Reactive Oxygen Species (ROS) Drive Osteocyte Dysfunction in Diabetic Osteoporosis by Impairing Autophagy and Triggering Apoptosis
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture and Viability Assay
2.2. Experimental Grouping
2.3. Measurement of Cellular ATP Levels
2.4. Isolation of Primary Mouse Osteocytes
2.5. ROS Measurement
2.6. Western Blot Analysis
2.7. Quantitative Reverse Transcription PCR (qRT-PCR)
2.8. Immunofluorescence
2.9. TUNEL Staining
2.10. Statistical Analyses
3. Results
- High Glucose Induces Insulin Resistance and Oxidative Stress in Osteocytes
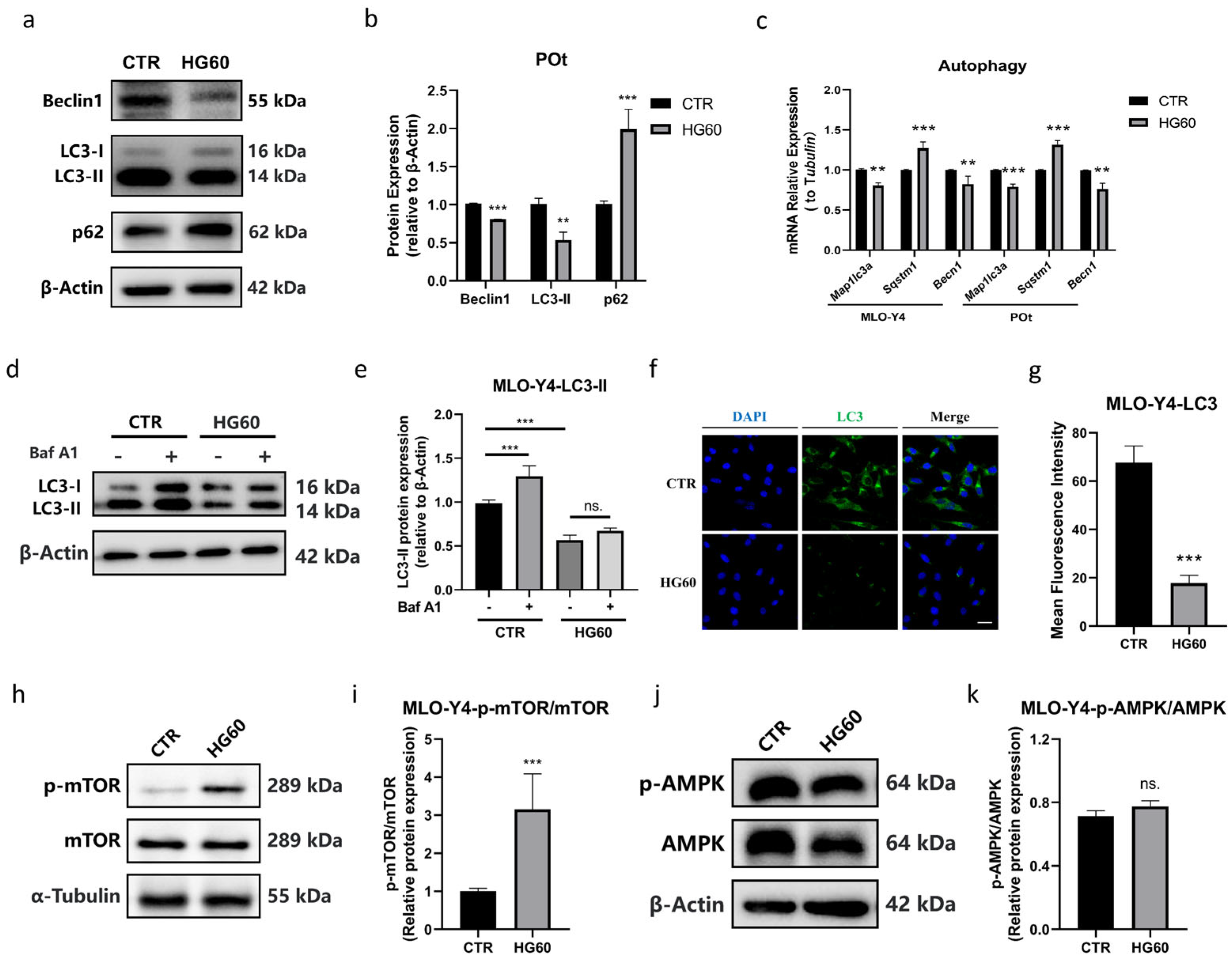
- High Glucose Activated mTOR and Inhibited Autophagy in Osteocytes
- High glucose induced the apoptosis of osteocytes
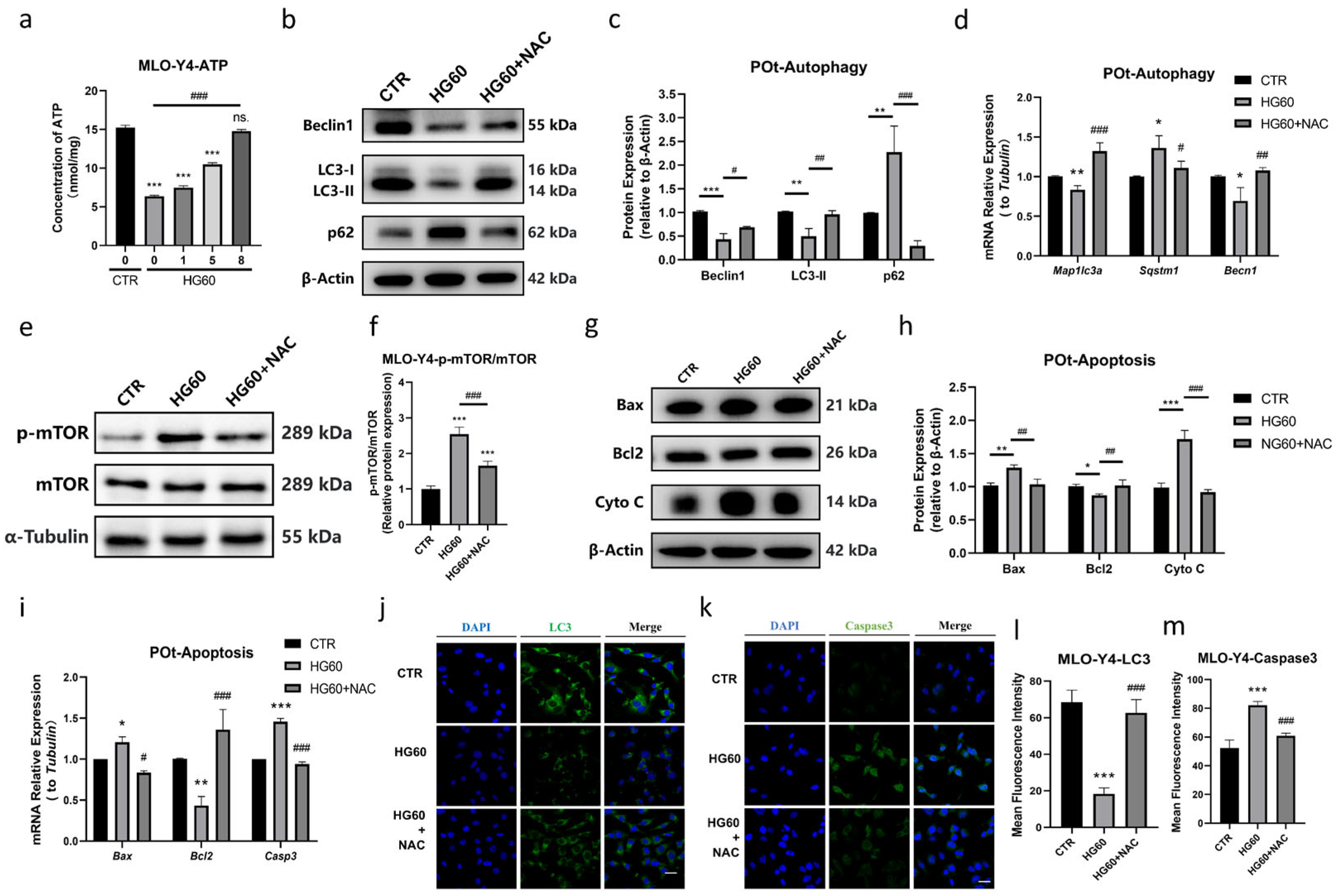
- NAC Ameliorated HG-Induced Decrease in Autophagy and Increase in Apoptosis in Osteocytes
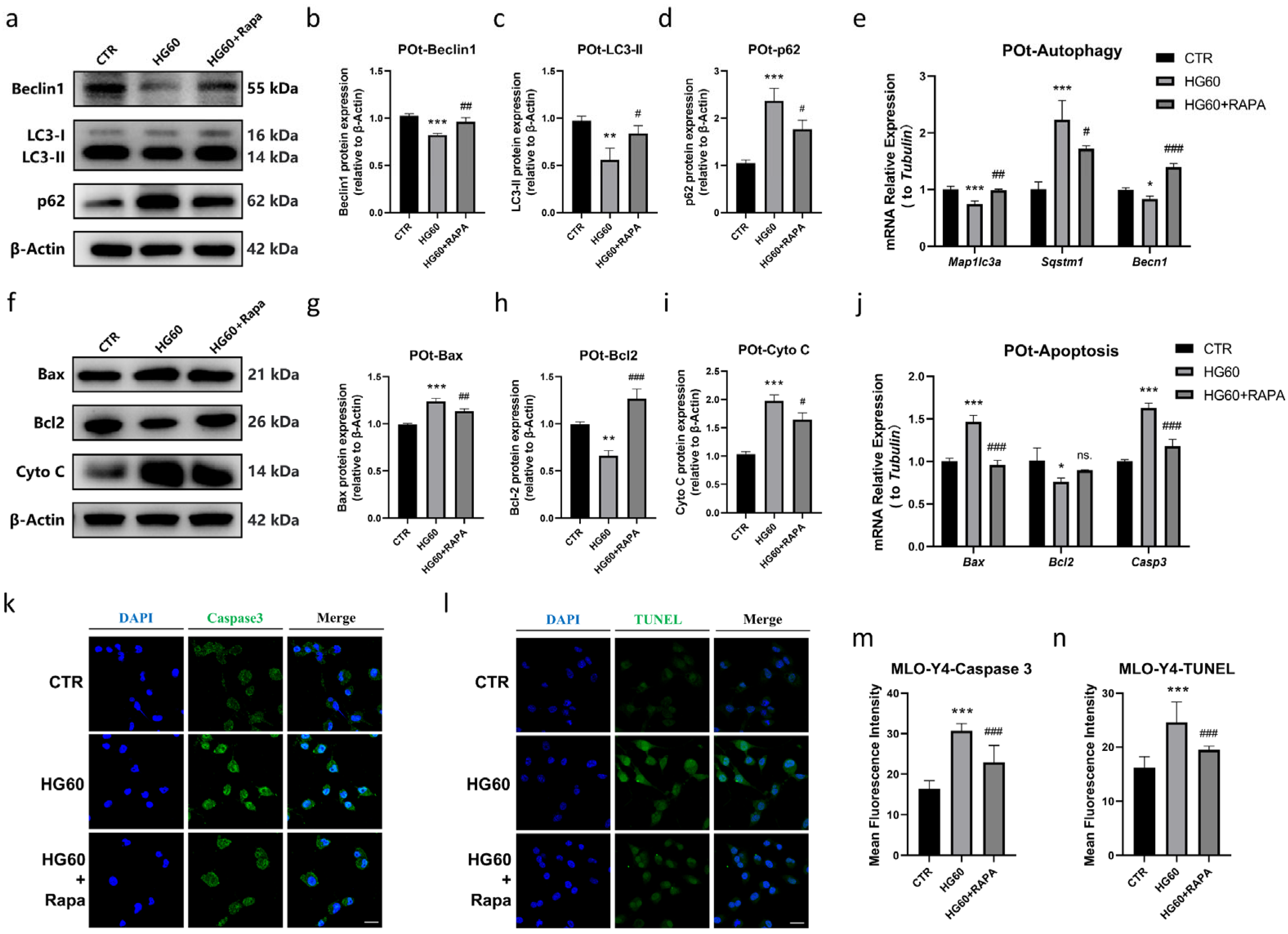
- Rapamycin Prevented HG-Induced Apoptosis in Osteocytes by Promoting Autophagy
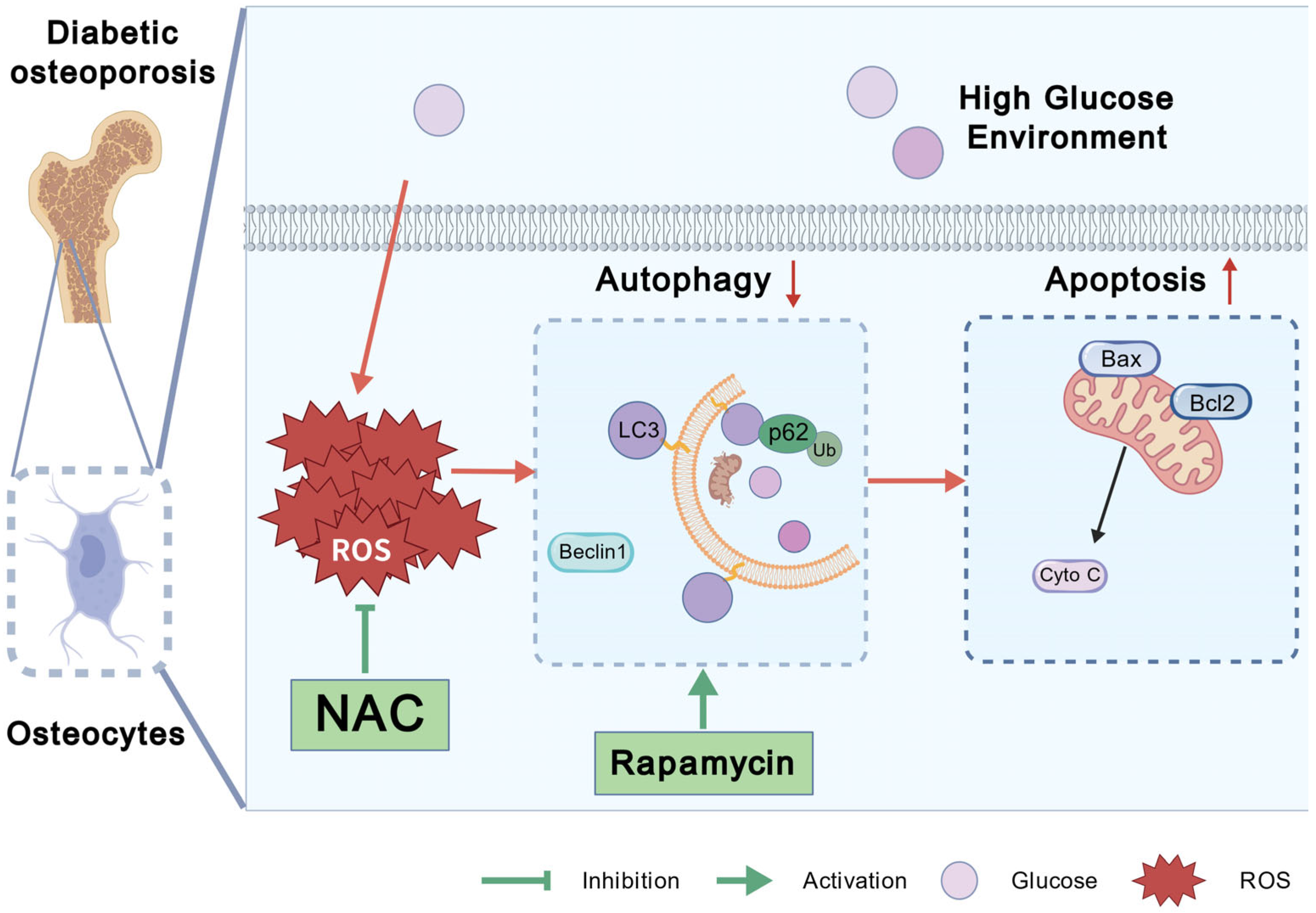
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| T2DM | Type 2 diabetes mellitus |
| ROS | Reactive oxygen species |
| HG | High glucose |
| NAC | N-acetylcysteine |
| EDTA | ethylenediaminetetraacetic acid |
| FBS | fetal bovine serum |
| Baf A1 | bafilomycin A1 |
| BSA | bovine serum albumin |
| Cyto C | Cytochrome C |
| POt | Primary osteocytes |
References
- Yamamoto, M.; Yamaguchi, T.; Yamauchi, M.; Kaji, H.; Sugimoto, T. Diabetic patients have an increased risk of vertebral fractures independent of BMD or diabetic complications. J. Bone Miner. Res. 2009, 24, 702–709. [Google Scholar] [CrossRef]
- Huang, X.; Li, S.; Lu, W.; Xiong, L. Metformin activates Wnt/beta-catenin for the treatment of diabetic osteoporosis. BMC Endocr. Disord. 2022, 22, 189. [Google Scholar] [CrossRef] [PubMed]
- Jiang, F.; Shan, H.; Pan, C.; Zhou, Z.; Cui, K.; Chen, Y.; Zhong, H.; Lin, Z.; Wang, N.; Yan, L.; et al. ATP6V1H facilitates osteogenic differentiation in MC3T3-E1 cells via Akt/GSK3beta signaling pathway. Organogenesis 2019, 15, 43–54. [Google Scholar] [CrossRef]
- Shu, A.; Yin, M.T.; Stein, E.; Cremers, S.; Dworakowski, E.; Ives, R.; Rubin, M.R. Bone structure and turnover in type 2 diabetes mellitus. Osteoporos. Int. 2012, 23, 635–641. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, A.G.; Sundh, D.; Johansson, L.; Nilsson, M.; Mellstrom, D.; Rudang, R.; Zoulakis, M.; Wallander, M.; Darelid, A.; Lorentzon, M. Type 2 Diabetes Mellitus Is Associated with Better Bone Microarchitecture But Lower Bone Material Strength and Poorer Physical Function in Elderly Women: A Population-Based Study. J. Bone Miner. Res. 2017, 32, 1062–1071. [Google Scholar] [CrossRef]
- Zeitoun, D.; Caliaperoumal, G.; Bensidhoum, M.; Constans, J.M.; Anagnostou, F.; Bousson, V. Microcomputed tomography of the femur of diabetic rats: Alterations of trabecular and cortical bone microarchitecture and vasculature-a feasibility study. Eur. Radiol. Exp. 2019, 3, 17. [Google Scholar] [CrossRef]
- Giner, M.; Miranda, C.; Vazquez-Gamez, M.A.; Altea-Manzano, P.; Miranda, M.J.; Casado-Diaz, A.; Perez-Cano, R.; Montoya-Garcia, M.J. Microstructural and Strength Changes in Trabecular Bone in Elderly Patients with Type 2 Diabetes Mellitus. Diagnostics 2021, 11, 577. [Google Scholar] [CrossRef] [PubMed]
- Jiao, H.; Xiao, E.; Graves, D.T. Diabetes and Its Effect on Bone and Fracture Healing. Curr. Osteoporos. Rep. 2015, 13, 327–335. [Google Scholar] [CrossRef]
- Farr, J.N.; Khosla, S. Determinants of bone strength and quality in diabetes mellitus in humans. Bone 2016, 82, 28–34. [Google Scholar] [CrossRef]
- Delgado-Calle, J.; Bellido, T. The osteocyte as a signaling cell. Physiol. Rev. 2022, 102, 379–410. [Google Scholar] [CrossRef]
- Moharrer, Y.; Boerckel, J.D. Tunnels in the rock: Dynamics of osteocyte morphogenesis. Bone 2021, 153, 116104. [Google Scholar] [CrossRef]
- Dallas, S.L.; Prideaux, M.; Bonewald, L.F. The osteocyte: An endocrine cell … and more. Endocr. Rev. 2013, 34, 658–690. [Google Scholar] [CrossRef]
- Plotkin, L.I.; Bellido, T. Osteocytic signalling pathways as therapeutic targets for bone fragility. Nat. Rev. Endocrinol. 2016, 12, 593–605. [Google Scholar] [CrossRef]
- Busse, B.; Djonic, D.; Milovanovic, P.; Hahn, M.; Puschel, K.; Ritchie, R.O.; Djuric, M.; Amling, M. Decrease in the osteocyte lacunar density accompanied by hypermineralized lacunar occlusion reveals failure and delay of remodeling in aged human bone. Aging Cell 2010, 9, 1065–1075. [Google Scholar] [CrossRef]
- Choi, J.U.A.; Kijas, A.W.; Lauko, J.; Rowan, A.E. The Mechanosensory Role of Osteocytes and Implications for Bone Health and Disease States. Front. Cell. Dev. Biol. 2021, 9, 770143. [Google Scholar] [CrossRef]
- Yvanoff, C.; Willaert, R.G. Development of bone cell microarrays in microfluidic chips for studying osteocyte-osteoblast communication under fluid flow mechanical loading. Biofabrication 2022, 14. [Google Scholar] [CrossRef]
- Qiu, S.; Rao, D.S.; Fyhrie, D.P.; Palnitkar, S.; Parfitt, A.M. The morphological association between microcracks and osteocyte lacunae in human cortical bone. Bone 2005, 37, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Shao, X.; Tian, Y.; Liu, J.; Yan, Z.; Ding, Y.; Hao, X.; Wang, D.; Shen, L.; Luo, E.; Guo, X.E.; et al. Rescuing SERCA2 pump deficiency improves bone mechano-responsiveness in type 2 diabetes by shaping osteocyte calcium dynamics. Nat. Commun. 2024, 15, 890. [Google Scholar] [CrossRef] [PubMed]
- Bolger, M.W.; Tekkey, T.; Kohn, D.H. Peripheral canalicular branching is decreased in streptozotocin-induced diabetes and correlates with decreased whole-bone ultimate load and perilacunar elastic work. JBMR Plus 2024, 8, ziad017. [Google Scholar] [CrossRef] [PubMed]
- Yao, Q.; Tsuboi, K.; Hongo, H.; Sakakibara, M.; Yamamoto, T.; Haraguchi-Kitakamae, M.; Ishizu, H.; Liu, X.; Shi, Y.; Li, W.; et al. Histochemical assessment of the femora of spontaneously diabetic torii-lepr(fa) (SDT-fa/fa) rats that mimic type II diabetes. J. Oral. Biosci. 2025, 67, 100602. [Google Scholar] [CrossRef]
- Eom, Y.S.; Gwon, A.R.; Kwak, K.M.; Kim, J.Y.; Yu, S.H.; Lee, S.; Kim, Y.S.; Park, I.B.; Kim, K.W.; Lee, K.; et al. Protective Effects of Vildagliptin against Pioglitazone-Induced Bone Loss in Type 2 Diabetic Rats. PLoS ONE 2016, 11, e0168569. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, X.; Zhou, R.; Lin, A.; Cao, M.; Lyu, Q.; Luo, P.; Zhang, J. Glycogen Metabolism Predicts the Efficacy of Immunotherapy for Urothelial Carcinoma. Front. Pharmacol. 2021, 12, 723066. [Google Scholar] [CrossRef]
- Weinstein, R.S.; Nicholas, R.W.; Manolagas, S.C. Apoptosis of osteocytes in glucocorticoid-induced osteonecrosis of the hip. J. Clin. Endocrinol. Metab. 2000, 85, 2907–2912. [Google Scholar] [CrossRef]
- Milovanovic, P.; Busse, B. Micropetrosis: Osteocyte Lacunar Mineralization in Aging and Disease. Curr. Osteoporos. Rep. 2023, 21, 750–757. [Google Scholar] [CrossRef]
- Zhong, C.; Zeng, X.; Yi, X.; Yang, Y.; Hu, J.; Yin, R.; Chen, X. The Function of Myostatin in Ameliorating Bone Metabolism Abnormalities in Individuals with Type 2 Diabetes Mellitus by Exercise. Curr. Issues Mol. Biol. 2025, 47, 158. [Google Scholar] [CrossRef]
- O’Brien, C.A.; Nakashima, T.; Takayanagi, H. Osteocyte control of osteoclastogenesis. Bone 2013, 54, 258–263. [Google Scholar] [CrossRef]
- Kitaura, H.; Marahleh, A.; Ohori, F.; Noguchi, T.; Shen, W.R.; Qi, J.; Nara, Y.; Pramusita, A.; Kinjo, R.; Mizoguchi, I. Osteocyte-Related Cytokines Regulate Osteoclast Formation and Bone Resorption. Int. J. Mol. Sci. 2020, 21, 5169. [Google Scholar] [CrossRef] [PubMed]
- Vahidi, G.; Moody, M.; Welhaven, H.D.; Davidson, L.; Rezaee, T.; Behzad, R.; Karim, L.; Roggenbeck, B.A.; Walk, S.T.; Martin, S.A.; et al. Germ-Free C57BL/6 Mice Have Increased Bone Mass and Altered Matrix Properties but Not Decreased Bone Fracture Resistance. J. Bone Miner. Res. 2023, 38, 1154–1174. [Google Scholar] [CrossRef]
- Li, J.; Sakisaka, Y.; Nemoto, E.; Maruyama, K.; Suzuki, S.; Xiong, K.; Tada, H.; Tenkumo, T.; Yamada, S. Cementocyte-derived extracellular vesicles regulate osteoclastogenesis and osteoblastogenesis. J. Dent. Sci. 2024, 19, 2236–2246. [Google Scholar] [CrossRef] [PubMed]
- Sies, H. Oxidative stress: A concept in redox biology and medicine. Redox. Biol. 2015, 4, 180–183. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Kukreti, R.; Saso, L.; Kukreti, S. Mechanistic Insight into Oxidative Stress-Triggered Signaling Pathways and Type 2 Diabetes. Molecules 2022, 27, 950. [Google Scholar] [CrossRef]
- Yaribeygi, H.; Mohammadi, M.T.; Sahebkar, A. Crocin potentiates antioxidant defense system and improves oxidative damage in liver tissue in diabetic rats. Biomed. Pharmacother. 2018, 98, 333–337. [Google Scholar] [CrossRef]
- Yaribeygi, H.; Lhaf, F.; Sathyapalan, T.; Sahebkar, A. Effects of novel antidiabetes agents on apoptotic processes in diabetes and malignancy: Implications for lowering tissue damage. Life Sci. 2019, 231, 116538. [Google Scholar] [CrossRef]
- Xu, N.; Liu, S.; Zhang, Y.; Chen, Y.; Zuo, Y.; Tan, X.; Liao, B.; Li, P.; Feng, J. Oxidative stress signaling in the pathogenesis of diabetic cardiomyopathy and the potential therapeutic role of antioxidant naringenin. Redox. Rep. 2023, 28, 2246720. [Google Scholar] [PubMed]
- Mody, N.; Parhami, F.; Sarafian, T.A.; Demer, L.L. Oxidative stress modulates osteoblastic differentiation of vascular and bone cells. Free Radic. Biol. Med. 2001, 31, 509–519. [Google Scholar] [CrossRef]
- Bai, X.C.; Lu, D.; Bai, J.; Zheng, H.; Ke, Z.Y.; Li, X.M.; Luo, S.Q. Oxidative stress inhibits osteoblastic differentiation of bone cells by ERK and NF-κB. Biochem. Biophys. Res. Commun. 2004, 314, 197–207. [Google Scholar] [CrossRef]
- Tao, Z.S.; Wu, X.J.; Yang, M.; Shen, C.L. Astaxanthin prevents bone loss in osteoporotic rats with palmitic acid through suppressing oxidative stress. Redox. Rep. 2024, 29, 2333096. [Google Scholar]
- Fatokun, A.A.; Stone, T.W.; Smith, R.A. Hydrogen peroxide-induced oxidative stress in MC3T3-E1 cells: The effects of glutamate and protection by purines. Bone 2006, 39, 542–551. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Li, Y.; Zhang, Z.; Chen, L.; Liu, Y.; Huang, S.; Zhang, X. Xianling Gubao attenuates high glucose-induced bone metabolism disorder in MG63 osteoblast-like cells. PLoS ONE 2022, 17, e0276328. [Google Scholar] [CrossRef] [PubMed]
- Starup-Linde, J.; Hygum, K.; Langdahl, B.L. Skeletal Fragility in Type 2 Diabetes Mellitus. Endocrinol. Metab. 2018, 33, 339–351. [Google Scholar] [CrossRef]
- Rendina-Ruedy, E.; Graef, J.L.; Lightfoot, S.A.; Ritchey, J.W.; Clarke, S.L.; Lucas, E.A.; Smith, B.J. Impaired glucose tolerance attenuates bone accrual by promoting the maturation of osteoblasts: Role of Beclin1-mediated autophagy. Bone Rep. 2016, 5, 199–207. [Google Scholar] [CrossRef]
- Kang, J.; Boonanantanasarn, K.; Baek, K.; Woo, K.M.; Ryoo, H.M.; Baek, J.H.; Kim, G.S. Hyperglycemia increases the expression levels of sclerostin in a reactive oxygen species- and tumor necrosis factor-alpha-dependent manner. J. Periodontal. Implant. Sci. 2015, 45, 101–110. [Google Scholar] [CrossRef]
- Amber, R.S.; Matthew, M.S.; Mark, E.V.D.; Katharina, J.; Matthew, P.; Lynda, F.B. Isolation and culture of primary osteocytes from the long bones of skeletally mature and aged mice. Biotechniques 2012, 52, 361–373. [Google Scholar]
- Yann, S.; Olivia, L. Endothelial Autophagy Dysregulation in Diabetes. Cells 2023, 12, 947. [Google Scholar] [CrossRef]
- Jiang, S.; Li, H.; Zhang, L.; Mu, W.; Zhang, Y.; Chen, T.; Wu, J.; Tang, H.; Zheng, S.; Liu, Y.; et al. Generic Diagramming Platform (GDP): A comprehensive database of high-quality biomedical graphics. Nucleic Acids Res. 2024, 53, D1670–D1676. [Google Scholar] [CrossRef]
- Forbes, J.M.; Cooper, M.E. Mechanisms of diabetic complications. Physiol. Rev. 2013, 93, 137–188. [Google Scholar] [CrossRef]
- Wu, B.; Fu, Z.; Wang, X.; Zhou, P.; Yang, Q.; Jiang, Y.; Zhu, D. A narrative review of diabetic bone disease: Characteristics, pathogenesis, and treatment. Front. Endocrinol. 2022, 13, 1052592. [Google Scholar] [CrossRef]
- Singh, H.; Singh, R.; Singh, A.; Singh, H.; Singh, G.; Kaur, S.; Singh, B. Role of oxidative stress in diabetes-induced complications and their management with antioxidants. Arch. Physiol. Biochem. 2024, 130, 616–641. [Google Scholar] [CrossRef] [PubMed]
- Dilworth, L.; Stennett, D.; Facey, A.; Omoruyi, F.; Mohansingh, S.; Omoruyi, F.O. Diabetes and the associated complications: The role of antioxidants in diabetes therapy and care. Biomed. Pharmacother. 2024, 181, 117641. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Kim, D.; Do, K.; Yang, C.B.; Jeon, S.W.; Jang, A. Effects of Horse Meat Hydrolysate on Oxidative Stress, Proinflammatory Cytokines, and the Ubiquitin-Proteasomal System of C2C12 Cells. Food Sci. Anim. Resour. 2024, 44, 132–145. [Google Scholar] [CrossRef] [PubMed]
- Sies, H.; Jones, D.P. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat. Rev. Mol. Cell. Biol. 2020, 21, 363–383. [Google Scholar] [CrossRef] [PubMed]
- Schroder, K. NADPH oxidases: Current aspects and tools. Redox. Biol. 2020, 34, 101512. [Google Scholar] [CrossRef]
- Wada, J.; Makino, H. Inflammation and the pathogenesis of diabetic nephropathy. Clin. Sci. 2013, 124, 139–152. [Google Scholar] [CrossRef]
- Ling, X.; Wang, C.; Feng, Q.; Zhang, T. Interleukin-17 prevents oxidative stress from damaging osteoblast formation by inhibiting autophagic degradation of metallothionein-2. Endocr. J. 2024, 71, 623–633. [Google Scholar] [CrossRef]
- Feng, Y.; Yang, D.; Zhi, X.; Deng, H.; Zhang, W.; Wang, R.; Wu, W. Role of interaction between reactive oxygen species and ferroptosis pathway in methylglyoxal-induced injury in mouse embryonic osteoblasts. Nan Fang Yi Ke Da Xue Xue Bao 2022, 42, 108–115. [Google Scholar] [PubMed]
- Li, W.; Jiang, W.S.; Su, Y.R.; Tu, K.W.; Zou, L.; Liao, C.R.; Wu, Q.; Wang, Z.H.; Zhong, Z.M.; Chen, J.T.; et al. PINK1/Parkin-mediated mitophagy inhibits osteoblast apoptosis induced by advanced oxidation protein products. Cell Death Dis. 2023, 14, 88. [Google Scholar] [CrossRef]
- Gao, J.; Wang, Z.; Gao, P.; Fan, Q.; Zhang, T.; Cui, L.; Shi, L.; Liu, Z.; Yang, Z.; He, L.; et al. Daphnetin Alleviates Senile and Disuse Osteoporosis by Distinct Modulations of Bone Formation and Resorption. Antioxidants 2022, 11, 2365. [Google Scholar] [CrossRef]
- Cao, F.; Yang, K.; Qiu, S.; Li, J.; Jiang, W.; Tao, L.; Zhu, Y. Metformin reverses oxidative stress-induced mitochondrial dysfunction in pre-osteoblasts via the EGFR/GSK-3beta/calcium pathway. Int. J. Mol. Med. 2023, 51, 36. [Google Scholar] [CrossRef]
- Ma, X.Y.; Cui, D.; Wang, Z.; Liu, B.; Yu, H.L.; Yuan, H.; Xiang, L.B.; Zhou, D.P. Silk Fibroin/Hydroxyapatite Coating Improved Osseointegration of Porous Titanium Implants under Diabetic Conditions via Activation of the PI3K/Akt Signaling Pathway. ACS Biomater. Sci. Eng. 2022, 8, 2908–2919. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, J.; Li, F. Gastrodin improves osteoblast function and adhesion to titanium surface in a high glucose environment. Biochem. Biophys. Rep. 2024, 37, 101623. [Google Scholar] [CrossRef] [PubMed]
- Mahboob, A.; Senevirathne, D.K.L.; Paul, P.; Nabi, F.; Khan, R.H.; Chaari, A. An investigation into the potential action of polyphenols against human Islet Amyloid Polypeptide aggregation in type 2 diabetes. Int. J. Biol. Macromol. 2023, 225, 318–350. [Google Scholar] [CrossRef]
- Rehan Ahmad, S.; Zeyaullah, M.; AlShahrani, A.M.; Qavi, D.; Altijani, A.A.G.; Khan, M.S.; Muzammil, K. An Unstable ATG2A Variant Causes a Neurodegenerative Disorder via Impaired Autophagy and Proteotoxic Stress in Brain Atrophy. Clin. Genet. 2025. [Google Scholar] [CrossRef]
- Alexander, A.; Cai, S.L.; Kim, J.; Nanez, A.; Sahin, M.; MacLean, K.H.; Inoki, K.; Guan, K.L.; Shen, J.; Person, M.D.; et al. ATM signals to TSC2 in the cytoplasm to regulate mTORC1 in response to, R.O.S. Proc. Natl. Acad. Sci. USA 2010, 107, 4153–4158. [Google Scholar] [CrossRef]
- Kim, Y.C.; Guan, K.L. mTOR: A pharmacologic target for autophagy regulation. J. Clin. Invest. 2015, 125, 25–32. [Google Scholar] [CrossRef]
- Bou-Teen, D.; Kaludercic, N.; Weissman, D.; Turan, B.; Maack, C.; Di Lisa, F.; Ruiz-Meana, M. Mitochondrial ROS and mitochondria-targeted antioxidants in the aged heart. Free Radic. Biol. Med. 2021, 167, 109–124. [Google Scholar] [CrossRef] [PubMed]
- Kalkavan, H.; Green, D.R. MOMP, cell suicide as a BCL-2 family business. Cell Death Differ. 2018, 25, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Wang, X. Cytochrome C-mediated apoptosis. Annu. Rev. Biochem. 2004, 73, 87–106. [Google Scholar] [CrossRef] [PubMed]
- Redza-Dutordoir, M.; Averill-Bates, D.A. Activation of apoptosis signalling pathways by reactive oxygen species. Biochim. Biophys. Acta 2016, 1863, 2977–2992. [Google Scholar] [CrossRef]
- Han, R.H.; Huang, H.M.; Han, H.; Chen, H.; Zeng, F.; Xie, X.; Liu, D.Y.; Cai, Y.; Zhang, L.Q.; Liu, X.; et al. Propofol postconditioning ameliorates hypoxia/reoxygenation induced H9c2 cell apoptosis and autophagy via upregulating forkhead transcription factors under hyperglycemia. Mil. Med. Res. 2021, 8, 58. [Google Scholar] [CrossRef]
- Zhao, Z.; Ming, Y.; Li, X.; Tan, H.; He, X.; Yang, L.; Song, J.; Zheng, L. Hyperglycemia Aggravates Periodontitis via Autophagy Impairment and ROS-Inflammasome-Mediated Macrophage Pyroptosis. Int. J. Mol. Sci. 2023, 24, 6309. [Google Scholar] [CrossRef]
- Demirtas, L.; Guclu, A.; Erdur, F.M.; Akbas, E.M.; Ozcicek, A.; Onk, D.; Turkmen, K. Apoptosis, autophagy & endoplasmic reticulum stress in diabetes mellitus. Indian J. Med. Res. 2016, 144, 515–524. [Google Scholar] [PubMed]
- Gao, G.; Chen, W.; Yan, M.; Liu, J.; Luo, H.; Wang, C.; Yang, P. Rapamycin regulates the balance between cardiomyocyte apoptosis and autophagy in chronic heart failure by inhibiting mTOR signaling. Int. J. Mol. Med. 2020, 45, 195–209. [Google Scholar] [CrossRef]
- Eastell, R.; Szulc, P. Use of bone turnover markers in postmenopausal osteoporosis. Lancet Diabetes Endocrinol. 2017, 5, 908–923. [Google Scholar] [CrossRef]
- Rahmani, D.; Faal, B.; Zali, H.; Tackallou, S.H.; Niknam, Z. The beneficial effects of simultaneous supplementation of Lactobacillus reuteri and calcium fluoride nanoparticles on ovariectomy-induced osteoporosis. BMC Complement. Med. Ther. 2023, 23, 340. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Huang, F.; Chen, X.; Wu, X.; Zhu, J. Ginsenoside Rg3 attenuates ovariectomy-induced osteoporosis via AMPK/mTOR signaling pathway. Drug. Dev. Res. 2020, 81, 875–884. [Google Scholar] [PubMed]
- Li, Y.R.; Li, S.; Lin, C.C. Effect of resveratrol and pterostilbene on aging and longevity. Biofactors 2018, 44, 69–82. [Google Scholar]


| Genes | Forward Primer Sequence | Reverse Primer Sequence |
|---|---|---|
| Nrf2 | CTTTAGTCAGCGACAGAAGGAC | AGGCATCTTGTTTGGGAATGTG |
| Sod2 | AGGCTCTGGCCAAGGGAGAT | CACGCTTGATAGCCTCCAGCA |
| Cat | CGCTGTAGATGTGAAACGCT | TCTCCTCCTCGTTCAACACC |
| Becn1 | AGGCTGAGGCGGAGAGATTG | TGTGGAAGGTGGCATTGAAGAC |
| Sqstm1 | GAACACAGCAAGCTCATCTTTC | AAAGTGTCCATGTTTCAGCTTC |
| Map1lc3a | GACGGCTTCCTGTACATGGTTT | TGGAGTCTTACACAGCCATTGC |
| Bax | GAAGCTGAGCGAGTGTCTCCGGC | TCAGCTGCCACCCGGAAGAAG |
| Bcl2 | CAACACTCCCTCTTGACCTATGC | GAAAATGTTCCCAAGTGAGTTAGA |
| Casp 3 | CTGGAGAAATTCAAAGGACGGG | TGAGCATGGACACAATACACGG |
| Tubulin | TGACTCCTTCAACACCTTCTTCA | GATCTCCTTGCCAATGGTGTAGT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, M.; Zhao, M.; Bai, F.; Wang, M.; Zhang, B.; Shi, J.; Liu, Z. Reactive Oxygen Species (ROS) Drive Osteocyte Dysfunction in Diabetic Osteoporosis by Impairing Autophagy and Triggering Apoptosis. Antioxidants 2025, 14, 1306. https://doi.org/10.3390/antiox14111306
Han M, Zhao M, Bai F, Wang M, Zhang B, Shi J, Liu Z. Reactive Oxygen Species (ROS) Drive Osteocyte Dysfunction in Diabetic Osteoporosis by Impairing Autophagy and Triggering Apoptosis. Antioxidants. 2025; 14(11):1306. https://doi.org/10.3390/antiox14111306
Chicago/Turabian StyleHan, Mengqi, Minyue Zhao, Furong Bai, Mengying Wang, Bo Zhang, Jianfeng Shi, and Zhongbo Liu. 2025. "Reactive Oxygen Species (ROS) Drive Osteocyte Dysfunction in Diabetic Osteoporosis by Impairing Autophagy and Triggering Apoptosis" Antioxidants 14, no. 11: 1306. https://doi.org/10.3390/antiox14111306
APA StyleHan, M., Zhao, M., Bai, F., Wang, M., Zhang, B., Shi, J., & Liu, Z. (2025). Reactive Oxygen Species (ROS) Drive Osteocyte Dysfunction in Diabetic Osteoporosis by Impairing Autophagy and Triggering Apoptosis. Antioxidants, 14(11), 1306. https://doi.org/10.3390/antiox14111306





