Genetic and Pharmacological Inhibition of NOX4 Protects Against Rhabdomyolysis-Induced Acute Kidney Injury Through Suppression of Endoplasmic Reticulum Stress
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Animals
2.3. Animal Experiments
2.4. Cell Culture and TCMK-1 Treatment
2.5. Immunohistochemistry (IHC)
2.6. Immunofluorescence Staining
2.7. Hematoxylin & Eosin (H&E) Staining and Histologic Scoring
2.8. Renal Function Assessment
2.9. Enzyme-Linked Immunosorbent Assay (ELISA)
2.10. Biochemical Tests
2.11. Terminal Deoxynucleotidyl Transferase-Mediated dUTP Nick End Labeling (TUNEL) Staining
2.12. Transmission Electron Microscopy
2.13. Cell Viability Assay
2.14. Calcein-AM/PI Double Staining Assay
2.15. Annexin V-FITC/PI Assay
2.16. ROS Detection
2.17. Quantitative Real-Time Polymerase Chain Reaction (RT-qPCR) Analysis
2.18. Western Blot Analysis
2.19. Statistical Analysis
3. Results
3.1. NOX4 Deficiency and GKT137831 Treatment Both Attenuated Glycerol-Induced RIAKI
3.2. NOX4 Inhibition Reduces Inflammation and Cell Apoptosis in Glycerol-Induced RIAKI Mice
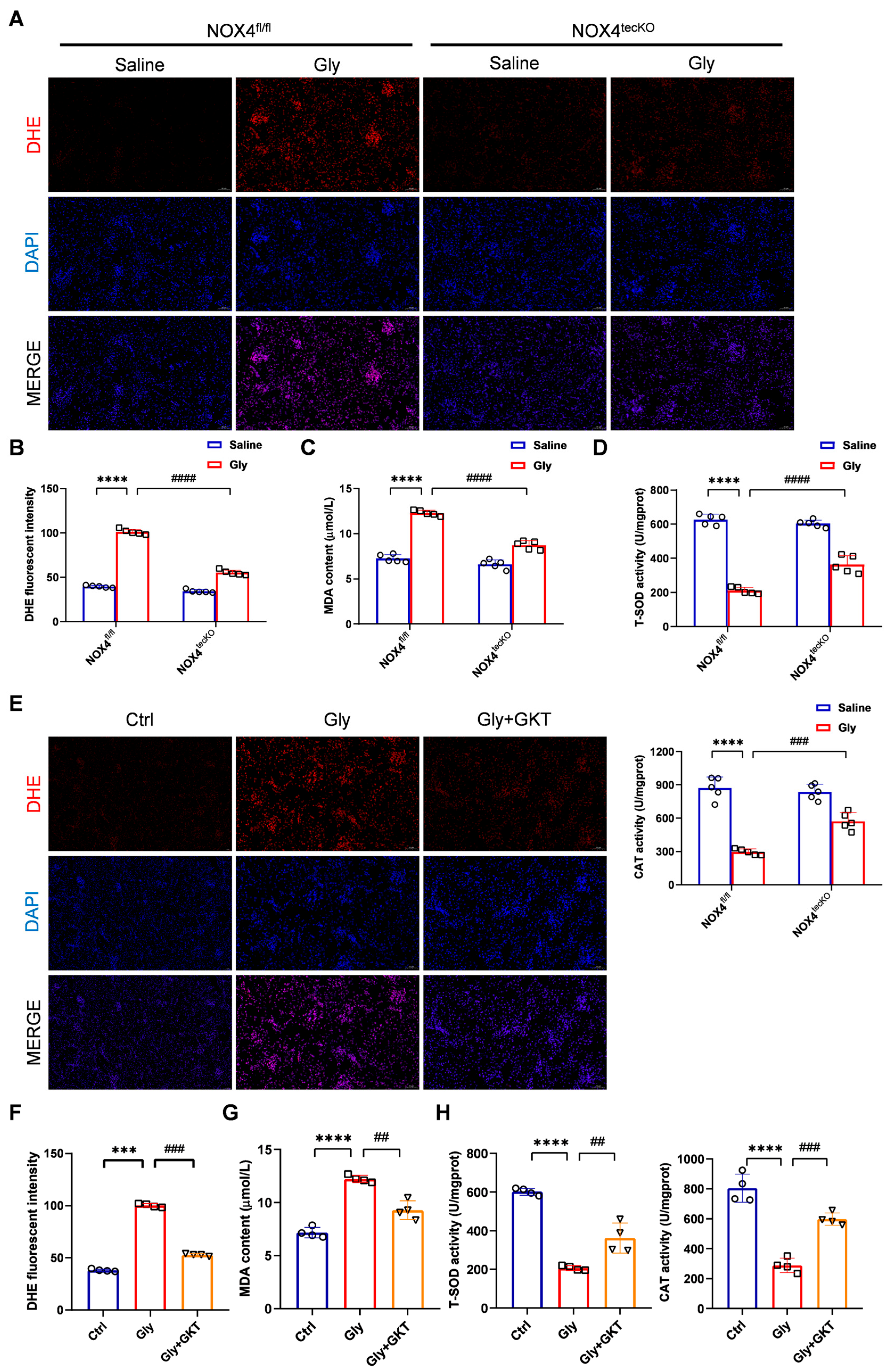
3.3. NOX4 Inhibition Suppresses Oxidative Stress in Glycerol-Induced RIAKI Mice
3.4. NOX4 Inhibition Suppresses Endoplasmic Reticulum Stress in Glycerol-Induced RIAKI Mice
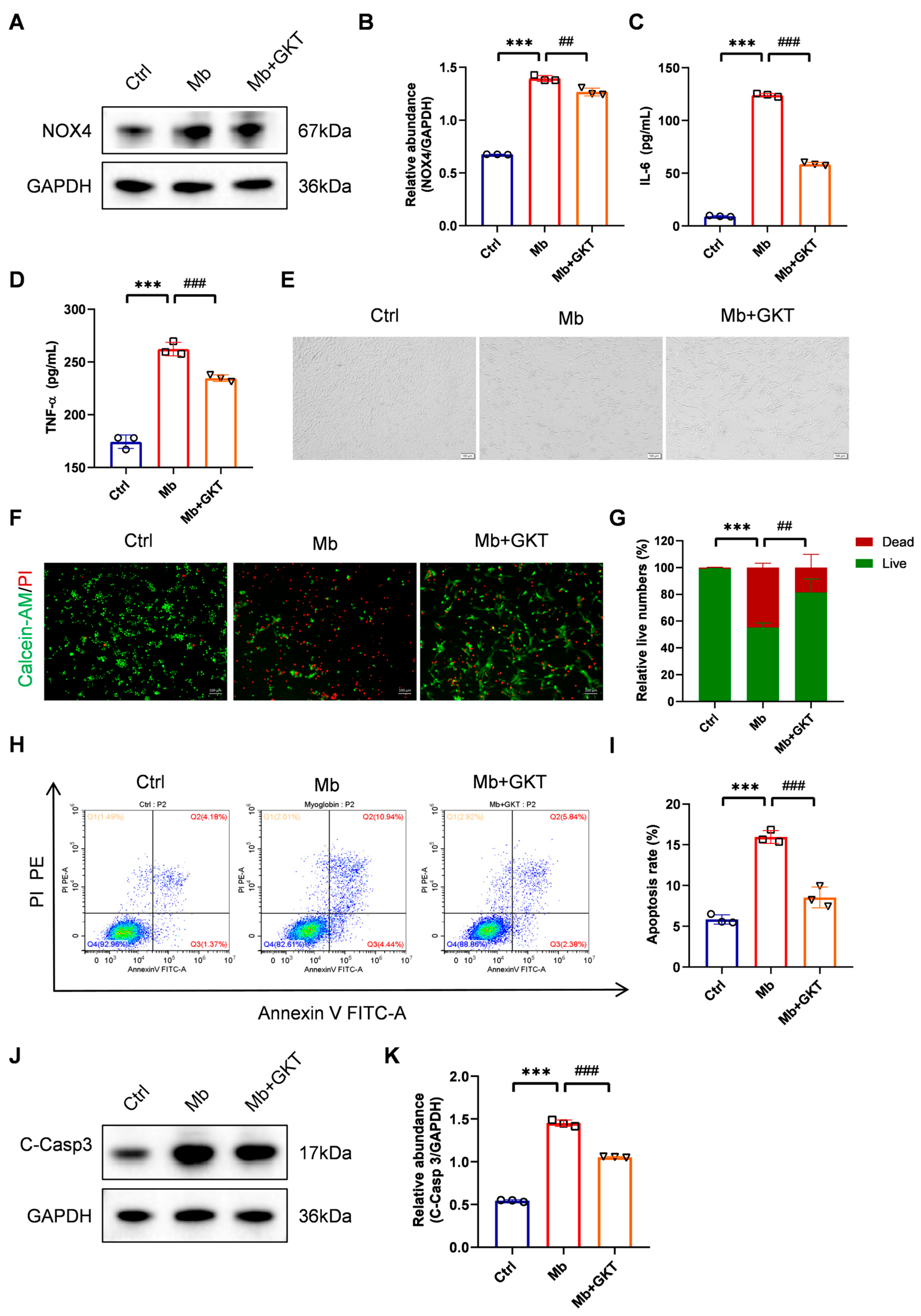
3.5. GKT137831 Suppressed NOX4 Expression in Ferrous Myoglobin-Stimulated TCMK-1 Cells
3.6. GKT137831 Mitigates Inflammation and Cell Apoptosis in Ferrous Myoglobin-Stimulated TCMK-1 Cells
3.7. GKT137831 Reduced ROS Levels and Suppressed ERS in Ferrous Myoglobin-Stimulated TCMK-1 Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cabral, B.M.I.; Edding, S.N.; Portocarrero, J.P.; Lerma, E.V. Rhabdomyolysis. Dis. Mon. 2020, 66, 101015. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Yuan, Q.; Mao, Z.; Hu, P.; Chi, K.; Geng, X.; Hong, Q.; Sun, X. The top 100 most cited articles on rhabdomyolysis: A bibliometric analysis. Am. J. Emerg. Med. 2020, 38, 1754–1759. [Google Scholar] [CrossRef] [PubMed]
- Petejova, N.; Martinek, A. Acute kidney injury due to rhabdomyolysis and renal replacement therapy: A critical review. Crit. Care 2014, 18, 224. [Google Scholar] [CrossRef]
- Delaney, K.A.; Givens, M.L.; Vohra, R.B. Use of RIFLE Criteria to Predict the Severity and Prognosis of Acute Kidney Injury in Emergency Department Patients with Rhabdomyolysis. J. Emerg. Med. 2012, 42, 521–528. [Google Scholar] [CrossRef]
- Candela, N.; Silva, S.; Georges, B.; Cartery, C.; Robert, T.; Moussi-Frances, J.; Rondeau, E.; Rebibou, J.-M.; Lavayssiere, L.; Belliere, J.; et al. Short- and long-term renal outcomes following severe rhabdomyolysis: A French multicenter retrospective study of 387 patients. Ann. Intensive Care 2020, 10, 27. [Google Scholar] [CrossRef] [PubMed]
- Bosch, X.; Poch, E.; Grau, J.M. Rhabdomyolysis and acute kidney injury. N. Engl. J. Med. 2009, 361, 62–72. [Google Scholar] [CrossRef]
- de Meijer, A.R.; Fikkers, B.G.; de Keijzer, M.H.; van Engelen, B.G.M.; Drenth, J.P.H. Serum creatine kinase as predictor of clinical course in rhabdomyolysis: A 5-year intensive care survey. Intensive Care Med. 2003, 29, 1121–1125. [Google Scholar] [CrossRef] [PubMed]
- McMahon, G.M.; Zeng, X.; Waikar, S.S. A risk prediction score for kidney failure or mortality in rhabdomyolysis. JAMA Intern. Med. 2013, 173, 1821–1828. [Google Scholar] [CrossRef] [PubMed]
- Torres, P.A.; Helmstetter, J.A.; Kaye, A.M.; Kaye, A.D. Rhabdomyolysis: Pathogenesis, diagnosis, and treatment. Ochsner J. 2015, 15, 58–69. [Google Scholar]
- Grivei, A.; Giuliani, K.T.K.; Wang, X.; Ungerer, J.; Francis, L.; Hepburn, K.; John, G.T.; Gois, P.F.H.; Kassianos, A.J.; Healy, H. Oxidative stress and inflammasome activation in human rhabdomyolysis-induced acute kidney injury. Free Radic. Biol. Med. 2020, 160, 690–695. [Google Scholar] [CrossRef]
- Scharman, E.J.; Troutman, W.G. Prevention of kidney injury following rhabdomyolysis: A systematic review. Ann. Pharmacother. 2013, 47, 90–105. [Google Scholar] [CrossRef] [PubMed]
- Joannidis, M.; Druml, W.; Forni, L.G.; Groeneveld, A.B.J.; Honore, P.; Oudemans-van Straaten, H.M.; Ronco, C.; Schetz, M.R.C.; Woittiez, A.J. Prevention of acute kidney injury and protection of renal function in the intensive care unit. Expert opinion of the Working Group for Nephrology, ESICM. Intensive Care Med. 2010, 36, 392–411. [Google Scholar] [CrossRef][Green Version]
- Chatzizisis, Y.S.; Misirli, G.; Hatzitolios, A.I.; Giannoglou, G.D. The syndrome of rhabdomyolysis: Complications and treatment. Eur. J. Intern. Med. 2008, 19, 568–574. [Google Scholar] [CrossRef] [PubMed]
- Zager, R.A.; Foerder, C.A. Effects of inorganic iron and myoglobin on in vitro proximal tubular lipid peroxidation and cytotoxicity. J. Clin. Investig. 1992, 89, 989–995. [Google Scholar] [CrossRef]
- Boutaud, O.; Moore, K.P.; Reeder, B.J.; Harry, D.; Howie, A.J.; Wang, S.; Carney, C.K.; Masterson, T.S.; Amin, T.; Wright, D.W.; et al. Acetaminophen inhibits hemoprotein-catalyzed lipid peroxidation and attenuates rhabdomyolysis-induced renal failure. Proc. Natl. Acad. Sci. USA 2010, 107, 2699–2704. [Google Scholar] [CrossRef]
- Sedeek, M.; Nasrallah, R.; Touyz, R.M.; Hébert, R.L. NADPH oxidases, reactive oxygen species, and the kidney: Friend and foe. J. Am. Soc. Nephrol. 2013, 24, 1512–1518. [Google Scholar] [CrossRef] [PubMed]
- Holterman, C.E.; Read, N.C.; Kennedy, C.R.J. Nox and renal disease. Clin. Sci. 2015, 128, 465–481. [Google Scholar] [CrossRef]
- Yang, Q.; Wu, F.-R.; Wang, J.-N.; Gao, L.; Jiang, L.; Li, H.-D.; Ma, Q.; Liu, X.-Q.; Wei, B.; Zhou, L.; et al. Nox4 in renal diseases: An update. Free Radic. Biol. Med. 2018, 124, 466–472. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Chen, Y.; Chabrashvili, T.; Aslam, S.; Borrego Conde, L.J.; Umans, J.G.; Wilcox, C.S. Role of oxidative stress in endothelial dysfunction and enhanced responses to angiotensin II of afferent arterioles from rabbits infused with angiotensin II. J. Am. Soc. Nephrol. 2003, 14, 2783–2789. [Google Scholar] [CrossRef] [PubMed]
- Eid, A.A.; Ford, B.M.; Bhandary, B.; de Cassia Cavaglieri, R.; Block, K.; Barnes, J.L.; Gorin, Y.; Choudhury, G.G.; Abboud, H.E. Mammalian target of rapamycin regulates Nox4-mediated podocyte depletion in diabetic renal injury. Diabetes 2013, 62, 2935–2947. [Google Scholar] [CrossRef] [PubMed]
- Cowley, A.W., Jr.; Yang, C.; Zheleznova, N.N.; Staruschenko, A.; Kurth, T.; Rein, L.; Kumar, V.; Sadovnikov, K.; Dayton, A.; Hoffman, M.; et al. Evidence of the Importance of Nox4 in Production of Hypertension in Dahl Salt-Sensitive Rats. Hypertension 2016, 67, 440–450. [Google Scholar] [CrossRef] [PubMed]
- Gil Lorenzo, A.F.; Costantino, V.V.; Appiolaza, M.L.; Cacciamani, V.; Benardon, M.E.; Bocanegra, V.; Vallés, P.G. Heat Shock Protein 70 and CHIP Promote Nox4 Ubiquitination and Degradation within the Losartan Antioxidative Effect in Proximal Tubule Cells. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 2015, 36, 2183–2197. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.M.; Ren, G.L.; Gao, L.; Yang, Q.; Li, H.D.; Wu, W.F.; Huang, C.; Zhang, L.; Lv, X.W.; Li, J. NADPH oxidase 4 promotes cisplatin-induced acute kidney injury via ROS-mediated programmed cell death and inflammation. Lab. Investig. J. Tech. Methods Pathol. 2018, 98, 63–78. [Google Scholar] [CrossRef] [PubMed]
- García-Caballero, C.; Guerrero-Hue, M.; Vallejo-Mudarra, M.; Palomino Antolin, A.; Decouty-Pérez, C.; Sánchez-Mendoza, L.M.; Villalba, J.M.; González-Reyes, J.A.; Opazo-Rios, L.; Vázquez-Carballo, C.; et al. Nox4 is involved in acute kidney injury associated to intravascular hemolysis. Free Radic. Biol. Med. 2024, 225, 430–444. [Google Scholar] [CrossRef]
- Gregg, J.L.; Turner, R.M., 2nd; Chang, G.; Joshi, D.; Zhan, Y.; Chen, L.; Maranchie, J.K. NADPH oxidase NOX4 supports renal tumorigenesis by promoting the expression and nuclear accumulation of HIF2α. Cancer Res. 2014, 74, 3501–3511. [Google Scholar] [CrossRef] [PubMed]
- Shanmugasundaram, K.; Nayak, B.K.; Friedrichs, W.E.; Kaushik, D.; Rodriguez, R.; Block, K. NOX4 functions as a mitochondrial energetic sensor coupling cancer metabolic reprogramming to drug resistance. Nat. Commun. 2017, 8, 997. [Google Scholar] [CrossRef]
- Wang, D.; Li, J.; Luo, G.; Zhou, J.; Wang, N.; Wang, S.; Zhao, R.; Cao, X.; Ma, Y.; Liu, G.; et al. Nox4 as a novel therapeutic target for diabetic vascular complications. Redox Biol. 2023, 64, 102781. [Google Scholar] [CrossRef]
- Li, J.; Zhang, Z.; Wang, L.; Jiang, L.; Qin, Z.; Zhao, Y.; Su, B. Maresin 1 Attenuates Lipopolysaccharide-Induced Acute Kidney Injury via Inhibiting NOX4/ROS/NF-κB Pathway. Front. Pharmacol. 2021, 12, 782660. [Google Scholar] [CrossRef]
- Li, J.; Wang, L.; Wang, B.; Zhang, Z.; Jiang, L.; Qin, Z.; Zhao, Y.; Su, B. NOX4 is a potential therapeutic target in septic acute kidney injury by inhibiting mitochondrial dysfunction and inflammation. Theranostics 2023, 13, 2863–2878. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Yang, L.; Wang, B.; Tang, L.; Li, J.; Liu, C.; Huang, Y.; Zhang, Z.; Zhang, D.; Zhang, L.; et al. Remote Ischemic Preconditioning Attenuates Mitochondrial Dysfunction and Ferroptosis of Tubular Epithelial Cells by Inhibiting NOX4-ROS Signaling in Acute Kidney Injury. Int. J. Biol. Sci. 2025, 21, 2313–2329. [Google Scholar] [CrossRef] [PubMed]
- Inagi, R. Endoplasmic reticulum stress as a progression factor for kidney injury. Curr. Opin. Pharmacol. 2010, 10, 156–165. [Google Scholar] [CrossRef]
- Liu, Z.; Lv, Y.; Zhao, N.; Guan, G.; Wang, J. Protein kinase R-like ER kinase and its role in endoplasmic reticulum stress-decided cell fate. Cell Death Dis. 2015, 6, e1822. [Google Scholar] [CrossRef]
- Huerta-Alardín, A.L.; Varon, J.; Marik, P.E. Bench-to-bedside review: Rhabdomyolysis—An overview for clinicians. Crit. Care 2005, 9, 158–169. [Google Scholar] [CrossRef] [PubMed]
- Gburek, J.; Verroust, P.J.; Willnow, T.E.; Fyfe, J.C.; Nowacki, W.; Jacobsen, C.; Moestrup, S.K.; Christensen, E.I. Megalin and cubilin are endocytic receptors involved in renal clearance of hemoglobin. J. Am. Soc. Nephrol. 2002, 13, 423–430. [Google Scholar] [CrossRef] [PubMed]
- Homsi, E.; Janino, P.; de Faria, J.B.L. Role of caspases on cell death, inflammation, and cell cycle in glycerol-induced acute renal failure. Kidney Int. 2006, 69, 1385–1392. [Google Scholar] [CrossRef] [PubMed]
- Panizo, N.; Rubio-Navarro, A.; Amaro-Villalobos, J.M.; Egido, J.; Moreno, J.A. Molecular Mechanisms and Novel Therapeutic Approaches to Rhabdomyolysis-Induced Acute Kidney Injury. Kidney Blood Press. Res. 2015, 40, 520–532. [Google Scholar] [CrossRef]
- Guerrero-Hue, M.; García-Caballero, C.; Palomino-Antolín, A.; Rubio-Navarro, A.; Vázquez-Carballo, C.; Herencia, C.; Martín-Sanchez, D.; Farré-Alins, V.; Egea, J.; Cannata, P.; et al. Curcumin reduces renal damage associated with rhabdomyolysis by decreasing ferroptosis-mediated cell death. FASEB J. 2019, 33, 8961–8975. [Google Scholar] [CrossRef]
- Murugan, R.; Kellum, J.A. Acute kidney injury: What’s the prognosis? Nat. Rev. Nephrol. 2011, 7, 209–217. [Google Scholar] [CrossRef]
- Gaudry, S.; Palevsky, P.M.; Dreyfuss, D. Extracorporeal Kidney-Replacement Therapy for Acute Kidney Injury. N. Engl. J. Med. 2022, 386, 964–975. [Google Scholar] [CrossRef]
- Bedard, K.; Krause, K.-H. The NOX family of ROS-generating NADPH oxidases: Physiology and pathophysiology. Physiol. Rev. 2007, 87, 245–313. [Google Scholar] [CrossRef]
- Geiszt, M.; Kopp, J.B.; Várnai, P.; Leto, T.L. Identification of renox, an NAD(P)H oxidase in kidney. Proc. Natl. Acad. Sci. USA 2000, 97, 8010–8014. [Google Scholar] [CrossRef]
- Barnes, J.L.; Gorin, Y. Myofibroblast differentiation during fibrosis: Role of NAD(P)H oxidases. Kidney Int. 2011, 79, 944–956. [Google Scholar] [CrossRef]
- Gao, Y.; Lu, X.; Zhang, G.; Liu, C.; Sun, S.; Mao, W.; Jiang, G.; Zhou, Y.; Zhang, N.; Tao, S.; et al. DRD4 alleviates acute kidney injury by suppressing ISG15/NOX4 axis-associated oxidative stress. Redox Biol. 2024, 70, 103078. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Ji, P.; Sun, R.; Zhou, H.; Huang, L.; Kong, L.; Li, W.; Li, W. Ginsenoside Rg1 attenuates LPS-induced chronic renal injury by inhibiting NOX4-NLRP3 signaling in mice. Biomed. Pharmacother. Biomed. Pharmacother. 2022, 150, 112936. [Google Scholar] [CrossRef] [PubMed]
- Feng, R.; Xiong, Y.; Lei, Y.; Huang, Q.; Liu, H.; Zhao, X.; Chen, Z.; Chen, H.; Liu, X.; Wang, L.; et al. Lysine-specific demethylase 1 aggravated oxidative stress and ferroptosis induced by renal ischemia and reperfusion injury through activation of TLR4/NOX4 pathway in mice. J. Cell. Mol. Med. 2022, 26, 4254–4267. [Google Scholar] [CrossRef] [PubMed]
- Mapuskar, K.A.; Pulliam, C.F.; Tomanek-Chalkley, A.; Rastogi, P.; Wen, H.; Dayal, S.; Griffin, B.R.; Zepeda-Orozco, D.; Sindler, A.L.; Anderson, C.M.; et al. The antioxidant and anti-inflammatory activities of avasopasem manganese in age-associated, cisplatin-induced renal injury. Redox Biol. 2024, 70, 103022. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.-N.; Yang, Q.; Yang, C.; Cai, Y.-T.; Xing, T.; Gao, L.; Wang, F.; Chen, X.; Liu, X.-Q.; He, X.-Y.; et al. Smad3 promotes AKI sensitivity in diabetic mice via interaction with p53 and induction of NOX4-dependent ROS production. Redox Biol. 2020, 32, 101479. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Qiu, Z.; Shao, Y.; Chen, Y.; Guan, Y.; Liu, M.; Li, Y.; Gao, N.; Wang, L.; Lu, X.; et al. Heritable gene targeting in the mouse and rat using a CRISPR-Cas system. Nat. Biotechnol. 2013, 31, 681–683. [Google Scholar] [CrossRef]
- Branda, C.S.; Dymecki, S.M. Talking about a revolution: The impact of site-specific recombinases on genetic analyses in mice. Dev. Cell 2004, 6. [Google Scholar] [CrossRef]
- Gurumurthy, C.B.; O’Brien, A.R.; Quadros, R.M.; Adams, J.; Alcaide, P.; Ayabe, S.; Ballard, J.; Batra, S.K.; Beauchamp, M.-C.; Becker, K.A.; et al. Reproducibility of CRISPR-Cas9 methods for generation of conditional mouse alleles: A multi-center evaluation. Genome Biol. 2019, 20, 171. [Google Scholar] [CrossRef]
- Suh, Y.A.; Arnold, R.S.; Lassegue, B.; Shi, J.; Xu, X.; Sorescu, D.; Chung, A.B.; Griendling, K.K.; Lambeth, J.D. Cell transformation by the superoxide-generating oxidase Mox1. Nature 1999, 401, 79–82. [Google Scholar] [CrossRef]
- Paravicini, T.M.; Touyz, R.M. NADPH oxidases, reactive oxygen species, and hypertension: Clinical implications and therapeutic possibilities. Diabetes Care 2008, 31 (Suppl. 2), S170–S180. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Chen, X.; Su, Y.; Paueksakon, P.; Hu, W.; Zhang, M.-Z.; Harris, R.C.; Blackwell, T.S.; Zent, R.; Pozzi, A. p47(phox) contributes to albuminuria and kidney fibrosis in mice. Kidney Int. 2015, 87, 948–962. [Google Scholar] [CrossRef] [PubMed]
- Geiszt, M. NADPH oxidases: New kids on the block. Cardiovasc. Res. 2006, 71, 289–299. [Google Scholar] [CrossRef] [PubMed]
- Begum, R.; Thota, S.; Abdulkadir, A.; Kaur, G.; Bagam, P.; Batra, S. NADPH oxidase family proteins: Signaling dynamics to disease management. Cell Mol. Immunol. 2022, 19, 660–686. [Google Scholar] [CrossRef] [PubMed]
- Kishi, S.; Nagasu, H.; Kidokoro, K.; Kashihara, N. Oxidative stress and the role of redox signalling in chronic kidney disease. Nat. Rev. Nephrol. 2024, 20, 101–119. [Google Scholar] [CrossRef] [PubMed]
- Rajaram, R.D.; Dissard, R.; Jaquet, V.; de Seigneux, S. Potential benefits and harms of NADPH oxidase type 4 in the kidneys and cardiovascular system. Nephrol. Dial. Transplant. 2019, 34, 567–576. [Google Scholar] [CrossRef]
- Nlandu Khodo, S.; Dizin, E.; Sossauer, G.; Szanto, I.; Martin, P.Y.; Feraille, E.; Krause, K.H.; de Seigneux, S. NADPH-oxidase 4 protects against kidney fibrosis during chronic renal injury. J. Am. Soc. Nephrol. 2012, 23, 1967–1976. [Google Scholar] [CrossRef]
- Santos, C.X.C.; Hafstad, A.D.; Beretta, M.; Zhang, M.; Molenaar, C.; Kopec, J.; Fotinou, D.; Murray, T.V.; Cobb, A.M.; Martin, D.; et al. Targeted redox inhibition of protein phosphatase 1 by Nox4 regulates eIF2α-mediated stress signaling. EMBO J. 2016, 35, 319–334. [Google Scholar] [CrossRef] [PubMed]
- Block, K.; Gorin, Y.; Abboud, H.E. Subcellular localization of Nox4 and regulation in diabetes. Proc. Natl. Acad. Sci. USA 2009, 106, 14385–14390. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Kirber, M.T.; Xiao, H.; Yang, Y.; Keaney, J.F. Regulation of ROS signal transduction by NADPH oxidase 4 localization. J. Cell Biol. 2008, 181, 1129–1139. [Google Scholar] [CrossRef]
- Wu, R.-F.; Ma, Z.; Liu, Z.; Terada, L.S. Nox4-derived H2O2 mediates endoplasmic reticulum signaling through local Ras activation. Mol. Cell Biol. 2010, 30, 3553–3568. [Google Scholar] [CrossRef]
- Byun, J.H.; Lebeau, P.F.; Trink, J.; Uppal, N.; Lanktree, M.B.; Krepinsky, J.C.; Austin, R.C. Endoplasmic reticulum stress as a driver and therapeutic target for kidney disease. Nat. Rev. Nephrol. 2025, 21, 299–313. [Google Scholar] [CrossRef]
- Chen, X.; Shi, C.; He, M.; Xiong, S.; Xia, X. Endoplasmic reticulum stress: Molecular mechanism and therapeutic targets. Signal Transduct. Target. Ther. 2023, 8, 352. [Google Scholar] [CrossRef]
- Wu, D.; Huang, L.-F.; Chen, X.-C.; Huang, X.-R.; Li, H.-Y.; An, N.; Tang, J.-X.; Liu, H.-F.; Yang, C. Research progress on endoplasmic reticulum homeostasis in kidney diseases. Cell Death Dis. 2023, 14, 473. [Google Scholar] [CrossRef] [PubMed]
- Qiu, L.; Zheng, X.; Jaishankar, D.; Green, R.; Fang, D.; Nadig, S.; Zhang, Z.J. Beyond UPR: Cell-specific roles of ER stress sensor IRE1α in kidney ischemic injury and transplant rejection. Kidney Int. 2023, 104, 463–469. [Google Scholar] [CrossRef]
- Marciniak, S.J.; Chambers, J.E.; Ron, D. Pharmacological targeting of endoplasmic reticulum stress in disease. Nat. Rev. Drug Discov. 2022, 21, 115–140. [Google Scholar] [CrossRef] [PubMed]
- Sciarretta, S.; Volpe, M.; Sadoshima, J. NOX4 regulates autophagy during energy deprivation. Autophagy 2014, 10, 699–701. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-K.; Lee, H.-Y.; Riaz, T.A.; Bhattarai, K.R.; Chaudhary, M.; Ahn, J.H.; Jeong, J.; Kim, H.-R.; Chae, H.-J. Chalcone suppresses tumor growth through NOX4-IRE1α sulfonation-RIDD-miR-23b axis. Redox Biol. 2021, 40, 101853. [Google Scholar] [CrossRef] [PubMed]
- Ochoa, C.D.; Wu, R.F.; Terada, L.S. ROS signaling and ER stress in cardiovascular disease. Mol. Aspects Med. 2018, 63, 18–29. [Google Scholar] [CrossRef] [PubMed]
- De Blasio, M.J.; Ramalingam, A.; Cao, A.H.; Prakoso, D.; Ye, J.-M.; Pickering, R.; Watson, A.M.D.; de Haan, J.B.; Kaye, D.M.; Ritchie, R.H. The superoxide dismutase mimetic tempol blunts diabetes-induced upregulation of NADPH oxidase and endoplasmic reticulum stress in a rat model of diabetic nephropathy. Eur. J. Pharmacol. 2017, 807, 12–20. [Google Scholar] [CrossRef] [PubMed]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Z.; Li, J.; Chen, S.; Peng, J.; Luo, X.; Wang, L.; Liao, R.; Zhao, Y.; Zhang, S.; Su, B. Genetic and Pharmacological Inhibition of NOX4 Protects Against Rhabdomyolysis-Induced Acute Kidney Injury Through Suppression of Endoplasmic Reticulum Stress. Antioxidants 2025, 14, 1162. https://doi.org/10.3390/antiox14101162
Zhang Z, Li J, Chen S, Peng J, Luo X, Wang L, Liao R, Zhao Y, Zhang S, Su B. Genetic and Pharmacological Inhibition of NOX4 Protects Against Rhabdomyolysis-Induced Acute Kidney Injury Through Suppression of Endoplasmic Reticulum Stress. Antioxidants. 2025; 14(10):1162. https://doi.org/10.3390/antiox14101162
Chicago/Turabian StyleZhang, Zhuyun, Jiameng Li, Shanshan Chen, Jing Peng, Xinyao Luo, Liya Wang, Ruoxi Liao, Yuliang Zhao, Shu Zhang, and Baihai Su. 2025. "Genetic and Pharmacological Inhibition of NOX4 Protects Against Rhabdomyolysis-Induced Acute Kidney Injury Through Suppression of Endoplasmic Reticulum Stress" Antioxidants 14, no. 10: 1162. https://doi.org/10.3390/antiox14101162
APA StyleZhang, Z., Li, J., Chen, S., Peng, J., Luo, X., Wang, L., Liao, R., Zhao, Y., Zhang, S., & Su, B. (2025). Genetic and Pharmacological Inhibition of NOX4 Protects Against Rhabdomyolysis-Induced Acute Kidney Injury Through Suppression of Endoplasmic Reticulum Stress. Antioxidants, 14(10), 1162. https://doi.org/10.3390/antiox14101162







