Red Pepper Powder Enhances Antioxidant and Immune Functions in the Sea Urchin Strongylocentrotus intermedius: Potential as a Functional Feed in Aquaculture
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Diets and Analysis
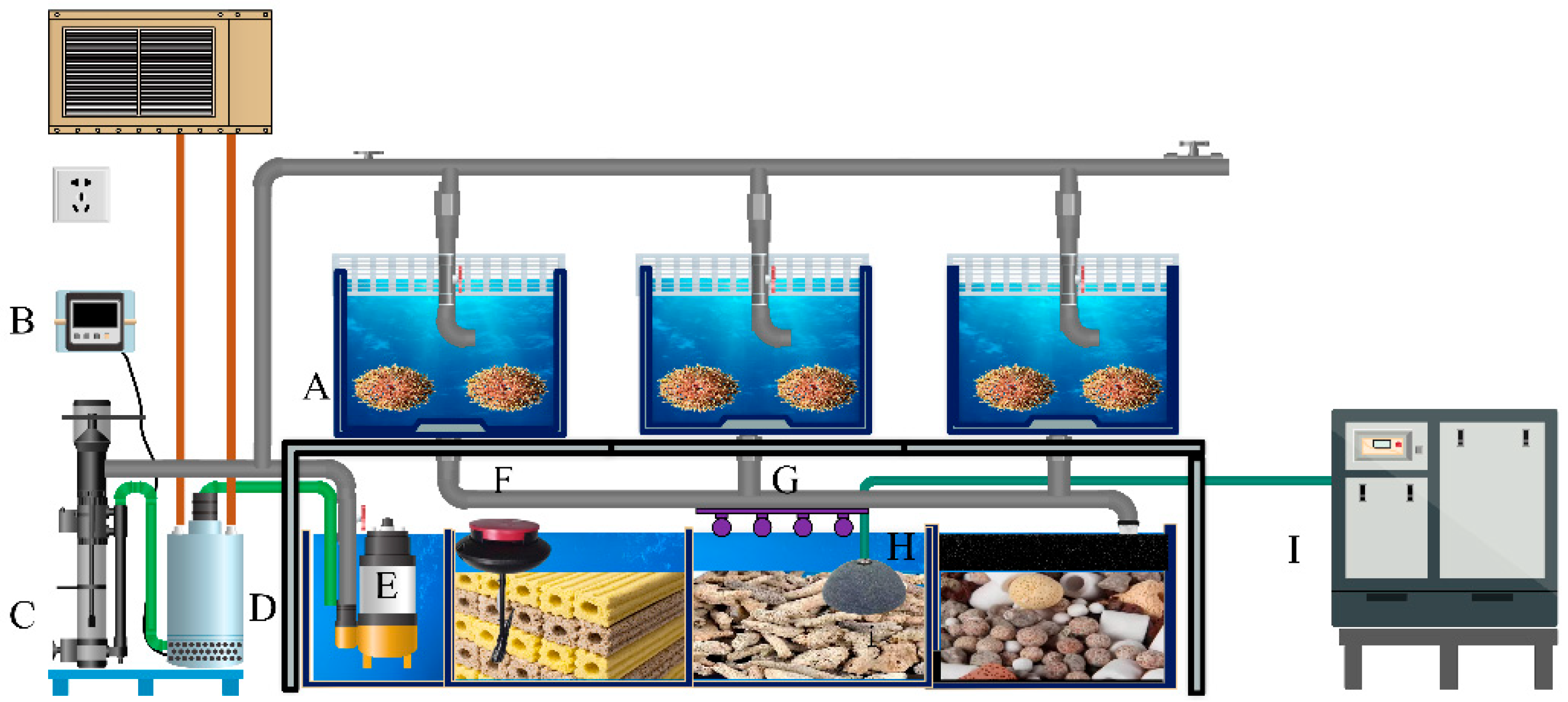
2.2. Experimental Design
2.3. Biological Sampling and Preservation
2.4. Evaluation of Growth Performance
2.5. Analysis of Gonad Proximate Composition and Color
2.6. Evaluation of Digestive Enzymes, Immune Capacity, and Antioxidant Responses
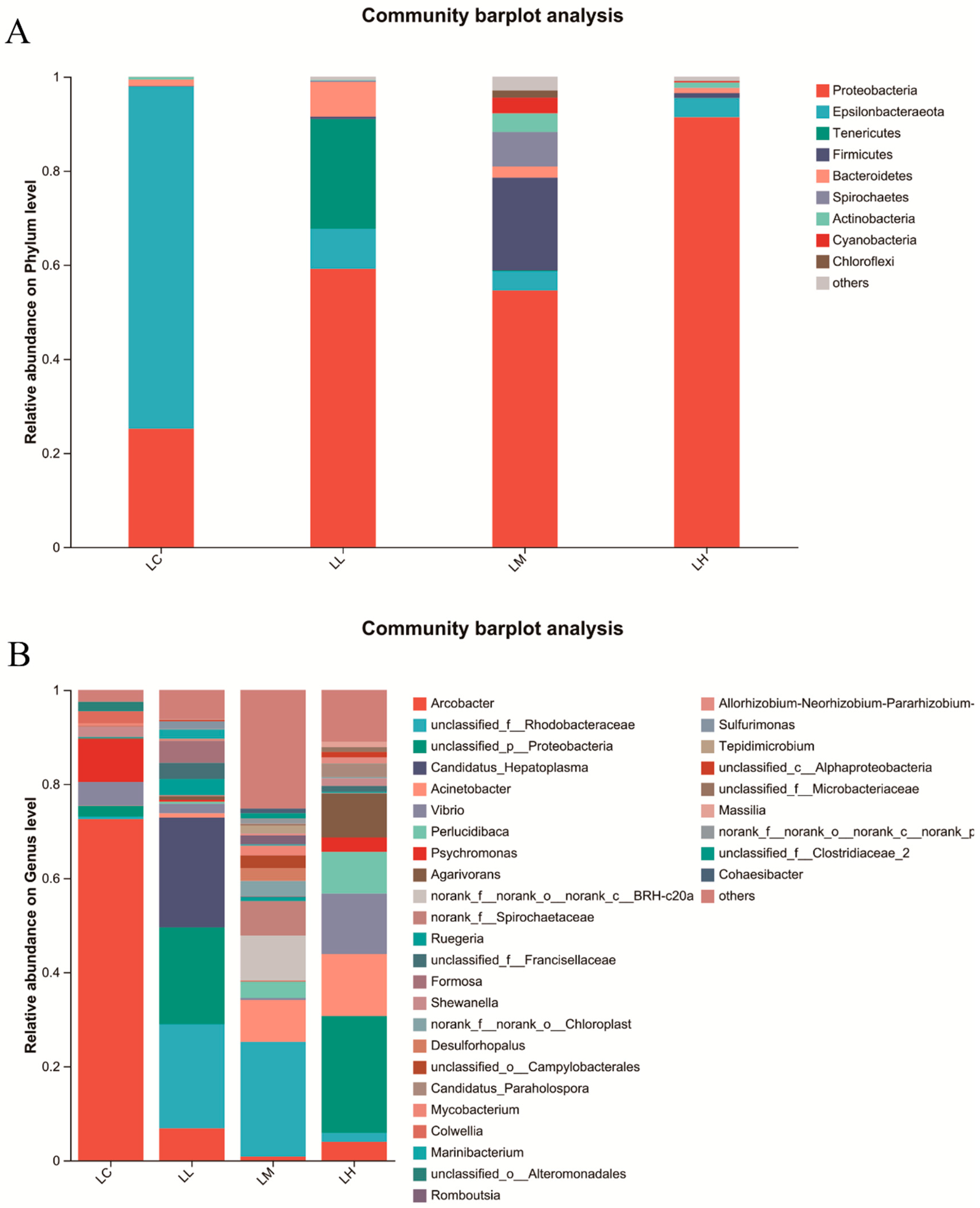
2.7. Microbial Community Diversity Assessment
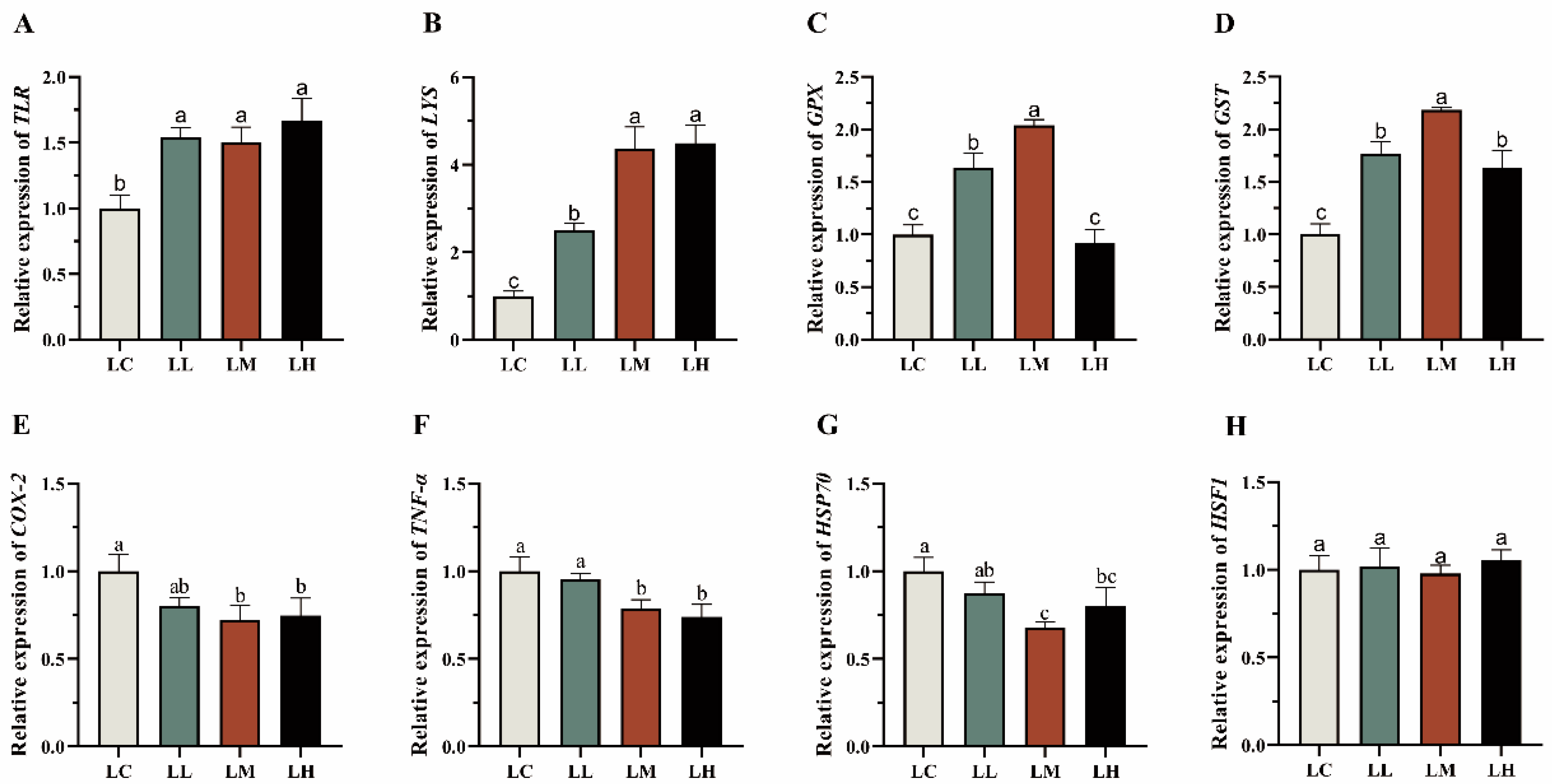
2.8. RNA Extraction and Quantitative Real-Time PCR
2.9. Statistical Analysis
3. Results
3.1. Growth Performance and Survival
3.2. Gonad Color and Proximate Composition
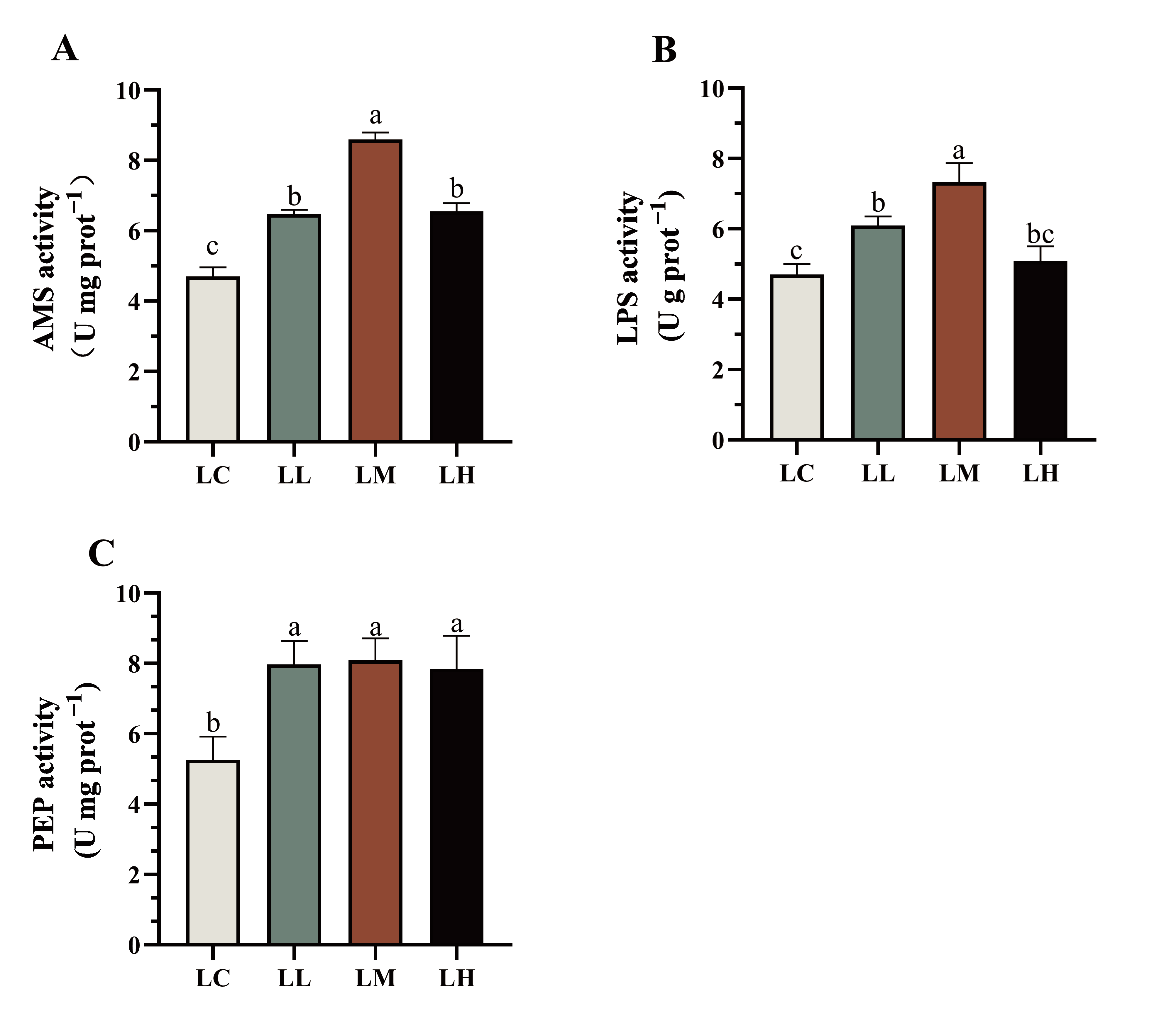
3.3. Digestive Enzyme Activities
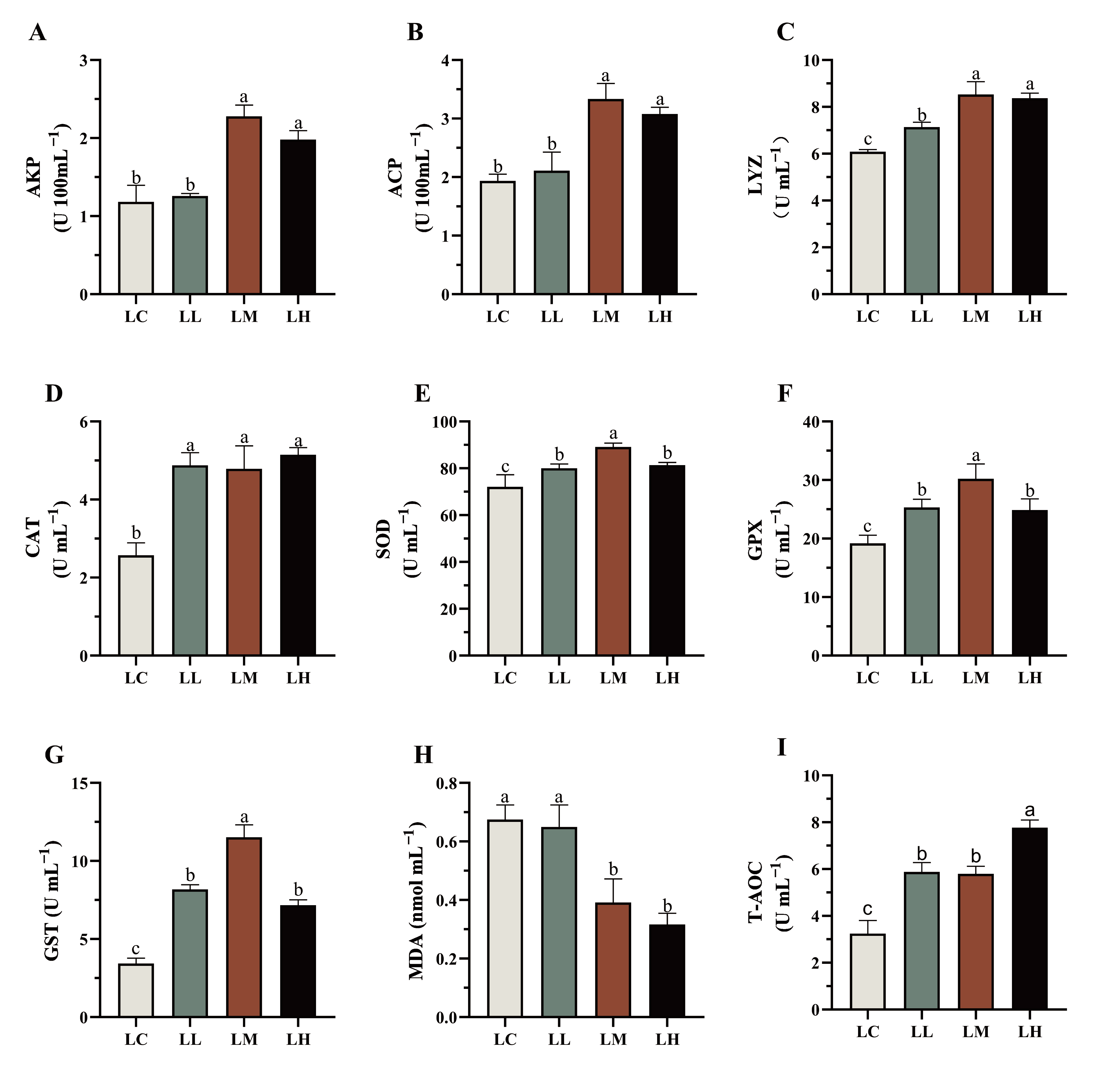
3.4. Immune and Antioxidation Related Parameters
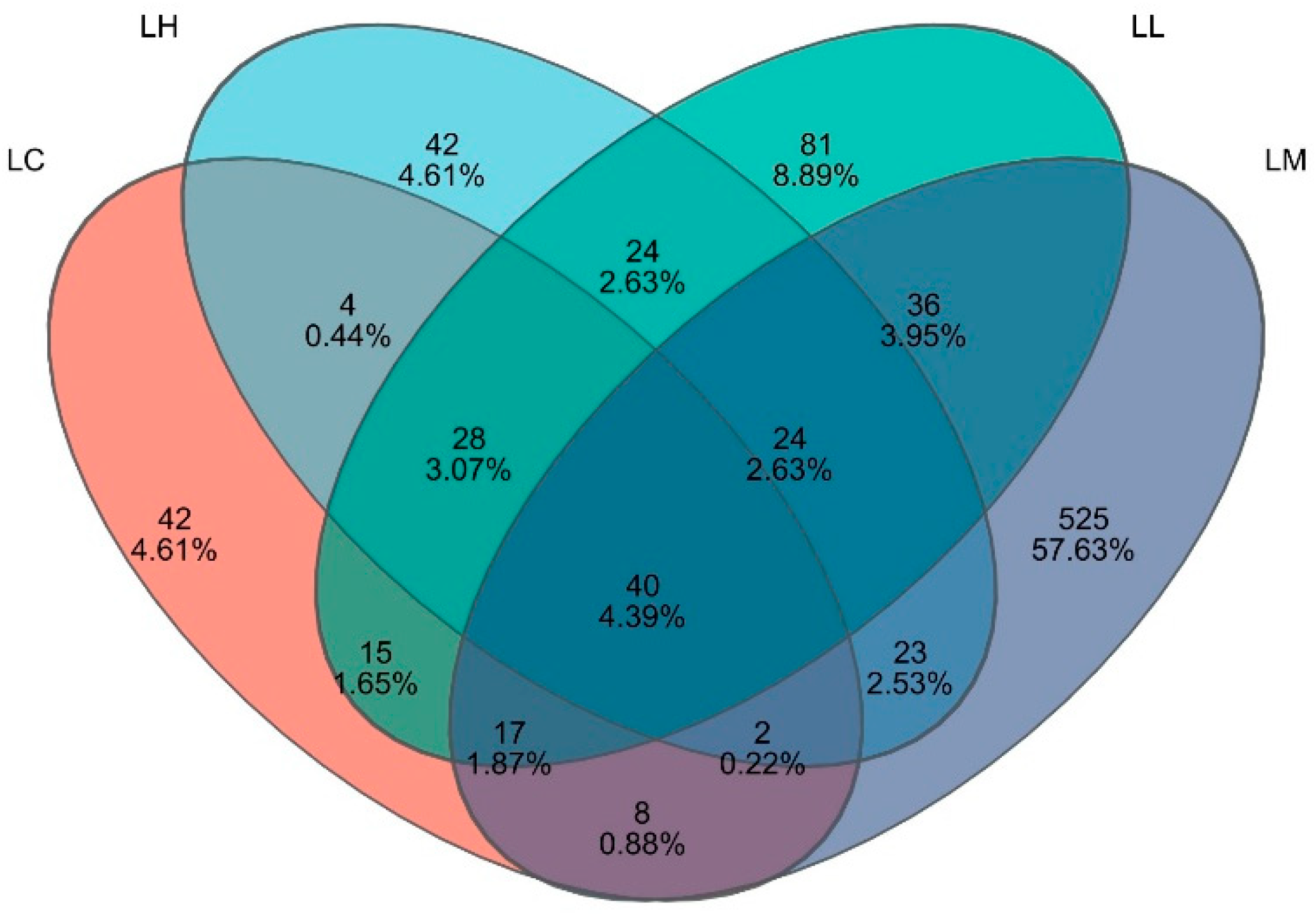
3.5. Intestinal Microflora
3.6. Physiological Response-Related Genes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, H.; Ding, J.; Ding, S.; Chang, Y. Metabolomic changes and polyunsaturated fatty acid biosynthesis during gonadal growth and development in the sea urchin Strongylocentrotus intermedius. Comp. Biochem. Physiol. Part D Genom. Proteom. 2019, 32, 100611. [Google Scholar] [CrossRef]
- Richardson, C.M.; Lawrence, J.M.; Watts, S.A. Factors leading to cannibalism in Lytechinus variegatus (Echinodermata: Echinoidia) held in intensive culture. J. Exp. Mar. Biol. Ecol. 2011, 399, 68–75. [Google Scholar] [CrossRef]
- Carrier, T.J.; Eddy, S.D.; Redmond, S. Solar-dried kelp as potential feed in sea urchin aquaculture. Aquac. Int. 2017, 25, 355–366. [Google Scholar] [CrossRef]
- Lushchak, V.I. Environmentally induced oxidative stress in aquatic animals. Aquat. Toxicol. 2011, 101, 13–30. [Google Scholar] [CrossRef] [PubMed]
- Khanna, P.; Ong, C.; Bay, B.H.; Baeg, G.H. Nanotoxicity: An Interplay of Oxidative Stress, Inflammation and Cell Death. Nanomaterials 2015, 5, 1163–1180. [Google Scholar] [CrossRef]
- Millar, B.C.; Rao, J.R.; Moore, J.E. Fighting antimicrobial resistance (AMR): Chinese herbal medicine as a source of novel antimicrobials—An update. Lett. Appl. Microbiol. 2021, 73, 400–407. [Google Scholar] [CrossRef]
- Parham, S.; Kharazi, A.Z.; Bakhsheshi-Rad, H.R.; Nur, H.; Ismail, A.F.; Sharif, S.; RamaKrishna, S.; Berto, F. Antioxidant, Antimicrobial and Antiviral Properties of Herbal Materials. Antioxidants 2020, 9, 1390. [Google Scholar] [CrossRef]
- Abdallah, A.; Zhang, P.; Zhong, Q.; Sun, Z. Application of Traditional Chinese Herbal Medicine By-products as Dietary Feed Supplements and Antibiotic Replacements in Animal Production. Curr. Drug Metab. 2019, 20, 54–64. [Google Scholar] [CrossRef] [PubMed]
- Safari, R.; Roosta, Z.; Vakili, F.; Rahmani, E.; Sakhawat Hossain, M.; Raeisi, M.; Van Doan, H.; Paolucci, M.; Hoseinifar, S.H. Dietary dragonhead effects on growth, immunity and antioxidant and related genes expression in zebrafish (Danio rerio). Aquac. Rep. 2022, 27, 101384. [Google Scholar] [CrossRef]
- Faheem, M.; Khaliq, S.; Abbas, R.Z.; Mansour, A.T. Moringa oleifera alleviated oxidative stress, physiological and molecular disruption induced by acute thermal stress in grass carp, Ctenopharyngodon idella. Fish Physiol. Biochem. 2022, 48, 1463–1473. [Google Scholar] [CrossRef]
- Ghafarifarsani, H.; Hoseinifar, S.H.; Javahery, S.; Yazici, M.; Van Doan, H. Growth performance, biochemical parameters, and digestive enzymes in common carp (Cyprinus carpio) fed experimental diets supplemented with vitamin C, thyme essential oil, and quercetin. Ital. J. Anim. Sci. 2022, 21, 291–302. [Google Scholar] [CrossRef]
- Kuebutornye, F.K.A.; Roy, K.; Folorunso, E.A.; Mraz, J. Plant-based feed additives in Cyprinus carpio aquaculture. Rev. Aquac. 2024, 16, 309–336. [Google Scholar] [CrossRef]
- Ibrahim, R.E.; Rhouma, N.R.; Elbealy, M.A.; Abdelwarith, A.A.; Younis, E.M.; Khalil, S.S.; Khamis, T.; Mansour, A.T.; Davies, S.J.; El-Murr, A.; et al. Effect of dietary intervention with Capsicum annuum extract on growth performance, physiological status, innate immune response, and related gene expression in Nile tilapia. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2024, 270, 110914. [Google Scholar] [CrossRef] [PubMed]
- Mohammad Salamatullah, A.; Hayat, K.; Mabood Husain, F.; Asif Ahmed, M.; Arzoo, S.; Musaad Althbiti, M.; Alzahrani, A.; Al-Zaied, B.A.M.; Kahlil Alyahya, H.; Albader, N.; et al. Effects of Different Solvents Extractions on Total Polyphenol Content, HPLC Analysis, Antioxidant Capacity, and Antimicrobial Properties of Peppers (Red, Yellow, and Green (Capsicum annum L.)). Evid. Based Complement. Altern. Med. 2022, 2022, 7372101. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Lee, W.M.; Rhee, H.C.; Kim, S. Red paprika (Capsicum annuum L.) and its main carotenoids, capsanthin and β-carotene, prevent hydrogen peroxide-induced inhibition of gap-junction intercellular communication. Chem. Biol. Interact. 2016, 254, 146–155. [Google Scholar] [CrossRef]
- Materska, M.; Perucka, I. Antioxidant Activity of the Main Phenolic Compounds Isolated from Hot Pepper Fruit (Capsicum annuum L.). J. Agric. Food Chem. 2005, 53, 1750–1756. [Google Scholar] [CrossRef]
- Taalab, H.A.; Mohammady, E.Y.; Hassan, T.M.M.; Abdella, M.M.; Hassaan, M.S. β-Carotene of Arthrospira platensis versus vitamin C and vitamin E as a feed supplement: Effects on growth, haemato-biochemical, immune-oxidative stress and related gene expression of Nile tilapia fingerlings. Aquac. Res. 2022, 53, 4832–4846. [Google Scholar] [CrossRef]
- Parrino, V.; Kesbiç, O.S.; Acar, Ü.; Fazio, F. Hot pepper (Capsicum sp.) oil and its effects on growth performance and blood parameters in rainbow trout (Oncorhynchus mykiss). Nat. Prod. Res. 2020, 34, 3226–3230. [Google Scholar] [CrossRef]
- Tepić Horecki, A.; Zekovic, Z.; Kravić, S.; Mandić, A. Pigment content and fatty acid composition of paprika oleoresins obtained by conventional and supercritical carbon dioxide extraction. Cienc. Tecnol. Aliment. 2009, 7, 95–102. [Google Scholar]
- Abd El-Hack, M.E.; El-Saadony, M.T.; Elbestawy, A.R.; Gado, A.R.; Nader, M.M.; Saad, A.M.; El-Tahan, A.M.; Taha, A.E.; Salem, H.M.; El-Tarabily, K.A. Hot red pepper powder as a safe alternative to antibiotics in organic poultry feed: An updated review. Poult. Sci. 2022, 101, 101684. [Google Scholar] [CrossRef]
- Mougin, J.; Lobanov, V.; Danion, M.; Roquigny, R.; Goardon, L.; Grard, T.; Morin, T.; Labbé, L.; Joyce, A. Effects of dietary co-exposure to fungal and herbal functional feed additives on immune parameters and microbial intestinal diversity in rainbow trout (Oncorhynchus mykiss). Fish Shellfish Immunol. 2023, 137, 108773. [Google Scholar] [CrossRef]
- Kop, A.; Durmaz, Y.; Hekimoglu, M. Effect of Natural Pigment Sources on Colouration of Cichlid (Cichlasoma severum sp. Heckel, 1840). J. Anim. Vet. Adv. 2012, 9, 566–569. [Google Scholar] [CrossRef]
- Parvari, S.; Ebrahimi-Mahmoudabad, S.R.; Kianfar, R. Performance, blood parameters, and immune response of Japanese quails fed turmeric and chili pepper powder. Anim. Prod. Res. 2022, 11, 39–53. [Google Scholar]
- Lourenço, S.; José, R.; Andrade, C.; Valente, L.M.P. Growth performance and gonad yield of sea urchin Paracentrotus lividus (Lamarck, 1816) fed with diets of increasing protein: Energy ratios. Anim. Feed. Sci. Technol. 2020, 270, 114690. [Google Scholar] [CrossRef]
- Ning, Y.; Zhang, F.; Tang, L.; Song, J.; Ding, J.; Chang, Y.; Zuo, R. Effects of dietary lipid sources on the growth, gonad development, nutritional and organoleptic quality, transcription of fatty acid synthesis related genes and antioxidant capacity during cold storage in adult sea urchin (Strongylocentrotus intermedius). Aquaculture 2022, 548, 737688. [Google Scholar] [CrossRef]
- Di, W.; Heqiu, Y.; Gou, D.; Gong, P.; Ding, J.; Chang, Y.; Zuo, R. Effects of Supplementary Kelp Feeding on the Growth, Gonad Yield, and Nutritional and Organoleptic Quality of Subadult Sea Urchin (Strongylocentrotus intermedius) with Soya Lecithin Intake History. Aquac. Nutr. 2023, 2023, 8894923. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis; Association of Official Analytical Chemists International: Arlington, VA, USA, 1990. [Google Scholar]
- Carneiro, M.D.D.; Medeiros, R.S.d.; Monserrat, J.M.; Rodrigues, R.V.; Sampaio, L.A. Growth and Oxidative Stress of Clownfish Amphiprion ocellaris Reared at Different Salinities. Fishes 2024, 9, 30. [Google Scholar] [CrossRef]
- Baião, L.F.; Rocha, F.; Costa, M.; Sá, T.; Oliveira, A.; Maia, M.R.G.; Fonseca, A.J.M.; Pintado, M.; Valente, L.M.P. Effect of protein and lipid levels in diets for adult sea urchin Paracentrotus lividus (Lamarck, 1816). Aquaculture 2019, 506, 127–138. [Google Scholar] [CrossRef]
- McBride, S.C.; Price, R.J.; Tom, P.D.; Lawrence, J.M.; Lawrence, A.L. Comparison of gonad quality factors: Color, hardness and resilience, of Strongylocentrotus franciscanus between sea urchins fed prepared feed or algal diets and sea urchins harvested from the Northern California fishery. Aquaculture 2004, 233, 405–422. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, R.; Chen, Y.; Guo, Z.; Meng, X.; Han, Y.; Zhao, X.; Ren, T. Growth performance, intestinal health, and non-specific immunity were significantly affected by feeding different compound lactic acid bacteria supplementation in sea urchin (Strongylocentrotus intermedius). Front. Mar. Sci. 2025, 12, 1525330. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Li, M.; Tang, L.; Heqiu, Y.; Lv, D.; Ding, J.; Chang, Y.; Zuo, R. Effects of Oxidized Fish Oil on the Growth, Immune and Antioxidant Capacity, Inflammation-Related Gene Expression, and Intestinal Microbiota Composition of Juvenile Sea Urchin (Strongylocentrotus intermedius). Aquac. Nutr. 2022, 2022, 2340308. [Google Scholar] [CrossRef]
- Dang, H.; Zhang, T.; Yi, F.; Ye, S.; Liu, J.; Li, Q.; Li, H.; Li, R. Enhancing the immune response in the sea cucumber Apostichopus japonicus by addition of Chinese herbs Houttuynia cordata Thunb as a food supplement. Aquac. Fish. 2019, 4, 114–121. [Google Scholar] [CrossRef]
- Van Hai, N. The use of medicinal plants as immunostimulants in aquaculture: A review. Aquaculture 2015, 446, 88–96. [Google Scholar] [CrossRef]
- Zhang, Y.; Bian, Y.; Cui, Q.; Yuan, C.; Lin, Y.; Li, J.; Meng, P. Effect of dietary complex Chinese herbal medicine on growth performance, digestive enzyme activities in tissues and expression of genes involved in the digestive enzymes and antioxidant enzymes and bacterial challenge in Litopenaeus vannamei. Aquac. Res. 2021, 52, 6741–6750. [Google Scholar] [CrossRef]
- Wang, N.; Zhou, X.; Zhang, T.; Jian, W.; Sun, Z.; Qi, P.; Feng, Y.; Liu, H.; Liu, L.; Yang, S. Capsaicin from chili peppers and its analogues and their valued applications: An updated literature review. Food Res. Int. 2025, 208, 116034. [Google Scholar] [CrossRef]
- Diler, I.; Hoşsu, B.; Dilek, K.; Emre, Y.; Sevgili, H. Effects of natural and synthetic pigments in diets on flesh coloration and growth of rainbow trout (Oncorhynchus mykiss W.). Isr. J. Aquac. Bamidgeh 2005, 57, 175–184. [Google Scholar] [CrossRef]
- Ergün, S.; Yilmaz, S.; Soytas, N. Enhancement of growth performance and pigmentation in red Oreochromis mossambicus associated with dietary intake of astaxanthin, paprika, or capsicum. Isr. J. Aquac. Bamidgeh 2013, 65. [Google Scholar] [CrossRef]
- Maniat, M.; Ghotbeddin, N.; Ghatrami, E.; Mohammadiazarm, H. Effect of Different Levels of Paprika on Some Growth Factors, Survival and Biochemical Body Composition of Benni fish (Mesopotamichthys Sharpeyi). Acad. J. Sci. 2014, 3, 223–229. [Google Scholar]
- Yigit, N.O.; Ozdal, A.M.; Bahadir Koca, S.; Ozmen, O. Effect on pigmentation, growth and liver histopathology of red pepper (Capsicum annuum) added in different ratios to jewel cichlid (Hemichromis guttatus) diet. Aquac. Res. 2021, 52, 5579–5584. [Google Scholar] [CrossRef]
- Khieokhajonkhet, A.; Suwannalers, P.; Aeksiri, N.; Ratanasut, K.; Chitmanat, C.; Inyawilert, W.; Phromkunthong, W.; Kaneko, G. Effects of dietary red pepper extracts on growth, hematology, pigmentation, disease resistance, and growth- and immune-related gene expressions of goldfish (Carassius auratus). Anim. Feed Sci. Technol. 2023, 301, 115658. [Google Scholar] [CrossRef]
- Lee, C.-R.; Pham, M.A.; Lee, S.-M. Effects of Dietary Paprika and Lipid Levels on Growth and Skin Pigmentation of Pale Chub (Zacco platypus). Asian Australas. J. Anim. Sci. 2010, 23, 724–732. [Google Scholar] [CrossRef]
- Li, Z.; Bao, N.; Ren, T.; Han, Y.; Jiang, Z.; Bai, Z.; Hu, Y.; Ding, J. The effect of a multi-strain probiotic on growth performance, non-specific immune response, and intestinal health of juvenile turbot, Scophthalmus maximus L. Fish Physiol. Biochem. 2019, 45, 1393–1407. [Google Scholar] [CrossRef]
- Wang, Y.-C.; Hu, S.-Y.; Chiu, C.-S.; Liu, C.-H. Multiple-strain probiotics appear to be more effective in improving the growth performance and health status of white shrimp, Litopenaeus vannamei, than single probiotic strains. Fish Shellfish Immunol. 2019, 84, 1050–1058. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Zhou, W.; Wang, H.; Li, C.; Wang, L.; Li, Y.; Wang, J. Bacillus baekryungensis MS1 regulates the growth, non-specific immune parameters and gut microbiota of the sea cucumber Apostichopus japonicus. Fish Shellfish Immunol. 2020, 102, 133–139. [Google Scholar] [CrossRef]
- Chen, Y.; Cui, S.; Wu, L.; Han, Y.; Zhao, X.; Ren, T. Dietary silicate minerals relieving cadmium or lead poisoning in juvenile sea cucumber, Apostichopus japonicus. Mar. Environ. Res. 2024, 202, 106795. [Google Scholar] [CrossRef]
- Patricia, J.J.; Dhamoon, A.S. Physiology, Digestion. In StatPearls; StatPearls Publishing Copyright © 2025; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2025. [Google Scholar]
- Tazikeh, T.; Abedian Kenari, A.; Esmaeili, N. Effects of fish meal replacement by meat and bone meal supplemented with garlic (Allium sativum) powder on biological indices, feeding, muscle composition, fatty acid and amino acid profiles of whiteleg shrimp (Litopenaeus vannamei). Aquac. Res. 2020, 51, 674–686. [Google Scholar] [CrossRef]
- Xu, A.; Shang-Guan, J.; Li, Z.; Gao, Z.; Huang, Y.; Chen, Q. Effects of garlic powder on feeding attraction activity, growth and digestive enzyme activities of Japanese seabass, Lateolabrax japonicus. Aquac. Nutr. 2020, 26, 390–399. [Google Scholar] [CrossRef]
- Sun, Y.; Fu, Z.; Ma, Z. The Effects of Acute Ammonia Nitrogen Stress on Antioxidant Ability, Phosphatases, and Related Gene Expression in the Kidney of Juvenile Yellowfin Tuna (Thunnus albacares). J. Mar. Sci. Eng. 2024, 12, 1009. [Google Scholar] [CrossRef]
- Chen, S.J.; Guo, Y.C.; Espe, M.; Yang, F.; Fang, W.P.; Wan, M.G.; Niu, J.; Liu, Y.J.; Tian, L.X. Growth performance, haematological parameters, antioxidant status and salinity stress tolerance of juvenile Pacific white shrimp (Litopenaeus vannamei) fed different levels of dietary myo-inositol. Aquac. Nutr. 2018, 24, 1527–1539. [Google Scholar] [CrossRef]
- AL-Megrin, W.A.; Alkhuriji, A.F.; Yousef, A.O.S.; Metwally, D.M.; Habotta, O.A.; Kassab, R.B.; Abdel Moneim, A.E.; El-Khadragy, M.F. Antagonistic Efficacy of Luteolin against Lead Acetate Exposure-Associated with Hepatotoxicity is Mediated via Antioxidant, Anti-Inflammatory, and Anti-Apoptotic Activities. Antioxidants 2020, 9, 10. [Google Scholar] [CrossRef]
- Wang, C.-Y.; Li, Z.-B.; Sun, Y.-Z.; Chen, Q.; Li, W.-J.; Huang, Y.-C.; Lu, J. Effects of Chinese herbal medicines mixture on growth performance digestive enzyme activity immune response of juvenile Japanese seabass, Lateolabrax japonicus. Aquac. Nutr. 2018, 24, 683–693. [Google Scholar] [CrossRef]
- Pan, Y.; Tian, L.; Zhao, Q.; Tao, Z.; Yang, J.; Zhou, Y.; Cao, R.; Zhang, G.; Wu, W. Evaluation of the acute toxic effects of crude oil on intertidal mudskipper (Boleophthalmus pectinirostris) based on antioxidant enzyme activity and the integrated biomarker response. Environ. Pollut. 2022, 292, 118341. [Google Scholar] [CrossRef]
- Wang, F.; Xue, Y.; Fu, L.; Wang, Y.; He, M.; Zhao, L.; Liao, X. Extraction, purification, bioactivity and pharmacological effects of capsaicin: A review. Crit. Rev. Food Sci. Nutr. 2022, 62, 5322–5348. [Google Scholar] [CrossRef]
- Zhang, M.; Yang, X.; Wang, C.; Shang, B.; Zhao, F.; Xu, H.; Xu, Q. Effects of Myo-Inositol on the Growth Performance, Digestive Enzyme Activity, and Antioxidation of Juvenile Hucho taimen. Fishes 2023, 8, 567. [Google Scholar] [CrossRef]
- Fernández-Boo, S.; Pedrosa-Oliveira, M.H.; Afonso, A.; Arenas, F.; Rocha, F.; Valente, L.M.P.; Costas, B. Annual assessment of the sea urchin (Paracentrotus lividus) humoral innate immune status: Tales from the north Portuguese coast. Mar. Environ. Res. 2018, 141, 128–137. [Google Scholar] [CrossRef] [PubMed]
- Molayemraftar, T.; Peyghan, R.; Razi Jalali, M.; Shahriari, A. Single and combined effects of ammonia and nitrite on common carp, Cyprinus carpio: Toxicity, hematological parameters, antioxidant defenses, acetylcholinesterase, and acid phosphatase activities. Aquaculture 2022, 548, 737676. [Google Scholar] [CrossRef]
- Wang, J.; Li, B.; Wang, Y.; Liao, M.; Rong, X.; Zhang, Z. Influences of immersion bathing in Bacillus velezensis DY-6 on growth performance, non-specific immune enzyme activities and gut microbiota of Apostichopus japonicus. J. Oceanol. Limnol. 2019, 37, 1449–1459. [Google Scholar] [CrossRef]
- Zhang, Z.-L.; Cao, Y.-L.; Xu, J.-R.; Zhang, X.-X.; Li, J.-J.; Li, J.-T.; Zheng, P.-H.; Xian, J.-A.; Lu, Y.-P. Effects of dietary chitosan oligosaccharide on the growth, intestinal microbiota and immunity of juvenile red claw crayfish (Cherax quadricarinatus). Fish Shellfish Immunol. 2024, 145, 109288. [Google Scholar] [CrossRef]
- Srichaiyo, N.; Tongsiri, S.; Hoseinifar, S.H.; Dawood, M.A.O.; Jaturasitha, S.; Esteban, M.Á.; Ringø, E.; Van Doan, H. The effects gotu kola (Centella asiatica) powder on growth performance, skin mucus, and serum immunity of Nile tilapia (Oreochromis niloticus) fingerlings. Aquac. Rep. 2020, 16, 100239. [Google Scholar] [CrossRef]
- Chen, X.; Yi, H.; Liu, S.; Zhang, Y.; Su, Y.; Liu, X.; Bi, S.; Lai, H.; Zeng, Z.; Li, G. Promotion of pellet-feed feeding in mandarin fish (Siniperca chuatsi) by Bdellovibrio bacteriovorus is influenced by immune and intestinal flora. Aquaculture 2021, 542, 736864. [Google Scholar] [CrossRef]
- Mehdinejad, N.; Imanpour, M.R.; Jafari, V. Combined or Individual Effects of Dietary Probiotic Pedicoccus acidilactici and Nucleotide on Growth Performance, Intestinal Microbiota, Hemato-biochemical Parameters, and Innate Immune Response in Goldfish (Carassius auratus). Probiotics Antimicrob. Proteins 2018, 10, 558–565. [Google Scholar] [CrossRef] [PubMed]
- Zan, Z.; Chen, K.; Wang, H.; Han, Z.; Sun, J. Effects of a multistrain probiotic on the growth, immune function and intestinal microbiota of the tongue sole Cynoglossus semilaevis. Aquaculture 2023, 575, 739813. [Google Scholar] [CrossRef]
- Butt, R.L.; Volkoff, H. Gut Microbiota and Energy Homeostasis in Fish. Front. Endocrinol. 2019, 10, 9. [Google Scholar] [CrossRef] [PubMed]
- Rosca, A.E.; Iesanu, M.I.; Zahiu, C.D.M.; Voiculescu, S.E.; Paslaru, A.C.; Zagrean, A.-M. Capsaicin and Gut Microbiota in Health and Disease. Molecules 2020, 25, 5681. [Google Scholar] [CrossRef]
- Rodríguez-Daza, M.C.; Pulido-Mateos, E.C.; Lupien-Meilleur, J.; Guyonnet, D.; Desjardins, Y.; Roy, D. Polyphenol-Mediated Gut Microbiota Modulation: Toward Prebiotics and Further. Front. Nutr. 2021, 8, 689456. [Google Scholar] [CrossRef]
- Chen, X.; Mo, L.; Zhang, L.; Huang, L.; Gao, Z.; Peng, J.; Yu, Z.; Zhang, X. Taxonomic Diversity, Predicted Metabolic Pathway, and Interaction Pattern of Bacterial Community in Sea Urchin Anthocidaris crassispina. Microorganisms 2024, 12, 2094. [Google Scholar] [CrossRef]
- Liu, L.; Wei, C.; Li, Y.; Wang, M.; Mao, Y.; Tian, X. A Comparative Study on Effects of Three Butyric Acid-Producing Additives on the Growth Performance, Non-specific Immunity, and Intestinal Microbiota of the Sea Cucumber Apostichopus japonicus. Aquac. Nutr. 2024, 2024, 6973951. [Google Scholar] [CrossRef]
- Luo, K.; Liu, Y.; Qin, G.; Wang, S.; Wei, C.; Pan, M.; Guo, Z.; Liu, Q.; Tian, X. A comparative study on effects of dietary three strains of lactic acid bacteria on the growth performance, immune responses, disease resistance and intestinal microbiota of Pacific white shrimp, Penaeus vannamei. Fish Shellfish Immunol. 2023, 136, 108707. [Google Scholar] [CrossRef]
- Zeng, F.; Wang, L.; Zhen, H.; Guo, C.; Liu, A.; Xia, X.; Pei, H.; Dong, C.; Ding, J. Nanoplastics affect the growth of sea urchins (Strongylocentrotus intermedius) and damage gut health. Sci. Total Environ. 2023, 869, 161576. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Kim, Y.-H.; Min, J. The potential of Rhodobacter sphaeroides extract as an alternative supplement for cell culture systems. Microbiol. Spectr. 2024, 12, e02456-23. [Google Scholar] [CrossRef]
- Kim, M.J.; Kim, J.G.; Sydara, K.M.; Lee, S.W.; Jung, S.K. Croton hirtus L’Hér Extract Prevents Inflammation in RAW264.7 Macrophages via Inhibition of NF-κB Signaling Pathway. J. Microbiol. Biotechnol. 2020, 30, 490–496. [Google Scholar] [CrossRef]
- Magnadóttir, B. Innate immunity of fish (overview). Fish Shellfish Immunol. 2006, 20, 137–151. [Google Scholar] [CrossRef]
- Guo, J.; Zhang, Y.; Chen, Y.; Zhang, R.; Han, Y.; Zhao, X.; Ren, T. Effects of Xiaochengqitang formulae on growth, immune function, intestinal microbiota, and resistance to Vibrio splendidus in Apostichopus japonicus. Aquac. Int. 2025, 33, 417. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, Y.; Guo, Z.; Zhang, R.; Guo, J.; Wang, F.; Han, Y.; Zhao, X.; Ren, T. Effects of a dietary probiotic on growth, gonadal development and quality, antioxidant capacity, intestinal health, and non-specific immunity of a sea urchin (Strongylocentrotus intermedius). Anim. Feed Sci. Technol. 2025, 319, 116184. [Google Scholar] [CrossRef]
- Ren, Q.; Sun, R.-R.; Zhao, X.-F.; Wang, J.-X. A selenium-dependent glutathione peroxidase (Se-GPx) and two glutathione S-transferases (GSTs) from Chinese shrimp (Fenneropenaeus chinensis). Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2009, 149, 613–623. [Google Scholar] [CrossRef] [PubMed]
- Ko, E.-Y.; Cho, S.-H.; Kwon, S.-H.; Eom, C.-Y.; Jeong, M.S.; Lee, W.; Kim, S.-Y.; Heo, S.-J.; Ahn, G.; Lee, K.P.; et al. The roles of NF-κB and ROS in regulation of pro-inflammatory mediators of inflammation induction in LPS-stimulated zebrafish embryos. Fish Shellfish Immunol. 2017, 68, 525–529. [Google Scholar] [CrossRef] [PubMed]
- Zou, J.; Secombes, C.J. The Function of Fish Cytokines. Biology 2016, 5, 23. [Google Scholar] [CrossRef] [PubMed]
- Kodagoda, Y.K.; Hanchapola, H.A.C.R.; Rodrigo, D.C.G.; Lim, C.; Liyanage, D.S.; Omeka, W.K.M.; Ganepola, G.A.N.P.; Dilshan, M.A.H.; Kim, J.; Lee, J.H.; et al. Expression profiling and functional role of cyclooxygenase-2 in the immune and inflammatory responses of red-spotted grouper (Epinephelus akaara). Fish Shellfish Immunol. 2025, 158, 110158. [Google Scholar] [CrossRef]
- Wang, T.; Yan, J.; Xu, W.; Ai, Q.; Mai, K. Characterization of Cyclooxygenase-2 and its induction pathways in response to high lipid diet-induced inflammation in Larmichthys crocea. Sci. Rep. 2016, 6, 19921. [Google Scholar] [CrossRef]
- Hwang, J.H.; Kim, K.J.; Ryu, S.J.; Lee, B.Y. Caffeine prevents LPS-induced inflammatory responses in RAW264.7 cells and zebrafish. Chem. Biol. Interact. 2016, 248, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Ryu, S.-J.; Choi, H.-S.; Yoon, K.-Y.; Lee, O.-H.; Kim, K.-J.; Lee, B.-Y. Oleuropein Suppresses LPS-Induced Inflammatory Responses in RAW 264.7 Cell and Zebrafish. J. Agric. Food Chem. 2015, 63, 2098–2105. [Google Scholar] [CrossRef]
- Zuo, R.; Hou, S.; Wu, F.; Song, J.; Zhang, W.; Zhao, C.; Chang, Y. Higher dietary protein increases growth performance, anti-oxidative enzymes activity and transcription of heat shock protein 70 in the juvenile sea urchin (Strongylocentrotus intermedius) under a heat stress. Aquac. Fish. 2017, 2, 18–23. [Google Scholar] [CrossRef]
- Sung, Y.Y.; Rahman, N.A.; Shazili, N.A.M.; Chen, S.; Lv, A.; Sun, J.; Shi, H.; MacRae, T.H. Non-lethal heat shock induces Hsp70 synthesis and promotes tolerance against heat, ammonia and metals in post-larvae of the white leg shrimp Penaeus vannamei (Boone, 1931). Aquaculture 2018, 483, 21–26. [Google Scholar] [CrossRef]
- Wu, T.; Sheng, Y.; Tian, Y.; Wang, C. Vitexin Regulates Heat Shock Protein Expression by Modulating ROS Levels Thereby Protecting against Heat-Stress-Induced Apoptosis. Molecules 2023, 28, 7639. [Google Scholar] [CrossRef]
- Keller, A. A Review on Heat Shock Proteins (HSP70 and HSP90) in Protein Folding and Stress Response. Int. J. Mol. Biol. Biochem. 2025, 7, 57–62. [Google Scholar] [CrossRef]
- Xu, D.; Sun, L.; Liu, S.; Zhang, L.; Yang, H. Molecular cloning of hsf1 and hsbp1 cDNAs, and the expression of hsf1, hsbp1 and hsp70 under heat stress in the sea cucumber Apostichopus japonicus. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2016, 198, 1–9. [Google Scholar] [CrossRef] [PubMed]
| Ingredient | Test Diets | |||
|---|---|---|---|---|
| LC | LL | LM | LH | |
| Fish meal 1 | 40 | 40 | 40 | 40 |
| Soybean meal 2 | 130 | 130 | 130 | 130 |
| Kelp meal 3 | 415 | 415 | 415 | 415 |
| Wheat meal 4 | 300 | 300 | 300 | 300 |
| Wheat gluten 5 | 50 | 50 | 50 | 50 |
| red pepper 6 | 0 | 5 | 10 | 20 |
| Soybean oil | 10 | 10 | 10 | 10 |
| Vitamin mixture 7 | 10 | 10 | 10 | 10 |
| Mineral mixture 8 | 10 | 10 | 10 | 10 |
| Monocalcium phosphate | 10 | 10 | 10 | 10 |
| Calcium propionate | 1.8 | 1.8 | 1.8 | 1.8 |
| Choline chloride | 1.2 | 1.2 | 1.2 | 1.2 |
| Microcrystalline cellulose | 22 | 17 | 12 | 2 |
| Total | 1000 | 1000 | 1000 | 1000 |
| Analyzed nutrients (% on a dry basis) | ||||
| Moisture | 9.35 | 9.33 | 9.37 | 9.35 |
| Crude lipid | 3.05 | 3.09 | 3.03 | 3.06 |
| Crude ash | 17.44 | 17.39 | 17.40 | 17.33 |
| Crude protein | 22.48 | 22.45 | 22.51 | 20.44 |
| Gross energy (kJ·g−1) | 16.12 | 16.13 | 16.17 | 16.16 |
| Genes | Primer Name | Primer Sequence (5′−3′) | Efficiency | R2 | Source or GeneBank |
|---|---|---|---|---|---|
| 18s | 18s-F | GTTCGAAGGCGATCAGATAC | 102.23 ± 4.84 | 0.997 | D14365.1 |
| 18s-R | CTGTCAATCCTCACTGTGTC | ||||
| TLR | TLR-F | GAGACGGTACAGGGCTACA | 103.2 ± 1.86 | 0.999 | [34] |
| TLR-R | CGGGCAAAATCCTCACAAG | ||||
| LYZ | LYZ-F | GAGACGGTACAGGGCTACA | 105.85 ± 2.84 | 0.997 | [34] |
| LYZ-R | CGGGCAAAATCCTCACAAG | ||||
| GPX | GPX-F | CGAGTTTGAGAAGCGTGGTG | 103.71 ± 2.48 | 0.996 | [34] |
| GPX-R | GGATCAGCTATGATTGGGTATGG | ||||
| GST | GST-F | CTCGGAGATTCGCTCACCA | 97.15 ± 1.75 | 0.997 | [34] |
| GST-R | GCTGGCTGGAGAAATGAACAA | ||||
| COX-2 | COX-2-F | GAGGTGGATAACCGATTGA | 105.83 ± 3.26 | 0.997 | MH516324 |
| COX-2-R | GAGGTGGATAACCGATTGA | ||||
| TNF-α | TNF-a-F | GCTGTAACGGCGTTCGTCTCC | 95.07 ± 4.46 | 0.998 | MH516331 |
| TNF-a-R | TGGTGTACTTGTGCTGGTTGTTGG | ||||
| HSP70 | HSP70-F | ACACTCATCTCGGAGGAG | 105.4 ± 5.04 | 0.99 | KF860046.1 |
| HSP70-R | ACACTCATCTCGGAGGAG | ||||
| HSF1 | HSF1-F | GGTCTGGAGGATGCGGGAGTAG | 105.88 ± 2.87 | 0.997 | 591088 |
| HSF1-R | TTGGTGGGTGAATCGCTTGTGAG |
| Growth Parameters | LC | LL | LM | LH |
|---|---|---|---|---|
| IBW (g) 1 | 24.32 ± 0.14 a | 23.97 ± 0.2 a | 24.27 ± 0.32 a | 23.34 ± 0.1 a |
| FBW (g) 2 | 34.35 ± 0.26 c | 35.86 ± 0.27 b | 38.12 ± 0.52 a | 36.44 ± 0.5 b |
| WGR (%) 3 | 41.28 ± 1.74 c | 49.59 ± 0.22 b | 57.1 ± 3.4 a | 49.69 ± 2.6 b |
| SGR (% day−1) 4 | 0.69 ± 0.02 c | 0.81 ± 0.00 b | 0.9 ± 0.04 a | 0.81 ± 0.03 b |
| DTW (g) 5 | 1.44 ± 0.06 b | 1.47 ± 0.15 ab | 1.61 ± 0.08 a | 1.45 ± 0.05 b |
| DTI (%) 6 | 4.2 ± 0.15 a | 4.09 ± 0.06 a | 4.22 ± 0.24 a | 3.97 ± 0.16 a |
| GWW (g) 7 | 7.64 ± 0.19 c | 8.53 ± 0.12 b | 9.3 ± 0.11 a | 8.68 ± 0.13 b |
| GSI (%) 8 | 22.24 ± 0.4 b | 23.8 ± 0.24 a | 24.39 ± 0.41 a | 23.82 ± 0.34 a |
| FCR 9 | 2.05 ± 0.07 a | 1.81 ± 0.01 b | 1.69 ± 0.08 b | 1.82 ± 0.08 b |
| SR (%) 10 | 100 ± 0.00 a | 100 ± 0.00 a | 100 ± 0.00 a | 100 ± 0.00 a |
| Parameter | Test Diets | |||
|---|---|---|---|---|
| LC | LL | LM | LH | |
| Moisture (%) | 78.09 ± 0.78 a | 77.37 ± 0.14 a | 77.14 ± 0.17 a | 77.09 ± 0.26 a |
| Crude protein (%) | 10.49 ± 0.12 c | 11.19 ± 0.18 b | 11.78 ± 0.13 a | 11.76 ± 0.1 a |
| Crude lipid (%) | 7.84 ± 0.2 b | 8.17 ± 0.23 ab | 8.53 ± 0.14 a | 8.57 ± 0.06 a |
| Crude ash (%) | 2.35 ± 0.02 a | 2.33 ± 0.06 a | 2.36 ± 0.04 a | 2.33 ± 0.03 a |
| Parameter | Test Diets | |||
|---|---|---|---|---|
| LC | LL | LM | LH | |
| L* 1 | 67.53 ± 0.17 a | 66.96 ± 0.73 a | 66.85 ± 0.49 a | 67.03 ± 0.49 a |
| a* 2 | 13.19 ± 0.31 c | 16.07 ± 0.32 b | 17.3 ± 0.58 ab | 18.36 ± 0.94 a |
| b* 3 | 30.22 ± 0.69 c | 32.45 ± 0.21 b | 35.83 ± 0.6 a | 36.3 ± 0.44 a |
| ΔE1 4 | 33.96 ± 0.73 a | 30.73 ± 0.21 b | 27.18 ± 0.32 c | 26.31 ± 0.45 c |
| ΔE2 5 | 39.72 ± 0.73 a | 36.75 ± 0.13 b | 33.27 ± 0.42 c | 32.45 ± 0.36 c |
| Parameter | Test Diets | |||
|---|---|---|---|---|
| LC | LL | LM | LH | |
| Shannon | 1.43 | 2.95 | 4.11 | 3.13 |
| Chao1 | 171.5 | 283 | 698.85 | 188.36 |
| Ace | 180.99 | 289.98 | 696.27 | 189.2 |
| Simpson | 0.53 | 0.12 | 0.05 | 0.1 |
| Coverage | 0.99 | 0.99 | 0.99 | 0.99 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, J.; Zhang, Y.; Chen, Y.; Zhang, Y.; Zhang, R.; Han, Y.; Zhao, X.; Ren, T. Red Pepper Powder Enhances Antioxidant and Immune Functions in the Sea Urchin Strongylocentrotus intermedius: Potential as a Functional Feed in Aquaculture. Antioxidants 2025, 14, 1173. https://doi.org/10.3390/antiox14101173
Guo J, Zhang Y, Chen Y, Zhang Y, Zhang R, Han Y, Zhao X, Ren T. Red Pepper Powder Enhances Antioxidant and Immune Functions in the Sea Urchin Strongylocentrotus intermedius: Potential as a Functional Feed in Aquaculture. Antioxidants. 2025; 14(10):1173. https://doi.org/10.3390/antiox14101173
Chicago/Turabian StyleGuo, Jiadong, Yuntian Zhang, Yi Chen, Yupeng Zhang, Rongwei Zhang, Yuzhe Han, Xiaoran Zhao, and Tongjun Ren. 2025. "Red Pepper Powder Enhances Antioxidant and Immune Functions in the Sea Urchin Strongylocentrotus intermedius: Potential as a Functional Feed in Aquaculture" Antioxidants 14, no. 10: 1173. https://doi.org/10.3390/antiox14101173
APA StyleGuo, J., Zhang, Y., Chen, Y., Zhang, Y., Zhang, R., Han, Y., Zhao, X., & Ren, T. (2025). Red Pepper Powder Enhances Antioxidant and Immune Functions in the Sea Urchin Strongylocentrotus intermedius: Potential as a Functional Feed in Aquaculture. Antioxidants, 14(10), 1173. https://doi.org/10.3390/antiox14101173






