Effects of Zanthoxyli Pericarpium Extracts on Ligature-Induced Periodontitis and Alveolar Bone Loss in Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Test Materials
2.2. Cell Culture
2.3. Measurement of Nitric Oxide (NO) Production
2.4. Immunoblot Analysis
2.5. Animal Husbandry
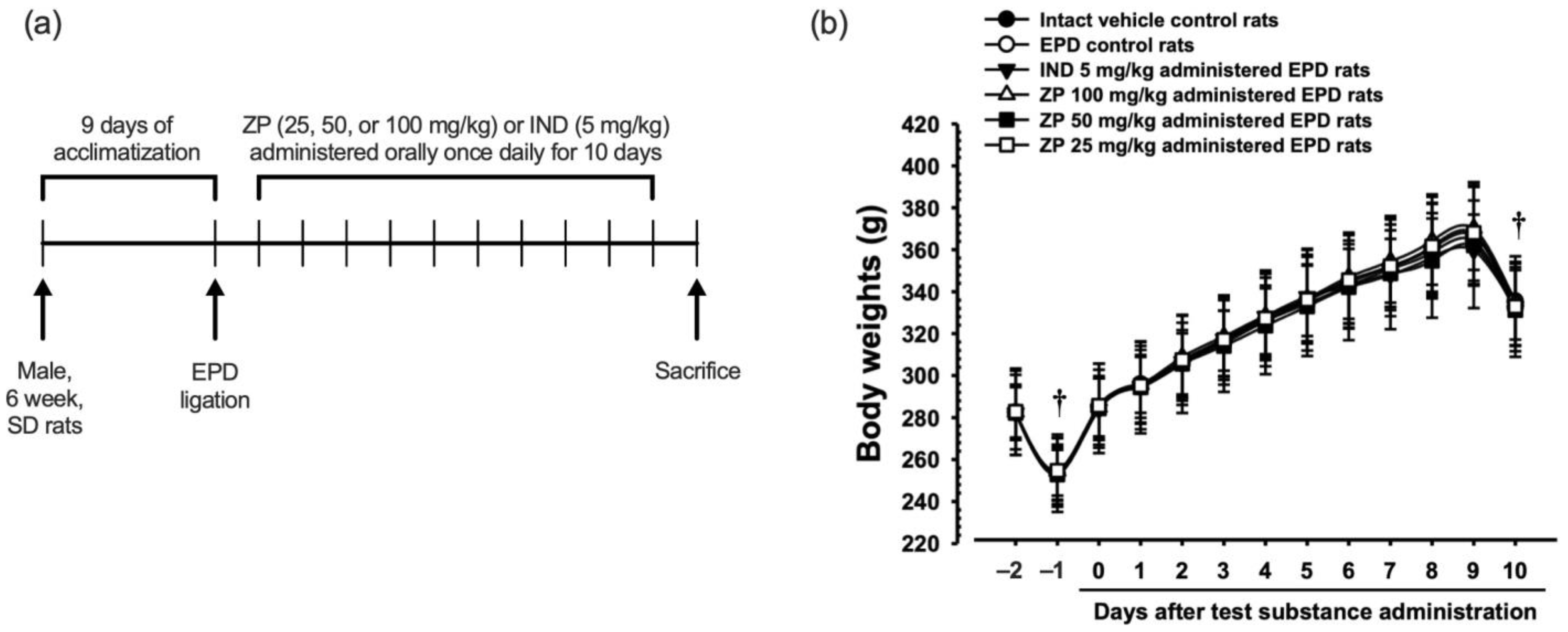
2.6. Induction of EPD
2.7. Measurement of Body Weights
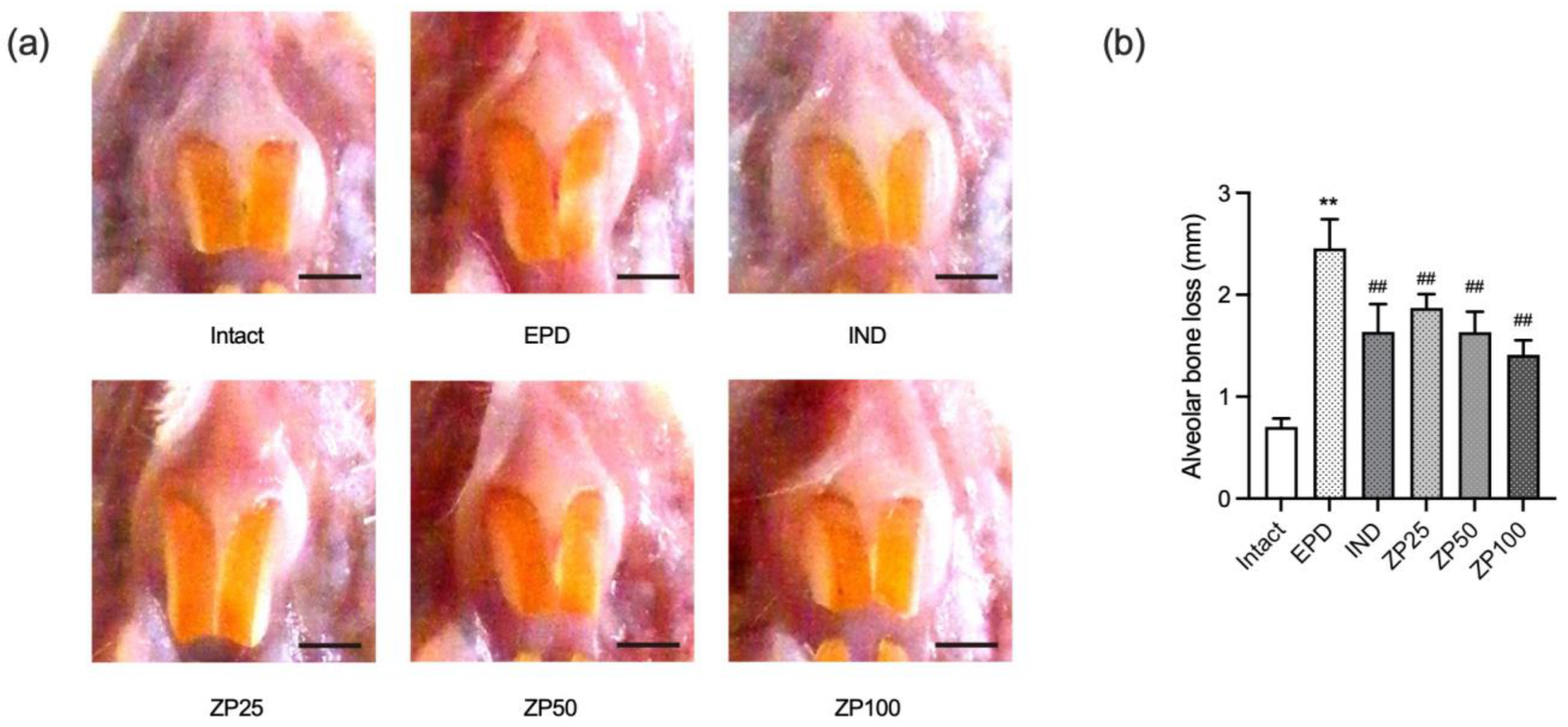
2.8. Measurement of Alveolar Bone Loss
2.9. Microbiological Analysis
2.10. Measurement of Myeloperoxidase (MPO) Activity
2.11. Radical Scavenging Assay
2.12. Measurement of Malondialdehyde (MDA) Levels
2.13. Measurement of Inducible Nitric Oxide Synthase (iNOS) Activity
2.14. Enzyme-Linked Immunosorbent Assay (ELISA)
2.15. Reverse Transcription Quantitative Polymerase Chain Reaction (RT-qPCR)
2.16. Histopathological Analysis
2.17. Statistical Analysis
3. Results
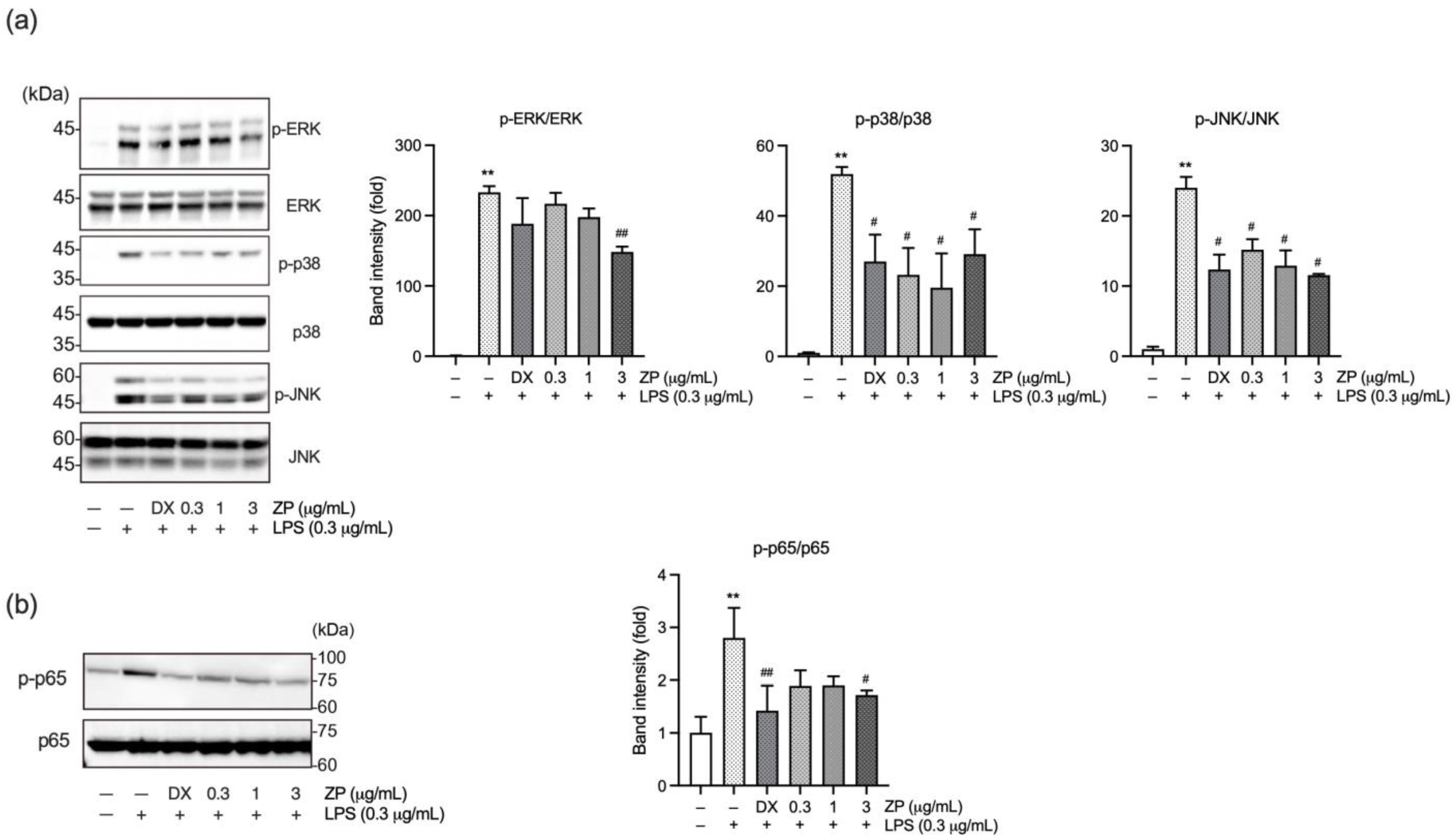
3.1. ZP Suppresses the Expression of Pro-Inflammatory Mediators in LPS-Stimulated RAW 264.7 Macrophages
3.2. ZP Suppresses LPS-Induced Inflammatory Response by Inhibiting Mitogen-Activated Protein Kinase (MAPK) and NF-κB Signaling Pathways
3.3. Experimental Schedule and Changes in Body Weight
3.4. ZP Attenuates Alveolar Bone Loss in EPD Rats
3.5. ZP Decreased the Gingival Anaerobic Bacterial Count and MPO Activity
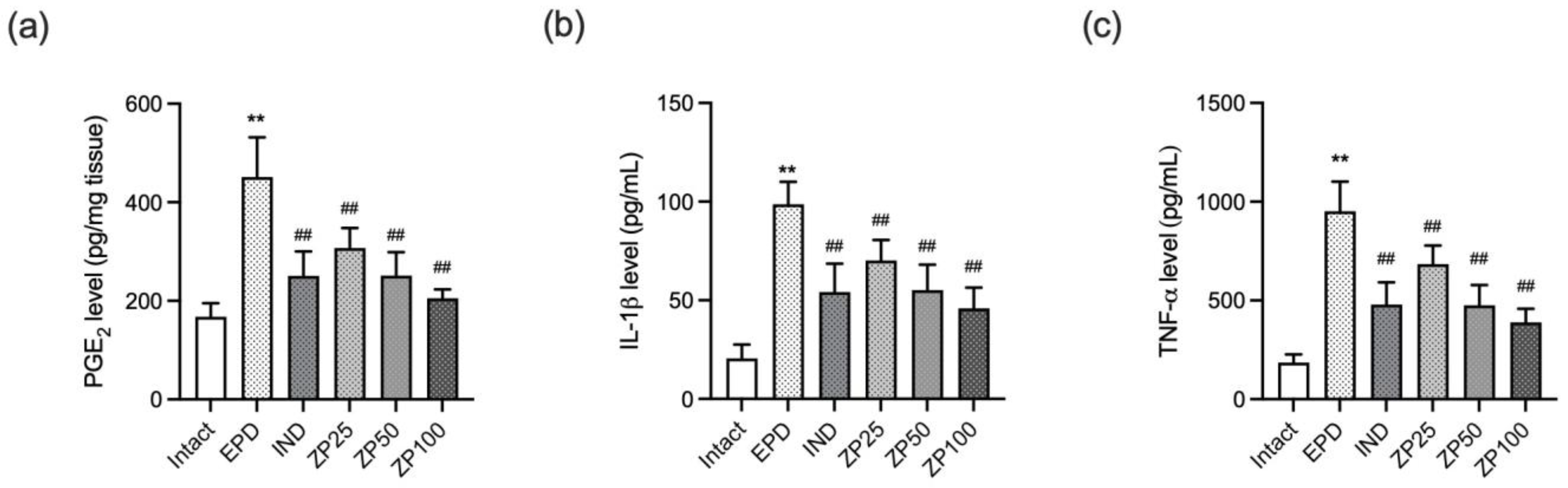
3.6. ZP Reduced the Gingival Expression of Pro-Inflammatory Mediators in EPD Rats
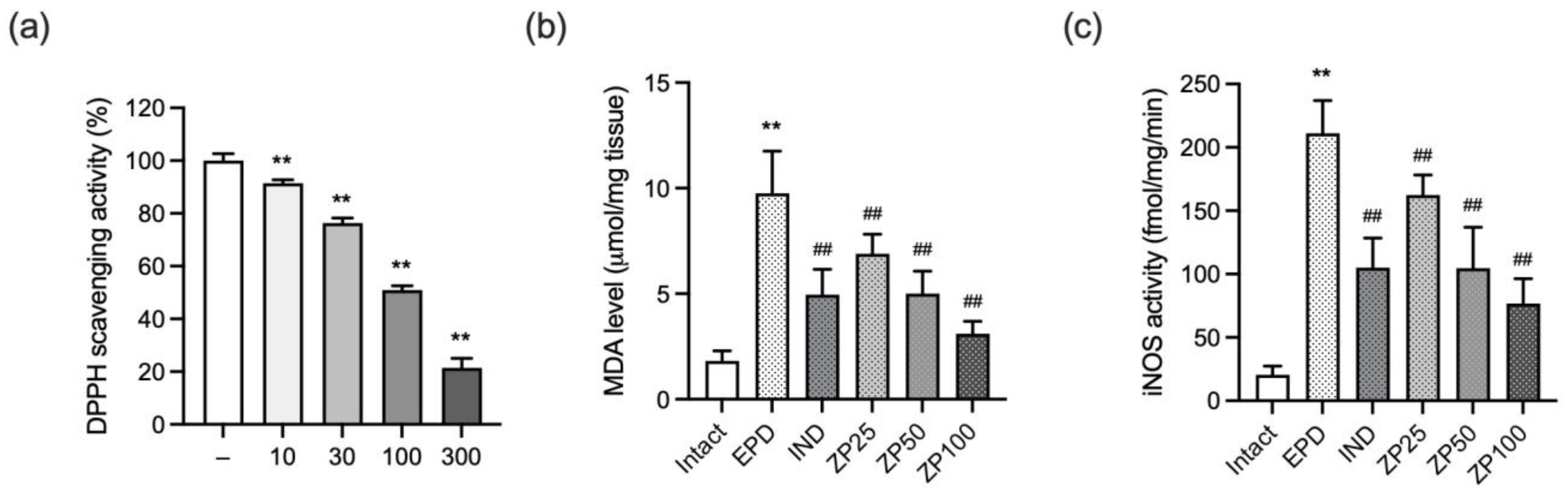
3.7. ZP Exhibits Antioxidant Effects by Reducing Free Radicals, Lipid Peroxidation, and Nitrosative Stress
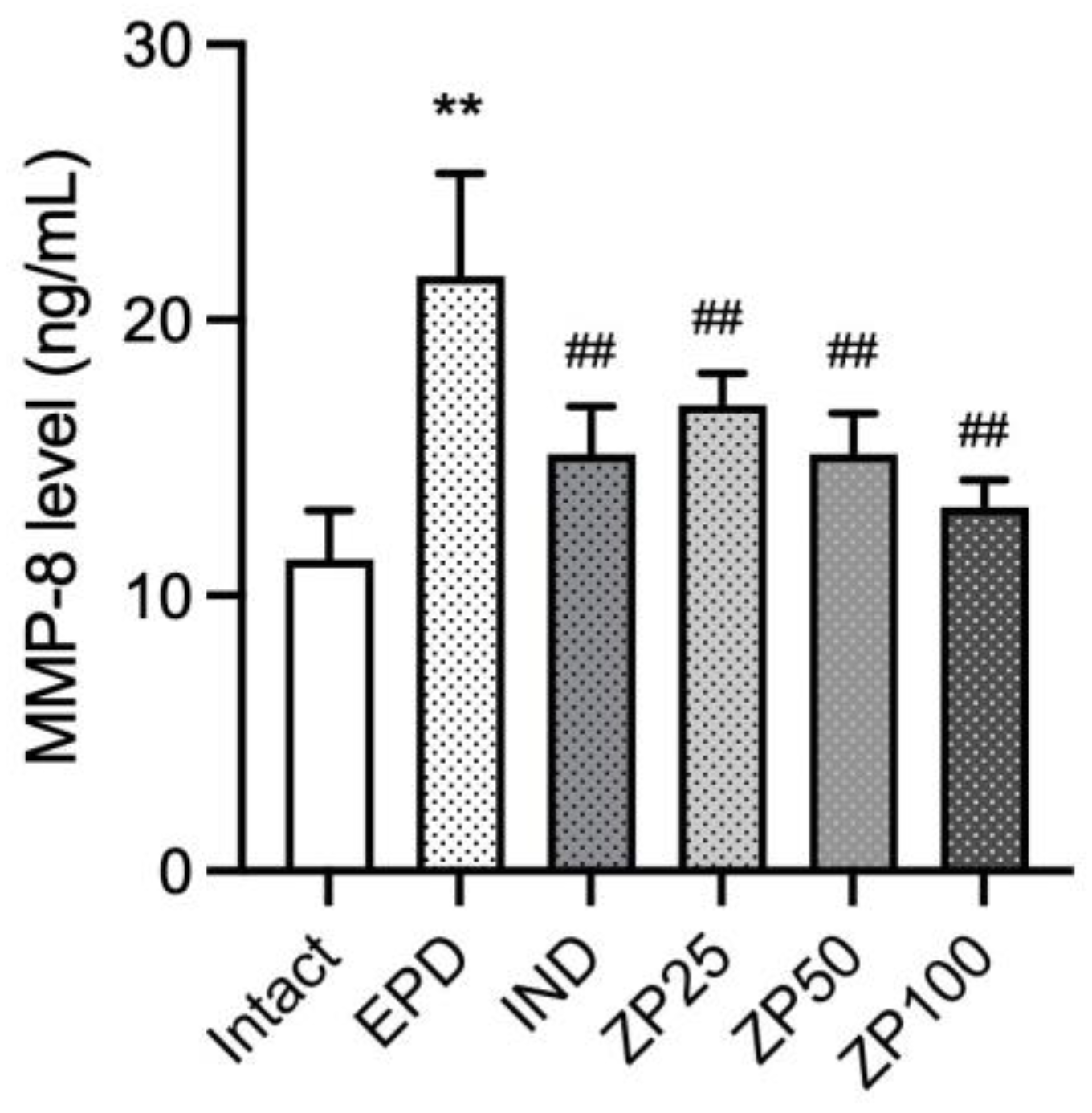
3.8. ZP Suppresses the Expression of MMP-8 in Gingival Tissues of EPD Rats
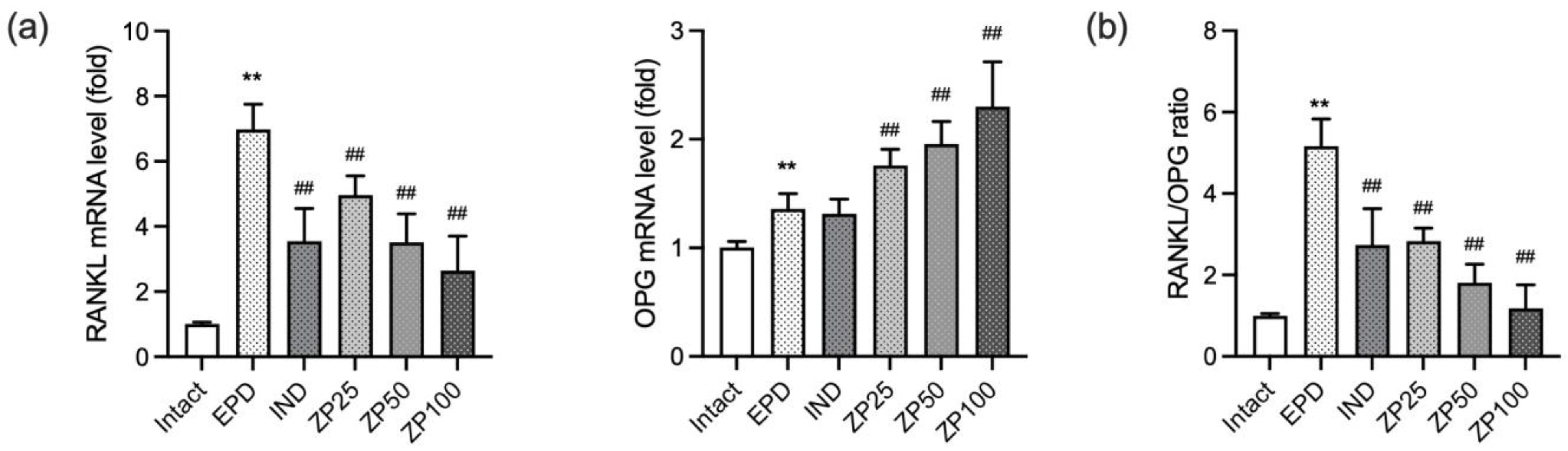
3.9. ZP Modulates Bone Remodeling in Periodontitis by Regulating the RANKL/Osteoprotegerin (OPG) Axis
3.10. Histopathological Changes in Maxillary Regions
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hajishengallis, G. Periodontitis: From microbial immune subversion to systemic inflammation. Nat. Rev. Immunol. 2015, 15, 30–44. [Google Scholar] [CrossRef]
- Jin, Q.; Cirelli, J.A.; Park, C.H.; Sugai, J.V.; Taba, M., Jr.; Kostenuik, P.J.; Giannobile, W.V. RANKL inhibition through osteoprotegerin blocks bone loss in experimental periodontitis. J. Periodontol. 2007, 78, 1300–1308. [Google Scholar] [CrossRef] [PubMed]
- Hajishengallis, G. New developments in neutrophil biology and periodontitis. Periodontol. 2000 2020, 82, 78–92. [Google Scholar] [CrossRef] [PubMed]
- Eke, P.I.; Borgnakke, W.S.; Genco, R.J. Recent epidemiologic trends in periodontitis in the USA. Periodontol. 2000 2020, 82, 257–267. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, G.G.; Alves-Costa, S.; Romandini, M. Burden of severe periodontitis and edentulism in 2021, with projections up to 2050: The Global Burden of Disease 2021 study. J. Periodontal Res. 2024, 59, 823–867. [Google Scholar] [CrossRef]
- Darby, I. Risk factors for periodontitis & peri-implantitis. Periodontol. 2000 2022, 90, 9–12. [Google Scholar] [CrossRef]
- Qin, X.; Zhao, Y.; Guo, Y. Periodontal disease and myocardial infarction risk: A meta-analysis of cohort studies. Am. J. Emerg. Med. 2021, 48, 103–109. [Google Scholar] [CrossRef]
- Zhang, Y.; Bian, C.E.; Yu, C.; Zhu, M.; Weir, M.D.; Xu, H.H.K.; Bai, Y.; Zhang, N. The Association between Periodontal Disease and Stroke Risk: A Systematic Review and Meta-Analysis. J. Evid. Based Dent. Pract. 2025, 25, 102172. [Google Scholar] [CrossRef]
- Graziani, F.; Karapetsa, D.; Alonso, B.; Herrera, D. Nonsurgical and surgical treatment of periodontitis: How many options for one disease? Periodontol. 2000 2017, 75, 152–188. [Google Scholar] [CrossRef]
- Jepsen, K.; Jepsen, S. Antibiotics/antimicrobials: Systemic and local administration in the therapy of mild to moderately advanced periodontitis. Periodontol. 2000 2016, 71, 82–112. [Google Scholar] [CrossRef]
- Yek, E.C.; Cintan, S.; Topcuoglu, N.; Kulekci, G.; Issever, H.; Kantarci, A. Efficacy of amoxicillin and metronidazole combination for the management of generalized aggressive periodontitis. J. Periodontol. 2010, 81, 964–974. [Google Scholar] [CrossRef]
- Heta, S.; Robo, I. The Side Effects of the Most Commonly Used Group of Antibiotics in Periodontal Treatments. Med. Sci. 2018, 6, 6. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Valverde, N.; Lopez-Valverde, A.; Montero, J.; Rodriguez, C.; Macedo de Sousa, B.; Aragoneses, J.M. Antioxidant, anti-inflammatory and antimicrobial activity of natural products in periodontal disease: A comprehensive review. Front. Bioeng. Biotechnol. 2023, 11, 1226907. [Google Scholar] [CrossRef]
- Mohammed, S.A.; Akram, H.M. Evaluating the Efficacy of Resveratrol-Containing Mouthwash as an Adjunct Treatment for Periodontitis: A Randomized Clinical Trial. Eur. J. Dent. 2025, 19, 354–365. [Google Scholar] [CrossRef] [PubMed]
- Park, S.M.; Kim, J.K.; Kim, E.O.; Jegal, K.H.; Jung, D.H.; Lee, S.G.; Cho, I.J.; Kim, S.; Byun, S.H.; Ku, S.K.; et al. Hepatoprotective Effect of Pericarpium zanthoxyli Extract Is Mediated via Antagonism of Oxidative Stress. Evid. Based Complement. Alternat. Med. 2020, 2020, 6761842. [Google Scholar] [CrossRef]
- Choi, W.G.; Ko, S.J.; Jung, D.; Kim, S.C.; Choi, N.R.; Park, J.W.; Kim, B.J. Therapeutic Effects of Zanthoxyli Pericarpium on Intestinal Inflammation and Network Pharmacological Mechanism Analysis in a Dextran Sodium Sulfate-Induced Colitis Mouse Model. Nutrients 2024, 16, 3521. [Google Scholar] [CrossRef]
- Bradley, P.P.; Christensen, R.D.; Rothstein, G. Cellular and extracellular myeloperoxidase in pyogenic inflammation. Blood 1982, 60, 618–622. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Song, H.; Lee, J.; Chung, H.; Kwon, Y.S.; Jegal, K.H.; Kim, J.K.; Ku, S.K. The Effect of the Root Bark of Lycium chinense (Lycii Radicis Cortex) on Experimental Periodontitis and Alveolar Bone Loss in Sprague-Dawley Rats. Antioxidants 2024, 13, 1332. [Google Scholar] [CrossRef]
- Cuzzocrea, S.; Zingarelli, B.; Hake, P.; Salzman, A.L.; Szabo, C. Antiinflammatory effects of mercaptoethylguanidine, a combined inhibitor of nitric oxide synthase and peroxynitrite scavenger, in carrageenan-induced models of inflammation. Free Radic. Biol. Med. 1998, 24, 450–459. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Kim, T.G.; Park, M.-R.; Ku, S.-K.; Heo, S.-M.; Kim, J.-L. Effects of Moringa oleifera L. and Eucommia ulmoides Oliver Mixed Formula on Ligation-Induced Experimental Periodontitis and Alveolar Bone Loss in Rats. J. Korean Soc. Food Sci. Nutr. 2022, 51, 765–779. [Google Scholar] [CrossRef]
- Park, S.-I.; Kang, S.-J.; Han, C.-H.; Kim, J.-W.; Song, C.-H.; Lee, S.-N.; Ku, S.-K.; Lee, Y.-J. The Effects of Topical Application of Polycal (a 2:98 (g/g) Mixture of Polycan and Calcium Gluconate) on Experimental Periodontitis and Alveolar Bone Loss in Rats. Molecules 2016, 21, 527. [Google Scholar] [CrossRef]
- Menezes, A.M.; Rocha, F.A.; Chaves, H.V.; Carvalho, C.B.; Ribeiro, R.A.; Brito, G.A. Effect of sodium alendronate on alveolar bone resorption in experimental periodontitis in rats. J. Periodontol. 2005, 76, 1901–1909. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Tang, E.; Guan, K.; Wang, C.Y. IKKβ plays an essential role in the phosphorylation of RelA/p65 on serine 536 induced by lipopolysaccharide. J. Immunol. 2003, 170, 5630–5635. [Google Scholar] [CrossRef]
- Toczewska, J.; Konopka, T.; Zalewska, A.; Maciejczyk, M. Nitrosative Stress Biomarkers in the Non-Stimulated and Stimulated Saliva, as well as Gingival Crevicular Fluid of Patients with Periodontitis: Review and Clinical Study. Antioxidants 2020, 9, 259. [Google Scholar] [CrossRef]
- Lin, P.; Niimi, H.; Ohsugi, Y.; Tsuchiya, Y.; Shimohira, T.; Komatsu, K.; Liu, A.; Shiba, T.; Aoki, A.; Iwata, T.; et al. Application of Ligature-Induced Periodontitis in Mice to Explore the Molecular Mechanism of Periodontal Disease. Int. J. Mol. Sci. 2021, 22, 8900. [Google Scholar] [CrossRef]
- Tomina, D.C.; Petrutiu, S.A.; Dinu, C.M.; Crisan, B.; Cighi, V.S.; Ratiu, I.A. Comparative Testing of Two Ligature-Induced Periodontitis Models in Rats: A Clinical, Histological and Biochemical Study. Biology 2022, 11, 634. [Google Scholar] [CrossRef]
- Duarte, P.M.; Tezolin, K.R.; Figueiredo, L.C.; Feres, M.; Bastos, M.F. Microbial profile of ligature-induced periodontitis in rats. Arch. Oral Biol. 2010, 55, 142–147. [Google Scholar] [CrossRef] [PubMed]
- Sczepanik, F.S.C.; Grossi, M.L.; Casati, M.; Goldberg, M.; Glogauer, M.; Fine, N.; Tenenbaum, H.C. Periodontitis is an inflammatory disease of oxidative stress: We should treat it that way. Periodontol. 2000 2020, 84, 45–68. [Google Scholar] [CrossRef] [PubMed]
- Chapple, I.L.; Matthews, J.B. The role of reactive oxygen and antioxidant species in periodontal tissue destruction. Periodontol. 2000 2007, 43, 160–232. [Google Scholar] [CrossRef]
- Hernandez, M.; Gamonal, J.; Tervahartiala, T.; Mantyla, P.; Rivera, O.; Dezerega, A.; Dutzan, N.; Sorsa, T. Associations between matrix metalloproteinase-8 and -14 and myeloperoxidase in gingival crevicular fluid from subjects with progressive chronic periodontitis: A longitudinal study. J. Periodontol. 2010, 81, 1644–1652. [Google Scholar] [CrossRef]
- Landzberg, M.; Doering, H.; Aboodi, G.M.; Tenenbaum, H.C.; Glogauer, M. Quantifying oral inflammatory load: Oral neutrophil counts in periodontal health and disease. J. Periodontal Res. 2015, 50, 330–336. [Google Scholar] [CrossRef] [PubMed]
- Cheng, R.; Wu, Z.; Li, M.; Shao, M.; Hu, T. Interleukin-1β is a potential therapeutic target for periodontitis: A narrative review. Int. J. Oral Sci. 2020, 12, 2. [Google Scholar] [CrossRef]
- Graves, D.T.; Oskoui, M.; Volejnikova, S.; Naguib, G.; Cai, S.; Desta, T.; Kakouras, A.; Jiang, Y. Tumor necrosis factor modulates fibroblast apoptosis, PMN recruitment, and osteoclast formation in response to P. gingivalis infection. J. Dent. Res. 2001, 80, 1875–1879. [Google Scholar] [CrossRef]
- Graves, D. Cytokines that promote periodontal tissue destruction. J. Periodontol. 2008, 79, 1585–1591. [Google Scholar] [CrossRef]
- Miyauchi, M.; Ijuhin, N.; Nikai, H.; Takata, T.; Ito, H.; Ogawa, I. Effect of exogenously applied prostaglandin E2 on alveolar bone losss—Histometric analysis. J. Periodontol. 1992, 63, 405–411. [Google Scholar] [CrossRef]
- Li, Q.; Valerio, M.S.; Kirkwood, K.L. MAPK usage in periodontal disease progression. J. Signal Transduct. 2012, 2012, 308943. [Google Scholar] [CrossRef]
- Arabaci, T.; Cicek, Y.; Canakci, V.; Canakci, C.F.; Ozgoz, M.; Albayrak, M.; Keles, O.N. Immunohistochemical and Stereologic Analysis of NF-κB Activation in Chronic Periodontitis. Eur. J. Dent. 2010, 4, 454–461. [Google Scholar]
- Birkedalhansen, H. Role of Matrix Metalloproteinases in Human Periodontal-Diseases. J. Periodontol. 1993, 64, 474–484. [Google Scholar] [CrossRef] [PubMed]
- Kinane, D.F.; Darby, I.B.; Said, S.; Luoto, H.; Sorsa, T.; Tikanoja, S.; Mantyla, P. Changes in gingival crevicular fluid matrix metalloproteinase-8 levels during periodontal treatment and maintenance. J. Periodontal Res. 2003, 38, 400–404. [Google Scholar] [CrossRef] [PubMed]
- Romanelli, R.; Mancini, S.; Laschinger, C.; Overall, C.M.; Sodek, J.; McCulloch, C.A. Activation of neutrophil collagenase in periodontitis. Infect. Immun. 1999, 67, 2319–2326. [Google Scholar] [CrossRef]
- Rai, B.; Kharb, S.; Jain, R.; Anand, S.C. Biomarkers of periodontitis in oral fluids. J. Oral Sci. 2008, 50, 53–56. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Rios, P.; Pussinen, P.J.; Vernal, R.; Hernandez, M. Oxidative Stress in the Local and Systemic Events of Apical Periodontitis. Front. Physiol. 2017, 8, 869. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.C.; Chen, H.S.; Chen, S.L.; Ho, Y.P.; Ho, K.Y.; Wu, Y.M.; Hung, C.C. Lipid peroxidation: A possible role in the induction and progression of chronic periodontitis. J. Periodontal Res. 2005, 40, 378–384. [Google Scholar] [CrossRef] [PubMed]
- Mohideen, K.; Chandrasekar, K.; Ramsridhar, S.; Rajkumar, C.; Ghosh, S.; Dhungel, S. Assessment of Oxidative Stress by the Estimation of Lipid Peroxidation Marker Malondialdehyde (MDA) in Patients with Chronic Periodontitis: A Systematic Review and Meta-Analysis. Int. J. Dent. 2023, 2023, 6014706. [Google Scholar] [CrossRef]
- Castro, M.M.L.; Duarte, N.N.; Nascimento, P.C.; Magno, M.B.; Fagundes, N.C.F.; Flores-Mir, C.; Monteiro, M.C.; Rosing, C.K.; Maia, L.C.; Lima, R.R. Antioxidants as Adjuvants in Periodontitis Treatment: A Systematic Review and Meta-Analysis. Oxid. Med. Cell. Longev. 2019, 2019, 9187978. [Google Scholar] [CrossRef]



| Target Gene | Orientation | Sequences (5′⟶3′) | NCBI Accession No. |
|---|---|---|---|
| RANKL | Sense | CTGATGAAAGGAGGGAGCAC, | NM_057149 |
| Antisense | GAAGGGTTGGACACCTGAATGC | ||
| OPG | Sense | TCCTGGCACCTACCTAAAACAGCA, | U94330 |
| Antisense | ACACTGGGCTGCAATACACA | ||
| β-actin | Sense | TCAGGTCATCACTATCGCCAAT, | NM_017008 |
| Antisense | AAAGAAAGGGTGTAAAACGCA | ||
| iNOS | Sense | GACAAGCTGCATGTGACATC, | NM_001313922.1 |
| Antisense | GCTGGTAGGTTCCTGTTGTT | ||
| COX-2 | Sense | TCCAGATCACATTTGATTGA, | NM_011198.5 |
| Antisense | TCTTTGACTGTGGGAGGATA | ||
| TNF-α | Sense | ATGAGCACAGAAAGCATGAT, | NM_013693.3 |
| Antisense | TACAGGCTTGTCACTCGAAT | ||
| IL-1β | Sense | ATGGCAACTGTTCCTGAACT, | NM_008361.4 |
| Antisense | CAGGACAGGTATAGATTCTT | ||
| MCP-1 | Sense | TGATCCCAATGAGTAGGCTGG, | NM_011333.3 |
| Antisense | ATGTCTGGACCCATTCCTTCT | ||
| GAPDH | Sense | AACGACCCCTTCATTGAC, | NM_001411843.1 |
| Antisense | TCCACGACATACTCAGCAC |
| Group | In Gingival Tissues | ||
|---|---|---|---|
| Histological Scores (Max = 3) | Inflammatory Cells (Cells/mm2) | Collagen Fibers (%/mm2) | |
| Intact vehicle | 0.40 ± 0.52 | 55.80 ± 16.29 | 77.42 ± 10.75 |
| EPD | 2.80 ± 0.42 ** | 734.60 ± 68.72 ** | 14.82 ± 3.74 ** |
| IND | 1.60 ± 0.52 ## | 262.80 ± 89.03 ## | 50.42 ± 7.83 ## |
| ZP (25 mg/kg) | 2.00 ± 0.47 # | 377.60 ± 114.03 ## | 34.93 ± 6.91 ## |
| ZP (50 mg/kg) | 1.60 ± 0.52 ## | 265.80 ± 99.82 ## | 50.11 ± 7.96 ## |
| ZP (100 mg/kg) | 1.00 ± 0.47 ## | 115.60 ± 35.54 ## | 60.85 ± 7.42 ## |
| Group | In Alveolar Bone Regions | ||
|---|---|---|---|
| Alveolar Bone Volume (%) | Osteoclast (Cells/mm2) | OC/BS (%) | |
| Intact vehicle | 78.08 ± 6.55 | 7.40 ± 2.84 | 3.73 ± 1.46 |
| EPD | 21.34 ± 6.16 ** | 50.40 ± 7.88 ** | 67.47 ± 7.01 ** |
| IND | 52.37 ± 7.49 ## | 27.20 ± 5.90 ## | 30.41 ± 9.20 ## |
| ZP (25 mg/kg) | 39.62 ± 8.18 ## | 35.20 ± 3.43 ## | 45.33 ± 5.96 ## |
| ZP (50 mg/kg) | 52.61 ± 8.98 ## | 27.60 ± 5.32 ## | 30.11 ± 8.76 ## |
| ZP (100 mg/kg) | 64.34 ± 10.21 ## | 16.20 ± 3.33 ## | 17.64 ± 5.72 ## |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.-S.; Choi, B.-R.; Choi, G.-L.; Park, H.-R.; Kwon, J.-G.; Seo, C.-G.; Kim, J.-K.; Ku, S.-K. Effects of Zanthoxyli Pericarpium Extracts on Ligature-Induced Periodontitis and Alveolar Bone Loss in Rats. Antioxidants 2025, 14, 1159. https://doi.org/10.3390/antiox14101159
Kim J-S, Choi B-R, Choi G-L, Park H-R, Kwon J-G, Seo C-G, Kim J-K, Ku S-K. Effects of Zanthoxyli Pericarpium Extracts on Ligature-Induced Periodontitis and Alveolar Bone Loss in Rats. Antioxidants. 2025; 14(10):1159. https://doi.org/10.3390/antiox14101159
Chicago/Turabian StyleKim, Jang-Soo, Beom-Rak Choi, Geun-Log Choi, Hye-Rim Park, Jin-Gwan Kwon, Chan-Gon Seo, Jae-Kwang Kim, and Sae-Kwang Ku. 2025. "Effects of Zanthoxyli Pericarpium Extracts on Ligature-Induced Periodontitis and Alveolar Bone Loss in Rats" Antioxidants 14, no. 10: 1159. https://doi.org/10.3390/antiox14101159
APA StyleKim, J.-S., Choi, B.-R., Choi, G.-L., Park, H.-R., Kwon, J.-G., Seo, C.-G., Kim, J.-K., & Ku, S.-K. (2025). Effects of Zanthoxyli Pericarpium Extracts on Ligature-Induced Periodontitis and Alveolar Bone Loss in Rats. Antioxidants, 14(10), 1159. https://doi.org/10.3390/antiox14101159








