Evaluating the Neuroprotective Potential of Caffeinated Coffee in the Context of Aluminum-Induced Neurotoxicity: Insights from a PC12 Cell Culture Model
Abstract
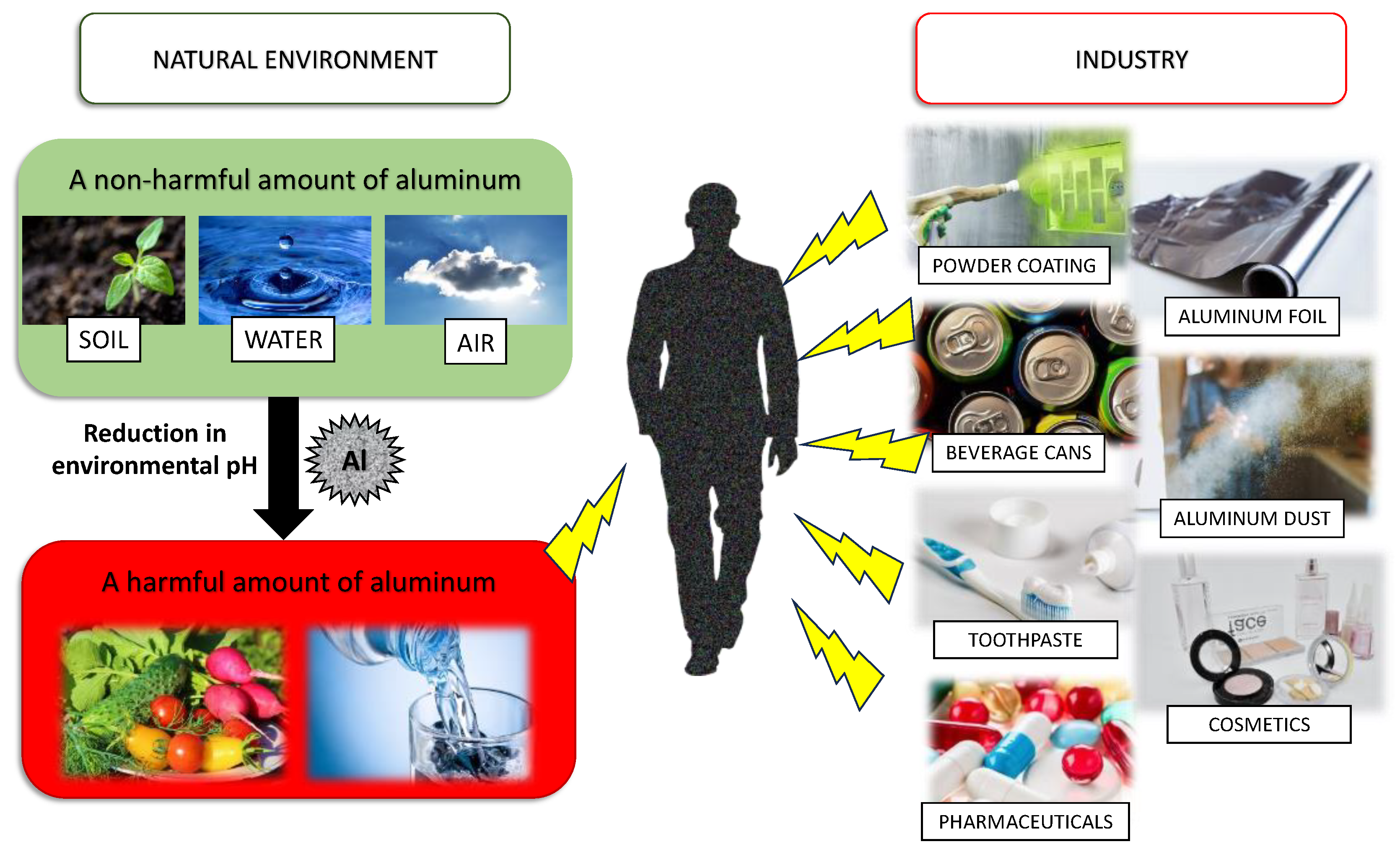
1. Introduction
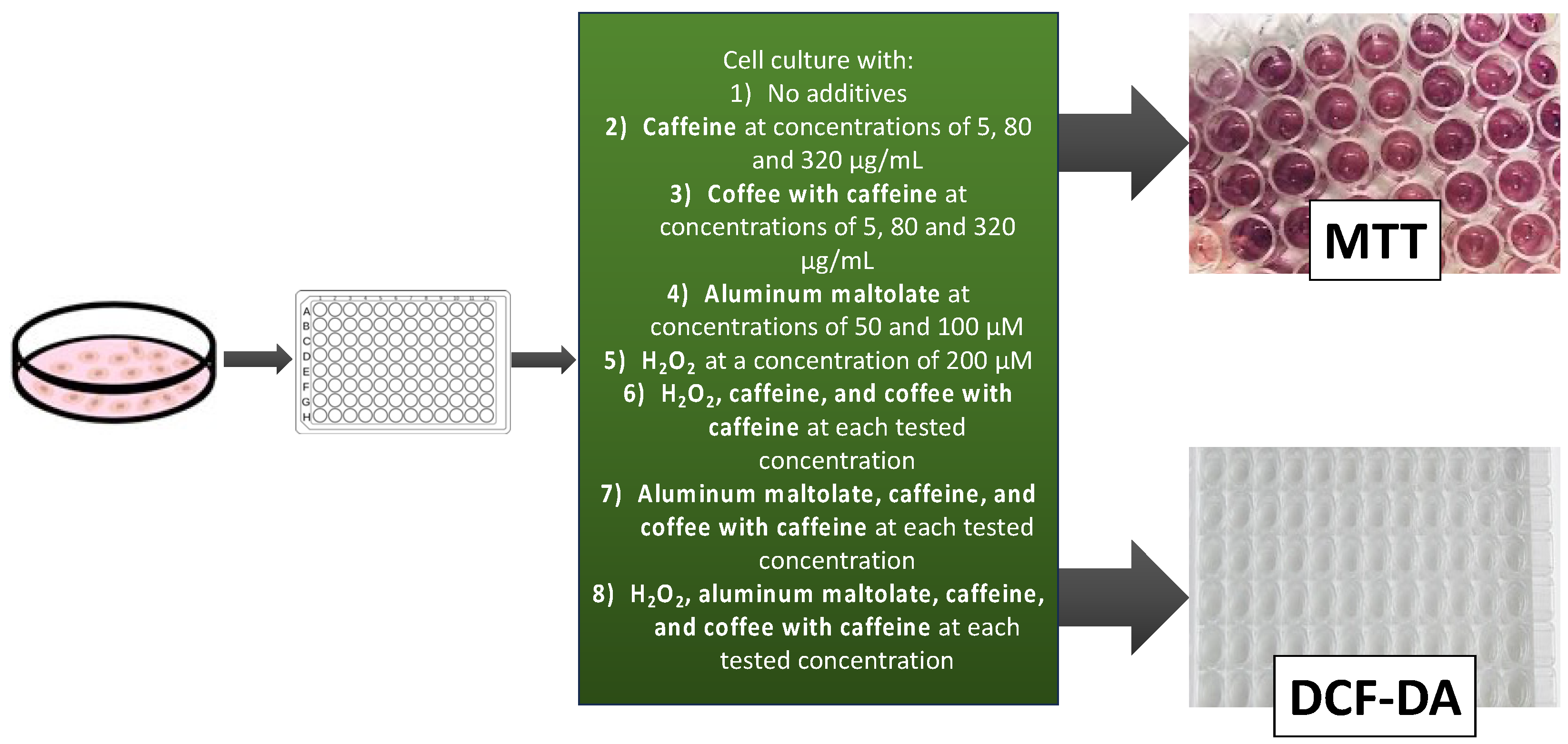
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Reagent Preparation
2.2.1. Coffee and Caffeine Solutions
2.2.2. Aluminum Maltolate Solution
2.3. Cell Culture Conditions
2.4. Cell Viability Assay
2.5. DCF-DA Assay
2.6. Statistical Analysis
3. Results
3.1. Effect of Tested Compounds and Their Combinations on the Viability of PC12 Cells
3.1.1. Effect of the Single Substances
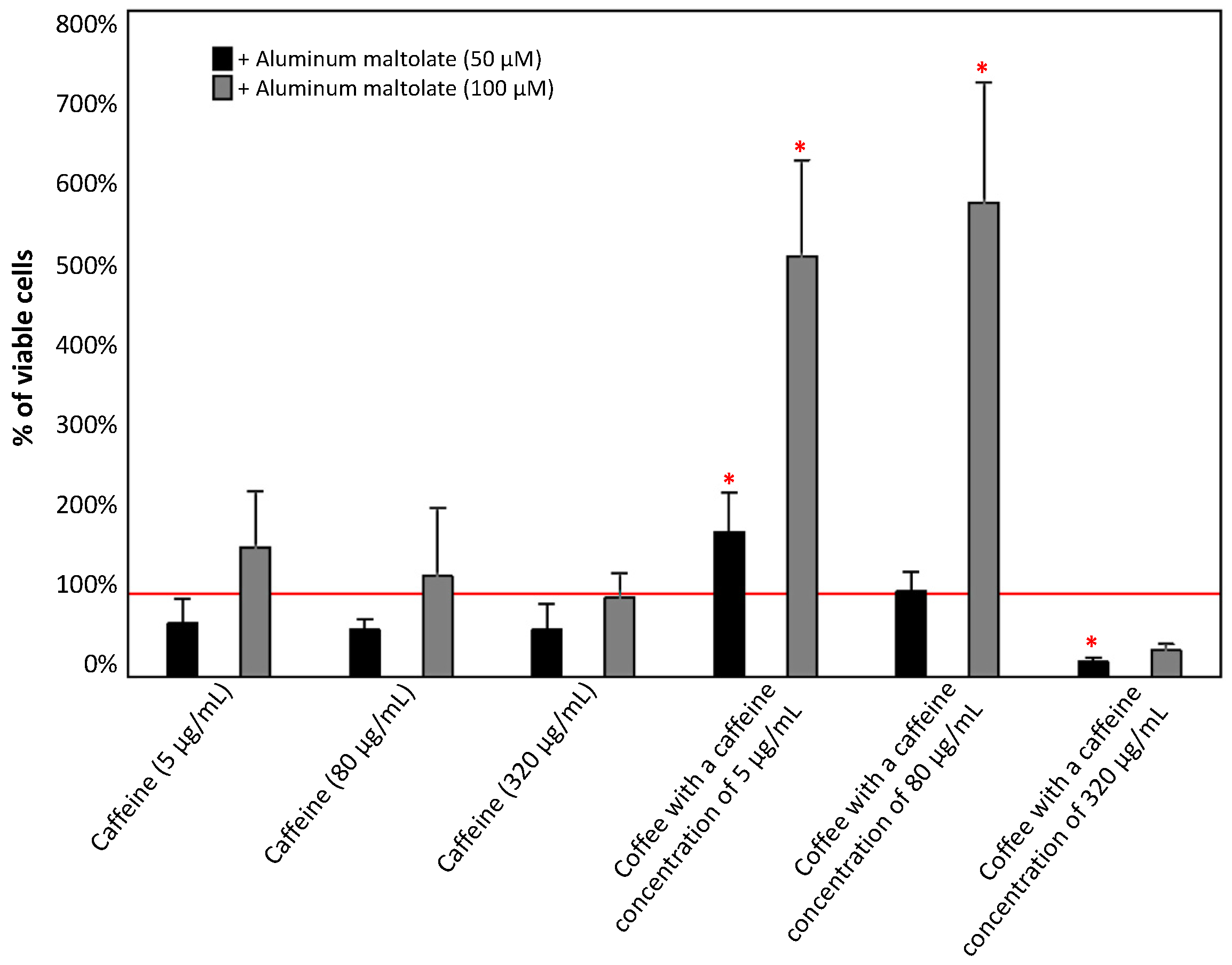
3.1.2. Effect of the Combinations of Caffeine or Coffee Containing Caffeine with Aluminum Maltolate
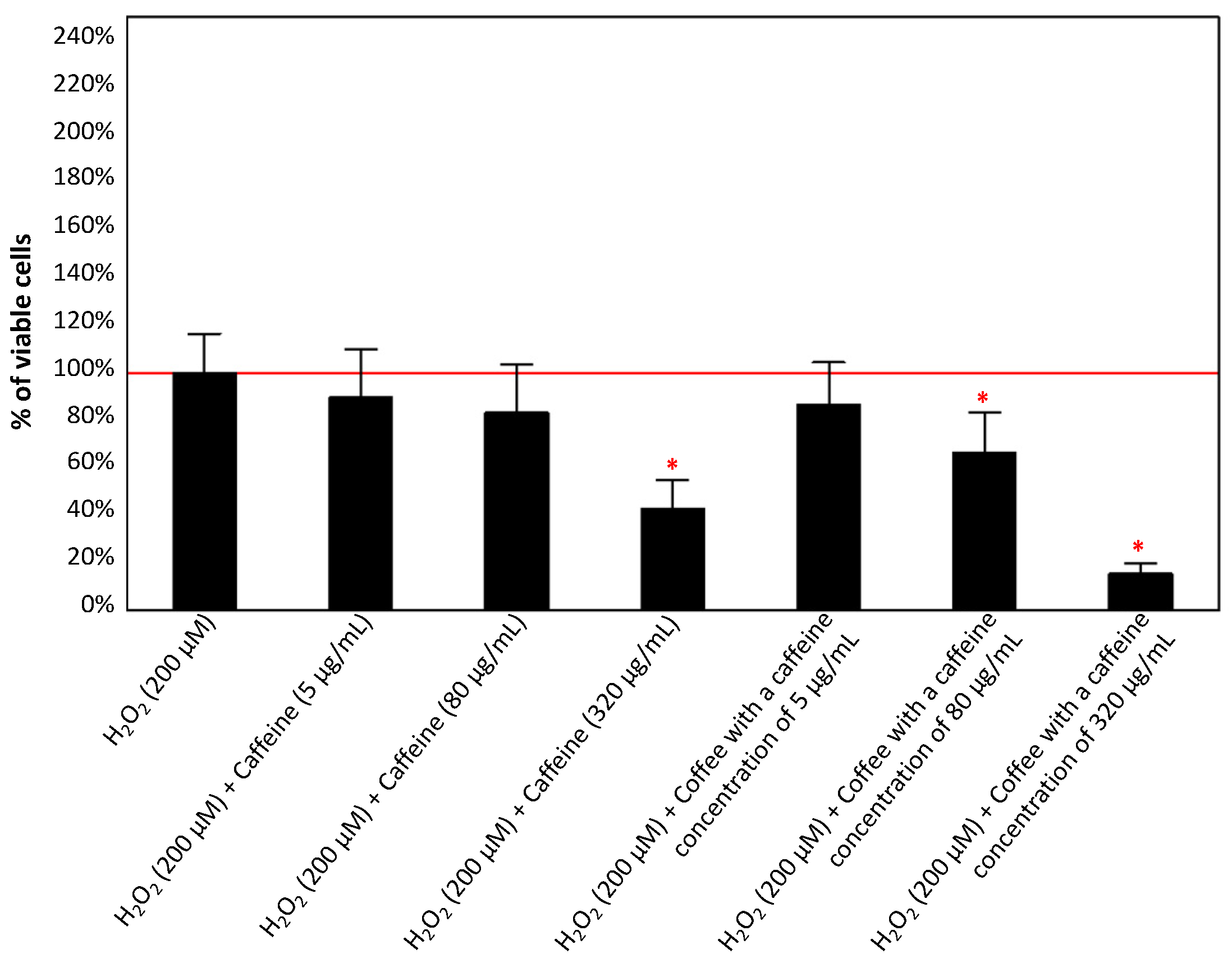
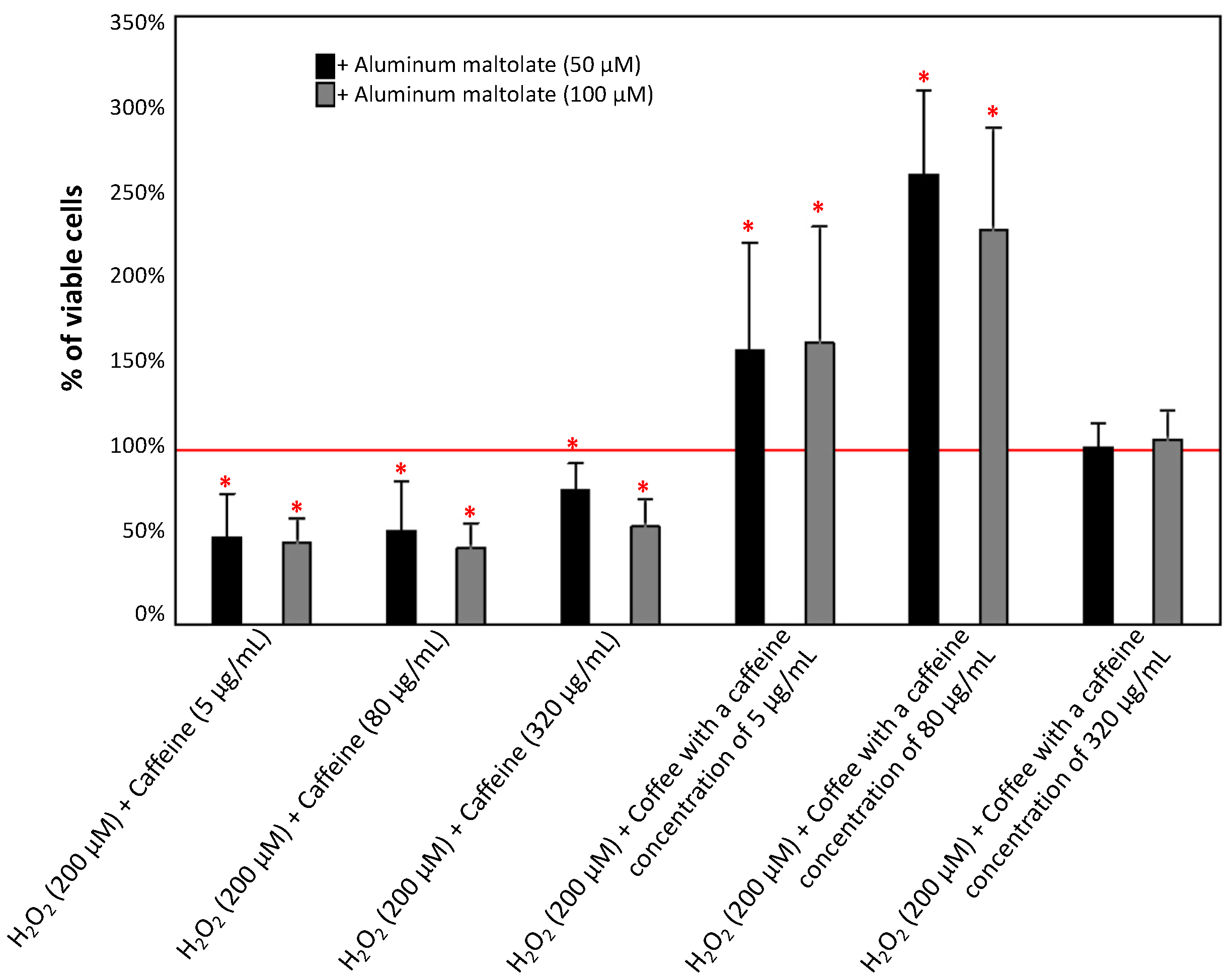
3.1.3. Effect of Caffeine or Coffee Containing Caffeine on Cells Exposed to H2O2 without or with the Addition of Aluminum Maltolate
3.2. Effect of Tested Compounds and Their Combinations on ROS Generation
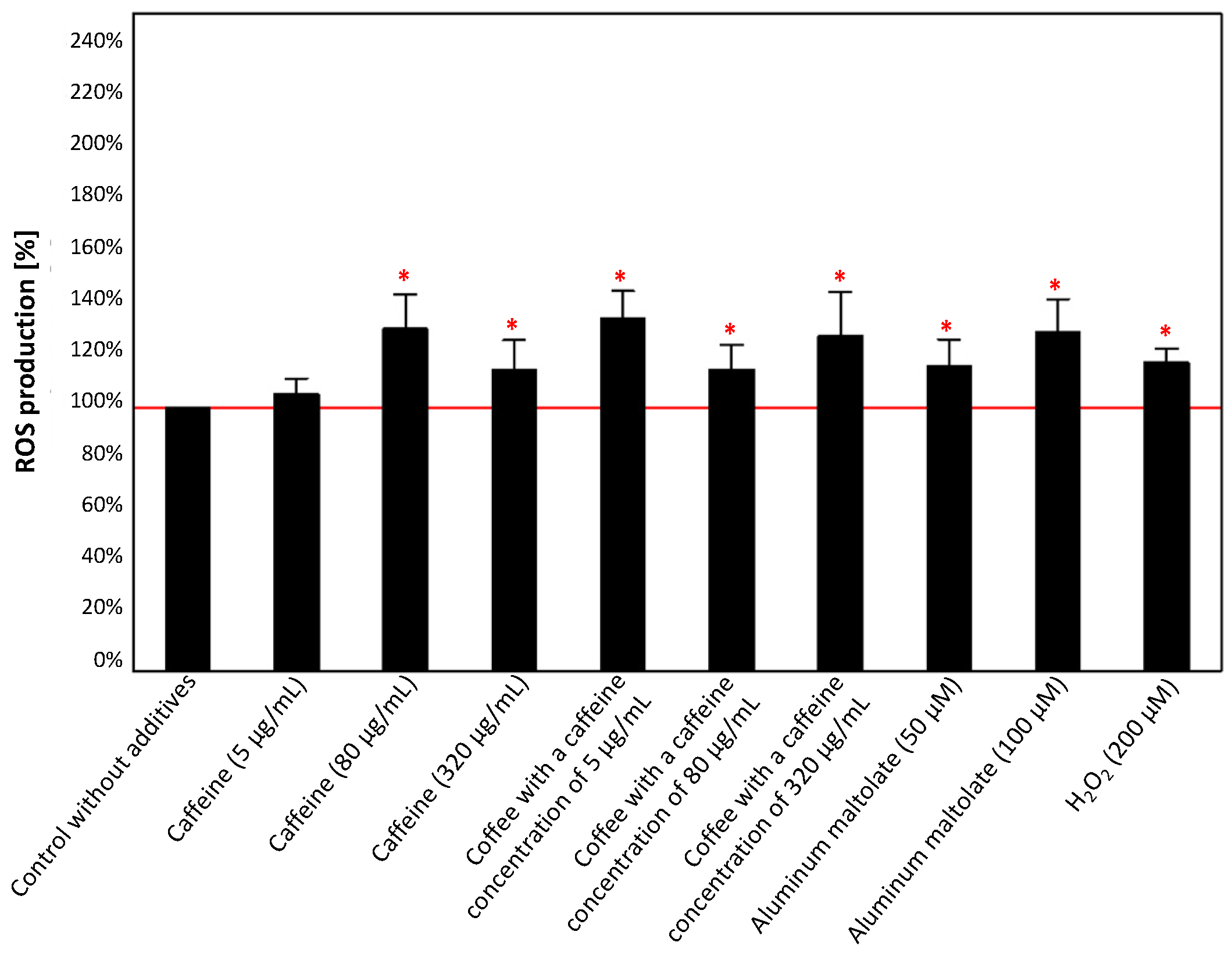
3.2.1. Effect of the Single Substances
3.2.2. Effect of the Combinations of Caffeine or Coffee Containing Caffeine with Aluminum Maltolate
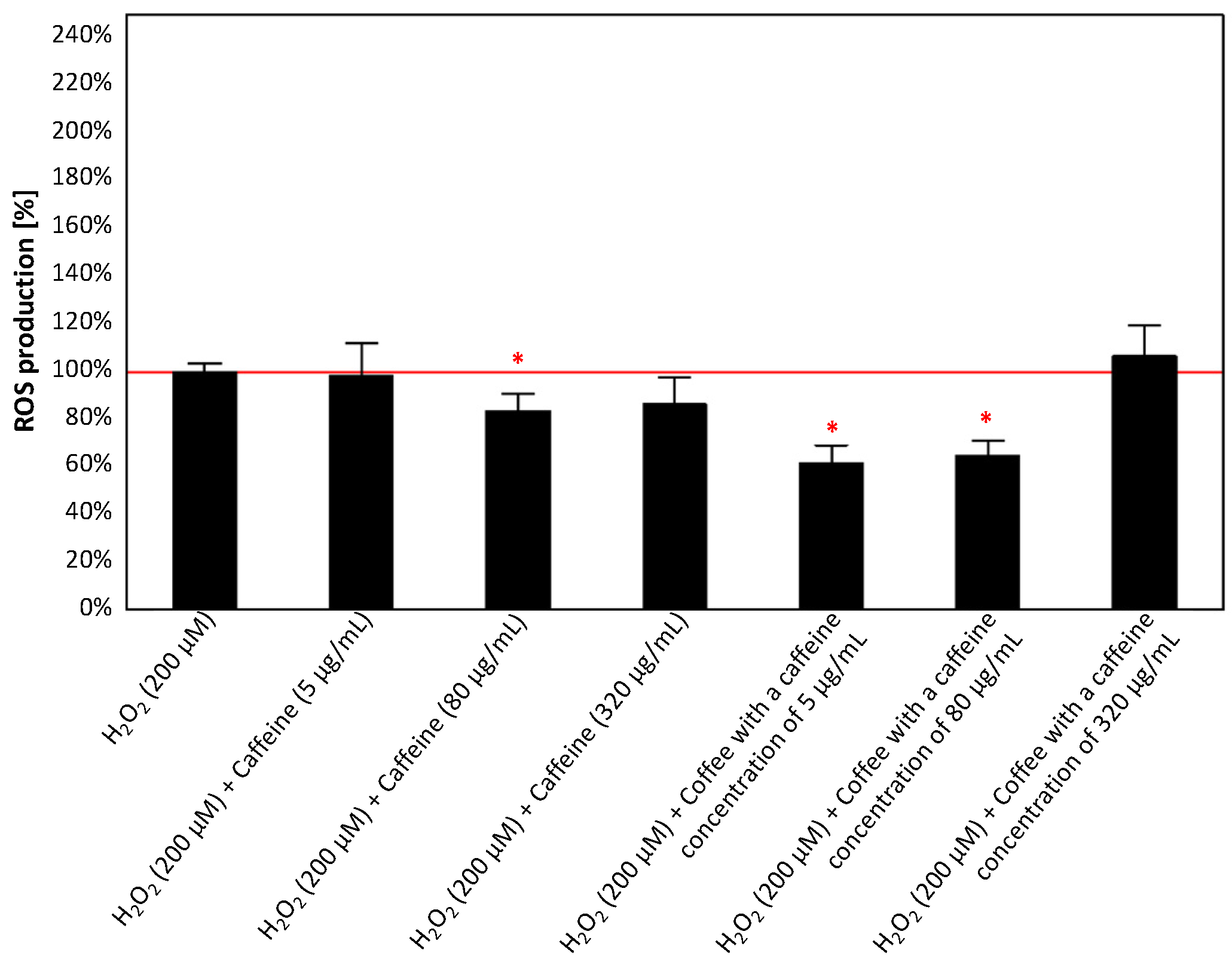
3.2.3. Effect of Caffeine or Coffee Containing Caffeine on Cells Exposed to H2O2 without or with the Addition of Aluminum Maltolate
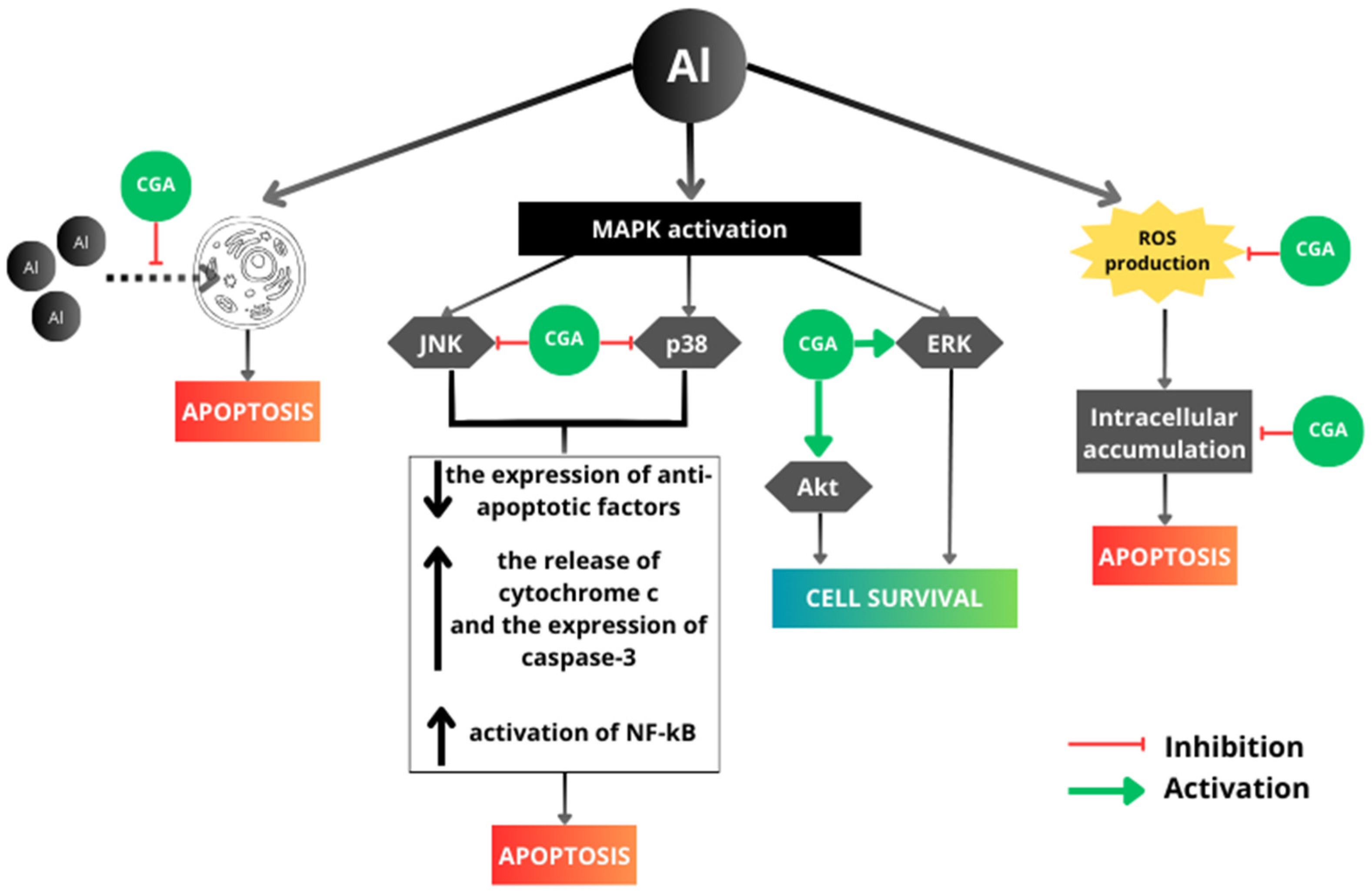
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ingerman, L.; Jones, D.G.; Keith, S.; Rosemond, Z.A. ATSDR Toxicological Profile for Aluminum. In ATSDR’s Toxicological Profiles; United States Environmental Protection Agency: Washington, DC, USA, 2002. [Google Scholar]
- Bondy, S.C. Low levels of aluminum can lead to behavioral and morphological changes associated with Alzheimer’s disease and age-related neurodegeneration. Neurotoxicology 2016, 52, 222–229. [Google Scholar] [CrossRef] [PubMed]
- Goullé, J.P.; Grangeot-Keros, L. Aluminum and vaccines: Current state of knowledge. Med. Mal. Infect. 2020, 50, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Wilhelm, M.; Jäger, D.E.; Ohnesorge, F.K. Aluminium Toxicokinetics. Pharmacol. Toxicol. 1990, 66, 4–9. [Google Scholar] [CrossRef] [PubMed]
- Krewski, D.; Yokel, R.A.; Nieboer, E.; Borchelt, D.; Cohen, J.; Harry, J.; Kacew, S.; Lindsay, J.; Mahfouz, A.M.; Rondeau, V. Human health risk assessment for aluminium, aluminium oxide, and aluminium hydroxide. J. Toxicol. Environ. Health Part B Crit. Rev. 2007, 10, 1–269. [Google Scholar] [CrossRef] [PubMed]
- Costa, L.G. Neurotoxicity testing: A discussion of in vitro alternatives. Environ. Health Perspect. 1998, 106, 505–510. [Google Scholar] [PubMed]
- Inan-Eroglu, E.; Ayaz, A. Is aluminum exposure a risk factor for neurological disorders? J. Res. Med. Sci. 2018, 23, 51. [Google Scholar] [CrossRef]
- Zatta, P.; Drago, D.; Bolognin, S.; Sensi, S.L. Alzheimer’s disease, metal ions and metal homeostatic therapy. Trends Pharmacol. Sci. 2009, 30, 346–355. [Google Scholar] [CrossRef]
- Stefanis, L. α-Synuclein in Parkinson’s disease. Cold Spring Harb. Perspect. Med. 2012, 2, a009399. [Google Scholar] [CrossRef]
- Banks, W.A.; Niehoff, M.L.; Drago, D.; Zatta, P. Aluminum complexing enhances amyloid β protein penetration of blood-brain barrier. Brain Res. 2006, 1116, 215–221. [Google Scholar] [CrossRef]
- Bolognin, S.; Messori, L.; Drago, D.; Gabbiani, C.; Cendron, L.; Zatta, P. Aluminum, copper, iron and zinc differentially alter amyloid-Aβ 1-42 aggregation and toxicity. Int. J. Biochem. Cell Biol. 2011, 43, 877–885. [Google Scholar] [CrossRef]
- Ribes, D.; Colomina, M.T.; Vicens, P.; Domingo, J.L. Impaired Spatial Learning and Unaltered Neurogenesis in a Transgenic Model of Alzheimers Disease After Oral Aluminum Exposure. Curr. Alzheimer Res. 2010, 7, 401–408. [Google Scholar] [CrossRef]
- Akiyama, H.; Hosokawa, M.; Kametani, F.; Kondo, H.; Chiba, M.; Fukushima, M.; Tabira, T. Long-term oral intake of aluminium or zinc does not accelerate Alzheimer pathology in AβPP and AβPP/tau transgenic mice. Neuropathology 2012, 32, 390–397. [Google Scholar] [CrossRef]
- Nübling, G.; Bader, B.; Levin, J.; Hildebrandt, J.; Kretzschmar, H.; Giese, A. Synergistic influence of phosphorylation and metal ions on tau oligomer formation and coaggregation with α-synuclein at the single molecule level. Mol. Neurodegener. 2012, 7, 35. [Google Scholar] [CrossRef]
- Yellamma, K.; Saraswathamma, S.; Kumari, B.N. Cholinergic system under aluminium toxicity in rat brain. Toxicol. Int. 2010, 17, 106–112. [Google Scholar] [CrossRef]
- Skalny, A.V.; Aschner, M.; Jiang, Y.; Gluhcheva, Y.G.; Tizabi, Y.; Lobinski, R.; Tinkov, A.A. Molecular mechanisms of aluminum neurotoxicity: Update on adverse effects and therapeutic strategies. In Advances in Neurotoxicology; Elsevier Inc.: Amsterdam, The Netherlands, 2021; Volume 5, pp. 1–34. ISBN 9780128237755. [Google Scholar]
- Morris, G.; Puri, B.K.; Frye, R.E. The putative role of environmental aluminium in the development of chronic neuropathology in adults and children. How strong is the evidence and what could be the mechanisms involved? Metab. Brain Dis. 2017, 32, 1335–1355. [Google Scholar] [CrossRef]
- Kawahara, M.; Kato-Negishi, M. Link between aluminum and the pathogenesis of Alzheimer’s disease: The integration of the aluminum and amyloid cascade hypotheses. Int. J. Alzheimers. Dis. 2011, 2011, 276393. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Ray, A.; Portengen, L.; Vermeulen, R.; Peters, S. Metal Exposure and Risk of Parkinson Disease: A Systematic Review and Meta-Analysis. Am. J. Epidemiol. 2023, 192, 1207–1223. [Google Scholar] [CrossRef] [PubMed]
- Cassidy, L.; Fernandez, F.; Johnson, J.B.; Naiker, M.; Owoola, A.G.; Broszczak, D.A. Oxidative stress in alzheimer’s disease: A review on emergent natural polyphenolic therapeutics. Complement. Ther. Med. 2020, 49, 102294. [Google Scholar] [CrossRef] [PubMed]
- Sies, H. Oxidative stress: Concept and some practical aspects. Antioxidants 2020, 9, 852. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Gill, K.D. Oxidative stress and mitochondrial dysfunction in aluminium neurotoxicity and its amelioration: A review. Neurotoxicology 2014, 41, 154–166. [Google Scholar] [CrossRef]
- Exley, C. The pro-oxidant activity of aluminum. Free Radic. Biol. Med. 2004, 36, 380–387. [Google Scholar] [CrossRef] [PubMed]
- Yuan, C.Y.; Lee, Y.J.; Hsu, G.S.W. Aluminum overload increases oxidative stress in four functional brain areas of neonatal rats. J. Biomed. Sci. 2012, 19, 51. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Du, Y.; Xue, H.; Wu, Y.; Zhou, B. Aluminum induces neurodegeneration and its toxicity arises from increased iron accumulation and reactive oxygen species (ROS) production. Neurobiol. Aging 2012, 33, 199.e1–199.e12. [Google Scholar] [CrossRef] [PubMed]
- Kremer, M.L. Mechanism of the Fenton reaction. Evidence for a new intermediate. Phys. Chem. Chem. Phys. 1999, 1, 3595–3605. [Google Scholar] [CrossRef]
- Ruipérez, F.; Mujika, J.I.; Ugalde, J.M.; Exley, C.; Lopez, X. Pro-oxidant activity of aluminum: Promoting the Fenton reaction by reducing Fe(III) to Fe(II). J. Inorg. Biochem. 2012, 117, 118–123. [Google Scholar] [CrossRef]
- Sánchez-Iglesias, S.; Méndez-Álvarez, E.; Iglesias-González, J.; Muñoz-Patiño, A.; Sánchez-Sellero, I.; Labandeira-García, J.L.; Soto-Otero, R. Brain oxidative stress and selective behaviour of aluminium in specific areas of rat brain: Potential effects in a 6-OHDA-induced model of Parkinson’s disease. J. Neurochem. 2009, 109, 879–888. [Google Scholar] [CrossRef]
- Fattoretti, P.; Bertoni-Freddari, C.; Balietti, M.; Mocchegiani, E.; Scancar, J.; Zambenedetti, P.; Zatta, P. The effect of chronic aluminum(III) administration on the nervous system of aged rats: Clues to understand its suggested role in Alzheimer’s disease. J. Alzheimer’s Dis. 2003, 5, 437–444. [Google Scholar] [CrossRef]
- International Coffee Day: Where Does Your Caffeine Fix Come from?|Interactive News|Al Jazeera. Available online: https://www.aljazeera.com/news/2023/10/1/international-coffee-day-where-does-your-caffeine-fix-come-from (accessed on 21 October 2023).
- Elsaid, F.G.; Shati, A.A.; Hafez, E.E. The protective role of coffea Arabica L. and Crocus sativus L. against the neurotoxicity induced by chronic administration of aluminium chloride. J. Pharmacol. Toxicol. 2011, 6, 647–663. [Google Scholar] [CrossRef]
- Ali, A.A.; I Ahmed, H. The Potential Effect of Caffeine and Nicotine Co-administration against Aluminum-induced Alzheimer’s disease in Rats. J. Alzheimer’s Dis. Park. 2016, 6, 236. [Google Scholar] [CrossRef]
- Rodak, K.; Kokot, I.; Kratz, E.M. Caffeine as a factor influencing the functioning of the human body—Friend or foe? Nutrients 2021, 13, 3088. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, J.A.; Sebastio, A.M. Caffeine and adenosine. In Proceedings of the Journal of Alzheimer’s Disease. J. Alzheimer Dis. 2010, 20, S3–S15. [Google Scholar] [CrossRef]
- Li, S.; Geiger, N.H.; Soliman, M.L.; Hui, L.; Geiger, J.D.; Chen, X. Caffeine, Through Adenosine A3 Receptor-Mediated Actions, Suppresses Amyloid-β Protein Precursor Internalization and Amyloid-β Generation. J. Alzheimer Dis. 2015, 47, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Rahman, A. The Role of Adenosine in Alzheimers Disease. Curr. Neuropharmacol. 2009, 7, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Eskelinen, M.H.; Ngandu, T.; Tuomilehto, J.; Soininen, H.; Kivipelto, M. Midlife coffee and tea drinking and the risk of late-life dementia: A population-based CAIDE study. J. Alzheimer Dis. 2009, 16, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Guo, X.; Park, Y.; Huang, X.; Sinha, R.; Freedman, N.D.; Hollenbeck, A.R.; Blair, A.; Chen, H. Caffeine intake, smoking, and risk of parkinson disease in men and women. Am. J. Epidemiol. 2012, 175, 1200–1207. [Google Scholar] [CrossRef] [PubMed]
- Słońska, A.; Cymerys, J. Application of three-dimensional neuronal cell cultures in the studies of mechanisms of neurodegenerative diseases. Postepy Hig. Med. Dosw. 2017, 71, 510–519. [Google Scholar] [CrossRef]
- Schlachetzki, J.C.M.; Saliba, S.W.; de Oliveira, A.C.P. Studying neurodegenerative diseases in culture models. Rev. Bras. Psiquiatr. 2013, 35, S92–S100. [Google Scholar] [CrossRef]
- Kawahara, M.; Kato-Negishi, M.; Hosoda, R.; Imamura, L.; Tsuda, M.; Kuroda, Y. Brain-derived neurotrophic factor protects cultured rat hippocampal neurons from aluminum maltolate neurotoxicity. J. Inorg. Biochem. 2003, 97, 124–131. [Google Scholar] [CrossRef]
- Bertholf, R.L.; Herman, M.M.; Savory, J.; Carpenter, R.M.; Sturgill, B.C.; Katsetos, C.D.; VandenBerg, S.R.; Wills, M.R. A long-term intravenous model of aluminum maltol toxicity in rabbits: Tissue distribution, hepatic, renal, and neuronal cytoskeletal changes associated with systemic exposure. Toxicol. Appl. Pharmacol. 1989, 98, 58–74. [Google Scholar] [CrossRef]
- Kinarivala, N.; Shah, K.; Abbruscato, T.J.; Trippier, P.C. Passage Variation of PC12 Cells Results in Inconsistent Susceptibility to Externally Induced Apoptosis. ACS Chem. Neurosci. 2017, 8, 82–88. [Google Scholar] [CrossRef]
- Yang, L.; Shen, K.; Ji, D. Natural compounds attenuate heavy metal-induced PC12 cell damage. J. Int. Med. Res. 2020, 48, 0300060520930847. [Google Scholar] [CrossRef]
- Chian, S.; Cheng, Z.; Jiang, L.; Wang, K.; Fan, Y.; Liao, T.; Chen, W.; Yao, W. Caffeine-induced neurotoxicity mediated by Nrf2 pathway in PC12 cells and zebrafish larvae. J. Appl. Toxicol. 2022, 42, 629–637. [Google Scholar] [CrossRef] [PubMed]
- Ng, N.S.; Ooi, L. A simple microplate assay for reactive oxygen species generation and rapid cellular protein normalization. Bio-Protoc. 2021, 11, e3877. [Google Scholar] [CrossRef]
- Liang, R. Cross Talk Between Aluminum and Genetic Susceptibility and Epigenetic Modification in Alzheimer’s Disease. In Advances in Experimental Medicine and Biology; Springer New York LLC: New York, NY, USA, 2018; Volume 1091, pp. 173–191. [Google Scholar]
- Wang, Z.; Wei, X.; Yang, J.; Suo, J.; Chen, J.; Liu, X.; Zhao, X. Chronic exposure to aluminum and risk of Alzheimer’s disease: A meta-analysis. Neurosci. Lett. 2016, 610, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Duan, X.-H.; Wang, W.-L.; Dai, R.; Yan, H.-W.; Han, C.-N.; Liu, L.-S. Current Situation of PC12 Cell Use in Neuronal Injury Study. Int. J. Biotechnol. Wellness Ind. 2015, 4, 61–66. [Google Scholar] [CrossRef]
- Mujika, J.I.; Rezabal, E.; Mercero, J.M.; Ruipérez, F.; Costa, D.; Ugalde, J.M.; Lopez, X. Aluminium in biological environments: A computational approach. Comput. Struct. Biotechnol. J. 2014, 9, e201403002. [Google Scholar] [CrossRef] [PubMed]
- Kono, Y.; Kashine, S.; Yoneyama, T.; Sakamoto, Y.; Matsui, Y.; Shibata, H. Iron chelation by chlorogenic acid as a natural antioxidant. Biosci. Biotechnol. Biochem. 1998, 62, 22–27. [Google Scholar] [CrossRef]
- Wang, H.; Shao, B.; Yu, H.; Xu, F.; Wang, P.; Yu, K.; Han, Y.; Song, M.; Li, Y.; Cao, Z. Neuroprotective role of hyperforin on aluminum maltolate-induced oxidative damage and apoptosis in PC12 cells and SH-SY5Y cells. Chem. Biol. Interact. 2019, 299, 15–26. [Google Scholar] [CrossRef]
- Yu, Q.; Zhu, K.; Ding, Y.; Han, R.; Cheng, D. Comparative study of aluminum (Al) speciation on apoptosis-promoting process in PC12 cells: Correlations between morphological characteristics and mitochondrial kinetic disorder. J. Inorg. Biochem. 2022, 232, 111835. [Google Scholar] [CrossRef]
- Cheng, L.; Liang, R.; Li, Z.; Ren, J.; Yang, S.; Bai, J.; Niu, Q.; Yu, H.; Zhang, H.; Xia, N.; et al. Aluminum maltolate triggers ferroptosis in neurons: Mechanism of action. Toxicol. Mech. Methods 2021, 31, 33–42. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, G.; Zhao, Y.; Bu, F.; Zhang, Y. Activation of PI3k/Akt/mTOR Signaling Induces Deposition of p-tau to Promote Aluminum Neurotoxicity. Neurotox. Res. 2022, 40, 1516–1525. [Google Scholar] [CrossRef]
- Kwon, J.T.; Seo, G.B.; Jo, E.; Lee, M.; Kim, H.M.; Shim, I.; Lee, B.W.; Yoon, B.; Kim, P.; Choi, K. Aluminum nanoparticles induce ERK and p38MAPK activation in rat brain. Toxicol. Res. 2013, 29, 181–185. [Google Scholar] [CrossRef]
- Pera, M.; Camps, P.; Muñoz-Torrero, D.; Perez, B.; Badia, A.; Clos Guillen, M.V. Undifferentiated and Differentiated PC12 Cells Protected by Huprines Against Injury Induced by Hydrogen Peroxide. PLoS ONE 2013, 8, e74344. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Liu, J.; Liu, A.; Wang, Y. Cytoprotective effect of selenium polysaccharide from Pleurotus ostreatus against H2O2-induced oxidative stress and apoptosis in PC12 cells. Arab. J. Chem. 2022, 15, 103686. [Google Scholar] [CrossRef]
- Li, P.; Li, Z. Neuroprotective effect of paeoniflorin on H2O2-induced apoptosis in PC12 cells by modulation of reactive oxygen species and the inflammatory response. Exp. Ther. Med. 2015, 9, 1768–1772. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Xiao, S.; Cai, Q.; Miao, J.; Li, J. Antioxidant Capacity and Protective Effects on H2O2-Induced Oxidative Damage in PC12 Cells of the Active Fraction of Brassica rapa L. Foods 2023, 12, 2075. [Google Scholar] [CrossRef]
- Hosny, E.N.; Sawie, H.G.; Elhadidy, M.E.; Khadrawy, Y.A. Evaluation of antioxidant and anti-inflammatory efficacy of caffeine in rat model of neurotoxicity. Nutr. Neurosci. 2019, 22, 789–796. [Google Scholar] [CrossRef]
- Mikami, Y.; Kakizawa, S.; Yamazawa, T. Essential roles of natural products and gaseous mediators on neuronal cell death or survival. Int. J. Mol. Sci. 2016, 17, 1652. [Google Scholar] [CrossRef]
- Gorjanović, S.; Komes, D.; Laličić-Petronijević, J.; Pastor, F.T.; Belščak-Cvitanović, A.; Veljović, M.; Pezo, L.; Sužnjević, D. Antioxidant efficiency of polyphenols from coffee and coffee substitutes-electrochemical versus spectrophotometric approach. J. Food Sci. Technol. 2017, 54, 2324–2331. [Google Scholar] [CrossRef] [PubMed]
- Rui, L.; Xie, M.; Hu, B.; Zhou, L.; Saeeduddin, M.; Zeng, X. Enhanced solubility and antioxidant activity of chlorogenic acid-chitosan conjugates due to the conjugation of chitosan with chlorogenic acid. Carbohydr. Polym. 2017, 170, 206–216. [Google Scholar] [CrossRef]
- Ohkawara, T.; Takeda, H.; Nishihira, J. Protective effect of chlorogenic acid on the inflammatory damage of pancreas and lung in mice with L-arginine-induced pancreatitis. Life Sci. 2017, 190, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Miyamae, Y.; Shigemori, H.; Isoda, H. Neuroprotective effect of 3,5-di-O-caffeoylquinic acid on SH-SY5Y cells and senescence-accelerated-prone mice 8 through the up-regulation of phosphoglycerate kinase. Neuroscience 2010, 169, 1039–1045. [Google Scholar] [CrossRef] [PubMed]
- Nabavi, S.F.; Tejada, S.; Setzer, W.N.; Gortzi, O.; Sureda, A.; Braidy, N.; Daglia, M.; Manayi, A.; Nabavi, S.M. Chlorogenic Acid and Mental Diseases: From Chemistry to Medicine. Curr. Neuropharmacol. 2016, 15, 471–479. [Google Scholar] [CrossRef]
- Torreggiani, A.; Jurasekova, Z.; Sanchez-Cortes, S.; Tamba, M. Spectroscopic and pulse radiolysis studies of the antioxidant properties of (+)catechin: Metal chelation and oxidizing radical scavenging. J. Raman Spectrosc. 2008, 39, 265–275. [Google Scholar] [CrossRef]
- Wang, X.; Xi, Y.; Zeng, X.; Zhao, H.; Cao, J.; Jiang, W. Effects of chlorogenic acid against aluminium neurotoxicity in ICR mice through chelation and antioxidant actions. J. Funct. Foods 2018, 40, 365–376. [Google Scholar] [CrossRef]
- Cheng, D.; Wang, G.; Wang, X.; Tang, J.; Yu, Q.; Zhang, X.; Wang, S. Neuro-protection of Chlorogenic acid against Al-induced apoptosis in PC12 cells via modulation of Al metabolism and Akt/GSK-3β pathway. J. Funct. Foods 2020, 70, 103984. [Google Scholar] [CrossRef]
- Elion, E.A. The Ste5p scaffold. J. Cell Sci. 2001, 114, 3967–3978. [Google Scholar] [CrossRef]
- Cargnello, M.; Roux, P.P. Activation and Function of the MAPKs and Their Substrates, the MAPK-Activated Protein Kinases. Microbiol. Mol. Biol. Rev. 2012, 76, 496. [Google Scholar] [CrossRef]
- Xia, Z.; Dickens, M.; Raingeaud, J.; Davis, R.J.; Greenberg, M.E. Opposing effects of ERK and JNK-p38 MAP kinases on apoptosis. Science 1995, 270, 1326–1331. [Google Scholar] [CrossRef] [PubMed]
- Cho, E.S.; Jang, Y.J.; Hwang, M.K.; Kang, N.J.; Lee, K.W.; Lee, H.J. Attenuation of oxidative neuronal cell death by coffee phenolic phytochemicals. Mutat. Res. Fundam. Mol. Mech. Mutagen. 2009, 661, 18–24. [Google Scholar] [CrossRef]
- Chassé, M.; Vasdev, N. Synthesis and Preclinical Positron Emission Tomography Imaging of the p38 MAPK Inhibitor [11C]Talmapimod: Effects of Drug Efflux and Sex Differences. ACS Chem. Neurosci. 2023, 14, 2193–2200. [Google Scholar] [CrossRef]
- Kim, E.K.; Choi, E.J. Pathological roles of MAPK signaling pathways in human diseases. Biochim. Biophys. Acta Mol. Basis Dis. 2010, 1802, 396–405. [Google Scholar] [CrossRef]
- Cheng, D.; Zhang, X.; Tang, J.; Kong, Y.; Wang, X.; Wang, S. Chlorogenic acid protects against aluminum toxicity via MAPK/Akt signaling pathway in murine RAW264.7 macrophages. J. Inorg. Biochem. 2019, 190, 113–120. [Google Scholar] [CrossRef]
- Tsao, L.T.; Tsai, P.S.; Lin, R.H.; Huang, L.J.; Kuo, S.C.; Wang, J.P. Inhibition of lipopolysaccharide-induced expression of inducible nitric oxide synthase by phenolic (3E)-4-(2-hydroxyphenyl)but-3-en-2-one in RAW 264.7 macrophages. Biochem. Pharmacol. 2005, 70, 618–626. [Google Scholar] [CrossRef]
- Ontawong, A.; Duangjai, A.; Vaddhanaphuti, C.S.; Amornlerdpison, D.; Pengnet, S.; Kamkaew, N. Chlorogenic acid rich in coffee pulp extract suppresses inflammatory status by inhibiting the p38, MAPK, and NF-κB pathways. Heliyon 2023, 9, e13917. [Google Scholar] [CrossRef]
- Li, D.; Li, Z.; Dong, L.; Zhang, Y.; Lu, Y.; Wang, J.; Sun, H.; Wang, S. Coffee prevents IQ-induced liver damage by regulating oxidative stress, inflammation, endoplasmic reticulum stress, autophagy, apoptosis, and the MAPK/NF-κB signaling pathway in zebrafish. Food Res. Int. 2023, 169, 112946. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Wang, C.; Yang, T.; Feng, G.; Tan, H.; Piao, X.; Chen, D.; Zhang, Y.; Jiao, W.; Chen, Y.; et al. Chlorogenic Acid Alleviates Chronic Stress-Induced Intestinal Damage by Inhibiting the P38MAPK/NF-κB Pathway. J. Agric. Food Chem. 2023, 71, 9381–9390. [Google Scholar] [CrossRef] [PubMed]
- Pavlica, S.; Gebhardt, R. Protective effects of ellagic and chlorogenic acids against oxidative stress in PC12 cells. Free Radic. Res. 2005, 39, 1377–1390. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Lee, S.; Shim, J.; Kim, H.W.; Kim, J.; Jang, Y.J.; Yang, H.; Park, J.; Choi, S.H.; Yoon, J.H.; et al. Caffeinated coffee, decaffeinated coffee, and the phenolic phytochemical chlorogenic acid up-regulate NQO1 expression and prevent H2O2-induced apoptosis in primary cortical neurons. Neurochem. Int. 2012, 60, 466–474. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Peng, S.; Xu, J.; Fang, J. Reversing ROS-mediated neurotoxicity by chlorogenic acid involves its direct antioxidant activity and activation of Nrf2-ARE signaling pathway. BioFactors 2019, 45, 616–626. [Google Scholar] [CrossRef] [PubMed]
- Bruggisser, R.; Von Daeniken, K.; Jundt, G.; Schaffner, W.; Tullberg-Reinert, H. Interference of plant extracts, phytoestrogens and antioxidants with the MTT tetrazolium assay. Planta Med. 2002, 68, 445–448. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi, M.; Liang, S.; Luu, Q.M.; Kempson, I. The MTT Assay: A Method for Error Minimization and Interpretation in Measuring Cytotoxicity and Estimating Cell Viability. Methods Mol. Biol. 2023, 2644, 15–33. [Google Scholar] [PubMed]
- Kalyanaraman, B.; Darley-Usmar, V.; Davies, K.J.A.; Dennery, P.A.; Forman, H.J.; Grisham, M.B.; Mann, G.E.; Moore, K.; Roberts, L.J.; Ischiropoulos, H. Measuring reactive oxygen and nitrogen species with fluorescent probes: Challenges and limitations. Free Radic. Biol. Med. 2012, 52, 1–6. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodak, K.; Bęben, D.; Birska, M.; Siwiela, O.; Kokot, I.; Moreira, H.; Radajewska, A.; Szyjka, A.; Kratz, E.M. Evaluating the Neuroprotective Potential of Caffeinated Coffee in the Context of Aluminum-Induced Neurotoxicity: Insights from a PC12 Cell Culture Model. Antioxidants 2024, 13, 342. https://doi.org/10.3390/antiox13030342
Rodak K, Bęben D, Birska M, Siwiela O, Kokot I, Moreira H, Radajewska A, Szyjka A, Kratz EM. Evaluating the Neuroprotective Potential of Caffeinated Coffee in the Context of Aluminum-Induced Neurotoxicity: Insights from a PC12 Cell Culture Model. Antioxidants. 2024; 13(3):342. https://doi.org/10.3390/antiox13030342
Chicago/Turabian StyleRodak, Kamil, Dorota Bęben, Monika Birska, Oliwia Siwiela, Izabela Kokot, Helena Moreira, Anna Radajewska, Anna Szyjka, and Ewa Maria Kratz. 2024. "Evaluating the Neuroprotective Potential of Caffeinated Coffee in the Context of Aluminum-Induced Neurotoxicity: Insights from a PC12 Cell Culture Model" Antioxidants 13, no. 3: 342. https://doi.org/10.3390/antiox13030342
APA StyleRodak, K., Bęben, D., Birska, M., Siwiela, O., Kokot, I., Moreira, H., Radajewska, A., Szyjka, A., & Kratz, E. M. (2024). Evaluating the Neuroprotective Potential of Caffeinated Coffee in the Context of Aluminum-Induced Neurotoxicity: Insights from a PC12 Cell Culture Model. Antioxidants, 13(3), 342. https://doi.org/10.3390/antiox13030342





