Abstract
The present study investigates the antioxidant potential of the stem bark of Elaeocarpus floribundus Blume through an integrated approach involving phytochemical isolation, in vitro radical scavenging assays, ADMET-based safety profiling, and molecular docking. Bioassay-guided fractionation of the ethanolic extract into hexane, chloroform, and ethyl acetate fractions revealed the ethyl acetate fraction to possess the highest antioxidant activity, with an IC50 value of 6.19 μg/mL in the DPPH assay, surpassing that of ascorbic acid (IC50 = 9.74 μg/mL). Subsequent isolation and characterization from the ethyl acetate fraction of the stem bark yielded five known compounds from this plant part for the first time, including gallic acid and epigallocatechin gallate. Both compounds showed potent radical scavenging activity in vitro. Among these, gallic acid exhibited superior pharmacokinetic and safety profiles based on in silico ADMET predictions, no Lipinski’s rule violations, and no predicted toxicity. Molecular docking studies showed that gallic acid had high binding affinities for glutathione reductase (GR) and superoxide dismutase (SOD), exceeding those of their reference inhibitors. A docking analysis further revealed stable interactions with catalytically relevant residues, suggesting a stabilizing modulatory effect on redox homeostasis. These findings identify E. floribundus stem bark as a novel source of antioxidant compounds and highlight gallic acid as a promising therapeutic candidate for oxidative stress-related disorders.
1. Introduction
Oxidative stress, characterized by an imbalance between reactive oxygen species (ROS) and the body’s antioxidant defenses, plays a central role in the pathogenesis of numerous chronic and degenerative diseases, including neurodegenerative disorders, diabetes, cardiovascular diseases, and cancer [1]. Naturally occurring antioxidants, particularly phenolic compounds from medicinal plants, have gained significant attention due to their ability to scavenge free radicals, modulate enzymatic activities, and offer protective effects with minimal toxicity [2].
The genus Elaeocarpus, comprising over 350 species widely distributed across tropical and subtropical regions, has been a valuable source of diverse secondary metabolites with promising biological activities [3]. Elaeocarpus floribundus Blume, commonly known as Indian olive and locally referred to as “jolphai,” is traditionally used in various regions of the world for the treatment of ulcers, dysentery, diabetes, and inflammatory conditions such as rheumatism and gingivitis [4,5]. Ethnopharmacological claims are supported by studies demonstrating the antimicrobial, antidiabetic, and antioxidant effects of its fruits [6], leaves [7], and seeds [8]. Previous investigations have identified several bioactive constituents, including flavonoids, triterpenoids, sterols, and phenolic acids, from different parts of the plant [9,10]. Notably, gallic acid, a well-characterized antioxidant, was recently isolated from the seeds of E. floribundus and shown to possess potent free radical scavenging and antimicrobial activities [8].
Despite these findings, the stem bark of E. floribundus has remained underexplored in terms of its phytochemical composition and biological potential. Previous research on the stem bark extract revealed considerable free radical scavenging potential; however, the investigation did not identify these bioactive constituents nor provide mechanistic insights into their mode of action [11]. Given the traditional use of the bark decoctions in inflammatory conditions and the established correlation between oxidative stress and inflammation, exploring the antioxidant profile of the stem bark is both pharmacologically and therapeutically relevant.
In this study, we investigated the antioxidant potential of the stem bark of E. floribundus, aiming to isolate and characterize its bioactive constituents, assess their radical scavenging capacity, and evaluate their drug-likeness and safety profile through in silico ADMET analysis. Furthermore, to elucidate the potential mechanism of action, molecular docking studies were conducted, targeting key antioxidant enzymes: glutathione reductase (GR) and superoxide dismutase (SOD). This integrated experimental and computational approach provides valuable insights into the pharmacological relevance of E. floribundus bark as a source of therapeutic antioxidants.
2. Materials and Methods
2.1. General Information
Nuclear magnetic resonance (NMR) spectra (1H and 13C NMR) were recorded in deuterated solvents using JEOL ECZ400R (Tokyo, Japan, 400 MHz) and Bruker Avance III FT (Billerica, MA, USA, 500 MHz) spectrometers, with tetramethylsilane (TMS) used as the internal standard. Chemical shifts (δ) are reported in ppm, and coupling constants (J) are given in hertz (Hz). Infrared (IR) spectra were obtained using a Perkin Elmer FT-IR 2000 spectrometer (Waltham, MA, USA). Optical rotation measurements were performed in methanol (MeOH) using an AUTOPOL I polarimeter (Cape Town, South Africa). Column chromatography was carried out on silica gel (60–120, 100–200, and 230–400 mesh) using solvent mixtures of increasing polarity as eluents. The purity of the eluates was monitored on pre-coated silica gel 60 F254 TLC plates (Merck, Bangalore, India), visualized with the aid of UV light (254 nm), exposure to iodine vapor, and spraying with p-anisaldehyde stain reagent followed by heating at 70 °C. Solvents and fractions were concentrated using a rotary evaporator at 45 °C. Solvents used in this study were distilled before use and dried over appropriate drying agents. High-resolution mass spectrometry (HRMS) was conducted using a Waters Xevo XS QTOF mass spectrometer, Bangalore, India.
2.2. Plant Material, Extraction, and Isolation
The stem bark of Elaeocarpus floribundus was collected in March 2019 from a mature tree on the campus of CSIR–North East Institute of Science and Technology, Jorhat, India (26°44′08.3″ N, 94°09′38.4″ E), during a period that coincided with favorable weather conditions for harvesting and drying. A voucher specimen (NEIST/1893) was deposited at the institute’s herbarium. The powdered bark (2.1 kg) was subjected to three 72 h maceration processes with ethanol (5 L) at room temperature (~25 °C). The extracts were pooled, filtered through Whatman No. 1 filter paper, and concentrated under reduced pressure using a rotary evaporator at 45 °C, yielding a blood-red flaky crude extract (135 g). The crude extract was subsequently partitioned between distilled water and solvents of increasing polarity (hexane, chloroform, and ethyl acetate), affording three fractions: hexane (HB, 3.0 g), chloroform (CB, 3.1 g), and ethyl acetate (EB, 4.0 g). The antioxidant potential of the crude extract and fractions was evaluated using the DPPH radical scavenging assay, with the ethyl acetate fraction exhibiting the highest activity. This fraction was subjected to silica gel column chromatography (100–200 mesh) and eluted with solvent gradients ranging from 100% hexane to 100% ethyl acetate (100:0 to 0:100, v/v). Based on TLC profiling, eluates were combined into six major fractions (EBA–EBF). Further purification of the EBB fraction (256 mg) using silica gel chromatography (hexane/chloroform gradient) yielded pentadecanoic acid (1) (14 mg). β-Sitosterol (2) (451 mg) precipitated from the EBC fraction and was purified by acetone washing. The EBC fraction (250 mg) was further purified via silica gel chromatography (hexane/chloroform gradient) to afford 2,4-di-tert-butylphenol (3) (16 mg). Purification of the EBD fraction (971 mg) via silica gel chromatography (chloroform/methanol gradient) yielded gallic acid (4) (205 mg) and epigallocatechin gallate (5) (388 mg).
2.3. Quantification of Total Phenolic and Total Flavonoid Contents
The total reducing capacity, commonly referred to as the total phenolic content (TPC) of the ethanolic extract and its fractions, was estimated using the Folin–Ciocalteu (F-C) reagent following standard protocols [12]. It is important to note that the F–C assay responds to a range of reducing substances, including but not limited to phenolic compounds. The TPC was quantified using a gallic acid calibration curve, and the results are expressed as milligrams of gallic acid equivalent (GAE) per milligram of dry weight (mg GAE/g d.w) of the extract or fractions. The TFC was assessed using a colorimetric aluminum chloride assay following a previously described method [13], which primarily detects flavonols and flavones based on their ability to form stable complexes with AlCl3. The TFC was determined using a rutin calibration curve and expressed as milligrams of rutin equivalent (RE) per milligram of dry weight (mg RE/g d.w) of the extract or fractions. Due to structural specificity, this method may not capture all subclasses of flavonoids uniformly. All measurements were performed in triplicate.
2.4. Radical Scavenging Potential Towards DPPH Free Radicals
The antioxidant potential of the stem bark as a cost-effective and natural alternative to synthetic antioxidants was evaluated using the DPPH free radical scavenging assay following the method detailed in [14]. Different concentrations (100 μg/mL to 5 mg/mL) of the samples were combined with an equal volume of 0.3 mM DPPH solution prepared in methanol. The mixture was thoroughly shaken and left to incubate in the dark at room temperature for 30 min. Absorbance readings were then taken at 517 nm using a spectrophotometer. Ascorbic acid served as the reference antioxidant, while a 0.3 mM methanol solution of DPPH acted as the control. The percentage inhibition was calculated using the following formula:
% inhibition = [(Absorbance of control − Absorbance of sample)/Absorbance of control] × 100.
2.5. Computational Toxicity Studies
The ADMET (absorption, distribution, metabolism, excretion, and toxicity) properties of the isolated compounds were predicted using in silico tools. The SwissADME server (http://www.swissadme.ch/, accessed on 10 December 2024) was employed to evaluate pharmacokinetic parameters, including absorption, distribution, and metabolism [15]. The toxicity profile of the compounds was further assessed using the pkCSM online tools (https://biosig.lab.uq.edu.au/pkcsm/prediction, accessed on 10 December 2024), which provide toxicity class predictions based on chemical structure [16]. These computational analyses were performed to predict the drug-likeness and potential toxicological risks associated with the isolated bioactive compounds.
2.6. Molecular Docking Studies
For molecular docking studies, the 3D structure of gallic acid and the standard were obtained from the PubChem database (https://pubchem.ncbi.nlm.nih.gov/, accessed on 02 December 2024) in SDF format and converted to PDB format using Open Babel software 2.4.1 [17]. Gallic acid was selected for molecular docking against GR and SOD based on its favorable pharmacokinetic profile, exhibiting no violations of Lipinski’s rules, no predicted toxicity risks, and superior in vitro antioxidant activity compared to the reference compound, ascorbic acid. The co-crystalized ajoene inhibitor was used as the reference ligand for GR, while quercetin served as the reference ligand for SOD [18].
The crystal structures of GR (PDB ID: 1BWC) and SOD (PDB ID: 2C9V) were retrieved from the Protein Data Bank (http://www.rcsb.org/pdb, accessed on 20 December 2024). The GR structure was co-crystallized with the ligand ajoene, which served as the reference inhibitor. This enabled targeted docking at the inhibitor binding site and provided insights into the key amino acid residues involved in GR inhibition. For SOD, the grid box center and size coordinates were obtained from a previous study [18], allowing for targeted docking at its active site.
The crystallized enzyme structures were processed using UCSF ChimeraX v1.8. Before analysis, water molecules and co-crystallized ligands were eliminated, followed by the addition of polar hydrogen atoms to account for the protonation states of ionizable amino acid residues under physiological conditions. After the preparation of the ligands and crystallized enzymes, molecular docking was performed using AutoDock Vina v1.1.2 [19]. The resulting docking poses were analyzed and visualized using BIOVIA Discovery Studio v21.1.0.20298.
2.7. Statistical Analysis
All experimental procedures were performed in triplicate using three independently prepared samples. Data are presented as mean ± standard deviation (SD). Statistical significance between groups was assessed using a one-way analysis of variance (ANOVA) followed by Tukey’s post hoc test, where applicable. A p-value of <0.05 was considered statistically significant. All analyses were performed using Microsoft Excel 2016 with the Real Statistics Resource Pack add-in.
3. Results
3.1. Isolation of Compounds
The structures of the compounds (1–5) were identified through a comparison of their MS and NMR data (Figures S1–S5) with those reported previously, including pentadecanoic acid (1) [20], β-Sitosterol (2) [21], 2,4-di-tert-butylphenol (3) [22], gallic acid (4) [23], and epigallocatechin gallate (5) [24], and the structures are shown in Figure 1.
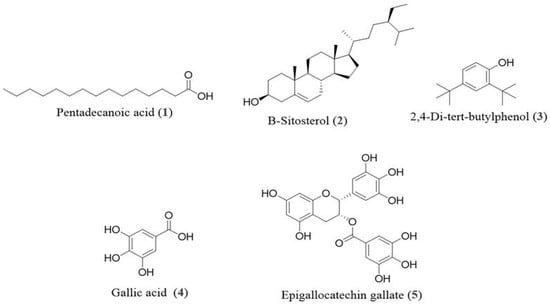
Figure 1.
Chemical structures of pentadecanoic acid (1), β-Sitosterol (2), 2,4-di-tert-butylphenol (3), gallic acid (4), and epigallocatechin gallate (5) from stem bark of E. floribundus.
3.2. Quantification of TPC, TFC, and DPPH Scavenging Activity of Extract and Fractions of E. floribundus
The stem bark of Elaeocarpus floribundus underwent ethanol extraction and was further fractionated using solvents of increasing polarity: hexane, chloroform, and ethyl acetate. The total reducing capacity (commonly interpreted as the total phenolic content, TPC), total flavonoid content (TFC), and 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging activity of the crude extract and its fractions were assessed and are summarized in Table 1. The crude extract displayed the highest TPC (65.46 ± 0.31 mg/g), followed by the ethyl acetate fraction with a value of 58.71 ± 2.05 mg/g. In contrast, the chloroform and hexane fractions had significantly lower values (p < 0.05). A similar trend was observed for the total flavonoid content (TFC) and DPPH scavenging activity, where the ethyl acetate fraction showed significantly stronger antioxidant activity (IC50 = 6.19 ± 0.03 μg/mL) compared to the other fractions (p < 0.05). No statistically significant difference was observed between the TPC of the ethanol extract and the ethyl acetate fraction (p > 0.05), suggesting that both are comparably rich in phenolic compounds.

Table 1.
Total phenolic content (TPC), total flavonoid content (TFC), and DPPH radical scavenging activity (expressed as IC50) of crude extract and solvent fractions of E. floribundus stem bark (mean ± standard deviation).
3.3. DPPH Antioxidant Inhibitory Activity of Isolated Components
To further investigate the potential applications of the isolated main compounds (Figure 1), pentadecanoic acid (1), β-Sitosterol (2), 2,4-di-tert-butylphenol (3), gallic acid (4), and epigallocatechin gallate (5) were evaluated for their radical scavenging activity. The isolated compounds demonstrated dose-dependent DPPH radical scavenging activity, as shown in Table 2. Notably, epigallocatechin gallate (5) and gallic acid (4) demonstrated significantly higher antioxidant activity, with IC50 values of 8.05 ± 0.17 μM and 16.04 ± 0.07 μM, respectively, which were significantly different (p < 0.05) from the weaker or inactive compounds. Their activities surpassed the standard, ascorbic acid (55.29 ± 0.22 μM), confirming their relevance to the extract’s potency. Compounds 1 and 2 showed negligible activity (>500 μM), while compound 3 exhibited moderate scavenging potential (IC50 = 125.05 ± 0.20 μM). Statistical analysis using a one-way ANOVA followed by Tukey’s post hoc test confirmed that the differences among compounds were statistically significant (p < 0.05). These findings suggest that compounds 4 and 5 are the primary contributors to the strong antioxidant properties of the ethyl acetate fraction.

Table 2.
Antiradical activity towards DPPH of isolated compounds.
3.4. Prediction of Pharmacokinetic and Toxicological Properties
The pharmacokinetic behavior and toxicological potential of the phytochemical constituents isolated from E. floribundus stem bark were evaluated using the SwissADME and pkCSM web tools. The results are summarized in Table 3, while the bioavailability radars in Figure 2 provide a graphical comparison of their physicochemical profiles. For these bioactive compounds to qualify as promising oral therapeutics targeting antioxidant pathways, they must satisfy two critical criteria: First, they should demonstrate favorable drug-likeness by exhibiting no more than one violation of Lipinski’s Rule of Five. Second, comprehensive toxicity screening should confirm that they pose no significant risks, showing no indications of mutagenic potential, carcinogenicity, tissue irritation, or adverse effects on reproductive function [25]. This two-tiered evaluation approach helps identify compounds with favorable bioavailability and safety profiles. Among the five isolated phytochemicals, only epigallocatechin gallate (5) exhibited multiple violations of Lipinski’s Rule of Five, with all four showing either complete compliance or only one violation of these established pharmacokinetic guidelines.

Table 3.
Predicted ADMET properties and drug-likeness profiles of isolated compounds.
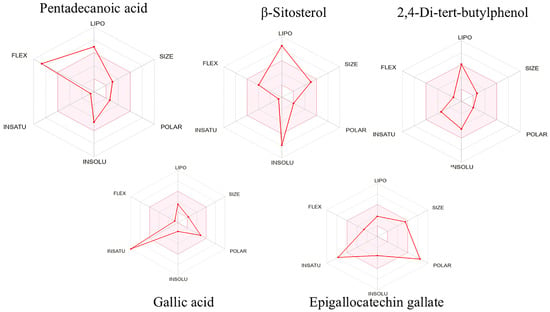
Figure 2.
Bioavailability radars of isolated compounds.
Gallic acid, which complied with Lipinski’s rules, exhibited no predicted toxicity risk, and demonstrated superior in vitro radical scavenging activity compared to the standard, was selected as a potential inhibitor of glutathione reductase (GR) and superoxide dismutase (SOD) for targeting antioxidant pathways. It was therefore evaluated through docking analysis to assess its inhibitory performance in comparison with the reference inhibitors: ajoene for GR and quercetin for SOD. Moreover, ADMET parameters revealed favorable drug-like properties, supporting its potential therapeutic applications.
3.5. Determination of Antioxidant Activity Through Molecular Docking
Given the superior in vitro antioxidant activity of gallic acid compared to the standard ascorbic acid, along with its excellent drug-likeness profile and absence of predicted toxicity as revealed by ADMET analysis, molecular docking was performed to further explore its potential mechanism of action. GR and SOD, two key enzymes involved in the antioxidant defense system, were selected as molecular targets. GR is a key enzyme that maintains the intracellular pool of reduced glutathione (GSH), essential for neutralizing ROS and preserving redox homeostasis. The inhibition or dysfunction of GR can impair glutathione recycling, leading to increased oxidative stress and cellular damage [26]. SOD, on the other hand, catalyzes the dismutation of the superoxide radical into hydrogen peroxide and molecular oxygen, thereby serving as the first line of enzymatic defense against ROS [27]. Together, GR and SOD form a crucial part of the antioxidant machinery, and their modulation can significantly influence oxidative stress-related pathways. The current therapeutic landscape for targeting antioxidant pathways is constrained by efficacy challenges and toxicity concerns, driving the search for improved compounds from natural origins that potentially exhibit fewer detrimental side effects. Hence, docking studies were conducted to assess the binding affinity and interaction patterns of gallic acid with the active sites of these enzymes in comparison with their respective reference inhibitors.
Molecular docking was performed to generate the ten most stable conformations of gallic acid from which the conformation with the highest binding affinity was selected for analysis. The docking results are summarized in Table 4. For GR, gallic acid exhibited a notable binding affinity of −10.04 kcal/mol, surpassing that of the reference inhibitor ajoene, which recorded a binding affinity of −8.98 kcal/mol. The binding pose of gallic acid within the catalytic pocket of GR and its spatial orientation in the active site are illustrated in Figure 3A and Figure 3B, respectively. The detailed interaction map is shown in Figure 3C. Gallic acid formed four conventional hydrogen bonds with key residues in the GR active site: one between the hydroxyl group on its phenyl ring and the side chain of Ser30; another between a separate hydroxyl group and Thr57; a third between a hydroxyl group and Thr339; and a fourth involving a hydrogen bond between a hydroxyl group and the carboxylate side chain of Asp331. In addition to hydrogen bonding, gallic acid established a π-alkyl interaction with Ala342 as well as a π-anion interaction between its aromatic ring and the negatively charged residue Asp331.

Table 4.
Docking analysis of gallic acid against two antioxidant enzymes compared to their reference inhibitors.
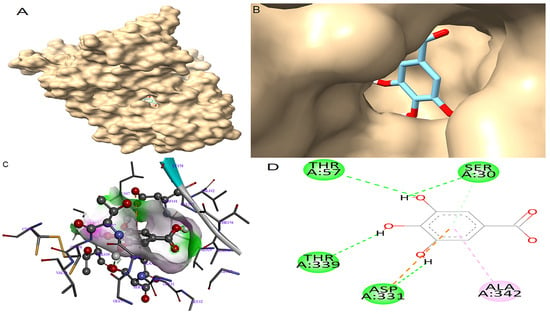
Figure 3.
Molecular docking between gallic acid and GR. (A) The adopted molecular geometry of gallic acid in the catalytic pocket of the GR enzyme; (B) a zoomed view of the geometry adopted by gallic acid in the catalytic pocket of GR; (C) an analysis of the hydrogen bonds of gallic acid–GR complex; (D) a map of predominant interactions of the molecular docking of gallic acid and GR.
In the case of SOD, gallic acid showed a theoretical binding energy of −9.84 kcal/mol, while the standard quercetin displayed a binding score of −7.54 kcal/mol. The binding pose of gallic acid within the catalytic pocket of SOD and its spatial orientation in the active site are illustrated in Figure 4A and Figure 4B, respectively. The detailed interaction map is shown in Figure 4C,D. Gallic acid interacts with SOD, forming H-bonds at the receptor site interacting region involving residues Cys111, Gly108, and Ala1. SOD residue Ser107 was involved in the π-σ interaction.
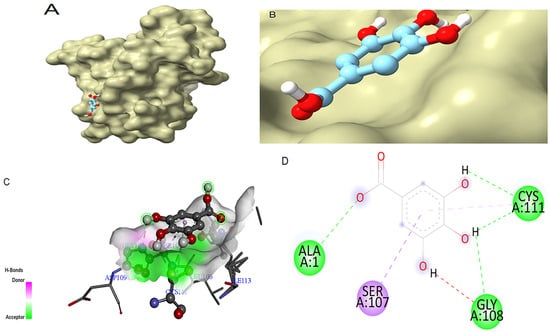
Figure 4.
Molecular docking between gallic acid and SOD. (A) The adopted molecular geometry of gallic acid in the catalytic pocket of the SOD enzyme; (B) a zoomed view of the geometry adopted by gallic acid in the catalytic pocket of SOD; (C) an analysis of the hydrogen bonds of gallic acid–SOD complex; (D) a map of predominant interactions of the molecular docking of gallic acid and SOD.
4. Discussion
This study provides a detailed evaluation of the antioxidant potential of Elaeocarpus floribundus stem bark, employing a multidisciplinary approach involving extraction, phytochemical isolation, in vitro antioxidant assays, pharmacokinetic and toxicity profiling, and molecular docking studies. The results highlight the plant’s potential as a valuable source of natural antioxidants, particularly phenolic compounds.
The ethanol extract and its solvent-partitioned fractions showed varying levels of TPC, TFC, and radical scavenging activity. The crude extract exhibited the highest TPC (65.46 mg GAE/g) and TFC (20.82 mg RE/g). Among the fractions, the ethyl acetate fraction stood out, not only for its high TPC (58.71 mg GAE/g) but also for its superior DPPH scavenging activity (IC50 = 6.19 μg/mL), outperforming the standard antioxidant ascorbic acid (IC50 = 9.74 μg/mL).
The antioxidant activity and polyphenolic content observed in this study are consistent with, and in some cases superior to, previous reports on various parts of the same species. For instance, Rahayu Utami et al. (2013) reported a significantly higher TPC of 161.5 ± 24.81 mg GAE/g DW for the methanolic extract of the stem bark compared to the value of 65.46 mg GAE/g extract in our study [11]. This difference may result from variations in solvent polarity and extraction efficiency, expression units (extract weight vs. dry weight), and other biological and environmental factors such as geographical origin, plant maturity, and the seasonal timing of collection, all of which are known to affect secondary metabolite composition. In terms of antioxidant activity, their reported DPPH IC50 value of 7.36 ± 0.01 μg/mL for the methanol stem bark extract closely matches the IC50 value of 6.19 μg/mL found in our ethyl acetate fraction, supporting the reproducibility of strong antioxidant potential in this plant part. Flavonoid content comparisons further support the relevance of our findings. Utami et al. (2023) reported a TFC of 60.59 ± 0.53 mg QE/g extract in ethyl acetate leaf extracts, whereas our ethyl acetate bark fraction exhibited a significantly lower TFC of 8.05 mg RE/g [28]. Our previous work on the fruit of the same plant showed a TPC of 0.5 mg GAE/mg and a TFC of 0.2 mg RE/mg in the crude extract, with significantly lower levels detected in the partitioned fractions [6]. In the current study, the TPC and TFC of the ethyl acetate bark fraction were notably higher (58.71 mg GAE/g and 8.05 mg RE/g, respectively), further establishing the bark as a richer source of antioxidant-relevant compounds. Similarly, Bijayanta and Shyamapada (2017) reported a TPC range of 0.087 to 1.39 mg/g for the ethanolic fruit extract, significantly lower than the values observed in this study for the bark extract and its fractions [29]. Likewise, the DPPH radical scavenging activity of the ethyl acetate fraction (IC50 = 6.19 μg/mL) was more potent than that of the fruit extract in our previous work, which exhibited an IC50 value of 2.05 mg/mL [6]. Although the seed extract showed stronger antioxidant activity (IC50 = 4.4 μg/mL), as reported in our previous study [8], the bark remains a valuable source due to its combination of significant in vitro antioxidant potential, high phenolic content, and superior pharmacokinetic and toxicity profiles demonstrated in this study. Collectively, these comparisons emphasize the antioxidant potential of the stem bark, particularly the ethyl acetate fraction, and validate the selection of this plant part for further phytochemical investigation.
It is worth emphasizing that the Folin–Ciocalteu assay used for estimating the TPC is not specific to phenolic compounds but rather reflects the total reducing capacity of a sample. This includes contributions from non-phenolic lowering agents such as ascorbic acid, certain amino acids, and reducing sugars [30,31]. As such, while high TPC values in the crude and ethyl acetate fractions likely reflect an enriched polyphenolic content, the low TPC recorded in the chloroform fraction may not necessarily indicate the absence of antioxidant-relevant compounds. Instead, it may reflect both the reduced polarity of the solvent, which selectively extracts fewer phenolic compounds and the limitations of the F–C assay itself in accurately distinguishing phenolics from other reductants. Additionally, the aluminum chloride assay used to estimate the total flavonoid content selectively detects flavonoids with certain structural features, such as flavonols and flavones. It may underestimate the presence of other flavonoid subclasses [32].
The subsequent isolation and characterization of compounds from the ethyl acetate fraction led to the identification of five compounds, among which gallic acid (4) and epigallocatechin gallate (5) exhibited potent radical scavenging activity in the DPPH assay (IC50 = 16.04 μM and 8.05 μM, respectively), exceeding that of ascorbic acid (IC50 = 55.29 μM). These results suggest that the antioxidant activity of the ethyl acetate fraction is largely attributed to its phenolic constituents, which are known for their electron-donating capacity and ability to stabilize free radicals [33].
ADMET predictions further supported the suitability of gallic acid for therapeutic development. It exhibited no violations of Lipinski’s Rule of Five, high gastrointestinal absorption, and no predicted toxicity. The pharmacokinetic simulation results obtained for gallic acid in this study, indicating high gastrointestinal absorption, the absence of hepatotoxicity and mutagenicity, and no violations of Lipinski’s rules, are consistent with existing experimental data in the literature. Gallic acid has been previously reported to exhibit favorable oral bioavailability and rapid intestinal absorption in animal studies, supporting its potential for systemic antioxidant effects [34,35]. Toxicological studies have shown that gallic acid has a high safety margin, with no observed adverse effects in rodents even at relatively high doses [36]. These findings corroborate the outcomes of our in silico analysis and support the suitability of gallic acid as a safe and effective antioxidant agent with translational relevance. In contrast, although epigallocatechin gallate showed strong in vitro activity, its poor drug-likeness profile due to multiple Lipinski violations made it less ideal for further docking analysis.
To investigate the mechanism behind gallic acid’s antioxidant action, molecular docking was performed against GR and SOD, key enzymes involved in intracellular redox regulation. GR regenerates reduced GSH [37], while SOD catalyzes the dismutation of superoxide radicals [38]. Both enzymes are vital for neutralizing ROS and preventing oxidative damage.
Docking poses were analyzed and compared to their respective antioxidant standards. In this study, gallic acid docked very well compared to the standards. The docking results show that gallic acid had strong binding affinities for both enzymes (−10.04 kcal/mol for GR and −9.84 kcal/mol for SOD), surpassing the reference inhibitors ajoene (−8.98 kcal/mol) and quercetin (−7.54 kcal/mol), respectively. Gallic acid formed stable interactions through multiple hydrogen bonds and π interactions with catalytically important residues, such as Thr57 and Thr339 in GR [39] and Cys111, Ala1, and Gly108 in SOD [18]. These interactions suggest that gallic acid may enhance or stabilize antioxidant enzyme function, and this stability is crucial for its potential inhibitory activity, thus contributing to its potent in vitro effects [40].
While five major compounds were isolated and structurally elucidated from the ethyl acetate fraction, their quantities within the fraction were not determined in this study. Nonetheless, based on their high radical scavenging capacity, particularly gallic acid and epigallocatechin gallate, and their consistent presence in bioactive polar fractions across similar studies [41,42], it is reasonable to infer that these compounds play a central role in the observed activity. However, the ethyl acetate fraction is likely to contain other polyphenolic constituents not captured in this study. High molecular weight tannins, polymeric flavonoids, and phenolic glycosides, commonly found in Elaeocarpus species and other medicinal plants, could also contribute significantly to the antioxidant potential [11,43]. Previous phytochemical studies on E. floribundus and related species have reported the presence of ellagitannins, proanthocyanidins, and other complex phenolic structures [44,45], which were not isolated here but may be present in minor or less extractable quantities. Future studies incorporating LC-MS or quantitative HPLC profiling are necessary to fully characterize the composition and relative abundance of antioxidant-active constituents in the extract.
Collectively, these findings establish Elaeocarpus floribundus Blume stem bark as a promising source of antioxidant compounds, with gallic acid emerging as a particularly potent, pharmacologically safe, and mechanistically validated lead compound. Although the results are promising, the lack of in vivo or ex vivo validation limits the extent to which the antioxidant effects of gallic acid can be extrapolated to physiological contexts. Therefore, further biological studies are necessary to confirm its therapeutic potential.
5. Conclusions
This study offers detailed insights into the antioxidant potential of the stem bark of Elaeocarpus floribundus Blume. The ethyl acetate fraction demonstrated remarkable radical scavenging activity, prompting the isolation of key bioactive constituents, with gallic acid exhibiting the highest antioxidant activity among them. Gallic acid exhibited superior in vitro antioxidant activity compared to ascorbic acid and met essential pharmacokinetic and safety criteria in silico based on ADMET predictions. Molecular docking further supported its high binding affinity and predicted stable interactions with GR and SOD, two central enzymes in cellular antioxidant defense. These findings suggest that E. floribundus stem bark is a valuable source of antioxidant compounds and highlight gallic acid as a promising lead compound for the development of therapeutic agents targeting oxidative stress-related diseases.
Beyond therapeutic prospects, the demonstrated antioxidant potential and safety profile of the extract and its constituents suggest possible applications in health protection and industry. These include incorporation as natural antioxidants in functional foods, nutraceuticals, and cosmeceutical products or use as natural preservatives in food and pharmaceutical formulations. While this study employed the DPPH assay, which primarily reflects hydrogen atom transfer (HAT)-based mechanisms, future studies should incorporate complementary assays such as FRAP, ORAC, CUPRAC, and ABTS to enable a broader evaluation of antioxidant capacity. Future studies involving in vivo validation and pharmacodynamic assessments are warranted to further explore its therapeutic potential against oxidative stress-related conditions. It should be noted that the present findings are based on material collected from a specific location and time point; future research should investigate seasonal and geographical influences on phytochemical composition and bioactivity.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/antiox14101161/s1. Copies of NMR and HRMS spectra of the isolated compounds.
Author Contributions
Conceptualization, data curation, methodology, software, and investigation, A.V.O.; validation, A.V.O., C.P. and A.M.D.; resources, A.V.O. and A.M.D.; writing—original draft preparation, A.V.O.; writing—review and editing, A.V.O., C.P. and A.M.D.; supervision, A.V.O. and A.M.D.; project administration, A.V.O. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the TWAS-CSIR 2015 PhD Fellowship Award and ANID Chile through Fondecyt Postdoctoral Grant 3240059. Universidad de La Frontera funded the article processing charge (APC).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The samples and any additional research data are available from the authors upon request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Olufunmilayo, E.O.; Gerke-Duncan, M.B.; Holsinger, R.M.D. Oxidative Stress and Antioxidants in Neurodegenerative Disorders. Antioxidants 2023, 12, 517. [Google Scholar] [CrossRef] [PubMed]
- Rudrapal, M.; Rakshit, G.; Singh, R.P.; Garse, S.; Khan, J.; Chakraborty, S. Dietary Polyphenols: Review on Chemistry/Sources, Bioavailability/Metabolism, Antioxidant Effects, and Their Role in Disease Management. Antioxidants 2024, 13, 429. [Google Scholar] [CrossRef] [PubMed]
- Jegadeshwari, B.; Thenmozhi, K.; Sanmuga Priya, E.; Saraswathy, S.D. An Overview on the Ethnopharmacological, Nutritional, and Phytochemical Perspectives of Elaeocarpus floribundus Blume. Curr. Pharmacol. Rep. 2023, 9, 377–389. [Google Scholar] [CrossRef]
- Amit Dadhich, A.D.; Anirudha Rishi, A.R.; Gargi Sharma, G.S.; Subhash Chandra, S.C. Phytochemicals of Elaeocarpus with Their Therapeutic Value: A Review. Int. J. Pharma Bio Sci. 2013, 4, P-591–P-598. [Google Scholar]
- Mahomoodally, M.F.; Sookhy, V. Ethnobotany and Pharmacological Uses of Elaeocarpus floribundus Blume (Elaeocarpaceae). In BT—Plant and Human Health; Ozturk, M., Hakeem, K.R., Eds.; Ethnobotany and Physiology; Springer International Publishing: Cham, Switzerland, 2018; Volume 1, pp. 125–137. [Google Scholar] [CrossRef]
- Ogundele, A.V.; Yadav, A.; Haldar, S.; Das, A.M. Antimicrobial Activities of Extract, Fractions and Isolated Compounds from the Fruits of Elaeocarpus floribundus Growing in North-East India. J. Herb. Med. 2021, 30, 100511. [Google Scholar] [CrossRef]
- Ogundele, A.V.; Yadav, A.; Das, A.M. Antimicrobial and α-Amylase Inhibitory Activities of Constituents from Elaeocarpus floribundus. Rev. Bras. Farmacogn. 2021, 31, 330–334. [Google Scholar] [CrossRef]
- Ogundele, A.V.; Haldar, S.; Yadav, A.; Das, A.M. Elaeocarpus floribundus Bl. Seeds as a New Source of Bioactive Compounds with Promising Antioxidant and Antimicrobial Properties. Z. Für Naturforschung C 2021, 76, 141–146. [Google Scholar] [CrossRef]
- Ogundele, A.V.; Das, A.M. Chemical Constituents from the Leaves of Elaeocarpus floribundus. Nat. Prod. Res. 2021, 35, 517–520. [Google Scholar] [CrossRef]
- Hossen, K.; Teruya, T.; Tojo, S.; Kato-Noguchi, H. Phytotoxicity and Identification of Active Compounds from Elaeocarpus floribundus Blume Plant for Controlling Weeds. Sci. World J. 2024, 2024, 4995447. [Google Scholar] [CrossRef]
- Utami, R.; Khalid, N.; Sukari, M.A.; Rahmani, M.; Abdul, A.B.; Dachriyanus, D. Phenolic Contents, Antioxidant and Cytotoxic Activities of Elaeocarpus floribundus Blume. Pak. J. Pharm. Sci. 2013, 26, 245–250. [Google Scholar]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M.B.T.-M. [14] Analysis of Total Phenols and Other Oxidation Substrates and Antioxidants by Means of Folin-Ciocalteu Reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar] [CrossRef]
- Stankovic, M.S.; Neda, N.; Marina, T.; Solujic, S. Total Phenolic Content, Flavonoid Concentrations and Antioxidant Activity, of the Whole Plant and Plant Parts Extracts from Teucrium montanum L. Var. Montanum, F. Supinum (L.) Reichenb. Biotechnol. Biotechnol. Equip. 2011, 25, 2222–2227. [Google Scholar] [CrossRef]
- Zhao, M.; Zhu, D.; Sun-Waterhouse, D.; Su, G.; Lin, L.; Wang, X.; Dong, Y. In Vitro and In Vivo Studies on Adlay-Derived Seed Extracts: Phenolic Profiles, Antioxidant Activities, Serum Uric Acid Suppression, and Xanthine Oxidase Inhibitory Effects. J. Agric. Food Chem. 2014, 62, 7771–7778. [Google Scholar] [CrossRef] [PubMed]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A Free Web Tool to Evaluate Pharmacokinetics, Drug-Likeness and Medicinal Chemistry Friendliness of Small Molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef]
- Banerjee, P.; Kemmler, E.; Dunkel, M.; Preissner, R. ProTox 3.0: A Webserver for the Prediction of Toxicity of Chemicals. Nucleic Acids Res. 2024, 52, W513–W520. [Google Scholar] [CrossRef]
- O’Boyle, N.M.; Banck, M.; James, C.A.; Morley, C.; Vandermeersch, T.; Hutchison, G.R. Open Babel: An Open Chemical Toolbox. J. Cheminform. 2011, 3, 33. [Google Scholar] [CrossRef]
- Alves, P.E.; Preet, G.; Dias, L.; Oliveira, M.; Silva, R.; Castro, I.; Silva, G.; Júnior, J.; Lima, N.; Silva, D.H.; et al. The Free Radical Scavenging Property of the Leaves, Branches, and Roots of Mansoa hirsuta DC: In Vitro Assessment, 3D Pharmacophore, and Molecular Docking Study. Molecules 2022, 27, 6016. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the Speed and Accuracy of Docking with a New Scoring Function, Efficient Optimization, and Multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef]
- Tanemossu, S.A.F.; Franke, K.; Arnold, N.; Schmidt, J.; Wabo, H.K.; Tane, P.; Wessjohann, L.A. Rare Biscoumarin Derivatives and Flavonoids from Hypericum riparium. Phytochemistry 2014, 105, 171–177. [Google Scholar] [CrossRef]
- Cha, J.M.; Kim, D.H.; Lee, T.H.; Subedi, L.; Kim, S.Y.; Lee, K.R. Phytochemical Constituents of Capsella bursa-Pastoris and Their Anti-Inflammatory Activity. Nat. Prod. Sci. 2018, 24, 132–138. [Google Scholar] [CrossRef]
- Nair, R.V.R.; Devi Velayudhan, J.; Prabath Gopalakrishnan, B.; Baby, S. Anti-Inflammatory and Anticancer Activities of Erythrodiol-3-Acetate and 2,4-Di-Tert-Butylphenol Isolated from Humboldtia unijuga. Nat. Prod. Res. 2020, 34, 2319–2322. [Google Scholar] [CrossRef] [PubMed]
- Abri, A.; Maleki, M. Isolation and Identification of Gallic Acid from the Elaeagnus Angustifolia Leaves and Determination of Total Phenolic, Flavonoids Contents and Investigation of Antioxidant Activity. Iran. Chem. Commun. 2016, 4, 146–154. [Google Scholar]
- Choi, S.J.; Hong, Y.D.; Lee, B.; Park, J.S.; Jeong, H.W.; Kim, W.G.; Shin, S.S.; Yoon, K.D. Separation of Polyphenols and Caffeine from the Acetone Extract of Fermented Tea Leaves (Camellia sinensis) Using High-Performance Countercurrent Chromatography. Molecules 2015, 20, 13216–13225. [Google Scholar] [CrossRef] [PubMed]
- Rani, J.M.S.; Akkarshana, P.; Neelaveni, V.; Mohan, S.; Rekha, P.D.; Rao, R.M.; Muthulakshmi, L. Evaluation of the Inhibitory Potential of Bioactive Compounds against SARS-CoV-2 by in Silico Approach. J. Mol. Model. 2024, 30, 60. [Google Scholar] [CrossRef]
- Castro, J.; Clauss, G.; Fontes, J.V.; Oliveira, L.S.; Abbehausen, C. Oxidative Stress Mechanism by Gold Compounds: A Close Look at Total ROS Increase and the Inhibition of Antioxidant Enzymes. Chem.—Asian J. 2025, 20, e202400792. [Google Scholar] [CrossRef]
- Chandimali, N.; Bak, S.G.; Park, E.H.; Lim, H.-J.; Won, Y.-S.; Kim, E.-K.; Park, S.-I.; Lee, S.J. Free Radicals and Their Impact on Health and Antioxidant Defenses: A Review. Cell Death Discov. 2025, 11, 19. [Google Scholar] [CrossRef]
- Utami, R.; Syahputra, R.; Dona, R.; Fadhli, H.; Furi, M.; Ikhtiarudin, I. Total flavonoid content and in vitro study on the sunscreen activity of extracts of leaves of Elaeocarpus floribundus blume. Pharm. Educ. 2023, 23, 118–121. [Google Scholar] [CrossRef]
- Sircar, B.; Mandal, S. Indian olive, Elaeocarpus floribundus fruit: Perspective to the antioxidative capacity and antibacterial activity. EC Microbiol. 2017, 12, 273–282. [Google Scholar]
- Pérez, M.; Dominguez-López, I.; Lamuela-Raventós, R.M. The chemistry behind the folin–ciocalteu method for the estimation of (poly) phenol content in food: Total phenolic intake in a Mediterranean dietary pattern. J. Agric. Food Chem. 2023, 71, 17543–17553. [Google Scholar] [CrossRef]
- Torres, P.; Osaki, S.; Silveira, E.; dos Santos, D.Y.; Chow, F. Comprehensive evaluation of Folin-Ciocalteu assay for total phenolic quantification in algae (Chlorophyta, Phaeophyceae, and Rhodophyta). Algal Res. 2024, 80, 103503. [Google Scholar] [CrossRef]
- Nicolescu, A.; Bunea, C.I.; Mocan, A. Total flavonoid content revised: An overview of past, present, and future determinations in phytochemical analysis. Anal. Biochem. 2025, 700, 115794. [Google Scholar] [CrossRef] [PubMed]
- Bhuyan, U.; Handique, J.G. Chapter 6—Plant Polyphenols as Potent Antioxidants: Highlighting the Mechanism of Antioxidant Activity and Synthesis/Development of Some Polyphenol Conjugates. Stud. Nat. Prod. Chem. 2022, 75, 243–266. [Google Scholar] [CrossRef]
- Zhou, Y.; Zheng, J.; Li, Y.; Xu, D.P.; Li, S.; Chen, Y.M.; Li, H.B. Natural polyphenols for prevention and treatment of cancer. Nutrients 2016, 8, 515. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Zou, F.; Liu, W. Recent advancement in prevention against hepatotoxicity, molecular mechanisms, and bioavailability of gallic acid, a natural phenolic compound: Challenges and perspectives. Front. Pharmacol. 2025, 16, 1549526. [Google Scholar] [CrossRef]
- Badhani, B.; Sharma, N.; Kakkar, R. Gallic acid: A versatile antioxidant with promising therapeutic and industrial applications. RSC Adv. 2015, 5, 27540–27557. [Google Scholar] [CrossRef]
- Ho, O.M.; Hoang, T.; Lee, C. Aromatic Residue F443 Modulates the Dimer Interface and Activity of Pseudomonas mandelii Glutathione Reductase. ACS Omega 2025, 10, 8709–8717. [Google Scholar] [CrossRef]
- Zheng, M.; Liu, Y.; Zhang, G.; Yang, Z.; Xu, W.; Chen, Q. The Applications and Mechanisms of Superoxide Dismutase in Medicine, Food, and Cosmetics. Antioxidants 2023, 12, 1675. [Google Scholar] [CrossRef]
- Lanez, E.; Mokhtar, S.; Lanez, T. Assessment of Antioxidant and DPPH Free Radical Scavenging Activity of 1,2-Dithiole-3-Thione Derivatives by Using Cyclic Voltammetry, Spectroscopic, and Molecular Docking Studies. J. Sulfur Chem. 2023, 44, 542–558. [Google Scholar] [CrossRef]
- Li, C.X.; Wang, F.R.; Zhang, B.; Deng, Z.Y.; Li, H.Y. Stability and Antioxidant Activity of Phenolic Compounds during in Vitro Digestion. J. Food Sci. 2023, 88, 696–716. [Google Scholar] [CrossRef]
- Soobrattee, M.A.; Neergheen, V.S.; Luximon-Ramma, A.; Aruoma, O.I.; Bahorun, T. Phenolics as potential antioxidant therapeutic agents: Mechanism and actions. Mutat. Res./Fundam. Mol. Mech. Mutagen. 2005, 579, 200–213. [Google Scholar] [CrossRef]
- Quideau, S.; Deffieux, D.; Douat-Casassus, C.; Pouységu, L. Plant polyphenols: Chemical properties, biological activities, and synthesis. Angew. Chem. Int. Ed. 2011, 50, 586–621. [Google Scholar] [CrossRef] [PubMed]
- Prasannan, P.; Jeyaram, Y.; Pandian, A.; Raju, R.; Sekar, S. A review on taxonomy, phytochemistry, pharmacology, threats and conservation of Elaeocarpus L. (Elaeocarpaceae). Bot. Rev. 2020, 86, 298–328. [Google Scholar] [CrossRef]
- Piao, M.J.; Kang, K.A.; Zhang, R.; Ko, D.O.; Wang, Z.H.; Lee, K.H.; Hyun, J.W. Antioxidant properties of 1, 2, 3, 4, 6-penta-O-galloyl-β-d-glucose from Elaeocarpus sylvestris var. ellipticus. Food Chem. 2009, 115, 412–418. [Google Scholar] [CrossRef]
- Sharma, S.; Hussain, S.; Rai, D.V.; Singh, A.N. A comprehensive analysis on the ecosystem services of Elaeocarpus L. (Elaeocarpaceae): A review. J. Phytol. 2023, 15, 12–37. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).