Abstract
The increasing world population means an increased demand for sustainable processes and products related to foods, particularly those with added health benefits. Plants can be an alternative source of nutritional and biofunctional ingredients. Cytisus plants are an underexploited bioresource, currently prevalent in the Mediterranean Basin and western Asia. This manuscript addresses the processing potential of Cytisus plants for the development of added-value products, including food formulations, food packaging, cosmetics, and therapeutic applications. Most research has reported that Cytisus spp. are a promising source of inexpensive bioactive polyphenol compounds. Cytisus flowers should be considered and exploited as raw materials for the development of new food ingredients (antioxidants, preservatives, additives, etc.), nutraceuticals, or even direct therapeutic agents (anticancer, antibacterial, etc.). In order to evaluate the socioeconomic effect of these underutilized plants, more research is needed to assess their valorization for therapeutic and dietary possibilities, as well as the economic impact.
1. Introduction
As the global population increases (by 2050, the world population may reach 9–10 billion), higher demand for more feedstock for food, pharmaceuticals, energetic alternatives, and attractive bioproducts is expected. Therefore, it is critical that industrial processes and resulting products grow according to sustainable practices relative to global demand [1,2].
Nowadays, the handling and processing of underexploited plants by-products and waste do not assure efficient use of these low-cost bioresources, even though some minor uses, such as biomass production and animal feed, has been reported. Plant by-products and waste can offer innovative possibilities for the development of added-value products and a reduction in their environmental impact [3]. It is indispensable to further reinforce the combination of suitable strategies to reduce, reprocess, and recycle organic agri-food biomass. These strategies will lead to their integral use and obtention of high-value ingredients, generating a minimal part of untreated and underused agro-industrial biomass, which can be discarded through incineration or uncontrolled landfill [4]. The reprocessing of plant biomass allows for added value of this bioresources and supports the bioeconomy. Plants and their by-products (e.g., bark, peels, pomace, seeds, flowers, etc.) are a potential source of nutritional and non-nutritional compounds. Among other constituents, the ones with the highest biofunctional and nutritional value should be highlighted, particularly primary metabolites including dietary fibers, proteins, and polysaccharides and bioactive secondary metabolites, i.e., phytochemicals like polyphenolics, carotenoids, alkaloids, and terpenes [5].
Plants of the genus Cytisus, better known as brooms, are the focus of this review article. The genus Cytisus includes about 70 species belonging to the family Fabaceae. Brooms are flowering shrubs, subshrubs, or small trees with alternate branches and unifoliate to trifoliate leaves, deciduous to evergreen. Flowers are single or paired up to four leaf axils, usually with a two-lipped, top lip minutely toothed calyx. The fruits are mostly typical pods with more or less explosive (and valvular) dehiscence via dorsal and ventral sutures (Figure 1) [6]. These plants are distributed all over the world, being prevalent to open sites (typically scrub and heathland) of the Mediterranean Basin (Europe and North Africa) and western Asia [7].
Cytisus plants are of agricultural interest, allowing nitrogen fixation in soils due to Rhizobium nodules on roots. However, in recent years, they are starting to present a problem for vegetation in many countries, and are considered weeds. It has been noted that these species have great expression worldwide due to their rapid growth, creating dense stands. This evidence can lead to problems such as inaccessibility for wildlife and the risk of fires [6]. Thus, in some countries, Cytisus plants are undervalued and considered a threat to other species; however, they can be an interesting source of valuable compounds [8,9]. In addition, plants of this genus have been traditionally used for infusions and decoctions, due to their significant number of therapeutic properties [10,11,12]. These properties seem to be related to their rich content of bioactive constituents, like polyphenolic compounds [9,13].
As mentioned before, the need for food products with a “clean label”, as well as nutraceuticals and natural drugs (pharmaceuticals), motivated by important factors such as health and sustainability, are the cause of the boom in research in this field. The consumer’s interest in natural, functional, and health-promoting products that are plant-based and obtained through environmentally friendly processes (green technologies) is pressuring this area of research. Therefore, the valorization of underexplored plants and the reuse of agricultural residues, in this case Cytisus plants, may be a viable option to develop products of interest for the food and pharmaceutical industries, such as antioxidants, dyes, flavorings, antimicrobial agents, or isolated drugs with highlighted therapeutic potential.
This review is dedicated to generating comprehensive information for sustainable valorization of the underexploited Cytisus spp. plants. Hence, this manuscript addresses different species of Cytisus plants and their sustainable processing for a green recovery of bioactive polyphenols, as well as the development of valuable products containing these compounds, that can be used worldwide for food and therapeutic applications.
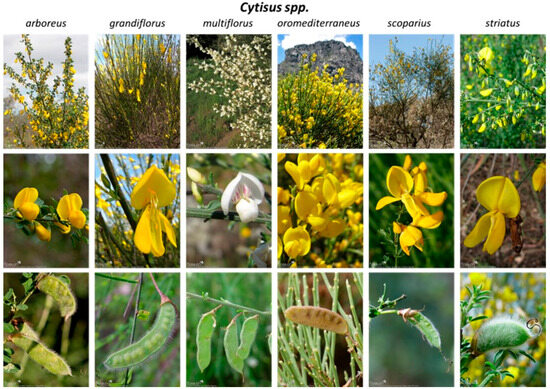
Figure 1.
Cytisus species most prevalent in the Mediterranean basin region [14].
Figure 1.
Cytisus species most prevalent in the Mediterranean basin region [14].

2. Recovery Strategies of Bioactive Polyphenols
The recovery of natural molecules (bioactives, pigments, proteins, biopolymers, etc.) is an imperative step to enable the (re)valorization of plant bioresources for consequent applications in food enrichment and preservatives, nutraceuticals, pharmaceutical, and cosmetic products.
Specifically, the effective recovery of bioactive molecules, e.g., polyphenols from natural sources, is highly influenced by the extraction technology applied. These methods must meet a number of necessities such as versatility, cost-effectiveness, environmental friendliness, and ease of operation, as well as guarantee high extraction yields and maintain the quality of the extracted phytochemicals [15].
Bioactive molecules are normally obtained using solid–liquid extraction (SLE). The efficiency of the extraction processes depends on several aspects related to the procedure used, like temperature, time, pH, and solvent, as well as raw materials composition [16]. Conventional approaches, such as Soxhlet extraction, hydrodistillation, and maceration, among others, are inarguably the most used for the recovery of valuable compounds [17]. These methods do have certain drawbacks, such as the destruction of thermolabile compounds caused by high temperatures maintained throughout the prolonged extraction period, limited target component recovery, low product quality, toxicological effects of resulting bioproducts, safety hazards due to a large amount of organic solvents used (harmful to the environment and human health), and high energy consumption (high energy inputs required for distillation) [18,19]. Therefore, there is the need to develop and optimize comprehensive methodologies (mainly using green solvents and technologies) to improve bioactive phenolic compound (and others) extraction, particularly from plants and vegetal biomass, where the cell wall can alter/impair the extraction efficiency.
New sustainable extraction techniques have been developed in an effort to address these problems and increase the quality and performance of the extraction process. When compared to conventional ones, green technologies have some advantages, including quicker heating and higher thermal efficiency, shorter extraction time, less solvent usage, and high selectivity [19,20]. In general, another important feature of these green technologies is their energy-saving efficiency (eco-friendly).
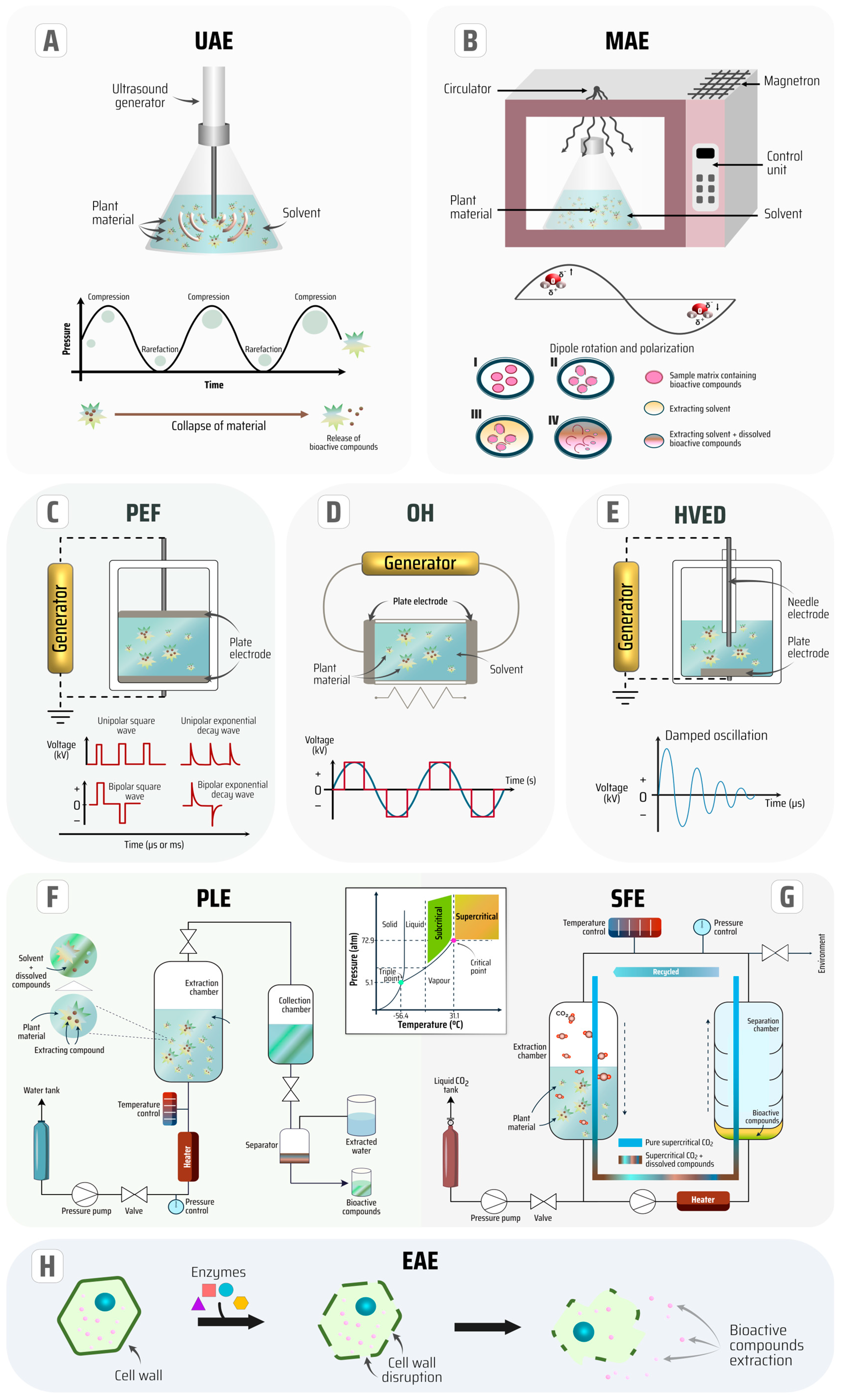
Thus, for the isolation of phenolic compounds, alternative green extraction technologies such as ultrasound-assisted extraction (UAE), microwave-assisted extraction (MAE), electrotechnologies-assisted extraction like pulsed-electric fields (PEF), high voltage electrical discharges (HVED) and ohmic heating (OH), pressurized liquid extraction (PLE), supercritical fluid extraction (SFE), and enzyme-assisted extraction (EAE) have been successfully applied for several bioresources [4,18,21,22]. These technologies are illustrated in Figure 2 to elucidate the extraction processes of bioactive phytochemicals from plant material. Furthermore, the concept of each innovative technique for the sustainable extraction process, as well as some advantages and disadvantages contrasting each of them, are summarized in Table 1.
Figure 2.
Illustration of innovative extraction technologies of bioactive compounds (A–H).
The next paragraphs address a few studies reported in the literature regarding the extraction of phenolic compounds from several Cytisus species. While using conventional extraction, numerous types of solvents (water or organic) have been tested for the effective extraction of these compounds from Cytisus biomass. Solvents such as ethanol, methanol, acetone, diethyl ether, isopropanol, ethyl acetate, chloroform, and ethyl lactate, as well as their combinations with water, have been the most used. For example, C. scoparius branches were previously treated with hexane to remove hydrophobic components in a Soxhlet extractor. The extracted solid was first processed with an acetone:water mixture (40 °C for 1 h) and its raffinate was extracted with acidified 70% ethanol (55 °C for 1 h) in an orbital shaker. The authors indicated that the extraction yield was 10 times higher in the acetone/water extracts than in the acidified ethanol extracts [23]. Sundararajan et al. [24] also used conventional SLE with ethanol:water (7:3 ratio) for the recovery of antioxidant compounds from C. scoparius aerial parts. Their results showed that the powdered extracts obtained from C. scoparius have a higher antioxidant capacity than the standard compounds used.
Barros et al. [25] determined the phenolic recovery and profiles of several plants used for traditional or folk medicine, including C. multiflorus. The results show that hydromethanolic extraction (80% methanol) was effective in extracting phenolic compounds, mainly flavonoids (54.5 mg/g plant). Recently, Larit et al. [26] has used hydroethanolic solvent (80% ethanol) for selective extraction of phenolic compounds from Algerian plant Cytisus villosus Pourr. The authors indicated that n-butanol fractioned ethanolic extract from C. villosus aerial parts had a higher total phenolic content (363 mg GAE/g extract) and antioxidant activity than the ethyl acetate (208 mg GAE/g extract) and chloroform (56 mg GAE/g extract) fractioned extracts.
Lores et al. [8] considered the viability of using ethyl lactate (an environmentally friendly solvent) to obtain solubilized phenolic compounds from different parts (flowers, seeds, pods, and branches) of C. scoparius, and evaluated the characteristics obtained extracts by PLE (120 °C; 10 min; hydro-organic mixtures of ethyl lactate) with those obtained with methanol (hazardous solvent). The results indicated that the PLE process was significantly better than the ambient-temperature columns, but it also consumed significantly more energy. In addition, a mixture of water (35%) and ethyl lactate (65%) proved to be a successful solvent for extracting C. scoparius polyphenols, resulting in extracts with high concentrations of natural polyphenols with great antioxidant activity (more than 35 mg GAE/g and around 4 mM Trolox/g of whole C. scoparius parts) comparatively to PLE of 100% ethyl lactate (35 mg GAE/g and 2 mM Trolox/g of whole C. scoparius parts) and conventional extraction with methanol (32 mg GAE/g and around 3.8 mM Trolox/g of whole C. scoparius parts).
González et al. [23] compared different extraction technologies to recover phenolics from branches of C. scoparius: conventional solvent extraction using ethanol, and extraction with PLE (water) or with SFE (SC-CO2). The authors found that PLE autohydrolysis can be a suitable technique with respect to conventional solvent methods in order to increase extraction yields. In addition, the stage by SC-CO2 extraction allows obtaining a raffinate with similar free radical scavenging properties to the ethanolic extract. Interestingly, microwave hydro-diffusion and gravity (MHG) may be an emergent solvent-free alternative to obtain selective phytochemicals in a sustainable manner [7]. In this context, López-Hortas et al. [7] evaluated the potential of MHG technology for recovering high-value-added compounds from C. scoparius flowers for sun cream applications. The results prove that the MHG extract has a higher content in lipids and carotenoids compared to the ethanolic extract obtained by SLE. On the other hand, this technology does not seem to be the most suitable for extracting phenolic compounds, proteins, and sugars from bioresources.
In the specific case of this review on Cytisus plants, as far as we know, in contrast to traditional SLE methods, only PLE, SFE, and MHG are used as alternative methodologies for recovering phenolic compounds. However, these methodologies must be optimized to maximize extraction yield, reduce the negative impact on target compounds, and increase energy efficiency. In this sense, more sustainable alternatives (other innovative technologies mentioned above), and their combination with alternative and non-harmful solvents (such as natural deep eutectic solvents (NADES)) should be explored to increase the application value of Cytisus spp. to obtain high-value products. These alternatives, if optimized, often involve the more sustainable valorization of renewable feedstocks with lower toxic solvents and reduced environmental impact, meeting the principles of Green Chemistry.

Table 1.
Concept, advantages, and drawbacks of innovative techniques for sustainable recovery of bioactive phytochemicals from plants.
Table 1.
Concept, advantages, and drawbacks of innovative techniques for sustainable recovery of bioactive phytochemicals from plants.
| Extraction Technique | Concept | Advantages (vs. Conventional Methods) | Drawbacks | Reference |
|---|---|---|---|---|
| Ultrasound-assisted extraction (UAE) |
|
|
| [27,28] |
| Microwave-assisted extraction (MAE) |
|
|
| [29,30] |
| Pulsed electric field (PEF) |
|
|
| [4,31,32,33] |
| Ohmic heating (OH) |
|
|
| [4] |
| High-voltage electrical discharges (HVED) |
|
|
| [34,35] |
| Pressurized liquid extraction (PLE) |
|
|
| [35,36] |
| Supercritical fluid extraction (SFE) |
|
|
| [36,37] |
| Enzyme-assisted extraction (EAE) |
|
|
| [36,38] |
3. Phytochemical Composition of Cytisus Extracts
Cytisus spp. contains more than 70 phytoconstituents belonging to different groups. It is reported that the stem extracts of C. multiflorus species are very rich in alkaloids, namely sparteine, with anti-arrhythmic properties. Alkaloids have been shown to provide the therapeutic effect of many plant materials; however, most of the alkaloids are toxic [39]. Over 200 alkaloids have been identified in 300 plant species of up to 13 families [40]. Alkaloids are the major chemical compound found in C. scoparius, specifically quinolizidine [41] and lupanine [8]. Other compounds were also identified in the extracts of C. scoparius, including alkenols, benzenoids, phenylpropanoids, carotenoids, alkanones, steroids, lipids, monoterpenes, and polyphenols [42]. The essential oils of C. triflorus L’Her are rich in monoterpenes and sesquiterpenes, especially in the flowering stage of the plant. The other identified compounds belong to the groups of hydrocarbons, ketones, alcohols, esters aldehydes, fatty acids, and polyphenols. The major oils components are b-linalool, geraniol, retinal, p-mentha-1,4-dien-8-ol, γ-terpinene, and eugenol [10].
Plant extracts are rich in phytochemical constituents, though their medicinal properties and bioactivities are a result of the synergetic effect of all the present molecules. Plant extracts are commonly rich in polyphenol compounds belonging to different groups, playing vital roles in human health. On the basis of their chemical structure, phenolic compounds can be divided into two major groups: flavonoid (including flavanols, flavonols, flavones, flavanones, anthocyanins, isoflavones, and tannins) and non-flavonoid (including phenolic acids like hydroxybenzoic acids and hydroxycinnamic acids, stilbenes, and lignans) [33]. For instance, polyphenol compounds are present in a small amount in nature, and display important actions in biofunctionalized products. Moreover, current research reports that part of the functional activity of polysaccharides and proteins is closely related to the bound polyphenolic compounds, that is, to the presence of molecular complexes [43]. Polyphenols are known to have diverse biological properties between them, including antioxidant, anti-inflammatory, anticancer, and many others, and appear to be essential in assisting the body’s redox balance (homeostasis).
The Cytisus spp. are used in folk medicine as diuretics, hypnotics, sedatives, anti-diabetics, and hepatoprotectives, among other purposes [40]. According to Luís et al. [40] ethanolic extract of Cytisus spp. Has more polyphenols than that of the aqueous extract. For the extracts of C. multiflorus, high content of polyphenol compounds has been reported: 142.4 mg GAE/g dry matter, 176.1 mg GAE/g dry matter, 120.4 mg GAE/g dry matter, and 155.1 mg GAE/g dry matter for stems, leaves, flowers, and fruits, respectively. Leaves, stems, flowers, and fruit extracts were analyzed for their individual polyphenol compounds and antioxidant activity. Leaf extracts were the richest in terms of concentration of identified polyphenolic compounds, with the highest concentrations observed for ellagic acid (16.1 mg/g dry matter). The most representative compound in the extracts of fruit and flowers was ferulic acid, at 25.4 mg/g and 13.0 mg/g dry matter, respectively. The stem extracts were richest in quercetin (37.1 mg/g dry matter). The antioxidant properties of the C. multiflorus extracts were considered to have strong to moderate activity.
Extracts from different parts of C. scoparius were evaluated for their polyphenol profile [8]. The most abundant polyphenols found in the extracts of the hole plant were: rutin (110 μg/g dw), isoquercetin (62.7 μg/g dw), chrysin (119 μg/g dw), and quercetin (70 μg/g dw). The compound orientin was specifically found in extracts from pods (220 μg/g dw). Nevertheless, kaempferol (309 μg/g dw) was the most abundant polyphenol in pod extracts, but was not found in the whole plant extracts. Rutin (562 μg/g dw) is mostly found in the extracts of flowers, together with quercetin (210 μg/g dw) and isoquercetin (242 μg/g dw). The flower extracts were the richest extracts in polyphenols, while the branch extracts showed low concentrations of polyphenols. All extracts demonstrated high levels of antioxidant activity. The antibacterial activity was dependent on the type of the solvent used. Moreover, antibacterial activity was registered only for Gram-positive bacteria. Sundararajan et al. [24] also studied the antioxidant activity of hydro-alcoholic extracts of the areal parts of C. scoparius, using different evaluation methods. The results demonstrated that these extracts are a potential source of natural antioxidants. The antimicrobial activities of an ethyl acetate extract from C. triflorus L’Her were also evaluated [44]. Once again, the extracts expressed antibacterial activity only against Gram-positive bacteria. Moreover, the extracts were rich in chrysin derivatives and had strong antioxidant activity.
The ethyl acetate and aqueous extracts obtained from the aerial parts of C. villosus Pourr were studied for their chemical composition, antioxidant, antimicrobial, and antiproliferative activities [9]. Both types of extracts had rich polyphenol composition. Epigallocatechin (111 μg/g dw) was the major polyphenol compound found in the aqueous extracts, and myricetin-O-rhamnoside (226 μg/g dw) was the main compound found in the ethyl acetate extracts. The aqueous extracts presented higher amounts of flavan-3-ols (496 μg/g dw), while the ethyl acetate extract presented higher flavonols (550 μg/g dw) content. The extracts demonstrated high antioxidant activity. The antimicrobial activity of the extracts was only against Gram-positive bacteria, but some antifungal activity was also registered. For the first time, the antiproliferative activities associated with C. villosus extracts were reported [9].
The aqueous extracts of C. scoparius were studied for their phytotoxic potential in two agricultural weed species [42]. The study demonstrated that the phytotoxic effects of the Cytisus aqueous complex extracts were greater than isolated phenolic compounds, perhaps due to synergistic effects. The final conclusion was that the phytotoxic activity of the C. scoparius aqueous extracts is a complex result from the combined action of the polyphenols components and the complex interactions with other constituents like proteins, carbohydrates, and lipids.
Cytisus multiflorus, C. scoparius, and C. striatus were evaluated for their total phenolic content and antioxidant activity by several in vitro methods (DPPH scavenging activity, reducing power, β-Carotene bleaching inhibition, TBARS inhibition) [11]. C. scoparius demonstrated the highest content in total phenolic compounds, followed by C. striatus and C. multiflorus, with values of 427.10 mg GAE/g extract, 389.04 mg GAE/g extract, and 313.87 mg GAE/g extract, respectively. The authors reported that these species are widely used in folk medicine, and C. multiflorus are indicated for the prevention and treatment of diabetes, hypertension, hypocholesterolemia, headache and migraine, heart failure, rheumatoid arthritis, sores and cutaneous eruptions, acne, and as anti-inflammatory. C. scoparius are indicated for the prevention and treatment of metabolic illness, gout and rheumatic disorders, heart failure and tachycardia, joint and muscles pain, renal problems, skin inflammations, anti-hemorrhagic, cholagogue, diuretic, and vasopressor, among others. C. striatus are also traditionally indicated for the prevention and treatment of gout and rheumatic illnesses, hypotension, heart failure, anti-inflammatory, cardiotonic, cholagogue, diuretic, depurative, and vasopressor. The experimental results obtained by Pinela et al. [11] gave scientific support to the uses of these plants in traditional medicine as antioxidant with anti-inflammatory properties.
Only the flowers of C. multiflorus were evaluated for their polyphenol profile [25]. The C. multiflorus did not present phenolic acids and derivatives. The identified polyphenol compounds belong mainly to the groups of flavonols and flavones, represented by quercetin derivatives and chrysin derivatives, respectively. Similar results were published by [13] for the ethanol extracts of flowers of C. multiflorus. The most abundant polyphenol compound found in the extract were chrysin derivatives (23.4 mg/g dw) that accounted for more than 50% of the total polyphenol content (41.8 mg/g dw). The antioxidant and cells protective effects of isolated ethanolic extracts of C. multiflorus were investigated and compared to the activities of the main individual polyphenolic constituents (chrysin, apigenin, quercetin, and luteolin) [45]. The authors suggested that the polyphenol chrysin (72.8 mg/g extract) is the main constituent responsible for the high antioxidant activity of the extracts, as it accounted for 56% of the total polyphenolic content (130.3 mg/g extract). In contrast, the cytoprotective action of the extracts could not be related to the major polyphenol compounds.
The acetone extracts of aerial parts of Algerian C. triflorus show antioxidant, antibacterial, and anti-inflammatory effects, as well as wound healing properties [46]. The scientific results supported and justified the traditional use of this plant. Moreover, according to the authors, further researches are necessary to identify the phytochemicals which are responsible for these biological activities. The antioxidant activities of another Cytisus specie from Algeria, C. monspessulanus, were studied [47]. According to the authors, this is the first study reporting the total phenolic and flavonoid contents for C. monspessulanus extracts. It was noted that the extracts had important higher amounts of flavonols and flavones (20.4 mg/g dw) than flavanones and di-hydroflavonols (5.5 mg/g dw). Moreover, C. monspessulanus extracts had the highest content of flavonols and flavones compared to the other seven plant extract in the study, namely Anthemis arvensis L., Haloxylon scoparium Pomel, Arbutus unedo L., Artemisia campestris L., Juniperus phoenicea L., Thymus algeriensis Boiss et Reut, Zizyphus lotus L. (Desf.), and C. villosus Pourr. The hydroethanolic extract of the aerial part of this specie were studied by Larit et al. [48], and the major identified polyphenolic compounds were ginestein and chrysin.
As final remarks, we can conclude that extracts of Cytisus species are rich in polyphenolic compounds, especially chrysin and quercetin and its derivatives, and with powerful antioxidant, antimicrobial, etc., biological activities (Section 4).
Table 2 summarizes the phenolic composition of some species of the genus Cytisus that are reported in the bibliography. It is noticeable that the amount of phenolic compounds reported depends on factors such as the species of Cytisus used for extraction, the age and growth conditions of the plants, the part of the plant used (branches, leaves, flowers or others), the applied technology of extraction, the solvent (water, ethanol, ethyl acetate, etc.), and other conditions used for phenolic recovery and isolation (time, temperature, solid–liquid ratio, etc.). In this sense, differences can be observed in the results reported by different authors, in addition to the units used to express the results.

Table 2.
Phenolic composition of some Cytisus species reported in the literature.
4. Emerging Applications of Cytisus Bioproducts
4.1. Potential Food and Food-Related Applications
The use of plant-based substitutes for meat, dairy, and others products of animal origin is well described in the literature. Its use was boosted due to an increase in vegan and/or vegetarian diets, environmental concerns, desire for sustainability, as well as an FAO recommendation for a healthier and sustainable and healthier diet, among other causes [49].
As mentioned in previous sections, plant and plant by-products have compounds in their composition that have a significant positive impact on human health, including polysaccharides, fibers, proteins, carotenoids, polyphenols, and sterols, among others. Cytisus plants have a rich composition of bioactive flavonoids and phenolic acids.
When discussing the possible applications of plant-based products in the food industry, there are several categories of food consumption products including proteins, whole grains, flour, oil, “milk”, sweeteners, and food additives (natural flavors and aromas, colorants, preservatives, etc.). Their incorporation into new food products or increasing the functionality of existing ones (functional foods) and in functionalized food packaging or films has also been explored [50,51,52,53].
As described by Zang et al. [54], natural food additives have drawn attention due to its green safety, health, and non-toxicity, this being an emerging field in the food industry.
Several species from the Cytisus genus have already been characterized in terms of their chemical and biological potential, as can be seen in Table 2 and Table 3, respectively. The phytochemical compounds detected in each species confers unique characteristics, with potential application in the food industry. As an example, Cytisus species, like C. multiflorus, C. villosus, and C. scoparius, which have in their composition compounds known for their antioxidant and anti-inflammatory properties, like gallic, protocatechuic, chlorogenic and caffeic acids, chrysin, rutin, kaempferol, and quercetin, can be incorporated into familiar foods like cookies and snacks to enhance their functional value. This addition of bioactive compounds can contribute to the prevention of several non-communicable diseases like diabetes, cardiovascular diseases, and even neurodegenerative diseases like Alzheimer’s [55,56,57].
Food preservatives are substances used to extend the shelf life of certain food products by preventing oxidation and avoid food degradation by microorganisms [54]. As reported previously, Cytisus extracts, independently of the species, have antioxidant and antimicrobial activities, making them an optimal source of food preservatives.
As described by Sahraee et al. [58], food deterioration can significantly alter the organoleptic qualities of food, therefore the food industry has been searching for ways to prevent this deterioration. For the past few years, synthetic antioxidants have been incorporated into food products and food packaging, although due to their toxicity, natural antioxidants have been sought, particularly plant-based ones [59]. Due to the rich composition in phenolic compounds of the Cytisus plants (Table 2), their extracts confer interesting antioxidant properties, which in combination with their antimicrobial characteristics makes them ideal for incorporation into edible films, for example. Therefore, plant-based products can be used as food additives, preservatives, or even food packing without being a hazard to the consumer, due to the low toxicity of most of the plant-based molecules [60].
The knowledge gathered in recent years regarding the beneficial effect of compounds present in the Cytisus genus opens the door to a widely vast array of applications for food and food-related applications, like safer and more efficient food additives, healthier diets to prevent non-communicable diseases (like cancer, diabetes, or cardiovascular diseases), and the improvement of food packaging materials, increasing their function, and reducing their carbon footprint by diminishing the use of synthetic alternatives.
4.2. Potential Health Applications
Phytochemicals have played an imperative role from ancient to recent times in the prevention and treatment of many illnesses with extensive effects, such as antioxidants which are directly linked with reduced risks of cancer, cardiovascular disease, diabetes, infectious diseases, and other disorders associated with age [61,62]. The benefit of many natural products, which have been part of the human diet for several thousand years, is that the human body has adjusted to them, which can lower the risk of damaging side effects [26].
Some Cytisus plants growing spontaneously in thermos-Mediterranean conditions and are widely used in local folk medicine and occasionally as condiments [11,13]. As reported before, for a long time, the extracts of these plants have been used for therapeutic purposes due to their hypnotic and sedative, diuretic, anti-diabetic, hepatoprotective, antioxidant, anti-inflammatory, antiparasitic, lithotriptic, cardiotonic, hypotensor, cathartic, and emetic properties.
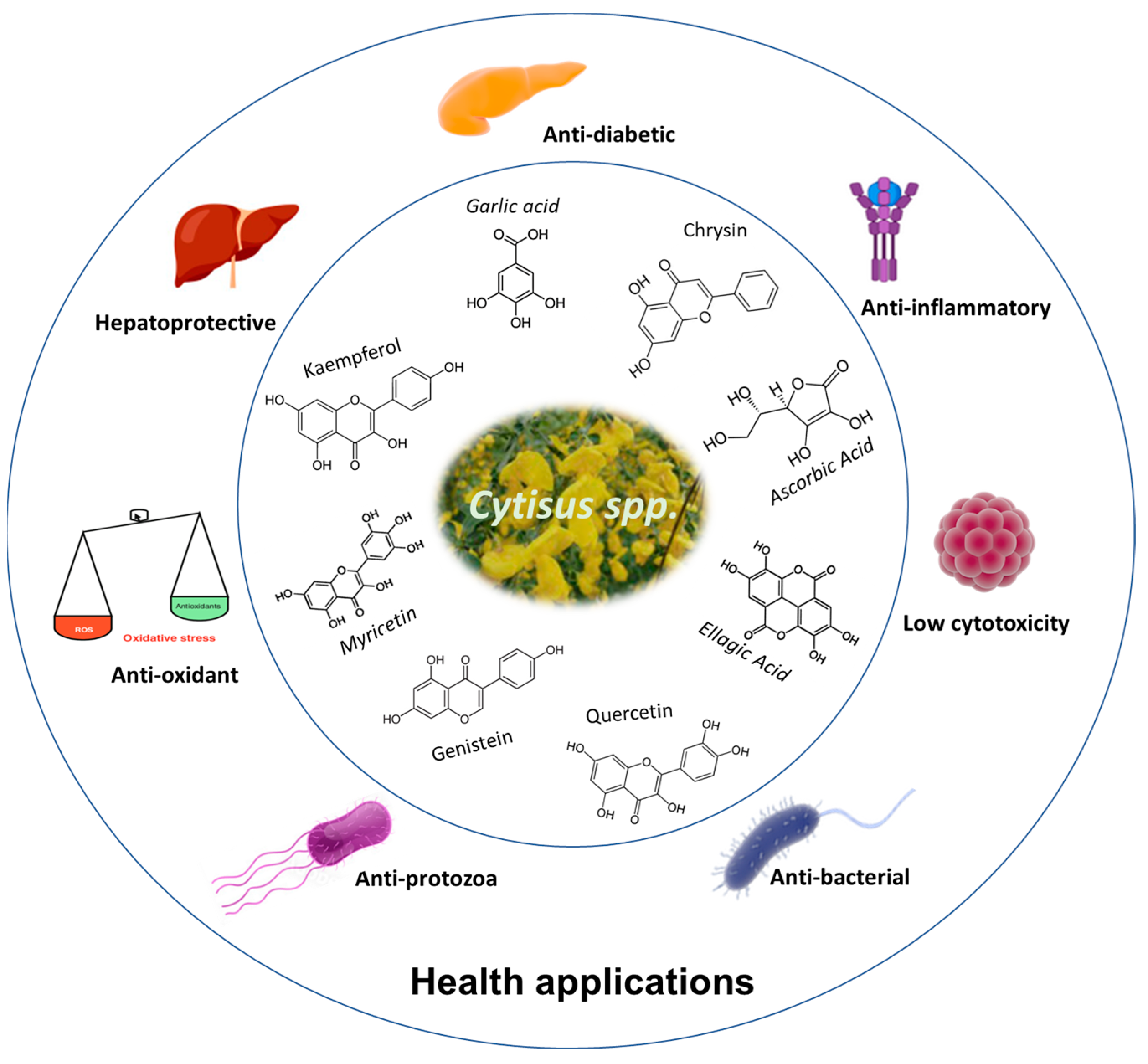
The therapeutic properties of Cytisus spp. are mainly associated with its high concentration of polyphenolic compounds, as reported in the previous sections. The scheme in Figure 3 presents the main therapeutic actions reported so far for products derived from plants of the genus Cytisus.
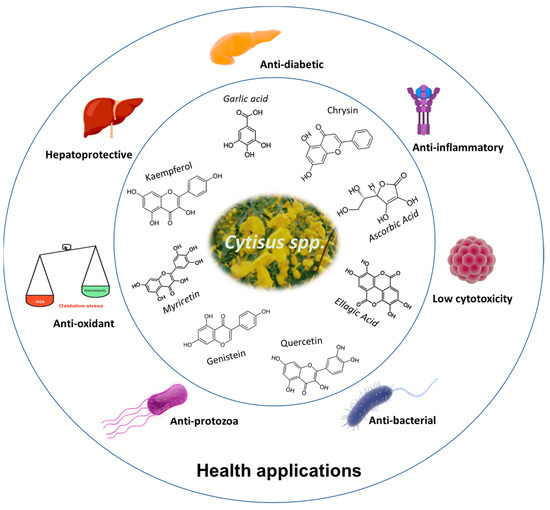
Figure 3.
Potential health applications of Cytisus polyphenolic extracts.
4.2.1. Antioxidant Activity
Antioxidant activity is one of the most studied biological properties of natural products, particularly medicinal and food plants.
Numerous studies have shown that the antioxidant action of plants could be related with protection against human diseases related to oxidative stress. In this regard, flavonoids and other polyphenol compounds have gained the most attention. The genus Cytisus contains the main components of phenolic acids and flavonoids with great antioxidant capacity [11,23,26,48]. In fact, Cytisus plants appear as an alternative to conventional plants as they have excellent antioxidant properties. This “opens the door” to providing added value from this bioresource to food and therapeutic applications, as a functional food, food supplement, or food additive (natural preservative).
Sundararajan et al. [24] reported that the extracts of C. scoparius aerial part (at the dose 104.0 µg/mL) present 50% protection against lipid peroxidation induced by Fe2+/ascorbate system in rat liver microsomal preparation. In addition, a decrease in the generation of the hydroxyl radical was visible with an IC50 value of 27.0 µg/mL for the Cytisus extracts when compared to the standard vitamin E (32.5 μg/mL).
Likewise, Raja et al. [63] have observed that the same plant extracts protect the liver from oxidative stress induced by carbon tetrachloride (CCl4) in rats at a dose of 250 and 500 mg/kg for 7 days. C. scoparius extracts also have anti-stress and moderate anxiolytic activity in rats at 125 and 250 mg/kg for 21 days. This effect may be due, in part, to its antioxidant effect [64]. In addition, González et al. [12] showed that these plant extracts present topical applications for skin protection against oxidative damage.
On the other hand, the antioxidant capacity of the C. villosus extracts and its isolated phenolics (genistein, chrysin, chrysin-7-O-β-D-glucopyranoside, and 2″-O-α-L-rhamnosylorientin) was measured in hepatocellular carcinoma cells (HepG2 cells) [26]. Results showed inhibition of intracellular oxidative stress (36% inhibition of ROS generation at 250 µg/mL). Concomitantly, the purified and isolated compounds were not effective, except for 2″-O-α-L-rhamnosylorientin from n-BuOH extract which presented 28% of ROS inhibition at 250 µg/mL. These results demonstrate the synergistic effect between natural compounds, in addition to the fact that the same phenomenon can occur between natural and synthetic compounds if administered at the same time.
By comparing the antioxidant activity of Cytisus extracts with other plants used in food and medicine, it is possible to perceive that the broom flower has similar or perhaps superior abilities to block and inhibit free radicals. For example, Ali-Jafri et al. [65] studied the in vitro antioxidant activity of different parts of traditional medicinal plants rich in polyphenols, and its results showed that 45 plant extracts (among them Thymus, Salvia, Melissa, Veronica officinalis, Mentha, etc.) have radical scavenging activity (IC50 values between 25–130 µg/mL for DPPH radical). In another study, Martins et al. [66] reported the in vitro antioxidant properties of edible wild greens in the traditional diet of the Iberian Peninsula (asparagus, white bryony, and black bryony). Their data showed DPPH radical scavenging effects with IC50 values of 423, 640, and 203 µg /mL for asparagus, white bryony, and black bryony, respectively. Comparing with Cytisus extracts, we observed that the antioxidant activity is similar (1.5 to 792 µg/mL for DPPH method) with these widely used plants. Furthermore, it is important to mention that the biological activity of the extracts cannot be literally compared with other studies because it depends on the antioxidant method (type of radical, reducing power, etc.), the species of Cytisus, the part of the plant used, and the extraction method used.
4.2.2. Cytotoxicity and Antiproliferative Activity
Toxicological studies are very important for both synthetic and natural products before they can be administered and/or consumed by humans and animals. These types of tests are crucial to assess the safety of compounds and study the appropriate dose to obtain therapeutic effects. For this purpose, pre-clinical toxicity studies are essential, and many categories of test can be conducted, such as acute, subacute and chronic toxicity, cytotoxicity, teratogenicity, genotoxicity, etc.
To the best of our knowledge, only three studies have reported the cytotoxic activity of Cytisus spp. extracts (Table 3). The cytotoxicity of extracts and isolated compounds from C. villosus was determined against four human cancer cell lines (SK-MEL, KB, BT-549, and SKOV-3) and two non-cancerous kidney cell lines (LLC-PK and Vero). The tested extract/molecules were not active against any cell lines used in this study at 100 μM [26]. In the second study, the authors reported that the in vitro cytotoxicity of C. scoparius methanolic extract showed the IC50 value of 4.5 μg/mL on human cervical cancer cells (HeLa), which shows that the isolated compound genistein is cytotoxic [67]. The results of the cell cycle assay revealed the cell arrest at a sub-G phase, affirming the apoptotic effect of isolated genistein. Moreover, the human topoisomerase II test proved that it is a considerable human topoisomerase II inhibitor at 10 μg/mL [67]. In the third study, the authors revealed the cytotoxic/antiproliferative potential of C. villosus aqueous and ethyl acetate extracts on three human cancer cell lines of breast (T47D, MCF7) and colon (HCT-116) cancers. The results showed that the ethyl acetate extract exhibited higher inhibitory activity (IC50 values of 1.57 ± 0.06 mg/mL, 2.2 ± 0.1 mg/mL, and 3.2 ± 0.2 mg/mL for T47D, MCF-7, and HCT-116 cell lines, respectively) [9].
Due to the scarcity of studies on the cytotoxic potential of extracts or isolated molecules obtained from Cytisus spp., more work should be undertaken to validate their use in food and therapeutic applications, taking into account the doses tested.
4.2.3. Anti-Inflammatory and Antidiabetic Activity
The genus Cytisus has been used for centuries and is reported to have anti-inflammatory properties. Ait-KaciAourahoun et al. [68] showed that the hydroalcoholic leaves extract from C. triflorus at a dose of 200 and 400 mg/kg body weight may be considered as a good anti-inflammatory agent in carrageenan-induced edema using a rat model. Likewise, extracts of C. villosus showed weak inhibition of iNOS with IC50 values of 48 µg/mL for EtOAc extracts to 90 µg/mL for n-BuOH in mouse macrophages (RAW 264 cells) [26]. These results encourage further investigation of the phytochemical compounds of Cytisus spp. regarding its anti-inflammatory potential and other biological actions.
Type 2 diabetes is one of the non-communicable diseases that is becoming a “pandemic” in the world. Traditional medicine has used herbs for the treatment of diabetes, either independently or in combination with food. Thus, scientific investigation on traditional herbal medicines for diabetes can provide valuable evidence for the development of alternative drugs and strategies, as shown by various studies carried out on plants from North Africa and Central Asia, including those of the genus Cytisus [61,69,70,71,72]. Recent studies have reported the effect of natural extracts rich in polyphenolics in the prevention and treatment of type 2 diabetes [73]. Some of these studies have confirmed the antidiabetic efficacy (antihyperglycemic activity) of extracts from various species of Fabaceae. Although there are no studies on the action of extracted compounds from Cytisus species, considering the composition in polyphenolics, they seem to have antidiabetic potential and more studies should be conducted both in vitro and in vivo.
4.2.4. Hepatoprotective Activity
Raja et al. [63] showed that pretreatment of Wistar albino rats with C. scoparius extract (250 and 500 mg/kg) significantly affects liver injury. The levels of antioxidant enzymes like superoxide dismutase (SOD), catalase (CAT), reduced glutathione (GSH), glutathione peroxidase (GPx), glutathione-s-transferase (GST), and glutathione reductase (GRD) parameters were considerably increased by C. scoparius extract treatment in a rat model of carbon tetrachloride (CCl4) hepatic toxicity. Moreover, the capacity of the extract at the dose of 500 mg/kg was comparable to that of the standard drug, silymarin (25 mg/kg).
In the same way, CCl4 induced liver injury in rabbits, and Cytisus extracts orally in a dose of 400 mg/kg/day for 14 days reduced levels of hepatic enzymes like alanine transaminase (ALT) and aspartate transaminase (AST), alkaline phosphatase (ALP), and total bilirubin, showing preventive liver damage and improving biological functions of liver [74].
4.2.5. Antimicrobial and Antiprotozoal Activities
Antibiotic resistance is one of the most critical situations facing humanity. Therefore, due to the increase in antibiotic resistance, it is very important to identify new and innovative antimicrobial agents. As mentioned previously, plants contain many phytochemicals that can be used for therapeutic purposes and generally have low toxicity [75,76,77].
The antibacterial activity of C. villosus extract was tested against Escherichia coli, Staphylococcus aureus, S. epidermidis, Bacillus subtilis, Pseudomonas aeruginosa, and Mycobacterium intracellular in comparison with standard antibiotics. However, the result was not significant against E. coli and P. aeruginosa [9,26]. Bouziane et al. [9] reported the selective action of Cytisus extracts and potent antimicrobial actions against the Gram-positive bacterium S. epidermidis (IC50 of 186 ± 9 μg/mL for aqueous extracts and 92 ± 3 μg/mL for ethyl acetate extracts). Likewise, the effect of the Cytisus extract against four fungi including Aspergillus fumigatus, Mucor specie, Aspergillus niger and Fusarium solani was explored, without finding significant results in its growth. Larit et al. [26] also evaluated the antifungal activity of this extract against a pathogenic fungus associated with opportunistic infections, including Candida albicans, C. glabrata, C. krusei, A. fumigatus, and Cryptococcus neoformans, and no relevant results were obtained. On the other hand, the same extracts presented an antitrypanosomal effect against Trypanosoma brucei at tested concentrations (7 to 19.48 µg/mL) [26].
Yahiaoui et al. [46] tested the antibacterial activity of C. triflorus extracts by microdilution method against E. coli, S. aureus, B. subtilis and P. aeruginosa. Their study demonstrated that all extracts were active against the tested bacteria, but more effective against Gram-positive ones. Benabderrahmane et al. [44] also suggested that C. triflorus polyphenols and flavonoids are responsible for its antioxidant capacity and antimicrobial properties against Gram-positive bacteria S. aureus.
In another study, Lores et al. [8] reported that the C. scoparius extracts obtained by PLE with ethyl lactate decreased the minimum inhibitory concentrations (MICs) from 3% to 2% for Gram-positive species (S. aureus and Bacillus spp.), demonstrating that the polyphenol-rich extract exerted a positive antibacterial activity. For a Gram-negative bacterium (E. coli) an increase in the MIC from 3% to 4% when using the extract was observed. On the other hand, experiments with the fungus C. albicans showed no anti-fungal activity for the C. scoparius extract.
In general, antimicrobial activity studies show that phytochemicals from different Cytisus extracts can confer some type of antibacterial action against various strains, and do not show antifungal activity.
The growing demand for new drugs with antibiotic effects has led to the study and adoption of new strategies to combat multidrug-resistant bacteria. Abreu et al. [78] tested 29 plant species as strategies to deal with methicillin-resistant S. aureus (MRSA). Interestingly, C. striatus extract presented antibacterial activity and acted as an antibiotic adjuvant, potentiating the effect of ciprofloxacin and erythromycin (commercial antibiotics) against MRSA strains. This study revealed the potential of C. striatus extracts for therapeutic applications in the control of infections caused by microorganisms’ resistant to antibiotics.

Table 3.
Potential health applications of Cytisus plant extracts.
Table 3.
Potential health applications of Cytisus plant extracts.
| Activity | Assay Model | Extracts | Effect/Methodology | Reference |
|---|---|---|---|---|
| Antioxidant | In vitro |
| IC50 activity: 1.5 µg/mL for DPPH radicals; 116 µg/mL nitric oxide; 4.7 µg/mL superoxide anion; 27.0 μg/mL hydroxyl radical; potent antioxidant reducing power | [24] |
| In vitro |
| DPPH radicals scavenging (IC50): 19.17 and 77.8 µg/mL for leaf and stem, respectively | [68] | |
| In vitro |
| DPPH radicals scavenging (IC50): 459 µg/mL CHCl3 extract; 425 µg/mL EtOAc extracts; 164 µg/mL for n-BuOH | [26] | |
| In vitro |
| DPPH radicals scavenging (IC50): 792 ± 10 µg/mL | [46] | |
| In vitro |
| DPPH radicals scavenging (IC50): 59 ± 2 µg/mL for aqueous extracts and 31 ± 2 for ethyl acetate extracts | [9] | |
| In vitro |
| Greater scavenging of DPPH radicals by ethyl lactate:water (65:35 (v:v)) extracts (approx. 6 mM Trolox/g plant) | [8] | |
| Rat liver microsomal preparation |
| Protection in lipid peroxidation at 104 µg/mL | [24] | |
| Rat liver |
| Protects liver from oxidative stress at 250 and 500 mg/kg for 7 days | [63] | |
| Rat |
| Anti-stress at 125 and 250 mg/kg for 21 days | [64] | |
| Human epidermis |
| Skin protection against oxidative damage. In vitro assays with the reconstituted human epidermis, topical use at 1% | [12] | |
| Human hepatocytes |
| Inhibition of intracellular oxidative stress in HepG2 cells at 250 µg/mL | [26] | |
| Cytotoxicity and Antiproliferative activity | Human cancer cells |
| Human cervical cancer cell line (HeLa); MTT assay, cell cycle analysis, and topoisomerase II inhibitory activity IC50 value of 4.5 μg/mL; cell arrest at a sub-G phase; promote apoptosis; excellent human topoisomerase II inhibitor at 10 μg/mL | [67] |
| Human cancer cells |
| No toxicity at 100 µM against human cancer cell lines (SK-MEL, KB, BT-549, and SKOV-3) and two noncancerous kidney cell lines (LLC-PK1 and Vero) | [26] | |
| Human cancer cells |
| Growth inhibition of the three different cell lines of breast (T47D, MCF7) and colon (HCT-116) cancers Ethyl acetate extract exhibited higher activity (IC50 values of 1.57 ± 0.06 mg/mL, 2.2 ± 0.1 mg/mL, and 3.2 ± 0.2 mg/mL for T47D, MCF7, and HCT-116 cells) | [9] | |
| Anti-Inflammatory | Rat |
| Anti-inflammatory agent at 200 and 400 mg/kg body weight | [68] |
| Mousemacrophages |
| EtOAc and n-ButOH extracts showed inhibition of iNOS with IC50 of 48 and 90 µg/mL, respectively. | [26] | |
| Hepatoprotective | Rats |
| Increase levels of SOD, GS, and GRD parameters in carbon tetrachloride treated wistar albino rats at 250 and 500 mg/kg/day for 7 days | [63] |
| Rabbits |
| Oral administration of extracts at 400 mg/kg/day for 14 days. Reduction in ALP, ALT, AST levels and total bilirubin in carbon tetrachloride treated rabbits | [74] | |
| Antimicrobial and Antiprotozoal | Staphylococcus aureus, Bacillus subtilis, Pasture llamultacida |
| Anti-bacterial activity by disc diffusion method (30 µg/disc): 13.8 ± 0.18 (maximum concentration) | [74] |
| Trypanosoma bruces |
| Antitrypanosomal activity (IC50): 19.48 µg/mL for EtOH extract; 7.99 µg/mL for n-BuOH | [26] | |
| Staphylococcus aureus, Bacillus subtilis, Pseudomonas aeruginosa, Escherichia coli |
| Minimal inhibitory concentration (MIC) assays S. aureus: 0.26 ± 0.09; B. subtilis: 0.42 ± 0.18; P. aeruginosa: 1.67 ± 0.72; E. coli: 1.04 ± 0.36 (values in mg/mL) | [46] | |
| Staphylococcus aureus, Bacillus spp., Escherichia coli, Candida albicans |
| MIC assayExtract exerted a positive antibacterial action | [8] | |
| Staphylococcus aureus |
| Act as antibiotic adjuvants | [78] | |
| Staphylococcus epidermidis, Escherichia coli, Pseudomonas aeruginosa, Candida glabrata |
| Selective and potent antimicrobial activities against the Gram-positive bacterium (S. epidermidis) IC50 of 186 ± 9 μg/mL for aqueous extracts and 92 ± 3 μg/mL for ethyl acetate | [9] | |
| Wound healing | Rats |
| Excision wound healing model for 14 days; results indicate re-epithelialization and good healing potential using 5% of the extract | [46] |
SK-MEL: Human malignant melanoma; KB: Human epidermoid carcinoma; BT-549: Human ductal carcinoma; SK-OV-3: Human ovary carcinoma; LLC-PK-1: Pig kidney epithelial cells; Vero: African green monkey kidney cell line.
5. Conclusions and Perspectives
The interest for “Clean label” products, such as functional foods, nutraceuticals, biocosmetics, and natural drugs (pharmaceuticals), is motivated by imperative factors such as health and sustainability. This phenomenon is expressed in the consumer’s interest in natural, functional, and health-promoting products that are plant-based. The processing of undervalued plant by-products by innovative and green technologies can satisfy this growing interest, in addition to minimizing the generation of biowastes, reducing energy consumption, and increasing the sustainability of the remaining industries. Therefore, the valorization of underexplored plants and the reuse of agricultural residues, in this case Cytisus plants, can be a viable option to obtain high-value products (e.g., bioactive polyphenols) for the food, cosmetic, and pharmaceutical industries, such as antioxidants, dyes, flavorings, antimicrobial agents, or isolated drugs with highlighted functional and therapeutic potential. On the other hand, future detailed technoeconomic, environmental, and social assessments of bioprocessing of underexploited plant materials and design of new or improved plant-based products are required.
Author Contributions
Conceptualization, P.F.-S. and C.M.B.; writing—original draft preparation, P.F.-S., D.F.-S., C.E.C., Z.G., M.J.R.-Y., B.G. and C.M.B.; writing—review and editing, P.F-S. and J.A.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Portuguese Foundation for Science and Technology (FCT) under the scope of the strategic funding of UIDB/04469/2020 unit, and by LABBELS—Associate Laboratory in Biotechnology, Bioengineering and Microelectromechanical Systems, LA/P/0029/2020. This work was also funded by the European Regional Development Fund (ERDF) through the Competitiveness factors Operational program—Norte 2020, COMPETE, and by National Funds through the FCT—under the project AgriFood XXI (NORTE-01-0145-FEDER-000041).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
Pedro Ferreira-Santos and Beatriz Gullón would like to express gratitude to the Spanish Ministry of Science, Innovation, and Universities and the European Union—NextGenerationEU/PRTR—for financial support (FJC2021-046978-I and RYC2018-026177-I, respectively).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Jimenez-Lopez, C.; Fraga-Corral, M.; Carpena, M.; García-Oliveira, P.; Echave, J.; Pereira, A.G.; Lourenço-Lopes, C.; Prieto, M.A.; Simal-Gandara, J. Agriculture Waste Valorisation as a Source of Antioxidant Phenolic Compounds within a Circular and Sustainable Bioeconomy. Food Funct. 2020, 11, 4853–4877. [Google Scholar] [CrossRef]
- FAO. The State of the World’s Land and Water Resources for Food and Agriculture—Systems at Breaking Point (SOLAW 2021); FAO: Rome, Italy, 2021. [Google Scholar]
- Dey, T.; Bhattacharjee, T.; Nag, P.; Ritika; Ghati, A.; Kuila, A. Valorization of Agro-Waste into Value Added Products for Sustainable Development. Bioresour. Technol. Rep. 2021, 16, 100834. [Google Scholar] [CrossRef]
- Rocha, C.M.R.; Genisheva, Z.; Ferreira-Santos, P.; Rodrigues, R.; Vicente, A.A.; Teixeira, J.A.; Pereira, R.N. Electric Field-Based Technologies for Valorization of Bioresources. Bioresour. Technol. 2018, 254, 325–339. [Google Scholar] [CrossRef]
- Hussein, R.A.; El-Anssary, A.A. Plants Secondary Metabolites: The Key Drivers of the Pharmacological Actions of Medicinal Plants. In Herbal Medicine; Builders, P.F., Ed.; IntechOpen: London, UK, 2019; p. 314. ISBN 978-1-78984-783-3. [Google Scholar]
- Caramelo, D.; Barroca, C.; Guiné, R.; Gallardo, E.; Anjos, O.; Gominho, J. Potential Applications of the Cytisus Shrub Species: Cytisus multiflorus, Cytisus scoparius, and Cytisus striatus. Processes 2022, 10, 1287. [Google Scholar] [CrossRef]
- López-Hortas, L.; Falqué, E.; Domínguez, H.; Torres, M.D. Microwave Hydrodiffusion and Gravity (MHG) Extraction from Different Raw Materials with Cosmetic Applications. Molecules 2019, 25, 92. [Google Scholar] [CrossRef] [PubMed]
- Lores, M.; Pájaro, M.; Álvarez-Casas, M.; Domínguez, J.; García-Jares, C. Use of Ethyl Lactate to Extract Bioactive Compounds from Cytisus scoparius: Comparison of Pressurized Liquid Extraction and Medium Scale Ambient Temperature Systems. Talanta 2015, 140, 134–142. [Google Scholar] [CrossRef]
- Bouziane, A.; Bakchiche, B.; Dias, M.; Barros, L.; Ferreira, I.; AlSalamat, H.; Bardaweel, S. Phenolic Compounds and Bioactivity of Cytisus villosus Pourr. Molecules 2018, 23, 1994. [Google Scholar] [CrossRef]
- Daghbouche, S.; Ammar, I.; Rekik, D.M.; Djazouli, Z.-E.; Zebib, B.; Merah, O. Effect of Phenological Stages on Essential Oil Composition of Cytisus triflorus L’Her. J. King Saud Univ.-Sci. 2020, 32, 2383–2387. [Google Scholar] [CrossRef]
- Pinela, J.; Barros, L.; Carvalho, A.M.; Ferreira, I.C.F.R. Influence of the Drying Method in the Antioxidant Potential and Chemical Composition of Four Shrubby Flowering Plants from the Tribe Genisteae (Fabaceae). Food Chem. Toxicol. 2011, 49, 2983–2989. [Google Scholar] [CrossRef]
- González, N.; Ribeiro, D.; Fernandes, E.; Nogueira, D.R.; Conde, E.; Moure, A.; Vinardell, M.P.; Mitjans, M.; Domínguez, H. Potential Use of Cytisus scoparius Extracts in Topical Applications for Skin Protection against Oxidative Damage. J. Photochem. Photobiol. B Biol. 2013, 125, 83–89. [Google Scholar] [CrossRef]
- Pereira, O.R.; Silva, A.M.S.; Domingues, M.R.M.; Cardoso, S.M. Identification of Phenolic Constituents of Cytisus multiflorus. Food Chem. 2012, 131, 652–659. [Google Scholar] [CrossRef]
- Flora-On Portugal Continental|Flora de Portugal Interactiva. Available online: https://flora-on.pt/ (accessed on 29 March 2023).
- Waseem, M.; Majeed, Y.; Nadeem, T.; Naqvi, L.H.; Khalid, M.A.; Sajjad, M.M.; Sultan, M.; Khan, M.U.; Khayrullin, M.; Shariati, M.A.; et al. Conventional and Advanced Extraction Methods of Some Bioactive Compounds with Health Benefits of Food and Plant Waste: A Comprehensive Review. Food Front. 2023, 4, 1681–1701. [Google Scholar] [CrossRef]
- Wen, L.; Zhang, Z.; Sun, D.-W.; Sivagnanam, S.P.; Tiwari, B.K. Combination of Emerging Technologies for the Extraction of Bioactive Compounds. Crit. Rev. Food Sci. Nutr. 2020, 60, 1826–1841. [Google Scholar] [CrossRef] [PubMed]
- Roth, G.A.; Johnson, C.; Abajobir, A.; Abd-Allah, F.; Abera, S.F.; Abyu, G.; Ahmed, M.; Aksut, B.; Alam, T.; Alam, K.; et al. Global, Regional, and National Burden of Cardiovascular Diseases for 10 Causes, 1990 to 2015. J. Am. Coll. Cardiol. 2017, 70, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Rico, X.; Gullón, B.; Alonso, J.L.; Yáñez, R. Recovery of High Value-Added Compounds from Pineapple, Melon, Watermelon and Pumpkin Processing By-Products: An Overview. Food Res. Int. 2020, 132, 109086. [Google Scholar] [CrossRef] [PubMed]
- Ameer, K.; Shahbaz, H.M.; Kwon, J.-H. Green Extraction Methods for Polyphenols from Plant Matrices and Their Byproducts: A Review. Compr. Rev. Food Sci. Food Saf. 2017, 16, 295–315. [Google Scholar] [CrossRef] [PubMed]
- Usman, I.; Hussain, M.; Imran, A.; Afzaal, M.; Saeed, F.; Javed, M.; Afzal, A.; Ashfaq, I.; Al Jbawi, E.; Saewan, S.A. Traditional and Innovative Approaches for the Extraction of Bioactive Compounds. Int. J. Food Prop. 2022, 25, 1215–1233. [Google Scholar] [CrossRef]
- Ferreira-Santos, P.; Genisheva, Z.; Pereira, R.N.; Teixeira, J.A.; Rocha, C.M.R. Moderate Electric Fields as a Potential Tool for Sustainable Recovery of Phenolic Compounds from Pinus pinaster Bark. ACS Sustain. Chem. Eng. 2019, 7, 8816–8826. [Google Scholar] [CrossRef]
- Ramos, M.; Laveriano, E.; San Sebastián, L.; Perez, M.; Jiménez, A.; Lamuela-Raventos, R.M.; Garrigós, M.C.; Vallverdú-Queralt, A. Rice Straw as a Valuable Source of Cellulose and Polyphenols: Applications in the Food Industry. Trends Food Sci. Technol. 2023, 131, 14–27. [Google Scholar] [CrossRef]
- González, N.; Otero, A.; Conde, E.; Falqué, E.; Moure, A.; Domínguez, H. Extraction of Phenolics from Broom Branches Using Green Technologies. J. Chem. Technol. Biotechnol. 2017, 92, 1345–1352. [Google Scholar] [CrossRef]
- Sundararajan, R.; Haja, N.A.; Venkatesan, K.; Mukherjee, K.; Saha, B.P.; Bandyopadhyay, A.; Mukherjee, P.K. Cytisus scoparius Link—A Natural Antioxidant. BMC Complement. Altern. Med. 2006, 6, 8. [Google Scholar] [CrossRef]
- Barros, L.; Dueñas, M.; Carvalho, A.M.; Ferreira, I.C.F.R.; Santos-Buelga, C. Characterization of Phenolic Compounds in Flowers of Wild Medicinal Plants from Northeastern Portugal. Food Chem. Toxicol. 2012, 50, 1576–1582. [Google Scholar] [CrossRef]
- Larit, F.; León, F.; Benyahia, S.; Cutler, S. Total Phenolic and Flavonoid Content and Biological Activities of Extracts and Isolated Compounds of Cytisus villosus Pourr. Biomolecules 2019, 9, 732. [Google Scholar] [CrossRef]
- Okolie, C.L.; Akanbi, T.O.; Mason, B.; Udenigwe, C.C.; Aryee, A.N.A. Influence of Conventional and Recent Extraction Technologies on Physicochemical Properties of Bioactive Macromolecules from Natural Sources: A Review. Food Res. Int. 2019, 116, 827–839. [Google Scholar] [CrossRef] [PubMed]
- Vinatoru, M.; Mason, T.J.; Calinescu, I. Ultrasonically Assisted Extraction (UAE) and Microwave Assisted Extraction (MAE) of Functional Compounds from Plant Materials. TrAC Trends Anal. Chem. 2017, 97, 159–178. [Google Scholar] [CrossRef]
- Carbonell-Capella, J.M.; Šic Žlabur, J.; Rimac Brnčić, S.; Barba, F.J.; Grimi, N.; Koubaa, M.; Brnčić, M.; Vorobiev, E. Electrotechnologies, Microwaves, and Ultrasounds Combined with Binary Mixtures of Ethanol and Water to Extract Steviol Glycosides and Antioxidant Compounds from Stevia rebaudiana Leaves. J. Food Process. Preserv. 2017, 41, e13179. [Google Scholar] [CrossRef]
- Soquetta, M.B.; Terra, L.d.M.; Bastos, C.P. Green Technologies for the Extraction of Bioactive Compounds in Fruits and Vegetables. CYTA—J. Food 2018, 16, 400–412. [Google Scholar] [CrossRef]
- bin Azmi, A.A.; Sankaran, R.; Show, P.L.; Ling, T.C.; Tao, Y.; Munawaroh, H.S.H.; Kong, P.S.; Lee, D.-J.; Chang, J.-S. Current Application of Electrical Pre-Treatment for Enhanced Microalgal Biomolecules Extraction. Bioresour. Technol. 2020, 302, 122874. [Google Scholar] [CrossRef]
- Puértolas, E.; Barba, F.J. Electrotechnologies Applied to Valorization of By-Products from Food Industry: Main Findings, Energy and Economic Cost of Their Industrialization. Food Bioprod. Process. 2016, 100, 172–184. [Google Scholar] [CrossRef]
- Ferreira-Santos, P.; Zanuso, E.; Genisheva, Z.; Rocha, C.M.R.; Teixeira, J.A. Green and Sustainable Valorization of Bioactive Phenolic Compounds from Pinus By-Products. Molecules 2020, 25, 2931. [Google Scholar] [CrossRef]
- Li, Z.; Fan, Y.; Xi, J. Recent Advances in High Voltage Electric Discharge Extraction of Bioactive Ingredients from Plant Materials. Food Chem. 2019, 277, 246–260. [Google Scholar] [CrossRef] [PubMed]
- Barba, F.J.; Zhu, Z.; Koubaa, M.; Sant’Ana, A.S.; Orlien, V. Green Alternative Methods for the Extraction of Antioxidant Bioactive Compounds from Winery Wastes and By-Products: A Review. Trends Food Sci. Technol. 2016, 49, 96–109. [Google Scholar] [CrossRef]
- Bonifácio-Lopes, T.; Teixeira, J.A.; Pintado, M. Current Extraction Techniques towards Bioactive Compounds from Brewer’s Spent Grain–A Review. Crit. Rev. Food Sci. Nutr. 2020, 60, 2730–2741. [Google Scholar] [CrossRef]
- Gallego, R.; Bueno, M.; Herrero, M. Sub- and Supercritical Fluid Extraction of Bioactive Compounds from Plants, Food-by-Products, Seaweeds and Microalgae—An Update. TrAC Trends Anal. Chem. 2019, 116, 198–213. [Google Scholar] [CrossRef]
- Dzah, C.S.; Duan, Y.; Zhang, H.; Wen, C.; Zhang, J.; Chen, G.; Ma, H. The Effects of Ultrasound Assisted Extraction on Yield, Antioxidant, Anticancer and Antimicrobial Activity of Polyphenol Extracts: A Review. Food Biosci. 2020, 35, 100547. [Google Scholar] [CrossRef]
- Tao, H.; Liu, X.; Tian, R.; Liu, Y.; Zeng, Y.; Meng, X.; Zhang, Y. A Review: Pharmacokinetics and Pharmacology of Aminoalcohol-Diterpenoid Alkaloids from Aconitum Species. J. Ethnopharmacol. 2023, 301, 115726. [Google Scholar] [CrossRef] [PubMed]
- Luís, Â.; Domingues, F.; Duarte, A.P. Bioactive Compounds, RP-HPLC Analysis of Phenolics, and Antioxidant Activity of Some Portuguese Shrub Species Extracts. Nat. Prod. Commun. 2011, 6, 1863–1872. [Google Scholar] [CrossRef] [PubMed]
- Ardekani, M.R.S.; Rahimi, R.; Javadi, B.; Abdi, L.; Khanavi, M. Relationship between Temperaments of Medicinal Plants and Their Major Chemical Compounds. J. Tradit. Chin. Med. 2011, 31, 27–31. [Google Scholar] [CrossRef]
- Pardo-Muras, M.; Puig, C.G.; Souto, X.C.; Pedrol, N. Water-Soluble Phenolic Acids and Flavonoids Involved in the Bioherbicidal Potential of Ulex Europaeus and Cytisus scoparius. S. Afr. J. Bot. 2020, 133, 201–211. [Google Scholar] [CrossRef]
- Li, Y.; Ji, S.; Xu, T.; Zhong, Y.; Xu, M.; Liu, Y.; Li, M.; Fan, B.; Wang, F.; Xiao, J.; et al. Chinese Yam (Dioscorea): Nutritional Value, Beneficial Effects, and Food and Pharmaceutical Applications. Trends Food Sci. Technol. 2023, 134, 29–40. [Google Scholar] [CrossRef]
- Benabderrahmane, W.; Amrani, A.; Benaissa, O.; Lores, M.; Lamas, J.P.; de Miguel, T.; Benayache, F.; Benayache, S. Chemical Constituents, in Vitro Antioxidant and Antimicrobial Properties of Ethyl Acetate Extract Obtained from Cytisus triflorus l’Her. Nat. Prod. Res. 2020, 34, 1586–1590. [Google Scholar] [CrossRef]
- Pereira, O.R.; Macias, R.I.R.; Perez, M.J.; Marin, J.J.G.; Cardoso, S.M. Protective Effects of Phenolic Constituents from Cytisus multiflorus, Lamium album L. and Thymus citriodorus on Liver Cells. J. Funct. Foods 2013, 5, 1170–1179. [Google Scholar] [CrossRef]
- Yahiaoui, F.; Zaouani, M.; Kardjadj, M.; Laghouati, A.; Kadour, R.; Bouzid, N.; Ben-Mahdi, M.H. Antibacterial, Antioxidant and Wound Healing Activities of Marrubium vulgare and Cytisus triflorus Extracts Native to Algeria. Phytothérapie 2018, 16, S32–S39. [Google Scholar] [CrossRef]
- Boulanouar, B.; Abdelaziz, G.; Aazza, S.; Gago, C.; Miguel, M.G. Antioxidant Activities of Eight Algerian Plant Extracts and Two Essential Oils. Ind. Crops Prod. 2013, 46, 85–96. [Google Scholar] [CrossRef]
- Larit, F.; Nael, M.A.; Benyahia, S.; Radwan, M.M.; León, F.; Jasicka-Misiak, I.; Poliwoda, A.; Wieczorek, D.; Benayache, F.; Benayache, S.; et al. Secondary Metabolites from the Aerial Parts of Cytisus villosus Pourr. Phytochem. Lett. 2018, 24, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Bryant, C.J. Plant-Based Animal Product Alternatives Are Healthier and More Environmentally Sustainable than Animal Products. Futur. Foods 2022, 6, 100174. [Google Scholar] [CrossRef]
- Donkor, L.; Kontoh, G.; Yaya, A.; Bediako, J.K.; Apalangya, V. Bio-Based and Sustainable Food Packaging Systems: Relevance, Challenges, and Prospects. Appl. Food Res. 2023, 3, 100356. [Google Scholar] [CrossRef]
- Langyan, S.; Yadava, P.; Khan, F.N.; Dar, Z.A.; Singh, R.; Kumar, A. Sustaining Protein Nutrition Through Plant-Based Foods. Front. Nutr. 2022, 8, 772573. [Google Scholar] [CrossRef]
- McClements, I.F.; McClements, D.J. Designing Healthier Plant-Based Foods: Fortification, Digestion, and Bioavailability. Food Res. Int. 2023, 169, 112853. [Google Scholar] [CrossRef] [PubMed]
- Iyer, A.; Bestwick, C.S.; Duncan, S.H.; Russell, W.R. Invasive Plants Are a Valuable Alternate Protein Source and Can Contribute to Meeting Climate Change Targets. Front. Sustain. Food Syst. 2021, 5, 575056. [Google Scholar] [CrossRef]
- Zang, E.; Jiang, L.; Cui, H.; Li, X.; Yan, Y.; Liu, Q.; Chen, Z.; Li, M. Only Plant-Based Food Additives: An Overview on Application, Safety, and Key Challenges in the Food Industry. Food Rev. Int. 2023, 39, 5132–5163. [Google Scholar] [CrossRef]
- Khan, H.; Ullah, H.; Aschner, M.; Cheang, W.S.; Akkol, E.K. Neuroprotective Effects of Quercetin in Alzheimer’s Disease. Biomolecules 2020, 10, 59. [Google Scholar] [CrossRef]
- Fernandes, M.Y.D.; Dobrachinski, F.; Silva, H.B.; Lopes, J.P.; Gonçalves, F.Q.; Soares, F.A.A.; Porciúncula, L.O.; Andrade, G.M.; Cunha, R.A.; Tomé, A.R. Neuromodulation and Neuroprotective Effects of Chlorogenic Acids in Excitatory Synapses of Mouse Hippocampal Slices. Sci. Rep. 2021, 11, 10488. [Google Scholar] [CrossRef] [PubMed]
- Chook, C.Y.B.; Cheung, Y.M.; Ma, K.Y.; Leung, F.P.; Zhu, H.; Niu, Q.J.; Wong, W.T.; Chen, Z.Y. Physiological Concentration of Protocatechuic Acid Directly Protects Vascular Endothelial Function against Inflammation in Diabetes through Akt/ENOS Pathway. Front. Nutr. 2023, 10, 1060226. [Google Scholar] [CrossRef]
- Sahraee, S.; Milani, J.M.; Regenstein, J.M.; Kafil, H.S. Protection of Foods against Oxidative Deterioration Using Edible Films and Coatings: A Review. Food Biosci. 2019, 32, 100451. [Google Scholar] [CrossRef]
- Rangaraj, V.M.; Rambabu, K.; Banat, F.; Mittal, V. Natural Antioxidants-Based Edible Active Food Packaging: An Overview of Current Advancements. Food Biosci. 2021, 43, 101251. [Google Scholar] [CrossRef]
- Jones, B.B.; Okokon, I.O.; Offiong, A.E. Advances in the Modification of Natural Polymers for Applications in Food Packaging and Preservation. Int. J. Res. Sci. Eng. 2022, 2, 1–9. [Google Scholar] [CrossRef]
- Naceiri Mrabti, H.; Bouyahya, A.; Naceiri Mrabti, N.; Jaradat, N.; Doudach, L.; Faouzi, M.E.A. Ethnobotanical Survey of Medicinal Plants Used by Traditional Healers to Treat Diabetes in the Taza Region of Morocco. Evid.-Based Complement. Altern. Med. 2021, 2021, 5515634. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, T.; Reker, D.; Schneider, P.; Schneider, G. Counting on Natural Products for Drug Design. Nat. Chem. 2016, 8, 531–541. [Google Scholar] [CrossRef] [PubMed]
- Raja, S.; Ahamed, K.F.H.N.; Kumar, V.; Mukherjee, K.; Bandyopadhyay, A.; Mukherjee, P.K. Antioxidant Effect of Cytisus scoparius against Carbon Tetrachloride Treated Liver Injury in Rats. J. Ethnopharmacol. 2007, 109, 41–47. [Google Scholar] [CrossRef]
- Nirmal, J.; Babu, C.S.; Harisudhan, T.; Ramanathan, M. Evaluation of Behavioural and Antioxidant Activity of Cytisus scoparius Link in Rats Exposed to Chronic Unpredictable Mild Stress. BMC Complement. Altern. Med. 2008, 8, 15. [Google Scholar] [CrossRef] [PubMed]
- Anees Ali Jafri, S.; Mehmood Khalid, Z.; Rizwan Khan, M.; Ashraf, S.; Ahmad, N.; Mahmoud Karami, A.; Rafique, E.; Ouladsmane, M.; Mohammad Saad Al Suliman, N.; Aslam, S. Evaluation of Some Essential Traditional Medicinal Plants for Their Potential Free Scavenging and Antioxidant Properties. J. King Saud Univ.-Sci. 2023, 35, 102562. [Google Scholar] [CrossRef]
- Martins, D.; Barros, L.; Carvalho, A.M.; Ferreira, I.C.F.R. Nutritional and in Vitro Antioxidant Properties of Edible Wild Greens in Iberian Peninsula Traditional Diet. Food Chem. 2011, 125, 488–494. [Google Scholar] [CrossRef]
- Chaitanya, M.; Papathoti, N.K.; Usamo, F.B.; Palanisami, D.; Selvaraj, J.; Thangavelu, P. Phytochemical Investigation and Cytotoxic Profile of Genistein Isolated from the Cytisus scoparius Linn. on Topoisomerase II. Curr. Bioact. Compd. 2022, 18, 54–63. [Google Scholar] [CrossRef]
- Ait-KaciAourahoun, K.; Fazouane, F.; Benayache, S. Pharmacological Potential of Cytisus triflorus l’Hérit. Extracts as Antioxidant and Anti-Inflammatory Agent. Pharm. Lett. 2015, 7, 104–110. [Google Scholar]
- Jouad, H.; Haloui, M.; Rhiouani, H.; El Hilaly, J.; Eddouks, M. Ethnobotanical Survey of Medicinal Plants Used for the Treatment of Diabetes, Cardiac and Renal Diseases in the North Centre Region of Morocco (Fez–Boulemane). J. Ethnopharmacol. 2001, 77, 175–182. [Google Scholar] [CrossRef]
- Pan, S.Y.; Zhou, S.F.; Gao, S.H.; Yu, Z.L.; Zhang, S.F.; Tang, M.K.; Sun, J.N.; Ma, D.L.; Han, Y.F.; Fong, W.F.; et al. New Perspectives on How to Discover Drugs from Herbal Medicines: CAM’s Outstanding Contribution to Modern Therapeutics. Evid. Based. Complement. Alternat. Med. 2013, 2013, 627375. [Google Scholar] [CrossRef]
- Barkaoui, M.; Katiri, A.; Boubaker, H.; Msanda, F. Ethnobotanical Survey of Medicinal Plants Used in the Traditional Treatment of Diabetes in Chtouka Ait Baha and Tiznit (Western Anti-Atlas), Morocco. J. Ethnopharmacol. 2017, 198, 338–350. [Google Scholar] [CrossRef]
- Yaseen, G.; Ahmad, M.; Zafar, M.; Sultana, S.; Kayani, S.; Cetto, A.A.; Shaheen, S. Traditional Management of Diabetes in Pakistan: Ethnobotanical Investigation from Traditional Health Practitioners. J. Ethnopharmacol. 2015, 174, 91–117. [Google Scholar] [CrossRef]
- Elbermawi, A.; Ali, A.R.; Amen, Y.; Ashour, A.; Ahmad, K.F.; Mansour, E.-S.S.; Halim, A.F. Anti-Diabetic Activities of Phenolic Compounds of Alternaria Sp., an Endophyte Isolated from the Leaves of Desert Plants Growing in Egypt. RSC Adv. 2022, 12, 24935–24945. [Google Scholar] [CrossRef]
- Rashid, N.; Siddique, W.; Zaheer, Z. Evaluation of Effect of Argyrolobiumroseum Aqueous Extract on Carbon Tetrachloride Induced Liver Injury in Rabbits. Pakistan J. Med. Health Sci. 2018, 12, 161–165. [Google Scholar]
- Ginovyan, M.; Trchounian, A. Novel Approach to Combat Antibiotic Resistance: Evaluation of Some Armenian Herb Crude Extracts for Their Antibiotic Modulatory and Antiviral Properties. J. Appl. Microbiol. 2019, 127, 472–480. [Google Scholar] [CrossRef]
- Abidullah, S.; Rauf, A.; Khan, S.W.; Ayaz, A.; Liaquat, F.; Saqib, S. A Comprehensive Review on Distribution, Paharmacological Uses and Biological Activities of Argyrolobium roseum (Cambess.) Jaub. & Spach. Acta Ecol. Sin. 2022, 42, 198–205. [Google Scholar] [CrossRef]
- Djeussi, D.E.; Noumedem, J.A.K.; Seukep, J.A.; Fankam, A.G.; Voukeng, I.K.; Tankeo, S.B.; Nkuete, A.H.L.; Kuete, V. Antibacterial Activities of Selected Edible Plants Extracts against Multidrug-Resistant Gram-Negative Bacteria. BMC Complement. Altern. Med. 2013, 13, 164. [Google Scholar] [CrossRef] [PubMed]
- Abreu, A.C.; Coqueiro, A.; Sultan, A.R.; Lemmens, N.; Kim, H.K.; Verpoorte, R.; Van Wamel, W.J.B.; Simões, M.; Choi, Y.H. Looking to Nature for a New Concept in Antimicrobial Treatments: Isoflavonoids from Cytisus striatus as Antibiotic Adjuvants against MRSA. Sci. Rep. 2017, 7, 3777. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).