Tiliacorinine as a Promising Candidate for Cholangiocarcinoma Therapy via Oxidative Stress Molecule Modulation: A Study Integrating Network Pharmacology, Molecular Docking and Molecular Dynamics Simulation
Abstract
1. Introduction
2. Materials and Methods
2.1. Assessment of Drug Likeness and ADMET Properties of Tiliacorinine
2.2. Target Proteins of Tiliacorinine
2.3. Potential Targets in Cholangiocarcinoma (CCA)
2.4. Protein–Protein Interaction Network (PPI)
2.5. Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) Enrichment Analysis
2.6. Molecular Docking Analysis
2.7. Molecular Dynamics Simulation Studies
3. Results
3.1. Prediction of Drug-Likeness and ADMET Characteristics of Tiliacorinine
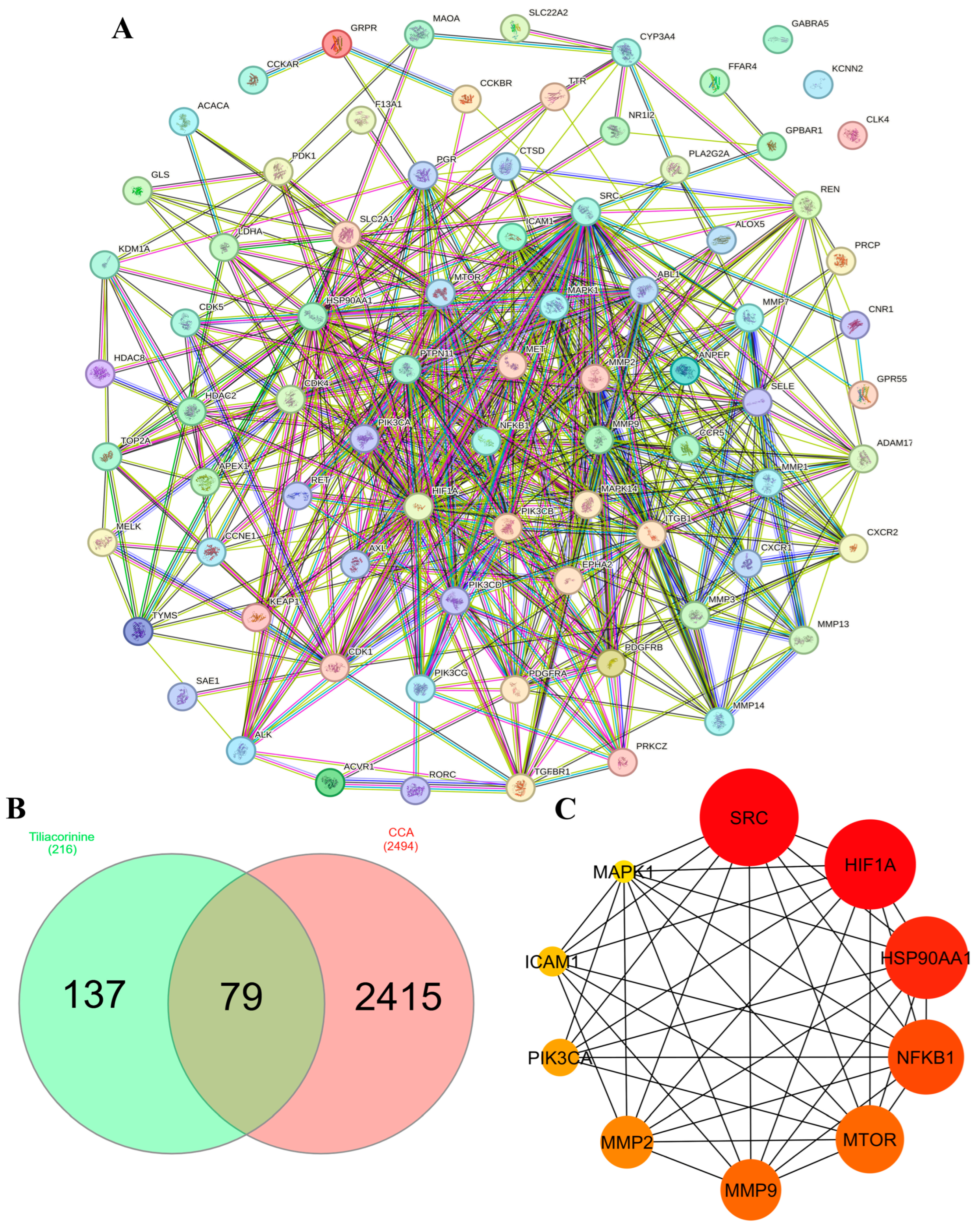
3.2. Target Proteins Identification and Analysis
3.3. PPI Network Construction and Hub Gene Identification
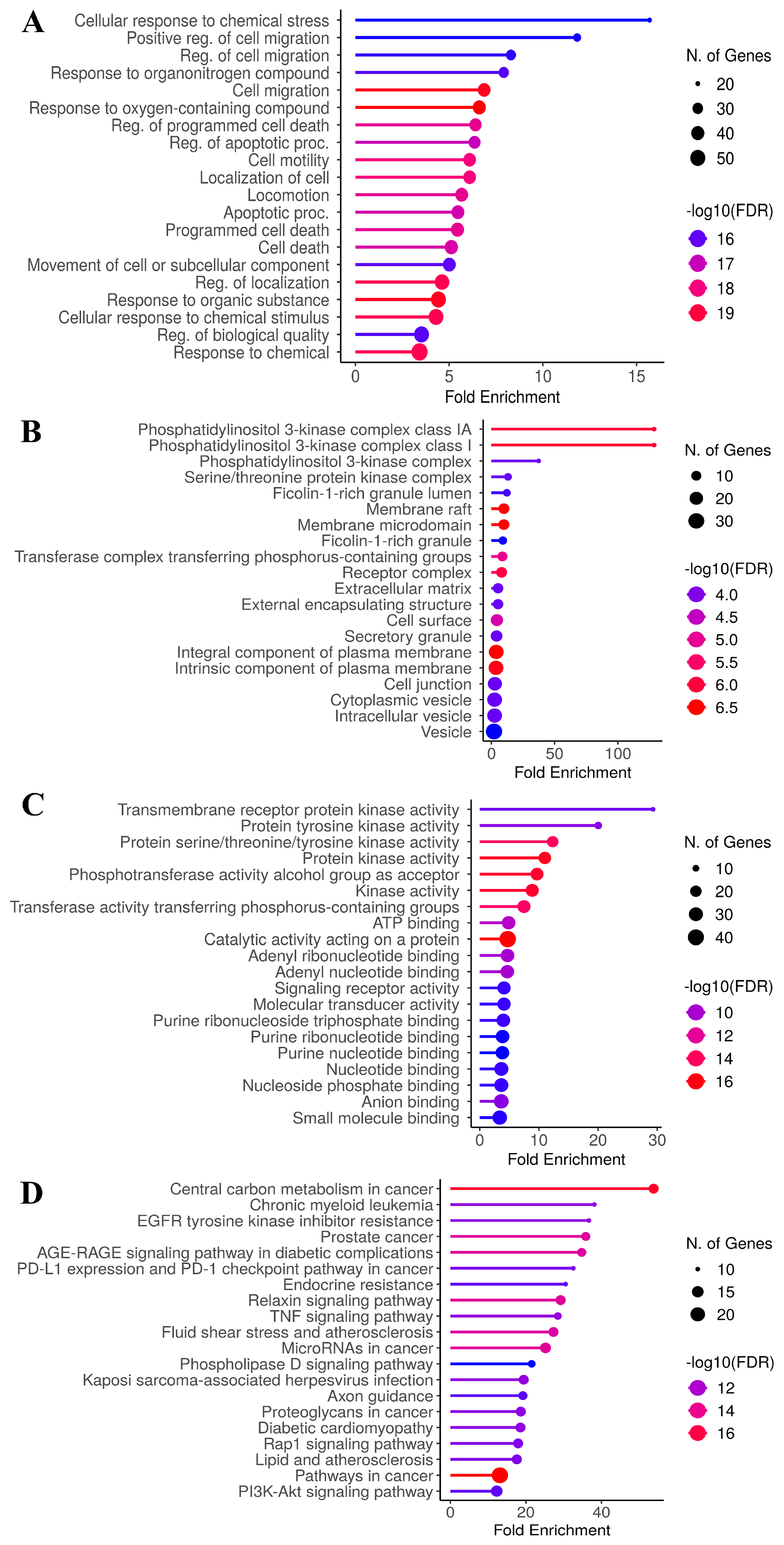
3.4. GO and KEGG Enrichment Analyses
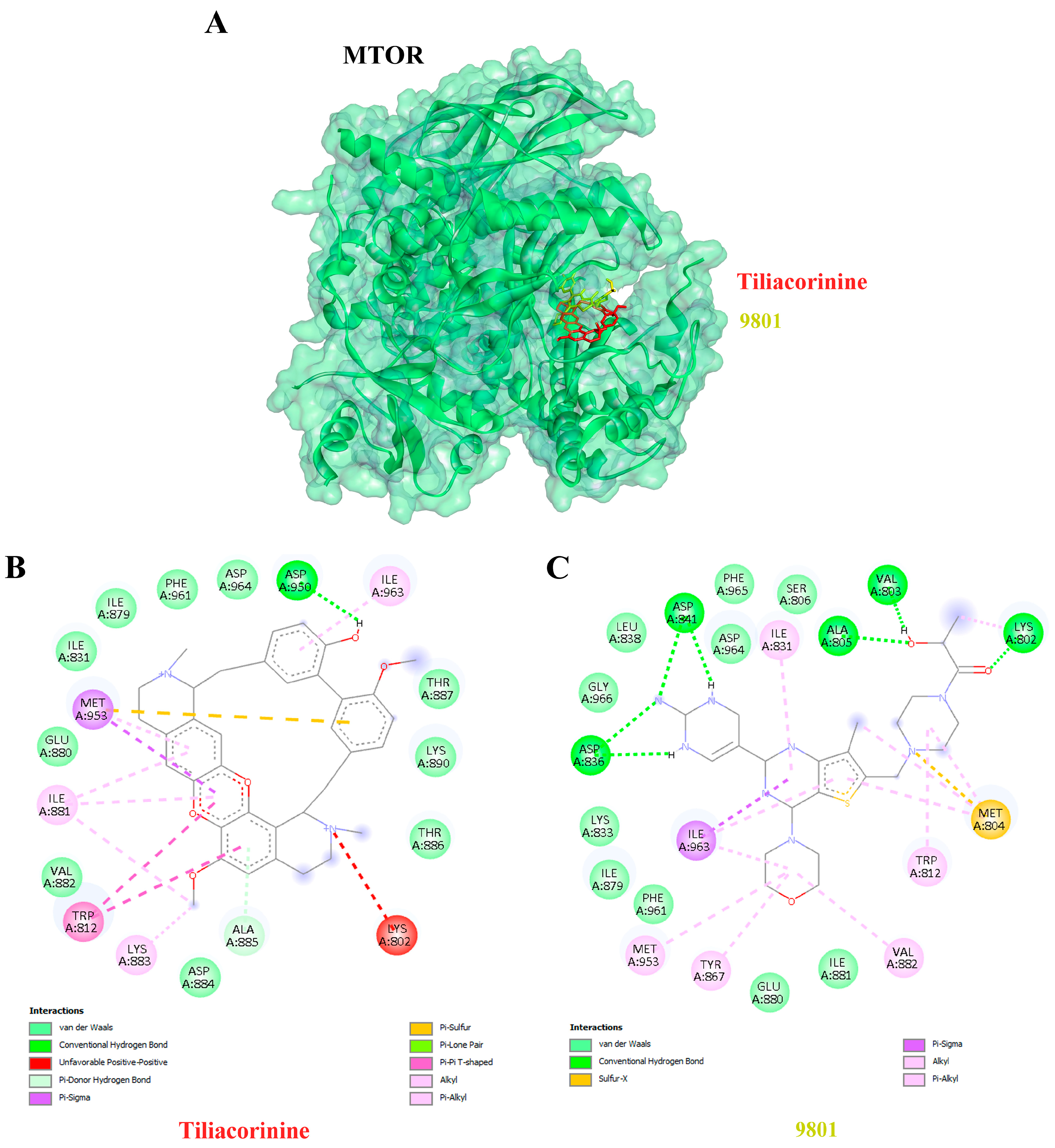
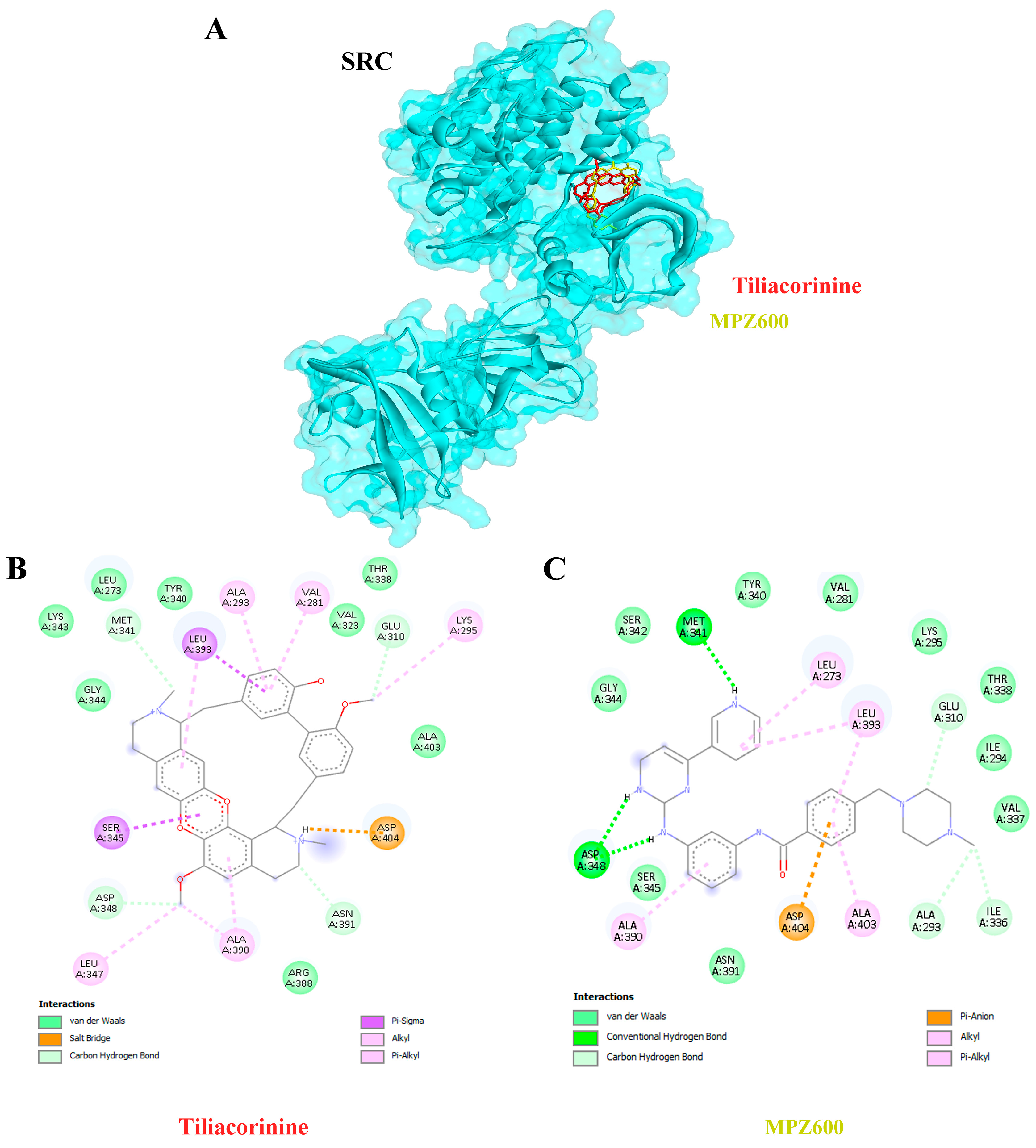
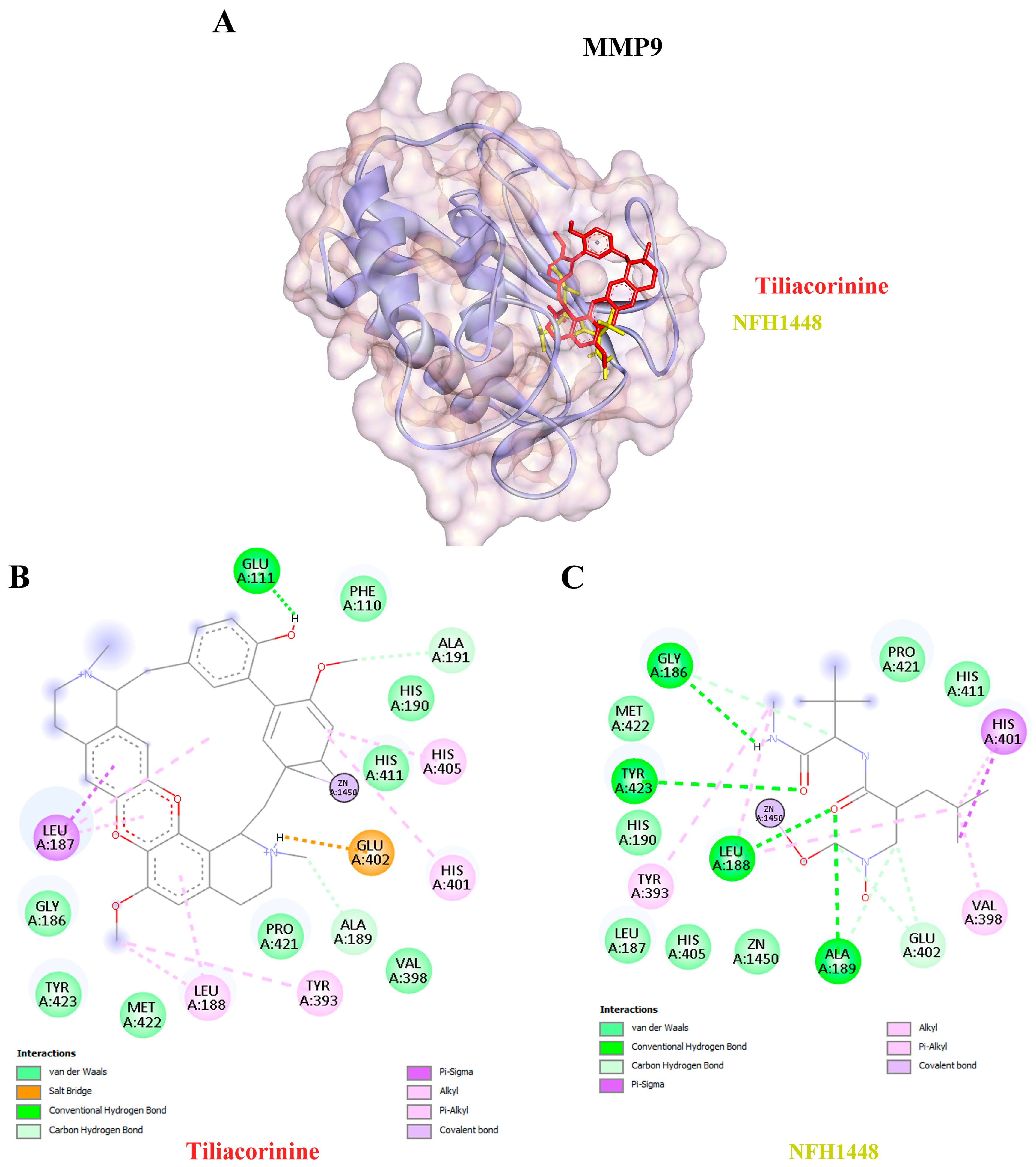
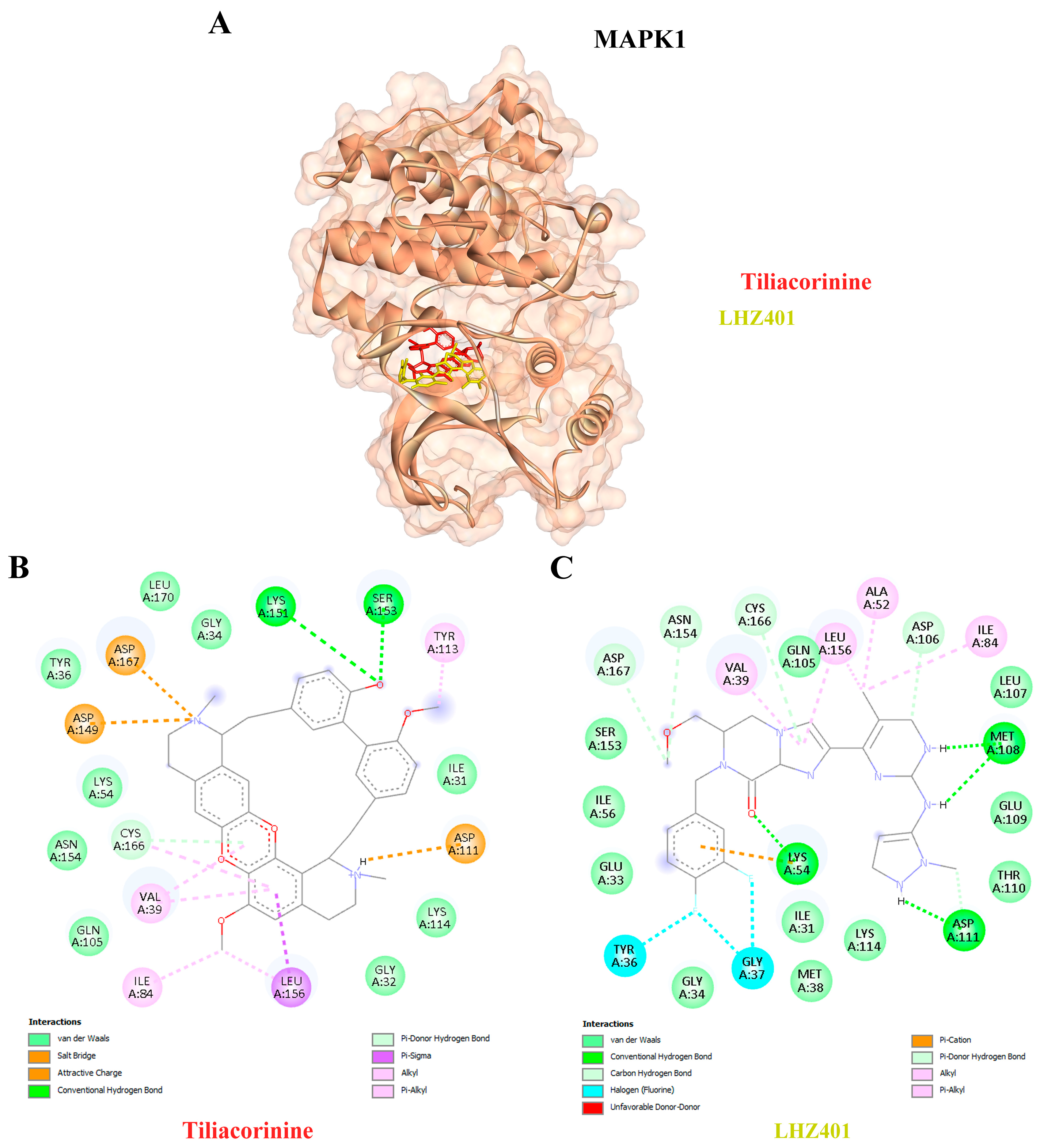
3.5. Ligand–Protein Docking Analysis
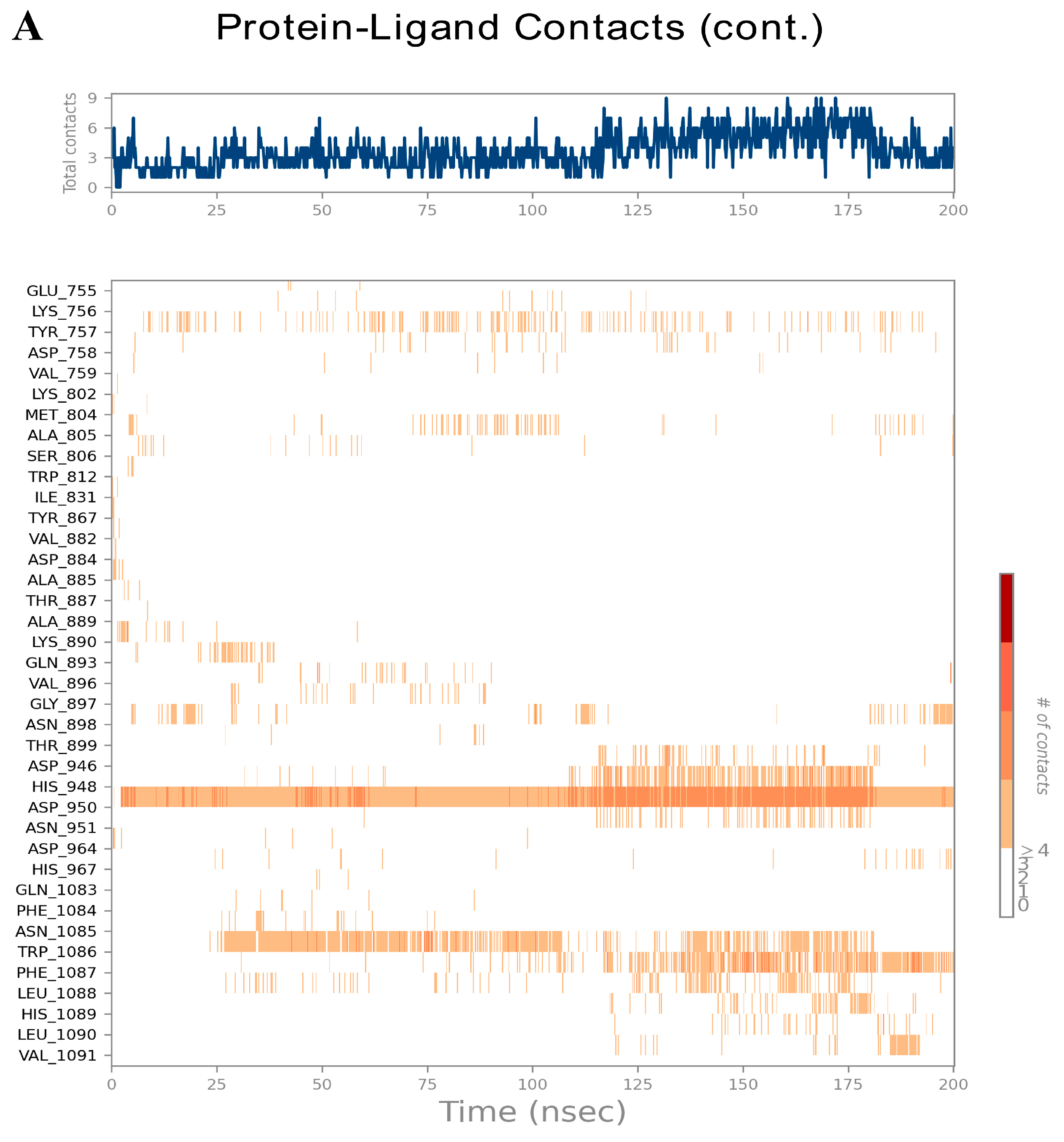
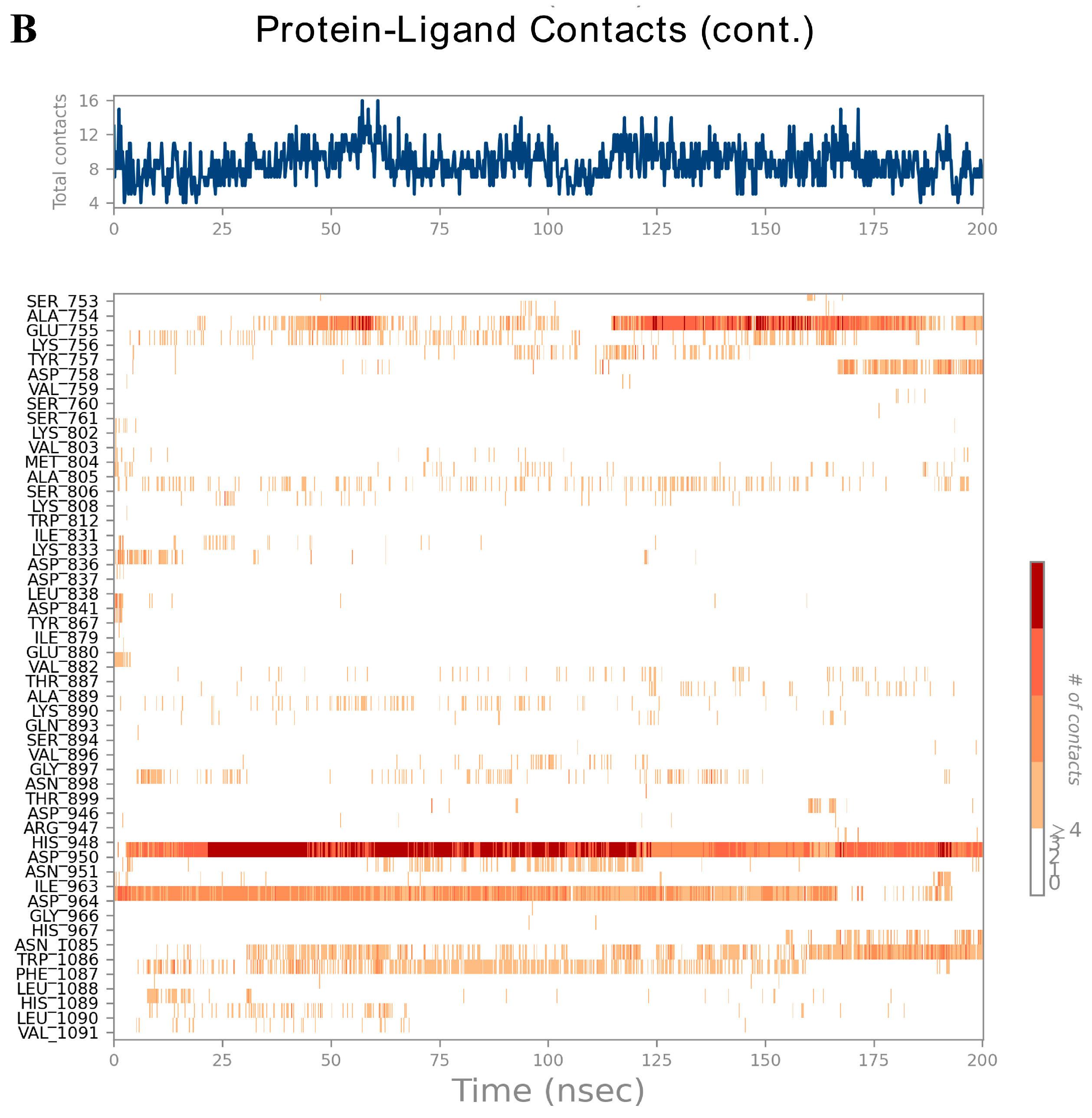
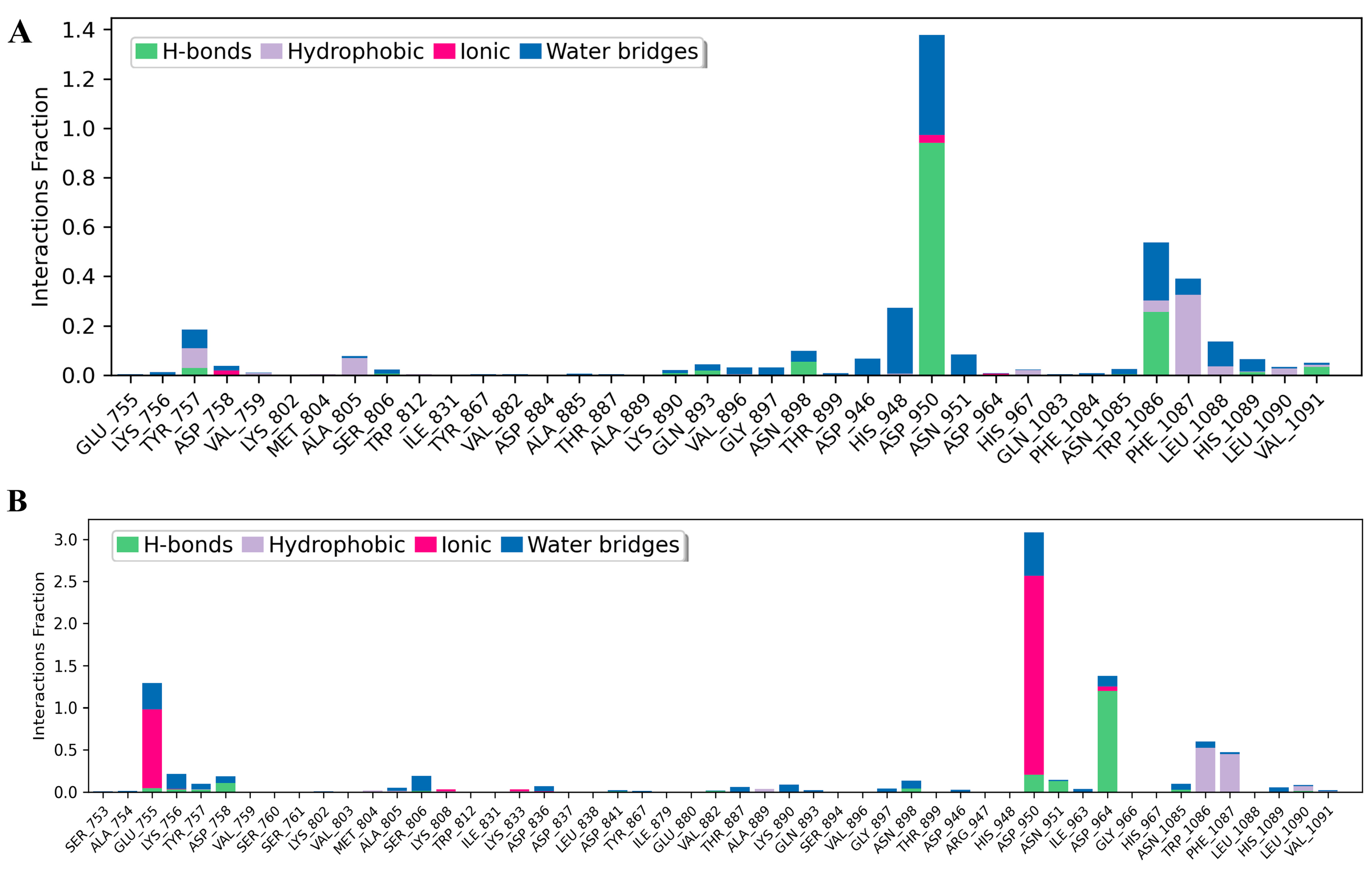
3.6. Analysis of Molecular Dynamics Trajectories
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Banales, J.M.; Marin, J.J.G.; Lamarca, A.; Rodrigues, P.M.; Khan, S.A.; Roberts, L.R.; Cardinale, V.; Carpino, G.; Andersen, J.B.; Braconi, C.; et al. Cholangiocarcinoma 2020: The next horizon in mechanisms and management. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 557–588. [Google Scholar] [CrossRef]
- Kirstein, M.M.; Vogel, A. Epidemiology and risk factors of cholangiocarcinoma. Visc. Med. 2016, 32, 395–400. [Google Scholar] [CrossRef] [PubMed]
- Bergquist, A.; von Seth, E. Epidemiology of cholangiocarcinoma. Best Pract. Res. Clin. Gastroenterol. 2015, 29, 221–232. [Google Scholar] [CrossRef]
- Plentz, R.R.; Malek, N.P. Clinical presentation, risk factors and staging systems of cholangiocarcinoma. Best Pract. Res. Clin. Gastroenterol. 2015, 29, 245–252. [Google Scholar] [CrossRef]
- Bertuccio, P.; Malvezzi, M.; Carioli, G.; Hashim, D.; Boffetta, P.; El-Serag, H.B.; La Vecchia, C.; Negri, E. Global trends in mortality from intrahepatic and extrahepatic cholangiocarcinoma. J. Hepatol. 2019, 71, 104–114. [Google Scholar] [CrossRef] [PubMed]
- Sripa, B.; Pairojkul, C. Cholangiocarcinoma: Lessons from Thailand. Curr. Opin. Gastroenterol. 2008, 24, 349–356. [Google Scholar] [CrossRef]
- Khan, A.; Dageforde, L. Cholangiocarcinoma. Surg. Oncol. Clin. N. Am. 2019, 99, 315–335. [Google Scholar] [CrossRef] [PubMed]
- Alsaleh, M.; Leftley, Z.; Barbera, T.A.; Sithithaworn, P.; Khuntikeo, N.; Loilome, W.; Yongvanit, P.; Cox, I.J.; Chamodol, N.; Syms, R.R.A.; et al. Cholangiocarcinoma: A guide for the nonspecialist. Int. J. Gen. Med. 2019, 12, 13–23. [Google Scholar] [CrossRef]
- Wongjarupong, N.; Assavapongpaiboon, B.; Susantitaphong, P.; Cheungpasitporn, W.; Treeprasertsuk, S.; Rerknimitr, R.; Chaiteerakij, R. Non-alcoholic fatty liver disease as a risk factor for cholangiocarcinoma: A systematic review and meta-analysis. BMC Gastroenterol. 2017, 17, 149. [Google Scholar] [CrossRef]
- Sripa, B.; Bethony, J.M.; Sithithaworn, P.; Kaewkes, S.; Mairiang, E.; Loukas, A.; Mulvenna, J.; Laha, T.; Hotez, P.J.; Brindley, P.J. Opisthorchiasis and Opisthorchis-associated cholangiocarcinoma in Thailand and Laos. Acta Trop. 2011, 120, S158–S168. [Google Scholar] [CrossRef] [PubMed]
- Perakanya, P.; Ungcharoen, R.; Worrabannakorn, S.; Ongarj, P.; Artchayasawat, A.; Boonmars, T.; Boueroy, P. Prevalence and risk factors of Opisthorchis viverrini infection in Sakon Nakhon Province, Thailand. Trop. Med. Infect. Dis. 2022, 7, 313, Erratum in Trop. Med. Infect. Dis. 2023, 8, 222. [Google Scholar] [CrossRef] [PubMed]
- Labib, P.L.; Goodchild, G.; Pereira, S.P. Molecular pathogenesis of cholangiocarcinoma. BMC Cancer 2019, 19, 185. [Google Scholar] [CrossRef] [PubMed]
- Esmail, A.; Badheeb, M.; Alnahar, B.W.; Almiqlash, B.; Sakr, Y.; Al-Najjar, E.; Awas, A.; Alsayed, M.; Khasawneh, B.; Alkhulaifawi, M.; et al. The recent trends of systemic treatments and locoregional therapies for cholangiocarcinoma. Pharmaceuticals 2024, 17, 910. [Google Scholar] [CrossRef]
- Nasim, N.; Sandeep, I.S.; Mohanty, S. Plant-derived natural products for drug discovery: Current approaches and prospects. Nucleus 2022, 65, 399–411. [Google Scholar] [CrossRef]
- Das, G.; Gouda, S.; Kerry, R.G.; Cortes, H.; Prado-Audelo, M.L.D.; Leyva-Gómez, G.; Tsouh Fokou, P.V.; Gutiérrez-Grijalva, E.P.; Heredia, J.B.; Shin, H.-S.; et al. Study of Traditional Uses, Extraction Procedures, Phytochemical Constituents, and Pharmacological Properties of Tiliacora Triandra. J. Chem. 2022, 2022, 754528. [Google Scholar] [CrossRef]
- Nutmakul, T. Phytochemical and pharmacological activity of Tiliacora Triandra (Colebr.) diels. Songklanakarin J. Sci. Technol. 2021, 43, 1264–1274. [Google Scholar]
- Lumlerdkij, N.; Boonrak, R.; Booranasubkajorn, S.; Akarasereenont, P.; Heinrich, M. In vitro protective effects of plants frequently used traditionally in cancer prevention in Thai traditional medicine: An ethnopharmacological study. J. Ethnopharmacol. 2020, 250, 112409. [Google Scholar] [CrossRef]
- Rattana, S.; Cushnie, B.; Taepongsorat, L.; Phadungkit, M. Chemical constituents and in vitro anticancer activity of Tiliacora triandra leaves. Pharmacogn. J. 2015, 8, 1–3. [Google Scholar] [CrossRef]
- Manosroi, A.; Akazawa, H.; Akihisa, T.; Jantrawut, P.; Kitdamrongtham, W.; Manosroi, W.; Manosroi, J. In vitro anti-proliferative activity on colon cancer cell line (HT-29) of Thai medicinal plants selected from Thai/Lanna medicinal plant recipe database “MANOSROI III”. J. Ethnopharmacol. 2015, 161, 11–17. [Google Scholar] [CrossRef]
- Jongrungraungchok, S.; Madaka, F.; Wunnakup, T.; Sudsai, T.; Pongphaew, C.; Songsak, T.; Pradubyat, N. In vitro antioxidant, anti-inflammatory, and anticancer activities of mixture Thai medicinal plants. BMC Complement. Med. Ther. 2023, 23, 43. [Google Scholar] [CrossRef]
- Janeklang, S.; Nakaew, A.; Vaeteewoottacharn, K.; Seubwai, W.; Boonsiri, P.; Kismali, G.; Suksamrarn, A.; Okada, S.; Wongkham, S. In vitro and in vivo antitumor activity of tiliacorinine in human cholangiocarcinoma. Asian Pac. J. Cancer Prev. 2014, 15, 7473–7478. [Google Scholar] [CrossRef]
- Wiriyachitra, P.; Phuriyakorn, B. Alkaloids of Tiliacora triandra. Aust. J. Chem. 1981, 34, 2001–2004. [Google Scholar] [CrossRef]
- Sureram, S.; Senadeera, S.P.D.; Hongmanee, P.; Mahidol, C.; Ruchirawat, S.; Kittakoop, P. Antimycobacterial activity of bisbenzylisoquinoline alkaloids from Tiliacora triandra against multidrug-resistant isolates of Mycobacterium tuberculosis. Bioorganic Med. Chem. Lett. 2012, 22, 2902–2905. [Google Scholar] [CrossRef]
- Pradubyat, N.; Madaka, F.; Songsak, T.; Jongrungruangchok, S. In Vitro Biological Activity of Tiliacora triandra (Colebr.) Diels Root Extract. J. Curr. Sci. Technol. 2024, 15, 77. [Google Scholar] [CrossRef]
- Detarya, M.; Mahalapbutr, P.; Waenphimai, O.; Kidoikhammouan, S.; Janeklang, S.; Sawanyawisuth, K.; Vaeteewoottacharn, K.; Seubwai, W.; Saengboonmee, C.; Thothaisong, T.; et al. Induction of apoptotic cell death of cholangiocarcinoma cells by tiliacorinine from Tiliacora triandra: A mechanistic insight. Biochim. Et Biophys. Acta General. Subj. 2023, 1867, 130486. [Google Scholar] [CrossRef]
- Yang, M.; Chen, J.L.; Xu, L.W.; Ji, G. Navigating traditional Chinese medicine network pharmacology and computational tools. Evid. Based Complement. Alternat. Med. 2013, 2013, 731969. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, A.L. Network Pharmacology. Nat. Biotechnol. 2007, 25, 1110–1111. [Google Scholar] [CrossRef]
- İslamoğlu, F. Molecular docking, bioactivity, ADME, toxicity risks, and quantum mechanical parameters of some 1,2-dihydroquinoline derivatives were calculated theoretically for investigation of their use as pharmaceutical active ingredients in the treatment of multiple sclerosis (MS). Prospect. Pharm. Sci. 2024, 22, 168–187. [Google Scholar] [CrossRef]
- Zuo, H.; Zhang, Q.; Su, S.; Chen, Q.; Yang, F.; Hu, Y. A network pharmacology-based approach to analyse potential targets of traditional herbal formulas: An example of Yu Ping Feng decoction. Sci. Rep. 2018, 8, 12349. [Google Scholar] [CrossRef] [PubMed]
- Macalino, S.J.Y.; Gosu, V.; Hong, S.; Choi, S. Role of computer-aided drug design in modern drug discovery. Arch. Pharm. Res. 2015, 38, 1686–1701. [Google Scholar] [CrossRef] [PubMed]
- Pinzi, L.; Rastelli, G. Molecular Docking: Shifting Paradigms in Drug Discovery. Int. J. Mol. Sci. 2019, 20, 4331. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef] [PubMed]
- Pires, D.E.V.; Blundell, T.L.; Ascher, D.B. pkCSM: Predicting small-molecule pharmacokinetic properties using graph-based signatures. J. Med. Chem. 2015, 58, 4066–4072. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. SwissTargetPrediction: Updated data and new features for efficient prediction of protein targets of small molecules. Nucleic Acids Res. 2019, 47, W357–W364. [Google Scholar] [CrossRef]
- Heberle, H.; Meirelles, G.V.; da Silva, F.R.; Telles, G.P.; Minghim, R. InteractiVenn: A web-based tool for the analysis of sets through Venn diagrams. BMC Bioinform. 2015, 16, 169. [Google Scholar] [CrossRef] [PubMed]
- Szklarczyk, D.; Kirsch, R.; Koutrouli, M.; Nastou, K.; Mehryary, F.; Hachilif, R.; Gable, A.L.; Fang, T.; Doncheva, N.T.; Pyysalo, S.; et al. The STRING database in 2023: Protein–protein association networks and functional enrichment analyses for any sequenced genome of interest. Nucleic Acids Res. 2023, 51, D638–D646. [Google Scholar] [CrossRef]
- Ge, S.X.; Jung, D.; Yao, R. ShinyGO: A graphical gene-set enrichment tool for animals and plants. Bioinformatics 2019, 36, 2628–2629. [Google Scholar] [CrossRef]
- Burley, S.K.; Bhikadiya, C.; Bi, C.; Bittrich, S.; Chen, L.; Crichlow, G.V.; Christie, C.H.; Dalenberg, K.; Di Costanzo, L.; Duarte, J.M.; et al. RCSB Protein Data Bank: Powerful new tools for exploring 3D structures of biological macromolecules for basic and applied research and education. Nucleic Acids Res. 2021, 49, D437–D451. [Google Scholar] [CrossRef]
- Kim, S.; Chen, J.; Cheng, T.; Gindulyte, A.; He, J.; He, S.; Li, Q.; Shoemaker, B.A.; Thiessen, P.A.; Yu, B.; et al. PubChem in 2021: New data content and improved web interfaces. Nucleic Acids Res. 2021, 49, D1388–D1395. [Google Scholar] [CrossRef]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera—A visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef]
- Sama-ae, I.; Pattaranggoon, N.C.; Tedasen, A. In silico prediction of antifungal compounds from natural sources towards lanosterol 14-alpha demethylase (CYP51) using molecular docking and molecular dynamics simulation. J. Mol. Graph. Model. 2023, 121, 108435. [Google Scholar] [CrossRef]
- Tedasen, A.; Chiabchalard, A.; Tencomnao, T.; Yamasaki, K.; Majima, H.J.; Phongphithakchai, A.; Chatatikun, M. Anti-melanogenic activity of ethanolic extract from Garcinia atroviridis fruits using in vitro experiments, network pharmacology, molecular docking, and molecular dynamics simulation. Antioxidants 2024, 13, 713. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Tareen, A.; Ashraf, S.U.; Mufti, I.S.; Karam, M.; Ahmed, F.I.; Rasool, S.N.; Akhtar, R.; Amin, A.; Khan, S.R. Intrahepatic cholangiocarcinoma mortality in the USA, 1999–2020: A 21-year population-based analysis. Cancer Causes Control 2025. [Google Scholar] [CrossRef] [PubMed]
- Rimassa, L.; Khan, S.; Groot Koerkamp, B.; Roessler, S.; Andersen, J.B.; Raggi, C.; Lleo, A.; Nault, J.C.; Calderaro, J.; Gabbi, C.; et al. Mapping the landscape of biliary tract cancer in Europe: Challenges and controversies. Lancet Reg. Health Eur. 2025, 50, 101171. [Google Scholar] [CrossRef] [PubMed]
- Lei, Z.N.; Tian, Q.; Teng, Q.X.; Wurpel, J.N.D.; Zeng, L.; Pan, Y.; Chen, Z.S. Understanding and targeting resistance mechanisms in cancer. MedComm. 2023, 4, e265. [Google Scholar] [CrossRef]
- Pillai, O.; Dhanikula, A.B.; Panchagnula, R. Drug delivery: An odyssey of 100 years. Curr. Opin. Chem. Biol. 2001, 5, 439–446. [Google Scholar] [CrossRef]
- Rai, M.; Singh, A.V.; Paudel, N.; Kanase, A.; Falletta, E.; Kerkar, P.; Heyda, J.; Barghash, R.F.; Singh, S.P.; Soos, M. Herbal concoction unveiled: A computational analysis of phytochemicals’ pharmacokinetic and toxicological profiles using novel approach methodologies (NAMs). Curr. Res. Toxicol. 2023, 5, 100118. [Google Scholar] [CrossRef]
- Ferreira, L.L.G.; Andricopulo, A.D. ADMET modeling approaches in drug discovery. Drug Discov. Today 2019, 24, 1157–1165. [Google Scholar] [CrossRef]
- Montanari, F.; Kuhnke, L.; ter Laak, A.; Clevert, D.-A. Modeling physico-chemical ADMET endpoints with multitask graph convolutional networks. Molecules 2019, 25, 44. [Google Scholar] [CrossRef] [PubMed]
- Pongchairerk, U.; Guan, J.L.; Leardkamolkarn, V. Focal adhesion kinase and Src phosphorylations in HGF-induced proliferation and invasion of human cholangiocarcinoma cell line, HuCCA-1. World J. Gastroenterol. 2005, 11, 5845–5852. [Google Scholar] [CrossRef]
- Wu, W.-S.; Ling, C.-H.; Lee, M.-C.; Cheng, C.-C.; Chen, R.-F.; Lin, C.-F.; You, R.-I.; Chen, Y.-C. Targeting Src-Hic-5 signal cascade for preventing migration of cholangiocarcinoma cell HuCCT1. Biomedicines 2022, 10, 1022. [Google Scholar] [CrossRef]
- Dash, S.; Hanson, S.; King, B.; Nyswaner, K.; Foss, K.; Tesi, N.; Harvey, M.J.B.; Navarro-Marchal, S.A.; Woods, A.; Poradosu, E.; et al. The SRC family kinase inhibitor NXP900 demonstrates potent antitumor activity in squamous cell carcinomas. J. Biol. Chem. 2024, 300, 107615. [Google Scholar] [CrossRef]
- Yu, A.; Zhao, L.; Kang, Q.; Li, J.; Chen, K.; Fu, H. Transcription factor HIF1α promotes proliferation, migration, and invasion of cholangiocarcinoma via long noncoding RNA H19/microRNA-612/Bcl-2 axis. Transl. Res. J. Lab. Clin. Med. 2020, 224, 26–39. [Google Scholar] [CrossRef] [PubMed]
- Thongchot, S.; Yongvanit, P.; Loilome, W.; Seubwai, W.; Phunicom, K.; Tassaneeyakul, W.; Namwat, N. High expression of HIF-1α, BNIP3 and PI3KC3: Hypoxia-induced autophagy predicts cholangiocarcinoma survival and metastasis. Asian Pac. J. Cancer Prev. 2014, 15, 5873–5878. [Google Scholar] [CrossRef]
- Shirota, T.; Ojima, H.; Hiraoka, N.; Shimada, K.; Rokutan, H.; Arai, Y.; Kanai, Y.; Miyagawa, S.; Shibata, T. Heat shock protein 90 is a potential therapeutic target in cholangiocarcinoma. Mol. Cancer Ther. 2015, 14, 1985–1993. [Google Scholar] [CrossRef]
- Yan, L.R.; Shen, S.X.; Wang, A.; Ding, H.X.; Liu, Y.N.; Yuan, Y.; Xu, Q. Comprehensive pan-cancer analysis of heat shock protein 110, 90, 70, and 60 families. Front. Mol. Biosci. 2021, 8, 726244. [Google Scholar] [CrossRef]
- Shahana, M.V.; Choudhary, B.H. HSP90 and the cancer transcriptome: A comprehensive review of inhibitors and mechanistic insights. Int. J. Clin. Oncol. 2025, 30, 1294–1308. [Google Scholar] [CrossRef]
- Seubwai, W.; Wongkham, C.; Puapairoj, A.; Khuntikeo, N.; Pugkhem, A.; Hahnvajanawong, C.; Chaiyagool, J.; Umezawa, K.; Okada, S.; Wongkham, S. Aberrant expression of NF-κB in liver fluke associated cholangiocarcinoma: Implications for targeted therapy. PLoS ONE 2014, 9, e106056. [Google Scholar] [CrossRef]
- Song, Z.; Feng, Z.; Wang, X.; Li, J.; Zhang, D. NFKB1 as a key player in tumor biology: From mechanisms to therapeutic implications. Cell Biol. Toxicol. 2025, 41, 29. [Google Scholar] [CrossRef]
- Daugan, C.; Boidot, R.; Ghiringhelli, F.; Borg, C.; Vienot, A. Targeting mTOR signaling for the treatment of intrahepatic cholangiocarcinoma with TSC1/ARID1A mutations: A case report with an unexpected response. Ther. Adv. Med. Oncol. 2024, 16, 17588359241271793. [Google Scholar] [CrossRef]
- Wu, C.-E.; Chen, M.-H.; Yeh, C.-N. mTOR inhibitors in advanced biliary tract cancers. Int. J. Mol. Sci. 2019, 20, 500. [Google Scholar] [CrossRef]
- Pan, S.; Hu, Y.; Hu, M.; Jian, H.; Chen, M.; Gan, L.; Zheng, P.; He, Y.; Wang, J. Platelet-derived PDGF promotes the invasion and metastasis of cholangiocarcinoma by upregulating MMP2/MMP9 expression and inducing EMT via the p38/MAPK signaling pathway. Am. J. Transl. Res. 2020, 12, 3577–3595. [Google Scholar]
- Joechle, K.; Jumaa, H.; Thriene, K.; Hellerbrand, C.; Kulemann, B.; Fichtner-Feigl, S.; Lang, S.A.; Guenzle, J. Dual inhibition of mTORC1/2 reduces migration of cholangiocarcinoma cells by regulation of matrix metalloproteinases. Front. Cell Dev. Biol. 2022, 9, 785979. [Google Scholar] [CrossRef]
- Ballin, N.; Ott, A.; Seibel-Kelemen, O.; Bonzheim, I.; Nann, D.; Beha, J.; Spahn, S.; Singer, S.; Ossowski, S.; Roggia, C.; et al. Case report: FGFR2 inhibitor resistance via PIK3CA and CDKN2A/B in an intrahepatic cholangiocarcinoma patient with FGFR2-SH3GLB1 fusion. Front. Oncol. 2025, 15, 1527484. [Google Scholar] [CrossRef]
- Deshpande, V.; Nduaguba, A.; Zimmerman, S.M.; Kehoe, S.M.; MacConaill, L.E.; Lauwers, G.Y.; Ferrone, C.; Bardeesy, N.; Zhu, A.X.; Hezel, A.F. Mutational profiling reveals PIK3CA mutations in gallbladder carcinoma. BMC Cancer 2011, 11, 60. [Google Scholar] [CrossRef]
- Janan, M.; Proungvitaya, S.; Limpaiboon, T.; Proungvitaya, T.; Roytrakul, S.; Wongkham, C.; Jearanaikoon, P.; Chur-in, S.; Wongkham, S. Serum adhesion molecule-1 (ICAM-1) as a potential prognostic marker for cholangiocarcinoma patients. Asian Pac. J. Cancer Prev. 2012, 13, 107–114. [Google Scholar]
- Zhu, B.; Wang, X.; Shimura, T.; Huang, A.C.; Kong, N.; Dai, Y.; Fang, J.; Guo, P.; Ying, J.E. Development of potent antibody drug conjugates against ICAM1⁺ cancer cells in preclinical models of cholangiocarcinoma. NPJ Precis. Oncol. 2023, 7, 93. [Google Scholar] [CrossRef]
- Zheng, Y.; Zhou, C.; Yu, X.X.; Wu, C.; Jia, H.L.; Gao, X.M.; Yang, J.M.; Wang, C.Q.; Luo, Q.; Zhu, Y.; et al. Osteopontin promotes metastasis of intrahepatic cholangiocarcinoma through recruiting MAPK1 and mediating Ser675 phosphorylation of β-catenin. Cell Death Dis. 2018, 9, 179. [Google Scholar] [CrossRef]
- Chen, C.; Nelson, L.J.; Ávila, M.A.; Cubero, F.J. Mitogen-Activated Protein Kinases (MAPKs) and Cholangiocarcinoma: The Missing Link. Cells 2019, 8, 1172. [Google Scholar] [CrossRef]
- Landskron, G.; De la Fuente, M.; Thuwajit, P.; Thuwajit, C.; Hermoso, A.M. Chronic inflammation and cytokines in the tumor microenvironment. J. Immunol. Res. 2014, 2014, 149185. [Google Scholar] [CrossRef]
- Caligiuri, A.; Becatti, M.; Porro, N.; Borghi, S.; Marra, F.; Pastore, M.; Taddei, N.; Fiorillo, C.; Gentilini, A. Oxidative Stress and Redox-Dependent Pathways in Cholangiocarcinoma. Antioxidants 2024, 13, 28. [Google Scholar] [CrossRef]
- Wu, Z. Transcriptomic analysis reveals oxidative stress-related signature and molecular subtypes in cholangio carcinoma. Mol. Genet. Genom. 2024, 299, 86. [Google Scholar] [CrossRef]
- Abraham, R.T.; Eng, C.H. Mammalian target of rapamycin as a therapeutic target in oncology. Expert. Opin. Ther. Targets 2008, 12, 209–222. [Google Scholar] [CrossRef]
- Wang, L.; Chen, L.; Yu, M.; Xu, L.H.; Cheng, B.; Lin, Y.S.; Gu, Q.; He, X.H.; Xu, J. Discovering new mTOR inhibitors for cancer treatment through virtual screening methods and in vitro assays. Sci. Rep. 2016, 6, 18987. [Google Scholar] [CrossRef]
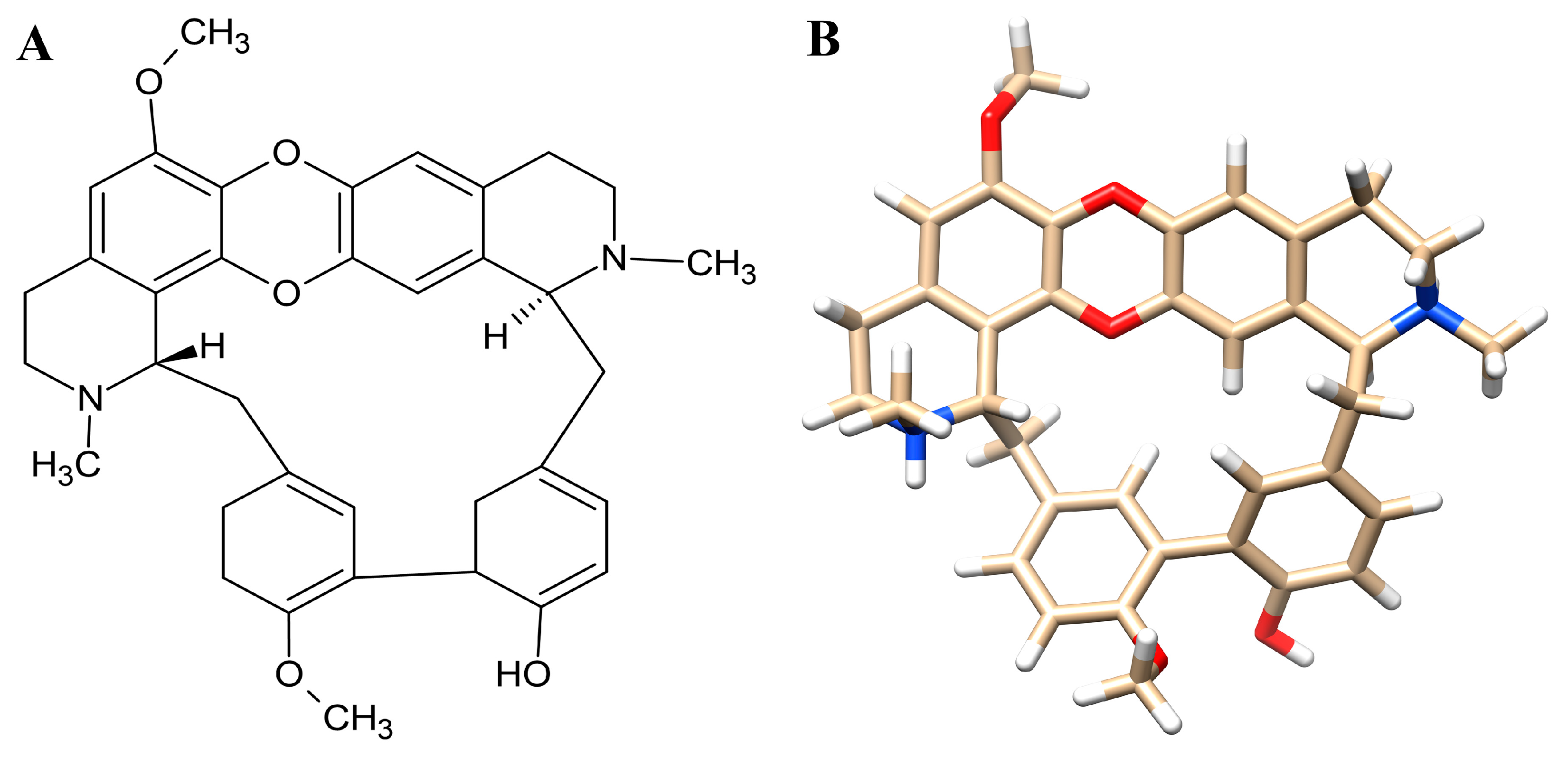

| Physicochemical Properties | |
| Formula | C36H36N2O5 |
| Molecular weight | 576.68 g/mol |
| Num. heavy atoms | 43 |
| Num. arom. heavy atoms | 24 |
| Fraction Csp3 | 0.33 |
| Num. rotatable bonds | 2 |
| Num. H-bond acceptors | 7 |
| Num. H-bond donors | 1 |
| Molar Refractivity | 174.11 |
| TPSA | 63.63 Å2 |
| Lipophilicity | |
| Log Po/w (iLOGP) | 4.96 |
| Log Po/w (XLOGP3) | 6.07 |
| Log Po/w (WLOGP) | 5.42 |
| Log Po/w (MLOGP) | 3.89 |
| Log Po/w (SILICOS-IT) | 5.85 |
| Consensus Log Po/w | 5.24 |
| Water Solubility | |
| Log S (ESOL) | −7.52 |
| Solubility | 1.74 × 10−5 mg/mL; 3.02 × 10−8 mol/L |
| Class | Poorly soluble |
| Log S (Ali) | −7.19 |
| Solubility | 3.76 × 10−5 mg/mL; 6.51 × 10−8 mol/L |
| Class | Poorly soluble |
| Log S (SILICOS-IT) | −10.03 |
| Solubility | 5.41 × 10−8 mg/mL; 9.38 × 10−11 mol/L |
| Class | Insoluble |
| Drug-likeness | |
| Lipinski | Yes; 1 violation: MW > 500 |
| Ghose | No; 3 violations: MW > 480, MR > 130, #atoms > 70 |
| Veber | Yes |
| Egan | Yes |
| Muegge | No; 2 violations: XLOGP3 > 5, #rings > 7 |
| Bioavailability Score | 0.55 |
| Property | Model Name | Predicted Value | Unit |
|---|---|---|---|
| Absorption | Water solubility | −3.62 | Numeric (log mol/L) |
| Caco2 permeability | 0.63 | Numeric (log Papp in 10−6 cm/s) | |
| Intestinal absorption (human) | 93.558 | Numeric (% Absorbed) | |
| Skin Permeability | −2.735 | Numeric (log Kp) | |
| P-glycoprotein substrate | Yes | Categorical (Yes/No) | |
| P-glycoprotein I inhibitor | Yes | Categorical (Yes/No) | |
| P-glycoprotein II inhibitor | Yes | Categorical (Yes/No) | |
| Distribution | VDss (human) | −1.152 | Numeric (log L/kg) |
| Fraction unbound (human) | 0.287 | Numeric (Fu) | |
| BBB permeability | −0.545 | Numeric (log BB) | |
| CNS permeability | −1.198 | Numeric (log PS) | |
| Metabolism | CYP2D6 substrate | No | Categorical (Yes/No) |
| CYP3A4 substrate | Yes | Categorical (Yes/No) | |
| CYP1A2 inhibitor | No | Categorical (Yes/No) | |
| CYP2C19 inhibitor | Yes | Categorical (Yes/No) | |
| CYP2C9 inhibitor | No | Categorical (Yes/No) | |
| CYP2D6 inhibitor | No | Categorical (Yes/No) | |
| CYP3A4 inhibitor | No | Categorical (Yes/No) | |
| Excretion | Total Clearance | 0.762 | Numeric (log mL/min/kg) |
| Renal OCT2 substrate | No | Categorical (Yes/No) | |
| Toxicity | AMES toxicity | Yes | Categorical (Yes/No) |
| Max. tolerated dose (human) | 0.244 | Numeric (log mg/kg/day) | |
| hERG I inhibitor | No | Categorical (Yes/No) | |
| hERG II inhibitor | Yes | Categorical (Yes/No) | |
| Oral Rat Acute Toxicity (LD50) | 2.527 | Numeric (mol/kg) | |
| Oral Rat Chronic Toxicity (LOAEL) | 0.513 | Numeric (log mg/kg_bw/day) | |
| Hepatotoxicity | No | Categorical (Yes/No) | |
| Skin Sensitization | No | Categorical (Yes/No) | |
| T. pyriformis toxicity | 0.285 | Numeric (log ug/L) | |
| Minnow toxicity | 1.623 | Numeric (log mM) |
| No. | Protein Name | PDB | Compound and Positive Control | Binding Energies (kcal/mol) | Inhibition Constant (nM) | Hydrogen Bond Interactions |
|---|---|---|---|---|---|---|
| 1 | SRC | 1Y57 | Tiliacorinine | −9.29 | 155.79 | GLU310, MET341, ASP348, ASN391 |
| MPZ600 | −8.77 | 369.78 | ALA293, ILE336, GLU310, MET341, ASP348 | |||
| 2 | HIF1A | 3KCX | Tiliacorinine | −7.32 | 4310.00 | TYR102 |
| CQL | −6.67 | 12,960.00 | HIS199, ASP201 | |||
| 3 | HSP90AA1 | 4AWQ | Tiliacorinine | −7.38 | 3910.00 | - |
| 5921224 | −13.91 | 0.06414 | GLN23, ASP93, MET98, ILE104 | |||
| 4 | NFKB1 | 8TQD | Tiliacorinine | −6.09 | 34,330.00 | HIS143, VAL144, THR145 |
| Dexamethasone | −6.99 | 7500.00 | THR126, GLY128, ASP131, VAL133, GLY135, ALA137 | |||
| 5 | MTOR | 3TL5 | Tiliacorinine | −10.78 | 12.62 | ALA885, ASP950 |
| GDC-0980 | −10.08 | 41.14 | LYS802, VAL803, ALA805, ASP836, ASP841 | |||
| 6 | MMP9 | 1GKC | Tiliacorinine | −9.42 | 125.43 | GLU111, ALA189, ALA191 |
| NFH1448 | −7.95 | 1490.00 | GLY186, LEU188, ALA189, GLU402, TYR423 | |||
| 7 | MMP2 | 8H78 | Tiliacorinine | −6.72 | 1183.00 | ASP77, LEU83, ALA86, VAL118 |
| L2U207 | −11.61 | 3.07 | TYR74, LEU83, ALA84, HIS85, VAL118, ILE142, THR144 | |||
| 8 | PIK3CA | 4JPS | Tiliacorinine | −8.99 | 259.27 | GLN589, ASP933 |
| 1LT1102 | −10.31 | 27.69 | LYS802, VAL851, SER854, GLN859 | |||
| 9 | ICAM1 | 1IAM | Tiliacorinine | −8.00 | 1360.00 | ILE10, GLU53 |
| BKA99414 | −6.64 | 1365.00 | LEU94, GLN181 | |||
| 10 | MAPK1 | 6SLG | Tiliacorinine | −9.30 | 151.80 | LYS151, SER153, CYS166 |
| LHZ401 | −8.23 | 923.74 | LYS54, MET108, ASP106, ASP111, ASN154, CYS166, ASP167 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boonsit, T.; Chatatikun, M.; Sirirattanakul, S.; Pattaranggoon, N.C.; Sama-ae, I.; Kawakami, F.; Imai, M.; Raungrut, P.; Phongphithakchai, A.; Tedasen, A.; et al. Tiliacorinine as a Promising Candidate for Cholangiocarcinoma Therapy via Oxidative Stress Molecule Modulation: A Study Integrating Network Pharmacology, Molecular Docking and Molecular Dynamics Simulation. Antioxidants 2025, 14, 1273. https://doi.org/10.3390/antiox14111273
Boonsit T, Chatatikun M, Sirirattanakul S, Pattaranggoon NC, Sama-ae I, Kawakami F, Imai M, Raungrut P, Phongphithakchai A, Tedasen A, et al. Tiliacorinine as a Promising Candidate for Cholangiocarcinoma Therapy via Oxidative Stress Molecule Modulation: A Study Integrating Network Pharmacology, Molecular Docking and Molecular Dynamics Simulation. Antioxidants. 2025; 14(11):1273. https://doi.org/10.3390/antiox14111273
Chicago/Turabian StyleBoonsit, Tavisa, Moragot Chatatikun, Suphasarang Sirirattanakul, Nawanwat C. Pattaranggoon, Imran Sama-ae, Fumitaka Kawakami, Motoki Imai, Pritsana Raungrut, Atthaphong Phongphithakchai, Aman Tedasen, and et al. 2025. "Tiliacorinine as a Promising Candidate for Cholangiocarcinoma Therapy via Oxidative Stress Molecule Modulation: A Study Integrating Network Pharmacology, Molecular Docking and Molecular Dynamics Simulation" Antioxidants 14, no. 11: 1273. https://doi.org/10.3390/antiox14111273
APA StyleBoonsit, T., Chatatikun, M., Sirirattanakul, S., Pattaranggoon, N. C., Sama-ae, I., Kawakami, F., Imai, M., Raungrut, P., Phongphithakchai, A., Tedasen, A., & Maungchanburi, S. (2025). Tiliacorinine as a Promising Candidate for Cholangiocarcinoma Therapy via Oxidative Stress Molecule Modulation: A Study Integrating Network Pharmacology, Molecular Docking and Molecular Dynamics Simulation. Antioxidants, 14(11), 1273. https://doi.org/10.3390/antiox14111273






