Antioxidant, Neuroprotective, and Antinociceptive Effects of Peruvian Black Maca (Lepidium meyenii Walp.)
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals, Drugs, and Solvents
2.2. Plant Material
2.3. Preparation of Lyophilized Aqueous Extract of Black Maca (BM)
2.4. Chemical Identification by HPLC-ESI-QTOFMS/ MS
2.5. Total Phenolic Content (TPC)
2.6. Antioxidant Capacity Assays
2.6.1. Cupric Reducing Antioxidant Capacity (CUPRAC) and Ferric-Reducing Antioxidant Power (FRAP) Assays
2.6.2. ABTS•+ Free-Radical Scavenging Assay
2.6.3. DPPH Free-Radical Scavenging Assay
2.7. Animals and Experimental Groups
2.8. Ovariectomy Procedure
2.9. Lipid Peroxidation Assay
2.10. Morris Water Maze (MWM) Experiment
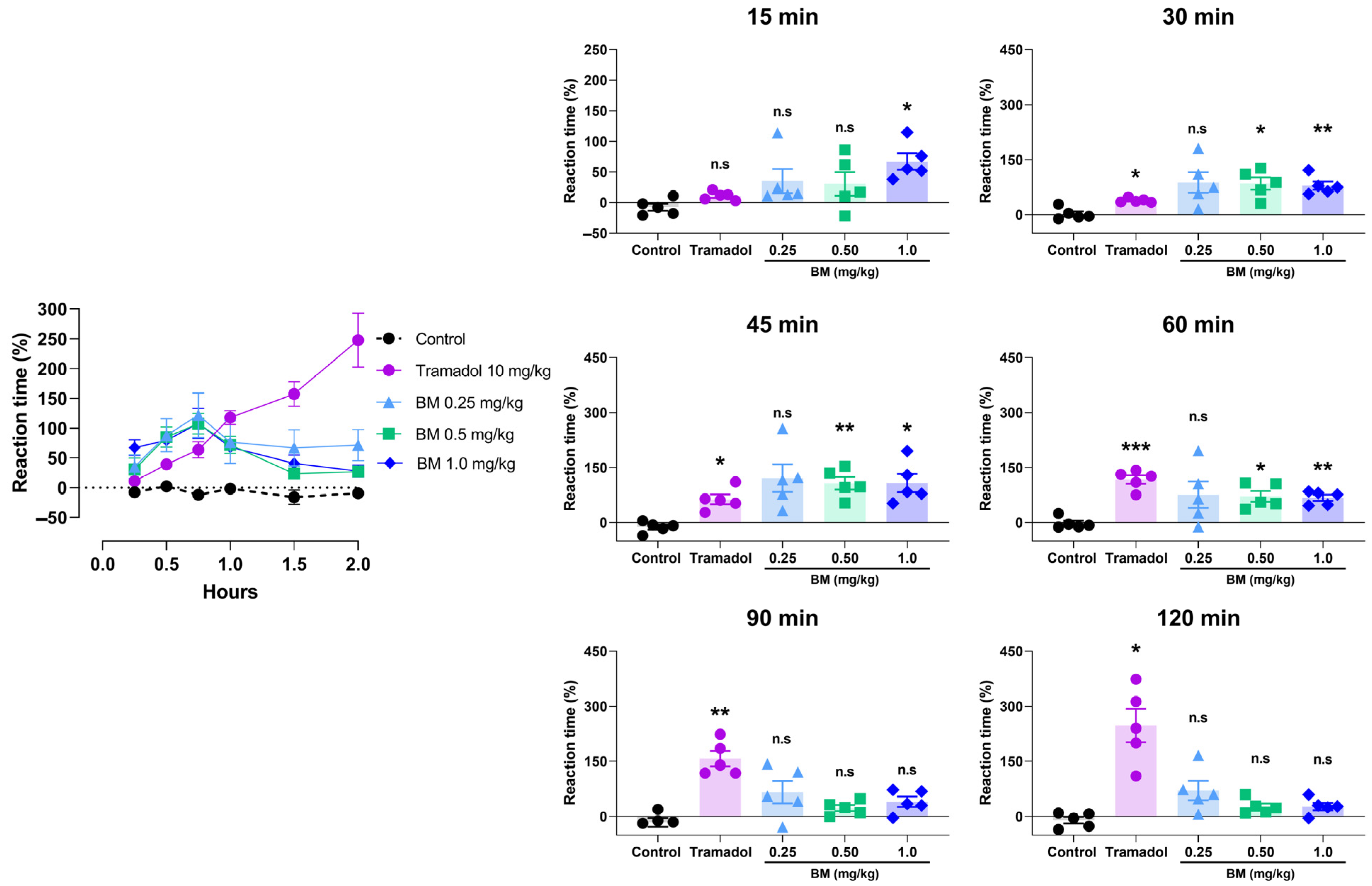
2.11. Hot/Cold Plate Test
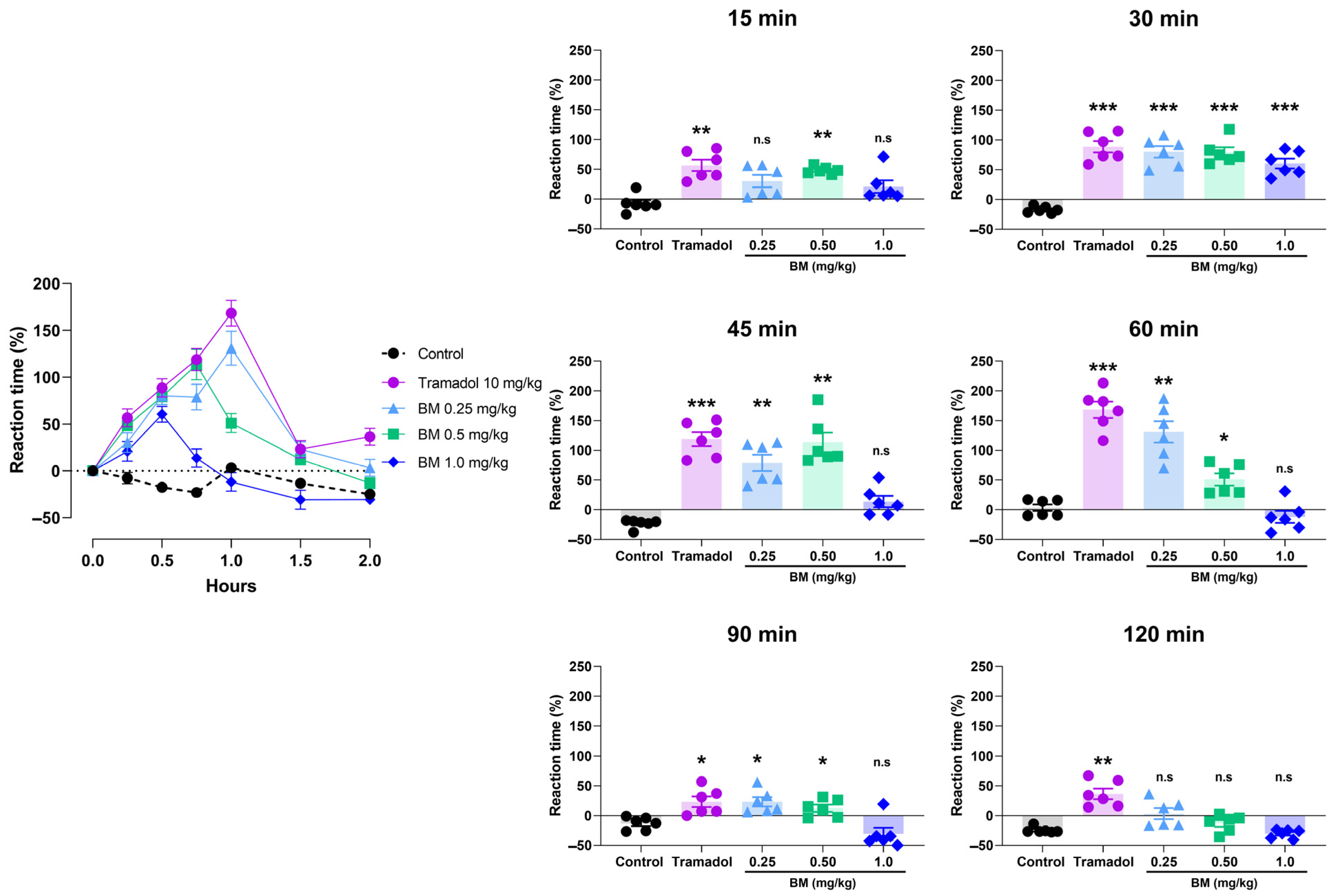
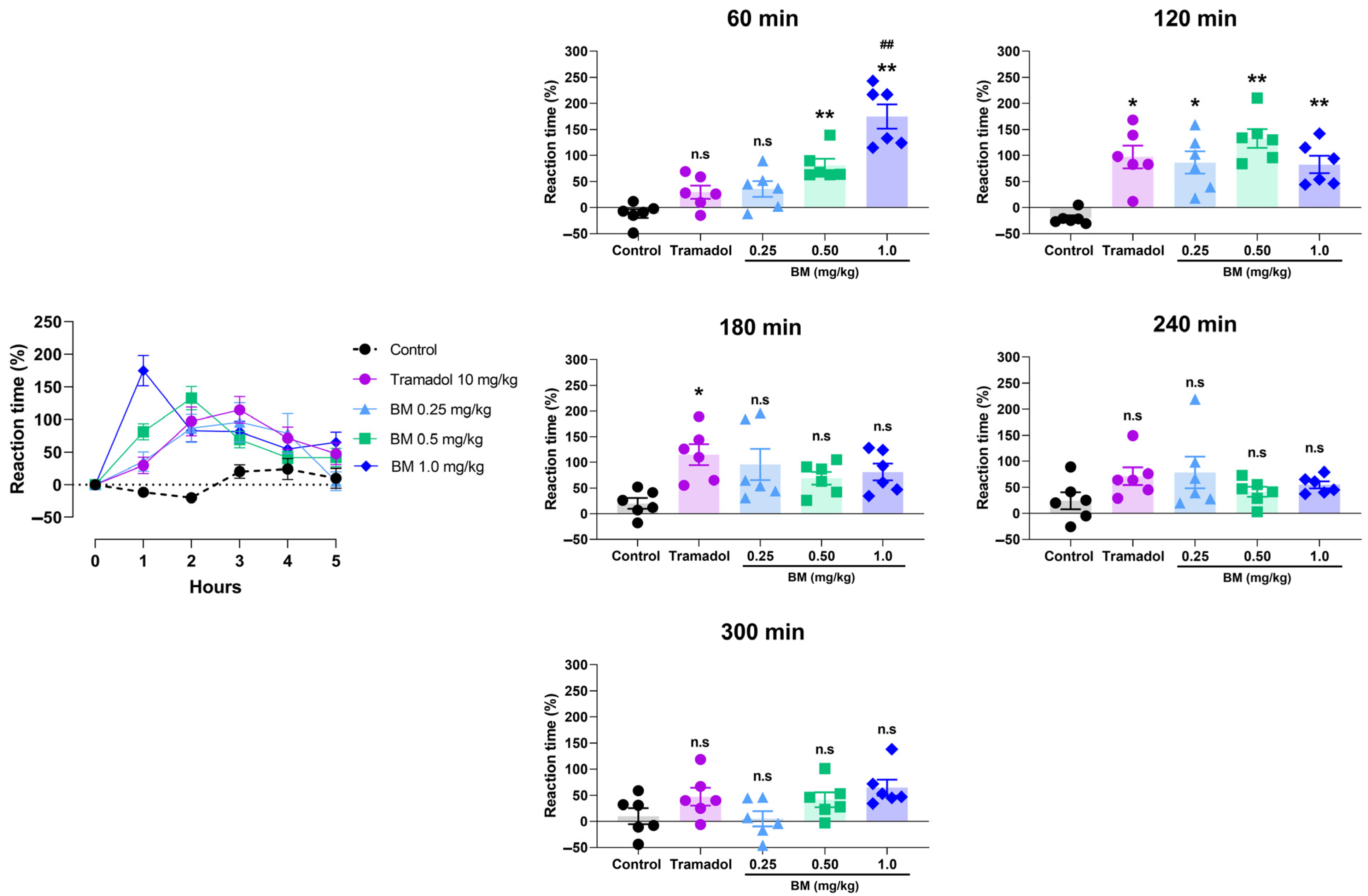
2.12. Tail Immersion Test
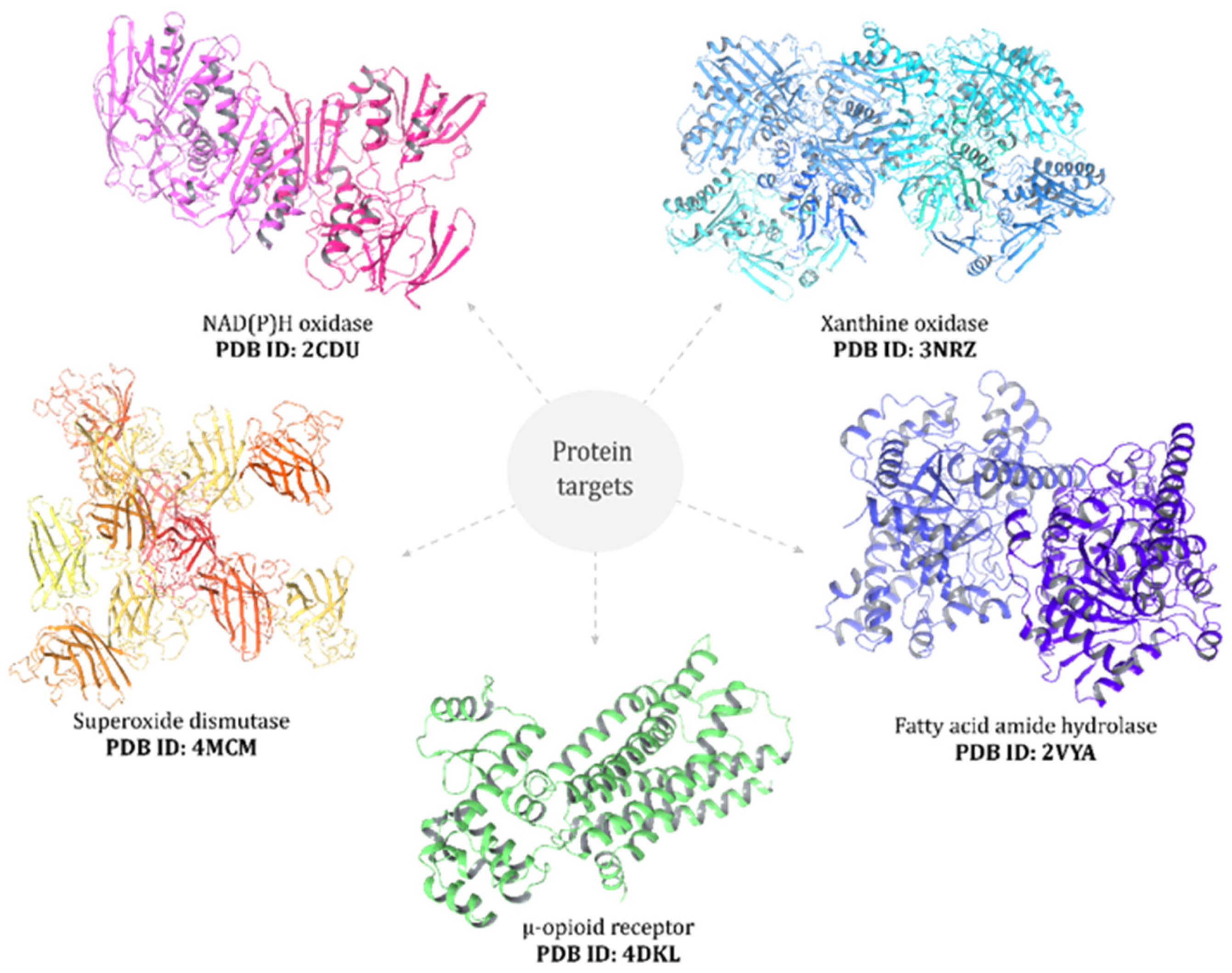
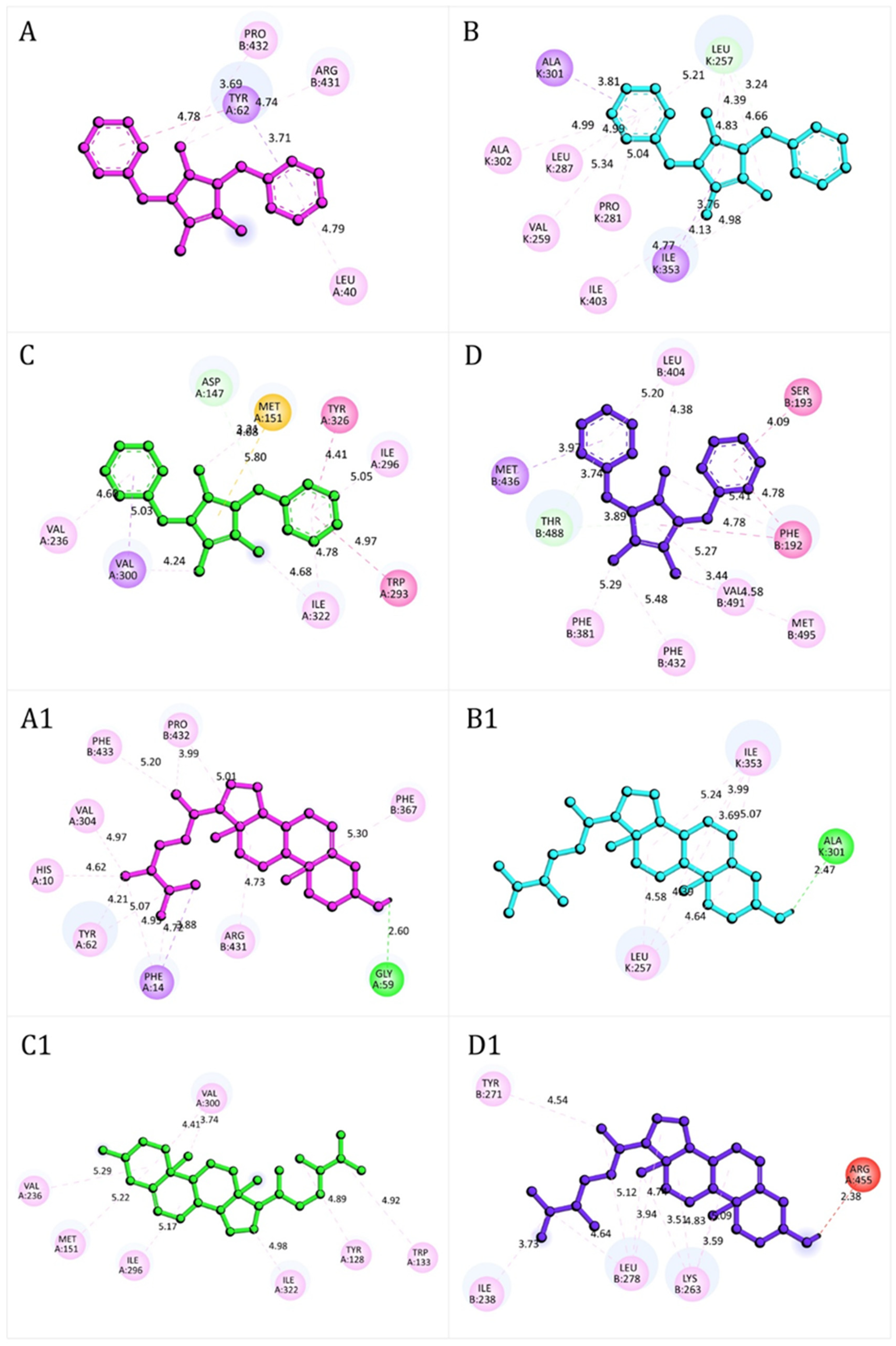
2.13. Molecular Docking
2.14. ADMET Prediction
2.15. Statistical Analysis
3. Results
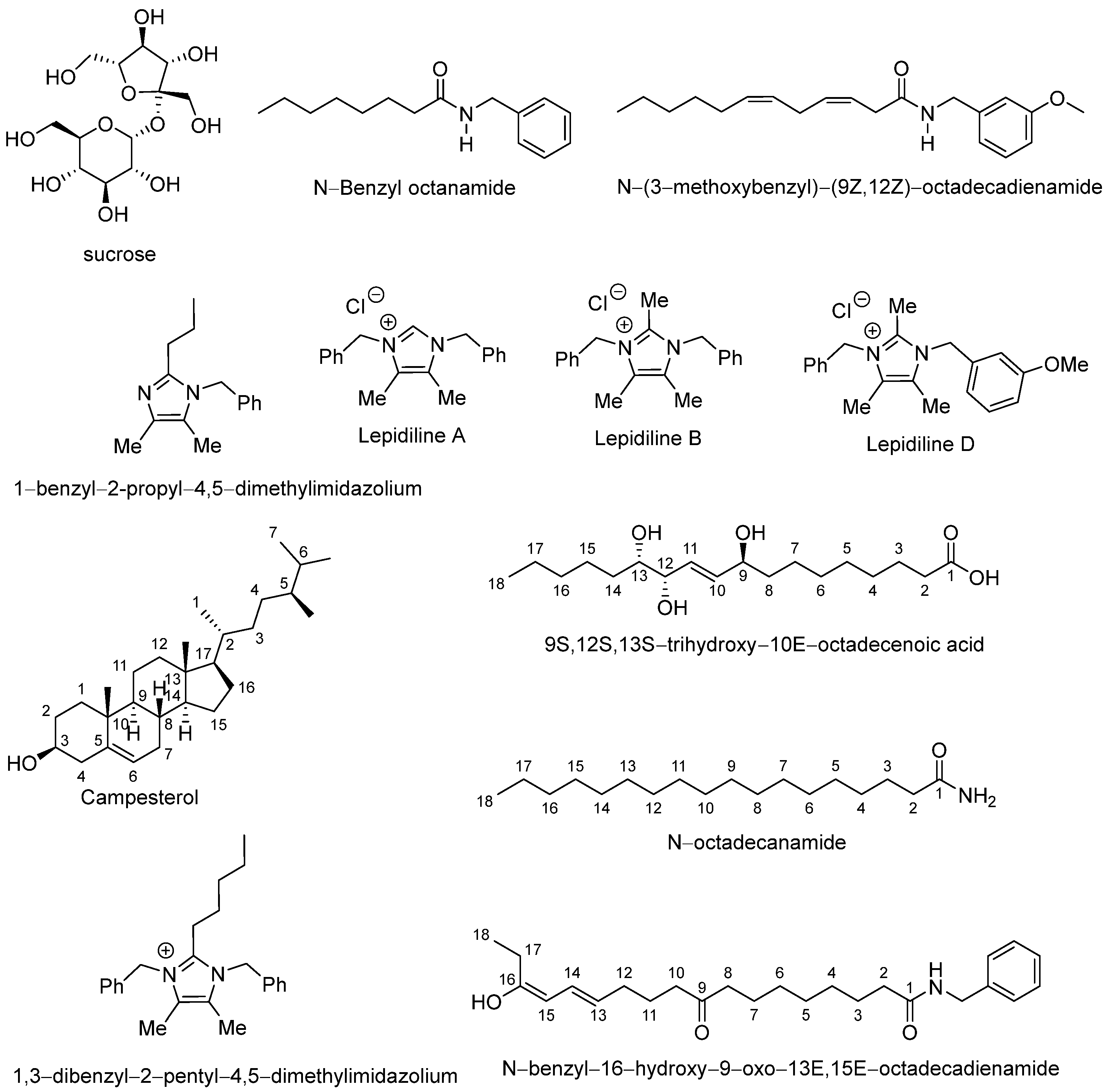
3.1. Chemical Composition of Lyophilized Hypocotyl Aqueous Extract of Black Maca
3.2. Total Phenolic Content and Antioxidant Capacity of BM
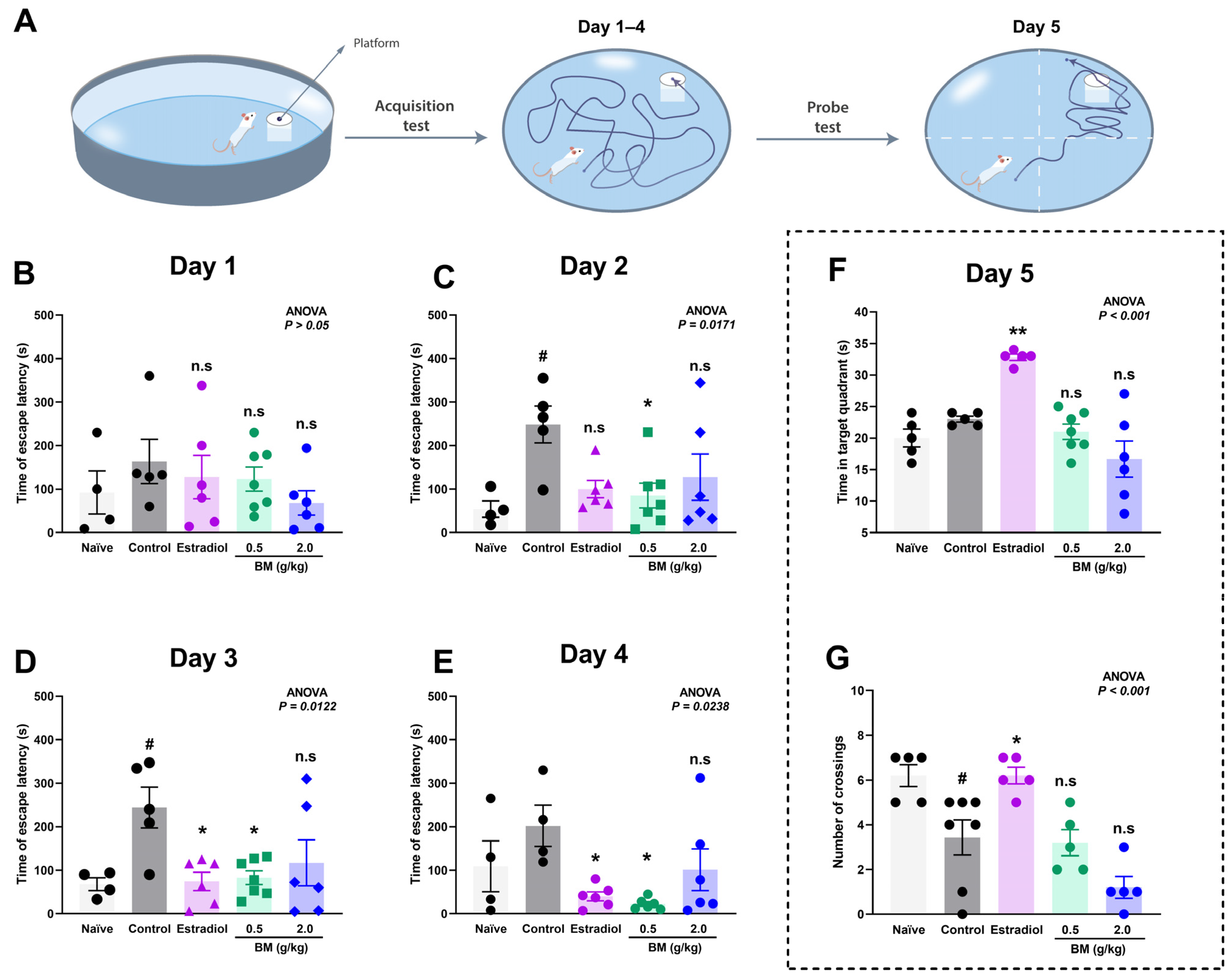
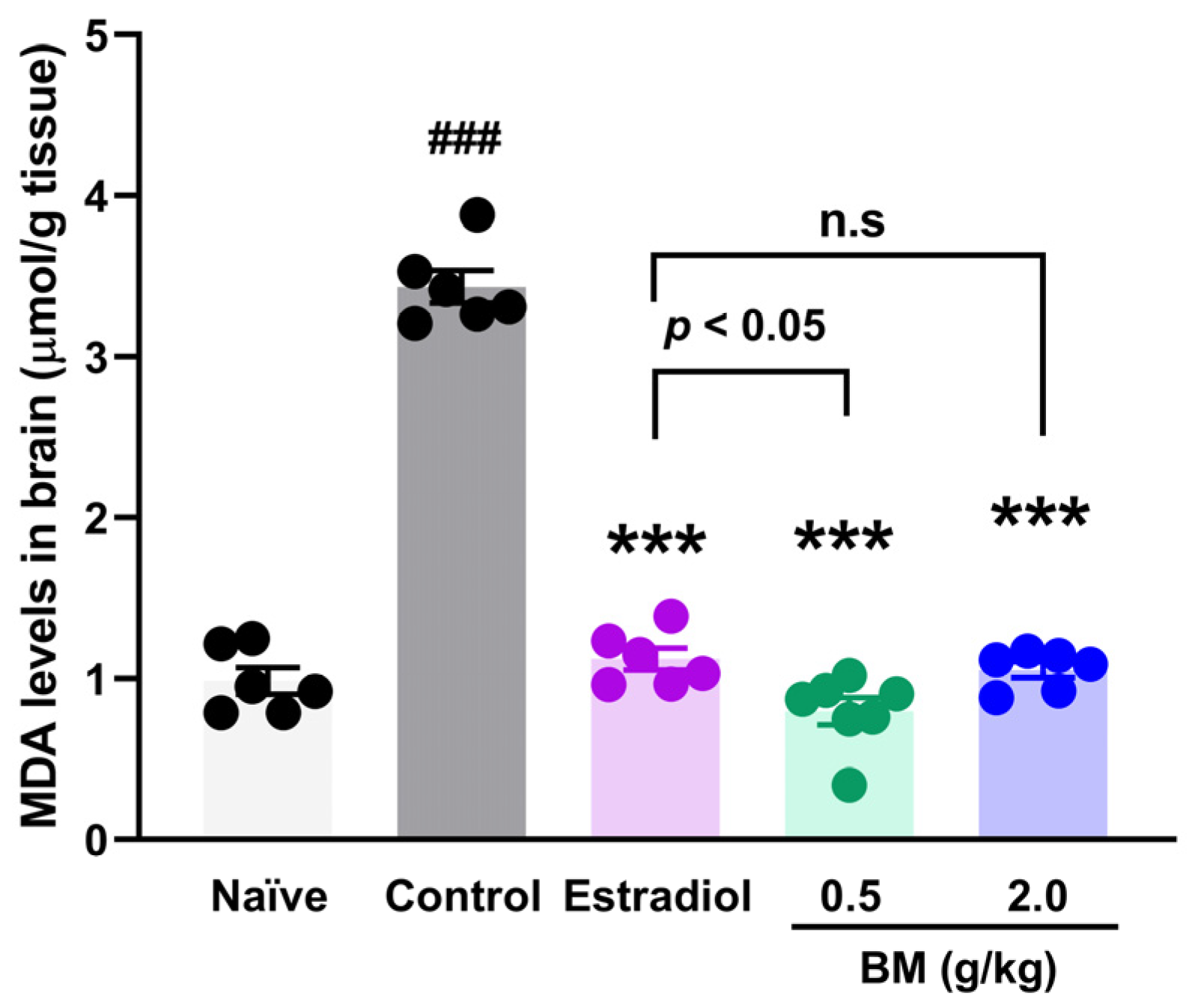
3.3. Spatial Memory and Brain Lipidic Peroxidation in Ovariectomized Rats
3.4. Antinociceptive Assays
3.5. Molecular Docking
3.6. ADMET Profiles of BM Metabolites
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- da Silva Leitão Peres, N.; Cabrera Parra Bortoluzzi, L.; Medeiros Marques, L.L.; Formigoni, M.; Fuchs, R.H.B.; Droval, A.A.; Reitz Cardoso, F.A. Medicinal effects of Peruvian maca (Lepidium meyenii): A review. Food Funct. 2020, 11, 83–92. [Google Scholar] [CrossRef]
- León, J. The “Maca” (Lepidium meyenii), a little known food plant of Peru. Econ. Bot. 1964, 18, 122–127. [Google Scholar] [CrossRef]
- Brinckmann, J.; Smith, E. Maca culture of the Junin Plateau. J. Altern. Complement. Med. 2004, 10, 426–430. [Google Scholar] [CrossRef]
- Gonzales, G.F.; Gonzales, C.; Gonzales-Castañeda, C. Lepidium meyenii (Maca): A plant from the highlands of Peru—From tradition to science. Forsch. Komplementmed. 2009, 16, 373–380. [Google Scholar] [CrossRef]
- Valentová, K.; Frcek, J.; Ulrichová, J. Yacon (Smallanthus sonchifolius) and Maca (Lepidium meyenii), traditional Andean crops as new functional foods on the European market. Chem. Listy 1997, 95, 594–601. [Google Scholar]
- Wang, Y.; Wang, Y.; McNeil, B.; Harvey, L.M. Maca: An Andean crop with multi-pharmacological functions. Food Res. Int. 2007, 40, 783–792. [Google Scholar] [CrossRef]
- Balick, M.J.; Lee, R. Maca: From traditional food crop to energy and libido stimulant. Altern. Ther. Health Med. 2002, 8, 96–98. [Google Scholar] [PubMed]
- Brooks, N.A.; Wilcox, G.; Walker, K.Z.; Ashton, J.F.; Cox, M.B.; Stojanovska, L. Beneficial effects of Lepidium meyenii, (Maca) on psychological symptoms and measures of sexual dysfunction in postmenopausal women are not related to estrogen or androgen content. Menopause 2008, 15, 1157–1162. [Google Scholar] [CrossRef]
- Alquraini, A.; Waggas, D.; Böhlke, M.; Maher, T.; Pino-Figueroa, A. Neuroprotective effects of Lepidium meyenii, (Maca) and macamides against amyloid-beta (25-35) induced toxicity in B-35 neuroblastoma cells. FASEB J. 2014, 28, 657.13. [Google Scholar] [CrossRef]
- Pino-Figueroa, A.; Nguyen, D.; Maher, T.J. Neuroprotective effects of Lepidium meyenii (Maca). Ann. N. Y. Acad. Sci. 2010, 1199, 77–85. [Google Scholar] [CrossRef]
- Rubio, J.; Caldas, M.; Dávila, S.; Gasco, M.; Gonzales, G.F. Effect of three different cultivars of Lepidium meyenii (Maca) on learning and depression in ovariectomized mice. BMC Complement. Altern. Med. 2006, 6, 23. [Google Scholar] [CrossRef]
- Bai, N.; He, K.; Roller, M.; Lai, C.-S.; Bai, L.; Pan, M.-H. Flavonolignans and other constituents from Lepidium meyenii with Activities in Anti-inflammation and Human Cancer Cell Lines. J. Agric. Food Chem. 2015, 63, 2458–2463. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Huamán, Á.; Casimiro-Gonzales, S.; Chávez-Pérez, J.A.; Gonzales-Arimborgo, C.; Cisneros-Fernández, R.; Aguilar-Mendoza, L.A. Antioxidant and neuroprotector effect of Lepidium meyenii (maca) methanol leaf extract against 6-hydroxy dopamine (6-OHDA)-induced toxicity in PC12 cells. Toxicol. Mech. Method. 2017, 27, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Cho, H.; Gil, M.; Kim, K.E. Anti-Inflammation and Anti-Melanogenic Effects of Maca Root Extracts Fermented Using Lactobacillus Strains. Antioxidants 2023, 12, 798. [Google Scholar] [CrossRef] [PubMed]
- Gonzales-Castaneda, C.; Gonzales, G.F. Hypocotyls of Lepidium meyenii (maca), a plant of the Peruvian highlands, prevent ultraviolet A-, B-, and C-induced skin damage in rats. Photodermatol. Photoimmunol. Photomed. 2007, 24, 24–31. [Google Scholar] [CrossRef]
- Gonzales, G.F. Ethnobiology and ethnopharmacology of Lepidium meyenii (Maca), a plant from the Peruvian Highlands. Evid. Based Complement. Altern. Med. 2012, 2012, 1–10. [Google Scholar] [CrossRef]
- Sandoval, M.; Okuhama, N.N.; Angeles, F.M.; Melchor, V.V.; Condezo, L.A.; Lao, J.; Miller, M.J.S. Antioxidant activity of the cruciferous vegetable Maca (Lepidium meyenii). Food Chem. 2002, 79, 207–213. [Google Scholar] [CrossRef]
- Guo, S.S.; Gao, X.F.; Gu, Y.R.; Wan, Z.X.; Lu, A.M.; Qin, Z.H.; Luo, L. Preservation of Cognitive Function by Lepidium meyenii (Maca) Is Associated with Improvement of Mitochondrial Activity and Upregulation of Autophagy-Related Proteins in Middle-Aged Mouse Cortex. Evid. Based Complement. Altern. Med. 2016, 2016, 4394261. [Google Scholar] [CrossRef]
- Rubio, J.; Qiong, W.; Liu, X.; Jiang, Z.; Dang, H.; Chen, S.L.; Gonzales, G.F. Aqueous Extract of Black Maca (Lepidium meyenii) on Memory Impairment Induced by Ovariectomy in Mice. Evid. Based Complement. Altern. Med. 2011, 2011, 253958. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J., Jr. Colorimetry of total phenolics with phosphomolybdic phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–153. [Google Scholar] [CrossRef]
- Baki, S.; Tufan, A.N.; Altun, M.; Özgökçe, F.; Güçlü, K.; Özyürek, M. Microwave-Assisted Extraction of Polyphenolics from Some Selected Medicinal Herbs Grown in Turkey. Rec. Nat. Prod. 2018, 12, 29–39. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Baran, A.; Karakılıç, E.; Faiz, Ö.; Özer, F. Synthesis of chalcone-containing zinc and cobalt metallophthalocyanines; investigation of their photochemical, DPPH radical scavenging and metal chelating characters. Org. Commun. 2020, 13, 65–78. [Google Scholar] [CrossRef]
- Ybañez-Julca, R.O.; Asunción-Alvarez, D.; Palacios, J.; Nwokocha, C.R. Maca extracts and estrogen replacement therapy in ovariectomized rats exposed at high altitude. Reprod. Med. Biol. 2020, 20, 88–95. [Google Scholar] [CrossRef]
- Esterbauer, H.; Cheeseman, K.H. Determination of aldehydic lipid peroxidation products: Malonaldehyde and 4-hydroxynonenal. Methods Enzymol. 1990, 186, 407–421. [Google Scholar] [CrossRef]
- Ybañez-Julca, R.O.; Quispe-Díaz, I.M.; Asunción- Alvarez, D.; Sánchez-Muñoz, K.; Vargas-Goñas, A.; Morote-Guzman, J.; Yaro- Marcelo, R.; Venegas- Casanova, E.A.; Jara-Aguilar, R.; Buc Calderon, P.; et al. Antidepressant-Like Behavioral and Spatial Memory Effects in Peruvian Red Maca (Lepidium meyenii)-Treated Rats. Pharmacogn. J. 2021, 13, 81–88. [Google Scholar] [CrossRef]
- Nunez, J. Morris water maze experiment. J. Vis. Exp. 2008, 19, 897–898. [Google Scholar] [CrossRef]
- Möller, M.; Möser, C.V.; Weiß, U.; Niederberger, E. The Role of AlphαSynuclein in Mouse Models of Acute, Inflammatory and Neuropathic Pain. Cells 2022, 11, 1967. [Google Scholar] [CrossRef] [PubMed]
- Udell, M.E.; Ni, J.; Garcia Martinez, A.; Mulligan, M.K.; Redei, E.E.; Chen, H. Tail Timer: A device for automating data collection in the rodent tail immersion assay. PLoS ONE 2021, 16, e0256264. [Google Scholar] [CrossRef]
- Lountos, G.T.; Jiang, R.; Wellborn, W.B.; Thaler, T.L.; Bommarius, A.S.; Orville, A.M. The crystal structure of NAD(P)H oxidase from Lactobacillus sanfranciscensis: Insights into the conversion of O2 into two water molecules by the flavoenzyme. Biochemistry 2006, 45, 9648–9659. [Google Scholar] [CrossRef]
- Cao, H.; Pauff, J.M.; Hille, R. Substrate orientation and catalytic specificity in the action of xanthine oxidase: The sequential hydroxylation of hypoxanthine to uric acid. J. Biol. Chem. 2010, 285, 28044–28053. [Google Scholar] [CrossRef]
- Sea, K.; Sohn, S.H.; Durazo, A.; Sheng, Y.; Shaw, B.F.; Cao, X.; Taylor, A.B.; Whitson, L.J.; Holloway, S.P.; Hart, P.J.; et al. Insights into the role of the unusual disulfide bond in copper-zinc superoxide dismutase. J. Biol. Chem. 2015, 290, 2405–2418. [Google Scholar] [CrossRef]
- Manglik, A.; Kruse, A.C.; Kobilka, T.S.; Thian, F.S.; Mathiesen, J.M.; Sunahara, R.K.; Pardo, L.; Weis, W.I.; Kobilka, B.K.; Granier, S. Crystal structure of the µ-opioid receptor bound to a morphinan antagonist. Nature 2012, 485, 321–326. [Google Scholar] [CrossRef] [PubMed]
- Mileni, M.; Johnson, D.S.; Wang, Z.; Everdeen, D.S.; Liimatta, M.; Pabst, B.; Bhattacharya, K.; Nugent, R.A.; Kamtekar, S.; Cravatt, B.F.; et al. Structure-guided inhibitor design for human FAAH by interspecies active site conversion. Proc. Natl. Acad. Sci. USA 2008, 105, 12820–12824. [Google Scholar] [CrossRef] [PubMed]
- Trott, O.; Olson, A. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Sanner, M.F. Python: A programming language for software integration and development. J. Mol. Graph. Model. 1999, 17, 55–84. [Google Scholar]
- Minchán-Herrera, P.; Ybañez-Julca, R.O.; Quispe-Díaz, I.M.; Venegas-Casanova, E.A.; Jara-Aguilar, R.; Salas, F.; Zevallos-Escobar, L.; Yáñez, O.; Pino-Rios, R.; Calderon, P.B.; et al. Valeriana pilosa Roots Essential Oil: Chemical Composition, Antioxidant Activities, and Molecular Docking Studies on Enzymes Involved in Redox Biological Processes. Antioxidants 2022, 11, 1337. [Google Scholar] [CrossRef]
- Pires, D.E.V.; Blundell, T.L.; Ascher, D.B. PkCSM: Predicting Small-Molecule Pharmacokinetic and Toxicity Properties Using Graph-Based Signatures. J. Med. Chem. 2015, 58, 4066–4072. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, P.; Brantner, A.; Wang, H.; Shu, X.; Yang, J.; Si, N.; Han, L.; Zhao, H.; Bian, B. Chemical profiling analysis of Maca using UHPLC−ESI−Orbitrap MS coupled with UHPLC−ESI−QqQ MS and the neuroprotective study on its active ingredients. Sci. Rep. 2017, 7, 44660. [Google Scholar] [CrossRef]
- Yang, S.; Zhan, L.; Liu, C.; Fu, L.; Chen, R.; Nie, Z. Mass spectrometry imaging of small molecule in situ in Lepidium meyenii (Maca) using gold nanoparticles matrix. Microchem. J. 2019, 150, 104190. [Google Scholar] [CrossRef]
- Gao, X.C.; Lv, J.W.; Li, C.N.; Zhang, N.X.; Tian, L.L.; Han, X.Y.; Zhang, H.; Sun, J.M. Screening of the Active Component Promoting Leydig Cell Proliferation from Lepidium meyenii Using HPLC-ESI-MS/MS Coupled with Multivariate Statistical Analysis. Molecules 2019, 24, 2101. [Google Scholar] [CrossRef]
- Tafuri, S.; Cocchia, N.; Carotenuto, D.; Vassetti, A.; Staropoli, A.; Mastellone, V.; Peretti, V.; Ciotola, F.; Albarella, S.; Del Prete, C.; et al. Chemical Analysis of Lepidium meyenii (Maca) and Its Effects on Redox Status and on Reproductive Biology in Stallions. Molecules 2019, 24, 1981. [Google Scholar] [CrossRef]
- Yang, S.; Sun, X.; Gao, Y.; Chen, R. Differentiation of Lepidium meyenii (Maca) from Different Origins by Electrospray Ionization Mass Spectrometry with Principal Component Analysis. ACS Omega 2019, 4, 16493–16500. [Google Scholar] [CrossRef]
- Carvalho, F.V.; Ribeiro, P.R. Structural diversity, biosynthetic aspects, and LC-HRMS data compilation for the identification of bioactive compounds of Lepidium meyenii. Food Res. Int. 2019, 125, 108615. [Google Scholar] [CrossRef]
- Tarabasz, D.; Szczeblewski, P.; Laskowski, T.; Płaziński, W.; Baranowska-Wójcik, E.; Szwajgier, D.; Kukula-Koch, W.; Meissner, H.O. The Distribution of Glucosinolates in Different Phenotypes of Lepidium peruvianum and Their Role as Acetyl- and Butyrylcholinesterase Inhibitors-In Silico and In Vitro Studies. Int. J. Mol. Sci. 2022, 23, 4858. [Google Scholar] [CrossRef] [PubMed]
- Ulloa Del Carpio, N.; Alvarado-Corella, D.; Quiñones-Laveriano, D.M.; Araya-Sibaja, A.; Vega-Baudrit, J.; Monagas-Juan, M.; Navarro-Hoyos, M.; Villar-López, M. Exploring the chemical and pharmacological variability of Lepidium meyenii: A comprehensive review of the effects of maca. Front. Pharmacol. 2024, 15, 1360422. [Google Scholar] [CrossRef] [PubMed]
- Minich, D.M.; Ross, K.; Frame, J.; Fahoum, M.; Warner, W.; Meissner, H.O. Not All Maca Is Created Equal: A Review of Colors, Nutrition, Phytochemicals, and Clinical Uses. Nutrients 2024, 16, 530. [Google Scholar] [CrossRef] [PubMed]
- Vera-López, K.J.; Davila-Del-Carpio, G.; Nieto-Montesinos, R. Macamides as Potential Therapeutic Agents in Neurological Disorders. Neurol. Int. 2024, 16, 1611–1625. [Google Scholar] [CrossRef]
- Yu, Z.; Li, D.; Zhai, S.; Xu, H.; Liu, H.; Ao, M.; Zhao, C.; Jin, W.; Yu, L. Neuroprotective effects of macamide from maca (Lepidium meyenii Walp.) on corticosterone-induced hippocampal impairments through its anti-inflammatory, neurotrophic, and synaptic protection properties. Food Funct. 2021, 19, 9211–9228. [Google Scholar] [CrossRef]
- Shen, M.; Yuan, L.; Zhang, J.; Wang, X.; Zhang, M.; Li, H.; Jing, Y.; Zeng, F.; Xie, J. Phytosterols: Physiological Functions and Potential Application. Foods 2024, 13, 1754. [Google Scholar] [CrossRef]
- Wang, S.; Zhu, F. Chemical composition and health effects of maca (Lepidium meyenii). Food Chem. 2019, 288, 422–443. [Google Scholar] [CrossRef]
- Cui, B.; Zheng, B.L.; He, K.; Zheng, Q.Y. Imidazole alkaloids from Lepidium meyenii. J. Nat. Prod. 2003, 66, 1101–1103. [Google Scholar] [CrossRef] [PubMed]
- Ezzili, C.; Otrubova, K.; Boger, D.L. Fatty acid amide signaling molecules. Bioorg Med. Chem. Lett. 2010, 20, 5959–5968. [Google Scholar] [CrossRef] [PubMed]
- Tuzcu, Z.; Koclar, G.; Agca, C.A.; Aykutoglu, G.; Dervisoglu, G.; Tartik, M.; Ozturk, Z.; Kaya, B.; Sahin, K. Antioxidant, antimicrobial and anticancer effects of different extracts from wild edible plant Eremurus spectabilis leaves and roots. Int. J. Clin. Exp. Med. 2017, 10, 4787–4797. [Google Scholar]
- Gulcin, İ. Antioxidants: A comprehensive review. Arch. Toxicol. 2025, 99, 1893–1997. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Wei, J.; Gao, Y.; Chen, R. Antioxidant and antitumoral activities of isolated macamide and macaene fractions from Lepidium meyenii (Maca). Talanta 2021, 221, 121635. [Google Scholar] [CrossRef] [PubMed]
- Pérez-González, A.; García-Hernández, E.; Chigo-Anota, E. The antioxidant capacity of an imidazole alkaloids family through single-electron transfer reactions. J. Mol. Model. 2020, 26, 321. [Google Scholar] [CrossRef]
- Bortz, J.; Klatt, K.C.; Wallace, T.C. Perspective: Estrogen and the Risk of Cognitive Decline: A Missing Choline(rgic) Link? Adv. Nutr. 2022, 13, 376–387. [Google Scholar] [CrossRef]
- Gibbs, R.B. Estrogen therapy and cognition: A review of the cholinergic hypothesis. Endocr. Rev. 2010, 31, 224–253. [Google Scholar] [CrossRef]
- Batallán Burrowes, A.A.; Olajide, O.J.; Iasenza, I.A.; Shams, W.M.; Carter, F.; Chapman, C.A. Ovariectomy reduces cholinergic modulation of excitatory synaptic transmission in the rat entorhinal cortex. PLoS ONE 2022, 17, e0271131. [Google Scholar] [CrossRef]
- Zhou, Y.; Xu, B.; Yu, H.; Zhao, W.; Song, X.; Liu, Y.; Wang, K.; Peacher, N.; Zhao, X.; Zhang, H.T. Biochanin A Attenuates Ovariectomy-Induced Cognition Deficit via Antioxidant Effects in Female Rats. Front. Pharmacol. 2021, 12, 603316. [Google Scholar] [CrossRef] [PubMed]
- Mading, A.; Chotritthirong, Y.; Chulikhit, Y.; Daodee, S.; Boonyarat, C.; Khamphukdee, C.; Sukketsiri, W.; Kwankhao, P.; Pitiporn, S.; Monthakantirat, O. Effectiveness of Tri-Kaysorn-Mas Extract in Ameliorating Cognitive-like Behavior Deficits in Ovariectomized Mice via Activation of Multiple Mechanisms. Pharmaceuticals 2024, 17, 1182. [Google Scholar] [CrossRef] [PubMed]
- Sobočanec, S.; Šarić, A.; Mačak Šafranko, Ž.; Popović Hadžija, M.; Abramić, M.; Balog, T. The role of 17β-estradiol in the regulation of antioxidant enzymes via the Nrf2-Keap1 pathway in the livers of CBA/H mice. Life Sci. 2015, 130, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Silva, V.G.; Silva, R.O.; Damasceno, S.R.; Carvalho, N.S.; Prudêncio, R.S.; Aragão, K.S.; Guimarães, M.A.; Campos, S.A.; Véras, L.M.; Godejohann, M.; et al. Anti-inflammatory and antinociceptive activity of epiisopiloturine, an imidazole alkaloid isolated from Pilocarpus microphyllus. J. Nat. Prod. 2013, 76, 1071–1077. [Google Scholar] [CrossRef]
- Tenci, B.; Di Cesare Mannelli, L.; Maresca, M.; Micheli, L.; Pieraccini, G.; Mulinacci, N.; Ghelardini, C. Effects of a water extract of Lepidium meyenii root in different models of persistent pain in rats. Z. Naturforsch. C J. Biosci. 2017, 72, 449–457. [Google Scholar] [CrossRef]
- Singh, N.; Barnych, B.; Morisseau, C.; Wagner, K.M.; Wan, D.; Takeshita, A.; Pham, H.; Xu, T.; Dandekar, A.; Liu, J.Y.; et al. N-Benzyl-linoleamide, a Constituent of Lepidium meyenii (Maca), Is an Orally Bioavailable Soluble Epoxide Hydrolase Inhibitor That Alleviates Inflammatory Pain. J. Nat. Prod. 2020, 83, 3689–3697. [Google Scholar] [CrossRef]
- Yu, Z.; Jin, W.; Cui, Y.; Ao, M.; Liu, H.; Xu, H.; Yu, L. Protective effects of macamides from Lepidium meyenii Walp. against corticosterone-induced neurotoxicity in PC12 cells. RSC Adv. 2019, 9, 23096–23108. [Google Scholar] [CrossRef]
- Ekowati, J.; Diyah, N.W.; Nofianti, K.A.; Hamid, I.S. Molecular Docking of Ferulic Acid Derivatives on P2Y12 Receptor and their ADMET Prediction. J. Math. Fundam. Sci. 2018, 50, 203–219. [Google Scholar] [CrossRef]
- Angelis, I.D.; Turco, L. Caco-2 Cells as a Model for Intestinal Absorption. Curr. Protoc. Toxicol. 2011, 47, 20–26. [Google Scholar] [CrossRef]
- Cheng, F.; Li, W.; Zhou, Y.; Shen, J.; Wu, Z.; Liu, G.; Lee, P.W.; Tang, Y. admetSAR: A Comprehensive Source and Free Tool for Assessment of Chemical ADMET Properties. J. Chem. Inf. Model. 2012, 52, 3099–3105. [Google Scholar] [CrossRef]
- Nau, R.; Sorgel, F.; Eiffert, H. Penetration of Drugs through the Blood-Cerebrospinal Fluid/Blood-Brain Barrier for Treatment of Central Nervous System Infections. Clin. Microbiol. Rev. 2010, 23, 858–883. [Google Scholar] [CrossRef]
- Morris-Schaffer, K.; McCoy, M.J. A Review of the LD50 and Its Current Role in Hazard Communication. ACS Chem. Health Saf. 2021, 28, 25–33. [Google Scholar] [CrossRef]
| Groups | Treatment Allocation | Oral Route Dose |
|---|---|---|
| Control | NaCl 0.9% | 0.1 mL/100 g |
| Reference standard | tramadol | 10 mg/kg |
| Test group I | 1–6 | 0.25 mg/kg |
| Test group II | 7–12 | 0.50 mg/kg |
| Test group III | 13–18 | 1.0 mg/kg |
| Groups | Treatment Allocation | Oral Route Dose |
|---|---|---|
| Control | NaCl 0.9% | 0.1 mL/100 g |
| Reference standard | tramadol | 10 mg/kg |
| Test group I | 1–6 | 0.25 mg/kg |
| Test group II | 7–12 | 0.50 mg/kg |
| Test group III | 13–18 | 1.0 mg/kg |
| N° | Proposed Compound | Molecular Formula | RT (min) | Mode of Ionization | Main Fragments (m/z) | References | |
|---|---|---|---|---|---|---|---|
| [M + H] | [M − H] | ||||||
| 1 | Sucrose | C12H22O11 | 0.8 | - | 341 | 179 | [39] |
| 2 | N-benzyloctanamide | C15H23NO | 1.4 | 270 | - | 202 | [40] |
| 3 | N-(3-methoxybenzyl)-(9Z,12Z)-octadecadienamide | C26H41NO2 | 4.8 | 399 | - | 292, 239, 150 | [41] |
| 4 | 1-benzyl-2-propyl-4,5-dimethylimidazilium | C15H20N2 | 10.7 | 227 | - | 186 | [42] |
| 5 | 1,3-dibenzyl-4,5-dimethylimidazilium chloride (Lepidiline A) | C19H21ClN2 | 17.3 | 277 | - | 239 | [43] |
| 6 | 1,3-dibenzyl-2,4,5-trimethylimidazilium chloride (Lepidiline B) | C20H23ClN2 | 17.9 | 291 | - | 239 | [42] |
| 7 | 3-benzyl-1-(3-methoxybenzyl)-2,4,5-trimethylimidazilium chloride (Lepidiline D) | C21H25ClN2O | 18.2 | 321 | - | 239 | [43] |
| 8 | Campesterol | C28H48O | 18.5 | 401 | - | 305, 190 | [44] |
| 9 | 9(S),12(S),13(S)-Trihydroxy-10(E)-octadecenoic acid (Pinellic acid) | C18H34O5 | 18.7 | - | 329 | 247, 151 | [45] |
| 10 | 1,3-dibenzyl-2-pentyl-4,5-dimethylimidazilium | C24H31N2+ | 19.6 | 347 | - | 309, 273, 239 | [39,42] |
| 11 | N-octadecanamide | C18H37NO | 20.9 | 284 | - | 239, 149, 121 | [39] |
| 12 | N-benzyl-16-hydroxy-9-oxo-13E,15E-octadecadienamide | C25H41NO2 | 22.4 | - | 387 | 345, 247, 177 | [39] |
| Samples | TPC (mg GAE/mL) | CUPRAC (mg TEAC/mL) | FRAP (mg TEAC/mL) | ABTS IC50 | DPPH IC50 |
|---|---|---|---|---|---|
| BM | 10.62 ± 0.56 | 14.06 ± 1.56 | 7.84 ± 0.49 | 15.26 ± 1.13 | 36.82 ± 9.57 |
| Quercetin | 1.23 ± 0.05 | 4.69 ± 0.24 | 3.14 ± 0.21 | 0.09 ± 0.03 | 0.09 ± 0.03 |
| Trolox® | - | - | - | 0.20 ± 0.06 | 0.18 ± 0.01 |
| Compounds | Free Binding Energy (kcal.mol−1) | ||||
|---|---|---|---|---|---|
| NAD(P)H Oxidase | Xanthine Oxidase | Superoxide Dismutase | μ-Opioid Receptor | Fatty Acid Amide Hydrolase | |
| 1 | −6.1 | −7.1 | −7.0 | −5.5 | −7.1 |
| 2 | −7.3 | −6.9 | −6.1 | −6.9 | −8.0 |
| 3 | −7.9 | −7.7 | −6.6 | −7.0 | −8.3 |
| 4 | −8.0 | −7.9 | −7.0 | −7.5 | −7.9 |
| 5 | −8.8 | −9.2 | −6.7 | −8.3 | −9.0 |
| 6 | −8.9 | −9.3 | −7.1 | −8.4 | −10.1 |
| 7 | −8.7 | −9.4 | −7.1 | −8.2 | −9.3 |
| 8 | −9.9 | −9.5 | −6.6 | −8.6 | −9.7 |
| 9 | −6.5 | −7.2 | −6.2 | −6.0 | −7.4 |
| 10 | −8.9 | −9.6 | −7.2 | −7.7 | −9.1 |
| 11 | −6.4 | −6.5 | −5.3 | −6.1 | −7.1 |
| 12 | −7.8 | −8.4 | −6.5 | −7.4 | −9.0 |
| tramadol | ND | ND | ND | −6.4 | ND |
| estradiol | ND | ND | ND | ND | −9.4 |
| Property | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Absorption | Distribution | Metabolism | Excretion | Toxicity | ||||||
| Model Name | ||||||||||
| Compounds | Caco-2 | IA | SP | VD ss | BBB | CNS | CYP2D6/ CYP3A4 Inhibitor | TC | Oral Rat Acute Tox. (LD50) | Oral Rat Chronic Tox. -LOAEL |
| 1 | −0.288 | 2.571 | −2.736 | 0.229 | −1.697 | −5.794 | No/No | 1.525 | 1.590 | 5.424 |
| 2 | 1.630 | 91.401 | −2.206 | 0.507 | 0.481 | −1.319 | No/No | 1.699 | 2.065 | 1.408 |
| 3 | 1.370 | 93.566 | −2.597 | 0.591 | −0.177 | −1.716 | No/No | 1.834 | 2.109 | 2.564 |
| 4 | 1.496 | 94.101 | −2.819 | 1.203 | 0.458 | −1.619 | No/No | 0.986 | 2.912 | 0.897 |
| 5 | 1.470 | 94.519 | −2.727 | 1.204 | 0.882 | −1.213 | Yes/No | 0.935 | 2.604 | 0.205 |
| 6 | 1.557 | 94.681 | −2.720 | 1.637 | 0.867 | −1.197 | Yes/No | 0.893 | 0.565 | 0.159 |
| 7 | 1.477 | 95.526 | −2.724 | 1.604 | 0.658 | −1.305 | Yes/Yes | 0.908 | 2.780 | 0.063 |
| 8 | 1.235 | 95.601 | −2.836 | 0.366 | 0.785 | −1.835 | No/No | 0.572 | 2.206 | 0.935 |
| 9 | 0.861 | 38.813 | −2.735 | −0.882 | −1.34 | −3.314 | No/No | 1.967 | 2.897 | 1.188 |
| 10 | 0.265 | 89.944 | −2.732 | 1.304 | 1.108 | −1.161 | Yes/No | 1.123 | 2.527 | 0.056 |
| 11 | 1.560 | 89.664 | −2.754 | 0.351 | −.424 | −1.704 | No/No | 1.906 | 1.786 | 0.903 |
| 12 | 0.411 | 87.941 | −2.681 | 0.22 | −0.633 | −2.517 | No/Yes | 1.990 | 3.265 | 2.693 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quispe-Díaz, I.M.; Ybañez-Julca, R.O.; Asunción-Alvarez, D.; Enriquez-Lara, C.; Polo-Bardales, J.L.; Jara-Aguilar, R.; Venegas-Casanova, E.A.; de Albuquerque, R.D.D.G.; Costilla-Sánchez, N.; Vásquez-Corales, E.; et al. Antioxidant, Neuroprotective, and Antinociceptive Effects of Peruvian Black Maca (Lepidium meyenii Walp.). Antioxidants 2025, 14, 1214. https://doi.org/10.3390/antiox14101214
Quispe-Díaz IM, Ybañez-Julca RO, Asunción-Alvarez D, Enriquez-Lara C, Polo-Bardales JL, Jara-Aguilar R, Venegas-Casanova EA, de Albuquerque RDDG, Costilla-Sánchez N, Vásquez-Corales E, et al. Antioxidant, Neuroprotective, and Antinociceptive Effects of Peruvian Black Maca (Lepidium meyenii Walp.). Antioxidants. 2025; 14(10):1214. https://doi.org/10.3390/antiox14101214
Chicago/Turabian StyleQuispe-Díaz, Iván M., Roberto O. Ybañez-Julca, Daniel Asunción-Alvarez, Cinthya Enriquez-Lara, José L. Polo-Bardales, Rafael Jara-Aguilar, Edmundo A. Venegas-Casanova, Ricardo D. D. G. de Albuquerque, Noé Costilla-Sánchez, Edison Vásquez-Corales, and et al. 2025. "Antioxidant, Neuroprotective, and Antinociceptive Effects of Peruvian Black Maca (Lepidium meyenii Walp.)" Antioxidants 14, no. 10: 1214. https://doi.org/10.3390/antiox14101214
APA StyleQuispe-Díaz, I. M., Ybañez-Julca, R. O., Asunción-Alvarez, D., Enriquez-Lara, C., Polo-Bardales, J. L., Jara-Aguilar, R., Venegas-Casanova, E. A., de Albuquerque, R. D. D. G., Costilla-Sánchez, N., Vásquez-Corales, E., Buc Calderon, P., & Benites, J. (2025). Antioxidant, Neuroprotective, and Antinociceptive Effects of Peruvian Black Maca (Lepidium meyenii Walp.). Antioxidants, 14(10), 1214. https://doi.org/10.3390/antiox14101214








