Abstract
Lepidium meyenii Walp. (black maca, BM) is a traditional Andean crop increasingly studied for its bioactive potential. This work characterized the phytochemical profile and evaluated the antioxidant, antinociceptive, and neuroprotective properties of a lyophilized aqueous extract of BM hypocotyls. UHPLC-ESI-QTOF-MS/MS identified twelve major compounds, including macamides, imidazole alkaloids, sterols, and fatty acid amides. BM showed a moderate total phenolic content but strong electron transfer-based antioxidant activity in CUPRAC and FRAP assays, together with moderate radical scavenging capacity in ABTS and DPPH systems. In ovariectomized rats, BM significantly reduced brain malondialdehyde levels, mitigated oxidative stress, and improved spatial learning during acquisition in the Morris water maze, confirming its neuroprotective effect. Antinociceptive assays (hot plate, cold plate, and tail immersion) further revealed a rapid but transient increase in nociceptive thresholds. This study provides experimental evidence supporting the analgesic effect of black maca. Molecular docking highlighted lepidiline B and campesterol as key metabolites with strong interactions with redox enzymes, the μ-opioid receptor, and the FAAH enzyme, supporting their role in the observed bioactivities. ADMET predictions indicated favorable oral bioavailability, CNS penetration, systemic clearance, and acceptable safety profiles. These results substantiate the role of black maca as a neuroprotective nutraceutical and highlight its promise as a novel source of rapidly acting natural analgesic compounds.
1. Introduction
Over the past two decades, there has been a significant increase in global interest and demand for Lepidium meyenii Walp, commonly referred to as “maca.” Consequently, maca has become one of Peru’s leading products, marketed in multiple presentations including powders, capsules, pills, flour, liquor, and extracts and commercialized in retail establishments such as health food stores and smoothie shops [1].
Maca roots are native to the Peruvian Andes region and belong to the Brassicaceae family. They thrive in high-altitude regions characterized by harsh environmental conditions such as rocky soils, intense solar radiation, and strong winds [2,3,4,5,6]. The availability of maca hypocotyls in various colors highlights their potential therapeutic versatility, which has been associated with diverse pharmacological effects [7]. These include the regulation of sexual dysfunction [8], neuroprotective effects [9,10], memory enhancement, and antidepressant properties [8,11]. Moreover, maca root supplements are rich in antioxidants, and possess anticancer, anti-inflammatory, and photoprotective properties [12,13,14,15]. Nutritionally, dried maca hypocotyls contain high levels of carbohydrates, proteins, lipids, essential amino acids, and free fatty acids. In addition, several secondary metabolites have been identified, including macamides, macaridine, alkaloids, and glucosinolates [16].
Within Peru, the Department of Junín (Carhuamayo) has recognized 13 distinct maca varieties ranging in color from white to black. Consumption of these varieties has been linked to enhanced energy levels, improved attentiveness, and hormonal balance. Among them, black maca is the rarest, representing approximately 15% of the annual harvest, yet it is also considered the most effective variety for men, particularly with respect to muscle gain, endurance, cognitive performance, and libido [7].
Importantly, maca has been consistently associated with biological activities relevant to the central nervous system. It has been shown to enhance memory [11], exert antioxidant effects [17], improve cognitive function [18], inhibit acetylcholinesterase activity, and maintain unaltered monoamine oxidase levels in male mice. In this context, ovariectomy is widely used as a female animal model of cognitive decline, since it induces memory impairment associated with increased oxidative stress, cholinergic dysfunction, and alterations in monoamine oxidase activity [19].
Based on this background, the present study aimed to investigate new pharmacological properties of this maca species and to confirm some of them previously reported. To this end, the chemical composition of the lyophilized aqueous extract of black maca hypocotyls was investigated, its total phenolic content was quantified, and its in vitro antioxidant activities was assessed using cupric-reducing antioxidant power (CUPRAC), ferric-reducing antioxidant power (FRAP), 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS•+), and 2,2-diphenyl-1-picrylhydrazyl (DPPH) assays. Furthermore, the evaluation of the extract’s analgesic effects was performed in rat models, while its impact on spatial memory was conducted in ovariectomized rats. These findings were complemented with in silico molecular docking analyses targeting proteins involved in redox regulation, and endocannabinoid metabolism involved in pain modulation and neuroprotection. Finally, pharmacokinetic and toxicity-related properties were predicted using the pkCSM online tool https://biosig.lab.uq.edu.au/pkcsm/ (accessed on 28 September 2025).
2. Materials and Methods
2.1. Chemicals, Drugs, and Solvents
All the solvents and reagents were purchased from different companies, such as Aldrich (St. Louis, MO, USA), Merck (Peruana S. A, Ate, Lima, Peru), and Eli Lilly (Indianapolis, IN, USA), and were used as supplied.
2.2. Plant Material
Hypocotyls of Lepidium meyenii Walp. (black maca) were collected in September 2024 in the Carhuamayo Province of Junin District, at 4000 m above sea level in Valle Mantaro, Peru. The specimen was taxonomically identified at the “Herbarium Truxillense (HUT) de la Facultad de Ciencias Biológicas de la Universidad Nacional de Trujillo,” where a voucher sample was deposited for reference.
2.3. Preparation of Lyophilized Aqueous Extract of Black Maca (BM)
The aqueous extract of Lepidium meyenii Walp. (black maca) hypocotyls was prepared following a standardized procedure. Briefly, 500 g of dried hypocotyls was weighed and added to 1.5 L of preheated distilled water at 50 °C. The mixture was refluxed for 2 h, and the extraction was repeated twice with another aqueous solution under the same conditions. The combined extracts were filtered to remove insoluble residues and evaporated under reduced pressure. The concentrated extract was frozen at −80 °C (Arctiko freezer, Nashville, TN, USA) and lyophilized using a freeze-dryer (Labconco, Kansas City, MO, USA), yielding 72.5 g of dried extract (14.5% w/w). Lyophilized samples were stored at +4 °C until further use.
2.4. Chemical Identification by HPLC-ESI-QTOFMS/ MS
Lyophilized BM was dissolved in methanol (1 mg/mL) and subjected to chromatographic separation using a UPLC Rapid Resolution System (Waters/Micromass, Milford, MA, USA) equipped with a binary pump, a degasser, and an automatic injector. The separation was performed on a ZORBAX Eclipse Plus C-18 column (2.1 × 150 mm, 5 μm) (Agilent Technologies Inc., Santa Clara, CA, USA) with a flow rate of 0.4 mL/min and a 2 μL injection volume. The mobile phase consisted of water with 0.1% acetic acid (A) and acetonitrile (B) using the following gradient: 0.0–4.7 min, 3–30% B; 4.7–7.8 min, 30–50% B; 7.8–11 min, 50–90% B; 11–12.5 min, 90% B; and 12.5–14 min, 90–93% B. The column effluent was split with a T-valve, and 20 μL/min was introduced into a micrO-TOF-Q™ orthogonal Q-TOF mass spectrometer (micrOTOF-QTM, Bruker Daltonics, Madison, WI, USA) with an electrospray ionization (ESI) source. Analyses were carried out in positive ion mode (m/z 100–1000) under the following conditions: capillary voltage: 4500 V, end-plate offset: −500 V, charging voltage: 2000 V, drying gas temperature: 200 °C, drying gas flow: 10.0 mL/min, nebulizer gas pressure: 4 bar, collision energy: 50 eV, and nitrogen as collision gas. Data acquisition and processing were performed with Bruker Compass Data Analysis 4.2 software (Bruker Daltonics, Madison, WI, USA).
2.5. Total Phenolic Content (TPC)
The TPC of BM was determined by the Folin–Ciocalteu method adapted from Singleton and Rossi [20]. Briefly, 25 μL of diluted extract was mixed with 125 μL of the Folin–Ciocalteu reagent and incubated for 20 min. Then, 100 μL of sodium carbonate (7%) was added, and the solution was incubated at 45 °C for 10 min. The absorbance of the resulting blue solution was measured at 760 nm. Results were expressed as mg gallic acid equivalents (GAE)/mL of BM.
2.6. Antioxidant Capacity Assays
2.6.1. Cupric Reducing Antioxidant Capacity (CUPRAC) and Ferric-Reducing Antioxidant Power (FRAP) Assays
CUPRAC and FRAP assays were performed following established methods with slight modifications [21]. For CUPRAC, ammonium acetate buffer (pH 7.0), CuCl2, and neocuproine were mixed in a 1:1:1 ratio. Then, 10 μL of standard/sample and 265 μL of H2O were added, followed by 30 min incubation in the dark. Absorbance was measured at 450 nm. For FRAP, the working solution consisted of acetate buffer (300 mM, pH 3.6), TPTZ (2,4,6-tripyridyl-S-triazine) solution (10 mM in 40 mM HCl), and FeCl3·6H2O (20 mM), prewarmed at 37 °C. A Trolox® (Sigma-Aldrich-Fluka, St. Louis, MO, USA) calibration curve (0.05–0.5 mM) was prepared. BM extract (8 μL) was incubated with 200 μL FRAP solution for 30 min in the dark, and absorbance was measured at 593 nm. The results were expressed as mg of Trolox® equivalents (TE)/mL of BM. A standard curve was prepared using the standard antioxidant Trolox®.
2.6.2. ABTS•+ Free-Radical Scavenging Assay
ABTS•+ scavenging activity was assessed according to Re et al. [22]. Trolox® (1 mg/mL in ethanol) was serially diluted (50–800 μM). Then, 10 μL of each dilution was mixed with 300 μL of the ABTS•+ solution, and absorbance was measured at 750 nm using a Fisherbrand AccuSkan GO UV/Vis Microplate Spectrophotometer (Hampton, VA, USA). The BM samples were analyzed under the same conditions. The % inhibition was plotted against concentration, and IC50 values were calculated.
2.6.3. DPPH Free-Radical Scavenging Assay
DPPH radical scavenging activity was determined following a modified protocol reported in the literature [23]. Trolox® (1 mg/mL in ethanol) was serially diluted (0.1–1 mM). Then, 20 μL of each dilution was combined with 300 μL of DPPH solution and incubated for 30 min at room temperature. Absorbance was measured at 517 nm using a Fisherbrand accuSkan GO UV/Vis Microplate Spectrophotometer (Hampton, VA, USA). For BM samples, 20 μL of extract replaced Trolox®. IC50 values (concentration required to scavenge 50% of radicals) were calculated. All assays were performed in triplicate.
2.7. Animals and Experimental Groups
All experiments were performed in accordance with the American Veterinary Medical Association (AVMA) guidelines and approved by the Ethics Committee of the Faculty of Pharmacy and Biochemistry, Universidad Nacional de Trujillo (COD.N_: P 012-19/CEIFYB).
For analgesic activity, adult male Rattus norvegicus (Holtzman strain) (10–12 weeks, 200–250 g) were used. For the ovariectomy (OVX)-induced estrogen depletion model, as well as for memory and lipid peroxidation studies, adult female rats of the same strain and age range were included. Animals were housed individually under controlled temperature (22–25 °C), 12 h light/dark cycle, with ad libitum access to water and standard chow (Molinorte S.A.C., Trujillo, Peru). Rats were randomly assigned to five groups (n = 7 per group): (a) naïve non-ovariectomized (no OVX) controls; (b) ovariectomized (OVX) controls receiving 0.9% NaCl; (c) OVX + estradiol valerate (200 μg/kg); (d) OVX + BM (0.5 g/kg); and (e) OVX + BM (2.0 g/kg). Treatments were administered orally for two months.
2.8. Ovariectomy Procedure
Three-month-old female rats were anesthetized with ketamine (110 mg/kg, i.p.) [24]. A ventral incision was made, and in OVX groups, the ovaries, oviducts, and upper fallopian tubes were removed. Muscles and skin were sutured, and animals recovered under standard conditions. Behavioral experiments began three months post-surgery.
2.9. Lipid Peroxidation Assay
Lipid peroxidation in brain tissue was determined using the thiobarbituric acid reactive substances (TBARS) assay, which quantifies malondialdehyde (MDA) levels, following modified protocols [25,26]. Briefly, rat brains were homogenized in phosphate buffer (20 mM, pH 7.4, 140 mM KCl), centrifuged, incubated at 37 °C, precipitated with trichloroacetic acid, and reacted with thiobarbituric acid. The organic phase was extracted with butanol/pyridine (15:1), centrifuged, and absorbance measured at 532 nm. Results were expressed as μmol MDA/g tissue.
2.10. Morris Water Maze (MWM) Experiment
Spatial memory was assessed using the MWM test [27]. Rats were trained for four days (four trials/day, 120 s max) to locate a hidden platform submerged 1 cm below the water. Escape latency was recorded. On day 5, a probe trial (platform removed) was conducted, measuring time spent in the target quadrant and platform crossings.
2.11. Hot/Cold Plate Test
Thermal sensitivity was measured using a hot/cold plate analgesiometer (Ugo Basile®, Gemonio, Italy), as previously described [28]. Male rats were habituated for 15 min at 25 °C. For the hot plate test, the surface was maintained at 55 ± 0.1 °C (cut-off time 10 s). For the cold plate test, the plate was set at 5 ± 0.1 °C (cut-off 60 s). Latency to paw lifting or shaking was recorded. Response latency was measured before treatment and at 15, 30, 45, 60, 90, and 120 min post-dosing.
The treatments administered to animals in each experimental test group followed the study design outlined in Table 1.

Table 1.
Experimental design of the hot/cold plate test in rats.
2.12. Tail Immersion Test
Analgesic activity was also assessed by the tail immersion test [29]. A 1 L beaker containing 900 mL water with 0.44% NaCl was maintained at 55–56 °C, stirred continuously for uniform heating. Response latency was measured before treatment and at 60, 120, 180, 240 and 300 min post-dosing. The animals assigned to different experimental groups were treated in accordance with the study design described in Table 2.

Table 2.
Experimental design of the tail immersion test in rats.
2.13. Molecular Docking
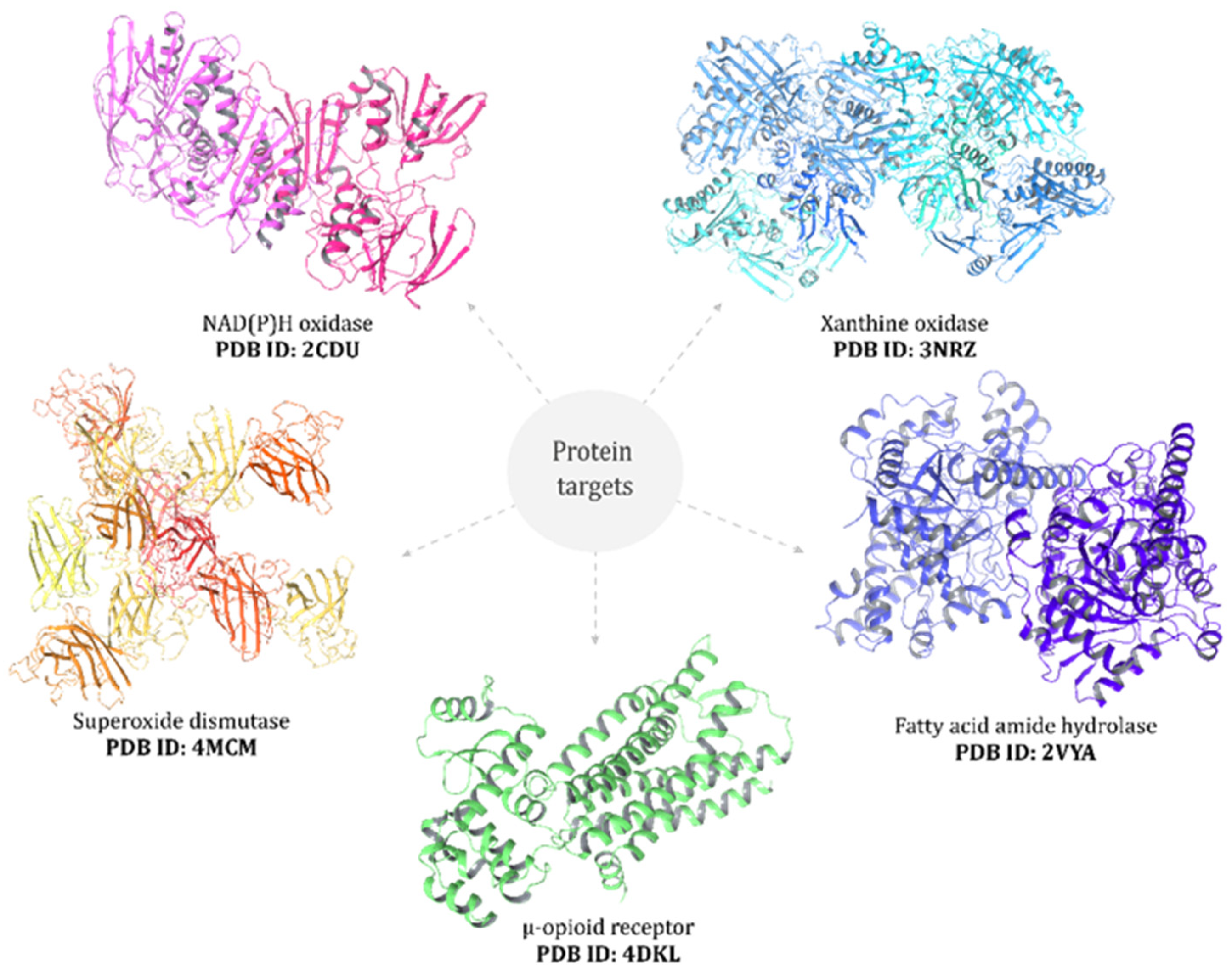
Docking studies were performed with selected BM compounds on protein targets including NADPH oxidase [30], xanthine oxidase [31], superoxide dismutase [32], μ-opioid receptor (PDB: 4DKL) [33], and fatty acid amide hydrolase [34] (Figure 1). Molecular docking was carried out using AutoDock (v 4.2.1, Scripps Research Institute, San Diego, CA, USA), AutoDock Vina (v 1.0.2 Scripps Research Institute, San Diego, CA, USA) [35], and the AutoDockTools package v 1.5.7 [36], following protocols describe by Minchán-Herrera et al. [37]. Validation was performed by re-docking co-crystallized ligands into their corresponding binding sites, yielding RMSD values < 2.0 Å, thereby confirming the reliability of the docking protocol. All other parameters were set to the AutoDock Vina default. Docking simulations were repeated 20 times with the search exhaustiveness parameter set to 100. The best binding energy values (kcal·mol−1) were selected for evaluation. Three-dimensional docking results were visualized using the Discovery Studio 3.1 (Accelrys, San Diego, CA, USA) molecular graphics system.
Figure 1.
Black maca extract was docked into the binding sites of selected protein targets: NAD(P)H oxidase, xanthine oxidase, superoxide dismutase, μ-opioid receptor, and fatty acid amide hydrolase (PDB IDs indicated in the figure).
2.14. ADMET Prediction
The pkCSM online tool (http://biosig.unimelb.edu.au/pkcsm/prediction, accessed on 26 March 2025) [38] was used to predict absorption, distribution, metabolism, excretion, and toxicity (ADMET) properties of the bioactive components of BM.
2.15. Statistical Analysis
Statistical analysis was conducted using the GraphPad Prism 8.0.2 software (San Diego, CA, USA). The data are presented as mean ± SEM. One-way or Two-way analysis of variance (ANOVA) with Tukey’s post hoc test was used for data analysis. Statistical significance was set at p ≤ 0.05.
3. Results
3.1. Chemical Composition of Lyophilized Hypocotyl Aqueous Extract of Black Maca
One of the objectives of this study was to characterize the phytochemical composition of the aqueous extract obtained from lyophilized hypocotyls of black maca (Lepidium meyenii Walp.). For this purpose, high-performance liquid chromatography coupled with electrospray ionization quadrupole time-of-flight tandem mass spectrometry (HPLC-ESI-QTOF-MS/MS) was employed. Representative spectra of the detected compounds are provided in the Supplementary Materials (Figure S1).
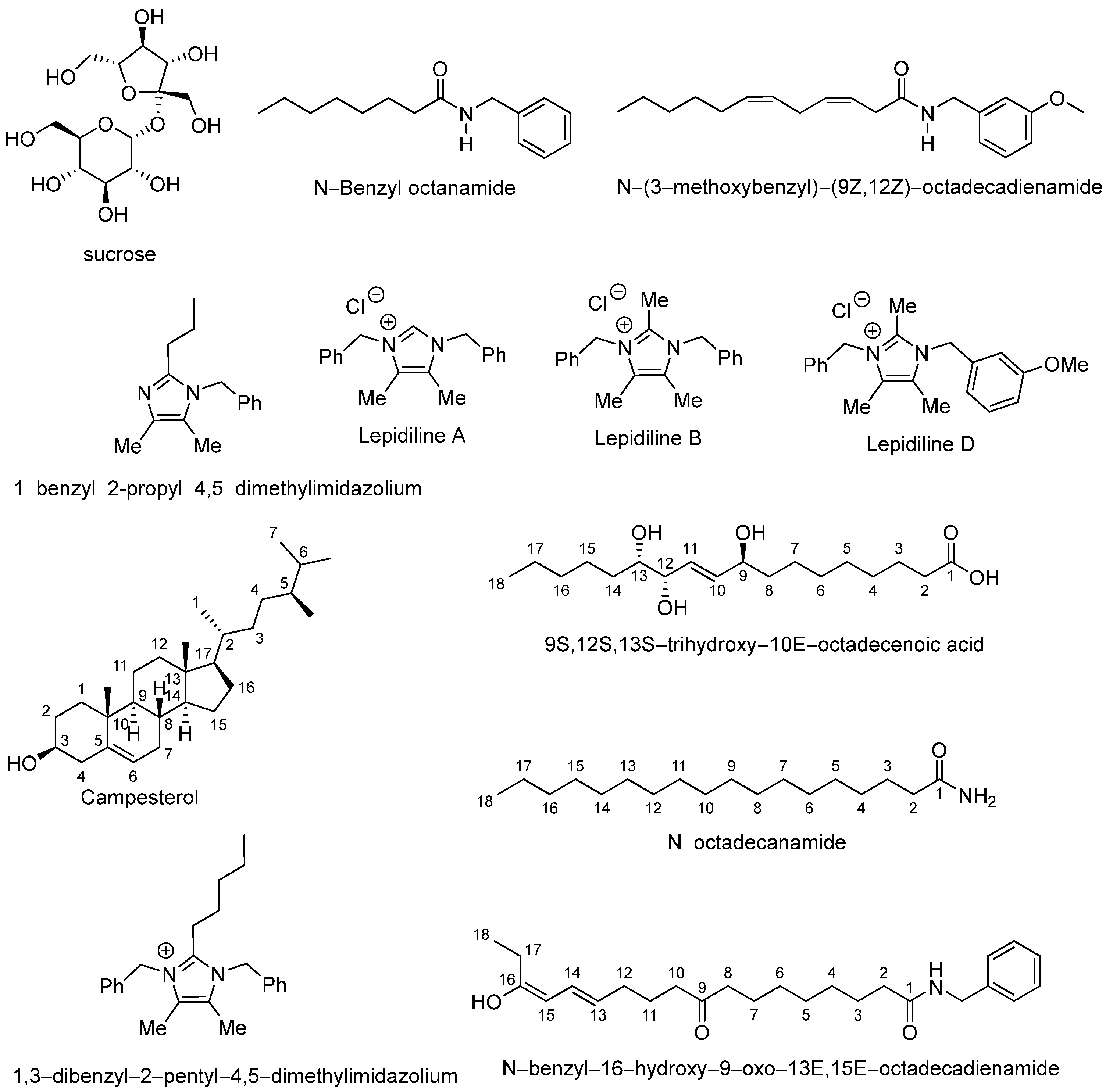
In total, twelve major compounds were identified (Table 3), whose corresponding mass spectra are also shown in the Supplementary Materials (Figure S1). These metabolites included sucrose, N-benzyloctanamide, N-(3-methoxybenzyl)-(9Z,12Z)-octadecadienamide, 1-benzyl-2-propyl-4,5-dimethylimidazilium, lepidiline A, lepidiline B, lepidiline D, campesterol, pinellic acid, 1,3-dibenzyl-2-pentyl-4,5-dimethylimidazilium, N-octadecanamide, and N-benzyl-16-hydroxy-9-oxo-13E,15E-octadecadienamide (Figure 2).

Table 3.
Chemical composition of lyophilized hypocotyl aqueous extract of black maca (Lepidium meyenii Walp.) determined by HPLC-ESI-QTOF-MS/MS.
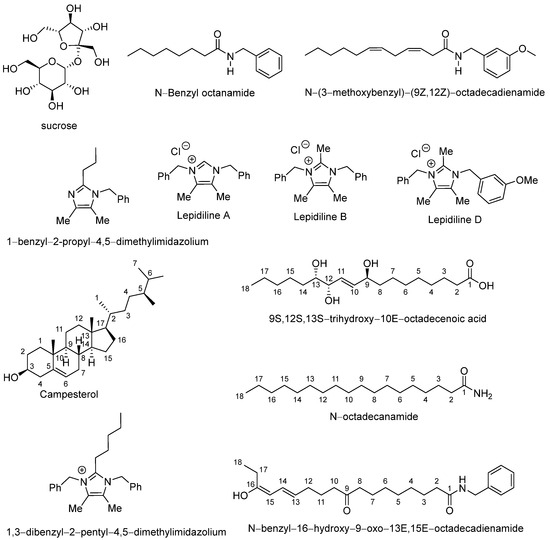
Figure 2.
Chemical structures of abundant compounds identified in the lyophilized hypocotyl aqueous extract of black maca (Lepidium meyenii Walp.).
3.2. Total Phenolic Content and Antioxidant Capacity of BM
Table 4 summarizes the total phenolic content (TPC) and antioxidant activities of BM. The TPC was expressed as gallic acid equivalents (GAE) per mL of extract. The analysis revealed a moderate phenolic content of 10.62 mg GAE/mL in the BM. The antioxidant potential of BM was further assessed using four complementary assays that capture distinct mechanisms of action: (1) cupric ion-reducing antioxidant capacity (CUPRAC), (2) ferric-reducing antioxidant power (FRAP), (3) ABTS•+ radical scavenging, and (4) DPPH radical scavenging.

Table 4.
Total phenolic content (TPC) and antioxidant activities of BM measured by CUPRAC, FRAP, ABTS, and DPPH assays.
In the CUPRAC assay, which measures the reduction of the Cu(II)–neocuproine complex to the Cu(I)–neocuproine chromophore, BM exhibited strong reducing capacity with a TEAC value of 14.06 mg/mL. This activity was markedly higher than that of the reference antioxidant quercetin (TEAC = 4.69 mg/mL).
Similarly, in the FRAP assay, which reflects the electron-donating ability of antioxidants through the reduction of the Fe3+–TPTZ complex to Fe2+–TPTZ, BM demonstrated substantial reducing power, with a TEAC value of 7.84 mg/mL, more than double that of quercetin (3.14 mg/mL).
The ABTS•+ assay, which evaluates the capacity of antioxidants to neutralize radical cations, showed that BM had an IC50 of 15.26 µg/mL. Although this value is higher than those of quercetin (IC50 = 0.09 µg/mL) and Trolox® (IC50 = 0.20 µg/mL), it nonetheless indicates a relevant ability to scavenge hydrophilic radicals.
Finally, the DPPH assay, which measures hydrogen- or electron-donating capacity toward the DPPH radical, revealed that BM exhibited an IC50 of 36.82 µg/mL. This demonstrates considerable radical scavenging activity, supporting the overall antioxidant potential of the extract.
3.3. Spatial Memory and Brain Lipidic Peroxidation in Ovariectomized Rats
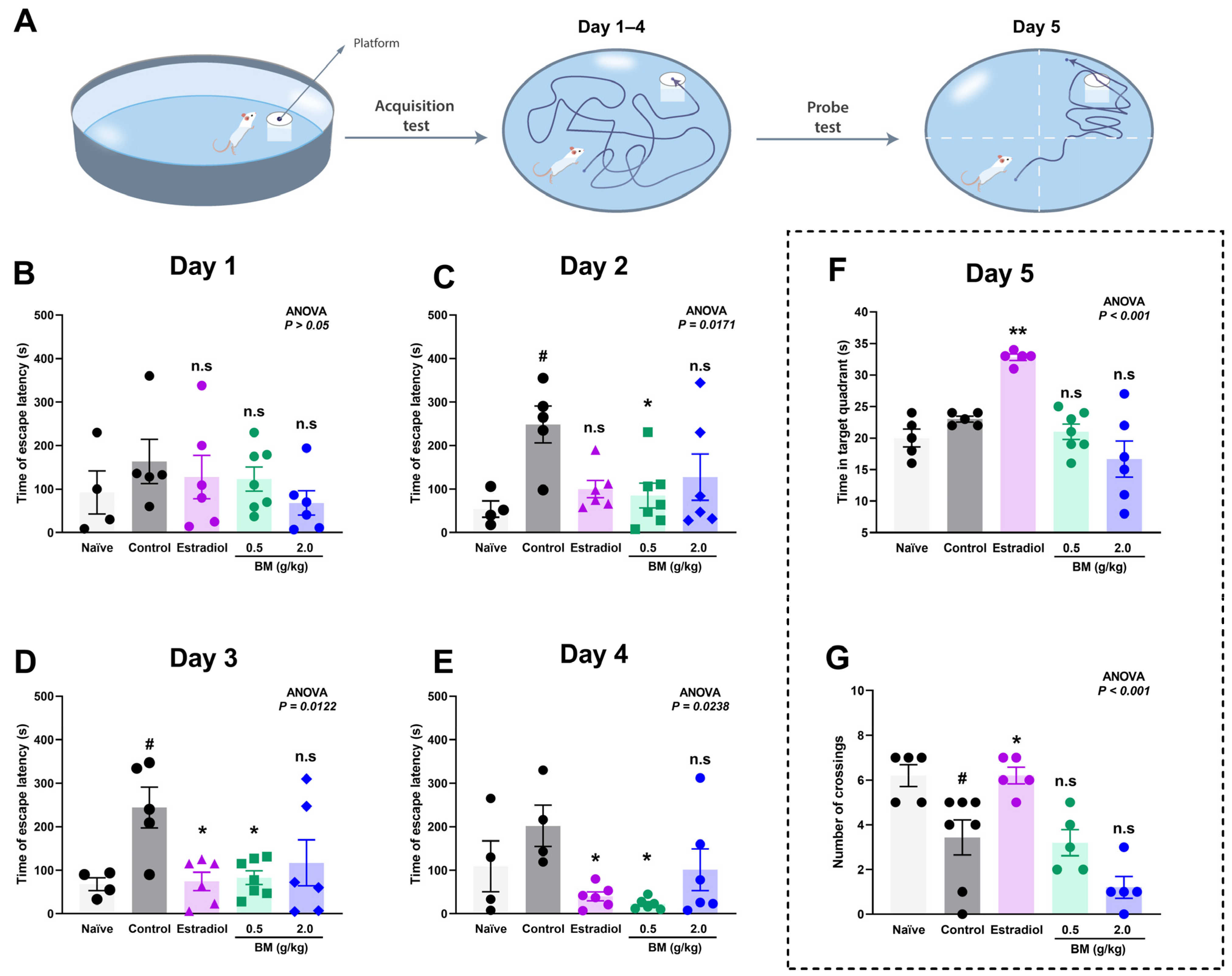
The effects of BM on spatial learning and memory were assessed using the Morris water maze. During the acquisition phase (days 1–4), escape latency to locate the hidden platform was measured (Figure 3A), while spatial memory retention was evaluated on day 5 through the probe test, recording the time spent in the target quadrant and the number of crossings over the former platform location.
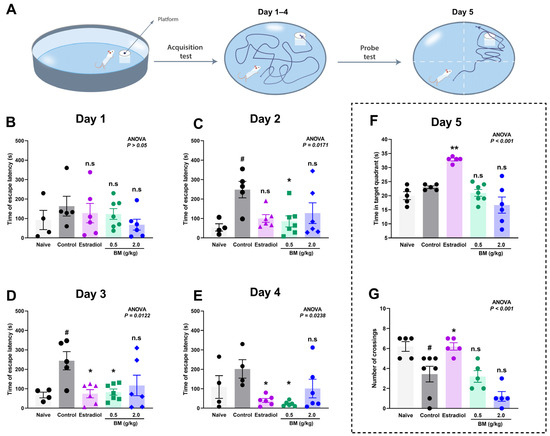
Figure 3.
Effect of the lyophilized aqueous extract of black maca (Lepidium meyenii Walp., BM) on spatial learning and memory performance in ovariectomized rats assessed using the Morris water maze. (A) Experimental design. Escape latency time (s) to find the hidden platform was recorded during the acquisition phase: (B) day 1, (C) day 2, (D) day 3, and (E) day 4. On day 5, memory retention was evaluated by measuring (F) the time spent in the target quadrant and (G) the number of crossings over the former platform location. Data are expressed as mean ± SEM (n = 7 per group). Statistical significance was determined by one-way ANOVA followed by Tukey’s post hoc test. # p < 0.05 vs. naïve group; * p < 0.05, ** p < 0.01 vs. control group; n.s., not significant vs. control group.
On day 1, no significant differences were observed between the groups (ANOVA, p > 0.05), confirming comparable baseline performance (Figure 3B). By day 2 (Figure 3C), the OVX control group exhibited a marked increase in escape latency (248.6 ± 42.5 s) compared with the naïve group (54.0 ± 18.7 s; p < 0.05), confirming the learning impairment induced by ovariectomy. Notably, BM at 0.5 g/kg (85.0 ± 28.2 s; p < 0.05 vs. control) significantly reduced escape latency, whereas estradiol (99.8 ± 19.8 s; p > 0.05) and BM at 2.0 g/kg (127.5 ± 53.2 s; p > 0.05) did not. On day 3 (Figure 3D), both estradiol (74.5 ± 21.1 s; p < 0.05) and BM 0.5 g/kg (82.7 ± 15.8 s; p < 0.05) significantly improved escape latency relative to controls (244.0 ± 46.7 s), while the 2.0 g/kg dose remained ineffective (116.8 ± 52.9 s; p > 0.05). On day 4 (Figure 3E), BM at 0.5 g/kg (22.3 ± 5.3 s; p < 0.05) and estradiol (40.0 ± 10.4 s; p < 0.05) maintained significantly reduced latencies compared to controls (202.0 ± 47.4 s), suggesting enhanced learning and consolidation. The BM 2.0 g/kg group (101.2 ± 47.9 s) again did not reach statistical significance. In the probe test (day 5), only estradiol-treated rats displayed significant improvements, with greater time in the target quadrant (32.8 ± 0.5 s vs. control: 23.0 ± 0.4 s; p < 0.05; Figure 3F) and increased platform crossings (6.20 ± 0.37 vs. control: 3.43 ± 0.78; p < 0.05; Figure 3G). Neither BM dose significantly improved probe test performance.
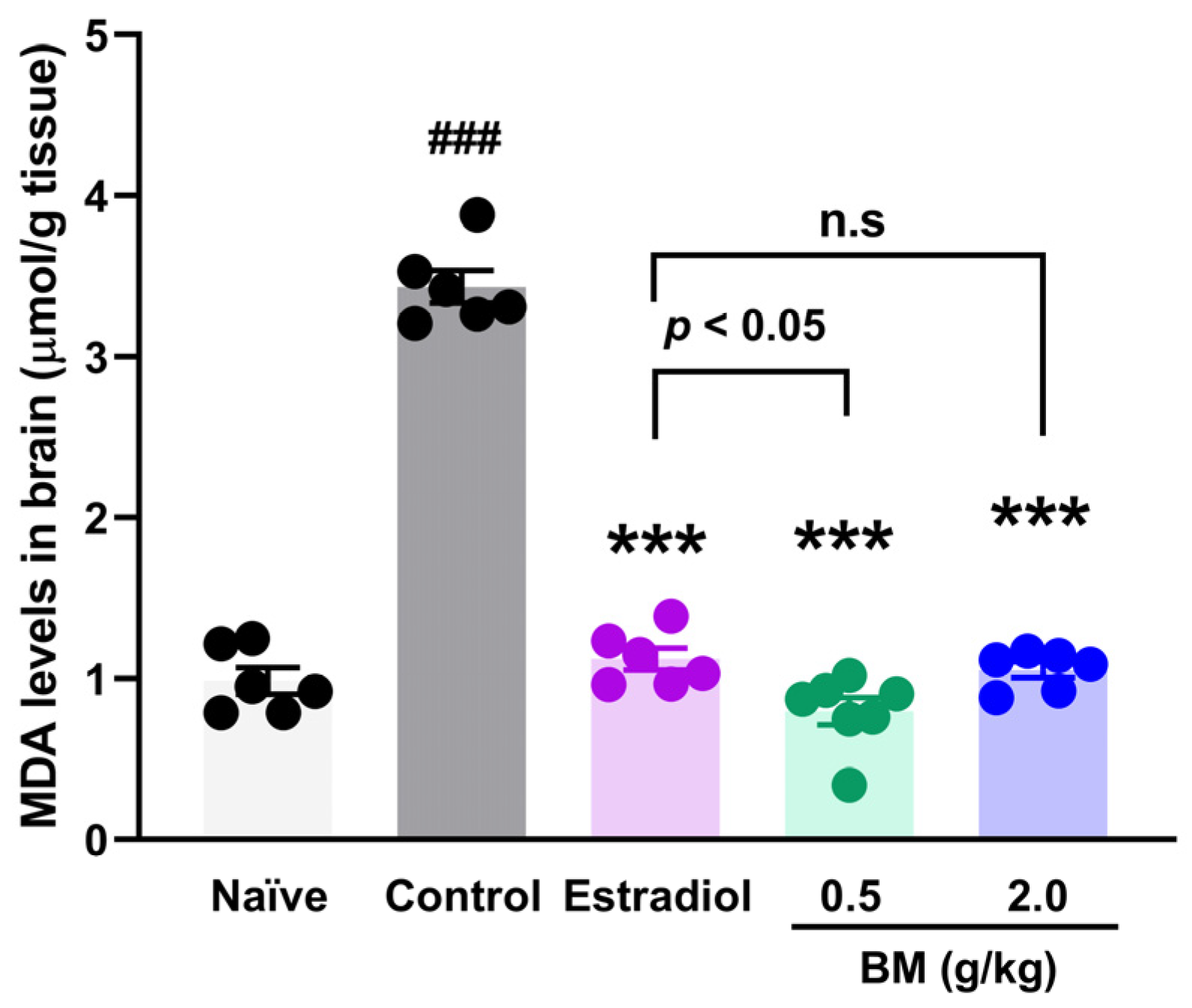
Given that lipid peroxidation is a free radical–driven process in which reactive oxygen species (ROS) attack polyunsaturated fatty acids in cellular membranes, we measured malondialdehyde (MDA) levels as a reliable biomarker of oxidative damage in neural tissue. In addition to behavioral outcomes, OVX animals exhibited a marked increase in brain MDA concentrations (3.44 ± 0.1 µmol/g tissue) compared with naïve rats (0.98 ± 0.08 µmol/g tissue; p < 0.001) (Figure 4). Estradiol treatment significantly reduced MDA concentrations (1.12 ± 0.07 µmol/g tissue; p < 0.001 vs. control), restoring values to those of naïve animals. Similarly, BM at both 0.5 g/kg (0.79 ± 0.08 µmol/g tissue) and 2.0 g/kg (1.06 ± 0.05 µmol/g tissue) significantly decreased MDA levels compared with OVX controls (p < 0.001). No significant differences were observed between the BM 2.0 g/kg and estradiol groups (p > 0.05), suggesting a comparable antioxidant effect. Although the 2.0 g/kg dose showed slightly higher MDA values than 0.5 g/kg, the difference was not statistically significant, indicating a plateau in the antioxidant efficacy of BM at higher concentrations.
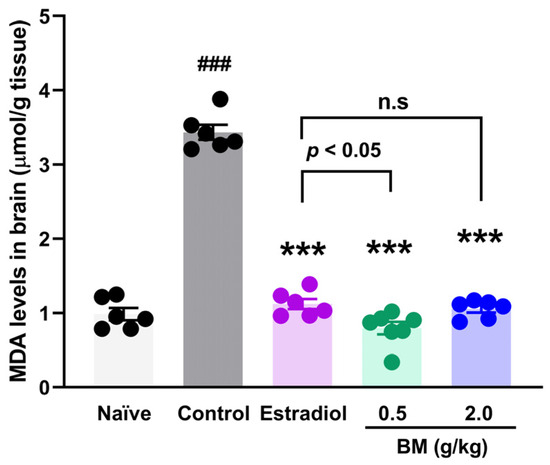
Figure 4.
Effect of the lyophilized aqueous extract of black maca (Lepidium meyenii Walp., BM) on brain lipid peroxidation, assessed by malondialdehyde (MDA) levels, in ovariectomized rats. Data are expressed as mean ± SEM (n = 7 per group). Statistical significance was determined by one-way ANOVA followed by Tukey’s post hoc test. *** p < 0.001 vs. control group; ### p < 0.001 vs. naïve group; n.s., not significant vs. estradiol group.
3.4. Antinociceptive Assays
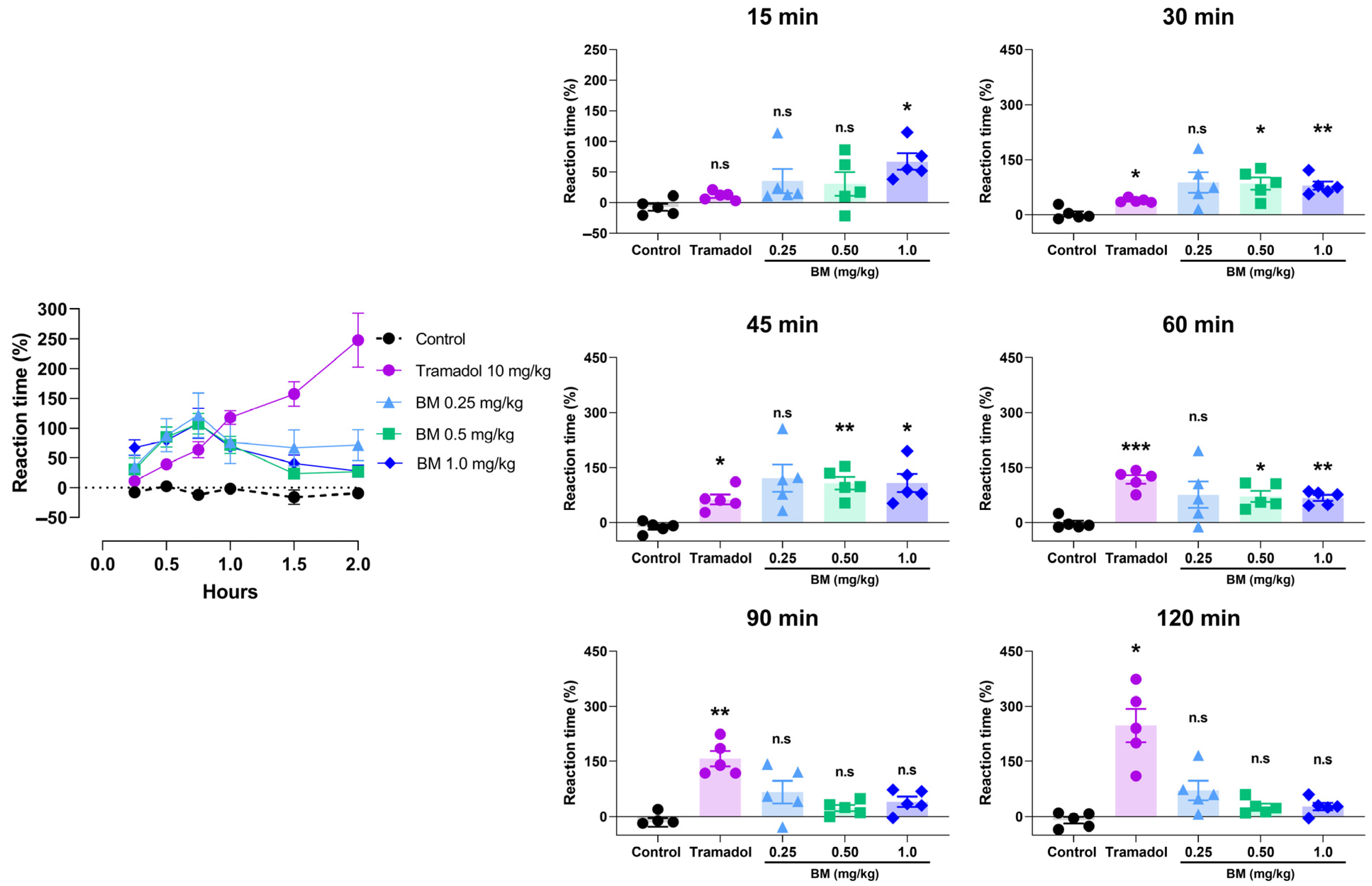
The antinociceptive effects of BM were first evaluated using the hot plate test following oral administration at doses of 0.25, 0.5, and 1.0 mg/kg. As shown in Figure 5, all doses of BM increased the reaction latency compared with the vehicle control group (0.9% NaCl), with effects evident as early as 15 min and peaking at 45 min. At 15 min, BM 1.0 mg/kg significantly prolonged reaction time by 67.2 ± 13.4% (p < 0.05 vs. control). The maximal effect occurred at 45 min, where BM 1.0 mg/kg produced the strongest response (108.2 ± 24.9%; p < 0.01), followed by BM 0.5 mg/kg (107.4 ± 17.3%; p < 0.05). At the same time point, tramadol (10 mg/kg) also increased response latency (63.6 ± 13.5%; p < 0.05). Between 15 and 45 min, all BM-treated groups (0.25–1.0 mg/kg) showed greater response latencies than tramadol, although the differences did not reach statistical significance. At 60 min, BM 0.5 mg/kg (71.8 ± 14.7%; p < 0.05) and BM 1.0 mg/kg (67.8 ± 8.2%; p < 0.01) maintained significant activity, while tramadol produced the highest latency (117.6 ± 11.7%; p < 0.001), not significantly different from BM. Interestingly, BM displayed an early onset of action (15–45 min) with transient efficacy, whereas tramadol produced a delayed but sustained response, reaching its maximum effect at 120 min (247.6 ± 45.4%; p < 0.05). After 90 min, BM-treated animals no longer exhibited significant increases in latency (p > 0.05).
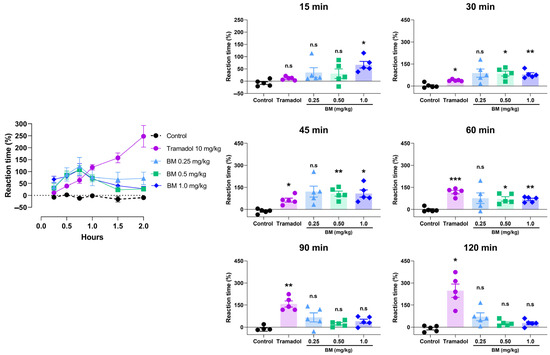
Figure 5.
Antinociceptive effect of the lyophilized aqueous extract of black maca (Lepidium meyenii Walp., BM) hypocotyl in the hot-plate test in rats. Reaction latency (time to paw licking or jumping) was recorded at baseline (0 min) and at 15, 30, 45, 60, 90, and 120 min after oral administration of BM at doses of 0.25, 0.5, and 1.0 mg/kg. Tramadol (10 mg/kg, p.o.) was used as the reference drug, and 0.9% NaCl served as the vehicle control. Data are expressed as mean ± SEM (n = 6 per group). Statistical significance was determined by two-way ANOVA followed by Tukey’s post hoc test. * p < 0.05, ** p < 0.01, *** p < 0.001 vs. control group; n.s., not significant vs. control group.
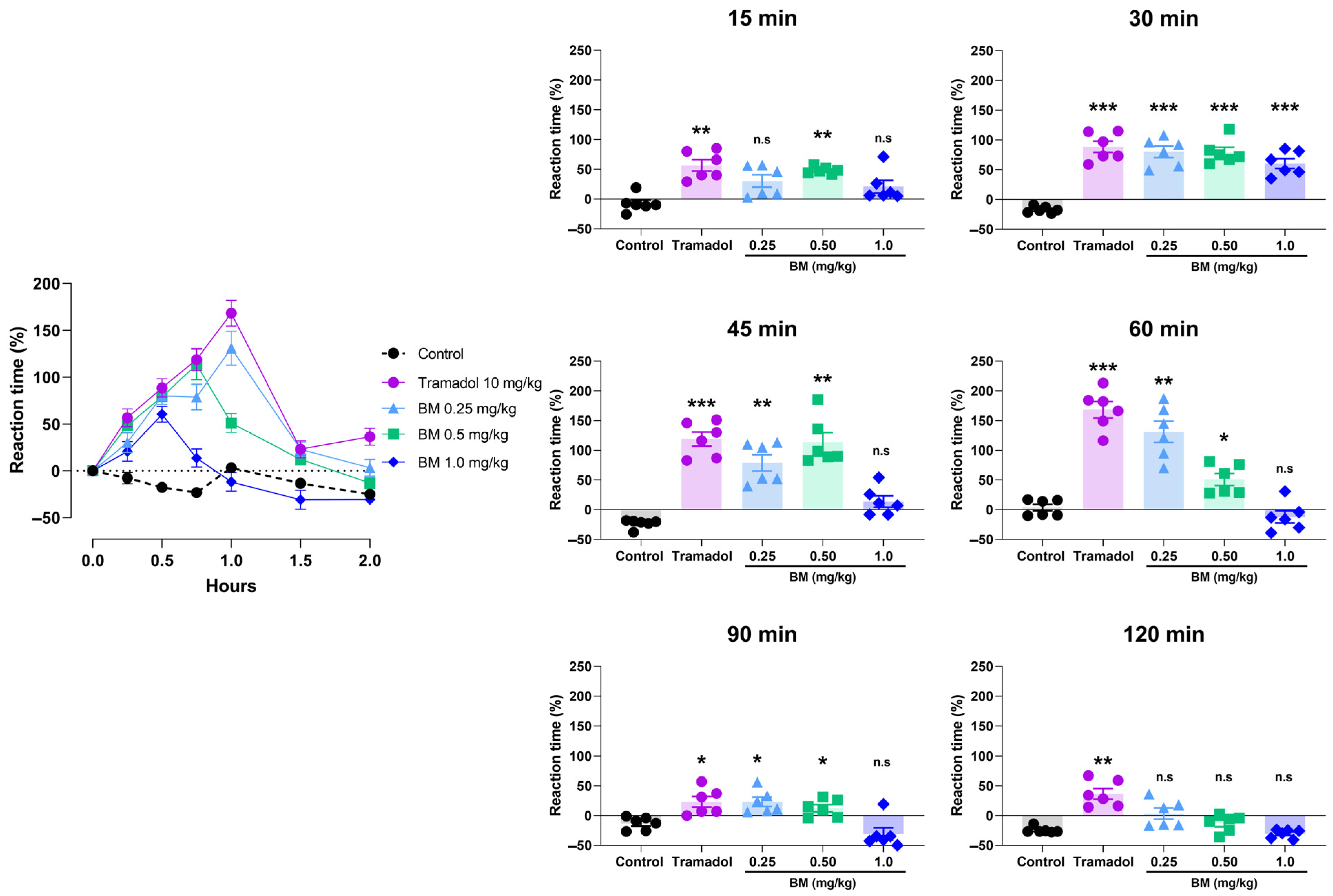
The cold plate test further confirmed the antinociceptive potential of BM (Figure 6). All doses significantly increased reaction latency compared with controls, with effects observed at 15 min after administration. At this time point, BM 0.25 mg/kg increased latency by 48.3 ± 2.5% (p < 0.01 vs. control), while tramadol elicited a comparable effect (56.7 ± 9.6%; p < 0.01). At 30 min, tramadol produced the greatest response (88.5 ± 9.6%; p < 0.001), followed by BM 1.0 mg/kg (80.2 ± 9.5%; p < 0.001), BM 0.5 mg/kg (79.2 ± 8.4%; p < 0.001), and BM 0.25 mg/kg (60.5 ± 8.3%; p < 0.001). No statistical differences were found between BM-treated groups and tramadol at this time point (p > 0.05). The maximum response was recorded at 60 min in the tramadol group (168.3 ± 13.6%; p < 0.001), followed by BM 0.25 mg/kg (131.0 ± 18.1%; p < 0.001) and BM 0.5 mg/kg (51.0 ± 10.1%; p < 0.001). Interestingly, BM 1.0 mg/kg failed to produce a significant effect at 60 min. By 90 min, efficacy declined in all groups, although tramadol and BM (0.25 and 0.5 mg/kg) still showed higher latencies than controls (p < 0.05). At 120 min, only tramadol maintained significant activity (36.3 9.0%; p < 0.05).
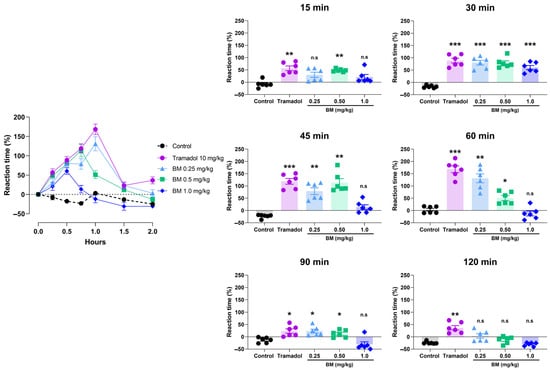
Figure 6.
Antinociceptive effect of the lyophilized aqueous extract of black maca (Lepidium meyenii Walp., BM) hypocotyl in the cold-plate test in rats. Reaction latency (time to paw licking or jumping) was recorded at baseline (0 min) and at 15, 30, 45, 60, 90, and 120 min after oral administration of BM at doses of 0.25, 0.5, and 1.0 mg/kg. Tramadol (10 mg/kg, p.o.) was used as the reference drug, and 0.9% NaCl served as the vehicle control. Data are expressed as mean ± SEM (n = 6 per group). Statistical significance was determined by two-way ANOVA followed by Tukey’s post hoc test. * p < 0.05, ** p < 0.01, *** p < 0.001 vs. control group; n.s., not significant vs. control group.
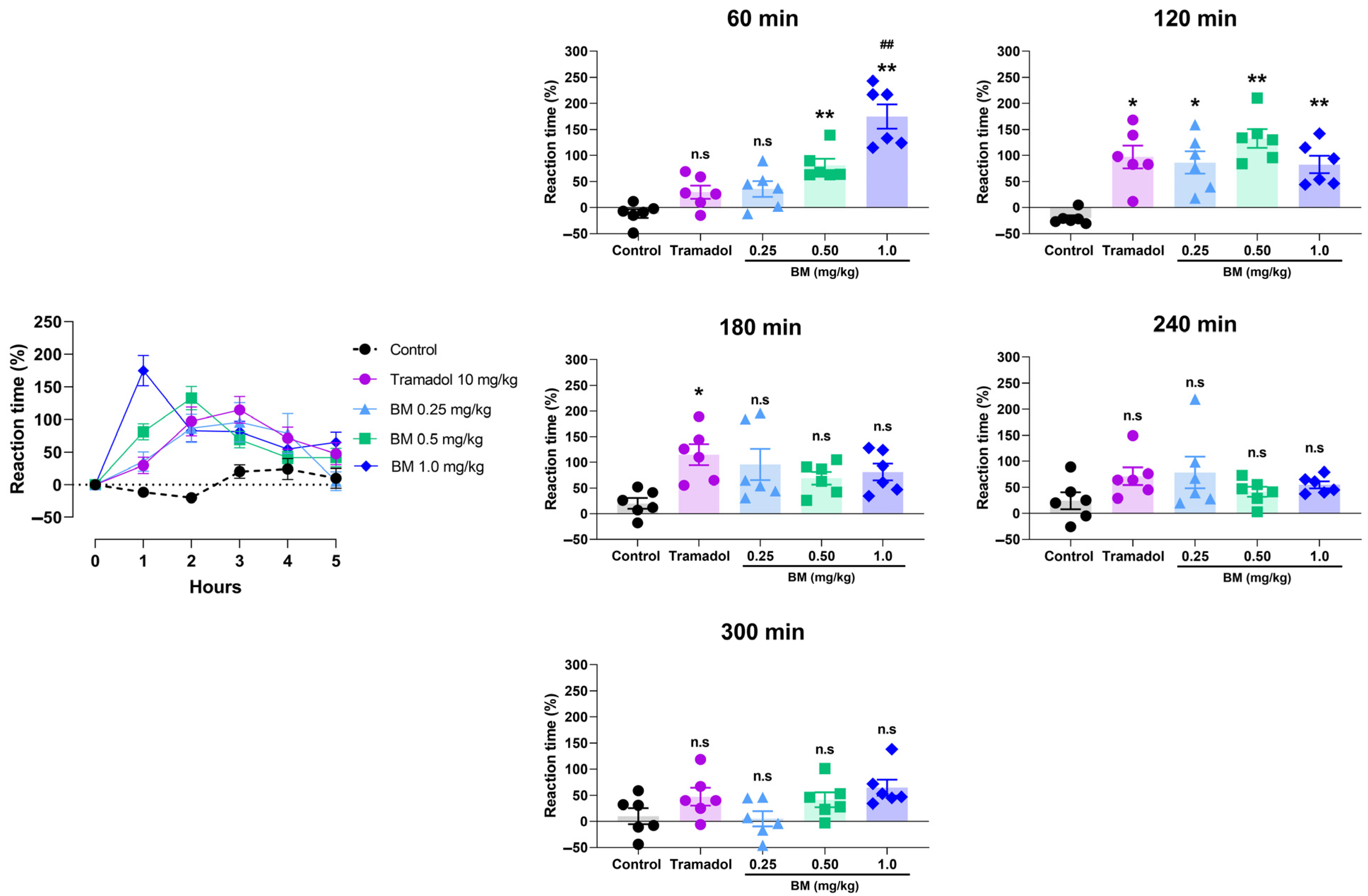
The tail immersion test provided additional evidence of BM’s antinociceptive activity (Figure 7). Oral administration of BM at 0.25, 0.5, and 1.0 mg/kg significantly increased tail-flick latency compared with vehicle-treated rats, with effects evident at 60 min post-treatment. The maximal response was observed at this time with BM 1.0 mg/kg (174.8 ± 23.2%; p < 0.01 vs. control), followed by BM 0.5 mg/kg (81.2 ± 12.3%; p < 0.01). Notably, BM 1.0 mg/kg was significantly more effective than tramadol (29.5 ± 12.7%; p < 0.01). At 120 min, all BM doses remained significantly active (BM 1.0 mg/kg: 82.5 ± 16.7%; BM 0.5 mg/kg: 132.7 ± 18.1%; BM 0.25 mg/kg: 86.5 ± 21.6%; p < 0.05–0.01), with no differences compared to tramadol (97.2 ± 21.9%). By 180 min, only tramadol-treated animals maintained significant activity (114.8 ± 20.5% vs. control: 20.0 ± 10.3%; p < 0.01). At 240 and 300 min, no significant antinociceptive effects were observed in any of the groups.
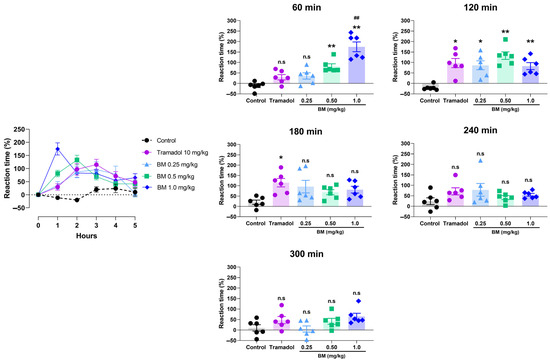
Figure 7.
Antinociceptive effect of the lyophilized aqueous extract of black maca (Lepidium meyenii Walp., BM) hypocotyl in the tail immersion test in rats. Tail-flick latency was recorded at baseline (0 h) and at 60, 120, 180, 240, and 300 min. after oral administration of BM at doses of 0.25, 0.5, and 1.0 mg/kg. Tramadol (10 mg/kg, p.o.) was used as the reference drug, and 0.9% NaCl served as the vehicle control. Data are expressed as mean ± SEM (n = 6 per group). Statistical significance was determined by two-way ANOVA followed by Tukey’s post hoc test. * p < 0.05, ** p < 0.01 vs. vehicle; ## p < 0.01 vs. tramadol; n.s., not significant vs. control group.
3.5. Molecular Docking
Molecular docking analysis was performed to investigate the potential interactions of black maca constituents with key protein targets relevant to redox regulation, analgesia, and neuroprotection. The selected targets included NADPH oxidase (PDB: 2CDU), xanthine oxidase (PDB: 3NRZ), and superoxide dismutase (PDB: 4MCM) as representative redox enzymes; the μ-opioid receptor (PDB: 4DKL) as an analgesic target; and fatty acid amide hydrolase (FAAH, PDB: 2VYA), a central enzyme in endocannabinoid metabolism involved in pain modulation and neuroprotection.
As summarized in Table 5, several compounds demonstrated strong binding affinity across multiple targets. Among the redox enzymes, campesterol (compound 8) exhibited the most robust docking scores, particularly with ROS-generating enzymes such as NADPH oxidase (−9.9 kcal/mol) and xanthine oxidase (−9.5 kcal/mol), suggesting strong inhibitory potential. Consequently, a low binding activity was observed with the antioxidant enzyme SOD (−6.6 kcal/mol). Similarly, lepidiline B (compound 6), lepidiline D (compound 7), and 1,3-dibenzyl-2-pentyl-4,5-dimethylimidazilium (compound 10) consistently displayed high binding affinities (−8.7 to −9.6 kcal/mol), reinforcing their role as redox candidates.

Table 5.
Free binding energy results (in kcal.mol−1) from molecular docking calculations of chemical constituents of black maca and reference compounds tramadol and estradiol with NAD(P)H oxidase (PDB ID: 2CDU), xanthine oxidase (PDB ID: 3NRZ, superoxide dismutase (PDB ID: 4MCM), μ-opioid receptor (PDB ID: 4DKL), and fatty acid amide hydrolase (PDB ID: 2VYA).
Docking results for the μ-opioid receptor further highlighted campesterol (−8.6 kcal/mol) as one of the top ligands, surpassing tramadol (−6.4 kcal/mol), which was used as a reference compound. Lepidilines (compounds 5–7) also showed strong interactions (−8.2 to −8.4 kcal/mol), supporting their potential contribution to the analgesic effects observed in vivo.
For FAAH, compound 6 displayed the strongest binding affinity (−10.1 kcal/mol), followed by compound 8 (−9.7 kcal/mol) and compound 7 (−9.3 kcal/mol). These values were comparable to estradiol (−9.4 kcal/mol), used as a reference ligand.
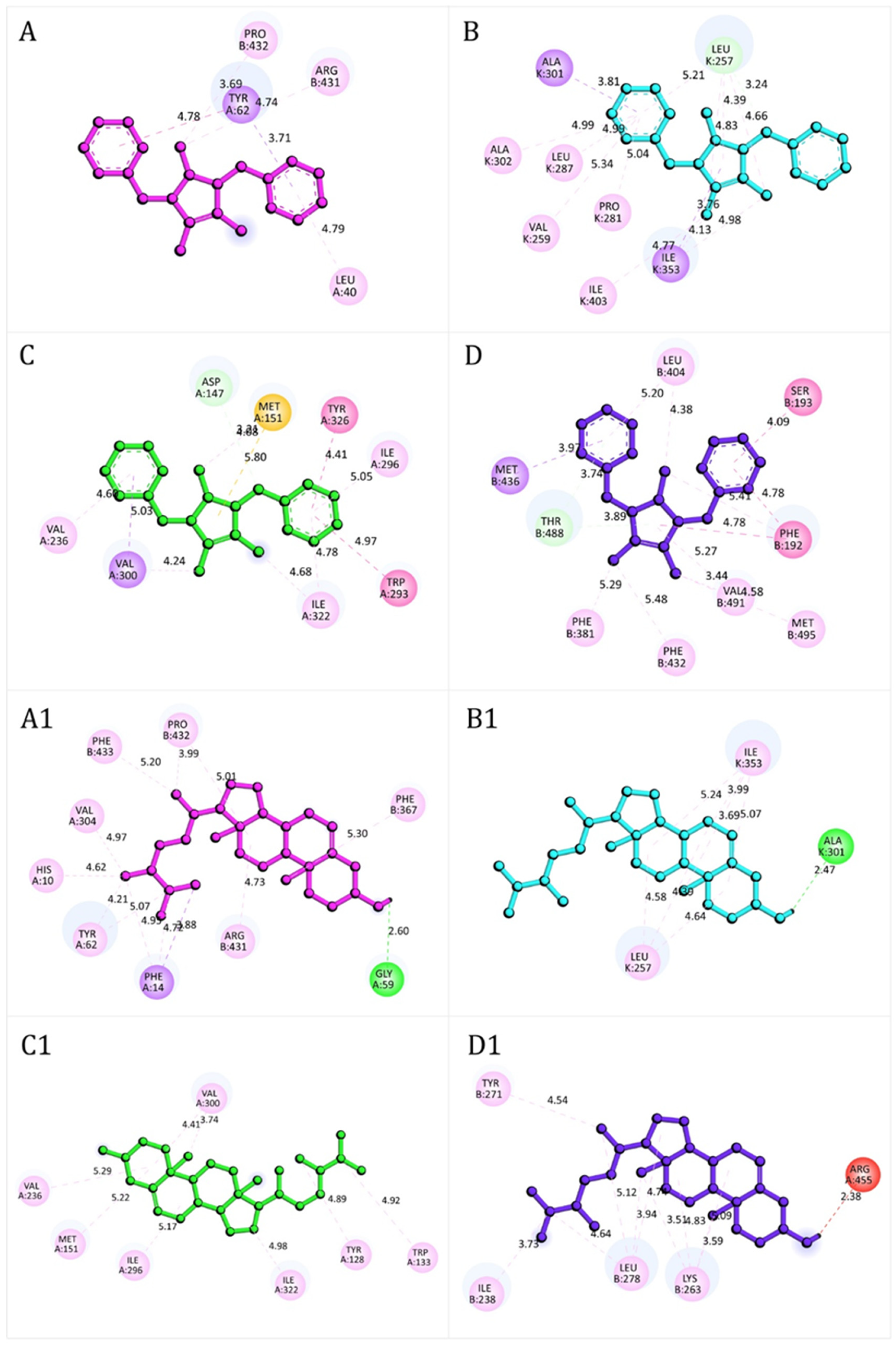
The docking interactions (Figure 8) illustrate the binding modes of lepidiline B (compound 6) and campesterol (compound 8) with key protein targets related to redox homeostasis, nociception, and neuroprotection.
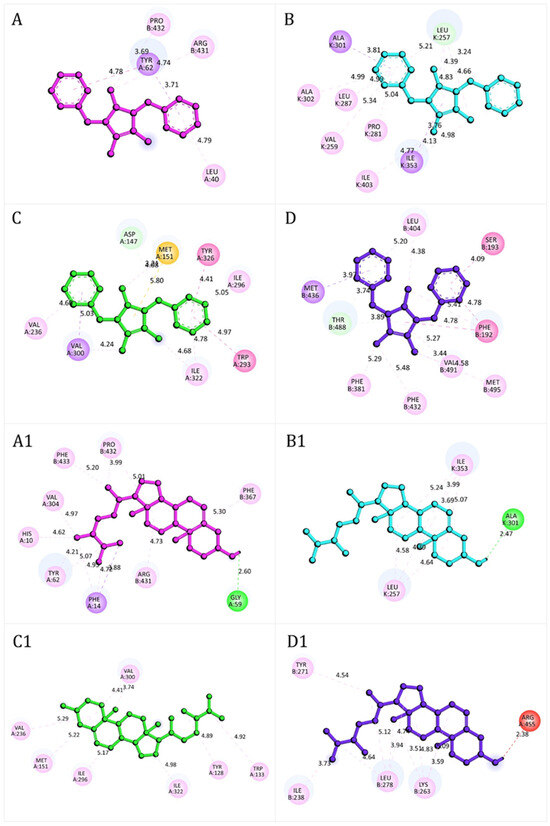
Figure 8.
Representative docking interactions of compound 6 (A–D) and compound 8 (A1–D1) with NADPH oxidase (A,A1), xanthine oxidase (B,B1), μ-opioid receptor (C,C1), and fatty acid amide hydrolase receptor (D,D1). Hydrogen atoms are omitted in some cases for clarity. Interaction types: green, conventional hydrogen bonds; light green, carbon-hydrogen bonds or π-donor hydrogen bonds; red, unfavorable donor–donor contacts; purple, π (aromatic)–σ interactions; yellow, π–sulfur interactions; fuchsia, amide–π stacked or π–π T-shaped interactions; pink, alkyl or π–alkyl interactions.
The stability of these complexes was mainly mediated by hydrogen bonds, π–sulfur interactions, amide–π stacked, and π–π or π–σ stacking interactions, which collectively compensated for less favorable donor–donor contacts. In NADPH oxidase (Figure 8A), compound 6 formed stable interactions with residues such as Leu40, Pro432, Arg431, and Tyr62. In contrast compound 8 (Figure 8A1) engaged a broader set, including His10, Phe14, Gly59, Tyr62, Val304, Phe367, Arg431, Pro432, and Phe433, with an additional hydrogen bond to Gly59 that further stabilized the complex. In xanthine oxidase, compound 6 (Figure 8B) primarily interacted with Ala301, Ala302, Ile353, Leu287, Val259, Pro281, Ile403, and Leu257 whereas compound 8 (Figure 8B1) established network of contacts, including Ile353, Leu257 and Ala301, consistent with stronger binding stability. At the μ-opioid receptor, compound 6 (Figure 8C) displayed π–σ stacking interactions with Val236, Ile296 and Ile322, together with π–σ interactions involving Val300. Additional π–alkyl interaction with Tyr326 and Trp293, along with π–sulfur interactions with Met151, further stabilized the complex. Compound 8 (Figure 8C1) engaged a broader set of residues, including Val236, Met151, Ile296, Ile322, Tyr128, Trp133 and Val300, reinforcing its high docking score. For FAAH, both compounds (Figure 8D,D1) formed a complex network of hydrogen bonding, and π–π stacking and π–δ interactions, consistent with the strong docking energies reported in Table 6. Detailed residue-level interaction profiles (Figures S2–S6) and the superimposition of experimental and re-docked ligand poses (Figures S7–S11) are provided in the Supplementary Materials.

Table 6.
ADMET properties of chemical constituents of black maca.
3.6. ADMET Profiles of BM Metabolites
Pharmacokinetic and toxicity parameters were predicted using the pkCSM online tool, and the results are summarized in Table 6.
Overall, all compounds complied with Lipinski’s rule of five, as their molecular weights were below 500 g/mol, supporting their predicted oral bioavailability. Caco-2 permeability values ranged from −0.288 to 1.630 log Papp (10−6 cm/s). Compounds 1 and 9 exhibited the lowest permeability (−0.288 and 0.861, respectively), whereas compound 2 displayed the highest value (1.630). Consistently, intestinal absorption (IA) exceeded 87% for most compounds, except for compounds 1 (2.57%) and 9 (38.81%), which showed markedly reduced absorption and therefore compromised oral bioavailability. In contrast, skin permeability was uniformly low (log Kp between −2.206 and −2.836 cm/h). Only compound 2 (−2.206) approached the threshold for potential dermal absorption, while the remaining compounds were unlikely to achieve meaningful transdermal penetration.
Regarding distribution, the predicted steady-state volumes of distribution (VDss) ranged from −0.882 to 1.637 log L/kg. Compounds 6 and 10 exhibited the highest values, suggesting broad systemic distribution. Blood-brain barrier (BBB) permeability values (log BB) ranged from −1.697 to 1.108. Notably, several compounds exceeded the threshold of 0.3, indicating the potential for central nervous system penetration, which may contribute to their neuroprotective activity.
With respect to metabolism, most compounds were not predicted to inhibit cytochrome P450 isoforms, suggesting a low likelihood of drug-drug interactions. Nevertheless, compounds 5, 6, 7, and 10 were predicted to inhibit CYP2D6, while compounds 7 and 12 inhibited CYP3A4, highlighting a possible risk of metabolism-based interactions that warrants further investigation.
Excretion analysis indicated total clearance values ranging from 0.572 (compound 8) to 1.990 (compound 12), consistent with efficient systemic elimination. Finally, acute oral toxicity (rat LD50) values ranged from 0.565 to 3.265 mol/kg, with compound 6 predicted as the most toxic and compound 12 as the least toxic.
4. Discussion
The chemical composition analysis of the lyophilized aqueous extract of black maca revealed a profile consistent with previous reports, confirming the presence of macamides, imidazole alkaloids, phytosterols, and fatty acid amides with potential biological relevance [46,47]. These constituents encompass a wide range of bioactive properties. For instance, macamides such as N-benzyloctanamide and its derivatives have been linked to neuroprotective, anti-inflammatory, and energizing effects, likely mediated through endocannabinoid modulation [48,49]. Their detection provides a plausible mechanistic basis for some of the cognitive and behavioral benefits traditionally attributed to this plant. Likewise, campesterol, a representative phytosterol, has been associated with cholesterol-lowering and anti-inflammatory activities [50], reinforcing the potential role of black maca in metabolic regulation and cardiovascular health. The identification of imidazole alkaloids, including lepidilines A, B, and D, further supports the hypothesis that black maca exerts adaptogenic and neuroactive effects, consistent with its ethnopharmacological use in Andean populations [51,52]. In addition, fatty acid amides such as N-octadecanamide and hydroxylated derivatives may contribute to neural signaling and energy metabolism [53].
BM exhibited a moderate total phenolic content (TPC), consistent with the variable accumulation of polyphenolic compounds in maca hypocotyls, which is known to be influenced by biosynthetic processes stimulated by environmental factors such as light exposure [54]. In antioxidant assays, BM showed strong activity in electron-transfer–based methods (CUPRAC and FRAP), indicating the presence of metabolites capable of stabilizing oxidants through single-electron donation [55]. In contrast, relatively high IC50 values in ABTS and DPPH assays indicated lower efficiency in direct radical scavenging. This profile may reflect both the chemical nature of the extract—imidazole alkaloids and macamides likely account for its reducing power, whereas the limited abundance of phenolic hydroxyl groups restricts hydrogen atom transfer [56,57]—and the aqueous matrix, which could limit the solubility and reactivity of lipophilic compounds effective in DPPH-type systems.
With respect to neurocognitive outcomes, the Morris water maze confirmed that OVX rats developed deficits in spatial learning and memory, consistent with hippocampal dysfunction and cholinergic decline in estrogen-deficient models [58,59,60]. As expected, estradiol treatment reversed these impairments. Interestingly, BM at 0.5 g/kg reproduced the cognitive benefits of estradiol during the acquisition phase, suggesting a role in learning acquisition and consolidation. However, BM did not improve performance in the probe test, indicating a limited impact on memory retrieval. Previous studies in mice have reported that black maca improves memory impairment in ovariectomized models, an effect associated with antioxidant activity and acetylcholinesterase inhibition [19]. In addition, another study demonstrated that black maca exerted the strongest benefits on latent learning when compared with red and yellow maca [11]. More recently, it has also been shown that maca enhances cognitive function in middle-aged mice, an effect linked to improved mitochondrial activity and the upregulation of autophagy-related proteins in the cortex [18]. In the present study, the absence of efficacy at 2.0 g/kg suggests paradoxical dose-dependent responses, possibly related to receptor desensitization or metabolic saturation. Because lipid peroxidation is a key mechanism of oxidative damage in the brain, we measured malondialdehyde (MDA) levels—a widely used biomarker of lipid peroxidation in ovariectomized animal models. Previous studies in OVX models have shown that elevated brain MDA concentrations are associated with cognitive impairments, and that both parameters can be attenuated by antioxidant interventions [61,62]. Biochemically, BM significantly reduced brain malondialdehyde (MDA) levels in OVX rats, reaching values comparable to estradiol. Notably, estradiol has been shown to act through the Nrf2 pathway in similar models [63]. Therefore, it is plausible that BM may also exert part of its antioxidant effects via Keap1–Nrf2 signaling, a hypothesis that warrants further investigation.
The antinociceptive assays provided complementary evidence of BM’s pharmacological activity. In the hot plate test, BM produced a dose-dependent analgesic effect with rapid onset and peak activity at 45 min. This earlier onset compared with tramadol, a μ-opioid receptor agonist, suggests the participation of non-opioid central pathways, potentially involving endocannabinoid, monoaminergic, or nitric oxide signaling [38,64]. The cold plate test corroborated the rapid onset but also revealed a transient efficacy, with a possible ceiling effect at higher doses. Similarly, the tail immersion test demonstrated robust activity at 1.0 g/kg, peaking at 1–2 h but declining after 180 min, indicating rapid metabolism or clearance of active constituents. Overall, BM exhibited a pharmacological profile characterized by fast-acting but short-lived analgesia, which could be advantageous in acute pain management. Previous studies have reported that maca alleviates articular and neuropathic pain by reducing mechanical hypersensitivity and postural imbalance in monoiodoacetate and sciatic nerve injury models [65]. In addition, macamides from maca have been identified as potent inhibitors of soluble epoxide hydrolase, and specific compounds such as N-benzyl-linoleamide have been shown to reduce inflammatory pain in vivo [66]. These findings are consistent with its phytochemical composition of BM, particularly macamides, lepidilines, and fatty acid amides, which are known to modulate endocannabinoid and monoaminergic pathways [67].
Molecular docking analysis provided further mechanistic insights. Compounds containing imidazilium or benzylamide scaffolds (e.g., lepidilines) displayed high binding affinities across multiple targets, while campesterol showed strong interactions consistent with its membrane-stabilizing and antioxidant properties. Notably, both lepidiline B and campesterol formed extensive stabilizing interactions with FAAH, suggesting a role in endocannabinoid modulation that could contribute to both analgesic and neuroprotective effects. This multitarget binding profile highlights their potential as lead scaffolds for the development of novel antioxidant and neuroactive agents. Molecular docking suggests that lepidiline B and campesterol may contribute to the observed effects, these compounds however were not directly tested. Future studies with isolated standards are needed to confirm their roles and possible synergistic interactions within the black maca extract.
The ADMET analysis revealed generally favorable pharmacokinetic and safety profiles. All compounds complied with Lipinski’s rule of five, with high intestinal absorption predicted for most molecules [68,69]. Skin permeability was limited, consistent with restricted transdermal diffusion [70], while several compounds displayed BBB permeability above the 0.3 threshold, suggesting potential CNS penetration [71]. Most compounds were not predicted to inhibit CYP450 isoforms; however some showed inhibition of CYP2D6 and CYP3A4. Such interactions may pose potential risks of altered drug metabolism, including reduced clearance, increased plasma concentrations, and enhanced toxicity. Predicted systemic clearance was efficient, and in silico acute oral toxicity values were within acceptable ranges according to OECD guidelines [72]. Among the tested metabolites, compounds 2, 3, 5, and 12 emerged as the most promising candidates, combining efficient absorption, wide distribution, acceptable clearance, and relatively low toxicity.
Taken together, these findings indicate that the bioactivity of black maca is likely mediated through a polypharmacological mechanism involving antioxidant, neuroprotective, and analgesic pathways. Although molecular docking shows that lepidiline B and campesterol may contribute to the observed effects, these metabolites were not directly tested in the bioassays. This constitutes a limitation of the present study, and future research should address the isolation and experimental assessment of these compounds to clarify their individual contributions as well as their potential synergistic effects within the extract.
5. Conclusions
Phytochemical profiling of black maca revealed a characteristic composition rich in macamides, imidazole alkaloids, sterols, and fatty acid derivatives. The extract demonstrated strong antioxidant activity through electron transfer mechanisms and moderate radical scavenging capacity. In vivo, BM improved spatial learning and reduced lipid peroxidation in brain tissue of ovariectomized rats, consolidating its neuroprotective role. In parallel, antinociceptive assays provided experimental evidence supporting its fast-acting but short-lasting analgesic effects. Docking studies identified lepidiline B and campesterol as lead candidates with multitarget interactions across redox enzymes, the μ-opioid receptor, and the FAAH enzyme. ADMET predictions supported their drug-likeness with favorable bioavailability and safety. Collectively, these findings reinforce the scientific basis for the neuroprotective effects of black maca and highlight its potential as a natural analgesic candidate. Future investigations should assess isolated metabolites to validate their specific contributions and to elucidate potential synergistic mechanisms.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/antiox14101214/s1, Figure S1: UHPLC-ESI-Q-TOF-MS total ion chromatograms of Lepidium meyenii Walp. Figure S2: 2D representation of the interactions between compounds 1–12 and residues of NADPH oxidase (PDB ID: 2CDU). Figure S3: 2D representation of the interactions between compounds 1–12 and residues of xanthine oxidase (PDB ID: 3NRZ). Figure S4: 2D representation of the interactions between compounds 1–12 and residues of superoxide dismutase (PDB ID: 4MCM). Figure S5: 2D representation of the interactions between compounds 1–12 and residues of the μ-opioid receptor (PDB ID: 4DKL). Figure S6: 2D representation of the interactions between compounds 1–12 and residues of the fatty acid amide hydrolase (FAAH, PDB ID: 2VYA). Figure S7: Superimposition of the crystallographic and re-docked ligand poses in the active site of NADPH oxidase (PDB ID: 2CDU). Figure S8: Superimposition of the crystallographic and re-docked ligand poses in the active site of xanthine oxidase (PDB ID: 3NRZ). Figure S9: Superimposition of the crystallographic and re-docked ligand poses in the active site of superoxide dismutase (PDB ID: 4MCM). Figure S10: Superimposition of the crystallographic and re-docked ligand poses in the active site of the μ-opioid receptor (PDB ID: 4DKL). Figure S11: Superimposition of the crystallographic and re-docked ligand poses in the active site of fatty acid amide hydrolase (FAAH) (PDB ID: 2VYA)
Author Contributions
Conceptualization, I.M.Q.-D.; R.O.Y.-J. and J.B.; methodology, I.M.Q.-D.; R.O.Y.-J.; D.A.-A.; C.E.-L.; J.L.P.-B.; R.J.-A.; E.A.V.-C.; R.D.D.G.d.A.; N.C.-S.; E.V.-C.; P.B.C. and J.B.; formal analysis, I.M.Q.-D.; R.O.Y.-J.; D.A.-A.; C.E.-L.; J.L.P.-B.; R.J.-A.; E.A.V.-C.; R.D.D.G.d.A.; N.C.-S.; E.V.-C.; P.B.C. and J.B.; investigation, I.M.Q.-D.; R.O.Y.-J.; D.A.-A.; C.E.-L.; J.L.P.-B.; R.J.-A.; E.A.V.-C.; R.D.D.G.d.A.; N.C.-S.; E.V.-C.; P.B.C. and J.B.; writing—original draft preparation, D.A.-A.; R.O.Y.-J. and J.B.; writing—review and editing, R.O.Y.-J.; P.B.C. and J.B.; funding acquisition, R.O.Y.-J. and J.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Proyecto Canon Minero (R.R. N◦ 0262-2021/UNT, Peru) andFondo Interno VRII-UNAP (grant number VRIIP0056-21, Chile).
Institutional Review Board Statement
The animal study protocol was approved by the Ethics Committee of the Faculty of Pharmacy and Biochemistry, Universidad Nacional de Trujillo (COD N° P 012-19/CEIFYB), and by the Institutional Committee (Res. Cons. Univ. N° 0361-2018/UNT).
Data Availability Statement
Data are contained within the article.
Acknowledgments
We thank the Analytical Methods Division, Subunit RPT13B, of the Drug Technology Institute (FIOCRUZ, Brazil) for performing the chemical analysis using UPLC-MS/MS.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- da Silva Leitão Peres, N.; Cabrera Parra Bortoluzzi, L.; Medeiros Marques, L.L.; Formigoni, M.; Fuchs, R.H.B.; Droval, A.A.; Reitz Cardoso, F.A. Medicinal effects of Peruvian maca (Lepidium meyenii): A review. Food Funct. 2020, 11, 83–92. [Google Scholar] [CrossRef]
- León, J. The “Maca” (Lepidium meyenii), a little known food plant of Peru. Econ. Bot. 1964, 18, 122–127. [Google Scholar] [CrossRef]
- Brinckmann, J.; Smith, E. Maca culture of the Junin Plateau. J. Altern. Complement. Med. 2004, 10, 426–430. [Google Scholar] [CrossRef]
- Gonzales, G.F.; Gonzales, C.; Gonzales-Castañeda, C. Lepidium meyenii (Maca): A plant from the highlands of Peru—From tradition to science. Forsch. Komplementmed. 2009, 16, 373–380. [Google Scholar] [CrossRef]
- Valentová, K.; Frcek, J.; Ulrichová, J. Yacon (Smallanthus sonchifolius) and Maca (Lepidium meyenii), traditional Andean crops as new functional foods on the European market. Chem. Listy 1997, 95, 594–601. [Google Scholar]
- Wang, Y.; Wang, Y.; McNeil, B.; Harvey, L.M. Maca: An Andean crop with multi-pharmacological functions. Food Res. Int. 2007, 40, 783–792. [Google Scholar] [CrossRef]
- Balick, M.J.; Lee, R. Maca: From traditional food crop to energy and libido stimulant. Altern. Ther. Health Med. 2002, 8, 96–98. [Google Scholar] [PubMed]
- Brooks, N.A.; Wilcox, G.; Walker, K.Z.; Ashton, J.F.; Cox, M.B.; Stojanovska, L. Beneficial effects of Lepidium meyenii, (Maca) on psychological symptoms and measures of sexual dysfunction in postmenopausal women are not related to estrogen or androgen content. Menopause 2008, 15, 1157–1162. [Google Scholar] [CrossRef]
- Alquraini, A.; Waggas, D.; Böhlke, M.; Maher, T.; Pino-Figueroa, A. Neuroprotective effects of Lepidium meyenii, (Maca) and macamides against amyloid-beta (25-35) induced toxicity in B-35 neuroblastoma cells. FASEB J. 2014, 28, 657.13. [Google Scholar] [CrossRef]
- Pino-Figueroa, A.; Nguyen, D.; Maher, T.J. Neuroprotective effects of Lepidium meyenii (Maca). Ann. N. Y. Acad. Sci. 2010, 1199, 77–85. [Google Scholar] [CrossRef]
- Rubio, J.; Caldas, M.; Dávila, S.; Gasco, M.; Gonzales, G.F. Effect of three different cultivars of Lepidium meyenii (Maca) on learning and depression in ovariectomized mice. BMC Complement. Altern. Med. 2006, 6, 23. [Google Scholar] [CrossRef]
- Bai, N.; He, K.; Roller, M.; Lai, C.-S.; Bai, L.; Pan, M.-H. Flavonolignans and other constituents from Lepidium meyenii with Activities in Anti-inflammation and Human Cancer Cell Lines. J. Agric. Food Chem. 2015, 63, 2458–2463. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Huamán, Á.; Casimiro-Gonzales, S.; Chávez-Pérez, J.A.; Gonzales-Arimborgo, C.; Cisneros-Fernández, R.; Aguilar-Mendoza, L.A. Antioxidant and neuroprotector effect of Lepidium meyenii (maca) methanol leaf extract against 6-hydroxy dopamine (6-OHDA)-induced toxicity in PC12 cells. Toxicol. Mech. Method. 2017, 27, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Cho, H.; Gil, M.; Kim, K.E. Anti-Inflammation and Anti-Melanogenic Effects of Maca Root Extracts Fermented Using Lactobacillus Strains. Antioxidants 2023, 12, 798. [Google Scholar] [CrossRef] [PubMed]
- Gonzales-Castaneda, C.; Gonzales, G.F. Hypocotyls of Lepidium meyenii (maca), a plant of the Peruvian highlands, prevent ultraviolet A-, B-, and C-induced skin damage in rats. Photodermatol. Photoimmunol. Photomed. 2007, 24, 24–31. [Google Scholar] [CrossRef]
- Gonzales, G.F. Ethnobiology and ethnopharmacology of Lepidium meyenii (Maca), a plant from the Peruvian Highlands. Evid. Based Complement. Altern. Med. 2012, 2012, 1–10. [Google Scholar] [CrossRef]
- Sandoval, M.; Okuhama, N.N.; Angeles, F.M.; Melchor, V.V.; Condezo, L.A.; Lao, J.; Miller, M.J.S. Antioxidant activity of the cruciferous vegetable Maca (Lepidium meyenii). Food Chem. 2002, 79, 207–213. [Google Scholar] [CrossRef]
- Guo, S.S.; Gao, X.F.; Gu, Y.R.; Wan, Z.X.; Lu, A.M.; Qin, Z.H.; Luo, L. Preservation of Cognitive Function by Lepidium meyenii (Maca) Is Associated with Improvement of Mitochondrial Activity and Upregulation of Autophagy-Related Proteins in Middle-Aged Mouse Cortex. Evid. Based Complement. Altern. Med. 2016, 2016, 4394261. [Google Scholar] [CrossRef]
- Rubio, J.; Qiong, W.; Liu, X.; Jiang, Z.; Dang, H.; Chen, S.L.; Gonzales, G.F. Aqueous Extract of Black Maca (Lepidium meyenii) on Memory Impairment Induced by Ovariectomy in Mice. Evid. Based Complement. Altern. Med. 2011, 2011, 253958. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J., Jr. Colorimetry of total phenolics with phosphomolybdic phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–153. [Google Scholar] [CrossRef]
- Baki, S.; Tufan, A.N.; Altun, M.; Özgökçe, F.; Güçlü, K.; Özyürek, M. Microwave-Assisted Extraction of Polyphenolics from Some Selected Medicinal Herbs Grown in Turkey. Rec. Nat. Prod. 2018, 12, 29–39. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Baran, A.; Karakılıç, E.; Faiz, Ö.; Özer, F. Synthesis of chalcone-containing zinc and cobalt metallophthalocyanines; investigation of their photochemical, DPPH radical scavenging and metal chelating characters. Org. Commun. 2020, 13, 65–78. [Google Scholar] [CrossRef]
- Ybañez-Julca, R.O.; Asunción-Alvarez, D.; Palacios, J.; Nwokocha, C.R. Maca extracts and estrogen replacement therapy in ovariectomized rats exposed at high altitude. Reprod. Med. Biol. 2020, 20, 88–95. [Google Scholar] [CrossRef]
- Esterbauer, H.; Cheeseman, K.H. Determination of aldehydic lipid peroxidation products: Malonaldehyde and 4-hydroxynonenal. Methods Enzymol. 1990, 186, 407–421. [Google Scholar] [CrossRef]
- Ybañez-Julca, R.O.; Quispe-Díaz, I.M.; Asunción- Alvarez, D.; Sánchez-Muñoz, K.; Vargas-Goñas, A.; Morote-Guzman, J.; Yaro- Marcelo, R.; Venegas- Casanova, E.A.; Jara-Aguilar, R.; Buc Calderon, P.; et al. Antidepressant-Like Behavioral and Spatial Memory Effects in Peruvian Red Maca (Lepidium meyenii)-Treated Rats. Pharmacogn. J. 2021, 13, 81–88. [Google Scholar] [CrossRef]
- Nunez, J. Morris water maze experiment. J. Vis. Exp. 2008, 19, 897–898. [Google Scholar] [CrossRef]
- Möller, M.; Möser, C.V.; Weiß, U.; Niederberger, E. The Role of AlphαSynuclein in Mouse Models of Acute, Inflammatory and Neuropathic Pain. Cells 2022, 11, 1967. [Google Scholar] [CrossRef] [PubMed]
- Udell, M.E.; Ni, J.; Garcia Martinez, A.; Mulligan, M.K.; Redei, E.E.; Chen, H. Tail Timer: A device for automating data collection in the rodent tail immersion assay. PLoS ONE 2021, 16, e0256264. [Google Scholar] [CrossRef]
- Lountos, G.T.; Jiang, R.; Wellborn, W.B.; Thaler, T.L.; Bommarius, A.S.; Orville, A.M. The crystal structure of NAD(P)H oxidase from Lactobacillus sanfranciscensis: Insights into the conversion of O2 into two water molecules by the flavoenzyme. Biochemistry 2006, 45, 9648–9659. [Google Scholar] [CrossRef]
- Cao, H.; Pauff, J.M.; Hille, R. Substrate orientation and catalytic specificity in the action of xanthine oxidase: The sequential hydroxylation of hypoxanthine to uric acid. J. Biol. Chem. 2010, 285, 28044–28053. [Google Scholar] [CrossRef]
- Sea, K.; Sohn, S.H.; Durazo, A.; Sheng, Y.; Shaw, B.F.; Cao, X.; Taylor, A.B.; Whitson, L.J.; Holloway, S.P.; Hart, P.J.; et al. Insights into the role of the unusual disulfide bond in copper-zinc superoxide dismutase. J. Biol. Chem. 2015, 290, 2405–2418. [Google Scholar] [CrossRef]
- Manglik, A.; Kruse, A.C.; Kobilka, T.S.; Thian, F.S.; Mathiesen, J.M.; Sunahara, R.K.; Pardo, L.; Weis, W.I.; Kobilka, B.K.; Granier, S. Crystal structure of the µ-opioid receptor bound to a morphinan antagonist. Nature 2012, 485, 321–326. [Google Scholar] [CrossRef] [PubMed]
- Mileni, M.; Johnson, D.S.; Wang, Z.; Everdeen, D.S.; Liimatta, M.; Pabst, B.; Bhattacharya, K.; Nugent, R.A.; Kamtekar, S.; Cravatt, B.F.; et al. Structure-guided inhibitor design for human FAAH by interspecies active site conversion. Proc. Natl. Acad. Sci. USA 2008, 105, 12820–12824. [Google Scholar] [CrossRef] [PubMed]
- Trott, O.; Olson, A. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Sanner, M.F. Python: A programming language for software integration and development. J. Mol. Graph. Model. 1999, 17, 55–84. [Google Scholar]
- Minchán-Herrera, P.; Ybañez-Julca, R.O.; Quispe-Díaz, I.M.; Venegas-Casanova, E.A.; Jara-Aguilar, R.; Salas, F.; Zevallos-Escobar, L.; Yáñez, O.; Pino-Rios, R.; Calderon, P.B.; et al. Valeriana pilosa Roots Essential Oil: Chemical Composition, Antioxidant Activities, and Molecular Docking Studies on Enzymes Involved in Redox Biological Processes. Antioxidants 2022, 11, 1337. [Google Scholar] [CrossRef]
- Pires, D.E.V.; Blundell, T.L.; Ascher, D.B. PkCSM: Predicting Small-Molecule Pharmacokinetic and Toxicity Properties Using Graph-Based Signatures. J. Med. Chem. 2015, 58, 4066–4072. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, P.; Brantner, A.; Wang, H.; Shu, X.; Yang, J.; Si, N.; Han, L.; Zhao, H.; Bian, B. Chemical profiling analysis of Maca using UHPLC−ESI−Orbitrap MS coupled with UHPLC−ESI−QqQ MS and the neuroprotective study on its active ingredients. Sci. Rep. 2017, 7, 44660. [Google Scholar] [CrossRef]
- Yang, S.; Zhan, L.; Liu, C.; Fu, L.; Chen, R.; Nie, Z. Mass spectrometry imaging of small molecule in situ in Lepidium meyenii (Maca) using gold nanoparticles matrix. Microchem. J. 2019, 150, 104190. [Google Scholar] [CrossRef]
- Gao, X.C.; Lv, J.W.; Li, C.N.; Zhang, N.X.; Tian, L.L.; Han, X.Y.; Zhang, H.; Sun, J.M. Screening of the Active Component Promoting Leydig Cell Proliferation from Lepidium meyenii Using HPLC-ESI-MS/MS Coupled with Multivariate Statistical Analysis. Molecules 2019, 24, 2101. [Google Scholar] [CrossRef]
- Tafuri, S.; Cocchia, N.; Carotenuto, D.; Vassetti, A.; Staropoli, A.; Mastellone, V.; Peretti, V.; Ciotola, F.; Albarella, S.; Del Prete, C.; et al. Chemical Analysis of Lepidium meyenii (Maca) and Its Effects on Redox Status and on Reproductive Biology in Stallions. Molecules 2019, 24, 1981. [Google Scholar] [CrossRef]
- Yang, S.; Sun, X.; Gao, Y.; Chen, R. Differentiation of Lepidium meyenii (Maca) from Different Origins by Electrospray Ionization Mass Spectrometry with Principal Component Analysis. ACS Omega 2019, 4, 16493–16500. [Google Scholar] [CrossRef]
- Carvalho, F.V.; Ribeiro, P.R. Structural diversity, biosynthetic aspects, and LC-HRMS data compilation for the identification of bioactive compounds of Lepidium meyenii. Food Res. Int. 2019, 125, 108615. [Google Scholar] [CrossRef]
- Tarabasz, D.; Szczeblewski, P.; Laskowski, T.; Płaziński, W.; Baranowska-Wójcik, E.; Szwajgier, D.; Kukula-Koch, W.; Meissner, H.O. The Distribution of Glucosinolates in Different Phenotypes of Lepidium peruvianum and Their Role as Acetyl- and Butyrylcholinesterase Inhibitors-In Silico and In Vitro Studies. Int. J. Mol. Sci. 2022, 23, 4858. [Google Scholar] [CrossRef] [PubMed]
- Ulloa Del Carpio, N.; Alvarado-Corella, D.; Quiñones-Laveriano, D.M.; Araya-Sibaja, A.; Vega-Baudrit, J.; Monagas-Juan, M.; Navarro-Hoyos, M.; Villar-López, M. Exploring the chemical and pharmacological variability of Lepidium meyenii: A comprehensive review of the effects of maca. Front. Pharmacol. 2024, 15, 1360422. [Google Scholar] [CrossRef] [PubMed]
- Minich, D.M.; Ross, K.; Frame, J.; Fahoum, M.; Warner, W.; Meissner, H.O. Not All Maca Is Created Equal: A Review of Colors, Nutrition, Phytochemicals, and Clinical Uses. Nutrients 2024, 16, 530. [Google Scholar] [CrossRef] [PubMed]
- Vera-López, K.J.; Davila-Del-Carpio, G.; Nieto-Montesinos, R. Macamides as Potential Therapeutic Agents in Neurological Disorders. Neurol. Int. 2024, 16, 1611–1625. [Google Scholar] [CrossRef]
- Yu, Z.; Li, D.; Zhai, S.; Xu, H.; Liu, H.; Ao, M.; Zhao, C.; Jin, W.; Yu, L. Neuroprotective effects of macamide from maca (Lepidium meyenii Walp.) on corticosterone-induced hippocampal impairments through its anti-inflammatory, neurotrophic, and synaptic protection properties. Food Funct. 2021, 19, 9211–9228. [Google Scholar] [CrossRef]
- Shen, M.; Yuan, L.; Zhang, J.; Wang, X.; Zhang, M.; Li, H.; Jing, Y.; Zeng, F.; Xie, J. Phytosterols: Physiological Functions and Potential Application. Foods 2024, 13, 1754. [Google Scholar] [CrossRef]
- Wang, S.; Zhu, F. Chemical composition and health effects of maca (Lepidium meyenii). Food Chem. 2019, 288, 422–443. [Google Scholar] [CrossRef]
- Cui, B.; Zheng, B.L.; He, K.; Zheng, Q.Y. Imidazole alkaloids from Lepidium meyenii. J. Nat. Prod. 2003, 66, 1101–1103. [Google Scholar] [CrossRef] [PubMed]
- Ezzili, C.; Otrubova, K.; Boger, D.L. Fatty acid amide signaling molecules. Bioorg Med. Chem. Lett. 2010, 20, 5959–5968. [Google Scholar] [CrossRef] [PubMed]
- Tuzcu, Z.; Koclar, G.; Agca, C.A.; Aykutoglu, G.; Dervisoglu, G.; Tartik, M.; Ozturk, Z.; Kaya, B.; Sahin, K. Antioxidant, antimicrobial and anticancer effects of different extracts from wild edible plant Eremurus spectabilis leaves and roots. Int. J. Clin. Exp. Med. 2017, 10, 4787–4797. [Google Scholar]
- Gulcin, İ. Antioxidants: A comprehensive review. Arch. Toxicol. 2025, 99, 1893–1997. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Wei, J.; Gao, Y.; Chen, R. Antioxidant and antitumoral activities of isolated macamide and macaene fractions from Lepidium meyenii (Maca). Talanta 2021, 221, 121635. [Google Scholar] [CrossRef] [PubMed]
- Pérez-González, A.; García-Hernández, E.; Chigo-Anota, E. The antioxidant capacity of an imidazole alkaloids family through single-electron transfer reactions. J. Mol. Model. 2020, 26, 321. [Google Scholar] [CrossRef]
- Bortz, J.; Klatt, K.C.; Wallace, T.C. Perspective: Estrogen and the Risk of Cognitive Decline: A Missing Choline(rgic) Link? Adv. Nutr. 2022, 13, 376–387. [Google Scholar] [CrossRef]
- Gibbs, R.B. Estrogen therapy and cognition: A review of the cholinergic hypothesis. Endocr. Rev. 2010, 31, 224–253. [Google Scholar] [CrossRef]
- Batallán Burrowes, A.A.; Olajide, O.J.; Iasenza, I.A.; Shams, W.M.; Carter, F.; Chapman, C.A. Ovariectomy reduces cholinergic modulation of excitatory synaptic transmission in the rat entorhinal cortex. PLoS ONE 2022, 17, e0271131. [Google Scholar] [CrossRef]
- Zhou, Y.; Xu, B.; Yu, H.; Zhao, W.; Song, X.; Liu, Y.; Wang, K.; Peacher, N.; Zhao, X.; Zhang, H.T. Biochanin A Attenuates Ovariectomy-Induced Cognition Deficit via Antioxidant Effects in Female Rats. Front. Pharmacol. 2021, 12, 603316. [Google Scholar] [CrossRef] [PubMed]
- Mading, A.; Chotritthirong, Y.; Chulikhit, Y.; Daodee, S.; Boonyarat, C.; Khamphukdee, C.; Sukketsiri, W.; Kwankhao, P.; Pitiporn, S.; Monthakantirat, O. Effectiveness of Tri-Kaysorn-Mas Extract in Ameliorating Cognitive-like Behavior Deficits in Ovariectomized Mice via Activation of Multiple Mechanisms. Pharmaceuticals 2024, 17, 1182. [Google Scholar] [CrossRef] [PubMed]
- Sobočanec, S.; Šarić, A.; Mačak Šafranko, Ž.; Popović Hadžija, M.; Abramić, M.; Balog, T. The role of 17β-estradiol in the regulation of antioxidant enzymes via the Nrf2-Keap1 pathway in the livers of CBA/H mice. Life Sci. 2015, 130, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Silva, V.G.; Silva, R.O.; Damasceno, S.R.; Carvalho, N.S.; Prudêncio, R.S.; Aragão, K.S.; Guimarães, M.A.; Campos, S.A.; Véras, L.M.; Godejohann, M.; et al. Anti-inflammatory and antinociceptive activity of epiisopiloturine, an imidazole alkaloid isolated from Pilocarpus microphyllus. J. Nat. Prod. 2013, 76, 1071–1077. [Google Scholar] [CrossRef]
- Tenci, B.; Di Cesare Mannelli, L.; Maresca, M.; Micheli, L.; Pieraccini, G.; Mulinacci, N.; Ghelardini, C. Effects of a water extract of Lepidium meyenii root in different models of persistent pain in rats. Z. Naturforsch. C J. Biosci. 2017, 72, 449–457. [Google Scholar] [CrossRef]
- Singh, N.; Barnych, B.; Morisseau, C.; Wagner, K.M.; Wan, D.; Takeshita, A.; Pham, H.; Xu, T.; Dandekar, A.; Liu, J.Y.; et al. N-Benzyl-linoleamide, a Constituent of Lepidium meyenii (Maca), Is an Orally Bioavailable Soluble Epoxide Hydrolase Inhibitor That Alleviates Inflammatory Pain. J. Nat. Prod. 2020, 83, 3689–3697. [Google Scholar] [CrossRef]
- Yu, Z.; Jin, W.; Cui, Y.; Ao, M.; Liu, H.; Xu, H.; Yu, L. Protective effects of macamides from Lepidium meyenii Walp. against corticosterone-induced neurotoxicity in PC12 cells. RSC Adv. 2019, 9, 23096–23108. [Google Scholar] [CrossRef]
- Ekowati, J.; Diyah, N.W.; Nofianti, K.A.; Hamid, I.S. Molecular Docking of Ferulic Acid Derivatives on P2Y12 Receptor and their ADMET Prediction. J. Math. Fundam. Sci. 2018, 50, 203–219. [Google Scholar] [CrossRef]
- Angelis, I.D.; Turco, L. Caco-2 Cells as a Model for Intestinal Absorption. Curr. Protoc. Toxicol. 2011, 47, 20–26. [Google Scholar] [CrossRef]
- Cheng, F.; Li, W.; Zhou, Y.; Shen, J.; Wu, Z.; Liu, G.; Lee, P.W.; Tang, Y. admetSAR: A Comprehensive Source and Free Tool for Assessment of Chemical ADMET Properties. J. Chem. Inf. Model. 2012, 52, 3099–3105. [Google Scholar] [CrossRef]
- Nau, R.; Sorgel, F.; Eiffert, H. Penetration of Drugs through the Blood-Cerebrospinal Fluid/Blood-Brain Barrier for Treatment of Central Nervous System Infections. Clin. Microbiol. Rev. 2010, 23, 858–883. [Google Scholar] [CrossRef]
- Morris-Schaffer, K.; McCoy, M.J. A Review of the LD50 and Its Current Role in Hazard Communication. ACS Chem. Health Saf. 2021, 28, 25–33. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).