Nrf2 Activated by PD-MSCs Attenuates Oxidative Stress in a Hydrogen Peroxide-Injured Retinal Pigment Epithelial Cell Line
Abstract
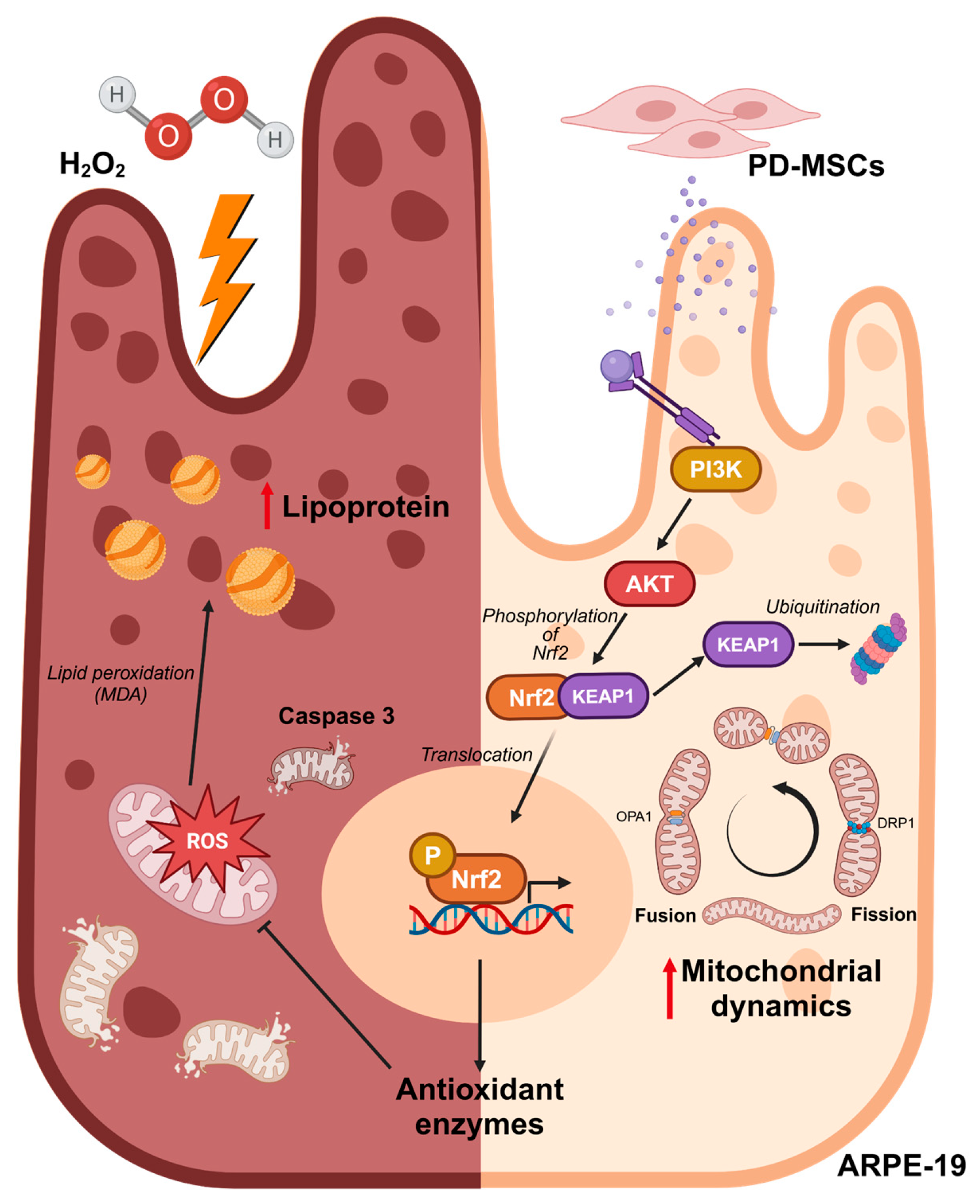
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. In Vitro Coculture System
2.3. RNA Isolation and Quantitative Real-Time Polymerase Chain Reaction
2.4. Protein Isolation and Western Blot
2.5. Enzyme-Linked Immunosorbent Assay (ELISA)
2.6. Immunofluorescence Staining
2.7. MitoSOX/MitoTracker
2.8. JC-1 Staining
2.9. TBARS Assay
2.10. BODIPY Staining
2.11. Statistical Analysis
3. Results
3.1. PD-MSC Cocultivation Decreased Lipid Accumulation in ARPE-19 Cells Exposed to H2O2
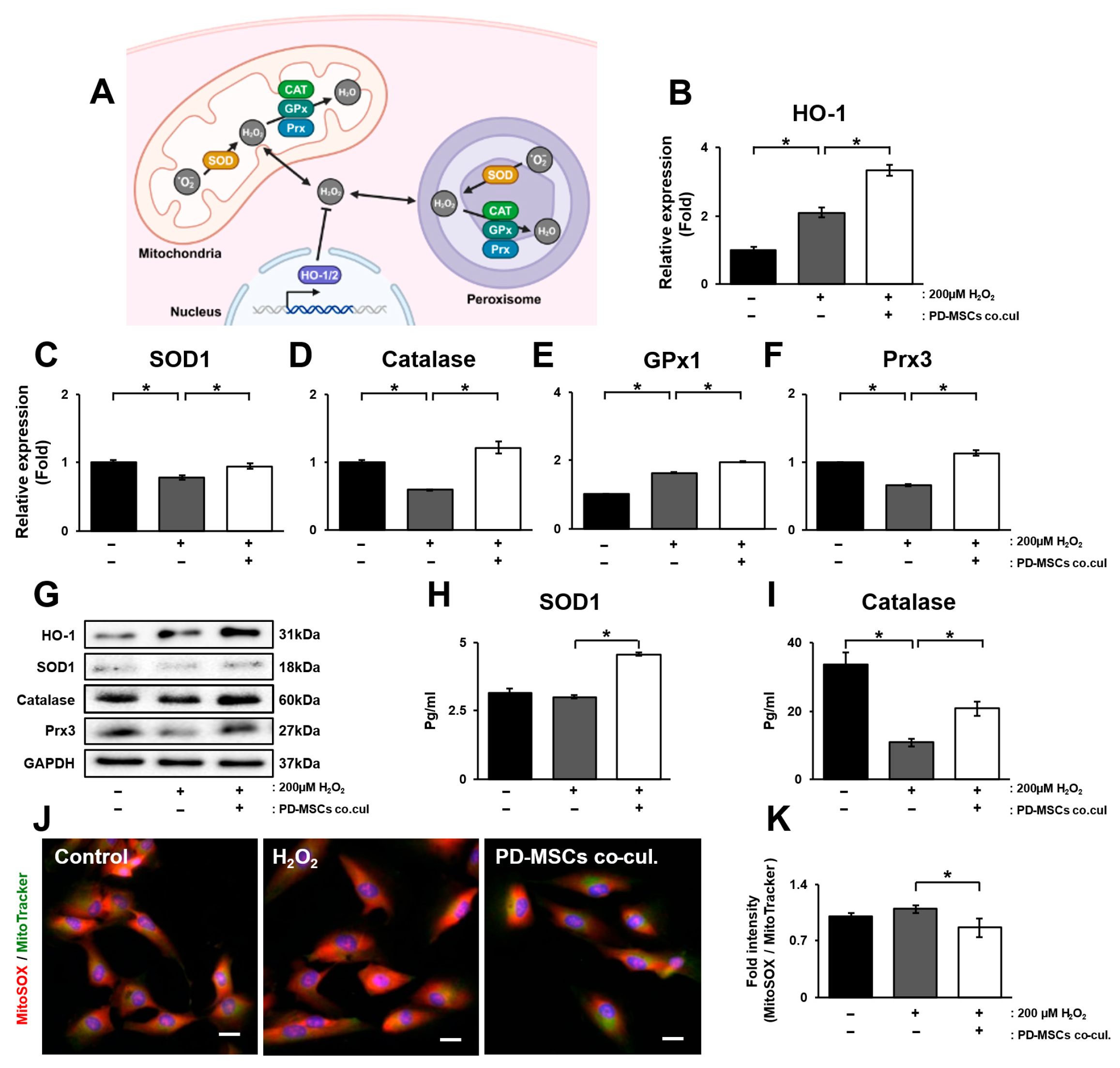
3.2. PD-MSC Cocultivation Ameliorates Oxidative Stress by Enhancing the Accumulation of Antioxidants in ARPE-19 Cells Exposed to H2O2
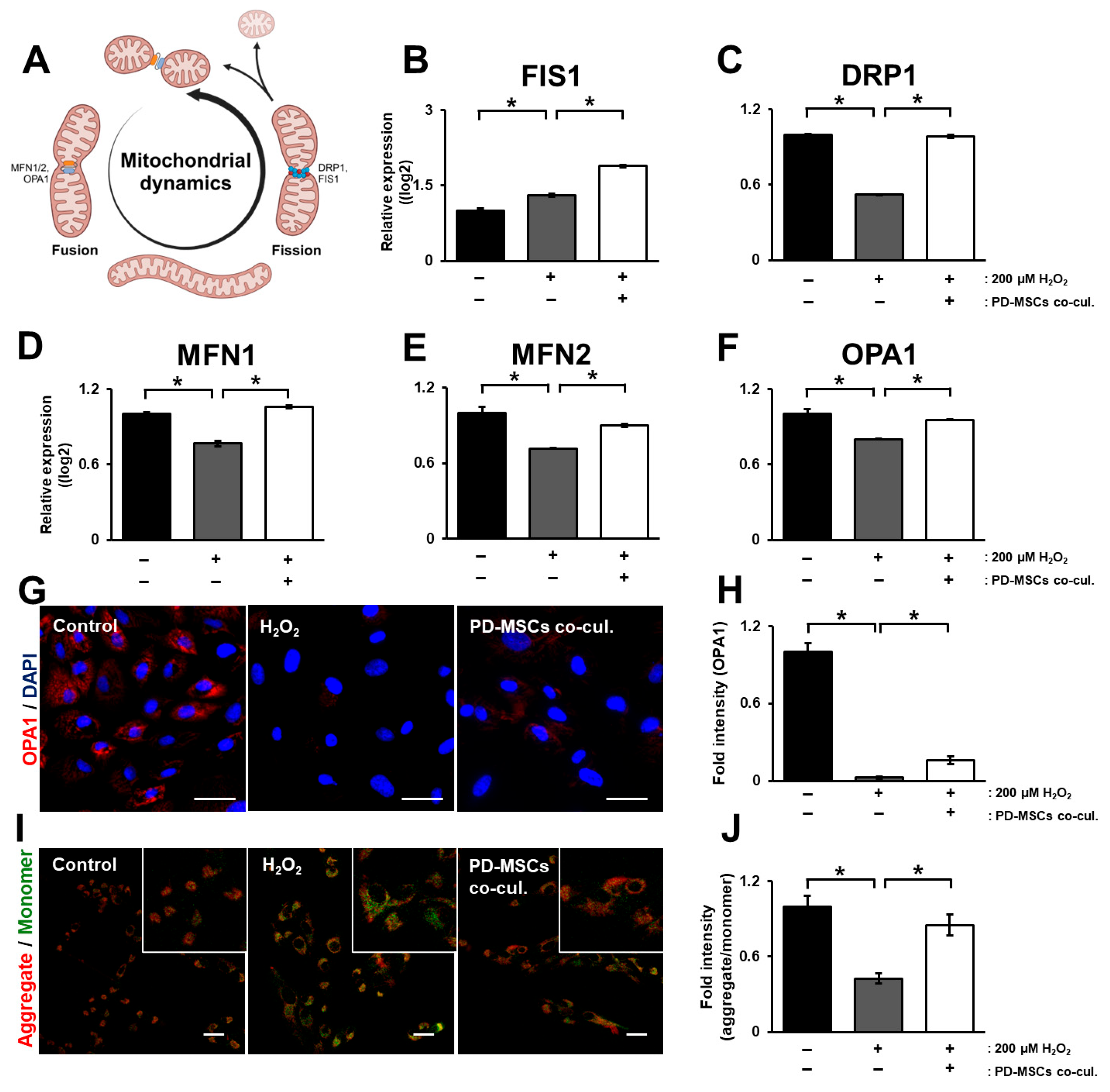
3.3. PD-MSC Cocultivation Enhances Mitochondrial Membrane Potential by Modulating Mitochondrial Dynamics in ARPE-19 Cells Exposed to H2O2
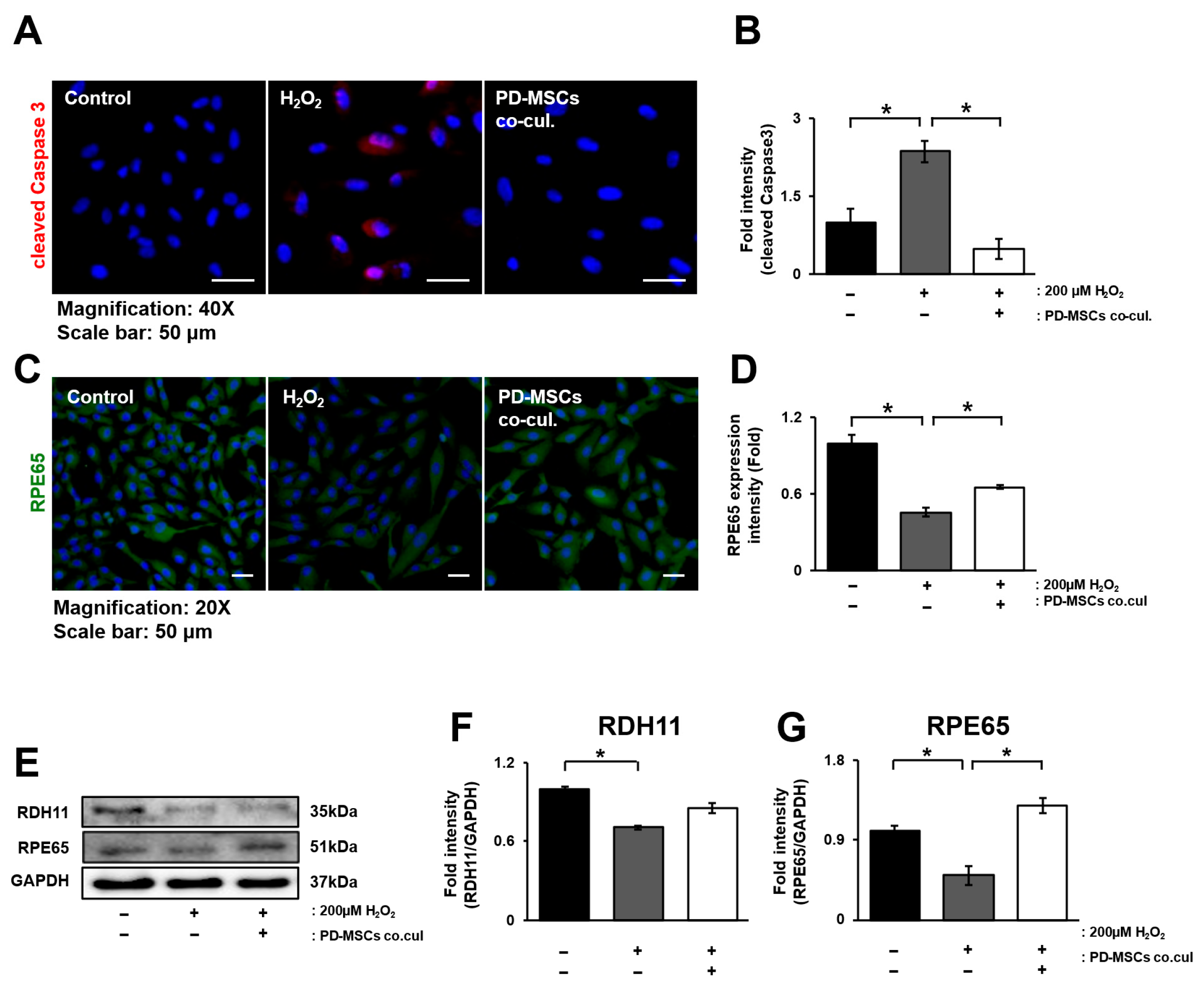
3.4. PD-MSC Cocultivation Improves the Visual Cycle in ARPE-19 Cells Exposed to H2O2
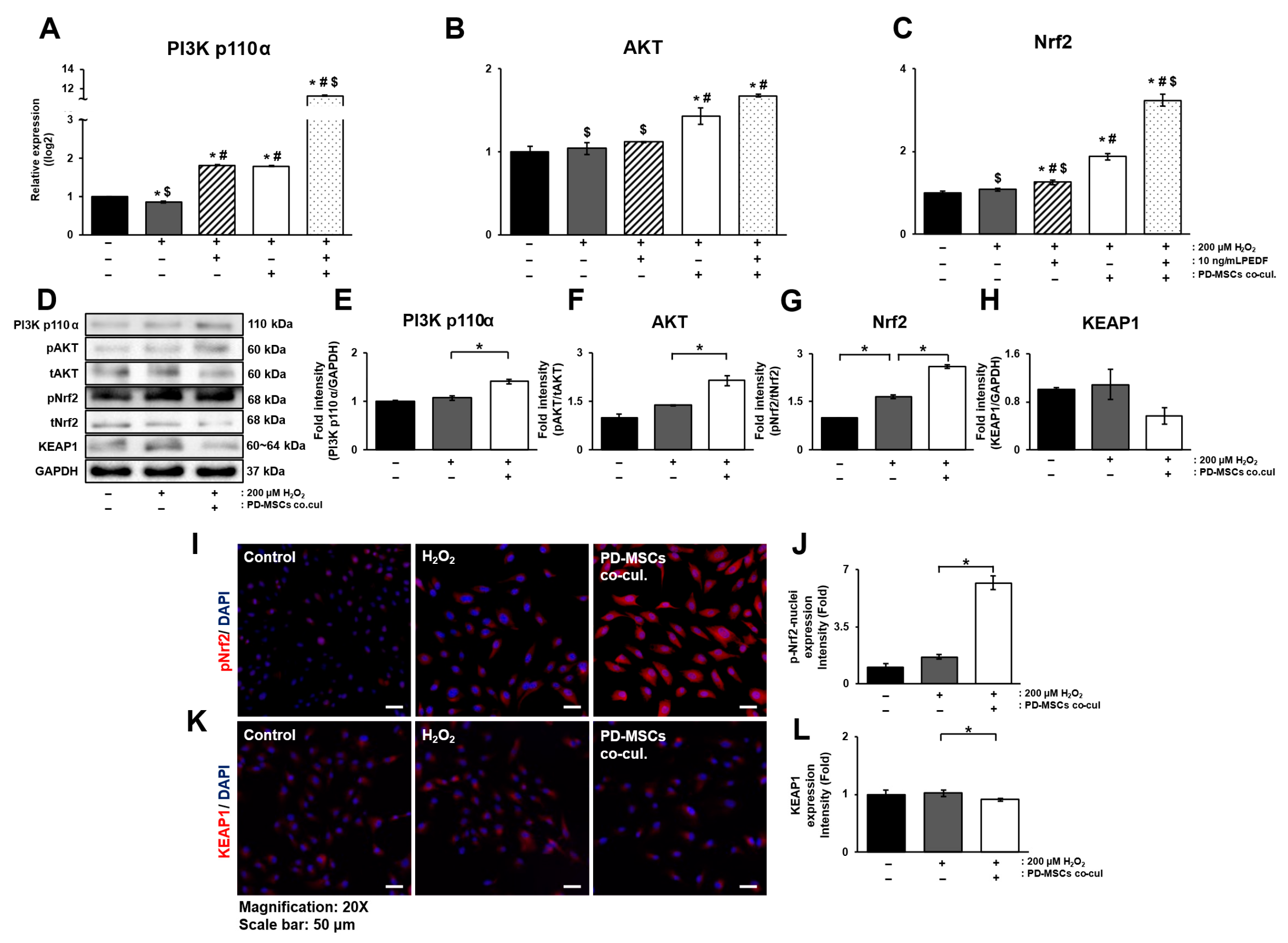
3.5. PD-MSC Cocultivation Activates the Nrf2 Signaling Pathway in ARPE-19 Cells Exposed to H2O2
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AMD | Age-related macular degeneration |
| PD-MSCs | Placenta-derived mesenchymal stem cells |
| RPE | Retinal pigment epithelium |
References
- Chen, Y.; Jiang, F.; Zeng, Y.; Zhang, M. The role of retinal pigment epithelial senescence and the potential of senotherapeutics in age-related macular degeneration. Surv. Ophthalmol. 2025, 70, 942–950. [Google Scholar] [CrossRef]
- Wang, J.; Li, M.; Geng, Z.; Khattak, S.; Ji, X.; Wu, D.; Dang, Y. Role of Oxidative Stress in Retinal Disease and the Early Intervention Strategies: A Review. Oxid. Med. Cell. Longev. 2022, 2022, 7836828. [Google Scholar] [CrossRef]
- Chiang, B.; Chung, Y.G.; Prausnitz, M.R. Suprachoroidal drug delivery for VEGF suppression in wet AMD and other diseases with choroidal neovascularization. Am. J. Ophthalmol. 2025, 277, 556–569. [Google Scholar] [CrossRef]
- Nissen, A.H.K.; Torp, T.L.; Vergmann, A.S. Clinical Outcomes of Treatment of Geographic Atrophy: A Narrative Review. Ophthalmol. Ther. 2025, 14, 1173–1181. [Google Scholar] [CrossRef]
- Ruan, Y.; Jiang, S.; Gericke, A. Age-Related Macular Degeneration: Role of Oxidative Stress and Blood Vessels. Int. J. Mol. Sci. 2021, 22, 1296. [Google Scholar] [CrossRef]
- Hyttinen, J.M.T.; Koskela, A.; Blasiak, J.; Kaarniranta, K. Autophagy in drusen biogenesis secondary to age-related macular degeneration. Acta Ophthalmol. 2024, 102, 759–772. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, J.; Liang, Y.; Huang, J.; Fu, Y.; Chen, N.; Lu, B.; Zhao, C. Clearance of lipid droplets by chimeric autophagy-tethering compound ameliorates the age-related macular degeneration phenotype in mice lacking APOE. Autophagy 2023, 19, 2668–2681. [Google Scholar] [CrossRef]
- Qu, S.; Lin, H.; Pfeiffer, N.; Grus, F.H. Age-Related Macular Degeneration and Mitochondria-Associated Autoantibodies: A Review of the Specific Pathogenesis and Therapeutic Strategies. Int. J. Mol. Sci. 2024, 25, 1624. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.M.; Fan, B.; Li, Y.L.; Zuo, Z.Y.; Li, G.Y. Oxidative Stress-Involved Mitophagy of Retinal Pigment Epithelium and Retinal Degenerative Diseases. Cell Mol. Neurobiol. 2023, 43, 3265–3276. [Google Scholar] [CrossRef]
- La Cunza, N.; Tan, L.X.; Thamban, T.; Germer, C.J.; Rathnasamy, G.; Toops, K.A.; Lakkaraju, A. Mitochondria-dependent phase separation of disease-relevant proteins drives pathological features of age-related macular degeneration. JCI Insight 2021, 6, e142254. [Google Scholar] [CrossRef] [PubMed]
- Si, Z.; Zheng, Y.; Zhao, J. The Role of Retinal Pigment Epithelial Cells in Age-Related Macular Degeneration: Phagocytosis and Autophagy. Biomolecules 2023, 13, 901. [Google Scholar] [CrossRef]
- Bryan, H.K.; Olayanju, A.; Goldring, C.E.; Park, B.K. The Nrf2 cell defence pathway: Keap1-dependent and -independent mechanisms of regulation. Biochem. Pharmacol. 2013, 85, 705–717. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, T.; Zeng, S.; Zhang, X.; Zhou, F.; Gillies, M.C.; Zhu, L. The Role of Nrf2/sMAF Signalling in Retina Ageing and Retinal Diseases. Biomedicines 2023, 11, 1512. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.Y.; Chang, S.F.; Chau, S.F.; Chiu, S.C. The Protective Effect of Hispidin against Hydrogen Peroxide-Induced Oxidative Stress in ARPE-19 Cells via Nrf2 Signaling Pathway. Biomolecules 2019, 9, 380. [Google Scholar] [CrossRef] [PubMed]
- Bellezza, I. Oxidative Stress in Age-Related Macular Degeneration: Nrf2 as Therapeutic Target. Front. Pharmacol. 2018, 9, 1280. [Google Scholar] [CrossRef] [PubMed]
- Kushwah, N.; Bora, K.; Maurya, M.; Pavlovich, M.C.; Chen, J. Oxidative Stress and Antioxidants in Age-Related Macular Degeneration. Antioxidants 2023, 12, 1379. [Google Scholar] [CrossRef]
- Corydon, T.J.; Bek, T. Multiple gene therapy as a tool for regulating the expression of molecules involved in neovascular age-related macular degeneration. Prog. Retin. Eye Res. 2025, 104, 101323. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, S.; Gao, S.; Li, N.; Huang, H.; Liu, X.; Yao, H.; Shen, X. Pigment epithelium-derived factor exerts neuroprotection in oxygen-induced retinopathy by targeting endoplasmic reticulum stress and oxidative stress. Exp. Eye Res. 2024, 249, 110147. [Google Scholar] [CrossRef]
- Tombran-Tink, J.; Barnstable, C.J. PEDF: A multifaceted neurotrophic factor. Nat. Rev. Neurosci. 2003, 4, 628–636. [Google Scholar] [CrossRef]
- Kim, J.Y.; Park, S.; Park, H.J.; Kim, S.H.; Lew, H.; Kim, G.J. PEDF-Mediated Mitophagy Triggers the Visual Cycle by Enhancing Mitochondrial Functions in a H2O2-Injured Rat Model. Cells 2021, 10, 1117. [Google Scholar] [CrossRef]
- Kim, M.J.; Shin, K.S.; Jeon, J.H.; Lee, D.R.; Shim, S.H.; Kim, J.K.; Cha, D.H.; Yoon, T.K.; Kim, G.J. Human chorionic-plate-derived mesenchymal stem cells and Wharton’s jelly-derived mesenchymal stem cells: A comparative analysis of their potential as placenta-derived stem cells. Cell Tissue Res. 2011, 346, 53–64. [Google Scholar] [CrossRef]
- Lyssenko, N.N.; Haider, N.; Picataggi, A.; Cipollari, E.; Jiao, W.; Phillips, M.C.; Rader, D.J.; Chavali, V.R.M. Directional ABCA1-mediated cholesterol efflux and apoB-lipoprotein secretion in the retinal pigment epithelium. J. Lipid Res. 2018, 59, 1927–1939. [Google Scholar] [CrossRef] [PubMed]
- Tilokani, L.; Nagashima, S.; Paupe, V.; Prudent, J. Mitochondrial dynamics: Overview of molecular mechanisms. Essays Biochem. 2018, 62, 341–360. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; Li, X.; Wood, J.W.; Ma, J.X. Mitochondrial dysfunctions, endothelial progenitor cells and diabetic retinopathy. J. Diabetes Complicat. 2018, 32, 966–973. [Google Scholar] [CrossRef] [PubMed]
- Tsin, A.; Betts-Obregon, B.; Grigsby, J. Visual cycle proteins: Structure, function, and roles in human retinal disease. J. Biol. Chem. 2018, 293, 13016–13021. [Google Scholar] [CrossRef]
- Kim, S.Y.; Kim, J.-H.; Kim, S.O.; Park, S.-K.; Jeong, J.-W.; Kim, M.-Y.; Lee, H.; Cheong, J.; Choi, Y.H. Ethanol Extract of Glycyrrhiza uralensis Protects Against Oxidative Stress-induced DNA Damage and Apoptosis in Retinal Pigment Epithelial Cells. J. Life Sci. 2019, 29, 1273–1280. [Google Scholar] [CrossRef]
- Kim, J.Y.; Park, S.; Park, S.H.; Lee, D.; Kim, G.H.; Noh, J.E.; Lee, K.J.; Kim, G.J. Overexpression of pigment epithelium-derived factor in placenta-derived mesenchymal stem cells promotes mitochondrial biogenesis in retinal cells. Lab. Investig. 2021, 101, 51–69. [Google Scholar] [CrossRef]
- Kaarniranta, K.; Pawlowska, E.; Szczepanska, J.; Jablkowska, A.; Blasiak, J. Role of Mitochondrial DNA Damage in ROS-Mediated Pathogenesis of Age-Related Macular Degeneration (AMD). Int. J. Mol. Sci. 2019, 20, 2374. [Google Scholar] [CrossRef]
- Pilat, A.; Herrnreiter, A.M.; Skumatz, C.M.; Sarna, T.; Burke, J.M. Oxidative stress increases HO-1 expression in ARPE-19 cells, but melanosomes suppress the increase when light is the stressor. Investig. Ophthalmol. Vis. Sci. 2013, 54, 47–56. [Google Scholar] [CrossRef][Green Version]
- Su, H.; Wang, Z.; Zhou, L.; Liu, D.; Zhang, N. Regulation of the Nrf2/HO-1 axis by mesenchymal stem cells-derived extracellular vesicles: Implications for disease treatment. Front. Cell Dev. Biol. 2024, 12, 1397954. [Google Scholar] [CrossRef]
- Shome, I.; Thathapudi, N.C.; Aramati, B.M.R.; Kowtharapu, B.S.; Jangamreddy, J.R. Stages, pathogenesis, clinical management and advancements in therapies of age-related macular degeneration. Int. Ophthalmol. 2023, 43, 3891–3909. [Google Scholar] [CrossRef]
- Fisher, C.R.; Shaaeli, A.A.; Ebeling, M.C.; Montezuma, S.R.; Ferrington, D.A. Investigating mitochondrial fission, fusion, and autophagy in retinal pigment epithelium from donors with age-related macular degeneration. Sci. Rep. 2022, 12, 21725. [Google Scholar] [CrossRef]
- Hartman, R.R.; Kompella, U.B. Intravitreal, Subretinal, and Suprachoroidal Injections: Evolution of Microneedles for Drug Delivery. J. Ocul. Pharmacol. Ther. 2018, 34, 141–153. [Google Scholar] [CrossRef]
- Kumaran, N.; Ripamonti, C.; Kalitzeos, A.; Rubin, G.S.; Bainbridge, J.W.B.; Michaelides, M. Severe Loss of Tritan Color Discrimination in RPE65 Associated Leber Congenital Amaurosis. Investig. Ophthalmol. Vis. Sci. 2018, 59, 85–93. [Google Scholar] [CrossRef]
- Shin, Y.; Moiseyev, G.; Petrukhin, K.; Cioffi, C.L.; Muthuraman, P.; Takahashi, Y.; Ma, J.X. A novel RPE65 inhibitor CU239 suppresses visual cycle and prevents retinal degeneration. Biochim. Biophys. Acta Mol. Basis Dis. 2018, 1864, 2420–2429. [Google Scholar] [CrossRef] [PubMed]
- Girgis, S.; Lee, L.R. Treatment of dry age-related macular degeneration: A review. Clin. Exp. Ophthalmol. 2023, 51, 835–852. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.M.; Jung, J.; Lee, H.J.; Jeong, S.J.; Cho, K.J.; Hwang, S.G.; Kim, G.J. Comparison of immunomodulatory effects of placenta mesenchymal stem cells with bone marrow and adipose mesenchymal stem cells. Int. Immunopharmacol. 2012, 13, 219–224. [Google Scholar] [CrossRef]
- Miotti, G.; Parodi, P.C.; Zeppieri, M. Stem cell therapy in ocular pathologies in the past 20 years. World J. Stem Cells 2021, 13, 366–385. [Google Scholar] [CrossRef] [PubMed]
- Salari, V.; Mengoni, F.; Del Gallo, F.; Bertini, G.; Fabene, P.F. The Anti-Inflammatory Properties of Mesenchymal Stem Cells in Epilepsy: Possible Treatments and Future Perspectives. Int. J. Mol. Sci. 2020, 21, 9683. [Google Scholar] [CrossRef]
- Tang, Y.; Kang, Y.; Zhang, X.; Cheng, C. Mesenchymal stem cell exosomes as nanotherapeutics for dry age-related macular degeneration. J. Control Release 2023, 357, 356–370. [Google Scholar] [CrossRef]
- Borkowska-Kuczkowska, A.; Sługocka, D.; Świątkowska-Flis, B.; Boruczkowski, D. The use of mesenchymal stem cells for the treatment of progressive retinal diseases: A review. Regen Med. 2019, 14, 321–329. [Google Scholar] [CrossRef] [PubMed]
- de Laorden, E.H.; Simon, D.; Milla, S.; Portela-Lomba, M.; Mellen, M.; Sierra, J.; de la Villa, P.; Moreno-Flores, M.T.; Iglesias, M. Human placenta-derived mesenchymal stem cells stimulate neuronal regeneration by promoting axon growth and restoring neuronal activity. Front. Cell Dev. Biol. 2023, 11, 1328261. [Google Scholar] [CrossRef]
- Sung, Y.; Lee, S.M.; Park, M.; Choi, H.J.; Kang, S.; Choi, B.I.; Lew, H. Treatment of traumatic optic neuropathy using human placenta-derived mesenchymal stem cells in Asian patients. Regen. Med. 2020, 15, 2163–2179. [Google Scholar] [CrossRef] [PubMed]
- Fan, R.; Su, L.; Zhang, H.; Jiang, Y.; Yu, Z.; Zhang, X.; Li, X. Enhanced therapeutic effect of PEDF-loaded mesenchymal stem cell-derived small extracellular vesicles against oxygen-induced retinopathy through increased stability and penetrability of PEDF. J. Nanobiotechnol. 2023, 21, 327. [Google Scholar] [CrossRef]
- Iloki-Assanga, S.B.; Lewis-Lujan, L.M.; Fernandez-Angulo, D.; Gil-Salido, A.A.; Lara-Espinoza, C.L.; Rubio-Pino, J.L. Retino-protective effect of Bucida buceras against oxidative stress induced by H2O2 in human retinal pigment epithelial cells line. BMC Complement. Altern. Med. 2015, 15, 254. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hong, S.J.; Lee, D.-H.; Choi, J.W.; Lee, H.; Sung, Y.; Kim, G.J. Nrf2 Activated by PD-MSCs Attenuates Oxidative Stress in a Hydrogen Peroxide-Injured Retinal Pigment Epithelial Cell Line. Antioxidants 2025, 14, 1279. https://doi.org/10.3390/antiox14111279
Hong SJ, Lee D-H, Choi JW, Lee H, Sung Y, Kim GJ. Nrf2 Activated by PD-MSCs Attenuates Oxidative Stress in a Hydrogen Peroxide-Injured Retinal Pigment Epithelial Cell Line. Antioxidants. 2025; 14(11):1279. https://doi.org/10.3390/antiox14111279
Chicago/Turabian StyleHong, Se Jin, Dae-Hyun Lee, Jeong Woo Choi, Hankyu Lee, Youngje Sung, and Gi Jin Kim. 2025. "Nrf2 Activated by PD-MSCs Attenuates Oxidative Stress in a Hydrogen Peroxide-Injured Retinal Pigment Epithelial Cell Line" Antioxidants 14, no. 11: 1279. https://doi.org/10.3390/antiox14111279
APA StyleHong, S. J., Lee, D.-H., Choi, J. W., Lee, H., Sung, Y., & Kim, G. J. (2025). Nrf2 Activated by PD-MSCs Attenuates Oxidative Stress in a Hydrogen Peroxide-Injured Retinal Pigment Epithelial Cell Line. Antioxidants, 14(11), 1279. https://doi.org/10.3390/antiox14111279







