Abstract
Papaya fruit is commonly known for its nutritional and medicinal value. It is a perennial, herbaceous, and trioecious cross-pollinated species with male, female, and hermaphrodite plants. The Chilean papaya, originating from South America, has been extensively spread throughout the Andean nations, cultivated primarily in the Coquimbo and Valparaíso valleys in Chile, between 34°41′ and 36°33′ latitude south. Its intense aroma, yellow color, and oblong shape characterize this fruit. It also stands out for its high content of carotenoids, vitamins, proteins, and polysaccharides, which make it a great functional food. Also, papaya contains bioactive compounds with antifungal, anti-inflammatory, and wound-healing effects. For years, the fruit has been used to produce canned fruit, juice, and candies to satisfy the local market. Chilean papaya has significant economic importance, supplying both local and international markets. This review aims to consolidate the evidence-based information on the native Chilean papaya (Vasconcellea pubescens) as a food matrix. The fruit’s ripening process, nutritional composition, industrial applications, and health-promoting properties, including its antioxidant and antidiabetic effects, are thoroughly examined. Additionally, the extraction of papaya oil, encapsulation of bioactive compounds, industrial and artisanal processing techniques, and patents are explored, highlighting the diverse applications and potential benefits of this fruit.
1. Introduction
The Chilean papaya is a native fruit of South America that has been widely distributed in the Andean countries. It belongs to the Caricaceae family and corresponds to Vasconcellea pubescens. This species was introduced in Chile more than 50 years ago and is cultivated in the Coquimbo and Valparaíso valleys, as well as on the coast of the Maule Region [1] between 34°41′ and 36°33′ latitude south. This fruit is characterized by its intense aroma, yellow color, and oblong shape. In addition, ripe papaya is an excellent source of carotenoids, vitamins, proteins, and polysaccharides [1]. Papaya is an essential fruit in Chile and has aroused great interest due to its wide use in preparing different food products, such as papaya gummies, canned papaya, juice, syrup, and jam [2], besides having antifungal properties, anti-inflammatory effects and wound-healing effects [3]. Additionally, this species produces latex with a high level of papain (Figure 1), an important and valuable proteolytic enzyme that plays a fundamental role in the digestive process by participating in the breakdown of strong protein fibers [4].
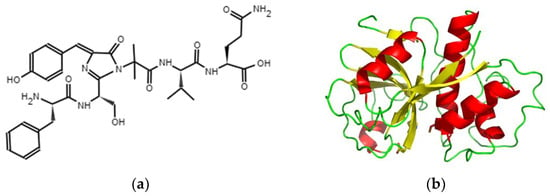
Figure 1.
Papain’s chemical structure (a), Caricaceae’s 3D papain structure (b) [5].
Papain is a cysteine protease isolated from papaya latex, a milky exudate that protects green fruits and young leaves. Structurally, papain is a globular protein containing around 212 amino acids with an approximate molecular weight of 23,406 Da. Its three-dimensional structure includes an alpha/beta domain, with a central beta sheet surrounded by alpha helices. The active site of papain consists of a catalytic triad formed by cysteine, histidine, and asparagine residues, which are crucial for its enzymatic activity. The greener the fruit, the more active the papain. This enzyme exhibits high activity in the hydrolysis of proteins, oligopeptides, amino acid esters, particularly in arginine, lysine, residues following phenylalanine, and amide bonds in general [5,6]. It is stable and active under various conditions, even at elevated temperatures. The protein is stabilized by three disulfide bridges, along which the molecule folds, creating strong interactions among the side chains, which contribute to the stability of the enzyme [6].
It is a component widely used in different medicinal lines, cell isolation, detergents, leather and textiles, cosmetics, the pharmaceutical industry, and dermatological products, and in foods mainly as a beer clarifier and meat tenderizer [7].
Only 225 hectares of Vasconcellea pubescens are cultivated in Chile, mainly concentrated between 30° and 33° latitude south (94%). Most orchards in this area are relatively large (10–40 ha) and maintained under standard fruit management practices (terracing, fertigation, pest management, pruning, etc.), supplying local and international markets. This species is also cultivated in southern Chile (35°–38° South Latitude) but in smaller orchards, primarily in home gardens [8]. Its life cycle begins after a brief fall-winter dormancy between June and August, when the average temperature exceeds >20 °C. New leaves, flowers, and fruits begin to grow in spring, with fruits from the previous season also starting to mature. This process continues annually between September and May [9].
The objective of this review is to highlight the potential of Chilean papaya by detailing its origin, nutritional characteristics, medicinal properties, industrial applications, and life cycle. This includes its role as a source of bioactive compounds (such as papain) and its multiple uses in food, cosmetics, and medicine, as well as its relevance in local and international markets and its potential in research.
2. Papaya Fruits Varieties
Papaya fruits grow in tropical and subtropical regions and are traded worldwide. They can be found in valleys at elevations up to 2000 m above sea level. Numerous studies have described the beneficial effects of this fruit against chronic diseases such as cancer, diabetes, and obesity [10].
The Caricaceae are a small family of six genera and 35 species (Table 1), and they have a disjunct distribution between Africa and the Neotropics. Cylicomorpha, the only African genus of Caricaceae, has two large tree species restricted to humid premontane forests in East and West Africa. The other five genera occur in South and Central America with representatives in wet and seasonally dry habitats. Vasconcellea, the largest genus within the family, comprises 20 species plus a naturally occurring hybrid, Vasconcellea × heilbornii. Due to its prominent economic importance, the unique species of Carica, the common papaya (C. papaya L.), has become pantropical [11]. The second-largest genus is Jacaratia, with seven species of trees; it is widespread in the lowlands of the Neotropics. Horovitzia and Jarilla are mainly herbaceous plants of tropical seasonal forests in Mexico. Horovitzia cnidoscoloides, the only species in the genus Horovitzia, is a small, thin tree. It is monotypic and endemic to the Sierra de Juárez in northern Oaxaca, Mexico. Jarilla comprises three species of herbs with perennial tubers that resprout and can be found in Guatemala.

Table 1.
Different types of Caricacea, based on genera, species, and origin.
3. Chilean Papayas
Chile is characterized as a long and narrow country with tremendous climatic variation, which makes it a suitable climate for papaya cultivation in certain regions. For this reason, in Chile, there are two varieties of papaya. One of these varieties is the introduced papaya (Vasconcellea pubescens), and the other is the Chilean origin papaya (Vasconcellea chilensis) [19]. It is still being determined if Vasconcellea chilensis was an evolution because of human selection or if it was present in the wild ancestors. Still, they also claim this variation has valuable introgression characteristics concerning other papaya species [19,20]. During this period, there was a lack of clarity regarding Vasconcellea pubescens, which was often used synonymously with the genus Carica (synonyms of Vasconcellea cundinamarcensis, Carica pubescens, initially called Carica candamarcensis).
The literature describes this confusion between the two genera due to their resemblance and typical ecological preference for higher altitudes. It is understood that there are differences in climate conditions, peak production months, and genetic evidence distinguishing them [21,22]. The Natural Resources Research Center, CIREN [23] reports that the regions of Arica and Parinacota, Coquimbo, Valparaíso, and Ñuble (Figure 2) have hectares of papaya plantations with 135.77 h total. The region of Coquimbo produces 83.81% of the area of papaya in Chile, where 100% of papaya production is destined for the agro-industry [24].
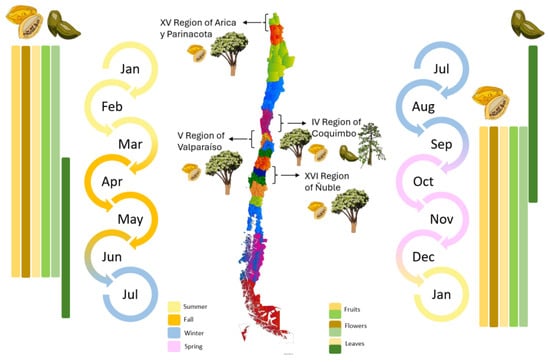
Figure 2.
Regional map of Chile showing papaya cultivation by geographical zone and life cycle of Vasconcellea pubescens ( —orange palette) and Vasconcellea chilensis (
—orange palette) and Vasconcellea chilensis ( —green palette) [9,23,25].
—green palette) [9,23,25].
3.1. Vasconcellea pubescens
Papaya (Vasconcellea pubescens) is a tropical species cultivated in Chile. It is assumed to be a trioecious plant [8] and is described as dioecious (with male and female plants) and monoecious, featuring scarce bisexual flowers that produce deformed fruits [26]. It reaches a height of 3–6 m, sometimes up to 10 m [27], and has succulent, medullose, and branched stems with an extensive basal part [28]. The fruit of Vasconcellea pubescens is yellow and obovoid or ellipsoidal, five-sided, 6–15 cm long, and 3–8 cm wide [29]. It is essential to mention that the edible yield is only 46% of the whole fruit, with approximately 5% sugar content [30]. Its taxonomy comes from the Plantae Kingdom, Brassicales Order, Caricaceae Family, Vasconcellea gender, Vasconcellea pubescens species. Vasconcellea pubescens is cultivated in several South American countries [25]. In Chile, it is grown from 29°54′ S–71°15′ W (La Serena city) to 36°08′ S–72°47′ W (Cobquecura city), always under moderate temperatures (10–25 °C) because it cannot tolerate frost or temperatures over 30 °C [9]. The timing of its introduction to northern Chile is still not precise, but the first Spanish chronicles indicate that native people had already cultivated it before the Spanish conquerors arrived in this region around 1535. [31]. V. pubescens is considered a food heritage site in the area and the country.
3.2. Vasconcellea chilensis
Vasconcellea chilensis is harvested from dioecious papaya, and has a branched succulent stem that is cylindrical, short, and thick, 1 to 4 m high, with flowers of 1–3 cm diameter. Its taxonomy comes from the Plantae Kingdom, Magnoliophyta Division, Magnoliopsida Class, Brassicales Orden, Caricaceae Family, and Vasconcellea Genus [32]. The fruit it produces measures from 1 and 2 to 3 cm and has a greenish-brown color, much smaller than the papaya (Vasconcellea pubescens) (Table 2) [19,33]. Its current fragmented natural populations are between 29°25′ S–71°17′ W and 30°42′ S –71°22′ W (Coquimbo, Chile) [25]. This papaya species is estimated to be in danger of extinction by the extreme that small populations meet and the destruction of its original habitat [19]. That is why it is estimated that they are not registered explicitly within Chile’s harvested fruit statistics.

Table 2.
Comparison between V. pubescens and V. chilensis.
4. Ripening and Shelf Life
The fruit has a strong and characteristic aroma. The ripe fruit is used to make preserves and drinks. Vasconcellea pubescens fruit has been classified as a climacteric fruit [22]. Climacteric fruits exhibit a characteristic rise in ethylene production and respiration during ripening [43,44]. Its life cycle begins after emerging from a brief fall-winter break between June and August (Figure 2) [9]. The first noticeable event during the ripening of Vasconcellea pubescens is the rapid degreening of the skin, which is followed by the climacteric ethylene rise, where the ethylene production reached values of 44 μL kg−1 h−1 after 11 days of storage at 20 °C [45]. In addition, a rapid softening of the flesh is apparent. Ethylene plays a key role during ripening, stimulating the development of ripening attributes such as color, texture, aroma, and flavor [22].
As the ripening develops, a slow increase in pH and a reduction in titratable acidity are observed during storage at 20 °C. While dramatic changes in skin color occur in the first 3 days of storage, a marked increase in total soluble solids occurs, and after that, a drop is observed [22].
The rate of degreening is not homogenous among different fruits. For almost total degreening (90% yellow), between 3 and 5 days at 20 °C are required, starting from green to 10% yellow fruit, as shown in Figure 3. This degreening rate is faster than reported for different Chilean papaya cultivars and related species, such as ‘Sunrise’ and ‘Kapolo’ [46].

Figure 3.
Evolution of the Vasconcellea pubescens fruit (unripe green fruits and ripe yellow fruit (180–200 g)) [9].
In the research by Caicedo Pineda and García Ortiz (2018), titled “Evaluation of Physicochemical Parameters During the Growth, Development, and Postharvest of the Species Vasconcellea pubescens, Solanum quitoense var. septentrionale, and Capsicum pubescens”, the polar diameter (length) and axial diameter (width) at different maturity stages was measured. They developed a growth curve for the species Vasconcellea pubescens during the development cycle over 22 days of evaluation and determined that both the diameter and length of the fruit increased proportionally, as shown in Figure 3 [47].
5. Nutritional Components
Papaya contains a critical number of vitamins (A, B1, B2, and C), minerals (calcium, iron, potassium, and sodium), and carotenoids (lycopene, β-carotene, and β-cryptoxanthin) while being low in sodium, fats, and calories. In addition to its striking aroma and high vitamin content, the fruit of Vasconcellea pubescens is attractive to consumers because it contains numerous phenolic compounds, specifically hydroxycinnamic acid and quercetin glycoside derivatives, which play an essential role in the anti-hyperglycemic, antioxidant, and insulin stimulating activities [48].
It has been determined that the nutritional properties expressed in Table 3 are important because they help to improve health and digestion, reduce harmful cholesterol levels, and prevent diabetes, among other benefits. On the other hand, it also highlights the qualities of latex for healing and protection against the development of gastric ulcers. In addition, oleic acid predominates in its seeds and benefits the blood vessels, reducing the risk of cardiovascular diseases [49]. In addition, papaya has a low content of lipids and proteins. However, it has a high moisture content; total carbohydrates are obtained by difference. The papaya fruit is generally rich in bioactive compounds such as phenolic compounds and vitamin C, contributing to antioxidant capacity [1].

Table 3.
Nutritional and proximal composition of fresh papaya.
6. Bioactive Compounds
Phenolic compounds are specialized metabolites primarily found in plant species, characterized by significant structural diversity. They are known for their antioxidant potential, which can prevent or repair damage caused by oxidation [52,53]. The most abundant antioxidants in fruits are polyphenols and vitamin C, while vitamins A, B, and E, and carotenoids, are present in lesser quantities in some fruits [54]. Moreover, papaya contains a significant concentration of carotenoids, vitamins, proteins, and polysaccharides [1].
Diverse studies have identified bioactive compounds in Chilean papaya following distinct processing methods and different parts that comprise the fruit (Table 4). Vega-Gálvez et al. (2021) [55] compare the bioactive components of fresh papaya with papaya subjected to various drying processes. Among the best results, vacuum-drying papaya maintains 5.83 (mg GAE g−1 D.M.) of total phenolic content and 2.50 (mg QE g−1 D.M.) of total flavonoid content, freeze-drying papaya maintains 21.16 (μmol TE g−1 D.M.), and infrared drying papaya maintains 66.83 (μmol TE g−1 D.M.). Conversely, an additional study using the same approach identified other compounds of interest in the peeled papaya, obtaining the best results of gallic acid 9.76 ± 0.61 (mg 100 g−1 D.M.), chlorogenic acid 4.38 ± 0.49 (mg 100 g−1 D.M.), Tyrosol 21.01 ± 0.74 (mg 100 g−1 D.M.), and naringin 3.34 ± 0.12 (mg 100 g−1 D.M.) in vacuum drying, and ρ-Coumaric acid 7.84 ± 0.12 (mg 100 g−1 D.M.), and trans-ferulic acid 5.56 ± 0.06 (mg 100 g−1 D.M.) in infrared drying [48]. Uribe et al. (2015) [1] determined extraction by agitation, ultrasound, high hydrostatic pressure, and a combination of them like high hydrostatic pressure-agitation, and high hydrostatic pressure-ultrasound extractions (HHPE-UE), HHPE-UE was the most considerable extractive process obtained from bioactive compounds. Briones-Labarca et al. (2015) [2] determined the bioactive compounds present in the seeds of the Chilean papaya by high hydrostatic pressure extraction (HHPE) and ultrasound-assisted extraction to different time processes. Then, 15 min for HHPE is the best process extraction, but 5 min for HHPE processes is more acceptable energetically.
On the other hand, it is interesting to estimate the bioactive compounds present in another component of the fruit plant. Halder et al. (2022) [56] studied the bioactive compounds present in the flower of the Carica papaya, where they obtained values of 108.66 ± 0.35 phenols (mg GAE 100 g−1), 13.7 ± 0.57 flavonoids (mg QE 100 g−1), 260.83 ± 0.57 CUPRAC (mg GAE g−1), and 59.33 ± 0.52 FRAP (µMFe2+ g−1), results that could be intriguing to investigate further in the plant of Chilean papaya. On the other hand, Robles-Apodaca et al. (2024) [57] successfully optimized the extraction process of bioactive compounds from papaya seeds using response surface methodology (RSM) combined with a central composite design (CCD). This approach maximized the total yield of polyphenols and flavonoids, and antioxidant capacity, as assessed through ABTS and DPPH assays.

Table 4.
Total phenolic, antioxidant activity, and other bioactive compounds of Chilean papaya (method with the best result).
Table 4.
Total phenolic, antioxidant activity, and other bioactive compounds of Chilean papaya (method with the best result).
| Reference | [55] | [48] | [1] | [2] | [58] | |
|---|---|---|---|---|---|---|
| Extraction | Pulp with Skin | Pulp | Pulp with Skin | Seeds | Mucilage and Seeds | |
| Fresh | Fresh | HHPE-UE | HHPE 15 min | ‡ PM | † PEA | |
| Total phenolic content | 7.29 ± 0.40 mg GAE g−1 D.M. | 7.02 ± 0.42 mg GAE g−1 D.M. | 129.1 ± 3.8 mg GAE 100 g−1 | <7 mg AG g−1 seed | 4.902 ± 0.702 g GAE 100 g−1 of extract | 6.244 ± 0.342 g GAE 100 g−1 of extract |
| Total flavonoid content | 2.51 ± 0.25 mg QE g−1 D.M. | 3.33 ± 0.26 mg QE g−1 D.M. | N/A | <2.5 mg quercetin g−1 seed | N/A | N/A |
| Sulforaphane | N/A | N/A | N/A | 54.97 ± 0.90 mg g−1 seed | N/A | N/A |
| DPPH 2 | 39.07 ± 5.68 μmol TE g−1 D.M. | 81.26 ± 1.23 µmol TE g−1 D.M. | 20.6 ± 0.2 mg TE g−1 | <110 μmol TE g−1 seed | 94.80 ± 2.69 μg mL−1 | 55.99 ± 3.55 μg mL−1 |
| FRAP 3 | N/A | N/A | 97.2 ± 4.3 mg TE g−1 | <115 μmol TE g−1 seed | N/A | N/A |
| ORAC 4 | 107.2 ± 5.17 μmol TE g−1 D.M. | 55.20 ± 0.58 µmol TE g−1 D.M. | N/A | N/A | N/A | N/A |
| Voltammetry | N/A | N/A | 141.0 ± 13.8 mM TE 100 g−1 | N/A | N/A | N/A |
| Phenolic | ||||||
| Caffeic acid | N/A | N/A | 1.5 ± 0.1 mg 100 g−1 | N/A | N/A | N/A |
| Trans-Ferulic acid | N/A | N/A | 0.86 ± 0.1 mg 100 g−1 | N/A | N/A | N/A |
| Rutin | N/A | N/A | 2.8 ± 0.3 mg 100 g−1 | N/A | N/A | N/A |
| Other | ||||||
| Vitamin C | N/A | 7.27 ± 0.11 mg g−1 D.M | 74.1 mg 100 g−1 FW | N/A | N/A | N/A |
| β-carotene | N/A | 2595 ± 65.0 µg 100 g−1 D.M. | N/A | N/A | N/A | N/A |
| Gallic acid | N/A | 2.41 ± 0.30 mg 100 g−1 D.M. | N/A | N/A | N/A | N/A |
N/A: Not applicable, 2 DPPH: 2,2-diphenyl-1-picrylhydrazyl, 3 FRAP: ferric reducing antioxidant power, 4 ORAC: radical absorbance capacity, ‡ PM: papaya methanol extract, † PEA: papaya ethyl acetate extract. Vega-Gálvez et al. (2021) [55]; Vega-Gálvez et al. (2019) [48]; Uribe et al. (2015) [1]; Briones-Labarca et al. (2014) [2]; Pino-Ramos et al. (2024) [58].
7. Papaya Latex
Latex corresponds to cytoplasm present in species that have a structure called laticifers. This cell forms a tubular network where the latex circulates, so a cross-section of the cell tissue will give off a milky substance known as latex [59]. Papaya latex is usually extracted from the immature fruit of green color and can be enveloped by various environmental and physiological variables [60]. According to Chen et al. (2012) [59], papaya latex contains 15% solid matter, of which 40% corresponds to enzymes such as cysteine endopeptidases (such as papain, glycyl endopeptidase, chymopapain, and caricain make up 80% of all enzymes), chitinolytic enzymes, glycosidase, glutaminyl cyclotransferase, and peroxidase, among others. Papain was the first cysteine to be characterized as a latex component of papaya fruit and is known as a 14 N-terminal structure of proteinases from the Caricaceae species [61,62,63].
It is essential to perform a thermal process on the enzyme papain before consumption. This process is characterized by scalding the tongue, reddening the area, and sensitizing it. However, this does not dismiss the multiple health properties attributed to it.
A study by Jiménez et al. (2003) [64] on Chilean papaya (V. pubescens) determined no statistical difference in the specific activity of latex extracted in different seasons of the year. Vidal et al. (2009) [60] performed the same experiment, finding significant differences only when analyzing different sizes of the green fruit in the other seasons, establishing that there is a higher yield in spring associated with an activity complementary to the production of the fruit along with an increase in height and diameter of the trunk of the plant that exerts the same effect. On the other hand, there is almost zero yield in autumn because there are no green fruits in that season.
Several studies detail that the method used to collect papaya enzymes is drying (Table 5), obtaining a dried power product between a white and brown color, to which different names will be attributed depending on the degree of purity obtained [60]. Jiménez et al. (2003) [64] determined a lower papaya color alteration in a freeze-drying process than in conventional or vacuum drying.

Table 5.
Latex component and enzyme activity of Vasconcellea pubescens.
The papain enzyme is a protein with a molecular weight of 24,500 Da and a polypeptide containing three disulfide bridges and a sulfhydryl group that is required for the enzyme’s activity [6].
The active site of the enzyme is located in a cleft between two unique structural domains in the three-dimensional structure [66].
The Cys-25 region of the active site, which attacks the carbonyl carbon in the peptide chain’s backbone to release the amino-terminal region and causes the protein to disassemble, enables Papain’s method of action [6].
Hydrophobic interactions influence the conformation of the protein and, hence, its functionality. The hydrophobic amino acids alanine, leucine, methionine, valine, and isoleucine are responsible for the hydrophobicity of the papain enzyme. The hydrophobicity of amino acids within a protein’s secondary sequence significantly determines its folding behavior and structural arrangement. The enzyme’s hydrophobic core plays a crucial role in stabilizing its tertiary structure through intramolecular interactions, while the polar regions on its surface ensure compatibility with the surrounding aqueous environment, maintaining both structural integrity and functionality [66].
The study [65] characterized the latex of papaya from three species of the genus Vasconcellea. It was observed that V. pubescens had a total latex value of 214.71 g, compared to V. x heilbornii and V. chachapoyensis, which exhibited values of 167.64 g and 334.32 g, respectively. Regarding enzymatic activity, V. pubescens showed an average value of 195.8 Upe, while V. x heilbornii and V. chachapoyensis demonstrated values of 112.45 Upe and 240.97 Upe, respectively, at pH 6.0.
The differences in latex value likely reflect inherent genetic and biochemical variations in the case of enzymatic activity, indicating variations in hydrophobic interactions and folding of the papain enzyme.
Papaya enzymes are highly studied and used in different areas such as food and pharmaceuticals, either to accelerate industrial processes, for their biological functions or their nutraceutical and antimicrobial properties, for their antidiabetic activity, and anticancer and antioxidant properties, among others reported by Ghaffar et al. (2023) [67].
8. Antidiabetic Potential
The antihyperglycemic effect of papaya is believed to target pancreatic beta cells by increasing their sensitivity to insulin while simultaneously inhibiting α-amylase and α-glucosidase. The contribution of flavonoids, tannins, and alkaloids in various parts of the papaya is also suspected to play a significant role [68].
The α-Glucosidase inhibitors are therapeutic agents for the treatment of type 2 diabetes. Acarbose and miglitol are notable FDA-approved drugs. However, their regular use may lead to various side effects, including diarrhea, vomiting, flatulence, severe abdominal pain, allergic reactions, and others [69,70].
α-Glucosidase is composed of 811 amino acids with a structural architecture comprising 35% helices, 25% β-sheets, and 38% coils. It exhibits a resolution of 2.04 Å, as determined through X-ray diffraction studies. Ramachandran plots indicated that 97.6% of the residues are in favored regions, demonstrating high precision in the phi (φ) and psi (ψ) angles of the target protein’s coordinates [71].
The enzyme α-glucosidase catalyzes the final step in carbohydrate digestion and breakdown, making it a critical target for studies investigating its inhibition through the polyphenolic composition of various fruits. These studies often correlate antioxidant capacity with antidiabetic activity [40,66,72]. This behavior is associated with the chemical structure of the compounds, particularly the presence of a methoxy group with electron-donating and electron-withdrawing properties [73].
Due to their structural similarity to carbohydrates, such as disaccharides or oligosaccharides, α-glucosidase inhibitors can form high-affinity complexes with the enzyme’s active site through competitive inhibition (Figure 4). This mechanism reduces the formation of carbon monomers and glucose absorption [70,74].
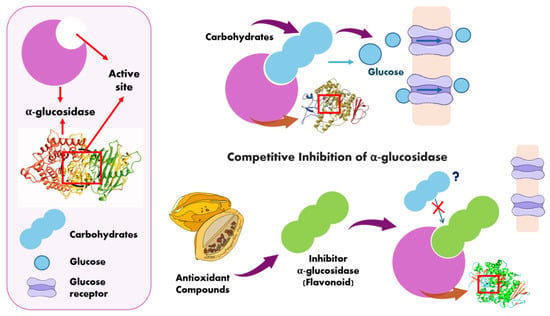
Figure 4.
Mechanism of competitive inhibition of α-Glucosidase [71,74].
Carbohydrates that are not hydrolyzed become undigested carbohydrates and are transported to the large intestine, where they are fermented into short-chain fatty acids or gases. Alternatively, they may act as prebiotics or simply reach the end of the digestive process as feces [75,76,77].
Vega-Gálvez et al. (2019) [48] studied α-glucosidase activity in Chilean papaya and the effects of different thermal preservation treatments on the fruit. The study determined that fresh papaya initially had an IC50 of 312 mg/mL. Notably, the sample treated with infrared drying was the most effective in inhibiting α-glucosidase, 24 times more effective than the fresh sample.
In a comparative analysis of Chilean Papaya (Vasconcellea pubescens) with the variety Carica papaya L., a clear difference in α-glucosidase IC50 values is observed (Table 6), which appears to be directly related to the total polyphenolic content (TPC) of the fruits [10,48,78]. Similarly, a study by Mohamed et al. (2024) [79] explored, through in vitro and in silico methods, the inhibitory effects of N. Latifolia fruit aqueous extract on carbohydrate-digesting enzymes. The study revealed the low fluctuation, high stability, and strong binding affinity of these enzymes with the compounds, as demonstrated by molecular dynamics simulations. These findings suggest promising inhibitory results compared to the natural inhibitor acarbose.

Table 6.
Comparative of total phenolic, total flavonoid, and DPPH radical-scavening of three fruits.
Although promising results have been reported on the α-glucosidase inhibitory effects of fruits such as Chilean papaya, further in vivo investigations are essential to confirm these findings and establish their potential as bioactive agents for preventing and treating type II diabetes. These inhibitors improve the metabolic profile and may potentially reduce the risk of long-term complications associated with hyperglycemia.
9. Papaya Oil
Around 13.9–30.7% of the fruit’s volume is comprised of seeds, which are abundant in protein, lipids, crude fiber, monounsaturated fatty acids, and various functional constituents, such as carotenoids, polyphenols, prunasin, benzyl glucosinolates, benzyl isothiocyanates (BITC), and cyanogenic substances [80,81]
The oil from Chilean papaya (V. pubescens) is extracted from its seeds. It is currently sold in “Elquimia”, a small shop, as a skin care product, attributing several antioxidant properties, essential fatty acids, and the small concentration of enzymes typical of papaya. Krist (2020) [82] indicates that the Carica papaya is 28% seed oil, 54% endosperm oil, and 7% sarcotesta oil. These percentages correlate with the information extracted from Figure 5, which indicates the lipid composition of the seeds and papaya pulp (V. pubescens) in different extraction and drying treatments.
The extraction method can significantly affect yield, quality, and antioxidant activity. Typically, oils are extracted through mechanical pressing and organic solvent extraction methods [83]. However, studies also use extraction methods for the extraction of supercritical fluid, and extraction assisted with high hydrostatic pressure (HHPE) or ultrasound, as well as the influence of drying pretreatments, to extract oil from the seed or pulp of the papayas (Figure 5) [2,84].
Although there is no documented evidence regarding the oxidative stability of Chilean papaya oil, a study indicates that papaya oil from the species Carica papaya L. contains 71.30% oleic acid with an oxidative stability of 77.97 h [85].
The fatty acid composition of Chilean papaya oil extracted from its seeds makes it comparable to well-known market and industrial oils, particularly due to its high oleic acid content, which exceeds 70% of the total. This is similar to olive oil, which has an oleic acid content of 70% [2,86] and FRAP antioxidant capacities of 120 (μmol TE g−1) and 107 (μmol TE g−1) for papaya and olive oil, respectively [2,87] Olive oil is known for its nutritional, health-promoting, functional, emulsifying, and antioxidant properties [88]. A study by Lu et al. (2023) [89] highlights the protective effects of oleic acid against cardiovascular diseases through multiple cellular mechanisms and signaling pathways, based on preclinical and clinical research. This provides a promising perspective on the potential properties that Chilean papaya oil may exhibit.
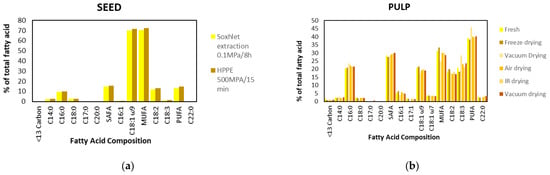
Figure 5.
Oil Chilean papaya (V. pubescens) fatty acid composition (% of total fatty acid), seed (a) [2] and pulp (b) [84].
10. Encapsulation of Chilean Papaya Compounds
Cañas-Sarazúa et al. (2024) [83] studied the effect of the encapsulation of Chilean papaya seed oil with alginate casein shell. They obtained microcapsules of high mechanical stability, demonstrating the protective effect obtained from components of microcapsules in gastric conditions, with a release of seed oil in the intestinal phase at 52.23%, and a bioaccessibility of 75.36%.
Fuentes et al. (2023) [90] investigated the microencapsulation of bioactive extracts from the seeds and skin of the Chilean papaya, which showed antimicrobial and antioxidant properties. Among the best results obtained were in the extract of microencapsulated seed with maltodextrin at 20%, reporting 44.20 ± 3.32 EAG g−1 for total phenols with an antioxidant capacity of 12.0 ± 0.32 mol ET g−1. In the case of the seed samples, the antimicrobial potential was particularly effective against Staphylococcus aureus, with a minimal inhibitory concentration of 0.03 mg mL−1 for the extract and 0.25 mg mL−1 for microencapsulation using all concentrations of maltodextrin.
11. Chilean Papaya Processing
Researchers have studied the conditions of various traditional preservation treatments in Chilean papaya, identifying the bioactive compounds of interest (Table 7). Additionally, they have developed mathematical models that best fit drying treatments under different parameters, moisture isotherms, and rehydration capacity [30,91]. They also determined how vacuum treatments enhance mass transfer, irrespective of the °Brix concentration of a solution [92].

Table 7.
Different processes of Chilean papaya.
12. Industrial and Craft Technologies
The papaya contains approximately between 7 and 9% of total sugars. It is mainly consumed as fresh fruit, for dessert or salad. There are flavor variations when they ripen in the summer months, since their sugar content is higher. The seeds have a pungent taste. Traditionally, the ways to industrialize papaya have been canning (fruit juice), juice and nectar, honey, jam, and candied fruit. Preferably, the mesocarp (pulp) of the fruit is processed, and in very few cases, the rest is taken as a surplus of the total industrialized volume; between 50 and 60% is done at an artisanal level with very elementary techniques and rudimentary infrastructure [94].
Papaya is the fruit that reaches the highest rate of industrialization in the national average, between 80 and 90% [95]. Some of the most commercialized products in Chile are observed in Figure 6.
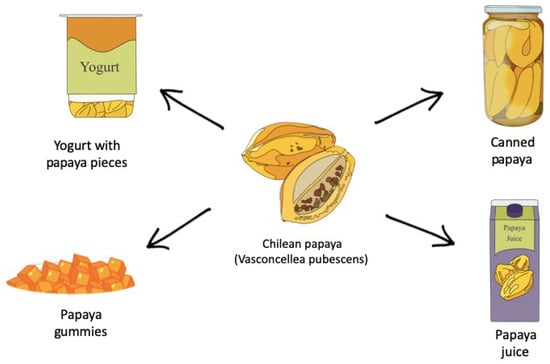
Figure 6.
Consumption forms of Chilean papaya.
The main challenges in large-scale processing of Vasconcellea pubescens focus on strategies to preserve its bioactive compounds (such as polyphenols, vitamin C, and carotenoids), which are highly susceptible to external conditions. This is where gentle methods, such as the encapsulation of Chilean papaya compounds [83,90], and non-thermal processing technologies, such as high-hydrostatic pressure processing [1,2], stand out. Furthermore, optimizing extraction and storage conditions is essential to minimize the degradation of these valuable compounds. However, the processing of Chilean papaya is limited to a small niche, which belongs to a small region of the country. For this reason, it is essential to conduct more studies related to how the processing of this product affects its characteristics.
In recent years, several products and procedures related to Vasconcellea pubescens for production, improving genetic and formulation purposes, as well as the extraction and use of the papain enzyme, have also been patented. Many patents focus primarily on methods for extracting, purification, and stabilization, and various health, chemical, and food applications of papain (approximately 4000 patents). This broad array of patents relating to papain predominantly encompasses the varieties Vasconcellea pubescens and Carica papaya, with a chronological search covering the period from 1910 to 2024 [96]. A brief description of the most relevant patents is presented in the following section (Table 8).

Table 8.
Patent title, code, and classifications for Vasconcellea pubescens.
13. Conclusions
Due to its production and consumption, Chilean papaya is an essential fruit in Chilean culture. Vasconcellea pubescens is rich in vitamins and nutrients and promotes a healthy digestive system thanks to papain enzymes. It also helps prevent cardiovascular diseases and diabetes, owing to its bioactive compounds such as phenols, flavonoids, and carotenoids, which possess antioxidant, anti-inflammatory, and antidiabetic potential, among other health benefits. As shown in this review, papaya latex is a vital enzyme extracted from unripe fruits, with proportions varying depending on the size of the papaya but remaining consistent regardless of the season. Papain is a proteolytic enzyme present in the latex of the fruit that not only facilitates digestion by breaking down proteins, but also has applications in cosmetics, food products, and wound treatments due to its regenerative effects. Additionally, this enzyme can inhibit α-glucosidase, offering the potential for controlling type II diabetes.
Chilean papaya also has a variety of processing methods that achieve different results in its nutritional composition, such as levels of antioxidants and phenolic compounds. Encapsulation technologies, such as alginate and maltodextrin, have been proven effective in protecting and releasing bioactive compounds under controlled conditions, opening possibilities for developing supplements and nutraceuticals. Furthermore, innovations in extraction processes, such as HHP and US, optimize the recovery of antioxidant and antimicrobial compounds. These advances could position Chilean papaya as a high-value functional product in international markets.
The main differences between papaya species (Vasconcellea) are their geographical distribution, morphology (size), resistance to environmental conditions, and papain content. Regarding the latter, papain content is higher in Andean species like V. pubescens, making it an essential molecule for the food industry. Finally, research on Chilean papaya must continue exploring methods to improve, scale up, patent, and optimize its production process, extend its shelf life and availability, and incorporate it more broadly into people’s diets.
Author Contributions
Writing—original draft preparation, A.C., K.L. and P.G.; review, editing and supervision R.L.-M. and L.P.-D. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare that there are no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Uribe, E.; Delgadillo, A.; Giovagnoli-Vicuña, C.; Quispe-Fuentes, I.; Zura-Bravo, L. Extraction techniques for bioactive compounds and antioxidant capacity determination of Chilean papaya (Vasconcellea pubescens) fruit. J. Chem. 2015, 2015, 347532. [Google Scholar] [CrossRef]
- Briones-Labarca, V.; Plaza-Morales, M.; Giovagnoli-Vicuña, C.; Jamett, F. High hydrostatic pressure and ultrasound extractions of antioxidant compounds, sulforaphane and fatty acids from Chilean papaya (Vasconcellea pubescens) seeds: Effects of extraction conditions and methods. LWT Food Sci. Technol. 2015, 60, 525–534. [Google Scholar] [CrossRef]
- Laily, A.N.; Daryono, B.S.; Purwantoro, A.; Purnomo. Plant segregation and pollen characteristics of highland papaya (Vasconcellea pubescens a.dc.) based on sex types. SABRAO J. Breed. Genet. 2023, 55, 1051–1064. [Google Scholar] [CrossRef]
- Torres Trujillo, A.E. Efecto de Vasconcellea pubescens “Papaya de Monte” Sobre la Calidad Espermática de Ratones Machos Tratados con Ciclofosfamida y el Efecto en el Desarrollo de Embriones Preimplantacionales. Bachelor’s Thesis, Universidad Nacional Mayor de San Marcos, Lima, Peru, 2017. Available online: https://core.ac.uk/download/pdf/323343898.pdf (accessed on 21 October 2024).
- Markovic, S.; Milosevic, J.; Djuric, M.; Lolic, A.; Polovic, N. One-step purification and freeze stability of papain at acidic pH values. Arch. Biol. Sci. 2021, 73, 57–64. [Google Scholar] [CrossRef]
- Jon, N. Papain, a plant enzyme of biological importance: A review. Am. J. Biochem. Biotechnol. 2012, 8, 99–104. [Google Scholar] [CrossRef]
- Muñoz Murillo, J.P.; Zambrano Vélez, M.I.; Párraga Álava, R.C.; Verduga López, C.D. Use of papain and bromelain and its effect on the organoleptic and bromatological characteristics of smoked pork chops. RECUS Rev. Electrónica Coop. Univ. Soc. 2019, 4, 38–42. [Google Scholar]
- Carrasco, B.; Avila, P.; Perez-Diaz, J.; Muñoz, P.; García, R.; Lavandero, B.; Zurita-Silva, A.; Retamales, J.B.; Caligari PD, S. Genetic structure of highland papayas (Vasconcellea pubescens (Lenné et C. Koch) Badillo) cultivated along a geographic gradient in Chile as revealed by Inter Simple Sequence Repeats (ISSR). Genet. Resour. Crop Evol. 2009, 56, 331–337. [Google Scholar] [CrossRef]
- Carrasco, B.; Arévalo, B.; Perez-Diaz, R.; Rodríguez-Alvarez, Y.; Gebauer, M.; Maldonado, J.E.; García-Gonzáles, R.; Chong-Pérez, B.; Pico-Mendoza, J.; Meisel, L.A.; et al. Descriptive genomic analysis and sequence genotyping of the two papaya species (Vasconcellea pubescens and Vasconcellea chilensis) using GBS tools. Plants 2022, 11, 2151. [Google Scholar] [CrossRef] [PubMed]
- Gironés-Vilaplana, A.; Baenas, N.; Villaño, D.; Speisky, H.; García-Viguera, C.; Moreno, D.A. Evaluation of Latin-American fruits rich in phytochemicals with biological effects. J. Funct. Foods 2014, 7, 599–608. [Google Scholar] [CrossRef]
- Scheldeman, X.; Kyndt, T.; d’Eeckenbrugge, G.C.; Ming, R.; Drew, R.; Van Droogenbroeck, B.; Van Damme, P.; Moore, P.H. Vasconcellea. In Wild Crop Relatives: Genomic and Breeding Resources; Kole, C., Ed.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 213–249. [Google Scholar] [CrossRef]
- Macedwardsproduce. Papaya Hawaiian. Available online: https://macedwardsproduce.com/product/hawaiian-papaya/ (accessed on 4 November 2024).
- X. Wild Pawpaw. Available online: https://x.com/EAHerbarium_NMK/status/1299604091774140418/photo/2 (accessed on 4 November 2024).
- Istockphoto. Berg-Papaya (Vasconcellea pubescens). Available online: https://www.istockphoto.com/de/foto/berg-papaya-gm669377102-122320347 (accessed on 4 November 2024).
- Tradewindsfruit. Jacaratia mexicana—Mexican Mountain Papaya. Available online: https://www.tradewindsfruit.com/jacaratia-mexicana-mexican-mountain-papaya-seeds (accessed on 4 November 2024).
- Rarepalmseeds. Carica cnidoscoloides. Available online: https://www.rarepalmseeds.com/es/carica-cnidoscoloides-es (accessed on 4 November 2024).
- Tradewindsfruit. Carica cnidoscoloides—Stinging Papaya. Available online: https://www.tradewindsfruit.com/carica-cnidoscoloides-stinging-papaya-seeds (accessed on 4 November 2024).
- Rarepalmseeds. Jarilla chocola. Available online: https://www.rarepalmseeds.com/es/jarilla-chocola-es (accessed on 4 November 2024).
- Carrasco, B.; García-Gonzáles, R.; Díaz, C.; Ávila, P.; Cáceres, P.; Lobos, G.A.; Silva, H.; Caligari, P.D.S. Genetic and morphological characterization of the endangered Austral papaya Vasconcellea chilensis (Planch. ex A. DC.) Solms. Genet. Resour. Crop Evol. 2014, 61, 1423–1432. [Google Scholar] [CrossRef]
- Miller, A.J.; Gross, B.L. From forest to field: Perennial fruit crop domestication. Am. J. Bot. 2011, 98, 1389–1414. [Google Scholar] [CrossRef] [PubMed]
- Kyndt, T.; Romeijn-Peeters, E.; Van Droogenbroeck, B.; Romero-Motochi, J.P.; Gheysen, G.; Goetghebeur, P. Species relationships in the genus Vasconcellea (Caricaceae) based on molecular and morphological evidence. Am. J. Bot. 2005, 92, 1033–1044. [Google Scholar] [CrossRef] [PubMed]
- Moya-León, M.A.; Moya, M.; Herrera, R. Ripening of mountain papaya (Vasconcellea pubescens) and ethylene dependence of some ripening events. Postharvest Biol. Technol. 2004, 34, 211–218. [Google Scholar] [CrossRef]
- Osores González, M.; Escobar Moreno, C.; Henríquez Armijo, G.; Beltrán Gómez, P.; Mendieta Pérez, B.; Peña Alburquenque, J.C.; Woywood Jeldrez, C.; Palomino Vásquez, M.; Avendaño Andrade, A.; Berrios Bascuñán, L.; et al. Catastro Frutícola, Principales Resultados. 2022. Available online: https://bibliotecadigital.odepa.gob.cl/bitstream/handle/20.500.12650/71978/Catastro_Frut_ARICA.pdf (accessed on 21 October 2024).
- Osores González, M.; Escobar Moreno, C.; Armijo, G.H.; Beltrán Gómez, P.; Mendieta Pérez, B.; Peña Alburquenque, J.C.; Woywood Jeldrez, C.; Palomino Vásquez, M.; Avendaño Andrade, A.; Berrios Bascuñán, L.; et al. Catastro Frutícola Principales Resultados. 2021. Available online: https://bibliotecadigital.odepa.gob.cl/bitstream/handle/20.500.12650/71117/Coquimbo202109.pdf (accessed on 21 October 2024).
- Chong-Pérez, B.; Carrasco, B.; Silva, H.; Herrera, F.; Quiroz, K.; Garcia-Gonzales, R. Regeneration of highland papaya (Vasconcellea pubescens) from another culture. Appl. Plant Sci. 2018, 6, e01182. [Google Scholar] [CrossRef]
- Muñoz, M. Nomenclatura del papayo cultivado en Chile. Agric. Técnica 1988, 48, 39–42. [Google Scholar]
- León, J. Botánica de los Cultivos Tropicales, 3rd ed.; Instituto Interamericano de Cooperación para la Agricultura (IICA): San José, Costa Rica, 2000; Available online: https://repositorio.iica.int/handle/11324/7228 (accessed on 11 October 2024).
- CABI. Vasconcellea pubescens; Compendio CABI: Wallingford, UK, 2022; Available online: https://doi.org/10.1079/cabicompendium.92836779 (accessed on 11 October 2024).
- Luza, J.; Lizana, A.; Fichet, T. Comparison of fruit and flowers from female and hermaphrodite papaya plants (Carica pubescens lenne et koch) grown commercially in Chile. Annu. Meet. Interam. Soc. Trop. Hortic. 1990, 36, 131–137. [Google Scholar]
- Vega, A.A.; Lemus, R.A. Modelado de la Cinética de Secado de la Papaya Chilena (Vasconcellea pubescens). Inf. Tecnológica 2006, 17, 23–31. [Google Scholar] [CrossRef]
- Latcham, R.E. La Agricultura Precolombina en Chile y los Países Vecinos; Ediciones de la Universidad de Chile, Ed.: Santiago, Chile, 1936. [Google Scholar]
- de Candolle, A.; de Candolle, A.P. Prodromus Systematis Naturalis Regni Vegetabilis, Sive, Enumeratio Contracta Ordinum Generum Specierumque Plantarum Huc Usque Cognitarium, Juxta Methodi Naturalis, Normas Digesta/Auctore Aug. Pyramo de Candolle; Sumptibus Sociorum Treuttel et Würtz: Paris, France, 1824. [Google Scholar] [CrossRef]
- Muñoz-Schick, M.; Serra, M.T. Ficha de Antecedentes de Especie 139. MNHN-CONAMA. 2006. Available online: https://clasificacionespecies.mma.gob.cl/wp-content/uploads/2019/10/Carica_chilensis_FINAL.pdf (accessed on 10 October 2024).
- Wikimedia. Mountain Papaya (Vasconcellea pubescens). Available online: https://commons.wikimedia.org/wiki/File:2011.09-385-158arp_Mountain_papaya(Vasconcellea_pubescens),fr(wh,TS)_Naivasha-Gilgil(Rift_Valley_Prov.),KE_tue13sep2011-1230h.jpg#globalusage (accessed on 21 October 2024).
- Chileflora. Image of Carica chilensis (Papayo Silvestre/Palo Gordo). Available online: https://www.chileflora.com/Florachilena/FloraSpanish/LowResPages/SL0457.htm (accessed on 21 October 2024).
- Inaturalist. Papayuela (Vasconcellea pubescens). Available online: https://inaturalist.mma.gob.cl/observations/93085227 (accessed on 21 October 2024).
- Chileflora. Image of Carica chilensis. Available online: https://www.chileflora.com/Florachilena/FloraEnglish/HighResPages/EH0457A.htm (accessed on 23 October 2024).
- Inaturalist. Papayuela (Vasconcellea pubescens). Available online: https://inaturalist.mma.gob.cl/observations/91908277 (accessed on 23 October 2024).
- Inaturalist. Papaya Chilena (Vasconcellea chilensis). Available online: https://inaturalist.mma.gob.cl/observations/105309033 (accessed on 23 October 2024).
- Flickr. Vasconcellea pubescens A.DC. (CARICACEAE)|Syn. Carica pubescens Lenné & K.Koch, Carica cundinamarcensis Linden ex Hook.f. Available online: https://www.flickr.com/photos/36517976@N06/3373968560 (accessed on 23 October 2024).
- Inaturalist. Papaya Chilena (Vasconcellea chilensis). Available online: https://inaturalist.mma.gob.cl/observations/89504716 (accessed on 23 October 2024).
- Elquimia. Aceite Semillas de Papaya Chilena—30 mL. Available online: https://elquimia.com/producto/aceite-semillas-de-papaya-30-ml/ (accessed on 23 October 2024).
- Tucker, G.A.; Grierson, D. Fruit Ripening. In The Biochemistry of Plants, Physiology of Metabolism; Academic Press: Cambridge, MA, USA, 1987; Volume 12, pp. 265–318. [Google Scholar]
- Abeles, F.B.; Morgan, P.W.; Saltveit, M.E., Jr. Ethylene in Plant Biology, 2nd ed.; Academic Press: Cambridge, MA, USA, 1992; Available online: https://ci.nii.ac.jp/ncid/BA18297606 (accessed on 6 December 2024).
- Balbontín, C.; Gaete-Eastman, C.; Vergara, M.; Herrera, R.; Moya-León, M.A. Treatment with 1-MCP and the role of ethylene in aroma development of mountain papaya fruit. Postharvest Biol. Technol. 2007, 43, 67–77. [Google Scholar] [CrossRef]
- Zhang, L.-X.; Paull, R.E. Ripening behavior of papaya genotypes. HortScience 1990, 25, 454–455. [Google Scholar] [CrossRef]
- Caicedo, M.; García, J. Evaluación de Parámetros Fisicoquímicos Durante el Crecimiento, Desarrollo y Postcosecha de las Especies Vasconcellea pubescens, Solanum quitoense var. septentrionale y Capsicum pubescens Ruiz & Pav, Cuantificación de Polifenoles Totales y Análisis Cualitativo de Metabolitos Secundarios Presentes en Estas y Otras Especies Priorizadas en el Proyecto “Biodiversidad Altoandina al Plato de Todos”; University of Distrital Francisco José de Caldas: Bogotá, Colombia, 2018. [Google Scholar]
- Vega-Gálvez, A.; Poblete, J.; Quispe-Fuentes, I.; Uribe, E.; Bilbao-Sainz, C.; Pastén, A. Chemical and bioactive characterization of papaya (Vasconcellea pubescens) under different drying technologies: Evaluation of antioxidant and antidiabetic potential. J. Food Meas. Charact. 2019, 13, 1980–1990. [Google Scholar] [CrossRef]
- Salvatierra, G.A.; Jana, A.C. Situación Actual del Cultivo de Papayos en las Principales Zonas de Producción; INIA: Santiago, Chile, 2014; Available online: https://biblioteca.inia.cl/server/api/core/bitstreams/d2cdda3c-0f08-438d-953e-f39cec3a275a/content#:~:text=El%20papayo%20que%20se%20cultiva,la%20llegada%20de%20los%20espa%C3%B1oles (accessed on 10 October 2024).
- Schmidt Hebbel, H.; Pennacchiotti Monti, I.; Masson Salaué, L.; Mella Rojas, M.A. Tabla de Composición Química de Alimentos Chilenos; University of Chile: Santiago, Chile, 1990; Available online: https://repositorio.uchile.cl/handle/2250/121427 (accessed on 21 October 2024).
- Nwofia, G.E.; Ojimelukwe, P.; Eji, C. Chemical composition of leaves, fruit pulp and seeds in some Carica papaya (L.) morphotypes. Int. J. Med. Arom. Plants 2012, 2, 200–206. [Google Scholar]
- Beta, T.; Duodu, K.G. Bioactives: Antioxidants. In Encyclopedia of Food Grains; Elsevier: Amsterdam, The Netherlands, 2016; pp. 277–282. [Google Scholar] [CrossRef]
- Crizel, R.L.; Zandoná, G.P.; Rossi, R.C.; Ferreira, C.D.; Hoffmann, J.F. Solid-phase extraction for determination of phenolic compounds in food and beverage. In Reference Module in Chemistry, Molecular Sciences and Chemical Engineering; Elsevier: Amsterdam, The Netherlands, 2023. [Google Scholar] [CrossRef]
- Lim, Y.Y.; Lim, T.T.; Tee, J.J. Antioxidant properties of several tropical fruits: A comparative study. Food Chem. 2007, 103, 1003–1008. [Google Scholar] [CrossRef]
- Vega-Gálvez, A.; Stucken, K.; Cantuarias, C.; Lamas, F.; García, V.; Pastén, A. Antimicrobial properties of papaya (Vasconcellea pubescens) subjected to low-temperature vacuum dehydration. Innov. Food Sci. Emerg. Technol. 2021, 67, 102563. [Google Scholar] [CrossRef]
- Halder, S.; Dutta, S.; Khaled, K.L. Evaluation of phytochemical content and in vitro antioxidant properties of methanol extract of Allium cepa, Carica papaya and Cucurbita maxima blossoms. Food Chem. Adv. 2022, 1, 100104. [Google Scholar] [CrossRef]
- Robles-Apodaca, S.M.; González-Vega, R.I.; Ruíz-Cruz, S.; Estrada-Alvarado, M.I.; Cira-Chávez, L.A.; Márquez-Ríos, E.; Del-Toro-Sánchez, C.L.; De Jesús Ornelas-Paz, J.; Suárez-Jiménez, G.M.; Ocaño-Higuera, V.M. Optimization of Extraction Process for Improving Polyphenols and Antioxidant Activity from Papaya Seeds (Carica papaya L.) Using Response Surface Methodology. Processes 2024, 12, 2729. [Google Scholar] [CrossRef]
- Pino-Ramos, L.L.; Farias, D.R.; Olivares-Caro, L.; Mitsi, C.; Mardones, C.; Echeverria, J.; Avila, F.; Gutierrez, M. Chilean papaya (Vasconcellea pubescens A. DC.) residues as a source of bioactive compounds: Chemical composition, antioxidant capacity, and antiglycation effects. Heliyon 2024, 10, e38837. [Google Scholar] [CrossRef]
- Chen, L.-C.; Chung, Y.-C.; Chang, C.-T. Characterisation of an acidic peroxidase from papaya (Carica papaya L. cv Tainung No. 2) latex and its application in the determination of micromolar hydrogen peroxide in milk. Food Chem. 2012, 135, 2529–2535. [Google Scholar] [CrossRef]
- Vidal, L.V.; Finot, V.L.; Mora, K.d.C.; Venegas, F.A. Características físico-químicas del látex de papayuelo (Vasconcellea cundinamarcensis Badillo, Caricaceae). Inf. Tecnológica 2009, 20, 93–103. [Google Scholar] [CrossRef]
- Salas, C.E.; Gomes, M.T.R.; Hernandez, M.; Lopes, M.T.P. Plant cysteine proteinases: Evaluation of the pharmacological activity. Phytochemistry 2008, 69, 2263–2269. [Google Scholar] [CrossRef] [PubMed]
- Karnjanapratum, S.; Benjakul, S. Glycyl endopeptidase from papaya latex: Partial purification and use for production of fish gelatin hydrolysate. Food Chem. 2014, 165, 403–411. [Google Scholar] [CrossRef]
- Teixeira, R.D.; Ribeiro, H.A.L.; Gomes, M.-T.R.; Lopes, M.T.P.; Salas, C.E. The proteolytic activities in latex from Carica candamarcensis. Plant Physiol. Biochem. 2008, 46, 956–961. [Google Scholar] [CrossRef] [PubMed]
- Jiménez, V.; Violeta, L.; Benavente, A.; Renán, O. Influence of the Dehydration Method and Extraction Time on the Enzyme Activity of Papaya Latex Grown in Cobquecura VIII Region, Chile; University of Concepción: Concepción, Chile, 2023. [Google Scholar]
- Rivera-Botonares, R.S.; Oliva-Cruz, S.M.; Flores, D.T. Extracción y purificación de papaína obtenida a partir de tres especies nativas del género Vasconcellea. J. High Andean Res. 2023, 25, 109–116. [Google Scholar] [CrossRef]
- Babalola, B.A.; Akinwande, A.I.; Otunba, A.A.; Adebami, G.E.; Babalola, O.; Nwufo, C. Therapeutic benefits of Carica papaya: A review on its pharmacological activities and characterization of papain. Arab. J. Chem. 2023, 17, 105369. [Google Scholar] [CrossRef]
- Ghaffar, A.; Munir, B.; Jahangeer, M.; Ashiq, M.; Qamar, S.A.; Ahmad, B. Bioactivity prospection, antimicrobial, nutraceutical, and pharmacological potentialities of Carica papaya. In Antiviral and Antimicrobial Smart Coatings; Elsevier: Amsterdam, The Netherlands, 2023; pp. 587–606. [Google Scholar] [CrossRef]
- Aruoma, O.I.; Somanah, J.; Bourdon, E.; Rondeau, P.; Bahorun, T. Diabetes as a risk factor to cancer: Functional role of fermented papaya preparation as phytonutraceutical adjunct in the treatment of diabetes and cancer. Mutat. Res./Fundam. Mol. Mech. Mutagen. 2014, 768, 60–68. [Google Scholar] [CrossRef]
- Akmal, M.; Patel, P.; Wadhwa, R. Alpha Glucosidase Inhibitors. StatPearls: Treasure Island, FL, USA, 2024. Available online: https://www.ncbi.nlm.nih.gov/books/NBK557848/?report=printable (accessed on 6 December 2024).
- Hossain, U.; Das, A.K.; Ghosh, S.; Sil, P.C. An overview on the role of bioactive α-glucosidase inhibitors in ameliorating diabetic complications. Food Chem. Toxicol. 2020, 145, 111738. [Google Scholar] [CrossRef]
- Tahir, T.; Shahzad, M.I.; Tabassum, R.; Rafiq, M.; Ashfaq, M.; Hassan, M.; Kotwica-Mojzych, K.; Mojzych, M. Diaryl azo derivatives as anti-diabetic and antimicrobial agents: Synthesis, in vitro, kinetic and docking studies. J. Enzym. Inhib. Med. Chem. 2021, 36, 1508–1519. [Google Scholar] [CrossRef]
- Lu, H.; Xie, T.; Wu, Q.; Hu, Z.; Luo, Y.; Luo, F. Alpha-Glucosidase Inhibitory Peptides: Sources, Preparations, Identifications, and Action Mechanisms. Nutrients 2023, 15, 4267. [Google Scholar] [CrossRef] [PubMed]
- Sharma, G.S.D.; Eswaran, S.V. Substituent effect of the methoxy group: A matter of give and take. Resonance 1997, 2, 73–75. [Google Scholar] [CrossRef]
- Rincon-Silva, N.G.; Rincon, J.D.; Acosta, J.S. Inhibition of α-glucosidase by naturally flavonoids as a control way in the development of diabetes mellitus. Universidad Libre Barranquilla. Biociencias 2019, 14, 129–148. [Google Scholar]
- Matus-Ortega, G.; Romero, A.L.; González, J.; Castillo, F.V. Capítulo 7. Metabolismo de los Carbohidratos. In Bioquímica para Ciencias de la Salud, 2nd ed.; BARKER & JULES™: Tijuana, Mexico, 2023; Volume 1, pp. 300–374. [Google Scholar]
- Chen, J.; Lan, M.; Zhang, X.; Jiao, W.; Chen, Z.; Li, L.; Li, B. Effects of Simulated In Vitro Digestion on the Structural Characteristics, Inhibitory Activity on α-Glucosidase, and Fermentation Behaviours of a Polysaccharide from Anemarrhena asphodeloides Bunge. Nutrients 2023, 15, 1965. [Google Scholar] [CrossRef] [PubMed]
- Dehghan-Kooshkghazi, M.; Mathers, J.C. Starch digestion, large-bowel fermentation and intestinal mucosal cell proliferation in rats treated with the α-glucosidase inhibitor acarbose. Br. J. Nutr. 2004, 91, 357–365. [Google Scholar] [CrossRef] [PubMed]
- Mishra, B.B.; Gautam, S.; Chander, R.; Sharma, A. Characterization of nutritional, organoleptic and functional properties of intermediate moisture shelf stable ready-to-eat Carica papaya cubes. Food Biosci. 2015, 10, 69–79. [Google Scholar] [CrossRef]
- Mohamed, A.I.; Erukainure, O.L.; Salau, V.F.; Msomi, N.Z.; Beseni, B.K.; Olofinsan, K.A.; Aljoundi, A.; Islam, M.S. An in vitro and in silico study of the antioxidant and antidiabetic activities of Nauclea latifolia fruit. Sci. Afr. 2024, 26, e02340. [Google Scholar] [CrossRef]
- Manaf Yanty, N.A.; Nazrim Marikkar, J.M.; Nusantoro, B.P.; Long, K.; Ghazali, H.M. Physico-chemical characteristics of papaya (Carica papaya L.) seed oil of the Hong Kong/Sekaki Variety. J. Oleo Sci. 2014, 63, 885–892. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Pan, Y.; Huang, W.; Chen, H.; Yang, H. Optimized ultrasonic-assisted extraction of papaya seed oil from Hainan/Eksotika variety. Food Sci. Nutr. 2019, 7, 2692–2701. [Google Scholar] [CrossRef] [PubMed]
- Krist, S. Vegetable Fats and Oils; Springer International Publishing: Cham, Switzerland, 2020. [Google Scholar] [CrossRef]
- Cañas-Sarazúa, R.; Briones-Labarca, V.; Giovagnoli-Vicuña, C. Encapsulation of papaya seed oil in casein-alginate-based shell materials. Future Foods 2024, 9, 100301. [Google Scholar] [CrossRef]
- Uribe, E.; Vega-Gálvez, A.; Pasten, A.; Cantuarias, C.; Stucken, K.; García, V.; Rodríguez, A.; Valenzuela-Barra, G.; Delporte, C. Effect of high- and low-temperature drying methods on fatty acid profile and antimicrobial and anti-inflammatory traits of papaya (Vasconcellea pubescens). ACS Food Sci. Technol. 2023, 3, 77–84. [Google Scholar] [CrossRef]
- Malacrida, C.R.; Kimura, M.; Jorge, N. Characterization of a high oleic oil extracted from papaya (Carica papaya L.) seeds. Food Sci. Technol. 2011, 31, 929–934. [Google Scholar] [CrossRef]
- Barba, L.; Arrighetti, G.; Calligaris, S. Crystallization and melting properties of extra virgin olive oil studied by synchrotron XRD and DSC. Eur. J. Lipid Sci. Technol. 2013, 115, 322–329. [Google Scholar] [CrossRef]
- Szydłowska-Czerniak, A.; Karlovits, G.; Dianoczki, C.; Recseg, K.; Szłyk, E. Comparison of two analytical methods for assessing antioxidant capacity of rapeseed and olive oils. J. Am. Oil Chem. Soc. 2008, 85, 141–149. [Google Scholar] [CrossRef]
- Samaram, S.; Mirhosseini, H.; Tan, C.; Ghazali, H. Ultrasound-assisted extraction (UAE) and solvent extraction of papaya seed oil: Yield, fatty acid composition and triacylglycerol profile. Molecules 2013, 18, 12474–12487. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Zhao, J.; Xin, Q.; Yuan, R.; Miao, Y.; Yang, M.; Mo, H.; Chen, K.; Cong, W. Protective effects of oleic acid and polyphenols in extra virgin olive oil on cardiovascular diseases. Food Sci. Hum. Wellness 2024, 13, 529–540. [Google Scholar] [CrossRef]
- Fuentes, Y.; Giovagnoli-Vicuña, C.; Faúndez, M.; Giordano, A. Microencapsulation of Chilean papaya waste extract and its impact on physicochemical and bioactive properties. Antioxidants 2023, 12, 1900. [Google Scholar] [CrossRef] [PubMed]
- Zura, L.; Uribe, E.; Lemus-Mondaca, R.; Saavedra-Torrico, J.; Vega-Gálvez, A.; Di Scala, K. Rehydration capacity of Chilean papaya (Vasconcellea pubescens): Effect of process temperature on kinetic parameters and functional properties. Food Bioprocess Technol. 2013, 6, 844–850. [Google Scholar] [CrossRef]
- Moreno, J.; Bugueño, G.; Velasco, V.; Petzold, G.; Tabilo-Munizaga, G. Osmotic dehydration and vacuum impregnation on physicochemical properties of Chilean papaya (Carica candamarcensis). J. Food Sci. 2004, 69, FEP102–FEP106. [Google Scholar] [CrossRef]
- Lemus-Mondaca, R.; Miranda, M.; Grau, A.A.; Briones, V.; Villalobos, R.; Vega-Gálvez, A. Effect of osmotic pretreatment on hot air drying kinetics and quality of Chilean papaya (Carica pubescens). Dry. Technol. 2009, 27, 1105–1115. [Google Scholar] [CrossRef]
- Montecino, S.; Alvear, A. Patrimonio Alimentario de Chile Productos y Preparaciones de la Región de Coquimbo. 2018. Available online: https://www.fia.cl/download/patrimonio-alimentario/Inventario-Patrimonio-Coquimbo.pdf (accessed on 13 October 2024).
- Kuncar Oneto, D. Investigación, Producción y Procesamiento de la Papaya en Cobquecura, VIII Región. 1994. Available online: https://bibliotecadigital.fia.cl/server/api/core/bitstreams/189b15f5-d774-4fd3-a134-2f53b056e071/content (accessed on 10 October 2024).
- OMPI. PATENTSCOPE Búsqueda Simple. Available online: https://patentscope.wipo.int/search/es/search.jsf (accessed on 6 December 2024).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).















