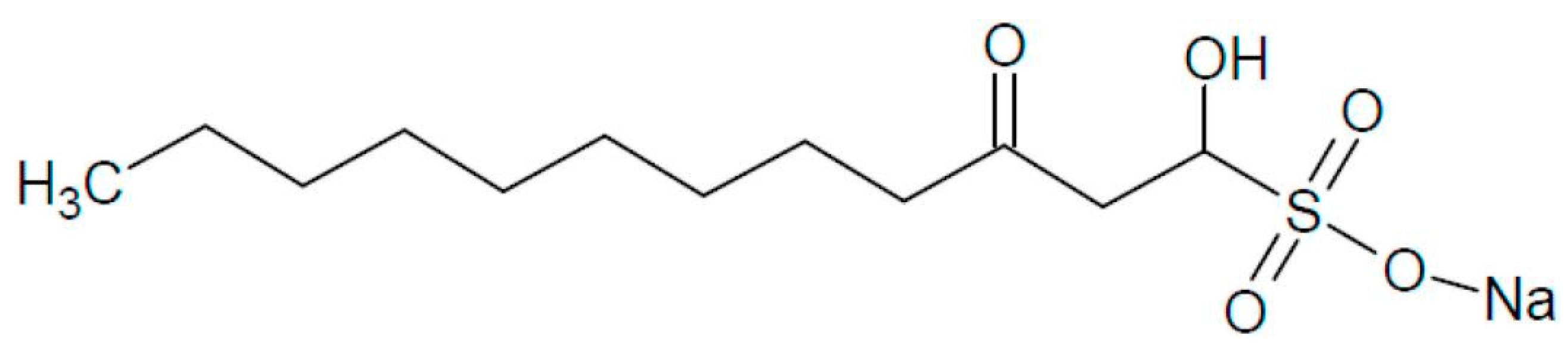
Sodium Houttuybonate Promotes the Browning of White Adipose Tissue by Inhibiting Ferroptosis via the AMPK-NRF2-HO1 Pathway
Abstract
1. Introduction
2. Materials and Methods
2.1. Mice and Treatments
2.2. Glucose Tolerance Tests (GTTs)
2.3. Metabolic and Biochemical Analyses
2.4. Histological and Immunohistochemical Analyses
2.5. Primary SVF Isolation, Maturation, and Treatment
2.6. Quantitative PCR (qPCR) Analysis
2.7. Western Blotting
2.8. Data Statistics
2.9. Data and Resource Availability
3. Results
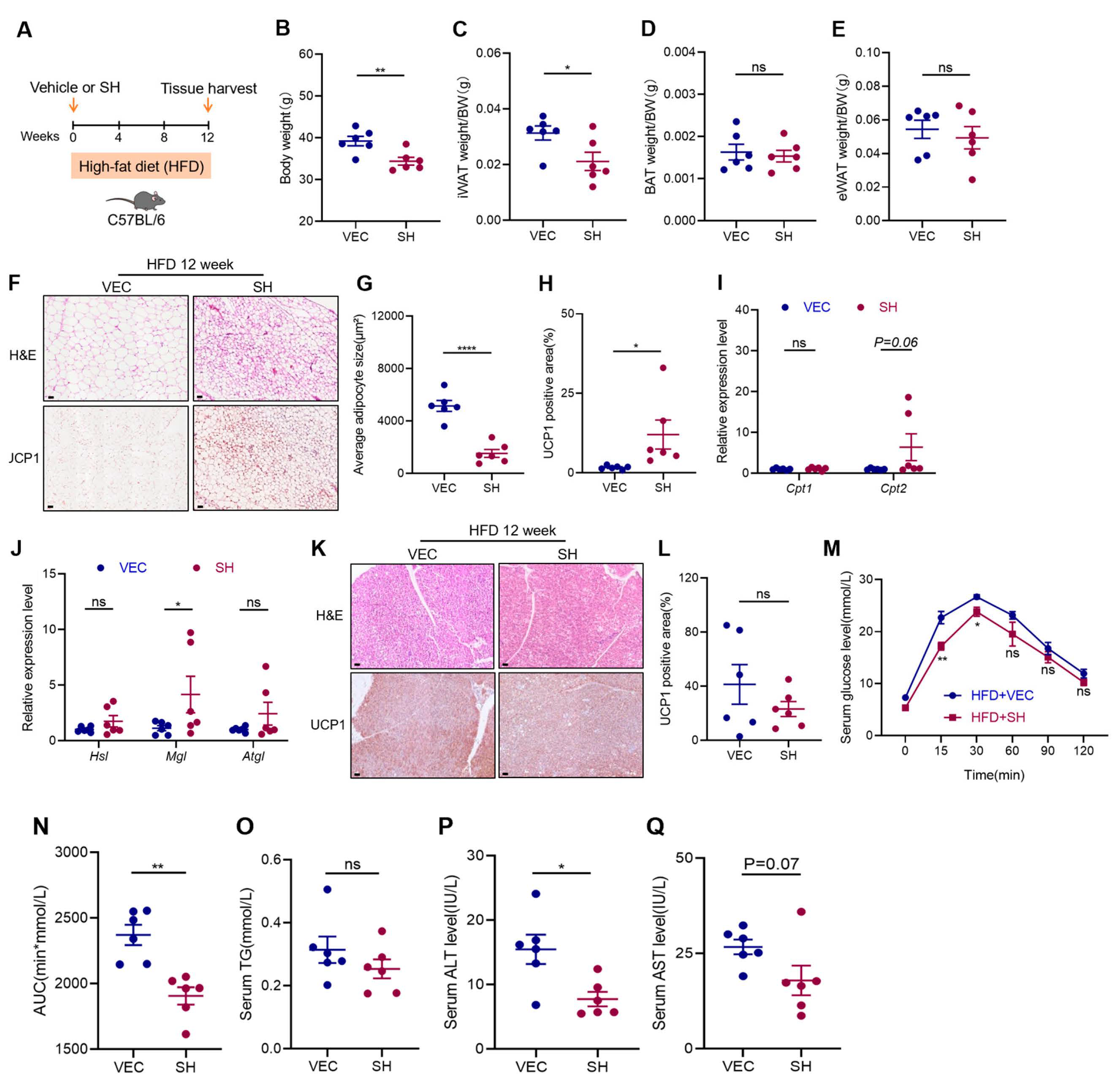
3.1. SH Promotes iWAT Browning and Prevents Diet-Induced Obesity
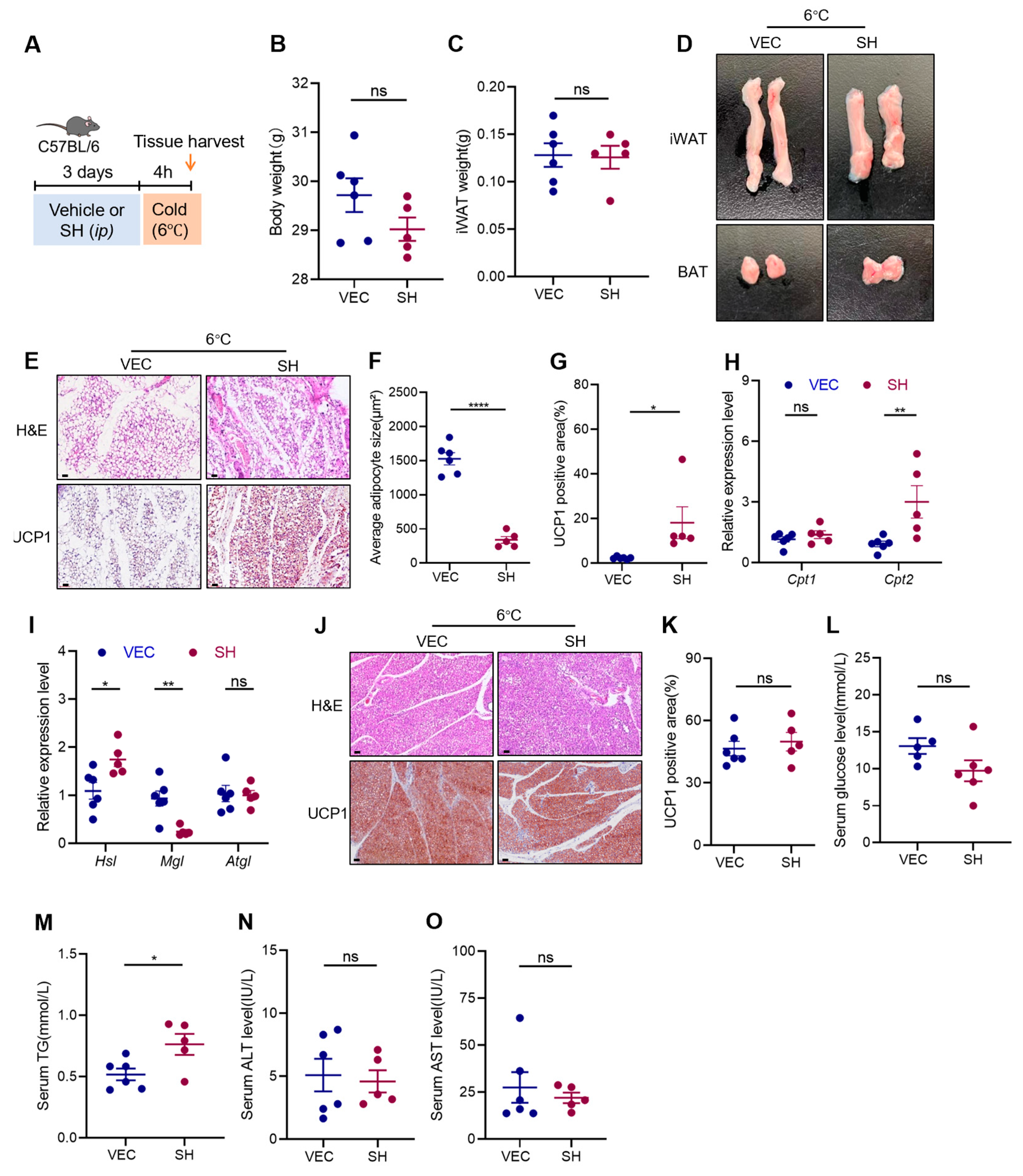
3.2. Effects of SH on Adipose Tissue under Cold Stimulation
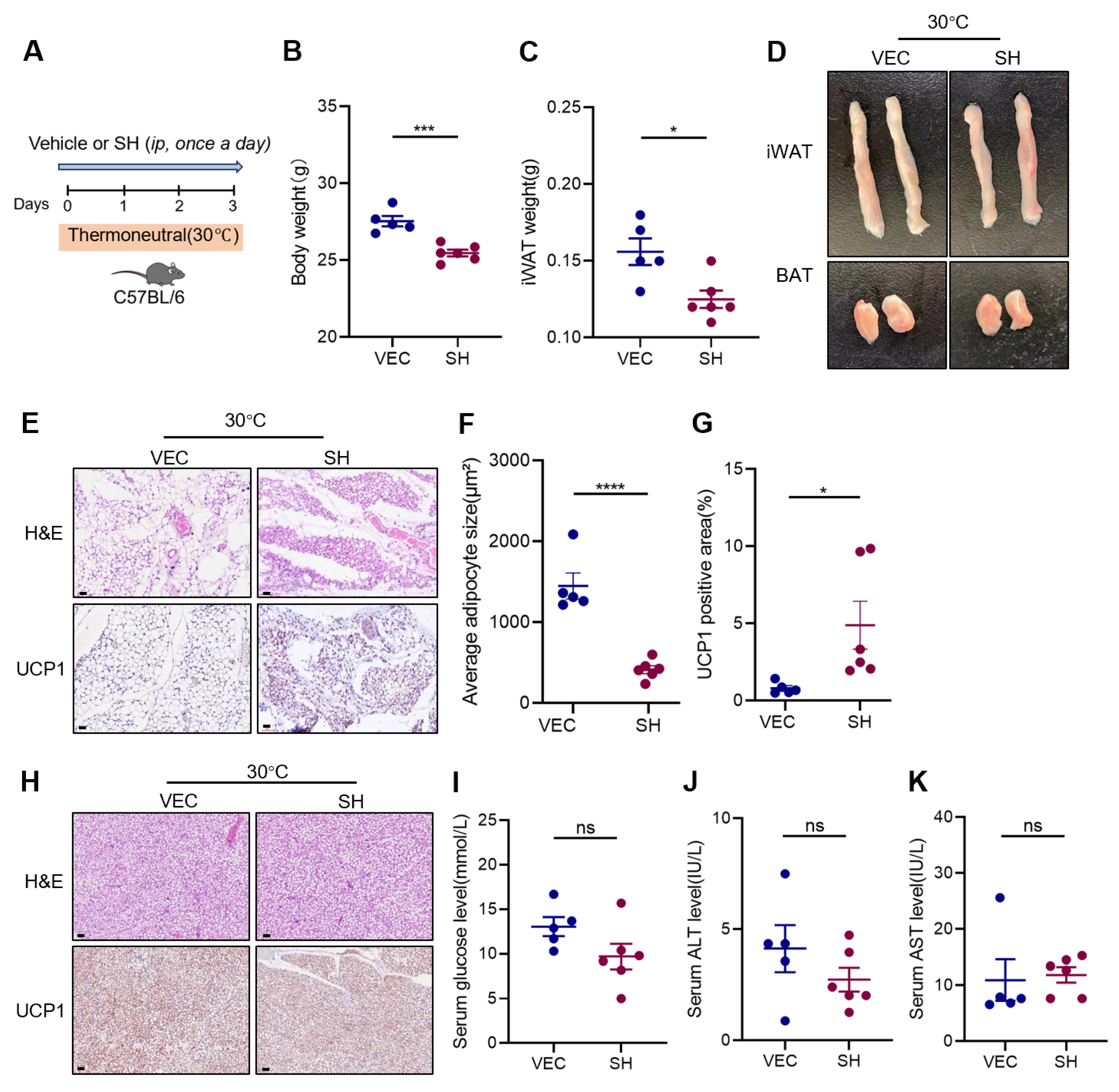
3.3. Effects of SH on Adipose Tissue at Thermoneutrality
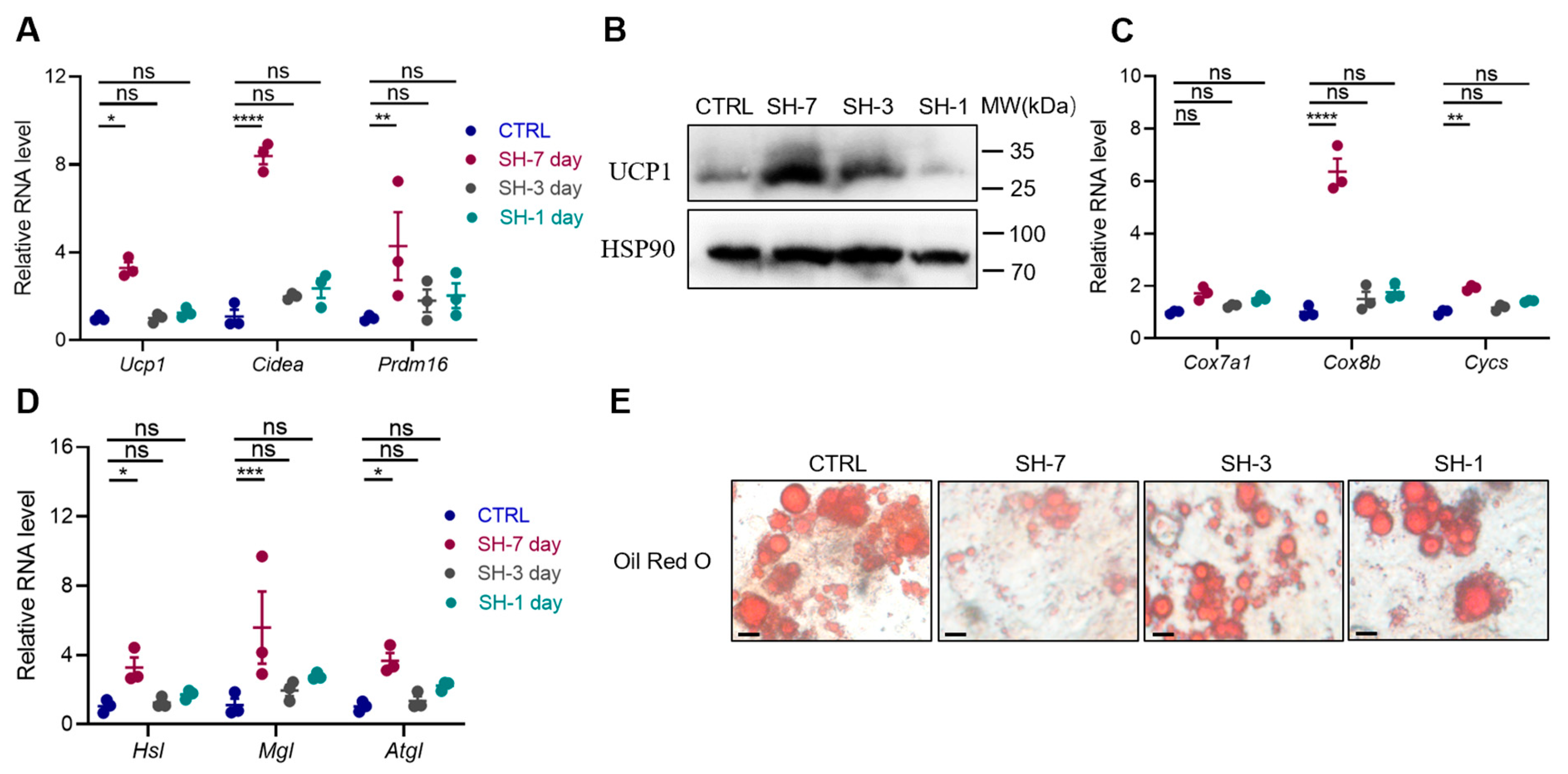
3.4. SH Directly Promotes the Browning of Adipocytes In Vitro
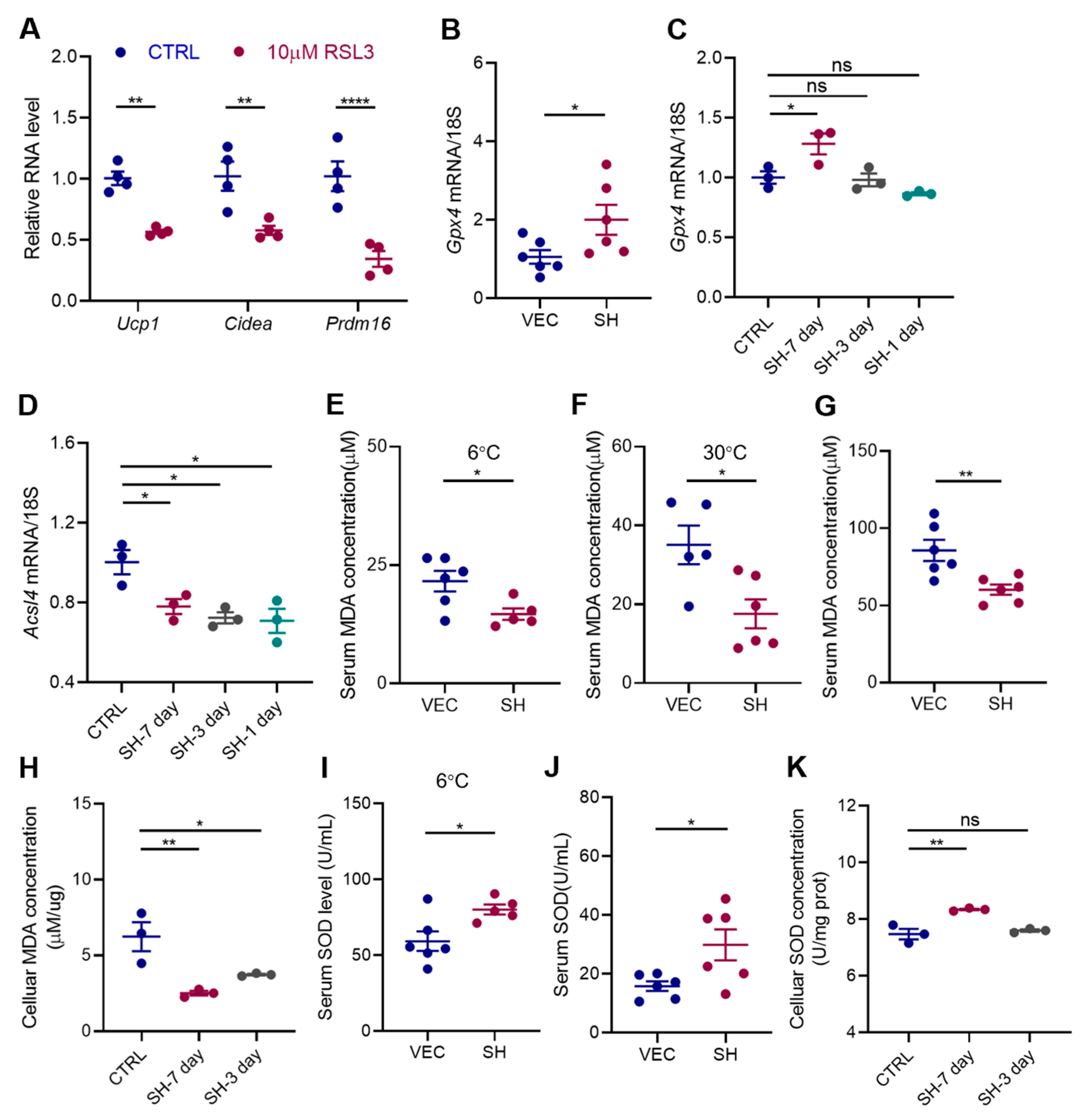
3.5. SH Enhances Adipocyte Browning by Inhibiting Ferroptosis
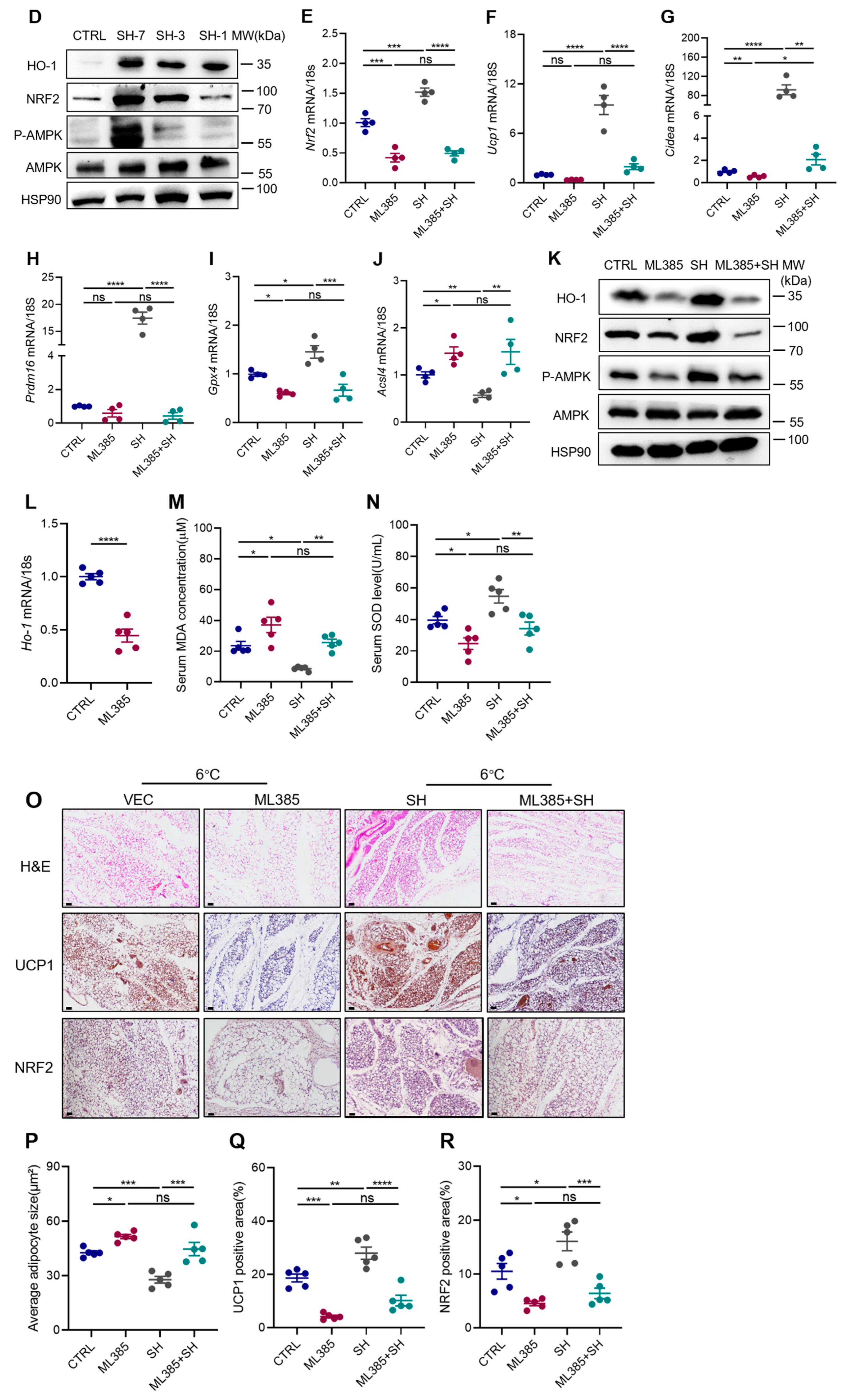
3.6. SH Promotes iWAT Browning via the AMPK-NRF2-HO1 Axis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Powell-Wiley, T.M.; Poirier, P.; Burke, L.E.; Després, J.-P.; Gordon-Larsen, P.; Lavie, C.J.; Lear, S.A.; Ndumele, C.E.; Neeland, I.J.; Sanders, P.; et al. Obesity and Cardiovascular Disease: A Scientific Statement from the American Heart Association. Circulation 2021, 143, e984–e1010. [Google Scholar] [CrossRef] [PubMed]
- Astrup, A.; Finer, N. Redefining Type 2 Diabetes: ‘Diabesity’ or ‘Obesity Dependent Diabetes Mellitus’? Obes. Rev. 2000, 1, 57–59. [Google Scholar] [CrossRef]
- Polyzos, S.A.; Kountouras, J.; Mantzoros, C.S. Obesity and Nonalcoholic Fatty Liver Disease: From Pathophysiology to Therapeutics. Metabolism 2019, 92, 82–97. [Google Scholar] [CrossRef]
- De Pergola, G.; Silvestris, F. Obesity as a Major Risk Factor for Cancer. J. Obes. 2013, 2013, 291546. [Google Scholar] [CrossRef]
- Hopkins, B.D.; Goncalves, M.D.; Cantley, L.C. Obesity and Cancer Mechanisms: Cancer Metabolism. J. Clin. Oncol. 2016, 34, 4277–4283. [Google Scholar] [CrossRef] [PubMed]
- Tchernof, A.; Després, J.-P. Pathophysiology of Human Visceral Obesity: An Update. Physiol. Rev. 2013, 93, 359–404. [Google Scholar] [CrossRef]
- Kuryłowicz, A.; Puzianowska-Kuźnicka, M. Induction of Adipose Tissue Browning as a Strategy to Combat Obesity. Int. J. Mol. Sci. 2020, 21, 6241. [Google Scholar] [CrossRef]
- Ng, R.; Hussain, N.A.; Zhang, Q.; Chang, C.; Li, H.; Fu, Y.; Cao, L.; Han, W.; Stunkel, W.; Xu, F. miRNA-32 Drives Brown Fat Thermogenesis and Trans-Activates Subcutaneous White Fat Browning in Mice. Cell Rep. 2017, 19, 1229–1246. [Google Scholar] [CrossRef] [PubMed]
- Das, S.K.; Mahanta, S.; Tanti, B.; Tag, H.; Hui, P.K. Identification of Phytocompounds from Houttuynia Cordata Thunb. as Potential Inhibitors for SARS-CoV-2 Replication Proteins through GC–MS/LC–MS Characterization, Molecular Docking and Molecular Dynamics Simulation. Mol. Divers. 2022, 26, 365–388. [Google Scholar] [CrossRef]
- Kim, G.S.; Kim, D.H.; Lim, J.J.; Lee, J.J.; Han, D.Y.; Lee, W.M.; Jung, W.C.; Min, W.G.; Won, C.G.; Rhee, M.H.; et al. Biological and Antibacterial Activities of the Natural Herb Houttuynia Cordata Water Extract against the Intracellular Bacterial Pathogen Salmonella within the RAW 264.7 Macrophage. Biol. Pharm. Bull. 2008, 31, 2012–2017. [Google Scholar] [CrossRef]
- Chou, S.-C.; Su, C.-R.; Ku, Y.-C.; Wu, T.-S. The Constituents and Their Bioactivities of Houttuynia Cordata. Chem. Pharm. Bull. 2009, 57, 1227–1230. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Yang, F.; Zhan, H.; Liu, B.; Cai, J.; Luo, Y.; Zhou, X. Houttuynia Cordata Extract Ameliorates Bladder Damage and Improves Bladder Symptoms via Anti-Inflammatory Effect in Rats with Interstitial Cystitis. Evid. Based Complement. Altern. Med. 2020, 2020, 1–7. [Google Scholar] [CrossRef]
- Wang, H.; Bao, J. Effect of Houttuynia Cordata Aetherolea on Adiponectin and Connective Tissue Growth Factor in a Rat Model of Diabetes Mellitus. J. Tradit. Chin. Med. 2012, 32, 58–62. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Miyata, M.; Koyama, T.; Yazawa, K. Water Extract of Houttuynia Cordata Thunb. Leaves Exerts Anti-Obesity Effects by Inhibiting Fatty Acid and Glycerol Absorption. J. Nutr. Sci. Vitaminol. 2010, 56, 150–156. [Google Scholar] [CrossRef]
- Sakuludomkan, W.; Yeewa, R.; Subhawa, S.; Khanaree, C.; Bonness, A.I.; Chewonarin, T. Effects of Fermented Houttuynia Cordata Thunb. on Diabetic Rats Induced by a High-Fat Diet with Streptozotocin and on Insulin Resistance in 3T3-L1 Adipocytes. J. Nutr. Metab. 2021, 2021, 6936025. [Google Scholar] [CrossRef]
- Schosserer, M.; Grillari, J.; Wolfrum, C.; Scheideler, M. Age-Induced Changes in White, Brite, and Brown Adipose Depots: A Mini-Review. Gerontology 2018, 64, 229–236. [Google Scholar] [CrossRef]
- Dixon, S.J.; Lemberg, K.M.; Lamprecht, M.R.; Skouta, R.; Zaitsev, E.M.; Gleason, C.E.; Patel, D.N.; Bauer, A.J.; Cantley, A.M.; Yang, W.S.; et al. Ferroptosis: An Iron-Dependent Form of Nonapoptotic Cell Death. Cell 2012, 149, 1060–1072. [Google Scholar] [CrossRef] [PubMed]
- Seibt, T.M.; Proneth, B.; Conrad, M. Role of GPX4 in Ferroptosis and Its Pharmacological Implication. Free Radic. Biol. Med. 2019, 133, 144–152. [Google Scholar] [CrossRef]
- Doll, S.; Proneth, B.; Tyurina, Y.Y.; Panzilius, E.; Kobayashi, S.; Ingold, I.; Irmler, M.; Beckers, J.; Aichler, M.; Walch, A.; et al. ACSL4 Dictates Ferroptosis Sensitivity by Shaping Cellular Lipid Composition. Nat. Chem. Biol. 2017, 13, 91–98. [Google Scholar] [CrossRef]
- Roh, J.-L.; Kim, E.H.; Jang, H.; Shin, D. Nrf2 Inhibition Reverses the Resistance of Cisplatin-Resistant Head and Neck Cancer Cells to Artesunate-Induced Ferroptosis. Redox Biol. 2017, 11, 254–262. [Google Scholar] [CrossRef]
- Dong, H.; Xia, Y.; Jin, S.; Xue, C.; Wang, Y.; Hu, R.; Jiang, H. Nrf2 Attenuates Ferroptosis-Mediated IIR-ALI by Modulating TERT and SLC7A11. Cell Death Dis. 2021, 12, 1027. [Google Scholar] [CrossRef] [PubMed]
- Rafiq, S.; Hao, H.; Ijaz, M.; Raza, A. Pharmacological Effects of Houttuynia Cordata Thunb (H. Cordata): A Comprehensive Review. Pharmaceuticals 2022, 15, 1079. [Google Scholar] [CrossRef] [PubMed]
- Marlatt, K.L.; Ravussin, E. Brown Adipose Tissue: An Update on Recent Findings. Curr. Obes. Rep. 2017, 6, 389–396. [Google Scholar] [CrossRef]
- Scheele, C.; Nielsen, S. Metabolic Regulation and the Anti-Obesity Perspectives of Human Brown Fat. Redox Biol. 2017, 12, 770–775. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Liu, J.; Kang, R.; Klionsky, D.J.; Kroemer, G.; Tang, D. Ferroptosis Is a Type of Autophagy-Dependent Cell Death. Semin. Cancer Biol. 2020, 66, 89–100. [Google Scholar] [CrossRef]
- Lettieri-Barbato, D. Redox Control of Non-Shivering Thermogenesis. Mol. Metab. 2019, 25, 11–19. [Google Scholar] [CrossRef]
- Jeanson, Y.; Carrière, A.; Casteilla, L. A New Role for Browning as a Redox and Stress Adaptive Mechanism? Front. Endocrinol. 2015, 6, 158. [Google Scholar] [CrossRef]
- Man, A.W.C.; Zhou, Y.; Xia, N.; Li, H. Perivascular Adipose Tissue Oxidative Stress in Obesity. Antioxid 2023, 12, 1595. [Google Scholar] [CrossRef]
- Peris, E.; Micallef, P.; Paul, A.; Palsdottir, V.; Enejder, A.; Bauzá-Thorbrügge, M.; Olofsson, C.S.; Wernstedt Asterholm, I. Antioxidant Treatment Induces Reductive Stress Associated with Mitochondrial Dysfunction in Adipocytes. J. Biol. Chem. 2019, 294, 2340–2352. [Google Scholar] [CrossRef]
- Castro, J.P.; Grune, T.; Speckmann, B. The Two Faces of Reactive Oxygen Species (ROS) in Adipocyte Function and Dysfunction. Biol. Chem. 2016, 397, 709–724. [Google Scholar] [CrossRef]
- Cataneo, A.H.D.; Tomiotto-Pellissier, F.; Miranda-Sapla, M.M.; Assolini, J.P.; Panis, C.; Kian, D.; Yamauchi, L.M.; Colado Simão, A.N.; Casagrande, R.; Pinge-Filho, P.; et al. Quercetin Promotes Antipromastigote Effect by Increasing the ROS Production and Anti-Amastigote by Upregulating Nrf2/HO-1 Expression, Affecting Iron Availability. Biomed. Pharmacother. 2019, 113, 108745. [Google Scholar] [CrossRef] [PubMed]
- Dodson, M.; Castro-Portuguez, R.; Zhang, D.D. NRF2 Plays a Critical Role in Mitigating Lipid Peroxidation and Ferroptosis. Redox Biol. 2019, 23, 101107. [Google Scholar] [CrossRef] [PubMed]
- Lou, Y.; Guo, Z.; Zhu, Y.; Kong, M.; Zhang, R.; Lu, L.; Wu, F.; Liu, Z.; Wu, J. Houttuynia Cordata Thunb. and Its Bioactive Compound 2-Undecanone Significantly Suppress Benzo(a)Pyrene-Induced Lung Tumorigenesis by Activating the Nrf2-HO-1/NQO-1 Signaling Pathway. J. Exp. Clin. Cancer Res. 2019, 38, 242. [Google Scholar] [CrossRef]
- Petsouki, E.; Cabrera, S.N.S.; Heiss, E.H. AMPK and NRF2: Interactive Players in the Same Team for Cellular Homeostasis? Free Radic. Biol. Med. 2022, 190, 75–93. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, W.; Zou, H.; You, D.; Zhang, H.; Xu, L. Sodium Houttuybonate Promotes the Browning of White Adipose Tissue by Inhibiting Ferroptosis via the AMPK-NRF2-HO1 Pathway. Antioxidants 2024, 13, 1057. https://doi.org/10.3390/antiox13091057
Liu W, Zou H, You D, Zhang H, Xu L. Sodium Houttuybonate Promotes the Browning of White Adipose Tissue by Inhibiting Ferroptosis via the AMPK-NRF2-HO1 Pathway. Antioxidants. 2024; 13(9):1057. https://doi.org/10.3390/antiox13091057
Chicago/Turabian StyleLiu, Wenhui, Huren Zou, Danming You, Huijie Zhang, and Lingling Xu. 2024. "Sodium Houttuybonate Promotes the Browning of White Adipose Tissue by Inhibiting Ferroptosis via the AMPK-NRF2-HO1 Pathway" Antioxidants 13, no. 9: 1057. https://doi.org/10.3390/antiox13091057
APA StyleLiu, W., Zou, H., You, D., Zhang, H., & Xu, L. (2024). Sodium Houttuybonate Promotes the Browning of White Adipose Tissue by Inhibiting Ferroptosis via the AMPK-NRF2-HO1 Pathway. Antioxidants, 13(9), 1057. https://doi.org/10.3390/antiox13091057






