Calafate (Berberis buxifolia Lam.) Berry as a Source of Bioactive Compounds with Potential Health-Promoting Effects: A Critical Review
Abstract
1. Introduction
2. General Aspects of Calafate Berry
2.1. Taxonomy and Botanical Aspects
2.2. Distribution, Habitat, and Cultivation Features
2.3. Traditional Use
3. Nutritional Composition of Calafate Berry
3.1. Carbohydrates
3.2. Proteins
3.3. Vitamin C
3.4. Lipids
3.5. Fatty Acids
| Berry | Calafate | Maqui | Cranberry | Blackberry | Strawberry | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| References | [38] | [39] | [2] | [40] | [41] | [42] | [43] | |||
| Location | Magallanes Region | Aysén Region | Punta Arenas | Ñuble Region | Aysén Region | Aysén Region | State of Washington | Panevėžys | ||
| Country | Chile | Chile | Chile | Chile | Chile | Chile | USA | Lithuania | ||
| Fatty acids | Pomace | Pomace | Fresh fruit | Seeds | Fresh fruit | Pomace | Seeds | Pomace | ||
| % | % | mg/g | % | % w/w | % w/w | % | % | % | ||
| C12:0 | Lauric acid | - | - | - | 0.23 ± 0.03 | 0.51 ± 0.02 | 0.48 ± 0.01 | 0.14 | - | - |
| C14:0 | Myristic acid | - | - | 0.16 ± 0.01 | 0.65 ± 0.02 | 1.07 ± 0.04 | 1.04 ± 0.00 | 0.08 | - | - |
| C15:0 | Pentadecanoic acid | - | - | - | - | 0.02 ± 0.00 | 0.03 ± 0.01 | - | - | - |
| C16:0 | Palmitic acid | 7.93 | 8.1 ± 0.8 | 0.08 ± 0.02 | 8.55 ± 0.01 | 8.63 ± 0.11 | 9.68 ± 0.02 | 5.38 | 4.56 ± 0.03 | 3.66 ± 0.46 |
| C16:1 | Palmitoleic acid | - | - | - | 0.26 ± 0.01 | 0.35 ± 0.01 | 0.50 ± 0.01 | - | 0.07 ± 0.00 | 0.14 ± 0.02 |
| C18:0 | Stearic acid | 2.58 | 2.7 ± 0.1 | 0.06 ± 0.00 | 2.63 ± 0.05 | 3.47 ± 0.09 | 3.46 ± 0.04 | 1.25 | 3.76 ± 0.06 | 1.28 ± 0.17 |
| C18:1 | Oleic acid | 19.18 | 22.2 ± 0.7 | 0.04 ± 0.01 | 39.71 ± 0.04 | 33.49 ± 0.11 | 32.26 ± 0.09 | 25.30 | 21.14 ± 0.36 | 12.91 ± 1.51 |
| C18:1 | Vaccenic acid | 0.61 | - | - | - | 3.81 ± 0.01 | 3.5 ± 0.05 | - | - | - |
| C18:2 | Linoleic acid | 31.79 | 30.0 ± 0.1 | 0.07 ± 0.03 | 45.81 ± 0.05 | 46.00 ± 0.35 | 44.89 ± 0.33 | 37.68 | 59.44 ± 0.93 | 40.67 ± 4.81 |
| C18:3 | Linolenic acid | 37.9 | 36.7 ± 0.2 | 0.09 ± 0.04 | 2.35 ± 0.02 | 1.96 ± 0.04 | 3.39 ± 0.21 | 30.09 | 9.14 ± 0.15 | 33.90 ± 3.98 |
| C20:1 | Eicosenoic acid | - | - | - | - | 0.24 ± 0.01 | 0.22 ± 0.00 | - | 0.55 ± 0.13 | 0.60 ± 0.09 |
| C20:4 | Arachidonic acid | - | - | - | - | - | - | 0.07 | - | - |
| C22:0 | Behenic acid | - | - | - | - | 0.18 ± 0.01 | 0.27 ± 0.02 | - | - | 0.13 ± 0.02 |
| C22:1 | Erucic acid | - | - | 0.08 ± 0.01 | - | - | - | - | - | - |
| C24:0 | Lignoceric acid | - | - | - | - | 0.13 ± 0.01 | 0.11 ± 0.13 | - | - | - |
| SFA | 10.52 | 10.8 | 0.3 | 12.06 | 14.01 | 15.07 | 6.92 | 8.32 | 5.07 | |
| MUFA | 19.18 | 22.2 | 0.12 | 39.97 | 37.89 | 36.48 | 25.14 | 21.76 | 13.65 | |
| PUFA | 70.31 | 66.7 | 0.16 | 48.16 | 47.96 | 48.28 | 67.98 | 68.58 | 74.57 | |
| PUFA/SFA ratio | 6.69 | 6.18 | 0.53 | 3.99 | 3.42 | 3.20 | 9.88 | 8.24 | 14.71 | |
3.6. Tocopherols
| Berry | Calafate | Maqui | Cranberry | Blueberry | Blackcurrant | ||||
|---|---|---|---|---|---|---|---|---|---|
| References | [38] | [39] | [66] | [40] | [67] | [38] | [68] | [42] | [69] |
| Location | Magallanes Region | Aysén Region | Bio-Bio Region | Ñuble Region | Lanco | Magallanes Region | State of Washington | Rijkevorsel | |
| Country | Chile | Chile | Chile | Chile | Chile | Chile | EC * | USA | Belgium |
| Compound | Pomace (oil) | Pomace (oil) | Dry fruit | Seeds (oil) | Dry fruit (oil) | Pomace (oil) | Press residues | Seed (oil) | Seed (oil) |
| ppm | ppm | ppm | ppm | ppm | ppm | ppm | ppm | ppm | |
| α-tocopherol | 112.10 ± 0.11 | 75.4 ± 3.8 | 4.5 ± 0.1 | 169.33 ± 11.39 | 735 ± 19 | 444.20 ± 0.13 | 8.7 | 4.4 ± 0.2 | 335.1 |
| β-tocopherol | 11.80 ± 0.14 | - | - | 7.78 ± 2.34 | 5 ± 1 | 14.79 ± 0.20 | 1.6 | - | 34.5 |
| γ-tocopherol | 3.03 ± 0.12 | - | - | 56.76 ± 2.98 | 97 ± 4 | 18.98 ± 0.09 | 1.5 | 34.4 ± 0.1 | 12.2 |
| δ-tocopherol | 4.28 ± 0.05 | - | - | 13.58 ± 3.5 | 7 ± 1 | 1.14 ± 0.05 | - | - | 239.9 |
| α-tocotrienol | 67.31 ± 0.05 | 127.7 ± 4.0 | - | 323.80 ± 20.3 | 53 ± 0 | 46.31 ± 0.05 | 10.1 | - | - |
| β-tocotrienol | 116.84 ± 0.1 | - | - | 20.20 ± 5.99 | - | 27.43 ± 0.10 | - | - | - |
| γ-tocotrienol | 13.84 ± 0.12 | 203.7 ± 10.5 | - | 5.74 ± 1.05 | - | 23.02 ± 0.15 | 39.2 | 330.4 ± 11.4 | - |
| δ-tocotrienol | - | - | - | 53.9 ± 7.42 | - | - | - | 6.0 ± 1.0 | - |
| Total tocols | 329.20 ± 0.11 | 406.4 | - | 327.3 ± 19.32 | 897 | 575.86 ± 0.12 | 61.1 | 375.2 ± 12.4 | 621.7 |
4. Phenolic Compounds in Calafate Berry
4.1. Polyphenols
4.2. Antioxidant Capacity
4.3. Anthocyanin
5. Calafate Berry and Its Health-Promoting Effects
5.1. Anti-Inflammatory Effect
5.2. Hypoglycemic Effect
5.3. Antioxidant Activity
5.4. Antimicrobial Activity
5.5. CVD Risk-Reducing Effects Potential
6. Potential Bioactivities Demonstrated in Preliminary Studies
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Coklar, H.; Akbulut, M. Anthocyanins and phenolic compounds of Mahonia aquifolium berries and their contributions to antioxidant activity. J. Funct. Food 2017, 35, 166–174. [Google Scholar] [CrossRef]
- Olivares-Caro, L.; Radojkovic, C.; Chau, S.Y.; Nova, D.; Bustamante, L.; Neira, J.Y.; Perez, A.J.; Mardones, C. Berberis microphylla G. forst (Calafate) berry extract reduces oxidative stress and lipid peroxidation of human LDL. Antioxidants 2020, 9, 1171. [Google Scholar] [CrossRef] [PubMed]
- Chamorro, M.; Ladio, A. Native and exotic plants with edible fleshy fruits utilized in Patagonia and their role as sources of local functional foods. BMC Complement. Med. Ther. 2020, 20, 155. [Google Scholar] [CrossRef] [PubMed]
- Armas-Ricard, M.; Quinán-Cárdenas, F.; Sanhueza, H.; Pérez-Vidal, R.; Mayorga-Lobos, C.; Ramírez-Rodríguez, O. Phytochemical screening and antioxidant activity of seven native species growing in the forests of southern Chilean Patagonia. Molecules 2021, 26, 6722. [Google Scholar] [CrossRef]
- Vidal-San Martín, C.; Bastías-Montes, J.M.; Villagra-Jorquera, C.; Salinas-Huenchulao, G.; Flores-Ríos, A.; Gonzáles-Díaz, N.; Tamarit-Pino, Y.; Muñoz-Fariña, O.; Quevedo-León, R. Effect of cryoconcentration assisted by centrifugation-filtration on bioactive compounds and microbiological quality of aqueous maqui (Aristotelia chilensis (Mol.) Stuntz) and Calafate (Berberis microphylla G. Forst) extracts pretreated with high-pressure homogenization. Processes 2021, 9, 692. [Google Scholar]
- Furrianca, M.; Alvear, M.; Zambrano, T.; Fajardo, V.; Salazar, L. Hypoglycemic effect of Berberis microphylla G forst root extract. Trop. J. Pharm. Res. 2007, 16, 2179–2184. [Google Scholar] [CrossRef]
- Olivares-Caro, L.; Nova-Baza, D.; Radojkovic, C.; Bustamante, L.; Duran, D.; Mennickent, D.; Melin, V.; Contreras, D.; Perez, A.J.; Mardones, C. Berberis microphylla G. Forst Intake Reduces the Cardiovascular Disease Plasmatic Markers Associated with a High-Fat Diet in a Mice Model. Antioxidants 2023, 12, 304. [Google Scholar] [CrossRef]
- Duarte, L.; Villanueva, V.; Barroux, R.; Orellana, J.F.; Poblete-Aro, C.; Gotteland, M.; Castro, M.; Magne, F.; Garcia-Diaz, D.F. Acute administration of Calafate (Berberis microphylla) extract induces the expression of thermogenic markers and modulates gut microbiota in mice fed a high-fat chow diet. Lifestyle Genom. 2024, 17, 72–81. [Google Scholar] [CrossRef]
- Brito, A.; Areche, C.; Sepúlveda, B.; Kennelly, E.J.; Simirgiotis, M.J. Anthocyanin characterization, total phenolic quantification and antioxidant features of some Chilean edible berry extracts. Molecules 2014, 19, 10936–10955. [Google Scholar] [CrossRef]
- Ojeda, A.; Hirzel, J.; Pino, M.T.; Mc Leod, C.; Águila, K. Composición y evolución nutricional del calafate en la Región de Magallanes, Punta Arenas, Chile: Informativo INIA Kampenaike, no. 68 (Código BIP30136585-0). Available online: https://hdl.handle.net/20.500.14001/4680 (accessed on 2 May 2025).
- Radice, S.; Alonso, M.; Arena, M.E. Berberis microphylla: A species with phenotypic plasticity in different climatic conditions. Int. J. Agric. Biol. 2018, 20, 2221–2229. [Google Scholar]
- McLeod, C.; Ojeda, A.; Moreno, S.; Molina, A.; Pino, M.T. Seed germination and in vitro propagation of different Berberis microphylla G. Forst accessions from Magellan’s Region in Chile. Acta Hortic. 2017, 1172, 225–230. [Google Scholar] [CrossRef]
- Pino, M.T.; Zamora, O.; Mc Leod, C.; Aguila, K.; Ojeda, A.; Vergara, C. Calafate: Propiedades del fruto y su potencial como ingredient. Inf. INIA Kampenaike 2018, 78, 1–4. [Google Scholar]
- Domínguez, E.; Mc Leod, C.; Pino, T.; Sepúlveda, P.; Aguila, K.; Ojeda, A. Funciones y servicios del calafate en la región del Magallanes. Inf. INIA Kampenaike 2017, 67, 1–6. [Google Scholar]
- Furrianca, M.; Alvear, M.; Zambrano, T.; Fajardo, V.; Salazar, L. Phytochemical constituents of the root of Berberis microphylla. Asian J. Pharm. Clin. Res. 2007, 10, 225–227. [Google Scholar]
- Valdebenito, G. Uso y valor de los Productos Forestales No Madereros (PFNM) en Chile. Cienc. Investig. For. CIFOR 2020, 26, 93–107. [Google Scholar] [CrossRef]
- Ruiz, A.; Hermosín-Gutiérrez, I.; Mardones, C.; Vergara, C.; Herlitz, E.; Vega, M.; Dorau, C.; Winterhalter, P.; von Baer, D. Polyphenols and antioxidant activity of calafate (Berberis microphylla) fruits and other native berries from Southern Chile. J. Agric. Food Chem. 2010, 58, 6081–6089. [Google Scholar] [CrossRef]
- Ruiz, A.; Meneses, M.; Varas, B.; Araya, J.; Vergara, C.; von Baer, D.; Hinrichsen, P.; Mardones, C. Calafate (Berberis microphylla G. Forst) Populations from Chilean Patagonia exhibit similar structuring at the genetic and metabolic levels. Horticulturae 2024, 10, 458. [Google Scholar] [CrossRef]
- Orellana-Palma, P.; Tobar-Bolaños, G.; Casas-Forero, N.; Zúñiga, R.N.; Petzold, G. Quality attributes of cryoconcentrated calafate (Berberis microphylla) juice during refrigerated storage. Foods 2020, 9, 1314. [Google Scholar] [CrossRef]
- Romero-Román, M.E.; Schoebitz, M.; Fuentealba, J.; García-Viguera, C.; Belchí, M.D.L. Phenolic compounds in calafate berries encapsulated by spray drying: Neuroprotection potential into the ingredient. Antioxidants 2021, 10, 1830. [Google Scholar] [CrossRef]
- Instituto Interamericano de Cooperación para la Agricultura. CALAFATE—Berberis microphylla; PROCISUR: Montevideo, Uruguay, 2018. [Google Scholar]
- Secreto de la Patagonia. Available online: https://www.secretodelapatagonia.cl/ (accessed on 27 July 2024).
- Alimag. Available online: https://alimag.cl (accessed on 27 July 2024).
- Cerveza Austral Calafate. Available online: https://cervezaaustral.cl/calafate/ (accessed on 27 July 2024).
- Nativ for Life. Available online: https://nativforlife.cl/products/ (accessed on 27 July 2024).
- Bayas del Sur. Available online: https://www.bayasdelsur.com (accessed on 27 July 2024).
- Boeri, P.; Piñuel, L.; Dalzotto, D.; Monasterio, R.; Fontana, A.; Sharry, S.; Carrillo, W. Argentine Patagonia barberry chemical composition and evaluation of its antioxidant capacity. J. Food Biochem. 2020, 44, e13254. [Google Scholar] [CrossRef]
- Mariangel, E.; Reyes-Diaz, M.; Lobos, W.; Bensch, E.; Schalchli, H.; Ibarra, P. The antioxidant properties of calafate (Berberis microphylla) fruits from four different locations in southern Chile. Cienc. Investig. Agrar. 2013, 40, 161–170. [Google Scholar] [CrossRef]
- Varas, B.; Castro, M.H.; Rodriguez, R.; Von Baer, D.; Mardones, C.; Hinrichsen, P. Identification and Characterization of Microsatellites from Calafate (Berberis microphylla, Berberidaceae). Appl. Plant Sci. 2013, 1, 5. [Google Scholar] [CrossRef]
- Ruiz, A.; Hermosín-Gutiérrez, I.; Vergara, C.; von Baer, D.; Zapata, M.; Hitschfeld, A.; Mardones, C. Anthocyanin profiles in south Patagonian wild berries by HPLC-DAD-ESI-MS/MS. Food Res. Int. 2013, 51, 706–713. [Google Scholar] [CrossRef]
- Bora, P.S.; Narain, N.; Rocha, R.V.M.; Queiroz Paulo, M. Characterization of the oils from the pulp and seeds of avocado (cultivar: Fuerte) fruits. Grasas Aceites 2001, 52, 171–174. [Google Scholar] [CrossRef]
- Alarcón, G.; Valoy, A.; Alzogaray, F.M.; Medina, A.; Van Nieuwenhove, C.; Medina, M.; Jerez, S. Consumption of a byproduct of chia seed oil extraction by cold pressing ameliorates cardiovascular risks factors in an experimental model of metabolically unhealthy normal weight. Plant Foods Hum. Nutr. 2024, 79, 292–299. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Zhang, H.; Zhai, L.; Ye, B.; Cheng, Y.; Zhai, C. ALA/LA ameliorates glucose toxicity on HK-2 cells by attenuating oxidative stress and apoptosis through the ROS/p38/TGF-β 1 pathway. Lipids Health Dis. 2017, 16, 216. [Google Scholar] [CrossRef]
- Kapoor, B.; Kapoor, D.; Gautam, S.; Singh, R.; Bhardwaj, S. Dietary Polyunsaturated Fatty Acids (PUFAs): Uses and Potential Health Benefits. Curr. Nutr. Rep. 2021, 10, 232–242. [Google Scholar] [CrossRef]
- Dos Santos Lopes, M.C.O.; Kaippert, V.C.; Crovesy, L.; de Carvalho, D.P.; Rosado, E.L. Monounsaturated fat-rich diet reduces body adiposity in women with obesity, but does not influence energy expenditure and substrate oxidation: A parallel randomized controlled clinical trial. Eur. J. Clin. Nutr. 2024, 78, 335–343. [Google Scholar] [CrossRef]
- Li, H.; Fan, Y.W.; Li, J.; Tang, L.; Hu, J.N.; Deng, Z.Y. Evaluating and predicting the oxidative stability of vegetable oils with different fatty acid compositions. J. Food Sci. 2013, 78, H633–H641. [Google Scholar] [CrossRef]
- FINUT. Grasas y Ácidos Grasos en Nutrición Humana. Consulta de Expertos, 10-14 de Noviembre de 2008, Ginebra; Publicado por la Organización de las Naciones Unidas para la Alimentación y la Agricultura (FAO) y la Fundación Iberoamericana de Nutrición (FINUT): Granada, Spain, 2012. [Google Scholar]
- Fraguela-Meissimilly, H.; Bastías-Montes, J.M.; Ortiz-Viedma, J.A.; Tamarit-Pino, Y.; Claret-Merino, M.; Araneda-Flores, J. Valorization of extracts from maqui (Aristotelia chilensis) and calafate (Berberis microphylla) biowaste blends by supercritical fluid and pressurized liquid extraction. J. Agric. Food Res. 2024, 15, 100950. [Google Scholar] [CrossRef]
- Ortiz-Viedma, J.; Bastias-Montes, J.M.; Char, C.; Vega, C.; Quintriqueo, A.; Gallón-Bedoya, M.; Flores, M.; Aguilera, J.M.; Miranda, J.M.; Barros-Velázquez, J. Sequential biorefining of bioactive compounds of high functional value from calafate pomace (Berberis microphylla) using supercritical CO2 and pressurized liquids. Antioxidants 2023, 12, 323. [Google Scholar] [CrossRef] [PubMed]
- Bastías-Montes, J.M.; Monterrosa, K.; Muñoz-Fariña, O.; García, O.; Acuña-Nelson, S.M.; Vidal-San Martín, C.; Quevedo-Leon, R.; Kubo, I.; Avila-Acevedo, J.G.; Domiguez-Lopez, M.; et al. Chemoprotective and antiobesity effects of tocols from seed oil of Maqui-berry: Their antioxidative and digestive enzyme inhibition potential. Food Chem. Toxicol. 2020, 136, 111036. [Google Scholar] [CrossRef] [PubMed]
- Brauch, J.E.; Buchweitz, M.; Schweiggert, R.M.; Carle, R. Detailed analyses of fresh and dried maqui (Aristotelia chilensis (Mol.) Stuntz) berries and juice. Food Chem. 2016, 190, 308–316. [Google Scholar] [CrossRef]
- Van Hoed, V.; De Clercq, N.; Echim, C.; Andjelkovic, M.; Leber, E.; Dewettinck, K.; Verhé, R. Berry seeds: A source of specialty oils with high content of bioactives and nutritional value. J. Food Lipids 2009, 16, 33–49. [Google Scholar] [CrossRef]
- Grabauskaitė, R.; Jūrienė, L.; Pukalskienė, M.; Šipailienė, A.; Skurkienė, R.; Venskutonis, P.R. Isolation of valuable substances from berry seeds and pomace by the green high-pressure methods, their evaluation and application in cosmetic creams. Ind. Crop Prod. 2024, 222, 119729. [Google Scholar] [CrossRef]
- Lu, D.; Yang, Y.; Li, Y.; Sun, C. Análisis de tocoferoles y tocotrienoles en productos farmacéuticos y alimentos: Una revisión crítica. Curr. Pharm. Anal. 2015, 11, 66–78. [Google Scholar] [CrossRef]
- Institute of Medicine (US) Panel on Dietary Antioxidants and Related Compounds. Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids; National Academies Press: Washington, DC, USA, 2000; Volume 6, Vitamin E. Available online: https://www.ncbi.nlm.nih.gov/books/NBK225461/ (accessed on 10 June 2025).
- Ghosh, N.; Das, A.; Khanna, S. Vitamin E: Tocopherols and tocotrienol and their role in health and disease. In En Essential and Toxic Trace Elements and Vitamins in Human Health; Academic Press: New York, NY, USA, 2020; pp. 283–293. [Google Scholar]
- Järvinen, R.; Erkkilä, A.T. Tocopherols: Physiology and Health Effects; Academic Press: New York, NY, USA, 2016. [Google Scholar]
- Kamal-Eldin, A.; Budilarto, E. Tocopherols and tocotrienols as antioxidants for food preservation. In Handbook of Antioxidants for Food Preservation; Woodhead Publishing: Sawston, UK, 2015; pp. 141–159. [Google Scholar]
- Zingg, J.M. Vitamin E: A role in signal transduction. Annu. Rev. Nutr. 2015, 35, 135–173. [Google Scholar] [CrossRef]
- Saldeen, K.; Saldeen, T. Importance of tocopherols beyond α-tocopherol: Evidence from animal and human studies. Nutr. Res. 2005, 25, 877–889. [Google Scholar] [CrossRef]
- Hazewindus, M.; Haenen, G.R.; Weseler, A.R.; Bast, A. The anti-inflammatory effect of lycopene complements the antioxidant action of ascorbic acid and α-tocopherol. Food Chem. 2012, 132, 954–958. [Google Scholar] [CrossRef]
- Beatty, S.; Koh, H.H.; Phil, M.; Henson, D.; Boulton, M. The role of oxidative stress in the pathogenesis of age-related macular degeneration. Surv. Ophthalmol. 2000, 45, 115–134. [Google Scholar] [CrossRef]
- Olchawa, M.M.; Herrnreiter, A.M.; Pilat, A.K.; Skumatz, C.M.; Niziolek-Kierecka, M.; Burke, J.M.; Sarna, T.J. Zeaxanthin and α-tocopherol reduce the inhibitory effects of photodynamic stress on phagocytosis by ARPE-19 cells. Free Radic. Biol. Med. 2015, 89, 873–882. [Google Scholar] [CrossRef]
- Kontush, A.; Schekatolina, S. Vitamin E in neurodegenerative disorders: Alzheimer’s disease. Ann. N. Y. Acad. Sci. 2004, 1031, 249–262. [Google Scholar] [CrossRef]
- Petersen, R.C.; Thomas, R.G.; Grundman, M.; Bennett, D.; Doody, R.; Ferris, S.; Galasko, D.; Jin, S.; Kaye, J.; Levey, A.; et al. Vitamin E and donepezil for the treatment of mild cognitive impairment. N. Engl. J. Med. 2005, 352, 2379–2388. [Google Scholar] [CrossRef]
- Lloret, A.; Badia, M.C.; Mora, N.J.; Pallardó, F.V.; Alonso, M.D.; Vina, J. Vitamin E paradox in Alzheimer’s disease: It does not prevent loss of cognition and may even be detrimental. J. Alzheimers Dis. 2009, 17, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Dysken, M.W.; Sano, M.; Asthana, S.; Vertrees, J.E.; Pallaki, M.; Llorente, M.; Love, S.; Schellenberg, G.D.; McCarten, J.R.; Malphurs, J.; et al. Effect of vitamin E and memantine on functional decline in Alzheimer disease: The TEAM-AD VA cooperative randomized trial. JAMA 2014, 311, 33–44. [Google Scholar] [CrossRef] [PubMed]
- Montero, D.; Walther, G.; Stehouwer, C.D.A.; Houben, A.J.H.M.; Beckman, J.A.; Vinet, A. Effect of antioxidant vitamin supplementation on endothelial function in type 2 diabetes mellitus: A systematic review and meta-analysis of randomized controlled trials. Obes. Rev. 2014, 15, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Sesso, H.D.; Buring, J.E.; Christen, W.G.; Kurth, T.; Belanger, C.; MacFadyen, J.; Bubes, V.; Manson, J.E.; Glynn, R.J.; Gaziano, J.M. Vitamins E and C in the prevention of cardiovascular disease in men: The Physicians’ Health Study II randomized controlled trial. JAMA 2008, 300, 2123–2133. [Google Scholar] [CrossRef]
- Zheng Selin, J.; Rautiainen, S.; Lindblad, B.E.; Morgenstern, R.; Wolk, A. High-dose supplements of vitamins C and E, low-dose multivitamins, and the risk of age-related cataract: A population-based prospective cohort study of men. Am. J. Epidemiol. 2013, 177, 548–555. [Google Scholar] [CrossRef]
- Slatore, C.G.; Littman, A.J.; Au, D.H.; Satia, J.A.; White, E. Long-term use of supplemental multivitamins, vitamin C, vitamin E, and folate does not reduce the risk of lung cancer. Am. J. Respir. Crit. Care Med. 2008, 177, 524–530. [Google Scholar] [CrossRef]
- Virtamo, J.; Taylor, P.R.; Kontto, J.; Männistö, S.; Utriainen, M.; Weinstein, S.J.; Huttunen, J.; Albanes, D. Effects of α-tocopherol and β-carotene supplementation on cancer incidence and mortality: 18-Year postintervention follow-up of the Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study. Int. J. Cancer 2014, 135, 178–185. [Google Scholar] [CrossRef]
- Lippman, S.M.; Klein, E.A.; Goodman, P.J.; Lucia, M.S.; Thompson, I.M.; Ford, L.G.; Parnes, H.L.; Minasian, L.M.; Gaziano, J.M.; Hartline, J.A.; et al. Effect of selenium and vitamin E on risk of prostate cancer and other cancers: The Selenium and Vitamin E Cancer Prevention Trial (SELECT). JAMA 2009, 301, 39–51. [Google Scholar] [CrossRef]
- Klein, E.A.; Thompson, I.M.; Tangen, C.M.; Crowley, J.J.; Lucia, M.S.; Goodman, P.J.; Minasian, L.M.; Ford, L.G.; Parnes, H.L.; Gaziano, J.M.; et al. Vitamin E and the risk of prostate cancer: The Selenium and Vitamin E Cancer Prevention Trial (SELECT). JAMA 2011, 306, 1549–1556. [Google Scholar] [CrossRef] [PubMed]
- Kristal, A.R.; Darke, A.K.; Morris, J.S.; Tangen, C.M.; Goodman, P.J.; Thompson, I.M.; Meyskens, F.L., Jr.; Goodman, G.E.; Minasian, L.M.; Parnes, H.L.; et al. Baseline selenium status and effects of selenium and vitamin e supplementation on prostate cancer risk. J. Natl. Cancer Inst. 2014, 106, djt456. [Google Scholar] [CrossRef] [PubMed]
- Quispe-Fuentes, I.; Vega-Gálvez, A.; Campos-Requena, V.H. Antioxidant compound extraction from maqui (Aristotelia chilensis [Mol] Stuntz) berries: Optimization by response surface methodology. Antioxidants 2017, 6, 10. [Google Scholar] [CrossRef]
- Sánchez, C.; Rodríguez, A.; Reinoso, F.; Dovale-Rosabal, G.; Romero, N.; Espinosa, A.; Pando, M.E.; Claria, B.; Valenzuela, R.; Char, C.; et al. Optimization of Oil and Tocopherol Extraction from Maqui (Aristotelia chilensis (Mol.) Stuntz) by Supercritical CO2 Procedure. Antioxidants 2024, 13, 845. [Google Scholar] [CrossRef]
- Helbig, D.; Böhm, V.; Wagner, A.; Schubert, R.; Jahreis, G. Berry seed press residues and their valuable ingredients with special regard to black currant seed press residues. Food Chem. 2008, 111, 1043–1049. [Google Scholar] [CrossRef]
- De Filette, M.; Schatteman, K.; Geuens, J. Characterization of six cold-pressed berry seed oils and their seed meals. Appl. Sci. 2024, 14, 439. [Google Scholar] [CrossRef]
- Ramírez, J.E.; Zambrano, R.; Sepúlveda, B.; Kennelly, E.J.; Simirgiotis, M.J. Anthocyanins and antioxidant capacities of six Chilean berries by HPLC-HR-ESI-ToF-MS. Food Chem. 2015, 176, 106–114. [Google Scholar] [CrossRef]
- Fredes, C.; Parada, A.; Salinas, J.; Robert, P. Phytochemicals and traditional use of two southernmost Chilean berry fruits: Murta (Ugni molinae Turcz) and calafate (Berberis buxifolia Lam.). Food 2020, 9, 54. [Google Scholar] [CrossRef]
- Rodoni, L.M.; Feuring, V.; Zaro, M.J.; Sozzi, G.O.; Vicente, A.R.; Arena, M.E. Ethylene responses and quality of antioxidant-rich stored barberry fruit (Berberis microphylla). Sci. Hortic. 2014, 179, 233–238. [Google Scholar] [CrossRef]
- Busso Casati, C.; Baeza, R.; Sánchez, V. Physicochemical properties and bioactive compounds content in encapsulated freeze-dried powders obtained from blueberry, elderberry, blackcurrant and maqui berry. J. Berry Res. 2019, 9, 431–447. [Google Scholar] [CrossRef]
- Reyes-Farias, M.; Vasquez, K.; Ovalle-Marin, A.; Fuentes, F.; Parra, C.; Quitral, V.; Garcia-Diaz, D. Chilean native fruit extracts inhibit inflammation linked to the pathogenic interaction between adipocytes and macrophages. J. Med. Food 2015, 18, 601–608. [Google Scholar] [CrossRef] [PubMed]
- Soto-Covasich, J.; Reyes-Farias, M.; Torres, R.F.; Vasquez, K.; Duarte, L.; Quezada, J.; Garcia-Diaz, D.F. A polyphenol-rich Calafate (Berberis microphylla) extract rescues glucose tolerance in mice fed with cafeteria diet. J. Funct. Food 2020, 67, 103856. [Google Scholar] [CrossRef]
- Chamorro, M.F.; Reiner, G.; Theoduloz, C.; Ladio, A.; Schmeda-Hirschmann, G.; Gómez-Alonso, S.; Jiménez-Aspee, F. Polyphenol composition and (bio)activity of Berberis species and wild strawberry from the Argentinean Patagonia. Molecules 2019, 24, 3331. [Google Scholar] [CrossRef] [PubMed]
- Calderón-Reyes, C.; Silva, R.; Leal, P.; Ribera-Fonseca, A.; Cáceres, C.; Riquelme, I.; Reyes-Díaz, M. Anthocyanin-rich extracts of Calafate (Berberis microphylla G. Forst.) fruits decrease in vitro viability and migration of human gastric and gallbladder cancer cell lines. J. Soil Sci. Plant Nutr. 2020, 20, 1891–4903. [Google Scholar] [CrossRef]
- Lazzè, M.C.; Savio, M.; Pizzala, R.; Cazzalini, O.; Perucca, P.; Scovassi, A.I.; Stivala, L.A.; Bianchi, L. Anthocyanins induce cell cycle perturbations and apoptosis in different human cell lines. Carcinogenesis 2004, 25, 1427–1433. [Google Scholar] [CrossRef]
- Correa-Betanzo, J.; Allen-Vercoe, E.; McDonald, J.; Schroeter, K.; Corredig, M.; Paliyath, G. Stability and biological activity of wild blueberry (Vaccinium angustifolium) polyphenols during simulated in vitro gastrointestinal digestion. Food Chem. 2014, 165, 522–531. [Google Scholar] [CrossRef]
- Lucas-Gonzalez, R.; Navarro-Coves, S.; Pérez-Álvarez, J.A.; Fernández-López, J.; Muñoz, L.A.; Viuda-Martos, M. Assessment of polyphenolic profile stability and changes in the antioxidant potential of maqui berry (Aristotelia chilensis (Molina) Stuntz) during in vitro gastrointestinal digestion. Ind. Crops Prod. 2016, 94, 774–782. [Google Scholar] [CrossRef]
- Viuda-Martos, M.; Lucas-Gonzalez, R.; Ballester-Costa, C.; Pérez-Álvarez, J.A.; Muñoz, L.A.; Fernández-López, J. Evaluation of protective effect of different dietary fibers on polyphenolic profile stability of maqui berry (Aristotelia chilensis (Molina) Stuntz) during in vitro gastrointestinal digestion. Food Funct. 2018, 9, 573–584. [Google Scholar] [CrossRef]
- Chang, S.K.; Alasalvar, C.; Shahidi, F. Superfruits: Phytochemicals, antioxidant efficacies, and health effects—A comprehensive review. Crit. Rev. Food Sci. 2019, 59, 1580–1604. [Google Scholar] [CrossRef]
- Reyes-Farias, M.; Vasquez, K.; Fuentes, F.; Ovalle-Marin, A.; Parra-Ruiz, C.; Zamora, O.; Garcia-Diaz, D. Extracts of Chilean native fruits inhibit oxidative stress, inflammation and insulin-resistance linked to the pathogenic interaction between adipocytes and macrophages. J. Funct. Food 2016, 27, 69–83. [Google Scholar] [CrossRef]
- Bottini, M.C.J.; De Bustos, A.; Sanso, A.M.; Jouve, N.; Poggio, L. Relationships in Patagonian species of Berberis (Berberidaceae) based on the characterization of rDNA internal transcribed spacer sequences. Bot. J. Linn. Soc. 2007, 153, 321–328. [Google Scholar] [CrossRef]
- Pitta-Alvarez, S.; Medina-Bolivar, F.; Alvarez, M.; Scambatto, A.; Marconi, P. In vitro shoot culture and antimicrobial activity of Berberis buxifolia Lam. In Vitro Cell. Dev. Biol.—Plant 2008, 44, 502–507. [Google Scholar] [CrossRef]
- Guzmán, C.; Sánchez, R. Effect of calafate (Berberis microphylla) supplementation on lipid profile in rats with diet-induced obesity. Funct. Foods Health Dis. 2021, 11, 512–521. [Google Scholar] [CrossRef]
- Anhê, F.F.; Nachbar, R.T.; Varin, T.V.; Vilela, V.; Dudonné, S.; Pilon, G.; Marette, A. A polyphenol-rich cranberry extract reverses insulin resistance and hepatic steatosis independently of body weight loss. Mol. Metab. 2017, 6, 1563–1573. [Google Scholar] [CrossRef] [PubMed]
- Bustamante, L.; Pastene, E.; Durán-Sandoval, D.; Vergara, C.; Von-Baer, D.; Mardones, C. Pharmacokinetics of low molecular weight phenolic compounds in gerbil plasma after the consumption of Calafate berry (Berberis microphylla) extract. Food Chem. 2018, 268, 347–354. [Google Scholar] [CrossRef]

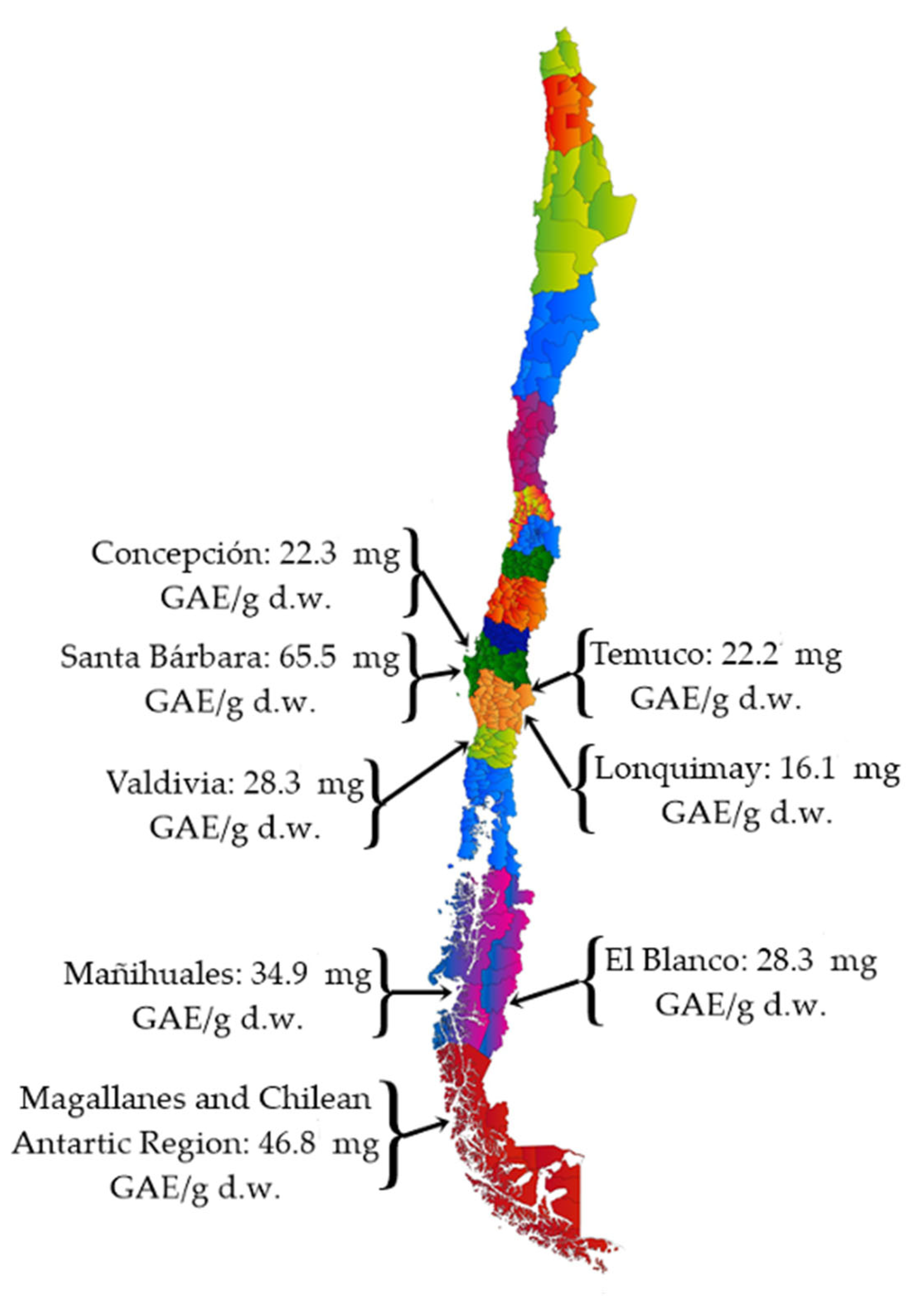
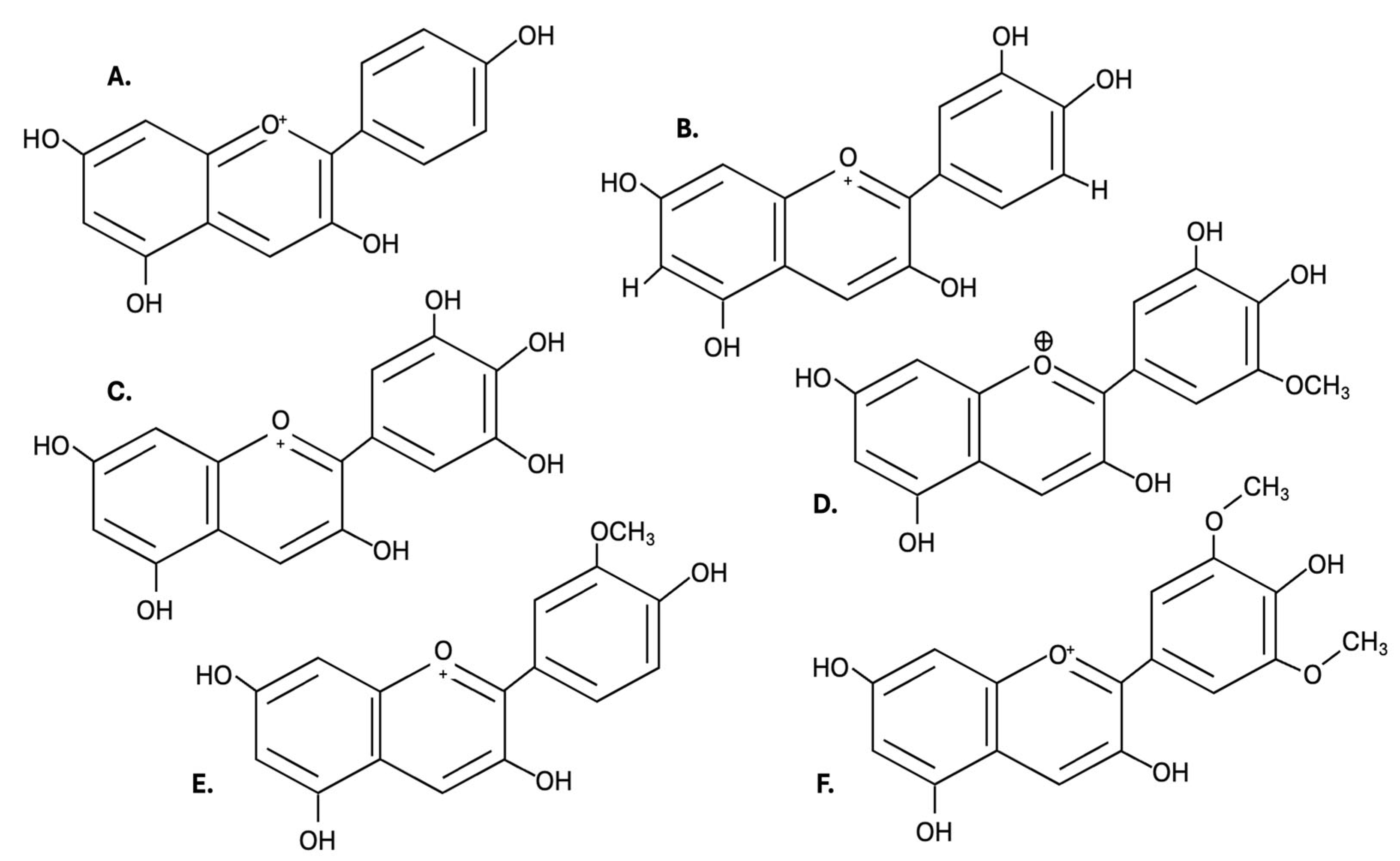
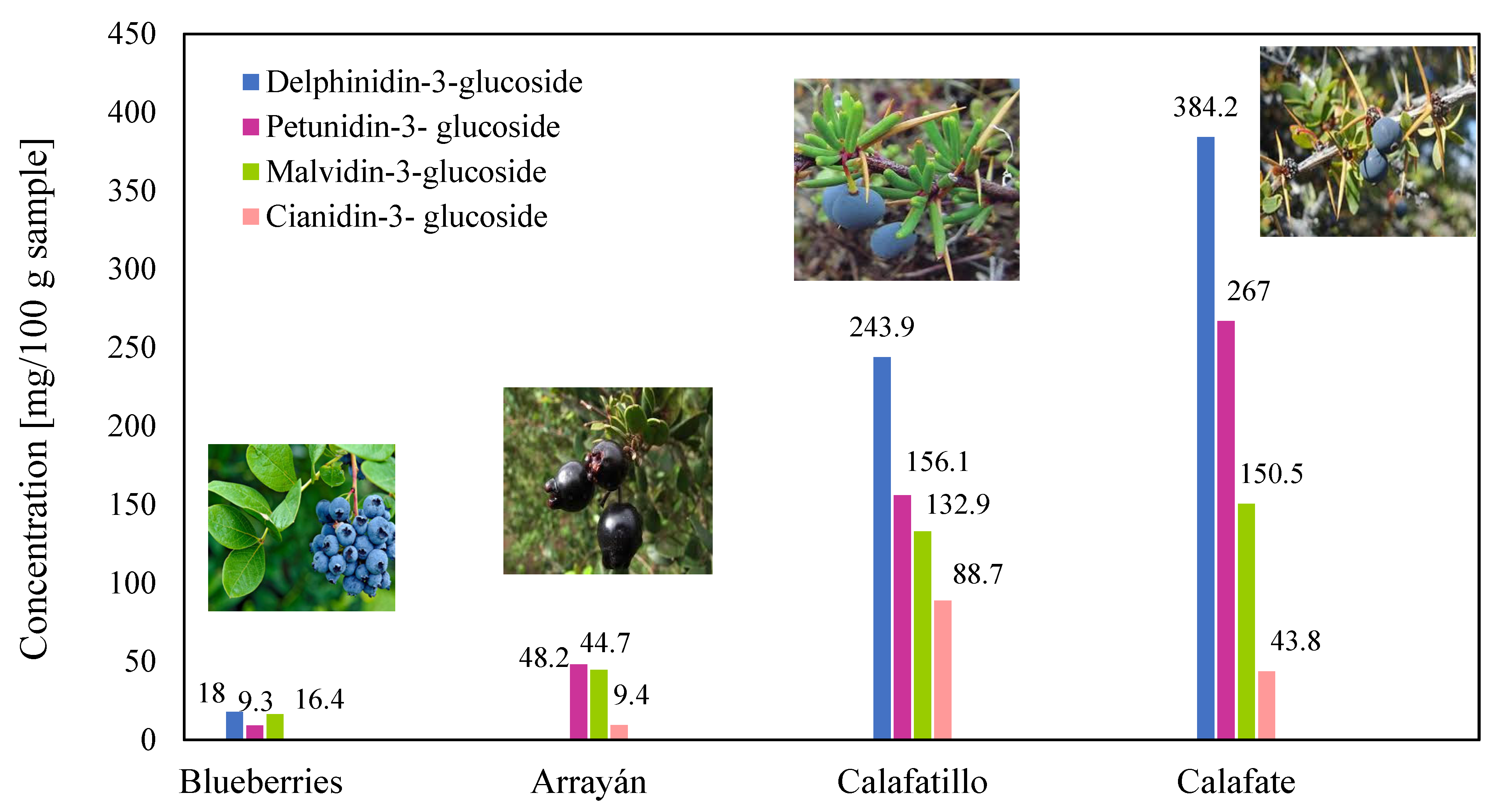
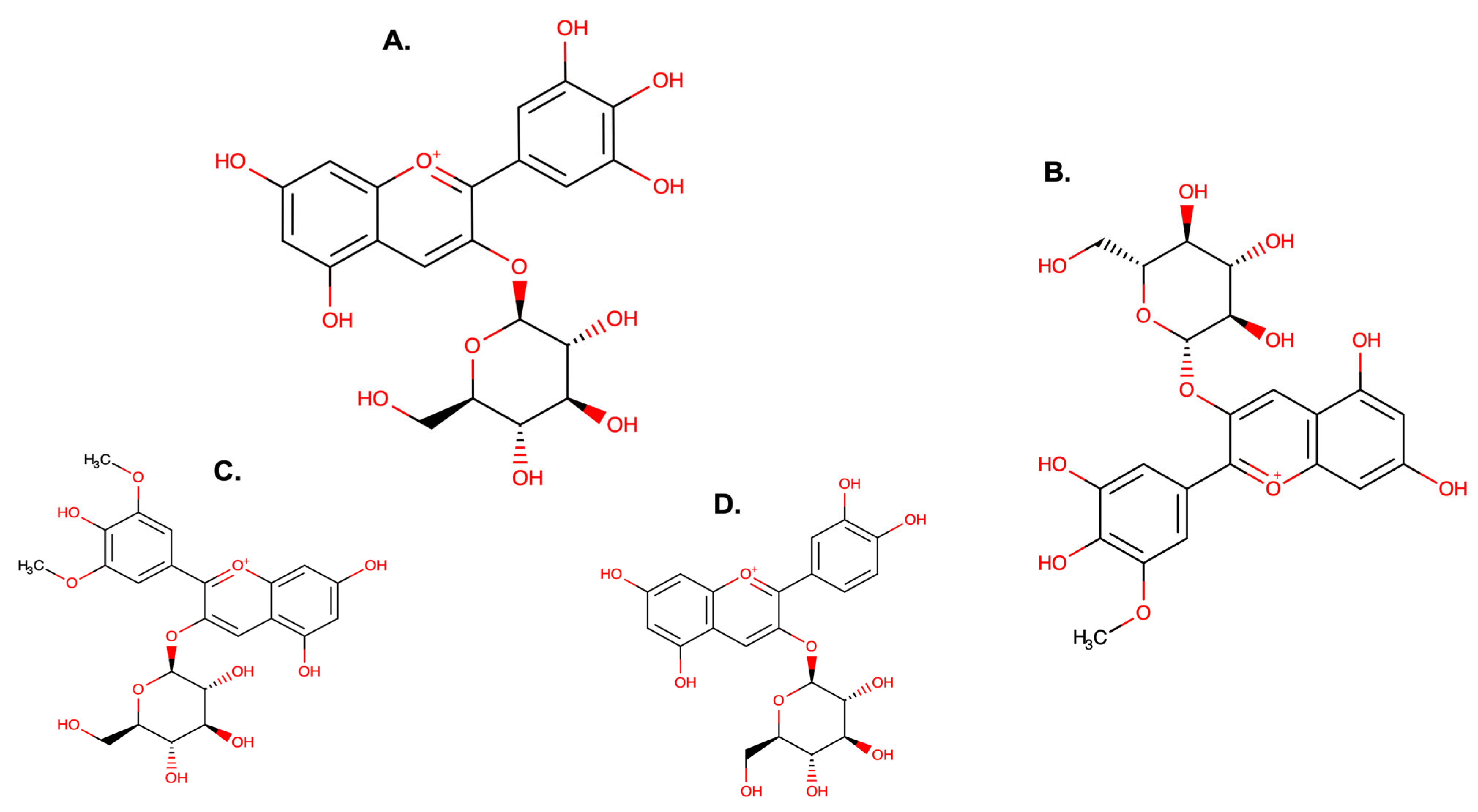
| References Location Country | [13] Punta Arenas Chile | [17] Aysén Region Chile | [27] Patagonia Argentina | |
|---|---|---|---|---|
| Nutritional Information | Pulp | Pulp | Pulp | Seeds |
| Energy (kcal/100 g) | 98.5 | n.m | 119.4 | 144.3 |
| Moisture content (%) | 75.9 | 75.0 | 93.3 | n.m |
| HC available (%) | 14.5 | n.m | 77.50 * | 0.06 |
| 9.80 | n.m | 74.40 * | n.m |
| n.m | n.m | 3.10 * | n.m |
| Proteins (%) | 2.80 | 2.60 | 1.33 | 13.65 |
| Fat (%) | 0.90 | n.m | 1.70 | 18.90 |
| Ash (%) | 0.60 | 0.90 | 3.65 | 2.21 |
| Crude fiber (%) | 0.10 | 7.00 | n.m | n.m |
| Total dietary fiber (%) | 5.30 | n.m | n.m | n.m |
| 0.75 | n.m | n.m | n.m |
| 4.55 | n.m | n.m | n.m |
| References | [74] | [75] | [70] | [76] | [30] | [28] | [39] |
|---|---|---|---|---|---|---|---|
| Location | Valdivia | Valdivia | Santa Bárbara | Patagonia | Magallanes | Aysén Region | Aysén Region |
| Country | Chile | Chile | Chile | Argentina | Chile | Chile | Chile |
| Total polyphenol (mg GAE/100 g d.w.) | 1344.2 ± 10.50 e | 2233 ± 6.39 d | 6553 ± 1.35 a | - | - | 2537.5 ± 0.22 c | 3600 ± 30 b |
| Total anthocyanin (mg C-3-G/100 g d.w.) | 31.5 ± 0.80 d | 66 ± 0.30 b | 51.62 ± 1.78 c | - | - | 26.5 ± 0.00 d | 80.0 ± 5.0 a |
| Antioxidant activity (mmol Fe2+/100 g d.w.) | 11.7 ± 1.80 b | 38.44 ± 0.08 a | - | - | - | - | - |
| Antioxidant activity (IC50, μg/mL) | - | - | 2.33 ± 0.21 c | 12.6 ± 0.38 b | - | - | 25.0 ± 1.0 a |
| Antioxidant activity (μmol TE/g d.w.) | - | - | 124.46 ± 6.54 a | - | 62.75 ± 3.10 b | - | 63.0 ± 5.0 b |
| Biological Activity | Preparation/Extract/Compound | Experimental Model | Major Outcomes | Reference |
|---|---|---|---|---|
 Anti- inflammatory | Polyphenol-rich berry extract | In vitro (macrophages stimulated with LPS); rodent models of diet-induced obesity | ↓ Pro-inflammatory markers; ↓ NO secretion; modulation of cytokine expression; ↓ fat deposits; improvement of insulin sensitivity | [74,75,83] |
| Leaves and roots (alkaloid fraction) | In vitro (antibiotic activity assays) | Reported antibiotic and anti-inflammatory properties; limited vasodilator evidence. | [84] | |
 Hypoglycemic | Ethanolic root extract | Hepatic cell culture | ↑ Glucose uptake correlated with dose | [15] |
| Berry extract (polyphenol-rich) | In vivo (diet-induced obesity, mice) | Restoration of glucose tolerance; improvement of insulin resistance | [75] | |
| Fruit extract | In vitro (adipocytes under insulin-resistant conditions) | Inhibition of glucose uptake by adipocytes | [83] | |
 Antioxidant | Fruit extract | In vitro assays (DPPH, ABTS, FRAP, ORAC); human erythrocytes (lipid peroxidation assay) | ↑ Radical scavenging activity; protection against lipid peroxidation; antiradical capacity correlated with phenolic and anthocyanin content | [17,70,71] |
| Pulp, peel, seeds (organ-specific) | Analytical assays | Pulp/peel: anthocyanins and hydrophilic phenolics; seeds: lipids and tocopherols; organ-dependent antioxidant roles | [20] | |
 Antimicrobial | Roots, stems, leaves (methanol/ethanol extracts, alkaloid fraction rich in berberine) | In vitro (Gram-positive bacteria: S. aureus, B. cereus, S. epidermidis, B. subtilis) | Inhibition of bacterial growth; activity linked to isoquinoline alkaloids (mainly berberine) | [9,11,85] |
 CVD risk-reducing/Metabolic modulation | Polyphenol-rich berry extract | In vivo (mice, chronic administration) | ↓ Plasma biomarkers (thrombomodulin, sE-selectin, sICAM-1, proMMP-9); ↑ adiponectin; ↓ OH radicals; protective effects against atherosclerosis | [7] |
| Freeze-dried calafate | In vivo (mice) | ↓ Atherogenic Index and CVD Risk Index; no significant effect on cholesterol or triglycerides | [86] | |
| Acute and chronic berry extract administration | In vivo (mice) | Modulation of thermogenic markers; prevention of mitochondrial and microbiota alterations in obesity models | [8] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ortiz-Viedma, J.; Vergara, C.; Toledo, T.; Zura-Bravo, L.; Flores, M.; Barrera, C.; Lemus-Mondaca, R. Calafate (Berberis buxifolia Lam.) Berry as a Source of Bioactive Compounds with Potential Health-Promoting Effects: A Critical Review. Antioxidants 2025, 14, 1272. https://doi.org/10.3390/antiox14111272
Ortiz-Viedma J, Vergara C, Toledo T, Zura-Bravo L, Flores M, Barrera C, Lemus-Mondaca R. Calafate (Berberis buxifolia Lam.) Berry as a Source of Bioactive Compounds with Potential Health-Promoting Effects: A Critical Review. Antioxidants. 2025; 14(11):1272. https://doi.org/10.3390/antiox14111272
Chicago/Turabian StyleOrtiz-Viedma, Jaime, Claudia Vergara, Tamar Toledo, Liliana Zura-Bravo, Marcos Flores, Constanza Barrera, and Roberto Lemus-Mondaca. 2025. "Calafate (Berberis buxifolia Lam.) Berry as a Source of Bioactive Compounds with Potential Health-Promoting Effects: A Critical Review" Antioxidants 14, no. 11: 1272. https://doi.org/10.3390/antiox14111272
APA StyleOrtiz-Viedma, J., Vergara, C., Toledo, T., Zura-Bravo, L., Flores, M., Barrera, C., & Lemus-Mondaca, R. (2025). Calafate (Berberis buxifolia Lam.) Berry as a Source of Bioactive Compounds with Potential Health-Promoting Effects: A Critical Review. Antioxidants, 14(11), 1272. https://doi.org/10.3390/antiox14111272










