Antioxidant Responses and Phytochemical Accumulation in Raphanus Species Sprouts through Elicitors and Predictive Models under High Temperature Stress
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Sprout Treatments
2.2. Physical Parameters and Sprouting Potential
2.3. Sprout Stress Response to Elicitors
2.4. Extraction of Bioactive Compounds
2.5. Analysis of Glucosinolates and Anthocyanins
2.6. Antioxidant Properties
2.6.1. Total Phenolic Contents
2.6.2. Antioxidant Capacity
2.7. Statistical Analysis
3. Results
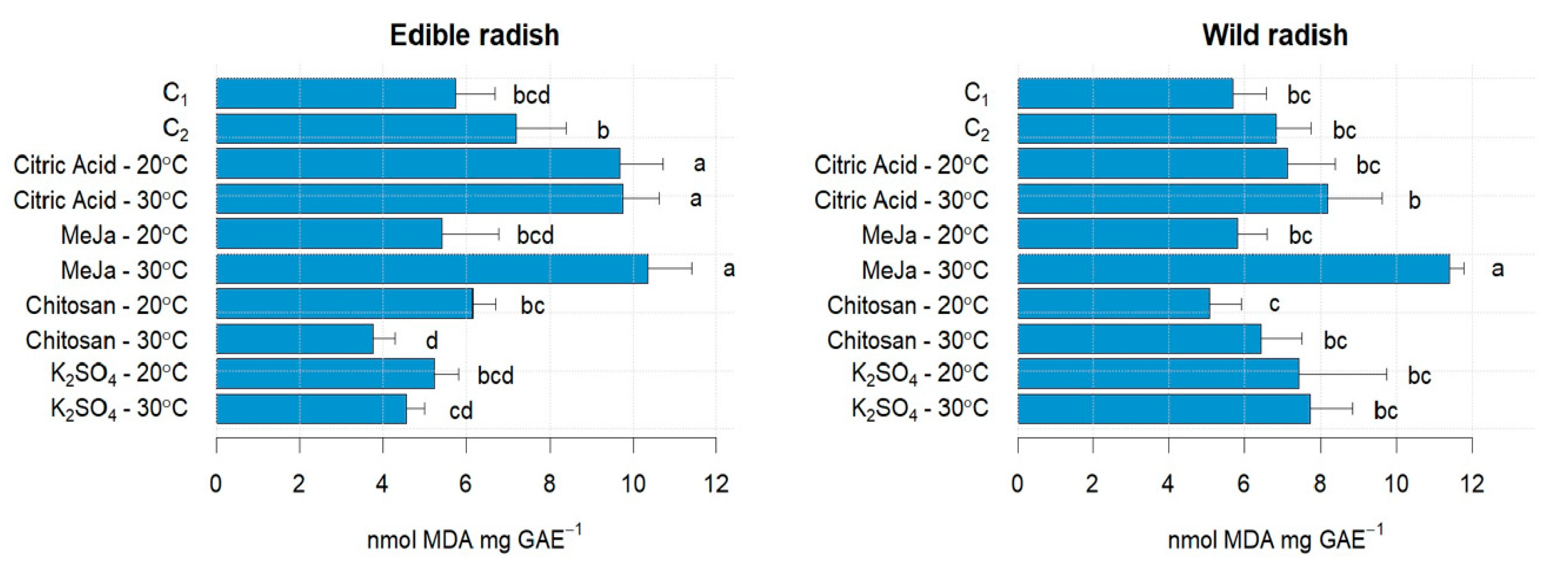
3.1. Sprouts Growth Performance and Malonyl-Dialdehyde Status Affected by Stress
3.2. Bioactive Compounds in ER and WR Sprouts
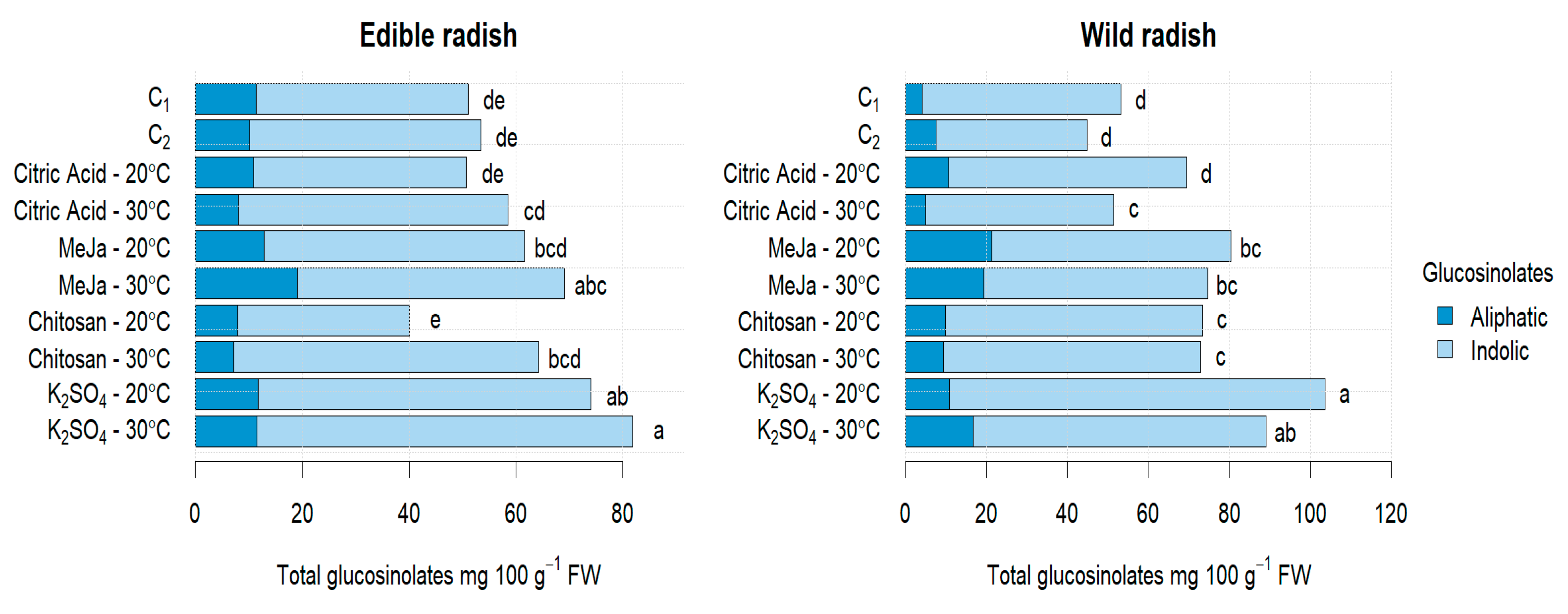
3.2.1. Glucosinolates
3.2.2. Anthocyanins
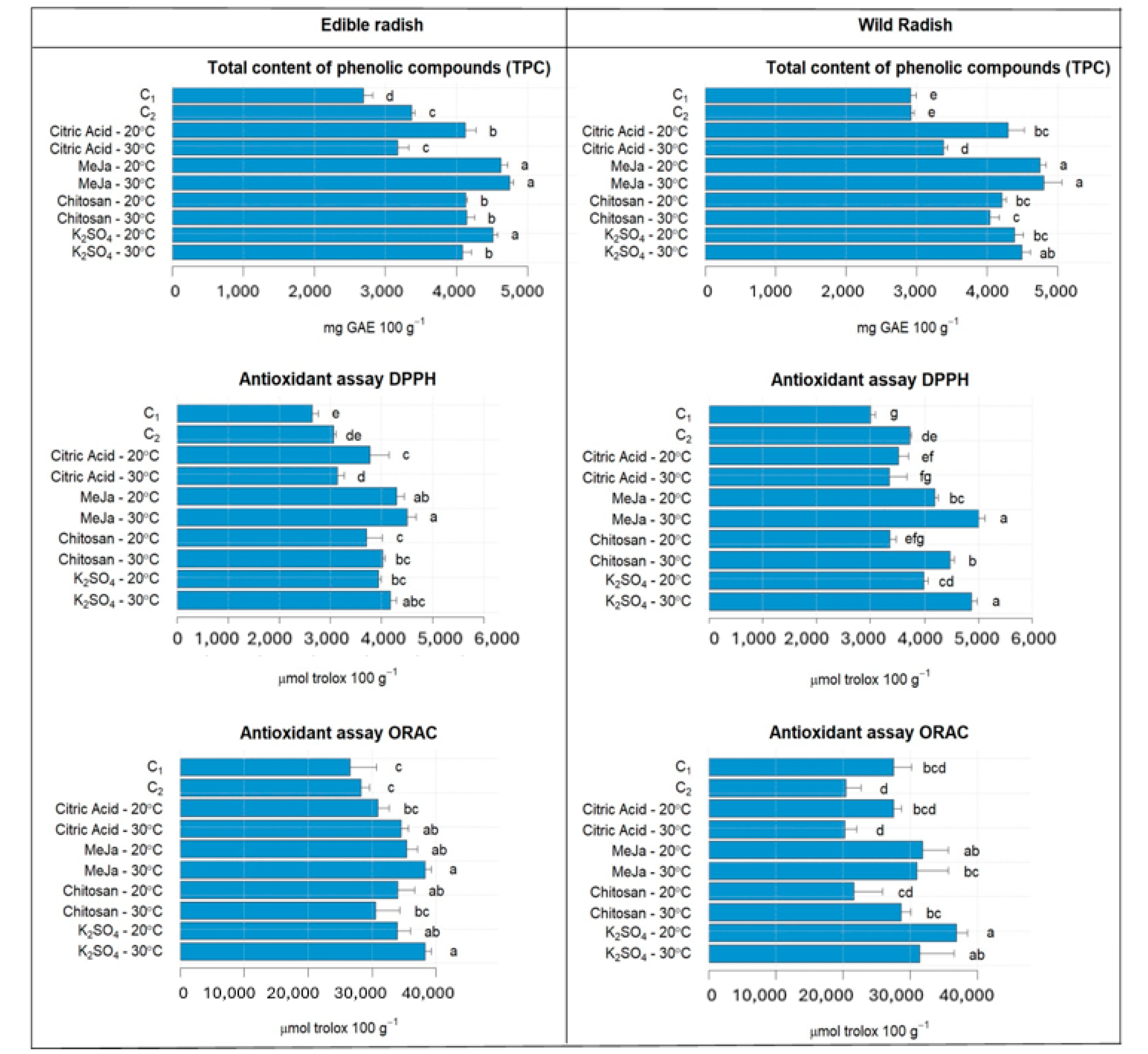
3.3. Antioxidant Properties of ER and WR Sprouts
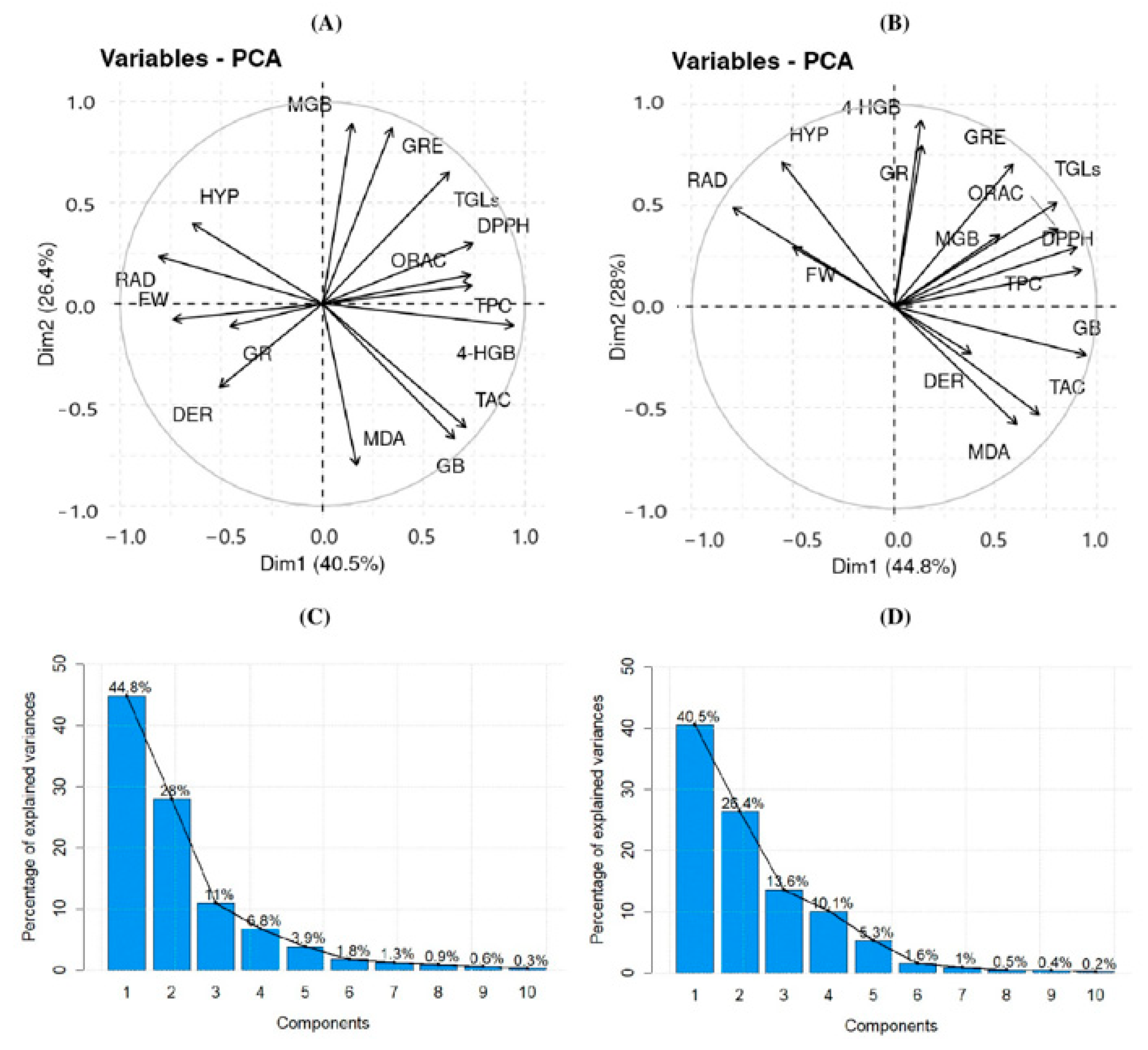
3.4. Interaction of Elicitors with Phytochemical Content: PCA Analysis at 30 °C
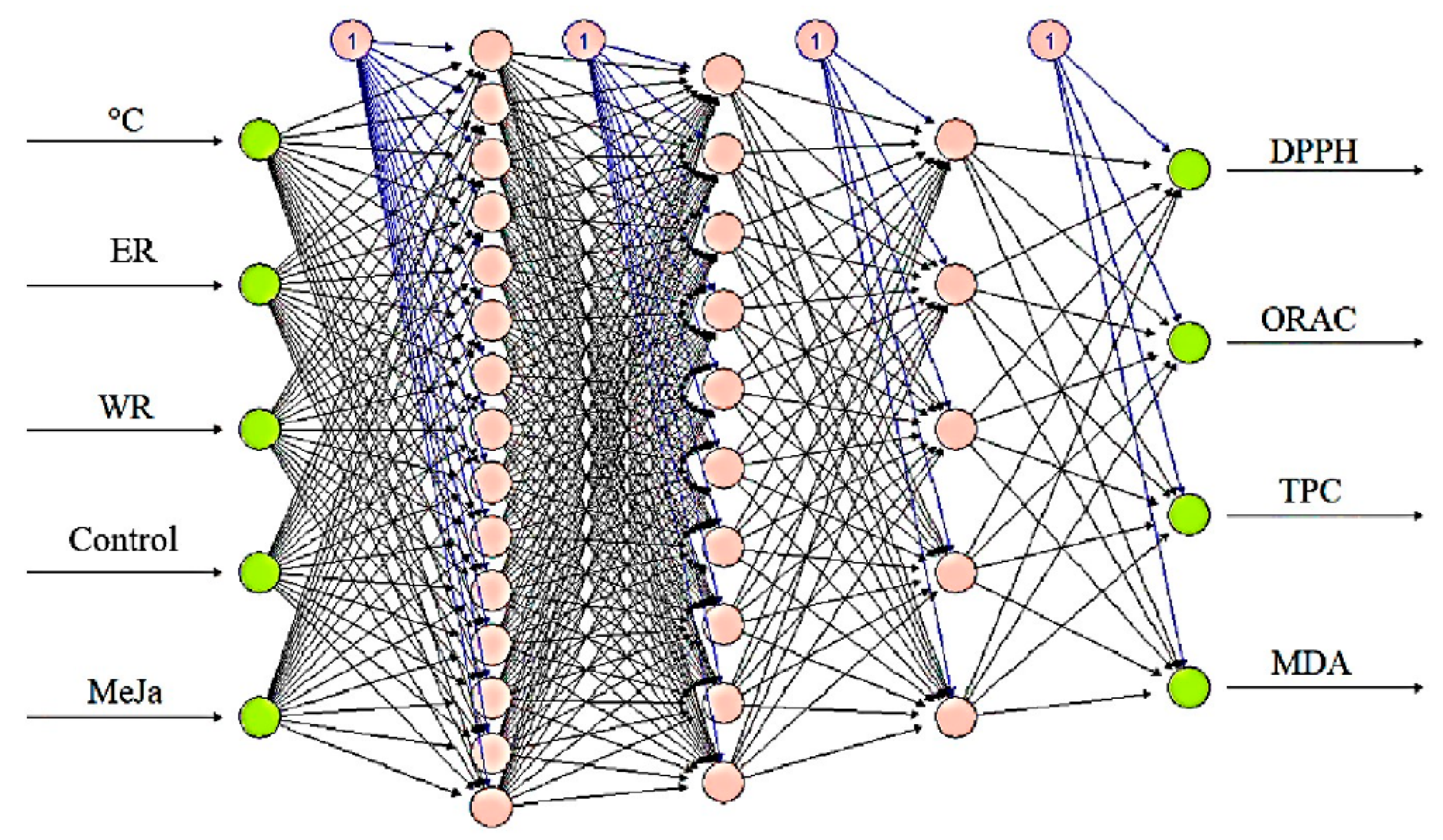
3.5. Artificial Neural Networks (ANNs) Modeling to Predict the Impact of High Temperatures and MeJa Exogenous Application
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schroeder, J. Wild radish (Raphanus raphanistrum) control in soft red winter wheat (Triticum aestivum). Weed Sci. 1989, 37, 112–116. [Google Scholar] [CrossRef]
- Hedge, I.; Vaughan, J.; Macleod, A.; Jones, B. The Biology and Chemistry of the Cruciferae; Vaughan, J.G., Ed.; Acadaemic Press: London, UK, 1976. [Google Scholar]
- Baenas, N.; García-Viguera, C.; Moreno, D.A. Biotic elicitors effectively increase the glucosinolates content in Brassicaceae sprouts. J. Agric. Food Chem. 2014, 62, 1881–1889. [Google Scholar] [CrossRef]
- Pérez-Balibrea, S.; Moreno, D.A.; García-Viguera, C. Influence of light on health-promoting phytochemicals of broccoli sprouts. J. Sci. Food Agric. 2008, 88, 904–910. [Google Scholar] [CrossRef]
- Natella, F.; Maldini, M.; Nardini, M.; Azzini, E.; Foddai, M.S.; Giusti, A.M.; Baima, S.; Morelli, G.; Scaccini, C. Improvement of the nutraceutical quality of broccoli sprouts by elicitation. Food Chem. 2016, 201, 101–109. [Google Scholar] [CrossRef]
- Plumb, G.W.; Lambert, N.; Chambers, S.J.; Wanigatunga, S.; Heaney, R.K.; Plumb, J.A.; Aruoma, O.I.; Halliwell, B.; Miller, N.J.; Williamson, G. Are whole extracts and purified glucosinolates from cruciferous vegetables antioxidants? Free Radic. Res. 1996, 25, 75–86. [Google Scholar] [CrossRef]
- Baenas, N.; Villaño, D.; García-Viguera, C.; Moreno, D.A. Optimizing elicitation and seed priming to enrich broccoli and radish sprouts in glucosinolates. Food Chem. 2016, 204, 314–319. [Google Scholar] [CrossRef] [PubMed]
- Dinkova-Kostova, A.T.; Kostov, R.V. Glucosinolates and isothiocyanates in health and disease. Trends Mol. Med. 2012, 18, 337–347. [Google Scholar] [CrossRef] [PubMed]
- Björkman, M.; Klingen, I.; Birch, A.N.; Bones, A.M.; Bruce, T.J.; Johansen, T.J.; Meadow, R.; Mølmann, J.; Seljåsen, R.; Smart, L.E. Phytochemicals of Brassicaceae in plant protection and human health–Influences of climate, environment and agronomic practice. Phytochemistry 2011, 72, 538–556. [Google Scholar] [CrossRef]
- de Pascual-Teresa, S.; Sanchez-Ballesta, M.T. Anthocyanins: From plant to health. Phytochem. Rev. 2008, 7, 281–299. [Google Scholar] [CrossRef]
- Verma, N.; Shukla, S. Impact of various factors responsible for fluctuation in plant secondary metabolites. J. Appl. Res. Med. Aromat. Plants 2015, 2, 105–113. [Google Scholar] [CrossRef]
- Liu, H.; Kang, Y.; Zhao, X.; Liu, Y.; Zhang, X.; Zhang, S. Effects of elicitation on bioactive compounds and biological activities of sprouts. J. Funct. Foods 2019, 53, 136–145. [Google Scholar] [CrossRef]
- Baenas, N.; García-Viguera, C.; Moreno, D.A. Elicitation: A tool for enriching the bioactive composition of foods. Molecules 2014, 19, 13541–13563. [Google Scholar] [CrossRef] [PubMed]
- Hoegh-Guldberg, O.; Jacob, D.; Bindi, M.; Brown, S.; Camilloni, I.; Diedhiou, A.; Djalante, R.; Ebi, K.; Engelbrecht, F.; Guiot, J. Impacts of 1.5 C global warming on natural and human systems. In Global Warming of 1.5 °C; IPCC: Geneva, Switzerland, 2018. [Google Scholar]
- Serrano, N.; Ling, Y.; Bahieldin, A.; Mahfouz, M.M. Thermopriming reprograms metabolic homeostasis to confer heat tolerance. Sci. Rep. 2019, 9, 181. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.-H.; Park, S.; Lim, Y.P.; Kim, S.-J.; Park, J.-T.; An, G. Metabolite profiles of glucosinolates in cabbage varieties (Brassica oleracea var. capitata) by season, color, and tissue position. Hortic. Environ. Biotechnol. 2014, 55, 237–247. [Google Scholar] [CrossRef]
- Sim, H.S.; Jo, J.S.; Woo, U.J.; Jo, W.J.; Moon, Y.H.; Lee, J.G.; Lee, H.J.; Wi, S.H.; Kim, S.K. Abscisic acid, carbohydrate, and Glucosinolate metabolite profiles in Kimchi cabbage treated with extremely high temperatures and chitosan foliar application. Sci. Hortic. 2022, 304, 111311. [Google Scholar]
- Toro, M.T.; Ortiz, J.; Becerra, J.; Zapata, N.; Fierro, P.; Illanes, M.; López, M.D. Strategies of elicitation to enhance bioactive compound content in edible plant sprouts: A bibliometric study. Plants 2021, 10, 2759. [Google Scholar] [CrossRef] [PubMed]
- Thakur, M.; Bhattacharya, S.; Khosla, P.K.; Puri, S. Improving production of plant secondary metabolites through biotic and abiotic elicitation. J. Appl. Res. Med. Aromat. Plants 2019, 12, 1–12. [Google Scholar] [CrossRef]
- Vale, A.P.; Santos, J.; Brito, N.V.; Fernandes, D.; Rosa, E.; Oliveira, M.B.P. Evaluating the impact of sprouting conditions on the glucosinolate content of Brassica oleracea sprouts. Phytochemistry 2015, 115, 252–260. [Google Scholar] [CrossRef]
- Pérez-Balibrea, S.; Moreno, D.A.; García-Viguera, C. Improving the phytochemical composition of broccoli sprouts by elicitation. Food Chem. 2011, 129, 35–44. [Google Scholar] [CrossRef]
- Mahdavian, K. Effect of citric acid on antioxidant activity of red bean (Phaseolus calcaratus L.) under Cr+ 6 stress. South Afr. J. Bot. 2021, 139, 83–91. [Google Scholar] [CrossRef]
- Hassini, I.; Rios, J.J.; Garcia-Ibañez, P.; Baenas, N.; Carvajal, M.; Moreno, D.A. Comparative effect of elicitors on the physiology and secondary metabolites in broccoli plants. J. Plant Physiol. 2019, 239, 1–9. [Google Scholar] [CrossRef]
- Hassini, I.; Baenas, N.; Moreno, D.A.; Carvajal, M.; Boughanmi, N.; Martinez Ballesta, M.D.C. Effects of seed priming, salinity and methyl jasmonate treatment on bioactive composition of Brassica oleracea var. capitata (white and red varieties) sprouts. J. Sci. Food Agric. 2017, 97, 2291–2299. [Google Scholar] [CrossRef]
- Ling, L.; Jiafeng, J.; Jiangang, L.; Minchong, S.; Xin, H.; Hanliang, S.; Yuanhua, D. Effects of cold plasma treatment on seed germination and seedling growth of soybean. Sci. Rep. 2014, 4, 5859. [Google Scholar] [CrossRef]
- Hodges, D.M.; DeLong, J.M.; Forney, C.F.; Prange, R.K. Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta 1999, 207, 604–611. [Google Scholar] [CrossRef]
- Heath, R.L.; Packer, L. Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Lopez, M.; Toro, M.; Riveros, G.; Illanes, M.; Noriega, F.; Schoebitz, M.; García-Viguera, C.; Moreno, D. Brassica sprouts exposed to microplastics: Effects on phytochemical constituents. Sci. Total Environ. 2022, 823, 153796. [Google Scholar] [CrossRef]
- Baenas, N.; Ferreres, F.; García-Viguera, C.; Moreno, D.A. Radish sprouts—Characterization and elicitation of novel varieties rich in anthocyanins. Food Res. Int. 2015, 69, 305–312. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- López, M.D.; Baenas, N.; Retamal-Salgado, J.; Zapata, N.; Moreno, D.A. Underutilized native Biobio berries: Opportunities for foods and trade. Nat. Prod. Commun. 2018, 13, 1934578X1801301226. [Google Scholar] [CrossRef]
- Mena, P.; García-Viguera, C.; Navarro-Rico, J.; Moreno, D.A.; Bartual, J.; Saura, D.; Martí, N. Phytochemical characterisation for industrial use of pomegranate (Punica granatum L.) cultivars grown in Spain. J. Sci. Food Agric. 2011, 91, 1893–1906. [Google Scholar] [CrossRef]
- Idris, S.A.; Markom, M.; Abd Rahman, N.; Ali, J.M. Prediction of overall yield of Gynura procumbens from ethanol-water+ supercritical CO2 extraction using artificial neural network model. Case Stud. Chem. Environ. Eng. 2022, 5, 100175. [Google Scholar] [CrossRef]
- Malik, M.S.; Riley, M.B.; Norsworthy, J.K.; Bridges Jr, W. Variation of glucosinolates in wild radish (Raphanus raphanistrum) accessions. J. Agric. Food Chem. 2010, 58, 11626–11632. [Google Scholar] [CrossRef] [PubMed]
- Tahjib-Ul-Arif, M.; Zahan, M.I.; Karim, M.M.; Imran, S.; Hunter, C.T.; Islam, M.S.; Mia, M.A.; Hannan, M.A.; Rhaman, M.S.; Hossain, M.A. Citric acid-mediated abiotic stress tolerance in plants. Int. J. Mol. Sci. 2021, 22, 7235. [Google Scholar] [CrossRef] [PubMed]
- Randhir, R.; Lin, Y.-T.; Shetty, K. Stimulation of phenolics, antioxidant and antimicrobial activities in dark germinated mung bean sprouts in response to peptide and phytochemical elicitors. Process Biochem. 2004, 39, 637–646. [Google Scholar] [CrossRef]
- Ayala, A.; Muñoz, M.F.; Argüelles, S. Lipid peroxidation: Production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxidative Med. Cell. Longev. 2014, 2014, 360438. [Google Scholar] [CrossRef]
- Ali, S.; Khan, A.S.; Anjum, M.A.; Nawaz, A.; Naz, S.; Ejaz, S.; Hussain, S. Aloe vera gel coating delays post-cut surface browning and maintains quality of cold stored lotus (Nelumbo nucifera Gaertn.) root slices. Sci. Hortic. 2019, 256, 108612. [Google Scholar] [CrossRef]
- Zhou, C.; Zhu, Y.; Luo, Y. Effects of sulfur fertilization on the accumulation of health-promoting phytochemicals in radish sprouts. J. Agric. Food Chem. 2013, 61, 7552–7559. [Google Scholar] [CrossRef]
- Shahrajabian, M.H.; Chaski, C.; Polyzos, N.; Tzortzakis, N.; Petropoulos, S.A. Sustainable agriculture systems in vegetable production using chitin and chitosan as plant biostimulants. Biomolecules 2021, 11, 819. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Arasu, M.V.; Lee, M.-K.; Chun, J.-H.; Seo, J.M.; Lee, S.-W.; Al-Dhabi, N.A.; Kim, S.-J. Quantification of glucosinolates, anthocyanins, free amino acids, and vitamin C in inbred lines of cabbage (Brassica oleracea L.). Food Chem. 2014, 145, 77–85. [Google Scholar] [CrossRef]
- Matera, R.; Gabbanini, S.; De Nicola, G.R.; Iori, R.; Petrillo, G.; Valgimigli, L. Identification and analysis of isothiocyanates and new acylated anthocyanins in the juice of Raphanus sativus cv. Sango sprouts. Food Chem. 2012, 133, 563–572. [Google Scholar] [CrossRef]
- De Pascale, S.; Maggio, A.; Pernice, R.; Fogliano, V.; Barbieri, G. Sulphur fertilization may improve the nutritional value of Brassica rapa L. subsp. sylvestris. Eur. J. Agron. 2007, 26, 418–424. [Google Scholar] [CrossRef]
- Kumar, I.; Sharma, R.K. Production of secondary metabolites in plants under abiotic stress: An overview. Significances Bioeng. Biosci. 2018, 2, 196–200. [Google Scholar] [CrossRef]
- Akula, R.; Ravishankar, G.A. Influence of abiotic stress signals on secondary metabolites in plants. Plant Signal. Behav. 2011, 6, 1720–1731. [Google Scholar] [CrossRef] [PubMed]
- Mondal, M.; Puteh, A.; Dafader, N.; Rafii, M.; Malek, M. Foliar application of chitosan improves growth and yield in maize. J. Food Agric. Environ. 2013, 11, 520–523. [Google Scholar]
- Sakamoto, M.; Suzuki, T. Methyl jasmonate and salinity increase anthocyanin accumulation in radish sprouts. Horticulturae 2019, 5, 62. [Google Scholar] [CrossRef]
- Liu, R.; Wang, Z.; Zheng, J.; Xu, Z.; Tang, X.; Huang, Z.; Zhang, N.; Dong, Y.; Li, T. The effects of methyl jasmonate on growth, gene expression and metabolite accumulation in Isatis indigotica Fort. Ind. Crops Prod. 2022, 177, 114482. [Google Scholar] [CrossRef]
- Świeca, M.; Sęczyk, Ł.; Gawlik-Dziki, U. Elicitation and precursor feeding as tools for the improvement of the phenolic content and antioxidant activity of lentil sprouts. Food Chem. 2014, 161, 288–295. [Google Scholar] [CrossRef]
- d’Hooghe, P.; Dubousset, L.; Gallardo, K.; Kopriva, S.; Avice, J.-C.; Trouverie, J. Evidence for proteomic and metabolic adaptations associated with alterations of seed yield and quality in sulfur-limited Brassica napus L. Mol. Cell. Proteom. 2014, 13, 1165–1183. [Google Scholar] [CrossRef] [PubMed]
- Falk, K.L.; Tokuhisa, J.G.; Gershenzon, J. The effect of sulfur nutrition on plant glucosinolate content: Physiology and molecular mechanisms. Plant Biol. 2007, 9, 573–581. [Google Scholar] [CrossRef]
- Narayan, O.P.; Kumar, P.; Yadav, B.; Dua, M.; Johri, A.K. Sulfur nutrition and its role in plant growth and development. Plant Signal. Behav. 2022, 18, 2030082. [Google Scholar] [CrossRef]
- Borpatragohain, P.; Rose, T.J.; Liu, L.; Barkla, B.J.; Raymond, C.A.; King, G.J. Remobilization and fate of sulphur in mustard. Ann. Bot. 2019, 124, 471–480. [Google Scholar] [CrossRef]
- Hoefgen, R.; Nikiforova, V.J. Metabolomics integrated with transcriptomics: Assessing systems response to sulfur-deficiency stress. Physiol. Plant. 2008, 132, 190–198. [Google Scholar] [CrossRef]
- Chiu, Y.-C.; Matak, K.; Ku, K.-M. Methyl jasmonate treated broccoli: Impact on the production of glucosinolates and consumer preferences. Food Chem. 2019, 299, 125099. [Google Scholar] [CrossRef]
- Berger, B. The Role of HIG1/MYB51 in the Regulation of Indolic Glucosinolate Biosynthesis; Universität zu Köln: Köln, Germany, 2007. [Google Scholar]
- Manivannan, A.; Kim, J.-H.; Kim, D.-S.; Lee, E.-S.; Lee, H.-E. Deciphering the nutraceutical potential of Raphanus sativus—A comprehensive overview. Nutrients 2019, 11, 402. [Google Scholar] [CrossRef]
- Ishida, M.; Hara, M.; Fukino, N.; Kakizaki, T.; Morimitsu, Y. Glucosinolate metabolism, functionality and breeding for the improvement of Brassicaceae vegetables. Breed. Sci. 2014, 64, 48–59. [Google Scholar] [CrossRef]
- Textor, S.; Gershenzon, J. Herbivore induction of the glucosinolate–myrosinase defense system: Major trends, biochemical bases and ecological significance. Phytochem. Rev. 2009, 8, 149–170. [Google Scholar] [CrossRef]
- Martínez-Zamora, L.; Castillejo, N.; Artés-Hernández, F. Postharvest UV-B and UV-C radiation enhanced the biosynthesis of glucosinolates and isothiocyanates in Brassicaceae sprouts. Postharvest Biol. Technol. 2021, 181, 111650. [Google Scholar] [CrossRef]
- Hanlon, P.R.; Barnes, D.M. Phytochemical composition and biological activity of 8 varieties of radish (Raphanus sativus L.) sprouts and mature taproots. J. Food Sci. 2011, 76, C185–C192. [Google Scholar] [CrossRef] [PubMed]
- Wasternack, C.; Strnad, M. Jasmonates are signals in the biosynthesis of secondary metabolites—Pathways, transcription factors and applied aspects—A brief review. New Biotechnol. 2019, 48, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Portu, J.; Santamaría, P.; López-Alfaro, I.; López, R.; Garde-Cerdán, T. Methyl jasmonate foliar application to Tempranillo vineyard improved grape and wine phenolic content. J. Agric. Food Chem. 2015, 63, 2328–2337. [Google Scholar] [CrossRef] [PubMed]
- Mubeen, B.; Hasnain, A.; Jie, W.; Zheng, H.; Peijnenburg, W.J.; Rozali, S.E.; Rasool, R.; Naqvi, S.A.H.; Rao, M.J.; Sohail, M.A. Enhanced Production of Active Photosynthetic and Biochemical Molecules in Silybum marianum L. Using Biotic and Abiotic Elicitors in Hydroponic Culture. Molecules 2023, 28, 1716. [Google Scholar] [CrossRef] [PubMed]
- Abou Yeo, N.; Gogbeu, D.G.L.; Tuo, S.; Coulibaly, I.; Konan, Y.K.F.; Silué, N.; Kouakou, T.H.; Zouzou, M. Effect of elicitation of cotton [(Gossypium hirsutum L.) cv. Y331B] with methyl jasmonate and ethephon in relation to secondary metabolites. Int. J. Multidiscip. Res. Growth Eval. 2022, 3, 278–284. [Google Scholar]

| Edible Radish | |||||||
| Treatments | Glucosinolates (mg 100 g −1 DW) | ||||||
| Elicitor | Temperature | GRE | 4-HGB | DER | GB | MGB | TGLs |
| C1 | 20 °C | 32.17 ± 7.016 cd | 5.20 ± 0.928 bc | 7.52 ± 0.627 ab | 2.93 ± 0.473 e | 3.19 ± 0.545 a | 51.01 ± 9.588 de |
| C2 | 30 °C | 34.92 ± 8.018 cd | 4.92 ± 0.589 bcd | 8.49 ± 0.990 a | 4.41 ± 0.285 d | 0.78 ± 0.303 c | 53.44 ± 9.233 de |
| Citric acid | 20 °C | 34.56 ± 3.285 cd | 5.17 ± 0.918 bc | 5.34 ± 0.147 d | 2.85 ± 0.377 e | 2.81 ± 0.115 a | 50.72 ± 4.842 de |
| Citric acid | 30 °C | 43.38 ± 3.421 bc | 3.96 ± 0.091 cd | 7.19 ± 0.743 abc | 2.46 ± 0.324 e | 1.56 ± 0.212 bc | 58.55 ± 4.791 cd |
| MeJa | 20 °C | 41.05 ± 4.536 bc | 3.01 ± 0.793 d | 7.63 ± 0.499 ab | 8.03 ± 0.585 b | 1.85 ± 0.312 ab | 61.56 ± 6.725 bcd |
| MeJa | 30 °C | 43.45 ± 3.595 bc | 7.84 ± 0.969 a | 6.58 ± 0.418 bcd | 10.22 ± 0.400 a | 1.01 ± 0.325 c | 69.10 ± 5.707 abc |
| Chitosan | 20 °C | 26.69 ± 3.540 d | 3.44 ± 1.189 cd | 5.35 ± 0.501 d | 2.09 ± 0.408 e | 2.418 ± 0.719 ab | 39.98 ± 6.358 e |
| Chitosan | 30 °C | 49.92 ± 8.414 b | 3.40 ± 0.581 cd | 7.15 ± 0.812 abc | 2.18 ± 0.190 e | 1.57 ± 0.267 bc | 64.24 ± 6.765 bcd |
| K2SO4 | 20 °C | 54.243 ± 2.804 ab | 3.05 ± 0.766 d | 8.03 ± 0.611 ab | 6.29 ± 0.931 c | 2.46 ± 0.300 ab | 74.08 ± 5.412 ab |
| K2SO4 | 30 °C | 64.43 ± 3.062 a | 6.78 ± 1.017 ab | 5.83 ± 0.262 cd | 2.25 ± 0.312 e | 2.47 ± 0.251 bc | 81.77 ± 4.904 a |
| Significance | |||||||
| Temperature (A) | *** | *** | NS | NS | *** | *** | |
| Elicitor (B) | *** | ** | *** | *** | *** | *** | |
| A × B | ** | *** | *** | *** | *** | * | |
| Wild Radish | |||||||
| Treatments | Glucosinolates (mg 100 g−1 DW) | ||||||
| Elicitor | Temperature | GRE | 4-HGB | DER | GB | MGB | TGLs |
| C1 | 20 °C | 46.56 ± 0.265 def | 0.95 ± 0.239 d | 2.52 ± 0.252 c | 2.50 ± 0.565 e | 0.73 ± 0.404 d | 53.27 ± 1.705 d |
| C2 | 30 °C | 36.17 ± 6.361 f | 0.86 ± 0.093 d | 1.13 ± 0.272 d | 5.33 ± 0.173 cd | 1.33 ± 0.231 cd | 44.82 ± 7.113 d |
| Citric acid | 20 °C | 57.16 ± 5.697 bcd | 3.57 ± 0.722 a | 1.53 ± 0.601 d | 5.93 ± 0.382 c | 1.22 ± 0.355 cd | 69.42 ± 7.740 c |
| Citric acid | 30 °C | 41.92 ± 3.859 ef | 1.07 ± 0.510 d | 4.58 ± 0.330 a | 2.98 ± 0.746 de | 0.92 ± 0.304 cd | 51.47 ± 5.732 d |
| MeJa | 20 °C | 54.65 ± 2.474 cde | 1.94 ± 0.520 cd | 4.43 ± 0.129 a | 17.64 ± 0.876 a | 1.74 ± 0.643 bcd | 80.01 ± 4.873 bc |
| MeJa | 30 °C | 50.97 ± 7.603 cde | 1.45 ± 0.680 cd | 4.18 ± 0.229 ab | 16.41 ± 0.876 a | 1.56 ± 0.613 bcd | 74.57 ± 9.002 bc |
| Chitosan | 20 °C | 60.88 ± 5.338 bc | 1.25 ± 0.360 cd | 2.61 ± 0.092 c | 5.67 ± 1.073 cd | 2.90 ± 0.603 a | 73.30 ± 7.453 c |
| Chitosan | 30 °C | 60.81 ± 5.547 bc | 3.83 ± 0.447 a | 2.71 ± 0.566 c | 4.08 ± 0.336 cde | 1.49 ± 0.502 bcd | 72.92 ± 7.372 c |
| K2SO4 | 20 °C | 89.22 ± 3.996 a | 3.37 ± 0.327 ab | 3.45 ± 0.415 bc | 4.92 ± 0.927 cde | 2.59 ± 0.436 ab | 103.54 ± 6.073 a |
| K2SO4 | 30 °C | 68.60 ± 6.657 b | 2.31 ± 0.273 bc | 3.70 ± 0.414 ab | 12.37 ± 2.051 b | 2.03 ± 0.592 abc | 89.01 ± 9.988 ab |
| Significance | |||||||
| Temperature (A) | *** | * | ** | * | ** | *** | |
| Elicitor (B) | *** | *** | *** | *** | *** | *** | |
| A × B | ** | *** | *** | *** | ** | * | |
| Edible Radish | ||||||||||
| Treatments | Anthocyanins (mg 100 g−1 DW) | |||||||||
| Elicitor | Temperature | 1-Cy | 2-Cy | 3-Cy | 4-Cy | 5-Cy | 6-Cy | 7-Cy | 8-Cy | TAC |
| C1 | 20 °C | 1.00 ± 0.045 a | 0.51 ± 0.030 ab | 0.42 ± 0.039 b | 1.80 ± 0.152 b | 0.27 ± 0.036 bc | 0.11 ± 0.012 bc | 0.16 ± 0.011 b | 0.05 ± 0.012 ab | 4.33 ± 0.302 c |
| C2 | 30 °C | 0.08 ± 0.032 d | 0.25 ± 0.132 cd | 0.12 ± 0.012 b | 0.92 ± 0.144 cd | 0.69 ± 0.493 a | 0.17 ± 0.056 b | NP | NP | 2.22 ± 0.768 de |
| Citric acid | 20 °C | 0.36 ± 0.042 bcd | 0.36 ± 0.062 bc | 0.34 ± 0.020 b | 1.13 ± 0.296 bc | 0.16 ± 0.073 bc | 0.08 ± 0.029 bc | 0.14 ± 0.039 b | 0.05 ± 0.029 ab | 2.61 ± 0.624 d |
| Citric acid | 30 °C | 0.12 ± 0.031 d | 0.12 ± 0.011 d | 0.26 ± 0.096 b | 0.21 ± 0.062 de | 0.03 ± 0.011 c | 0.01 ± 0.004 c | 0.03 ± 0.022 d | NP | 0.78 ± 0.364 f |
| MeJa | 20 °C | 0.71 ± 0.101 ab | 0.48 ± 0.086 ab | 0.82 ± 0.060 a | 3.85 ± 0.489 a | 0.27 ± 0.021 bc | 0.38 ± 0.057 a | 0.33 ± 0.053 a | 0.07 ± 0.010 a | 6.91 ± 0.500 a |
| MeJa | 30 °C | 0.31 ± 0.056 cd | 0.57 ± 0.017 a | 0.40 ± 0.141 b | 3.44 ± 0.521 a | 0.40 ± 0.113 ab | 0.34 ± 0.108 a | 0.02 ± 0.012 d | 0.01 ± 0.011 cd | 5.49 ± 0.696 b |
| Chitosan | 20 °C | 0.57 ± 0.153 bc | 0.16 ± 0.043 d | 0.24 ± 0.031 b | 1.09 ± 0.282 bc | 0.11 ± 0.017 bc | 0.08 ± 0.013 bc | 0.14 ± 0.032 b | 0.04 ± 0.006 abc | 2.42 ± 0.391 d |
| Chitosan | 30 °C | 0.17 ± 0.020 d | 0.11 ± 0.010 d | 0.10 ± 0.018 b | 0.14 ± 0.010 de | 0.04 ± 0.013 c | 0.03 ± 0.013 c | 0.03 ± 0.007 d | NP | 0.62 ± 0.067 f |
| K2SO4 | 20 °C | 0.13 ± 0.032 d | 0.25 ± 0.095 cd | 0.24 ± 0.081 b | 0.78 ± 0.291 cde | 0.09 ± 0.037 bc | 0.05 ± 0.024 c | 0.11 ± 0.029 bc | 0.02 ± 0.015 bcd | 1.67 ± 0.393 def |
| K2SO4 | 30 °C | 0.04 ± 0.015 d | 0.13 ± 0.022 d | 0.17 ± 0.033 b | 0.51 ± 0.091 cde | 0.15 ± 0.022 bc | 0.10 ± 0.009 bc | 0.05 ± 0.010 cd | NP | 1.13 ± 0.098 ef |
| Significance | ||||||||||
| Temperature (A) | *** | *** | ** | *** | NS | NS | *** | *** | *** | |
| Elicitor (B) | *** | *** | *** | *** | *** | *** | *** | ** | *** | |
| A × B | ** | *** | NS | NS | * | * | *** | NS | * | |
| Treatments | Anthocyanins (mg 100 g−1 DW) | |||||||||
| Elicitor | Temperature | 1-Cy | 2-Cy | 3-Cy | 4-Cy | 5-Cy | 6-Cy | 7-Cy | 8-Cy | TAC |
| C1 | 20 °C | 0.04 ± 0.017 d | 0.08 ± 0.025 bc | 0.39 ± 0.077 b | 1.47 ± 0.342 bc | 0.02 ± 0.021 d | 0.09 ± 0.011 cd | 0.01 ± 0.006 bc | 0.06 ± 0.021 ab | 2.16 ± 0.426 bcd |
| C2 | 30 °C | 0.28 ± 0.043 ab | 0.12 ± 0.033 ab | 0.57 ± 0.106 ab | 1.86 ± 0.571 b | 0.09 ± 0.01 bcd | 0.07 ± 0.004 cd | 0.04 ± 0.015 ab | 0.03 ± 0.004 abc | 3.06 ± 0.676 b |
| Citric acid | 20 °C | 0.14 ± 0.011 cd | 0.04 ± 0.009 c | 0.30 ± 0.055 b | 1.22 ± 0.276 bcd | 0.05 ± 0.014 cd | 0.11 ± 0.018 bcd | 0.02 ± 0.006 bc | 0.01 ± 0.001 cd | 1.87 ± 0.367 cd |
| Citric acid | 30 °C | 0.35 ± 0.063 a | 0.17 ± 0.011 a | 0.70 ± 0.145 a | 0.86 ± 0.155 cd | 0.15 ± 0.019 b | 0.18 ± 0.067 ab | 0.04 ± 0.013 ab | 0.03 ± 0.027 abc | 2.50 ± 0.303 bc |
| MeJa | 20 °C | 0.14 ± 0.029 cd | 0.08 ± 0.025 bc | 0.70 ± 0.168 a | 3.61 ± 0.360 a | 0.12 ± 0.028 bc | 0.20 ± 0.056 a | 0.07 ± 0.022 a | 0.04 ± 0.006 ab | 4.96 ± 0.500 a |
| MeJa | 30 °C | 0.11 ± 0.015 cd | 0.08 ± 0.013 bc | 0.75 ± 0.138 a | 4.14 ± 0.571 a | 0.23 ± 0.086 a | 0.22 ± 0.052 a | 0.05 ± 0.02 ab | 0.06 ± 0.015 a | 5.64 ± 0.649 a |
| Chitosan | 20 °C | 0.33 ± 0.039 a | 0.12 ± 0.011 ab | 0.42 ± 0.054 b | 0.44 ± 0.093 d | 0.05 ± 0.006 cd | 0.02 ± 0.006 d | NP | NP | 1.39 ± 0.060 d |
| Chitosan | 30 °C | 0.27 ± 0.079 ab | 0.13 ± 0.047 ab | 0.71 ± 0.120 a | 0.74 ± 0.159 cd | 0.07 ± 0.008 cd | 0.14 ± 0.005 abc | 0.02 ± 0.005 bc | 0.03 ± 0.01 bcd | 2.11 ± 0.274 bcd |
| K2SO4 | 20 °C | 0.19 ± 0.029 bc | 0.08 ± 0.020 bc | 0.42 ± 0.047 b | 1.45 ± 0.197 bc | 0.08 ± 0.017 bcd | 0.04 ± 0.005 d | 0.03 ± 0.017 abc | NP | 2.29 ± 0.231 bcd |
| K2SO4 | 30 °C | 0.05 ± 0.021 d | 0.08 ± 0.011 bc | 0.36 ± 0.058 b | 1.38 ± 0.114 bc | 0.08 ± 0.013 bcd | 0.19 ± 0.046 ab | 0.06 ± 0.025 a | NP | 2.22 ± 0.079 bcd |
| Significance | ||||||||||
| Temperature (A) | *** | *** | *** | NS | NS | NS | * | NS | * | |
| Elicitor (B) | *** | ** | *** | *** | *** | *** | *** | *** | *** | |
| A × B | *** | *** | ** | * | *** | *** | ** | ** | ** | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Toro, M.-T.; Fustos-Toribio, R.; Ortiz, J.; Becerra, J.; Zapata, N.; López-Belchí, M.D. Antioxidant Responses and Phytochemical Accumulation in Raphanus Species Sprouts through Elicitors and Predictive Models under High Temperature Stress. Antioxidants 2024, 13, 333. https://doi.org/10.3390/antiox13030333
Toro M-T, Fustos-Toribio R, Ortiz J, Becerra J, Zapata N, López-Belchí MD. Antioxidant Responses and Phytochemical Accumulation in Raphanus Species Sprouts through Elicitors and Predictive Models under High Temperature Stress. Antioxidants. 2024; 13(3):333. https://doi.org/10.3390/antiox13030333
Chicago/Turabian StyleToro, María-Trinidad, Roberto Fustos-Toribio, Jaime Ortiz, José Becerra, Nelson Zapata, and María Dolores López-Belchí. 2024. "Antioxidant Responses and Phytochemical Accumulation in Raphanus Species Sprouts through Elicitors and Predictive Models under High Temperature Stress" Antioxidants 13, no. 3: 333. https://doi.org/10.3390/antiox13030333
APA StyleToro, M.-T., Fustos-Toribio, R., Ortiz, J., Becerra, J., Zapata, N., & López-Belchí, M. D. (2024). Antioxidant Responses and Phytochemical Accumulation in Raphanus Species Sprouts through Elicitors and Predictive Models under High Temperature Stress. Antioxidants, 13(3), 333. https://doi.org/10.3390/antiox13030333