Preliminary Investigation of Astragalus arpilobus subsp. hauarensis: LC-MS/MS Chemical Profiling, In Vitro Evaluation of Antioxidant, Anti-Inflammatory Properties, Cytotoxicity, and In Silico Analysis against COX-2
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Extraction
2.2. Total Bioactive Compounds
2.3. Equipment and Chromatographic Parameters
2.4. Antioxidant Activity
- Ac: Absorbance of the control (this is the absorbance of the blank containing only DPPH).
- At: Absorbance of the test (this is the absorbance of the DPPH solution containing the extract).
- Ae: Absorbance of the blank (this is the absorbance of the extract solution without DPPH).
- The IC50 value, which represents the concentration of the sample required to scavenge 50% of the DPPH radicals, can be determined from the regression line where the inhibition ratio is 50%.
2.5. Anti Inflammatory Assay
- As: Absorbance of samples: 0.5 mL extract + 0.5 mL BSA.
- Aw: Absorbance of white: 0.5 mL extract + 0.5 mL Tris-HCL (pH: 6.8).
- Ac: Absorbance of control: 0.5 H2O + 0.5 mL BSA (The control represents 100% of proteins denaturation).
2.6. Cytotoxic Effect by Brine Shrimp Lethality Test (BST)
2.7. Virtual Screening
2.7.1. Retrieval and Preparation of Protein Structure
2.7.2. Phytochemicals Preparation
2.7.3. Molecular Docking Protocol
2.7.4. Molecular Dynamics Simulation
2.8. Statistical Analysis
3. Results
3.1. Total Bioactive Compounds, Antioxidant, Anti-Inflammatory, and Cytotoxic Activities
3.2. LC-MS/MS Analysis
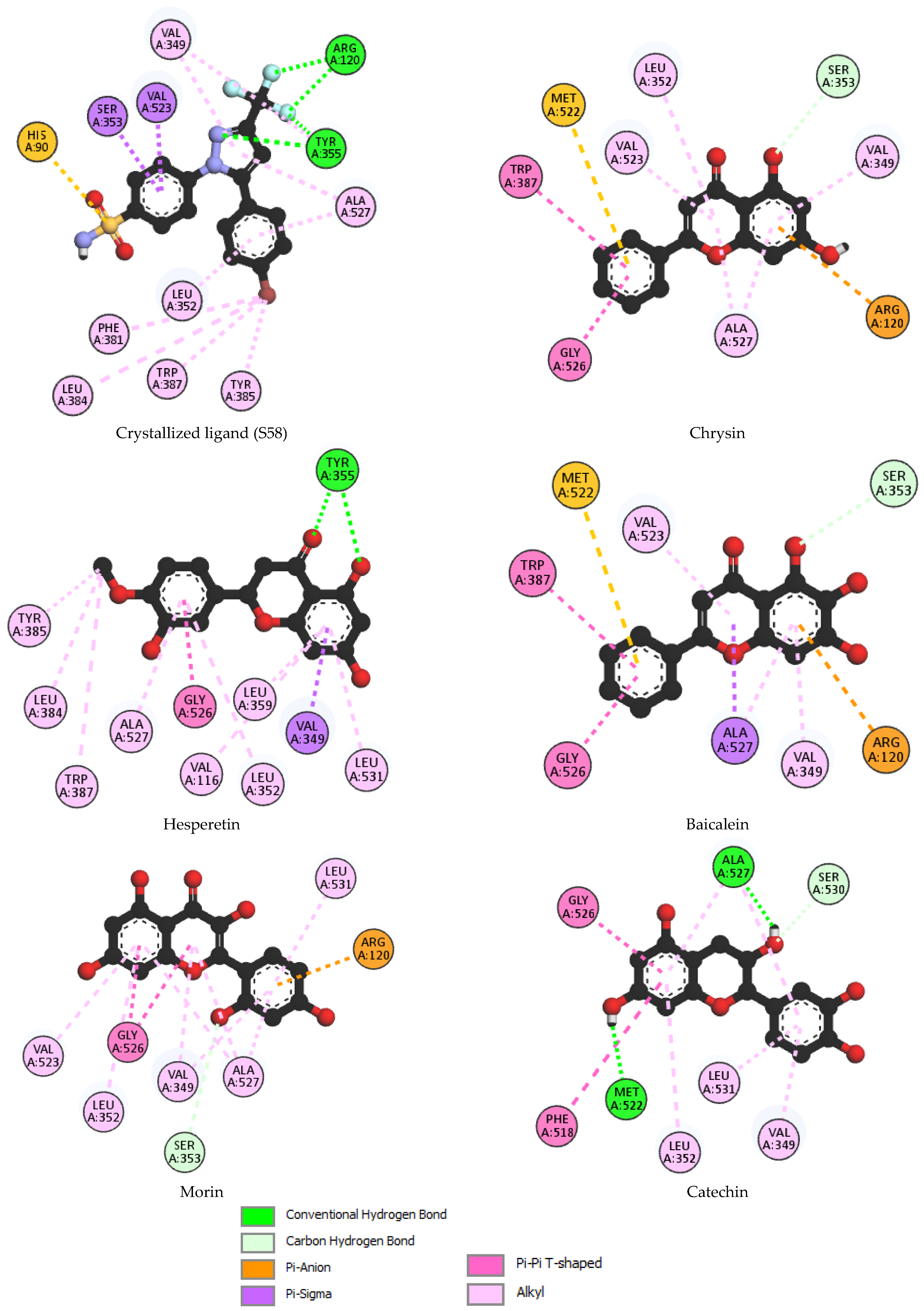
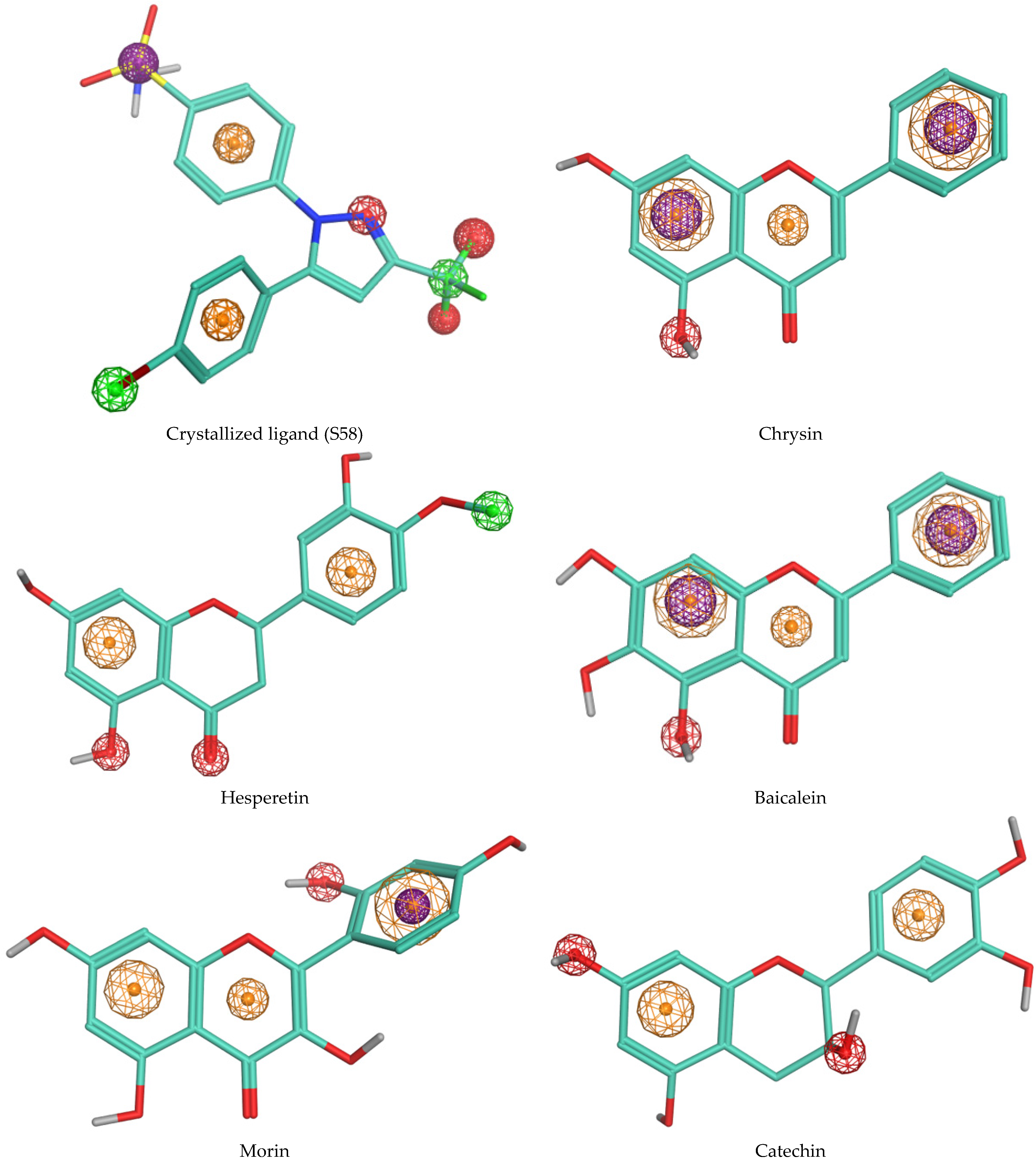
3.3. Molecular Docking
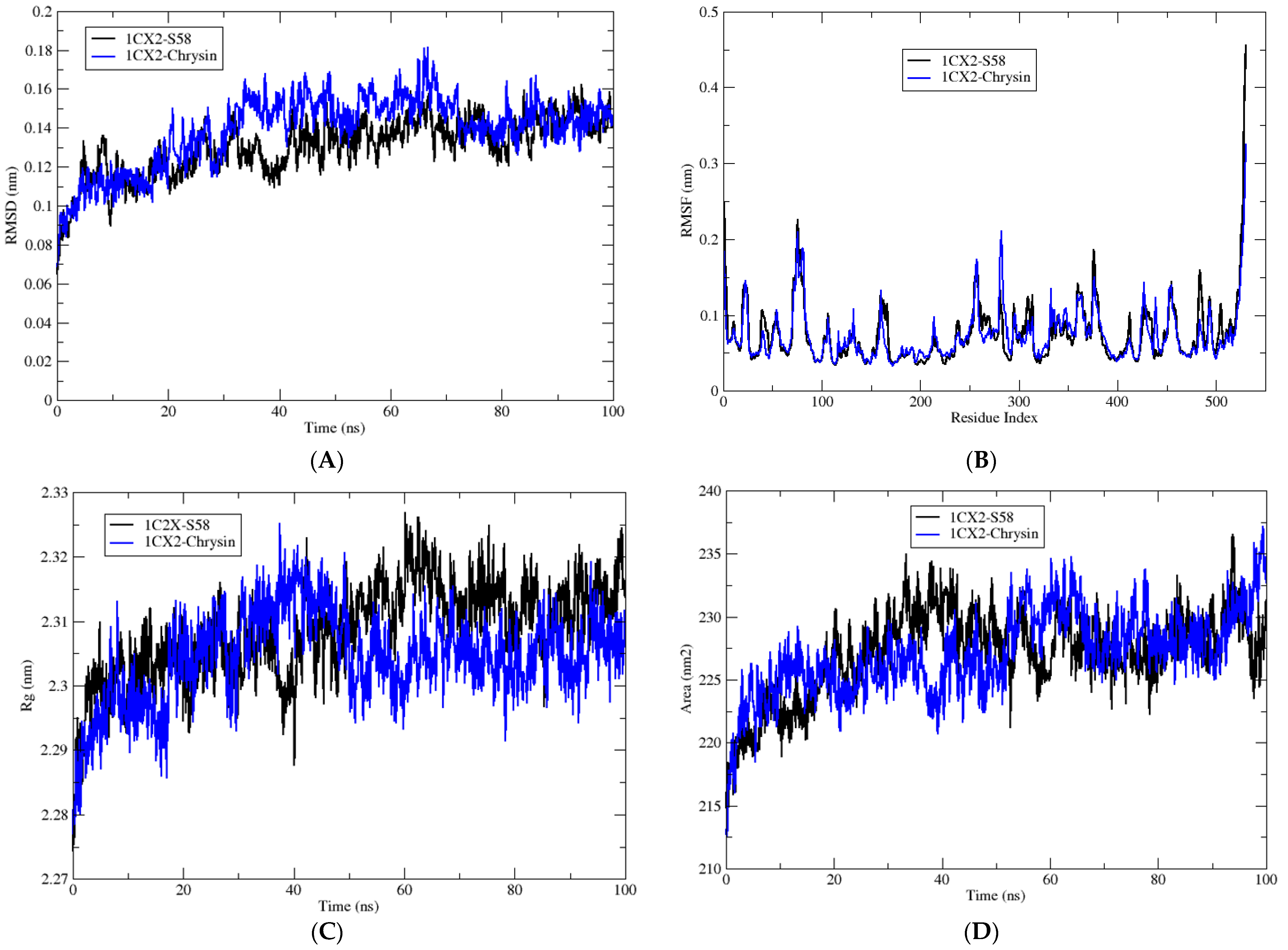
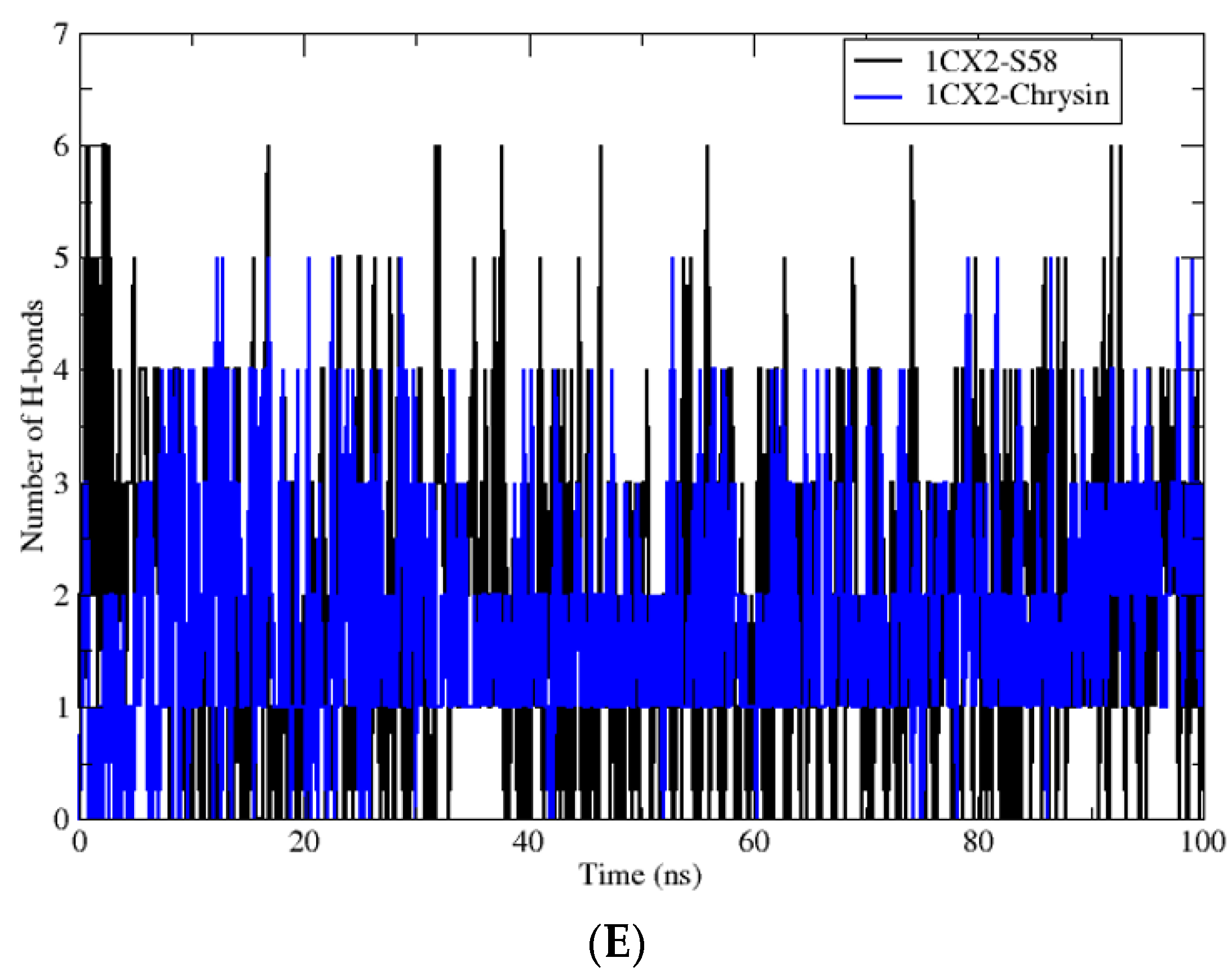
3.4. Molecular Dynamic Simulations
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhu, Y.; Wang, K.; Jia, X.; Fu, C.; Yu, H.; Wang, Y. Antioxidant Peptides, the Guardian of Life from Oxidative Stress. Med. Res. Rev. 2024, 44, 275–364. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Ouyang, Y.; Zhao, T.; Wang, Z.; Yan, Q.; Qian, Q.; Wang, W.; Wang, S. Antioxidant Hydrogels: Antioxidant Mechanisms, Design Strategies, and Applications in the Treatment of Oxidative Stress-Related Diseases. Adv. Healthc. Mater. 2024, 13, 2303817. [Google Scholar] [CrossRef] [PubMed]
- Boussekine, S.; Lekmine, S.; Gasmi, S.; Benkhedir, A.; Saker, H.; Lidoughi, A. The Protective Effect of Selenium on Diabetic Nephropathy in Wistar Rats. J. Microbiol. Biotechnol. Food Sci. 2022, 12, e5960. [Google Scholar] [CrossRef]
- Benslama, O.; Lekmine, S.; Mansouri, N. Phytochemical Constituents of Astragalus monspessulanus and Integrative Analysis for Its Antioxidant, Photoprotective, and Antityrosinase Activities: Experimental and Computational Investigation. Eur. J. Integr. Med. 2023, 60, 102247. [Google Scholar] [CrossRef]
- Lekmine, S.; Bendjedid, S.; Benslama, O.; Martín-García, A.I.; Boussekine, S.; Kadi, K.; Akkal, S.; Nieto, G.; Sami, R.; Al-Mushhin, A.A.M. Ultrasound-Assisted Extraction, LC–MS/MS Analysis, Anticholinesterase, and Antioxidant Activities of Valuable Natural Metabolites from Astragalus armatus Willd.: In Silico Molecular Docking and In Vitro Enzymatic Studies. Antioxidants 2022, 11, 2000. [Google Scholar] [CrossRef] [PubMed]
- Kadi, K.; Mrah, R.; Hamli, S.; Lekmine, S.; Dib, D.; Addad, D.; Boukeria, S.; Gueboudji, Z.; Hafsaoui, I. Evaluation of the Anticoagulant Activity of Margins from Olives Extraction in the Khenchela Region. J. Fundam. Appl. Sci. 2020, 12, 634–649. [Google Scholar] [CrossRef]
- Lekmine, S.; Boussekine, S.; Akkal, S.; Martín-García, A.I.; Boumegoura, A.; Kadi, K.; Djeghim, H.; Mekersi, N.; Bendjedid, S.; Bensouici, C.; et al. Investigation of Photoprotective, Anti-Inflammatory, Antioxidant Capacities and LC–ESI–MS Phenolic Profile of Astragalus gombiformis Pomel. Foods 2021, 10, 1937. [Google Scholar] [CrossRef] [PubMed]
- De-Hong, Y.U.; Yong-Ming, B.A.O.; Li-Jia, A.N.; Ming, Y. Protection of PC12 Cells against Superoxide-Induced Damage by Isoflavonoids from Astragalus mongholicus. Biomed. Environ. Sci. 2009, 22, 50–54. [Google Scholar]
- Chouana, T.; Pierre, G.; Vial, C.; Gardarin, C.; Wadouachi, A.; Cailleu, D.; Le Cerf, D.; Boual, Z.; El Hadj, M.D.O.; Michaud, P. Structural Characterization and Rheological Properties of a Galactomannan from Astragalus gombo Bunge Seeds Harvested in Algerian Sahara. Carbohydr. Polym. 2017, 175, 387–394. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.-J.; Chiang, C.-C.; Lin, C.-Y.; Chen, Y.-L.; Leu, Y.-L.; Sie, J.-Y.; Chen, W.-L.; Hsu, C.-Y.; Kuo, J.-J.; Hwang, T.-L. Astragalus mongholicus Bunge Water Extract Exhibits Anti-Inflammatory Effects in Human Neutrophils and Alleviates Imiquimod-Induced Psoriasis-like Skin Inflammation in Mice. Front. Pharmacol. 2021, 12, 762829. [Google Scholar] [CrossRef] [PubMed]
- Amiri, M.; Raeisi-Dehkordi, H.; Verkaar, A.J.C.F.; Wu, Y.; Van Westing, A.C.; Berk, K.A.; Bramer, W.M.; Aune, D.; Voortman, T. Circulating Lipoprotein (a) and All-Cause and Cause-Specific Mortality: A Systematic Review and Dose-Response Meta-Analysis. Eur. J. Epidemiol. 2023, 38, 485–499. [Google Scholar] [CrossRef] [PubMed]
- Lekmine, S.; Benslama, O.; Kadi, K.; Ignacio Martín-García, A.; Shamsul Ola, M.; Abdullah Yilmaz, M.; Ali, A. Therapeutic Potential of Hyoscyamus niger-Derived Compounds: Targeting Ovarian Cancer through Antioxidant Activity and EGFR Tyrosine Kinase Inhibition. J. King Saud Univ.-Sci. 2024, 36, 103103. [Google Scholar] [CrossRef]
- Smara, M.; Khalladi, R.; Moulai-Mostefa, N.; Madi, K.; Mansour, D.; Lekmine, S.; Benslama, O.; Tahraoui, H.; Zhang, J.; Amrane, A. Efficiency of Hydrogen Peroxide and Fenton Reagent for Polycyclic Aromatic Hydrocarbon Degradation in Contaminated Soil: Insights from Experimental and Predictive Modeling. Processes 2024, 12, 621. [Google Scholar] [CrossRef]
- Auyeung, K.K.; Han, Q.-B.; Ko, J.K. Astragalus membranaceus: A Review of Its Protection against Inflammation and Gastrointestinal Cancers. Am. J. Chin. Med. 2016, 44, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Dong, M.; Li, J.; Yang, D.; Li, M.; Wei, J. Biosynthesis and Pharmacological Activities of Flavonoids, Triterpene Saponins and Polysaccharides Derived from Astragalus membranaceus. Molecules 2023, 28, 5018. [Google Scholar] [CrossRef] [PubMed]
- Gorai, D.; Jash, S.K.; Roy, R. Flavonoids from Astragalus Genus. Int. J. Pharm. Sci. Res. 2016, 7, 2732. [Google Scholar]
- Chen, R.-Z.; Tan, L.; Jin, C.-G.; Lu, J.; Tian, L.; Chang, Q.-Q.; Wang, K. Extraction, Isolation, Characterization and Antioxidant Activity of Polysaccharides from Astragalus membranaceus. Ind. Crops Prod. 2015, 77, 434–443. [Google Scholar] [CrossRef]
- Tedjani, A.; Boual, Z.; El Hadj, M.D.O.; Lanez, T.; Belkhalfa, H.; El Alaoui-Talibi, Z.; El Modafar, C.; Abdelkafi, S.; Fendri, I.; Le Cerf, D. Antidiabetic Potential of Mucilage Fraction Extracted from Astragalus gyzensis Seeds. Eur. J. Biol. Res. 2023, 13, 18–30. [Google Scholar]
- Qandeel, N.A.; El-Damasy, A.K.; Sharawy, M.H.; Bayomi, S.M.; El-Gohary, N.S. Synthesis, In Vivo Anti-Inflammatory, COX-1/COX-2 and 5-LOX Inhibitory Activities of New 2, 3, 4-Trisubstituted Thiophene Derivatives. Bioorg. Chem. 2020, 102, 103890. [Google Scholar] [CrossRef] [PubMed]
- Parente, L.; Perretti, M. Advances in the Pathophysiology of Constitutive and Inducible Cyclooxygenases: Two Enzymes in the Spotlight. Biochem. Pharmacol. 2003, 65, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Rowlinson, S.W.; Kiefer, J.R.; Prusakiewicz, J.J.; Pawlitz, J.L.; Kozak, K.R.; Kalgutkar, A.S.; Stallings, W.C.; Kurumbail, R.G.; Marnett, L.J. A Novel Mechanism of Cyclooxygenase-2 Inhibition Involving Interactions with Ser-530 and Tyr-385. J. Biol. Chem. 2003, 278, 45763–45769. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Ayoola, G.A.; Coker, H.A.; Adesegun, S.A.; Adepoju-Bello, A.A.; Obaweya, K.; Ezennia, E.C.; Atangbayila, T.O. Phytochemical Screening and Antioxidant Activities of Some Selected Medicinal Plants Used for Malaria Therapy in Southwestern Nigeria. Trop. J. Pharm. Res. 2008, 7, 1019–1024. [Google Scholar]
- Oyedemi, S.O.; Afolayan, A.J. Antibacterial and Antioxidant Activities of Hydroalcoholic Stem Bark Extract of Schotia latifolia Jacq. Asian Pac. J. Trop. Med. 2011, 4, 952–958. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Garcia, M.A.; Calleja, E.; Monroy, E.; Sanchez, F.J.; Calle, F.; Munoz, E.; Beresford, R. The Effect of the III/V Ratio and Substrate Temperature on the Morphology and Properties of GaN-and AlN-Layers Grown by Molecular Beam Epitaxy on Si (1 1 1). J. Cryst. Growth 1998, 183, 23–30. [Google Scholar] [CrossRef]
- Karthik, K.; Kumar, B.R.P.; Priya, V.R.; Kumar, S.K.; Rathore, R.S.B. Evaluation of Anti-Inflammatory Activity of Canthium parviflorum by In-Vitro Method. Indian J. Res. Pharm. Biotechnol. 2013, 1, 729–731. [Google Scholar]
- Weli, A.M.; Al-Harrasi, A.; Al Baiti, N.H.; Philip, A.; Hossain, A.; Gilani, S.A.; Banioraba, N. Biological and Toxicological Evaluation of Aerial Parts Extracts of Locally Grown Cleome austroarabica. J. King Saud Univ.-Sci. 2020, 32, 753–757. [Google Scholar] [CrossRef]
- Padmaja, R.; Arun, P.C.; Prashanth, D.; Deepak, M.; Amit, A.; Anjana, M. Brine Shrimp Lethality Bioassay of Selected Indian Medicinal Plants. Fitoterapia 2002, 73, 508–510. [Google Scholar] [CrossRef] [PubMed]
- Spinelli, R.; Rietmann, Á.; Sanchis, I.; Goicoechea, H.; Siano, Á. Toxicity Evaluation of Anti-cholinesterasic Amphibian Extracts by MTT and an Optimized Artemia salina Test. Chem. Biodivers. 2024, 21, e202301367. [Google Scholar] [CrossRef] [PubMed]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the Speed and Accuracy of Docking with a New Scoring Function, Efficient Optimization, and Multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera—A Visualization System for Exploratory Research and Analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef] [PubMed]
- Shoaib, S.; Ansari, M.A.; Al Fatease, A.; Safhi, A.Y.; Hani, U.; Jahan, R.; Alomary, M.N.; Ansari, M.N.; Ahmed, N.; Wahab, S. Plant-Derived Bioactive Compounds in the Management of Neurodegenerative Disorders: Challenges, Future Directions and Molecular Mechanisms Involved in Neuroprotection. Pharmaceutics 2023, 15, 749. [Google Scholar] [CrossRef] [PubMed]
- Haşimi, N.; Ertaş, A.; Yılmaz, M.A.; Boğa, M.; Temel, H.; Demirci, S.; Özden, T.Y.; Yener, İ.; Kolak, U. LC-MS/MS and GC-MS Analyses of Three Endemic Astragalus Species from Anatolia towards Their Total Phenolic-Flavonoid Contents and Biological Activities. Biol. Divers. Conserv. 2017, 10, 18–30. [Google Scholar]
- Zhang, Q.; Mao, Z.; Zhang, Q.; Qiu, J.; Jia, Z.; Qin, L. Acute and Sub-Chronic Toxicological Studies of the Iridoid Glycosides Extract of Lamiophlomis rotata (Benth.) Kudo in Rats. Regul. Toxicol. Pharmacol. 2018, 92, 315–323. [Google Scholar] [CrossRef]
- Qi, L.; Yu, Q.; Yi, L.; Ren, M.; Wen, X.; Wang, Y.; Li, P. Simultaneous Determination of 15 Marker Constituents in Various Radix Astragali Preparations by Solid-phase Extraction and High-performance Liquid Chromatography. J. Sep. Sci. 2008, 31, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Pu, W.; Wang, D.; Zhou, D. Structural Characterization and Evaluation of the Antioxidant Activity of Phenolic Compounds from Astragalus taipaishanensis and Their Structure-Activity Relationship. Sci. Rep. 2015, 5, 13914. [Google Scholar] [CrossRef]
- Lobanova, E. PhytochAmical Description of Astragalus glycyphyllos (Fabaceae). Veg. World Asian Russ. 2011, 1, 87–90. [Google Scholar]
- Mollaei, S.; Ebadi, M.; Hazrati, S.; Habibi, B.; Gholami, F.; Sourestani, M.M. Essential Oil Variation and Antioxidant Capacity of Mentha pulegium Populations and Their Relation to Ecological Factors. Biochem. Syst. Ecol. 2020, 91, 104084. [Google Scholar] [CrossRef]
- Henderson, I.R.; Salt, D.E. Natural Genetic Variation and Hybridization in Plants. J. Exp. Bot. 2017, 68, 5415–5417. [Google Scholar] [CrossRef] [PubMed]
- Mehalaine, S.; Chenchouni, H. Plants of the Same Place Do Not Have the Same Metabolic Pace: Soil Properties Affect Differently Essential Oil Yields of Plants Growing Wild in Semiarid Mediterranean Lands. Arab. J. Geosci. 2020, 13, 1263. [Google Scholar] [CrossRef]
- Li, L.; Yang, X.; Tong, B.; Wang, D.; Tian, X.; Liu, J.; Chen, J.; Xiao, X.; Wang, S. Rhizobacterial Compositions and Their Relationships with Soil Properties and Medicinal Bioactive Ingredients in Cinnamomum migao. Front. Microbiol. 2023, 14, 1078886. [Google Scholar] [CrossRef] [PubMed]
- Lekmine, S.; Boussekine, S.; Kadi, K.; Martín-García, A.I.; Kheddouma, A.; Nagaz, K.; Bensouici, C. A Comparative Study on Chemical Profile and Biological Activities of Aerial Parts (Stems, Flowers, Leaves, Pods and Seeds) of Astragalus gombiformis. Biocatal. Agric. Biotechnol. 2020, 27, 101668. [Google Scholar] [CrossRef]
- Kishore, N. Influence of Amino Acids on Alkaline PH Induced Partially Folded Molten Globule like Intermediate of Bovine Serum Albumin: Conformational and Thermodynamic Insights. J. Mol. Liq. 2022, 368, 120599. [Google Scholar]
- Saeed, K.; Rafiq, M.; Khalid, M.; Hussain, A.; Siddique, F.; Hanif, M.; Hussain, S.; Mahmood, K.; Ameer, N.; Ahmed, M.M. Synthesis, Characterization, Computational Assay and Anti-Inflammatory Activity of Thiosemicarbazone Derivatives: Highly Potent and Efficacious for COX Inhibitors. Int. Immunopharmacol. 2024, 126, 111259. [Google Scholar] [CrossRef] [PubMed]
- Varghese, R.M.; Kumar, A.; Shanmugam, R. Comparative Anti-Inflammatory Activity of Silver and Zinc Oxide Nanoparticles Synthesized Using Ocimum tenuiflorum and Ocimum gratissimum Herbal Formulations. Cureus 2024, 16, e52995. [Google Scholar] [CrossRef] [PubMed]
- Tavanappanavar, A.N.; Mulla, S.I.; Seth, C.S.; Bagewadi, Z.K.; Rahamathulla, M.; Ahmed, M.M.; Farhana, S.A. Phytochemical Analysis, GC–MS Profile and Determination of Antibacterial, Antifungal, Anti-Inflammatory, Antioxidant Activities of Peel and Seeds Extracts (Chloroform and Ethyl Acetate) of Tamarindus indica L. Saudi J. Biol. Sci. 2024, 31, 103878. [Google Scholar] [CrossRef] [PubMed]
- Ramadan, R.M.; Youssef, F.S.; Fouad, E.A.; Orabi, A.; Khalifa, M.M. The Pharmacological Impact of Astragalus membranaceus against Coccidial and Bacterial Infection In Vitro. Egypt. Pharm. J. 2023, 22, 324–335. [Google Scholar] [CrossRef]
- Hwang, Y.S.; Lim, J.; Yoon, H.R.; Park, S.-H.; Kim, A.; Jang, J.-P.; Cho, H.J.; Lee, H.G. Astragalus complanatus Ethanol Attenuates Septic Shock by Exerting Anti-Inflammatory Effects on Macrophages. Int. J. Mol. Sci. 2023, 25, 384. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.-B.; Chen, G.-L.; Guo, M.-Q. Antioxidant and Anti-Inflammatory Activities of the Crude Extracts of Moringa oleifera from Kenya and Their Correlations with Flavonoids. Antioxidants 2019, 8, 296. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.M.; Rahaman, M.S.; Islam, M.R.; Rahman, F.; Mithi, F.M.; Alqahtani, T.; Almikhlafi, M.A.; Alghamdi, S.Q.; Alruwaili, A.S.; Hossain, M.S. Role of Phenolic Compounds in Human Disease: Current Knowledge and Future Prospects. Molecules 2021, 27, 233. [Google Scholar] [CrossRef]
- Kim, H.H.; Jeong, S.H.; Park, M.Y.; Bhosale, P.B.; Abusaliya, A.; Kim, H.W.; Seong, J.K.; Ahn, M.; Park, K.I.; Kim, G.S. Author Correction: Antioxidant Effects of Phenolic Compounds in through the Distillation of Lonicera japonica & Chenpi Extract and Anti-Inflammation on Skin Keratinocyte. Sci. Rep. 2023, 13, 22761. [Google Scholar] [PubMed]
- Miya, G.M.; Oriola, A.O.; Payne, B.; Cuyler, M.; Lall, N.; Oyedeji, A.O. Steroids and Fatty Acid Esters from Cyperus sexangularis Leaf and Their Antioxidant, Anti-Inflammatory and Anti-Elastase Properties. Molecules 2023, 28, 3434. [Google Scholar] [CrossRef] [PubMed]
- Vaou, N.; Stavropoulou, E.; Voidarou, C.; Tsakris, Z.; Rozos, G.; Tsigalou, C.; Bezirtzoglou, E. Interactions between Medical Plant-Derived Bioactive Compounds: Focus on Antimicrobial Combination Effects. Antibiotics 2022, 11, 1014. [Google Scholar] [CrossRef]
- Efferth, T.; Koch, E. Complex Interactions between Phytochemicals. The Multi-Target Therapeutic Concept of Phytotherapy. Curr. Drug Targets 2011, 12, 122–132. [Google Scholar] [CrossRef] [PubMed]
- van Vuuren, S.; Viljoen, A. Plant-Based Antimicrobial Studies–Methods and Approaches to Study the Interaction between Natural Products. Planta Med. 2011, 77, 1168–1182. [Google Scholar] [CrossRef] [PubMed]
- Ungureanu, A.R.; Popovici, V.; Oprean, C.; Danciu, C.; Schröder, V.; Olaru, O.T.; Mihai, D.P.; Popescu, L.; Luță, E.-A.; Chițescu, C.L. Cytotoxicity Analysis and In Silico Studies of Three Plant Extracts with Potential Application in Treatment of Endothelial Dysfunction. Pharmaceutics 2023, 15, 2125. [Google Scholar] [CrossRef]
- Kalaycı, B.; Özek, N.Ş.; Aysin, F.; Özbek, H.; Kazaz, C.; Önal, M.; Güvenalp, Z. Evaluation of Cytotoxic and Apoptotic Effects of the Extracts and Phenolic Compounds of Astragalus globosus Vahl and Astragalus breviflorus DC. Saudi Pharm. J. 2023, 31, 101682. [Google Scholar] [CrossRef] [PubMed]
- Kurt-Celep, İ.; Zengin, G.; Uba, A.I.; Caprioli, G.; Mustafa, A.M.; Angeloni, S.; Cakilcioglu, U.; Guler, O.; Kaplan, A.; Sharmeen, J. Unraveling the Chemical Profile, Antioxidant, Enzyme Inhibitory, Cytotoxic Potential of Different Extracts from Astragalus caraganae. Arch. Pharm. 2023, 356, 2300263. [Google Scholar] [CrossRef] [PubMed]
- Hinkov, A.; Tsvetkov, V.; Shkondrov, A.; Krasteva, I.; Shishkov, S.; Shishkova, K. Effect of a Total Extract and Saponins from Astragalus glycyphyllos L. on Human Coronavirus Replication In Vitro. Int. J. Mol. Sci. 2023, 24, 16525. [Google Scholar] [CrossRef]
- Ji, H.; Lou, X.; Jiao, J.; Li, Y.; Dai, K.; Jia, X. Preliminary Structural Characterization of Selenium Nanoparticle Composites Modified by Astragalus Polysaccharide and the Cytotoxicity Mechanism on Liver Cancer Cells. Molecules 2023, 28, 1561. [Google Scholar] [CrossRef]
- Luiz-Ferreira, A.; Pacifico, T.; Cruz, Á.C.; Laudisi, F.; Monteleone, G.; Stolfi, C. TRAIL-Sensitizing Effects of Flavonoids in Cancer. Int. J. Mol. Sci. 2023, 24, 16596. [Google Scholar] [CrossRef] [PubMed]
- Felice, M.R.; Maugeri, A.; De Sarro, G.; Navarra, M.; Barreca, D. Molecular Pathways Involved in the Anti-Cancer Activity of Flavonols: A Focus on Myricetin and Kaempferol. Int. J. Mol. Sci. 2022, 23, 4411. [Google Scholar] [CrossRef]
- Shahrajabian, M.H.; Sun, W.; Cheng, Q. The Importance of Flavonoids and Phytochemicals of Medicinal Plants with Antiviral Activities. Mini Rev. Org. Chem. 2022, 19, 293–318. [Google Scholar] [CrossRef]
- Eldahshan, O.A.; Azab, S.S. Anti-Inflammatory Effect of Apigenin-7-Neohesperidoside (Rhoifolin) in Carrageenin-Induced Rat Oedema Model. J. Appl. Pharm. Sci. 2012, 2, 74–79. [Google Scholar] [CrossRef]
- Mostafa, N.M.; Ashour, M.L.; Eldahshan, O.A.; Singab, A.N.B. Cytotoxic Activity and Molecular Docking of a Novel Biflavonoid Isolated from Jacaranda acutifolia (Bignoniaceae). Nat. Prod. Res. 2016, 30, 2093–2100. [Google Scholar] [CrossRef] [PubMed]
- Eldahshan, O.A.; Ayoub, N.A.; Singab, A.-N.B.; Al-Azizi, M.M. Potential Antioxidant Phenolic Metabolites from Doum Palm Leaves. Afr. J. Pharm. Pharmacol. 2009, 3, 158–164. [Google Scholar]
| TPC mg GAE/g DM | TFC mg QE/g DM | TTC mg CE/g DM | DPPH IC50 µg/mL | Anti-Inflammatory IC50 µg/mL | Cytotoxic LC50 µg/mL | |
|---|---|---|---|---|---|---|
| AAH extract | 191 ± 0.03 | 80.82 ± 0.02 | 51.91 ± 0.01 | 19.44 ± 1.1 d | 35.73 ± 1.3 a | 28.84 ± 2.1 b |
| BHA | / | / | / | 11.94 ± 1.2 b | / | / |
| BHT | / | / | / | 5.5 ± 2.3 a | / | / |
| Ascorbic acid | / | / | / | 15.03 ± 1.6 c | / | / |
| Alpha tocopherol | / | / | / | 14.71 ± 0.9 c | / | / |
| Ketoprofen | / | / | / | / | 164.79 ± 2.3 c | / |
| Diclofenac | / | / | / | / | 63.78 ± 2.1 b | / |
| BP | / | / | / | / | / | 15.73 ± 2.1 a |
| No | Name | (M-H) | M2 | Retention Time | Phytochemical Class | Ion Mod |
|---|---|---|---|---|---|---|
| 1 | Gallic acid | 169 | 125 | 3.218 | Phenols | Negative |
| 2 | Protocatechuic acid | 153 | 112, 109 | 5.449 | Phenols | Negative |
| 3 | Catechin | 288 | 245 | 6.904 | Phenols | Negative |
| 4 | Chlorogenic acid | 353 | 191 | 7.378 | Phenols | Negative |
| 5 | o-Hydroxybenzaldehyde | 121 | 108, 102 | 7.679 | Aldehyde | Negative |
| 6 | Vanillic acid | 167 | 153, 122 | 7.791 | Phenols | Negative |
| 7 | Syringic acid | 197 | 183, 166 | 8.401 | Phenols | Negative |
| 8 | Salicylic acid | 137 | 94, 64, 75 | 9.539 | Phenols | Negative |
| 9 | trans-Ferulic acid | 193 | 133, 115, 104 | 10.132 | Phenols | Negative |
| 10 | Sinapic acid | 223 | 210 | 10.414 | Phenols | Negative |
| 11 | Scutellarein-O-hexouronide (Scutellarin) | 461 | 369, 286 | 11.151 | Flavone glycoside | Negative |
| 12 | P-Coumaric acid | 163 | 141 | 11.502 | Phenols | Negative |
| 13 | Protocatechuic acid ethyl ester | 181 | 162 | 11.622 | Phenols | Negative |
| 14 | Hesperetin-O-deoxyhexosyl hexoside (Hesperetin rutinoside) | 609 | 304, 365 | 11.842 | Flavanone glycoside | Negative |
| 15 | Quercetin-O-deoxyhexosyl hexoside (Rutin) | 609 | 301, 286 | 12.293 | Flavonol glycoside | Negative |
| 16 | Quarcetin pentoside | 432 | 315 | 12.433 | Flavonol glycoside | Negative |
| 17 | Kaempferol-O-hexoside (Kaempferol-O-glucoside) | 447 | 284, 255 | 12.500 | Flavonol glycoside | Negative |
| 18 | Baicalein-O-hexouronide (Baicalin) | 445 | 425 | 13.287 | Flavone glycoside | Negative |
| 19 | Morin | 300 | 215 | 13.327 | Flavonol aglycone | Negative |
| 20 | Fisetin | 285 | 137, 131 | 13.900 | Flavonol aglycone | Negative |
| 21 | Chrysin | 253 | 188 | 14.230 | Flavonone aglycone | Negative |
| 22 | Quercetin | 301 | 216 | 14.821 | Flavonol aglycone | Negative |
| 23 | Naringenin | 271 | 177 | 14.999 | Flavanone aglycone | Negative |
| 24 | Hesperetin | 301 | 286, 242, 199 | 15.815 | Flavanone aglycone | Negative |
| 25 | Kaempferol | 285 | 217, 133 | 16.431 | Flavonol aglycone | Negative |
| 26 | Baicalein | 269 | 147 | 17.084 | Flavone aglycone | Negative |
| 27 | Luteolin | 285 | 155, 161 | 17.909 | Flavone aglycone | Negative |
| 28 | Biochanin A | 283 | 121 | 17.91 | Isoflavone aglycone | Negative |
| Binding Energy (Kcal/mol) | Hydrogen Interactions (Distance Å) | Hydrophobic Interactions | Electrostatic Interactions | ||
|---|---|---|---|---|---|
| 1 | Crystallized ligand (S58) | −10.8 | F2-Arg120 (2.32), F3-Arg120 (2.44) F3-Tyr355 (2.58) N2-Tyr355 (3.06) | Val349, Val523, Ser353, Ala527, Leu352, Phe381, Leu384, Trp387, Tyr385 | His90 |
| 2 | Chrysin | −10.3 | Ser353 (2.56) | Leu352, Val523, Trp387, Gly526, ala527, Val349 | Met522, Arg120 |
| 3 | Hesperetin | −9.8 | O2-Tyr355 (1.86) O5-Tyr355 (2.71) | Tyr385, Leu384, Trp387, Ala527, Gly526, Val116, Leu352, Leu359, Val349, Leu531 | - |
| 4 | Baicalein | −9.7 | Ser353 (2.47) | Val523, Trp387, Gly526, Ala527, Val349 | Met522, Arg120 |
| 5 | Morin | −9.5 | Ser353 (3.40) | Val523, Leu352, Gly526, Val349, Ala527, Leu531 | Arg120 |
| 6 | Catechin | −9.4 | Met522 (2.29), Ala527 (2.82), Ser530 (3.42) | Gly526, Ala527, Phe518, Leu352, Leu531, Val349 | - |
| Complex | Average RMSD (nm) | Average RMSF (nm) | Average Rg (nm) | Average SASA (nm2) | H-Bond |
|---|---|---|---|---|---|
| 1CX2-S58 | 0.13 ± 0.01 | 0.07 ± 0.03 | 2.30 ± 0.06 | 231.29 ± 3.47 | 6 |
| 1CX2-Chrysin | 0.14 ± 0.01 | 0.07 ± 0.04 | 2.30 ± 0.07 | 232.67 ± 2.73 | 5 |
| Protein–Ligand Complex | Van der Waals Energy | Electrostatic Energy | Polar Salvation | SASA Energy | Total Energy (kJ mol−1) |
|---|---|---|---|---|---|
| 1CX2-S58 | −151.45 ± 9.03 | −15.07 ± 1.22 | 26.57 ± 7.84 | −7.16 ± 0.30 | −147.11 ± 11.38 |
| 1CX2-Chrysin | −139.11 ± 12.45 | −12.66 ± 1.17 | 17.41 ± 5.71 | −4.73 ± 0.25 | −139.09 ± 13.21 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lekmine, S.; Benslama, O.; Kadi, K.; Brik, A.; Djeffali, O.; Ounissi, M.; Slimani, M.; Ola, M.S.; Eldahshan, O.A.; Martín-García, A.I.; et al. Preliminary Investigation of Astragalus arpilobus subsp. hauarensis: LC-MS/MS Chemical Profiling, In Vitro Evaluation of Antioxidant, Anti-Inflammatory Properties, Cytotoxicity, and In Silico Analysis against COX-2. Antioxidants 2024, 13, 654. https://doi.org/10.3390/antiox13060654
Lekmine S, Benslama O, Kadi K, Brik A, Djeffali O, Ounissi M, Slimani M, Ola MS, Eldahshan OA, Martín-García AI, et al. Preliminary Investigation of Astragalus arpilobus subsp. hauarensis: LC-MS/MS Chemical Profiling, In Vitro Evaluation of Antioxidant, Anti-Inflammatory Properties, Cytotoxicity, and In Silico Analysis against COX-2. Antioxidants. 2024; 13(6):654. https://doi.org/10.3390/antiox13060654
Chicago/Turabian StyleLekmine, Sabrina, Ouided Benslama, Kenza Kadi, Abir Brik, Ouidad Djeffali, Manar Ounissi, Meriem Slimani, Mohammad Shamsul Ola, Omayma A. Eldahshan, Antonio Ignacio Martín-García, and et al. 2024. "Preliminary Investigation of Astragalus arpilobus subsp. hauarensis: LC-MS/MS Chemical Profiling, In Vitro Evaluation of Antioxidant, Anti-Inflammatory Properties, Cytotoxicity, and In Silico Analysis against COX-2" Antioxidants 13, no. 6: 654. https://doi.org/10.3390/antiox13060654
APA StyleLekmine, S., Benslama, O., Kadi, K., Brik, A., Djeffali, O., Ounissi, M., Slimani, M., Ola, M. S., Eldahshan, O. A., Martín-García, A. I., & Ali, A. (2024). Preliminary Investigation of Astragalus arpilobus subsp. hauarensis: LC-MS/MS Chemical Profiling, In Vitro Evaluation of Antioxidant, Anti-Inflammatory Properties, Cytotoxicity, and In Silico Analysis against COX-2. Antioxidants, 13(6), 654. https://doi.org/10.3390/antiox13060654











