A Bibenzyl from Dendrobium pachyglossum Exhibits Potent Anti-Cancer Activity Against Glioblastoma Multiforme
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
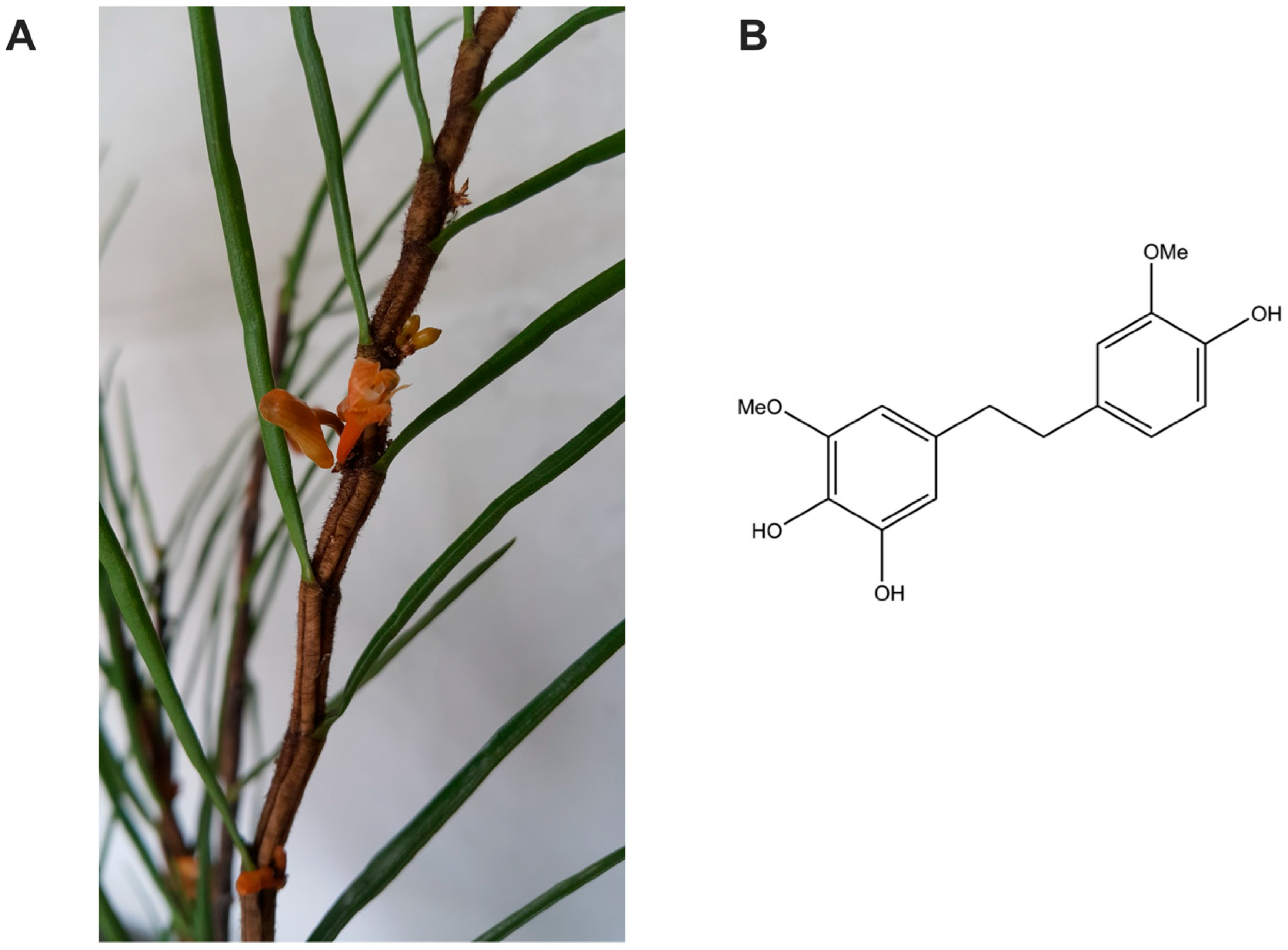
2.2. Isolation of 4,5,4′-Trihydroxy-3,3′-dimethoxybibenzyl (TDB)
2.3. Ferric Reducing Antioxidant Power (FRAP) Assay
2.4. DPPH Free Radical Scavenging Assay
2.5. Cell Culture
2.6. Treatment with TDB or Temozolomide
2.7. Cell Viability Assay
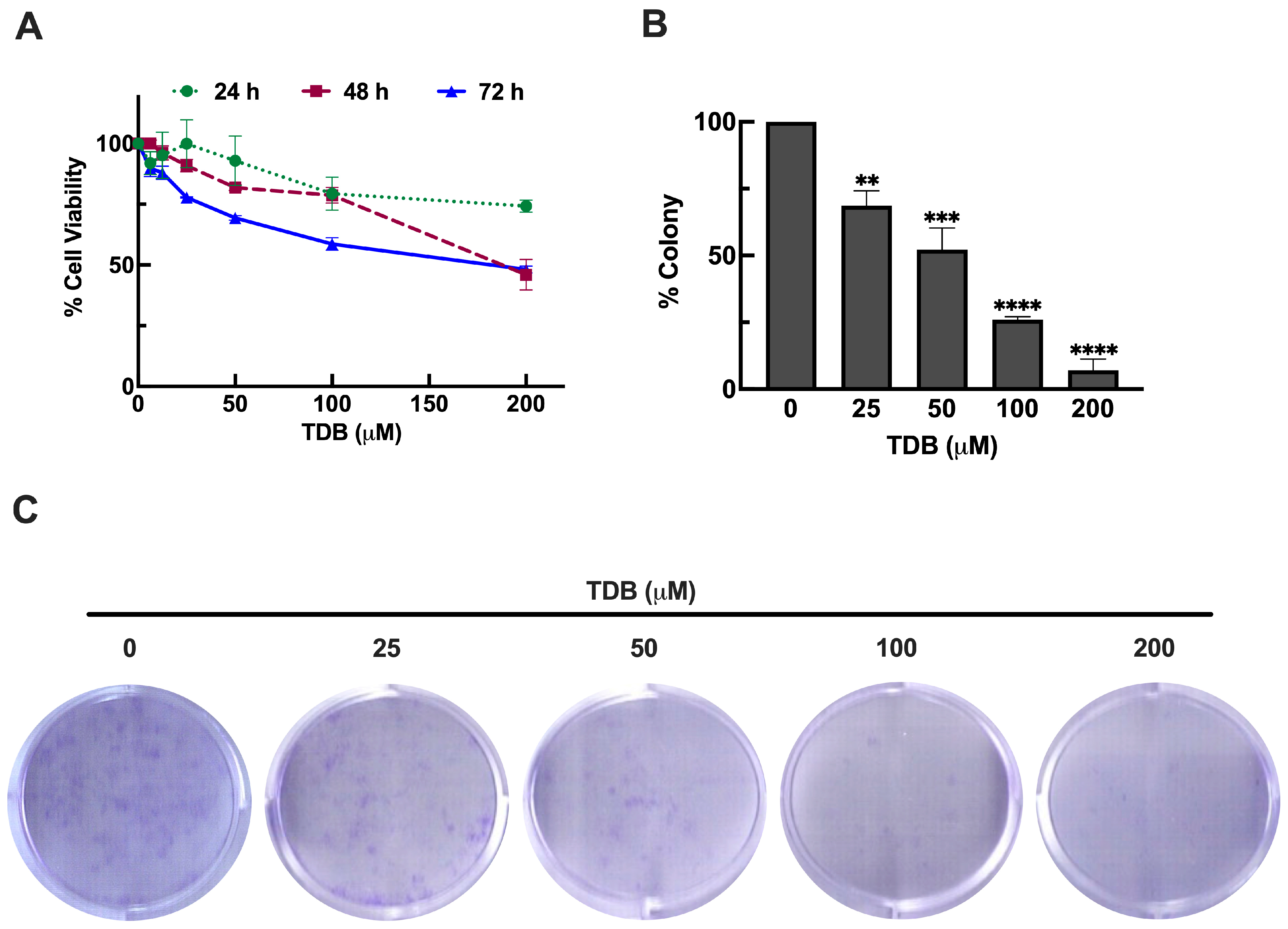
2.8. Colony Formation Assay
2.9. Flow Cytometry Analysis
2.10. Scratch-Wound Assay
2.11. Western Blot Analysis
2.12. In Silico Prediction of Blood–Brain Barrier Permeability
- ADMETlab 3.0 (https://admetlab3.scbdd.com accessed on 29 July 2025):
- LogBB_Pred (http://ssbio.cau.ac.kr/software/logbb_pred/ accessed on 29 July 2025):
- LightBBB (http://ssbio.cau.ac.kr/software/bbb/ accessed on 29 July 2025):
- BBB Predictor (Tree2C) (https://www.ddl.unimi.it/vegaol/bbbp.htm accessed on 29 July 2025):
2.13. Statistical Analysis
3. Results
3.1. 4,5,4′-Trihydroxy-3,3′-dimethoxybibenzyl is a Potent Antioxidant Isolated from Dendrobium pachyglossum
3.2. TDB Reduces Cell Viability and Clonogenic Survival in U87MG Glioblastoma Cells
3.3. TDB Induces Apoptosis Cell Death in U87MG Glioblastoma Cells
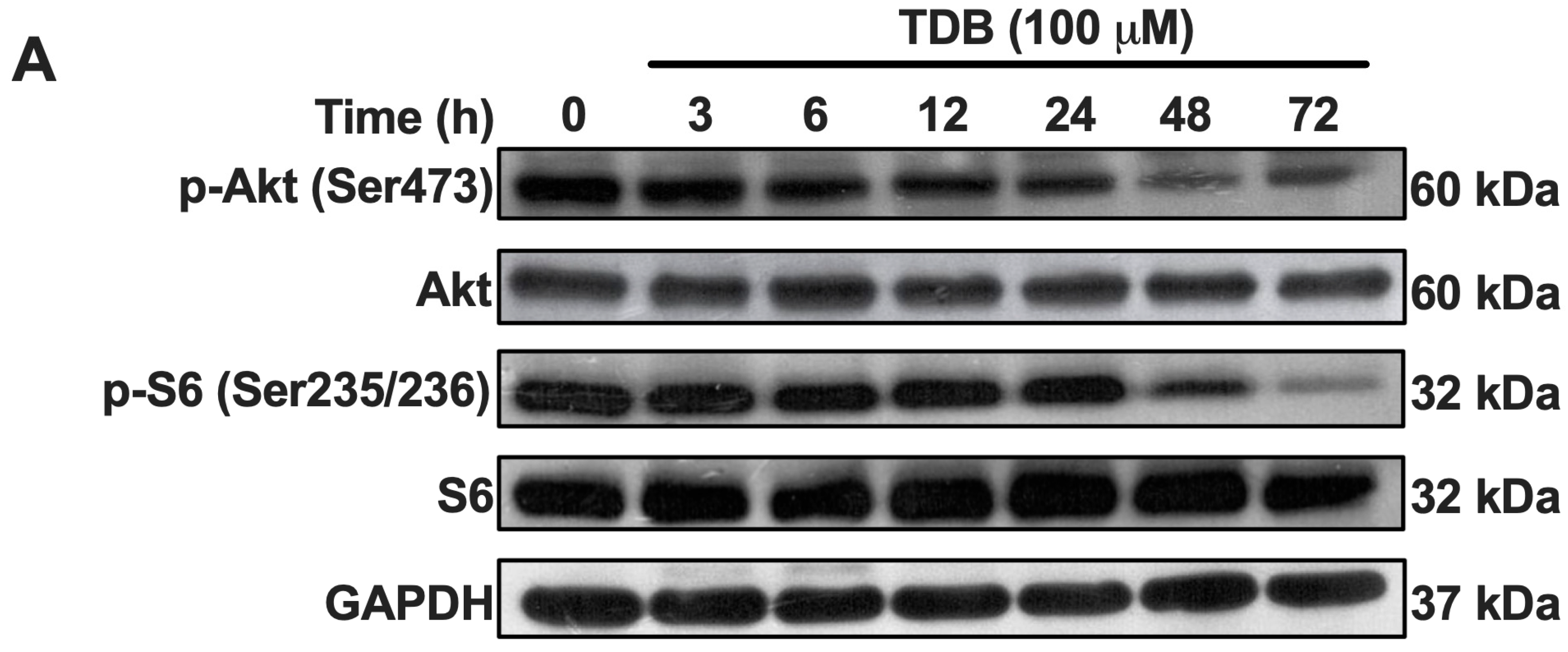
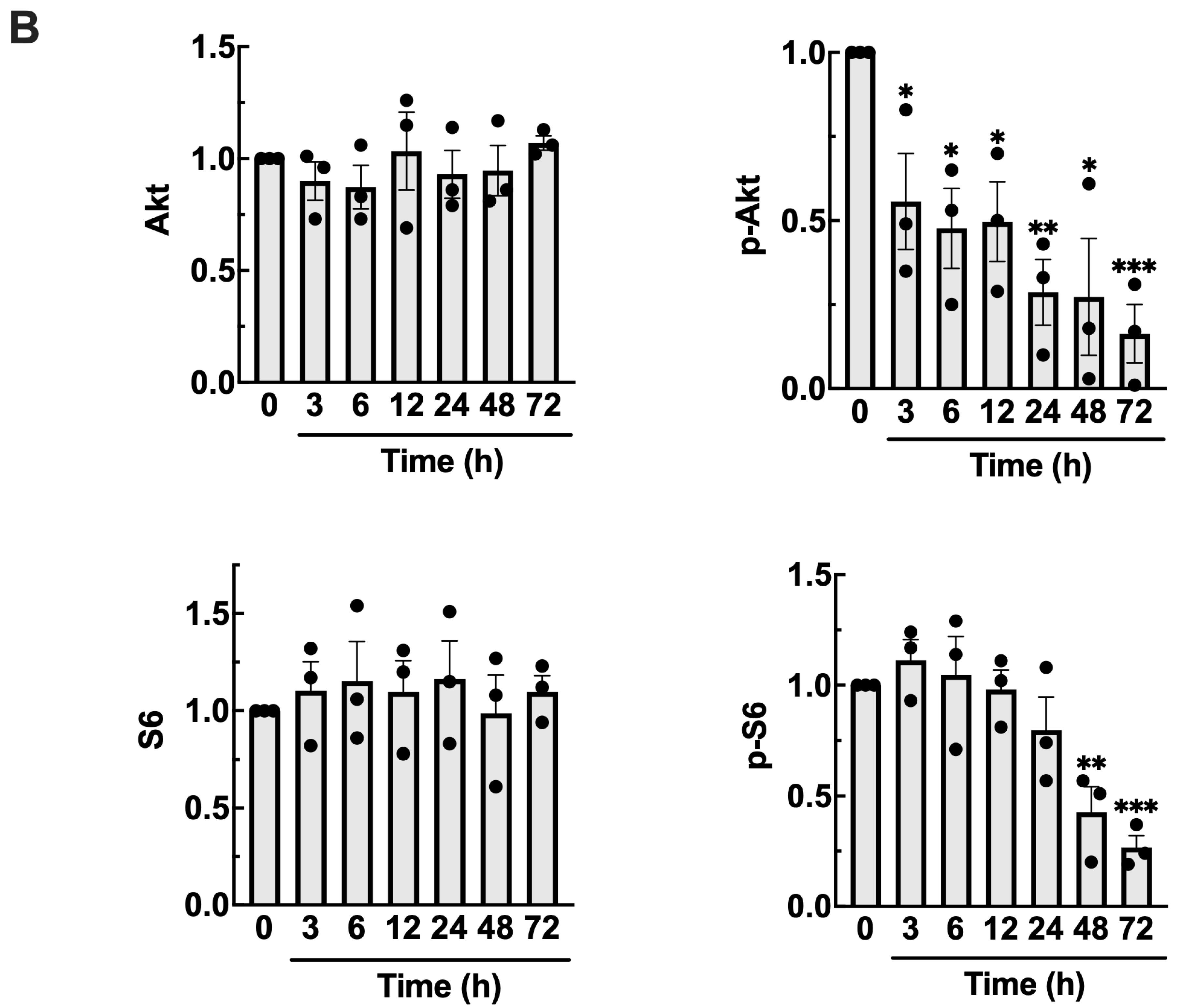
3.4. TDB Suppresses mTORC1/mTORC2 Activity in U87MG Glioblastoma Cells
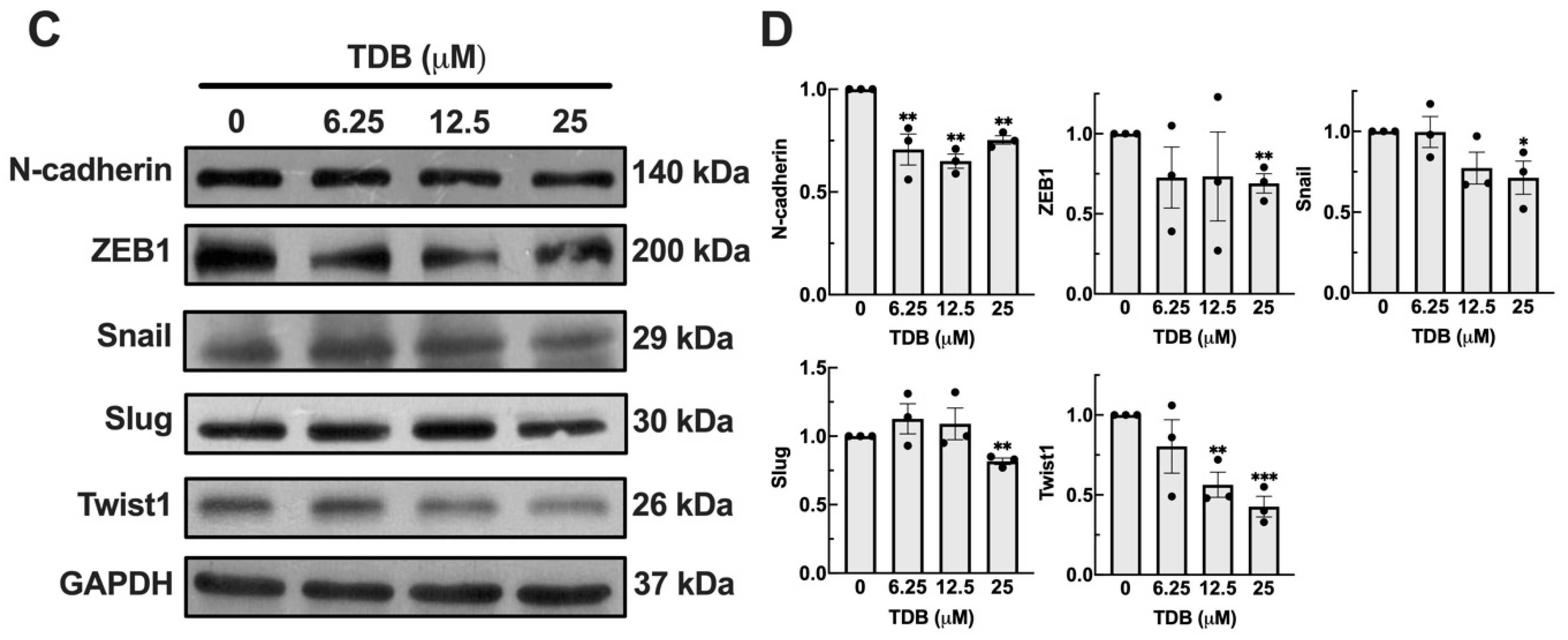
3.5. TDB Attenuates Cell Migration and Mesenchymal Transition in U87MG Glioblastoma Cells
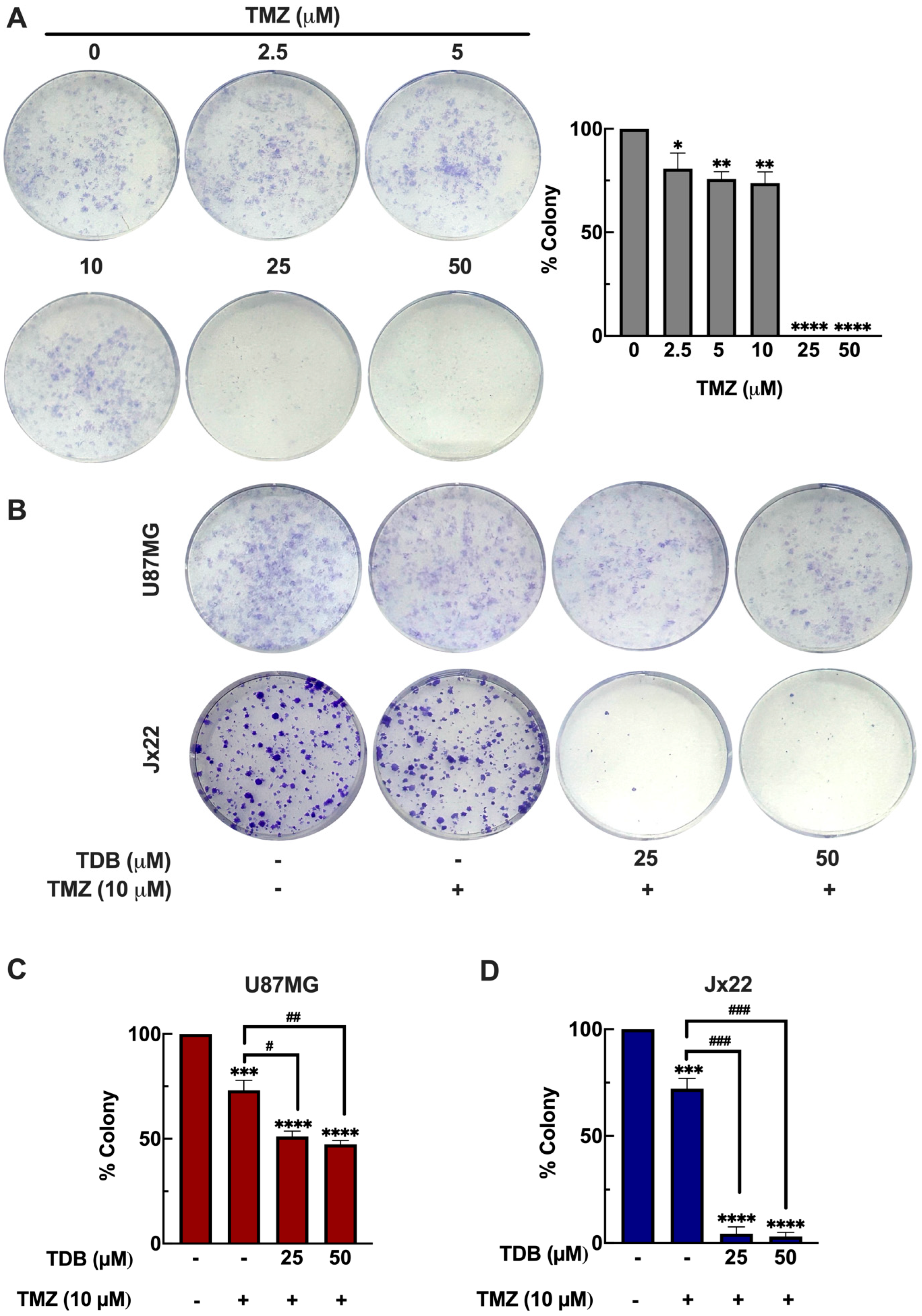
3.6. TDB Improves the Temozolomide Efficacy Against GBM Cells
3.7. TDB Shows Potential to Permeate the Blood–Brain Barrier
4. Discussions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stupp, R.; Mason, W.P.; Bent, M.J.v.d.; Weller, M.; Fisher, B.; Taphoorn, M.J.B.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; et al. Radiotherapy plus Concomitant and Adjuvant Temozolomide for Glioblastoma. N. Engl. J. Med. 2005, 352, 987–996. [Google Scholar] [CrossRef] [PubMed]
- Ostrom, Q.T.; Gittleman, H.; Liao, P.; Vecchione-Koval, T.; Wolinsky, Y.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS Statistical Report: Primary brain and other central nervous system tumors diagnosed in the United States in 2010–2014. Neuro. Oncol. 2017, 19, v1–v88. [Google Scholar] [CrossRef]
- Poon, M.T.C.; Sudlow, C.L.M.; Figueroa, J.D.; Brennan, P.M. Longer-term (≥2 years) survival in patients with glioblastoma in population-based studies pre- and post-2005: A systematic review and meta-analysis. Sci. Rep. 2020, 10, 11622. [Google Scholar] [CrossRef]
- Ali Abdalla, Y.O.; Subramaniam, B.; Nyamathulla, S.; Shamsuddin, N.; Arshad, N.M.; Mun, K.S.; Awang, K.; Nagoor, N.H. Natural Products for Cancer Therapy: A Review of Their Mechanism of Actions and Toxicity in the Past Decade. J. Trop. Med. 2022, 2022, 5794350. [Google Scholar] [CrossRef]
- Chen, P.; Wu, Q.; Feng, J.; Yan, L.; Sun, Y.; Liu, S.; Xiang, Y.; Zhang, M.; Pan, T.; Chen, X.; et al. Erianin, a novel dibenzyl compound in Dendrobium extract, inhibits lung cancer cell growth and migration via calcium/calmodulin-dependent ferroptosis. Signal Transduct. Target. Ther. 2020, 5, 51. [Google Scholar] [CrossRef]
- Chaotham, C.; Pongrakhananon, V.; Sritularak, B.; Chanvorachote, P. A Bibenzyl from Dendrobium ellipsophyllum inhibits epithelial-to-mesenchymal transition and sensitizes lung cancer cells to anoikis. Anticancer Res. 2014, 34, 1931–1938. [Google Scholar] [PubMed]
- Hlosrichok, A.; Sumkhemthong, S.; Sritularak, B.; Chanvorachote, P.; Chaotham, C. A bibenzyl from Dendrobium ellipsophyllum induces apoptosis in human lung cancer cells. J. Nat. Med. 2018, 72, 615–625. [Google Scholar] [CrossRef]
- Liu, R.; Qiu, M.; Deng, X.; Zhang, M.; Gao, Z.; Wang, Y.; Mei, H.; Zhai, M.; Zhang, Q.; Hao, J.; et al. Erianin inhibits the progression of pancreatic cancer by directly targeting AKT and ASK1. Cancer Cell Int. 2024, 24, 348. [Google Scholar] [CrossRef]
- Wei, X.; Liu, Q.; Liu, L.; Wang, D.; Liu, J.; Zhu, Q.; Xu, Z.; Chen, Q.; Xu, W. Discovery of the Natural Bibenzyl Compound Erianin in Dendrobium Inhibiting the Growth and EMT of Gastric Cancer through Downregulating the LKB1-SIK2/3-PARD3 Pathway. Int. J. Mol. Sci. 2024, 25, 7973. [Google Scholar] [CrossRef]
- Aljeldah, M.M. Evaluation of the anticancer and antibacterial activities of moscatilin. Heliyon 2024, 10, e31131. [Google Scholar] [CrossRef] [PubMed]
- Taweecheep, P.; Khine, H.E.E.; Hlosrichok, A.; Ecoy, G.A.U.; Sritularak, B.; Prompetchara, E.; Chanvorachote, P.; Chaotham, C. Stemness-Suppressive Effect of Bibenzyl from Dendrobium ellipsophyllum in Human Lung Cancer Stem-Like Cells. Evid. Based Complement. Altern. Med. 2021, 2021, 5516655. [Google Scholar] [CrossRef] [PubMed]
- Warinhomhoun, S.; Muangnoi, C.; Buranasudja, V.; Mekboonsonglarp, W.; Rojsitthisak, P.; Likhitwitayawuid, K.; Sritularak, B. Antioxidant Activities and Protective Effects of Dendropachol, a New Bisbibenzyl Compound from Dendrobium pachyglossum, on Hydrogen Peroxide-Induced Oxidative Stress in HaCaT Keratinocytes. Antioxidants 2021, 10, 252. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Xu, J.K.; Wang, J.; Wang, N.L.; Kurihara, H.; Kitanaka, S.; Yao, X.S. Bioactive bibenzyl derivatives and fluorenones from Dendrobium nobile. J. Nat. Prod. 2007, 70, 24–28. [Google Scholar] [CrossRef] [PubMed]
- Chu, C.; Li, T.; Pedersen, H.A.; Kongstad, K.T.; Yan, J.; Staerk, D. Antidiabetic constituents of Dendrobium officinale as determined by high-resolution profiling of radical scavenging and α-glucosidase and α-amylase inhibition combined with HPLC-PDA-HRMS-SPE-NMR analysis. Phytochem. Lett. 2019, 31, 47–52. [Google Scholar] [CrossRef]
- Khine, H.E.E.; Sungthong, R.; Sritularak, B.; Prompetchara, E.; Chaotham, C. Untapped Pharmaceutical Potential of 4,5,4′-Trihydroxy-3,3′-dimethoxybibenzyl for Regulating Obesity: A Cell-Based Study with a Focus on Terminal Differentiation in Adipogenesis. J. Nat. Prod. 2022, 85, 1591–1602. [Google Scholar] [CrossRef]
- Chantaravisoot, N.; Sanookpan, K.; Wattanathamsan, O.; Bootsri, R.; Banlue, T.; Chuenjit, C.; Kalpongnukul, N.; Oliva, C.R.; Griguer, C.E.; Buranasudja, V. Targeting glioblastoma multiforme cells with pharmacological ascorbate: Disrupting DNA damage response and mTOR cascades via extracellular H2O2. Free Radic Biol. Med. 2025, 237, 326–343. [Google Scholar] [CrossRef]
- Singh, S.; Dey, D.; Barik, D.; Mohapatra, I.; Kim, S.; Sharma, M.; Prasad, S.; Wang, P.; Singh, A.; Singh, G. Glioblastoma at the crossroads: Current understanding and future therapeutic horizons. Signal Transduct. Target. Ther. 2025, 10, 213. [Google Scholar] [CrossRef]
- Munteanu, I.G.; Apetrei, C. Analytical Methods Used in Determining Antioxidant Activity: A Review. Int. J. Mol. Sci. 2021, 22, 3380. [Google Scholar] [CrossRef]
- Vaubel, R.A.; Tian, S.; Remonde, D.; Schroeder, M.A.; Mladek, A.C.; Kitange, G.J.; Caron, A.; Kollmeyer, T.M.; Grove, R.; Peng, S.; et al. Genomic and Phenotypic Characterization of a Broad Panel of Patient-Derived Xenografts Reflects the Diversity of Glioblastoma. Clin. Cancer Res. 2020, 26, 1094–1104. [Google Scholar] [CrossRef]
- Fu, L.; Shi, S.; Yi, J.; Wang, N.; He, Y.; Wu, Z.; Peng, J.; Deng, Y.; Wang, W.; Wu, C.; et al. ADMETlab 3.0: An updated comprehensive online ADMET prediction platform enhanced with broader coverage, improved performance, API functionality and decision support. Nucleic Acids Res. 2024, 52, W422–w431. [Google Scholar] [CrossRef]
- Shaker, B.; Lee, J.; Lee, Y.; Yu, M.-S.; Lee, H.-M.; Lee, E.; Kang, H.-C.; Oh, K.-S.; Kim, H.W.; Na, D. A machine learning-based quantitative model (LogBB_Pred) to predict the blood–brain barrier permeability (logBB value) of drug compounds. Bioinformatics 2023, 39, btad577. [Google Scholar] [CrossRef]
- Shaker, B.; Yu, M.S.; Song, J.S.; Ahn, S.; Ryu, J.Y.; Oh, K.S.; Na, D. LightBBB: Computational prediction model of blood-brain-barrier penetration based on LightGBM. Bioinformatics 2021, 37, 1135–1139. [Google Scholar] [CrossRef]
- Pedretti, A.; Mazzolari, A.; Gervasoni, S.; Vistoli, G. Tree2C: A Flexible Tool for Enabling Model Deployment with Special Focus on Cheminformatics Applications. Appl. Sci. 2020, 10, 7704. [Google Scholar] [CrossRef]
- Moazzen, A.; Öztinen, N.; Ak-Sakalli, E.; Koşar, M. Structure-antiradical activity relationships of 25 natural antioxidant phenolic compounds from different classes. Heliyon 2022, 8, e10467. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Garcia, M.; Gracia-Vitoria, J.; Vanbroekhoven, K.; Dejonghe, W.; Satyawali, Y. Selective Enzymatic Esterification of Lignin-Derived Phenolics for the Synthesis of Lipophilic Antioxidants. Antioxidants 2023, 12, 657. [Google Scholar] [CrossRef] [PubMed]
- Karamać, M.; Koleva, L.; Kancheva, V.D.; Amarowicz, R. The Structure-Antioxidant Activity Relationship of Ferulates. Molecules 2017, 22, 527. [Google Scholar] [CrossRef] [PubMed]
- Venkatadri, R.; Muni, T.; Iyer, A.K.V.; Yakisich, J.S.; Azad, N. Role of apoptosis-related miRNAs in resveratrol-induced breast cancer cell death. Cell Death Dis. 2016, 7, e2104. [Google Scholar] [CrossRef]
- Rivera Rivera, A.; Castillo-Pichardo, L.; Gerena, Y.; Dharmawardhane, S. Anti-Breast Cancer Potential of Quercetin via the Akt/AMPK/Mammalian Target of Rapamycin (mTOR) Signaling Cascade. PLoS ONE 2016, 11, e0157251. [Google Scholar] [CrossRef]
- Namiki, K.; Wongsirisin, P.; Yokoyama, S.; Sato, M.; Rawangkan, A.; Sakai, R.; Iida, K.; Suganuma, M. (−)-Epigallocatechin gallate inhibits stemness and tumourigenicity stimulated by AXL receptor tyrosine kinase in human lung cancer cells. Sci. Rep. 2020, 10, 2444. [Google Scholar] [CrossRef]
- Panwar, V.; Singh, A.; Bhatt, M.; Tonk, R.K.; Azizov, S.; Raza, A.S.; Sengupta, S.; Kumar, D.; Garg, M. Multifaceted role of mTOR (mammalian target of rapamycin) signaling pathway in human health and disease. Signal Transduct. Target Ther. 2023, 8, 375. [Google Scholar] [CrossRef]
- Rosén, E.; Mangukiya, H.B.; Elfineh, L.; Stockgard, R.; Krona, C.; Gerlee, P.; Nelander, S. Inference of glioblastoma migration and proliferation rates using single time-point images. Commun. Biol. 2023, 6, 402. [Google Scholar] [CrossRef] [PubMed]
- Hara, A.; Kanayama, T.; Noguchi, K.; Niwa, A.; Miyai, M.; Kawaguchi, M.; Ishida, K.; Hatano, Y.; Niwa, M.; Tomita, H. Treatment Strategies Based on Histological Targets against Invasive and Resistant Glioblastoma. J. Oncol. 2019, 2019, 2964783. [Google Scholar] [CrossRef]
- Dongre, A.; Weinberg, R.A. New insights into the mechanisms of epithelial–mesenchymal transition and implications for cancer. Nat. Rev. Mol. Cell Biol. 2019, 20, 69–84. [Google Scholar] [CrossRef] [PubMed]
- Majc, B.; Sever, T.; Zarić, M.; Breznik, B.; Turk, B.; Lah, T.T. Epithelial-to-mesenchymal transition as the driver of changing carcinoma and glioblastoma microenvironment. Biochim. Biophys. Acta (BBA) Mol. Cell Res. 2020, 1867, 118782. [Google Scholar] [CrossRef]
- Noh, M.-G.; Oh, S.-J.; Ahn, E.-J.; Kim, Y.-J.; Jung, T.-Y.; Jung, S.; Kim, K.-K.; Lee, J.-H.; Lee, K.-H.; Moon, K.-S. Prognostic significance of E-cadherin and N-cadherin expression in Gliomas. BMC Cancer 2017, 17, 583. [Google Scholar] [CrossRef]
- Lee, S.Y. Temozolomide resistance in glioblastoma multiforme. Genes Dis. 2016, 3, 198–210. [Google Scholar] [CrossRef]
- Wu, D.; Chen, Q.; Chen, X.; Han, F.; Chen, Z.; Wang, Y. The blood–brain barrier: Structure, regulation and drug delivery. Signal Transduct. Target. Ther. 2023, 8, 217. [Google Scholar] [CrossRef]
- Kongkatitham, V.; Muangnoi, C.; Kyokong, N.; Thaweesest, W.; Likhitwitayawuid, K.; Rojsitthisak, P.; Sritularak, B. Anti-oxidant and anti-inflammatory effects of new bibenzyl derivatives from Dendrobium parishii in hydrogen peroxide and lipopolysaccharide treated RAW264.7 cells. Phytochem. Lett. 2018, 24, 31–38. [Google Scholar] [CrossRef]
- Wu, X.; Luo, Q.; Liu, Z. Ubiquitination and deubiquitination of MCL1 in cancer: Deciphering chemoresistance mechanisms and providing potential therapeutic options. Cell Death Dis. 2020, 11, 556. [Google Scholar] [CrossRef]
- Chantaravisoot, N.; Wongkongkathep, P.; Kalpongnukul, N.; Pacharakullanon, N.; Kaewsapsak, P.; Ariyachet, C.; Loo, J.A.; Tamanoi, F.; Pisitkun, T. mTORC2 interactome and localization determine aggressiveness of high-grade glioma cells through association with gelsolin. Sci. Rep. 2023, 13, 7037. [Google Scholar] [CrossRef] [PubMed]
- Chandrika, G.; Natesh, K.; Ranade, D.; Chugh, A.; Shastry, P. Suppression of the invasive potential of Glioblastoma cells by mTOR inhibitors involves modulation of NFκB and PKC-α signaling. Sci. Rep. 2016, 6, 22455. [Google Scholar] [CrossRef]
- Huang, J.; Liu, C.; Duan, S.; Lin, J.; Luo, Y.; Tao, S.; Xing, S.; Zhang, X.; Du, H.; Wang, H.; et al. Gigantol inhibits proliferation and enhances DDP-induced apoptosis in breast-cancer cells by downregulating the PI3K/Akt/mTOR signaling pathway. Life Sci. 2021, 274, 119354. [Google Scholar] [CrossRef]
- Chen, H.; Huang, Y.; Huang, J.; Lin, L.; Wei, G. Gigantol attenuates the proliferation of human liver cancer HepG2 cells through the PI3K/Akt/NF-κB signaling pathway. Oncol. Rep. 2017, 37, 865–870. [Google Scholar] [CrossRef]
- Kang, H.; Sun, Y.; Hu, X.; Liu, L. Gigantol inhibits proliferation and enhanced oxidative stress-mediated apoptosis through modulating of Wnt/β-catenin signaling pathway in HeLa cells. J. Biochem. Mol. Toxicol. 2022, 36, e22944. [Google Scholar] [CrossRef]
- Hu, A.; Li, K. Erianin Impedes the Proliferation and Metastatic Migration Through Suppression of STAT-3 Phosphorylation in Human Esophageal Cancer Cells. Appl. Biochem. Biotechnol. 2024, 196, 5859–5874. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, T.; Sun, W.; Wang, Z.; Zuo, D.; Zhou, Z.; Li, S.; Xu, J.; Yin, F.; Hua, Y.; et al. Erianin induces G2/M-phase arrest, apoptosis, and autophagy via the ROS/JNK signaling pathway in human osteosarcoma cells in vitro and in vivo. Cell Death Dis. 2016, 7, e2247. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.H.; Pan, S.L.; Guh, J.H.; Liao, C.H.; Huang, D.Y.; Chen, C.C.; Teng, C.M. Moscatilin induces apoptosis in human colorectal cancer cells: A crucial role of c-Jun NH2-terminal protein kinase activation caused by tubulin depolymerization and DNA damage. Clin. Cancer Res. 2008, 14, 4250–4258. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.; Han, A.R.; Nam, B.; Kim, Y.R.; Jin, C.H.; Kim, J.B.; Eun, Y.G.; Jung, C.H. Moscatilin Induces Apoptosis in Human Head and Neck Squamous Cell Carcinoma Cells via JNK Signaling Pathway. Molecules 2020, 25, 901. [Google Scholar] [CrossRef]
- Zhang, L.; Fang, Y.; Xu, X.F.; Jin, D.Y. Moscatilin induces apoptosis of pancreatic cancer cells via reactive oxygen species and the JNK/SAPK pathway. Mol. Med. Rep. 2017, 15, 1195–1203. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Yang, J.; Chen, L.; Chen, P.; Liu, C.; Long, X.; Chen, B.; Long, J. Moscatilin Reverses EMT Progression and its Resulting Enhanced Invasion and Migration by Affecting the TGF-β Signaling Pathway in Bladder Cancer. Anticancer Agents Med. Chem. 2024, 24, 1074–1084. [Google Scholar] [CrossRef]
- Unahabhokha, T.; Chanvorachote, P.; Sritularak, B.; Kitsongsermthon, J.; Pongrakhananon, V. Gigantol Inhibits Epithelial to Mesenchymal Process in Human Lung Cancer Cells. Evid. Based Complement. Altern. Med. 2016, 2016, 4561674. [Google Scholar] [CrossRef]
- Karve, A.S.; Desai, J.M.; Gadgil, S.N.; Dave, N.; Wise-Draper, T.M.; Gudelsky, G.A.; Phoenix, T.N.; DasGupta, B.; Yogendran, L.; Sengupta, S.; et al. A Review of Approaches to Potentiate the Activity of Temozolomide against Glioblastoma to Overcome Resistance. Int. J. Mol. Sci. 2024, 25, 3217. [Google Scholar] [CrossRef]
- Mikitsh, J.L.; Chacko, A.-M. Pathways for Small Molecule Delivery to the Central Nervous System across the Blood-Brain Barrier. Perspect. Med. Chem. 2014, 6, 11–24. [Google Scholar] [CrossRef]
- Patrizii, M.; Bartucci, M.; Pine, S.R.; Sabaawy, H.E. Utility of Glioblastoma Patient-Derived Orthotopic Xenografts in Drug Discovery and Personalized Therapy. Front. Oncol. 2018, 8, 23. [Google Scholar] [CrossRef]
- Nigim, F.; Cavanaugh, J.; Patel, A.P.; Curry, W.T., Jr.; Esaki, S.; Kasper, E.M.; Chi, A.S.; Louis, D.N.; Martuza, R.L.; Rabkin, S.D.; et al. Targeting Hypoxia-Inducible Factor 1α in a New Orthotopic Model of Glioblastoma Recapitulating the Hypoxic Tumor Microenvironment. J. Neuropathol. Exp. Neurol. 2015, 74, 710–722. [Google Scholar] [CrossRef]
- Doroszko, M.; Stockgard, R.; Uppman, I.; Heinold, J.; Voukelatou, F.; Mangukiya, H.B.; Millner, T.O.; Skeppås, M.; Ballester Bravo, M.; Elgendy, R.; et al. The invasion phenotypes of glioblastoma depend on plastic and reprogrammable cell states. Nat. Commun. 2025, 16, 6662. [Google Scholar] [CrossRef]
- Kong, B.H.; Shin, H.D.; Kim, S.H.; Mok, H.S.; Shim, J.K.; Lee, J.H.; Shin, H.J.; Huh, Y.M.; Kim, E.H.; Park, E.K.; et al. Increased in vivo angiogenic effect of glioma stromal mesenchymal stem-like cells on glioma cancer stem cells from patients with glioblastoma. Int. J. Oncol. 2013, 42, 1754–1762. [Google Scholar] [CrossRef]
- Garcia, C.; Dubois, L.G.; Xavier, A.L.; Geraldo, L.H.; da Fonseca, A.C.; Correia, A.H.; Meirelles, F.; Ventura, G.; Romão, L.; Canedo, N.H.; et al. The orthotopic xenotransplant of human glioblastoma successfully recapitulates glioblastoma-microenvironment interactions in a non-immunosuppressed mouse model. BMC Cancer 2014, 14, 923. [Google Scholar] [CrossRef] [PubMed]
- Georgiou, C.J.; Cai, Z.; Alsaden, N.; Cho, H.; Behboudi, M.; Winnik, M.A.; Rutka, J.T.; Reilly, R.M. Treatment of Orthotopic U251 Human Glioblastoma Multiforme Tumors in NRG Mice by Convection-Enhanced Delivery of Gold Nanoparticles Labeled with the β-Particle-Emitting Radionuclide, (177)Lu. Mol. Pharm. 2023, 20, 582–592. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.; Liu, J.; Zhang, C.; Shao, Y.; Li, X.; Yu, Z.; Huang, Y. Apatinib inhibits glioma cell malignancy in patient-derived orthotopic xenograft mouse model by targeting thrombospondin 1/myosin heavy chain 9 axis. Cell Death Dis. 2021, 12, 927. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Wang, P.; Zhao, J.; Fan, X.; Jiang, J.; Mu, X.; Wang, Y.; Yang, A.; Zhang, R.; Hu, S.; et al. Overexpression of miR-124 enhances the therapeutic benefit of TMZ treatment in the orthotopic GBM mice model by inhibition of DNA damage repair. Cell Death Dis. 2025, 16, 47. [Google Scholar] [CrossRef] [PubMed]
- Di, L.; Kerns, E.H.; Fan, K.; McConnell, O.J.; Carter, G.T. High throughput artificial membrane permeability assay for blood-brain barrier. Eur. J. Med. Chem. 2003, 38, 223–232. [Google Scholar] [CrossRef] [PubMed]
- Cho, C.F.; Wolfe, J.M.; Fadzen, C.M.; Calligaris, D.; Hornburg, K.; Chiocca, E.A.; Agar, N.Y.R.; Pentelute, B.L.; Lawler, S.E. Blood-brain-barrier spheroids as an in vitro screening platform for brain-penetrating agents. Nat. Commun. 2017, 8, 15623. [Google Scholar] [CrossRef] [PubMed]


| In Silico Model | Prediction Outcome | Quantitative Value/Category Rule |
|---|---|---|
| ADMETlab 3.0 | BBB+ (Category 1) | Probability = 0.022 |
| LogBB_Pred | BBB Permeable | LogBB value = −0.42844 (LogBB ≥ −1 for Permeable) |
| LightBBB | Permeable | Categorical prediction |
| BBB Predictor (Tree2C) | BBB permeant: Yes | Binary classification |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aung, H.M.; Wattanathamsan, O.; Sanookpan, K.; Hongprasit, A.; Muangnoi, C.; Phumsuay, R.; Rojpitikul, T.; Sritularak, B.; Bunlue, T.; Chantaravisoot, N.; et al. A Bibenzyl from Dendrobium pachyglossum Exhibits Potent Anti-Cancer Activity Against Glioblastoma Multiforme. Antioxidants 2025, 14, 1212. https://doi.org/10.3390/antiox14101212
Aung HM, Wattanathamsan O, Sanookpan K, Hongprasit A, Muangnoi C, Phumsuay R, Rojpitikul T, Sritularak B, Bunlue T, Chantaravisoot N, et al. A Bibenzyl from Dendrobium pachyglossum Exhibits Potent Anti-Cancer Activity Against Glioblastoma Multiforme. Antioxidants. 2025; 14(10):1212. https://doi.org/10.3390/antiox14101212
Chicago/Turabian StyleAung, Hnin Mon, Onsurang Wattanathamsan, Kittipong Sanookpan, Aphinan Hongprasit, Chawanphat Muangnoi, Rianthong Phumsuay, Thanawan Rojpitikul, Boonchoo Sritularak, Tankun Bunlue, Naphat Chantaravisoot, and et al. 2025. "A Bibenzyl from Dendrobium pachyglossum Exhibits Potent Anti-Cancer Activity Against Glioblastoma Multiforme" Antioxidants 14, no. 10: 1212. https://doi.org/10.3390/antiox14101212
APA StyleAung, H. M., Wattanathamsan, O., Sanookpan, K., Hongprasit, A., Muangnoi, C., Phumsuay, R., Rojpitikul, T., Sritularak, B., Bunlue, T., Chantaravisoot, N., Oliva, C. R., Griguer, C. E., & Buranasudja, V. (2025). A Bibenzyl from Dendrobium pachyglossum Exhibits Potent Anti-Cancer Activity Against Glioblastoma Multiforme. Antioxidants, 14(10), 1212. https://doi.org/10.3390/antiox14101212








