Involvement of Neuroinflammation and Oxidative Stress in L-DOPA-Induced Dyskinesia in Parkinson’s Disease: Role of Renin–Angiotensin System and ROCK Pathway
Abstract
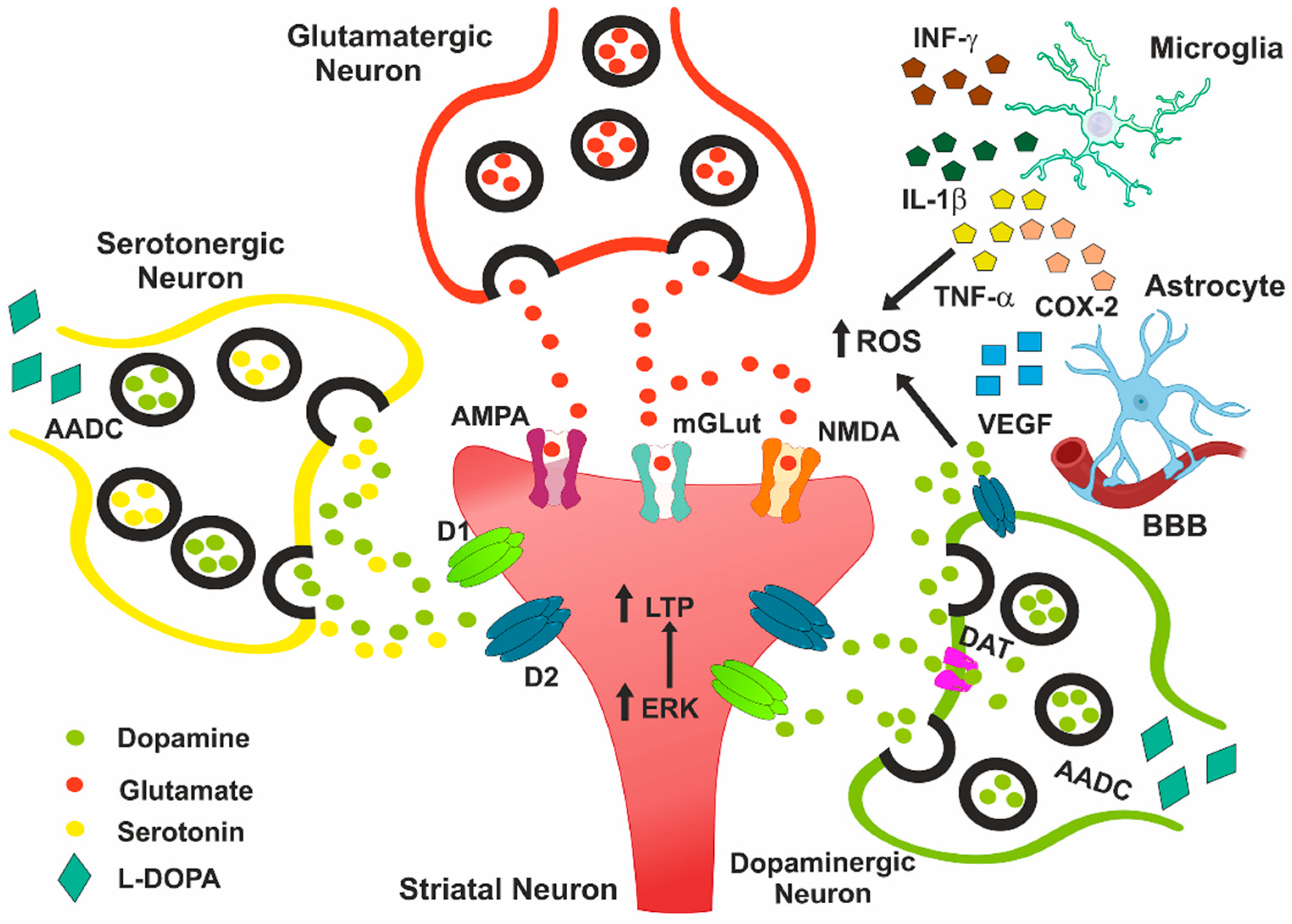
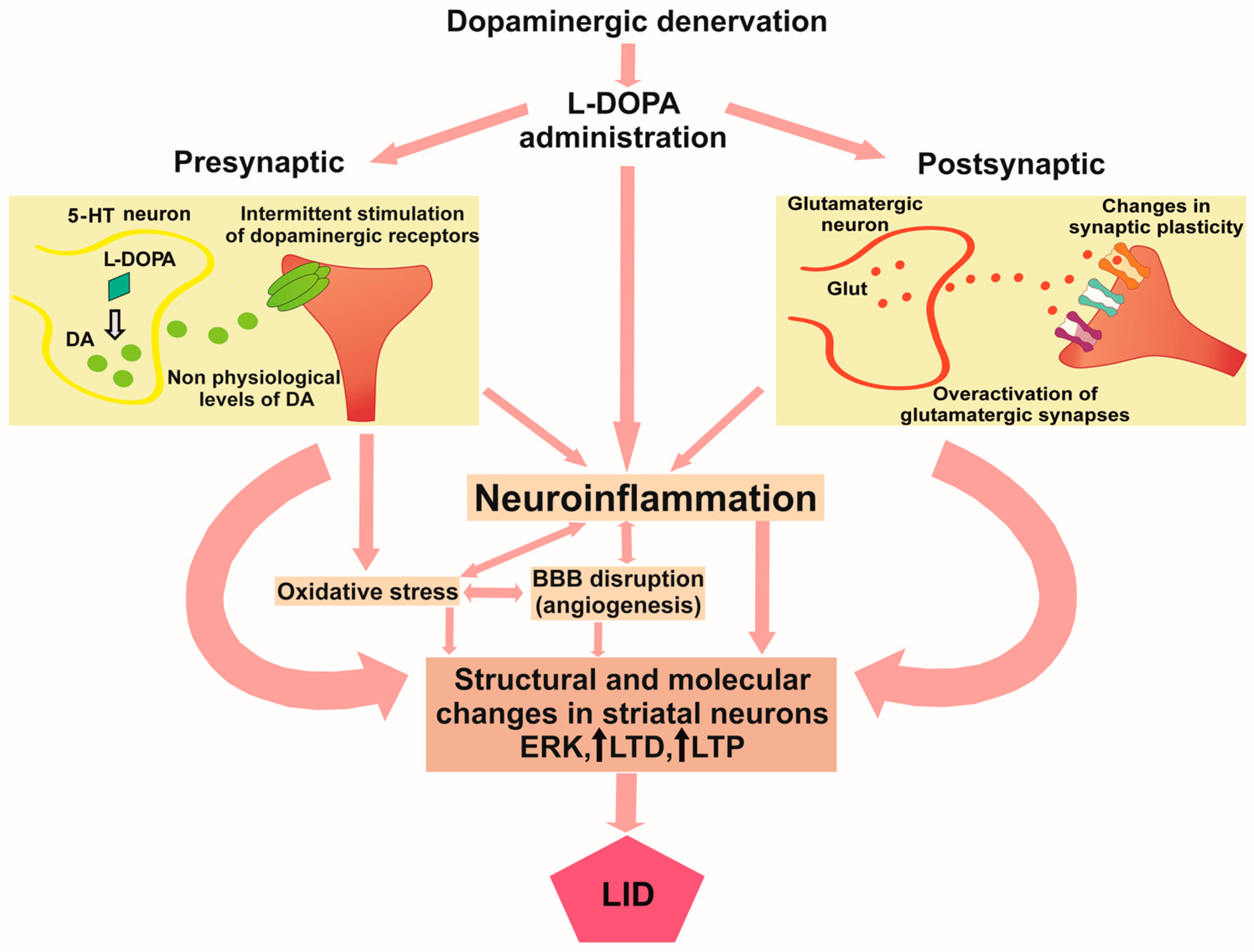
1. Introduction
2. Role of Oxidative Stress in Pathophysiology of LID
3. Role of Neuroinflammation in LID
3.1. Interleukin-1β
3.2. Tumor Necrosis Factor-Alpha
3.3. Interferon-Gamma
3.4. Cyclooxygenase-2
3.5. Vascular Endothelial Growth Factor
4. Therapeutic Perspectives for LID Targeting Inflammation and Oxidative Stress
5. Renin–Angiotensin System and ROCK Pathway as Therapeutic Targets of LID
5.1. Effect of Renin–Angiotensin System on LID
5.2. Effect of Rho Kinase Pathway on LID
6. Concluding Remarks and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 5-HTP | 5-hydroxy-tryptophan |
| 6-OHDA | 6-hydroxydopamine |
| AADC | L-amino acid decarboxylase |
| ACE | Angiotensin-converting enzyme |
| ALS | Amyotrophic lateral sclerosis |
| AMPA | α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid |
| Ang II | Angiotensin II |
| AT1 | Angiotensin II type-1 receptor |
| AAV | Adeno-associated virus |
| BBB | Blood–brain barrier |
| BDNF | Brain-derived neurotrophic factor |
| COMT | Catechol-O-methyltransferase |
| COX2 | Cyclooxygenase-2 |
| CRBN | Cereblon |
| CSF | Cerebrospinal fluid |
| DA | Dopamine |
| ERK | Extracellular-regulated kinase |
| GAD | Glutamic acid decarboxylase |
| GDNF | Glial-derived neurotrophic factor |
| GFAP | Glial fibrillary acidic protein |
| GSH | Glutathione |
| IFN-γ | Interferon-gamma |
| IL-1β | Interleukin-1 beta |
| iNOS | Inducible nitric oxide synthase |
| KO | Knockout |
| LID | L-DOPA-induced dyskinesia |
| LTD | Long-term depression |
| LTP | Long-term potentiation |
| MAO-B | Monoamine oxidase B |
| MDA | Malondialdehyde |
| MPTP | 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine |
| NMDA | N-methyl-D-aspartate glutamate |
| NO | Nitric oxide |
| NOS | Nitric oxide synthase |
| P75NTR | p75 neurotrophic receptor |
| PD | Parkinson’s disease |
| PG | Prostaglandin |
| RAS | Renin–angiotensin system |
| RNAi | Ribonucleic acid interference |
| ROCK | Rho kinase |
| ROS | Reactive oxygen species |
| TFG-β | Transforming growth factor-beta |
| TNF-α | Tumor necrosis factor-alpha |
| VEGF | Vascular endothelial growth factor |
| WT | Wild type |
References
- Van Schependom, J.; D’haeseleer, M. Advances in Neurodegenerative Diseases. J. Clin. Med. 2023, 12, 1709. [Google Scholar] [CrossRef]
- Ou, Z.; Pan, J.; Tang, S.; Duan, D.; Yu, D.; Nong, H.; Wang, Z. Global Trends in the Incidence, Prevalence, and Years Lived With Disability of Parkinson’s Disease in 204 Countries/Territories from 1990 to 2019. Front. Public Health 2021, 9, 776847. [Google Scholar] [CrossRef]
- McGregor, M.M.; Nelson, A.B. Circuit Mechanisms of Parkinson’s Disease. Neuron 2019, 101, 1042–1056. [Google Scholar] [CrossRef]
- Hu, W.; Wang, W.; Liao, H.; Bulloch, G.; Zhang, X.; Shang, X.; Huang, Y.; Hu, Y.; Yu, H.; Yang, X.; et al. Metabolic profiling reveals circulating biomarkers associated with incident and prevalent Parkinson’s disease. npj Park. Dis. 2024, 10, 130. [Google Scholar] [CrossRef]
- Janssen Daalen, J.M.; van den Bergh, R.; Prins, E.M.; Moghadam, M.S.C.; van den Heuvel, R.; Veen, J.; Mathur, S.; Meijerink, H.; Mirelman, A.; Darweesh, S.K.L.; et al. Digital biomarkers for non-motor symptoms in Parkinson’s disease: The state of the art. npj Digit. Med. 2024, 7, 186. [Google Scholar] [CrossRef]
- Quattrone, A.; Zappia, M.; Quattrone, A. Simple biomarkers to distinguish Parkinson’s disease from its mimics in clinical practice: A comprehensive review and future directions. Front. Neurol. 2024, 15, 1460576. [Google Scholar] [CrossRef]
- Stocchi, F.; Bravi, D.; Emmi, A.; Antonini, A. Parkinson disease therapy: Current strategies and future research priorities. Nat. Rev. Neurol. 2024, 20, 695–707. [Google Scholar] [CrossRef]
- Cilia, R.; Cereda, E.; Akpalu, A.; Sarfo, F.S.; Cham, M.; Laryea, R.; Obese, V.; Oppon, K.; Del Sorbo, F.; Bonvegna, S.; et al. Natural history of motor symptoms in Parkinson’s disease and the long-duration response to levodopa. Brain 2020, 143, 2490–2501. [Google Scholar] [CrossRef]
- Cenci, M.A.; Kumar, A. Cells, pathways, and models in dyskinesia research. Curr. Opin. Neurobiol. 2024, 84, 102833. [Google Scholar] [CrossRef]
- Cesaroni, V.; Blandini, F.; Cerri, S. Dyskinesia and Parkinson’s disease: Animal model, drug targets, and agents in preclinical testing. Expert. Opin. Ther. Targets. 2022, 26, 837–851. [Google Scholar] [CrossRef]
- Kwon, D.K.; Kwatra, M.; Wang, J.; Ko, H.S. Levodopa-Induced Dyskinesia in Parkinson’s Disease: Pathogenesis and Emerging Treatment Strategies. Cells 2022, 11, 3736. [Google Scholar] [CrossRef]
- Zhang, F.; Liu, M.; Tuo, J.; Zhang, L.; Zhang, J.; Yu, C.; Xu, Z. Levodopa-induced dyskinesia: Interplay between the N-methyl-D-aspartic acid receptor and neuroinflammation. Front. Immunol. 2023, 14, 1253273. [Google Scholar] [CrossRef]
- Eusebi, P.; Romoli, M.; Paoletti, F.P.; Tambasco, N.; Calabresi, P.; Parnetti, L. Risk factors of levodopa-induced dyskinesia in Parkinson’s disease: Results from the PPMI cohort. npj Park. Dis. 2018, 4, 33. [Google Scholar] [CrossRef]
- Cenci, M.A.; Crossman, A.R. Animal models of l-dopa-induced dyskinesia in Parkinson’s disease. Mov. Disord. 2018, 33, 889–899. [Google Scholar] [CrossRef]
- Lane, E.; Dunnett, S. Animal models of Parkinson’s disease and L-dopa induced dyskinesia: How close are we to the clinic? Psychopharmacology 2008, 199, 303–312. [Google Scholar] [CrossRef]
- Servillo, F.; De Carluccio, M.; Di Lazzaro, G.; Campanelli, F.; Marino, G.; Natale, G.; Ledonne, A.; Massaro Cenere, M.; Paldino, E.; Di Giuda, D.; et al. Molecular and cellular determinants of L-Dopa-induced dyskinesia in Parkinson’s Disease. npj Park. Dis. 2024, 10, 228. [Google Scholar] [CrossRef]
- Bove, F.; Angeloni, B.; Sanginario, P.; Rossini, P.M.; Calabresi, P.; Di Iorio, R. Neuroplasticity in levodopa-induced dyskinesias: An overview on pathophysiology and therapeutic targets. Prog. Neurobiol. 2024, 232, 102548. [Google Scholar] [CrossRef]
- Campanelli, F.; Natale, G.; Marino, G.; Ghiglieri, V.; Calabresi, P. Striatal glutamatergic hyperactivity in Parkinson’s disease. Neurobiol. Dis. 2022, 168, 105697. [Google Scholar] [CrossRef]
- Mellone, M.; Gardoni, F. Glutamatergic mechanisms in L-DOPA-induced dyskinesia and therapeutic implications. J. Neural Transm. 2018, 125, 1225–1236. [Google Scholar] [CrossRef]
- Kang, W.; Frouni, I.; Kwan, C.; Desbiens, L.; Hamadjida, A.; Huot, P. Activation of mGlu 2/3 receptors with the orthosteric agonist LY-404,039 alleviates dyskinesia in experimental parkinsonism. Behav. Pharmacol. 2024, 35, 185–192. [Google Scholar] [CrossRef]
- Yabumoto, T.; Kochoian, B.A.; Coletta, S.; Laur, O.; Huang, X.; Bure, C.A.; Ware, C.; Jin, P.; Traynelis, S.F.; Papa, S.M. Striatal GluN2A gene suppression reduces L-DOPA-induced abnormal involuntary movements in parkinsonian rats. Neuropharmacology 2025, 279, 110616. [Google Scholar] [CrossRef]
- Huang, Y.T.; Chen, Y.W.; Lin, T.Y.; Chen, J.C. Suppression of presynaptic corticostriatal glutamate activity attenuates L-dopa-induced dyskinesia in 6-OHDA-lesioned Parkinson’s disease mice. Neurobiol. Dis. 2024, 193, 106452. [Google Scholar] [CrossRef]
- Hattori, N.; Kamei, T.; Ishida, T.; Suzuki, I.; Nomoto, M.; Tsuboi, Y. Correction: Long-term effects of safinamide adjunct therapy on levodopa-induced dyskinesia in Parkinson’s disease: Post-hoc analysis of a Japanese phase III study. J. Neural Transm. 2022, 129, 1393–1394. [Google Scholar] [CrossRef]
- Li, J.; Zhang, J.; Meng, P. A meta-analysis of the effectiveness and safety of safinamide for levodopa-induced motor complications in Parkinson’s disease. Biotechnol. Genet. Eng. Rev. 2024, 40, 4627–4638. [Google Scholar] [CrossRef]
- Carta, M.; Carlsson, T.; Kirik, D.; Björklund, A. Dopamine released from 5-HT terminals is the cause of L-DOPA-induced dyskinesia in parkinsonian rats. Brain 2007, 130 Pt 7, 1819–1833. [Google Scholar] [CrossRef]
- Lopez, A.; Muñoz, A.; Guerra, M.J.; Labandeira-Garcia, J.L. Mechanisms of the effects of exogenous levodopa on the dopamine-denervated striatum. Neuroscience 2001, 103, 639–651. [Google Scholar] [CrossRef]
- Miguelez, C.; Benazzouz, A.; Ugedo, L.; De Deurwaerdère, P. Impairment of Serotonergic Transmission by the Antiparkinsonian Drug L-DOPA: Mechanisms and Clinical Implications. Front. Cell Neurosci. 2017, 11, 274. [Google Scholar] [CrossRef]
- Rylander, D.; Parent, M.; O’Sullivan, S.S.; Dovero, S.; Lees, A.J.; Bezard, E.; Descarries, L.; Cenci, M.A. Maladaptive plasticity of serotonin axon terminals in levodopa-induced dyskinesia. Ann. Neurol. 2010, 68, 619–628. [Google Scholar] [CrossRef]
- Corsi, S.; Stancampiano, R.; Carta, M. Serotonin/dopamine interaction in the induction and maintenance of L-DOPA-induced dyskinesia: An update. Prog. Brain Res. 2021, 261, 287–302. [Google Scholar] [CrossRef]
- Lanza, K.; Bishop, C. Serotonergic targets for the treatment of L-DOPA-induced dyskinesia. J. Neural Transm. 2018, 125, 1203–1216. [Google Scholar] [CrossRef]
- Muñoz, A.; Li, Q.; Gardoni, F.; Marcello, E.; Qin, C.; Carlsson, T.; Kirik, D.; Di Luca, M.; Björklund, A.; Bezard, E.; et al. Combined 5-HT1A and 5-HT1B receptor agonists for the treatment of L-DOPA-induced dyskinesia. Brain 2008, 131 Pt 12, 3380–3394. [Google Scholar] [CrossRef]
- Muñoz, A.; Lopez-Lopez, A.; Labandeira, C.M.; Labandeira-Garcia, J.L. Interactions Between the Serotonergic and Other Neurotransmitter Systems in the Basal Ganglia: Role in Parkinson’s Disease and Adverse Effects of L-DOPA. Front. Neuroanat. 2020, 14, 26. [Google Scholar] [CrossRef]
- Lipari, N.; Galfano, A.; Venkatesh, S.; Grezenko, H.; Sandoval, I.M.; Manfredsson, F.P.; Bishop, C. The effects of chemogenetic targeting of serotonin-projecting pathways on L-DOPA-induced dyskinesia and psychosis in a bilateral rat model of Parkinson’s disease. Front. Neural Circuits 2024, 18, 1463941. [Google Scholar] [CrossRef]
- Goetz, C.G.; Damier, P.; Hicking, C.; Laska, E.; Müller, T.; Olanow, C.W.; Rascol, O.; Russ, H. Sarizotan as a treatment for dyskinesias in Parkinson’s disease: A double-blind placebo-controlled trial. Mov. Disord. 2007, 22, 179–186. [Google Scholar] [CrossRef]
- Chen, Y.H.; Kuo, T.T.; Wang, V.; Cheng, P.W.; Huang, E.Y.; Ma, K.H.; Greig, N.H.; Olson, L.; Hoffer, B.J.; Tseng, K.Y.J. Serotonergic Regulation of Synaptic Dopamine Levels Mitigates L-DOPA-Induced Dyskinesia in a Mouse Model of Parkinson’s Disease. J. Park. Dis. 2024, 14, 941–964. [Google Scholar] [CrossRef]
- Zajdel, P.; Matłoka, M.; Konieczny, J.; Kos, T.; Lammers, J.C.; Cavalco, N.G.; Clark, A.A.; Lenda, T.; Satała, G.; Canale, V.; et al. Simultaneous 5-HT(1B)R agonist/5-HT(6)R antagonist action as a potential treatment of Parkinson’s disease and its comorbidities. J. Pharmacol. Exp. Ther. 2025, 392, 100055. [Google Scholar] [CrossRef]
- Ohno, Y.; Okita, E.; Kawai-Uchida, M.; Shoukei, Y.; Soshiroda, K.; Kanda, T.; Uchida, S. The adenosine A2A receptor antagonist/inverse agonist, KW-6356 enhances the anti-parkinsonian activity of L-DOPA with a low risk of dyskinesia in MPTP-treated common marmosets. J. Pharmacol. Sci. 2023, 52, 193–199. [Google Scholar] [CrossRef]
- Carta, A.R.; Mulas, G.; Bortolanza, M.; Duarte, T.; Pillai, E.; Fisone, G.; Vozari, R.R.; Del-Bel, E. l-DOPA-induced dyskinesia and neuroinflammation: Do microglia and astrocytes play a role? Eur. J. Neurosci. 2017, 45, 73–91. [Google Scholar] [CrossRef]
- Aron Badin, R.; Spinnewyn, B.; Gaillard, M.C.; Jan, C.; Malgorn, C.; Van Camp, N.; Dollé, F.; Guillermier, M.; Boulet, S.; Bertrand, A.; et al. IRC-082451, a novel multitargeting molecule, reduces L-DOPA-induced dyskinesias in MPTP Parkinsonian primates. PLoS ONE 2013, 8, e52680. [Google Scholar] [CrossRef]
- Lopez-Lopez, A.; Labandeira, C.M.; Labandeira-Garcia, J.L.; Muñoz, A. Rho kinase inhibitor fasudil reduces l-DOPA-induced dyskinesia in a rat model of Parkinson’s disease. Br. J. Pharmacol. 2020, 177, 5622–5641. [Google Scholar] [CrossRef]
- Muñoz, A.; Garrido-Gil, P.; Dominguez-Meijide, A.; Labandeira-Garcia, J.L. Angiotensin type 1 receptor blockage reduces l-dopa-induced dyskinesia in the 6-OHDA model of Parkinson’s disease. Involvement of vascular endothelial growth factor and interleukin-1beta. Exp. Neurol. 2014, 261, 720–732. [Google Scholar] [CrossRef]
- Zheng, C.Q.; Fan, H.X.; Li, X.X.; Li, J.J.; Sheng, S.; Zhang, F. Resveratrol Alleviates Levodopa-Induced Dyskinesia in Rats. Front. Immunol. 2021, 12, 683577. [Google Scholar] [CrossRef]
- Labandeira-Garcia, J.L.; Rodríguez-Perez, A.I.; Villar-Cheda, B.; Borrajo, A.; Dominguez-Meijide, A.; Guerra, M.J. Rho Kinase and Dopaminergic Degeneration: A Promising Therapeutic Target for Parkinson’s Disease. Neuroscientist 2015, 21, 616–629. [Google Scholar] [CrossRef]
- Labandeira-Garcia, J.L.; Labandeira, C.M.; Guerra, M.J.; Rodriguez-Perez, A.I. The role of the brain renin-angiotensin system in Parkinson’s disease. Transl. Neurodegener. 2024, 13, 22. [Google Scholar] [CrossRef]
- Lopez-Lopez, A.; Valenzuela, R.; Rodriguez-Perez, A.I.; Guerra, M.J.; Labandeira-Garcia, J.L.; Muñoz, A. Interactions between Angiotensin Type-1 Antagonists, Statins, and ROCK Inhibitors in a Rat Model of L-DOPA-Induced Dyskinesia. Antioxidants 2023, 12, 1454. [Google Scholar] [CrossRef]
- Park, H.Y.; Lee, G.S.; Go, J.; Ryu, Y.K.; Lee, C.H.; Moon, J.H.; Kim, K.S. Angiotensin-converting enzyme inhibition prevents l-dopa-induced dyskinesia in a 6-ohda-induced mouse model of Parkinson’s disease. Eur. J. Pharmacol. 2024, 973, 176573. [Google Scholar] [CrossRef]
- Rivas-Santisteban, R.; Rodriguez-Perez, A.I.; Muñoz, A.; Reyes-Resina, I.; Labandeira-García, J.L.; Navarro, G.; Franco, R. Angiotensin AT1 and AT2 receptor heteromer expression in the hemilesioned rat model of Parkinson’s disease that increases with levodopa-induced dyskinesia. J. Neuroinflamm. 2020, 17, 243. [Google Scholar] [CrossRef]
- Rivas-Santisteban, R.; Muñoz, A.; Lillo, J.; Raïch, I.; Rodríguez-Pérez, A.I.; Navarro, G.; Labandeira-García, J.L.; Franco, R. Cannabinoid regulation of angiotensin II-induced calcium signaling in striatal neurons. npj Park. Dis. 2024, 10, 220. [Google Scholar] [CrossRef]
- Mulas, G.; Espa, E.; Fenu, S.; Spiga, S.; Cossu, G.; Pillai, E.; Carboni, E.; Simbula, G.; Jadžić, D.; Angius, F.; et al. Differential induction of dyskinesia and neuroinflammation by pulsatile versus continuous l-DOPA delivery in the 6-OHDA model of Parkinson’s disease. Exp. Neurol. 2016, 286, 83–92. [Google Scholar] [CrossRef]
- Zhang, S.F.; Xie, C.L.; Lin, J.Y.; Wang, M.H.; Wang, X.J.; Liu, Z.G. Lipoic acid alleviates L–DOPA–induced dyskinesia in 6–OHDA parkinsonian rats via anti–oxidative stress. Mol. Med. Rep. 2018, 17, 1118–1124. [Google Scholar] [CrossRef]
- Li, D.W.; Wang, Y.D.; Zhou, S.Y.; Sun, W.P. alpha-lipoic acid exerts neuroprotective effects on neuronal cells by upregulating the expression of PCNA via the P53 pathway in neurodegenerative conditions. Mol. Med. Rep. 2016, 14, 4360–4366. [Google Scholar] [CrossRef]
- Zhang, J.; Gao, Y.; Zhang, L.; Zhang, C.; Zhao, Y.; Zhang, Y.; Li, S.; Chang, C.; Zhang, X.; Yang, G. Alpha-Lipoic Acid Attenuates MPTP/MPP(+)-Induced Neurotoxicity: Roles of SIRT1-Dependent PGC-1alpha Signaling Pathways. Neurotox. Res. 2022, 40, 410–419. [Google Scholar] [CrossRef]
- Arbo, B.D.; André-Miral, C.; Nasre-Nasser, R.G.; Schimith, L.E.; Santos, M.G.; Costa-Silva, D.; Muccillo-Baisch, A.L.; Hort, M.A. Resveratrol Derivatives as Potential Treatments for Alzheimer’s and Parkinson’s Disease. Front. Aging Neurosci. 2020, 12, 103. [Google Scholar] [CrossRef]
- da Silva Barroso, S.; Lopes, L.E.S.; Dos Santos, A.M.G.; Neto, R.V.B.; Dos Santos Lima, B.; de Souza Araújo, A.A.; Cardoso, J.C.; Severino, P.; Souto, E.B.; Gomes, M.Z. Liquiritigenin-Rich Hydroalcoholic Extract of Brazilian Red Propolis Reduces Dyskinesia Induced by 3,4- Dihydroxyphenylalanine in Hemiparkinsonian Rats. Basic Clin. Pharmacol. Toxicol. 2025, 137, e70062. [Google Scholar] [CrossRef]
- Li, Q.M.; Xu, H.; Zha, X.Q.; Zhang, F.Y.; Luo, J.P. Polygonatum cyrtonema polysaccharide alleviates dopaminergic neuron apoptosis in Parkinson’s disease mouse model via inhibiting oxidative stress and endoplasmic reticulum stress. Int. J. Biol. Macromol. 2025, 311 Pt 3, 143986. [Google Scholar] [CrossRef]
- Sarkar, S.; Roy, A.; Choudhury, S.; Banerjee, R.; Dey, S.; Kumar, H. Levodopa-induced Dyskinesia in Parkinson’s Disease: Plausible Inflammatory and Oxidative Stress Biomarkers. Can. J. Neurol. Sci. 2024, 51, 104–109. [Google Scholar] [CrossRef] [PubMed]
- Padovan-Neto, F.E.; Ferreira, N.R.; de Oliveira-Tavares, D.; de Aguiar, D.; da Silva, C.A.; Raisman-Vozari, R.; Del Bel, E. Anti-dyskinetic effect of the neuronal nitric oxide synthase inhibitor is linked to decrease of FosB/deltaFosB expression. Neurosci. Lett. 2013, 541, 126–131. [Google Scholar] [CrossRef]
- Takuma, K.; Tanaka, T.; Takahashi, T.; Hiramatsu, N.; Ota, Y.; Ago, Y.; Matsuda, T. Neuronal nitric oxide synthase inhibition attenuates the development of L-DOPA-induced dyskinesia in hemi-Parkinsonian rats. Eur. J. Pharmacol. 2012, 683, 166–173. [Google Scholar] [CrossRef]
- Bortolanza, M.; Cavalcanti-Kiwiatkoski, R.; Padovan-Neto, F.E.; da-Silva, C.A.; Mitkovski, M.; Raisman-Vozari, R.; Del-Bel, E. Glial activation is associated with l-DOPA induced dyskinesia and blocked by a nitric oxide synthase inhibitor in a rat model of Parkinson’s disease. Neurobiol. Dis. 2015, 73, 377–387. [Google Scholar] [CrossRef] [PubMed]
- Solís, O.; Espadas, I.; Del-Bel, E.A.; Moratalla, R. Nitric oxide synthase inhibition decreases l-DOPA-induced dyskinesia and the expression of striatal molecular markers in Pitx3(-/-) aphakia mice. Neurobiol. Dis. 2015, 73, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Kaya, I.; Vallianatou, T.; Nilsson, B.P.; Shariatgorji, R.; Svenningsson, P.; Bezard, E.; Andrén, P.E. Brain-region-specific lipid dysregulation in L-DOPA-induced dyskinesia in a primate model of Parkinson’s disease. npj Park. Dis. 2025, 11, 258. [Google Scholar] [CrossRef]
- Keeney, M.T.; Hoffman, E.K.; Farmer, K.; Bodle, C.R.; Fazzari, M.; Zharikov, A.; Castro, S.L.; Hu, X.; Mortimer, A.; Kofler, J.K.; et al. NADPH oxidase 2 activity in Parkinson’s disease. Neurobiol. Dis. 2022, 170, 105754. [Google Scholar] [CrossRef]
- Rodriguez-Pallares, J.; Parga, J.A.; Muñoz, A.; Rey, P.; Guerra, M.J.; Labandeira-Garcia, J.L. Mechanism of 6-hydroxydopamine neurotoxicity: The role of NADPH oxidase and microglial activation in 6-hydroxydopamine-induced degeneration of dopaminergic neurons. J. Neurochem. 2007, 103, 145–156. [Google Scholar] [CrossRef]
- Rodriguez-Pallares, J.; Rey, P.; Parga, J.A.; Muñoz, A.; Guerra, M.J.; Labandeira-Garcia, J.L. Brain angiotensin enhances dopaminergic cell death via microglial activation and NADPH-derived ROS. Neurobiol. Dis. 2008, 31, 58–73. [Google Scholar] [CrossRef]
- Boonpraman, N.; Yi, S.S. NADPH oxidase 4 (NOX4) as a biomarker and therapeutic target in neurodegenerative diseases. Neural Regen. Res. 2023, 19, 1961–1966. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Ying, C.; Si, X.; Xue, N.; Liu, Y.; Zheng, R.; Chen, Y.; Pu, J.; Zhang, B. NOX4 exacerbates Parkinson’s disease pathology by promoting neuronal ferroptosis and neuroinflammation. Neural Regen. Res. 2025, 20, 2038–2052. [Google Scholar] [CrossRef]
- Spencer, J.P.; Jenner, P.; Halliwell, B. Superoxide-dependent depletion of reduced glutathione by L-DOPA and dopamine. Relevance to Parkinson’s disease. Neuroreport 1995, 6, 1480–1484. [Google Scholar] [CrossRef] [PubMed]
- Ben-Shachar, D.; Zuk, R.; Gazawi, H.; Ljubuncic, P. Dopamine toxicity involves mitochondrial complex I inhibition: Implications to dopamine-related neuropsychiatric disorders. Biochem. Pharmacol. 2004, 67, 1965–1974. [Google Scholar] [CrossRef] [PubMed]
- Naydenov, A.V.; Vassoler, F.; Luksik, A.S.; Kaczmarska, J.; Konradi, C. Mitochondrial abnormalities in the putamen in Parkinson’s disease dyskinesia. Acta Neuropathol. 2010, 120, 623–631. [Google Scholar] [CrossRef]
- Zhang, F.; Ye, Z.; Xie, Y.; Liu, M.; Zhang, L.; Zhang, J.; Xu, Z. Levodopa-induced dyskinesia: Brain iron deposition as a new hypothesis. Biometals 2024, 37, 1307–1323. [Google Scholar] [CrossRef]
- Bishop, C. Neuroinflammation: Fanning the fire of l-dopa-induced dyskinesia. Mov. Disord. 2019, 34, 1758–1760. [Google Scholar] [CrossRef]
- Pisanu, A.; Boi, L.; Mulas, G.; Spiga, S.; Fenu, S.; Carta, A.R. Neuroinflammation in L-DOPA-induced dyskinesia: Beyond the immune function. J. Neural Transm. 2018, 125, 1287–1297. [Google Scholar] [CrossRef]
- Cardinale, A.; de Iure, A.; Picconi, B. Neuroinflammation and Dyskinesia: A Possible Causative Relationship? Brain Sci. 2024, 14, 514. [Google Scholar] [CrossRef] [PubMed]
- Barnum, C.J.; Eskow, K.L.; Dupre, K.; Blandino, P., Jr.; Deak, T.; Bishop, C. Exogenous corticosterone reduces L-DOPA-induced dyskinesia in the hemi-parkinsonian rat: Role for interleukin-1beta. Neuroscience 2008, 156, 30–41. [Google Scholar] [CrossRef] [PubMed]
- Lanza, K.; Perkins, A.E.; Deak, T.; Bishop, C. Late aging-associated increases in L-DOPA-induced dyskinesia are accompanied by heightened neuroinflammation in the hemi-parkinsonian rat. Neurobiol. Aging 2019, 81, 190–199. [Google Scholar] [CrossRef]
- Ferrari, D.P.; Bortolanza, M.; Del Bel, E.A. Interferon-gamma Involvement in the Neuroinflammation Associated with Parkinson’s Disease and L-DOPA-Induced Dyskinesia. Neurotox. Res. 2021, 39, 705–719. [Google Scholar] [CrossRef]
- Morissette, M.; Bourque, M.; Tremblay, M.È.; Di Paolo, T. Prevention of L-Dopa-Induced Dyskinesias by MPEP Blockade of Metabotropic Glutamate Receptor 5 Is Associated with Reduced Inflammation in the Brain of Parkinsonian Monkeys. Cells 2022, 11, 691. [Google Scholar] [CrossRef] [PubMed]
- Santos-Lobato, B.L.; Bortolanza, M.; Pinheiro, L.C.; Batalhão, M.E.; Pimentel, Â.V.; Capellari-Carnio, E.; Del-Bel, E.A.; Tumas, V. Levodopa-induced dyskinesias in Parkinson’s disease increase cerebrospinal fluid nitric oxide metabolites’ levels. J. Neural Transm. 2022, 129, 55–63. [Google Scholar] [CrossRef]
- Yan, A.; Song, L.; Zhang, Y.; Wang, X.; Liu, Z. Systemic Inflammation Increases the Susceptibility to Levodopa-Induced Dyskinesia in 6-OHDA Lesioned Rats by Targeting the NR2B-Medicated PKC/MEK/ERK Pathway. Front. Aging Neurosci. 2021, 12, 625166. [Google Scholar] [CrossRef]
- Kuter, K.Z.; Cenci, M.A.; Carta, A.R. The role of glia in Parkinson’s disease: Emerging concepts and therapeutic applications. Prog. Brain Res. 2020, 252, 131–168. [Google Scholar] [CrossRef]
- Ryu, Y.K.; Park, H.Y.; Kim, J.E.; Seo, H.H.; Lee, C.H.; Kim, K.S. Sci Rep. Regulating astrocytic activity in the dorsal striatum mitigates L-dopa-induced dyskinesia in Parkinson’s disease. Sci. Rep. 2025, 15, 26635. [Google Scholar] [CrossRef]
- Favetta, G. Bubacco. L. Beyond neurons: How does dopamine signaling impact astrocytic functions and pathophysiology. Prog. Neurobiol. 2025, 251, 102798. [Google Scholar] [CrossRef]
- Lanza, K.; Bishop, C. Dopamine D3 Receptor Plasticity in Parkinson’s Disease and L-DOPA-Induced Dyskinesia. Biomedicines 2021, 9, 314. [Google Scholar] [CrossRef]
- Dyavar, S.R.; Potts, L.F.; Beck, G.; Dyavar Shetty, B.L.; Lawson, B.; Podany, A.T.; Fletcher, C.V.; Amara, R.R.; Papa, S.M. Transcriptomic approach predicts a major role for transforming growth factor beta type 1 pathway in L-Dopa-induced dyskinesia in parkinsonian rats. Genes Brain Behav. 2020, 19, e12690. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.; Rong, L.; Li, Q.; Liu, S.; Li, W.; Ye, M.; Luo, W.; Xie, A.; Shao, J.; Guo, D.; et al. Integrative Approaches Identify Genetic Determinants of Levodopa Induced Dyskinesia. Mol. Neurobiol. 2025, 62, 11572–11580. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos Pereira, M.; Abreu, G.H.D.; Rocca, J.; Hamadat, S.; Raisman-Vozari, R.; Michel, P.P.; Del Bel, E. Contributive Role of TNF-α to L-DOPA-Induced Dyskinesia in a Unilateral 6-OHDA Lesion Model of Parkinson’s Disease. Front. Pharmacol. 2021, 11, 617085. [Google Scholar] [CrossRef] [PubMed]
- Brahadeeswaran, S.; Sivagurunathan, N.; Calivarathan, L. Inflammasome Signaling in the Aging Brain and Age-Related Neurodegenerative Diseases. Mol. Neurobiol. 2022, 59, 2288–2304. [Google Scholar] [CrossRef]
- Villar-Cheda, B.; Valenzuela, R.; Rodriguez-Perez, A.I.; Guerra, M.J.; Labandeira-Garcia, J.L. Aging-related changes in the nigral angiotensin system enhances proinflammatory and pro-oxidative markers and 6-OHDA-induced dopaminergic degeneration. Neurobiol. Aging 2012, 33, e1–e11. [Google Scholar] [CrossRef]
- Yuan, Y.; Zhang, Y.; Cheng, Y.; Hou, Y.; Huang, Z.; Ma, J.; Li, N.; Zhan, S. Cerebrospinal Fluid TNF-alpha and Orexin in Patients With Parkinson’s Disease and Rapid Eye Movement Sleep Behavior Disorder. Front. Neurol. 2022, 13, 826013. [Google Scholar] [CrossRef]
- Zamanian, M.Y.; Golmohammadi, M.; Gardanova, Z.R.; Rahimi, M.; Khachatryan, L.G.; Khazaei, M. The Roles of Neuroinflammation in l-DOPA-Induced Dyskinesia: Dissecting the Roles of NF-kappaB and TNF-alpha for Novel Pharmacological Therapeutic Approaches. Eur. J. Neurosci. 2025, 61, e70034. [Google Scholar] [CrossRef]
- Mogi, M.; Kondo, T.; Mizuno, Y.; Nagatsu, T. p53 protein, interferon-gamma, and NF-kappaB levels are elevated in the parkinsonian brain. Neurosci. Lett. 2007, 414, 94–97. [Google Scholar] [CrossRef]
- Azar, Y.O.; Badawi, G.A.; Zaki, H.F.; Ibrahim, S.M. Agmatine-mediated inhibition of NMDA receptor expression and amelioration of dyskinesia via activation of Nrf2 and suppression of HMGB1/RAGE/TLR4/MYD88/NF-κB signaling cascade in rotenone lesioned rats. Life Sci. 2022, 311 Pt A, 121049. [Google Scholar] [CrossRef]
- Rentsch, P.; Stayte, S.; Egan, T.; Clark, I.; Vissel, B. Targeting the cannabinoid receptor CB2 in a mouse model of l-dopa induced dyskinesia. Neurobiol. Dis. 2020, 134, 104646. [Google Scholar] [CrossRef]
- Dos Santos Pereira, M.; Dias de Abreu, G.H.; Vanderlei, L.C.A.; Raisman-Vozari, R.; Guimarães, F.S.; Lu, H.C.; Michel, P.P.; Del Bel, E. 4’-fluorocannabidiol associated with capsazepine restrains L-DOPA-induced dyskinesia in hemiparkinsonian mice: Contribution of anti-inflammatory and anti-glutamatergic mechanisms. Neuropharmacology 2024, 251, 109926. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos Pereira, M.; Del Bel, E. Persistent COX-2 upregulation in L-DOPA-induced dyskinesia is unaffected by inhibition with celecoxib. Neuropharmacology 2025, 278, 110560. [Google Scholar] [CrossRef]
- Dos Santos Pereira, M.; do Nascimento, G.C.; Bortolanza, M.; Michel, P.P.; Raisman-Vozari, R.; Del Bel, E. Doxycycline attenuates l-DOPA-induced dyskinesia through an anti-inflammatory effect in a hemiparkinsonian mouse model. Front. Pharmacol. 2022, 13, 1045465. [Google Scholar] [CrossRef]
- Boi, L.; Pisanu, A.; Greig, N.H.; Scerba, M.T.; Tweedie, D.; Mulas, G.; Fenu, S.; Carboni, E.; Spiga, S.; Carta, A.R. Immunomodulatory drugs alleviate l-dopa-induced dyskinesia in a rat model of Parkinson’s disease. Mov. Disord. 2019, 34, 1818–1830. [Google Scholar] [CrossRef]
- Ohlin, K.E.; Francardo, V.; Lindgren, H.S.; Sillivan, S.E.; O’Sullivan, S.S.; Luksik, A.S.; Vassoler, F.M.; Lees, A.J.; Konradi, C.; Cenci, M.A. Vascular endothelial growth factor is upregulated by L-dopa in the parkinsonian brain: Implications for the development of dyskinesia. Brain 2011, 134 Pt 8, 2339–2357. [Google Scholar] [CrossRef]
- Teismann, P.; Tieu, K.; Choi, D.K.; Wu, D.C.; Naini, A.; Hunot, S.; Vila, M.; Jackson-Lewis, V.; Przedborski, S. Cyclooxygenase-2 is instrumental in Parkinson’s disease neurodegeneration. Proc. Natl. Acad. Sci. USA 2003, 100, 5473–5478. [Google Scholar] [CrossRef] [PubMed]
- Bortolanza, M.; Padovan-Neto, F.E.; Cavalcanti-Kiwiatkoski, R.; Dos Santos-Pereira, M.; Mitkovski, M.; Raisman-Vozari, R.; Del-Bel, E. Are cyclooxygenase-2 and nitric oxide involved in the dyskinesia of Parkinson’s disease induced by L-DOPA? Philos. Trans. R. Soc. Lond. B Biol. Sci. 2015, 370, 20140190. [Google Scholar] [CrossRef] [PubMed]
- Lerner, R.P.; Francardo, V.; Fujita, K.; Bimpisidis, Z.; Jourdain, V.A.; Tang, C.C.; Dewey, S.L.; Chaly, T.; Cenci, M.A.; Eidelberg, D. Levodopa-induced abnormal involuntary movements correlate with altered permeability of the blood-brain-barrier in the basal ganglia. Sci. Rep. 2017, 7, 16005. [Google Scholar] [CrossRef] [PubMed]
- Ohlin, K.E.; Sebastianutto, I.; Adkins, C.E.; Lundblad, C.; Lockman, P.R.; Cenci, M.A. Impact of L-DOPA treatment on regional cerebral blood flow and metabolism in the basal ganglia in a rat model of Parkinson’s disease. Neuroimage 2012, 61, 228–239. [Google Scholar] [CrossRef]
- Balzano, T.; Del Rey, N.L.; Esteban-García, N.; Reinares-Sebastián, A.; Pineda-Pardo, J.A.; Trigo-Damas, I.; Obeso, J.A.; Blesa, J. Neurovascular and immune factors of vulnerability of substantia nigra dopaminergic neurons in non-human primates. npj Park. Dis. 2024, 10, 118. [Google Scholar] [CrossRef]
- Lindgren, H.S.; Ohlin, K.E.; Cenci, M.A. Differential involvement of D1 and D2 dopamine receptors in L-DOPA-induced angiogenic activity in a rat model of Parkinson’s disease. Neuropsychopharmacology 2009, 34, 2477–2488. [Google Scholar] [CrossRef]
- Espa, E.; Song, L.; Skovgård, K.; Fanni, S.; Cenci, M.A. Dopamine Agonist Cotreatment Alters Neuroplasticity and Pharmacology of Levodopa-Induced Dyskinesia. Mov. Disord. 2023, 38, 410–422. [Google Scholar] [CrossRef]
- Elabi, O.F.; Espa, E.; Skovgård, K.; Fanni, S.; Cenci, M.A. Ropinirole Cotreatment Prevents Perivascular Glial Recruitment in a Rat Model of L-DOPA-Induced Dyskinesia. Cells 2023, 12, 1859. [Google Scholar] [CrossRef]
- Del-Bel, E.; Bortolanza, M.; Dos-Santos-Pereira, M.; Bariotto, K.; Raisman-Vozari, R. l-DOPA-induced dyskinesia in Parkinson’s disease: Are neuroinflammation and astrocytes key elements? Synapse 2016, 70, 479–500. [Google Scholar] [CrossRef]
- Hubsher, G.; Haider, M.; Okun, M.S. Amantadine: The journey from fighting flu to treating Parkinson disease. Neurology 2012, 78, 1096–1099. [Google Scholar] [CrossRef]
- Bortolanza, M.; do Nascimento, G.C.; Raisman-Vozari, R.; Del-Bel, E. Doxycycline and its derivative, COL-3, decrease dyskinesia induced by l-DOPA in hemiparkinsonian rats. Br. J. Pharmacol. 2021, 178, 2595–2616. [Google Scholar] [CrossRef] [PubMed]
- Lazzarini, M.; Martin, S.; Mitkovski, M.; Vozari, R.R.; Stühmer, W.; Bel, E.D. Doxycycline restrains glia and confers neuroprotection in a 6-OHDA Parkinson model. Glia 2013, 61, 1084–1100. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Yan, A.; Zhao, J.; Yang, S.; Song, L.; Liu, Z. The p75 neurotrophin receptor as a novel intermediate in L-dopa-induced dyskinesia in experimental Parkinson’s disease. Exp. Neurol. 2021, 342, 113740. [Google Scholar] [CrossRef]
- Pinna, A.; Costa, G.; Serra, M.; Contu, L.; Morelli, M. Neuroinflammation and L-dopa-induced abnormal involuntary movements in 6-hydroxydopamine-lesioned rat model of Parkinson’s disease are counteracted by combined administration of a 5-HT(1A/1B) receptor agonist and A(2A) receptor antagonist. Neuropharmacology 2021, 196, 108693. [Google Scholar] [CrossRef]
- Steece-Collier, K.; Caulfield, M.E.; Vander Werp, M.J.; Muller, S.J.; Stancati, J.A.; Chu, Y.; Sandoval, I.M.; Collier, T.J.; Kordower, J.H.; Manfredsson, F.P. Disease-modifying, multidimensional efficacy of putaminal Ca(V)1.3-shRNA gene therapy in aged parkinsonism male and female macaques. Mol. Ther. 2025, 33, 4338–4359. [Google Scholar] [CrossRef] [PubMed]
- Christine, C.W.; Richardson, R.M.; Van Laar, A.D.; Thompson, M.E.; Fine, E.M.; Khwaja, O.S.; Li, C.; Liang, G.S.; Meier, A.; Roberts, E.W.; et al. Safety of AADC Gene Therapy for Moderately Advanced Parkinson Disease: Three-Year Outcomes From the PD-1101 Trial. Neurology 2022, 98, e40–e50. [Google Scholar] [CrossRef] [PubMed]
- Van Laar, A.D.; Christine, C.W.; Phielipp, N.; Larson, P.S.; Elder, J.B.; Merola, A.; San Sebastian, W.; Fiandaca, M.S.; Kells, A.P.; Wisniewski, M.E.; et al. Intraputaminal Delivery of Adeno-Associated Virus Serotype 2-Glial Cell Line-Derived Neurotrophic Factor in Mild or Moderate Parkinson’s Disease. Mov. Disord. 2025, 40, 1297–1306. [Google Scholar] [CrossRef]
- Heiss, J.D.; Ray-Chaudhury, A.; Kleiner, D.E.; Ehrlich, D.J.; Scott, G.; Edwards, N.A.; Goldstein, D.S.; Hammoud, D.A.; Hadaczek, P.; Van Laar, V.S.; et al. Persistent GDNF Expression 45 Months after Putaminal Infusion of AAV2-GDNF in a Patient with Parkinson’s Disease. Mov. Disord. 2024, 39, 1412–1417. [Google Scholar] [CrossRef]
- Niethammer, M.; Tang, C.C.; LeWitt, P.A.; Rezai, A.R.; Leehey, M.A.; Ojemann, S.G.; Flaherty, A.W.; Eskandar, E.N.; Kostyk, S.K.; Sarkar, A.; et al. Long-term follow-up of a randomized AAV2-GAD gene therapy trial for Parkinson’s disease. JCI Insight 2017, 2, e90133. [Google Scholar] [CrossRef] [PubMed]
- Kamath, T.; Abdulraouf, A.; Burris, S.J.; Langlieb, J.; Gazestani, V.; Nadaf, N.M.; Balderrama, K.; Vanderburg, C.; Macosko, E.Z. Single-cell genomic profiling of human dopamine neurons identifies a population that selectively degenerates in Parkinson’s disease. Nat. Neurosci. 2022, 25, 588–595. [Google Scholar] [CrossRef]
- Garrido-Gil, P.; Joglar, B.; Rodriguez-Perez, A.I.; Guerra, M.J.; Labandeira-Garcia, J.L. Involvement of PPAR-γ in the neuroprotective and anti-inflammatory effects of angiotensin type 1 receptor inhibition: Effects of the receptor antagonist telmisartan and receptor deletion in a mouse MPTP model of Parkinson’s disease. J. Neuroinflamm. 2012, 9, 38. [Google Scholar] [CrossRef]
- Joglar, B.; Rodriguez-Pallares, J.; Rodriguez-Perez, A.I.; Rey, P.; Guerra, M.J.; Labandeira-Garcia, J.L. The inflammatory response in the MPTP model of Parkinson’s disease is mediated by brain angiotensin: Relevance to progression of the disease. J. Neurochem. 2009, 109, 656–669. [Google Scholar] [CrossRef]
- Lage, L.; Rodriguez-Perez, A.I.; Villar-Cheda, B.; Labandeira-Garcia, J.L.; Dominguez-Meijide, A. Angiotensin type 1 receptor activation promotes neuronal and glial alpha-synuclein aggregation and transmission. npj Park. Dis. 2024, 10, 37. [Google Scholar] [CrossRef] [PubMed]
- Rey, P.; Lopez-Real, A.; Sanchez-Iglesias, S.; Muñoz, A.; Soto-Otero, R.; Labandeira-Garcia, J.L. Angiotensin type-1-receptor antagonists reduce 6-hydroxydopamine toxicity for dopaminergic neurons. Neurobiol. Aging 2007, 28, 555–567. [Google Scholar] [CrossRef]
- Rodriguez-Perez, A.I.; Sucunza, D.; Pedrosa, M.A.; Garrido-Gil, P.; Kulisevsky, J.; Lanciego, J.L.; Labandeira-Garcia, J.L. Angiotensin Type 1 Receptor Antagonists Protect Against Alpha-Synuclein-Induced Neuroinflammation and Dopaminergic Neuron Death. Neurotherapeutics 2018, 15, 1063–1081. [Google Scholar] [CrossRef]
- Quan, W.; Zhang, S.X.; Zhang, X.Y.; Chen, X.; Yang, C.; Li, Z.Y.; Hu, R. The application of telmisartan in central nervous system disorders. Pharmacol. Rep. 2025, 77, 1196–1216. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Garrote, M.; Parga, J.A.; Labandeira, P.J.; Labandeira-Garcia, J.L.; Rodriguez-Pallares, J. Dopamine regulates adult neurogenesis in the ventricular-subventricular zone via dopamine D3 angiotensin type 2 receptor interactions. Stem Cells 2021, 39, 1778–1794. [Google Scholar] [CrossRef]
- Rodríguez-Pallares, J.; Garcia-Crivaro, L.A.; Parga, J.A.; Labandeira-Garcia, J.L. Renin-angiotensin system as an emerging target to modulate adult neurogenesis in health and disease. Stem Cell Res. Ther. 2025, 16, 332. [Google Scholar] [CrossRef]
- Sonsalla, P.K.; Coleman, C.; Wong, L.Y.; Harris, S.L.; Richardson, J.R.; Gadad, B.S.; Li, W.; German, D.C. The angiotensin converting enzyme inhibitor captopril protects nigrostriatal dopamine neurons in animal models of parkinsonism. Exp. Neurol. 2013, 250, 376–383. [Google Scholar] [CrossRef]
- Lopez-Real, A.; Rey, P.; Soto-Otero, R.; Mendez-Alvarez, E.; Labandeira-Garcia, J.L. Angiotensin-converting enzyme inhibition reduces oxidative stress and protects dopaminergic neurons in a 6-hydroxydopamine rat model of Parkinsonism. J. Neurosci. Res. 2005, 81, 865–873. [Google Scholar] [CrossRef]
- Muñoz, A.; Rey, P.; Guerra, M.J.; Mendez-Alvarez, E.; Soto-Otero, R.; Labandeira-Garcia, J.L. Reduction of dopaminergic degeneration and oxidative stress by inhibition of angiotensin converting enzyme in a MPTP model of parkinsonism. Neuropharmacology 2006, 51, 112–120. [Google Scholar] [CrossRef]
- Dominguez-Meijide, A.; Villar-Cheda, B.; Garrido-Gil, P.; Sierrra-Paredes, G.; Guerra, M.J.; Labandeira-Garcia, J.L. Effect of chronic treatment with angiotensin type 1 receptor antagonists on striatal dopamine levels in normal rats and in a rat model of Parkinson’s disease treated with L-DOPA. Neuropharmacology 2014, 76 Pt A, 156–168. [Google Scholar] [CrossRef] [PubMed]
- Labandeira, C.M.; Pedrosa, M.A.; Quijano, A.; Valenzuela, R.; Garrido-Gil, P.; Sanchez-Andrade, M.; Suarez-Quintanilla, J.A.; Rodriguez-Perez, A.I.; Labandeira-Garcia, J.L. Angiotensin type-1 receptor and ACE2 autoantibodies in Parkinsons disease. npj Park. Dis. 2022, 8, 76. [Google Scholar] [CrossRef]
- Labandeira, C.M.; Camacho-Meño, L.; Aracil-Pastor, P.; Suárez-Quintanilla, J.A.; Labandeira-García, J.L.; Rodríguez-Pérez, A.I. Renin-Angiotensin System Autoantibody Network in Parkinson’s Disease Patients. Antioxidants 2025, 14, 706. [Google Scholar] [CrossRef]
- Kulisevsky, J.; Martínez-Horta, S.; Campolongo, A.; Pascual-Sedano, B.; Marín-Lahoz, J.; Bejr-Kasem, H.; Labandeira-Garcia, J.L.; Lanciego, J.L.; Puig-Davi, A.; Horta-Barba, A.; et al. A randomized clinical trial of candesartan for cognitive impairment in Parkinson’s disease. Parkinsonism Relat. Disord. 2023, 110, 105367. [Google Scholar] [CrossRef]
- Rivas-Santisteban, R.; Lillo, J.; Muñoz, A.; Rodríguez-Pérez, A.I.; Labandeira-García, J.L.; Navarro, G.; Franco, R. Novel Interactions Involving the Mas Receptor Show Potential of the Renin-Angiotensin system in the Regulation of Microglia Activation: Altered Expression in Parkinsonism and Dyskinesia. Neurotherapeutics 2021, 18, 998–1016. [Google Scholar] [CrossRef]
- Rivas-Santisteban, R.; Lillo, J.; Raïch, I.; Muñoz, A.; Lillo, A.; Rodríguez-Pérez, A.I.; Labandeira-García, J.L.; Navarro, G.; Franco, R. The cannabinoid CB(1) receptor interacts with the angiotensin AT(2) receptor. Overexpression of AT(2)-CB(1) receptor heteromers in the striatum of 6-hydroxydopamine hemilesioned rats. Exp. Neurol. 2023, 362, 114319. [Google Scholar] [CrossRef] [PubMed]
- Labandeira-Garcia, J.L.; Rodríguez-Perez, A.I.; Garrido-Gil, P.; Rodriguez-Pallares, J.; Lanciego, J.L.; Guerra, M.J. Brain Renin-Angiotensin System and Microglial Polarization: Implications for Aging and Neurodegeneration. Front. Aging Neurosci. 2017, 9, 129. [Google Scholar] [CrossRef]
- He, Q.W.; Xia, Y.P.; Chen, S.C.; Wang, Y.; Huang, M.; Huang, Y.; Li, J.Y.; Li, Y.N.; Gao, Y.; Mao, L.; et al. Astrocyte-derived sonic hedgehog contributes to angiogenesis in brain microvascular endothelial cells via RhoA/ROCK pathway after oxygen-glucose deprivation. Mol. Neurobiol. 2013, 47, 976–987. [Google Scholar] [CrossRef] [PubMed]
- Moskal, N.; Riccio, V.; Bashkurov, M.; Taddese, R.; Datti, A.; Lewis, P.N.; Angus McQuibban, G. ROCK inhibitors upregulate the neuroprotective Parkin-mediated mitophagy pathway. Nat. Commun. 2020, 11, 88. [Google Scholar] [CrossRef]
- Tatenhorst, L.; Tönges, L.; Saal, K.A.; Koch, J.C.; Szegő, É.M.; Bähr, M.; Lingor, P. Rho kinase inhibition by fasudil in the striatal 6-hydroxydopamine lesion mouse model of Parkinson disease. J. Neuropathol. Exp. Neurol. 2014, 73, 770–779. [Google Scholar] [CrossRef] [PubMed]
- Koch, J.C.; Tatenhorst, L.; Roser, A.E.; Saal, K.A.; Tönges, L.; Lingor, P. ROCK inhibition in models of neurodegeneration and its potential for clinical translation. Pharmacol. Ther. 2018, 189, 1–21. [Google Scholar] [CrossRef]
- Borrajo, A.; Rodriguez-Perez, A.I.; Villar-Cheda, B.; Guerra, M.J.; Labandeira-Garcia, J.L. Inhibition of the microglial response is essential for the neuroprotective effects of Rho-kinase inhibitors on MPTP-induced dopaminergic cell death. Neuropharmacology 2014, 85, 1–8. [Google Scholar] [CrossRef]
- Rodriguez-Perez, A.I.; Dominguez-Meijide, A.; Lanciego, J.L.; Guerra, M.J.; Labandeira-Garcia, J.L. Inhibition of Rho kinase mediates the neuroprotective effects of estrogen in the MPTP model of Parkinson’s disease. Neurobiol. Dis. 2013, 58, 209–219. [Google Scholar] [CrossRef]
- Villar-Cheda, B.; Dominguez-Meijide, A.; Joglar, B.; Rodriguez-Perez, A.I.; Guerra, M.J.; Labandeira-Garcia, J.L. Involvement of microglial RhoA/Rho-kinase pathway activation in the dopaminergic neuron death. Role of angiotensin via angiotensin type 1 receptors. Neurobiol. Dis. 2012, 47, 268–279. [Google Scholar] [CrossRef] [PubMed]
- Saal, K.A.; Koch, J.C.; Tatenhorst, L.; Szegő, E.M.; Ribas, V.T.; Michel, U.; Bähr, M.; Tönges, L.; Lingor, P. AAV.shRNA-mediated downregulation of ROCK2 attenuates degeneration of dopaminergic neurons in toxin-induced models of Parkinson’s disease in vitro and in vivo. Neurobiol. Dis. 2015, 73, 150–162. [Google Scholar] [CrossRef] [PubMed]
- Lage, L.; Rodriguez-Perez, A.I.; Labandeira-Garcia, J.L.; Dominguez-Meijide, A. Fasudil inhibits α-synuclein aggregation through ROCK-inhibition-mediated mechanisms. Neurotherapeutics 2025, 22, e00544. [Google Scholar] [CrossRef]
- Carbajal, C.; Rodriguez, M.; Owens, F.; Stone, N.; Veeragoni, D.; Fan, R.Z.; Tieu, K.; El-Hage, N. Therapeutic Efficacy of Small Extracellular Vesicles Loaded with ROCK Inhibitor in Parkinson’s Disease. Pharmaceutics 2025, 17, 365. [Google Scholar] [CrossRef]
- Lingor, P.; Weber, M.; Camu, W.; Friede, T.; Hilgers, R.; Leha, A.; Neuwirth, C.; Günther, R.; Benatar, M.; Kuzma-Kozakiewicz, M.; et al. ROCK-ALS: Protocol for a Randomized, Placebo-Controlled, Double-Blind Phase IIa Trial of Safety, Tolerability and Efficacy of the Rho Kinase (ROCK) Inhibitor Fasudil in Amyotrophic Lateral Sclerosis. Front. Neurol. 2019, 10, 293. [Google Scholar] [CrossRef]
- Koch, J.C.; Kuttler, J.; Maass, F.; Lengenfeld, T.; Zielke, E.; Bähr, M.; Lingor, P. Compassionate Use of the ROCK Inhibitor Fasudil in Three Patients With Amyotrophic Lateral Sclerosis. Front. Neurol. 2020, 11, 173. [Google Scholar] [CrossRef]
- Koch, J.C.; Leha, A.; Bidner, H.; Cordts, I.; Dorst, J.; Günther, R.; Zeller, D.; Braun, N.; Metelmann, M.; Corcia, P.; et al. ROCK-ALS Study group. Safety, tolerability, and efficacy of fasudil in amyotrophic lateral sclerosis (ROCK-ALS): A phase 2, randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2024, 23, 1133–1146. [Google Scholar] [CrossRef]
- Wolff, A.W.; Bidner, H.; Remane, Y.; Zimmer, J.; Aarsland, D.; Rascol, O.; Wyse, R.K.; Hapfelmeier, A.; Lingor, P. Protocol for a randomized, placebo-controlled, double-blind phase IIa study of the safety, tolerability, and symptomatic efficacy of the ROCK-inhibitor Fasudil in patients with Parkinson’s disease (ROCK-PD). Front. Aging Neurosci. 2024, 16, 1308577. [Google Scholar] [CrossRef] [PubMed]
- Wolff, A.W.; Peine, J.; Höfler, J.; Zurek, G.; Hemker, C.; Lingor, P. SAFE-ROCK: A Phase I Trial of an Oral Application of the ROCK Inhibitor Fasudil to Assess Bioavailability, Safety, and Tolerability in Healthy Participants. CNS Drugs 2024, 38, 291–302. [Google Scholar] [CrossRef]
| Compound | Target | Reference | Experimental Model | Results | Mechanism |
|---|---|---|---|---|---|
| Agmatine | NMDA receptors Nrf2 | Azar et al., 2022 [92] | Rotenone lesioned rats | ↓ LID | ↑ Nrf2 ↓ NF-κB |
| Amantadine | NMDA/AMPA receptors | Rentsch et al., 2020 [93] | 6-OHDA mouse model | ↓ LID | ↓ TNF-α, IL-1β, IL-6 |
| Candesartan | AT1 receptors | Muñoz et al., 2014 [41] | 6-OHDA rat model | ↓ LID | ↓ VEGF, IL-1β |
| Capsazepine | Competitive antagonist of capsaicin | Dos Santos Pereira et al. 2021; 2024 [86,94] | 6-OHDA mouse model | ↓ LID | ↓ TNF-α release in glutamate-activated astrocytes, ↓ microglial and astroglial activation |
| Captopril, enalapril, perindopril | ACE | Park et al., 2024 [46] | 6-OHDA mouse model | ↓ LID | ↓ astrocyte and microglial transcripts |
| Doxycicline | Semisynthetic tetracycline antibiotic | Dos Santos Pereira et al., 2022; 2025 [95,96] | 6-OHDA mouse model | ↓ LID ↓ Fos B | ↓ PGE2, TNF-α, IL-1β |
| Corticosterone | COX-2 | Barnum et al., 2008 [74] | 6-OHDA rat model | ↓ LID | ↓ IL-1β |
| IRC-082451 | sodium channel blocker, oxidative stress, COX-2 | Aron Badin et al., 2013 [39] | MPTP Parkinsonian primates | ↓ LID ↓ cFOS, FosB | ↓ sodium channel blocker, oxidative stress, and COX-2 |
| Fasudil | ROCK | López-López et al., 2020 [40] | 6-OHDA rat model | ↓ LID | ↓ IL-1β, TNF-α |
| Resveratrol | SIRT1 and AMPK | Zheng et al., 2021 [42] | 6-OHDA rat model | ↓ LID ↓ ΔFOS B, ERK | ↓ microglia, astroglia activation and inflammation |
| Thalidomide | CRBN | Boi et al., 2019 [97] | 6-OHDA rat model | ↓ LID ↓ angiogenesis | ↓ TNF-α, restored levels of IL-10 |
| Vandetanib | VEGF | Ohlin et al., 2011 [98] | 6-OHDA rat model | ↓ LID ↓ angiogenesis | ↓ VEGF |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muñoz, A.; López-López, A.; Rodríguez-Pallares, J.; Labandeira-Garcia, J.L. Involvement of Neuroinflammation and Oxidative Stress in L-DOPA-Induced Dyskinesia in Parkinson’s Disease: Role of Renin–Angiotensin System and ROCK Pathway. Antioxidants 2025, 14, 1154. https://doi.org/10.3390/antiox14101154
Muñoz A, López-López A, Rodríguez-Pallares J, Labandeira-Garcia JL. Involvement of Neuroinflammation and Oxidative Stress in L-DOPA-Induced Dyskinesia in Parkinson’s Disease: Role of Renin–Angiotensin System and ROCK Pathway. Antioxidants. 2025; 14(10):1154. https://doi.org/10.3390/antiox14101154
Chicago/Turabian StyleMuñoz, Ana, Andrea López-López, Jannette Rodríguez-Pallares, and José Luis Labandeira-Garcia. 2025. "Involvement of Neuroinflammation and Oxidative Stress in L-DOPA-Induced Dyskinesia in Parkinson’s Disease: Role of Renin–Angiotensin System and ROCK Pathway" Antioxidants 14, no. 10: 1154. https://doi.org/10.3390/antiox14101154
APA StyleMuñoz, A., López-López, A., Rodríguez-Pallares, J., & Labandeira-Garcia, J. L. (2025). Involvement of Neuroinflammation and Oxidative Stress in L-DOPA-Induced Dyskinesia in Parkinson’s Disease: Role of Renin–Angiotensin System and ROCK Pathway. Antioxidants, 14(10), 1154. https://doi.org/10.3390/antiox14101154








