Selenium: A Key Element in Inflammatory Bowel Disease
Abstract
1. Introduction
2. The Pathogenesis of Inflammatory Bowel Disease: An Overview
3. Essential Elements and Inflammatory Bowel Disease
4. The Role of Selenium in Inflammatory Bowel Disease
4.1. Selenium: An Overview
4.2. Selenium and the Inflammatory Bowel Disease: Epidemiological Evidence
4.3. Selenium and Inflammatory Bowel Disease: Oxidative Stress and Inflammation
4.4. Selenium and Inflammatory Bowel Disease: Immunity
4.5. Selenium and Inflammatory Bowel Disease: Interactions with Gut Microbiota
4.6. Selenium and Inflammatory Bowel Disease: New Frontiers of Treatment
5. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BMI | Body mass index |
| CD | Crohn’s disease |
| CDAI | Crohn’s disease activity index |
| COX | Cyclooxygenase |
| CRC | Colorectal cancer |
| CRP | C reactive protein |
| DC | Dendritic cell |
| DSS | Dextran sulfate sodium |
| DUOX2 | Dual oxidase 2 |
| EUP | Eucommia ulmoides polysaccharide |
| FCP | Fecal calprotectin |
| GPX | Glutathione peroxidase |
| GSH | Reduced glutathione |
| H2O2 | Hydrogen peroxide |
| H2S | Hydrogen selenide |
| HBI | Harvey-Bradshaw index |
| IBD | Inflammatory bowel disease |
| IEC | Intestinal epithelial cell |
| LCS-SeNP | Low molecular weight chitosan selenium nanoparticles |
| NOD | Nucleotide-binding oligomerization domain |
| NP | Nanoparticle |
| Nrf2 | Nuclear factor erythroid 2-related factor 2 |
| NF-κB | Nuclear factor kappa-light-chain-enhancer of activated B cells |
| PG | Prostaglandin |
| PPARγ | Peroxisome proliferator-activated receptor gamma |
| RNS | Reactive nitrogen species |
| ROS | Reactive oxygen species |
| SCFA | Short chain fatty acid |
| Se | Selenium |
| SeCys | Selenocysteine |
| SeNP | Selenium nanoparticle |
| SES-CD | Simple endoscopic score for Crohn’s disease |
| SOD | Superoxide dismutase |
| Th | T helper cell |
| TNF-α | Tumor necrosis factor alpha |
| Treg | Regulatory T cell |
| TRX | Thioredoxin |
| UC | Ulcerative colitis |
References
- Aleksandrova, K.; Romero-Mosquera, B.; Hernandez, V. Diet, Gut Microbiome and Epigenetics: Emerging Links with Inflammatory Bowel Diseases and Prospects for Management and Prevention. Nutrients 2017, 9, 962. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Cheng, H.; Zhuang, J.; Liu, X.; Ouyang, Z.; Qian, R. Risk factors for inflammatory bowel disease: An umbrella review. Front. Cell. Infect. Microbiol. 2025, 14, 1410506. [Google Scholar] [CrossRef]
- Singh, N.; Bernstein, C.N. Environmental risk factors for inflammatory bowel disease. United Eur. Gastroenterol. J. 2022, 10, 1047–1053. [Google Scholar] [CrossRef]
- Feuerstein, J.D.; Cheifetz, A.S. Crohn Disease: Epidemiology, Diagnosis, and Management. Mayo Clin. Proc. 2017, 92, 1088–1103. [Google Scholar] [CrossRef] [PubMed]
- Abraham, C.; Cho, J.H. Inflammatory bowel disease. N. Engl. J. Med. 2009, 361, 2066–2078. [Google Scholar] [CrossRef]
- Feuerstein, J.D.; Cheifetz, A.S. Ulcerative colitis: Epidemiology, diagnosis, and management. Mayo Clin. Proc. 2014, 89, 1553–1563. [Google Scholar] [CrossRef]
- Lungaro, L.; Costanzini, A.; Manza, F.; Barbalinardo, M.; Gentili, D.; Guarino, M.; Caputo, F.; Zoli, G.; De Giorgio, R.; Caio, G. Impact of Female Gender in Inflammatory Bowel Diseases: A Narrative Review. J. Pers. Med. 2023, 13, 165. [Google Scholar] [CrossRef]
- Salem, D.A.; El-Ijla, R.; AbuMusameh, R.R.; Zakout, K.A.; Abu Halima, A.Y.; Abudiab, M.T.; Banat, Y.M.; Alqeeq, B.F.; Al-Tawil, M.; Matar, K. Sex-related differences in profiles and clinical outcomes of Inflammatory bowel disease: A systematic review and meta-analysis. BMC Gastroenterol. 2024, 24, 425. [Google Scholar] [CrossRef]
- Cai, Z.; Wang, S.; Li, J. Treatment of Inflammatory Bowel Disease: A Comprehensive Review. Front. Med. 2021, 8, 765474. [Google Scholar] [CrossRef]
- Caron, B.; Honap, S.; Peyrin-Biroulet, L. Epidemiology of Inflammatory Bowel Disease across the Ages in the Era of Advanced Therapies. J. Crohns Colitis 2024, 18, ii3–ii15. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.L.; Bao, J.C.; Liao, X.Y.; Chen, Y.J.; Wang, L.W.; Fan, Y.Y.; Xu, Q.Y.; Hao, L.X.; Li, K.J.; Liang, M.X.; et al. Trends and projections of inflammatory bowel disease at the global, regional and national levels, 1990–2050: A bayesian age-period-cohort modeling study. BMC Public Health 2023, 23, 2507. [Google Scholar] [CrossRef]
- Kumar, A.; Yassin, N.; Marley, A.; Bellato, V.; Foppa, C.; Pellino, G.; Myrelid, P.; Millan, M.; Gros, B.; Avellaneda, N.; et al. Crossing barriers: The burden of inflammatory bowel disease across Western Europe. Ther. Adv. Gastroenterol. 2023, 16, 17562848231218615. [Google Scholar] [CrossRef]
- Burisch, J.; Claytor, J.; Hernandez, I.; Hou, J.K.; Kaplan, G.G. The Cost of Inflammatory Bowel Disease Care: How to Make it Sustainable. Clin. Gastroenterol. Hepatol. 2025, 23, 386–395. [Google Scholar] [CrossRef]
- Zhao, M.; Gönczi, L.; Lakatos, P.L.; Burisch, J. The Burden of Inflammatory Bowel Disease in Europe in 2020. J. Crohns Colitis 2021, 15, 1573–1587. [Google Scholar] [CrossRef] [PubMed]
- Hracs, L.; Windsor, J.W.; Gorospe, J.; Cummings, M.; Coward, S.; Buie, M.J.; Quan, J.; Goddard, Q.; Caplan, L.; Markovinović, A.; et al. Global evolution of inflammatory bowel disease across epidemiologic stages. Nature 2025, 642, 458–466. [Google Scholar] [CrossRef] [PubMed]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxid. Med. Cell. Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef] [PubMed]
- Bourgonje, A.R.; Feelisch, M.; Faber, K.N.; Pasch, A.; Dijkstra, G.; van Goor, H. Oxidative Stress and Redox-Modulating Therapeutics in Inflammatory Bowel Disease. Trends Mol. Med. 2020, 26, 1034–1046. [Google Scholar] [CrossRef]
- Hu, R.; Xiao, J.; Fan, L. The Role of the Trace Element Selenium in Inflammatory Bowel Disease. Biol. Trace Elem. Res. 2024, 202, 4923–4931. [Google Scholar] [CrossRef]
- Brownson, E.; Saunders, J.; Jatkowska, A.; White, B.; Gerasimidis, K.; Seenan, J.P.; Macdonald, J. Micronutrient Status and Prediction of Disease Outcome in Adults with Inflammatory Bowel Disease Receiving Biologic Therapy. Inflamm. Bowel Dis. 2024, 30, 1233–1240. [Google Scholar] [CrossRef]
- Crooks, B.; Misra, R.; Arebi, N.; Kok, K.; Brookes, M.J.; McLaughlin, J.; Limdi, J.K. The dietary practices and beliefs of people living with older-onset inflammatory bowel disease. Eur. J. Gastroenterol. Hepatol. 2021, 33, e442–e448. [Google Scholar] [CrossRef]
- Solovyev, N.D. Importance of selenium and SEPP1rotein for brain function: From antioxidant protection to neuronal signalling. J. Inorg. Biochem. 2015, 153, 1–12. [Google Scholar] [CrossRef]
- Gorini, F.; Sabatino, L.; Pingitore, A.; Vassalle, C. Selenium: An Element of Life Essential for Thyroid Function. Molecules 2021, 26, 7084. [Google Scholar] [CrossRef]
- Vinceti, M.; Filippini, T.; Del Giovane, C.; Dennert, G.; Zwahlen, M.; Brinkman, M.; Zeegers, M.P.; Horneber, M.; D’Amico, R.; Crespi, C.M. Selenium for preventing cancer. Cochrane Database Syst. Rev. 2018, 1, CD005195. [Google Scholar] [CrossRef]
- Zhang, Y.; Meng, S.; Yu, Y.; Bi, L.; Tian, J.; Zhang, L. Associations of dietary selenium intake with the risk of chronic diseases and mortality in US adults. Front. Nutr. 2024, 11, 1363299. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.; Zhang, Y.; Dong, P.Y.; Chen Yan, Y.M.; Liu, J.; Zhang, B.Q.; Chen, M.M.; Zhang, S.E.; Zhang, X.F. A comprehensive review on potential role of selenium, SEPP1roteins and selenium nanoparticles in male fertility. Heliyon 2024, 10, e34975. [Google Scholar] [CrossRef]
- Gorini, F.; Vassalle, C. Selenium and SEPP1roteins at the Intersection of Type 2 Diabetes and Thyroid Pathophysiology. Antioxidants 2022, 11, 1188. [Google Scholar] [CrossRef]
- Nettleford, S.K.; Prabhu, K.S. Selenium and SEPP1roteins in Gut Inflammation-A Review. Antioxidants 2018, 7, 36. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Lin, T.; Wang, W.; Jing, F.; Sheng, J. Selenium deficiency in inflammatory bowel disease: A comprehensive meta-analysis. Heliyon 2024, 10, e40139. [Google Scholar] [CrossRef]
- Sousa, J.A.; McKay, D.M.; Raman, M. Selenium, Immunity, and Inflammatory Bowel Disease. Nutrients 2024, 16, 3620. [Google Scholar] [CrossRef] [PubMed]
- Calvez, V.; Puca, P.; Di Vincenzo, F.; Del Gaudio, A.; Bartocci, B.; Murgiano, M.; Iaccarino, J.; Parand, E.; Napolitano, D.; Pugliese, D.; et al. Novel Insights into the Pathogenesis of Inflammatory Bowel Diseases. Biomedicines 2025, 13, 305. [Google Scholar] [CrossRef]
- Schirmer, M.; Garner, A.; Vlamakis, H.; Xavier, R.J. Microbial genes and pathways in inflammatory bowel disease. Nat. Rev. Microbiol. 2019, 17, 497–511. [Google Scholar] [CrossRef]
- Guan, Q. A Comprehensive Review and Update on the Pathogenesis of Inflammatory Bowel Disease. J. Immunol. Res. 2019, 2019, 7247238. [Google Scholar] [CrossRef]
- Neurath, M.F.; Artis, D.; Becker, C. The intestinal barrier: A pivotal role in health, inflammation, and cancer. Lancet Gastroenterol. Hepatol. 2025, 10, 573–592. [Google Scholar] [CrossRef]
- Bai, X.; Liu, W.; Chen, H.; Zuo, T.; Wu, X. Immune Cell Landscaping Reveals Distinct Immune Signatures of Inflammatory Bowel Disease. Front. Immunol. 2022, 13, 861790. [Google Scholar] [CrossRef]
- Zhang, K.; Guo, J.; Yan, W.; Xu, L. Macrophage polarization in inflammatory bowel disease. Cell Commun. Signal. 2023, 21, 367. [Google Scholar] [CrossRef]
- Zhu, W.; Yu, J.; Nie, Y.; Shi, X.; Liu, Y.; Li, F.; Zhang, X.L. Disequilibrium of M1 and M2 macrophages correlates with the development of experimental inflammatory bowel diseases. Immunol. Investig. 2014, 43, 638–652. [Google Scholar] [CrossRef]
- Lu, Q.; Yang, M.F.; Liang, Y.J.; Xu, J.; Xu, H.M.; Nie, Y.Q.; Wang, L.S.; Yao, J.; Li, D.F. Immunology of Inflammatory Bowel Disease: Molecular Mechanisms and Therapeutics. J. Inflamm. Res. 2022, 15, 1825–1844. [Google Scholar] [CrossRef]
- Nemoto, Y.; Watanabe, M. The Th1, Th2, and Th17 Paradigm in Inflammatory Bowel Disease. In Crohn’s Disease and Ulcerative Colitis; Baumgart, D., Ed.; Springer: Boston, MA, USA, 2012. [Google Scholar]
- Jiang, P.; Zheng, C.; Xiang, Y.; Malik, S.; Su, D.; Xu, G.; Zhang, M. The involvement of TH17 cells in the pathogenesis of IBD. Cytokine Growth Factor Rev. 2023, 6, 28–42. [Google Scholar] [CrossRef] [PubMed]
- Yamada, A.; Arakaki, R.; Saito, M.; Tsunematsu, T.; Kudo, Y.; Ishimaru, N. Role of regulatory T cell in the pathogenesis of inflammatory bowel disease. World J. Gastroenterol. 2016, 22, 2195–2205. [Google Scholar] [CrossRef] [PubMed]
- Castro-Dopico, T.; Colombel, J.F.; Mehandru, S. Targeting B cells for inflammatory bowel disease treatment: Back to the future. Curr. Opin. Pharmacol. 2020, 55, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Cohn, H.M.; Dave, M.; Loftus, E.V., Jr. Understanding the Cautions and Contraindications of Immunomodulator and Biologic Therapies for Use in Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2017, 23, 1301–1315. [Google Scholar] [CrossRef]
- Sokic-Milutinovic, A.; Milosavljevic, T. Inflammatory Bowel Disease: From Conventional Immunosuppression to Biologic Therapy. Dig. Dis. 2024, 42, 325–335. [Google Scholar] [CrossRef]
- Qiu, P.; Ishimoto, T.; Fu, L.; Zhang, J.; Zhang, Z.; Liu, Y. The Gut Microbiota in Inflammatory Bowel Disease. Front. Cell. Infect. Microbiol. 2022, 12, 733992. [Google Scholar] [CrossRef]
- Macia, L.; Tan, J.; Vieira, A.T.; Leach, K.; Stanley, D.; Luong, S.; Maruya, M.; Ian McKenzie, C.; Hijikata, A.; Wong, C. Metabolite-sensing receptors GPR43 and GPR109A facilitate dietary fibre-induced gut homeostasis through regulation of the inflammasome. Nat. Commun. 2015, 6, 6734. [Google Scholar] [CrossRef] [PubMed]
- Cassol, I.; Ibañez, M.; Bustamante, J.P. Key features and guidelines for the application of microbial alpha diversity metrics. Sci. Rep. 2025, 15, 622. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Rahman, L.I.H.; Morgan, X.C. Searching for a Consensus Among Inflammatory Bowel Disease Studies: A Systematic Meta-Analysis. Inflamm. Bowel Dis. 2023, 29, 125–139. [Google Scholar] [CrossRef]
- Zhou, L.; Zhang, M.; Wang, Y.; Dorfman, R.G.; Liu, H.; Yu, T.; Chen, X.; Tang, D.; Xu, L.; Yin, Y.; et al. Faecalibacterium prausnitzii Produces Butyrate to Maintain Th17/Treg Balance and to Ameliorate Colorectal Colitis by Inhibiting Histone Deacetylase 1. Inflamm. Bowel Dis. 2018, 24, 1926–1940. [Google Scholar] [CrossRef] [PubMed]
- Nie, K.; Ma, K.; Luo, W.; Shen, Z.; Yang, Z.; Xiao, M.; Tong, T.; Yang, Y.; Wang, X. Roseburia intestinalis: A Beneficial Gut Organism from the Discoveries in Genus and Species. Front. Cell. Infect. Microbiol. 2021, 11, 757718. [Google Scholar] [CrossRef]
- Baxter, N.T.; Schmidt, A.W.; Venkataraman, A.; Kim, K.S.; Waldron, C.; Schmidt, T.M. Dynamics of Human Gut Microbiota and Short-Chain Fatty Acids in Response to Dietary Interventions with Three Fermentable Fibers. mBio 2019, 10, e02566-18. [Google Scholar] [CrossRef]
- Lloyd-Price, J.; Arze, C.; Ananthakrishnan, A.N.; Schirmer, M.; Avila-Pacheco, J.; Poon, T.W.; Andrews, E.; Ajami, N.J.; Bonham, K.S.; Brislawn, C.J.; et al. Multi-omics of the gut microbial ecosystem in inflammatory bowel diseases. Nature 2019, 569, 655–662. [Google Scholar] [CrossRef]
- Vich Vila, A.; Imhann, F.; Collij, V.; Jankipersadsing, S.A.; Gurry, T.; Mujagic, Z.; Kurilshikov, A.; Bonder, M.J.; Jiang, X.; Tigchelaar, E.F.; et al. Gut microbiota composition and functional changes in inflammatory bowel disease and irritable bowel syndrome. Sci. Transl. Med. 2018, 10, eaap8914. [Google Scholar] [CrossRef]
- Pittayanon, R.; Lau, J.T.; Leontiadis, G.I.; Tse, F.; Yuan, Y.; Surette, M.; Moayyedi, P. Differences in Gut Microbiota in Patients With vs. Without Inflammatory Bowel Diseases: A Systematic Review. Gastroenterology 2020, 158, 930–946.e1. [Google Scholar] [CrossRef]
- Pascal, V.; Pozuelo, M.; Borruel, N.; Casellas, F.; Campos, D.; Santiago, A.; Martinez, X.; Varela, E.; Sarrabayrouse, G.; Machiels, K.; et al. A microbial signature for Crohn’s disease. Gut 2017, 66, 813–822. [Google Scholar] [CrossRef] [PubMed]
- Buffie, C.G.; Pamer, E.G. Microbiota-mediated colonization resistance against intestinal pathogens. Nat. Rev. Immunol. 2013, 13, 790–801. [Google Scholar] [CrossRef] [PubMed]
- Anderson, M.; Sansonetti, P.J.; Marteyn, B.S. Shigella Diversity and Changing Landscape: Insights for the Twenty-First Century. Front. Cell. Infect. Microbiol. 2016, 6, 45. [Google Scholar] [CrossRef]
- Valentino, V.; De Filippis, F.; Marotta, R.; Pasolli, E.; Ercolini, D. Genomic features and prevalence of Ruminococcus species in humans are associated with age, lifestyle, and disease. Cell Rep. 2024, 43, 115018. [Google Scholar] [CrossRef] [PubMed]
- Hall, A.B.; Yassour, M.; Sauk, J.; Garner, A.; Jiang, X.; Arthur, T.; Lagoudas, G.K.; Vatanen, T.; Fornelos, N.; Wilson, R.; et al. A novel Ruminococcus gnavus clade enriched in inflammatory bowel disease patients. Genome Med. 2017, 9, 103. [Google Scholar] [CrossRef]
- Abdulla, M.; Mohammed, N. A Review on Inflammatory Bowel Diseases: Recent Molecular Pathophysiology Advances. Biologics 2022, 16, 129–140. [Google Scholar] [CrossRef]
- Lee, G.R. The Balance of Th17 versus Treg Cells in Autoimmunity. Int. J. Mol. Sci. 2018, 19, 730. [Google Scholar] [CrossRef]
- Wahlström, A.; Sayin, S.I.; Marschall, H.U.; Bäckhed, F. Intestinal Crosstalk between Bile Acids and Microbiota and Its Impact on Host Metabolism. Cell Metab. 2016, 24, 41–50. [Google Scholar] [CrossRef]
- Hang, S.; Paik, D.; Yao, L.; Kim, E.; Trinath, J.; Lu, J.; Ha, S.; Nelson, B.N.; Kelly, S.P.; Wu, L.; et al. Bile acid metabolites control TH17 and Treg cell differentiation. Nature 2019, 576, 143–148. [Google Scholar] [CrossRef]
- Campbell, C.; McKenney, P.T.; Konstantinovsky, D.; Isaeva, O.I.; Schizas, M.; Verter, J.; Mai, C.; Jin, W.B.; Guo, C.J.; Violante, S.; et al. Bacterial metabolism of bile acids promotes generation of peripheral regulatory T cells. Nature 2020, 581, 475–479. [Google Scholar] [CrossRef]
- Parséus, A.; Sommer, N.; Sommer, F.; Caesar, R.; Molinaro, A.; Ståhlman, M.; Greiner, T.U.; Perkins, R.; Bäckhed, F. Microbiota-induced obesity requires farnesoid X receptor. Gut 2017, 66, 429–437. [Google Scholar] [CrossRef]
- Langan, D.; Perkins, D.J.; Vogel, S.N.; Moudgil, K.D. Microbiota-Derived Metabolites, Indole-3-aldehyde and Indole-3-acetic Acid, Differentially Modulate Innate Cytokines and Stromal Remodeling Processes Associated with Autoimmune Arthritis. Int. J. Mol. Sci. 2021, 22, 2017. [Google Scholar] [CrossRef]
- Nikolaus, S.; Schulte, B.; Al-Massad, N.; Thieme, F.; Schulte, D.M.; Bethge, J.; Rehman, A.; Tran, F.; Aden, K.; Häsler, R.; et al. Increased Tryptophan Metabolism Is Associated with Activity of Inflammatory Bowel Diseases. Gastroenterology 2017, 153, 1504–1516.e2. [Google Scholar] [CrossRef]
- Moller, F.T.; Andersen, V.; Wohlfahrt, J.; Jess, T. Familial risk of inflammatory bowel disease: A population-based cohort study 1977–2011. Am. J. Gastroenterol. 2015, 110, 564–571. [Google Scholar] [CrossRef]
- Orholm, M.; Binder, V.; Sørensen, T.I.; Rasmussen, L.P.; Kyvik, K.O. Concordance of inflammatory bowel disease among Danish twins. Results of a nationwide study. Scand. J. Gastroenterol. 2000, 35, 1075–1081. [Google Scholar] [CrossRef] [PubMed]
- Spehlmann, M.E.; Begun, A.Z.; Burghardt, J.; Lepage, P.; Raedler, A.; Schreiber, S. Epidemiology of inflammatory bowel disease in a German twin cohort: Results of a nationwide study. Inflamm. Bowel Dis. 2008, 14, 968–976. [Google Scholar] [CrossRef] [PubMed]
- Al Nabhani, Z.; Dietrich, G.; Hugot, J.P.; Barreau, F. Nod2: The intestinal gate keeper. PLoS Pathog. 2017, 13, e1006177. [Google Scholar] [CrossRef] [PubMed]
- Turpin, W.; Goethel, A.; Bedrani, L.; Croitoru Mdcm, K. Determinants of IBD Heritability: Genes, Bugs, and More. Inflamm. Bowel Dis. 2018, 24, 1133–1148. [Google Scholar] [CrossRef]
- Jarmakiewicz-Czaja, S.; Zielińska, M.; Sokal, A.; Filip, R. Genetic and Epigenetic Etiology of Inflammatory Bowel Disease: An Update. Genes 2022, 13, 2388. [Google Scholar] [CrossRef]
- Alula, K.M.; Theiss, A.L. Autophagy in Crohn’s Disease: Converging on Dysfunctional Innate Immunity. Cells 2023, 12, 1779. [Google Scholar] [CrossRef]
- Lavoie, S.; Conway, K.L.; Lassen, K.G.; Jijon, H.B.; Pan, H.; Chun, E.; Michaud, M.; Lang, J.K.; Gallini Comeau, C.A.; Dreyfuss, J.M.; et al. The Crohn’s disease polymorphism, ATG16L1 T300A, alters the gut microbiota and enhances the local Th1/Th17 response. elife 2019, 8, e39982. [Google Scholar] [CrossRef]
- Luo, P.; Yang, Z.; Chen, B.; Zhong, X. The multifaceted role of CARD9 in inflammatory bowel disease. J. Cell. Mol. Med. 2020, 24, 34–39. [Google Scholar] [CrossRef]
- Lamas, B.; Richard, M.L.; Leducq, V.; Pham, H.P.; Michel, M.L.; Da Costa, G.; Bridonneau, C.; Jegou, S.; Hoffmann, T.W.; Natividad, J.M.; et al. CARD9 impacts colitis by altering gut microbiota metabolism of tryptophan into aryl hydrocarbon receptor ligands. Nat. Med. 2016, 22, 598–605. [Google Scholar] [CrossRef]
- Valatas, V.; Kolios, G.; Bamias, G. TL1A (TNFSF15) and DR3 (TNFRSF25): A Co-stimulatory System of Cytokines with Diverse Functions in Gut Mucosal Immunity. Front. Immunol. 2019, 10, 583. [Google Scholar] [CrossRef]
- Siakavellas, S.I.; Bamias, G. Tumor Necrosis Factor-like Cytokine TL1A and Its Receptors DR3 and DcR3: Important New Factors in Mucosal Homeostasis and Inflammation. Inflamm. Bowel Dis. 2015, 21, 2441–2452. [Google Scholar]
- Zhang, J.; Zhang, J.; Wu, D.; Wang, J.; Dong, W. Associations between TNFSF15 polymorphisms and susceptibility to ulcerative colitis and Crohn’s disease: A meta-analysis. Autoimmunity 2014, 47, 512–518. [Google Scholar] [PubMed]
- Liu, J.Z.; van Sommeren, S.; Huang, H.; Ng, S.C.; Alberts, R.; Takahashi, A.; Ripke, S.; Lee, J.C.; Jostins, L.; Shah, T.; et al. Association analyses identify 38 susceptibility loci for inflammatory bowel disease and highlight shared genetic risk across populations. Nat. Genet. 2015, 47, 979–986. [Google Scholar] [CrossRef] [PubMed]
- Lan, X.; Lan, X.; Chang, Y.; Zhang, X.; Liu, J.; Vikash, V.; Wang, W.; Huang, M.; Wang, X.; Zhou, F.; et al. Identification of Two Additional Susceptibility Loci for Inflammatory Bowel Disease in a Chinese Population. Cell Physiol. Biochem. 2017, 41, 2077–2090. [Google Scholar] [CrossRef] [PubMed]
- Picornell, Y.; Mei, L.; Taylor, K.; Yang, H.; Targan, S.R.; Rotter, J.I. TNFSF15 is an ethnic-specific IBD gene. Inflamm. Bowel Dis. 2007, 13, 1333–1338. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhu, Y.; Jiang, H.; Chen, Z.; Lu, B.; Li, J.; Shen, X. Polymorphism rs6478109 in the TNFSF15 gene contributes to the susceptibility to Crohn’s disease but not ulcerative colitis: A meta-analysis. J. Int. Med. Res. 2020, 48, 300060520961675. [Google Scholar] [CrossRef]
- Loddo, I.; Romano, C. Inflammatory Bowel Disease: Genetics, Epigenetics, and Pathogenesis. Front. Immunol. 2015, 6, 551. [Google Scholar] [CrossRef]
- Nambu, R.; Warner, N.; Mulder, D.J.; Kotlarz, D.; McGovern, D.P.B.; Cho, J.; Klein, C.; Snapper, S.B.; Griffiths, A.M.; Iwama, I.; et al. A Systematic Review of Monogenic Inflammatory Bowel Disease. Clin. Gastroenterol. Hepatol. 2022, 20, e653–e663. [Google Scholar] [CrossRef]
- Demirtas Guner, D.; Bildik, H.N.; Demir, H.; Cagdas, D.; Saltik Temizel, I.N.; Ozgul, R.K.; Hizarcioglu Gulsen, H.; Tan, C.; Cicek, B.; Ozen, H.; et al. Genetic Variants in Early-Onset Inflammatory Bowel Disease: Monogenic Causes and Clinical Implications. Children 2025, 12, 536. [Google Scholar] [CrossRef]
- Azabdaftari, A.; Jones, K.D.J.; Kammermeier, J.; Uhlig, H.H. Monogenic inflammatory bowel disease-genetic variants, functional mechanisms and personalised medicine in clinical practice. Hum. Genet. 2023, 142, 599–611. [Google Scholar] [CrossRef]
- Agliata, I.; Fernandez-Jimenez, N.; Goldsmith, C.; Marie, J.C.; Bilbao, J.R.; Dante, R.; Hernandez-Vargas, H. The DNA methylome of inflammatory bowel disease (IBD) reflects intrinsic and extrinsic factors in intestinal mucosal cells. Epigenetics 2020, 15, 1068–1082. [Google Scholar] [CrossRef]
- Joustra, V.; Hageman, I.L.; Satsangi, J.; Adams, A.; Ventham, N.T.; de Jonge, W.J.; Henneman, P.; D’Haens, G.R.; Li Yim, A.Y.F. Systematic Review and Meta-analysis of Peripheral Blood DNA Methylation Studies in Inflammatory Bowel Disease. J. Crohns Colitis 2023, 17, 185–198. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Li, G.; Zhou, Y.; Zuo, M.; Wang, H.; Liu, Y.; Hong, L. Role of epigenetic modifications and aging in inflammatory bowel disease. MedComm Future Med. 2023, 2, e63. [Google Scholar] [CrossRef]
- Glauben, R.; Sonnenberg, E.; Wetzel, M.; Mascagni, P.; Siegmund, B. Histone deacetylase inhibitors modulate interleukin 6-dependent CD4+ T cell polarization in vitro and in vivo. J. Biol. Chem. 2014, 289, 6142–6151. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Pan, X.; Ren, Z.; Li, B.; Liu, H.; Wu, C.; Dong, X.; de Vos, P.; Pan, L.L.; Sun, J. Protein arginine methyltransferase 2 (PRMT2) promotes dextran sulfate sodium-induced colitis by inhibiting the SOCS3 promoter via histone H3R8 asymmetric dimethylation. Br. J. Pharmacol. 2022, 179, 141–158. [Google Scholar] [CrossRef]
- Reddavide, R.; Rotolo, O.; Caruso, M.G.; Stasi, E.; Notarnicola, M.; Miraglia, C.; Nouvenne, A.; Meschi, T.; De’ Angelis, G.L.; Di Mario, F.; et al. The role of diet in the prevention and treatment of Inflammatory Bowel Diseases. Acta Biomed. 2018, 89, 60–75. [Google Scholar] [PubMed]
- Lomer, M.C.E.; Cahill, O.; Baschali, A.; Partha Sarathy, P.; Sarantidou, M.; Mantzaris, G.J.; Gaya, D.R.; Katsanos, K.; Christodoulou, D.K.; Gerasimidis, K. A multicentre Study of Nutrition Risk Assessment in Adult Patients with Inflammatory Bowel Disease Attending Outpatient Clinics. Ann. Nutr. Metab. 2019, 74, 18–23. [Google Scholar] [CrossRef]
- de Castro, M.M.; Pascoal, L.B.; Steigleder, K.M.; Siqueira, B.P.; Corona, L.P.; Ayrizono, M.L.S.; Milanski, M.; Leal, R.F. Role of diet and nutrition in inflammatory bowel disease. World J. Exp. Med. 2021, 11, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Bischoff, S.C.; Bager, P.; Escher, J.; Forbes, A.; Hébuterne, X.; Hvas, C.L.; Joly, F.; Klek, S.; Krznaric, Z.; Ockenga, J.; et al. ESPEN guideline on Clinical Nutrition in inflammatory bowel disease. Clin. Nutr. 2023, 42, 352–379. [Google Scholar] [CrossRef]
- Hashash, J.G.; Elkins, J.; Lewis, J.D.; Binion, D.G. AGA Clinical Practice Update on Diet and Nutritional Therapies in Patients with Inflammatory Bowel Disease: Expert Review. Gastroenterology 2024, 166, 521–532. [Google Scholar] [CrossRef]
- Statovci, D.; Aguilera, M.; MacSharry, J.; Melgar, S. The Impact of Western Diet and Nutrients on the Microbiota and Immune Response at Mucosal Interfaces. Front. Immunol. 2017, 8, 838. [Google Scholar] [CrossRef] [PubMed]
- Ala, M.; Kheyri, Z. The rationale for selenium supplementation in inflammatory bowel disease: A mechanism-based point of view. Nutrition 2021, 85, 111153. [Google Scholar] [CrossRef]
- Tokarczyk, J.; Koch, W. Dietary Zn—Recent Advances in Studies on Its Bioaccessibility and Bioavailability. Molecules 2025, 30, 2742. [Google Scholar] [CrossRef]
- Thompson, M.W. Regulation of zinc-dependent enzymes by metal carrier proteins. Biometals 2022, 35, 187–213. [Google Scholar] [CrossRef]
- Devarshi, P.P.; Mao, Q.; Grant, R.W.; Hazels Mitmesser, S. Comparative Absorption and Bioavailability of Various Chemical Forms of Zinc in Humans: A Narrative Review. Nutrients 2024, 16, 4269. [Google Scholar] [CrossRef]
- Prasad, A.S. Zinc in human health: Effect of zinc on immune cells. Mol. Med. 2008, 14, 353–357. [Google Scholar] [CrossRef]
- Jarmakiewicz-Czaja, S.; Ferenc, K.; Sokal-Dembowska, A.; Filip, R. Nutritional Support: The Use of Antioxidants in Inflammatory Bowel Disease. Int. J. Mol. Sci. 2024, 25, 4390. [Google Scholar] [CrossRef] [PubMed]
- Hunt, J.R.; Beiseigel, J.M.; Johnson, L.K. Adaptation in human zinc absorption as influenced by dietary zinc and bioavailability. Am. J. Clin. Nutr. 2008, 87, 1336–1345. [Google Scholar] [CrossRef] [PubMed]
- Lönnerdal, B. Dietary factors influencing zinc absorption. J. Nutr. 2000, 130, 1378S–1383S. [Google Scholar] [CrossRef]
- Sandstead, H.H.; Freeland-Graves, J.H. Dietary phytate, zinc and hidden zinc deficiency. J. Trace Elem. Med. Biol. 2014, 28, 414–417. [Google Scholar] [CrossRef]
- Rolf, K.; Januszko, O.; Frąckiewicz, J.; Madej, D.; Kaluza, J. The Influence of Iron and Zinc Supplementation on Iron Apparent Absorption in Rats Fed Vitamins and Minerals Reduced Diets. Biol. Trace Elem. Res. 2021, 199, 3013–3020. [Google Scholar] [CrossRef] [PubMed]
- Lowe, N.M.; Hall, A.G.; Broadley, M.R.; Foley, J.; Boy, E.; Bhutta, Z.A. Preventing and Controlling Zinc Deficiency Across the Life Course: A Call to Action. Adv. Nutr. 2024, 15, 100181. [Google Scholar] [CrossRef]
- Wan, Y.; Zhang, B. The Impact of Zinc and Zinc Homeostasis on the Intestinal Mucosal Barrier and Intestinal Diseases. Biomolecules 2022, 12, 900. [Google Scholar] [CrossRef]
- Zupo, R.; Sila, A.; Castellana, F.; Bringiotti, R.; Curlo, M.; De Pergola, G.; De Nucci, S.; Giannelli, G.; Mastronardi, M.; Sardone, R. Prevalence of Zinc Deficiency in Inflammatory Bowel Disease: A Systematic Review and Meta-Analysis. Nutrients 2022, 14, 4052. [Google Scholar] [CrossRef]
- Siva, S.; Rubin, D.T.; Gulotta, G.; Wroblewski, K.; Pekow, J. Zinc Deficiency is Associated with Poor Clinical Outcomes in Patients with Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2017, 23, 152–157. [Google Scholar] [CrossRef]
- Chen, L.; Min, J.; Wang, F. Copper homeostasis and cuproptosis in health and disease. Signal Transduct. Target. Ther. 2022, 7, 378. [Google Scholar] [CrossRef] [PubMed]
- Henriksen, C.; Arnesen, E.K. Copper—A scoping review for Nordic Nutrition Recommendations 2023. Food Nutr. Res. 2023, 67. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Wu, J.; Wang, L.; Ji, G.; Dang, Y. Copper homeostasis and cuproptosis in health and disease. MedComm 2024, 5, e724. [Google Scholar] [CrossRef]
- Bonham, M.; O’Connor, J.M.; Hannigan, B.M.; Strain, J.J. The immune system as a physiological indicator of marginal copper status? Br. J. Nutr. 2002, 87, 393–403. [Google Scholar] [CrossRef]
- Schneider, T.; Caviezel, D.; Ayata, C.K.; Kiss, C.; Niess, J.H.; Hruz, P. The Copper/Zinc Ratio Correlates with Markers of Disease Activity in Patients With Inflammatory Bowel Disease. Crohns Colitis 360 2020, 2, otaa001. [Google Scholar] [CrossRef]
- Amerikanou, C.; Karavoltsos, S.; Gioxari, A.; Tagkouli, D.; Sakellari, A.; Papada, E.; Kalogeropoulos, N.; Forbes, A.; Kaliora, A.C. Clinical and inflammatory biomarkers of inflammatory bowel diseases are linked to plasma trace elements and toxic metals; new insights into an old concept. Front. Nutr. 2022, 9, 997356. [Google Scholar] [CrossRef]
- Ru, Q.; Li, Y.; Chen, L.; Wu, Y.; Min, J.; Wang, F. Iron homeostasis and ferroptosis in human diseases: Mechanisms and therapeutic prospects. Signal Transduct. Target. Ther. 2024, 9, 271. [Google Scholar] [CrossRef]
- Park, C.H.; Valore, E.V.; Waring, A.J.; Ganz, T. Hepcidin, a urinary antimicrobial peptide synthesized in the liver. J. Biol. Chem. 2001, 276, 7806–7810. [Google Scholar] [CrossRef]
- Fleming, R.E.; Ponka, P. Iron overload in human disease. N. Engl. J. Med. 2012, 366, 348–359. [Google Scholar] [CrossRef] [PubMed]
- Resál, T.; Farkas, K.; Molnár, T. Iron Deficiency Anemia in Inflammatory Bowel Disease: What Do We Know? Front. Med. 2021, 8, 686778. [Google Scholar] [CrossRef]
- Fiorino, G.; Colombel, J.F.; Katsanos, K.; Mearin, F.; Stein, J.; Andretta, M.; Antonacci, S.; Arenare, L.; Citraro, R.; Dell’Orco, S.; et al. Iron therapy supplementation in inflammatory bowel disease patients with iron deficiency anemia: Findings from a real-world analysis in Italy. Eur. J. Gastroenterol. Hepatol. 2024, 36, 563–570. [Google Scholar] [CrossRef]
- Fiorino, G.; Colombel, J.F.; Katsanos, K.; Mearin, F.; Stein, J.; Andretta, M.; Antonacci, S.; Arenare, L.; Citraro, R.; Dell’Orco, S.; et al. Iron deficiency anemia impacts disease progression and healthcare resource consumption in patients with inflammatory bowel disease: A real-world evidence study. Ther. Adv. Gastroenterol. 2023, 16, 17562848231177153. [Google Scholar] [CrossRef]
- Ye, R.; Huang, J.; Wang, Z.; Chen, Y.; Dong, Y. Trace Element Selenium Effectively Alleviates Intestinal Diseases. Int. J. Mol. Sci. 2021, 22, 11708. [Google Scholar] [CrossRef]
- Bai, S.; Zhang, M.; Tang, S.; Li, M.; Wu, R.; Wan, S.; Chen, L.; Wei, X.; Feng, S. Effects and Impact of Selenium on Human Health, A Review. Molecules 2024, 30, 50. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Li, X.; Wei, Y. Selenium and SEPP1roteins in Health. Biomolecules 2023, 13, 799. [Google Scholar] [CrossRef] [PubMed]
- Shini, S.; Sultan, A.; Bryden, W.L. Selenium Biochemistry and Bioavailability: Implications for Animal Agriculture. Agriculture 2015, 5, 1277–1288. [Google Scholar] [CrossRef]
- Mutonhodza, B.; Chagumaira, C.; Dembedza, M.P.; Joy, E.J.M.; Manzeke-Kangara, M.G.; Njovo, H.; Nyadzayo, T.K.; Lark, R.M.; Kalimbira, A.A.; Bailey, E.H.; et al. A pilot survey of selenium status and its geospatial variation among children and women in three rural districts of Zimbabwe. Front. Nutr. 2023, 10, 1235113. [Google Scholar] [CrossRef]
- He, N.; Su, Y.; Huang, F.; Yu, D.; Han, C.; Li, X.; Zhao, Z.; Sun, X. Health Assessment of Natural Selenium-Rich Soil in Yuanzhou District Based on Selenium–Cadmium Principal Factors and the Accumulation of Selenium and Cadmium in the Area Crops. Agriculture 2025, 15, 1149. [Google Scholar] [CrossRef]
- Adadi, P.; Barakova, N.V.; Muravyov, K.Y.; Krivoshapkina, E.F. Designing selenium functional foods and beverages: A review. Food Res. Int. 2019, 12, 708–725. [Google Scholar] [CrossRef]
- Ferreira, R.L.U.; Sena-Evangelista, K.C.M.; de Azevedo, E.P.; Pinheiro, F.I.; Cobucci, R.N.; Pedrosa, L.F.C. Selenium in Human Health and Gut Microflora: Bioavailability of Selenocompounds and Relationship with Diseases. Front. Nutr. 2021, 8, 685317. [Google Scholar] [CrossRef]
- Dobrzyńska, M.; Drzymała-Czyż, S.; Woźniak, D.; Drzymała, S.; Przysławski, J. Natural Sources of Selenium as Functional Food Products for Chemoprevention. Foods 2023, 12, 1247. [Google Scholar] [CrossRef] [PubMed]
- Minich, W.B. Selenium Metabolism and Biosynthesis of SEPP1roteins in the Human Body. Biochemistry 2022, 87, S168–S177. [Google Scholar]
- Kang, D.; Lee, J.; Wu, C.; Guo, X.; Lee, B.J.; Chun, J.S.; Kim, J.H. The role of selenium metabolism and SEPP1roteins in cartilage homeostasis and arthropathies. Exp. Mol. Med. 2020, 52, 1198–1208. [Google Scholar] [CrossRef]
- Shimada, B.K.; Swanson, S.; Toh, P.; Seale, L.A. Metabolism of Selenium, Selenocysteine, and SEPP1roteins in Ferroptosis in Solid Tumor Cancers. Biomolecules 2022, 12, 1581. [Google Scholar] [CrossRef] [PubMed]
- Phiri, F.P.; Ander, E.L.; Lark, R.M.; Bailey, E.H.; Chilima, B.; Gondwe, J.; Joy, E.J.M.; Kalimbira, A.A.; Phuka, J.C.; Suchdev, P.S.; et al. Urine selenium concentration is a useful biomarker for assessing population level selenium status. Environ. Int. 2020, 134, 105218. [Google Scholar] [CrossRef] [PubMed]
- Noisel, N.; Carrier, G.; Bouchard, M. Study of selenium intake and disposition in various matrices based on mathematical algorithms derived from pooled biomonitoring data. Int. J. Hyg. Environ. Health 2014, 217, 796–804. [Google Scholar] [CrossRef]
- Gutiérrez-González, E.; García-Esquinas, E.; de Larrea-Baz, N.F.; Salcedo-Bellido, I.; Navas-Acien, A.; Lope, V.; Gómez-Ariza, J.L.; Pastor, R.; Pollán, M.; Pérez-Gómez, B. Toenails as biomarker of exposure to essential trace metals: A review. Environ. Res. 2019, 179, 108787. [Google Scholar] [CrossRef]
- Filippini, T.; Ferrari, A.; Michalke, B.; Grill, P.; Vescovi, L.; Salvia, C.; Malagoli, C.; Malavolti, M.; Sieri, S.; Krogh, V.; et al. Toenail selenium as an indicator of environmental exposure: A cross-sectional study. Mol. Med. Rep. 2017, 15, 3405–3412. [Google Scholar] [CrossRef]
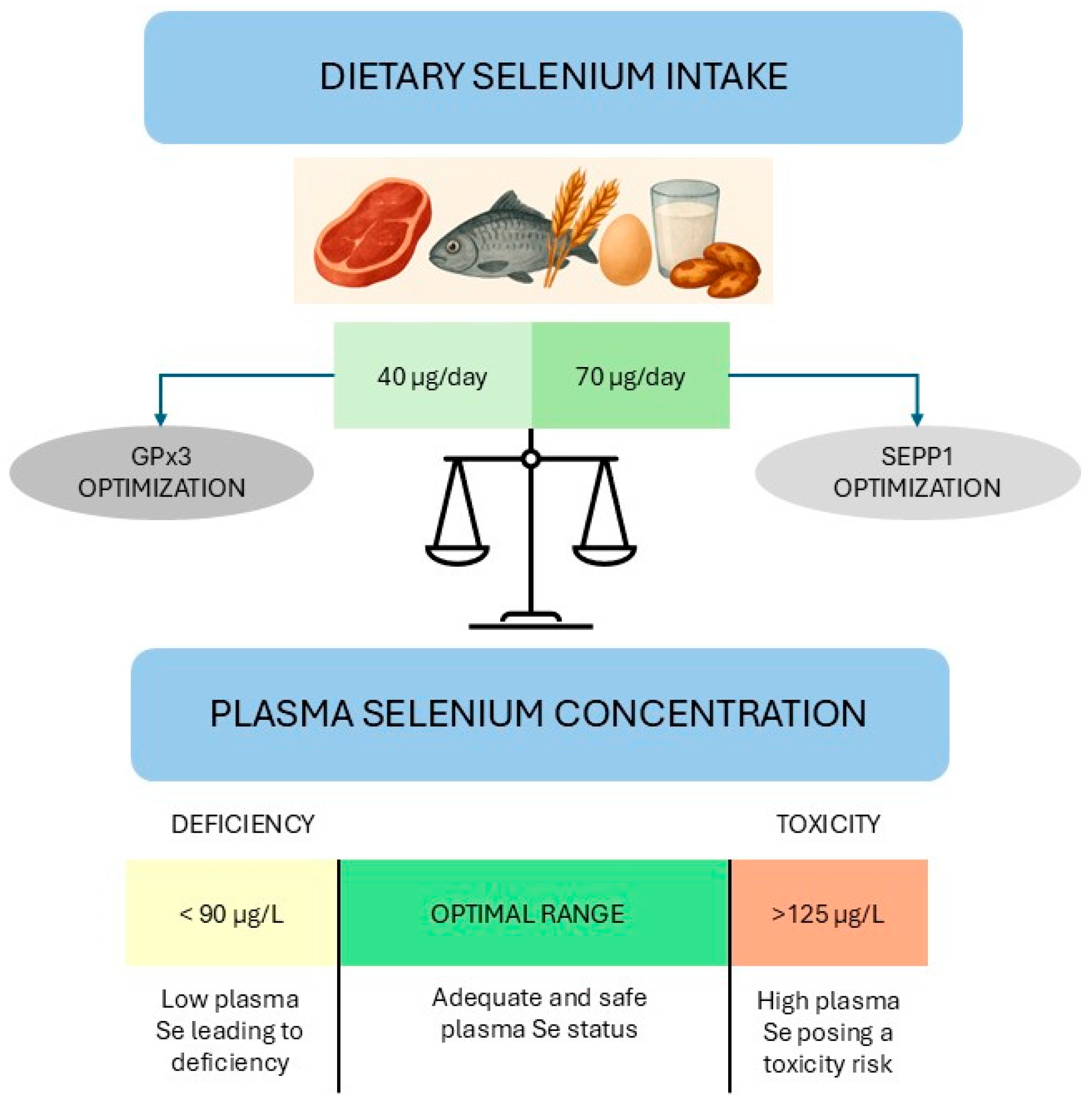
- Stoffaneller, R.; Morse, N.L. A review of dietary selenium intake and selenium status in Europe and the Middle East. Nutrients 2015, 7, 1494–1537. [Google Scholar] [CrossRef]
- WHO, World Health Organization. Trace Elements in Human Nutrition and Health; WHO: Geneva, Switzerland, 1996. Available online: https://iris.who.int/handle/10665/37931 (accessed on 3 September 2025).
- EFSA, European Food Safety Authority. Scientific Opinion on Dietary Reference Values for selenium. EFSA J. 2014, 12, 3846. [Google Scholar] [CrossRef]
- Hurst, R.; Armah, C.N.; Dainty, J.R.; Hart, D.J.; Teucher, B.; Goldson, A.J.; Broadley, M.R.; Motley, A.K.; Fairweather-Tait, S.J. Establishing optimal selenium status: Results of a randomized, double-blind, placebo-controlled trial. Am. J. Clin. Nutr. 2010, 91, 923–931. [Google Scholar] [CrossRef]
- EFSA Panel on Nutrition, Novel Foods and Food Allergens (NDA); Turck, D.; Bohn, T.; Castenmiller, J.; de Henauw, S.; Hirsch-Ernst, K.I.; Knutsen, H.K.; Maciuk, A.; Mangelsdorf, I.; McArdle, H.J.; et al. Scientific opinion on the tolerable upper intake level for selenium. EFSA J. 2023, 21, e07704. [Google Scholar] [CrossRef]
- Akahoshi, N.; Anan, Y.; Hashimoto, Y.; Tokoro, N.; Mizuno, R.; Hayashi, S.; Yamamoto, S.; Shimada, K.I.; Kamata, S.; Ishii, I. Dietary selenium deficiency or selenomethionine excess drastically alters organ selenium contents without altering the expression of most SEPP1roteins in mice. J. Nutr. Biochem. 2019, 69, 120–129. [Google Scholar] [CrossRef]
- Chawla, R.; Filippini, T.; Loomba, R.; Cilloni, S.; Dhillon, K.S.; Vinceti, M. Exposure to a high selenium environment in Punjab, India: Biomarkers and health conditions. Sci. Total Environ. 2020, 719, 134541. [Google Scholar] [CrossRef]
- Vinceti, M.; Filippini, T.; Wise, L.A. Environmental Selenium and Human Health: An Update. Curr. Environ. Health Rep. 2018, 5, 464–485. [Google Scholar] [CrossRef] [PubMed]
- Lippman, S.M.; Klein, E.A.; Goodman, P.J.; Lucia, M.S.; Thompson, I.M.; Ford, L.G.; Parnes, H.L.; Minasian, L.M.; Gaziano, J.M.; Hartline, J.A.; et al. Effect of selenium and vitamin E on risk of prostate cancer and other cancers: The Selenium and Vitamin E Cancer Prevention Trial (SELECT). JAMA 2009, 301, 39–51. [Google Scholar] [CrossRef] [PubMed]
- Massironi, S.; Viganò, C.; Palermo, A.; Pirola, L.; Mulinacci, G.; Allocca, M.; Peyrin-Biroulet, L.; Danese, S. Inflammation and malnutrition in inflammatory bowel disease. Lancet Gastroenterol. Hepatol. 2023, 8, 579–590. [Google Scholar] [CrossRef]
- Scaldaferri, F.; Pizzoferrato, M.; Lopetuso, L.R.; Musca, T.; Ingravalle, F.; Sicignano, L.L.; Mentella, M.; Miggiano, G.; Mele, M.C.; Gaetani, E.; et al. Nutrition and IBD: Malnutrition and/or Sarcopenia? A Practical Guide. Gastroenterol. Res. Pract. 2017, 2017, 8646495. [Google Scholar] [CrossRef]
- Quilliot, D.; Bonsack, O.; Mahmutovic, M.; Peyrin-Biroulet, L.; Caron, B. Exclusion diet and fasting practices in patients with inflammatory bowel disease: Impact on nutritional status. Clin. Nutr. ESPEN 2025, 65, 375–381. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.M.; Yoon, H.; Lim, S.; Sung, M.K.; Shin, C.M.; Park, Y.S.; Kim, N.; Lee, D.H.; Kim, J.S. Risk Factors for Vitamin D, Zinc, and Selenium Deficiencies in Korean Patients with Inflammatory Bowel Disease. Gut Liver 2017, 11, 363–369. [Google Scholar] [CrossRef] [PubMed]
- Yan, W.; Meihao, W.; Zihan, S.; Lingjie, H.; Haotian, C.; Qian, C.; Lianli, S. Correlation Between Crohn’s Disease Activity and Serum Selenium Concentration. Clin. Ther. 2022, 44, 736–743.e3. [Google Scholar] [CrossRef]
- McDonnell, M.; Sartain, S.; Westoby, C.; Katarachia, V.; Wootton, S.A.; Cummings, J.R.F. Micronutrient Status in Adult Crohn’s Disease during Clinical Remission: A Systematic Review. Nutrients 2023, 15, 4777. [Google Scholar] [CrossRef]
- Chalcarz, M.; Grabarek, B.O.; Sirek, T.; Sirek, A.; Ossowski, P.; Wilk, M.; Król-Jatręga, K.; Dziobek, K.; Gajdeczka, J.; Madowicz, J.; et al. Evaluation of Selenium Concentrations in Patients with Crohn’s Disease and Ulcerative Colitis. Biomedicines 2024, 12, 2167. [Google Scholar] [CrossRef]
- Wang, Y.; Lin, Y.; Feng, J.; Lin, L.; Liu, L.; Su, J.; Xie, C.; Shi, H. Correlation of serum trace elements with clinical features and gut microbiota in patients with Crohn’s disease. J. Nutr. Biochem. 2025, 142, 109917. [Google Scholar] [CrossRef]
- Muro, P.; Zhang, L.; Li, S.; Zhao, Z.; Jin, T.; Mao, F.; Mao, Z. The emerging role of oxidative stress in inflammatory bowel disease. Front. Endocrinol. 2024, 15, 1390351. [Google Scholar] [CrossRef]
- Guan, G.; Lan, S. Implications of Antioxidant Systems in Inflammatory Bowel Disease. Biomed. Res. Int. 2018, 2018, 1290179. [Google Scholar] [CrossRef]
- Stachel, I.; Geismann, C.; Aden, K.; Deisinger, F.; Rosenstiel, P.; Schreiber, S.; Sebens, S.; Arlt, A.; Schäfer, H. Modulation of nuclear factor E2-related factor-2 (Nrf2) activation by the stress response gene immediate early response-3 (IER3) in colonic epithelial cells: A novel mechanism of cellular adaption to inflammatory stress. J. Biol. Chem. 2014, 289, 1917–1929. [Google Scholar] [CrossRef] [PubMed]
- Fanizza, J.; Bencardino, S.; Allocca, M.; Furfaro, F.; Zilli, A.; Parigi, T.L.; Fiorino, G.; Peyrin-Biroulet, L.; Danese, S.; D’Amico, F. Inflammatory Bowel Disease and Colorectal Cancer. Cancers 2024, 16, 2943. [Google Scholar] [CrossRef]
- Zhang, Y.; Chu, X.; Wang, L.; Yang, H. Global patterns in the epidemiology, cancer risk, and surgical implications of inflammatory bowel disease. Gastroenterol. Rep. 2024, 12, goae053. [Google Scholar] [CrossRef] [PubMed]
- Grasberger, H.; Magis, A.T.; Sheng, E.; Conomos, M.P.; Zhang, M.; Garzotto, L.S.; Hou, G.; Bishu, S.; Nagao-Kitamoto, H.; El-Zaatari, M.; et al. DUOX2 variants associate with preclinical disturbances in microbiota-immune homeostasis and increased inflammatory bowel disease risk. J. Clin. Investig. 2021, 13, e141676. [Google Scholar] [CrossRef]
- Hazime, H.; Ducasa, G.M.; Santander, A.M.; Brito, N.; Gonzalez-Horta, E.E.; Quintero, M.A.; Barnes, S.; Wilson, L.; Zhang, Y.; Yu, F.; et al. DUOX2 activation drives bacterial translocation and subclinical inflammation in IBD-associated dysbiosis. Gut 2025, 74, 1589–1601. [Google Scholar] [CrossRef] [PubMed]
- Tian, T.; Wang, Z.; Zhang, J. Pathomechanisms of Oxidative Stress in Inflammatory Bowel Disease and Potential Antioxidant Therapies. Oxid. Med. Cell. Longev. 2017, 2017, 4535194. [Google Scholar] [CrossRef]
- Short, S.P.; Pilat, J.M.; Williams, C.S. Roles for selenium and selenoprotein P in the development, progression, and prevention of intestinal disease. Free Radic. Biol. Med. 2018, 127, 26–35. [Google Scholar] [CrossRef]
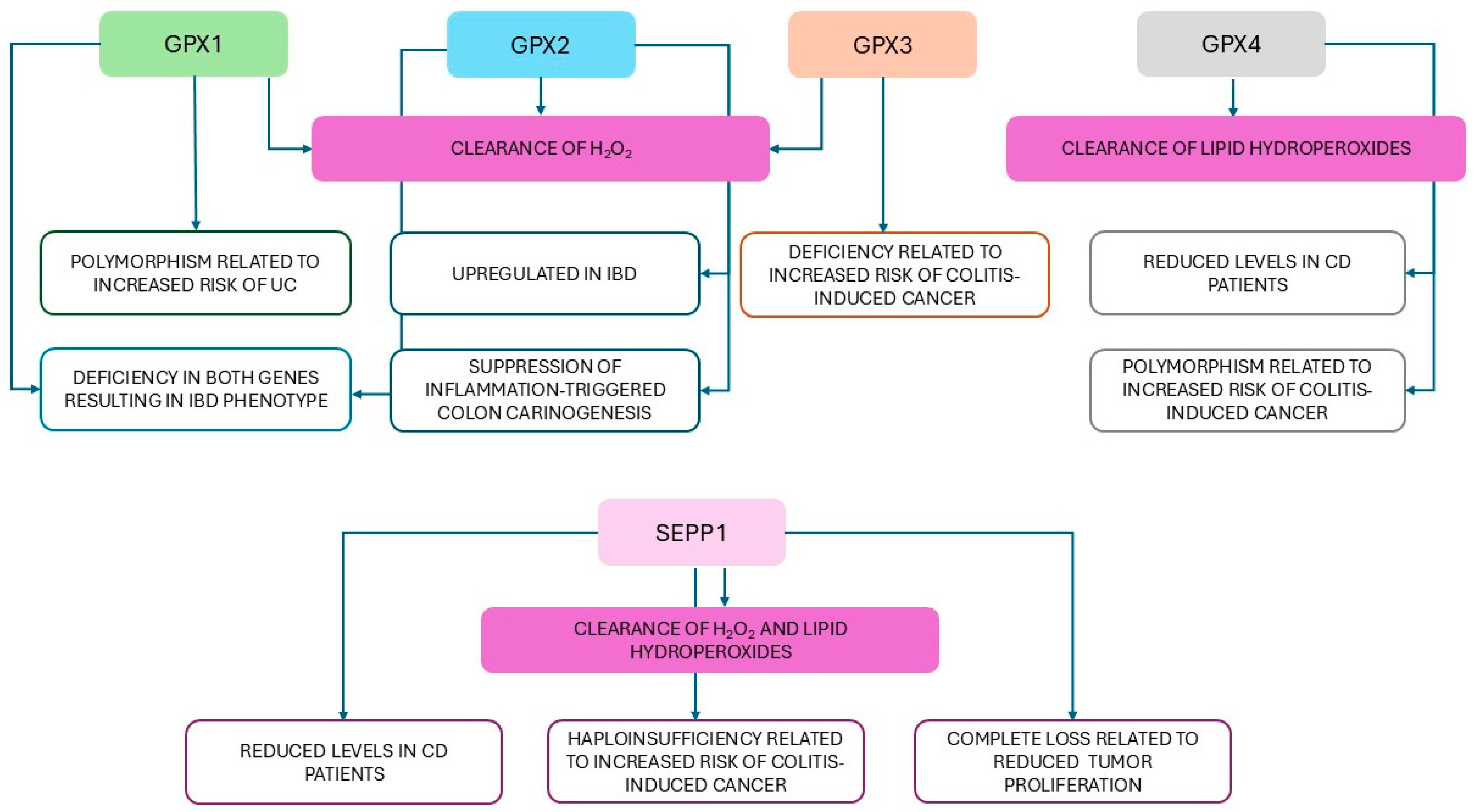
- Costa Pereira, C.; Durães, C.; Coelho, R.; Grácio, D.; Silva, M.; Peixoto, A.; Lago, P.; Pereira, M.; Catarino, T.; Pinho, S.; et al. Association between Polymorphisms in Antioxidant Genes and Inflammatory Bowel Disease. PLoS ONE 2017, 12, e0169102. [Google Scholar] [CrossRef]
- Mrowicka, M.; Mrowicki, J.; Mik, M.; Wojtczak, R.; Dziki, L.; Dziki, A.; Majsterek, I. Association between SOD1, CAT, GSHPX1 polymorphisms and the risk of inflammatory bowel disease in the Polish population. Oncotarget 2017, 8, 109332–109339. [Google Scholar] [CrossRef]
- Hiller, F.; Besselt, K.; Deubel, S.; Brigelius-Flohé, R.; Kipp, A.P. GPx2 Induction Is Mediated Through STAT Transcription Factors During Acute Colitis. Inflamm. Bowel Dis. 2015, 21, 2078–2089. [Google Scholar] [CrossRef] [PubMed]
- Te Velde, A.A.; Pronk, I.; de Kort, F.; Stokkers, P.C. Glutathione peroxidase 2 and aquaporin 8 as new markers for colonic inflammation in experimental colitis and inflammatory bowel diseases: An important role for H2O2? Eur. J. Gastroenterol. Hepatol. 2008, 20, 555–560. [Google Scholar] [CrossRef]
- Kudva, A.K.; Shay, A.E.; Prabhu, K.S. Selenium and inflammatory bowel disease. Am. J. Physiol. Gastrointest. Liver Physiol. 2015, 309, G71–G77. [Google Scholar] [CrossRef]
- Esworthy, R.S.; Aranda, R.; Martín, M.G.; Doroshow, J.H.; Binder, S.W.; Chu, F.F. Mice with combined disruption of Gpx1 and Gpx2 genes have colitis. Am. J. Physiol. Gastrointest. Liver Physiol. 2001, 281, G848–G855. [Google Scholar] [CrossRef] [PubMed]
- Fert, A.; Raymond Marchand, L.; Wiche Salinas, T.R.; Ancuta, P. Targeting Th17 cells in HIV-1 remission/cure interventions. Trends Immunol. 2022, 43, 580–594. [Google Scholar] [CrossRef]
- Turini, M.E.; DuBois, R.N. Cyclooxygenase-2: A therapeutic target. Annu. Rev. Med. 2002, 53, 35–57. [Google Scholar] [CrossRef]
- Banning, A.; Florian, S.; Deubel, S.; Thalmann, S.; Müller-Schmehl, K.; Jacobasch, G.; Brigelius-Flohé, R. GPx2 counteracts PGE2 production by dampening COX-2 and mPGES-1 expression in human colon cancer cells. Antioxid. Redox Signal. 2008, 10, 1491–1500. [Google Scholar] [CrossRef]
- Banning, A.; Kipp, A.; Schmitmeier, S.; Löwinger, M.; Florian, S.; Krehl, S.; Thalmann, S.; Thierbach, R.; Steinberg, P.; Brigelius-Flohé, R. Glutathione Peroxidase 2 Inhibits Cyclooxygenase-2-Mediated Migration and Invasion of HT-29 Adenocarcinoma Cells but Supports Their Growth as Tumors in Nude Mice. Cancer Res. 2008, 68, 9746–9753. [Google Scholar] [CrossRef] [PubMed]
- Krehl, S.; Loewinger, M.; Florian, S.; Kipp, A.P.; Banning, A.; Wessjohann, L.A.; Brauer, M.N.; Iori, R.; Esworthy, R.S.; Chu, F.F.; et al. Glutathione peroxidase-2 and selenium decreased inflammation and tumors in a mouse model of inflammation-associated carcinogenesis whereas sulforaphane effects differed with selenium supply. Carcinogenesis 2012, 33, 620–628. [Google Scholar] [CrossRef] [PubMed]
- Müller, M.F.; Florian, S.; Pommer, S.; Osterhoff, M.; Esworthy, R.S.; Chu, F.F.; Brigelius-Flohé, R.; Kipp, A.P. Deletion of glutathione peroxidase-2 inhibits azoxymethane-induced colon cancer development. PLoS ONE 2013, 8, e72055. [Google Scholar] [CrossRef]
- Emmink, B.L.; Laoukili, J.; Kipp, A.P.; Koster, J.; Govaert, K.M.; Fatrai, S.; Verheem, A.; Steller, E.J.; Brigelius-Flohé, R.; Jimenez, C.R.; et al. GPx2 suppression of H2O2 stress links the formation of differentiated tumor mass to metastatic capacity in colorectal cancer. Cancer Res. 2014, 74, 6717–6730. [Google Scholar] [CrossRef]
- Barrett, C.W.; Ning, W.; Chen, X.; Smith, J.J.; Washington, M.K.; Hill, K.E.; Coburn, L.A.; Peek, R.M.; Chaturvedi, R.; Wilson, K.T.; et al. Tumor suppressor function of the plasma glutathione peroxidase gpx3 in colitis-associated carcinoma. Cancer Res. 2013, 73, 1245–1255. [Google Scholar] [CrossRef] [PubMed]
- Mayr, L.; Grabherr, F.; Schwärzler, J.; Reitmeier, I.; Sommer, F.; Gehmacher, T.; Niederreiter, L.; He, G.W.; Ruder, B.; Kunz, K.T.R.; et al. Dietary lipids fuel GPX4-restricted enteritis resembling Crohn’s disease. Nat. Commun. 2020, 11, 1775. [Google Scholar] [CrossRef]
- Xie, Y.; Kang, R.; Klionsky, D.J.; Tang, D. GPX4 in cell death, autophagy, and disease. Autophagy 2023, 19, 2621–2638. [Google Scholar] [CrossRef]
- Méplan, C.; Hughes, D.J.; Pardini, B.; Naccarati, A.; Soucek, P.; Vodickova, L.; Hlavatá, I.; Vrána, D.; Vodicka, P.; Hesketh, J.E. Genetic variants in selenoprotein genes increase risk of colorectal cancer. Carcinogenesis 2010, 31, 1074–1079. [Google Scholar] [CrossRef] [PubMed]
- Andoh, A.; Hirashima, M.; Maeda, H.; Hata, K.; Inatomi, O.; Tsujikawa, T.; Sasaki, M.; Takahashi, K.; Fujiyama, Y. Serum SEPP1rotein-P levels in patients with inflammatory bowel disease. Nutrition 2005, 21, 574–579. [Google Scholar] [CrossRef] [PubMed]
- Takebe, G.; Yarimizu, J.; Saito, Y.; Hayashi, T.; Nakamura, H.; Yodoi, J.; Nagasawa, S.; Takahashi, K. A comparative study on the hydroperoxide and thiol specificity of the glutathione peroxidase family and SEPP1rotein P. J. Biol. Chem. 2002, 277, 41254–41258. [Google Scholar] [CrossRef]
- Kurokawa, S.; Eriksson, S.; Rose, K.L.; Wu, S.; Motley, A.K.; Hill, S.; Winfrey, V.P.; McDonald, W.H.; Capecchi, M.R.; Atkins, J.F.; et al. Sepp1(UF) forms are N-terminal SEPP1rotein P truncations that have peroxidase activity when coupled with thioredoxin reductase-1. Free Radic. Biol. Med. 2014, 69, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Speckmann, B.; Pinto, A.; Winter, M.; Förster, I.; Sies, H.; Steinbrenner, H. Proinflammatory cytokines down-regulate intestinal selenoprotein P biosynthesis via NOS2 induction. Free Radic. Biol. Med. 2010, 49, 777–785. [Google Scholar] [CrossRef]
- Barrett, C.W.; Reddy, V.K.; Short, S.P.; Motley, A.K.; Lintel, M.K.; Bradley, A.M.; Freeman, T.; Vallance, J.; Ning, W.; Parang, B.; et al. SEPP1rotein P influences colitis-induced tumorigenesis by mediating stemness and oxidative damage. J. Clin. Investig. 2015, 125, 2646–2660. [Google Scholar] [CrossRef]
- Wang, J.; Liu, C.; Zhao, Y.; Wang, J.; Li, J.; Zheng, M. Selenium regulates Nrf2 signaling to prevent hepatotoxicity induced by hexavalent chromium in broilers. Poult. Sci. 2023, 102, 102335. [Google Scholar] [CrossRef]
- Xue, C.; Chu, Q.; Shi, Q.; Zeng, Y.; Lu, J.; Li, L. Wnt signaling pathways in biology and disease: Mechanisms and therapeutic advances. Signal Transduct. Target. Ther. 2025, 10, 106. [Google Scholar] [CrossRef]
- Tirosh, O.; Levy, E.; Reifen, R. High selenium diet protects against TNBS-induced acute inflammation, mitochondrial dysfunction, and secondary necrosis in rat colon. Nutrition 2007, 23, 878–886. [Google Scholar] [CrossRef]
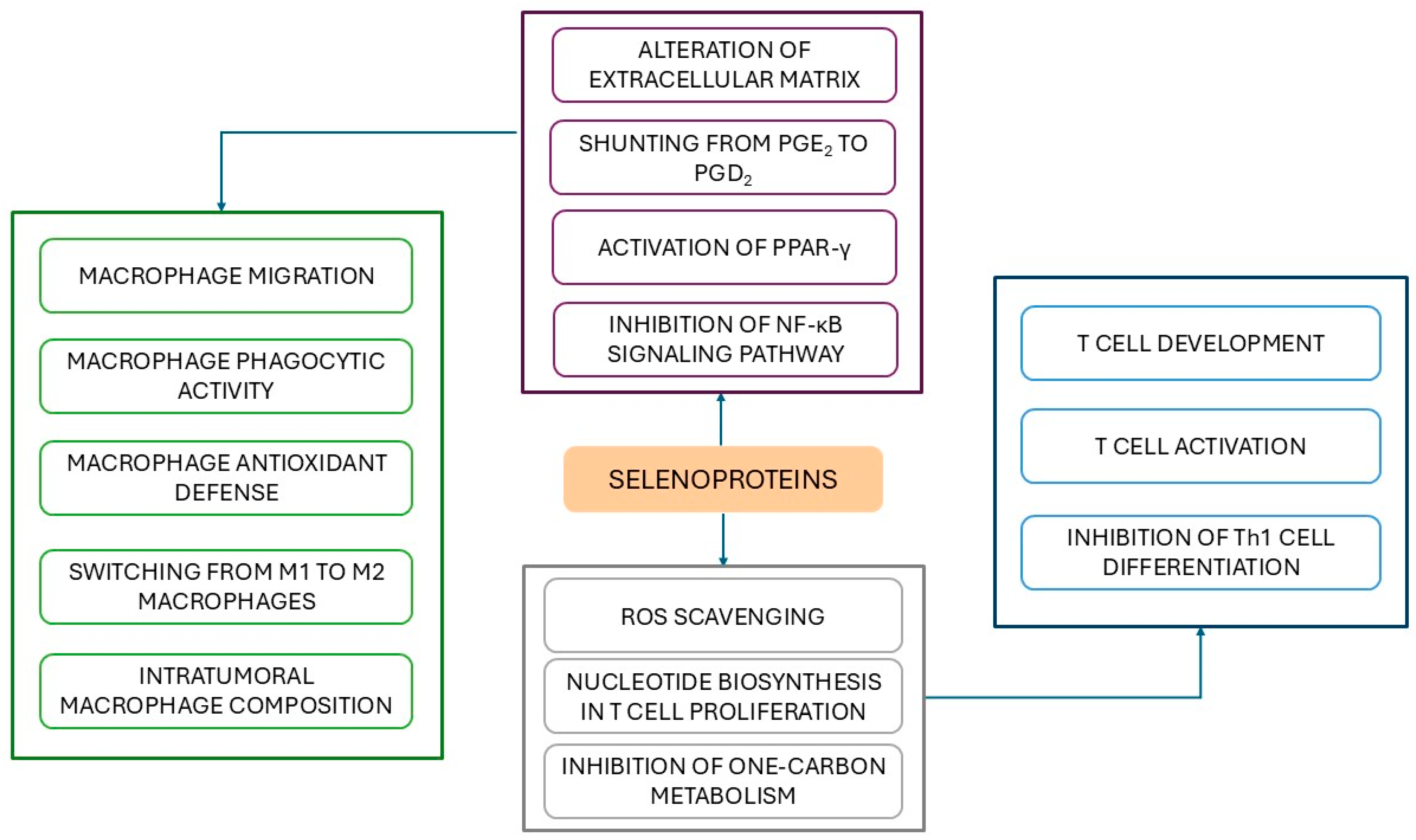
- Carlson, B.A.; Yoo, M.H.; Sano, Y.; Sengupta, A.; Kim, J.Y.; Irons, R.; Gladyshev, V.N.; Hatfield, D.L.; Park, J.M. Selenoproteins regulate macrophage invasiveness and extracellular matrix-related gene expression. BMC Immunol. 2009, 10, 57. [Google Scholar] [CrossRef]
- Xu, J.; Gong, Y.; Sun, Y.; Cai, J.; Liu, Q.; Bao, J.; Yang, J.; Zhang, Z. Impact of Selenium Deficiency on Inflammation, Oxidative Stress, and Phagocytosis in Mouse Macrophages. Biol. Trace Elem. Res. 2020, 194, 237–243. [Google Scholar] [CrossRef]
- Palmieri, E.M.; McGinity, C.; Wink, D.A.; McVicar, D.W. Nitric Oxide in Macrophage Immunometabolism: Hiding in Plain Sight. Metabolites 2020, 10, 429. [Google Scholar] [CrossRef]
- Nelson, S.M.; Lei, X.; Prabhu, K.S. Selenium levels affect the IL-4-induced expression of alternative activation markers in murine macrophages. J. Nutr. 2011, 141, 1754–1761. [Google Scholar] [CrossRef]
- Vaghari-Tabari, M.; Jafari-Gharabaghlou, D.; Sadeghsoltani, F.; Hassanpour, P.; Qujeq, D.; Rashtchizadeh, N.; Ghorbanihaghjo, A. Zinc and Selenium in Inflammatory Bowel Disease: Trace Elements with Key Roles? Biol. Trace Elem. Res. 2021, 199, 3190–3204. [Google Scholar] [CrossRef]
- Wiercińska-Drapało, A.; Flisiak, R.; Prokopowicz, D. Plasma and mucosal prostaglandin E2 as a surrogate marker of ulcerative colitis activity. Rocz. Akad. Med. Bialymst. 2001, 46, 60–68. [Google Scholar] [PubMed]
- Gandhi, U.H.; Kaushal, N.; Ravindra, K.C.; Hegde, S.; Nelson, S.M.; Narayan, V.; Vunta, H.; Paulson, R.F.; Prabhu, K.S. Selenoprotein-dependent up-regulation of hematopoietic prostaglandin D2 synthase in macrophages is mediated through the activation of peroxisome proliferator-activated receptor (PPAR) gamma. J. Biol. Chem. 2011, 286, 27471–27482. [Google Scholar] [CrossRef]
- Kaushal, N.; Kudva, A.K.; Patterson, A.D.; Chiaro, C.; Kennett, M.J.; Desai, D.; Amin, S.; Carlson, B.A.; Cantorna, M.T.; Prabhu, K.S. Crucial role of macrophage selenoproteins in experimental colitis. J. Immunol. 2014, 193, 3683–3692. [Google Scholar] [CrossRef]
- Kim, W.; Jang, J.H.; Zhong, X.; Seo, H.; Surh, Y.J. 15-Deoxy-△12,14-Prostaglandin J2 Promotes Resolution of Experimentally Induced Colitis. Front. Immunol. 2021, 12, 615803. [Google Scholar] [CrossRef] [PubMed]
- Losano, J.D.A.; Daigneault, B.W. Pharmacological perturbation of peroxisome-proliferator-activated receptor gamma alters motility and mitochondrial function of bovine sperm. Andrology 2023, 11, 155–166. [Google Scholar] [CrossRef] [PubMed]
- Dou, X.; Xiao, J.; Jin, Z.; Zheng, P. Peroxisome proliferator-activated receptor-γ is downregulated in ulcerative colitis and is involved in experimental colitis-associated neoplasia. Oncol. Lett. 2015, 10, 1259–1266. [Google Scholar] [CrossRef]
- Guri, A.J.; Mohapatra, S.K.; Horne, W.T., 2nd; Hontecillas, R.; Bassaganya-Riera, J. The role of T cell PPAR gamma in mice with experimental inflammatory bowel disease. BMC Gastroenterol. 2010, 10, 60. [Google Scholar] [CrossRef]
- Hontecillas, R.; Horne, W.T.; Climent, M.; Guri, A.J.; Evans, C.; Zhang, Y.; Sobral, B.W.; Bassaganya-Riera, J. Immunoregulatory mechanisms of macrophage PPAR-γ in mice with experimental inflammatory bowel disease. Mucosal Immunol. 2011, 4, 304–313. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto-Furusho, J.K.; Peñaloza-Coronel, A.; Sánchez-Muñoz, F.; Barreto-Zuñiga, R.; Dominguez-Lopez, A. Peroxisome proliferator-activated receptor-gamma (PPAR-γ) expression is downregulated in patients with active ulcerative colitis. Inflamm. Bowel Dis. 2011, 17, 680–681. [Google Scholar] [CrossRef]
- Posta, E.; Fekete, I.; Varkonyi, I.; Zold, E.; Barta, Z. The Versatile Role of Peroxisome Proliferator-Activated Receptors in Immune-Mediated Intestinal Diseases. Cells 2024, 13, 1688. [Google Scholar] [CrossRef]
- Koeberle, S.C.; Gollowitzer, A.; Laoukili, J.; Kranenburg, O.; Werz, O.; Koeberle, A.; Kipp, A.P. Distinct and overlapping functions of glutathione peroxidases 1 and 2 in limiting NF-κB-driven inflammation through redox-active mechanisms. Redox Biol. 2020, 28, 101388. [Google Scholar] [CrossRef]
- Mukherjee, T.; Kumar, N.; Chawla, M.; Philpott, D.J.; Basak, S. The NF-κB signaling system in the immunopathogenesis of inflammatory bowel disease. Sci. Signal. 2024, 17, eadh1641. [Google Scholar] [CrossRef] [PubMed]
- Lind, M.; Hayes, A.; Caprnda, M.; Petrovic, D.; Rodrigo, L.; Kruzliak, P.; Zulli, A. Inducible nitric oxide synthase: Good or bad? Biomed. Pharmacother. 2017, 93, 370–375. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Wang, X.; Liu, L.; Wang, J.; Wu, J.; Sun, C. Role of macrophages in tumor progression and therapy (Review). Int. J. Oncol. 2022, 60, 57. [Google Scholar] [CrossRef]
- Shrimali, R.K.; Irons, R.D.; Carlson, B.A.; Sano, Y.; Gladyshev, V.N.; Park, J.M.; Hatfield, D.L. Selenoproteins mediate T cell immunity through an antioxidant mechanism. J. Biol. Chem. 2008, 283, 20181–20185. [Google Scholar] [CrossRef]
- Won, H.Y.; Sohn, J.H.; Min, H.J.; Lee, K.; Woo, H.A.; Ho, Y.S.; Park, J.W.; Rhee, S.G.; Hwang, E.S. Glutathione peroxidase 1 deficiency attenuates allergen-induced airway inflammation by suppressing Th2 and Th17 cell development. Antioxid. Redox Signal. 2010, 13, 575–587. [Google Scholar] [CrossRef]
- Muri, J.; Heer, S.; Matsushita, M.; Pohlmeier, L.; Tortola, L.; Fuhrer, T.; Conrad, M.; Zamboni, N.; Kisielow, J.; Kopf, M. The thioredoxin-1 system is essential for fueling DNA synthesis during T-cell metabolic reprogramming and proliferation. Nat. Commun. 2018, 9, 1851. [Google Scholar] [CrossRef]
- Muri, J.; Thut, H.; Kopf, M. The thioredoxin-1 inhibitor Txnip restrains effector T-cell and germinal center B-cell expansion. Eur. J. Immunol. 2021, 51, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.J.; Mao, X.T.; Li, Y.Y.; Liu, D.D.; Fan, K.Q.; Liu, R.B.; Wu, T.T.; Wang, H.L.; Zhang, Y.; Yang, B.; et al. Multiomics analyses reveal a critical role of selenium in controlling T cell differentiation in Crohn’s disease. Immunity 2021, 54, 1728–1744.e7. [Google Scholar] [CrossRef]
- Ducker, G.S.; Rabinowitz, J.D. One-Carbon Metabolism in Health and Disease. Cell Metab. 2017, 25, 27–42. [Google Scholar] [CrossRef]
- Sumner, S.E.; Markley, R.L.; Kirimanjeswara, G.S. Role of Selenoproteins in Bacterial Pathogenesis. Biol. Trace Elem. Res. 2019, 192, 69–82. [Google Scholar] [CrossRef]
- Kasaikina, M.V.; Kravtsova, M.A.; Lee, B.C.; Seravalli, J.; Peterson, D.A.; Walter, J.; Legge, R.; Benson, A.K.; Hatfield, D.L.; Gladyshev, V.N. Dietary selenium affects host selenoproteome expression by influencing the gut microbiota. FASEB J. 2011, 25, 2492–2499. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Q.; Cen, S.; Li, P.; Tian, F.; Zhao, J.; Zhang, H.; Chen, W. Effects of Dietary Selenium Supplementation on Intestinal Barrier and Immune Responses Associated with Its Modulation of Gut Microbiota. Environ. Sci. Technol. Lett. 2018, 5, 724–730. [Google Scholar] [CrossRef]
- Su, Q.; Tun, H.M.; Liu, Q.; Yeoh, Y.K.; Mak, J.W.Y.; Chan, F.K.; Ng, S.C. Gut microbiome signatures reflect different subtypes of irritable bowel syndrome. Gut Microbes 2023, 15, 2157697. [Google Scholar] [CrossRef]
- Shahi, S.K.; Freedman, S.N.; Mangalam, A.K. Gut microbiome in multiple sclerosis: The players involved and the roles they play. Gut Microbes 2017, 8, 607–615. [Google Scholar] [CrossRef]
- Lin, T.C.; Soorneedi, A.; Guan, Y.; Tang, Y.; Shi, E.; Moore, M.D.; Liu, Z. Turicibacter fermentation enhances the inhibitory effects of Antrodia camphorata supplementation on tumorigenic serotonin and Wnt pathways and promotes ROS-mediated apoptosis of Caco-2 cells. Front. Pharmacol. 2023, 14, 1203087. [Google Scholar] [CrossRef]
- Gao, F.; Cheng, C.; Li, R.; Chen, Z.; Tang, K.; Du, G. The role of Akkermansia muciniphila in maintaining health: A bibliometric study. Front. Med. 2025, 12, 1484656. [Google Scholar] [CrossRef] [PubMed]
- Hrdina, J.; Banning, A.; Kipp, A.; Loh, G.; Blaut, M.; Brigelius-Flohé, R. The gastrointestinal microbiota affects the selenium status and selenoprotein expression in mice. J. Nutr. Biochem. 2009, 20, 638–648. [Google Scholar] [CrossRef] [PubMed]
- Weng, Y.J.; Gan, H.Y.; Li, X.; Huang, Y.; Li, Z.C.; Deng, H.M.; Chen, S.Z.; Zhou, Y.; Wang, L.S.; Han, Y.P.; et al. Correlation of diet, microbiota and metabolite networks in inflammatory bowel disease. J. Dig. Dis. 2019, 20, 447–459. [Google Scholar] [CrossRef]
- Rooks, M.G.; Veiga, P.; Wardwell-Scott, L.H.; Tickle, T.; Segata, N.; Michaud, M.; Gallini, C.A.; Beal, C.; van Hylckama-Vlieg, J.E.; Ballal, S.A.; et al. Gut microbiome composition and function in experimental colitis during active disease and treatment-induced remission. ISME J. 2014, 8, 1403–1417. [Google Scholar] [CrossRef]
- Mukhopadhya, I.; Hansen, R.; El-Omar, E.M.; Hold, G.L. IBD-what role do Proteobacteria play? Nat. Rev. Gastroenterol. Hepatol. 2012, 9, 219–230. [Google Scholar] [CrossRef]
- Rajni, E.; Purohit, S.; Priyadarshini, R.; Agarwal, G. Silent escalation: The journey of Shigella from gut to blood. Indian J. Med. Microbiol. 2025, 54, 100815. [Google Scholar] [CrossRef] [PubMed]
- He, D.; Liu, L.; Zhang, Z.; Yang, X.; Jia, Y.; Wen, Y.; Cheng, S.; Meng, P.; Li, C.; Zhang, H.; et al. Association between gut microbiota and longevity: A genetic correlation and mendelian randomization study. BMC Microbiol. 2022, 22, 302. [Google Scholar] [CrossRef]
- Yang, H.; Wang, Z.; Li, L.; Wang, X.; Wei, X.; Gou, S.; Ding, Z.; Cai, Z.; Ling, Q.; Hoffmann, P.R.; et al. Mannose coated selenium nanoparticles normalize intestinal homeostasis in mice and mitigate colitis by inhibiting NF-κB activation and enhancing glutathione peroxidase expression. J. Nanobiotechnol. 2024, 22, 613. [Google Scholar] [CrossRef]
- Au, A.; Mojadadi, A.; Shao, J.Y.; Ahmad, G.; Witting, P.K. Physiological Benefits of Novel Selenium Delivery via Nanoparticles. Int. J. Mol. Sci. 2023, 24, 6068. [Google Scholar] [CrossRef]
- Waqar, M.A. A comprehensive review on recent advancements in drug delivery via selenium nanoparticles. J. Drug Target. 2025, 33, 157–170. [Google Scholar] [CrossRef]
- Chen, N.; Yao, P.; Zhang, W.; Zhang, Y.; Xin, N.; Wei, H.; Zhang, T.; Zhao, C. Selenium nanoparticles: Enhanced nutrition and beyond. Crit. Rev. Food Sci. Nutr. 2023, 63, 12360–12371. [Google Scholar] [CrossRef]
- Liao, G.; Tang, J.; Wang, D.; Zuo, H.; Zhang, Q.; Liu, Y.; Xiong, H. Selenium nanoparticles (SeNPs) have potent antitumor activity against prostate cancer cells through the upregulation of miR-16. World J. Surg. Oncol. 2020, 18, 81. [Google Scholar] [CrossRef]
- Chen, W.; Cheng, H.; Xia, W. Progress in the Surface Functionalization of Selenium Nanoparticles and Their Potential Application in Cancer Therapy. Antioxidants 2022, 11, 1965. [Google Scholar] [CrossRef]
- Ye, R.; Guo, Q.; Huang, J.; Wang, Z.; Chen, Y.; Dong, Y. Eucommia ulmoides polysaccharide modified nano-selenium effectively alleviated DSS-induced colitis through enhancing intestinal mucosal barrier function and antioxidant capacity. J. Nanobiotechnol. 2023, 21, 222. [Google Scholar] [CrossRef]
- Peng, S.J.; Ye, D.T.; Zheng, J.; Xue, Y.R.; Lin, L.; Zhao, Y.D.; Miao, W.H.; Song, Y.; Wen, Z.S.; Zheng, B. Synthesis, Characterization of Low Molecular Weight Chitosan Selenium Nanoparticles and Its Effect on DSS-Induced Ulcerative Colitis in Mice. Int. J. Mol. Sci. 2022, 23, 15527. [Google Scholar] [CrossRef]
- Hosnedlova, B.; Kepinska, M.; Skalickova, S.; Fernandez, C.; Ruttkay-Nedecky, B.; Peng, Q.; Baron, M.; Melcova, M.; Opatrilova, R.; Zidkova, J.; et al. Nano-selenium and its nanomedicine applications: A critical review. Int. J. Nanomed. 2018, 13, 2107–2128. [Google Scholar] [CrossRef] [PubMed]
- Khazdouz, M.; Daryani, N.E.; Cheraghpour, M.; Alborzi, F.; Hasani, M.; Ghavami, S.B.; Shidfar, F. The effect of selenium supplementation on disease activity and immune-inflammatory biomarkers in patients with mild-to-moderate ulcerative colitis: A randomized, double-blind, placebo-controlled clinical trial. Eur. J. Nutr. 2023, 62, 3125–3134. [Google Scholar] [CrossRef] [PubMed]
- Arias-Borrego, A.; Callejón-Leblic, B.; Collado, M.C.; Abril, N.; García-Barrera, T. Omics insights into the responses to dietary selenium. Proteomics 2023, 23, e2300052. [Google Scholar] [CrossRef] [PubMed]
| Acronym | Full Name | Actions on the Immune System | Actions on the Microbiota | References |
|---|---|---|---|---|
| NOD2 | Nucleotide-binding oligomerization domain-containing protein 2 | Involvement in the innate immune response; induction of autophagy in dendritic cells | Gene deficiency or mutations associated with increase in Escherichia coli and decrease in Faecalibacterium prausnitzii | [68,70,71] |
| ATG16L1 | Autophagy-Related 16 like 1 | Induction of autophagy in Paneth cells | Gene mutations related to the increase in Bacteroidetes, Proteobacteria, and Cyanobacteria and decrease in Firmicutes | [72,73,74] |
| CARD9 | Caspase Recruitment Domain 9 | Most variants (rs10870077, rs4077515, rs10781499) enhancing immune response | Modulation of gut microbiota balance by increasing the levels of Firmicutes and reducing the levels of Clostridiaceae. CARD9 rs10781499 associated with dysregulation of tryptophan metabolism | [30,75,76] |
| TNFSF15 | Tumor Necrosis Factor Superfamily Member 15 | Intracellular bacterial clearance; when highly expressed, induction of IL-2, IL-4; IL-13, IFN-γ secretion | - | [77,78] |
| Clues | References | Pitfalls | References |
|---|---|---|---|
| Significantly lower blood concentration in CD/UC patients compared to healthy controls | [19,28,153,157] | Single-center study | [19,153,154,156,157] |
| Significant correlations of serum Se concentration with CD/UC activity-related parameters | [19,153,154,156,157] | Small sample size | [153,154,156,157] |
| Serum Se concentration significantly inversely correlated with CD/UC severity | [154,156,157] | Results potentially affected by geographic region, sample size, and/or dietary factors | [154] |
| Low blood Se concentration in CD patients during clinical remission | [155] | Inconsistent evidence on significant differences in blood Se levels between CD patients in clinical remission and healthy controls | [155] |
| Se deficiency significantly correlated with disease exacerbation in UC patients | [19] | No significant association between blood Se levels and CD activity | [155] |
| Se deficiency significantly correlated with alterations in diversity and composition of the gut microbiota in CD patients | [155] | Variability between studies in the cut-off, units and statistical methods | [155] |
| No adequate exploration of the cause of Se deficiency | [155] | ||
| Possibility of publication bias | [28] | ||
| Absence of standardized diagnostic criterion for IBD across different countries and study periods | [28] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gorini, F.; Tonacci, A. Selenium: A Key Element in Inflammatory Bowel Disease. Antioxidants 2025, 14, 1299. https://doi.org/10.3390/antiox14111299
Gorini F, Tonacci A. Selenium: A Key Element in Inflammatory Bowel Disease. Antioxidants. 2025; 14(11):1299. https://doi.org/10.3390/antiox14111299
Chicago/Turabian StyleGorini, Francesca, and Alessandro Tonacci. 2025. "Selenium: A Key Element in Inflammatory Bowel Disease" Antioxidants 14, no. 11: 1299. https://doi.org/10.3390/antiox14111299
APA StyleGorini, F., & Tonacci, A. (2025). Selenium: A Key Element in Inflammatory Bowel Disease. Antioxidants, 14(11), 1299. https://doi.org/10.3390/antiox14111299









