Vitamin B12 Protects the Exacerbated Ischemia–Reperfusion Injury-Induced Chronic Kidney Disease in Mice with Genetically Increased Elmo1
Abstract
1. Introduction
2. Methods
3. Results
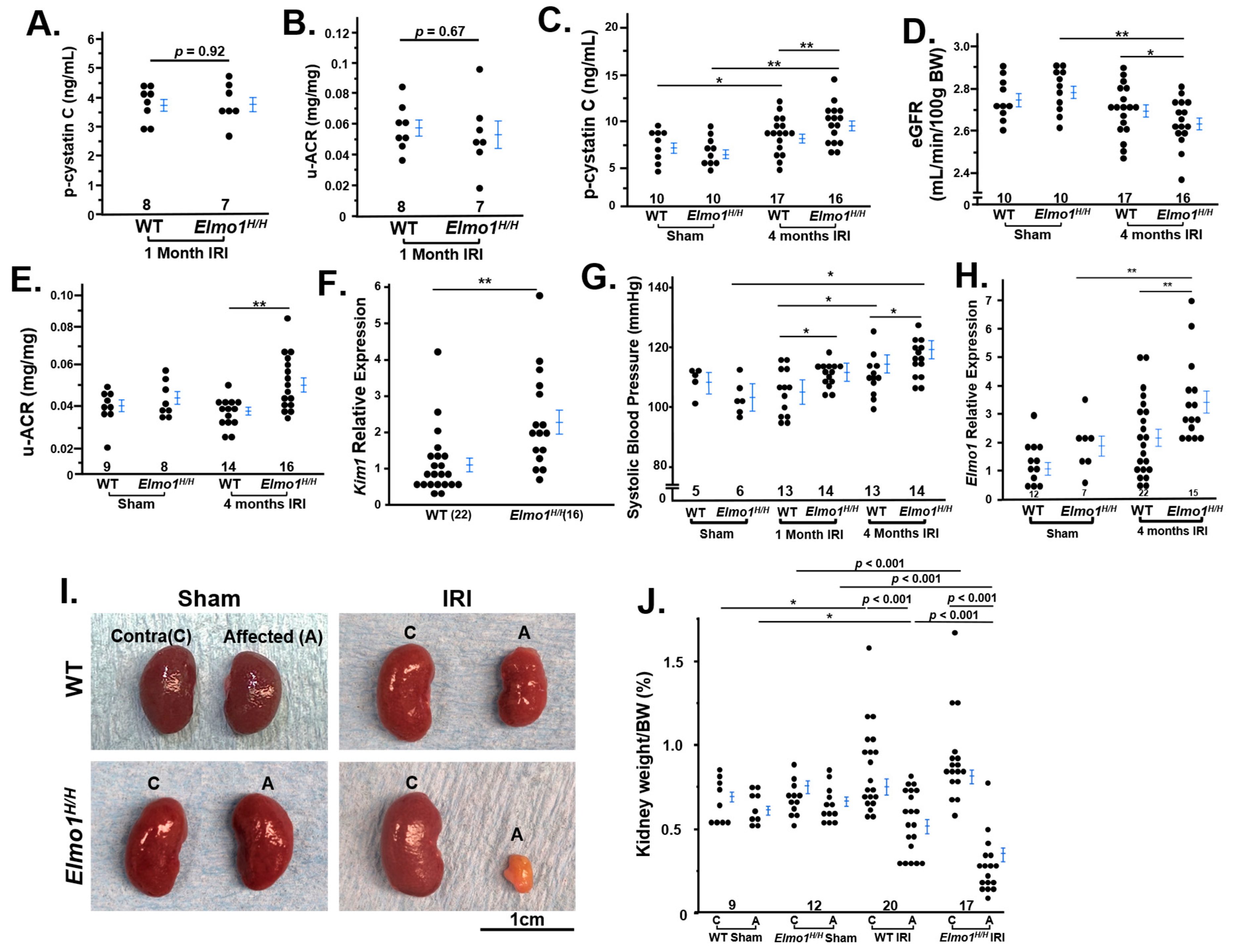
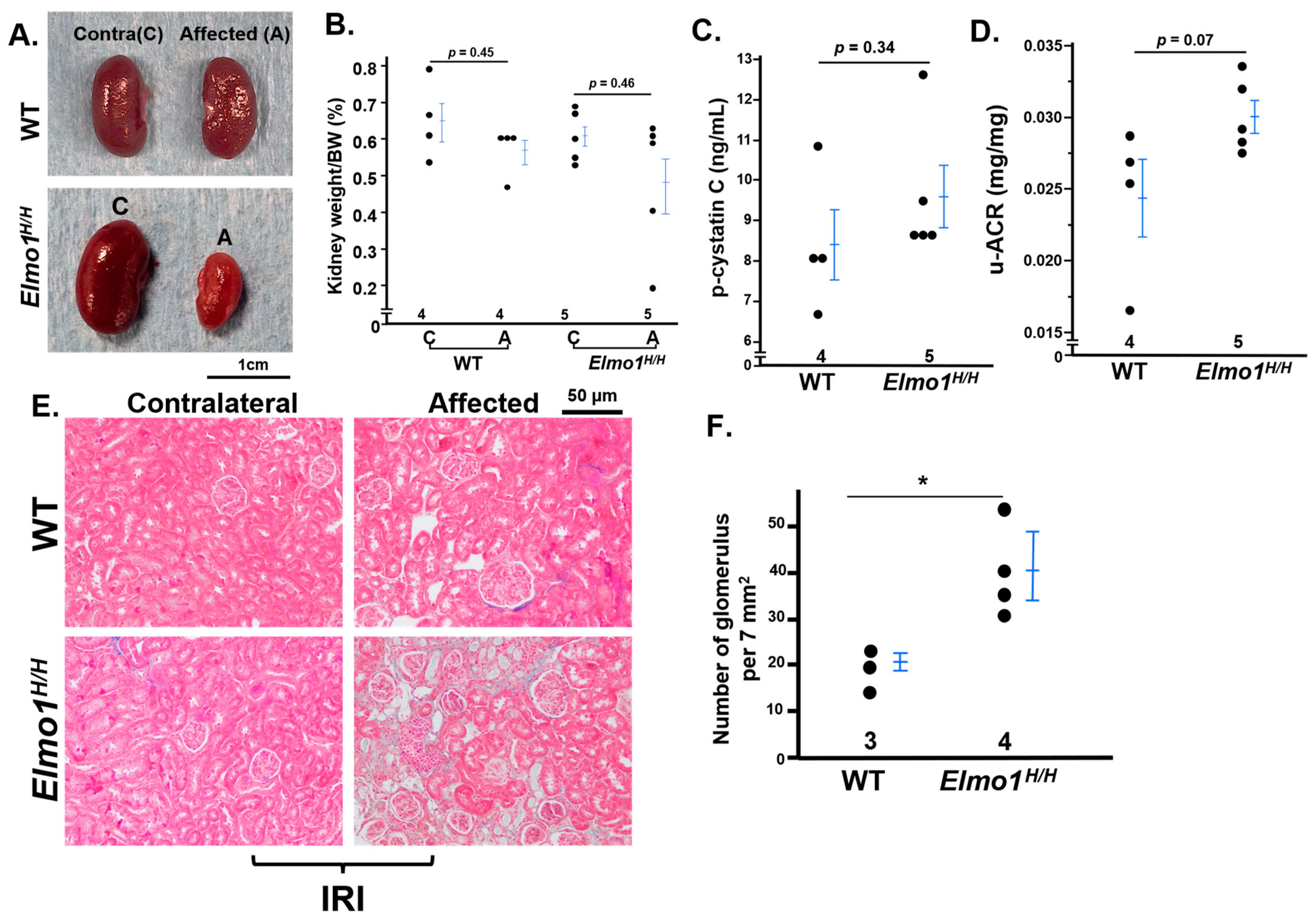
3.1. Elmo1H/H Mice Had Severely Compromised Kidney Function than WT Counterparts Four Months After IRI
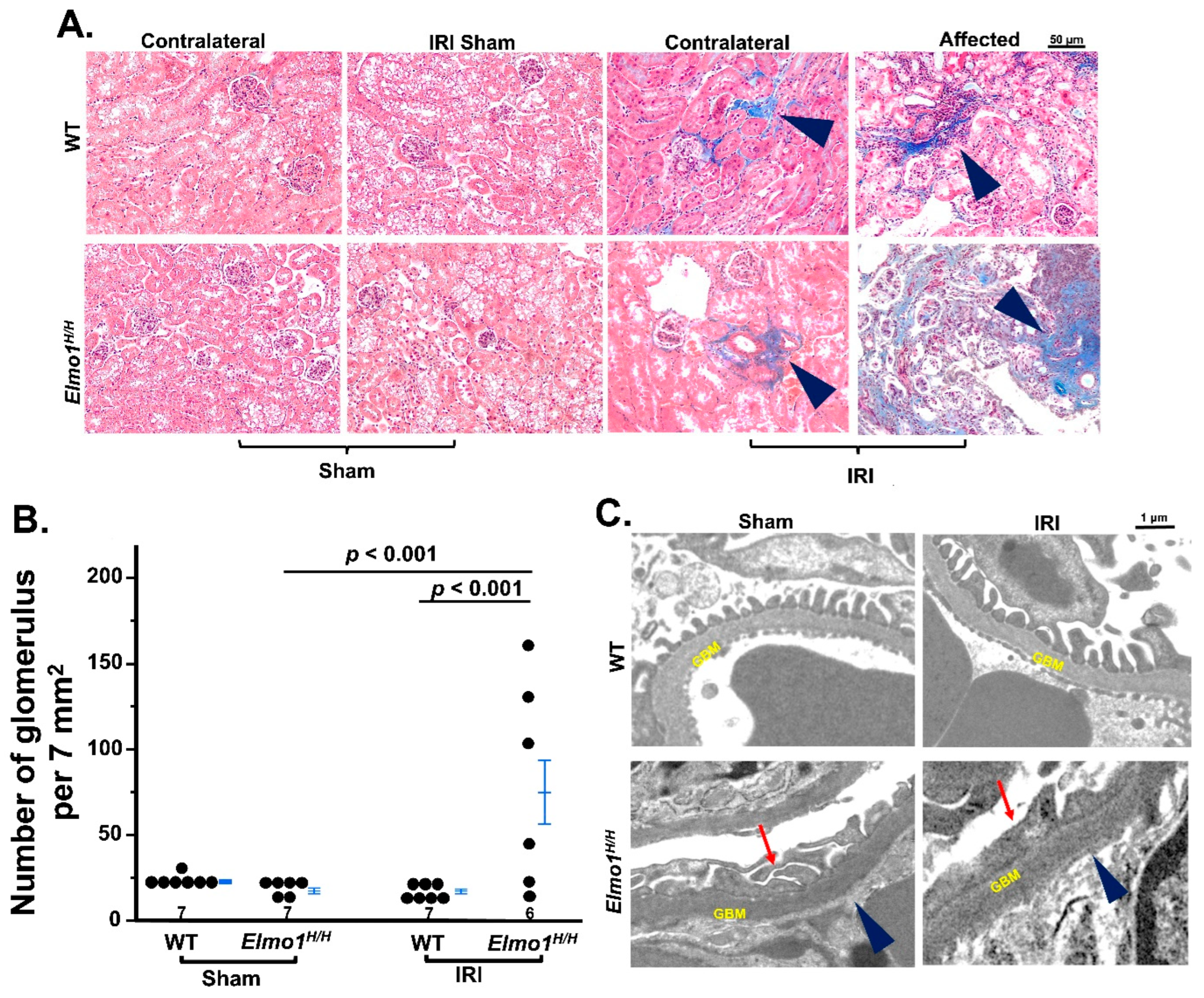
3.2. Elmo1H/H Mice Had More Severe CKD-like Morphological Changes, Especially in the Affected Kidneys
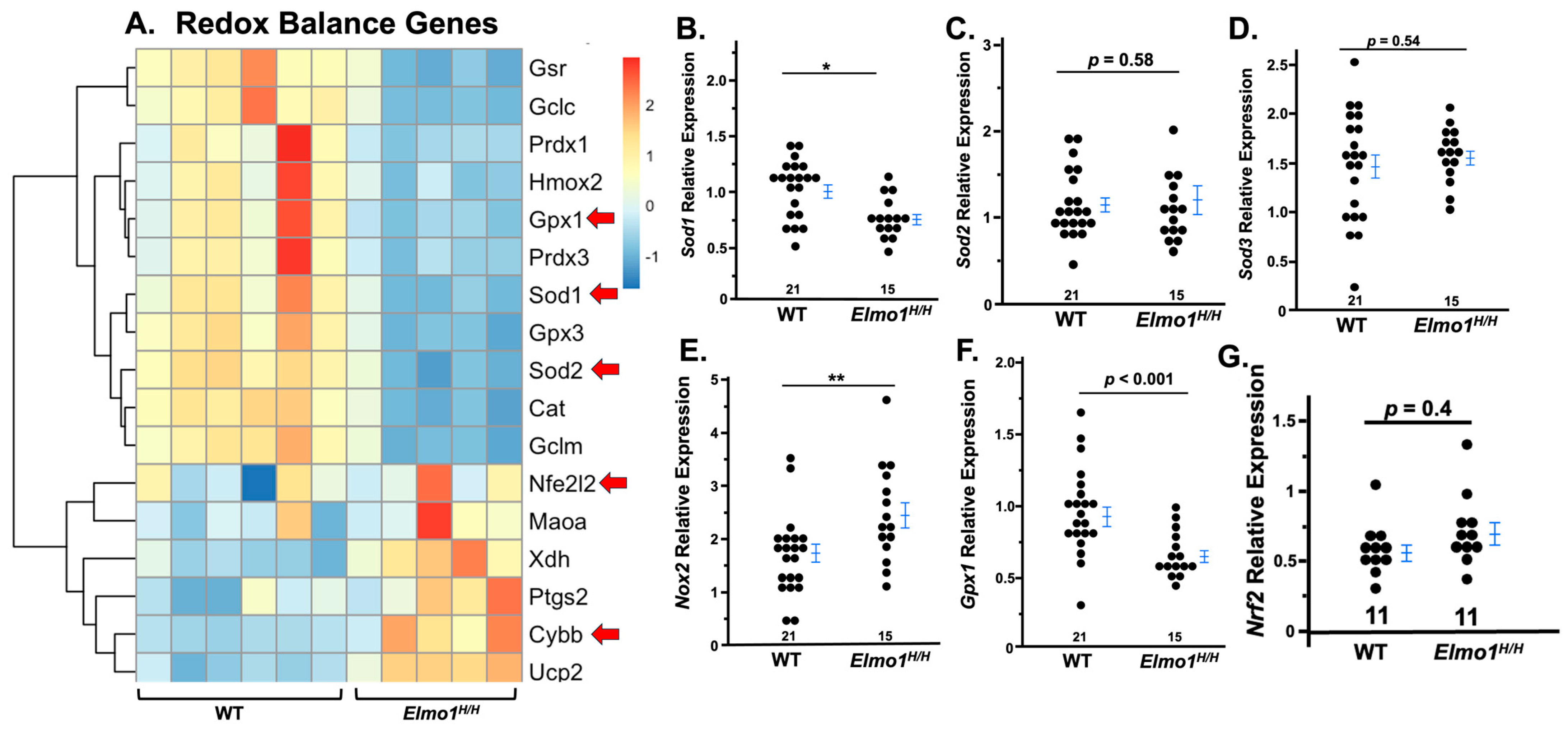
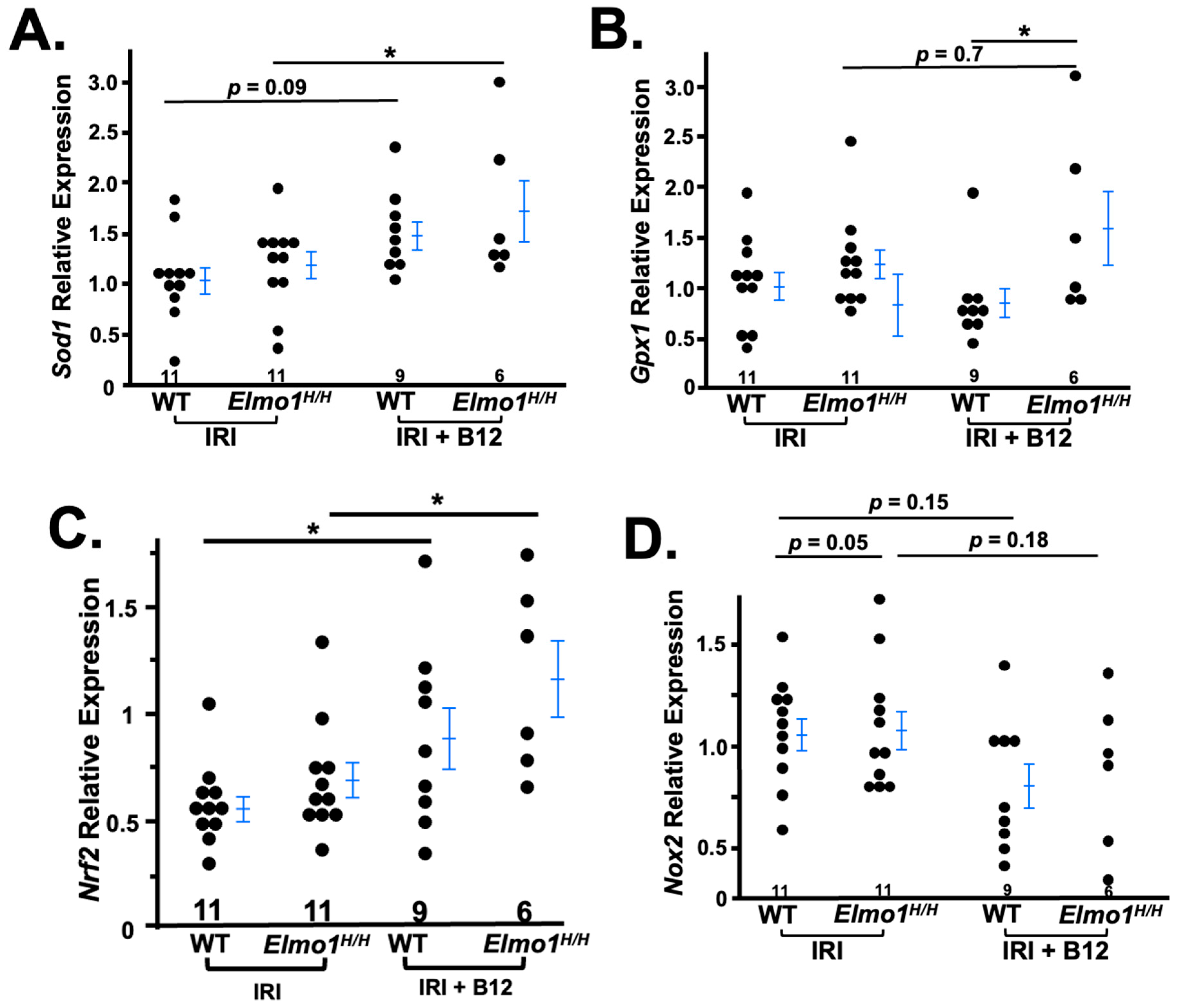
3.3. Elmo1H/H Mouse Kidneys Showed Redox Imbalance Four Months After IR Surgery
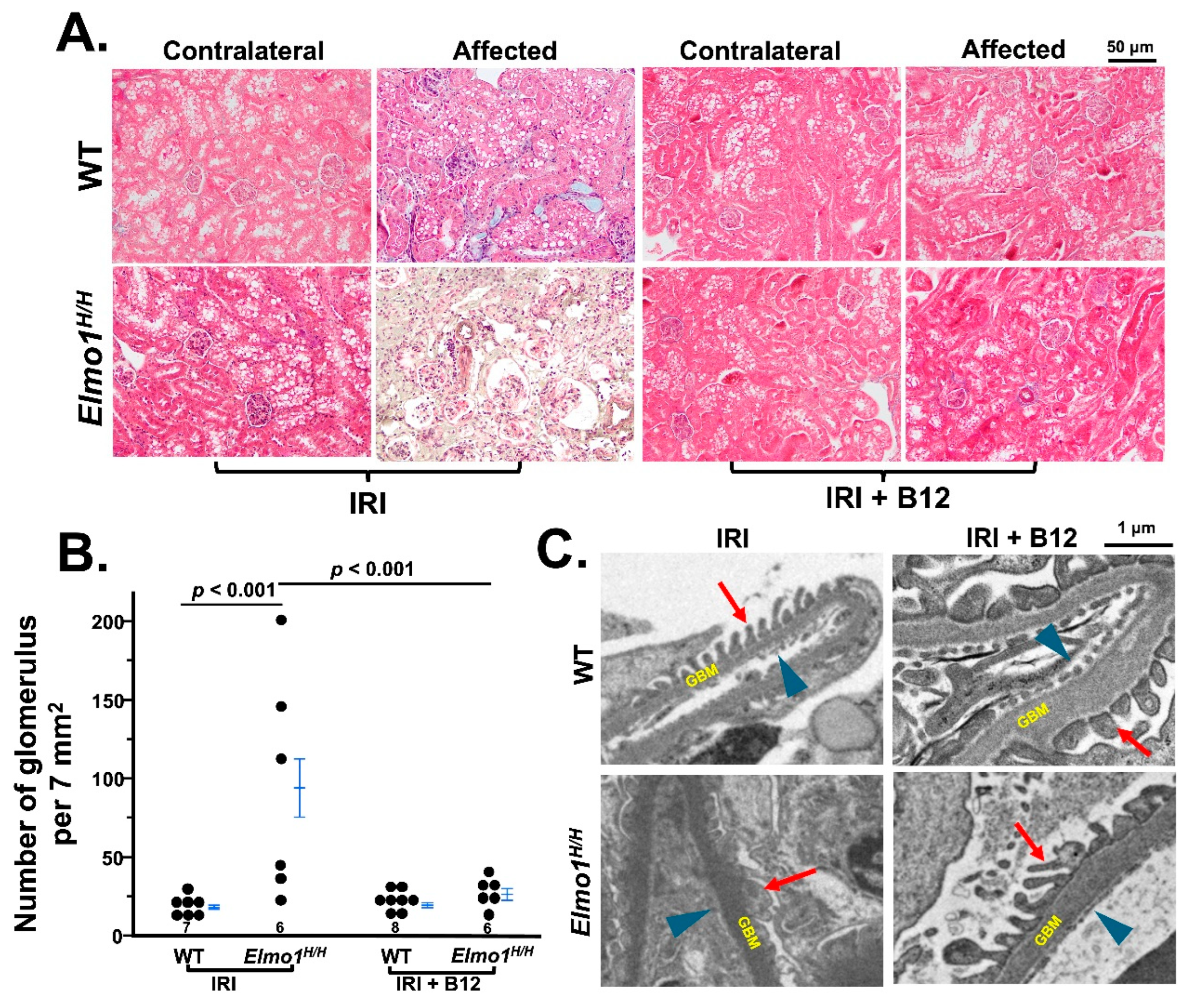
3.4. Vitamin B12 (B12) Markedly Improved the Kidney Function and Structure Four Months After IR Surgery
3.5. Female Mice Developed Less Severe CKD-like Phenotypes After IRI than Males Four Months After IRI, While Effects of High Elmo1 Persisted
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kovesdy, C.P. Epidemiology of chronic kidney disease: An update 2022. Kidney Int. Suppl. 2022, 12, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Jankowski, J.; Floege, J.; Fliser, D.; Bohm, M.; Marx, N. Cardiovascular Disease in Chronic Kidney Disease: Pathophysiological Insights and Therapeutic Options. Circulation 2021, 143, 1157–1172. [Google Scholar] [CrossRef]
- Harwood, R.; Bridge, J.; Ressel, L.; Scarfe, L.; Sharkey, J.; Czanner, G.; Kalra, P.A.; Odudu, A.; Kenny, S.; Wilm, B.; et al. Murine models of renal ischemia reperfusion injury: An opportunity for refinement using noninvasive monitoring methods. Physiol. Rep. 2022, 10, e15211. [Google Scholar] [CrossRef] [PubMed]
- Gameiro, J.; Marques, F.; Lopes, J.A. Long-term consequences of acute kidney injury: A narrative review. Clin. Kidney J. 2020, 14, 789–804. [Google Scholar] [CrossRef]
- Zhang, T.; Widdop, R.E.; Ricardo, S.D. Transition from acute kidney injury to chronic kidney disease: Mechanisms, models, and biomarkers. Am. J. Physiol. Ren. Physiol. 2024, 327, F788–F805. [Google Scholar] [CrossRef]
- Stevens, P.E.; Ahmed, S.B.; Carrero, J.J.; Foster, B.; Francis, A.; Hall, R.K.; Herrington, W.G.; Hill, G.; Inker, L.A.; Kazancıoğlu, R.; et al. KDIGO 2024 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int. 2024, 105, S117–S314. [Google Scholar] [CrossRef]
- Menshikh, A.; Scarfe, L.; Delgado, R.; Finney, C.; Zhu, Y.; Yang, H.; de Caestecker, M.P. Capillary rarefaction is more closely associated with CKD progression after cisplatin, rhabdomyolysis, and ischemia-reperfusion-induced AKI than renal fibrosis. Am. J. Physiol. Ren. Physiol. 2019, 317, F1383–F1397. [Google Scholar] [CrossRef]
- Mira, F.S.; Oliveiros, B.; Carreira, I.M.; Alves, R.; Ribeiro, I.P. Genetic Variants Related to Increased CKD Progression–A Systematic Review. Biology 2025, 14, 68. [Google Scholar] [CrossRef]
- Hassan, E.A.; Elsaid, A.M.; Abou-Elzahab, M.M.; El-Refaey, A.M.; Elmougy, R.; Youssef, M.M. The Potential Impact of MYH9 (rs3752462) and ELMO1 (rs741301) Genetic Variants on the Risk of Nephrotic Syndrome Incidence. Biochem. Genet. 2024, 62, 1304–1324. [Google Scholar] [CrossRef]
- Shimazaki, A.; Kawamura, Y.; Kanazawa, A.; Sekine, A.; Saito, S.; Tsunoda, T.; Koya, D.; Babazono, T.; Tanaka, Y.; Matsuda, M.; et al. Genetic variations in the gene encoding ELMO1 are associated with susceptibility to diabetic nephropathy. Diabetes 2005, 54, 1171–1178. [Google Scholar] [CrossRef]
- Hathaway, C.K.; Chang, A.S.; Grant, R.; Kim, H.S.; Madden, V.J.; Bagnell, C.R., Jr.; Jennette, J.C.; Smithies, O.; Kakoki, M. High Elmo1 expression aggravates and low Elmo1 expression prevents diabetic nephropathy. Proc. Natl. Acad. Sci. USA 2016, 113, 2218–2222. [Google Scholar] [CrossRef]
- Kakoki, M.; Bahnson, E.M.; Hagaman, J.R.; Siletzky, R.M.; Grant, R.; Kayashima, Y.; Li, F.; Lee, E.Y.; Sun, M.T.; Taylor, J.M.; et al. Engulfment and cell motility protein 1 potentiates diabetic cardiomyopathy via Rac-dependent and Rac-independent ROS production. JCI Insight 2019, 4, e127660. [Google Scholar] [CrossRef]
- He, L.; Wei, Q.; Liu, J.; Yi, M.; Liu, Y.; Liu, H.; Sun, L.; Peng, Y.; Liu, F.; Venkatachalam, M.A.; et al. AKI on CKD: Heightened injury, suppressed repair, and the underlying mechanisms. Kidney Int. 2017, 92, 1071–1083. [Google Scholar] [CrossRef]
- Li, F.; Bahnson, E.M.; Wilder, J.; Siletzky, R.; Hagaman, J.; Nickekeit, V.; Hiller, S.; Ayesha, A.; Feng, L.; Levine, J.S.; et al. Oral high dose vitamin B12 decreases renal superoxide and post-ischemia/reperfusion injury in mice. Redox Biol. 2020, 32, 101504. [Google Scholar] [CrossRef]
- Cohen, A.H. Masson’s trichrome stain in the evaluation of renal biopsies. An appraisal. Am. J. Clin. Pathol. 1976, 65, 631–643. [Google Scholar] [CrossRef] [PubMed]
- Feldman, A.T.; Wolfe, D. Tissue processing and hematoxylin and eosin staining. Methods Mol. Biol. 2014, 1180, 31–43. [Google Scholar] [CrossRef]
- Solez, K.; Axelsen, R.A.; Benediktsson, H.; Burdick, J.F.; Cohen, A.H.; Colvin, R.B.; Croker, B.P.; Droz, D.; Dunnill, M.S.; Halloran, P.F.; et al. International standardization of criteria for the histologic diagnosis of renal allograft rejection: The Banff working classification of kidney transplant pathology. Kidney Int. 1993, 44, 411–422. [Google Scholar] [CrossRef]
- Krege, J.H.; Hodgin, J.B.; Hagaman, J.R.; Smithies, O. A Noninvasive Computerized Tail-Cuff System for Measuring Blood Pressure in Mice. Hypertension 1995, 25, 1111–1115. [Google Scholar] [CrossRef]
- Reynolds, E.S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J. Cell Biol. 1963, 17, 208–212. [Google Scholar] [CrossRef]
- Gottlieb, E.R.; Estiverne, C.; Tolan, N.V.; Melanson, S.E.F.; Mendu, M.L. Estimated GFR With Cystatin C and Creatinine in Clinical Practice: A Retrospective Cohort Study. Kidney Med. 2023, 5, 100600. [Google Scholar] [CrossRef]
- Ichimura, T.; Bonventre, J.V.; Bailly, V.; Wei, H.; Hession, C.A.; Cate, R.L.; Sanicola, M. Kidney injury molecule-1 (KIM-1), a putative epithelial cell adhesion molecule containing a novel immunoglobulin domain, is up-regulated in renal cells after injury. J. Biol. Chem. 1998, 273, 4135–4142. [Google Scholar] [CrossRef]
- Sabbisetti, V.S.; Waikar, S.S.; Antoine, D.J.; Smiles, A.; Wang, C.; Ravisankar, A.; Ito, K.; Sharma, S.; Ramadesikan, S.; Lee, M.; et al. Blood kidney injury molecule-1 is a biomarker of acute and chronic kidney injury and predicts progression to ESRD in type I diabetes. J. Am. Soc. Nephrol. 2014, 25, 2177–2186. [Google Scholar] [CrossRef] [PubMed]
- Wadei, H.M.; Textor, S.C. The role of the kidney in regulating arterial blood pressure. Nat. Rev. Nephrol. 2012, 8, 602–609. [Google Scholar] [CrossRef] [PubMed]
- Kala, P.; Vanourkova, Z.; Skaroupkova, P.; Kompanowska-Jezierska, E.; Sadowski, J.; Walkowska, A.; Veselka, J.; Taborsky, M.; Maxova, H.; Vaneckova, I.; et al. Endothelin type A receptor blockade increases renoprotection in congestive heart failure combined with chronic kidney disease: Studies in 5/6 nephrectomized rats with aorto-caval fistula. Biomed. Pharmacother. 2023, 158, 114157. [Google Scholar] [CrossRef]
- McLarnon, S.C.; Johnson, C.; Giddens, P.; O’Connor, P.M. Hidden in Plain Sight: Does Medullary Red Blood Cell Congestion Provide the Explanation for Ischemic Acute Kidney Injury? Semin. Nephrol. 2022, 42, 3151280. [Google Scholar] [CrossRef]
- Polichnowski, A.J.; Griffin, K.A.; Licea-Vargas, H.; Lan, R.; Picken, M.M.; Long, J.; Williamson, G.A.; Rosenberger, C.; Mathia, S.; Venkatachalam, M.A.; et al. Pathophysiology of unilateral ischemia-reperfusion injury: Importance of renal counterbalance and implications for the AKI-CKD transition. Am. J. Physiol. Ren. Physiol. 2020, 318, F1086–F1099. [Google Scholar] [CrossRef]
- Finch, N.C.; Neal, C.R.; Welsh, G.I.; Foster, R.R.; Satchell, S.C. The unique structural and functional characteristics of glomerular endothelial cell fenestrations and their potential as a therapeutic target in kidney disease. Am. J. Physiol. Ren. Physiol. 2023, 325, F465–F478. [Google Scholar] [CrossRef]
- Diaz-Ricart, M.; Torramade-Moix, S.; Pascual, G.; Palomo, M.; Moreno-Castano, A.B.; Martinez-Sanchez, J.; Vera, M.; Cases, A.; Escolar, G. Endothelial Damage, Inflammation and Immunity in Chronic Kidney Disease. Toxins 2020, 12, 361. [Google Scholar] [CrossRef]
- Nezu, M.; Suzuki, N. Roles of Nrf2 in Protecting the Kidney from Oxidative Damage. Int. J. Mol. Sci. 2020, 21, 2951. [Google Scholar] [CrossRef]
- Zhang, D.; Li, Z.; Gao, Y.; Sun, H. MiR-556-3p mediated repression of klotho under oxidative stress promotes fibrosis of renal tubular epithelial cells. Sci. Rep. 2025, 15, 12182. [Google Scholar] [CrossRef]
- Gyurászová, M.; Gurecká, R.; Bábíčková, J.; Tóthová, Ľ. Oxidative Stress in the Pathophysiology of Kidney Disease: Implications for Noninvasive Monitoring and Identification of Biomarkers. Oxid. Med. Cell Longev. 2020, 2020, 5478708. [Google Scholar] [CrossRef]
- Barwinska, D.; El-Achkar, T.M.; Melo Ferreira, R.; Syed, F.; Cheng, Y.H.; Winfree, S.; Ferkowicz, M.J.; Hato, T.; Collins, K.S.; Dunn, K.W.; et al. Molecular characterization of the human kidney interstitium in health and disease. Sci. Adv. 2021, 7, eabd3359. [Google Scholar] [CrossRef] [PubMed]
- Ando, M.; Shibuya, A.; Tsuchiya, K.; Akiba, T.; Nitta, K. Reduced expression of Toll-like receptor 4 contributes to impaired cytokine response of monocytes in uremic patients. Kidney Int. 2006, 70, 358–362. [Google Scholar] [CrossRef]
- Deng, J.; Wu, Z.; He, Y.; Lin, L.; Tan, W.; Yang, J. Interaction Between Intrinsic Renal Cells and Immune Cells in the Progression of Acute Kidney Injury. Front. Med. 2022, 9, 954574. [Google Scholar] [CrossRef]
- Tirichen, H.; Yaigoub, H.; Xu, W.; Wu, C.; Li, R.; Li, Y. Mitochondrial Reactive Oxygen Species and Their Contribution in Chronic Kidney Disease Progression Through Oxidative Stress. Front. Physiol. 2021, 12, 627837. [Google Scholar] [CrossRef]
- Schupp, N.; Stopper, H.; Heidland, A. DNA Damage in Chronic Kidney Disease: Evaluation of Clinical Biomarkers. Oxid. Med. Cell Longev. 2016, 2016, 3592042. [Google Scholar] [CrossRef] [PubMed]
- Aguila, T.; Madan, R.; Palmer, M.B.; Derk, C.T. Zebra Bodies in Kidney Biopsy: Drug-Induced Phospholipidosis in a Patient With Systemic Lupus Erythematosus. J. Rheumatol. 2025, 52, 10. [Google Scholar] [CrossRef] [PubMed]
- van de Lagemaat, E.E.; de Groot, L.; van den Heuvel, E. Vitamin B(12) in Relation to Oxidative Stress: A Systematic Review. Nutrients 2019, 11, 482. [Google Scholar] [CrossRef]
- Aydin, E.; Hallner, A.; Grauers Wiktorin, H.; Staffas, A.; Hellstrand, K.; Martner, A. NOX2 inhibition reduces oxidative stress and prolongs survival in murine KRAS-induced myeloproliferative disease. Oncogene 2019, 38, 1534–1543. [Google Scholar] [CrossRef]
- Lima-Posada, I.; Portas-Cortes, C.; Perez-Villalva, R.; Fontana, F.; Rodriguez-Romo, R.; Prieto, R.; Sanchez-Navarro, A.; Rodriguez-Gonzalez, G.L.; Gamba, G.; Zambrano, E.; et al. Gender Differences in the Acute Kidney Injury to Chronic Kidney Disease Transition. Sci. Rep. 2017, 7, 12270. [Google Scholar] [CrossRef]
- Stowers, K.; Rudman-Melnick, V.; Ma, Q.; Devarajan, P. Prolonged unilateral renal ischemia-reperfusion as a model for acute to chronic kidney injury in female mice. Am. J. Physiol. Ren. Physiol. 2025, 328, F684–F690. [Google Scholar] [CrossRef] [PubMed]
- Hosszu, A.; Fekete, A.; Szabo, A.J. Sex differences in renal ischemia-reperfusion injury. Am. J. Physiol. Ren. Physiol. 2020, 319, F149–F154. [Google Scholar] [CrossRef] [PubMed]
- Irazabal, M.V.; Torres, V.E. Reactive Oxygen Species and Redox Signaling in Chronic Kidney Disease. Cells 2020, 9, 1342. [Google Scholar] [CrossRef] [PubMed]
- Sedeek, M.; Nasrallah, R.; Touyz, R.M.; Hébert, R.L. NADPH oxidases, reactive oxygen species, and the kidney: Friend and foe. J. Am. Soc. Nephrol. 2013, 24, 1512–1518. [Google Scholar] [CrossRef]
- Zhu, Z.; Hu, J.; Chen, Z.; Feng, J.; Yang, X.; Liang, W.; Ding, G. Transition of acute kidney injury to chronic kidney disease: Role of metabolic reprogramming. Metabolism 2022, 131, 155194. [Google Scholar] [CrossRef]
- Lu, H.; Hou, L.; Zhang, Y.; Guo, T.; Wang, Y.; Xing, M. Polystyrene microplastics mediate cell cycle arrest, apoptosis, and autophagy in the G2/M phase through ROS in grass carp kidney cells. Environ. Toxicol. 2024, 39, 1923–1935. [Google Scholar] [CrossRef]
- Osman, D.; Cooke, A.; Young, T.R.; Deery, E.; Robinson, N.J.; Warren, M.J. The requirement for cobalt in vitamin B(12): A paradigm for protein metalation. Biochim. Biophys. Acta Mol. Cell Res. 2021, 1868, 118896. [Google Scholar] [CrossRef]
- Kapadia, C.R. Vitamin B12 in health and disease: Part I--inherited disorders of function, absorption, and transport. Gastroenterologist 1995, 3, 329–344. Available online: https://www.ncbi.nlm.nih.gov/pubmed/8775094 (accessed on 20 September 2025).
- Suarez-Moreira, E.; Yun, J.; Birch, C.S.; Williams, J.H.; McCaddon, A.; Brasch, N.E. Vitamin B(12) and redox homeostasis: Cob(II)alamin reacts with superoxide at rates approaching superoxide dismutase (SOD). J. Am. Chem. Soc. 2009, 131, 15078–15079. [Google Scholar] [CrossRef]
- Froese, D.S.; Fowler, B.; Baumgartner, M.R. Vitamin B(12), folate, and the methionine remethylation cycle-biochemistry, pathways, and regulation. J. Inherit. Metab. Dis. 2019, 42, 673–685. [Google Scholar] [CrossRef]
- Lyon, P.; Strippoli, V.; Fang, B.; Cimmino, L. B Vitamins and One-Carbon Metabolism: Implications in Human Health and Disease. Nutrients 2020, 12, 2867. [Google Scholar] [CrossRef]
- Li, F. The beneficial role of vitamin B12 in injury induced by ischemia/reperfusion: Beyond scavenging superoxide? J. Exp. Nephrol. 2021, 2, 3–6. [Google Scholar] [CrossRef]
- Spence, J.D.; Yi, Q.; Hankey, G.J. B vitamins in stroke prevention: Time to reconsider. Lancet Neurol. 2017, 16, 750–760. [Google Scholar] [CrossRef]
- Kakoki, M.; Ramanathan, P.V.; Hagaman, J.R.; Grant, R.; Wilder, J.C.; Taylor, J.M.; Charles Jennette, J.; Smithies, O.; Maeda-Smithies, N. Cyanocobalamin prevents cardiomyopathy in type 1 diabetes by modulating oxidative stress and DNMT-SOCS1/3-IGF-1 signaling. Commun. Biol. 2021, 4, 775. [Google Scholar] [CrossRef]
- Park, C.H.; Yoo, T.H. TGF-β Inhibitors for Therapeutic Management of Kidney Fibrosis. Pharmaceuticals 2022, 15, 1485. [Google Scholar] [CrossRef] [PubMed]
- Kierulf-Lassen, C.; Nielsen, P.M.; Qi, H.; Damgaard, M.; Laustsen, C.; Pedersen, M.; Krag, S.; Birn, H.; Nørregaard, R.; Jespersen, B. Unilateral nephrectomy diminishes ischemic acute kidney injury through enhanced perfusion and reduced pro-inflammatory and pro-fibrotic responses. PLoS ONE 2017, 12, e0190009. [Google Scholar] [CrossRef] [PubMed]
- Dupont, V.; Berg, A.H.; Yamashita, M.; Huang, C.; Covarrubias, A.E.; Ali, S.; Stotland, A.; Van Eyk, J.E.; Jim, B.; Thadhani, R.; et al. Impaired renal reserve contributes to preeclampsia via the kynurenine and soluble fms-like tyrosine kinase 1 pathway. J. Clin. Investig. 2022, 132, e158346. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, J.; Wang, Y.; Hagaman, J.; Ma, Q.; Jennette, J.C.; Chen, M.; Yi, X.; Kayashima, Y.; Maeda-Smithies, N.; Li, F. Vitamin B12 Protects the Exacerbated Ischemia–Reperfusion Injury-Induced Chronic Kidney Disease in Mice with Genetically Increased Elmo1. Antioxidants 2025, 14, 1277. https://doi.org/10.3390/antiox14111277
Zhou J, Wang Y, Hagaman J, Ma Q, Jennette JC, Chen M, Yi X, Kayashima Y, Maeda-Smithies N, Li F. Vitamin B12 Protects the Exacerbated Ischemia–Reperfusion Injury-Induced Chronic Kidney Disease in Mice with Genetically Increased Elmo1. Antioxidants. 2025; 14(11):1277. https://doi.org/10.3390/antiox14111277
Chicago/Turabian StyleZhou, Jiayi, Yuye Wang, John Hagaman, Qing Ma, J. Charles Jennette, Meitong Chen, Xianwen Yi, Yukako Kayashima, Nobuyo Maeda-Smithies, and Feng Li. 2025. "Vitamin B12 Protects the Exacerbated Ischemia–Reperfusion Injury-Induced Chronic Kidney Disease in Mice with Genetically Increased Elmo1" Antioxidants 14, no. 11: 1277. https://doi.org/10.3390/antiox14111277
APA StyleZhou, J., Wang, Y., Hagaman, J., Ma, Q., Jennette, J. C., Chen, M., Yi, X., Kayashima, Y., Maeda-Smithies, N., & Li, F. (2025). Vitamin B12 Protects the Exacerbated Ischemia–Reperfusion Injury-Induced Chronic Kidney Disease in Mice with Genetically Increased Elmo1. Antioxidants, 14(11), 1277. https://doi.org/10.3390/antiox14111277






