Redox System and Oxidative Stress-Targeted Therapeutic Approaches in Bladder Cancer
Abstract
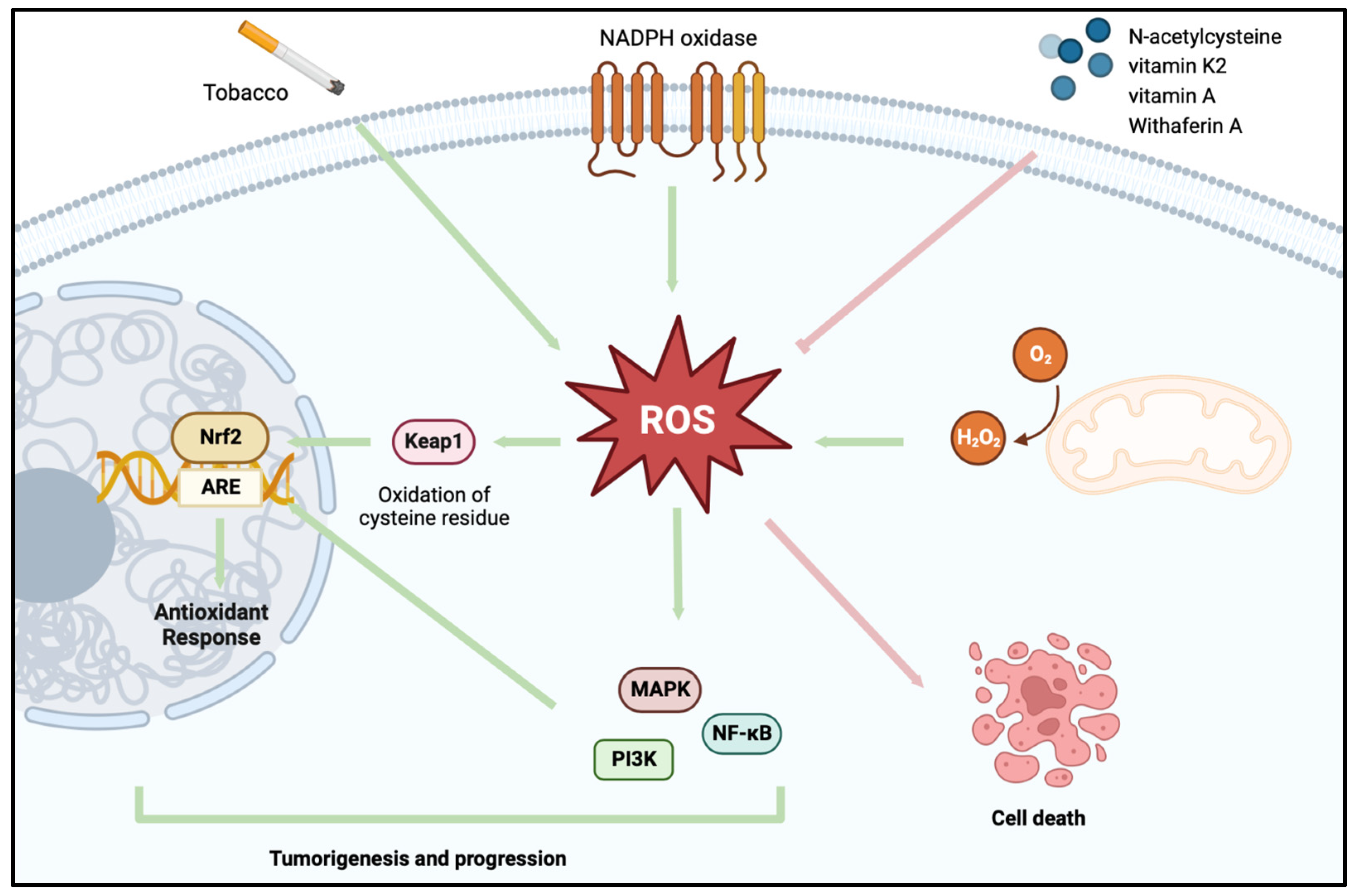
1. Introduction
2. Redox Homeostasis
A Shift in the Redox System in BCa
3. Utilizing Oxidative Stress-Based Strategies in the Treatment of BCa
3.1. Eliminating the ROS-Facilitated Tumor Cell Survival Advantage
3.2. Utilizing ROS to Induce Tumor Cell Self-Destruction
| Pro-Oxidant Agent | Model System | Observed Effect | Reference |
|---|---|---|---|
| Emodin (1,3,8-trihydroxy-6-methylanthraquinone) | In vitro human BCa cells | Enhanced susceptibility to cisplatin | [88] |
| Cordycepin | In vitro human BCa cells | Increased tumor cell apoptotic cell death | [89] |
| Withaferin A | In vitro human BCa cells | Increased tumor cell DNA damage and apoptotic cell death | [90] |
| Vitamin K2 | Mouse, tumor site injection | Decreased tumor burden and increased tumor cell apoptosis | [91] |
| Jolkinolide B | In vitro cisplatin resistant-BCa cells | Induced apoptosis and paraptosis | [95] |
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Richters, A.; Aben, K.K.H.; Kiemeney, L.A.L.M. The global burden of urinary bladder cancer: An update. World J. Urol. 2019, 38, 1895–1904. [Google Scholar] [CrossRef]
- Saginala, K.; Barsouk, A.; Aluru, J.S.; Rawla, P.; Padala, S.A.; Barsouk, A. Epidemiology of Bladder Cancer. Med. Sci. 2020, 8, 15. [Google Scholar] [CrossRef]
- Dobruch, J.; Daneshmand, S.; Fisch, M.; Lotan, Y.; Noon, A.P.; Resnick, M.J.; Shariat, S.F.; Zlotta, A.R.; Boorjian, S.A. Gender and Bladder Cancer: A Collaborative Review of Etiology, Biology, and Outcomes. Eur. Urol. 2016, 69, 300–310. [Google Scholar] [CrossRef]
- Lenis, A.T.; Lec, P.M.; Chamie, K.; Mshs, M.D. Bladder Cancer: A Review. JAMA 2020, 324, 1980–1991. [Google Scholar] [CrossRef] [PubMed]
- Kamat, A.M.; Hahn, N.M.; Efstathiou, J.A.; Lerner, S.P.; Malmström, P.U.; Choi, W.; Guo, C.C.; Lotan, Y.; Kassouf, W. Bladder cancer. Lancet 2016, 388, 2796–2810. [Google Scholar] [CrossRef] [PubMed]
- Freedman, N.D.; Silverman, D.T.; Hollenbeck, A.R.; Schatzkin, A.; Abnet, C.C. Association between smoking and risk of bladder cancer among men and women. JAMA 2011, 306, 737–745. [Google Scholar] [CrossRef] [PubMed]
- Wigner, P.; Grębowski, R.; Bijak, M.; Saluk-Bijak, J.; Szemraj, J. The Interplay between Oxidative Stress, Inflammation and Angiogenesis in Bladder Cancer Development. Int. J. Mol. Sci. 2021, 22, 4483. [Google Scholar] [CrossRef] [PubMed]
- Makena, P.; Chung, K.T. Evidence that 4-aminobiphenyl, benzidine, and benzidine congeners produce genotoxicity through reactive oxygen species. Environ. Mol. Mutagen. 2007, 48, 404–413. [Google Scholar] [CrossRef]
- Hu, Y.; Li, J.; Lou, B.; Wu, R.; Wang, G.; Lu, C.; Wang, H.; Pi, J.; Xu, Y. The Role of Reactive Oxygen Species in Arsenic Toxicity. Biomolecules 2020, 10, 240. [Google Scholar] [CrossRef] [PubMed]
- Ohnishi, S.; Murata, M.; Kawanishi, S. Oxidative DNA damage induced by a metabolite of 2-naphthylamine, a smoking-related bladder carcinogen. Jpn. J. Cancer Res. 2002, 93, 736–743. [Google Scholar] [CrossRef]
- Mushtaq, J.; Thurairaja, R.; Nair, R. Bladder cancer. Surgery 2019, 37, 529–537. [Google Scholar] [CrossRef]
- Batista, R.; Vinagre, N.; Meireles, S.; Vinagre, J.; Prazeres, H.; Leão, R.; Máximo, V.; Soares, P. Biomarkers for Bladder Cancer Diagnosis and Surveillance: A Comprehensive Review. Diagnostics 2020, 10, 39. [Google Scholar] [CrossRef] [PubMed]
- Soorojebally, Y.; Neuzillet, Y.; Roumiguié, M.; Lamy, P.J.; Allory, Y.; Descotes, F.; Ferlicot, S.; Kassab-Chahmi, D.; Oudard, S.; Rébillard, X.; et al. Urinary biomarkers for bladder cancer diagnosis and NMIBC follow-up: A systematic review. World J. Urol. 2023, 41, 345–359. [Google Scholar] [CrossRef]
- Anastasiadis, A.; de Reijke, T.M. Best practice in the treatment of nonmuscle invasive bladder cancer. Ther. Adv. Urol. 2011, 4, 13–32. [Google Scholar] [CrossRef] [PubMed]
- Flaig, T.W.; Spiess, P.E.; Agarwal, N.; Bangs, R.; Boorjian, S.A.; Buyyounouski, M.K.; Chang, S.; Downs, T.M.; Efstathiou, J.A.; Friedlander, T.; et al. Bladder Cancer, Version 3.2020, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2020, 18, 329–354. [Google Scholar] [CrossRef]
- Sylvester, R.J.; van der Meijden, A.P.; Witjes, J.A.; Kurth, K. Bacillus calmette-guerin versus chemotherapy for the intravesical treatment of patients with carcinoma in situ of the bladder: A meta-analysis of the published results of randomized clinical trials. J. Urol. 2005, 174, 86–91. [Google Scholar] [CrossRef]
- Chang, S.S.; Bochner, B.H.; Chou, R.; Dreicer, R.; Kamat, A.M.; Lerner, S.P.; Lotan, Y.; Meeks, J.J.; Michalski, J.M.; Morgan, T.M.; et al. Treatment of Non-Metastatic Muscle-Invasive Bladder Cancer: AUA/ASCO/ASTRO/SUO Guideline. J. Urol. 2017, 198, 552–559. [Google Scholar] [CrossRef]
- Herr, H.W.; Morales, A. History of bacillus Calmette-Guerin and bladder cancer: An immunotherapy success story. J. Urol. 2008, 179, 53–56. [Google Scholar] [CrossRef]
- Messing, E.M. The BCG Shortage. Bladder Cancer 2017, 3, 227–228. [Google Scholar] [CrossRef]
- Witjes, J.A. Management of BCG failures in superficial bladder cancer: A review. Eur. Urol. 2006, 49, 790–797. [Google Scholar] [CrossRef]
- Kamat, A.M.; Colombel, M.; Sundi, D.; Lamm, D.; Boehle, A.; Brausi, M.; Buckley, R.; Persad, R.; Palou, J.; Soloway, M.; et al. BCG-unresponsive non-muscle-invasive bladder cancer: Recommendations from the IBCG. Nat. Rev. Urol. 2017, 14, 244–255. [Google Scholar] [CrossRef]
- Suh, J.; Yoo, S. Role of immunotherapy in Bacillus Calmette-Guerin unresponsive: Non-muscle invasive bladder cancer. Transl. Cancer Res. 2020, 9, 6537–6545. [Google Scholar] [CrossRef]
- Andreyev, A.Y.; Kushnareva, Y.E.; Starkov, A.A. Mitochondrial metabolism of reactive oxygen species. Biochemistry 2005, 70, 200–214. [Google Scholar] [CrossRef]
- Dunn, J.D.; Alvarez, L.A.; Zhang, X.; Soldati, T. Reactive oxygen species and mitochondria: A nexus of cellular homeostasis. Redox Biol. 2015, 6, 472–485. [Google Scholar] [CrossRef]
- Fukai, T.; Ushio-Fukai, M. Cross-talk between NADPH oxidase and mitochondria: Role of ROS signaling and angiogenesis. Cells 2020, 9, 1849. [Google Scholar] [CrossRef]
- Wong, H.S.; Benoit, B.; Brand, M.D. Mitochondrial and cytosolic sources of hydrogen peroxide in resting C2C12 myoblasts. Free Radic. Biol. Med. 2019, 130, 140–150. [Google Scholar] [CrossRef] [PubMed]
- Forrester, S.J.; Kikuchi, D.S.; Hernandes, M.S.; Xu, Q.; Griendling, K.K. Reactive oxygen species in metabolic and inflammatory signaling. Circ. Res. 2018, 122, 877–902. [Google Scholar] [CrossRef] [PubMed]
- Trachootham, D.; Lu, W.; Ogasawara, M.A.; Nilsa, R.D.; Huang, P. Redox regulation of cell survival. Antioxid. Redox Signal. 2008, 10, 1343–1374. [Google Scholar] [CrossRef] [PubMed]
- Sauer, H.; Wartenberg, M.; Hescheler, J. Reactive oxygen species as intracellular messengers during cell growth and differentiation. Cell. Physiol. Biochem. 2001, 11, 173–186. [Google Scholar] [CrossRef] [PubMed]
- Hayes, J.D.; Dinkova-Kostova, A.T.; Tew, K.D. Oxidative stress in cancer. Cancer Cell 2020, 38, 167–197. [Google Scholar] [CrossRef]
- Jung, K.A.; Kwak, M.K. The NRF2 system as a potential target for the development of indirect antioxidants. Molecules 2010, 15, 7266–7291. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, X.; Cueto, R.; Effi, C.; Zhang, Y.; Tan, H.; Qin, X.; Ji, Y.; Yang, X.; Wang, H. Biochemical basis and metabolic interplay of redox regulation. Redox Biol. 2019, 26, 101284. [Google Scholar] [CrossRef]
- Kamp, D.W.; Shacter, E.; Weitzman, S.A. Chronic inflammation and cancer: The role of the mitochondria. Oncology 2011, 25, 400–410, 413. [Google Scholar] [PubMed]
- Yang, H.; Villani, R.M.; Wang, H.; Simpson, M.J.; Roberts, M.S.; Tang, M.; Liang, X. The role of cellular reactive oxygen species in cancer chemotherapy. J. Exp. Clin. Cancer Res. 2018, 37, 266. [Google Scholar] [CrossRef]
- Liu, D.; Qui, X.; Xiong, X.; Chen, X.; Pan, F. Current updates on the role of reactive oxygen species in bladder cancer pathogenesis and therapeutics. Clin. Transl. Oncol. 2020, 22, 1687–1697. [Google Scholar] [CrossRef]
- Szymańska, B.; Sawicka, E.; Matuszewski, M.; Dembowski, J.; Piwowar, A. The Dependence between Urinary Levels of Angiogenesis Factors, 8-Iso-prostaglandin F2α, ɣ-Synuclein, and Interleukin-13 in Patients with Bladder Cancer: A Pilot Study. J. Oncol. 2020, 2020, 4848752. [Google Scholar] [CrossRef]
- Jelic, M.D.; Mandic, A.D.; Maricic, S.M.; Srdjenovic, B.U. Oxidative stress and its role in cancer. J. Cancer Res. Ther. 2021, 17, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Lepara, Z.; Lepara, O.; Fajkić, A.; Rebić, D.; Alić, J.; Spahović, H. Serum malondialdehyde (MDA) level as a potential biomarker of cancer progression for patients with bladder cancer. Rom. J. Intern. Med. 2020, 58, 146–152. [Google Scholar] [CrossRef] [PubMed]
- Gecit, İ.; Eryılmaz, R.; Kavak, S.; Meral, İ.; Demir, H.; Pirinççi, N.; Güneş, M.; Taken, K. The Prolidase Activity, Oxidative Stress, and Nitric Oxide Levels of Bladder Tissues with or Without Tumor in Patients with Bladder Cancer. J. Membr. Biol. 2017, 250, 455–459. [Google Scholar] [CrossRef]
- Jeon, S.H.; Park, J.H.; Chang, S.G. Expression of Antioxidant Enzymes (Catalase, Superoxide Dismutase, and Glutathione Peroxidase) in Human Bladder Cancer. Korean J. Urol. 2007, 48, 921–926. [Google Scholar] [CrossRef]
- Bayraktar, N.; Kilic, S.; Bayraktar, M.R.; Aksoy, N. Lipid peroxidation and antioxidant enzyme activities in cancerous bladder tissue and their relation with bacterial infection: A controlled clinical study. J. Clin. Lab. Anal. 2010, 24, 25–30. [Google Scholar] [CrossRef]
- Durak, I.; Perk, H.; Kavutçu, M.; Canbolat, O.; Akyol, O.; Bedük, Y. Adenosine deaminase, 5’nucleotidase, xanthine oxidase, superoxide dismutase, and catalase activities in cancerous and noncancerous human bladder tissues. Free Radic. Biol. Med. 1994, 16, 825–831. [Google Scholar] [CrossRef] [PubMed]
- Tafani, M.; Sansone, L.; Limana, F.; Arcangeli, T.; De Santis, E.; Polese, M.; Fini, M.; Russo, M.A. The interplay of reactive oxygen species, hypoxia, inflammation, and sirtuins in cancer initiation and progression. Oxid. Med. Cell. Longev. 2015, 2016, 3907147. [Google Scholar] [CrossRef] [PubMed]
- Harris, I.S.; DeNicola, G.M. The complex interplay between antioxidants and ROS in cancer. Trends Cell Biol. 2020, 30, 440–451. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Chun, F.K.H.; Rutz, J.; Blaheta, R.A. Sulforaphane impact on reaction oxygen species (ROS) in bladder carcinoma. Int. J. Mol. Sci. 2021, 22, 5938. [Google Scholar] [CrossRef]
- Robertson, H.; Dinkova-Kostova, A.T.; Hayes, J.D. NRF2 and the ambiguous consequences of its activation during initiation and the subsequent stages of tumorigenesis. Cancers 2020, 12, 3609. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Liu, H.; Lv, C.; Du, J.; Lian, F.; Zhang, S.; Wang, Z.; Zeng, Y. Gypenoside-Induced Apoptosis via the PI3K/AKT/mTOR Signaling Pathway in Bladder Cancer. BioMed Res Int. 2022, 2022, 9304552. [Google Scholar] [CrossRef] [PubMed]
- Bellamri, M.; Walmsley, S.J.; Brown, C.; Brandt, K.; Konorev, D.; Day, A.; Wu, C.F.; Wu, M.T.; Turesky, R.J. DNA damage and oxidative stress of tobacco smoke condensate in human bladder epithelial cells. Chem. Res. Toxicol. 2022, 35, 1863–1880. [Google Scholar] [CrossRef]
- Zeegers, M.P.; Tan, F.E.; Dorant, E.; van Den Brandt, P.A. The impact of characteristics of cigarette smoking on urinary tract cancer risk: A meta-analysis of epidemiologic studies. Cancer 2000, 89, 630–639. [Google Scholar] [CrossRef]
- Ciccarese, C.; Massari, F.; Blanca, A.; Tortora, G.; Montironi, R.; Cheng, L.; Scarpelli, M.; Raspollini, M.R.; Vau, N.; Fonseca, J.; et al. Tp53 and its potential therapeutic role as a target in bladder cancer. Expert Opin. Ther. Targets 2017, 21, 401–414. [Google Scholar] [CrossRef]
- Kim, J.; Akbani, R.; Creighton, C.J.; Lerner, S.P.; Weinstein, J.N.; Getz, G.; Kwiatkowski, D.J. Invasive bladder cancer: Genomic insights and therapeutic promise. Clin. Cancer Res. 2015, 21, 4514–4524. [Google Scholar] [CrossRef] [PubMed]
- Kopnin, P.B.; Agapova, L.S.; Kopnin, B.P.; Chumakov, P.M. Repression of Sestrin Family Genes Contributes to Oncogenic Ras-Induced Reactive Oxygen Species Up-regulation and Genetic Instability. Cancer Res. 2007, 67, 4671–4678. [Google Scholar] [CrossRef] [PubMed]
- Kung-Chun Chiu, D.; Pui-Wah Tse, A.; Law, C.T.; Ming-Jing Xu, I.; Lee, D.; Chen, M.; Kit-Ho Lai, R.; Wai-Hin Yuen, V.; Wing-Sum Cheu, J.; Wai-Hung Ho, D.; et al. Hypoxia regulates the mitochondrial activity of hepatocellular carcinoma cells through HIF/HEY1/PINK1 pathway. Cell Death Dis. 2019, 10, 934. [Google Scholar] [CrossRef] [PubMed]
- Brown, N.S.; Streeter, E.H.; Jones, A.; Harris, A.L.; Bicknell, R. Cooperative stimulation of vascular endothelial growth factor expression by hypoxia and reactive oxygen species: The effect of targeting vascular endothelial growth factor and oxidative stress in an orthotopic xenograft model of bladder carcinoma. Br. J. Cancer 2005, 92, 1696–1701. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Liang, S.; Dong, S.; Liu, W.; Wang, M.; Tian, S.; Ai, Y.; Wang, H. Accumulated ROS Activates HIF-1α-Induced Glycolysis and Exerts a Protective Effect on Sensory Hair Cells Against Noise-Induced Damage. Front. Mol. Biosci. 2022, 8, 806650. [Google Scholar] [CrossRef]
- Keith, B.; Simon, M.C. Hypoxia-Inducible Factors, Stem Cells, and Cancer. Cell 2007, 129, 465–472. [Google Scholar] [CrossRef] [PubMed]
- Theodoropoulos, V.E.; Lazaris, A.C.; Sofras, F.; Gerzelis, I.; Tsoukala, V.; Ghikonti, I.; Manikas, K.; Kastriotis, I. Hypoxia-Inducible Factor 1α Expression Correlates with Angiogenesis and Unfavorable Prognosis in Bladder Cancer. Eur. Urol. 2004, 46, 200–208. [Google Scholar] [CrossRef]
- Liu, Z.; Tang, Q.; Qi, T.; Othmane, B.; Yang, Z.; Chen, J.; Hu, J.; Zu, X. A robust hypoxia risk score predicts the clinical outcomes and tumor microenvironment immune characters in bladder cancer. Front. Immunol. 2021, 12, 725223. [Google Scholar] [CrossRef]
- Gecit, I.; Aslan, M.; Gunes, M.; Pirincci, N.; Esen, R.; Demir, H.; Ceylan, K. Serum prolidase activity, oxidative stress, and nitric oxide levels in patients with bladder cancer. J. Cancer Res. Clin. Oncol. 2012, 138, 739–743. [Google Scholar] [CrossRef]
- Mendes, F.; Pereira, E.; Martins, D.; Tavares-Silva, E.; Pires, A.S.; Abrantes, A.M.; Figueiredo, A.; Botelho, M.F. Oxidative stress in bladder cancer: An ally or an enemy? Mol. Biol. Rep. 2021, 48, 2791–2802. [Google Scholar] [CrossRef]
- Utangac, M.M.; Yeni, E.; Savas, M.; Altunkol, A.; Ciftci, H.; Gumus, K.; Demir, M. Paraoxonase and arylesterase activity in bladder cancer. Turk. J. Urol. 2017, 43, 147–151. [Google Scholar] [CrossRef]
- Islam, M.O.; Bacchetti, T.; Ferretti, G. Alterations of Antioxidant Enzymes and Biomarkers of Nitro-oxidative Stress in Tissues of Bladder Cancer. Oxid. Med. Cell. Longev. 2019, 2019, 2730896. [Google Scholar] [CrossRef]
- Sawicka, E.; Kratz, E.M.; Szymanska, B.; Guzik, A.; Wesolowski, A.; Kowal, P.; Pawlik-Sobecka, L.; Piwowar, A. Preliminary study on selected markers of oxidative stress, inflammation and angiogenesis in patients with bladder cancer. Pathol. Oncol. Res. 2020, 26, 821–831. [Google Scholar] [CrossRef]
- Wigner, P.; Szymanska, B.; Bijak, M.; Sawicka, E.; Kowal, P.; Marchewka, Z.; Saluk-Bijak, J. Oxidative stress parameters as biomarkers of bladder cancer development and progression. Sci. Rep. 2021, 11, 15134. [Google Scholar] [CrossRef]
- Zhang, M.; Du, G.; Li, Z.; Li, D.; Li, W.; Li, H.; Gao, X.; Tang, Z. An oxidative stress-related genes signature for predicting survival in bladder cancer: Based on TCGA database and bioinformatics. Int. J. Gen. Med. 2022, 15, 2645–2667. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, Y. Bladder cancer cells prevent cisplatin-induced oxidative stress by upregulating Nestin1 expression. Am. J. Transl. Res. 2021, 13, 11178–11193. [Google Scholar] [PubMed]
- Wang, J.; Lu, Q.; Cai, J.; Wang, Y.; Lai, X.; Qiu, Y.; Huang, Y.; Ke, Q.; Zhang, Y.; Guan, Y.; et al. Nestin regulates cellular redox homeostasis in lung cancer through the Keap1–Nrf2 feedback loop. Nat. Commun. 2019, 10, 5043. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q. Role of Nrf2 in Oxidative Stress and Toxicity. Annu. Rev. Pharmacol. Toxicol. 2013, 53, 401–426. [Google Scholar] [CrossRef] [PubMed]
- Liou, G.Y.; Storz, P. Reactive oxygen species in cancer. Free Radic. Res. 2010, 44, 479–496. [Google Scholar] [CrossRef] [PubMed]
- Supabphol, A.; Muangman, V.; Chavasiri, W.; Supabphol, R.; Gritsanapan, W. N-acetylcysteine inhibits proliferation, adhesion, migration and invasion of human bladder cancer cells. J. Med. Assoc. Thail. 2009, 92, 1171–1177. [Google Scholar]
- Zupancic, D.; Korac-Prlic, J.; Kreft, M.E.; Frankovic, L.; Vilovic, K.; Jeruc, J.; Romih, R.; Terzic, J. Vitamin A rich diet diminishes early urothelial carcinogenesis by altering retinoic acid signaling. Cancers 2020, 12, 1712. [Google Scholar] [CrossRef]
- Luo, K.W.; Chen, W.; Lung, W.Y.; Wei, X.Y.; Cheng, B.H.; Cai, Z.M.; Huang, W.R. EGCG inhibited bladder cancer SW780 cell proliferation and migration both in vitro and in vivo via down-regulation of NF-kB and MMP-9. J. Nutr. Biochem. 2017, 41, 56–64. [Google Scholar] [CrossRef]
- Qiu, Z.; Zhou, B.; Jin, L.; Yu, H.; Liu, L.; Liu, Y.; Qin, C.; Xie, S.; Zhu, F. In vitro antioxidant and antiproliferative effects of ellagic acid and its colonic metabolite, urolithins, on human bladder cancer T24 cells. Food Chem. Toxicol. 2013, 59, 428–437. [Google Scholar] [CrossRef]
- Günes, M.; Eryilmaz, R.; Aslan, R.; Taken, K.; Demir, H.; Demir, C. Oxidant-antioxidant levels in patients with bladder tumours. Aging Male 2020, 23, 1176–1181. [Google Scholar] [CrossRef] [PubMed]
- Bryan, R.T.; Pirrie, S.J.; Abbotts, B.; Maycock, S.; During, V.; Lewis, C.; Grant, M.; Bird, D.; Devall, A.J.; Wallace, D.M.A.; et al. SELENIB Investigators Selenium and Vitamin E for Prevention of Non-Muscle-Invasive Bladder Cancer Recurrence and Progression: A Randomized Clinical Trial. JAMA Netw. Open 2023, 6, e2337494. [Google Scholar] [CrossRef]
- Lepara, Z.; Alić, J.; Lepara, O.; Spahović, H.; Fajkić, A. Antioxidant status in patients with bladder cancer regarding cancer stage and grade. Asian J. Urol. 2023, 10, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Zuo, J.; Li, B.; Chen, R.; Luo, K.; Xiang, X.; Lu, S.; Huang, C.; Liu, L.; Tang, J.; et al. Drug-induced oxidative stress in cancer treatments: Angel or devil? Redox Biol. 2023, 63, 102754. [Google Scholar] [CrossRef] [PubMed]
- McElree, I.M.; Steinberg, R.L.; Mott, S.L.; O’Donnell, M.A.; Packiam, V.T. Comparison of Sequential Intravesical Gemcitabine and Docetaxel vs Bacillus Calmette-Guérin for the Treatment of Patients with High-Risk Non–Muscle-Invasive Bladder Cancer. JAMA Netw. Open 2023, 6, e230849. [Google Scholar] [CrossRef] [PubMed]
- Mediavilla-Varela, M.; Pacheco, F.J.; Almaguel, F.; Perez, J.; Sahakian, E.; Daniels, T.R.; Leoh, L.S.; Padilla, A.; Wall, N.R.; Lilly, M.B.; et al. Docetaxel-induced prostate cancer cell death involves concomitant activation of caspase and lysosomal pathways and is attenuated by LEDGF/p75. Mol. Cancer 2009, 8, 68. [Google Scholar] [CrossRef] [PubMed]
- Cao, D.; Qiao, B.; Ge, Z.; Yuan, Y. Amplification loop cascade for increasing caspase activity induced by docetaxel. J. Cell. Biochem. 2005, 96, 810–820. [Google Scholar] [CrossRef] [PubMed]
- Farr, S.E.; Chess-Williams, R.; McDermott, C.M. Gemcitabine: Selective cytotoxicity, induction of inflammation and effects on urothelial function. Toxicol. Appl. Pharmacol. 2017, 316, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, S.; Wang, H.C.R. Role of reactive oxygen species in proapoptotic ability of oncogenic H-Ras to increase human bladder cancer cell susceptibility to histone deacetylase inhibitor for caspase induction. J. Cancer Res. Clin. Oncol. 2009, 135, 1601–1613. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, S.; Rathore, K.; Wang, H.C.R. Differential induction of reactive oxygen species through Erk1/2 and Nox-1 by FK228 for selective apoptosis of oncogenic H-Ras-expressing human urinary bladder cancer J82 cells. J. Cancer Res. Clin. Oncol. 2010, 137, 471–480. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Gao, J.; Hu, S.; Zeng, W.; Yang, H.; Chen, H.; Wang, S. SLC25A21 suppresses cell growth in bladder cancer via an oxidative stress-mediated mechanism. Front. Oncol. 2021, 11, 682710. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Xu, Z.; Zhu, Z.; Chen, A.; Fu, G.; Wang, Y.; Pan, H.; Jin, B. Modulation of G6PD affects bladder cancer via ROS accumulation and the AKT pathway in vitro. Int. J. Oncol. 2018, 53, 1703–1712. [Google Scholar] [CrossRef]
- Budihardjo, I.I.; Walker, D.L.; Svingen, P.A.; Buckwalter, C.A.; Desnoyers, S.; Eckdahl, S.; Shah, G.M.; Poirier, G.G.; Reid, J.M.; Ames, M.M.; et al. 6-Aminonicotinamide sensitizes human tumor cell lines to cisplatin. Clin. Cancer Res. 1998, 4, 117–130. [Google Scholar]
- Li, X.; Wang, H.; Wang, J.; Chen, Y.; Yin, X.; Shi, G.; Li, H.; Hu, Z.; Liang, X. Emodin enhances cisplatin-induced cytotoxicity in human bladder cancer cells through ROS elevation and MRP1 downregulation. BMC Cancer 2016, 16, 578. [Google Scholar] [CrossRef]
- Kim, S.O.; Cha, H.J.; Park, C.; Lee, H.; Hong, S.H.; Jeong, S.J.; Park, S.H.; Kim, G.Y.; Leem, S.H.; Jin, C.Y.; et al. Cordycepin induces apoptosis in human bladder cancer T24 cells through ROS-dependent inhibition of the PI3K/Akt signaling pathway. Biosci. Trends 2019, 13, 324–333. [Google Scholar] [CrossRef]
- Chien, T.M.; Wu, K.H.; Chuang, Y.T.; Yeh, Y.C.; Wang, H.R.; Yeh, B.W.; Yen, C.H.; Yu, T.J.; Wu, W.J.; Chang, H.W. Withaferin A triggers apoptosis and DNA damage in bladder cancer J82 cells through oxidative stress. Antioxidants 2021, 10, 1063. [Google Scholar] [CrossRef]
- Duan, F.; Yu, Y.; Guan, R.; Xu, Z.; Liang, H.; Hong, L. Vitamin K2 induces mitochondrial-related apoptosis in human bladder cancer cells via ROS and JNK/p38 MAPK signal pathways. PLoS ONE 2016, 11, e0161886. [Google Scholar] [CrossRef] [PubMed]
- Yao, W.; Qian, X.; Ochsenreither, S.; Soldano, F.; DeLeo, A.B.; Sudhoff, H.; Oppel, F.; Kuppig, A.; Klinghammer, K.; Kaufmann, A.M.; et al. Disulfiram Acts as a Potent Radio-Chemo Sensitizer in Head and Neck Squamous Cell Carcinoma Cell Lines and Transplanted Xenografts. Cells 2021, 10, 517. [Google Scholar] [CrossRef] [PubMed]
- Vogg, A.T.J.; Drude, N.; Mottaghy, F.M.; Morgenroth, A.; Miran, T. Modulation of glutathione promotes apoptosis in triple-negative breast cancer cells. FASEB J. 2018, 32, 2803–2813. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, H.; Shimozato, O.; Ishii, T.; Kamoda, H.; Hagiwara, Y.; Ohtori, S.; Yonemoto, T. The Thioredoxin-1 Inhibitor, PX-12, Suppresses Local Osteosarcoma Progression. Anticancer Res. 2021, 41, 6013–6021. [Google Scholar] [CrossRef]
- Sang, J.; Li, W.; Diao, H.J.; Fan, R.Z.; Huang, J.L.; Gan, L.; Zou, M.F.; Tang, G.H.; Yin, S. Jolkinolide B targets thioredoxin and glutathione systems to induce ROS-mediated paraptosis and apoptosis in bladder cancer cells. Cancer Lett. 2021, 509, 13–25. [Google Scholar] [CrossRef]
| Antioxidant Agent | Model System | Observed Effect | Reference |
|---|---|---|---|
| N-acetylcysteine | In vitro human BCa cells | Depleted cancer cell viability, adhesiveness, migratory abilities, and invasiveness | [71] |
| Vitamin A (retinol) | Mouse, oral administration | Diminished early BCa progression | [72] |
| Epigallocatechin gallate (EGCG) | Mouse, IP injection | Decreased tumor burden, downregulated NF-κB and MMP-9 | [73] |
| Ellagic acid | In vitro human BCa cells | Decreased cell proliferation | [74] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dugbartey, G.J.; Relouw, S.; McFarlane, L.; Sener, A. Redox System and Oxidative Stress-Targeted Therapeutic Approaches in Bladder Cancer. Antioxidants 2024, 13, 287. https://doi.org/10.3390/antiox13030287
Dugbartey GJ, Relouw S, McFarlane L, Sener A. Redox System and Oxidative Stress-Targeted Therapeutic Approaches in Bladder Cancer. Antioxidants. 2024; 13(3):287. https://doi.org/10.3390/antiox13030287
Chicago/Turabian StyleDugbartey, George J., Sydney Relouw, Liam McFarlane, and Alp Sener. 2024. "Redox System and Oxidative Stress-Targeted Therapeutic Approaches in Bladder Cancer" Antioxidants 13, no. 3: 287. https://doi.org/10.3390/antiox13030287
APA StyleDugbartey, G. J., Relouw, S., McFarlane, L., & Sener, A. (2024). Redox System and Oxidative Stress-Targeted Therapeutic Approaches in Bladder Cancer. Antioxidants, 13(3), 287. https://doi.org/10.3390/antiox13030287






