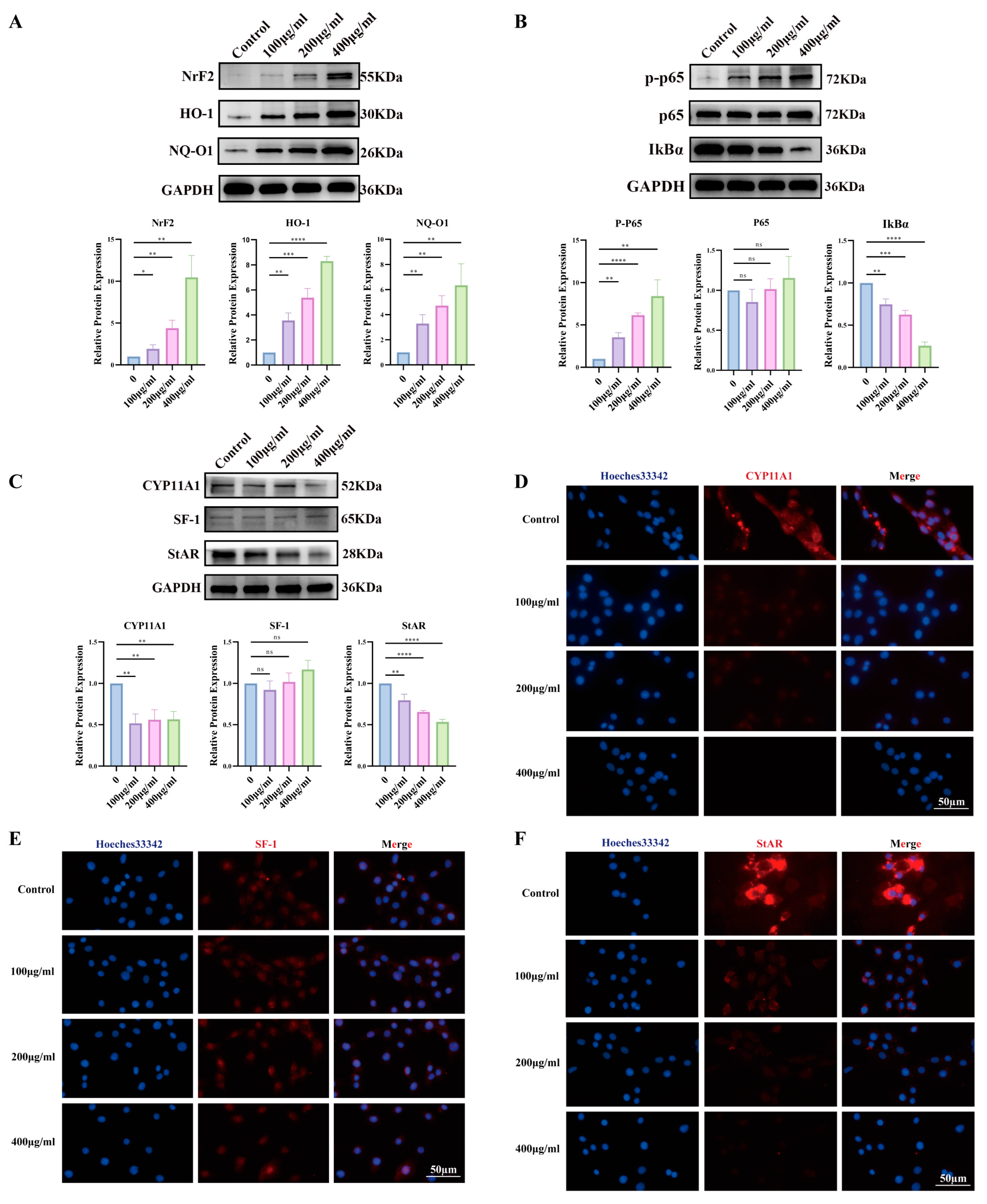
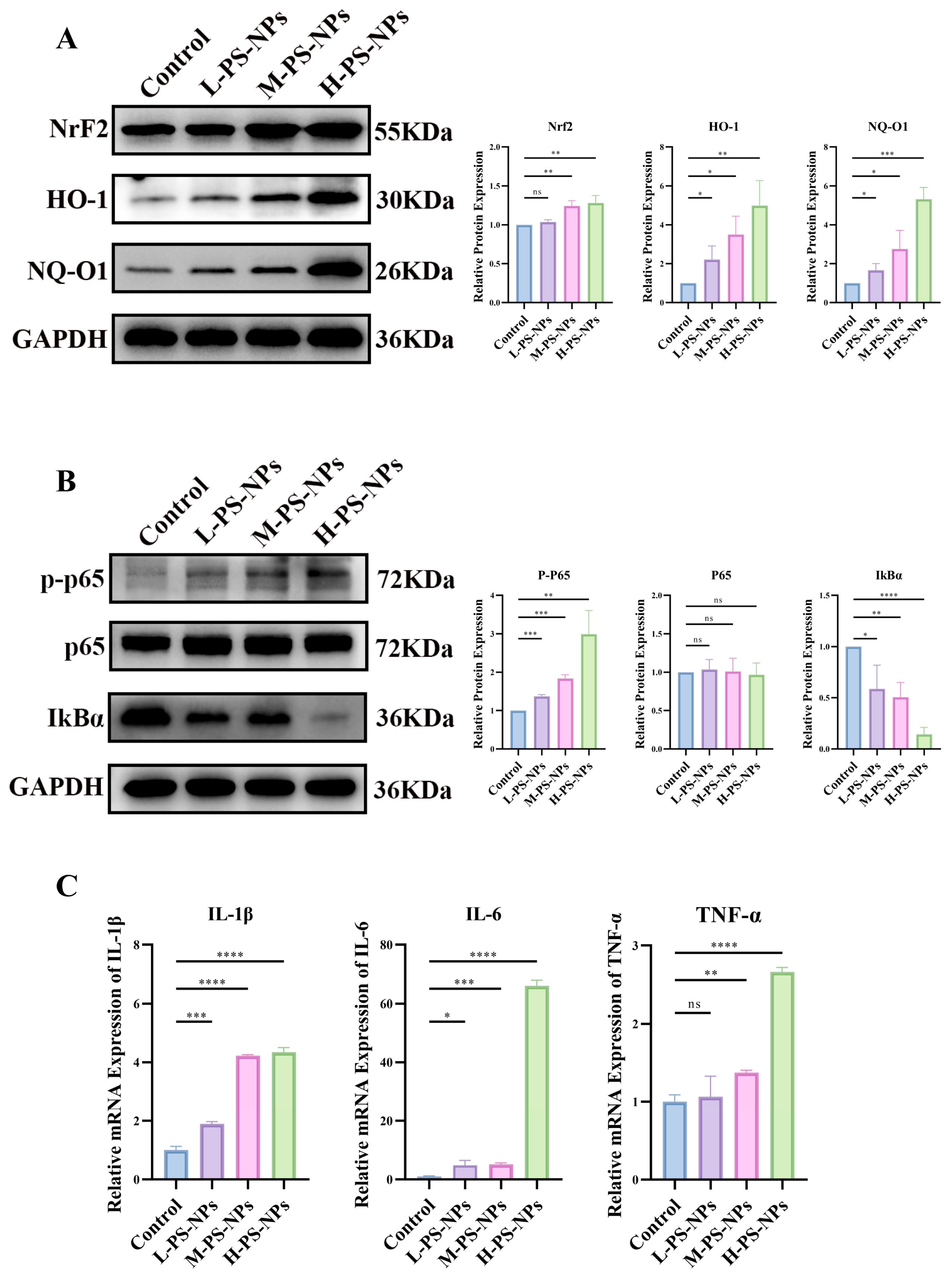
3.4. PS-NPs Activate the ROS/NF-κB Pathway in TM3 Cells and Reduce Testosterone Production
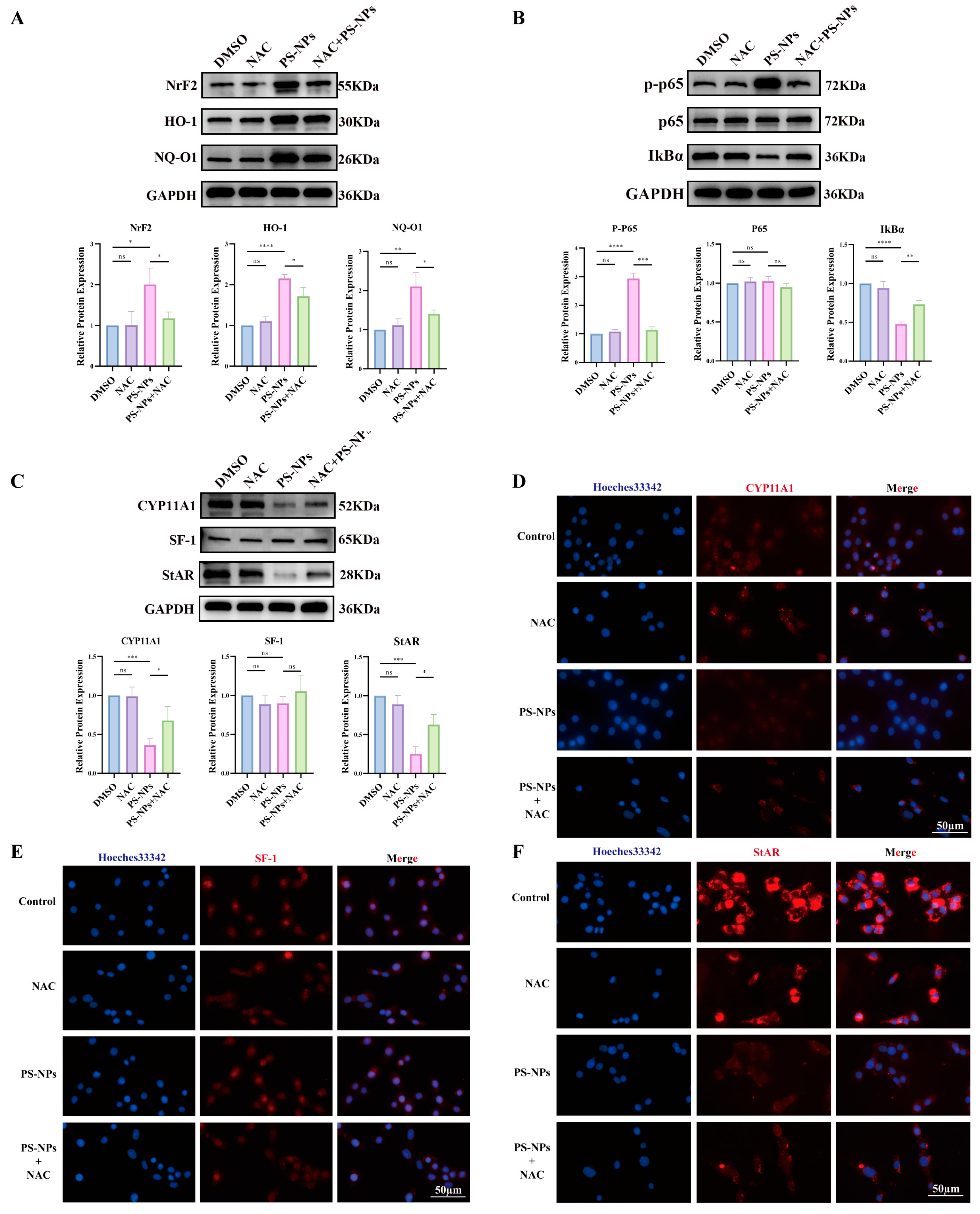
3.5. NAC Mitigates PS-NP-Induced ROS/NF-κB Activation and Restores Testosterone Synthesis
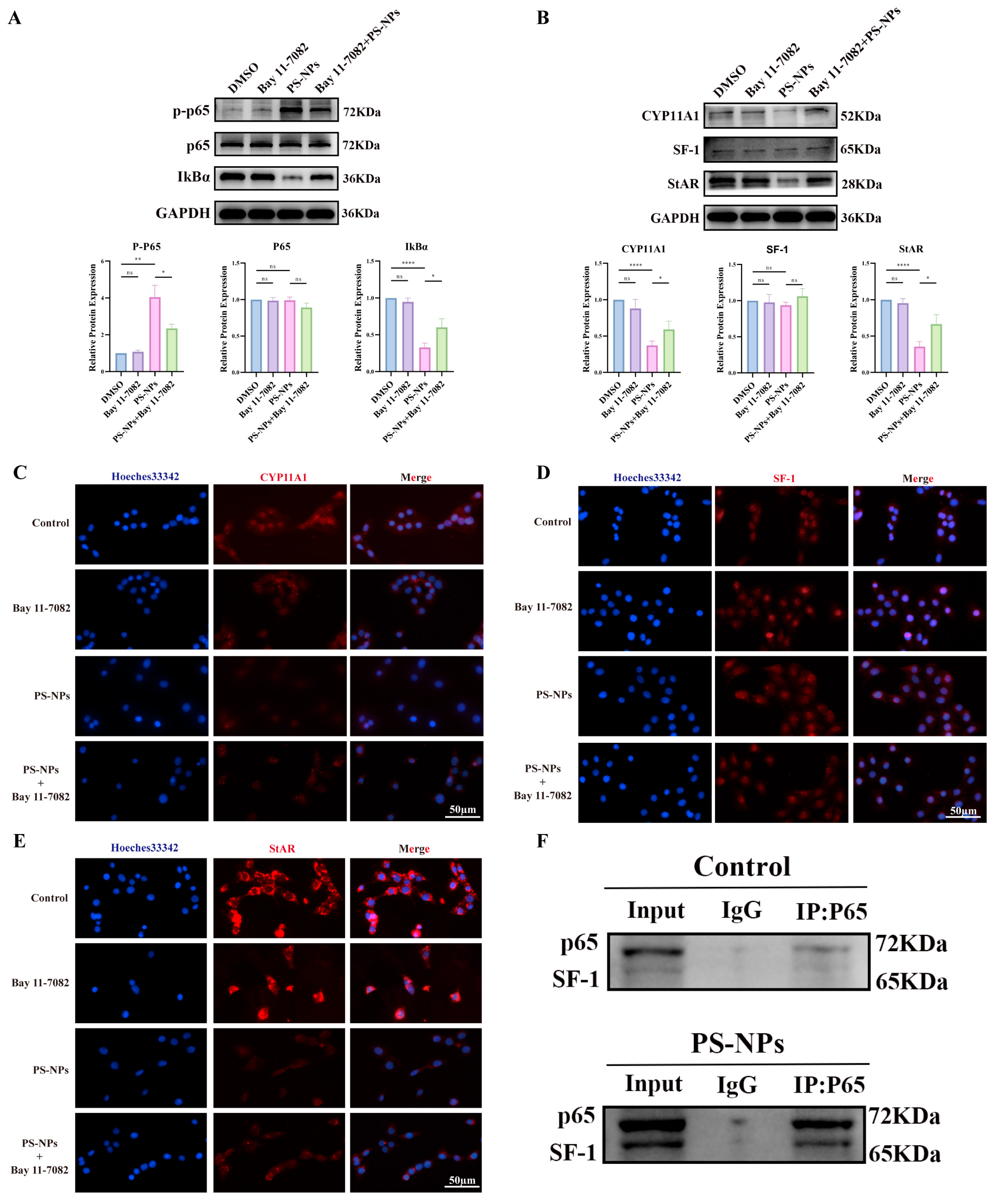
3.6. Bay 11-7082 Inhibits NF-κB Activation and Restores Testosterone Synthesis
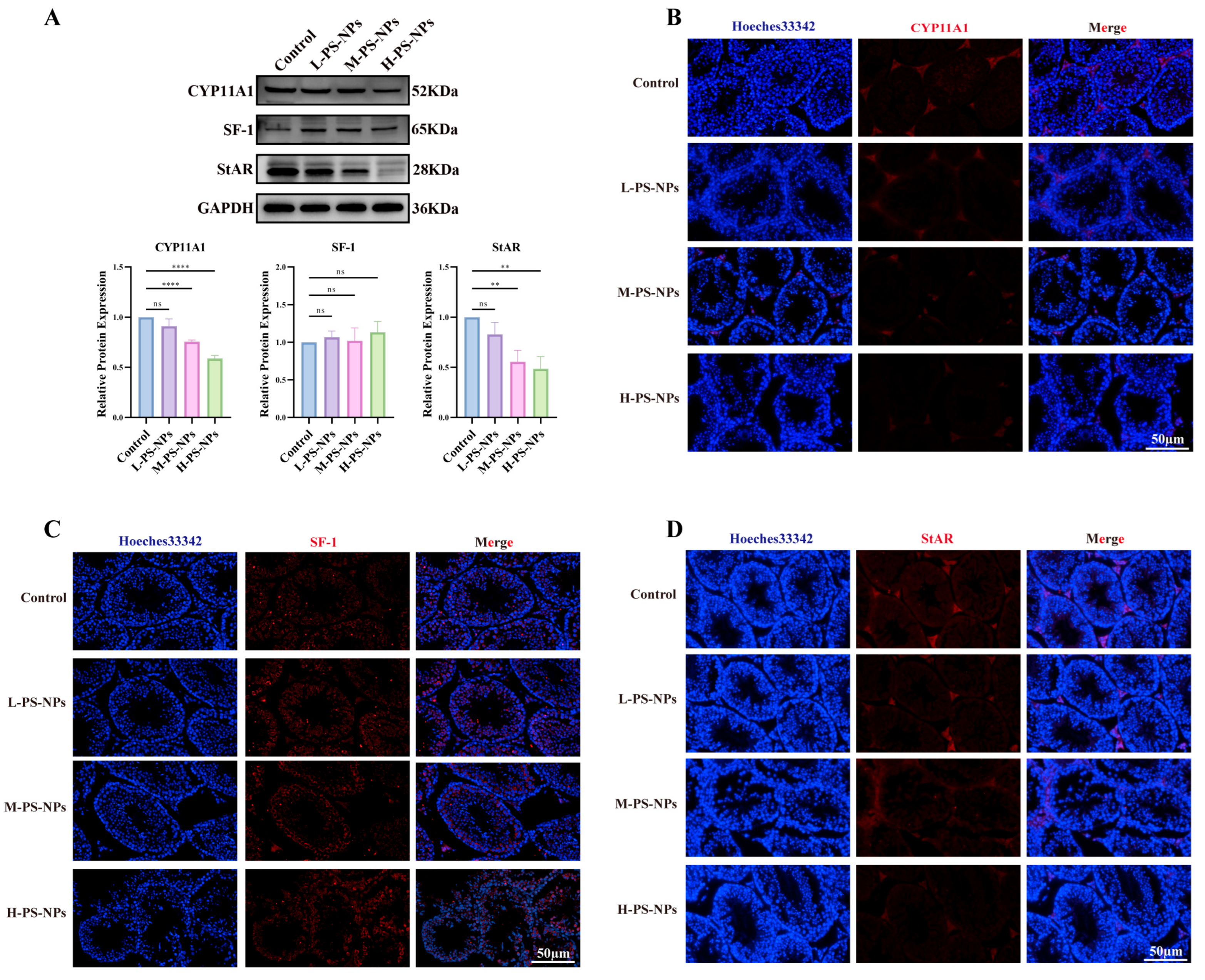
3.7. PS-NPs Activate the ROS/NF-κB Pathway in Testicular Tissue
3.8. PTL Rescues PS-NP-Induced Testicular Damage
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chao, H.H.; Zhang, Y.; Dong, P.Y.; Gurunathan, S.; Zhang, X.F. Comprehensive review on the positive and negative effects of various important regulators on male spermatogenesis and fertility. Front. Nutr. 2022, 9, 1063510. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Wang, Z.; Kong, Y.; Jin, M.; Ma, L. Global, regional and national burden of male infertility in 204 countries and territories between 1990 and 2019: An analysis of global burden of disease study. BMC Public Health 2023, 23, 2195. [Google Scholar] [CrossRef]
- Daniels, D.; Berger Eberhardt, A. Climate change, microplastics, and male infertility. Curr. Opin. Urol. 2024, 34, 366–370. [Google Scholar] [CrossRef]
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, use, and fate of all plastics ever made. Sci. Adv. 2017, 3, e1700782. [Google Scholar] [CrossRef] [PubMed]
- Rai, P.K.; Lee, J.; Brown, R.J.C.; Kim, K.H. Environmental fate, ecotoxicity biomarkers, and potential health effects of micro- and nano-scale plastic contamination. J. Hazard. Mater. 2021, 403, 123910. [Google Scholar] [CrossRef]
- Liu, Z.; Huang, Y.; Jiao, Y.; Chen, Q.; Wu, D.; Yu, P.; Li, Y.; Cai, M.; Zhao, Y. Polystyrene nanoplastic induces ROS production and affects the MAPK-HIF-1/NFkB-mediated antioxidant system in Daphnia pulex. Aquat. Toxicol. 2020, 220, 105420. [Google Scholar] [CrossRef]
- Sarasamma, S.; Audira, G.; Siregar, P.; Malhotra, N.; Lai, Y.H.; Liang, S.T.; Chen, J.R.; Chen, K.H.; Hsiao, C.D. Nanoplastics Cause Neurobehavioral Impairments, Reproductive and Oxidative Damages, and Biomarker Responses in Zebrafish: Throwing up Alarms of Wide Spread Health Risk of Exposure. Int. J. Mol. Sci. 2020, 21, 1410. [Google Scholar] [CrossRef]
- Xu, D.; Ma, Y.; Han, X.; Chen, Y. Systematic toxicity evaluation of polystyrene nanoplastics on mice and molecular mechanism investigation about their internalization into Caco-2 cells. J. Hazard. Mater. 2021, 417, 126092. [Google Scholar] [CrossRef]
- Sun, S.C. The non-canonical NF-κB pathway in immunity and inflammation. Nat. Rev. Immunol. 2017, 17, 545–558. [Google Scholar] [CrossRef]
- Chen, X.; Xuan, Y.; Chen, Y.; Yang, F.; Zhu, M.; Xu, J.; Chen, J. Polystyrene nanoplastics induce intestinal and hepatic inflammation through activation of NF-κB/NLRP3 pathways and related gut-liver axis in mice. Sci. Total Environ. 2024, 935, 173458. [Google Scholar] [CrossRef] [PubMed]
- Pei, J.; Chen, S.; Ke, Q.; Pang, A.; Niu, M.; Li, N.; Li, J.; Wang, Z.; Wu, H.; Nie, P. Immune response to polystyrene microplastics: Regulation of inflammatory response via the ROS-driven NF-κB pathway in zebrafish (Danio rerio). Aquat. Toxicol. 2025, 282, 107308. [Google Scholar] [CrossRef]
- Xu, W.; Yuan, Y.; Tian, Y.; Cheng, C.; Chen, Y.; Zeng, L.; Yuan, Y.; Li, D.; Zheng, L.; Luo, T. Oral exposure to polystyrene nanoplastics reduced male fertility and even caused male infertility by inducing testicular and sperm toxicities in mice. J. Hazard. Mater. 2023, 454, 131470. [Google Scholar] [CrossRef]
- Gong, D.Y.; Chen, X.Y.; Guo, S.X.; Wang, B.C.; Li, B. Recent advances and new insights in biosynthesis of dendrobine and sesquiterpenes. Appl. Microbiol. Biotechnol. 2021, 105, 6597–6606. [Google Scholar] [CrossRef]
- Ghantous, A.; Sinjab, A.; Herceg, Z.; Darwiche, N. Parthenolide: From plant shoots to cancer roots. Drug Discov. Today 2013, 18, 894–905. [Google Scholar] [CrossRef]
- Deller, R.C.; Diamanti, P.; Morrison, G.; Reilly, J.; Ede, B.C.; Richardson, R.; Le Vay, K.; Collins, A.M.; Blair, A.; Perriman, A.W. Functionalized Triblock Copolymer Vectors for the Treatment of Acute Lymphoblastic Leukemia. Mol. Pharm. 2017, 14, 722–732. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Gao, L.; Hu, K.; Wang, Q.M. Parthenolide enhances the metronomic chemotherapy effect of cyclophosphamide in lung cancer by inhibiting the NF-kB signaling pathway. World J. Clin. Oncol. 2024, 15, 895–907. [Google Scholar] [CrossRef] [PubMed]
- Toraman, E.; Budak, B.; Bayram, C.; Sezen, S.; Mokhtare, B.; Hacımüftüoğlu, A. Role of parthenolide in paclitaxel-induced oxidative stress injury and impaired reproductive function in rat testicular tissue. Chem. Biol. Interact. 2024, 387, 110793. [Google Scholar] [CrossRef] [PubMed]
- Danaei, M.; Dehghankhold, M.; Ataei, S.; Hasanzadeh Davarani, F.; Javanmard, R.; Dokhani, A.; Khorasani, S.; Mozafari, M.R. Impact of Particle Size and Polydispersity Index on the Clinical Applications of Lipidic Nanocarrier Systems. Pharmaceutics 2018, 10, 57. [Google Scholar] [CrossRef]
- Clogston, J.D.; Patri, A.K. Zeta potential measurement. Methods Mol. Biol. 2011, 697, 63–70. [Google Scholar]
- Bhattacharjee, S. DLS and zeta potential—What they are and what they are not? J. Control. Release 2016, 235, 337–351. [Google Scholar]
- Patel, V.R.; Agrawal, Y.K. Nanosuspension: An approach to enhance solubility of drugs. J. Adv. Pharm. Technol. Res. 2011, 2, 81–87. [Google Scholar] [CrossRef]
- Nair, A.B.; Jacob, S. A simple practice guide for dose conversion between animals and human. J. Basic Clin. Pharm. 2016, 7, 27–31. [Google Scholar] [CrossRef]
- Zuccarello, P.; Ferrante, M.; Cristaldi, A.; Copat, C.; Grasso, A.; Sangregorio, D.; Fiore, M.; Oliveri Conti, G. Exposure to microplastics (<10 μm) associated to plastic bottles mineral water consumption: The first quantitative study. Water Res. 2019, 157, 365–371. [Google Scholar] [CrossRef] [PubMed]
- Senathirajah, K.; Attwood, S.; Bhagwat, G.; Carbery, M.; Wilson, S.; Palanisami, T. Estimation of the mass of microplastics ingested—A pivotal first step towards human health risk assessment. J. Hazard. Mater. 2021, 404, 124004. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Chen, L.; Kong, X.; Huang, T.; Cai, Y.D. Analysis of tumor suppressor genes based on gene ontology and the KEGG pathway. PLoS ONE 2014, 9, e107202. [Google Scholar] [CrossRef]
- Glass, K.; Girvan, M. Annotation enrichment analysis: An alternative method for evaluating the functional properties of gene sets. Sci. Rep. 2014, 4, 4191. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.; Zhang, J.; Wang, B.; Xu, G.; Yang, X.; Zou, Z.; Yu, C. Ferritinophagy is involved in the zinc oxide nanoparticles-induced ferroptosis of vascular endothelial cells. Autophagy 2021, 17, 4266–4285. [Google Scholar] [CrossRef]
- Zhang, L.; Xie, J.; Gan, R.; Wu, Z.; Luo, H.; Chen, X.; Lu, Y.; Wu, L.; Zheng, D. Synergistic inhibition of lung cancer cells by EGCG and NF-κB inhibitor BAY11-7082. J. Cancer 2019, 10, 6543–6556. [Google Scholar] [CrossRef]
- Nasilli, G.; de Waal, T.M.; Marchal, G.A.; Bertoli, G.; Veldkamp, M.W.; Rothenberg, E.; Casini, S.; Remme, C.A. Decreasing microtubule detyrosination modulates Nav1.5 subcellular distribution and restores sodium current in mdx cardiomyocytes. Cardiovasc. Res. 2024, 120, 723–734. [Google Scholar] [CrossRef]
- Qu, X.A.; Rajpal, D.K. Applications of Connectivity Map in drug discovery and development. Drug Discov. Today 2012, 17, 1289–1298. [Google Scholar] [CrossRef]
- Gao, Y.; Kim, S.; Lee, Y.I.; Lee, J. Cellular Stress-Modulating Drugs Can Potentially Be Identified by in Silico Screening with Connectivity Map (CMap). Int. J. Mol. Sci. 2019, 20, 5601. [Google Scholar] [CrossRef]
- Mozaffari, S.; Moen, A.; Ng, C.Y.; Nicolaes, G.A.F.; Wichapong, K. Structural bioinformatics for rational drug design. Res. Pract. Thromb. Haemost. 2025, 9, 102691. [Google Scholar] [CrossRef] [PubMed]
- Parenti, M.D.; Rastelli, G. Advances and applications of binding affinity prediction methods in drug discovery. Biotechnol. Adv. 2012, 30, 244–250. [Google Scholar] [CrossRef]
- Nayarisseri, A. Experimental and Computational Approaches to Improve Binding Affinity in Chemical Biology and Drug Discovery. Curr. Top. Med. Chem. 2020, 20, 1651–1660. [Google Scholar] [CrossRef]
- Zou, H.; Qu, H.; Bian, Y.; Sun, J.; Wang, T.; Ma, Y.; Yuan, Y.; Gu, J.; Bian, J.; Liu, Z. Polystyrene Microplastics Induce Oxidative Stress in Mouse Hepatocytes in Relation to Their Size. Int. J. Mol. Sci. 2023, 24, 7382. [Google Scholar] [CrossRef]
- Huang, J.; Sun, X.; Wang, Y.; Su, J.; Li, G.; Wang, X.; Yang, Y.; Zhang, Y.; Li, B.; Zhang, G.; et al. Biological interactions of polystyrene nanoplastics: Their cytotoxic and immunotoxic effects on the hepatic and enteric systems. Ecotoxicol. Environ. Saf. 2023, 264, 115447. [Google Scholar] [CrossRef]
- Wang, W.; Guan, J.; Feng, Y.; Nie, L.; Xu, Y.; Xu, H.; Fu, F. Polystyrene microplastics induced nephrotoxicity associated with oxidative stress, inflammation, and endoplasmic reticulum stress in juvenile rats. Front. Nutr. 2022, 9, 1059660. [Google Scholar] [CrossRef]
- Qiao, J.; Chen, R.; Wang, M.; Bai, R.; Cui, X.; Liu, Y.; Wu, C.; Chen, C. Perturbation of gut microbiota plays an important role in micro/nanoplastics-induced gut barrier dysfunction. Nanoscale 2021, 13, 8806–8816. [Google Scholar] [CrossRef]
- Zhang, T.; Yang, S.; Ge, Y.; Wan, X.; Zhu, Y.; Yang, F.; Li, J.; Gong, S.; Cheng, Y.; Hu, C.; et al. Multi-dimensional evaluation of cardiotoxicity in mice following respiratory exposure to polystyrene nanoplastics. Part. Fibre Toxicol. 2023, 20, 46. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.; Zhou, D.; Yang, S.; Shi, Z.; Pan, H.; Jin, Q.; Ding, Z. Neurotoxicity of polystyrene nanoplastics with different particle sizes at environment-related concentrations on early zebrafish embryos. Sci. Total Environ. 2023, 872, 162096. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wu, Y.; Fu, X.; He, S.; Shi, L.; Xu, H.; Shi, X.; Yang, Y.; Zhu, Y.; Wang, Y.; et al. Toxicity to the Male Reproductive System after Exposure to Polystyrene Nanoplastics: A Macrogenomic and Metabolomic Analysis. Toxics 2024, 12, 531. [Google Scholar] [CrossRef]
- Zhang, C.; Li, L.; Alava, J.J.; Yan, Z.; Chen, P.; Gul, Y.; Wang, L.; Xiong, D. Female zebrafish (Danio rerio) exposure to polystyrene nanoplastics induces reproductive toxicity in mother and their offspring. Aquat. Toxicol. 2024, 273, 107023. [Google Scholar] [CrossRef]
- Feng, M.; Luo, J.; Wan, Y.; Zhang, J.; Lu, C.; Wang, M.; Dai, L.; Cao, X.; Yang, X.; Wang, Y. Polystyrene Nanoplastic Exposure Induces Developmental Toxicity by Activating the Oxidative Stress Response and Base Excision Repair Pathway in Zebrafish (Danio rerio). ACS Omega 2022, 7, 32153–32163. [Google Scholar] [CrossRef]
- Merlo, B.; Volsa, A.M.; Tovar, L.; Gaiani, M.; Gugole, P.M.; Attolini, E.; Iacono, E. Effects of polystyrene nanoparticles on bovine oocyte in vitro maturation. Theriogenology 2025, 244, 117482. [Google Scholar] [CrossRef]
- Liu, X.; Lu, B.; Fu, J.; Zhu, X.; Song, E.; Song, Y. Amorphous silica nanoparticles induce inflammation via activation of NLRP3 inflammasome and HMGB1/TLR4/MYD88/NF-kb signaling pathway in HUVEC cells. J. Hazard. Mater. 2021, 404, 124050. [Google Scholar] [PubMed]
- Lingappan, K. NF-κB in Oxidative Stress. Curr. Opin. Toxicol. 2018, 7, 81–86. [Google Scholar] [CrossRef]
- Gülow, K.; Tümen, D.; Heumann, P.; Schmid, S.; Kandulski, A.; Müller, M.; Kunst, C. Unraveling the Role of Reactive Oxygen Species in T Lymphocyte Signaling. Int. J. Mol. Sci. 2024, 25, 6114. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Fan, G.; Wang, D. Akebia Saponin D ameliorated kidney injury and exerted anti-inflammatory and anti-apoptotic effects in diabetic nephropathy by activation of NRF2/HO-1 and inhibition of NF-KB pathway. Int. Immunopharmacol. 2020, 84, 106467. [Google Scholar] [PubMed]
- Liao, Y.; Tan, R.Z.; Li, J.C.; Liu, T.T.; Zhong, X.; Yan, Y.; Yang, J.K.; Lin, X.; Fan, J.M.; Wang, L. Isoliquiritigenin Attenuates UUO-Induced Renal Inflammation and Fibrosis by Inhibiting Mincle/Syk/NF-Kappa B Signaling Pathway. Drug Des. Dev. Ther. 2020, 14, 1455–1468. [Google Scholar]
- Zhang, Y.; Zhong, Y.; Zeng, Q.; Tang, M. Redox-architected signaling networks in cardiac fibrosis. Biochem. Biophys. Res. Commun. 2025, 781, 152553. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Quan, H.; Zhang, H.; Lin, L.; Ou, Q.; Chen, K. Silencing cyclophilin A improves insulin secretion, reduces cell apoptosis, and alleviates inflammation as well as oxidant stress in high glucose-induced pancreatic β-cells via MAPK/NF-kb signaling pathway. Bioengineered 2020, 11, 1047–1057. [Google Scholar] [CrossRef]
- Hu, X.; Ding, C.; Ding, X.; Fan, P.; Zheng, J.; Xiang, H.; Li, X.; Qiao, Y.; Xue, W.; Li, Y. Inhibition of myeloid differentiation protein 2 attenuates renal ischemia/reperfusion-induced oxidative stress and inflammation via suppressing TLR4/TRAF6/NF-kB pathway. Life Sci. 2020, 256, 117864. [Google Scholar]
- Yang, Y.; Wang, J.; Peng, W.; Shu, Y.; Mo, X. Cytochrome P450 Epoxygenase 2J2 Protects Against Lung Ischemia/Reperfusion Injury by Activating the P13K/Akt/GSK-3-β/NF-kB Signaling Pathway During Deep Hypothermic Low Flow in Mice. J. Surg. Res. 2020, 253, 8–17. [Google Scholar] [CrossRef]
- Luo, J.; Zhang, M.; Deng, Y.; Li, H.; Bu, Q.; Liu, R.; Yu, J.; Liu, S.; Zeng, Z.; Sun, W.; et al. Copper nanoparticles lead to reproductive dysfunction by affecting key enzymes of ovarian hormone synthesis and metabolism in female rats. Ecotoxicol. Environ. Saf. 2023, 254, 114704. [Google Scholar] [CrossRef]
- Yi, X.; Tang, D.; Cao, S.; Li, T.; Gao, H.; Ma, T.; Yao, T.; Li, J.; Chang, B. Effect of Different Exercise Loads on Testicular Oxidative Stress and Reproductive Function in Obese Male Mice. Oxidative Med. Cell. Longev. 2020, 2020, 3071658. [Google Scholar] [CrossRef]
- Nasiri, K.; Akbari, A.; Nimrouzi, M.; Ruyvaran, M.; Mohamadian, A. Safflower seed oil improves steroidogenesis and spermatogenesis in rats with type II diabetes mellitus by modulating the genes expression involved in steroidogenesis, inflammation and oxidative stress. J. Ethnopharmacol. 2021, 275, 114139. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Y.; Feng, R.; Zheng, P.; Huang, H.; Zhou, S.; Ji, W.; Huang, F.; Liu, H.; Zhang, G. Cadmium induces testosterone synthesis disorder by testicular cell damage via TLR4/MAPK/NF-κB signaling pathway leading to reduced sexual behavior in piglets. Ecotoxicol. Environ. Saf. 2022, 233, 113345. [Google Scholar]
- Jin, H.; Ding, J.; Liu, H.; Yang, L.; Li, D.; Han, X. Chronic exposure to polystyrene microplastics induced LHR reduction and decreased testosterone levels through NF-κB pathway. Environ. Pollut. 2024, 358, 124543. [Google Scholar]
- Hong, C.Y.; Park, J.H.; Seo, K.H.; Kim, J.M.; Im, S.Y.; Lee, J.W.; Choi, H.S.; Lee, K. Expression of MIS in the testis is downregulated by tumor necrosis factor alpha through the negative regulation of SF-1 transactivation by NF-kappa B. Mol. Cell. Biol. 2003, 23, 6000–6012. [Google Scholar] [CrossRef]
- Na, S.; Duan, X.; Wang, R.; Fan, Y.; Xue, K.; Tian, S.; Yang, Z.; Li, K.; Yue, J. Chronic Neuroinflammation Induced by Lipopolysaccharide Injection into the Third Ventricle Induces Behavioral Changes. J. Mol. Neurosci. 2021, 71, 1306–1319. [Google Scholar] [CrossRef]
- Zhu, M.; Wei, J.; Li, Y.; Wang, Y.; Ren, J.; Li, B.; Ma, B.; Wang, X.; Qiao, L.; Zhou, C.; et al. Efficacy and Mechanism of Buyang Huanwu Decoction in Patients With Ischemic Heart Failure: A Randomized, Double-Blind, Placebo-Controlled Trial Combined With Proteomic Analysis. Front. Pharmacol. 2022, 13, 831208. [Google Scholar] [CrossRef]
- Yang, L.; Liu, Q.; Lu, Q.; Xiao, J.J.; Fu, A.Y.; Wang, S.; Ni, L.; Hu, J.W.; Yu, H.; Wu, X.; et al. Scavenger Receptor Class B Type I Deficiency Induces Iron Overload and Ferroptosis in Renal Tubular Epithelial Cells via Hypoxia-Inducible Factor-1α/Transferrin Receptor 1 Signaling Pathway. Antioxid. Redox Signal. 2024, 41, 56–73. [Google Scholar] [CrossRef]
- Wieczfinska, J.; Sitarek, P.; Kowalczyk, T.; Skała, E.; Pawliczak, R. The Anti-inflammatory Potential of Selected Plant-derived Compounds in Respiratory Diseases. Curr. Pharm. Des. 2020, 26, 2876–2884. [Google Scholar] [CrossRef] [PubMed]
- Fan, M.; Wang, C.; Zhao, X.; Jiang, Y.; Wang, C. Parthenolide alleviates microglia-mediated neuroinflammation via MAPK/TRIM31/NLRP3 signaling to ameliorate cognitive disorder. Int. Immunopharmacol. 2023, 120, 110287. [Google Scholar] [CrossRef] [PubMed]

| Antibodies Application | Application | Campany |
|---|---|---|
| CYP11A1 | WB (1:1000), IF (1:100) | 13363-1-AP, Proteintech (Rosemont, IL, USA) |
| SF-1 | WB (1:500), IF (1:100) | 18658-1-AP, Proteintech (Rosemont, IL, USA) |
| StAR | WB (1:500), IF (1:100) | 8449T, Cell Signaling (Danvers, MA, USA) |
| NRF2 | WB (1:1000), IF (1:100) | 16396-1-AP, Proteintech (Rosemont, IL, USA) |
| HO-1 | WB (1:1000), IF (1:100) | 10701-1-AP, Proteintech (Rosemont, IL, USA) |
| NQ-O1 | WB (1:1000), IF (1:100) | 11451-1-AP, Proteintech (Rosemont, IL, USA) |
| P-P65 | WB (1:1000), IF (1:100) | 3033T, Cell Signaling (Danvers, MA, USA) |
| P65 | WB (1:1000), IF (1:100) | 10745-1-AP, Proteintech (Rosemont, IL, USA) |
| IkBα | WB (1:1000), IF (1:100) | 10268-1-AP, Proteintech (Rosemont, IL, USA) |
| GAPDH | WB (1:20,000) | 10494-1-AP, Proteintech (Rosemont, IL, USA) |
| goat anti-mouse | WB (1:20,000) | RGAM001, Proteintech (Rosemont, IL, USA) |
| goat anti-rabbit | WB (1:20,000) | RGAG001, Proteintech (Rosemont, IL, USA) |
| Cy3-conjugated goat anti-mouse antibody | IF (1:200) | SA00009-1, Proteintech (Rosemont, IL, USA) |
| Cy3-conjugated goat anti-rabbit antibody | IF (1:200) | SA00009-2, Proteintech (Rosemont, IL, USA) |
| FITC-conjugated mouse anti-mouse antibody | IF (1:200) | SA00003-1, Proteintech (Rosemont, IL, USA) |
| Gene | Primers | Sequence 5′→3′ |
|---|---|---|
| IL-1β | Sense | TGCAAGTGTCTGAAGCAGCTATG |
| Antisense | TAGCCACTGAAGGAGATGAGTTG | |
| IL-6 | Sense | AACCGCTATGAAGTTCCTCTCTG |
| Antisense | TGGTATCCTCTGTGAAGTCTCCT | |
| TNF-α | Sense | CCAGACCCTCACACTCAGATCAT |
| Antisense | AGAACCTGGGAGTAGACAAGGTA | |
| GAPDH | Sense | AGGTCGGTGTGAACGGATTTG |
| Antisense | TGTAGACCATGTAGTTGAGGTCA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, P.; Yan, H.; Wang, R.; Zhao, J.; Zheng, X.; Li, D.; Guo, X.; Ji, F.; Long, C.; Shen, L.; et al. Parthenolide Restores Testosterone Biosynthesis After Nanoplastic Exposure by Blocking ROS-Driven NF-κB Nuclear Translocation. Antioxidants 2025, 14, 1315. https://doi.org/10.3390/antiox14111315
Zhao P, Yan H, Wang R, Zhao J, Zheng X, Li D, Guo X, Ji F, Long C, Shen L, et al. Parthenolide Restores Testosterone Biosynthesis After Nanoplastic Exposure by Blocking ROS-Driven NF-κB Nuclear Translocation. Antioxidants. 2025; 14(11):1315. https://doi.org/10.3390/antiox14111315
Chicago/Turabian StyleZhao, Peng, Hao Yan, Runchang Wang, Jie Zhao, Xiangqin Zheng, Dinggang Li, Xitong Guo, Fengming Ji, Chunlan Long, Lianju Shen, and et al. 2025. "Parthenolide Restores Testosterone Biosynthesis After Nanoplastic Exposure by Blocking ROS-Driven NF-κB Nuclear Translocation" Antioxidants 14, no. 11: 1315. https://doi.org/10.3390/antiox14111315
APA StyleZhao, P., Yan, H., Wang, R., Zhao, J., Zheng, X., Li, D., Guo, X., Ji, F., Long, C., Shen, L., Wei, G., & Wu, S. (2025). Parthenolide Restores Testosterone Biosynthesis After Nanoplastic Exposure by Blocking ROS-Driven NF-κB Nuclear Translocation. Antioxidants, 14(11), 1315. https://doi.org/10.3390/antiox14111315








