Physiological Responses and Serum Metabolite Alterations in Grass Carp (Ctenopharyngodon idellus) Under Chronic Salinity Exposure
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals, Experimental Design and Sampling
2.2. Growth Performance
2.3. Serum Physiological Parameter Analysis
2.4. Oxidative Stress Parameter Analyses
2.5. Serum Metabolomics Analysis
2.6. Statistical Analysis
3. Results
3.1. Growth Performance
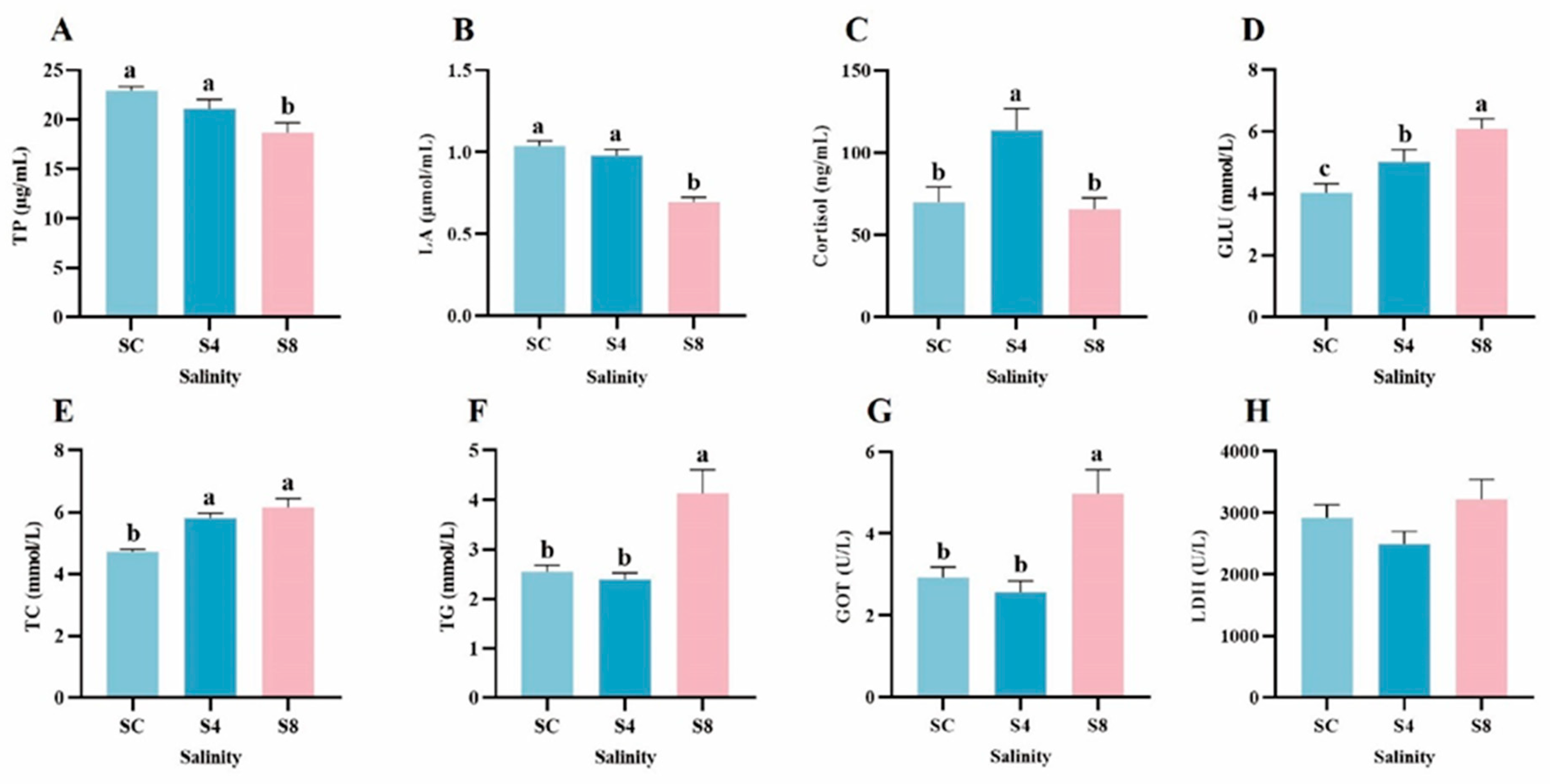
3.2. Physiological Parameters
3.3. Serum Ion Content
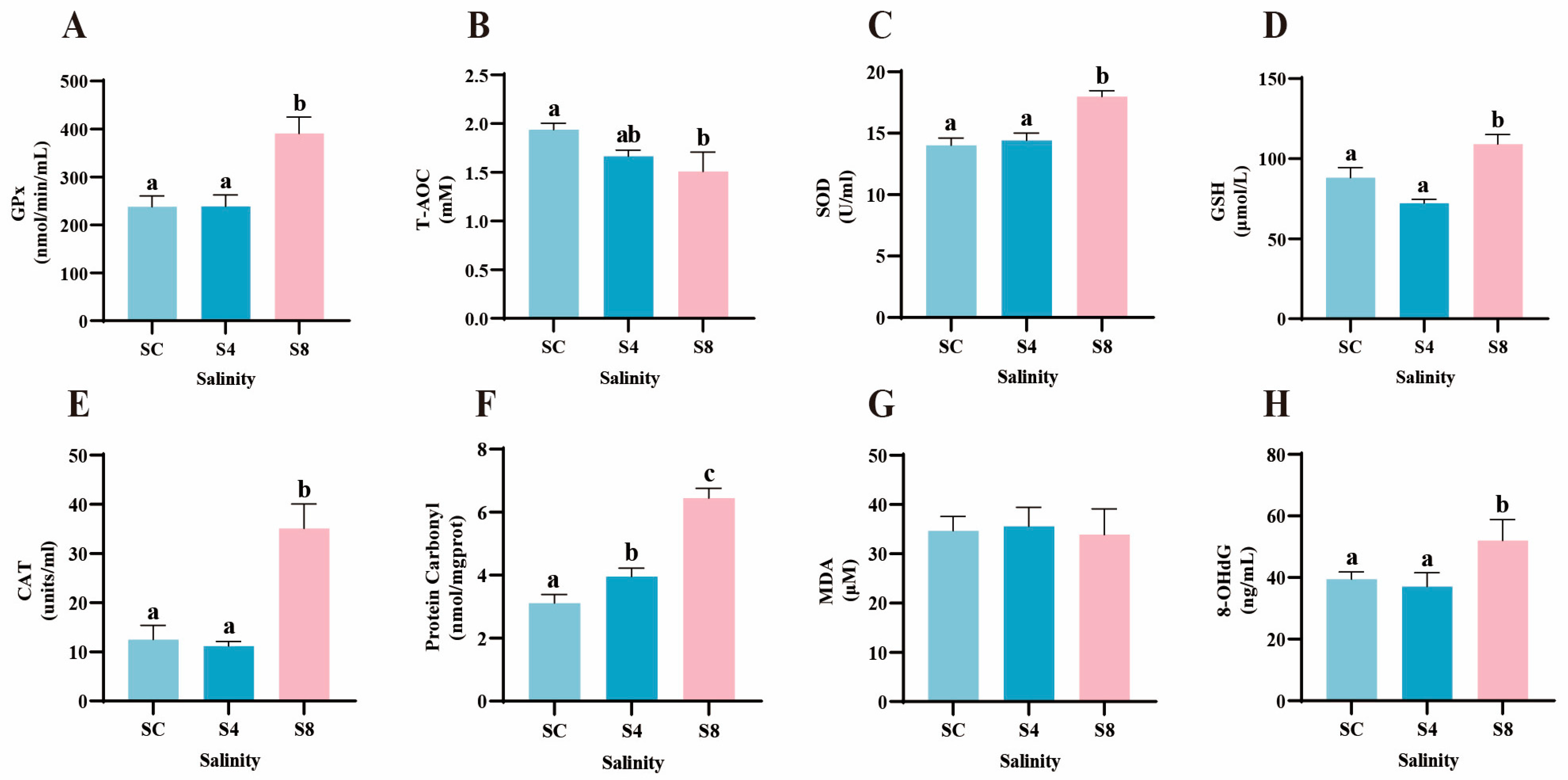
3.4. Oxidative Stress Parameters
3.5. Serum Metabolomics
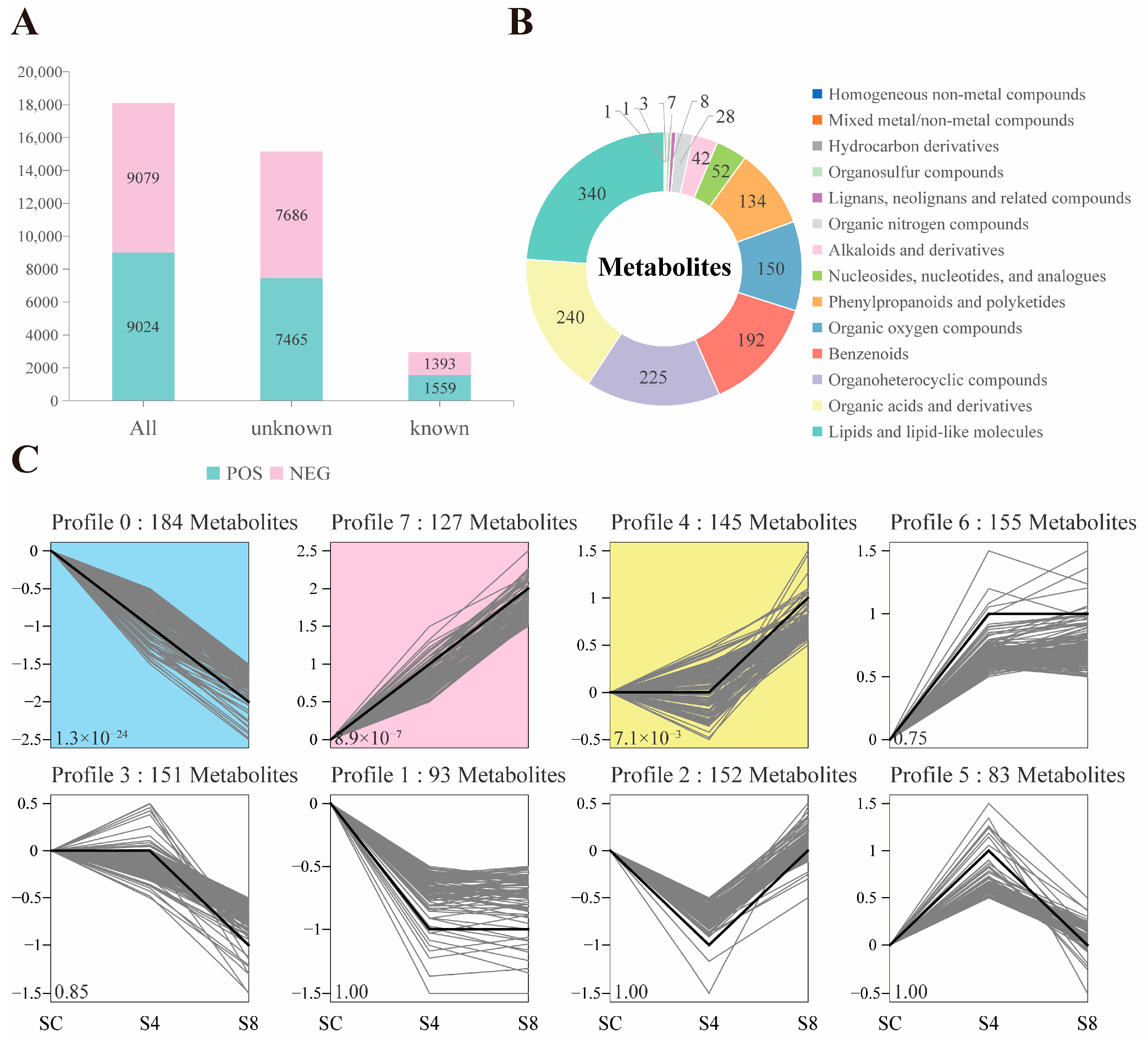
3.5.1. Serum Metabolites Composition
3.5.2. Differential Metabolites Analysis
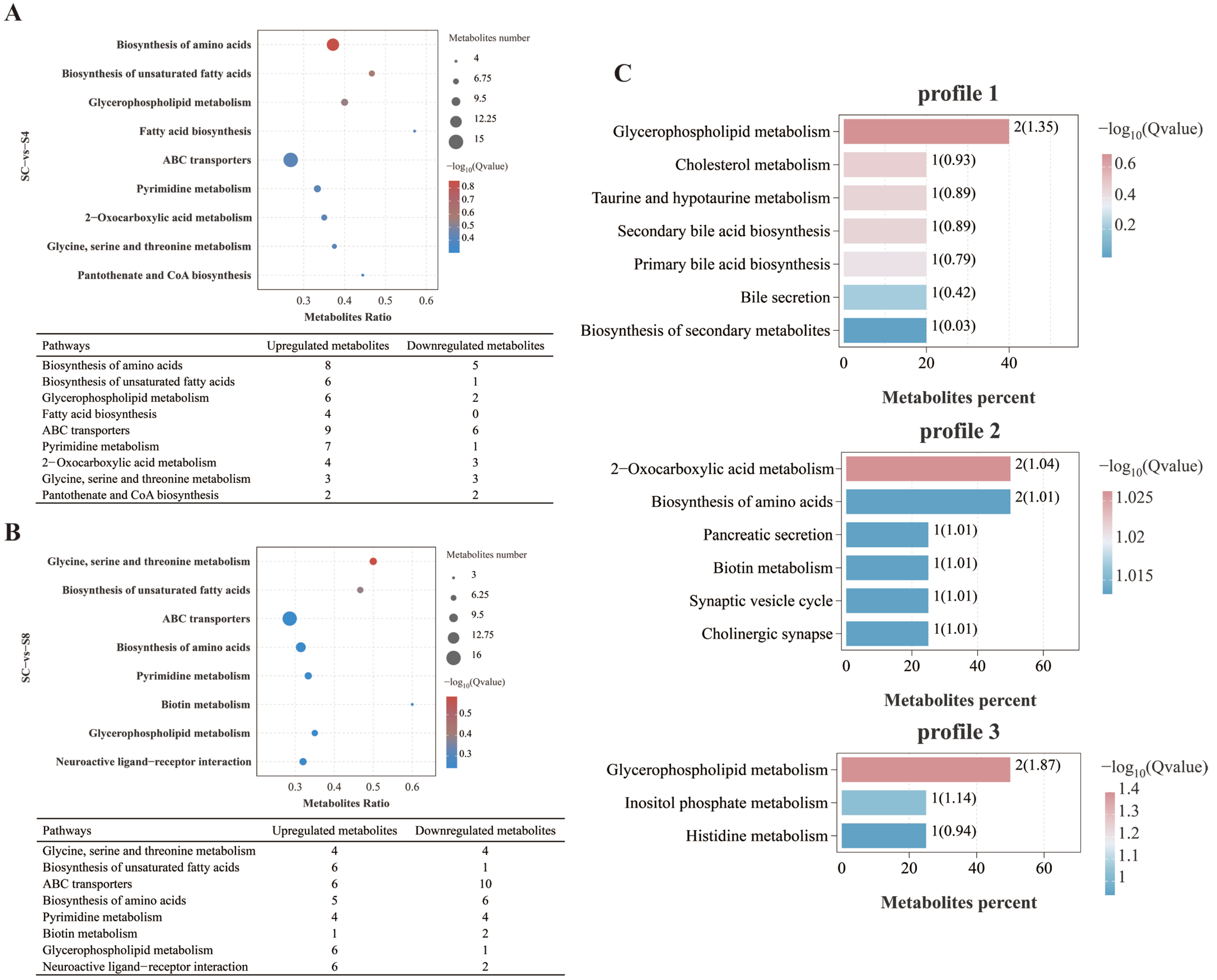
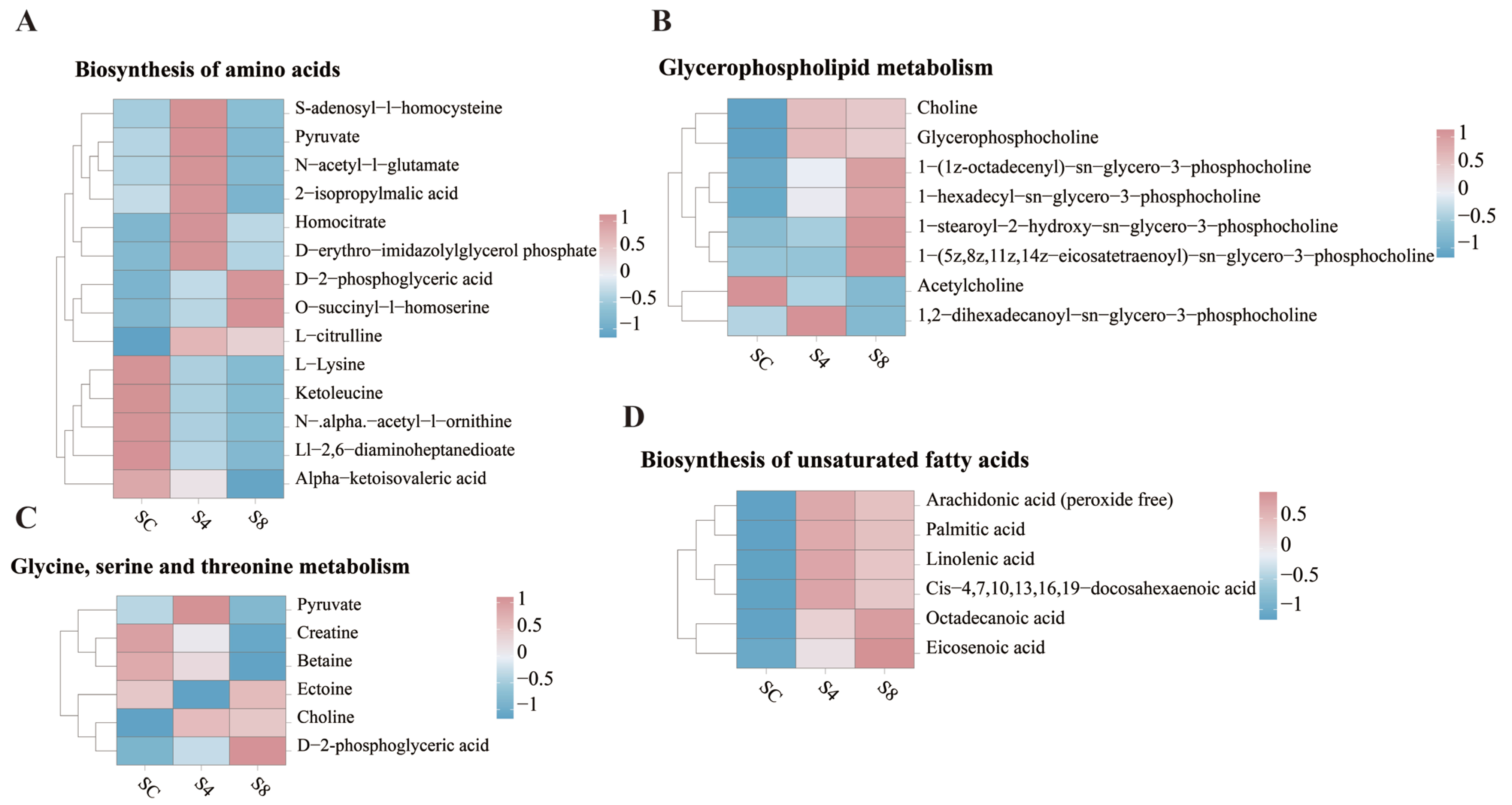
3.5.3. Metabolic Pathway Analysis
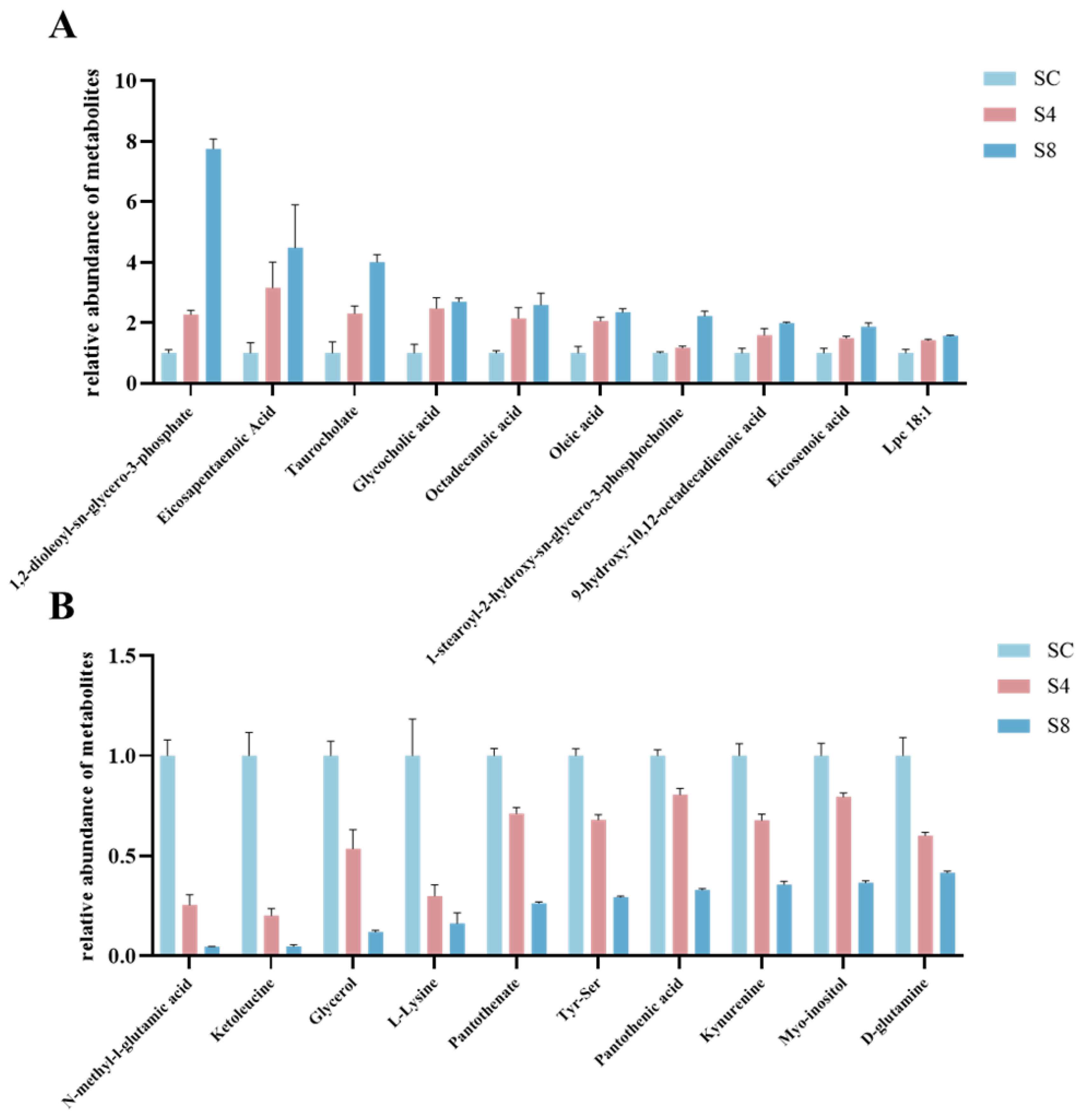
3.5.4. Signature Differential Metabolites
4. Discussion
4.1. Salinity Stress Inhibited Growth Performance
4.2. The Effect of Salinity Stress on Physiological Parameters
4.3. The Effect of Salinity Stress on Ion Content
4.4. Salinity Stress-Induced Oxidative Stress
4.5. The Effect of Salinity Stress on Metabolic Functions
4.6. The Effect of Salinity Stress on Key Metabolites
4.7. Integrative Physiology and Metabolomics Informing the Precision Breeding of Salt-Tolerant Grass Carp
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mifsud, C.; Rowland, S.J. Use of salt to control ichthyophthiriosis and prevent saprolegniosis in silver perch, Bidyanus bidyanus. Aquac. Res. 2008, 39, 1175–1180. [Google Scholar] [CrossRef]
- Luz, R.K.; Favero, G.C. Use of salt, anesthetics, and stocking density in transport of live fish: A review. Fishes 2024, 9, 286. [Google Scholar] [CrossRef]
- Ramírez-Duarte, W.F.; Pineda-Quiroga, C.; Martínez, N.; Eslava-Mocha, P.R. Use of sodium chloride and zeolite during shipment of Ancistrus triradiatus under high temperature. Neotrop. Ichthyol. 2011, 9, 909–914. [Google Scholar] [CrossRef]
- Li, X.; Liu, X.; Leng, X.; Wang, X. Effect of salinity on growth and flesh quality of snakehead Channa argus. Oceanol. Limnol. Sin. Sin. 2008, 5, 14. [Google Scholar]
- Abou Anni, I.S.; Bianchini, A.; Barcarolli, I.F.; Junior, A.S.V.; Robaldo, R.B.; Tesser, M.B.; Sampaio, L.A. Salinity influence on growth, osmoregulation and energy turnover in juvenile pompano Trachinotus marginatus Cuvier 1832. Aquaculture 2016, 455, 63–72. [Google Scholar] [CrossRef]
- Djiba, P.K.; Zhang, J.; Xu, Y.; Zhang, P.; Zhou, J.; Zhang, Y.; Luo, Y. Correlation between metabolic rate and salinity tolerance and metabolic response to salinity in grass carp (Ctenopharyngodon idella). Animals 2021, 11, 3445. [Google Scholar] [CrossRef]
- Ma, S.; Tian, X.H. Experience in raising freshwater fish in semi-salty water on the beach. Sci. Fish Farming 1998, 33. [Google Scholar]
- Ahmmed, M.K.; Ahmmed, F.; Kabir, K.A.; Faisal, M.; Ahmed, S.I.; Ahsan, M.N. Biochemical impacts of salinity on the catfish, Heteropneustes fossilis (Bloch, 1794), and possibility of their farming at low saline water. Aquac. Res. 2017, 48, 4251–4261. [Google Scholar] [CrossRef]
- Fabregat, T.; Damian, J.; Fialho, N.S.; Costa, D.; Broggi, J.A.; Pereira, R.G.; Takata, R. Acute toxicity of common salt and intensive larviculture of silver catfish Rhamdia quelen in brackish water. Arq. Bras. Med. Vet. Zootec. 2015, 67, 547–554. [Google Scholar] [CrossRef]
- Wang, J.; Lui, H.; Po, H.; Fan, L. Influence of salinity on food consumption, growth and energy conversion efficiency of common carp (Cyprinus carpio) fingerlings. Aquaculture 1997, 148, 115–124. [Google Scholar] [CrossRef]
- Wang, G.D.; Tian, X.L.; Dong, S.L.; Gong, Q.L. Effects of Different Salinities on the Growth, Osmoregulation and Energy Budget of the Bester (Husohuso×Acipenser ruthenus). J. Ocean Univ. China 2007, 37, 189–194. [Google Scholar]
- Zhang, Y.; Yu, H.; Chen, H.; Wang, X.; Tan, Y.; Sun, J.; Luo, J.; Song, F. Integrative transcriptomic and metabolomic analyses reveal preliminary molecular mechanisms of gills response to salinity stress in Micropterus salmoides. Aquaculture 2025, 606, 742600. [Google Scholar] [CrossRef]
- Li, X.; Shen, Y.; Bao, Y.; Wu, Z.; Yang, B.; Jiao, L.; Zhang, C.; Tocher, D.R.; Zhou, Q.; Jin, M. Physiological responses and adaptive strategies to acute low-salinity environmental stress of the euryhaline marine fish black seabream (Acanthopagrus schlegelii). Aquaculture 2022, 554, 738117. [Google Scholar] [CrossRef]
- Jiang, Y.; Yuan, C.; Qi, M.; Liu, Q.; Hu, Z. The effect of salinity stress on enzyme activities, histology, and transcriptome of silver carp (Hypophthalmichthys molitrix). Biology 2022, 11, 1580. [Google Scholar] [CrossRef]
- Qiang, J.; Wang, H.; Kpundeh, M.D.; He, J.; Xu, P. Effect of water temperature, salinity, and their interaction on growth, plasma osmolality, and gill Na+, K+-ATPase activity in juvenile GIFT tilapia Oreochromis niloticus (L.). J. Therm. Biol. 2013, 38, 331–338. [Google Scholar] [CrossRef]
- Khairnar, S.O.; Tian, X.; Fang, Z.; Dong, S. Effects of the amplitude and frequency of salinity fluctuation on the body composition and energy budget of juvenile tongue sole (Cynoglossus semilaevis). J. Ocean Univ. 2015, 14, 127–134. [Google Scholar] [CrossRef]
- Tavares-Dias, M. Toxicity, physiological, histopathological, handling, growth and antiparasitic effects of the sodium chloride (salt) in the freshwater fish aquaculture. Aquac. Res. 2022, 53, 715–734. [Google Scholar] [CrossRef]
- Samuelsson, L.M.; Förlin, L.; Karlsson, G.; Adolfsson-Erici, M.; Larsson, D.J. Using NMR metabolomics to identify responses of an environmental estrogen in blood plasma of fish. Aquat. Toxicol. 2006, 78, 341–349. [Google Scholar] [CrossRef]
- Giebułtowicz, J.; Grabicová, K.; Brooks, B.W.; Grabic, R. Influence of time-dependent sampling on the plasma metabolome and exposome of fish collected from an effluent-dependent pond. Sci. Total Environ. 2024, 906, 167446. [Google Scholar] [CrossRef]
- Li, W.T.; Liu, B.; Wang, J.; Zhang, Y.A.; Zhang, X.J. Genomic localization and immune response of IgT in grass carp (Ctenopharyngodon idellus). Fish Shellfish Immunol. 2025, 163, 110422. [Google Scholar] [CrossRef]
- Tian, Y. Effects of salinity on Ctenopharyngodon idella growth. Heilongjiang Fish. 2022, 41, 23–25. [Google Scholar]
- Peyghan, R.; Khadjeh, G.H.; Enayati, A. Effect of water salinity on total protein and electrophoretic pattern of serum proteins of grass carp, Ctenopharyngodon idella. Vet. Res. Forum Int. Q. J. 2014, 5, 225–229. [Google Scholar]
- Liu, D.; Zhang, Z.; Song, Y.; Yang, J.; Lu, Y.; Lai, W.; Wu, Z.; Zhao, D.; Lin, H.; Zhang, Y. Effects of salinity on growth, physiology, biochemistry and gut microbiota of juvenile grass carp (Ctenopharyngodon idella). Aquat. Toxicol. 2023, 258, 106482. [Google Scholar] [CrossRef] [PubMed]
- Jia, R.; Hou, Y.; Feng, W.; Nomingerel, M.; Li, B.; Zhu, J. Multi-omics analysis to understand the effects of dietary proanthocyanidins on antioxidant capacity, muscle nutrients, lipid metabolism, and intestinal microbiota in Cyprinus carpio. Antioxidants 2023, 12, 2095. [Google Scholar] [CrossRef] [PubMed]
- Tubino, M.; Souza, R.L.D.; Hoehr, N.F. Rapid quantitative turbidimetric spot test analysis of potassium in blood serum. J. Braz. Chem. Soc. 2004, 15, 635–639. [Google Scholar] [CrossRef]
- Zhou, H.; Liu, Y.; Fan, J.; Huang, H.; Deng, J.; Tan, B. Feeding Rainbow Trout with Different Types of Non-Starch Polysaccharides: Impacts on Serum Metabolome and Gut Microbiota. Metabolites 2022, 12, 1167. [Google Scholar] [CrossRef]
- Cai, R.; Chen, L.; Xin, Y.; Zhao, Z.; Yu, X.; Huang, J.; Liao, Z.; Li, W. Effects of salinity stress on immune-related parameters of the Nile tilapia (Oreochromis niloticus). J. Fish. China 2020, 44, 978–986. [Google Scholar]
- Tang, L.; Duan, Y.; Xie, B.; Liu, H.; Zhong, L.; Wang, M.; Liu, J.; Su, C.; Chen, X.; Zhang, S. Effects of salinity stress on the growth performance, histological characteristics, and expression of genes related to apoptosis and immunity in channel catfish (Ictalurus punctatus). J. Fish Biol. 2025, 106, 1112–1123. [Google Scholar] [CrossRef]
- Kultz, D. Molecular and evolutionary basis of the cellular stress response. Annu. Rev. Physiol. 2005, 67, 225–257. [Google Scholar] [CrossRef]
- Dawood, M.A.; Koshio, S.; Ishikawa, M.; Yokoyama, S. Effects of partial substitution of fish meal by soybean meal with or without heat—killed Lactobacillus plantarum (LP20) on growth performance, digestibility, and immune response of amberjack, Seriola dumerili juveniles. Biomed Res. Int. 2015, 2015, 514196. [Google Scholar] [CrossRef]
- Dawood, M.A.; Koshio, S.; Ishikawa, M.; Yokoyama, S. Effects of heat killed Lactobacillus plantarum (LP20) supplemental diets on growth performance, stress resistance and immune response of red sea bream, Pagrus major. Aquaculture 2015, 442, 29–36. [Google Scholar] [CrossRef]
- Patel, R.K.; Verma, A.K.; Krishnani, K.K.; Sreedharan, K.; Chandrakant, M.H. Growth performance, physio-metabolic, and haemato-biochemical status of Labeo rohita (Hamilton, 1822) juveniles reared at varying salinity levels using inland saline groundwater. Aquaculture 2022, 559, 738408. [Google Scholar] [CrossRef]
- Iffat, J.; Tiwari, V.K.; Pavan Kumar, A.; Verma, A.K.; Harikrishna, V.; Babitha Rani, A.M.; Chadha, N.K.; Anand, G. The effect of inland saline groundwater on growth, maturation, and osmoregulation of common carp. N. Am. J. Aquac. 2021, 83, 15–25. [Google Scholar] [CrossRef]
- Dawood, M.A.; Alkafafy, M.; Sewilam, H. The antioxidant responses of gills, intestines and livers and blood immunity of common carp (Cyprinus carpio) exposed to salinity and temperature stressors. Fish Physiol. Biochem. 2022, 48, 397–408. [Google Scholar] [CrossRef]
- Rahmah, S.; Liew, H.J.; Napi, N.; Rahmat, S.A. Metabolic cost of acute and chronic salinity response of hybrid red tilapia Oreochromis sp. larvae. Aquac. Rep. 2020, 16, 100233. [Google Scholar] [CrossRef]
- Abdel-Tawwab, M.; Monier, M.N. Stimulatory effect of dietary taurine on growth performance, digestive enzymes activity, antioxidant capacity, and tolerance of common carp, Cyprinus carpio L., fry to salinity stress. Fish Physiol. Biochem. 2018, 44, 639–649. [Google Scholar] [CrossRef]
- Sun, J.; Liu, Q.; Zhao, L.; Cui, C.; Wu, H.; Liao, L.; Tang, G.; Yang, S.; Yang, S. Potential regulation by miRNAs on glucose metabolism in liver of common carp (Cyprinus carpio) at different temperatures. Comp. Biochem. Physiol. D Genom. Proteom. 2019, 32, 100628. [Google Scholar] [CrossRef]
- Shen, S.; Zhang, S.; Xie, H.; Wang, B.; He, X.; Wang, W. Effects of salinity domestication on serum biochemistry and osmotic pressure of carp Cyprinus carpio. Chin. J. Ecol. 2013, 32, 3261. [Google Scholar]
- Ding, L.; Liu, Y.; Wei, X.; Geng, C.; Liu, W.; Han, L.; Yuan, F.; Wang, P.; Sun, Y. Effects of saline-alkaline stress on metabolome, biochemical parameters, and histopathology in the kidney of crucian carp (Carassius auratus). Metabolites 2023, 13, 159. [Google Scholar] [CrossRef]
- Wang, L.; Lin, W.; Zha, Q.; Guo, H.; Zhang, D.; Yang, L.; Li, L.; Li, D.; Tang, R. Persistent exposure to environmental levels of microcystin-LR disturbs cortisol production via hypothalamic-pituitary-interrenal (HPI) axis and subsequently liver glucose metabolism in adult male zebrafish (Danio rerio). Toxins 2020, 12, 282. [Google Scholar] [CrossRef]
- Mccormick, S.D.; Taylor, M.L.; Regish, A.M. Cortisol is an osmoregulatory and glucose-regulating hormone in Atlantic sturgeon, a basal ray-finned fish. J. Exp. Biol. 2020, 223, jeb220251. [Google Scholar] [CrossRef]
- De Boeck, G.; Vlaeminck, A.; Van der Linden, A.; Blust, R. The energy metabolism of common carp (Cyprinus carpio) when exposed to salt stress: An increase in energy expenditure or effects of starvation? Physiol. Biochem. Zool. 2000, 73, 102–111. [Google Scholar] [CrossRef]
- Dawood, M.A.O.; Koshio, S.; Ishikawa, M.; El-Sabagh, M.; Yokoyama, S.; Wang, W.L.; Yukun, Z.; Olivier, A. Physiological response, blood chemistry profile and mucus secretion of red sea bream (Pagrus major) fed diets supplemented with Lactobacillus rhamnosus under low salinity stress. Fish Physiol. Biochem. 2017, 43, 179–192. [Google Scholar] [CrossRef]
- Salati, A.P.; Baghbanzadeh, A.; Soltani, M.; Peyghan, R.; Riazi, G.H. The response of plasma glucose, lactate, protein and hematological parameters to osmotic challenge in common carp (Cyprinus carpio). Int. J. Vet. Res. 2010, 4, 49–52. [Google Scholar]
- Kammerer, B.D.; Cech, J.J., Jr.; Kültz, D. Rapid changes in plasma cortisol, osmolality, and respiration in response to salinity stress in tilapia (Oreochromis mossambicus). Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2010, 157, 260–265. [Google Scholar] [CrossRef] [PubMed]
- Laurent, P.; Perry, S.F. Effects of cortisol on gill chloride cell morphology and ionic uptake in the freshwater trout, Salmo gairdneri. Cell. Tissue Res. 1990, 259, 429–442. [Google Scholar] [CrossRef]
- Lin, C.; Tsai, I.; Su, C.; Tseng, D.; Hwang, P. Reverse effect of mammalian hypocalcemic cortisol in fish: Cortisol stimulates Ca2+ uptake via glucocorticoid receptor-mediated vitamin D3 metabolism. PLoS ONE 2011, 6, e23689. [Google Scholar] [CrossRef]
- Mossambicus, O. Cortisol Increases Na+/K+-ATPase Density in Plasma Membranes of Gill Chloride Cells in the Freshwater Tilapia. J. Exp. Biol. 2000, 203, 2349–2355. [Google Scholar] [CrossRef]
- Imanpoor, M.R.; Najafi, E.; Kabir, M. Effects of different salinity and temperatures on the growth, survival, haematocrit and blood biochemistry of Goldfish (Carassius auratus). Aquac. Res. 2012, 43, 332–338. [Google Scholar] [CrossRef]
- Mansourghanaei, A.; Khara, H.; Vahabzadeh Roudsari, H.; Pajand, Z.; Ahmadnezhad, M. Alterations in Hematological indices, histopathology and p450 gene expression in stellate sturgeon (Acipenser stellatus Pallas, 1811) fingerlings exposed to different salinities levels and ammonia. Iran. J. Fish. Sci. 2022, 21, 1343–1366. [Google Scholar]
- Al-Khashali, M.S.; Al-Shawi, S. Effect of salt stress on ALT and AST enzymes activity and cortisol level in adults of Carassius auratus. Pak. J. Nutr. 2013, 12, 97. [Google Scholar] [CrossRef]
- Kim, J.; Choi, H.; Sung, G.; Seo, S.; Kim, K.I.; Kang, Y.J.; Kang, J. Toxic effects on hematological parameters and oxidative stress in juvenile olive flounder, Paralichthys olivaceus exposed to waterborne zinc. Aquacult. Rep. 2019, 15, 100225. [Google Scholar] [CrossRef]
- Lee, D.; Choi, Y.J.; Kim, J. Toxic effects of waterborne cadmium exposure on hematological parameters, oxidative stress, neurotoxicity, and heat shock protein 70 in juvenile olive flounder, Paralichthys olivaceus. Fish Shellfish Immunol. 2022, 122, 476–483. [Google Scholar] [CrossRef] [PubMed]
- öner, M.; Atli, G.; Canli, M. Changes in serum biochemical parameters of freshwater fish Oreochromis niloticus following prolonged metal (Ag, Cd, Cr, Cu, Zn) exposures. Environ. Toxicol. Chem. 2008, 27, 360–366. [Google Scholar] [CrossRef]
- Wang, N.; Gao, C.; Zhang, P.; Guan, L.; Wang, Y.; Qin, Y.; Li, Y. Effect of Bacillus cereus against cadmium induced hematological disturbances and immunosuppression in Carassius auratus gibelio. Fish Shellfish Immunol. 2019, 89, 141–148. [Google Scholar] [CrossRef]
- Roychowdhury, P.; Aftabuddin, M.; Pati, M.K. Thermal stress altered growth performance and metabolism and induced anaemia and liver disorder in Labeo rohita. Aquac. Res. 2020, 51, 1406–1414. [Google Scholar] [CrossRef]
- Zhao, F.; Qu, L.; Zhuang, P.; Zhang, L.; Liu, J.; Zhang, T. Salinity tolerance as well as osmotic and ionic regulation in juvenile Chinese sturgeon (Acipenser sinensis Gray, 1835) exposed to different salinities. J. Appl. Ichthyol. 2011, 27, 231–234. [Google Scholar] [CrossRef]
- Salati, A.P.; Baghbanzadeh, A.; Soltani, M.; Peyghan, R.; Riazi, G. Effect of different levels of salinity on gill and kidney function in common carp Cyprinus carpio (Pisces: Cyprinidae). Ital. J. Zool. 2011, 78, 298–303. [Google Scholar] [CrossRef]
- Zhu, J.; Wang, X.; Bu, X.; Wang, C.; Pan, J.; Li, E.; Shi, Q.; Zhang, M.; Qin, J.G.; Chen, L. Relationship between myo-inositol synthesis and carbohydrate metabolism changes in Mozambique tilapia (Oreochromis mossambicus) under acute hypersaline stress. Aquaculture 2021, 532, 736005. [Google Scholar] [CrossRef]
- Tam, W.L.; Wong, W.P.; Loong, A.M.; Hiong, K.C.; Chew, S.F.; Ballantyne, J.S.; Ip, Y.K. The osmotic response of the Asian freshwater stingray (Himantura signifer) to increased salinity: A comparison with marine (Taeniura lymma) and Amazonian freshwater (Potamotrygon motoro) stingrays. J. Exp. Biol. 2003, 206, 2931–2940. [Google Scholar] [CrossRef]
- Zhu, Z.; Li, S.; Lei, C.; Zhu, T.; Tian, J.; Du, J.; Wei, S.; Song, H. Survival and acute osmoregulatory response of grass carp under salinity stress. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2025, 308, 111905. [Google Scholar] [CrossRef]
- Mohamed, N.A.; Saad, M.F.; Shukry, M.; El-Keredy, A.M.; Nasif, O.; Van Doan, H.; Dawood, M.A. Physiological and ion changes of Nile tilapia (Oreochromis niloticus) under the effect of salinity stress. Aquac. Rep. 2021, 19, 100567. [Google Scholar] [CrossRef]
- Enayati, A.; Peyghan, R.; Papahn, A.A.; Khadjeh, G. Study on effect of salinity level of water on electrocardiogram and some of blood serum minerals in grass carp, Ctenopharyngodon idella. Vet. Res. Forum. Int. Q. J. 2013, 4, 49–53. [Google Scholar]
- Vonck, A.; Bonga, S.W.; Flik, G. Sodium and calcium balance in Mozambique tilapia, Oreochromis mossambicus, raised at different salinities. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 1998, 119, 441–449. [Google Scholar] [CrossRef] [PubMed]
- Graham, D.E.; Graupner, M.; Xu, H.; White, R.H. Identification of coenzyme M biosynthetic 2—Phosphosulfolactate phosphatase. A member of a new class of Mg2+—Dependent acid phosphatases. Eur. J. Biochem. 2001, 268, 5176–5188. [Google Scholar] [CrossRef]
- Olorunniji, F.J.; Igunnu, A.; Adebayo, J.O.; Arise, R.O.; Malomo, S.O. Cofactor interactions in the activation of tissue non-specific alkaline phosphatase: Synergistic effects of Zn2+ and Mg2+ ions. Biokemistri 2007, 19, 43–48. [Google Scholar]
- Moniruzzaman, M.; Mukherjee, M.; Kumar, S.; Chakraborty, S.B. Effects of salinity stress on antioxidant status and inflammatory responses in females of a “Near Threatened” economically important fish species Notopterus chitala: A mechanistic approach. Environ. Sci. Pollut. Res. 2022, 29, 75031–75042. [Google Scholar] [CrossRef]
- Chowdhury, S.; Saikia, S.K. Oxidative stress in fish: A review. J. Sci. Res. 2020, 12, 145–160. [Google Scholar] [CrossRef]
- Chien, Y.; Pan, C.; Hunter, B. The resistance to physical stresses by Penaeus monodon juveniles fed diets supplemented with astaxanthin. Aquaculture 2003, 216, 177–191. [Google Scholar] [CrossRef]
- Roche, H.; Bogé, G. Fish blood parameters as a potential tool for identification of stress caused by environmental factors and chemical intoxication. Mar. Environ. Res. 1996, 41, 27–43. [Google Scholar] [CrossRef]
- Yin, F.; Peng, S.; Sun, P.; Shi, Z. Effects of low salinity on antioxidant enzymes activities in kidney and muscle of juvenile silver pomfret Pampus argenteus. Acta Ecol. Sin. 2011, 31, 55–60. [Google Scholar] [CrossRef]
- Vinodhini, R.; Narayanan, M. Biochemical changes of antioxidant enzymes in common carp (Cyprinus carpio L.) after heavy metal exposure. Turk. J. Vet. Anim. Sci. 2009, 33, 273–278. [Google Scholar] [CrossRef]
- Monteiro, D.A.; Rantin, F.T.; Kalinin, A.L. Inorganic mercury exposure: Toxicological effects, oxidative stress biomarkers and bioaccumulation in the tropical freshwater fish matrinxã, Brycon amazonicus (Spix and Agassiz, 1829). Ecotoxicology 2010, 19, 105–123. [Google Scholar] [CrossRef] [PubMed]
- Lushchak, V.I. Environmentally induced oxidative stress in aquatic animals. Aquat. Toxicol. 2011, 101, 13–30. [Google Scholar] [CrossRef]
- Ma, Y.; Meng, X.; Sowanou, A.; Wang, J.; Li, H.; Li, A.; Zhong, N.; Yao, Y.; Pei, J. Effect of fluoride on the Expression of 8-Hydroxy-2′-deoxyguanosine in the blood, kidney, liver, and brain of rats. Biol. Trace Elem. Res. 2023, 201, 2904–2916. [Google Scholar] [CrossRef]
- Honda, M.; Yamada, Y.; Tomonaga, M.; Ichinose, H.; Kamihira, S. Correlation of urinary 8-hydroxy-2′-deoxyguanosine (8-OHdG), a biomarker of oxidative DNA damage, and clinical features of hematological disorders: A pilot study. Leuk. Res. 2000, 24, 461–468. [Google Scholar] [CrossRef]
- Taysı, M.R. Assessing the effects of cadmium on antioxidant enzymes and histological structures in rainbow trout liver and kidney. Sci. Rep. 2024, 14, 27453. [Google Scholar] [CrossRef]
- Jiang, W.; Liu, Y.; Hu, K.; Jiang, J.; Li, S.; Feng, L.; Zhou, X. Copper exposure induces oxidative injury, disturbs the antioxidant system and changes the Nrf2/ARE (CuZnSOD) signaling in the fish brain: Protective effects of myo-inositol. Aquat. Toxicol. 2014, 155, 301–313. [Google Scholar] [CrossRef]
- Bopp, S.K.; Abicht, H.K.; Knauer, K. Copper-induced oxidative stress in rainbow trout gill cells. Aquat. Toxicol. 2008, 86, 197–204. [Google Scholar] [CrossRef]
- Sánchez-Aceves, L.; Pérez-Alvarez, I.; Gómez-Oliván, L.M.; Islas-Flores, H.; Barceló, D. Long-term exposure to environmentally relevant concentrations of ibuprofen and aluminum alters oxidative stress status on Danio rerio. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2021, 248, 109071. [Google Scholar] [CrossRef]
- Sivakumar, S.; Khatiwada, C.P.; Sivasubramanian, J. Bioaccumulations of aluminum and the effects of chelating agents on different organs of Cirrhinus mrigala. Environ. Toxicol. Pharmacol. 2012, 34, 791–800. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Dong, M.; Jiang, W.; Wu, P.; Liu, Y.; Jin, X.; Kuang, S.; Tang, L.; Zhang, L.; Feng, L. Acute nitrite exposure-induced oxidative damage, endoplasmic reticulum stress, autophagy and apoptosis caused gill tissue damage of grass carp (Ctenopharyngodon idella): Relieved by dietary protein. Ecotoxicol. Environ. Saf. 2022, 243, 113994. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.; Kwon, Y.; Nam, M.; Vaidya, B.; Kim, S.R.; Lee, S.; Kwon, J.; Kim, D.; Hwang, G. Integrated profiling of global metabolomic and transcriptomic responses to viral hemorrhagic septicemia virus infection in olive flounder. Fish Shellfish Immunol. 2017, 71, 220–229. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Luo, W.; Chen, P.; Wang, Y.; Liu, D.; Lan, Y.; Chen, X.; Zhou, L.; Yang, S.; Du, Z. Study on the physiological responses and tolerance mechanisms to subchronic carbonate alkalinity exposure in the gills of Paramisgurnus dabryanus. Ecotoxicol. Environ. Saf. 2024, 287, 117319. [Google Scholar] [CrossRef]
- Wang, S.; Li, X.; Zhang, M.; Jiang, H.; Wang, R.; Qian, Y.; Li, M. Ammonia stress disrupts intestinal microbial community and amino acid metabolism of juvenile yellow catfish (Pelteobagrus fulvidraco). Ecotoxicol. Environ. Saf. 2021, 227, 112932. [Google Scholar] [CrossRef]
- Ren, X.; Jia, S.; Gao, B.; Zhou, Q.; Xu, Y.; Liu, P.; Li, J. Application of proteomics and metabolomics to assess ammonia stress response and tolerance mechanisms of juvenile ornate rock lobster Panulirus ornatus. Sci. Total Environ. 2022, 837, 155751. [Google Scholar] [CrossRef]
- Duan, Y.; Xiong, D.; Wang, Y.; Li, H.; Dong, H.; Zhang, J. Toxic effects of ammonia and thermal stress on the intestinal microbiota and transcriptomic and metabolomic responses of Litopenaeus vannamei. Sci. Total Environ. 2021, 754, 141867. [Google Scholar] [CrossRef]
- Guo, R.; Yu, K.; Huang, K.; Jiang, S.; Pang, L.; Huang, J.; Yang, X.; Wang, D. Effects of cholesterol and soy lecithin interaction on growth performance, physiology and biochemistry, and serum metabolomics of Cyprinus carpio var. Aquacult. Rep. 2024, 37, 102283. [Google Scholar] [CrossRef]
- Xie, S.; Tian, L.; Jin, Y.; Yang, H.; Liang, G.; Liu, Y. Effect of glycine supplementation on growth performance, body composition and salinity stress of juvenile Pacific white shrimp, Litopenaeus vannamei fed low fishmeal diet. Aquaculture 2014, 418, 159–164. [Google Scholar] [CrossRef]
- Zhou, Q.; Wang, Y.; Wang, H.; Tan, B. Dietary threonine requirements of juvenile Pacific white shrimp, Litopenaeus vannamei. Aquaculture 2013, 392, 142–147. [Google Scholar] [CrossRef]
- Glover, C.N. Cellular and molecular approaches to the investigation of piscine osmoregulation: Current and future perspectives. In Fish Osmoregulation; CRC Press: Boca Raton, FL, USA, 2019; pp. 177–234. [Google Scholar]
- Tseng, Y.; Hwang, P. Some insights into energy metabolism for osmoregulation in fish. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2008, 148, 419–429. [Google Scholar] [CrossRef]
- Andersen, S.M.; Waagbø, R.; Espe, M. Functional amino acids in fish health and welfare. Front. Biosci. 2016, 8, 143–169. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Zheng, S.; Zheng, C.; Shi, Y.; Xie, X.; Wang, K.; Liu, H. The immune-related fatty acids are responsive to CO2 driven seawater acidification in a crustacean brine shrimp Artemia sinica. Dev. Comp. Immunol. 2018, 81, 342–347. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Yu, Z.; Xu, Y.; Zhang, Y.; Mu, C.; Liu, P.; Li, J. Integrated transcriptomic and metabolomic responses in the hepatopancreas of kuruma shrimp (Marsupenaeus japonicus) under cold stress. Ecotoxicol. Environ. Saf. 2020, 206, 111360. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Jayasundara, N.; Zhang, J.; Ren, X.; Gao, B.; Li, J.; Liu, P. Integrated physiological, transcriptome and metabolome analyses of the hepatopancreas of the female swimming crab Portunus trituberculatus under ammonia exposure. Ecotoxicol. Environ. Saf. 2021, 228, 113026. [Google Scholar] [CrossRef]
- Cheng, Y.; Zhao, J.; Ayisi, C.L.; Cao, X. Effects of salinity and alkalinity on fatty acids, free amino acids and related substance anabolic metabolism of Nile tilapia. Aquac. Fish. 2022, 7, 389–395. [Google Scholar] [CrossRef]
- Huang, M.; Dong, Y.; Zhang, Y.; Chen, Q.; Xie, J.; Xu, C.; Zhao, Q.; Li, E. Growth and lipidomic responses of juvenile pacific white shrimp Litopenaeus vannamei to low salinity. Front. Physiol. 2019, 10, 1087. [Google Scholar] [CrossRef]
- Song, L.; Zhao, Y.; Song, Y.; Zhao, L.; Ma, C.; Zhao, J. Effects of saline-alkaline water on growth performance, nutritional processing, and immunity in Nile tilapia (Oreochromis niloticus). Aquaculture 2021, 544, 737036. [Google Scholar] [CrossRef]
- Wang, W.; Pang, J.; Zhang, F.; Sun, L.; Yang, L.; Siddique, K.H. Transcriptomic and metabolomics-based analysis of key biological pathways reveals the role of lipid metabolism in response to salt stress in the root system of Brassica napus. Plant Growth Regul. 2022, 97, 127–141. [Google Scholar] [CrossRef]
- Yao, H.; Li, X.; Tang, L.; Wang, H.; Wang, C.; Mu, C.; Shi, C. Metabolic mechanism of the mud crab (Scylla paramamosain) adapting to salinity sudden drop based on GC-MS technology. Aquac. Rep. 2020, 18, 100533. [Google Scholar] [CrossRef]
- Shi, H.; Li, J.; Li, X.; Ru, X.; Huang, Y.; Zhu, C.; Li, G. Survival pressure and tolerance of juvenile greater amberjack (Seriola dumerili) under acute hypo-and hyper-salinity stress. Aquac. Rep. 2024, 36, 102150. [Google Scholar] [CrossRef]
- Mansour, M.M.F.; Salama, K.H.; Allam, H.Y. Role of the plasma membrane in saline conditions: Lipids and proteins. Bot. Rev. 2015, 81, 416–451. [Google Scholar] [CrossRef]
- Kurniawan, J.; Suga, K.; Kuhl, T.L. Interaction forces and membrane charge tunability: Oleic acid containing membranes in different pH conditions. BBA-Biomembr. 2017, 1859, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Zhou, Y.; Ge, J.; Agustsson, T.; Li, L.; Gao, Q.; Dong, S. Fatty acid composition and digestive enzyme activities of rainbow trout in response to dietary docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA) during salinity acclimation. J. Ocean Univ. 2020, 19, 1430–1440. [Google Scholar] [CrossRef]
- Cui, W.; Ma, A.; Farhadi, A.; Saqib, H.S.A.; Liu, S.; Chen, H.; Ma, H. How myo-inositol improves the physiological functions of aquatic animals: A review. Aquaculture 2022, 553, 738118. [Google Scholar] [CrossRef]
- Gardell, A.M.; Yang, J.; Sacchi, R.; Fangue, N.A.; Hammock, B.D.; Kültz, D. Tilapia (Oreochromis mossambicus) brain cells respond to hyperosmotic challenge by inducing myo-inositol biosynthesis. J. Exp. Biol. 2013, 216, 4615–4625. [Google Scholar]
- Ma, A.; Cui, W.; Wang, X.; Zhang, W.; Liu, Z.; Zhang, J.; Zhao, T. Osmoregulation by the myo-inositol biosynthesis pathway in turbot Scophthalmus maximus and its regulation by anabolite and c-Myc. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2020, 242, 110636. [Google Scholar] [CrossRef]
- Harpaz, S. L-carnitine and its attributed functions in fish culture and nutrition—A review. Aquaculture 2005, 249, 3–21. [Google Scholar] [CrossRef]
- Su, H.; Ma, D.; Fan, J.; Zhong, Z.; Li, Y.; Zhu, H. Metabolism response mechanism in the gill of Oreochromis mossambicus under salinity, alkalinity and saline-alkalinity stresses. Ecotoxicol. Environ. Saf. 2023, 251, 114523. [Google Scholar] [CrossRef]


| Parameters | SC | S4 | S8 |
|---|---|---|---|
| IBW (g) | 103.08 ± 0.70 | 105.17 ± 1.71 | 102.31 ± 0.91 |
| FBW (g) | 261.55 ± 18.06 a | 248.34 ± 15.65 a | 133.73 ± 9.91 b |
| SGR (%/d) | 1.51 ± 0.11 a | 1.39 ± 0.11 a | 0.49 ± 0.11 b |
| WGR (%) | 139.92 ± 11.80 a | 145.40 ± 12.11 a | 30.76 ± 5.48 b |
| SR (%) | 100 | 100 | 97.22 |
| FCR | 1.54 ± 0.25 b | 1.70 ± 0.36 b | 5.81 ± 1.90 a |
| Tendency | Metabolites | Metabolite Class |
|---|---|---|
| Upregulation | 1,2-dioleoyl-sn-glycero-3-phosphate | Lipids and lipid-like molecules |
| Eicosapentaenoic Acid | Lipids and lipid-like molecules | |
| Taurocholate | Lipids and lipid-like molecules | |
| Glycocholic acid | Lipids and lipid-like molecules | |
| Octadecanoic acid | Lipids and lipid-like molecules | |
| Oleic acid | Lipids and lipid-like molecules | |
| 1-stearoyl-2-hydroxy-sn-glycero-3-phosphocholine | Lipids and lipid-like molecules | |
| 9-hydroxy-10,12-octadecadienoic acid | Lipids and lipid-like molecules | |
| Eicosenoic acid | Lipids and lipid-like molecules | |
| Lpc 18:1 | Lipids and lipid-like molecules | |
| Downregulation | N-methyl-l-glutamic acid | Organic acids and derivatives |
| Ketoleucine | Organic acids and derivatives | |
| L-Lysine | Organic acids and derivatives | |
| Pantothenate | Organic acids and derivatives | |
| D-glutamine | Organic acids and derivatives | |
| Tyr-Ser | Organic acids and derivatives | |
| Myo-inositol | Organic oxygen compounds | |
| Glycerol | Organic oxygen compounds | |
| Kynurenine | Organic oxygen compounds | |
| Pantothenic acid | Organic oxygen compounds |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, X.; Li, B.; Hou, Y.; Wei, K.; Zhou, L.; Zhang, C.; Zhang, L.; Zhu, J.; Jia, R. Physiological Responses and Serum Metabolite Alterations in Grass Carp (Ctenopharyngodon idellus) Under Chronic Salinity Exposure. Antioxidants 2025, 14, 1287. https://doi.org/10.3390/antiox14111287
Chen X, Li B, Hou Y, Wei K, Zhou L, Zhang C, Zhang L, Zhu J, Jia R. Physiological Responses and Serum Metabolite Alterations in Grass Carp (Ctenopharyngodon idellus) Under Chronic Salinity Exposure. Antioxidants. 2025; 14(11):1287. https://doi.org/10.3390/antiox14111287
Chicago/Turabian StyleChen, Xiajie, Bing Li, Yiran Hou, Kepeng Wei, Linjun Zhou, Chengfeng Zhang, Liqiang Zhang, Jian Zhu, and Rui Jia. 2025. "Physiological Responses and Serum Metabolite Alterations in Grass Carp (Ctenopharyngodon idellus) Under Chronic Salinity Exposure" Antioxidants 14, no. 11: 1287. https://doi.org/10.3390/antiox14111287
APA StyleChen, X., Li, B., Hou, Y., Wei, K., Zhou, L., Zhang, C., Zhang, L., Zhu, J., & Jia, R. (2025). Physiological Responses and Serum Metabolite Alterations in Grass Carp (Ctenopharyngodon idellus) Under Chronic Salinity Exposure. Antioxidants, 14(11), 1287. https://doi.org/10.3390/antiox14111287







