Lung Ischemia–Reperfusion Injury in Lung Transplant Surgery: Where Do We Stand?
Abstract
1. Introduction
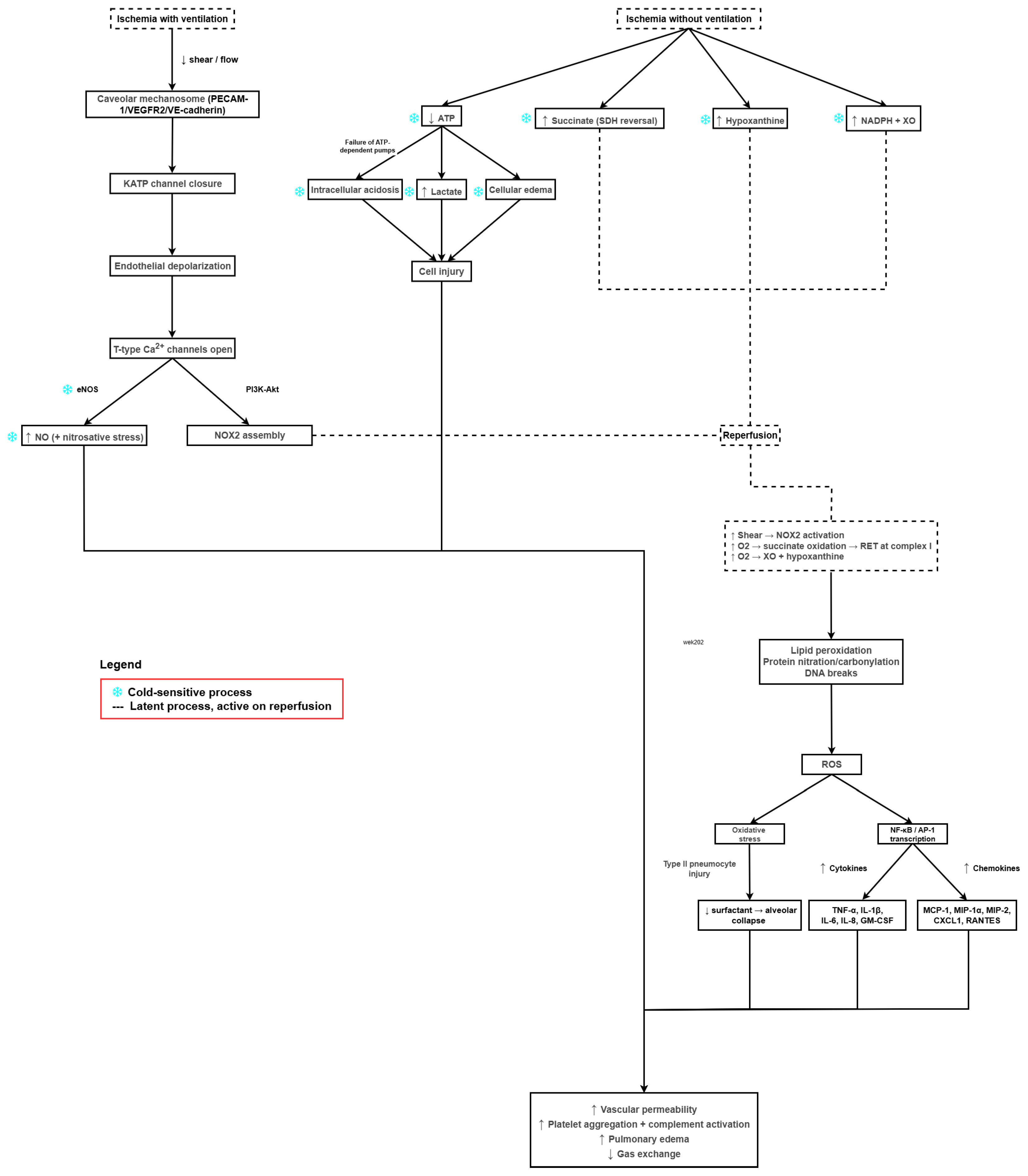
2. Pathophysiology of LIRI
3. Clinical Impact of LIRI–Lung Transplantation
3.1. Lung Transplantation and PGD
3.2. Risk Factors and Selection of Donor/Recipient
3.3. Donor Procedure and Preservation
3.4. Lung Implantation Procedure
3.5. LIRI in Donation After Circulatory Death
4. Treatment Strategies
4.1. Lung-Protective Ventilation and Hypercapnia
4.2. EVLP
4.3. Therapeutic Gases–NO, Carbon Monoxide, and Hydrogen
4.4. Pharmacological Therapies
4.5. Anti-Inflammatory and Immunomodulatory Approaches
4.6. Antioxidant Therapies
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ALI | Acute Lung Injury |
| ARDS | Acute Respiratory Distress Syndrome |
| ATP | Adenosine Triphosphate |
| BAL | Bronchoalveolar lavage |
| BMI | Body Mass Index |
| cAMP | Cyclic Adenosine Monophosphate |
| CD26 | Cluster of Differentiation 26 |
| cGMP | Cyclic Guanosine Monophosphate |
| CLAD | Chronic Lung Allograft Dysfunction |
| CO | Carbon Monoxide |
| COPD | Chronic Obstructive Pulmonary Disease |
| COR | Controlled Oxygenated Rewarming |
| CPB | Cardiopulmonary Bypass |
| CVP | Central Venous Pressure |
| CXCL | C-X-C Ligand |
| DAMP | Damage-associated Molecular Patterns |
| DBD | Donation after Brain Death |
| DCD | Donation after Circulatory Death |
| DNA | Deoxyribonucleic Acid |
| DPP-4 | Dipeptidyl Peptidase-4 |
| ECMO | Extracorporeal Membrane Oxygenation |
| EV | Extracellular Vesicles |
| EVLP | Ex Vivo Lung Perfusion |
| FiO2 | Fraction of Inspired Oxygen |
| GLP | Glucagon-like Peptide |
| GM-CSF | Granulocyte-Macrophage Colony-Stimulating Factor |
| HIF | Hypoxia-inducible Factor |
| HMG-CoA | 3-Hydroxy-3-Methylglutaryl Coenzyme A |
| HMGB1 | High Mobility Group Box 1 |
| HOPE | Hypothermic Oxygenated Perfusion |
| ICU | Intensive Care Unit |
| IL | Interleukin |
| iNOS | Inducible Nitric Oxide Synthase |
| IR | Ischemia–Reperfusion |
| IRI | Ischemia–Reperfusion Injury |
| IV | Intravenous |
| KATP | ATP-Sensitive Potassium Channel |
| LIRI | Lung Ischemia–Reperfusion Injury |
| MaR1 | Maresin-1 |
| MLKL | Mixed Lineage Kinase Domain Like Pseudokinase |
| MOF | Multiple Organ Failure |
| MSC | Mesenchymal Stem Cell |
| NAC | N-acetylcysteine |
| NADPH | Nicotinamide Adenine Dinucleotide Phosphate |
| NET | Neutrophil Extracellular Traps |
| NF-κB | Nuclear Factor Kappa B |
| NLRP3 | NOD-like Receptor Family Pyrin Domain Containing 3 |
| NMP | Normothermic Machine Perfusion |
| NO | Nitric Oxide |
| NOS | Nitric Oxide Synthase |
| NOX | NADPH Oxidase 2 |
| NRP | Normothermic Regional Perfusion |
| OCS | Organ Care System |
| OLV | One-lung Ventilation |
| PAP | Pulmonary Artery Pressure |
| PECAM-1 | Platelet Endothelial Cell Adhesion Molecule |
| PEEP | Positive End-Expiratory Pressure |
| PGD | Primary Graft Dysfunction |
| PI3K-Akt | Phosphoinositide 3-Kinase–Akt pathway |
| PINK1 | PTEN-induced kinase 1 |
| RANTES | Regulated upon Activation, Normal T cell Expressed and Secreted |
| RIC | Remote Ischemic Conditioning |
| RIPK1/3 | Receptor-interacting Serine/Threonine-Protein Kinases 1/3 |
| RNA | Ribonucleic Acid |
| ROS | Reactive Oxygen Species |
| SDF | Stromal-cell Derived Factor |
| SIRT1 | Sirtuin 1 |
| TLR | Toll-like Receptor |
| TNF | Tumor Necrosis Factor |
| tPA | Tissue Plasminogen Activator |
| uDCD | Uncontrolled Donation after Circulatory Death |
| VEGFR2 | Vascular Endothelial Growth Factor Receptor 2 |
| VIP | Vasoactive Intestinal Peptide |
| V/Q | Ventilation/Perfusion |
| XO | Xanthine Oxidase |
References
- Vlastos, D.; Zeinah, M.; Ninkovic-Hall, G.; Vlachos, S.; Salem, A.; Asonitis, A.; Chavan, H.; Kalampalikis, L.; Al Shammari, A.; Alvarez Gallesio, J.M.; et al. The effects of ischaemic conditioning on lung ischaemia–reperfusion injury. Respir. Res. 2022, 23, 351. [Google Scholar] [CrossRef] [PubMed]
- Den Hengst, W.A.; Gielis, J.F.; Lin, J.Y.; Van Schil, P.E.; De Windt, L.J.; Moens, A.L. Lung ischemia-reperfusion injury: A molecular and clinical view on a complex pathophysiological process. Am. J. Physiol.-Heart Circ. Physiol. 2010, 299, H1283–H1299. [Google Scholar] [CrossRef] [PubMed]
- Laubach, V.E.; Sharma, A.K. Mechanisms of lung ischemia-reperfusion injury. Curr. Opin. Organ. Transplant. 2016, 21, 246–252. [Google Scholar] [CrossRef]
- Ferrari, R.S.; Andrade, C.F. Oxidative Stress and Lung Ischemia-Reperfusion Injury. Oxid. Med. Cell. Longev. 2015, 2015, 590987. [Google Scholar] [CrossRef]
- Rowe, C.J.; Walsh, S.A.; Dragon, A.H.; Rhodes, A.M.; Pak, O.L.; Ronzier, E.; Levi, B.; Potter, B.K.; Spreadborough, P.J.; Davis, T.A. Tourniquet-induced ischemia creates increased risk of organ dysfunction and mortality following delayed limb amputation. Injury 2023, 54, 1792–1803. [Google Scholar] [CrossRef]
- Dulu, A.; Pastores, S.M.; Park, B.; Riedel, E.; Rusch, V.; Halpern, N.A. Prevalence and mortality of acute lung injury and ARDS after lung resection. Chest 2006, 130, 73–78. [Google Scholar] [CrossRef]
- Li, C.; Li, Y.S.; Xu, M.; Wen, S.H.; Yao, X.; Wu, Y.; Huang, C.Y.; Huang, W.Q.; Liu, K.X. Limb remote ischemic preconditioning for intestinal and pulmonary protection during elective open infrarenal abdominal aortic aneurysm repair: A randomized controlled trial. Anesthesiology 2013, 118, 842–852. [Google Scholar] [CrossRef]
- Hu, Q.; Luo, W.; Huang, L.; Huang, R.; Chen, R.; Gao, Y. Multiorgan protection of remote ischemic perconditioning in valve replacement surgery. J. Surg. Res. 2016, 200, 13–20. [Google Scholar] [CrossRef]
- Suzuki, T. Additional lung-protective perfusion techniques during cardiopulmonary bypass. Ann. Thorac. Cardiovasc. Surg. 2010, 16, 150–155. [Google Scholar]
- Chen-Yoshikawa, T.F. Ischemia-Reperfusion Injury in Lung Transplantation. Cells 2021, 10, 1333. [Google Scholar] [CrossRef] [PubMed]
- Sayah, D.M.; Mallavia, B.; Liu, F.; Ortiz-Muñoz, G.; Caudrillier, A.; DerHovanessian, A.; Ross, D.J.; Lynch III, J.P.; Saggar, R.; Ardehali, A.; et al. Neutrophil Extracellular Traps Are Pathogenic in Primary Graft Dysfunction after Lung Transplantation. Am. J. Respir. Crit. Care Med. 2015, 191, 455–463. [Google Scholar] [CrossRef] [PubMed]
- Talaie, T.; DiChiacchio, L.; Prasad, N.K.; Pasrija, C.; Julliard, W.; Kaczorowski, D.J.; Zhao, Y.; Lau, C.L. Ischemia-reperfusion Injury in the Transplanted Lung: A Literature Review. Transplant. Direct 2021, 7, e652. [Google Scholar] [CrossRef] [PubMed]
- Carter, Y.M.; Gelman, A.E.; Kreisel, D. Pathogenesis, Management, and Consequences of Primary Graft Dysfunction. Semin. Thorac. Cardiovasc. Surg. 2008, 20, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Miller, C.L.; O, J.M.; Allan, J.S.; Madsen, J.C. Novel approaches for long-term lung transplant survival. Front. Immunol. 2022, 13, 931251. [Google Scholar] [CrossRef]
- Van Slambrouck, J.; Van Raemdonck, D.; Vos, R.; Vanluyten, C.; Vanstapel, A.; Prisciandaro, E.; Willems, L.; Orlitová, M.; Kaes, J.; Jin, X.; et al. A Focused Review on Primary Graft Dysfunction after Clinical Lung Transplantation: A Multilevel Syndrome. Cells 2022, 11, 745. [Google Scholar] [CrossRef]
- Forgie, K.A.; Fialka, N.; Freed, D.H.; Nagendran, J. Lung Transplantation, Pulmonary Endothelial Inflammation, and Ex-Situ Lung Perfusion: A Review. Cells 2021, 10, 1417. [Google Scholar] [CrossRef]
- Beckers, P.A.J.; Gielis, J.F.; Van Schil, P.E.; Adriaensen, D. Lung ischemia reperfusion injury: The therapeutic role of dipeptidyl peptidase 4 inhibition. Ann. Transl. Med. 2017, 5, 129. [Google Scholar] [CrossRef]
- De Sousa, S.G.; Nascimento da Silva, G.V.; Costa Rodrigues, A.M.; Meireles Fernandes da Silva, T.M.; Costa, F.C.; Freitas Teixeira da Silva, A.; Santana de Macedo, B.F.; Brito, M.V.H. Organ Preservation Solutions in Transplantation: A Literature Review. Exp. Clin. Transplant. 2021, 19, 511–521. [Google Scholar] [CrossRef]
- Latchana, N.; Peck, J.R.; Whitson, B.; Black, S.M. Preservation solutions for cardiac and pulmonary donor grafts: A review of the current literature. J. Thorac. Dis. 2014, 6, 1143–1149. [Google Scholar] [CrossRef]
- Ahmad, K.; Pluhacek, J.L.; Brown, A.W. Ex Vivo Lung Perfusion: A Review of Current and Future Application in Lung Transplantation. Pulm. Ther. 2022, 8, 149–165. [Google Scholar] [CrossRef]
- Cenik, I.; Van Slambrouck, J.; Provoost, A.L.; Barbarossa, A.; Vanluyten, C.; Boelhouwer, C.; Vanaudenaerde, B.M.; Vos, R.; Pirenne, J.; Van Raemdonck, D.E.; et al. Controlled Hypothermic Storage for Lung Preservation: Leaving the Ice Age Behind. Transpl. Int. 2024, 37, 12601. [Google Scholar] [CrossRef]
- Hofmann, J.; Pühringer, M.; Steinkellner, S.; Holl, A.-S.; Meszaros, A.T.; Schneeberger, S.; Troppmair, J.; Hautz, T. Novel, Innovative Models to Study Ischemia/Reperfusion-Related Redox Damage in Organ Transplantation. Antioxidants 2023, 12, 31. [Google Scholar] [CrossRef]
- Mathis, S.; Putzer, G.; Schneeberger, S.; Martini, J. The Endothelial Glycocalyx and Organ Preservation-From Physiology to Possible Clinical Implications for Solid Organ Transplantation. Int. J. Mol. Sci. 2021, 22, 4019. [Google Scholar] [CrossRef] [PubMed]
- Ta, H.Q.; Kuppusamy, M.; Sonkusare, S.K.; Roeser, M.E.; Laubach, V.E. The endothelium: Gatekeeper to lung ischemia-reperfusion injury. Respir. Res. 2024, 25, 172. [Google Scholar] [CrossRef] [PubMed]
- Jungraithmayr, W. Novel Strategies for Endothelial Preservation in Lung Transplant Ischemia-Reperfusion Injury. Front. Physiol. 2020, 11, 581420. [Google Scholar] [CrossRef] [PubMed]
- Yamada, Y.; Sato, T.; Oda, H.; Harada, N.; Yoshizawa, A.; Nishikawa, S.; Kayawake, H.; Tanaka, S.; Yutaka, Y.; Hamaji, M.; et al. Favorable effect of CD26/DPP-4 inhibitors on postoperative outcomes after lung transplantation: A propensity-weighted analysis. J. Heart Lung Transplant. 2024, 43, 66–76. [Google Scholar] [CrossRef]
- Jang, J.-H.; Yamada, Y.; Janker, F.; De Meester, I.; Baerts, L.; Vliegen, G.; Inci, I.; Chatterjee, S.; Weder, W.; Jungraithmayr, W. Anti-inflammatory effects on ischemia/reperfusion-injured lung transplants by the cluster of differentiation 26/dipeptidylpeptidase 4 (CD26/DPP4) inhibitor vildagliptin. J. Thorac. Cardiovasc. Surg. 2017, 153, 713–724.e714. [Google Scholar] [CrossRef]
- Pak, O.; Sydykov, A.; Kosanovic, D.; Schermuly, R.T.; Dietrich, A.; Schröder, K.; Brandes, R.P.; Gudermann, T.; Sommer, N.; Weissmann, N. Lung Ischaemia–Reperfusion Injury: The Role of Reactive Oxygen Species. In Pulmonary Vasculature Redox Signaling in Health and Disease; Wang, Y.-X., Ed.; Springer International Publishing: Cham, Switzerland, 2017; pp. 195–225. [Google Scholar]
- Kalogeris, T.; Baines, C.P.; Krenz, M.; Korthuis, R.J. Cell biology of ischemia/reperfusion injury. Int. Rev. Cell Mol. Biol. 2012, 298, 229–317. [Google Scholar] [CrossRef]
- Granger, D.N.; Kvietys, P.R. Reperfusion injury and reactive oxygen species: The evolution of a concept. Redox Biol. 2015, 6, 524–551. [Google Scholar] [CrossRef]
- Xiang, M.; Lu, Y.; Xin, L.; Gao, J.; Shang, C.; Jiang, Z.; Lin, H.; Fang, X.; Qu, Y.; Wang, Y.; et al. Role of Oxidative Stress in Reperfusion following Myocardial Ischemia and Its Treatments. Oxid. Med. Cell. Longev. 2021, 2021, 6614009. [Google Scholar] [CrossRef]
- Chatterjee, S.; Nieman, G.F.; Christie, J.D.; Fisher, A.B. Shear stress-related mechanosignaling with lung ischemia: Lessons from basic research can inform lung transplantation. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2014, 307, L668–L680. [Google Scholar] [CrossRef]
- Li, C.; Jackson, R.M. Reactive species mechanisms of cellular hypoxia-reoxygenation injury. Am. J. Physiol.-Cell Physiol. 2002, 282, C227–C241. [Google Scholar] [CrossRef]
- Vishwakarma, V.K.; Upadhyay, P.K.; Gupta, J.K.; Yadav, H.N. Pathophysiologic role of ischemia reperfusion injury: A review. J. Indian Coll. Cardiol. 2017, 7, 97–104. [Google Scholar] [CrossRef]
- Rogers, L.K.; Cismowski, M.J. Oxidative stress in the lung—The essential paradox. Curr. Opin. Toxicol. 2018, 7, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Mittal, M.; Siddiqui, M.R.; Tran, K.; Reddy, S.P.; Malik, A.B. Reactive oxygen species in inflammation and tissue injury. Antioxid. Redox Signal 2014, 20, 1126–1167. [Google Scholar] [CrossRef] [PubMed]
- Qiao, X.; Yin, J.; Zheng, Z.; Li, L.; Feng, X. Endothelial cell dynamics in sepsis-induced acute lung injury and acute respiratory distress syndrome: Pathogenesis and therapeutic implications. Cell Commun. Signal 2024, 22, 241. [Google Scholar] [CrossRef]
- Matthay, M.A.; Zemans, R.L.; Zimmerman, G.A.; Arabi, Y.M.; Beitler, J.R.; Mercat, A.; Herridge, M.; Randolph, A.G.; Calfee, C.S. Acute respiratory distress syndrome. Nat. Rev. Dis. Primers 2019, 5, 18. [Google Scholar] [CrossRef]
- Kulkarni, H.S.; Ramphal, K.; Ma, L.; Brown, M.; Oyster, M.; Speckhart, K.N.; Takahashi, T.; Byers, D.E.; Porteous, M.K.; Kalman, L.; et al. Local complement activation is associated with primary graft dysfunction after lung transplantation. JCI Insight 2020, 5, e138358. [Google Scholar] [CrossRef]
- Shah, R.J.; Emtiazjoo, A.M.; Diamond, J.M.; Smith, P.A.; Roe, D.W.; Wille, K.M.; Orens, J.B.; Ware, L.B.; Weinacker, A.; Lama, V.N.; et al. Plasma Complement Levels Are Associated with Primary Graft Dysfunction and Mortality after Lung Transplantation. Am. J. Respir. Crit. Care Med. 2014, 189, 1564–1567. [Google Scholar] [CrossRef]
- Westall, G.P.; Snell, G.I.; McLean, C.; Kotsimbos, T.; Williams, T.; Magro, C. C3d and C4d deposition early after lung transplantation. J. Heart Lung Transplant. 2008, 27, 722–728. [Google Scholar] [CrossRef]
- Ruaro, B.; Salton, F.; Braga, L.; Wade, B.; Confalonieri, P.; Volpe, M.C.; Baratella, E.; Maiocchi, S.; Confalonieri, M. The History and Mystery of Alveolar Epithelial Type II Cells: Focus on Their Physiologic and Pathologic Role in Lung. Int. J. Mol. Sci. 2021, 22, 2566. [Google Scholar] [CrossRef] [PubMed]
- Duni, A.; Liakopoulos, V.; Koutlas, V.; Pappas, C.; Mitsis, M.; Dounousi, E. The Endothelial Glycocalyx as a Target of Ischemia and Reperfusion Injury in Kidney Transplantation—Where Have We Gone So Far? Int. J. Mol. Sci. 2021, 22, 2157. [Google Scholar] [CrossRef] [PubMed]
- Foote, C.A.; Soares, R.N.; Ramirez-Perez, F.I.; Ghiarone, T.; Aroor, A.; Manrique-Acevedo, C.; Padilla, J.; Martinez-Lemus, L. Endothelial Glycocalyx. Compr. Physiol. 2022, 12, 3781–3811. [Google Scholar] [CrossRef] [PubMed]
- Kimura, A.; Suehiro, K.; Yamada, T.; Shinta, Y.; Juri, T.; Fujimoto, Y.; Hirano, S.; Mori, T. Protective Effects of Hydrogen-Rich Saline Against Hemorrhagic Shock in Rats via an Endothelial Glycocalyx Pathway. Biomedicines 2025, 13, 833. [Google Scholar] [CrossRef]
- Moskowitzova, K.; Orfany, A.; Liu, K.; Ramirez-Barbieri, G.; Thedsanamoorthy, J.K.; Yao, R.; Guariento, A.; Doulamis, I.P.; Blitzer, D.; Shin, B.; et al. Mitochondrial transplantation enhances murine lung viability and recovery after ischemia-reperfusion injury. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2020, 318, L78–L88. [Google Scholar] [CrossRef]
- Li, Q.; Nie, H. Advances in lung ischemia/reperfusion injury: Unraveling the role of innate immunity. Inflamm. Res. 2024, 73, 393–405. [Google Scholar] [CrossRef]
- Fard, N.; Saffari, A.; Emami, G.; Hofer, S.; Kauczor, H.-U.; Mehrabi, A. Acute respiratory distress syndrome induction by pulmonary ischemia–reperfusion injury in large animal models. J. Surg. Res. 2014, 189, 274–284. [Google Scholar] [CrossRef]
- Christie, J.D.; Kotloff, R.M.; Ahya, V.N.; Tino, G.; Pochettino, A.; Gaughan, C.; DeMissie, E.; Kimmel, S.E. The effect of primary graft dysfunction on survival after lung transplantation. Am. J. Respir. Crit. Care Med. 2005, 171, 1312–1316. [Google Scholar] [CrossRef]
- Morrison, M.I.; Pither, T.L.; Fisher, A.J. Pathophysiology and classification of primary graft dysfunction after lung transplantation. J. Thorac. Dis. 2017, 9, 4084–4097. [Google Scholar] [CrossRef]
- Avtaar Singh, S.S.; Das De, S.; Al-Adhami, A.; Singh, R.; Hopkins, P.M.; Curry, P.A. Primary graft dysfunction following lung transplantation: From pathogenesis to future frontiers. World J. Transplant. 2023, 13, 58–85. [Google Scholar] [CrossRef]
- Diamond, J.M.; Cantu, E.; Calfee, C.S.; Anderson, M.R.; Clausen, E.S.; Shashaty, M.G.S.; Courtwright, A.M.; Kalman, L.; Oyster, M.; Crespo, M.M.; et al. The Impact of Donor Smoking on Primary Graft Dysfunction and Mortality after Lung Transplantation. Am. J. Respir. Crit. Care Med. 2024, 209, 91–100. [Google Scholar] [CrossRef]
- Lowery, E.M.; Kuhlmann, E.A.; Mahoney, E.L.; Dilling, D.F.; Kliethermes, S.A.; Kovacs, E.J. Heavy alcohol use in lung donors increases the risk for primary graft dysfunction. Alcohol Clin. Exp. Res. 2014, 38, 2853–2861. [Google Scholar] [CrossRef]
- Roesel, M.J.; Sharma, N.S.; Schroeter, A.; Matsunaga, T.; Xiao, Y.; Zhou, H.; Tullius, S.G. Primary Graft Dysfunction: The Role of Aging in Lung Ischemia-Reperfusion Injury. Front. Immunol. 2022, 13, 891564. [Google Scholar] [CrossRef]
- Bennett, R.M.; Reilly, J.P.; Diamond, J.M.; Cantu, E.; Shashaty, M.; Benvenuto, L.; Singer, J.P.; Palmer, S.M.; Christie, J.D.; Anderson, M.R. Body mass index and mortality following primary graft dysfunction: A Lung Transplant Outcomes Group study. JHLT Open 2024, 5, 100107. [Google Scholar] [CrossRef] [PubMed]
- Fang, A.; Studer, S.; Kawut, S.M.; Ahya, V.N.; Lee, J.; Wille, K.; Lama, V.; Ware, L.; Orens, J.; Weinacker, A.; et al. Elevated pulmonary artery pressure is a risk factor for primary graft dysfunction following lung transplantation for idiopathic pulmonary fibrosis. Chest 2011, 139, 782–787. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, J.A.; Verdiner, R.; Omar, A.; Farina, J.M.; Wilson, R.; D’Cunha, J.; Reck Dos Santos, P.A. Donor and recipient risk factors for the development of primary graft dysfunction following lung transplantation. Front. Immunol. 2024, 15, 1341675. [Google Scholar] [CrossRef] [PubMed]
- Lederer, D.J.; Kawut, S.M.; Wickersham, N.; Winterbottom, C.; Bhorade, S.; Palmer, S.M.; Lee, J.; Diamond, J.M.; Wille, K.M.; Weinacker, A.; et al. Obesity and primary graft dysfunction after lung transplantation: The Lung Transplant Outcomes Group Obesity Study. Am. J. Respir. Crit. Care Med. 2011, 184, 1055–1061. [Google Scholar] [CrossRef]
- Loor, G.; Warnecke, G.; Villavicencio, M.A.; Smith, M.A.; Zhou, X.; Kukreja, J.; Ardehali, A.; Hartwig, M.G.; Daneshmand, M.A.; Hertz, M.I.; et al. Long-term outcomes of the international EXPAND trial of Organ Care System (OCS) Lung preservation for lung transplantation. eClinicalMedicine 2025, 85, 103334. [Google Scholar] [CrossRef]
- Seay, T.; Guinn, N.; Maisonave, Y.; Fuller, M.; Poisson, J.; Pollak, A.; Bryner, B.; Haney, J.; Klapper, J.; Hartwig, M.; et al. The Association of Increased FFP:RBC Transfusion Ratio to Primary Graft Dysfunction in Bleeding Lung Transplantation Patients. J. Cardiothorac. Vasc. Anesth. 2020, 34, 3024–3032. [Google Scholar] [CrossRef]
- Vandervelde, C.M.; Vos, R.; Vanluyten, C.; Fieuws, S.; Verleden, S.E.; Van Slambrouck, J.; De Leyn, P.; Coosemans, W.; Nafteux, P.; Decaluwé, H.; et al. Impact of anastomosis time during lung transplantation on primary graft dysfunction. Am. J. Transplant. 2022, 22, 1418–1429. [Google Scholar] [CrossRef]
- Hunt, M.L.; Cantu, E. Primary graft dysfunction after lung transplantation. Curr. Opin. Organ. Transplant. 2023, 28, 180–186. [Google Scholar] [CrossRef]
- Jennekens, J.; Braithwaite, S.A.; Luijk, B.; van der Kaaij, N.P.; Vrisekoop, N.; de Jager, S.C.A.; de Heer, L.M. Primary Graft Dysfunction in Lung Transplantation: An Overview of the Molecular Mechanisms. Int. J. Mol. Sci. 2025, 26, 6776. [Google Scholar] [CrossRef]
- Jin, Z.; Suen, K.C.; Wang, Z.; Ma, D. Review 2: Primary graft dysfunction after lung transplant-pathophysiology, clinical considerations and therapeutic targets. J. Anesth. 2020, 34, 729–740. [Google Scholar] [CrossRef]
- Shaver, C.M.; Ware, L.B. Primary graft dysfunction: Pathophysiology to guide new preventive therapies. Expert Rev. Respir. Med. 2017, 11, 119–128. [Google Scholar] [CrossRef]
- Wong, K.H.M.; Hsin, K.Y.M. Primary graft dysfunction in lung transplantation: Still a thorn in the side of lung transplant. J. Thorac. Dis. 2024, 16, 1–5. [Google Scholar] [CrossRef]
- Snell, G.I.; Yusen, R.D.; Weill, D.; Strueber, M.; Garrity, E.; Reed, A.; Pelaez, A.; Whelan, T.P.; Perch, M.; Bag, R.; et al. Report of the ISHLT Working Group on Primary Lung Graft Dysfunction, part I: Definition and grading—A 2016 Consensus Group statement of the International Society for Heart and Lung Transplantation. J. Heart Lung Transplant. 2017, 36, 1097–1103. [Google Scholar] [CrossRef] [PubMed]
- Hyun, S.; Haam, S. Donation after Circulatory Death in Lung Transplantation. J. Chest Surg. 2022, 55, 283–287. [Google Scholar] [CrossRef] [PubMed]
- Avlonitis, V.S.; Wigfield, C.H.; Kirby, J.A.; Dark, J.H. The Hemodynamic Mechanisms of Lung Injury and Systemic Inflammatory Response Following Brain Death in the Transplant Donor. Am. J. Transplant. 2005, 5, 684–693. [Google Scholar] [CrossRef] [PubMed]
- Tague, L.K.; Bedair, B.; Witt, C.; Byers, D.E.; Vazquez-Guillamet, R.; Kulkarni, H.; Alexander-Brett, J.; Nava, R.; Puri, V.; Kreisel, D.; et al. Lung protective ventilation based on donor size is associated with a lower risk of severe primary graft dysfunction after lung transplantation. J. Heart Lung Transplant. 2021, 40, 1212–1222. [Google Scholar] [CrossRef]
- Upala, S.; Panichsillapakit, T.; Wijarnpreecha, K.; Jaruvongvanich, V.; Sanguankeo, A. Underweight and obesity increase the risk of mortality after lung transplantation: A systematic review and meta-analysis. Transpl. Int. 2016, 29, 285–296. [Google Scholar] [CrossRef]
- Porteous, M.K.; Lee, J.C.; Lederer, D.J.; Palmer, S.M.; Cantu, E.; Shah, R.J.; Bellamy, S.L.; Lama, V.N.; Bhorade, S.M.; Crespo, M.M.; et al. Clinical Risk Factors and Prognostic Model for Primary Graft Dysfunction after Lung Transplantation in Patients with Pulmonary Hypertension. Ann. Am. Thorac. Soc. 2017, 14, 1514–1522. [Google Scholar] [CrossRef]
- Catelli, C.; D’Alessandro, M.; Lloret Madrid, A.; Fossi, A.; Franchi, F.; Bennett, D.; Paladini, P.; Bargagli, E.; Luzzi, L. Donor-Recipient Mismatch in Lung Transplantation: The Role of Graft Sizing in Clinical Outcomes. Transpl. Int. 2025, 38, 14387. [Google Scholar] [CrossRef]
- Eberlein, M.; Reed, R.M.; Bolukbas, S.; Diamond, J.M.; Wille, K.M.; Orens, J.B.; Brower, R.G.; Christie, J.D. Lung size mismatch and primary graft dysfunction after bilateral lung transplantation. J. Heart Lung Transplant. 2015, 34, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Neizer, H.; Singh, G.B.; Gupta, S.; Singh, S.K. Addressing donor-organ shortages using extended criteria in lung transplantation. Ann. Cardiothorac. Surg. 2019, 9, 49–50. [Google Scholar] [CrossRef] [PubMed]
- D’Alessandro, D.; Schroder, J.; Meyer, D.M.; Vidic, A.; Shudo, Y.; Silvestry, S.; Leacche, M.; Sciortino, C.M.; Rodrigo, M.E.; Pham, S.M.; et al. Impact of controlled hypothermic preservation on outcomes following heart transplantation. J. Heart Lung Transplant. 2024, 43, 1153–1161. [Google Scholar] [CrossRef] [PubMed]
- Hoetzenecker, K.; Ali, A.; Campo-Cañaveral de la Cruz, J.; Schwarz, S.; Crowley Carrasco, S.; Romero Roman, A.; Aladaileh, M.; Benazzo, A.; Jaksch, P.; Wakeam, E.; et al. Prolonged Preservation of up to 24 Hours at 10 °C Does Not Impact Outcomes after Lung Transplantation. Ann. Surg. 2025, 281, 664–670. [Google Scholar] [CrossRef]
- Kukreja, J.; Campo-Canaveral de la Cruz, J.L.; Van Raemdonck, D.; Cantu, E.; Date, H.; D’Ovidio, F.; Hartwig, M.; Klapper, J.A.; Kelly, R.F.; Lindstedt, S.; et al. The 2024 American Association for Thoracic Surgery expert consensus document: Current standards in donor lung procurement and preservation. J. Thorac. Cardiovasc. Surg. 2025, 169, 484–504. [Google Scholar] [CrossRef]
- Bobba, C.M.; Saha, B.; Stukov, Y.; Kugler, L.; Aladaileh, M.A.; Oduntan, O.; Weir, W.; Jacobs, J.P.; Gries, C.; Emtiazjoo, A.; et al. One-year clinical outcomes of an observational study of static lung preservation at 10° centigrade and semi-elective lung transplantation. JHLT Open 2025, 9, 100241. [Google Scholar] [CrossRef]
- Abdelnour-Berchtold, E.; Ali, A.; Baciu, C.; Beroncal, E.L.; Wang, A.; Hough, O.; Kawashima, M.; Chen, M.; Zhang, Y.; Liu, M.; et al. Evaluation of 10 °C as the optimal storage temperature for aspiration-injured donor lungs in a large animal transplant model. J. Heart Lung Transplant. 2022, 41, 1679–1688. [Google Scholar] [CrossRef]
- Ali, A.; Wang, A.; Ribeiro, R.V.P.; Beroncal, E.L.; Baciu, C.; Galasso, M.; Gomes, B.; Mariscal, A.; Hough, O.; Brambate, E.; et al. Static lung storage at 10 °C maintains mitochondrial health and preserves donor organ function. Sci. Transl. Med. 2021, 13, eabf7601. [Google Scholar] [CrossRef]
- Wang, A.; Ali, A.; Baciu, C.; Bellissimo, C.; Siebiger, G.; Yamanashi, K.; Montagne, J.; Garza, G.; Goligher, E.; Keshavjee, S.; et al. Metabolomic studies reveal an organ-protective hibernation state in donor lungs preserved at 10 °C. J. Thorac. Cardiovasc. Surg. 2025, 169, 796–810.e791. [Google Scholar] [CrossRef]
- Yamanashi, K.; Wang, A.; Bellissimo, C.A.; Siebiger, G.; Oliveira, P.; Zhang, Y.; Montagne, J.; Garza, G.; Furie, N.; Pal, P.; et al. Protective effects of 10 °C preservation on donor lungs with lipopolysaccharide-induced acute lung injury. J. Thorac. Cardiovasc. Surg. 2025, 169, e74–e87. [Google Scholar] [CrossRef]
- Noda, K.; Philips, B.J.; Snyder, M.E.; Phillippi, J.A.; Sullivan, M.; Stolz, D.B.; Ren, X.; Luketich, J.D.; Sanchez, P.G. Heparanase inhibition preserves the endothelial glycocalyx in lung grafts and improves lung preservation and transplant outcomes. Sci. Rep. 2021, 11, 12265. [Google Scholar] [CrossRef]
- Sladden, T.M.; Yerkovich, S.; Wall, D.; Tan, M.; Hunt, W.; Hill, J.; Smith, I.; Hopkins, P.; Chambers, D.C. Endothelial Glycocalyx Shedding Occurs during Ex Vivo Lung Perfusion: A Pilot Study. J. Transplant. 2019, 2019, 6748242. [Google Scholar] [CrossRef]
- Ali, A.; Hoetzenecker, K.; Cruz, J.L.C.-C.d.l.; Schwarz, S.; Barturen, M.G.; Tomlinson, G.; Yeung, J.; Donahoe, L.; Yasufuku, K.; Pierre, A.; et al. Extension of Cold Static Donor Lung Preservation at 10 °C. NEJM Evid. 2023, 2, EVIDoa2300008. [Google Scholar] [CrossRef] [PubMed]
- Chouchani, E.T.; Pell, V.R.; Gaude, E.; Aksentijević, D.; Sundier, S.Y.; Robb, E.L.; Logan, A.; Nadtochiy, S.M.; Ord, E.N.J.; Smith, A.C.; et al. Ischaemic accumulation of succinate controls reperfusion injury through mitochondrial ROS. Nature 2014, 515, 431–435. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, D.C.; Loor, G.; Carrott, P.; Shafii, A. Review of donor and recipient surgical procedures in lung transplantation. J. Thorac. Dis. 2019, 11, S1810–S1816. [Google Scholar] [CrossRef]
- Kuipers, M.T.; van der Poll, T.; Schultz, M.J.; Wieland, C.W. Bench-to-bedside review: Damage-associated molecular patterns in the onset of ventilator-induced lung injury. Crit. Care 2011, 15, 235. [Google Scholar] [CrossRef]
- Mehew, J.D.; Hogg, R.; Clark, S.; Santhanakrishnan, K.; Catarino, P.; Mascaro, J.; Stock, U.; Dark, J. Risk of prolonged ischemic time linked to use of cardiopulmonary bypass during implantation for lung transplantation in the United Kingdom. J. Heart Lung Transplant. 2023, 42, 1378–1396. [Google Scholar] [CrossRef] [PubMed]
- Iske, J.; Hinze, C.A.; Salman, J.; Haverich, A.; Tullius, S.G.; Ius, F. The potential of ex vivo lung perfusion on improving organ quality and ameliorating ischemia reperfusion injury. Am. J. Transplant. 2021, 21, 3831–3839. [Google Scholar] [CrossRef]
- Casillan, A.J.; Zhou, A.L.; Ruck, J.M.; Larson, E.L.; Etchill, E.W.; Ha, J.S.; Shah, P.D.; Merlo, C.A.; Bush, E.L. The effect of allograft ischemic time on outcomes following bilateral, single, and reoperative lung transplantation. J. Thorac. Cardiovasc. Surg. 2024, 167, 556–565.e558. [Google Scholar] [CrossRef]
- Ahmed, H.; Saez, D.G.; Zych, B.; Dunning, J.; Khoshbin, E. Moderately Prolonged Cold Ischemic Time. Does It Impact Outcome of Lung Transplantation. J. Heart Lung Transplant. 2023, 42, S527–S528. [Google Scholar] [CrossRef]
- Elgharably, H.; Javorski, M.J.; McCurry, K.R. Bilateral sequential lung transplantation: Technical aspects. J. Thorac. Dis. 2021, 13, 6564–6575. [Google Scholar] [CrossRef] [PubMed]
- Beller, J.P.; Byler, M.R.; Money, D.T.; Chancellor, W.Z.; Zhang, A.; Zhao, Y.; Stoler, M.H.; Narahari, A.K.; Shannon, A.; Mehaffey, J.H.; et al. Reduced-flow ex vivo lung perfusion to rehabilitate lungs donated after circulatory death. J. Heart Lung. Transplant. 2020, 39, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Barnes, L.; Reed, R.M.; Parekh, K.R.; Bhama, J.K.; Pena, T.; Rajagopal, S.; Schmidt, G.A.; Klesney-Tait, J.A.; Eberlein, M. Mechanical ventilation for the lung transplant recipient. Curr. Pulmonol. Rep. 2015, 4, 88–96. [Google Scholar] [CrossRef]
- Rijn, R.v.; Schurink, I.J.; Vries, Y.d.; Berg, A.P.v.d.; Cerisuelo, M.C.; Murad, S.D.; Erdmann, J.I.; Gilbo, N.; Haas, R.J.d.; Heaton, N.; et al. Hypothermic Machine Perfusion in Liver Transplantation—A Randomized Trial. N. Engl. J. Med. 2021, 384, 1391–1401. [Google Scholar] [CrossRef]
- Schlegel, A.; Porte, R.; Dutkowski, P. Protective mechanisms and current clinical evidence of hypothermic oxygenated machine perfusion (HOPE) in preventing post-transplant cholangiopathy. J. Hepatol. 2022, 76, 1330–1347. [Google Scholar] [CrossRef]
- Minor, T.; von Horn, C.; Zlatev, H.; Saner, F.; Grawe, M.; Lüer, B.; Huessler, E.M.; Kuklik, N.; Paul, A. Controlled oxygenated rewarming as novel end-ischemic therapy for cold stored liver grafts. A randomized controlled trial. Clin. Transl. Sci. 2022, 15, 2918–2927. [Google Scholar] [CrossRef]
- Hoyer, D.P.; Benkö, T.; Manka, P.; von Horn, C.; Treckmann, J.W.; Paul, A.; Minor, T. Long-term Outcomes After Controlled Oxygenated Rewarming of Human Livers Before Transplantation. Transplant. Direct 2020, 6, e542. [Google Scholar] [CrossRef]
- Collins, M.G.; Chadban, S.J. Dealing With Delayed Graft Function. Transplantation 2024, 108, 1273–1274. [Google Scholar] [CrossRef]
- Beyersdorf, F. The use of controlled reperfusion strategies in cardiac surgery to minimize ischaemia/reperfusion damage. Cardiovasc. Res. 2009, 83, 262–268. [Google Scholar] [CrossRef]
- Schnickel, G.T.; Ross, D.J.; Beygui, R.; Shefizadeh, A.; Laks, H.; Saggar, R.; Lynch, J.P., 3rd; Ardehali, A. Modified reperfusion in clinical lung transplantation: The results of 100 consecutive cases. J. Thorac. Cardiovasc. Surg. 2006, 131, 218–223. [Google Scholar] [CrossRef]
- Shaver, C.M.; Wickersham, N.; McNeil, J.B.; Nagata, H.; Miller, A.; Landstreet, S.R.; Kuck, J.L.; Diamond, J.M.; Lederer, D.J.; Kawut, S.M.; et al. Cell-free hemoglobin promotes primary graft dysfunction through oxidative lung endothelial injury. JCI Insight 2018, 3, e98546. [Google Scholar] [CrossRef] [PubMed]
- Ellman, P.I.; Alvis, J.S.; Tache-Leon, C.; Singh, R.; Reece, T.B.; Kern, J.A.; Tribble, C.G.; Kron, I.L. Hyperoxic ventilation exacerbates lung reperfusion injury. J. Thorac. Cardiovasc. Surg. 2005, 130, 1440.e1–1440.e8. [Google Scholar] [CrossRef] [PubMed]
- Beer, A.; Reed, R.M.; Bölükbas, S.; Budev, M.; Chaux, G.; Zamora, M.R.; Snell, G.; Orens, J.B.; Klesney-Tait, J.A.; Schmidt, G.A.; et al. Mechanical Ventilation after Lung Transplantation. An International Survey of Practices and Preferences. Ann. Am. Thorac. Soc. 2014, 11, 546–553. [Google Scholar] [CrossRef] [PubMed]
- Dezube, R.; Arnaoutakis, G.J.; Reed, R.M.; Bolukbas, S.; Shah, A.S.; Orens, J.B.; Brower, R.G.; Eberlein, M. The effect of lung-size mismatch on mechanical ventilation tidal volumes after bilateral lung transplantation. Interact. Cardiovasc. Thorac. Surg. 2013, 16, 275–281. [Google Scholar] [CrossRef]
- Chapin, K.C.; Dragnich, A.G.; Gannon, W.D.; Martel, A.K.; Bacchetta, M.; Erasmus, D.B.; Shaver, C.M.; Trindade, A.J. Risk factors and clinical consequences of early extubation failure in lung transplant recipients. JHLT Open 2024, 4, 100046. [Google Scholar] [CrossRef]
- Habib, A.; Gouchoe, D.A.; Rosenheck, J.P.; Mokadam, N.A.; Henn, M.C.; Nunley, D.R.; Ramsammy, V.; Whitson, B.A.; Ganapathi, A.M. Early Extubation: Who Qualifies Postoperatively in Lung Transplantation? J. Surg. Res. 2024, 299, 303–312. [Google Scholar] [CrossRef]
- Kao, C.C.; Parulekar, A.D. Postoperative management of lung transplant recipients. J. Thorac. Dis. 2019, 11, S1782–S1788. [Google Scholar] [CrossRef]
- Duan, Q.; Zhang, Y.; Yang, D. Perioperative fluid management for lung transplantation is challenging. Heliyon 2023, 9, e14704. [Google Scholar] [CrossRef]
- Currey, J.; Pilcher, D.V.; Davies, A.; Scheinkestel, C.; Botti, M.; Bailey, M.; Snell, G. Implementation of a management guideline aimed at minimizing the severity of primary graft dysfunction after lung transplant. J. Thorac. Cardiovasc. Surg. 2010, 139, 154–161. [Google Scholar] [CrossRef]
- Laskey, D.; Housman, B.; Dawodu, G.; Scheinin, S. Intraoperative Extracorporeal Support during Lung Transplantation: Not Just for the High-Risk Patient. J. Clin. Med. 2024, 13, 192. [Google Scholar] [CrossRef]
- Coster, J.N.; Loor, G. Extracorporeal life support during lung transplantation. Indian J. Thorac. Cardiovasc. Surg. 2021, 37, 476–483. [Google Scholar] [CrossRef]
- Bermudez, C.A.; Shiose, A.; Esper, S.A.; Shigemura, N.; D’Cunha, J.; Bhama, J.K.; Richards, T.J.; Arlia, P.; Crespo, M.M.; Pilewski, J.M. Outcomes of Intraoperative Venoarterial Extracorporeal Membrane Oxygenation Versus Cardiopulmonary Bypass During Lung Transplantation. Ann. Thorac. Surg. 2014, 98, 1936–1943. [Google Scholar] [CrossRef]
- Gray, A.L.; Mulvihill, M.S.; Hartwig, M.G. Lung transplantation at Duke. J. Thorac. Dis. 2016, 8, E185–E196. [Google Scholar] [CrossRef] [PubMed]
- Luu, H.Y.; Santos, J.; Isaza, E.; Brzezinski, M.; Kukreja, J. Management of primary graft dysfunction after lung transplantation with extracorporeal life support: An evidence-based review. J. Thorac. Dis. 2023, 15, 4090–4100. [Google Scholar] [CrossRef] [PubMed]
- Paulo, N.; Prunet, H.; Armoiry, X.; Hugon-Vallet, E.; Mocan, R.; Portran, P.; Sebbag, L.; Pozzi, M.; Baudry, G. Outcome of primary graft dysfunction rescued by venoarterial extracorporeal membrane oxygenation after heart transplantation. Arch. Cardiovasc. Dis. 2022, 115, 426–435. [Google Scholar] [CrossRef] [PubMed]
- Boffini, M.; Simonato, E.; Ricci, D.; Scalini, F.; Marro, M.; Pidello, S.; Attisani, M.; Solidoro, P.; Lausi, P.O.; Fanelli, V.; et al. Extracorporeal membrane oxygenation after lung transplantation: Risk factors and outcomes analysis. Ann. Cardiothorac. Surg. 2019, 8, 54–61. [Google Scholar] [CrossRef]
- Gulack, B.C.; Hirji, S.A.; Hartwig, M.G. Bridge to lung transplantation and rescue post-transplant: The expanding role of extracorporeal membrane oxygenation. J. Thorac. Dis. 2014, 6, 1070–1079. [Google Scholar]
- Hatami, S.; Conway, J.; Freed, D.H.; Urschel, S. Thoracic organ donation after circulatory determination of death. Transplant. Rep. 2023, 8, 100125. [Google Scholar] [CrossRef]
- Keshavamurthy, S.; Rodgers-Fischl, P. Donation after circulatory death (DCD)-lung procurement. Indian J. Thorac. Cardiovasc. Surg. 2021, 37, 425–432. [Google Scholar] [CrossRef]
- Carteaux, G.; Parfait, M.; Combet, M.; Haudebourg, A.F.; Tuffet, S.; Mekontso Dessap, A. Patient-Self Inflicted Lung Injury: A Practical Review. J. Clin. Med. 2021, 10, 2738. [Google Scholar] [CrossRef] [PubMed]
- Chi, Y.; Zhao, Z.; Frerichs, I.; Long, Y.; He, H. Prevalence and prognosis of respiratory pendelluft phenomenon in mechanically ventilated ICU patients with acute respiratory failure: A retrospective cohort study. Ann. Intensive Care 2022, 12, 22. [Google Scholar] [CrossRef] [PubMed]
- Sklienka, P.; Frelich, M.; Burša, F. Patient Self-Inflicted Lung Injury-A Narrative Review of Pathophysiology, Early Recognition, and Management Options. J. Pers. Med. 2023, 13, 593. [Google Scholar] [CrossRef] [PubMed]
- Stengl, M.; Ledvinova, L.; Chvojka, J.; Benes, J.; Jarkovska, D.; Holas, J.; Soukup, P.; Sviglerová, J.; Matejovic, M. Effects of clinically relevant acute hypercapnic and metabolic acidosis on the cardiovascular system: An experimental porcine study. Crit. Care 2013, 17, R303. [Google Scholar] [CrossRef]
- Sylvester, J.T.; Shimoda, L.A.; Aaronson, P.I.; Ward, J.P. Hypoxic pulmonary vasoconstriction. Physiol. Rev. 2012, 92, 367–520. [Google Scholar] [CrossRef]
- Prag, H.A.; Murphy, M.P.; Krieg, T. Preventing mitochondrial reverse electron transport as a strategy for cardioprotection. Basic Res. Cardiol. 2023, 118, 34. [Google Scholar] [CrossRef]
- Gwoździńska, P.; Buchbinder, B.A.; Mayer, K.; Herold, S.; Morty, R.E.; Seeger, W.; Vadász, I. Hypercapnia Impairs ENaC Cell Surface Stability by Promoting Phosphorylation, Polyubiquitination and Endocytosis of β-ENaC in a Human Alveolar Epithelial Cell Line. Front. Immunol. 2017, 8, 591. [Google Scholar] [CrossRef]
- Huppert, L.A.; Matthay, M.A. Alveolar Fluid Clearance in Pathologically Relevant Conditions: In Vitro and In Vivo Models of Acute Respiratory Distress Syndrome. Front. Immunol. 2017, 8, e00371. [Google Scholar] [CrossRef]
- Vadász, I.; Sznajder, J.I. Gas Exchange Disturbances Regulate Alveolar Fluid Clearance during Acute Lung Injury. Front. Immunol. 2017, 8, 757. [Google Scholar] [CrossRef]
- Alzahrani, A.; Noda, K.; Chan, E.G.; Ryan, J.P.; Furukawa, M.; Sanchez, P.G. The length of the warm ischemic interval in lung donation after circulatory death does not impact post-transplantation outcomes. JHLT Open 2025, 8, 100244. [Google Scholar] [CrossRef]
- Berg, A.R.; Choi, A.Y.; Zhou, A.; MacArthur, J.W. From proxy to precision: The growing need to capture true warm ischemic time in donation-after-circulatory-death heart transplantation. J. Heart Lung Transplant. 2025, in press. [Google Scholar] [CrossRef] [PubMed]
- Van Raemdonck, D.; Ceulemans, L.J.; Neyrinck, A.; Levvey, B.; Snell, G.I. Donation After Circulatory Death in lung transplantation. Thorac. Surg. Clin. 2022, 32, 153–165. [Google Scholar] [CrossRef] [PubMed]
- Lazzeri, C.; Bonizzoli, M.; Di Valvasone, S.; Peris, A. Uncontrolled Donation after Circulatory Death Only Lung Program: An Urgent Opportunity. J. Clin. Med. 2023, 12, 6492. [Google Scholar] [CrossRef] [PubMed]
- Bello, I.; Palleschi, A.; Cypel, M.; Argudo, E.; Sandiumenge, A. Unlocking the Potential of Uncontrolled DCD in Lung Transplantation: A Review of Two Decades of Experience. JHLT Open 2025, 10, 100374. [Google Scholar] [CrossRef]
- Wong, A.; Liu, M. Inflammatory responses in lungs from donation after brain death: Mechanisms and potential therapeutic targets. J. Heart Lung Transplant. 2021, 40, 890–896. [Google Scholar] [CrossRef]
- Baciu, C.; Sage, A.; Zamel, R.; Shin, J.; Bai, X.-H.; Hough, O.; Bhat, M.; Yeung, J.C.; Cypel, M.; Keshavjee, S.; et al. Transcriptomic investigation reveals donor-specific gene signatures in human lung transplants. Eur. Respir. J. 2021, 57, 2000327. [Google Scholar] [CrossRef]
- Sommer, S.P.; Sommer, S.; Sinha, B.; Wiedemann, J.; Otto, C.; Aleksic, I.; Schimmer, C.; Leyh, R.G. Ischemia-reperfusion injury-induced pulmonary mitochondrial damage. J. Heart Lung Transplant. 2011, 30, 811–818. [Google Scholar] [CrossRef]
- Baniene, R.; Trumbeckas, D.; Kincius, M.; Pauziene, N.; Raudone, L.; Jievaltas, M.; Trumbeckaite, S. Short ischemia induces rat kidney mitochondria dysfunction. J. Bioenerg. Biomembr. 2016, 48, 77–85. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, S.N.; Li, L.; Zhang, X.B.; Wu, R.C.; Liu, J.H.; Huang, Z.Y.; Li, W.; Ran, J.H. Prolonged warm ischemia aggravates hepatic mitochondria damage and apoptosis in DCD liver by regulating Ca(2+)/CaM/CaMKII signaling pathway. Int. J. Clin. Exp. Pathol. 2019, 12, 217–228. [Google Scholar]
- Parrilla, G.A.; Hunt, W.R.; Daneshmand, M.A. Lung transplantation following donation after circulatory death. Transplant. Rep. 2022, 7, 100110. [Google Scholar] [CrossRef]
- Malas, J.; Chen, Q.; Thomas, J.; Emerson, D.; Megna, D.; Esmailian, F.; Bowdish, M.E.; Chikwe, J.; Catarino, P. The impact of thoracoabdominal normothermic regional perfusion on early outcomes in donation after circulatory death lung transplantation. J. Heart Lung Transplant. 2023, 42, 1040–1044. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.H.; Geraci, T.C.; Piper, G.L.; Chan, J.; James, L.; Paone, D.; Sommer, P.M.; Natalini, J.; Rudym, D.; Lesko, M.; et al. Outcomes of lung and heart-lung transplants utilizing donor after circulatory death with thoracoabdominal normothermic regional perfusion. JHLT Open 2024, 4, 100058. [Google Scholar] [CrossRef] [PubMed]
- Alderete, I.S.; Pontula, A.; Halpern, S.E.; Patel, K.J.; Klapper, J.A.; Hartwig, M.G. Thoracoabdominal Normothermic Regional Perfusion and Donation After Circulatory Death Lung Use. JAMA Netw. Open 2025, 8, e2460033. [Google Scholar] [CrossRef]
- Goff, R.R.; Daly, R.C.; Lease, E.D. Ex Vivo Lung Perfusion on Donor Lungs in the United States: National Trends and Post-Transplant Outcomes. J. Heart Lung Transplant. 2020, 39, S217. [Google Scholar] [CrossRef]
- Divithotawela, C.; Cypel, M.; Martinu, T.; Singer, L.G.; Binnie, M.; Chow, C.-W.; Chaparro, C.; Waddell, T.K.; de Perrot, M.; Pierre, A.; et al. Long-term Outcomes of Lung Transplant With Ex Vivo Lung Perfusion. JAMA Surg. 2019, 154, 1143–1150. [Google Scholar] [CrossRef]
- Abul Kashem, M.; Loor, G.; Hartwig, M.; Van Raemdonck, D.; Villavicencio, M.; Ius, F.; Ghadimi, K.; Salman, J.; Chandrashekaran, S.; Machuca, T.; et al. A multicenter analysis of lung transplantation outcomes comparing donation after circulatory death and donation after brain death. JHLT Open 2024, 6, 100132. [Google Scholar] [CrossRef]
- Gandhi, N.N.; DeSantis, J.; Miskoff, J.A. Breathing New Life Into Donation After Circulatory Death (DCD) Lung Transplantation: The Role of Machine Perfusion. Cureus 2025, 17, e87196. [Google Scholar] [CrossRef]
- Al Salihi, M.; Alsalihi, Y.; Cahill, J. Boosting lung transplantation, a narrative review of the impact of donor after cardiac death, organ care system and ex vivo lung perfusion in expanding donor pool. Curr. Chall. Thorac. Surg. 2022, 5, 19. [Google Scholar] [CrossRef]
- Curley, G.F.; Laffey, J.G.; Zhang, H.; Slutsky, A.S. Biotrauma and Ventilator-Induced Lung Injury: Clinical Implications. Chest 2016, 150, 1109–1117. [Google Scholar] [CrossRef]
- Lohser, J.; Slinger, P. Lung Injury After One-Lung Ventilation: A Review of the Pathophysiologic Mechanisms Affecting the Ventilated and the Collapsed Lung. Anesth. Analg. 2015, 121, 302–318. [Google Scholar] [CrossRef]
- Sha, Y.; Xu, R.; Shao, S.; Yang, J.; Tang, B.; Liang, Q.; Wang, Z. Tailored single-lung ventilation approaches and postoperative pulmonary outcomes in thoracic surgery. J. Thorac. Dis. 2025, 17, 5371–5387. [Google Scholar] [CrossRef] [PubMed]
- De Perrot, M.; McRae, K. Left ventricular lusitropy and primary graft dysfunction in lung transplantation. J. Heart Lung Transplant. 2019, 38, 719–720. [Google Scholar] [CrossRef] [PubMed]
- Jin, Z.; Hana, Z.; Alam, A.; Rajalingam, S.; Abayalingam, M.; Wang, Z.; Ma, D. Review 1: Lung transplant-from donor selection to graft preparation. J. Anesth. 2020, 34, 561–574. [Google Scholar] [CrossRef] [PubMed]
- Brower, R.G.; Matthay, M.A.; Morris, A.; Schoenfeld, D.; Thompson, B.T.; Wheeler, A. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N. Engl. J. Med. 2000, 342, 1301–1308. [Google Scholar] [CrossRef]
- Briel, M.; Meade, M.; Mercat, A.; Brower, R.G.; Talmor, D.; Walter, S.D.; Slutsky, A.S.; Pullenayegum, E.; Zhou, Q.; Cook, D.; et al. Higher vs lower positive end-expiratory pressure in patients with acute lung injury and acute respiratory distress syndrome: Systematic review and meta-analysis. JAMA 2010, 303, 865–873. [Google Scholar] [CrossRef]
- Ranieri, V.M.; Suter, P.M.; Tortorella, C.; De Tullio, R.; Dayer, J.M.; Brienza, A.; Bruno, F.; Slutsky, A.S. Effect of mechanical ventilation on inflammatory mediators in patients with acute respiratory distress syndrome: A randomized controlled trial. JAMA 1999, 282, 54–61. [Google Scholar] [CrossRef]
- O’Croinin, D.; Ni Chonghaile, M.; Higgins, B.; Laffey, J.G. Bench-to-bedside review: Permissive hypercapnia. Crit. Care 2004, 9, 51. [Google Scholar] [CrossRef]
- Peltekova, V.; Engelberts, D.; Otulakowski, G.; Uematsu, S.; Post, M.; Kavanagh, B.P. Hypercapnic acidosis in ventilator-induced lung injury. Intensive Care Med. 2010, 36, 869–878. [Google Scholar] [CrossRef]
- Wu, S.Y.; Li, M.H.; Ko, F.C.; Wu, G.C.; Huang, K.L.; Chu, S.J. Protective effect of hypercapnic acidosis in ischemia-reperfusion lung injury is attributable to upregulation of heme oxygenase-1. PLoS ONE 2013, 8, e74742. [Google Scholar] [CrossRef]
- Wu, S.Y.; Wu, C.P.; Kang, B.H.; Li, M.H.; Chu, S.J.; Huang, K.L. Hypercapnic acidosis attenuates reperfusion injury in isolated and perfused rat lungs. Crit. Care Med 2012, 40, 553–559. [Google Scholar] [CrossRef] [PubMed]
- Steen, S.; Liao, Q.; Wierup, P.N.; Bolys, R.; Pierre, L.; Sjöberg, T. Transplantation of lungs from non-heart-beating donors after functional assessment ex vivo. Ann. Thorac. Surg. 2003, 76, 244–252; discussion 252. [Google Scholar] [CrossRef] [PubMed]
- Steen, S.; Sjöberg, T.; Pierre, L.; Liao, Q.; Eriksson, L.; Algotsson, L. Transplantation of lungs from a non-heart-beating donor. Lancet 2001, 357, 825–829. [Google Scholar] [CrossRef] [PubMed]
- Cypel, M.; Yeung, J.C.; Liu, M.; Anraku, M.; Chen, F.; Karolak, W.; Sato, M.; Laratta, J.; Azad, S.; Madonik, M.; et al. Normothermic Ex Vivo Lung Perfusion in Clinical Lung Transplantation. N. Engl. J. Med. 2011, 364, 1431–1440. [Google Scholar] [CrossRef]
- Prasad, N.K.; Pasrija, C.; Talaie, T.; Krupnick, A.S.; Zhao, Y.; Lau, C.L. Ex Vivo Lung Perfusion: Current Achievements and Future Directions. Transplantation 2021, 105, 979–985. [Google Scholar] [CrossRef]
- Kanou, T.; Nakahira, K.; Choi, A.M.; Yeung, J.C.; Cypel, M.; Liu, M.; Keshavjee, S. Cell-free DNA in human ex vivo lung perfusate as a potential biomarker to predict the risk of primary graft dysfunction in lung transplantation. J. Thorac. Cardiovasc. Surg. 2021, 162, 490–499.e492. [Google Scholar] [CrossRef]
- Boffini, M.; Marro, M.; Simonato, E.; Scalini, F.; Costamagna, A.; Fanelli, V.; Barbero, C.; Solidoro, P.; Brazzi, L.; Rinaldi, M. Cytokines Removal During Ex-Vivo Lung Perfusion: Initial Clinical Experience. Transpl. Int. 2023, 36, 10777. [Google Scholar] [CrossRef]
- Ponholzer, F.; Dumfarth, J.; Krapf, C.; Pircher, A.; Hautz, T.; Wolf, D.; Augustin, F.; Schneeberger, S. The impact and relevance of techniques and fluids on lung injury in machine perfusion of lungs. Front. Immunol. 2024, 15, e1358153. [Google Scholar] [CrossRef]
- Hasenauer, A.; Bédat, B.; Parapanov, R.; Lugrin, J.; Debonneville, A.; Abdelnour-Berchtold, E.; Gonzalez, M.; Perentes, J.Y.; Piquilloud, L.; Szabo, C.; et al. Effects of cold or warm ischemia and ex-vivo lung perfusion on the release of damage associated molecular patterns and inflammatory cytokines in experimental lung transplantation. J. Heart Lung Transplant. 2021, 40, 905–916. [Google Scholar] [CrossRef]
- Gouchoe, D.A.; Cui, E.Y.; Satija, D.; Henn, M.C.; Choi, K.; Rosenheck, J.P.; Nunley, D.R.; Mokadam, N.A.; Ganapathi, A.M.; Whitson, B.A. Ex Vivo Lung Perfusion and Primary Graft Dysfunction Following Lung Transplantation: A Contemporary United Network for Organ Sharing Database Analysis. J. Clin. Med. 2024, 13, 4440. [Google Scholar] [CrossRef]
- Benazzo, A.; Peel, J.; Mariscal, A.; Yeung, J.; Aversa, M.; Keshavjee, S.; Cypel, M. Differential outcomes of ISHLT PGD 3 after ex-vivo lung perfusion compared to PGD 3 after direct transplantation. J. Heart Lung Transplant. 2025, in press. [Google Scholar] [CrossRef] [PubMed]
- Lightle, W.; Daoud, D.; Loor, G. Breathing lung transplantation with the Organ Care System (OCS) Lung: Lessons learned and future implications. J. Thorac. Dis. 2019, 11, S1755–S1760. [Google Scholar] [CrossRef] [PubMed]
- Warnecke, G.; Van Raemdonck, D.; Smith, M.A.; Massard, G.; Kukreja, J.; Rea, F.; Loor, G.; De Robertis, F.; Nagendran, J.; Dhital, K.K.; et al. Normothermic ex-vivo preservation with the portable Organ Care System Lung device for bilateral lung transplantation (INSPIRE): A randomised, open-label, non-inferiority, phase 3 study. Lancet Respir. Med. 2018, 6, 357–367. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Kahan, R.; Gonzalez, T.J.; Zhang, M.; Alderete, I.S.; DeLaura, I.; Kesseli, S.J.; Song, M.; Asokan, A.; Barbas, A.S.; et al. Gene delivery followed by ex vivo lung perfusion using an adeno-associated viral vector in a rodent lung transplant model. J. Thorac. Cardiovasc. Surg. 2024, 167, e131–e139. [Google Scholar] [CrossRef]
- Nykänen, A.I.; Mariscal, A.; Duong, A.; Estrada, C.; Ali, A.; Hough, O.; Sage, A.; Chao, B.T.; Chen, M.; Gokhale, H.; et al. Engineered mesenchymal stromal cell therapy during human lung ex vivo lung perfusion is compromised by acidic lung microenvironment. Mol. Ther. Methods Clin. Dev. 2021, 23, 184–197. [Google Scholar] [CrossRef]
- Machuca, T.N.; Cypel, M.; Bonato, R.; Yeung, J.C.; Chun, Y.M.; Juvet, S.; Guan, Z.; Hwang, D.M.; Chen, M.; Saito, T.; et al. Safety and Efficacy of Ex Vivo Donor Lung Adenoviral IL-10 Gene Therapy in a Large Animal Lung Transplant Survival Model. Hum. Gene Ther. 2017, 28, 757–765. [Google Scholar] [CrossRef]
- Oishi, H.; Juvet, S.C.; Martinu, T.; Sato, M.; Medin, J.A.; Liu, M.; Keshavjee, S. A novel combined ex vivo and in vivo lentiviral interleukin-10 gene delivery strategy at the time of transplantation decreases chronic lung allograft rejection in mice. J. Thorac. Cardiovasc. Surg. 2018, 156, 1305–1315. [Google Scholar] [CrossRef]
- Nakata, K.; Alderete, I.S.; Hughes, B.A.; Hartwig, M.G. Ex vivo lung perfusion: Recent advancements and future directions. Front. Immunol. 2025, 16, e1513546. [Google Scholar] [CrossRef]
- Coggins, M.P.; Bloch, K.D. Nitric Oxide in the Pulmonary Vasculature. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 1877–1885. [Google Scholar] [CrossRef]
- Klinger, J.R.; Kadowitz, P.J. The Nitric Oxide Pathway in Pulmonary Vascular Disease. Am. J. Cardiol. 2017, 120, S71–S79. [Google Scholar] [CrossRef]
- Radi, R. Oxygen radicals, nitric oxide, and peroxynitrite: Redox pathways in molecular medicine. Proc. Natl. Acad. Sci. USA 2018, 115, 5839–5848. [Google Scholar] [CrossRef]
- Kumar, A.; Noda, K.; Philips, B.; Velayutham, M.; Stolz, D.B.; Gladwin, M.T.; Shiva, S.; D’Cunha, J. Nitrite attenuates mitochondrial impairment and vascular permeability induced by ischemia-reperfusion injury in the lung. Am. J. Physiol. Lung Cell. Mol. Physiol. 2020, 318, L580–L591. [Google Scholar] [CrossRef]
- Meade, M.O.; Granton, J.T.; Matte-Martyn, A.; McRae, K.; Weaver, B.; Cripps, P.; Keshavjee, S.H. A randomized trial of inhaled nitric oxide to prevent ischemia-reperfusion injury after lung transplantation. Am. J. Respir. Crit. Care Med. 2003, 167, 1483–1489. [Google Scholar] [CrossRef] [PubMed]
- Cerier, E.; Lung, K.; Bharat, A. Role of Pulmonary Vasodilators in Ameliorating Primary Graft Dysfunction Following Lung Transplant. JAMA Surg. 2022, 157, e215857. [Google Scholar] [CrossRef] [PubMed]
- Chin, B.Y.; Jiang, G.; Wegiel, B.; Wang, H.J.; Macdonald, T.; Zhang, X.C.; Gallo, D.; Cszimadia, E.; Bach, F.H.; Lee, P.J.; et al. Hypoxia-inducible factor 1alpha stabilization by carbon monoxide results in cytoprotective preconditioning. Proc. Natl. Acad. Sci. USA 2007, 104, 5109–5114. [Google Scholar] [CrossRef] [PubMed]
- Kohmoto, J.; Nakao, A.; Kaizu, T.; Tsung, A.; Ikeda, A.; Tomiyama, K.; Billiar, T.R.; Choi, A.M.; Murase, N.; McCurry, K.R. Low-dose carbon monoxide inhalation prevents ischemia/reperfusion injury of transplanted rat lung grafts. Surgery 2006, 140, 179–185. [Google Scholar] [CrossRef]
- Ryter, S.W.; Choi, A.M. Targeting heme oxygenase-1 and carbon monoxide for therapeutic modulation of inflammation. Transl. Res. 2016, 167, 7–34. [Google Scholar] [CrossRef]
- Kang, I.S.; Kim, R.I.; Kim, C. Carbon Monoxide Regulates Macrophage Differentiation and Polarization toward the M2 Phenotype through Upregulation of Heme Oxygenase 1. Cells 2021, 10, 3444. [Google Scholar] [CrossRef]
- Chhikara, M.; Wang, S.; Kern, S.J.; Ferreyra, G.A.; Barb, J.J.; Munson, P.J.; Danner, R.L. Carbon monoxide blocks lipopolysaccharide-induced gene expression by interfering with proximal TLR4 to NF-kappaB signal transduction in human monocytes. PLoS ONE 2009, 4, e8139. [Google Scholar] [CrossRef]
- Rosas, I.O.; Goldberg, H.J.; Collard, H.R.; El-Chemaly, S.; Flaherty, K.; Hunninghake, G.M.; Lasky, J.A.; Lederer, D.J.; Machado, R.; Martinez, F.J.; et al. A Phase II Clinical Trial of Low-Dose Inhaled Carbon Monoxide in Idiopathic Pulmonary Fibrosis. Chest 2018, 153, 94–104. [Google Scholar] [CrossRef]
- Goebel, U.; Wollborn, J. Carbon monoxide in intensive care medicine—time to start the therapeutic application?! Intensive Care Med. Exp. 2020, 8, 2. [Google Scholar] [CrossRef]
- Li, H.; Zhou, R.; Liu, J.; Li, Q.; Zhang, J.; Mu, J.; Sun, X. Hydrogen-Rich Saline Attenuates Lung Ischemia-Reperfusion Injury in Rabbits. J. Surg. Res. 2012, 174, e11–e16. [Google Scholar] [CrossRef]
- Kawamura, T.; Wakabayashi, N.; Shigemura, N.; Huang, C.S.; Masutani, K.; Tanaka, Y.; Noda, K.; Peng, X.; Takahashi, T.; Billiar, T.R.; et al. Hydrogen gas reduces hyperoxic lung injury via the Nrf2 pathway in vivo. Am. J. Physiol. Lung Cell. Mol. Physiol. 2013, 304, L646–L656. [Google Scholar] [CrossRef]
- Yang, F.; Lei, Y.; Liu, R.; Luo, X.; Li, J.; Zeng, F.; Lu, S.; Huang, X.; Lan, Y. Hydrogen: Potential Applications in Solid Organ Transplantation. Oxid. Med. Cell. Longev. 2021, 2021, 6659310. [Google Scholar] [CrossRef]
- Obara, T.; Naito, H.; Nojima, T.; Hirayama, T.; Hongo, T.; Ageta, K.; Aokage, T.; Hisamura, M.; Yumoto, T.; Nakao, A. Hydrogen in Transplantation: Potential Applications and Therapeutic Implications. Biomedicines 2024, 12, 118. [Google Scholar] [CrossRef] [PubMed]
- Kayawake, H.; Chen-Yoshikawa, T.F.; Saito, M.; Yamagishi, H.; Yoshizawa, A.; Hirano, S.-i.; Kurokawa, R.; Date, H. Protective Effects of a Hydrogen-Rich Preservation Solution in a Canine Lung Transplantation Model. Ann. Thorac. Surg. 2021, 111, 246–252. [Google Scholar] [CrossRef] [PubMed]
- Oesterle, A.; Laufs, U.; Liao, J.K. Pleiotropic Effects of Statins on the Cardiovascular System. Circ. Res. 2017, 120, 229–243. [Google Scholar] [CrossRef] [PubMed]
- Raphael, J.; Collins, S.R.; Wang, X.-Q.; Scalzo, D.C.; Singla, P.; Lau, C.L.; Kozower, B.D.; Durieux, M.E.; Blank, R.S. Perioperative statin use is associated with decreased incidence of primary graft dysfunction after lung transplantation. J. Heart Lung Transplant. 2017, 36, 948–956. [Google Scholar] [CrossRef]
- Bello, I.; Coll, E.; Sandiumenge, A.; Romero, L.; Jauregui, A.; Pérez, J.; Ochoa, J.; Peñafiel, S.; Sánchez, L.; Ascanio, F.; et al. Do the Pretransplant Use of Statins in a Recipient Reduce the Incidence of Primary Graft Dysfunction after Lung Transplant? J. Heart Lung Transplant. 2019, 38, S226. [Google Scholar] [CrossRef]
- Dashti-Khavidaki, S.; Saidi, R.; Lu, H. Current status of glucocorticoid usage in solid organ transplantation. World J. Transplant. 2021, 11, 443–465. [Google Scholar] [CrossRef]
- Araujo, L.F.; Holand, A.R.; Paludo Ade, O.; Silva É, F.; Forgiarini, L.A.; Forgiarini, L.F.; Barbachan, E.S.M.; Andrade, C.F. Effect of the systemic administration of methylprednisolone on the lungs of brain-dead donor rats undergoing pulmonary transplantation. Clinics 2014, 69, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, B.J.; Sharma, N.S. Immunosuppression in lung transplantation: A narrative review. Curr. Chall. Thorac. Surg. 2023, 5, 21. [Google Scholar] [CrossRef]
- Paulus, P.; Holfeld, J.; Urbschat, A.; Mutlak, H.; Ockelmann, P.A.; Tacke, S.; Zacharowski, K.; Reissig, C.; Stay, D.; Scheller, B. Prednisolone as Preservation Additive Prevents from Ischemia Reperfusion Injury in a Rat Model of Orthotopic Lung Transplantation. PLoS ONE 2013, 8, e73298. [Google Scholar] [CrossRef] [PubMed]
- Van Zanden, J.E.; Leuvenink, H.G.D.; Verschuuren, E.A.M.; Veldhuis, Z.J.; Ottens, P.J.; Erasmus, M.E.; Hottenrott, M.C. Ex Vivo Perfusion With Methylprednisolone Attenuates Brain Death-induced Lung Injury in Rats. Transplant. Direct 2021, 7, e682. [Google Scholar] [CrossRef]
- Birk, A.V.; Liu, S.; Soong, Y.; Mills, W.; Singh, P.; Warren, J.D.; Seshan, S.V.; Pardee, J.D.; Szeto, H.H. The mitochondrial-targeted compound SS-31 re-energizes ischemic mitochondria by interacting with cardiolipin. J. Am. Soc. Nephrol. 2013, 24, 1250–1261. [Google Scholar] [CrossRef]
- Mitchell, W.; Pharaoh, G.; Tyshkovskiy, A.; Campbell, M.; Marcinek, D.J.; Gladyshev, V.N. The Mitochondria-Targeted Peptide Therapeutic Elamipretide Improves Cardiac and Skeletal Muscle Function During Aging Without Detectable Changes in Tissue Epigenetic or Transcriptomic Age. Aging Cell 2025, 24, e70026. [Google Scholar] [CrossRef]
- Du, X.; Zeng, Q.; Luo, Y.; He, L.; Zhao, Y.; Li, N.; Han, C.; Zhang, G.; Liu, W. Application research of novel peptide mitochondrial-targeted antioxidant SS-31 in mitigating mitochondrial dysfunction. Mitochondrion 2024, 75, 101846. [Google Scholar] [CrossRef]
- Prag, H.A.; Aksentijevic, D.; Dannhorn, A.; Giles, A.V.; Mulvey, J.F.; Sauchanka, O.; Du, L.; Bates, G.; Reinhold, J.; Kula-Alwar, D.; et al. Ischemia-Selective Cardioprotection by Malonate for Ischemia/Reperfusion Injury. Circ. Res. 2022, 131, 528–541. [Google Scholar] [CrossRef]
- Jiang, T.; Liu, T.; Deng, X.; Ding, W.; Yue, Z.; Yang, W.; Lv, X.; Li, W. Adiponectin ameliorates lung ischemia-reperfusion injury through SIRT1-PINK1 signaling-mediated mitophagy in type 2 diabetic rats. Respir. Res. 2021, 22, 258. [Google Scholar] [CrossRef]
- Lee, S.E.; Kim, I.H.; Kang, Y.C.; Kim, Y.; Yu, S.H.; Yeo, J.S.; Kwon, I.; Lim, J.H.; Kim, J.H.; Han, K.; et al. Mitochondrial transplantation attenuates lipopolysaccharide-induced acute respiratory distress syndrome. BMC Pulm. Med. 2024, 24, 477. [Google Scholar] [CrossRef]
- Miao, X.; Jiang, P.; Wang, Z.; Kong, W.; Feng, L. Mitochondrial Transplantation: A Novel Therapeutic Approach for Treating Diseases. MedComm (2020) 2025, 6, e70253. [Google Scholar] [CrossRef] [PubMed]
- Tian, D.; Zheng, X.; Tang, H.; Huang, H.; Wang, J.; Xu, L.; Li, C.; Yan, H.; Yu, R.; Nan, J.; et al. Metformin attenuates chronic lung allograft dysfunction: Evidence in rat models. Respir. Res. 2023, 24, 192. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Wei, H.; Zhang, L.; Ma, C.; Wei, Y.; Jiang, T.; Li, W. Metformin attenuates lung ischemia-reperfusion injury and necroptosis through AMPK pathway in type 2 diabetic recipient rats. BMC Pulm. Med. 2024, 24, 237. [Google Scholar] [CrossRef] [PubMed]
- Nemeth, D.V.; Iannelli, L.; Gangitano, E.; D’Andrea, V.; Bellini, M.I. Energy Metabolism and Metformin: Effects on Ischemia-Reperfusion Injury in Kidney Transplantation. Biomedicines 2024, 12, 1534. [Google Scholar] [CrossRef]
- Wei, H.; Liu, T.-H.; Zhang, L.-J.; Yan, W.; Ma, C.; Lv, S.-H.; Zeng, X.-Z.; Li, W.-Z. Metformin alleviates lung ischemia-reperfusion injury via the SIRT1 pathway following lung transplantation in diabetic rats. Mol. Med. Rep. 2025, 32, 287. [Google Scholar] [CrossRef]
- Gazoni, L.M.; Walters, D.M.; Unger, E.B.; Linden, J.; Kron, I.L.; Laubach, V.E. Activation of A1, A2A, or A3 adenosine receptors attenuates lung ischemia-reperfusion injury. J. Thorac. Cardiovasc. Surg. 2010, 140, 440–446. [Google Scholar] [CrossRef]
- Gazoni, L.M.; Laubach, V.E.; Mulloy, D.P.; Bellizzi, A.; Unger, E.B.; Linden, J.; Ellman, P.I.; Lisle, T.C.; Kron, I.L. Additive protection against lung ischemia-reperfusion injury by adenosine A2A receptor activation before procurement and during reperfusion. J. Thorac. Cardiovasc. Surg. 2008, 135, 156–165. [Google Scholar] [CrossRef]
- Stone, M.L.; Sharma, A.K.; Mas, V.R.; Gehrau, R.C.; Mulloy, D.P.; Zhao, Y.; Lau, C.L.; Kron, I.L.; Huerter, M.E.; Laubach, V.E. Ex Vivo Perfusion With Adenosine A2A Receptor Agonist Enhances Rehabilitation of Murine Donor Lungs After Circulatory Death. Transplantation 2015, 99, 2494–2503. [Google Scholar] [CrossRef]
- Mulloy, D.P.; Stone, M.L.; Crosby, I.K.; Lapar, D.J.; Sharma, A.K.; Webb, D.V.; Lau, C.L.; Laubach, V.E.; Kron, I.L. Ex vivo rehabilitation of non-heart-beating donor lungs in preclinical porcine model: Delayed perfusion results in superior lung function. J. Thorac. Cardiovasc. Surg. 2012, 144, 1208–1215. [Google Scholar] [CrossRef]
- Wagner, C.E.; Pope, N.H.; Charles, E.J.; Huerter, M.E.; Sharma, A.K.; Salmon, M.D.; Carter, B.T.; Stoler, M.H.; Lau, C.L.; Laubach, V.E.; et al. Ex vivo lung perfusion with adenosine A2A receptor agonist allows prolonged cold preservation of lungs donated after cardiac death. J. Thorac. Cardiovasc. Surg. 2016, 151, 538–546. [Google Scholar] [CrossRef]
- Fleischmann, E.; Conaway, M.; Rabin, J.; Vecere, K.; Masih, K.; Zhao, Y.; Zhang, A.; Khan, M.; Mas, V.; Krupnick, A.; et al. Regadenoson in the rehabilitation of marginal donor lungs on ex-vivo lung perfusion: A blinded multi-center randomized controlled clinical trial. JTCVS Open 2025. [Google Scholar] [CrossRef]
- Iyer, S.S.; Cheng, G. Role of interleukin 10 transcriptional regulation in inflammation and autoimmune disease. Crit. Rev. Immunol. 2012, 32, 23–63. [Google Scholar] [CrossRef] [PubMed]
- Yeung, J.C.; Wagnetz, D.; Cypel, M.; Rubacha, M.; Koike, T.; Chun, Y.-M.; Hu, J.; Waddell, T.K.; Hwang, D.M.; Liu, M.; et al. Ex Vivo Adenoviral Vector Gene Delivery Results in Decreased Vector-associated Inflammation Pre- and Post–lung Transplantation in the Pig. Mol. Ther. 2012, 20, 1204–1211. [Google Scholar] [CrossRef] [PubMed]
- Nykänen, A.I.; Keshavjee, S.; Liu, M. Creating superior lungs for transplantation with next-generation gene therapy during ex vivo lung perfusion. J. Heart Lung Transplant. 2024, 43, 838–848. [Google Scholar] [CrossRef]
- Mulvihill, E.E.; Drucker, D.J. Pharmacology, physiology, and mechanisms of action of dipeptidyl peptidase-4 inhibitors. Endocr. Rev. 2014, 35, 992–1019. [Google Scholar] [CrossRef]
- Zhang, T.; Tong, X.; Zhang, S.; Wang, D.; Wang, L.; Wang, Q.; Fan, H. The Roles of Dipeptidyl Peptidase 4 (DPP4) and DPP4 Inhibitors in Different Lung Diseases: New Evidence. Front. Pharmacol. 2021, 12, e731453. [Google Scholar] [CrossRef]
- Zhai, W.; Cardell, M.; De Meester, I.; Augustyns, K.; Hillinger, S.; Inci, I.; Arni, S.; Jungraithmayr, W.; Scharpé, S.; Weder, W.; et al. Ischemia/reperfusion injury: The role of CD26/dipeptidyl-peptidase-IV-inhibition in lung transplantation. Transplant. Proc. 2006, 38, 3369–3371. [Google Scholar] [CrossRef]
- Jungraithmayr, W.; De Meester, I.; Matheeussen, V.; Inci, I.; Augustyns, K.; Scharpé, S.; Weder, W.; Korom, S. Inhibition of CD26/DPP IV attenuates ischemia/reperfusion injury in orthotopic mouse lung transplants: The pivotal role of vasoactive intestinal peptide. Peptides 2010, 31, 585–591. [Google Scholar] [CrossRef]
- Zhai, W.; Cardell, M.; De Meester, I.; Augustyns, K.; Hillinger, S.; Inci, I.; Arni, S.; Jungraithmayr, W.; Scharpé, S.; Weder, W.; et al. Intragraft DPP IV inhibition attenuates post-transplant pulmonary ischemia/reperfusion injury after extended ischemia. J Heart Lung Transplant. 2007, 26, 174–180. [Google Scholar] [CrossRef]
- Delgado, M.; Ganea, D. Vasoactive intestinal peptide: A neuropeptide with pleiotropic immune functions. Amino Acids 2013, 45, 25–39. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, C.-Y.; Duan, J.-X.; Li, Q.; Yang, H.-H.; Sun, C.-C.; Zhang, J.; Luo, X.-Q.; Liu, S.-K. Vasoactive intestinal peptide suppresses the NLRP3 inflammasome activation in lipopolysaccharide-induced acute lung injury mice and macrophages. Biomed. Pharmacother. 2020, 121, 109596. [Google Scholar] [CrossRef]
- Delgado, M.; Ganea, D. VIP and PACAP inhibit activation induced apoptosis in T lymphocytes. Ann. N. Y. Acad. Sci. 2000, 921, 55–67. [Google Scholar] [CrossRef] [PubMed]
- Fadeel, B.; Åhlin, A.; Henter, J.-I.; Orrenius, S.; Hampton, M.B. Involvement of Caspases in Neutrophil Apoptosis: Regulation by Reactive Oxygen Species. Blood 1998, 92, 4808–4818. [Google Scholar] [CrossRef] [PubMed]
- Lataillade, J.-J.; Clay, D.; Bourin, P.; Hérodin, F.i.; Dupuy, C.; Jasmin, C.; Le Bousse-Kerdilès, M.-C. Stromal cell–derived factor 1 regulates primitive hematopoiesis by suppressing apoptosis and by promoting G0/G1 transition in CD34+ cells: Evidence for an autocrine/paracrine mechanism. Blood 2002, 99, 1117–1129. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, T.; Guo, H.; Shi, J.; Yang, J.; Fu, S.; Pan, X.; Li, F.; Zhang, H.; Zhang, D.; et al. Ex vivo lung perfusion with GLP-1R agonist mitigates ischemia/reperfusion injury through pyroptosis modulation in lung transplantation- an experimental study. Int. J. Surg. 2025, 111, 3781–3797. [Google Scholar] [CrossRef]
- Mehdi, S.F.; Pusapati, S.; Anwar, M.S.; Lohana, D.; Kumar, P.; Nandula, S.A.; Nawaz, F.K.; Tracey, K.; Yang, H.; LeRoith, D.; et al. Glucagon-like peptide-1: A multi-faceted anti-inflammatory agent. Front. Immunol. 2023, 14, e1148209. [Google Scholar] [CrossRef]
- Deacon, C.F. Physiology and Pharmacology of DPP-4 in Glucose Homeostasis and the Treatment of Type 2 Diabetes. Front. Endocrinol. 2019, 10, e00080. [Google Scholar] [CrossRef]
- Fandiño, J.; Toba, L.; González-Matías, L.C.; Diz-Chaves, Y.; Mallo, F. GLP-1 receptor agonist ameliorates experimental lung fibrosis. Sci. Rep. 2020, 10, 18091. [Google Scholar] [CrossRef]
- Shiraki, A.; Oyama, J.; Komoda, H.; Asaka, M.; Komatsu, A.; Sakuma, M.; Kodama, K.; Sakamoto, Y.; Kotooka, N.; Hirase, T.; et al. The glucagon-like peptide 1 analog liraglutide reduces TNF-α-induced oxidative stress and inflammation in endothelial cells. Atherosclerosis 2012, 221, 375–382. [Google Scholar] [CrossRef]
- Steven, S.; Jurk, K.; Kopp, M.; Kröller-Schön, S.; Mikhed, Y.; Schwierczek, K.; Roohani, S.; Kashani, F.; Oelze, M.; Klein, T.; et al. Glucagon-like peptide-1 receptor signalling reduces microvascular thrombosis, nitro-oxidative stress and platelet activation in endotoxaemic mice. Br. J. Pharmacol. 2017, 174, 1620–1632. [Google Scholar] [CrossRef]
- Steven, S.; Hausding, M.; Kröller-Schön, S.; Mader, M.; Mikhed, Y.; Stamm, P.; Zinßius, E.; Pfeffer, A.; Welschof, P.; Agdauletova, S.; et al. Gliptin and GLP-1 analog treatment improves survival and vascular inflammation/dysfunction in animals with lipopolysaccharide-induced endotoxemia. Basic Res. Cardiol. 2015, 110, 6. [Google Scholar] [CrossRef] [PubMed]
- Sakanoue, I.; Nakajima, D. Mesenchymal stem cell therapy against ischemia-reperfusion injury in lung transplantation. Curr. Opin. Organ. Transplant. 2025, 30, 372–378. [Google Scholar] [CrossRef] [PubMed]
- Park, K.-S.; Lässer, C.; Lötvall, J. Extracellular vesicles and the lung: From disease pathogenesis to biomarkers and treatments. Physiol. Rev. 2025, 105, 1733–1821. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Ma, F.; Song, H.; He, N.; Zhang, H.; Li, J.; Liu, Q.; Xu, C. Mesenchymal Stem Cell-Derived Extracellular Vesicles for Regenerative Applications and Radiotherapy. Cell Transplant. 2025, 34, 09636897241311019. [Google Scholar] [CrossRef]
- Nykänen, A.I.; Liu, M.; Keshavjee, S. Mesenchymal Stromal Cell Therapy in Lung Transplantation. Bioengineering 2023, 10, 728. [Google Scholar] [CrossRef]
- Matthay, M.A.; Calfee, C.S.; Zhuo, H.; Thompson, B.T.; Wilson, J.G.; Levitt, J.E.; Rogers, A.J.; Gotts, J.E.; Wiener-Kronish, J.P.; Bajwa, E.K.; et al. Treatment with allogeneic mesenchymal stromal cells for moderate to severe acute respiratory distress syndrome (START study): A randomised phase 2a safety trial. Lancet Respir. Med. 2019, 7, 154–162. [Google Scholar] [CrossRef]
- Niroomand, A.; Hirdman, G.; Olm, F.; Lindstedt, S. Current Status and Future Perspectives on Machine Perfusion: A Treatment Platform to Restore and Regenerate Injured Lungs Using Cell and Cytokine Adsorption Therapy. Cells 2022, 11, 91. [Google Scholar] [CrossRef]
- Yang, X.; Hong, S.; Yan, T.; Liu, M.; Liu, M.; Zhao, J.; Yue, B.; Wu, D.; Shao, J.; Huang, M.; et al. MiR-146a engineered extracellular vesicles derived from mesenchymal stromal cells more potently attenuate ischaemia–reperfusion injury in lung transplantation. Clin. Transl. Med. 2025, 15, e70298. [Google Scholar] [CrossRef]
- Egan, T.M.; Haithcock, B.E.; Lobo, J.; Mody, G.; Love, R.B.; Requard III, J.J.; Espey, J.; Ali, M.H. Donation after circulatory death donors in lung transplantation. J. Thorac. Dis. 2021, 13, 6536–6549. [Google Scholar] [CrossRef]
- Motoyama, H.; Chen, F.; Ohsumi, A.; Hijiya, K.; Okita, K.; Nakajima, D.; Sakamoto, J.; Yamada, T.; Sato, M.; Aoyama, A.; et al. Protective effect of plasmin in marginal donor lungs in an ex vivo lung perfusion model. J. Heart Lung Transplant. 2013, 32, 505–510. [Google Scholar] [CrossRef]
- Liersch-Nordqvist, A.; Fakhro, M.; Pierre, L.; Hlebowicz, J.; Malmsjo, M.; Ingemansson, R.; Lindstedt, S. The impact of alteplase on pulmonary graft function in donation after circulatory death—An experimental study. Ann. Med. Surg. 2017, 22, 1–6. [Google Scholar] [CrossRef]
- Van Raemdonck, D.; Neyrinck, A.; Cypel, M.; Keshavjee, S. Ex-vivo lung perfusion. Transpl. Int. 2015, 28, 643–656. [Google Scholar] [CrossRef]
- Morgan, M.J.; Kim, Y.-S. Roles of RIPK3 in necroptosis, cell signaling, and disease. Exp. Mol. Med. 2022, 54, 1695–1704. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Liang, F.; Lou, Z.; Li, Y.; Li, J.; Chen, Y.; Ding, J.; Jiang, B.; Wu, C.; Yu, H.; et al. Necrostatin-1 Alleviates Lung Ischemia-Reperfusion Injury via Inhibiting Necroptosis and Apoptosis of Lung Epithelial Cells. Cells 2022, 11, 3139. [Google Scholar] [CrossRef] [PubMed]
- Ueda, S.; Chen-Yoshikawa, T.F.; Tanaka, S.; Yamada, Y.; Nakajima, D.; Ohsumi, A.; Date, H. Protective effect of necrosulfonamide on rat pulmonary ischemia-reperfusion injury via inhibition of necroptosis. J. Thorac. Cardiovasc. Surg. 2022, 163, e113–e122. [Google Scholar] [CrossRef] [PubMed]
- Teke, Z.; Kabay, B.; Ozden, A.; Yenisey, C.; Bir, F.; Demirkan, N.C.; Bicakci, T.; Erdem, E. Effects of tempol, a membrane-permeable radical scavenger, on local and remote organ injuries caused by intestinal ischemia/reperfusion in rats. J. Surg. Res. 2008, 149, 259–271. [Google Scholar] [CrossRef]
- Baltalarli, A.; Ozcan, V.; Bir, F.; Aybek, H.; Sacar, M.; Onem, G.; Goksin, I.; Demir, S.; Teke, Z. Ascorbic acid (vitamin C) and iloprost attenuate the lung injury caused by ischemia/reperfusion of the lower extremities of rats. Ann. Vasc. Surg. 2006, 20, 49–55. [Google Scholar] [CrossRef]
- Inci, I.; Zhai, W.; Arni, S.; Hillinger, S.; Vogt, P.; Weder, W. N-acetylcysteine attenuates lung ischemia-reperfusion injury after lung transplantation. Ann. Thorac. Surg. 2007, 84, 240–246; discussion 246. [Google Scholar] [CrossRef]
- Rega, F.R.; Wuyts, W.A.; Vanaudenaerde, B.M.; Jannis, N.C.; Neyrinck, A.P.; Verleden, G.M.; Lerut, T.E.; Van Raemdonck, D.E. Nebulized N-acetyl cysteine protects the pulmonary graft inside the non-heart-beating donor. J. Heart Lung Transplant. 2005, 24, 1369–1377. [Google Scholar] [CrossRef]
- Okamoto, T.; Tang, X.; Janocha, A.; Farver, C.F.; Gladwin, M.T.; McCurry, K.R. Nebulized nitrite protects rat lung grafts from ischemia reperfusion injury. J. Thorac. Cardiovasc. Surg. 2013, 145, 1108–1116.e1101. [Google Scholar] [CrossRef]
- Sugimoto, R.; Okamoto, T.; Nakao, A.; Zhan, J.; Wang, Y.; Kohmoto, J.; Tokita, D.; Farver, C.F.; Tarpey, M.M.; Billiar, T.R.; et al. Nitrite reduces acute lung injury and improves survival in a rat lung transplantation model. Am. J. Transplant. 2012, 12, 2938–2948. [Google Scholar] [CrossRef]
- Egemnazarov, B.; Sydykov, A.; Schermuly, R.T.; Weissmann, N.; Stasch, J.-P.; Sarybaev, A.S.; Seeger, W.; Grimminger, F.; Ghofrani, H.A. Novel soluble guanylyl cyclase stimulator BAY 41-2272 attenuates ischemia-reperfusion-induced lung injury. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2009, 296, L462–L469. [Google Scholar] [CrossRef]
- Zhang, Y.; Ding, S.; Li, C.; Wang, Y.; Chen, Z.; Wang, Z. Effects of N-acetylcysteine treatment in acute respiratory distress syndrome: A meta-analysis. Exp. Ther. Med. 2017, 14, 2863–2868. [Google Scholar] [CrossRef]
- Liu, X.; Pan, B.; Wang, X.; Xu, J.; Wang, X.; Song, Z.; Zhang, E.; Wang, F.; Wang, W. Ischemia/reperfusion-activated ferroptosis in the early stage triggers excessive inflammation to aggregate lung injury in rats. Front. Med. 2023, 10, 1181286. [Google Scholar] [CrossRef]
- Deng, P.; Wu, Y.; Wan, L.; Sun, X.; Sun, Q. Maresin1 Alleviates Ischemia Reperfusion Injury After Lung Transplantation by Inhibiting Ferroptosis via the PKA-Hippo-YAP Signaling Pathway. Biomedicines 2025, 13, 1594. [Google Scholar] [CrossRef]
- Zhao, J.; Li, J.; Wei, D.; Gao, F.; Yang, X.; Yue, B.; Xiong, D.; Liu, M.; Xu, H.; Hu, C.; et al. Liproxstatin-1 Alleviates Lung Transplantation-induced Cold Ischemia-Reperfusion Injury by Inhibiting Ferroptosis. Transplantation 2023, 107, 2190–2202. [Google Scholar] [CrossRef]
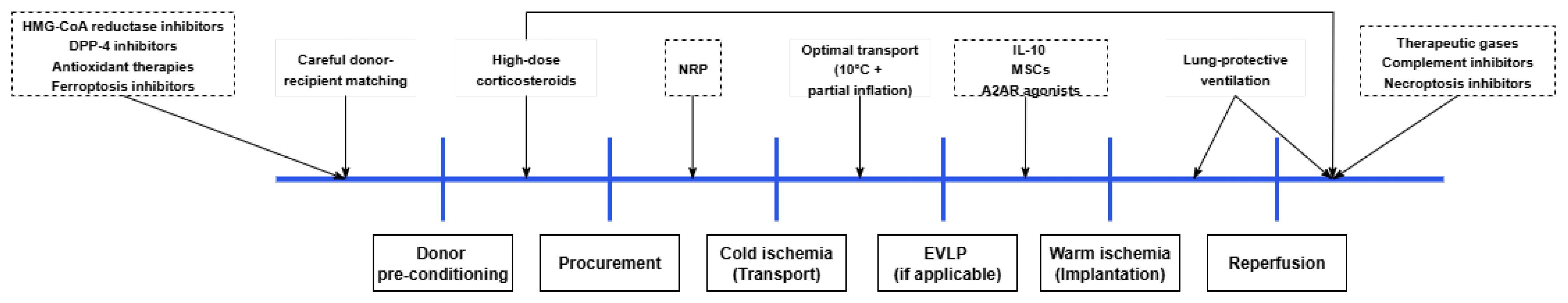
| Intervention | Proposed Mechanism(s) | Evidence Classes | Net Signal | Translational Status |
|---|---|---|---|---|
| LPV ± permissive hypercapnia | - ↓ Volutrauma/atelectrauma - Hypercapnia → ↓ NF-κB signaling and ROS injury | - Small animal ✓ - Human observational ✓ | - LPV favorable - Hypercapnia preclinical favorable | - LPV standard of care - Hypercapnia investigational |
| Controlled/gradual reperfusion at implantation | Pressure/flow-limited reperfusion → ↓ endothelial shear/ROS burst | - Small animal ✓ - Large animal ✓ - Human observational ✓ | Favorable | Standard of care |
| EVLP | - Normothermic assessment and reconditioning - Reduces ischemic time - Delivery vehicle for therapies | - Large animal ✓ - Human observational ✓ - RCT non-inferior vs. conventional preservation | Favorable | Established adjunct in many centers |
| iNO | - Selective pulmonary vasodilation - Improves V/Q matching - Theoretical anti-inflammatory effects | - Human RCT | - Neutral for PGD prevention - Useful as rescue/support | Supportive therapy |
| iCO | - Cytoprotective gas - Anti-inflammatory/anti-apoptotic via HO-1 and mito-signaling | - Small animal ✓ - Large animal ✓ | Favorable (preclinical) | Investigational |
| iH2 | - Scavenges •OH/ONOO− - Activates Nrf2/HO-1 - Anti-apoptotic | - Large animal ✓ - EVLP (DCD) ✓ | Favorable (preclinical/EVLP) | - Investigational - EVLP add-on candidate |
| Statins (pre-LTx exposure) | Pleiotropic anti-inflammatory and endothelial stabilizing | - Human observational ✓ | Mixed/neutral | Not recommended specifically for PGD prevention |
| High-dose corticosteroids (peri-operative) | General anti-inflammatory part of standard immunosuppression | - Human observational ✓ | Neutral for PGD prevention | Standard immunosuppression; not targeted PGD therapy |
| Mitochondria targeted agents | - Preserve mitochondrial integrity - Reduce mPTP opening - Reverse-electron-transport ROS | - Small animal ✓ - Large animal ✓ - EVLP model ✓ | Favorable (preclinical) | Investigational |
| Metformin | - Activates AMPK - Dampens inflammation/oxidative stress - May limit necroptosis | - Small animal ✓ | Favorable (preclinical) | Investigational (repurposing candidate) |
| DPP-4i | - Preserve VIP/chemokine tone - Anti-inflammatory/anti-apoptotic | - Small animal ✓ - Large animal ✓ - Human observational ✓ | Favorable signal | Investigational (early clinical signal) |
| A2A RA | - Immune-cell deactivation - Limits neutrophil-endothelium interactions | - Small animal ✓ - EVLP ✓ - RCT ✓ | Biomarker signal; clinical efficacy unproven | Early-phase clinical; more trials needed |
| IL-10 therapy | - Potent anti-inflammatory cytokine - Shifts macrophage/T-cell responses | - Large animal ✓ - EVLP model ✓ | Favorable (preclinical) | Investigational (EVLP delivery under refinement) |
| MSCs | - Paracrine immunomodulation - Antioxidant & endothelial protection | - Small animal ✓ - Large animal ✓ - EVLP model ✓ | Favorable (preclinical); early human safety | Investigational/early clinical |
| “Classic” antioxidants | - Direct ROS scavenging - Glutathione repletion | - Small animal ✓ - EVLP model ✓ | Inconsistent; no proven clinical benefit for PGD | Stalled—insufficient evidence for routine use |
| TLR pathway antagonists | Dampen innate immune activation to DAMPs | - Small animal ✓ | Favorable (preclinical) | Investigational |
| Fibrinolytics | - Dissolve donor pulmonary thrombi - Improve perfusion/oxygenation | - EVLP model ✓ | Favorable in selected EVLP cases | Selective EVLP tool; not PGD-directed therapy |
| Necroptosis inhibitors | - Block RIPK1/RIPK3/MLKL-mediated regulated necrosis | - Small animal ✓ | Favorable (preclinical) | Investigational |
| Ferroptosis inhibitors | - Limit iron-dependent lipid peroxidation and cell death | - Small animal ✓ | Favorable (preclinical) | Investigational |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Berzenji, L.; Hendriks, J.M.H.; Verleden, S.E.; Yogeswaran, S.K.; Wen, W.; Lauwers, P.; Verleden, G.; Paep, R.D.; Mertens, P.; Rodrigus, I.; et al. Lung Ischemia–Reperfusion Injury in Lung Transplant Surgery: Where Do We Stand? Antioxidants 2025, 14, 1295. https://doi.org/10.3390/antiox14111295
Berzenji L, Hendriks JMH, Verleden SE, Yogeswaran SK, Wen W, Lauwers P, Verleden G, Paep RD, Mertens P, Rodrigus I, et al. Lung Ischemia–Reperfusion Injury in Lung Transplant Surgery: Where Do We Stand? Antioxidants. 2025; 14(11):1295. https://doi.org/10.3390/antiox14111295
Chicago/Turabian StyleBerzenji, Lawek, Jeroen M. H. Hendriks, Stijn E. Verleden, Suresh Krishan Yogeswaran, Wen Wen, Patrick Lauwers, Geert Verleden, Rudi De Paep, Pieter Mertens, Inez Rodrigus, and et al. 2025. "Lung Ischemia–Reperfusion Injury in Lung Transplant Surgery: Where Do We Stand?" Antioxidants 14, no. 11: 1295. https://doi.org/10.3390/antiox14111295
APA StyleBerzenji, L., Hendriks, J. M. H., Verleden, S. E., Yogeswaran, S. K., Wen, W., Lauwers, P., Verleden, G., Paep, R. D., Mertens, P., Rodrigus, I., Adriaensen, D., & Van Schil, P. (2025). Lung Ischemia–Reperfusion Injury in Lung Transplant Surgery: Where Do We Stand? Antioxidants, 14(11), 1295. https://doi.org/10.3390/antiox14111295







