Deoxycholic Acid Impairs Human Sperm Quality and Function Through Oxidative Stress-Driven Damage
Abstract
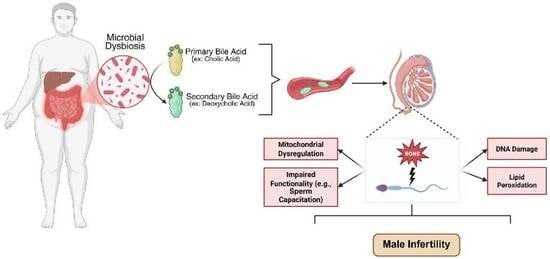
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Subjects and Sperm Sample Preparation
2.3. Sperm Viability and Motility Determinations
2.4. Acrosome Reaction (AR) Determination
2.5. SDS-PAGE and Immunoblotting
2.6. Sperm DNA Oxidation
2.7. Mitochondrial Membrane Potential
2.8. Lipid Peroxidation Determination
2.9. Determination of Mitochondrial Superoxide Production
2.10. Statistical Analysis
3. Results
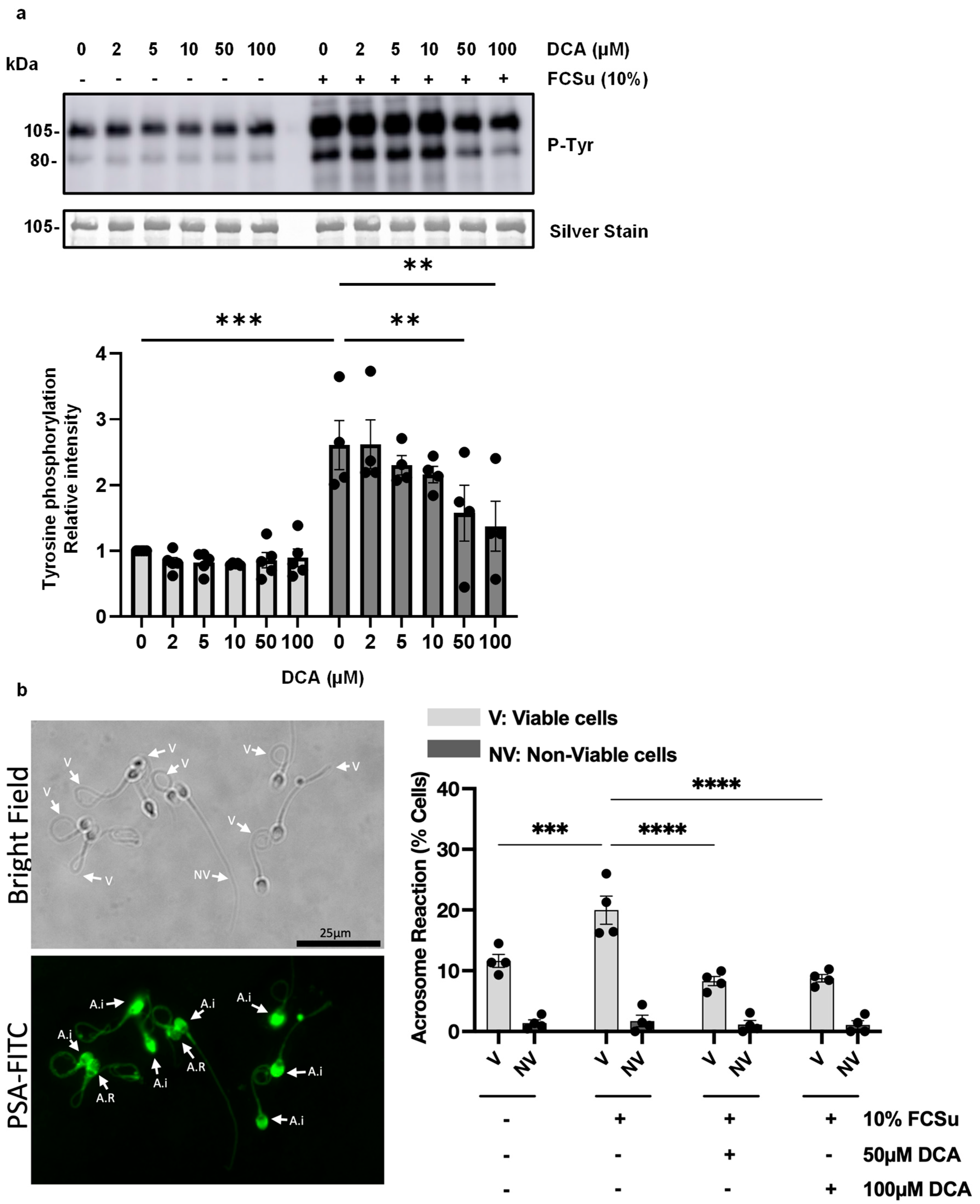
3.1. Deoxycholic Acid Impairs Sperm Capacitation
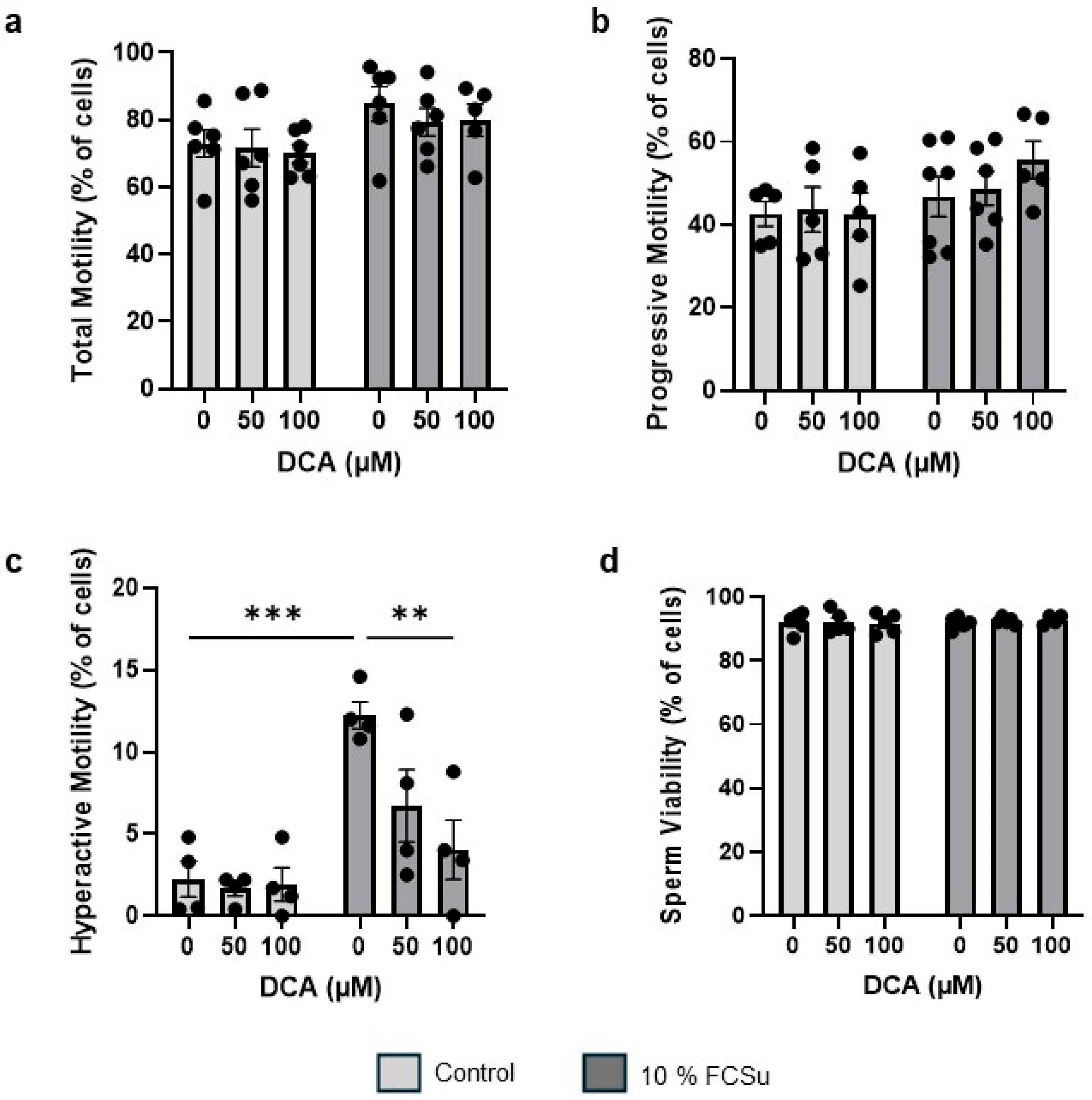
3.2. DCA Treatment Does Not Affect Sperm Total and Progressive Motility and Viability, Yet Impairs Hyperactive Motility
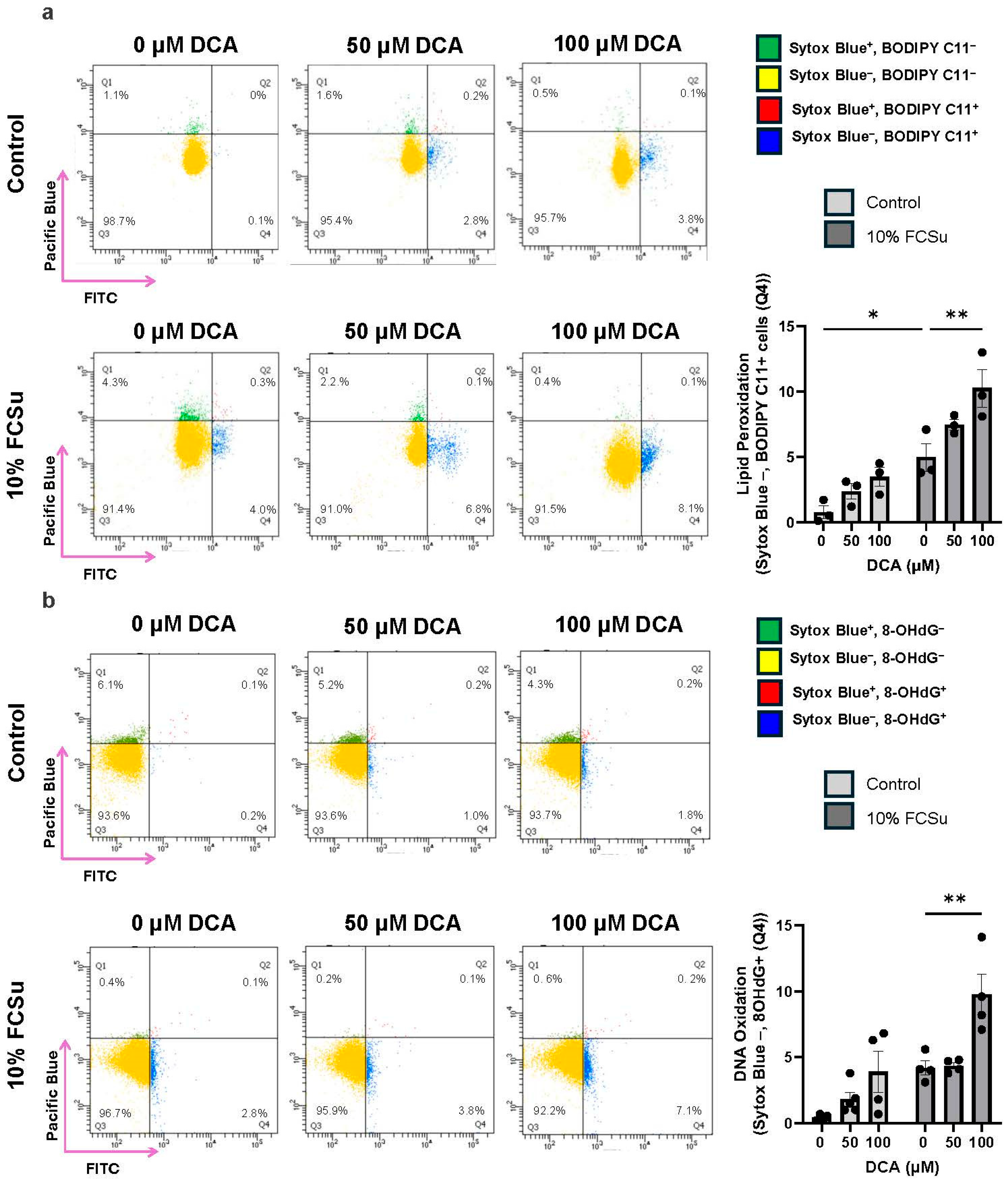
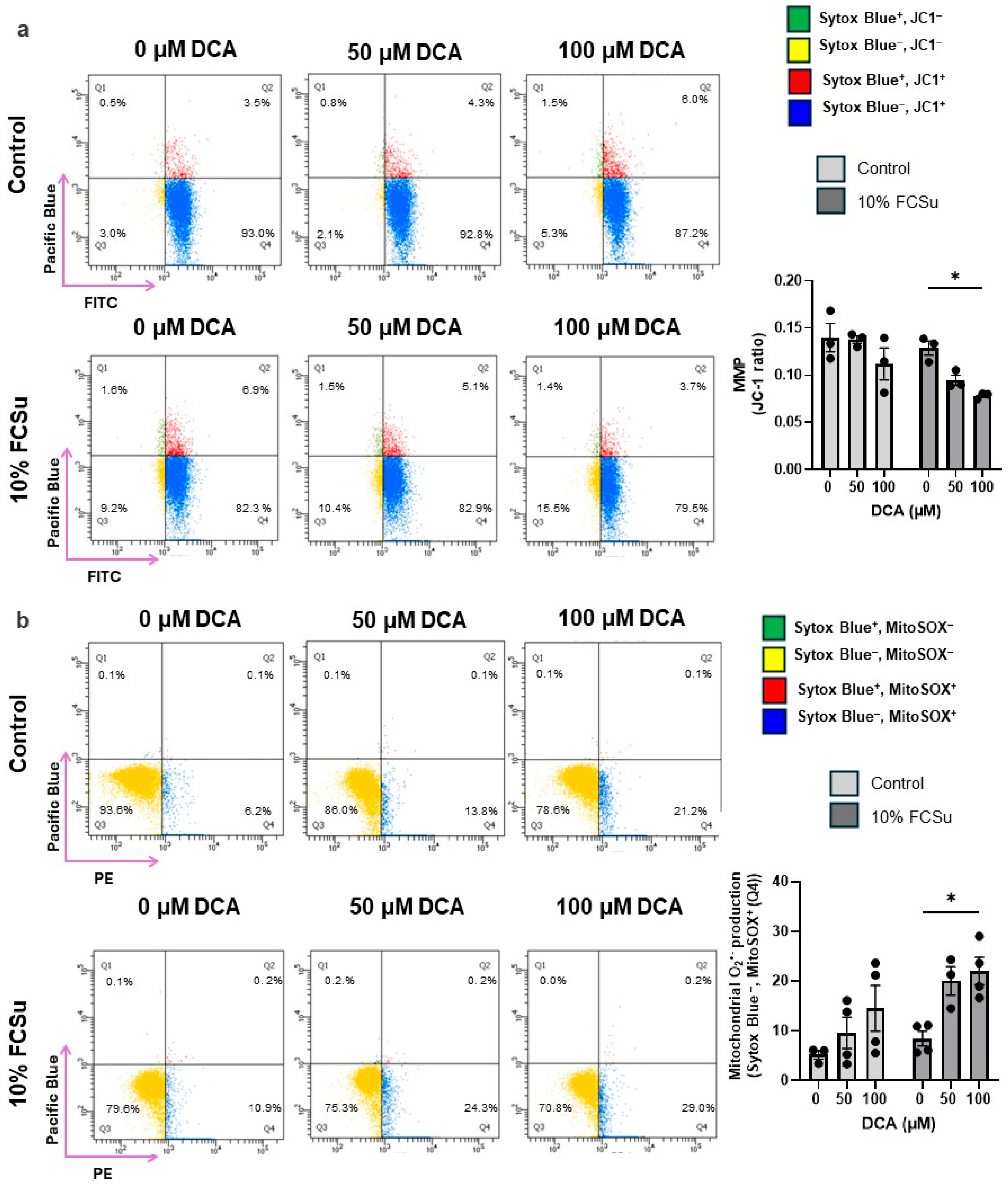
3.3. DCA Exposure Increases Oxidative Stress in Human Spermatozoa
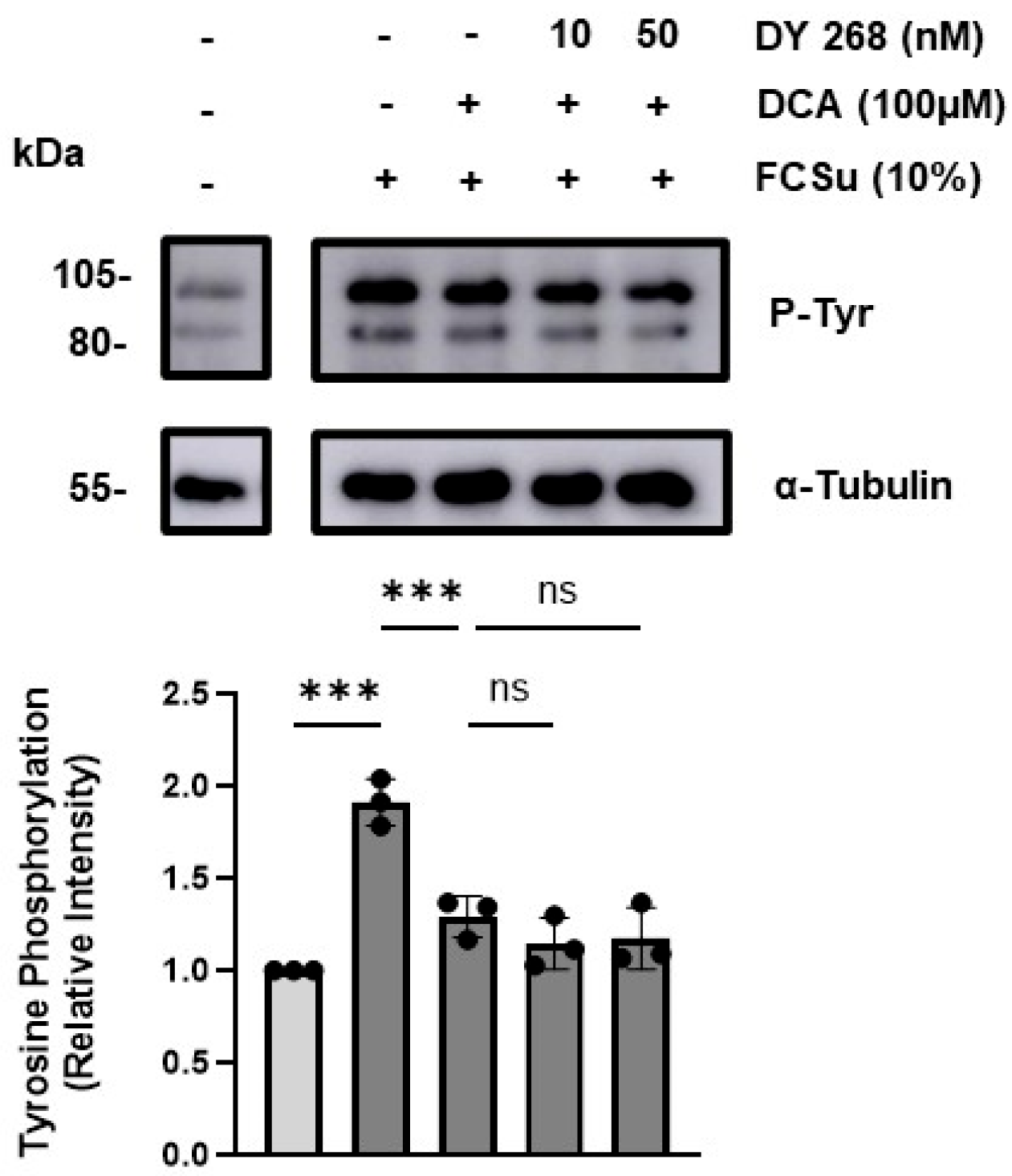
3.4. DCA Does Not Exert Its Effects via the Farnesoid X Receptor (FXR)
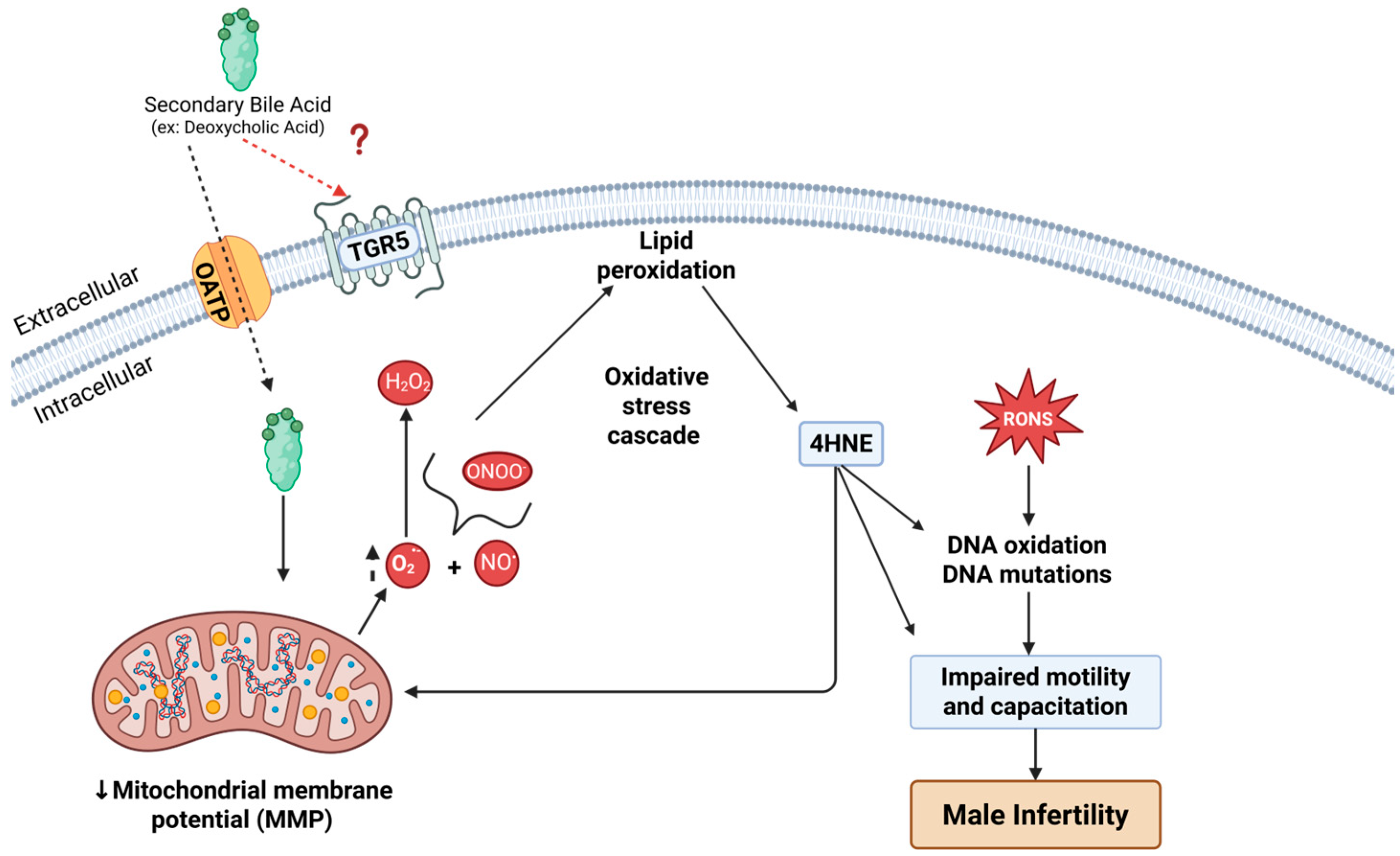
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 8-OHdG | 8-hydroxy-2′-deoxyguanosine |
| 4-HNE | 4-hydroxynonenal |
| ALH | Amplitude of lateral head displacement |
| AR | Acrosome reaction |
| ASBT | Apical sodium-dependent bile acid transporter |
| BSA | Bovine serum albumin |
| BWW | Biggers, Whitten, and Whittingham medium |
| CASA | Computer-assisted semen analysis |
| DAPI | 4′,6-diamidino-2-phenylindole |
| DCA | Deoxycholic acid |
| DNA | Deoxyribonucleic acid |
| DTT | Dithiothreitol |
| ECL | Enhanced chemiluminescence |
| FCSu | Fetal cord serum ultrafiltrate |
| FITC | Fluorescein isothiocyanate |
| FXR | Farnesoid X receptor |
| HBS | HEPES-buffered saline |
| HEPES | 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid |
| HOS | Hypo-osmotic swelling test |
| IC50 | Half maximal inhibitory concentration |
| IgG | Immunoglobulin G |
| IVF | In vitro fertilization |
| JC-1 | 5,5′,6,6′-Tetrachloro-1,1′,3,3′-tetraethylbenzimidazolylcarbocyanine iodide |
| LIN | Linearity |
| MMP | Mitochondrial membrane potential |
| NO | Nitric oxide radical |
| O2•− | Superoxide anion |
| OATP | Organic anion transporting polypeptides |
| OXPHOS | Oxidative phosphorylation |
| PBS | Phosphate-buffered saline |
| PSA | Pisum sativum agglutinin |
| PUFA | Polyunsaturated fatty acids |
| P-Tyr | Phospho-tyrosine |
| RNS | Reactive nitrogen species |
| ROOH | Lipid hydroperoxides |
| ROS | Reactive oxygen species |
| RONS | Reactive oxygen and nitrogen species |
| SEM | Standard error of the mean |
| STR | Straightness |
| TGR5 | G-protein coupled bile acid receptor 1 (Takeda G-protein receptor 5) |
| TTBS | Tris-buffered saline with Tween 20 |
| VAP | Average path velocity |
| VCL | Curvilinear velocity |
| WHO | World Health Organization |
References
- Collins, M.E. The impact of infertility on daily occupations and roles. J. Reprod. Infertil. 2019, 20, 24. [Google Scholar]
- Cox, C.; Thoma, M.; Tchangalova, N.; Mburu, G.; Bornstein, M.; Johnson, C.; Kiarie, J. Infertility prevalence and the methods of estimation from 1990 to 2021: A systematic review and meta-analysis. Hum. Reprod. Open 2022, 2022, hoac051. [Google Scholar] [CrossRef]
- World Health Organization. Infertility Prevalence Estimates, 1990–2021; World Health Organization: Geneva, Switzerland, 2023. [Google Scholar]
- Singh, K.; Jaiswal, D. Human male infertility: A complex multifactorial phenotype. Reprod. Sci. 2011, 18, 418–425. [Google Scholar] [CrossRef]
- Longo, V.; Forleo, A.; Provenzano, S.P.; Coppola, L.; Zara, V.; Ferramosca, A.; Siciliano, P.; Capone, S. Seminal VOCs Analysis Investigating Sperm Quality Decline—New Studies to Improve Male Fertility Contrasting Population Ageing. In Ambient Assisted Living: Italian Forum 2018; Springer: Cham, Switzerland, 2019; pp. 501–508. [Google Scholar]
- Aitken, R.J. Falling sperm counts twenty years on: Where are we now? Asian J. Androl. 2013, 15, 204. [Google Scholar] [CrossRef] [PubMed]
- Sahu, A.; Pajai, S. The impact of obesity on reproductive health and pregnancy outcomes. Cureus 2023, 15, e48882. [Google Scholar] [CrossRef] [PubMed]
- Davidson, L.M.; Millar, K.; Jones, C.; Fatum, M.; Coward, K. Deleterious effects of obesity upon the hormonal and molecular mechanisms controlling spermatogenesis and male fertility. Hum. Fertil. 2015, 18, 184–193. [Google Scholar] [CrossRef]
- Kyrou, I.; Randeva, H.S.; Tsigos, C.; Kaltsas, G.; Weickert, M.O. Clinical problems caused by obesity. In Endotext; Feingold, K.R., Anawalt, B., Blackman, M.R., Boyce, A., Chrousos, G., Corpas, E., de Herder, W.W., Dhatariya, K., Dungan, K., Hofland, J., et al., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2000. [Google Scholar]
- Mu, Y.; Yan, W.-J.; Yin, T.-L.; Yang, J. Curcumin ameliorates high-fat diet-induced spermatogenesis dysfunction. Mol. Med. Rep. 2016, 14, 3588–3594. [Google Scholar] [PubMed]
- Mu, Y.; Yan, W.-J.; Yin, T.-L.; Zhang, Y.; Li, J.; Yang, J. Diet-induced obesity impairs spermatogenesis: A potential role for autophagy. Sci. Rep. 2017, 7, 43475. [Google Scholar] [CrossRef] [PubMed]
- Bashandy, A.S. Effect of fixed oil of Nigella sativa on male fertility in normal and hyperlipidemic rats. Int. J. Pharmacol. 2007, 3, 27–33. [Google Scholar]
- Saez Lancellotti, T.E.; Boarelli, P.V.; Monclus, M.A.; Cabrillana, M.E.; Clementi, M.A.; Espínola, L.S.; Cid Barria, J.L.; Vincenti, A.E.; Santi, A.G.; Fornés, M.W. Hypercholesterolemia impaired sperm functionality in rabbits. PLoS ONE 2010, 5, e13457. [Google Scholar]
- Saez Lancellotti, T.E.; Boarelli, P.V.; Romero, A.A.; Funes, A.K.; Cid-Barria, M.; Cabrillana, M.E.; Monclus, M.A.; Simón, L.; Vicenti, A.E.; Fornés, M.W. Semen quality and sperm function loss by hypercholesterolemic diet was recovered by addition of olive oil to diet in rabbit. PLoS ONE 2013, 8, e52386. [Google Scholar] [CrossRef]
- Aziz, N.; Saleh, R.A.; Sharma, R.K.; Lewis-Jones, I.; Esfandiari, N.; Thomas, A.J., Jr.; Agarwal, A. Novel association between sperm reactive oxygen species production, sperm morphological defects, and the sperm deformity index. Fertil. Steril. 2004, 81, 349–354. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Gong, L.; Xu, J. Probiotics attenuate sperm damage induced by oxidative stress in rats. In Proceedings of the 2012 International Conference on Biomedical Engineering and Biotechnology, Macau, China, 28–30 May 2012; pp. 446–448. [Google Scholar]
- Ferramosca, A.; Conte, A.; Moscatelli, N.; Zara, V. A high-fat diet negatively affects rat sperm mitochondrial respiration. Andrology 2016, 4, 520–525. [Google Scholar] [CrossRef]
- Leisegang, K.; Sengupta, P.; Agarwal, A.; Henkel, R. Obesity and male infertility: Mechanisms and management. Andrologia 2021, 53, e13617. [Google Scholar] [CrossRef] [PubMed]
- Pushpendra, A.; Jain, G. Hyper-lipidemia and male fertility: A critical review of literature. Andrology 2015, 4, 141. [Google Scholar] [CrossRef]
- Zhang, K.; Lv, Z.; Jia, X.; Huang, D. Melatonin prevents testicular damage in hyperlipidaemic mice. Andrologia 2012, 44, 230–236. [Google Scholar] [CrossRef]
- Nenicu, A.; Lüers, G.H.; Kovacs, W.; Bergmann, M.; Baumgart-Vogt, E. Peroxisomes in human and mouse testis: Differential expression of peroxisomal proteins in germ cells and distinct somatic cell types of the testis. Biol. Reprod. 2007, 77, 1060–1072. [Google Scholar] [CrossRef]
- De Jonge, C. Biological basis for human capacitation—Revisited. Hum. Reprod. Update 2017, 23, 289–299. [Google Scholar] [CrossRef]
- O’Flaherty, C.; de Lamirande, E.; Gagnon, C. Positive role of reactive oxygen species in mammalian sperm capacitation: Triggering and modulation of phosphorylation events. Free Radic. Biol. Med. 2006, 41, 528–540. [Google Scholar] [CrossRef]
- Serafini, S.; O’Flaherty, C. Redox regulation to modulate phosphorylation events in human spermatozoa. Antioxid. Redox Signal. 2022, 37, 437–450. [Google Scholar] [CrossRef]
- Tamburrino, L.; Marchiani, S.; Muratori, M.; Luconi, M.; Baldi, E. Progesterone, spermatozoa and reproduction: An updated review. Mol. Cell. Endocrinol. 2020, 516, 110952. [Google Scholar] [CrossRef] [PubMed]
- de Lamirande, E.; Gagnon, C. Capacitation-associated production of superoxide anion by human spermatozoa. Free Radic. Biol. Med. 1995, 18, 487–495. [Google Scholar] [PubMed]
- Zini, A.; De Lamirande, E.; Gagnon, C. Reactive oxygen species in semen of infertile patients: Levels of superoxide dismutase-and catalase-like activities in seminal plasma and spermatozoa. Int. J. Androl. 1993, 16, 183–188. [Google Scholar] [PubMed]
- Storey, B.T. Biochemistry of the induction and prevention of lipoperoxidative damage in human spermatozoa. Mol. Hum. Reprod. 1997, 3, 203–213. [Google Scholar] [CrossRef]
- Aitken, R.J.; Gordon, E.; Harkiss, D.; Twigg, J.P.; Milne, P.; Jennings, Z.; Irvine, D.S. Relative impact of oxidative stress on the functional competence and genomic integrity of human spermatozoa. Biol. Reprod. 1998, 59, 1037–1046. [Google Scholar] [CrossRef]
- Sikka, S.C.; Rajasekaran, M.; Hellstrom, W.J. Role of oxidative stress and antioxidants in male infertility. J. Androl. 1995, 16, 464–468. [Google Scholar] [CrossRef]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative stress: Harms and benefits for human health. Oxidative Med. Cell. Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef]
- Crovesy, L.; Masterson, D.; Rosado, E.L. Profile of the gut microbiota of adults with obesity: A systematic review. Eur. J. Clin. Nutr. 2020, 74, 1251–1262. [Google Scholar] [CrossRef]
- Bäckhed, F.; Ding, H.; Wang, T.; Hooper, L.V.; Koh, G.Y.; Nagy, A.; Semenkovich, C.F.; Gordon, J.I. The gut microbiota as an environmental factor that regulates fat storage. Proc. Natl. Acad. Sci. USA 2004, 101, 15718–15723. [Google Scholar] [CrossRef]
- Turnbaugh, P.J.; Ley, R.E.; Mahowald, M.A.; Magrini, V.; Mardis, E.R.; Gordon, J.I. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 2006, 444, 1027–1031. [Google Scholar] [CrossRef]
- Zeyad, A.; Hamad, M.; Amor, H.; Hammadeh, M.E. Relationships between bacteriospermia, DNA integrity, nuclear protamine alteration, sperm quality and ICSI outcome. Reprod. Biol. 2018, 18, 115–121. [Google Scholar] [CrossRef]
- Ricci, S.; De Giorgi, S.; Lazzeri, E.; Luddi, A.; Rossi, S.; Piomboni, P.; De Leo, V.; Pozzi, G. Impact of asymptomatic genital tract infections on in vitro Fertilization (IVF) outcome. PLoS ONE 2018, 13, e0207684. [Google Scholar] [CrossRef]
- Chen, M.L.; Takeda, K.; Sundrud, M.S. Emerging roles of bile acids in mucosal immunity and inflammation. Mucosal Immunol. 2019, 12, 851–861. [Google Scholar] [CrossRef]
- Xu, J.; Huang, D.; Xu, X.; Wu, X.; Liu, L.; Niu, W.; Lu, L.; Zhou, H. An elevated deoxycholic acid level induced by high-fat feeding damages intestinal stem cells by reducing the ileal IL-22. Biochem. Biophys. Res. Commun. 2021, 579, 153–160. [Google Scholar] [CrossRef]
- Barrasa, J.I.; Olmo, N.; Pérez-Ramos, P.; Santiago-Gómez, A.; Lecona, E.; Turnay, J.; Antonia Lizarbe, M. Deoxycholic and chenodeoxycholic bile acids induce apoptosis via oxidative stress in human colon adenocarcinoma cells. Apoptosis 2011, 16, 1054–1067. [Google Scholar] [CrossRef] [PubMed]
- de Lamirande, E.; Lamothe, G. Reactive oxygen-induced reactive oxygen formation during human sperm capacitation. Free Radic. Biol. Med. 2009, 46, 502–510. [Google Scholar] [CrossRef] [PubMed]
- Biggers, J.; Whitten, W.; Whittingham, D. The culture of mouse embryos in vitro. In Methods in Mammalian Enbryology; W-H-Freeman-Co.: San Francisco, CA, USA, 1971. [Google Scholar]
- de Lamirande, E.; Jiang, H.; Zini, A.; Kodama, H.; Gagnon, C. Reactive oxygen species and sperm physiology. Rev. Reprod. 1997, 2, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Baptissart, M.; Vega, A.; Martinot, E.; Pommier, A.J.; Houten, S.M.; Marceau, G.; de Haze, A.; Baron, S.; Schoonjans, K.; Lobaccaro, J.M.; et al. Bile acids alter male fertility through G-protein-coupled bile acid receptor 1 signaling pathways in mice. Hepatology 2014, 60, 1054–1065. [Google Scholar] [CrossRef]
- Zhao, S.; Gong, Z.; Zhou, J.; Tian, C.; Gao, Y.; Xu, C.; Chen, Y.; Cai, W.; Wu, J. Deoxycholic Acid Triggers NLRP3 Inflammasome Activation and Aggravates DSS-Induced Colitis in Mice. Front. Immunol. 2016, 7, 536. [Google Scholar] [CrossRef]
- Leclerc, P.; de Lamirande, E.; Gagnon, C. Cyclic adenosine 3′, 5′ monophosphate-dependent regulation of protein tyrosine phosphorylation in relation to human sperm capacitation and motility. Biol. Reprod. 1996, 55, 684–692. [Google Scholar] [CrossRef]
- WHO. WHO Laboratory Manual for the Examination and Processing of Human Semen; World Health Organization: Geneva, Switzerland, 2021. [Google Scholar]
- Ramu, S.; Jeyendran, R.S. The Hypo-Osmotic Swelling Test for Evaluation of Sperm Membrane Integrity. In Spermatogenesis. Methods in Molecular Biology; Carrell, D., Aston, K., Eds.; Humana Press: Totowa, NJ, USA, 2013; Volume 927, pp. 21–25. [Google Scholar]
- Serafini, S.; O’Flaherty, C. Sphingolipids modulate redox signalling during human sperm capacitation. Hum. Reprod. 2025, 40, 210–225. [Google Scholar] [CrossRef]
- Serafini, S.; O’Flaherty, C. Dysregulation of sphingolipid and cholesterol homeostasis imposes oxidative stress in human spermatozoa. Redox Biol. 2025, 84, 103669. [Google Scholar] [CrossRef] [PubMed]
- Mortimer, S.T.; Mortimer, D. Kinematics of human spermatozoa incubated under capacitating conditions. J. Androl. 1990, 11, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Baldi, E.; Casano, R.; Falsetti, C.; Krausz, C.; Maggi, M.; Forti, G. Intracellular calcium accumulation and responsiveness to progesterone in capacitating human spermatozoa. J. Androl. 1991, 12, 323–330. [Google Scholar] [CrossRef]
- Fernandez, M.C.; Yu, A.; Moawad, A.R.; O’Flaherty, C. Peroxiredoxin 6 regulates the phosphoinositide 3-kinase/AKT pathway to maintain human sperm viability. Mol. Hum. Reprod. 2019, 25, 787–796. [Google Scholar] [CrossRef] [PubMed]
- Brouwers, J.F.; Gadella, B.M. In situ detection and localization of lipid peroxidation in individual bovine sperm cells. Free Radic. Biol. Med. 2003, 35, 1382–1391. [Google Scholar] [CrossRef]
- Drummen, G.P.; Gadella, B.M.; Post, J.A.; Brouwers, J.F. Mass spectrometric characterization of the oxidation of the fluorescent lipid peroxidation reporter molecule C11-BODIPY(581/591). Free Radic. Biol. Med. 2004, 36, 1635–1644. [Google Scholar] [CrossRef]
- Lee, D.; Moawad, A.R.; Morielli, T.; Fernandez, M.C.; O’Flaherty, C. Peroxiredoxins prevent oxidative stress during human sperm capacitation. Mol. Hum. Reprod. 2017, 23, 106–115. [Google Scholar] [CrossRef]
- Moawad, A.R.; Fernandez, M.C.; Scarlata, E.; Dodia, C.; Feinstein, S.I.; Fisher, A.B.; O’Flaherty, C. Deficiency of peroxiredoxin 6 or inhibition of its phospholipase A2 activity impair the in vitro sperm fertilizing competence in mice. Sci. Rep. 2017, 7, 12994. [Google Scholar] [CrossRef]
- Guthrie, H.D.; Welch, G.R. Using Fluorescence-Activated Flow Cytometry to Determine Reactive Oxygen Species Formation and Membrane Lipid Peroxidation in Viable Boar Spermatozoa. In Advanced Protocols in Oxidative Stress II; Armstrong, D., Ed.; Humana Press: Totowa, NJ, USA, 2010; pp. 163–171. [Google Scholar]
- Koppers, A.J.; De Iuliis, G.N.; Finnie, J.M.; McLaughlin, E.A.; Aitken, R.J. Significance of mitochondrial reactive oxygen species in the generation of oxidative stress in spermatozoa. J. Clin. Endocrinol. Metab. 2008, 93, 3199–3207. [Google Scholar] [CrossRef]
- O’Flaherty, C.; de Lamirande, E.; Gagnon, C. Reactive oxygen species modulate independent protein phosphorylation pathways during human sperm capacitation. Free Radic. Biol. Med. 2006, 40, 1045–1055. [Google Scholar] [CrossRef]
- Agarwal, A.; Makker, K.; Sharma, R. Clinical Relevance of Oxidative Stress in Male Factor Infertility: An Update. Am. J. Reprod. Immunol. 2008, 59, 2–11. [Google Scholar] [CrossRef] [PubMed]
- Aitken, R. Sperm function tests and fertility. Int. J. Androl. 2006, 29, 69–75. [Google Scholar] [CrossRef]
- Jean-Louis, S.; Akare, S.; Ali, M.A.; Mash, E.A., Jr.; Meuillet, E.; Martinez, J.D. Deoxycholic acid induces intracellular signaling through membrane perturbations. J. Biol. Chem. 2006, 281, 14948–14960. [Google Scholar] [CrossRef]
- Zhou, Y.; Maxwell, K.N.; Sezgin, E.; Lu, M.; Liang, H.; Hancock, J.F.; Dial, E.J.; Lichtenberger, L.M.; Levental, I. Bile acids modulate signaling by functional perturbation of plasma membrane domains. J. Biol. Chem. 2013, 288, 35660–35670. [Google Scholar] [CrossRef] [PubMed]
- Dawson, P.A.; Lan, T.; Rao, A. Bile acid transporters. J. Lipid Res. 2009, 50, 2340–2357. [Google Scholar] [CrossRef] [PubMed]
- Klein, D.M.; Cherrington, N.J. Organic and inorganic transporters of the testis: A review. Spermatogenesis 2014, 4, e979653. [Google Scholar] [CrossRef][Green Version]
- Sousa, T.; Castro, R.E.; Pinto, S.N.; Coutinho, A.; Lucas, S.D.; Moreira, R.; Rodrigues, C.M.P.; Prieto, M.; Fernandes, F. Deoxycholic acid modulates cell death signaling through changes in mitochondrial membrane properties. J. Lipid Res. 2015, 56, 2158–2171. [Google Scholar] [CrossRef]
- Malivindi, R.; Santoro, M.; De Rose, D.; Panza, S.; Gervasi, S.; Rago, V.; Aquila, S. Activated-farnesoid X receptor (FXR) expressed in human sperm alters its fertilising ability. Reproduction 2018, 156, 249–259. [Google Scholar] [CrossRef]
- Abrigo, J.; Olguín, H.; Tacchi, F.; Orozco-Aguilar, J.; Valero-Breton, M.; Soto, J.; Castro-Sepúlveda, M.; Elorza, A.A.; Simon, F.; Cabello-Verrugio, C. Cholic and deoxycholic acids induce mitochondrial dysfunction, impaired biogenesis and autophagic flux in skeletal muscle cells. Biol. Res. 2023, 56, 30. [Google Scholar] [CrossRef]
- Yin, H.; Xu, L.; Porter, N.A. Free radical lipid peroxidation: Mechanisms and analysis. Chem. Rev. 2011, 111, 5944–5972. [Google Scholar] [CrossRef] [PubMed]
- Guéraud, F.; Atalay, M.; Bresgen, N.; Cipak, A.; Eckl, P.M.; Huc, L.; Jouanin, I.; Siems, W.; Uchida, K. Chemistry and biochemistry of lipid peroxidation products. Free Radic. Res. 2010, 44, 1098–1124. [Google Scholar] [CrossRef] [PubMed]
- Romero, F.J.; Bosch-Morell, F.; Romero, M.J.; Jareño, E.J.; Romero, B.; Marín, N.; Romá, J. Lipid peroxidation products and antioxidants in human disease. Environ. Health Perspect. 1998, 106, 1229–1234. [Google Scholar] [PubMed]
- Bakos, H.; Mitchell, M.; Setchell, B.; Lane, M. The effect of paternal diet-induced obesity on sperm function and fertilization in a mouse model. Int. J. Androl. 2011, 34, 402–410. [Google Scholar] [CrossRef]
- Baker, M.A.; Weinberg, A.; Hetherington, L.; Villaverde, A.-I.; Velkov, T.; Baell, J.; Gordon, C.P. Defining the mechanisms by which the reactive oxygen species by-product, 4-hydroxynonenal, affects human sperm cell function. Biol. Reprod. 2015, 92, 108. [Google Scholar] [CrossRef]
- Alvarez, J.G.; Aitken, R.J. Lipid peroxidation in human spermatozoa. In Studies on Men’s Health and Fertility; Humana Press: Totowa, NJ, USA, 2012; pp. 119–130. [Google Scholar]
- Zhong, H.; Yin, H. Role of lipid peroxidation derived 4-hydroxynonenal (4-HNE) in cancer: Focusing on mitochondria. Redox Biol. 2015, 4, 193–199. [Google Scholar] [CrossRef]
- Kino, K.; Sugiyama, H. GC→CG transversion mutation might be caused by 8-oxoguanine oxidation product. Nucleic Acids Symp. Ser. 2000, 44, 139–140. [Google Scholar] [CrossRef]
- Wyck, S.; Herrera, C.; Requena, C.E.; Bittner, L.; Hajkova, P.; Bollwein, H.; Santoro, R. Oxidative stress in sperm affects the epigenetic reprogramming in early embryonic development. Epigenetics Chromatin 2018, 11, 60. [Google Scholar] [CrossRef]
- Kaltsas, A.; Zikopoulos, A.; Kojovic, V.; Dimitriadis, F.; Sofikitis, N.; Chrisofos, M.; Zachariou, A. Paternal Contributions to Recurrent Pregnancy Loss: Mechanisms, Biomarkers, and Therapeutic Approaches. Medicina 2024, 60, 1920. [Google Scholar] [CrossRef]
- Moazamian, A.; Saez, F.; Drevet, J.R.; Aitken, R.J.; Gharagozloo, P. Redox-Driven Epigenetic Modifications in Sperm: Unraveling Paternal Influences on Embryo Development and Transgenerational Health. Antioxidants 2025, 14, 570. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Serafini, S.; Pranov, E.; Timova Bauer, K.; Onochie, C.; O’Flaherty, C. Deoxycholic Acid Impairs Human Sperm Quality and Function Through Oxidative Stress-Driven Damage. Antioxidants 2025, 14, 1271. https://doi.org/10.3390/antiox14111271
Serafini S, Pranov E, Timova Bauer K, Onochie C, O’Flaherty C. Deoxycholic Acid Impairs Human Sperm Quality and Function Through Oxidative Stress-Driven Damage. Antioxidants. 2025; 14(11):1271. https://doi.org/10.3390/antiox14111271
Chicago/Turabian StyleSerafini, Steven, Elizabeth Pranov, Kaya Timova Bauer, Chika Onochie, and Cristian O’Flaherty. 2025. "Deoxycholic Acid Impairs Human Sperm Quality and Function Through Oxidative Stress-Driven Damage" Antioxidants 14, no. 11: 1271. https://doi.org/10.3390/antiox14111271
APA StyleSerafini, S., Pranov, E., Timova Bauer, K., Onochie, C., & O’Flaherty, C. (2025). Deoxycholic Acid Impairs Human Sperm Quality and Function Through Oxidative Stress-Driven Damage. Antioxidants, 14(11), 1271. https://doi.org/10.3390/antiox14111271