Mechanisms of Phytoremediation by Resveratrol against Cadmium Toxicity
Abstract
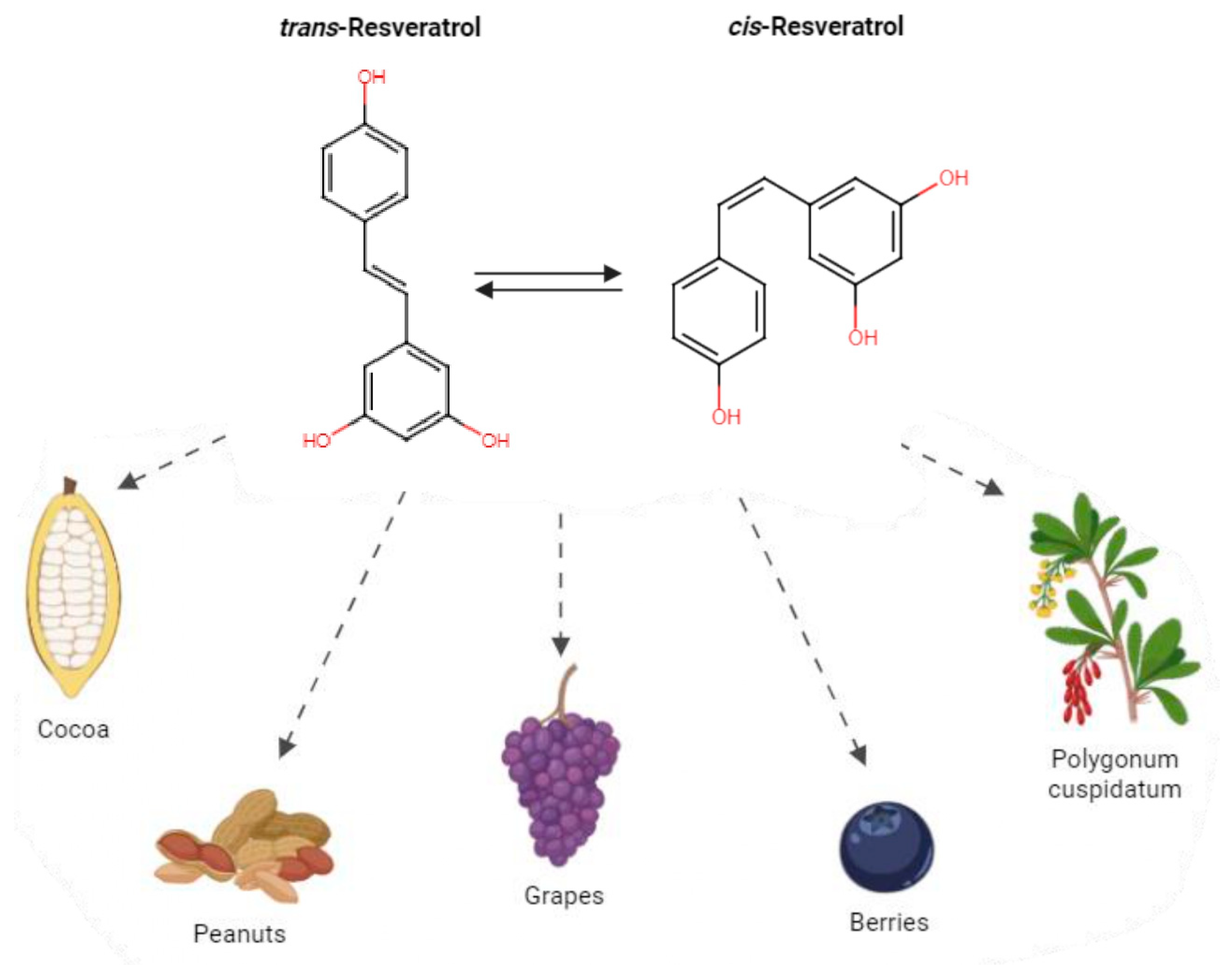
1. Introduction
2. Materials and Methods
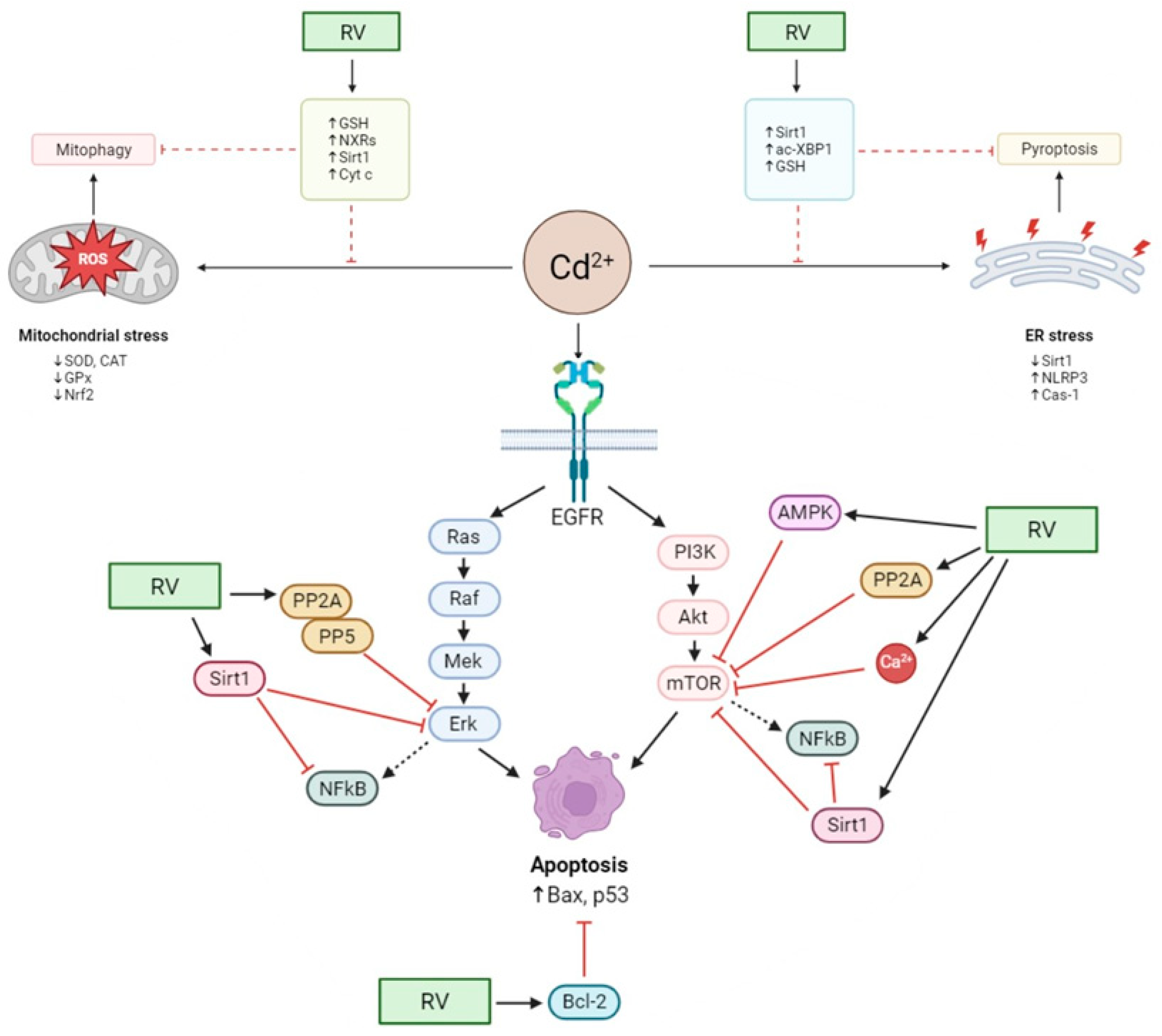
3. Central Nervous System
4. Reproductive System
5. Kidneys and Liver
6. Thyroid
7. Multiorgan Impact
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Genchi, G.; Sinicropi, M.S.; Lauria, G.; Carocci, A.; Catalano, A. The Effects of Cadmium Toxicity. Int. J. Environ. Res. Public Health 2020, 17, 3782. [Google Scholar] [CrossRef] [PubMed]
- Mezynska, M.; Brzóska, M.M. Environmental Exposure to Cadmium—A Risk for Health of the General Population in Industrialized Countries and Preventive Strategies. Environ. Sci. Pollut. Res. 2018, 25, 3211–3232. [Google Scholar] [CrossRef] [PubMed]
- Joint FAO/WHO Expert Committee on Food Additives (JECFA). Cadmium. In Proceedings of the Virtual Meeting, Virtual, 1–12 February 2021. [Google Scholar]
- Zhang, Y.; Wang, J. Advances in Effects of Cadmium on Calcium Metabolism and Its Associated Potential Mechanisms. J. Environ. Health 2004, 21, 269–271. [Google Scholar]
- Brzóska, M.M.; Moniuszko-Jakoniuk, J. Interactions between Cadmium and Zinc in the Organism. Food Chem. Toxicol. 2001, 39, 967–980. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Zhen, J.; Leng, J.; Cai, L.; Ji, H.; Keller, B.B. Zinc as a Countermeasure for Cadmium Toxicity. Acta Pharmacol. Sin. 2021, 42, 340–346. [Google Scholar] [CrossRef] [PubMed]
- Martin, P.; Fareh, M.; Poggi, M.C.; Boulukos, K.E.; Pognonec, P. Manganese Is Highly Effective in Protecting Cells from Cadmium Intoxication. Biochem. Biophys. Res. Commun. 2006, 351, 294–299. [Google Scholar] [CrossRef] [PubMed]
- Choong, G.; Liu, Y.; Templeton, D.M. Interplay of Calcium and Cadmium in Mediating Cadmium Toxicity. Chem. Biol. Interact. 2014, 211, 54–65. [Google Scholar] [CrossRef]
- Satarug, S. Dietary Cadmium Intake and Its Effects on Kidneys. Toxics 2018, 6, 15. [Google Scholar] [CrossRef]
- Siewit, C.L.; Gengler, B.; Vegas, E.; Puckett, R.; Louie, M.C. Cadmium Promotes Breast Cancer Cell Proliferation by Potentiating the Interaction between ERα and C-Jun. Mol. Endocrinol. 2010, 24, 981–992. [Google Scholar] [CrossRef]
- Kippler, M.; Hoque, A.M.W.; Raqib, R.; Öhrvik, H.; Ekström, E.-C.; Vahter, M. Accumulation of Cadmium in Human Placenta Interacts with the Transport of Micronutrients to the Fetus. Toxicol. Lett. 2010, 192, 162–168. [Google Scholar] [CrossRef]
- Kippler, M.; Lönnerdal, B.; Goessler, W.; Ekström, E.-C.; El Arifeen, S.; Vahter, M. Cadmium Interacts with the Transport of Essential Micronutrients in the Mammary Gland—A Study in Rural Bangladeshi Women. Toxicology 2009, 257, 64–69. [Google Scholar] [CrossRef]
- Margrete Meltzer, H.; Lise Brantsæter, A.; Borch-Iohnsen, B.; Ellingsen, D.G.; Alexander, J.; Thomassen, Y.; Stigum, H.; Ydersbond, T.A. Low Iron Stores Are Related to Higher Blood Concentrations of Manganese, Cobalt and Cadmium in Non-Smoking, Norwegian Women in the HUNT 2 Study. Environ. Res. 2010, 110, 497–504. [Google Scholar] [CrossRef]
- Charkiewicz, A.E.; Omeljaniuk, W.J.; Nowak, K.; Garley, M.; Nikliński, J. Cadmium Toxicity and Health Effects—A Brief Summary. Molecules 2023, 28, 6620. [Google Scholar] [CrossRef]
- Chen, X.-X.; Xu, Y.-M.; Lau, A.T.Y. Metabolic Effects of Long-Term Cadmium Exposure: An Overview. Environ. Sci. Pollut. Res. 2022, 29, 89874–89888. [Google Scholar] [CrossRef] [PubMed]
- Branca, J.J.V.; Fiorillo, C.; Carrino, D.; Paternostro, F.; Taddei, N.; Gulisano, M.; Pacini, A.; Becatti, M. Cadmium-Induced Oxidative Stress: Focus on the Central Nervous System. Antioxidants 2020, 9, 492. [Google Scholar] [CrossRef]
- Cirmi, S.; Maugeri, A.; Micali, A.; Marini, H.R.; Puzzolo, D.; Santoro, G.; Freni, J.; Squadrito, F.; Irrera, N.; Pallio, G.; et al. Cadmium-Induced Kidney Injury in Mice Is Counteracted by a Flavonoid-Rich Extract of Bergamot Juice, Alone or in Association with Curcumin and Resveratrol, via the Enhancement of Different Defense Mechanisms. Biomedicines 2021, 9, 1797. [Google Scholar] [CrossRef]
- Yuan, Y.; Zhang, Y.; Zhao, S.; Chen, J.; Yang, J.; Wang, T.; Zou, H.; Wang, Y.; Gu, J.; Liu, X.; et al. Cadmium-Induced Apoptosis in Neuronal Cells Is Mediated by Fas/FasL-Mediated Mitochondrial Apoptotic Signaling Pathway. Sci. Rep. 2018, 8, 8837. [Google Scholar] [CrossRef]
- Gao, D.; Xu, Z.; Qiao, P.; Liu, S.; Zhang, L.; He, P.; Zhang, X.; Wang, Y.; Min, W. Cadmium Induces Liver Cell Apoptosis through Caspase-3A Activation in Purse Red Common Carp (Cyprinus carpio). PLoS ONE 2013, 8, e83423. [Google Scholar] [CrossRef]
- Korotkov, S.M. Mitochondrial Oxidative Stress Is the General Reason for Apoptosis Induced by Different-Valence Heavy Metals in Cells and Mitochondria. Int. J. Mol. Sci. 2023, 24, 14459. [Google Scholar] [CrossRef] [PubMed]
- Yiming, L.; Yanfei, H.; Hang, Y.; Yimei, C.; Guangliang, S.; Shu, L. Cadmium Induces Apoptosis of Pig Lymph Nodes by Regulating the PI3K/AKT/HIF-1α Pathway. Toxicology 2021, 451, 152694. [Google Scholar] [CrossRef] [PubMed]
- Thévenod, F. Cadmium and Cellular Signaling Cascades: To Be or Not to Be? Toxicol. Appl. Pharmacol. 2009, 238, 221–239. [Google Scholar] [CrossRef]
- Brown, K.; Theofanous, D.; Britton, R.G.; Aburido, G.; Pepper, C.; Sri Undru, S.; Howells, L. Resveratrol for the Management of Human Health: How Far Have We Come? A Systematic Review of Resveratrol Clinical Trials to Highlight Gaps and Opportunities. Int. J. Mol. Sci. 2024, 25, 747. [Google Scholar] [CrossRef]
- Meng, X.; Zhou, J.; Zhao, C.-N.; Gan, R.-Y.; Li, H.-B. Health Benefits and Molecular Mechanisms of Resveratrol: A Narrative Review. Foods 2020, 9, 340. [Google Scholar] [CrossRef]
- Michio, T. The Phenolic Substances of White Helleboro (Veratrum grandiflorum Hoes. Fil.) III. Nippon. Kagaku Kaishi 1940, 61, 1067–1069. [Google Scholar]
- Xiao, K.; Xuan, L.; Xu, Y.; Bai, D.-L. Studies on the Chemical Constituents of Polygonum cuspidatum. Chin. Pharm. J. 2003, 38, 12–14. [Google Scholar]
- Burns, J.; Yokota, T.; Ashihara, H.; Lean, M.E.J.; Crozier, A. Plant Foods and Herbal Sources of Resveratrol. J. Agric. Food Chem. 2002, 50, 3337–3340. [Google Scholar] [CrossRef] [PubMed]
- Soleas, G.J.; Diamandis, E.P.; Goldberg, D.M. Resveratrol: A Molecule Whose Time Has Come? And Gone? Clin. Biochem. 1997, 30, 91–113. [Google Scholar] [CrossRef]
- Langcake, P.; Pryce, R.J. The Production of Resveratrol by Vitis Vinifera and Other Members of the Vitaceae as a Response to Infection or Injury. Physiol. Plant Pathol. 1976, 9, 77–86. [Google Scholar] [CrossRef]
- Crozier, A.; Jaganath, I.B.; Clifford, M.N. Dietary Phenolics: Chemistry, Bioavailability and Effects on Health. Nat. Prod. Rep. 2009, 26, 1001. [Google Scholar] [CrossRef]
- Pervaiz, S.; Holme, A.L. Resveratrol: Its Biologic Targets and Functional Activity. Antioxid. Redox Signal. 2009, 11, 2851–2897. [Google Scholar] [CrossRef]
- Meng, T.; Xiao, D.; Muhammed, A.; Deng, J.; Chen, L.; He, J. Anti-Inflammatory Action and Mechanisms of Resveratrol. Molecules 2021, 26, 229. [Google Scholar] [CrossRef] [PubMed]
- Kassumeh, S.; Wertheimer, C.M.; Ohlmann, A.; Priglinger, S.G.; Wolf, A. Cytoprotective Effect of Crocin and Trans-Resveratrol on Photodamaged Primary Human Retinal Pigment Epithelial Cells. Eur. J. Ophthalmol. 2021, 31, 630–637. [Google Scholar] [CrossRef] [PubMed]
- Buryanovskyy, L.; Fu, Y.; Boyd, M.; Ma, Y.; Hsieh, T.; Wu, J.M.; Zhang, Z. Crystal Structure of Quinone Reductase 2 in Complex with Resveratrol. Biochemistry 2004, 43, 11417–11426. [Google Scholar] [CrossRef] [PubMed]
- Directive 2002/46/EC of the European Parliament and of the Council of 10 June 2002 on the Approximation of the Laws of the Member States Relating to Food Supplements. Off. J. Eur. Communities 2002, 45, 51–57.
- Hausenblas, H.A.; Schoulda, J.A.; Smoliga, J.M. Resveratrol Treatment as an Adjunct to Pharmacological Management in Type 2 Diabetes Mellitus—Systematic Review and Meta-analysis. Mol. Nutr. Food Res. 2015, 59, 147–159. [Google Scholar] [CrossRef] [PubMed]
- Dyck, G.; Raj, P.; Zieroth, S.; Dyck, J.; Ezekowitz, J. The Effects of Resveratrol in Patients with Cardiovascular Disease and Heart Failure: A Narrative Review. Int. J. Mol. Sci. 2019, 20, 904. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Du, Y. Cadmium and Its Neurotoxic Effects. Oxid. Med. Cell Longev. 2013, 2013, 898034. [Google Scholar] [CrossRef] [PubMed]
- Arruebarrena, M.A.; Hawe, C.T.; Lee, Y.M.; Branco, R.C. Mechanisms of Cadmium Neurotoxicity. Int. J. Mol. Sci. 2023, 24, 16558. [Google Scholar] [CrossRef] [PubMed]
- Hao, R.; Ge, J.; Song, X.; Li, F.; Sun-Waterhouse, D.; Li, D. Cadmium Induces Ferroptosis and Apoptosis by Modulating MiR-34a-5p/Sirt1axis in PC12 Cells. Environ. Toxicol. 2022, 37, 41–51. [Google Scholar] [CrossRef]
- Ali, M.M.; Mathur, N.; Chandra, S. V Effect of Chronic Cadmium Exposure on Locomotor Behaviour of Rats. Indian. J. Exp. Biol. 1990, 28, 653–656. [Google Scholar]
- Viaene, M.K. Neurobehavioural Effects of Occupational Exposure to Cadmium: A Cross Sectional Epidemiological Study. Occup. Environ. Med. 2000, 57, 19–27. [Google Scholar] [CrossRef]
- Shati, A.A. Resveratrol Protects against Cadmium Chloride-Induced Hippocampal Neurotoxicity by Inhibiting ER Stress and GAAD 153 and Activating Sirtuin 1/AMPK/Akt. Environ. Toxicol. 2019, 34, 1340–1353. [Google Scholar] [CrossRef] [PubMed]
- Shati, A.A.; Alfaifi, M.Y. Trans-Resveratrol Inhibits Tau Phosphorylation in the Brains of Control and Cadmium Chloride-Treated Rats by Activating PP2A and PI3K/Akt Induced-Inhibition of GSK3β. Neurochem. Res. 2019, 44, 357–373. [Google Scholar] [CrossRef] [PubMed]
- Toral-Rios, D.; Pichardo-Rojas, P.S.; Alonso-Vanegas, M.; Campos-Peña, V. GSK3β and Tau Protein in Alzheimer’s Disease and Epilepsy. Front. Cell Neurosci. 2020, 14, 19. [Google Scholar] [CrossRef] [PubMed]
- Lin, F.; Peng, Y.H.; Yang, Q.H.; Mi, X.J. Resveratrol Inhibits Cadmium Induced Neuronal Apoptosis by Modulating Calcium Signalling Pathway via Regulation of MAPK/MTOR Network. Bangladesh J. Pharmacol. 2015, 10, 366–376. [Google Scholar] [CrossRef][Green Version]
- Liu, C.; Zhang, R.; Sun, C.; Zhang, H.; Xu, C.; Liu, W.; Gao, W.; Huang, S.; Chen, L. Resveratrol Prevents Cadmium Activation of Erk1/2 and JNK Pathways from Neuronal Cell Death via Protein Phosphatases 2A and 5. J. Neurochem. 2015, 135, 466–478. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, R.; Yang, L.; Ji, T.; Zhu, C.; Liu, B.; Zhang, H.; Xu, C.; Zhang, N.; Huang, S.; et al. Neuroprotection of Resveratrol against Cadmium-Poisoning Acts through Dual Inhibition of MTORC1/2 Signaling. Neuropharmacology 2022, 219. [Google Scholar] [CrossRef]
- Chen, L.; Liu, L.; Luo, Y.; Huang, S. MAPK and MTOR Pathways Are Involved in Cadmium-induced Neuronal Apoptosis. J. Neurochem. 2008, 105, 251–261. [Google Scholar] [CrossRef]
- Lv, M.W.; Zhang, C.; Ge, J.; Sun, X.H.; Li, J.Y.; Li, J.L. Resveratrol Protects against Cadmium-Induced Cerebrum Toxicity through Modifications of the Cytochrome P450 Enzyme System in Microsomes. J. Sci. Food Agric. 2023, 103, 5883–5892. [Google Scholar] [CrossRef]
- He, X.; Li, Z.; Rizak, J.D.; Wu, S.; Wang, Z.; He, R.; Su, M.; Qin, D.; Wang, J.; Hu, X. Resveratrol Attenuates Formaldehyde Induced Hyperphosphorylation of Tau Protein and Cytotoxicity in N2a Cells. Front. Neurosci. 2017, 10, 598. [Google Scholar] [CrossRef]
- Xu, C.; Wang, X.; Gu, C.; Zhang, H.; Zhang, R.; Dong, X.; Liu, C.; Hu, X.; Ji, X.; Huang, S.; et al. Celastrol Ameliorates Cd-induced Neuronal Apoptosis by Targeting NOX2-derived ROS-dependent PP5-JNK Signaling Pathway. J. Neurochem. 2017, 141, 48–62. [Google Scholar] [CrossRef]
- Biagioli, M.; Pifferi, S.; Ragghianti, M.; Bucci, S.; Rizzuto, R.; Pinton, P. Endoplasmic Reticulum Stress and Alteration in Calcium Homeostasis Are Involved in Cadmium-Induced Apoptosis. Cell Calcium 2008, 43, 184–195. [Google Scholar] [CrossRef]
- Brazert, J.; Piekarski, T.; Mitkowska, H.; Miedzianowski, J.; Biczysko, R. The Evaluation of the Efficiency of Treatment in Ectopic Pregnancy with the Application of Various Surgical Procedures. Ginekol. Pol. 1997, 68, 302–307. [Google Scholar]
- Xu, B.; Chen, S.; Luo, Y.; Chen, Z.; Liu, L.; Zhou, H.; Chen, W.; Shen, T.; Han, X.; Chen, L.; et al. Calcium Signaling Is Involved in Cadmium-Induced Neuronal Apoptosis via Induction of Reactive Oxygen Species and Activation of MAPK/MTOR Network. PLoS ONE 2011, 6, e19052. [Google Scholar] [CrossRef]
- Yuan, Y.; Jiang, C.; Xu, H.; Sun, Y.; Hu, F.; Bian, J.; Liu, X.; Gu, J.; Liu, Z. Cadmium-Induced Apoptosis in Primary Rat Cerebral Cortical Neurons Culture Is Mediated by a Calcium Signaling Pathway. PLoS ONE 2013, 8, e64330. [Google Scholar] [CrossRef]
- Benoff, S. Male Infertility and Environmental Exposure to Lead and Cadmium. Hum. Reprod. Update 2000, 6, 107–121. [Google Scholar] [CrossRef]
- Xiong, L.; Zhou, B.; Liu, H.; Cai, L. Comprehensive Review of Cadmium Toxicity Mechanisms in Male Reproduction and Therapeutic Strategies. In Reviews of Environmental Contamination and Toxicology; Springer: Cham, Switzerland, 2021; pp. 151–193. [Google Scholar]
- Gao, X.; Li, G.; Pan, X.; Xia, J.; Yan, D.; Xu, Y.; Ruan, X.; He, H.; Wei, Y.; Zhai, J. Environmental and Occupational Exposure to Cadmium Associated with Male Reproductive Health Risk: A Systematic Review and Meta-Analysis Based on Epidemiological Evidence. Environ. Geochem. Health 2023, 45, 7491–7517. [Google Scholar] [CrossRef]
- Akinloye, O.; Arowojolu, A.O.; Shittu, O.B.; Anetor, J.I. Cadmium Toxicity: A Possible Cause of Male Infertility in Nigeria. Reprod. Biol. 2006, 6, 17–30. [Google Scholar]
- Mitra, S.; Bhattacharyya, S.; Ray, S.; Saha, R.; Ghosh, P.; Rauth, S.; Mandal, S.; Banerjee, S.; Murmu, N. Resveratrol Alleviates Cadmium-Induced Damage and Overexpression of Epidermal Growth Factor Receptor and Its Downstream Signaling Proteins in the Reproductive System of Male Swiss Albino Mice. J. Environ. Pathol. Toxicol. Oncol. 2016, 35, 73–90. [Google Scholar] [CrossRef]
- Mason, K.E.; Brown, J.A.; Young, J.O.; Nesbit, R.R. Cadmium-induced Injury of the Rat Testis. Anat. Rec. 1964, 149, 135–147. [Google Scholar] [CrossRef]
- El-Maraghy, S.A.; Gad, M.Z.; Fahim, A.T.; Hamdy, M.A. Effect of Cadmium and Aluminum Intake on the Antioxidant Status and Lipid Peroxidation in Rat Tissues. J. Biochem. Mol. Toxicol. 2001, 15, 207–214. [Google Scholar] [CrossRef]
- Song, T.; Wang, L.; Mo, Z.; Mao, L.; Ma, X.; Niu, R.; Gu, K.; Yan, R.; Ma, P.; Qi, Y.; et al. Expression of P-Akt in Ovarian Serous Carcinoma and Its Association with Proliferation and Apoptosis. Oncol. Lett. 2014, 7, 59–64. [Google Scholar] [CrossRef][Green Version]
- Ali, I.; Damdimopoulou, P.; Stenius, U.; Halldin, K. Cadmium at Nanomolar Concentrations Activates Raf–MEK–ERK1/2 MAPKs Signaling via EGFR in Human Cancer Cell Lines. Chem. Biol. Interact. 2015, 231, 44–52. [Google Scholar] [CrossRef]
- Lian, S.; Xia, Y.; Khoi, P.N.; Ung, T.T.; Yoon, H.J.; Kim, N.H.; Kim, K.K.; Jung, Y. Do Cadmium Induces Matrix Metalloproteinase-9 Expression via ROS-Dependent EGFR, NF-КB, and AP-1 Pathways in Human Endothelial Cells. Toxicology 2015, 338, 104–116. [Google Scholar] [CrossRef]
- Kundu, S.; Sengupta, S.; Bhattacharyya, A. EGFR Upregulates Inflammatory and Proliferative Responses in Human Lung Adenocarcinoma Cell Line (A549), Induced by Lower Dose of Cadmium Chloride. Inhal. Toxicol. 2011, 23, 339–348. [Google Scholar] [CrossRef]
- Chakraborty, P.K.; Scharner, B.; Jurasovic, J.; Messner, B.; Bernhard, D.; Thévenod, F. Chronic Cadmium Exposure Induces Transcriptional Activation of the Wnt Pathway and Upregulation of Epithelial-to-Mesenchymal Transition Markers in Mouse Kidney. Toxicol. Lett. 2010, 198, 69–76. [Google Scholar] [CrossRef]
- Mitra, S.; Patra, T.; Saha, D.; Ghosh, P.; Mustafi, S.M.; Varghese, A.C.; Murmu, N. Sub-Chronic Cadmium and Lead Compound Exposure Induces Reproductive Toxicity and Development of Testicular Germ Cell Neoplasia in Situ in Murine Model: Attenuative Effects of Resveratrol. J. Biochem. Mol. Toxicol. 2022, 36, e23058. [Google Scholar] [CrossRef]
- Micheli, L.; Cerretani, D.; Collodel, G.; Menchiari, A.; Moltoni, L.; Fiaschi, A.I.; Moretti, E. Evaluation of Enzymatic and Non-enzymatic Antioxidants in Seminal Plasma of Men with Genitourinary Infections, Varicocele and Idiopathic Infertility. Andrology 2016, 4, 456–464. [Google Scholar] [CrossRef]
- Vara, J.Á.F.; Casado, E.; de Castro, J.; Cejas, P.; Belda-Iniesta, C.; González-Barón, M. PI3K/Akt Signalling Pathway and Cancer. Cancer Treat. Rev. 2004, 30, 193–204. [Google Scholar] [CrossRef]
- Matsuoka, M.; Igisu, H. Cadmium Induces Phosphorylation of P53 at Serine 15 in MCF-7 Cells. Biochem. Biophys. Res. Commun. 2001, 282, 1120–1125. [Google Scholar] [CrossRef]
- Gülçin, İ. Antioxidant Properties of Resveratrol: A Structure–Activity Insight. Innov. Food Sci. Emerg. Technol. 2010, 11, 210–218. [Google Scholar] [CrossRef]
- Li, D.; Wang, G.; Jin, G.; Yao, K.; Zhao, Z.; Bie, L.; Guo, Y.; Li, N.; Deng, W.; Chen, X.; et al. Resveratrol Suppresses Colon Cancer Growth by Targeting the AKT/STAT3 Signaling Pathway. Int. J. Mol. Med. 2018, 43, 630–640. [Google Scholar] [CrossRef]
- Ferlazzo, N.; Micali, A.; Marini, H.R.; Freni, J.; Santoro, G.; Puzzolo, D.; Squadrito, F.; Pallio, G.; Navarra, M.; Cirmi, S.; et al. A Flavonoid-Rich Extract from Bergamot Juice, Alone or in Association with Curcumin and Resveratrol, Shows Protective Effects in a Murine Model of Cadmium-Induced Testicular Injury. Pharmaceuticals 2021, 14, 386. [Google Scholar] [CrossRef] [PubMed]
- Shaito, A.; Posadino, A.M.; Younes, N.; Hasan, H.; Halabi, S.; Alhababi, D.; Al-Mohannadi, A.; Abdel-Rahman, W.M.; Eid, A.H.; Nasrallah, G.K.; et al. Potential Adverse Effects of Resveratrol: A Literature Review. Int. J. Mol. Sci. 2020, 21, 2084. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Liu, J.; He, L.; Liu, L.; Cheng, B.; Zhou, F.; Cao, D.; He, Y. A Comprehensive Review on the Benefits and Problems of Curcumin with Respect to Human Health. Molecules 2022, 27, 4400. [Google Scholar] [CrossRef]
- Perna, S.; Spadaccini, D.; Botteri, L.; Girometta, C.; Riva, A.; Allegrini, P.; Petrangolini, G.; Infantino, V.; Rondanelli, M. Efficacy of Bergamot: From Anti-inflammatory and Anti-oxidative Mechanisms to Clinical Applications as Preventive Agent for Cardiovascular Morbidity, Skin Diseases, and Mood Alterations. Food Sci. Nutr. 2019, 7, 369–384. [Google Scholar] [CrossRef]
- Eleawa, S.M.; Alkhateeb, M.A.; Alhashem, F.H.; Bin-Jaliah, I.; Sakr, H.F.; Elrefaey, H.M.; Elkarib, A.O.; Alessa, R.M.; Haidara, M.A.; Shatoor, A.S.; et al. Resveratrol Reverses Cadmium Chloride-Induced Testicular Damage and Subfertility by Downregulating P53 and Bax and Upregulating Gonadotropins and Bcl-2 Gene Expression. J. Reprod. Dev. 2014, 60, 115–127. [Google Scholar] [CrossRef]
- Crowell, J.A. Resveratrol-Associated Renal Toxicity. Toxicol. Sci. 2004, 82, 614–619. [Google Scholar] [CrossRef]
- Lagouge, M.; Argmann, C.; Gerhart-Hines, Z.; Meziane, H.; Lerin, C.; Daussin, F.; Messadeq, N.; Milne, J.; Lambert, P.; Elliott, P.; et al. Resveratrol Improves Mitochondrial Function and Protects against Metabolic Disease by Activating SIRT1 and PGC-1α. Cell 2006, 127, 1109–1122. [Google Scholar] [CrossRef]
- Juan, M.E.; González-Pons, E.; Munuera, T.; Ballester, J.; Rodríguez-Gil, J.E.; Planas, J.M. Trans-Resveratrol, a Natural Antioxidant from Grapes, Increases Sperm Output in Healthy Rats. J. Nutr. 2005, 135, 757–760. [Google Scholar] [CrossRef]
- Thompson, J.; Banningan, J. Cadmium: Toxic Effects on the Reproductive System and the Embryo. Reprod. Toxicol. 2008, 25, 304–315. [Google Scholar] [CrossRef]
- Akar, Y.; Ahmad, N.; Khalıd, M. The Effect of Cadmium on the Bovine in Vitro Oocyte Maturation and Early Embryo Development. Int. J. Vet. Sci. Med. 2018, 6, S73–S77. [Google Scholar] [CrossRef]
- Leoni, G.; Bogliolo, L.; Deiana, G.; Berlinguer, F.; Rosati, I.; Pintus, P.P.; Ledda, S.; Naitana, S. Influence of Cadmium Exposure on in Vitro Ovine Gamete Dysfunction. Reprod. Toxicol. 2002, 16, 371–377. [Google Scholar] [CrossRef] [PubMed]
- Nandi, S.; Gupta, P.S.P.; Selvaraju, S.; Roy, S.C.; Ravindra, J.P. Effects of Exposure to Heavy Metals on Viability, Maturation, Fertilization, and Embryonic Development of Buffalo (Bubalus Bubalis) Oocytes In Vitro. Arch. Environ. Contam. Toxicol. 2010, 58, 194–204. [Google Scholar] [CrossRef]
- Tessaro, I.; Modina, S.C.; Crotti, G.; Franciosi, F.; Colleoni, S.; Lodde, V.; Galli, C.; Lazzari, G.; Luciano, A.M. Transferability and Inter-Laboratory Variability Assessment of the in Vitro Bovine Oocyte Fertilization Test. Reprod. Toxicol. 2015, 51, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Zhang, J.; Wu, T.; Jiang, X.; Jia, H.; Qing, S.; An, Q.; Zhang, Y.; Su, J. Reproductive Toxicity of Acute Cd Exposure in Mouse: Resulting in Oocyte Defects and Decreased Female Fertility. Toxicol. Appl. Pharmacol. 2019, 379, 114684. [Google Scholar] [CrossRef]
- Dong, F.; Li, J.; Lei, W.-L.; Wang, F.; Wang, Y.; Ouyang, Y.-C.; Hou, Y.; Wang, Z.-B.; Schatten, H.; Sun, Q.-Y. Chronic Cadmium Exposure Causes Oocyte Meiotic Arrest by Disrupting Spindle Assembly Checkpoint and Maturation Promoting Factor. Reprod. Toxicol. 2020, 96, 141–149. [Google Scholar] [CrossRef]
- Liu, M.-J.; Sun, A.-G.; Zhao, S.-G.; Liu, H.; Ma, S.-Y.; Li, M.; Huai, Y.-X.; Zhao, H.; Liu, H.-B. Resveratrol Improves in Vitro maturation of Oocytes in Aged Mice and Humans. Fertil. Steril. 2018, 109, 900–907. [Google Scholar] [CrossRef]
- Piras, A.R.; Ariu, F.; Falchi, L.; Zedda, M.T.; Pau, S.; Schianchi, E.; Paramio, M.; Bogliolo, L. Resveratrol Treatment during Maturation Enhances Developmental Competence of Oocytes after Prolonged Ovary Storage at 4 °C in the Domestic Cat Model. Theriogenology 2020, 144, 152–157. [Google Scholar] [CrossRef]
- Piras, A.-R.; Menéndez-Blanco, I.; Soto-Heras, S.; Catalá, M.-G.; Izquierdo, D.; Bogliolo, L.; Paramio, M.-T. Resveratrol Supplementation during in Vitro Maturation Improves Embryo Development of Prepubertal Goat Oocytes Selected by Brilliant Cresyl Blue Staining. J. Reprod. Dev. 2019, 65, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Zabihi, A.; Shabankareh, H.K.; Hajarian, H.; Foroutanifar, S. Resveratrol Addition to in Vitro Maturation and in Vitro Culture Media Enhances Developmental Competence of Sheep Embryos. Domest. Anim. Endocrinol. 2019, 68, 25–31. [Google Scholar] [CrossRef]
- Sovernigo, T.; Adona, P.; Monzani, P.; Guemra, S.; Barros, F.; Lopes, F.; Leal, C. Effects of Supplementation of Medium with Different Antioxidants during in Vitro Maturation of Bovine Oocytes on Subsequent Embryo Production. Reprod. Domest. Anim. 2017, 52, 561–569. [Google Scholar] [CrossRef]
- Piras, A.R.; Ariu, F.; Maltana, A.; Leoni, G.G.; Martino, N.A.; Mastrorocco, A.; Dell’Aquila, M.E.; Bogliolo, L. Protective Effect of Resveratrol against Cadmium-Induced Toxicity on Ovine Oocyte in Vitro Maturation and Fertilization. J. Anim. Sci. Biotechnol. 2022, 13, 83. [Google Scholar] [CrossRef]
- Han, Y.; Luo, H.; Wang, H.; Cai, J.; Zhang, Y. SIRT1 Induces Resistance to Apoptosis in Human Granulosa Cells by Activating the ERK Pathway and Inhibiting NF-ΚB Signaling with Anti-Inflammatory Functions. Apoptosis 2017, 22, 1260–1272. [Google Scholar] [CrossRef]
- Itami, N.; Shirasuna, K.; Kuwayama, T.; Iwata, H. Resveratrol Improves the Quality of Pig Oocytes Derived from Early Antral Follicles through Sirtuin 1 Activation. Theriogenology 2015, 83, 1360–1367. [Google Scholar] [CrossRef] [PubMed]
- Nishijo, M.; Satarug, S.; Honda, R.; Tsuritani, I.; Aoshima, K. The Gender Differences in Health Effects of Environmental Cadmium Exposure and Potential Mechanisms. Mol. Cell Biochem. 2004, 255, 87–92. [Google Scholar] [CrossRef]
- Geng, H.-X.; Wang, L. Cadmium: Toxic Effects on Placental and Embryonic Development. Environ. Toxicol. Pharmacol. 2019, 67, 102–107. [Google Scholar] [CrossRef]
- Moynihan, M.; Peterson, K.E.; Cantoral, A.; Song, P.X.K.; Jones, A.; Solano-González, M.; Meeker, J.D.; Basu, N.; Téllez-Rojo, M.M. Dietary Predictors of Urinary Cadmium among Pregnant Women and Children. Sci. Total Environ. 2017, 575, 1255–1262. [Google Scholar] [CrossRef]
- Wang, H.; Wang, Y.; Bo, Q.-L.; Ji, Y.-L.; Liu, L.; Hu, Y.-F.; Chen, Y.-H.; Zhang, J.; Zhao, L.-L.; Xu, D.-X. Maternal Cadmium Exposure Reduces Placental Zinc Transport and Induces Fetal Growth Restriction in Mice. Reprod. Toxicol. 2016, 63, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Johnston, J.E.; Valentiner, E.; Maxson, P.; Miranda, M.L.; Fry, R.C. Maternal Cadmium Levels during Pregnancy Associated with Lower Birth Weight in Infants in a North Carolina Cohort. PLoS ONE 2014, 9, e109661. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Wu, Z.; Xi, Y.; Wang, L. Epigenetic Regulation of Placental Glucose Transporters Mediates Maternal Cadmium-Induced Fetal Growth Restriction. Toxicology 2016, 372, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Wu, Z.; Yang, W.; Wang, L. Dysregulation of DNA Methylation and Expression of Imprinted Genes in Mouse Placentas of Fetal Growth Restriction Induced by Maternal Cadmium Exposure. Toxicology 2017, 390, 109–116. [Google Scholar] [CrossRef]
- Levin, A.A.; Plautz, J.R.; di Sant’Agnese, P.A.; Miller, R.K. Cadmium: Placental Mechanisms of Fetal Toxicity. Placenta Suppl. 1981, 3, 303–318. [Google Scholar] [PubMed]
- Zhu, H.-L.; Shi, X.-T.; Xu, X.-F.; Xiong, Y.-W.; Yi, S.-J.; Zhou, G.-X.; Liu, W.-B.; Huang, M.-M.; Gao, L.; Zhang, C.; et al. Environmental Cadmium Exposure Induces Fetal Growth Restriction via Triggering PERK-Regulated Mitophagy in Placental Trophoblasts. Environ. Int. 2021, 147, 106319. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, H.; Xu, Z.M.; Ji, Y.-L.; Chen, Y.-H.; Zhang, Z.-H.; Zhang, C.; Meng, X.-H.; Zhao, M.; Xu, D.-X. Cadmium-Induced Teratogenicity: Association with ROS-Mediated Endoplasmic Reticulum Stress in Placenta. Toxicol. Appl. Pharmacol. 2012, 259, 236–247. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Wang, H.; Hu, Y.-F.; Xu, X.-F.; Chen, Y.-H.; Xia, M.-Z.; Zhang, C.; Xu, D.-X. Cadmium Induces Inflammatory Cytokines through Activating Akt Signaling in Mouse Placenta and Human Trophoblast Cells. Placenta 2018, 65, 7–14. [Google Scholar] [CrossRef]
- Mestan, K.; Yu, Y.; Matoba, N.; Cerda, S.; Demmin, B.; Pearson, C.; Ortiz, K.; Wang, X. Placental Inflammatory Response Is Associated With Poor Neonatal Growth: Preterm Birth Cohort Study. Pediatrics 2010, 125, e891–e898. [Google Scholar] [CrossRef] [PubMed]
- Vilahur, N.; Vahter, M.; Broberg, K. The Epigenetic Effects of Prenatal Cadmium Exposure. Curr. Environ. Health Rep. 2015, 2, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Aldawsari, F.S.; Aguayo-Ortiz, R.; Kapilashrami, K.; Yoo, J.; Luo, M.; Medina-Franco, J.L.; Velázquez-Martínez, C.A. Resveratrol-Salicylate Derivatives as Selective DNMT3 Inhibitors and Anticancer Agents. J. Enzyme Inhib. Med. Chem. 2016, 31, 695–703. [Google Scholar] [CrossRef] [PubMed]
- Jiang, G.; Xu, L.; Song, S.; Zhu, C.; Wu, Q.; Zhang, L.; Wu, L. Effects of Long-Term Low-Dose Cadmium Exposure on Genomic DNA Methylation in Human Embryo Lung Fibroblast Cells. Toxicology 2008, 244, 49–55. [Google Scholar] [CrossRef]
- Wang, W.; Liu, G.; Jiang, X.; Wu, G. Resveratrol Ameliorates Toxic Effects of Cadmium on Placental Development in Mouse Placenta and Human Trophoblast Cells. Birth Defects Res. 2021, 113, 1470–1483. [Google Scholar] [CrossRef] [PubMed]
- Roberts, V.H.J.; Pound, L.D.; Thorn, S.R.; Gillingham, M.B.; Thornburg, K.L.; Friedman, J.E.; Frias, A.E.; Grove, K.L. Beneficial and Cautionary Outcomes of Resveratrol Supplementation in Pregnant Nonhuman Primates. FASEB J. 2014, 28, 2466–2477. [Google Scholar] [CrossRef] [PubMed]
- Souza-Arroyo, V.; Fabián, J.J.; Bucio-Ortiz, L.; Miranda-Labra, R.U.; Gomez-Quiroz, L.E.; Gutiérrez-Ruiz, M.C. The Mechanism of the Cadmium-Induced Toxicity and Cellular Response in the Liver. Toxicology 2022, 480, 153339. [Google Scholar] [CrossRef] [PubMed]
- Johri, N.; Jacquillet, G.; Unwin, R. Heavy Metal Poisoning: The Effects of Cadmium on the Kidney. BioMetals 2010, 23, 783–792. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.-J.; Allen, D.C. Cadmium-Induced Kidney Injury: Oxidative Damage as a Unifying Mechanism. Biomolecules 2021, 11, 1575. [Google Scholar] [CrossRef]
- Smereczański, N.M.; Brzóska, M.M. Current Levels of Environmental Exposure to Cadmium in Industrialized Countries as a Risk Factor for Kidney Damage in the General Population: A Comprehensive Review of Available Data. Int. J. Mol. Sci. 2023, 24, 8413. [Google Scholar] [CrossRef]
- Hu, J.; Zhang, B.; Du, L.; Chen, J.; Lu, Q. Resveratrol Ameliorates Cadmium Induced Renal Oxidative Damage and Inflammation. Int. J. Clin. Exp. Med. 2017, 10, 7563–7572. [Google Scholar]
- Fu, B.; Zhao, J.; Peng, W.; Wu, H.; Zhang, Y. Resveratrol Rescues Cadmium-Induced Mitochondrial Injury by Enhancing Transcriptional Regulation of PGC-1α and SOD2 via the Sirt3/FoxO3a Pathway in TCMK-1 Cells. Biochem. Biophys. Res. Commun. 2017, 486, 198–204. [Google Scholar] [CrossRef] [PubMed]
- Granata, S.; Dalla Gassa, A.; Tomei, P.; Lupo, A.; Zaza, G. Mitochondria: A New Therapeutic Target in Chronic Kidney Disease. Nutr. Metab. 2015, 12, 49. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, C.; Ge, J.; Lv, M.-W.; Talukder, M.; Guo, K.; Li, Y.; Li, J.-L. Ameliorative Effects of Resveratrol against Cadmium-Induced Nephrotoxicity via Modulating Nuclear Xenobiotic Receptor Response and PINK1/Parkin-Mediated Mitophagy. Food Funct. 2020, 11, 1856–1868. [Google Scholar] [CrossRef]
- Lugrin, J.; Rosenblatt-Velin, N.; Parapanov, R.; Liaudet, L. The Role of Oxidative Stress during Inflammatory Processes. Biol. Chem. 2014, 395, 203–230. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, A.M.; Wilkinson, F.L.; Sandhu, M.A.; Lightfoot, A.P. The Interplay of Oxidative Stress and Inflammation: Mechanistic Insights and Therapeutic Potential of Antioxidants. Oxid. Med. Cell Longev. 2021, 2021, 9851914. [Google Scholar] [CrossRef]
- Chou, X.; Ding, F.; Zhang, X.; Ding, X.; Gao, H.; Wu, Q. Sirtuin-1 Ameliorates Cadmium-Induced Endoplasmic Reticulum Stress and Pyroptosis through XBP-1s Deacetylation in Human Renal Tubular Epithelial Cells. Arch. Toxicol. 2019, 93, 965–986. [Google Scholar] [CrossRef] [PubMed]
- Sharma, B.R.; Kanneganti, T.-D. NLRP3 Inflammasome in Cancer and Metabolic Diseases. Nat. Immunol. 2021, 22, 550–559. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Gao, J.; Hou, H.; Qi, Z.; Chen, H.; Zhang, X.-X. Inhibition of Mitochondrial Fatty Acid Oxidation Contributes to Development of Nonalcoholic Fatty Liver Disease Induced by Environmental Cadmium Exposure. Environ. Sci. Technol. 2019, 53, 13992–14000. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Zhu, Y.; Lu, Z.; Guo, W.; Tumen, B.; He, Y.; Chen, C.; Hu, S.; Xu, K.; Wang, Y.; et al. Cadmium Induces Acute Liver Injury by Inhibiting Nrf2 and the Role of NF-ΚB, NLRP3, and MAPKs Signaling Pathway. Int. J. Environ. Res. Public. Health 2019, 17, 138. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.; Wang, Q.; Liu, S.; Qu, Z.; Li, K.; Geng, X.; Wang, T.; Gao, J. Characterization the Performances of Twofold Resveratrol Integrated Compounds in Binding with SIRT1 by Molecular Dynamics Simulation and Molecular Mechanics/Generalized Born Surface Area (MM/GBSA) Calculation. Chem. Phys. 2021, 544, 111108. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, Y.; Fu, L.; Wang, B.; Ji, Y.; Wang, H.; Xu, D. Chronic Cadmium Exposure Induced Hepatic Cellular Stress and Inflammation in Aged Female Mice. J. Appl. Toxicol. 2019, 39, 498–509. [Google Scholar] [CrossRef]
- Rafati, A.; Hoseini, L.; Babai, A.; Noorafshan, A.; Haghbin, H.; Karbalay-Doust, S. Mitigating Effect of Resveratrol on the Structural Changes of Mice Liver and Kidney Induced by Cadmium; A Stereological Study. Prev. Nutr. Food Sci. 2015, 20, 266–275. [Google Scholar] [CrossRef]
- Eybl, V.; Kotyzova, D.; Koutensky, J. Comparative Study of Natural Antioxidants—Curcumin, Resveratrol and Melatonin—In Cadmium-Induced Oxidative Damage in Mice. Toxicology 2006, 225, 150–156. [Google Scholar] [CrossRef]
- Al-Baqami, N.; Hamza, R. Protective Effect of Resveratrol against Hepatotoxicity of Cadmium in Male Rats: Antioxidant and Histopathological Approaches. Coatings 2021, 11, 594. [Google Scholar] [CrossRef]
- Takiguchi, M.; Yoshihara, S. New Aspects of Cadmium as Endocrine Disruptor. Environ. Sci. 2006, 13, 107–116. [Google Scholar]
- Duntas, L.H. Chemical Contamination and the Thyroid. Endocrine 2015, 48, 53–64. [Google Scholar] [CrossRef] [PubMed]
- Maqbool, F.; Mostafalou, S.; Bahadar, H.; Abdollahi, M. Review of Endocrine Disorders Associated with Environmental Toxicants and Possible Involved Mechanisms. Life Sci. 2016, 145, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Jancic, S.A.; Stosic, B.Z. Cadmium Effects on the Thyroid Gland; Vitamins & Hormones; Academic Press: Cambridge, MA, USA, 2014; pp. 391–425. [Google Scholar]
- Buha, A.; Matovic, V.; Antonijevic, B.; Bulat, Z.; Curcic, M.; Renieri, E.; Tsatsakis, A.; Schweitzer, A.; Wallace, D. Overview of Cadmium Thyroid Disrupting Effects and Mechanisms. Int. J. Mol. Sci. 2018, 19, 1501. [Google Scholar] [CrossRef] [PubMed]
- Piłat-Marcinkiewicz, B.; Sawicki, B.; Brzóska, M.M.; Moniuszko-Jakoniuk, J. Effect of Chronic Administration of Cadmium on the Rat Thyroid: Radioimmunological and Immunohistochemical Studies. Folia Histochem. Cytobiol. 2002, 40, 189–190. [Google Scholar] [PubMed]
- Czykier, E.; Moniuszko-Jakoniuk, J.; Sawicki, B. Effect of Acute Exposure to Cadmium on the Expression of Calcitonin Gene-Related Peptide (CGRP), Calcitonin (CT), Somatostatin (SST) and Synaptophysin (SYN) in the C Cells of the Rat Thyroid—A Preliminary Study. Folia Morphol. 2004, 63, 217–219. [Google Scholar]
- Benvenga, S.; Marini, H.R.; Micali, A.; Freni, J.; Pallio, G.; Irrera, N.; Squadrito, F.; Altavilla, D.; Antonelli, A.; Ferrari, S.M.; et al. Protective Effects of Myo-Inositol and Selenium on Cadmium-Induced Thyroid Toxicity in Mice. Nutrients 2020, 12, 1222. [Google Scholar] [CrossRef]
- Benvenga, S.; Micali, A.; Ieni, A.; Antonelli, A.; Fallahi, P.; Pallio, G.; Irrera, N.; Squadrito, F.; Picciolo, G.; Puzzolo, D.; et al. The Association of Myo-Inositol and Selenium Contrasts Cadmium-Induced Thyroid C Cell Hyperplasia and Hypertrophy in Mice. Front. Endocrinol. 2021, 12, 608697. [Google Scholar] [CrossRef]
- Reyes-Hinojosa, D.; Lozada-Pérez, C.A.; Zamudio Cuevas, Y.; López-Reyes, A.; Martínez-Nava, G.; Fernández-Torres, J.; Olivos-Meza, A.; Landa-Solis, C.; Gutiérrez-Ruiz, M.C.; Rojas del Castillo, E.; et al. Toxicity of Cadmium in Musculoskeletal Diseases. Environ. Toxicol. Pharmacol. 2019, 72, 103219. [Google Scholar] [CrossRef]
- Krishnan, S.S.; Lui, S.M.W.; Jervis, R.E.; Harrison, J.E. Studies of Cadmium Uptake in Bone and Its Environmental Distribution. Biol. Trace Elem. Res. 1990, 26–27, 257–261. [Google Scholar] [CrossRef]
- Brzóska, M.M. Low-level Chronic Exposure to Cadmium Enhances the Risk of Long Bone Fractures: A Study on a Female Rat Model of Human Lifetime Exposure. J. Appl. Toxicol. 2012, 32, 34–44. [Google Scholar] [CrossRef]
- Chen, X.; Zhu, G.; Gu, S.; Jin, T.; Shao, C. Effects of Cadmium on Osteoblasts and Osteoclasts in Vitro. Environ. Toxicol. Pharmacol. 2009, 28, 232–236. [Google Scholar] [CrossRef]
- Rodríguez, J.; Mandalunis, P.M. Effect of Cadmium on Bone Tissue in Growing Animals. Exp. Toxicol. Pathol. 2016, 68, 391–397. [Google Scholar] [CrossRef]
- Taha, M.M.; Mahdy-Abdallah, H.; Shahy, E.M.; Ibrahim, K.S.; Elserougy, S. Impact of Occupational Cadmium Exposure on Bone in Sewage Workers. Int. J. Occup. Environ. Health 2018, 24, 101–108. [Google Scholar] [CrossRef]
- Ma, Y.; Ran, D.; Shi, X.; Zhao, H.; Liu, Z. Cadmium Toxicity: A Role in Bone Cell Function and Teeth Development. Sci. Total Environ. 2021, 769, 144646. [Google Scholar] [CrossRef] [PubMed]
- Mei, W.; Song, D.; Wu, Z.; Yang, L.; Wang, P.; Zhang, R.; Zhu, X. Resveratrol Protects MC3T3-E1 Cells against Cadmium-Induced Suppression of Osteogenic Differentiation by Modulating the ERK1/2 and JNK Pathways. Ecotoxicol. Environ. Saf. 2021, 214, 112080. [Google Scholar] [CrossRef]
- Bodo, M.; Balloni, S.; Lumare, E.; Bacci, M.; Calvitti, M.; Dell’Omo, M.; Murgia, N.; Marinucci, L. Effects of Sub-Toxic Cadmium Concentrations on Bone Gene Expression Program: Results of an in Vitro Study. Toxicol. Vitr. 2010, 24, 1670–1680. [Google Scholar] [CrossRef]
- Arbon, K.S.; Christensen, C.M.; Harvey, W.A.; Heggland, S.J. Cadmium Exposure Activates the ERK Signaling Pathway Leading to Altered Osteoblast Gene Expression and Apoptotic Death in Saos-2 Cells. Food Chem. Toxicol. 2012, 50, 198–205. [Google Scholar] [CrossRef]
- Sasikumar, S.; Yuvraj, S.; Veilumuthu, P.; Godwin Christopher, J.S.; Anandkumar, P.; Nagarajan, T.; Sureshkumar, S.; Selvam, G.S. Ascorbic Acid Attenuates Cadmium-Induced Myocardial Hypertrophy and Cardiomyocyte Injury through Nrf2 Signaling Pathways Comparable to Resveratrol. 3 Biotech 2023, 13, 108. [Google Scholar] [CrossRef]
- Feki-Tounsi, M.; Hamza-Chaffai, A. Cadmium as a Possible Cause of Bladder Cancer: A Review of Accumulated Evidence. Environ. Sci. Pollut. Res. 2014, 21, 10561–10573. [Google Scholar] [CrossRef]
- Eriksen, K.T.; Halkjær, J.; Meliker, J.R.; McElroy, J.A.; Sørensen, M.; Tjønneland, A.; Raaschou-Nielsen, O. Dietary Cadmium Intake and Risk of Prostate Cancer: A Danish Prospective Cohort Study. BMC Cancer 2015, 15, 177. [Google Scholar] [CrossRef]
- Chen, C.; Xun, P.; Nishijo, M.; He, K. Cadmium Exposure and Risk of Lung Cancer: A Meta-Analysis of Cohort and Case–Control Studies among General and Occupational Populations. J. Expo. Sci. Environ. Epidemiol. 2016, 26, 437–444. [Google Scholar] [CrossRef]
- Wang, H.; Gan, X.; Tang, Y. Mechanisms of Heavy Metal Cadmium (Cd)-Induced Malignancy. Biol. Trace Elem. Res. 2024. [Google Scholar] [CrossRef]
- Tran, F.; Lee, E.; Cuddapah, S.; Choi, B.H.; Dai, W. MicroRNA-Gene Interactions Impacted by Toxic Metal(Oid)s during EMT and Carcinogenesis. Cancers 2022, 14, 5818. [Google Scholar] [CrossRef]
- Qian, Y.; Wang, R.; Wei, W.; Wang, M.; Wang, S. Resveratrol Reverses the Cadmium-Promoted Migration, Invasion, and Epithelial-Mesenchymal Transition Procession by Regulating the Expression of ZEB1. Hum. Exp. Toxicol. 2021, 40, S331–S338. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Preventing Disease through Healthy Environments: Exposure to Cadmium: A Major Public Health Concern; World Health Organization: Geneva, Switzerland, 2019. [Google Scholar]
- Järup, L. Cadmium Overload and Toxicity. Nephrol. Dial. Transplant. 2002, 17 (Suppl. S2), 35–39. [Google Scholar] [CrossRef]
- Godt, J.; Scheidig, F.; Grosse-Siestrup, C.; Esche, V.; Brandenburg, P.; Reich, A.; Groneberg, D.A. The Toxicity of Cadmium and Resulting Hazards for Human Health. J. Occup. Med. Toxicol. 2006, 1, 22. [Google Scholar] [CrossRef]
- Brzóska, M.M.; Borowska, S.; Tomczyk, M. Antioxidants as a Potential Preventive and Therapeutic Strategy for Cadmium. Curr. Drug Targets 2016, 17, 1350–1384. [Google Scholar] [CrossRef] [PubMed]
- Moon, S.; Lee, J.; Yu, J.M.; Choi, H.; Choi, S.; Park, J.; Choi, K.; Kim, E.; Kim, H.; Kim, M.J.; et al. Association between Environmental Cadmium Exposure and Increased Mortality in the U.S. National Health and Nutrition Examination Survey (1999–2018). J. Expo. Sci. Environ. Epidemiol. 2023, 33, 874–882. [Google Scholar] [CrossRef] [PubMed]
- Nawrot, T.S.; Van Hecke, E.; Thijs, L.; Richart, T.; Kuznetsova, T.; Jin, Y.; Vangronsveld, J.; Roels, H.A.; Staessen, J.A. Cadmium-Related Mortality and Long-Term Secular Trends in the Cadmium Body Burden of an Environmentally Exposed Population. Environ. Health Perspect. 2008, 116, 1620–1628. [Google Scholar] [CrossRef]
- Frémont, L. Biological Effects of Resveratrol. Life Sci. 2000, 66, 663–673. [Google Scholar] [CrossRef] [PubMed]
- Baur, J.A.; Sinclair, D.A. Therapeutic Potential of Resveratrol: The in Vivo Evidence. Nat. Rev. Drug Discov. 2006, 5, 493–506. [Google Scholar] [CrossRef]
- Gu, T.; Wang, N.; Wu, T.; Ge, Q.; Chen, L. Antioxidative Stress Mechanisms behind Resveratrol: A Multidimensional Analysis. J. Food Qual. 2021, 2021, 5571733. [Google Scholar] [CrossRef]
- Ali, M.; Benfante, V.; Di Raimondo, D.; Salvaggio, G.; Tuttolomondo, A.; Comelli, A. Recent Developments in Nanoparticle Formulations for Resveratrol Encapsulation as an Anticancer Agent. Pharmaceuticals 2024, 17, 126. [Google Scholar] [CrossRef]
- Bohara, R.A.; Tabassum, N.; Singh, M.P.; Gigli, G.; Ragusa, A.; Leporatti, S. Recent Overview of Resveratrol’s Beneficial Effects and Its Nano-Delivery Systems. Molecules 2022, 27, 5154. [Google Scholar] [CrossRef]
- Li, C.; Wang, Z.; Lei, H.; Zhang, D. Recent Progress in Nanotechnology-Based Drug Carriers for Resveratrol Delivery. Drug Deliv. 2023, 30, 2174206. [Google Scholar] [CrossRef]
- Silva, P.M.; Gonçalves, C.; Pastrana, L.M.; Coimbra, M.A.; Vicente, A.A.; Cerqueira, M.A. Recent Advances in Oral Delivery Systems of Resveratrol: Foreseeing Their Use in Functional Foods. Food Funct. 2023, 14, 10286–10313. [Google Scholar] [CrossRef]
| Article | Experimental Target System | Dosage | Exposure Time | Administration Route | Ameliorative Effects of Resveratrol | |||
|---|---|---|---|---|---|---|---|---|
| Cd | RV | Cd | RV | Cd | RV | |||
| Shati et al. (2019) [44] * | AMPK/PI3K/Akt pathway, PP2A, and GSK3β | 5 mg/kg | 300 mg/kg | 45 days | 45 days | Oral | Oral | ↑ cell survival; SIRT1 activity, mRNA, and protein; p-AMPK (Thr172) and p-Akt (Ser473); GSH and Bcl-2 ↓ cell apoptosis and ROS content; GSSG and MDA; Bax, cleaved caspase-3, cleaved caspase-12 and p-JNK; GAAD 153, GRP78, and ATF-6 |
| Shati et al. (2019) [43] * | SIRT1/AMPK/Akt, ROS, Bcl-2, ER stress, GAAD 153 | 5 mg/kg | 300 mg/kg | 45 days | 45 days | Oral | Oral | ↑ learning and memory formation; GSH; Bcl-2; Ach and ChAT activity; p-PI3K and p-Akt; AMPKα1 and p-AMPKα1/2; activation ratio of p-PP2A ↓ ROS, MDA, and GSSG; Bax and cleaved caspase-3; AchE activity |
| Lin et al. (2015) [46] § | MAPK/mTOR | 10–20 μM | 5–10-20 μM | 12 h | 12 h | NA | NA | ↑ cell viability, PTEN ↓ apoptosis; [Ca2+]I; ROS, pAkt, S6K, and 4E-BP1; phosphorylation of JNK, ERK1/2, c-Jun, and p38 MAPK |
| Liu et al. (2015) [47] § | Erk1/2, JNK, PP2A/5 | 10–20 μM | 0–400 μM | 24 h | 24 h | NA | NA | ↑ cell viability, PTEN, PP2A, PP5 ↓ nuclear fragmentation and condensation; TUNEL-positive cells, cleaved caspase-3; phosphorylation of JNK, c-Jun, p38, and Erk1/2 |
| Liu et al. (2022) [48] § | mTOR and neuronal apoptosis | 10–20 μM | 100 μM | 4 h | 1 h | NA | NA | ↓ apoptotic cells; ROS; p-Akt; p-mTOR; p-S6K1; p-S6; p-4E-BP1; caspase-3 cleavage; caspases 3/7 activation; p-Erk1/2; p-JNK; TUNEL-positive cells |
| Lv et al. (2023) [50] * | antioxidant enzymes, CYP450 activity, and NXR-AHR-CYP1 pathway | 140 mg/kg | 400 mg/kg | 90 days | 90 days | Oral | Oral | ↑ antioxidant enzymes (Cu-Zn SOD and T-SOD); Cyt b5; AH and NCR activities; AHR, CYP1A1/2, CAR/PXR, and CYP2&3 mRNA and protein levels ↓ ERND and APND activities |
| Article | Experimental Target System | Dosage | Exposure Time | Administration Route | Ameliorative Effects of Resveratrol | |||
|---|---|---|---|---|---|---|---|---|
| Cd | RV | Cd | RV | Cd | RV | |||
| Mitra et al. (2016) [61] * | EGFR, Akt, NF-κB | 1.25–2.5 mg/kg | 10 mg/kg | 3 times/wk | 14 days | IP | Oral | ↑ body weight; motile cells, live sperm cells ↓ morphologically abnormal cells, necrosis, germ cell derangement, and epithelium vacuolization; EGFR, p-AKT, AKT1/2/3, NF-κβ (p50), COX-2 |
| Ferlazzo et al. (2021) [75] * | ROS, inflammation, apoptosis | 2 mg/kg | 20 mg/kg | Daily | 14 days | IP | Oral | ↑ body weight; testes weight; Bcl-2 ↓ edema in extratubular compartment; TUNEL-positive cells in seminiferous tubules; expression of TNF-α and IL-1β mRNA; Bax |
| Mitra et al. (2022) [69] * | AKT and GCNIS markers | 0.25–0.5 mg/kg | 20 mg/kg | 2 times/wk | 16 weeks | IP | Oral | ↑ body weight; testes weight, sperm motility, viability, and morphology; GSH ↓ Akt, p-Akt, NF-kB, Cox-2; MDA, GSH ratio; expression of GCNIS markers |
| Eleawa et al. (2014) [79] * | Bcl-2, p53, Bax | 1 mg/kg | 20 mg/kg | Single | 15 days | IP | Oral | ↑ sperm parameters (count, motility, daily production); hormonal levels (FSH, LH, testosterone); diameter of seminiferous tubules; increased SOD activity, Bcl-2 mRNA expression ↓ testicular degeneration and necrosis, p53 and Bax mRNA expression |
| Piras et al. (2022) [95] § | Maturation process and fertilization | 2 μM | 1–2 μM | - | - | NA | NA | ↑ oocyte maturation and fertilization rates; SIRT1, SOD1, GPX1; ↓ polyspermic fertilization; mitochondrial activity; ROS |
| Wang et al. (2021) [113] * | DNMT3 and PI3K/Akt | 4.5 mg/kg | 300 mg/kg | Single | 18 days | IP | Oral | ↑ fetal weight and growth; placental diameter; estradiol secretion ↓ apoptosis and inflammatory response in placental cells; DNMT activity and expression; Akt signaling pathway; endoplasmic reticulum stress |
| Article | Experimental Target System | Dosage | Exposure Time | Administration Route | Ameliorative Effects of Resveratrol | |||
|---|---|---|---|---|---|---|---|---|
| Cd | RV | Cd | RV | Cd | RV | |||
| Cirmi et al. (2021) [17] * | ROS, inflammation, apoptosis | 2 mg/kg | 20 mg/kg | Daily | 14 days | IP | Oral | ↑ GSH and GPx levels; Bcl2 expression; SIRT1 ↓ apoptosis, oxidative stress, and inflammation; urea nitrogen and creatinine levels; tp53, Bax, DNMT3B, DNMT3L, Nos2, and Il1b gene expression; Akt signaling pathway |
| Hu et al. (2017) [119] * | Oxidative stress, inflammation | 5 mg/kg | 20 mg/kg | 4 weeks | 4 weeks | Oral | IG | ↑ kidney and body weight; SOD, CAT, GPx, GR, GSH, Nrf-2, HO-1, and γ-GCLC expression /activity ↓ blood urea nitrogen and serum creatinine; glomerulus shrinkage, tubule dilation, collagen deposition, and renal inflammation; COX-2, iNOS, PGE2, NO, EMT markers (TGF-β1, Twist, fibronectin) |
| Fu et al. (2017) [120] * | Sirt3/FoxO3a pathway | 2 mg/kg | 10 mg/kg | 7 days | 7 days | IP | IP | ↑ mitochondrial biogenesis, membrane potential, mtDNA copy number and mass; ATP levels, COX IV, Sirt3, FoxO3a, PGC-1α, and SOD2 expression/activity ↓ mROS, Cd-induced apoptosis, and mitochondrial damage, caspase-3 activity, Bax expression, and ERK1/2 phosphorylation |
| Zhang et al. (2020) [122] * | Nuclear xenobiotic receptor (NXR) response and mitophagy | 140 mg/kg | 400 mg/kg | 90 days | 90 days | Oral | Oral | ↑ CYP450 content, APND and ERND activity; activated NXRs response; mitochondrial function and structure; Sirt3, FoxO3a, PGC-1α, and SOD2 expression; T-SOD, Cu-Zn SOD, GSH-Px, GST, and CAT activities ↓ atrophy, enlargement, exfoliation, vacuolation, and nuclear damage; AH and NCR activities; oxidative stress markers; caspase-3 activity, Bax expression, ERK1/2 phosphorylation |
| Chou et al. (2019) [125] § | Pyroptosis, ER stress | 2–10 μM | 10 μM | 48 h | 12 h | NA | NA | ↑ SIRT1 protein levels and activity ↓ NLRP3, cleaved-caspase-1, cleaved-IL-1β; IL-6, IL-18, IL-1β, TNF-α; LDH release, PI-positive cells; ER stress markers; XBP-1s mRNA and protein levels, Edem1, P58ipk |
| Rafati et al. (2015) [131] * | [histological study] | 1 mg/kg | 20 mg/kg | Daily | 4 weeks | IP | IP | ↑ restoration of total hepatocyte volume and number, sinusoid and central vein volume. Restoration of total glomeruli volume, mean glomerulus volume, PCT and DCT volumes, and lengths. Restoration of glomerular changes and intact DCT length ↓ hepatocyte nuclei number, fibrous tissue accumulation and bridges, perisinusoidal fibrosis, canal narrowing. Degenerated glomeruli and tubules, fibrous tissue volume. Degenerative glomerular changes and dilatation of PCT and DCT lumen, fibrous tissue accumulation |
| Eybl et al. (2006) [132] * | Lipid peroxidation, GSH | 7 mg/kg | 20 mg/kg | Single | 3 days | SC | Oral | ↓ liver lipid peroxidation; glutathione levels and GPx activity; catalase activity |
| Al-Baqami et al. (2021) [133] * | Biomarkers of hepatic functions and antioxidant enzymes | 5 mg/kg | 20 mg/kg | 30 days | 30 days | IP | IP | ↑ SOD, GPx, CAT ↑ALT, AST, LDH, ALP, γ-GT |
| Article | Experimental Target System | Dosage | Exposure Time | Administration Route | Ameliorative Effects of Resveratrol | |||
|---|---|---|---|---|---|---|---|---|
| Cd | RV | Cd | RV | Cd | RV | |||
| Benvenga et al. (2020) [141] * | MCP-1; CXCL10 | 2 mg/kg | 20 mg/kg | Daily | 14 days | IP | Oral | Follicular area and epithelial height restored to control levels ↓ MCP-1/CCL2 and CXCL10 levels; perifollicular connective tissue |
| Benvenga et al. (2021) [142] * | [histological study] | 2 mg/kg | 20 mg/kg | Daily | 14 days | IP | Oral | ↑ follicular thyroid diameters ↓ number of CT-positive cells; TUNEL-positive C cells |
| Article | Experimental Target System | Dosage | Exposure Time | Administration Route | Ameliorative Effects of Resveratrol | |||
|---|---|---|---|---|---|---|---|---|
| Cd | RV | Cd | RV | Cd | RV | |||
| Mei et al. (2021) [150] § | ERK1/2 and JNK pathways | 10 μM | 10 μM | 48 h | 48 h | NA | NA | ↑ cell viability; differentiation; ALP, RUNX2, COL1, and BMP-2 activity/expression; p-ERK1/2 and p-JNK ↓ apoptosis and necrosis; phosphorylation of ERK1/2 and JNK |
| Sasikumar et al. (2023) [153] § | Oxidative stress, MMP, Nrf2, and downstream genes | 3 μM | 20 μM | 24 h | 24 h | NA | NA | ↑ cell viability; Nrf2 and downstream genes (HO-1, NQO1, SOD, CAT) ↓ Cd-induced structural abnormalities; expression of hypertrophic markers (ANP, BNP, βMHC) |
| Qian et al. (2021) [159] § | EMT (in colorectal cancer) | 1–10 μM | 10–200 μM | 24 h | 24 h | NA | NA | ↑ E-cadherin ↓ invasive ability of CRC cells; ZEB1, vimentin, and N-cadherin levels |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mognetti, B.; Franco, F.; Castrignano, C.; Bovolin, P.; Berta, G.N. Mechanisms of Phytoremediation by Resveratrol against Cadmium Toxicity. Antioxidants 2024, 13, 782. https://doi.org/10.3390/antiox13070782
Mognetti B, Franco F, Castrignano C, Bovolin P, Berta GN. Mechanisms of Phytoremediation by Resveratrol against Cadmium Toxicity. Antioxidants. 2024; 13(7):782. https://doi.org/10.3390/antiox13070782
Chicago/Turabian StyleMognetti, Barbara, Francesco Franco, Chiara Castrignano, Patrizia Bovolin, and Giovanni Nicolao Berta. 2024. "Mechanisms of Phytoremediation by Resveratrol against Cadmium Toxicity" Antioxidants 13, no. 7: 782. https://doi.org/10.3390/antiox13070782
APA StyleMognetti, B., Franco, F., Castrignano, C., Bovolin, P., & Berta, G. N. (2024). Mechanisms of Phytoremediation by Resveratrol against Cadmium Toxicity. Antioxidants, 13(7), 782. https://doi.org/10.3390/antiox13070782








