Genetic and Transcriptomic Background of Oxidative Stress and Antioxidative Therapies in Late Complications of Type 2 Diabetes Mellitus: A Systematic Review
Abstract
1. Introduction
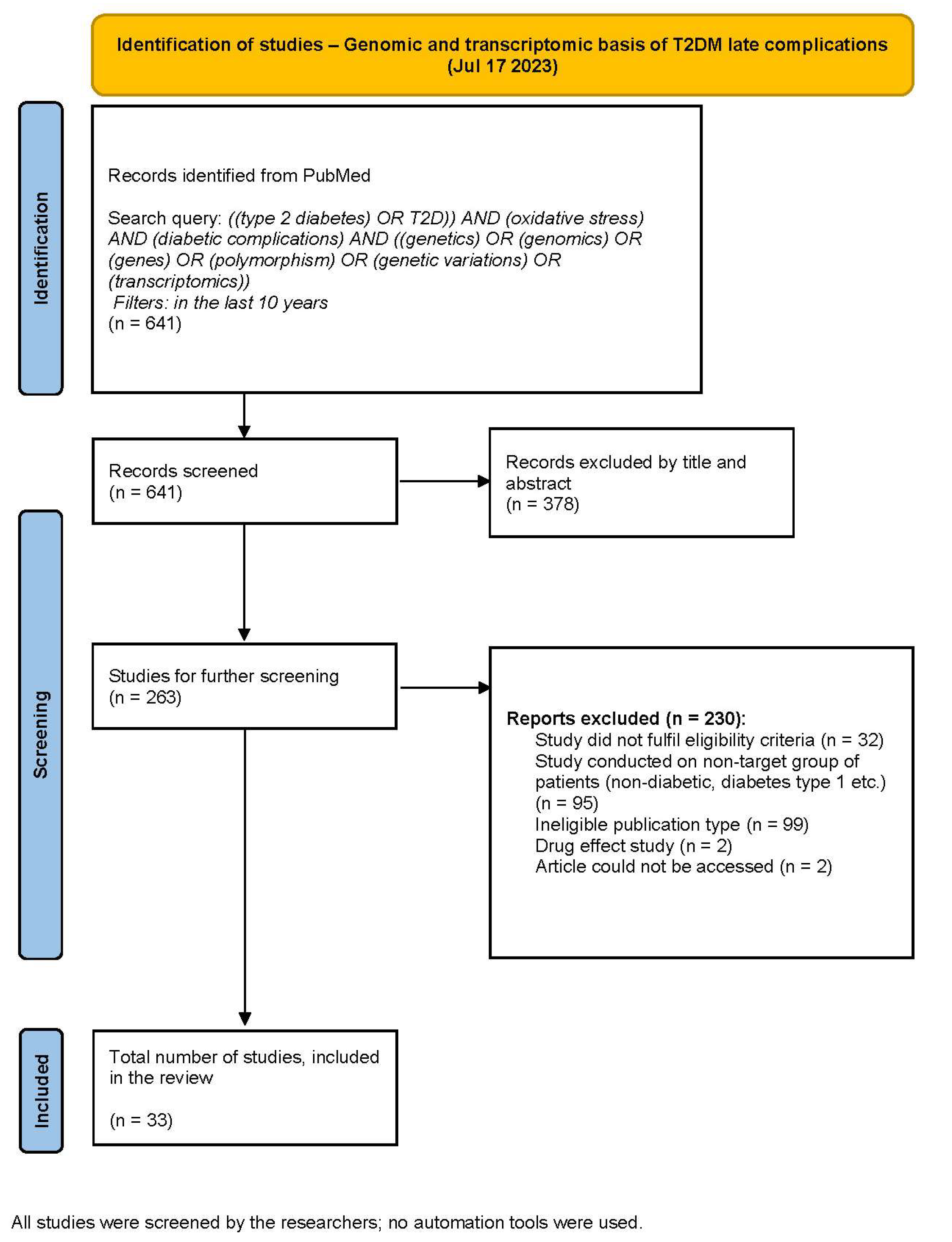
2. Materials and Methods
2.1. Eligibility Criteria
2.2. Search Strategy
2.3. Study Selection
2.4. Data Collection and Extraction
2.5. Risk of Bias Assessment
2.6. Data Synthesis Methodology
3. Results
3.1. Genomic and Transcriptomic Basis of T2DM Late Complications—Overview of the Studies’ Characteristics
3.2. Antioxidants as an Intervention in T2DM Late Complications—Overview of the Studies’ Characteristics
Risk of Bias Assessment
4. Discussion
4.1. Genetic Background of T2DM Late Complications
4.1.1. Genetic Variability in Antioxidant Systems and T2DM Late Complications
4.1.2. Genetic Variability in ROS-Generating Enzymes and T2DM Late Complications
4.1.3. Genetic Variability in RNS Generating Enzymes and T2DM Late Complications
4.1.4. Genomic Approaches in Elucidating the Genetic Basis of T2DM Late Complications
4.1.5. Transcriptome and Gene Expression Studies in Patients with T2DM Late Complications
4.1.6. Genetic and Transcriptomic Biomarkers of T2DM Late Complications—Commentary on Studies’ Characteristics and Limitations
4.1.7. Future Perspectives on Genetic and Transcriptomic Studies of T2DM Late Complications
4.2. Antioxidants as an Intervention in T2DM Late Complications
4.2.1. Dietary Antioxidants and Lifestyle
Polyphenols
Carotenoids
4.2.2. Antioxidant Supplements
Resveratrol
γ-Linolenic Acid
Vitamin E
α-Lipoic Acid
Melatonin
4.2.3. Antioxidants as Treatment in T2DM Late Complications—Commentary on Studies’ Characteristics and Limitations
4.2.4. Future Perspectives on the Use of Antioxidant Therapy in T2DM Late Complications
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Galicia-Garcia, U.; Benito-Vicente, A.; Jebari, S.; Larrea-Sebal, A.; Siddiqi, H.; Uribe, K.B.; Ostolaza, H.; Martín, C. Pathophysiology of Type 2 Diabetes Mellitus. Int. J. Mol. Sci. 2020, 21, 6275. [Google Scholar] [CrossRef] [PubMed]
- Gæde, P.; Vedel, P.; Larsen, N.; Jensen, G.V.H.; Parving, H.-H.; Pedersen, O. Multifactorial Intervention and Cardiovascular Disease in Patients with Type 2 Diabetes. N. Engl. J. Med. 2003, 348, 383–393. [Google Scholar] [CrossRef] [PubMed]
- Kang, Q.; Yang, C. Oxidative Stress and Diabetic Retinopathy: Molecular Mechanisms, Pathogenetic Role and Therapeutic Implications. Redox Biol. 2020, 37, 101799. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Ma, J.; Leng, T.; Yuan, Z.; Hu, T.; Liu, Q.; Shen, T. Advances in Oxidative Stress in Pathogenesis of Diabetic Kidney Disease and Efficacy of TCM Intervention. Ren. Fail. 2023, 45, 2146512. [Google Scholar] [CrossRef] [PubMed]
- Jin, Q.; Liu, T.; Qiao, Y.; Liu, D.; Yang, L.; Mao, H.; Ma, F.; Wang, Y.; Peng, L.; Zhan, Y. Oxidative Stress and Inflammation in Diabetic Nephropathy: Role of Polyphenols. Front. Immunol. 2023, 14, 1185317. [Google Scholar] [CrossRef] [PubMed]
- Feldman, E.L.; Callaghan, B.C.; Pop-Busui, R.; Zochodne, D.W.; Wright, D.E.; Bennett, D.L.; Bril, V.; Russell, J.W.; Viswanathan, V. Diabetic Neuropathy. Nat. Rev. Dis. Primers 2019, 5, 42. [Google Scholar] [CrossRef] [PubMed]
- Yun, J.-S.; Ko, S.-H. Current Trends in Epidemiology of Cardiovascular Disease and Cardiovascular Risk Management in Type 2 Diabetes. Metabolism 2021, 123, 154838. [Google Scholar] [CrossRef]
- González, P.; Lozano, P.; Ros, G.; Solano, F. Hyperglycemia and Oxidative Stress: An Integral, Updated and Critical Overview of Their Metabolic Interconnections. Int. J. Mol. Sci. 2023, 24, 9352. [Google Scholar] [CrossRef]
- Schieber, M.; Chandel, N.S. ROS Function in Redox Signaling and Oxidative Stress. Curr. Biol. 2014, 24, R453–R462. [Google Scholar] [CrossRef]
- Forman, H.J.; Zhang, H. Targeting Oxidative Stress in Disease: Promise and Limitations of Antioxidant Therapy. Nat. Rev. Drug Discov. 2021, 20, 689–709. [Google Scholar] [CrossRef]
- Evans, J.L.; Goldfine, I.D.; Maddux, B.A.; Grodsky, G.M. Oxidative Stress and Stress-Activated Signaling Pathways: A Unifying Hypothesis of Type 2 Diabetes. Endocr. Rev. 2002, 23, 599–622. [Google Scholar] [CrossRef] [PubMed]
- Gray, S.P.; Di Marco, E.; Okabe, J.; Szyndralewiez, C.; Heitz, F.; Montezano, A.C.; De Haan, J.B.; Koulis, C.; El-Osta, A.; Andrews, K.L.; et al. NADPH Oxidase 1 Plays a Key Role in Diabetes Mellitus–Accelerated Atherosclerosis. Circulation 2013, 127, 1888–1902. [Google Scholar] [CrossRef] [PubMed]
- Weydert, C.J.; Cullen, J.J. Measurement of Superoxide Dismutase, Catalase and Glutathione Peroxidase in Cultured Cells and Tissue. Nat. Protoc. 2010, 5, 51–66. [Google Scholar] [CrossRef]
- Mehri, F.; Rahbar, A.H.; Ghane, E.T.; Souri, B.; Esfahani, M. Changes in Oxidative Markers in COVID-19 Patients. Arch. Med. Res. 2021, 52, 843–849. [Google Scholar] [CrossRef] [PubMed]
- Rosa, A.C.; Corsi, D.; Cavi, N.; Bruni, N.; Dosio, F. Superoxide Dismutase Administration: A Review of Proposed Human Uses. Molecules 2021, 26, 1844. [Google Scholar] [CrossRef] [PubMed]
- Trist, B.G.; Hilton, J.B.; Hare, D.J.; Crouch, P.J.; Double, K.L. Superoxide Dismutase 1 in Health and Disease: How a Frontline Antioxidant Becomes Neurotoxic. Angew. Chem. Int. Ed. 2021, 60, 9215–9246. [Google Scholar] [CrossRef]
- Ali, S.S.; Ahsan, H.; Zia, M.K.; Siddiqui, T.; Khan, F.H. Understanding Oxidants and Antioxidants: Classical Team with New Players. J. Food Biochem. 2020, 44, e13145. [Google Scholar] [CrossRef]
- Dworzański, J.; Strycharz-Dudziak, M.; Kliszczewska, E.; Kiełczykowska, M.; Dworzańska, A.; Drop, B.; Polz-Dacewicz, M. Glutathione Peroxidase (GPx) and Superoxide Dismutase (SOD) Activity in Patients with Diabetes Mellitus Type 2 Infected with Epstein-Barr Virus. PLoS ONE 2020, 15, e0230374. [Google Scholar] [CrossRef]
- Ngoye Briggs, O.; Brown, H.; Elechi-amadi, K.; Ezeiruaku, F.; Nduka, N. Superoxide Dismutase and Glutathione Peroxidase Levels in Patients with Long Standing Type 2 Diabetes in Port Harcourt, Rivers State, Nigeria. Int. J. Sci. Res. 2016, 5, 1282–1288. [Google Scholar] [CrossRef]
- Dato, S.; Crocco, P.; D’Aquila, P.; De Rango, F.; Bellizzi, D.; Rose, G.; Passarino, G. Exploring the Role of Genetic Variability and Lifestyle in Oxidative Stress Response for Healthy Aging and Longevity. Int. J. Mol. Sci. 2013, 14, 16443–16472. [Google Scholar] [CrossRef]
- Iacobini, C.; Vitale, M.; Pesce, C.; Pugliese, G.; Menini, S. Diabetic Complications and Oxidative Stress: A 20-Year Voyage Back in Time and Back to the Future. Antioxidants 2021, 10, 727. [Google Scholar] [CrossRef] [PubMed]
- Reddy, V.P. Oxidative Stress in Health and Disease. Biomedicines 2023, 11, 2925. [Google Scholar] [CrossRef] [PubMed]
- Caturano, A.; D’Angelo, M.; Mormone, A.; Russo, V.; Mollica, M.P.; Salvatore, T.; Galiero, R.; Rinaldi, L.; Vetrano, E.; Marfella, R.; et al. Oxidative Stress in Type 2 Diabetes: Impacts from Pathogenesis to Lifestyle Modifications. Curr. Issues Mol. Biol. 2023, 45, 6651–6666. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association Professional Practice Committee. Erratum. 10. Cardiovascular Disease and Risk Management: Standards of Medical Care in Diabetes—2022. Diabetes Care 2022, 45 (Suppl. S1), S144–S174, Erratum in: Diabetes Care 2022, 45, 1296. [Google Scholar] [CrossRef]
- Darenskaya, M.A.; Kolesnikova, L.I.; Kolesnikov, S.I. Oxidative Stress: Pathogenetic Role in Diabetes Mellitus and Its Complications and Therapeutic Approaches to Correction. Bull. Exp. Biol. Med. 2021, 171, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Chen, R.; Yang, Z.; Sun, G.; Wang, M.; Ma, X.; Yang, L.; Sun, X. Taxifolin Prevents Diabetic Cardiomyopathy in Vivo and in Vitro by Inhibition of Oxidative Stress and Cell Apoptosis. Food Chem. Toxicol. 2014, 63, 221–232. [Google Scholar] [CrossRef] [PubMed]
- Mohammadshahi, M.; Haidari, F.; Soufi, F.G. Chronic Resveratrol Administration Improves Diabetic Cardiomyopathy in Part by Reducing Oxidative Stress. Cardiol. J. 2014, 21, 39–46. [Google Scholar] [CrossRef]
- Bai, Y.; Cui, W.; Xin, Y.; Miao, X.; Barati, M.T.; Zhang, C.; Chen, Q.; Tan, Y.; Cui, T.; Zheng, Y.; et al. Prevention by Sulforaphane of Diabetic Cardiomyopathy Is Associated with Up-Regulation of Nrf2 Expression and Transcription Activation. J. Mol. Cell Cardiol. 2013, 57, 82–95. [Google Scholar] [CrossRef]
- Huynh, K.; Bernardo, B.C.; McMullen, J.R.; Ritchie, R.H. Diabetic Cardiomyopathy: Mechanisms and New Treatment Strategies Targeting Antioxidant Signaling Pathways. Pharmacol. Ther. 2014, 142, 375–415. [Google Scholar] [CrossRef]
- Dludla, P.V.; Dias, S.C.; Obonye, N.; Johnson, R.; Louw, J.; Nkambule, B.B. A Systematic Review on the Protective Effect of N-Acetyl Cysteine Against Diabetes-Associated Cardiovascular Complications. Am. J. Cardiovasc. Drugs 2018, 18, 283–298. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, n71. [Google Scholar] [CrossRef] [PubMed]
- Hermans, N.; Cos, P.; Maes, L.; De Bruyne, T.; Vanden Berghe, D.; Vlietinck, A.J.; Pieters, L. Challenges and Pitfalls in Antioxidant Research. Curr. Med. Chem. 2007, 14, 417–430. [Google Scholar] [CrossRef] [PubMed]
- Kotha, R.R.; Tareq, F.S.; Yildiz, E.; Luthria, D.L. Oxidative Stress and Antioxidants—A Critical Review on In Vitro Antioxidant Assays. Antioxidants 2022, 11, 2388. [Google Scholar] [CrossRef] [PubMed]
- Brainina, K.; Stozhko, N.; Vidrevich, M. Antioxidants: Terminology, Methods, and Future Considerations. Antioxidants 2019, 8, 297. [Google Scholar] [CrossRef] [PubMed]
- KA-04151; NLM Customer Support Center. Available online: https://support.nlm.nih.gov/knowledgebase/article/KA-04151/en-us (accessed on 29 October 2023).
- Risk of Bias Tools—Current Version of RoB 2. Available online: https://sites.google.com/site/riskofbiastool/welcome/rob-2-0-tool/current-version-of-rob-2 (accessed on 24 October 2023).
- Mohammedi, K.; Bellili-Muñoz, N.; Marklund, S.L.; Driss, F.; Le Nagard, H.; Patente, T.A.; Fumeron, F.; Roussel, R.; Hadjadj, S.; Marre, M.; et al. Plasma Extracellular Superoxide Dismutase Concentration, Allelic Variations in the SOD3 Gene and Risk of Myocardial Infarction and All-Cause Mortality in People with Type 1 and Type 2 Diabetes. Cardiovasc. Diabetol. 2015, 14, 845. [Google Scholar] [CrossRef] [PubMed]
- Kasznicki, J.; Sliwinska, A.; Kosmalski, M.; Merecz, A.; Majsterek, I.; Drzewoski, J. Genetic Polymorphisms (Pro197Leu of Gpx1, +35A/C of SOD1, −262C/T of CAT), the Level of Antioxidant Proteins (GPx1, SOD1, CAT) and the Risk of Distal Symmetric Polyneuropathy in Polish Patients with Type 2 Diabetes Mellitus. Adv. Med. Sci. 2016, 61, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Katakami, N.; Kaneto, H.; Matsuoka, T.; Takahara, M.; Osonoi, T.; Saitou, M.; Kawai, K.; Ishibashi, F.; Kashiwagi, A.; Kawamori, R.; et al. Accumulation of Oxidative Stress-Related Gene Polymorphisms and the Risk of Coronary Heart Disease Events in Patients with Type 2 Diabetes—An 8-Year Prospective Study. Atherosclerosis 2014, 235, 408–414. [Google Scholar] [CrossRef]
- Klen, J.; Goričar, K.; Janež, A.; Dolžan, V. Common Polymorphisms in Antioxidant Genes Are Associated with Diabetic Nephropathy in Type 2 Diabetes Patients. Pers. Med. 2015, 12, 187–198. [Google Scholar] [CrossRef]
- Abaidi, H.; Denden, S.; Ghazouani, A.; Trimèche, A.; Snoussi, C.; Haj Khelil, A.; Ben Chibani, J.; Hamdaoui, M.H. Mn-SOD 47 CC Genotype in Combination with High Tea Consumption May Prevent Complications in Tunisian Type-2 Diabetes. Genet. Mol. Res. 2015, 14, 8613–8622. [Google Scholar] [CrossRef]
- Liu, D.; Liu, L.; Hu, Z.; Song, Z.; Wang, Y.; Chen, Z. Evaluation of the Oxidative Stress–Related Genes ALOX5, ALOX5AP, GPX1, GPX3 and MPO for Contribution to the Risk of Type 2 Diabetes Mellitus in the Han Chinese Population. Diabetes Vasc. Dis. Res. 2018, 15, 336–339. [Google Scholar] [CrossRef]
- Roumeliotis, A.K.; Roumeliotis, S.K.; Panagoutsos, S.A.; Tsetsos, F.; Georgitsi, M.; Manolopoulos, V.; Paschou, P.; Passadakis, P.S. Association of ALOX12 Gene Polymorphism with All-Cause and Cardiovascular Mortality in Diabetic Nephropathy. Int. Urol. Nephrol. 2018, 50, 321–329. [Google Scholar] [CrossRef] [PubMed]
- Shahid, S.U.; Shabana; Humphries, S. The SNP Rs10911021 Is Associated with Oxidative Stress in Coronary Heart Disease Patients from Pakistan. Lipids Health Dis. 2018, 17, 6. [Google Scholar] [CrossRef] [PubMed]
- Stoian, A.; Bănescu, C.; Bălaşa, R.I.; Moţăţăianu, A.; Stoian, M.; Moldovan, V.G.; Voidăzan, S.; Dobreanu, M. Influence of GSTM1, GSTT1, and GSTP1 Polymorphisms on Type 2 Diabetes Mellitus and Diabetic Sensorimotor Peripheral Neuropathy Risk. Dis. Markers 2015, 2015, 638693. [Google Scholar] [CrossRef] [PubMed]
- Narne, P.; Ponnaluri, K.C.; Siraj, M.; Ishaq, M. Polymorphisms in Oxidative Stress Pathway Genes and Risk of Diabetic Nephropathy in S Outh I Ndian Type 2 Diabetic Patients. Nephrology 2014, 19, 623–629. [Google Scholar] [CrossRef] [PubMed]
- Roumeliotis, A.; Roumeliotis, S.; Tsetsos, F.; Georgitsi, M.; Georgianos, P.I.; Stamou, A.; Vasilakou, A.; Kotsa, K.; Tsekmekidou, X.; Paschou, P.; et al. Oxidative Stress Genes in Diabetes Mellitus Type 2: Association with Diabetic Kidney Disease. Oxidative Med. Cell. Longev. 2021, 2021, 2531062. [Google Scholar] [CrossRef] [PubMed]
- Sadati, S.M.; Radfar, M.; Hamidi, A.K.; Abdollahi, M.; Qorbani, M.; Esfahani, E.N.; Amoli, M.M. Association Between the Polymorphism of Glu298Asp in Exon 7 of the eNOS Gene with Foot Ulcer and Oxidative Stress in Adult Patients with Type 2 Diabetes. Can. J. Diabetes 2018, 42, 18–22. [Google Scholar] [CrossRef] [PubMed]
- Narne, P.; Ponnaluri, K.C.; Siraj, M.; Ishaq, M. Association Analysis of Polymorphisms in Genes Related to Oxidative Stress in South Indian Type 2 Diabetic Patients with Retinopathy. Ophthalmic Genet. 2014, 37, 1–8. [Google Scholar] [CrossRef]
- Sharma, M.; Mehndiratta, M.; Gupta, S.; Kalra, O.P.; Shukla, R.; Gambhir, J.K. Genetic Association of NAD(P)H Quinone Oxidoreductase (NQO1*2) Polymorphism with NQO1 Levels and Risk of Diabetic Nephropathy. Biol. Chem. 2016, 397, 725–730. [Google Scholar] [CrossRef]
- Ramus, S.M.; Cilensek, I.; Petrovic, M.G.; Soucek, M.; Kruzliak, P.; Petrovic, D. Single Nucleotide Polymorphisms in the Trx2/TXNIP and TrxR2 Genes of the Mitochondrial Thioredoxin Antioxidant System and the Risk of Diabetic Retinopathy in Patients with Type 2 Diabetes Mellitus. J. Diabetes Its Complicat. 2016, 30, 192–198. [Google Scholar] [CrossRef]
- Kariž, S.; Mankoč, S.; Petrovič, D. Association of Thioredoxin Reductase 2 (TXNRD2) Gene Polymorphisms with Myocardial Infarction in Slovene Patients with Type 2 Diabetes Mellitus. Diabetes Res. Clin. Pract. 2015, 108, 323–328. [Google Scholar] [CrossRef]
- Lee, E.Y.; Lee, Y.; Kim, S.H.; Jung, K.S.; Kwon, O.; Kim, B.S.; Nam, C.M.; Park, C.S.; Lee, B.-W.; Kang, E.S.; et al. Association Between Heme Oxygenase-1 Promoter Polymorphisms and the Development of Albuminuria in Type 2 Diabetes: A Case–Control Study. Medicine 2015, 94, e1825. [Google Scholar] [CrossRef] [PubMed]
- Petrovic, M.G.; Kruzliak, P.; Petrovic, D. The Rs6060566 of the Reactive Oxygen Species Modulator 1 ( R Omo-1) Gene Affects R Omo-1 Expression and the Development of Diabetic Retinopathy in C Aucasians with Type 2 Diabetes. Acta Ophthalmol. 2015, 93, e654–e657. [Google Scholar] [CrossRef] [PubMed]
- Dabhi, B.; Mistry, K.N. Oxidative Stress and Its Association with TNF-α-308 G/C and IL-1α-889 C/T Gene Polymorphisms in Patients with Diabetes and Diabetic Nephropathy. Gene 2015, 562, 197–202. [Google Scholar] [CrossRef] [PubMed]
- Klen, J.; Goričar, K.; Janež, A.; Dolžan, V. NLRP3 Inflammasome Polymorphism and Macrovascular Complications in Type 2 Diabetes Patients. J. Diabetes Res. 2015, 2015, 616747. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Azarova, I.; Klyosova, E.; Polonikov, A. Single Nucleotide Polymorphisms of the RAC1 Gene as Novel Susceptibility Markers for Neuropathy and Microvascular Complications in Type 2 Diabetes. Biomedicines 2023, 11, 981. [Google Scholar] [CrossRef] [PubMed]
- Khalili, F.; Vaisi-Raygani, A.; Shakiba, E.; Kohsari, M.; Dehbani, M.; Naseri, R.; Asadi, S.; Rahimi, Z.; Rahimi, M.; Rahimi, Z. Oxidative Stress Parameters and Keap 1 Variants in T2DM: Association with T2DM, Diabetic Neuropathy, Diabetic Retinopathy, and Obesity. Clin. Lab. Anal. 2022, 36, e24163. [Google Scholar] [CrossRef]
- Morita, K.; Saruwatari, J.; Miyagawa, H.; Uchiyashiki, Y.; Oniki, K.; Sakata, M.; Kajiwara, A.; Yoshida, A.; Jinnouchi, H.; Nakagawa, K. Association between Aldehyde Dehydrogenase 2 Polymorphisms and the Incidence of Diabetic Retinopathy among Japanese Subjects with Type 2 Diabetes Mellitus. Cardiovasc. Diabetol. 2013, 12, 132. [Google Scholar] [CrossRef]
- Dieter, C.; Lemos, N.E.; Corrêa, N.R.D.F.; Pellenz, F.M.; Canani, L.H.; Crispim, D.; Bauer, A.C. The A Allele of the Rs759853 Single Nucleotide Polymorphism in the AKR1B1 Gene Confers Risk for Diabetic Kidney Disease in Patients with Type 2 Diabetes from a Brazilian Population. Arch. Endocrinol. Metab. 2022, 66, 12–18. [Google Scholar] [CrossRef]
- Klashami, Z.N.; Ahrabi, N.Z.; Ahrabi, Y.S.; Hasanzad, M.; Asadi, M.; Amoli, M.M. The Vitamin D Receptor Gene Variants, ApaI, TaqI, BsmI, and FokI in Diabetic Foot Ulcer and Their Association with Oxidative Stress. Mol. Biol. Rep. 2022, 49, 8627–8639. [Google Scholar] [CrossRef]
- Yesil-Devecioglu, T.; Dayan, A.; Demirtunc, R.; Sardas, S. Role of DNA Repair Genes XRCC3 and XRCC1 in Predisposition to Type 2 Diabetes Mellitus and Diabetic Nephropathy. Endocrinol. Diabetes Y Nutr. 2019, 66, 90–98. [Google Scholar] [CrossRef]
- Merecz, A.; Markiewicz, L.; Sliwinska, A.; Kosmalski, M.; Kasznicki, J.; Drzewoski, J.; Majsterek, I. Analysis of Oxidative DNA Damage and Its Repair in Polish Patients with Diabetes Mellitus Type 2: Role in Pathogenesis of Diabetic Neuropathy. Adv. Med. Sci. 2015, 60, 220–230. [Google Scholar] [CrossRef] [PubMed]
- Oropeza-Valdez, J.J.; Hernandez, J.D.L.C.M.; Jaime-Sánchez, E.; López-Ramos, E.; Lara-Ramírez, E.E.; Hernández, Y.L.; Castañeda-Delgado, J.E.; Moreno, J.A.E. Transcriptome Analysis Identifies Oxidative Stress Injury Biomarkers for Diabetic Nephropathy. Arch. Med. Res. 2023, 54, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Pinazo-Durán, M.D.; Shoaie-Nia, K.; Sanz-González, S.M.; Raga-Cervera, J.; García-Medina, J.J.; López-Gálvez, M.I.; Galarreta-Mira, D.; Duarte, L.; Campos-Borges, C.; Zanón-Moreno, V. Identificación de nuevos genes candidatos para la retinopatía en diabéticos tipo 2. Estudio Valencia sobre Retinopatía Diabética (EVRD). Informe n.° 3. Arch. De La Soc. Española De Oftalmol. 2018, 93, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Victor, P.; Umapathy, D.; George, L.; Juttada, U.; Ganesh, G.V.; Amin, K.N.; Viswanathan, V.; Ramkumar, K.M. Crosstalk between Endoplasmic Reticulum Stress and Oxidative Stress in the Progression of Diabetic Nephropathy. Cell Stress. Chaperones 2021, 26, 311–321. [Google Scholar] [CrossRef] [PubMed]
- Fath El-Bab, M.; Zaki, N.S.; Mojaddidi, M.; AL-Barry, M.; El-Beshbishy, H. Diabetic Retinopathy Is Associated with Oxidative Stress and Mitigation of Gene Expression of Antioxidant Enzymes. IJGM 2013, 2013, 799–806. [Google Scholar] [CrossRef] [PubMed][Green Version]
- El-Horany, H.E.; Abd-Ellatif, R.N.; Watany, M.; Hafez, Y.M.; Okda, H.I. NLRP3 Expression and Urinary HSP72 in Relation to Biomarkers of Inflammation and Oxidative Stress in Diabetic Nephropathy Patients. IUBMB Life 2017, 69, 623–630. [Google Scholar] [CrossRef] [PubMed]
- Chawla, D.; Bansal, S.; Banerjee, B.D.; Madhu, S.V.; Kalra, O.P.; Tripathi, A.K. Role of Advanced Glycation End Product (AGE)-Induced Receptor (RAGE) Expression in Diabetic Vascular Complications. Microvasc. Res. 2014, 95, 1–6. [Google Scholar] [CrossRef]
- Jaafarinia, A.; Kafami, B.; Sahebnasagh, A.; Saghafi, F. Evaluation of Therapeutic Effects of Crocin in Attenuating the Progression of Diabetic Nephropathy: A Preliminary Randomized Triple-Blind Placebo-Controlled Trial. BMC Complement. Med. Ther. 2022, 22, 262. [Google Scholar] [CrossRef]
- Chuar, P.F.; Ng, Y.T.; Phang, S.C.W.; Koay, Y.Y.; Ho, J.-I.; Ho, L.S.; Botross Henien, N.P.; Ahmad, B.; Abdul Kadir, K. Tocotrienol-Rich Vitamin E (Tocovid) Improved Nerve Conduction Velocity in Type 2 Diabetes Mellitus Patients in a Phase II Double-Blind, Randomized Controlled Clinical Trial. Nutrients 2021, 13, 3770. [Google Scholar] [CrossRef]
- Fakhari, M.; Fakhari, M.; BamBaeichi, E. The Effects of Pilates and Flavanol-Rich Dark Chocolate Consumption on the Total Antioxidant Capacity, Glycemic Control and BMI in Diabetic Females with Neuropathy Complications. J. Bodyw. Mov. Ther. 2021, 26, 294–299. [Google Scholar] [CrossRef]
- Yarahmadi, A.; Saeed Modaghegh, M.-H.; Mostafavi-Pour, Z.; Azarpira, N.; Mousavian, A.; Bonakdaran, S.; Jarahi, L.; Samadi, A.; Peimani, M.; Hamidi Alamdari, D. The Effect of Platelet-Rich Plasma-Fibrin Glue Dressing in Combination with Oral Vitamin E and C for Treatment of Non-Healing Diabetic Foot Ulcers: A Randomized, Double-Blind, Parallel-Group, Clinical Trial. Expert. Opin. Biol. Ther. 2021, 21, 687–696. [Google Scholar] [CrossRef] [PubMed]
- Essmat, A.; Hussein, M.S. Green Tea Extract for Mild-to-Moderate Diabetic Peripheral Neuropathy A Randomized Controlled Trial. Complement. Ther. Clin. Pract. 2021, 43, 101317. [Google Scholar] [CrossRef] [PubMed]
- Koay, Y.Y.; Tan, G.C.J.; Phang, S.C.W.; Ho, J.-I.; Chuar, P.F.; Ho, L.S.; Ahmad, B.; Abdul Kadir, K. A Phase IIb Randomized Controlled Trial Investigating the Effects of Tocotrienol-Rich Vitamin E on Diabetic Kidney Disease. Nutrients 2021, 13, 258. [Google Scholar] [CrossRef] [PubMed]
- Mokhtari, M.; Razzaghi, R.; Momen-Heravi, M. The Effects of Curcumin Intake on Wound Healing and Metabolic Status in Patients with Diabetic Foot Ulcer: A Randomized, Double-blind, Placebo-controlled Trial. Phytother. Res. 2021, 35, 2099–2107. [Google Scholar] [CrossRef] [PubMed]
- Shafabakhsh, R.; Mobini, M.; Raygan, F.; Aghadavod, E.; Ostadmohammadi, V.; Amirani, E.; Mansournia, M.A.; Asemi, Z. Curcumin Administration and the Effects on Psychological Status and Markers of Inflammation and Oxidative Damage in Patients with Type 2 Diabetes and Coronary Heart Disease. Clin. Nutr. ESPEN 2020, 40, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Didangelos, T.; Karlafti, E.; Kotzakioulafi, E.; Kontoninas, Z.; Margaritidis, C.; Giannoulaki, P.; Kantartzis, K. Efficacy and Safety of the Combination of Superoxide Dismutase, Alpha Lipoic Acid, Vitamin B12, and Carnitine for 12 Months in Patients with Diabetic Neuropathy. Nutrients 2020, 12, 3254. [Google Scholar] [CrossRef] [PubMed]
- Ng, Y.T.; Phang, S.C.W.; Tan, G.C.J.; Ng, E.Y.; Botross Henien, N.P.; Palanisamy, U.D.M.; Ahmad, B.; Abdul Kadir, K. The Effects of Tocotrienol-Rich Vitamin E (Tocovid) on Diabetic Neuropathy: A Phase II Randomized Controlled Trial. Nutrients 2020, 12, 1522. [Google Scholar] [CrossRef]
- Won, J.C.; Kwon, H.-S.; Moon, S.-S.; Chun, S.W.; Kim, C.H.; Park, I.B.; Kim, I.J.; Lee, J.; Cha, B.Y.; Park, T.S. γ-Linolenic Acid versus α-Lipoic Acid for Treating Painful Diabetic Neuropathy in Adults: A 12-Week, Double-Placebo, Randomized, Noninferiority Trial. Diabetes Metab. J. 2020, 44, 542. [Google Scholar] [CrossRef]
- Moon, S.W.; Shin, Y.U.; Cho, H.; Bae, S.H.; Kim, H.K. Effect of Grape Seed Proanthocyanidin Extract on Hard Exudates in Patients with Non-Proliferative Diabetic Retinopathy. Medicine 2019, 98, e15515. [Google Scholar] [CrossRef]
- Asadi, S.; Gholami, M.S.; Siassi, F.; Qorbani, M.; Khamoshian, K.; Sotoudeh, G. Nano Curcumin Supplementation Reduced the Severity of Diabetic Sensorimotor Polyneuropathy in Patients with Type 2 Diabetes Mellitus: A Randomized Double-Blind Placebo- Controlled Clinical Trial. Complement. Ther. Med. 2019, 43, 253–260. [Google Scholar] [CrossRef]
- Sattarinezhad, A.; Roozbeh, J.; Shirazi Yeganeh, B.; Omrani, G.R.; Shams, M. Resveratrol Reduces Albuminuria in Diabetic Nephropathy: A Randomized Double-Blind Placebo-Controlled Clinical Trial. Diabetes Metab. 2019, 45, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Aghadavod, E.; Soleimani, A.; Hamidi, G.; Keneshlou, F.; Heidari, A.; Asemi, Z. Effects of High-Dose Vitamin E Supplementation on Markers of Cardiometabolic Risk and Oxidative Stress in Patients with Diabetic Nephropathy: A Randomized Double-Blinded Controlled Trial. Iran. J. Kidney Dis. 2018, 12, 156–162. [Google Scholar] [PubMed]
- Raygan, F.; Ostadmohammadi, V.; Bahmani, F.; Reiter, R.J.; Asemi, Z. Melatonin Administration Lowers Biomarkers of Oxidative Stress and Cardio-Metabolic Risk in Type 2 Diabetic Patients with Coronary Heart Disease: A Randomized, Double-Blind, Placebo-Controlled Trial. Clin. Nutr. 2019, 38, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Soleimani, A.; Taghizadeh, M.; Bahmani, F.; Badroj, N.; Asemi, Z. Metabolic Response to Omega-3 Fatty Acid Supplementation in Patients with Diabetic Nephropathy: A Randomized, Double-Blind, Placebo-Controlled Trial. Clin. Nutr. 2017, 36, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Miraghajani, M.; Zaghian, N.; Mirlohi, M.; Feizi, A.; Ghiasvand, R. The Impact of Probiotic Soy Milk Consumption on Oxidative Stress Among Type 2 Diabetic Kidney Disease Patients: A Randomized Controlled Clinical Trial. J. Ren. Nutr. 2017, 27, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Osorio, A.S.; García-Niño, W.R.; González-Reyes, S.; Álvarez-Mejía, A.E.; Guerra-León, S.; Salazar-Segovia, J.; Falcón, I.; Montes de Oca-Solano, H.; Madero, M.; Pedraza-Chaverri, J. The Effect of Dietary Supplementation with Curcumin on Redox Status and Nrf2 Activation in Patients with Nondiabetic or Diabetic Proteinuric Chronic Kidney Disease: A Pilot Study. J. Ren. Nutr. 2016, 26, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Soleimani, Z.; Hashemdokht, F.; Bahmani, F.; Taghizadeh, M.; Memarzadeh, M.R.; Asemi, Z. Clinical and Metabolic Response to Flaxseed Oil Omega-3 Fatty Acids Supplementation in Patients with Diabetic Foot Ulcer: A Randomized, Double-Blind, Placebo-Controlled Trial. J. Diabetes Complicat. 2017, 31, 1394–1400. [Google Scholar] [CrossRef]
- Garcia-Alcala, H.; Santos Vichido, C.I.; Islas Macedo, S.; Genestier-Tamborero, C.N.; Minutti-Palacios, M.; Hirales Tamez, O.; García, C.; Ziegler, D. Treatment with α-Lipoic Acid over 16 Weeks in Type 2 Diabetic Patients with Symptomatic Polyneuropathy Who Responded to Initial 4-Week High-Dose Loading. J. Diabetes Res. 2015, 2015, 189857. [Google Scholar] [CrossRef][Green Version]
- Rodríguez-Carrizalez, A.D.; Castellanos-González, J.A.; Martínez-Romero, E.C.; Miller-Arrevillaga, G.; Pacheco-Moisés, F.P.; Román-Pintos, L.M.; Miranda-Díaz, A.G. The Effect of Ubiquinone and Combined Antioxidant Therapy on Oxidative Stress Markers in Non-Proliferative Diabetic Retinopathy: A Phase IIa, Randomized, Double-Blind, and Placebo-Controlled Study. Redox Report. 2016, 21, 155–163. [Google Scholar] [CrossRef]
- Zhao, L.; Hu, F.-X. α-Lipoic Acid Treatment of Aged Type 2 Diabetes Mellitus Complicated with Acute Cerebral Infarction. Eur. Rev. Med. Pharmacol. Sci. 2014, 18, 3715–3719. [Google Scholar]
- Akbari Fakhrabadi, M.; Zeinali Ghotrom, A.; Mozaffari-Khosravi, H.; Hadi Nodoushan, H.; Nadjarzadeh, A. Effect of Coenzyme Q10 on Oxidative Stress, Glycemic Control and Inflammation in Diabetic Neuropathy: A Double Blind Randomized Clinical Trial. Int. J. Vitam. Nutr. Res. 2014, 84, 252–260. [Google Scholar] [CrossRef]
- Saxena, P.; Selvaraj, K.; Khare, S.K.; Chaudhary, N. Superoxide Dismutase as Multipotent Therapeutic Antioxidant Enzyme: Role in Human Diseases. Biotechnol. Lett. 2022, 44, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Matuz-Mares, D.; Riveros-Rosas, H.; Vilchis-Landeros, M.M.; Vázquez-Meza, H. Glutathione Participation in the Prevention of Cardiovascular Diseases. Antioxidants 2021, 10, 1220. [Google Scholar] [CrossRef] [PubMed]
- Polonikov, A.; Bocharova, I.; Azarova, I.; Klyosova, E.; Bykanova, M.; Bushueva, O.; Polonikova, A.; Churnosov, M.; Solodilova, M. The Impact of Genetic Polymorphisms in Glutamate-Cysteine Ligase, a Key Enzyme of Glutathione Biosynthesis, on Ischemic Stroke Risk and Brain Infarct Size. Life 2022, 12, 602. [Google Scholar] [CrossRef] [PubMed]
- Horstkotte, J.; Perisic, T.; Schneider, M.; Lange, P.; Schroeder, M.; Kiermayer, C.; Hinkel, R.; Ziegler, T.; Mandal, P.K.; David, R.; et al. Mitochondrial Thioredoxin Reductase Is Essential for Early Postischemic Myocardial Protection. Circulation 2011, 124, 2892–2902. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, P.; Upadhyay, N.; Tiwari, A.; P Singh, L. Genetic and Epigenetic Modifications in the Pathogenesis of Diabetic Retinopathy: A Molecular Link to Regulate Gene Expression. New Front. Ophthalmol. 2016, 2, 192–204. [Google Scholar] [CrossRef] [PubMed]
- Imamura, M.; Maeda, S. Perspectives on Genetic Studies of Type 2 Diabetes from the Genome-wide Association Studies Era to Precision Medicine. J. Diabetes Investig. 2024. [Google Scholar] [CrossRef]
- Dallali, H.; Boukhalfa, W.; Kheriji, N.; Fassatoui, M.; Jmel, H.; Hechmi, M.; Gouiza, I.; Gharbi, M.; Kammoun, W.; Mrad, M.; et al. The First Exome Wide Association Study in Tunisia: Identification of Candidate Loci and Pathways with Biological Relevance for Type 2 Diabetes. Front. Endocrinol. 2023, 14, 1293124. [Google Scholar] [CrossRef]
- Holcar, M.; Ferdin, J.; Sitar, S.; Tušek-Žnidarič, M.; Dolžan, V.; Plemenitaš, A.; Žagar, E.; Lenassi, M. Enrichment of Plasma Extracellular Vesicles for Reliable Quantification of Their Size and Concentration for Biomarker Discovery. Sci. Rep. 2020, 10, 21346. [Google Scholar] [CrossRef]
- Park, S.; Kim, O.-H.; Lee, K.; Park, I.B.; Kim, N.H.; Moon, S.; Im, J.; Sharma, S.P.; Oh, B.-C.; Nam, S.; et al. Plasma and Urinary Extracellular Vesicle microRNAs and Their Related Pathways in Diabetic Kidney Disease. Genomics 2022, 114, 110407. [Google Scholar] [CrossRef]
- Zapała, B.; Kamińska, A.; Piwowar, M.; Paziewska, A.; Gala-Błądzińska, A.; Stępień, E.Ł. miRNA Signature of Urine Extracellular Vesicles Shows the Involvement of Inflammatory and Apoptotic Processes in Diabetic Chronic Kidney Disease. Pharm. Res. 2023, 40, 817–832. [Google Scholar] [CrossRef] [PubMed]
- Grabež, M.; Škrbić, R.; Stojiljković, M.P.; Vučić, V.; Rudić Grujić, V.; Jakovljević, V.; Djuric, D.M.; Suručić, R.; Šavikin, K.; Bigović, D.; et al. A Prospective, Randomized, Double-Blind, Placebo-Controlled Trial of Polyphenols on the Outcomes of Inflammatory Factors and Oxidative Stress in Patients with Type 2 Diabetes Mellitus. Rev. Cardiovasc. Med. 2022, 23, 057. [Google Scholar] [CrossRef] [PubMed]
- Pandey, K.B.; Rizvi, S.I. Plant Polyphenols as Dietary Antioxidants in Human Health and Disease. Oxidative Med. Cell. Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Mahjabeen, W.; Khan, D.A.; Mirza, S.A. Role of Resveratrol Supplementation in Regulation of Glucose Hemostasis, Inflammation and Oxidative Stress in Patients with Diabetes Mellitus Type 2: A Randomized, Placebo-Controlled Trial. Complement. Ther. Med. 2022, 66, 102819. [Google Scholar] [CrossRef] [PubMed]
- Brown, V.A.; Patel, K.R.; Viskaduraki, M.; Crowell, J.A.; Perloff, M.; Booth, T.D.; Vasilinin, G.; Sen, A.; Schinas, A.M.; Piccirilli, G.; et al. Repeat Dose Study of the Cancer Chemopreventive Agent Resveratrol in Healthy Volunteers: Safety, Pharmacokinetics, and Effect on the Insulin-like Growth Factor Axis. Cancer Res. 2010, 70, 9003–9011. [Google Scholar] [CrossRef] [PubMed]
- Jeyaraman, M.M.; Al-Yousif, N.S.H.; Singh Mann, A.; Dolinsky, V.W.; Rabbani, R.; Zarychanski, R.; Abou-Setta, A.M. Resveratrol for Adults with Type 2 Diabetes Mellitus. Cochrane Database Syst. Rev. 2020, 1, CD011919. [Google Scholar] [CrossRef]
- Bruckbauer, A.; Zemel, M.B. Synergistic Effects of Metformin, Resveratrol, and Hydroxymethylbutyrate on Insulin Sensitivity. Diabetes Metab. Syndr. Obes. 2013, 6, 93–102. [Google Scholar] [CrossRef][Green Version]
- Yang, A.J.T.; Frendo-Cumbo, S.; MacPherson, R.E.K. Resveratrol and Metformin Recover Prefrontal Cortex AMPK Activation in Diet-Induced Obese Mice but Reduce BDNF and Synaptophysin Protein Content. JAD 2019, 71, 945–956. [Google Scholar] [CrossRef]
- Miguel Angel, D.V.; Maria Antonieta, G.S. Effects of Combined Resveratrol Plus Metformin Therapy in Db/Db Diabetic Mice. J. Metab. Synd. 2016, 05. [Google Scholar] [CrossRef]
- Chawla, A.; Chawla, R.; Jaggi, S. Microvasular and Macrovascular Complications in Diabetes Mellitus: Distinct or Continuum? Indian. J. Endocrinol. Metab. 2016, 20, 546–551. [Google Scholar] [CrossRef]
- Ashok, A.; Andrabi, S.S.; Mansoor, S.; Kuang, Y.; Kwon, B.K.; Labhasetwar, V. Antioxidant Therapy in Oxidative Stress-Induced Neurodegenerative Diseases: Role of Nanoparticle-Based Drug Delivery Systems in Clinical Translation. Antioxidants 2022, 11, 408. [Google Scholar] [CrossRef] [PubMed]
- Kang, L.; Liu, S.; Li, J.; Tian, Y.; Xue, Y.; Liu, X. The Mitochondria-Targeted Anti-Oxidant MitoQ Protects against Intervertebral Disc Degeneration by Ameliorating Mitochondrial Dysfunction and Redox Imbalance. Cell Prolif. 2020, 53, e12779. [Google Scholar] [CrossRef] [PubMed]
- Young, M.L.; Franklin, J.L. The Mitochondria-Targeted Antioxidant MitoQ Inhibits Memory Loss, Neuropathology, and Extends Lifespan in Aged 3xTg-AD Mice. Mol. Cell. Neurosci. 2019, 101, 103409. [Google Scholar] [CrossRef] [PubMed]
- Akpoveso, O.-O.P.; Ubah, E.E.; Obasanmi, G. Antioxidant Phytochemicals as Potential Therapy for Diabetic Complications. Antioxidants 2023, 12, 123. [Google Scholar] [CrossRef] [PubMed]
- Islam, F.; Khadija, J.F.; Islam, M.R.; Shohag, S.; Mitra, S.; Alghamdi, S.; Babalghith, A.O.; Theyab, A.; Rahman, M.T.; Akter, A.; et al. Investigating Polyphenol Nanoformulations for Therapeutic Targets against Diabetes Mellitus. Evid. -Based Complement. Altern. Med. 2022, 2022, e5649156. [Google Scholar] [CrossRef]
- De Oliveira, W.Q.; Neri-Numa, I.A.; Arruda, H.S.; McClements, D.J.; Pastore, G.M. Encapsulation of Flavonoids in Foods for Diabetics: The Emerging Paradigm for an Effective Therapy. Trends Food Sci. Technol. 2022, 127, 198–206. [Google Scholar] [CrossRef]
- Haider, M.; Abdin, S.M.; Kamal, L.; Orive, G. Nanostructured Lipid Carriers for Delivery of Chemotherapeutics: A Review. Pharmaceutics 2020, 12, 288. [Google Scholar] [CrossRef]
- Medici, S.; Peana, M.; Pelucelli, A.; Zoroddu, M.A. An Updated Overview on Metal Nanoparticles Toxicity. Semin. Cancer Biol. 2021, 76, 17–26. [Google Scholar] [CrossRef]
- Liu, Y.; Zeng, S.; Ji, W.; Yao, H.; Lin, L.; Cui, H.; Santos, H.A.; Pan, G. Emerging Theranostic Nanomaterials in Diabetes and Its Complications. Adv. Sci. 2022, 9, 2102466. [Google Scholar] [CrossRef]


| Gene/Biomarker | Participants | Ethnicity | Type of T2D Late Complications | Key Findings | Reference Year |
|---|---|---|---|---|---|
| SOD3 locus: rs2284659, rs2695234, rs17552548, rs758946, rs2270224, rs1799895 (Arg213Gly) | 3137 T2DM patients from DIABHYCAR study; 5 year follow-up | French | Macrovascular (MI) | SOD3 rs2284659 T allele associated with lower incidence of myocardial infarction. | [37] 2015 |
| GPX1 Pro197Leu, SOD1 +35A/C, CAT -262C/T; SOD1, GPX1 and CAT plasma level | 401 T2DM patients: 110 with DSPN, 135 without DSPN, 156 controls without T2DM and DSPN | Polish | Microvascular (DSPN) | No association with DSPN among T2DM patients; GPX1 and SOD1 levels were decreased in T2DM + DSPN patients compared to controls. | [38] 2016 |
| GCLM C-588T, SOD2 Val16Ala, NOS3 G894T, CYBA C242T, MPO G-463A | 1977 T2DM patients without a history of CVD; 85 had CHD events within 7.5 years (median) follow-up | Japanese | Macrovascular (CHD) | Carriers of ≥8 pro-oxidant alleles had an increased risk for CHD compared to the carriers of <8 pro-oxidant alleles, with or without adjustments for clinical variables. | [39] 2014 |
| SOD2, CAT, GPX1, GSTP1, GSTM1*0, GSTT1*0, GCLC, and GCLM | 181 T2DM patients | Slovenian | Macrovascular and microvascular | CAT rs1001179 and GSTP1 rs1138272 associated with end-stage kidney failure, GSTP1 rs1695, and GSTP1 haplotypes associated with moderately increased albuminuria. | [40] 2015 |
| SOD1 +35A/C, SOD2 T47C, and CAT -21A/T; Plasma MDA; Urine UPDs | 366 T2DM patients; consumption of ≥ or < than 3 cups of green tea per day | Tunisian | Macrovascular and microvascular | SOD2 47 CC is associated with a lower risk of T2DM complications and higher UPD levels are protective in high tea consumers (>3 cups/day). | [41] 2015 |
| ALOX5 rs10900213, ALOX5AP rs4293222, GPX1 rs1050450, GPX3 rs3828599, and MPO rs2107545 | 396 T2DM patients and 678 controls | Han Chinese | Macrovascular (carotid plaques) | GPX1 rs1050450 and MPO rs2107545 are associated with an increased risk of carotid plaques in T2DM patients. | [42] 2015 |
| ALOX12 rs14309 | 145 T2DM patients: 108 with DN and 37 without DN | Greek | Macrovascular (MI, stroke, or PAD), CV- and overall mortality | ALOX12 rs14309 GG genotype is associated with MI, higher cIMT, increased CV events, CV- and overall mortality. | [43] 2018 |
| GLUL rs10911021 Plasma levels of MDA, GSH and GSSG | 650 CHD patients (275 with T2DM) and 225 controls (30 with T2DM) | Pakistani | Macrovascular (CHD) | GLUL rs10911021 is associated with CHD only among patients with T2DM but is associated with MDA, GSH, and GSSG levels. | [44] 2018 |
| GSTM1, GSTT1, and GSTP1 | 84 T2DM patients: 42 with DN and 42 without DN; 98 healthy controls | Romanian | Microvascular (DSPN) | No association with DN in T2DM patients. | [45] 2015 |
| NOS3 894G>T, CYBA C242T, PARP-1 Val762Ala, and XRCC1 | 217 T2DM patients: 55 with DKD and 162 without DKD | South Indian | Microvascular (DKD) | NOS3 894T allele associated with increased risk for DKD; PARP-1 762Ala allele protected against DKD. | [46] 2014 |
| 1449 SNPs in OS pathway analyzed on Illumina Human PsychArray-24 v.1.1 BeadChip | 331 T2DM patients: 121 with DKD, 220 without DKD | Greek | Microvascular (DKD) | 43 SNPs in 21 genes in the OS pathway associated with DKD: SPP1, TPO, TTN, SGO2, NOS3, PDLIM1, CLU, CCS, GPX4, TXNRD2, EPHX2, MTL5, EPX, GPX3, ALOX12, IPCEF1, GSTA1, OXR1, GPX6, AOX1, and PRN. | [47] 2021 |
| NOS3 Glu298Asp | 257 T2DM patients: 123 with DFU, 134 without DFU | Iranian | PVD (DFU) | NOS3 298T allele protective against DFU. | [48] 2018 |
| ACE ins/del, NOS3 4a4b, CYBA C242T, PARP-1 Val762Ala, and XRCC1 Arg399Gln | 460 T2DM patients: 149 with DR and 162 with no evidence of DR. | Indian | Microvascular (DR) | PARP-1 762Ala allele protected against DR, while XRCC1 399Gln allele increased the risk for DR. | [49] 2016 |
| NQO1*2 (rs1800566) and plasma NQO1 levels | 400 T2DM patients: 200 with DN and 200 without complications and 200 healthy controls | North Indian | Microvascular (DKD) | NQO1*2 allele is associated with a higher risk for DKD and lower plasma NQO1 levels. | [50] 2016 |
| Trx2/TXNIP and TrxR2 rs8140110, rs7211, rs7212, rs4755, rs1548357, rs4485648 and rs5748469 | 802 T2DM patients: 277 with DR and 525 with no DR | Slovenian | Microvascular (DR) | TrxR2 rs4485648 polymorphism associated with increased risk for DR. | [51] 2016 |
| TXNRD2 rs1548357, rs4485648, and rs5748469 | 972 T2DM patients: 161 patients with MI and 811 patients with no history of CAD | Slovenian | Macrovascular (MI) | TXNRD2 rs 1548357 CC+CT genotypes are associated with a lower prevalence of MI compared with TT genotype group. | [52] 2015 |
| HO-1 T(-413)A SNP and (GT)n repeat | 536 T2DM patients | Korean | Microvascular (albuminuria) | HO-1 -413 TT genotype associated with albuminuria compared to A allele carriers, especially with longer T2DM duration and poor glycemic control. | [53] 2015 |
| ROMO-1 rs6060566 | 806 T2DM patients: 278 with DR and 528 without clinical signs of DR | Slovenian | Microvascular (DR) | ROMO-1 rs6060566 CC genotype associated with increased risk for DR. | [54] 2015 |
| TNF-α-308 G/C and IL-1α -889 C/T; plasma MDA, GSH, SOD, and CAT levels | 402 T2DM patients: 188 with DN and 214 without DN; 188 patients with NDN; 235 controls | West Indian | Microvascular (DKD) | IL-1α -889 C/T polymorphism may be associated with diabetic nephropathy. MDA was increased, while GSH and SOD were decreased in the patients group compared to controls. | [55] 2015 |
| NLRP3 rs35829419, CARD8 rs2043211 | 181 clinically well-characterized T2DM patients | Slovenian | Macrovascular (MI, PAD, stroke) | NLRP3 rs35829419 is associated with an increased risk for macrovascular complications, in particular with MI. | [56] 2015 |
| RAC1 rs4724800, rs7784465, rs10951982, rs10238136, rs836478, and rs9374; plasma H2O2 levels in 489, and GSSG levels in 258 T2DM patients | 1470 T2DM patients; 1007 with and 407 without DR; 553 with and 887 without DKD; 1309 with and 109 without DN; 968 with and 502 without DA; 107 with and 1309 without DFS | Russian | Microvascular (DKD, DR) Macrovascular (DA, DFU) | RAC1 rs7784465 T/C was associated with increased risk of DR and DA in males, and DR in females. RAC1 rs836478 was associated with DR and DN in males, whereas SNP rs10238136 was associated with DA in females. | [57] 2023 |
| KEAP1 rs11085735; GSH, GPX, MDA, and TAC | 300 T2DM patients: 100 with DR, 100 with DN, 100 without complication; and 100 healthy controls | Iranian | Microvascular (DN, DR) | KEAP1 rs11085735 AA genotype associated with DN and lower GPX activity compared to CC genotype. | [58] 2022 |
| ALDH2*1/*2 alleles; drinking habits, serum GGT | 234 T2DM patients with no DR at baseline; follow-up 5.5 ± 2.5 years | Japanese | Microvascular (DR) | The incidence of DR is significantly higher in the ALDH2*2 allele carriers with a high GGT level than in the non-carriers with a high or low GGT level. | [59] 2013 |
| AKR1B1 rs759853 | 1005 T2DM patients: 695 with DKD and 310 patients without DKD | Brazilian | Microvascular (DKD) | A/A genotype was associated with risk for DKD after adjustment for gender, triglycerides, BMI, presence of hypertension and DR, and duration of DM. | [60] 2022 |
| VDR ApaI, TaqI, BsmI, and FokI variants, plasma vitamin D, homocysteine, and TBARS levels | 262 2DM patients: 127 with DFU and 135 without DFU | Iranian | Macrovascular PVD (DFU) | VDR ApaI associated with DFU; ApaI GG and BsmI CC carriers had lower levels of TBARS compared to other genotypes. | [61] 2022 |
| XRCC3 T241M and XRCC1 A399G | 238 subjects: 116 with T2DM, 50 with DKD, and 72 with normal glucose metabolism | Turkish | Microvascular (DKD) | XRCC1 Gln allele associated with T2DM and DKD. | [62] 2019 |
| ADPRT 726 Val/Ala, MUTYH 324 His/Glu, APE 148 Asp/Glu; comet assay | 355 subjects: 89 with T2DM + DSPN, 120 with T2DM and without DSPN; 146 healthy subjects without T2DM and neuropathy | Polish | Microvascular (DSPN) | None of the 3 polymorphisms associated with the risk of DSPN; ADPRT 726 Ala allele increased T2DM risk. | [63] 2015 |
| Biomarkers Studied | Participants and Design | Ethnicity/Region | Type of T2D Late Complications | Key Findings | Reference Year |
|---|---|---|---|---|---|
| Microarray expression analysis (26,803 unique gene sequences and 30,606 lncRNA), validation by qPCR | Total RNA from PB of 15 patients with T2DM, 15 with DKD, 15 with prediabetes, and 15 controls | Mexican | Microvascular (DKD) | 300 genes higher expressed in DKD compared to other groups. qPCR confirmed higher METLL22, PFKL, CCNB1, and CASP2 expression in DKD compared to other groups | [64] 2023 |
| qPCR analysis of expression of TP53, MMP9, and SLC23A2 | Total RNA from PBMCs in 49 patients with T2DM: 14 with DR, 35 without DR, and 32 controls | Spanish | Microvascular (DR) | Expression of MMP9 was higher and SLC23A2 was lower in T2DM compared to controls. TP53, MMP9, and SLC23A2 expression differed between the T2DM-RD, T2DM+RD, and controls | [65] 2018 |
| mRNA levels of endoplasmic reticulum stress, oxidative stress, and crosstalk markers | Total RNA from PBMCs in 30 patients with T2DM, 30 patients with DKD, and 30 healthy controls | Indian | Microvascular (DKD) | ATF6, IRE1α, PERK, CHOP, GRP78, CYBA, TXNIP, PDI, and ERO1A expression is higher in T2DM and DKD patients compared to other groups | [66] 2021 |
| Serum SOD, GPX, and CAT gene expression and enzyme level | Total RNA from PB of 67 patients with T2DM: 22 with DR, and 45 without DR | Saudi Arabian | Microvascular (DR) | Poor glycemic control and altered SOD, GPX, and CAT expression associated with DR | [67] 2013 |
| NLRP3 mRNA expression—qPCR; Serum 8-OHdG, IL-1β, uHSP72 levels | Total RNA from PB of 45 T2DM patients: 15 normoalbuminuric, 15 microalbuminuric, 15 macroalbuminuric; and 15 healthy controls | Egyptian | Microvascular (albuminuria) | NLRP3 mRNA expression, serum 8-OHdG, IL-1β, and uHSP72, and CHIT 1 activity increased in macroalbuminuric patients compared to other groups | [68] 2017 |
| RAGE qPCR and WB, serum AGEs; PCO, AOP, and MDA | Total RNA from PBMCs of 75 T2DM patients: 25 without vascular complications, 25 with microvascular complications; 25 with macrovascular complications, and 25 healthy controls | Indian | Microvascular and macrovascular | RAGE mRNA expression and serum AGE levels are higher in T2DM patients with microvascular and macrovascular complications compared to T2DM without complications and controls | [69] 2014 |
| Type of T2D Late Complications | Antioxidant | Comparator | Duration | Participants and Design | Key Findings | The Overall Risk of Bias | Reference Year |
|---|---|---|---|---|---|---|---|
| Microvascular DKD | crocin tablets (15 mg/day) | control group (placebo pill) | 90 days | 44 Iranian patients (52% men) were randomly assigned to the crocin (22) or placebo (22) group, and 40 participants completed the trial | No statistically significant differences in terms of baseline anthropometric, clinical, and biochemical parameters. | low risk | [70] 2022 |
| Microvascular DPN | 200 mg Tocovid capsule (400 mg/day) | control group (placebo capsules; identical in size, shape, and color) | 12 months, followed by a 6-month washout period | 70 of 88 Malaysian patients completed this study treatment (33 vs. 37 in the placebo group); 72 participants were included in the washout analysis | Highly significant improvements in the CV of both median and sural sensory nerves. No significant differences from baseline between groups in nerve conduction parameters of all three nerves after 6 months of washout. No significant changes in TGFβ-1, or VEGF-A. | some concerns | [71] 2021 |
| Microvascular DPN | two Pilates groups consumed 25 g dark (450 mg flavonoids per dose) or white chocolate 10 min before each Pilates training session (60 min), three times per week | control group consumed 25 g of the same dark chocolate at a pre-determined time 3 times per week, without any regular exercise | 8 weeks (Pilates three times a week) | 36 female Iranian patients (no other diabetic complications) in three groups: control (12); Pilates and dark chocolate (12); Pilates and flavanol-free white chocolate (12) | Significant increases in the TAC status in all groups. The Post hoc Bonferroni test showed a statistically significant increase in TAC for the dark chocolate group. | some concerns | [72] 2021 |
| Macrovascular PVD (non-healing DFU) | PRP-FG dressing plus oral vitamin E (200 IU/2 Day) and C (250 IU/2Day), the dressing was changed 3 times a week | PRP-FG dressing plus oral placebo of vitamin E and C | 8 weeks | 25 Iranian patients with non-healing DFU, 13 in the intervention group, 12 in a placebo group | Significant wound size reduction and decrease in PAB, ESR, and hs-CRP were observed in the intervention group compared to the control group (p < 0.05). | low risk | [73] 2021 |
| Microvascular DPN (mild-to-moderate) | decaffeinated GTE capsules 500 mg 30 min after meals three times daily | placebo material in a similar package | 16 weeks (assessment made at baseline and after 4, 8, and 16 weeks of treatment) | 194 Egyptian patients, 96 in GTE, and 98 in placebo group | After 8 weeks of treatment, patients in the GTE group expressed lower VAS scores, significantly lower TCSS scores and significantly lower VPT. The difference became more evident at 16 weeks. | some concerns | [74] 2021 |
| Microvascular DKD | tocotrienol-rich vitamin E 200 mg (Tocovid SupraBioTM; Hovid Berhad, Ipoh, Malaysia) twice daily (400 mg/day) | identical-looking capsules (tocotrienol-free palm oil capsules) twice daily | 12 months (2-month interval visits) | 31 Malaysian patients in the intervention group and 28 in the control group | Reduced serum creatinine levels (p = 0.029) and significantly improved eGFR (p = 0.011) after eight months. Subgroup analysis (patients with stage 3 CKD) demonstrated persistent renoprotective effects over 12 months; Tocovid improved eGFR (p = 0.022) and serum creatinine (p = 0.042) but not UACR. | some concerns | [75] 2021 |
| Macrovascular PVD (non-healing DFU) | nano curcumin supplements (80 mg/day) | tablet with placebo, similar in size and shape and odor | 12 weeks | 50 Iranian patients, 25 in curcumin and 25 in a placebo group | Significant improvement of TAC, GSH, total and LDL-cholesterol, and glycemic control but did not affect the ulcer size. | low risk | [76] 2021 |
| Macrovascular CHD | curcumin (1000 mg/day) | placebo tablet (starch) | 12 weeks | 60 Iranian patients with T2DM and CHD, aged 45–85 years with 2- and 3-vessel CHD | Reduced MDA (p = 0.01), significantly increased TAC (p = 0.04) and GSH levels (p = 0.001); curcumin intake upregulated PPAR-γ and decreased PSQI (p = 0.01). | some concerns | [77] 2020 |
| Microvascular DPN | tablet with a combination of four elements (SOD 10 mg, ALA 570 mg, B12 250 mcg, and Acetyl L-Carnitine 300 mg) | placebo (tablet) | 12 months | 85 Greek patients, 43 in the intervention group, 42 in the placebo group | BIO, MNSIQ, QL, pain, and SNCV, SNAP, and B12 levels had significantly improved in the intervention group, whereas in the placebo group, MCR and pain deteriorated. The changes in MNSIQ, QL, SNCV, BIO, and pain differed significantly between groups. | low risk | [78] 2020 |
| Microvascular DPN | 200 mg of Tocovid (tocotrienol-rich vitamin E) capsule twice a day | placebo capsule | 8 weeks | 80 Malaysian patients, 39 in the intervention group, 41 in a placebo group | Tocovid significantly improves the CV of all observed nerves (p < 0.001). Significantly higher levels of serum NGF. | low risk | [79] 2020 |
| Microvascular DPN | GLA (320 mg/day) | ALA (600 mg/day) | 12 weeks | Out of 100 Korean patients, 73 patients were included in the final analysis, 35 in the GLA group and 38 in the ALA group | Per-protocol analyses revealed significant decreases in the mean VAS and TSS scores compared to baseline in both groups, but there were no significant differences between the groups. | some concerns | [80] 2020 |
| Microvascular NPDR | grape seed proanthocyanidin extract (GSPE) (150 mg/day in three tablets) or calcium dobesilate (CD; 750 mg/day) | placebo tablets, similar to GSPE tablets | 12 months (follow-up visits at 3, 6, 9, and 12 months) | Korean patients with retinal thickening with HEs; 32 in the GSPE group, 35 in the CD group, and 19 in the placebo group were included in the analysis | Significantly greater improvement in HE severity in the GSPE group compared to placebo or CD group. In the GSPE group, TMV after 9 months of treatment was significantly decreased compared to baseline. | low risk | [81] 2019 |
| Microvascular DPN | nanocurcumin (72% curcumin, 25% demethoxycurcumin, and bisdesmethoxycurcumin 3%) supplements (80 mg/day) | placebo capsules (polysorbate 80) | 8 weeks | 80 Iranian patients with non–insulin-dependent T2DM, 40 in the intervention group and 40 in the control group | Significant reduction in HbA1c (p < 0.001) and FBS (p = 0.004), total score of neuropathy (p < 0.001), total reflex score (p = 0.04), and temperature sensation (p = 0.01) compared to the placebo group. | some concerns | [82] 2019 |
| Microvascular DKD | Resveratrol (500 mg/day) | carboxymethylcellulose as placebo; capsules were identical to the resveratrol ones | 90 days | Out of 64 Iranian patients, 60 patients were analyzed, 30 in the intervention group and 30 in the placebo group. | The mean urine albumin/creatinine ratio was significantly reduced (p < 0.001), whereas eGFR (p = 0.08) and serum creatinine (p = 0.13) were unchanged. Serum antioxidant enzymes were significantly increased with resveratrol. After adjusting for confounding variables, the reduction in urinary albumin excretion was still significant. | some concern | [83] 2019 |
| Microvascular DKD | vitamin E supplement (800 IU/day) | placebo, similar in shape and size capsule | 12 weeks | 27 Iranian patients in the intervention group and 27 in the placebo group | Significant elevation in vitamin E levels p < 0.001) and plasma glutathione levels. Significant reduction in serum total cholesterol (p = 0.03), LDL-cholesterol (p = 0.01), and ratio of total cholesterol to HDL-cholesterol ratio (p = 0.001), and HDL-cholesterol levels (p = 0.006). No favorable effect for TGA, very low-density lipoprotein cholesterol, NO, and TAC levels. | some concerns | [84] 2018 |
| Macrovascular CHD | melatonin (10 mg/day) | placebo | 12 weeks | Iranian patients with CHD, 30 in an intervention group, 30 in a placebo group | Significant increases in plasma GSH, NO, and HDL-cholesterol. Significant decreases in MDA, PCO, and serum hs-CRP levels, total-/HDL-cholesterol ratio, and systolic and diastolic blood pressure. | some concerns | [85] 2019 |
| Macrovascular PVD (grade 3 DFU) | omega-3 fatty acids from flaxseed oil supplements (1000 mg/day) | placebo | 12 weeks | 60 Iranian patients (aged 40–85 years old) with grade 3 DFU; 30 in an intervention group and 30 in a placebo group. | Significant decreases in ulcer length (p = 0.03), width (p = 0.02), and depth (p = 0.01). Significant increase in plasma TAC (p < 0.001) and GSH concentrations (p = 0.03) compared to the placebo. | some concerns | [86] 2017 |
| Microvascular DKD | 200 mL/ day probiotic (Lactobacillus plantarum A7) fortified soy milk | soy milk in the control group | 8 weeks | Out of 48 randomized Iranian patients with stage 1 or 2 nephropathy, 20 in the intervention group and 20 in the control group were included in the final analysis | Higher mean value of GSH, and increase in activity of GSH-Px and GR. Oxidized GSH concentration was significantly reduced in the probiotic soy milk group. However, no significant reduction of the serum 8-iso-prostaglandin F2a or MDA and no induction of TAC concentrations within and between the 2 groups. | some concerns | [87] 2017 |
| Microvascular DKD | CUR (320 mg/day) | placebo supplement containing starch | 8 weeks | 51 Mexican patients with diabetic proteinuric CKD, 28 in an intervention group and 23 in a placebo group | No improvement in proteinuria, estimated GFR, or lipid profile. Enhanced antioxidant capacity in subjects with diabetic proteinuric CKD. No effect of CUR was observed on the antioxidant enzyme activities or Nrf2 activation. | some concerns | [88] 2016 |
| Microvascular DKD | omega-3 fatty acid (1000 mg/day) from flaxseed oil | placebo | 12 weeks | Out of 60 Iranian patients with DN, 30 were analyzed in the intervention group and 30 in the placebo group. | No significant effects on other lipid subfractions, biomarkers of inflammation, and oxidative stress compared with the placebo. | some concerns | [89] 2017 |
| Microvascular DPN (symptomatic) | Two phases. First phase: ALA (600 mg tid); second phase: ALA (600 mg qd). | First phase: no comparator; Second phase: ALA withdrawal | First phase: 4 weeks; Second phase: 16 weeks | 45 Mexican patients with polyneuropathy in the first phase and 16 patients in the intervention group and 17 patients in the control group in the second phase. | In patients who responded to the initial 4-week high-dose administration of ALA, subsequent treatment with ALA improved neuropathic symptoms, whereas ALA withdrawal was associated with a higher use of rescue analgesic drugs. | some concerns | [90] 2015 |
| Microvascular DR (non-proliferative) | Group 1: Coenzyme Q10 (400 mg/day); Group 2: combined antioxidant therapy (composed of 10 mg of lutein, 4 mg of astaxanthin, 1 mg of zeaxanthin, 180 mg of vitamin C, 30 mg of vitamin E, 20 mg of zinc, and 1 mg of copper) once per day | Group 3: placebo; Group 4: without intervention. | 6 months | 60 Mexican patients in Group 1 (n = 20), Group 2 (n = 20), and Group 3 (n = 20), but healthy subjects of similar age and gender (blood donors) in Group 4 (n = 20). | LPO levels decreased significantly in groups exposed to antioxidant therapy (p < 0.0001). Levels of nitrites/nitrates decreased significantly (p < 0.0001) in the coenzyme Q10 group and CAT group. TAC increased significantly in groups subjected to pharmacological antioxidant therapy (p < 0.0001). The activity of CAT decreased significantly (p < 0.002) in groups exposed to antioxidant therapy. The activity of the GSH-px was decreased significantly with a tendency to normalize in groups subjected to antioxidant therapy (p < 0.0001). | high risk | [91] 2016 |
| Macrovascular cerebrovascular complications (acute cerebral infarction) | ALA injection (600 mg in 250 mL 0.9% sodium chloride, iv. gtt once per day) | Vitamin C (3 g, solved in 250 mL 0.9% sodium chloride for iv. gtt injection, once per day) | 3 weeks | 90 Chinese patients, 46 patients in the intervention group and 44 in the control group. | After the treatment, the plasma SOD and GSH-Px levels increased, while MDA decreased (p < 0.05), compared to the control group (p < 0.01). NIHSS score, blood glucose, blood lipids, and HOMA-IA of the experiment group decreased significantly (p < 0.01). | high risk | [92] 2014 |
| Microvascular DPN | Coenzyme Q10 (200 mg/day); as a 100 mg capsule taken twice daily | placebo | 12 weeks | Out of 74 Iranian patients, 32 were analyzed in an intervention group and 30 in a placebo group. | CoQ10 did not improve the neuropathy signs compared to placebo, although it had some beneficial effects on TAC and hsCRP. | high risk | [93] 2014 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tonin, G.; Dolžan, V.; Klen, J. Genetic and Transcriptomic Background of Oxidative Stress and Antioxidative Therapies in Late Complications of Type 2 Diabetes Mellitus: A Systematic Review. Antioxidants 2024, 13, 277. https://doi.org/10.3390/antiox13030277
Tonin G, Dolžan V, Klen J. Genetic and Transcriptomic Background of Oxidative Stress and Antioxidative Therapies in Late Complications of Type 2 Diabetes Mellitus: A Systematic Review. Antioxidants. 2024; 13(3):277. https://doi.org/10.3390/antiox13030277
Chicago/Turabian StyleTonin, Gašper, Vita Dolžan, and Jasna Klen. 2024. "Genetic and Transcriptomic Background of Oxidative Stress and Antioxidative Therapies in Late Complications of Type 2 Diabetes Mellitus: A Systematic Review" Antioxidants 13, no. 3: 277. https://doi.org/10.3390/antiox13030277
APA StyleTonin, G., Dolžan, V., & Klen, J. (2024). Genetic and Transcriptomic Background of Oxidative Stress and Antioxidative Therapies in Late Complications of Type 2 Diabetes Mellitus: A Systematic Review. Antioxidants, 13(3), 277. https://doi.org/10.3390/antiox13030277







