The Preparation and Evaluation of Carvacrol-Added Hyaluronic Acid for Early Osteoarthritis Treatment
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of HA-Carvacrol Solution
2.2. Free Radical Scavenging Assay
2.3. Cell Culture and Treatment
2.4. Chondrocyte Viability Under Inflammatory Conditions
2.5. Chondrocyte Cytotoxicity Under Inflammatory Conditions
2.6. Gene Expression Analysis of OA-Related Markers Under Inflammatory Conditions
2.7. Quantification of Inflammation-Related Proteins Under Inflammatory Conditions
2.8. Quantification of Cartilage Matrix Molecules Under Inflammatory Conditions
2.9. Establishment of Monosodium Iodoacetate (MIA)-Induced OA Model in Rats
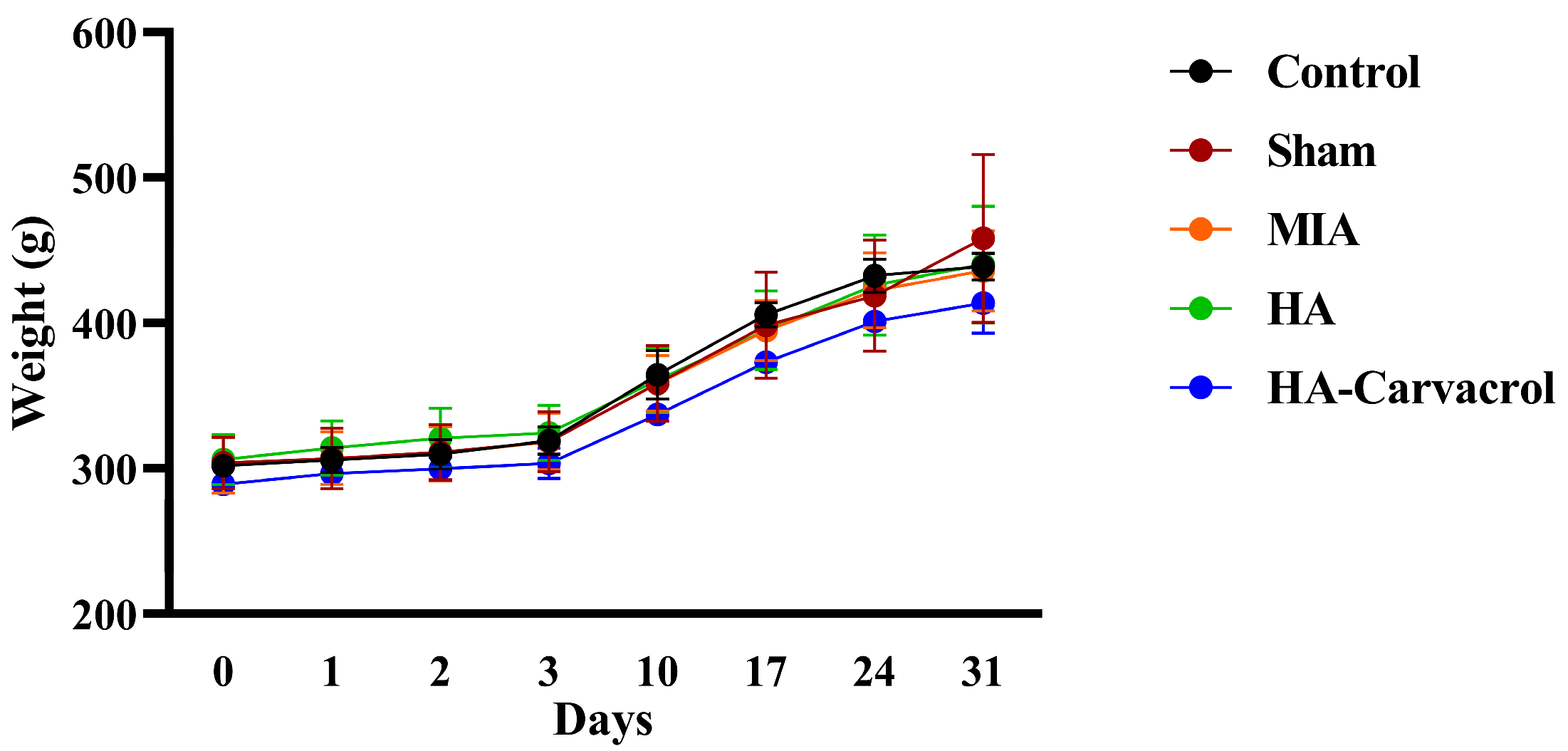
2.10. Body Weight Monitoring
2.11. Evaluation of Hematological and Biochemical Parameters
2.12. Assessment of Mechanical Allodynia
2.13. Thermal Hyperalgesia Test Using Hot Plate Method
2.14. Histological Examination of Articular Cartilage
2.15. Statistical Analysis
3. Results
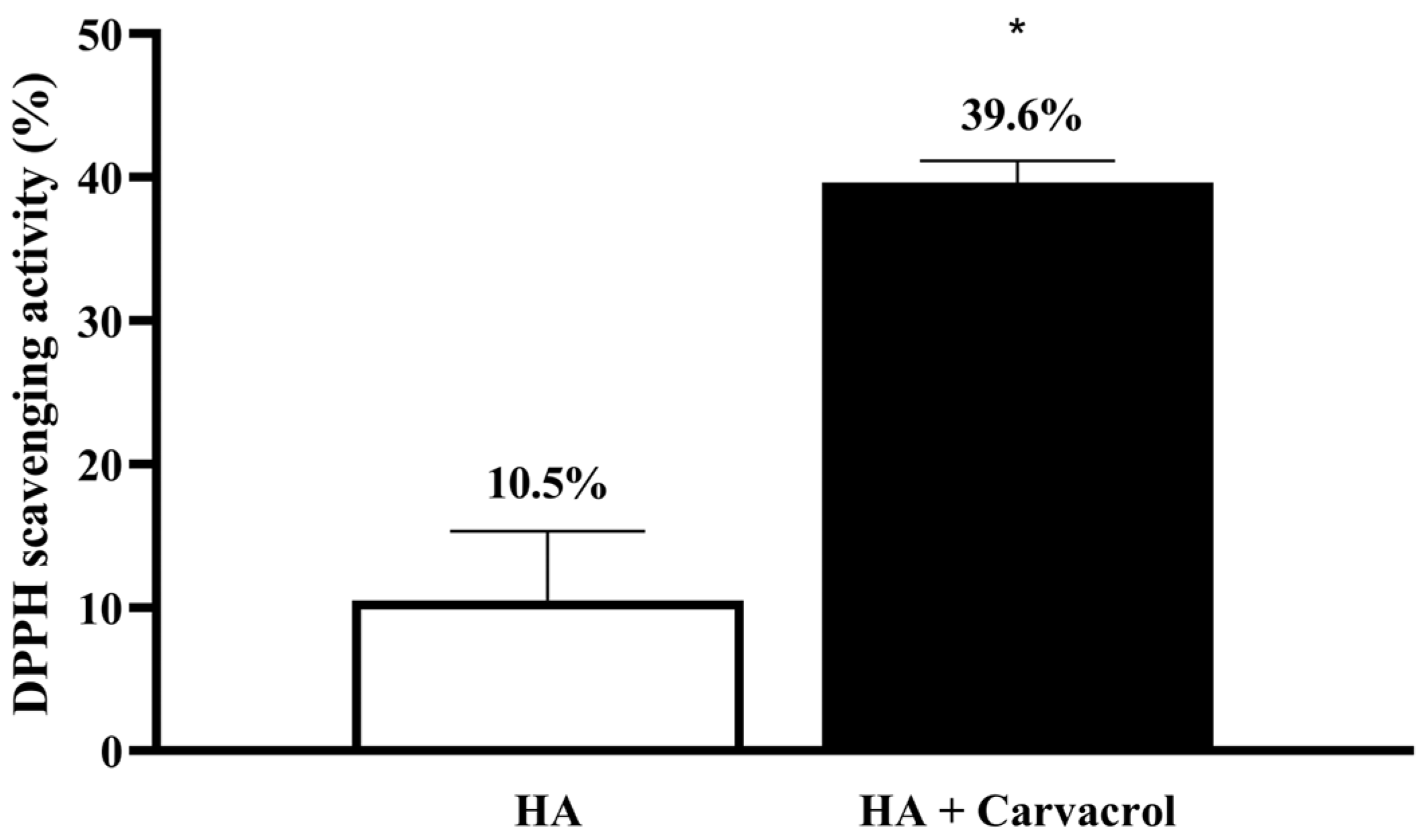
3.1. Antioxidant Activity of HA-Carvacrol
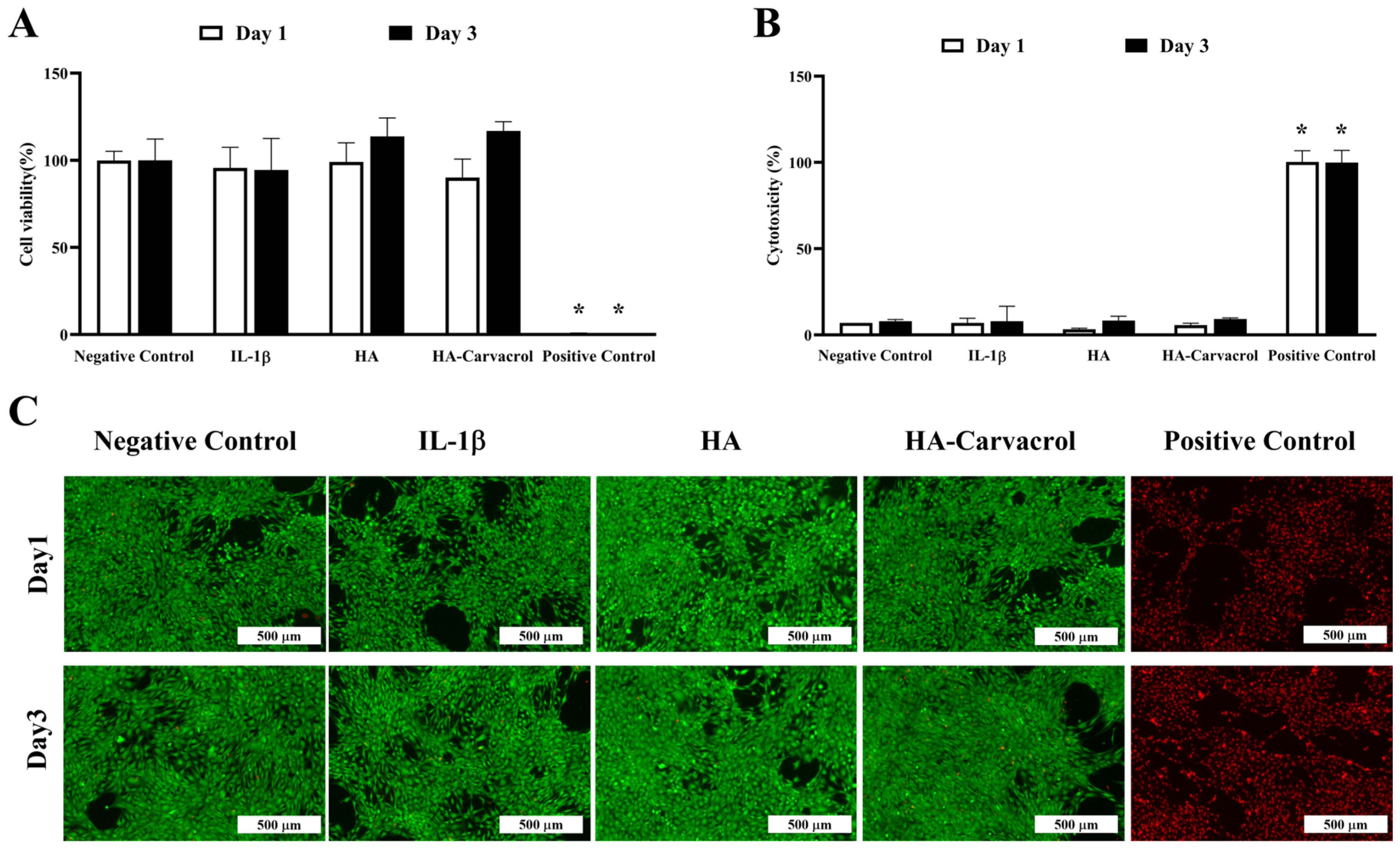
3.2. Cytocompatibility and Cytotoxicity Under Inflammatory Conditions
3.3. Effects on Inflammatory Molecular Responses in IL-1-Stimulated Chondrocytes
3.4. Effects on Antioxidant Molecular Responses in IL-1-Stimulated Chondrocytes
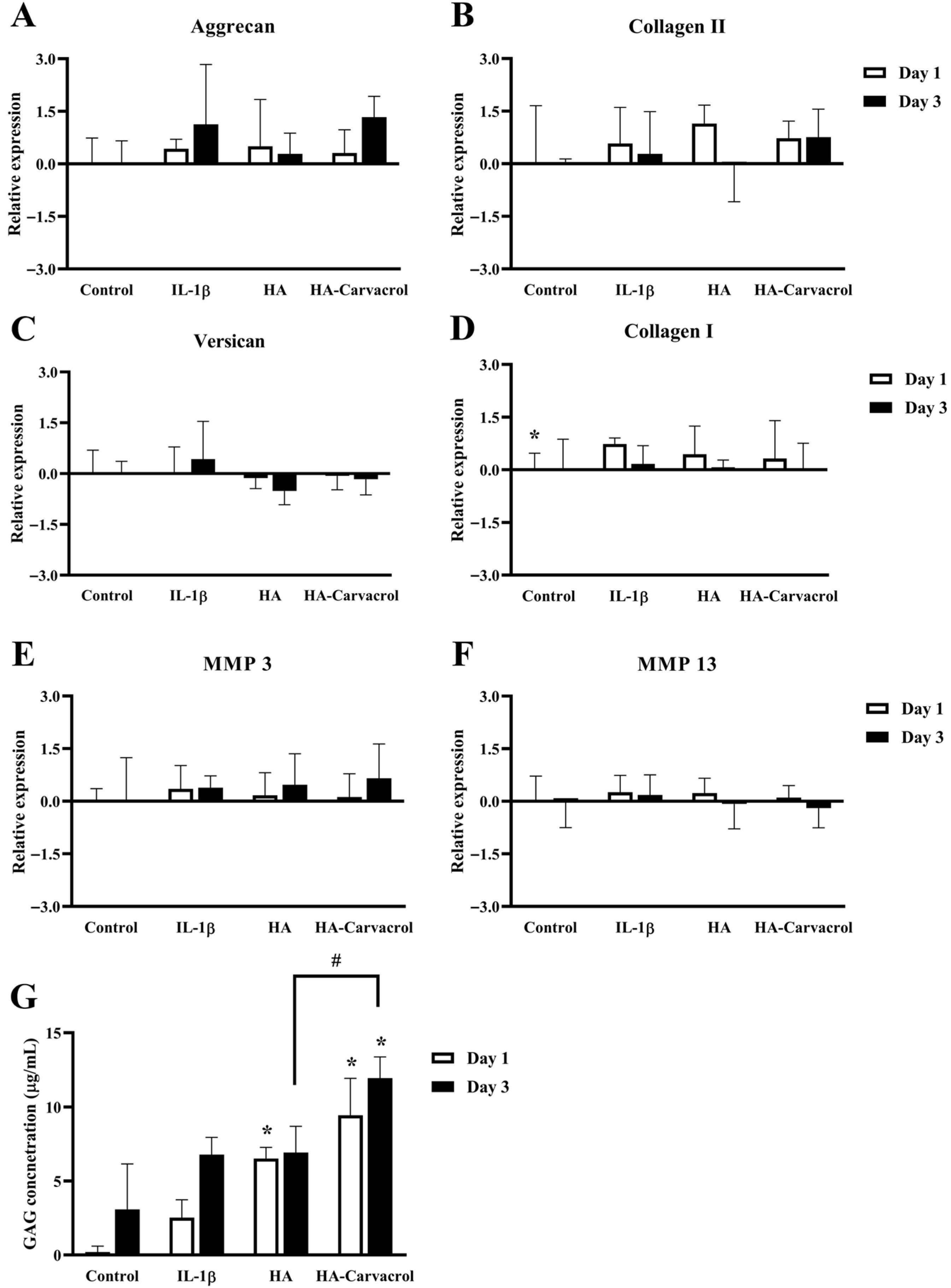
3.5. Effects on ECM-Related Molecular Responses in IL-1-Stimulated Chondrocytes
3.6. In Vivo Biosafety Evaluation
3.7. Behavioral Assessments and Histological Evaluation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| OA | Osteoarthritis |
| MIA | Monosodium iodoacetate |
| ECM | Extracellular matrix |
| ROS | Reactive oxygen species |
| IL-1β | Interleukin-1β |
| IL-6 | Interleukin-6 |
| TNF-α | Tumor necrosis factor-α |
| IL-1RA | Interleukin-1 receptor antagonist |
| MMP | Matrix metalloproteinases |
| Collagen II | Collagen type II |
| Collagen I | Collagen type I |
| iNOS | Inducible nitric oxide synthase |
| SOD | Superoxide dismutase |
| CAT | Catalase |
| GPX | Glutathione peroxidase |
| RBC | Red blood cell |
| HGB | Hemoglobin |
| HCT | Hematocrit |
| MCV | Mean corpuscular volume |
| MCH | Mean corpuscular hemoglobin |
| MCHC | Mean corpuscular hemoglobin concentration |
| PLT | Platelet |
| WBC | White blood cell |
| MO | Monocyte |
| LY | Lymphocyte |
| AST | Aspartate aminotransferase |
| ALT | Alanine aminotransferase |
| ALP | Akaline phosphatase |
| BUN | Bood urea nitrogen |
| CRE | Creatinine |
| PWT | Paw withdrawal threshold |
| PWL | Paw withdrawal latency |
References
- Leifer, V.P.; Katz, J.N.; Losina, E. The burden of OA-health services and economics. Osteoarthr. Cartil. 2022, 30, 10–16. [Google Scholar] [CrossRef]
- Hsu, H.; Siwiec, R.M. Knee Osteoarthritis. 2018. Available online: https://www.ncbi.nlm.nih.gov/books/NBK507884/ (accessed on 12 October 2025).
- Geng, R.; Li, J.; Yu, C.; Zhang, C.; Chen, F.; Chen, J.; Ni, H.; Wang, J.; Kang, K.; Wei, Z.; et al. Knee osteoarthritis: Current status and research progress in treatment. Exp. Ther. Med. 2023, 26, 481. [Google Scholar] [CrossRef]
- Palazzo, C.; Nguyen, C.; Lefevre-Colau, M.-M.; Rannou, F.; Poiraudeau, S. Risk factors and burden of osteoarthritis. Ann. Phys. Rehabil. Med. 2016, 59, 134–138. [Google Scholar] [CrossRef] [PubMed]
- Ansari, M.Y.; Ahmad, N.; Haqqi, T.M. Oxidative stress and inflammation in osteoarthritis pathogenesis: Role of polyphenols. Biomed. Pharmacother. 2020, 129, 110452. [Google Scholar] [CrossRef] [PubMed]
- Sokolove, J.; Lepus, C.M. Role of inflammation in the pathogenesis of osteoarthritis: Latest findings and interpretations. Ther. Adv. Musculoskelet. Dis. 2013, 5, 77–94. [Google Scholar] [CrossRef] [PubMed]
- Molnar, V.; Matišić, V.; Kodvanj, I.; Bjelica, R.; Jeleč, Ž.; Hudetz, D.; Rod, E.; Čukelj, F.; Vrdoljak, T.; Vidović, D.; et al. Cytokines and chemokines involved in osteoarthritis pathogenesis. Int. J. Mol. Sci. 2021, 22, 9208. [Google Scholar] [CrossRef]
- Razak, H.R.B.A.; Chew, D.; Kazezian, Z.; Bull, A.M. Autologous protein solution: A promising solution for osteoarthritis? EFORT Open Rev. 2021, 6, 716–726. [Google Scholar] [CrossRef]
- Tudorachi, N.B.; Totu, E.E.; Fifere, A.; Ardeleanu, V.; Mocanu, V.; Mircea, C.; Isildak, I.; Smilkov, K.; Cărăuşu, E.M. The implication of reactive oxygen species and antioxidants in knee osteoarthritis. Antioxidants 2021, 10, 985. [Google Scholar] [CrossRef]
- Bolduc, J.A.; Collins, J.A.; Loeser, R.F. Reactive oxygen species, aging and articular cartilage homeostasis. Free Radic. Biol. Med. 2019, 132, 73–82. [Google Scholar] [CrossRef]
- Lepetsos, P.; Papavassiliou, A.G. ROS/oxidative stress signaling in osteoarthritis. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2016, 1862, 576–591. [Google Scholar] [CrossRef]
- Berdiaki, A.; Neagu, M.; Spyridaki, I.; Kuskov, A.; Perez, S.; Nikitovic, D. Hyaluronan and reactive oxygen species signaling—Novel cues from the matrix? Antioxidants 2023, 12, 824. [Google Scholar] [CrossRef] [PubMed]
- Tavianatou, A.G.; Caon, I.; Franchi, M.; Piperigkou, Z.; Galesso, D.; Karamanos, N.K. Hyaluronan: Molecular size-dependent signaling and biological functions in inflammation and cancer. FEBS J. 2019, 286, 2883–2908. [Google Scholar] [CrossRef] [PubMed]
- Ansari, M.Y.; Khan, N.M.; Ahmad, I.; Haqqi, T.M. Parkin clearance of dysfunctional mitochondria regulates ROS levels and increases survival of human chondrocytes. Osteoarthr. Cartil. 2018, 26, 1087–1097. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Luo, P.; Yang, M.; Wang, J.; Hou, W.; Xu, P. The role of oxidative stress in the development of knee osteoarthritis: A comprehensive research review. Front. Mol. Biosci. 2022, 9, 1001212. [Google Scholar] [CrossRef]
- Gupta, R.C.; Lall, R.; Srivastava, A.; Sinha, A. Hyaluronic acid: Molecular mechanisms and therapeutic trajectory. Front. Vet. Sci. 2019, 6, 192. [Google Scholar] [CrossRef]
- Band, P.; Heeter, J.; Wisniewski, H.-G.; Liublinska, V.; Pattanayak, C.; Karia, R.; Stabler, T.; Balazs, E.; Kraus, V. Hyaluronan molecular weight distribution is associated with the risk of knee osteoarthritis progression. Osteoarthr. Cartil. 2015, 23, 70–76. [Google Scholar] [CrossRef]
- Smith, A.E.; Sigurbjörnsdóttir, E.S.; Steingrímsson, E.; Sigurbjörnsdóttir, S. Hedgehog signalling in bone and osteoarthritis: The role of Smoothened and cholesterol. FEBS J. 2023, 290, 3059–3075. [Google Scholar] [CrossRef]
- Yunus, M.H.M.; Nordin, A.; Kamal, H. Pathophysiological perspective of osteoarthritis. Medicina 2020, 56, 614. [Google Scholar] [CrossRef]
- Jenei-Lanzl, Z.; Meurer, A.; Zaucke, F. Interleukin-1β signaling in osteoarthritis–chondrocytes in focus. Cell. Signal. 2019, 53, 212–223. [Google Scholar] [CrossRef]
- Testa, G.; Giardina, S.M.C.; Culmone, A.; Vescio, A.; Turchetta, M.; Cannavò, S.; Pavone, V. Intra-articular injections in knee osteoarthritis: A review of literature. J. Funct. Morphol. Kinesiol. 2021, 6, 15. [Google Scholar] [CrossRef]
- Choueiri, M.; Chevalier, X.; Eymard, F. Intraarticular corticosteroids for hip osteoarthritis: A review. Cartilage 2021, 13, 122S–131S. [Google Scholar] [CrossRef]
- Kompel, A.J.; Roemer, F.W.; Murakami, A.M.; Diaz, L.E.; Crema, M.D.; Guermazi, A. Intra-articular corticosteroid injections in the hip and knee: Perhaps not as safe as we thought? Radiology 2019, 293, 656–663. [Google Scholar] [CrossRef] [PubMed]
- Ayhan, E.; Kesmezacar, H.; Akgun, I. Intraarticular injections (corticosteroid, hyaluronic acid, platelet rich plasma) for the knee osteoarthritis. World J. Orthop. 2014, 5, 351. [Google Scholar] [CrossRef] [PubMed]
- Lacci, K.M.; Dardik, A. Platelet-rich plasma: Support for its use in wound healing. Yale J. Biol. Med. 2010, 83, 1. [Google Scholar]
- Bayer, I.S. Hyaluronic acid and controlled release: A review. Molecules 2020, 25, 2649. [Google Scholar] [CrossRef]
- Sinusas, K. Osteoarthritis: Diagnosis and treatment. Am. Fam. Physician 2012, 85, 49–56. [Google Scholar]
- Altman, R.; Manjoo, A.; Fierlinger, A.; Niazi, F.; Nicholls, M. The mechanism of action for hyaluronic acid treatment in the osteoarthritic knee: A systematic review. BMC Musculoskelet. Disord. 2015, 16, 321. [Google Scholar] [CrossRef]
- Chen, W.; Ye, Q.; Zhang, M.; Xie, R.; Xu, C. Lubrication for Osteoarthritis: From Single-Function to Multifunctional Lubricants. Int. J. Mol. Sci. 2025, 26, 1856. [Google Scholar] [CrossRef]
- Xu, Z.; He, Z.; Shu, L.; Li, X.; Ma, M.; Ye, C. Intra-articular platelet-rich plasma combined with hyaluronic acid injection for knee osteoarthritis is superior to platelet-rich plasma or hyaluronic acid alone in inhibiting inflammation and improving pain and function. Arthrosc. J. Arthrosc. Relat. Surg. 2021, 37, 903–915. [Google Scholar] [CrossRef]
- Kim, Y.S.; Guilak, F. Engineering hyaluronic acid for the development of new treatment strategies for osteoarthritis. Int. J. Mol. Sci. 2022, 23, 8662. [Google Scholar] [CrossRef]
- Ji, X.; Zhang, H. Current strategies for the treatment of early stage osteoarthritis. Front. Mech. Eng. 2019, 5, 57. [Google Scholar] [CrossRef]
- Jin, Y.; Koh, R.H.; Kim, S.-H.; Kim, K.M.; Park, G.K.; Hwang, N.S. Injectable anti-inflammatory hyaluronic acid hydrogel for osteoarthritic cartilage repair. Mater. Sci. Eng. C 2020, 115, 111096. [Google Scholar] [CrossRef] [PubMed]
- Tian, Z.; Zhang, X.; Sun, M. Phytochemicals mediate autophagy against osteoarthritis by maintaining cartilage homeostasis. Front. Pharmacol. 2021, 12, 795058. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-Z.; Zhang, S.-N. Recent advance in treatment of osteoarthritis by bioactive components from herbal medicine. Chin. Med. 2020, 15, 80. [Google Scholar] [CrossRef]
- Hassanpour, S.H.; Doroudi, A. Review of the antioxidant potential of flavonoids as a subgroup of polyphenols and partial substitute for synthetic antioxidants. Avicenna J. Phytomed. 2023, 13, 354. [Google Scholar]
- Pourreza, N. Phenolic compounds as potential antioxidant. Jundishapur J. Nat. Pharm. Prod. 2013, 8, 149. [Google Scholar] [CrossRef]
- Sirše, M. Effect of dietary polyphenols on osteoarthritis—Molecular mechanisms. Life 2022, 12, 436. [Google Scholar] [CrossRef]
- Srivastava, S.; Saksena, A.K.; Khattri, S.; Kumar, S.; Dagur, R.S. Curcuma longa extract reduces inflammatory and oxidative stress biomarkers in osteoarthritis of knee: A four-month, double-blind, randomized, placebo-controlled trial. Inflammopharmacology 2016, 24, 377–388. [Google Scholar] [CrossRef]
- Liang, Q.; Wang, X.-P.; Chen, T.-S. Resveratrol protects rabbit articular chondrocyte against sodium nitroprusside-induced apoptosis via scavenging ROS. Apoptosis 2014, 19, 1354–1363. [Google Scholar] [CrossRef]
- Park, S.; Lee, L.R.; Seo, J.H.; Kang, S. Curcumin and tetrahydrocurcumin both prevent osteoarthritis symptoms and decrease the expressions of pro-inflammatory cytokines in estrogen-deficient rats. Genes Nutr. 2016, 11, 2. [Google Scholar] [CrossRef]
- Wei, Y.; Jia, J.; Jin, X.; Tong, W.; Tian, H. Resveratrol ameliorates inflammatory damage and protects against osteoarthritis in a rat model of osteoarthritis. Mol. Med. Rep. 2018, 17, 1493–1498. [Google Scholar] [CrossRef]
- Qiu, L.; Luo, Y.; Chen, X. Quercetin attenuates mitochondrial dysfunction and biogenesis via upregulated AMPK/SIRT1 signaling pathway in OA rats. Biomed. Pharmacother. 2018, 103, 1585–1591. [Google Scholar] [CrossRef] [PubMed]
- Mahmoodi, M.; Amiri, H.; Ayoobi, F.; Rahmani, M.; Taghipour, Z.; Ghavamabadi, R.T.; Jafarzadeh, A.; Sankian, M. Carvacrol ameliorates experimental autoimmune encephalomyelitis through modulating pro-and anti-inflammatory cytokines. Life Sci. 2019, 219, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Dezfuli, S.A.T.; Ehsani, M.; Azar, O.L. Effects of Diabetes on Inflammation and Oxidation by Modulation in Gene Expression of Inflammatory and Antioxidant System in Diabetic Rat Model. GMJ Med. 2017, 1, 15–20. [Google Scholar] [CrossRef]
- Arigesavan, K.; Sudhandiran, G. Carvacrol exhibits anti-oxidant and anti-inflammatory effects against 1,2-dimethyl hydrazine plus dextran sodium sulfate induced inflammation associated carcinogenicity in the colon of Fischer 344 rats. Biochem. Biophys. Res. Commun. 2015, 461, 314–320. [Google Scholar] [CrossRef]
- Bağcı, B.; Aydın, Ş.; Dalkılınç, E.; Çomaklı, S.; Küçükler, S.; Özdemir, S. Neuroprotective potential of carvacrol: Restoration of oxidative balance and mitigation of brain injury markers in isoproterenol-induced rats. Metab. Brain Dis. 2025, 40, 211. [Google Scholar] [CrossRef]
- Li, Y.; Xu, J.Z.; Gu, C.X.; Liu, G.L.; Tian, K. Carvacrol suppresses inflammatory responses in rheumatoid arthritis fibroblast-like synoviocytes. J. Cell. Biochem. 2019, 120, 8169–8176. [Google Scholar] [CrossRef]
- Khazdair, M.R.; Alavinezhad, A.; Boskabady, M.H. Carvacrol ameliorates haematological parameters, oxidant/antioxidant biomarkers and pulmonary function tests in patients with sulphur mustard-induced lung disorders: A randomized double-blind clinical trial. J. Clin. Pharm. Ther. 2018, 43, 664–674. [Google Scholar] [CrossRef]
- Ghorani, V.; Alavinezhad, A.; Rajabi, O.; Mohammadpour, A.H.; Boskabady, M.H. Safety and tolerability of carvacrol in healthy subjects: A phase I clinical study. Drug Chem. Toxicol. 2021, 44, 177–189. [Google Scholar] [CrossRef]
- Calabrese, G.; Ardizzone, A.; Campolo, M.; Conoci, S.; Esposito, E.; Paterniti, I. Beneficial effect of tempol, a membrane-permeable radical scavenger, on inflammation and osteoarthritis in in vitro models. Biomolecules 2021, 11, 352. [Google Scholar] [CrossRef]
- Imran, M.; Aslam, M.; Alsagaby, S.A.; Saeed, F.; Ahmad, I.; Afzaal, M.; Arshad, M.U.; Abdelgawad, M.A.; El-Ghorab, A.H.; Khames, A.; et al. Therapeutic application of carvacrol: A comprehensive review. Food Sci. Nutr. 2022, 10, 3544–3561. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Davidson, P.M.; Zhong, Q. Impacts of sample preparation methods on solubility and antilisterial characteristics of essential oil components in milk. Appl. Environ. Microbiol. 2014, 80, 907–916. [Google Scholar] [CrossRef] [PubMed]
- Marinelli, L.; Fornasari, E.; Eusepi, P.; Ciulla, M.; Genovese, S.; Epifano, F.; Fiorito, S.; Turkez, H.; Örtücü, S.; Mingoia, M.; et al. Carvacrol prodrugs as novel antimicrobial agents. Eur. J. Med. Chem. 2019, 178, 515–529. [Google Scholar] [CrossRef] [PubMed]
- Gulcin, I.; Alwasel, S.H. DPPH radical scavenging assay. Processes 2023, 11, 2248. [Google Scholar] [CrossRef]
- Lu, Z.; Wang, D.; Sun, Y.; Dai, Y. ENO1 regulates IL-1β-induced chondrocyte inflammation, apoptosis and matrix degradation possibly through the potential binding to CRLF1. Tissue Cell 2024, 90, 102504. [Google Scholar] [CrossRef]
- Lu, J.; Bian, J.; Wang, Y.; Zhao, Y.; Zhao, X.; Wang, G.; Yang, J. Oxymatrine protects articular chondrocytes from IL-1β-induced damage through autophagy activation via AKT/mTOR signaling pathway inhibition. J. Orthop. Surg. Res. 2024, 19, 178. [Google Scholar] [CrossRef]
- Milaras, C.; Lepetsos, P.; Dafou, D.; Potoupnis, M.; Tsiridis, E. Association of matrix metalloproteinase (MMP) gene polymorphisms with knee osteoarthritis: A review of the literature. Cureus 2021, 13, e18607. [Google Scholar] [CrossRef]
- Sanchez-Lopez, E.; Coras, R.; Torres, A.; Lane, N.E.; Guma, M. Synovial inflammation in osteoarthritis progression. Nat. Rev. Rheumatol. 2022, 18, 258–275. [Google Scholar] [CrossRef]
- Mora, J.C.; Przkora, R.; Cruz-Almeida, Y. Knee osteoarthritis: Pathophysiology and current treatment modalities. J. Pain Res. 2018, 11, 2189–2196. [Google Scholar] [CrossRef]
- Ahmad, N.; Ansari, M.Y.; Haqqi, T.M. Role of iNOS in osteoarthritis: Pathological and therapeutic aspects. J. Cell. Physiol. 2020, 235, 6366–6376. [Google Scholar] [CrossRef]
- Ostojic, M.; Soljic, V.; Vukojevic, K.; Dapic, T. Immunohistochemical characterization of early and advanced knee osteoarthritis by NF-κB and iNOS expression. J. Orthop. Res. 2017, 35, 1990–1997. [Google Scholar] [CrossRef]
- Ighodaro, O.M.; Akinloye, O.A. First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): Their fundamental role in the entire antioxidant defence grid. Alex. J. Med. 2018, 54, 287–293. [Google Scholar] [CrossRef]
- Laavola, M.; Leppänen, T.; Hämäläinen, M.; Vuolteenaho, K.; Moilanen, T.; Nieminen, R.; Moilanen, E. IL-6 in osteoarthritis: Effects of pine stilbenoids. Molecules 2018, 24, 109. [Google Scholar] [CrossRef] [PubMed]
- Haseeb, A.; Ansari, M.Y.; Haqqi, T.M. Harpagoside suppresses IL-6 expression in primary human osteoarthritis chondrocytes. J. Orthop. Res. 2017, 35, 311–320. [Google Scholar] [CrossRef] [PubMed]
- Wojdasiewicz, P.; Poniatowski, L.A.; Szukiewicz, D. The role of inflammatory and anti-inflammatory cytokines in the pathogenesis of osteoarthritis. Mediat. Inflamm. 2014, 2014, 561459. [Google Scholar] [CrossRef] [PubMed]
- Xia, B.; Chen, D.; Zhang, J.; Hu, S.; Jin, H.; Tong, P. Osteoarthritis pathogenesis: A review of molecular mechanisms. Calcif. Tissue Int. 2014, 95, 495–505. [Google Scholar] [CrossRef]
- Sun, Y.; Tsui, Y.-K.; Yu, M.; Lyu, M.; Cheung, K.; Kao, R.; Leung, V. Integration of a miniaturized DMMB assay with high-throughput screening for identifying regulators of proteoglycan metabolism. Sci. Rep. 2022, 12, 1083. [Google Scholar] [CrossRef]
- Mehana, E.-S.E.; Khafaga, A.F.; El-Blehi, S.S. The role of matrix metalloproteinases in osteoarthritis pathogenesis: An updated review. Life Sci. 2019, 234, 116786. [Google Scholar] [CrossRef]
- Primorac, D.; Molnar, V.; Rod, E.; Jeleč, Ž.; Čukelj, F.; Matišić, V.; Vrdoljak, T.; Hudetz, D.; Hajsok, H.; Borić, I. Knee osteoarthritis: A review of pathogenesis and state-of-the-art non-operative therapeutic considerations. Genes 2020, 11, 854. [Google Scholar] [CrossRef]
- Roughley, P.J.; Mort, J.S. The role of aggrecan in normal and osteoarthritic cartilage. J. Exp. Orthop. 2014, 1, 8. [Google Scholar] [CrossRef]
- Watanabe, H. Aggrecan and versican: Two brothers close or apart. Am. J. Physiol. -Cell Physiol. 2022, 322, C967–C976. [Google Scholar] [CrossRef]
- Alcaide-Ruggiero, L.; Molina-Hernández, V.; Granados, M.M.; Domínguez, J.M. Main and minor types of collagens in the articular cartilage: The role of collagens in repair tissue evaluation in chondral defects. Int. J. Mol. Sci. 2021, 22, 13329. [Google Scholar] [CrossRef]
- Xu, J.; Yan, L.; Yan, B.; Zhou, L.; Tong, P.; Shan, L. Osteoarthritis pain model induced by intra-articular injection of mono-iodoacetate in rats. J. Vis. Exp. (JoVE) 2020, 159, e60649. [Google Scholar]
- Bowles, R.D.; Mata, B.A.; Bell, R.D.; Mwangi, T.K.; Huebner, J.L.; Kraus, V.B.; Setton, L.A. In vivo luminescence imaging of NF-κB activity and serum cytokine levels predict pain sensitivities in a rodent model of osteoarthritis. Arthritis Rheumatol. 2014, 66, 637–646. [Google Scholar] [CrossRef]
- Boettger, M.K.; Leuchtweis, J.; Kümmel, D.; Gajda, M.; Bräuer, R.; Schaible, H.-G. Differential effects of locally and systemically administered soluble glycoprotein 130 on pain and inflammation in experimental arthritis. Arthritis Res. Ther. 2010, 12, R140. [Google Scholar] [CrossRef]



| Item | Unit | Control | Sham | MIA | HA | HA-Carvacrol |
|---|---|---|---|---|---|---|
| RBC | 106/mL | 7.93 ± 0.18 | 7.81 ± 0.73 | 7.77 ± 0.33 | 7.82 ± 0.1 | 7.89 ± 0.1 |
| HGB | g/dL | 15.6 ± 0.36 | 15.83 ± 0.83 | 15.8 ± 0.81 | 16.03 ± 0.25 | 16.06 ± 0.23 |
| HCT | % | 42.77 ± 0.55 | 43.17 ± 1.83 | 42.25 ± 1.73 | 43.37 ± 0.85 | 43.94 ± 0.5 |
| MCV | fL | 53.9 ± 1.85 | 55.47 ± 3.07 | 54.43 ± 0.31 | 55.47 ± 0.68 | 55.42 ± 1.12 |
| MCH | pg | 19.67 ± 0.84 | 20.37 ± 0.96 | 20.33 ± 0.26 | 20.5 ± 0.2 | 20.28 ± 0.34 |
| MCHC | g/dL | 36.47 ± 0.83 | 36.7 ± 0.4 | 37.38 ± 0.41 | 36.93 ± 0.15 | 36.66 ± 0.3 |
| PLT | 103/mL | 1023.67 ± 96.34 | 983.33 ± 424.51 | 1092.75 ± 114.21 | 1044.67 ± 223.31 | 1035 ± 142.95 |
| WBC | 103/mL | 11.05 ± 0.98 | 14.48 ± 2.4 | 13.44 ± 3.15 | 13.46 ± 4.08 | 11.17 ± 1.63 |
| MO | 103/mL | 0.69 ± 0.3 | 0.82 ± 0.23 | 0.72 ± 0.27 | 0.89 ± 0.41 | 0.57 ± 0.06 |
| LY | 103/mL | 8.86 ± 0.08 | 12.3 ± 2.6 | 11.3 ± 2.58 | 10.9 ± 3.65 | 9.1 ± 1.06 |
| AST | U/L | 106 ± 18.52 | 96.33 ± 23.97 | 93.75 ± 12.18 | 71.67 ± 10.79 | 76 ± 22.08 |
| ALT | U/L | 40 ± 7 | 40.33 ± 1.15 | 39.5 ± 8.35 | 35.67 ± 8.96 | 33.8 ± 3.9 |
| ALP | U/L | 442.33 ± 53.26 | 451.67 ± 51.03 | 422.5 ± 84.23 | 431 ± 74.36 | 546.4 ± 192.99 |
| BUN | mg/dL | 18.87 ± 2.18 | 18.7 ± 1.32 | 18 ± 2.82 | 17.17 ± 2.56 | 18.7 ± 1.35 |
| CRE | mg/dL | 0.29 ± 0.02 | 0.27 ± 0.03 | 0.27 ± 0.03 | 0.24 ± 0.04 | 0.25 ± 0.03 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.-P.; Lin, J.-N.; Chang, C.-T.; Lin, Y.-Y.; Kuan, C.-Y.; Chen, Y.-C.; Lin, F.-H. The Preparation and Evaluation of Carvacrol-Added Hyaluronic Acid for Early Osteoarthritis Treatment. Antioxidants 2025, 14, 1265. https://doi.org/10.3390/antiox14101265
Chen Y-P, Lin J-N, Chang C-T, Lin Y-Y, Kuan C-Y, Chen Y-C, Lin F-H. The Preparation and Evaluation of Carvacrol-Added Hyaluronic Acid for Early Osteoarthritis Treatment. Antioxidants. 2025; 14(10):1265. https://doi.org/10.3390/antiox14101265
Chicago/Turabian StyleChen, Yu-Ping, Jhih-Ni Lin, Chia-Tien Chang, Yu-Ying Lin, Che-Yung Kuan, Yu-Chun Chen, and Feng-Huei Lin. 2025. "The Preparation and Evaluation of Carvacrol-Added Hyaluronic Acid for Early Osteoarthritis Treatment" Antioxidants 14, no. 10: 1265. https://doi.org/10.3390/antiox14101265
APA StyleChen, Y.-P., Lin, J.-N., Chang, C.-T., Lin, Y.-Y., Kuan, C.-Y., Chen, Y.-C., & Lin, F.-H. (2025). The Preparation and Evaluation of Carvacrol-Added Hyaluronic Acid for Early Osteoarthritis Treatment. Antioxidants, 14(10), 1265. https://doi.org/10.3390/antiox14101265









