Plasma Glycated and Oxidized Amino Acid-Based Screening Test for Clinical Early-Stage Osteoarthritis
Abstract
1. Introduction
2. Methods
2.1. Subjects with Early-Stage Osteoarthritis, Asymptomatic Control Subjects, Blood Sampling, and Analysis
2.2. Machine Learning Analysis
2.3. Statistical Analyses
3. Results
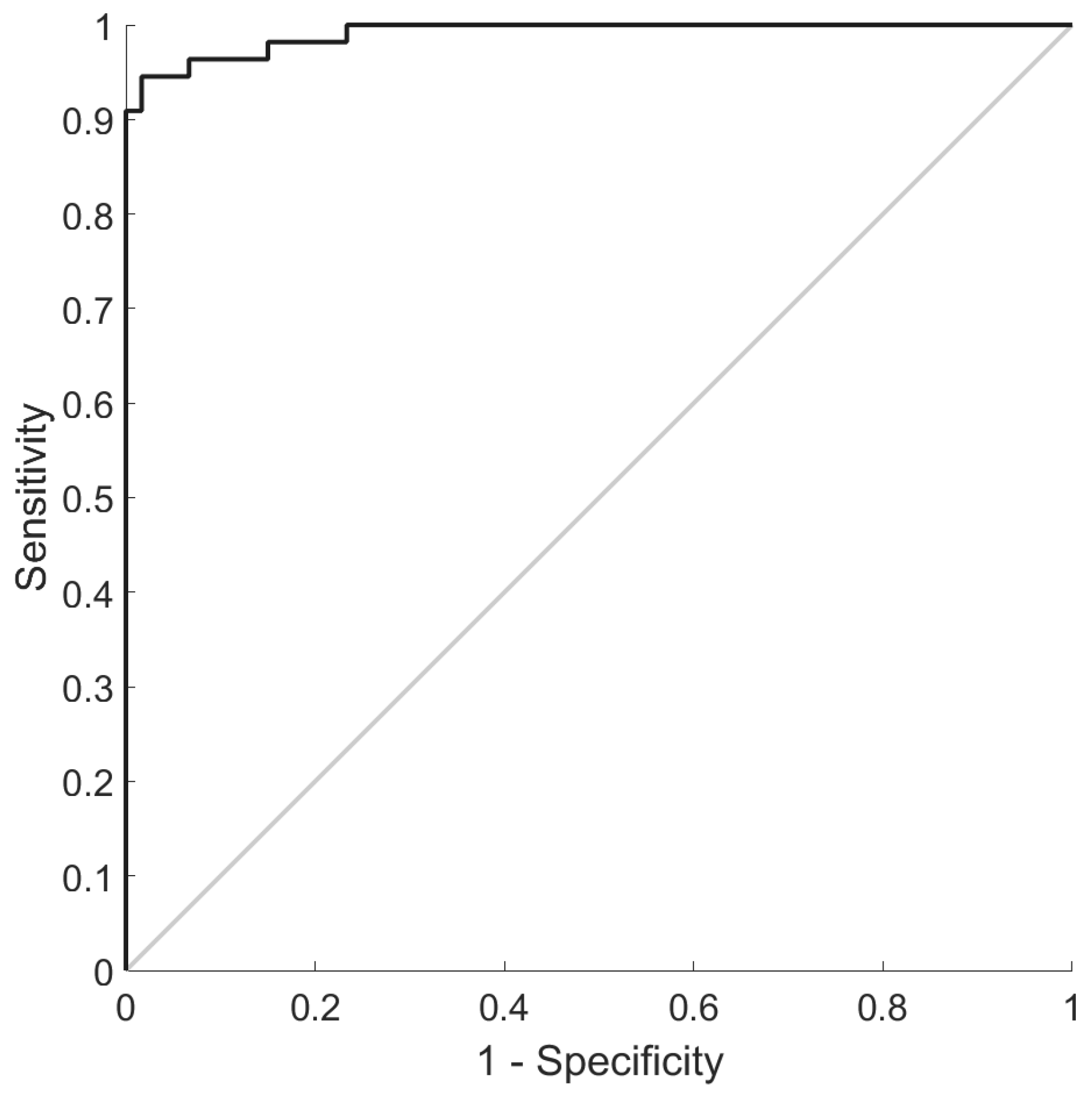
Classification of Subjects with Early-Stage Osteoarthritis and Asymptomatic Controls Using Algorithms Developed Using Plasma Glycated and Oxidized Amino Acids and Hydroxyproline as Features
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hunter, D.J.; Bierma-Zeinstra, S. Osteoarthritis. Lancet 2019, 393, 1745–1759. [Google Scholar] [CrossRef] [PubMed]
- Kloppenburg, M.; Namane, M.; Cicuttini, F. Osteoarthritis. Lancet 2025, 405, 71–85. [Google Scholar] [CrossRef] [PubMed]
- Scott, C.E.H.; MacDonald, D.J.; Howie, C.R. ‘Worse than death’ and waiting for a joint arthroplasty. Bone Jt. J. 2019, 101, 941–950. [Google Scholar] [CrossRef] [PubMed]
- Clynes, M.A.; Jameson, K.A.; Edwards, M.H.; Cooper, C.; Dennison, E.M. Impact of osteoarthritis on activities of daily living: Does joint site matter? Aging Clin. Exp. Res. 2019, 31, 1049–1056. [Google Scholar] [CrossRef] [PubMed]
- Chu, C.R.; Williams, A.A.; Coyle, C.H.; Bowers, M.E. Early diagnosis to enable early treatment of pre-osteoarthritis. Arthritis Res. Ther. 2012, 14, 212. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudian, A.; Lohmander, L.S.; Mobasheri, A.; Englund, M.; Luyten, F.P. Early-stage symptomatic osteoarthritis of the knee—Time for action. Nat. Rev. Rheumatol. 2021, 17, 621–632. [Google Scholar] [CrossRef] [PubMed]
- Kundu, S.; Ashinsky, B.G.; Bouhrara, M.; Dam, E.B.; Demehri, S.; Shifat-E-Rabbi, M.; Spencer, R.G.; Urish, K.L.; Rohde, G.K. Enabling early detection of osteoarthritis from presymptomatic cartilage texture maps via transport-based learning. Proc. Natl. Acad. Sci. USA 2020, 117, 24709–24719. [Google Scholar] [CrossRef] [PubMed]
- Henrotin, Y. Osteoarthritis in year 2021: Biochemical markers. Osteoarthr. Cartil. 2022, 30, 237–248. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, U.; Anwar, A.; Savage, R.S.; Thornalley, P.J.; Rabbani, N. Protein oxidation, nitration and glycation biomarkers for early-stage diagnosis of osteoarthritis of the knee and typing and progression of arthritic disease. Arthritis Res. Ther. 2016, 18, 250. [Google Scholar] [CrossRef] [PubMed]
- Legrand, C.; Ahmed, U.; Anwar, A.; Rajpoot, K.; Pasha, S.; Lambert, C.; Davidson, R.K.; Clark, I.M.; Thornalley, P.J.; Henrotin, Y.; et al. Glycation marker glucosepane increases with the progression of osteoarthritis and correlates with morphological and functional changes of cartilage in vivo. Arthritis Res. Ther. 2018, 20, 131. [Google Scholar] [CrossRef] [PubMed]
- Rabbani, N.; Thornalley, P.J. Reading patterns of proteome damage by glycation, oxidation and nitration: Quantitation by stable isotopic dilution analysis LC-MS/MS. Essays Biochem. 2020, 64, 169–183. [Google Scholar] [CrossRef] [PubMed]
- Mohtadi, N.G.H.; Griffin, D.R.; Pedersen, M.E.; Chan, D.; Safran, M.R.; Parsons, N.; Sekiya, J.K.; Kelly, B.T.; Werle, J.R.; Leunig, M.; et al. The Development and Validation of a Self-Administered Quality-of-Life Outcome Measure for Young, Active Patients with Symptomatic Hip Disease: The International Hip Outcome Tool (iHOT-33). Arthroscopy 2012, 28, 595–610.e1. [Google Scholar] [CrossRef] [PubMed]
- McLean, M.; Whyte, T.; Millar, N.L. Outerbridge grade IV cartilage lesions in the hip identified at arthroscopy. Ann. Transl. Med. 2017, 5, 226. [Google Scholar] [CrossRef] [PubMed]
- Fawcett, T. An introduction to ROC analysis. Pattern Recognit. Lett. 2006, 27, 861–874. [Google Scholar] [CrossRef]
- Robin, X.; Turck, N.; Hainard, A.; Tiberti, N.; Lisacek, F.; Sanchez, J.C.; Muller, M. pROC: An open-source package for R and S plus to analyze and compare ROC curves. BMC Bioinform. 2011, 12, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Collins, G.S.; Reitsma, J.B.; Altman, D.G.; Moons, K.G.M. Transparent Reporting of a multivariable prediction model for Individual Prognosis or Diagnosis (TRIPOD): The TRIPOD Statement. Ann. Intern. Med. 2015, 162, 55–63. [Google Scholar] [CrossRef] [PubMed]
- McCord, J.M. Free Radicals and Inflammation: Protection of Synovial Fluid by Superoxide Dismutase. Science 1974, 185, 529–531. [Google Scholar] [CrossRef] [PubMed]
- Kaur, H.; Halliwell, B. Evidence for nitric oxide-mediated oxidative damage in chronic inflammation Nitrotyrosine in serum and synovial fluid from rheumatoid patients. FEBS Lett. 1994, 350, 9–12. [Google Scholar] [CrossRef] [PubMed]
- Miyata, T.; Ishiguro, N.; Yasuda, Y.; Ito, T.; Nangaku, M.; Iwata, H.; Kurokawa, K. Increased pentosidine, an advanced glycation end product, in plasma and synovial fluid from patients with rheumatoid arthritis and its relation with inflammatory markers. Biochem. Biophys. Res. Commun. 1998, 244, 45–49. [Google Scholar] [CrossRef] [PubMed]
- Martel-Pelletier, J. Pathophysiology of osteoarthritis. Osteoarthr.Cartil. 2004, 12, 31–33. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Marchand, N.E.; Driban, J.B.; McAlindon, T.; Eaton, C.B.; Lu, B. Dietary Patterns and Progression of Knee Osteoarthritis: Data from the Osteoarthritis Initiative. Am. J. Clin. Nutr. 2020, 111, 667–676. [Google Scholar] [CrossRef] [PubMed]
- Visconti, G.; Boccard, J.; Feinberg, M.; Rudaz, S. From fundamentals in calibration to modern methodologies: A tutorial for small molecules quantification in liquid chromatography–mass spectrometry bioanalysis. Anal. Chim. Acta 2023, 1240, 340711. [Google Scholar] [CrossRef] [PubMed]
- Jannetto, P.J.; Fitzgerald, R.L. Effective Use of Mass Spectrometry in the Clinical Laboratory. Clin. Chem. 2016, 62, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Steinmetz, J.D.; Culbreth, G.T.; Haile, L.M.; Rafferty, Q.; Lo, J.; Fukutaki, K.G.; Cruz, J.A.; Smith, A.E.; Vollset, S.E.; Brooks, P.M.; et al. Global, regional, and national burden of osteoarthritis, 1990–2020 and projections to 2050: A systematic analysis for the Global Burden of Disease Study 2021. Lancet Rheumatol. 2023, 5, e508–e522. [Google Scholar] [CrossRef] [PubMed]
- Quintana, J.M.; Arostegui, I.; Escobar, A.; Lafuente, I.; Arenaza, J.C.; Garcia, I.; Aguirre, U. Validation of a screening questionnaire for hip and knee osteoarthritis in old people. BMC Musculoskelet. Disord. 2007, 8, 84. [Google Scholar] [CrossRef] [PubMed]
- Rabbani, N.; Xue, M.; Thornalley, P.J. Methylglyoxal-induced dicarbonyl stress in aging and disease: First steps towards glyoxalase 1-based treatments. Clin. Sci. 2016, 130, 1677–1696. [Google Scholar] [CrossRef] [PubMed]
- Vos, P.A.J.M.; DeGroot, J.; Huisman, A.M.; Oostveen, J.C.M.; Marijnissen, A.C.A.; Bijlsma, J.W.J.; van El, B.; Zuurmond, A.M.; Lafeber, F.P.J.G. Skin and urine pentosidine weakly correlate with joint damage in a cohort of patients with early signs of osteoarthritis (CHECK). Osteoarthr. Cartil. 2010, 18, 1329–1336. [Google Scholar] [CrossRef] [PubMed]
- Trellu, S.; Courties, A.; Jaisson, S.; Gorisse, L.; Gillery, P.; Kerdine-Römer, S.; Vaamonde-Garcia, C.; Houard, X.; Ekhirch, F.-P.; Sautet, A.; et al. Impairment of glyoxalase-1, an advanced glycation end-product detoxifying enzyme, induced by inflammation in age-related osteoarthritis. Arthritis Res. Ther. 2019, 21, 18. [Google Scholar] [CrossRef] [PubMed]
| Study Group | N | Age (yr) | Gender (M/F) | BMI (kg/m2) | iHOT-33 |
|---|---|---|---|---|---|
| Asymptomatic controls | 120 | 33 ± 9 | 60/60 | 28 ± 5 | |
| Subjects with eOA | 110 | 39 ± 12 | 54/56 | 25 ± 6 | 39 ± 17 |
| [Analytes]Plasma (nM) | |||
|---|---|---|---|
| Analyte | Asymptomatic Controls (n = 120) | eOA (n = 110) | p-Value |
| Hydroxyproline (Hyp) | 340 (70–1870) | 1570 (45–6440) | <0.001 *** |
| Nε-Fructosyl-lysine (FL) | 543 (170–2770) | 262 (79.1–12,259) | <0.001 *** |
| Nε-Carboxymethyl-lysine (CML) | 77 (29–209) | 50.4 (32.5–87.5) | <0.001 *** |
| Nε(1-Carboxyethyl)lysine (CEL) | 145 (27–543) | 58.5 (20.0–146.0) | <0.001 *** |
| Glyoxal-derived hydroimidazolone (G-H1) | 3.53 (1.27–7.27) | 2.66 (0.366–5.86) | <0.001 *** |
| Methylglyoxal-derived hydroimidazolone (MG-H1) | 446 (104–2343) | 614 (142–3502) | 0.014 * |
| 3-Deoxyglucosone-derived hydroimidazolone (3DG-H) | 70 (37–262) | 143 (130–501) | <0.001 *** |
| Nω-Carboxymethyl-arginine (CMA) | 24.6 (9.3–86.9) | 19.4 (5.22–45.8) | <0.001 *** |
| Glucosepane (GSP) | 17.3 (6.7–86.7) | 28.4 (6.81–64.9) | <0.001 *** |
| Methionine sulfoxide (MetSO) | 95 (30–1118) | 199 (44.3–944) | <0.001 *** |
| Dityrosine (DT) | 0.81 (0.44–1.28) | 0.835 (0.276–1.72) | |
| N-Formyl-kynurenine (NFK) | 6.64 (0.104–270) | 10.0 (1.03–62.0) | <0.01 ** |
| 3-Nitrotyrosine (3-NT) | 1.10 (0.13–1.63) | 1.68 (0.302–4.98) | <0.001 *** |
| Features | CMA, G-H1, MG-H1, 3DG-H, and GSP Free Adducts |
|---|---|
| nCorrect | 219/230 |
| Sensitivity (%) | 96 (95–98) |
| Specificity (%) | 94 (91–98) |
| AUROC (%) | 99 (99–100) |
| Positive predictive value (%) | 94 |
| Negative predictive value (%) | 97 |
| Positive likelihood ratio (LR+) | 21.4 |
| Negative likelihood ratio (LR−) | 0.04 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Saei, A.N.J.M.; Ahmed, U.; Dickenson, E.J.; Rajpoot, K.; Xue, M.; Abdelalim, E.M.; Arredouani, A.; Albagha, O.M.E.; Griffin, D.R.; Thornalley, P.J.; et al. Plasma Glycated and Oxidized Amino Acid-Based Screening Test for Clinical Early-Stage Osteoarthritis. Antioxidants 2025, 14, 1146. https://doi.org/10.3390/antiox14101146
Al-Saei ANJM, Ahmed U, Dickenson EJ, Rajpoot K, Xue M, Abdelalim EM, Arredouani A, Albagha OME, Griffin DR, Thornalley PJ, et al. Plasma Glycated and Oxidized Amino Acid-Based Screening Test for Clinical Early-Stage Osteoarthritis. Antioxidants. 2025; 14(10):1146. https://doi.org/10.3390/antiox14101146
Chicago/Turabian StyleAl-Saei, Aisha Nasser J. M., Usman Ahmed, Edward J. Dickenson, Kashif Rajpoot, Mingzhan Xue, Essam M. Abdelalim, Abdelilah Arredouani, Omar M. E. Albagha, Damian R. Griffin, Paul J. Thornalley, and et al. 2025. "Plasma Glycated and Oxidized Amino Acid-Based Screening Test for Clinical Early-Stage Osteoarthritis" Antioxidants 14, no. 10: 1146. https://doi.org/10.3390/antiox14101146
APA StyleAl-Saei, A. N. J. M., Ahmed, U., Dickenson, E. J., Rajpoot, K., Xue, M., Abdelalim, E. M., Arredouani, A., Albagha, O. M. E., Griffin, D. R., Thornalley, P. J., & Rabbani, N. (2025). Plasma Glycated and Oxidized Amino Acid-Based Screening Test for Clinical Early-Stage Osteoarthritis. Antioxidants, 14(10), 1146. https://doi.org/10.3390/antiox14101146








