Exploring the Epigenetic Landscape of Spermatozoa: Impact of Oxidative Stress and Antioxidant Supplementation on DNA Methylation and Hydroxymethylation
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Tissue Sampling
2.2. DNA Slot Blot Analysis
2.3. Immunofluorescence for 5mC and 5hmC Nuclear Localization
2.4. DNA Sequencing: RREM-Seq for 5mC and RREhM-Seq for 5hmC Analysis
2.5. Bioinformatics and Statistics
3. Results
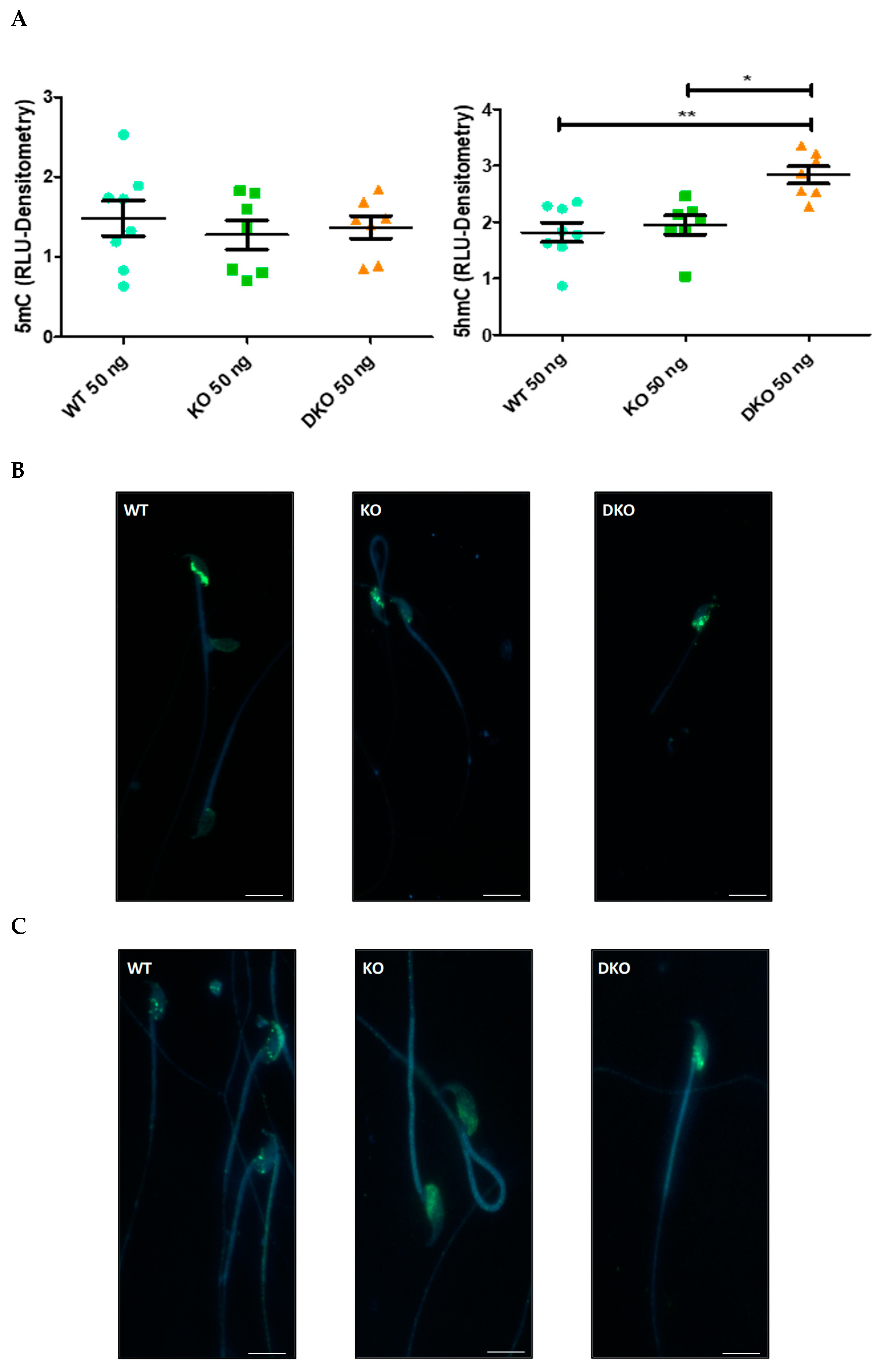
3.1. Observation and Quantification of DNA Methylation and Hydroxymethylation
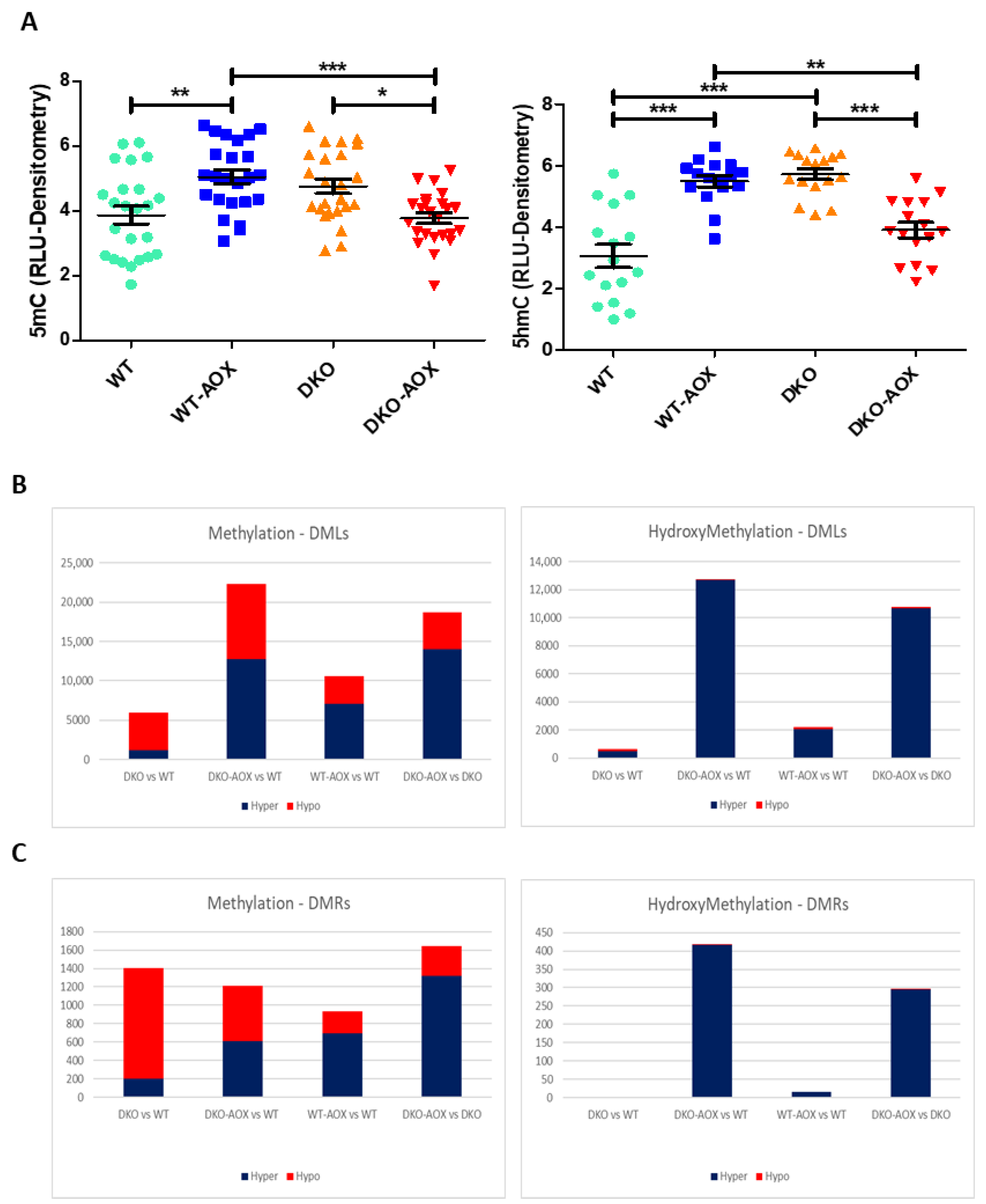
3.2. Impact of Oral Antioxidant Supplementation (AOX) on Sperm DNA (Hydroxy)-Methylation
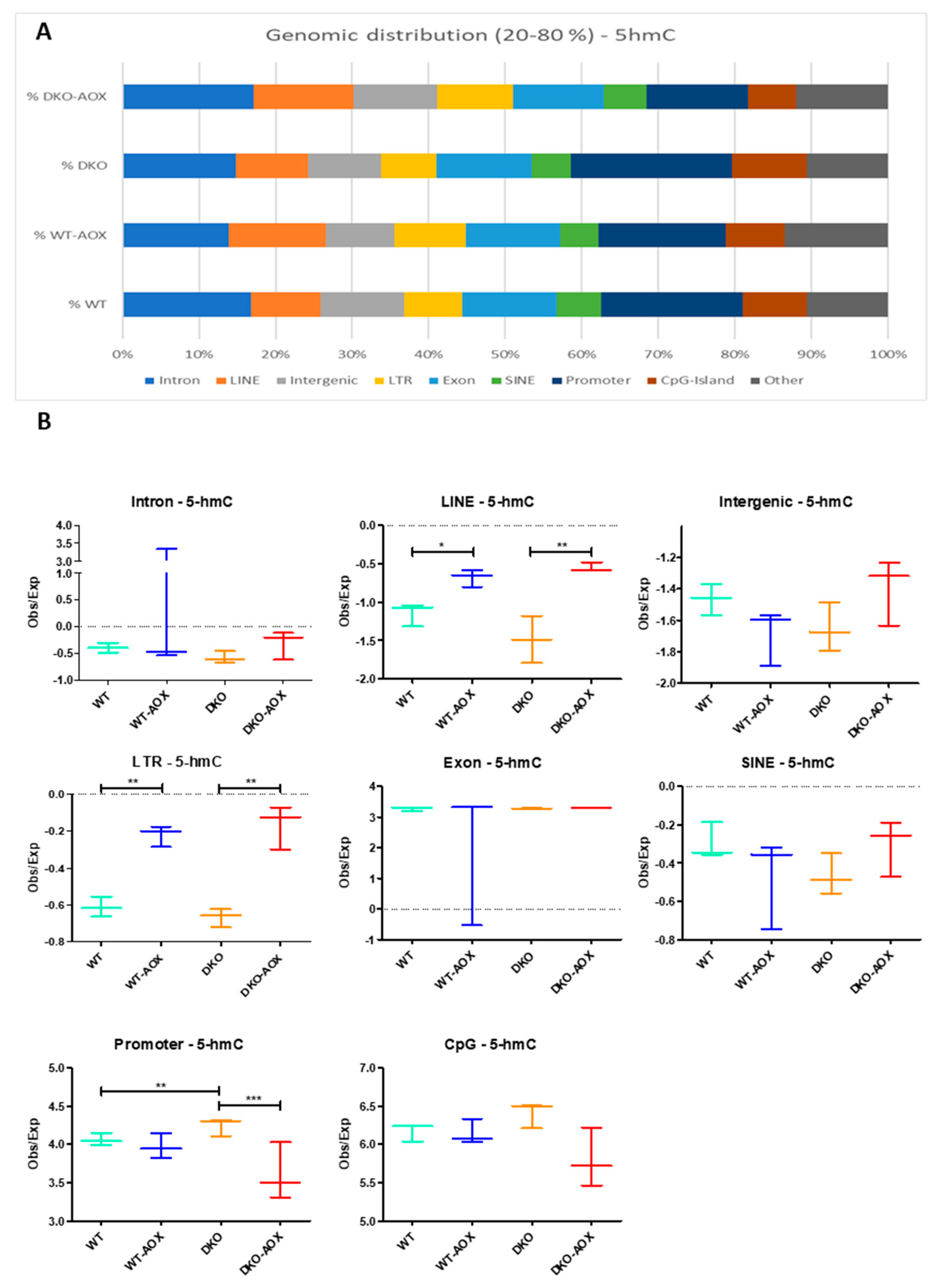
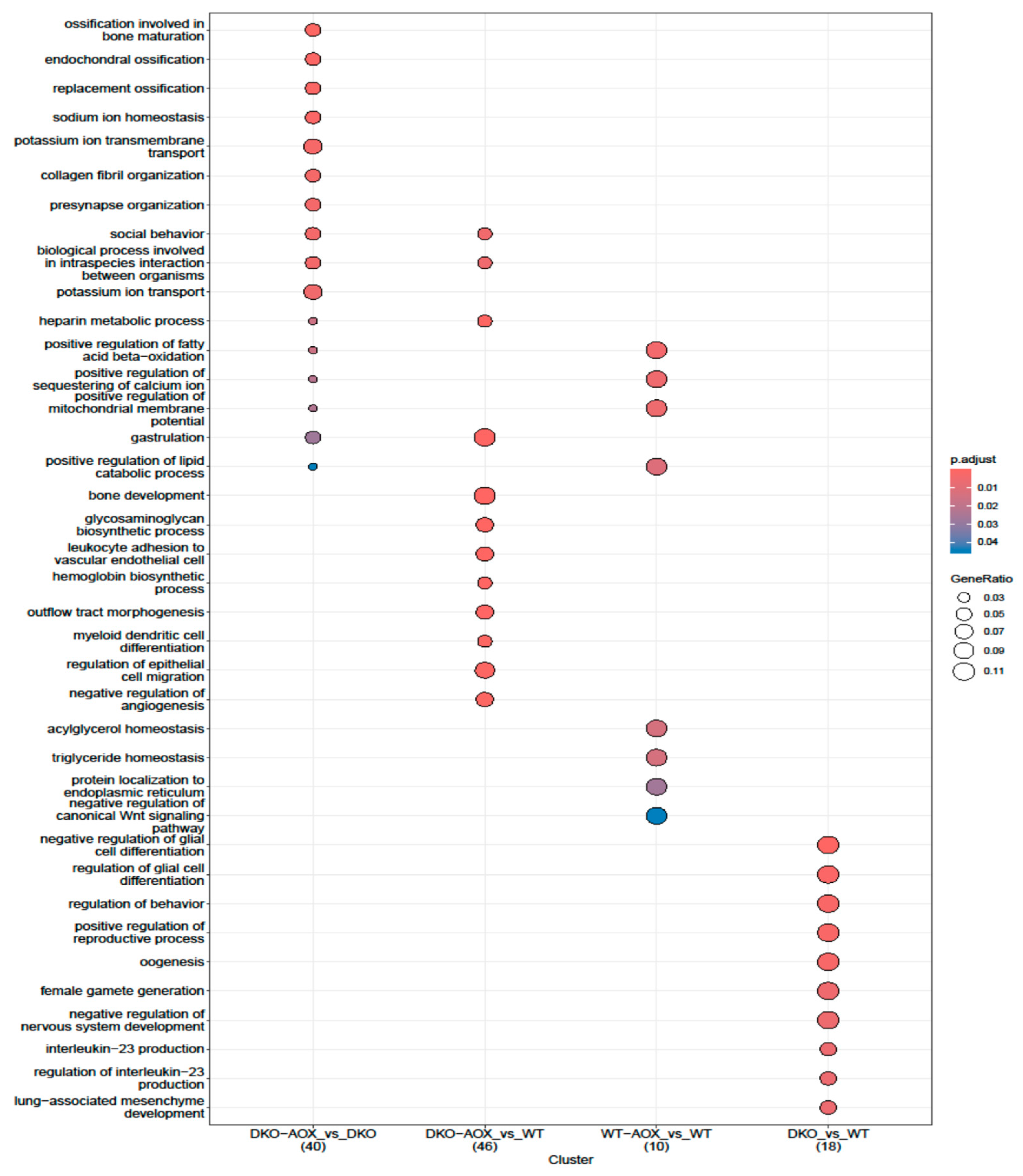
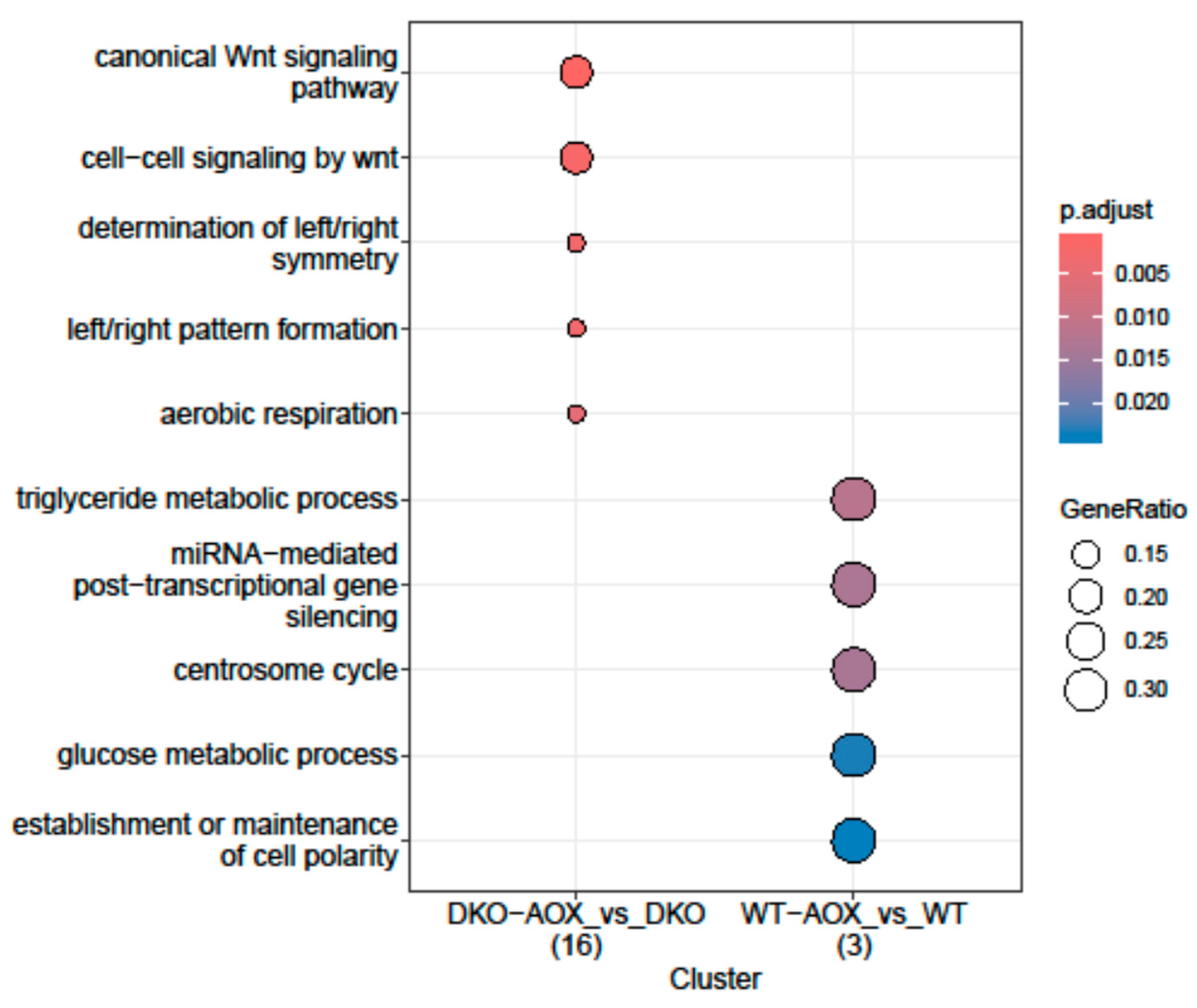
3.3. RREM-Seq for 5mC and 5hmC Characterization
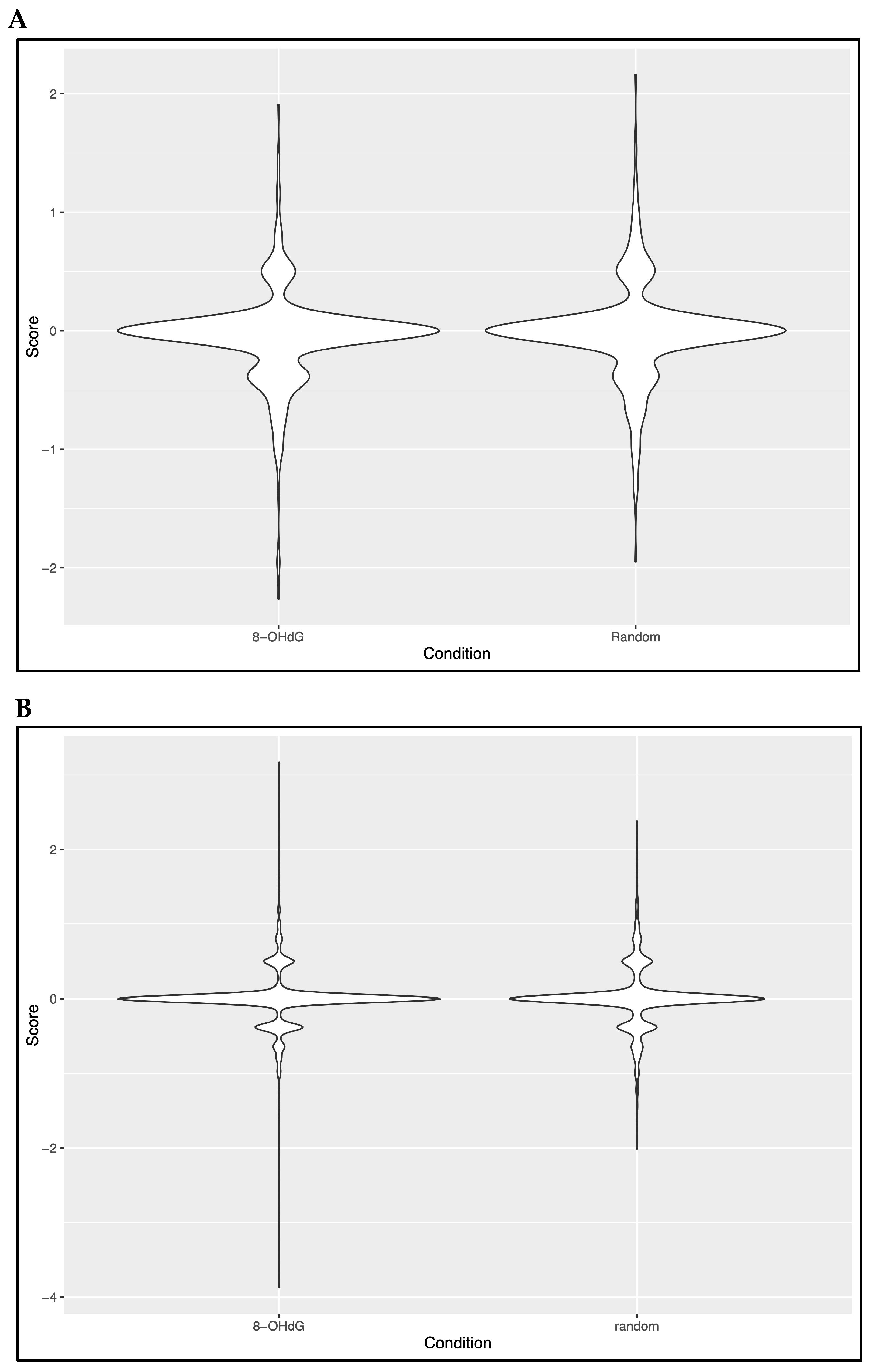
3.4. Evaluation of a Potential Co-Localization Between 8-OHdG and 5hmC
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ribas-Maynou, J.; Nguyen, H.; Wu, H.; Ward, W.S. Functional Aspects of Sperm Chromatin Organization. Results Probl. Cell Differ. 2022, 70, 295–311. [Google Scholar] [CrossRef] [PubMed]
- Ward, W.S.; Coffey, D.S. DNA packaging and organization in mammalian spermatozoa: Comparison with somatic cells. Biol. Reprod. 1991, 44, 569–574. [Google Scholar] [CrossRef]
- Champroux, A.; Cocquet, J.; Henry-Berger, J.; Drevet, J.R.; Kocer, A. A Decade of Exploring the Mammalian Sperm Epigenome: Paternal Epigenetic and Transgenerational Inheritance. Front. Cell Dev. Biol. 2018, 6, 50. [Google Scholar] [CrossRef]
- Chen, Q.; Yan, M.; Cao, Z.; Li, X.; Zhang, Y.; Shi, J.; Feng, G.H.; Peng, H.; Zhang, X.; Zhang, Y.; et al. Sperm tsRNAs contribute to intergenerational inheritance of an acquired metabolic disorder. Science 2016, 351, 397–400. [Google Scholar] [CrossRef] [PubMed]
- Grandjean, V.; Fourre, S.; De Abreu, D.A.; Derieppe, M.A.; Remy, J.J.; Rassoulzadegan, M. RNA-mediated paternal heredity of diet-induced obesity and metabolic disorders. Sci. Rep. 2015, 5, 18193. [Google Scholar] [CrossRef] [PubMed]
- Pepin, A.S.; Lafleur, C.; Lambrot, R.; Dumeaux, V.; Kimmins, S. Sperm histone H3 lysine 4 tri-methylation serves as a metabolic sensor of paternal obesity and is associated with the inheritance of metabolic dysfunction. Mol. Metab. 2022, 59, 101463. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Pan, D.; Zheng, X.; Ding, H.; Ma, Z.; Xie, M.; Ge, S. Long-term effects of neonatal exposure to bisphenol A on testes structure and the expression of Boule in testes of male mice. Wei Sheng Yan Jiu 2017, 46, 975–980. [Google Scholar]
- Conine, C.C.; Sun, F.; Song, L.; Rivera-Perez, J.A.; Rando, O.J. Small RNAs Gained during Epididymal Transit of Sperm Are Essential for Embryonic Development in Mice. Dev. Cell 2018, 46, 470–480 e473. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Zeng, Q.X.; Liu, J.C.; Yeung, W.S.; Zhang, J.V.; Duan, Y.G. Role of small RNAs harbored by sperm in embryonic development and offspring phenotype. Andrology 2023, 11, 770–782. [Google Scholar] [CrossRef] [PubMed]
- Lismer, A.; Kimmins, S. Emerging evidence that the mammalian sperm epigenome serves as a template for embryo development. Nat. Commun. 2023, 14, 2142. [Google Scholar] [CrossRef]
- Rando, O.J. Intergenerational Transfer of Epigenetic Information in Sperm. Cold Spring Harb. Perspect. Med. 2016, 6, a022988. [Google Scholar] [CrossRef] [PubMed]
- Molaro, A.; Hodges, E.; Fang, F.; Song, Q.; McCombie, W.R.; Hannon, G.J.; Smith, A.D. Sperm methylation profiles reveal features of epigenetic inheritance and evolution in primates. Cell 2011, 146, 1029–1041. [Google Scholar] [CrossRef] [PubMed]
- Shea, J.M.; Serra, R.W.; Carone, B.R.; Shulha, H.P.; Kucukural, A.; Ziller, M.J.; Vallaster, M.P.; Gu, H.; Tapper, A.R.; Gardner, P.D.; et al. Genetic and Epigenetic Variation, but Not Diet, Shape the Sperm Methylome. Dev. Cell 2015, 35, 750–758. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, M.V.C.; Bourc’his, D. The diverse roles of DNA methylation in mammalian development and disease. Nat. Rev. Mol. Cell Biol. 2019, 20, 590–607. [Google Scholar] [CrossRef] [PubMed]
- McSwiggin, H.M.; O’Doherty, A.M. Epigenetic reprogramming during spermatogenesis and male factor infertility. Reproduction 2018, 156, R9–R21. [Google Scholar] [CrossRef]
- Iqbal, K.; Jin, S.G.; Pfeifer, G.P.; Szabo, P.E. Reprogramming of the paternal genome upon fertilization involves genome-wide oxidation of 5-methylcytosine. Proc. Natl. Acad. Sci. USA 2011, 108, 3642–3647. [Google Scholar] [CrossRef]
- Oswald, J.; Engemann, S.; Lane, N.; Mayer, W.; Olek, A.; Fundele, R.; Dean, W.; Reik, W.; Walter, J. Active demethylation of the paternal genome in the mouse zygote. Curr. Biol. 2000, 10, 475–478. [Google Scholar] [CrossRef]
- Wossidlo, M.; Nakamura, T.; Lepikhov, K.; Marques, C.J.; Zakhartchenko, V.; Boiani, M.; Arand, J.; Nakano, T.; Reik, W.; Walter, J. 5-Hydroxymethylcytosine in the mammalian zygote is linked with epigenetic reprogramming. Nat. Commun. 2011, 2, 241. [Google Scholar] [CrossRef]
- Amouroux, R.; Nashun, B.; Shirane, K.; Nakagawa, S.; Hill, P.W.; D’Souza, Z.; Nakayama, M.; Matsuda, M.; Turp, A.; Ndjetehe, E.; et al. De novo DNA methylation drives 5hmC accumulation in mouse zygotes. Nat. Cell Biol. 2016, 18, 225–233. [Google Scholar] [CrossRef]
- Yan, R.; Cheng, X.; Gu, C.; Xu, Y.; Long, X.; Zhai, J.; Sun, F.; Qian, J.; Du, Y.; Wang, H.; et al. Dynamics of DNA hydroxymethylation and methylation during mouse embryonic and germline development. Nat. Genet. 2023, 55, 130–143. [Google Scholar] [CrossRef]
- Zheng, H.; Zhou, X.; Li, D.K.; Yang, F.; Pan, H.; Li, T.; Miao, M.; Li, R.; Yuan, W. Genome-wide alteration in DNA hydroxymethylation in the sperm from bisphenol A-exposed men. PLoS ONE 2017, 12, e0178535. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Tang, Q.; Lu, Y.; Li, M.; Zhou, Y.; Wu, P.; Li, J.; Pan, F.; Han, X.; Chen, M.; et al. Semen quality and sperm DNA methylation in relation to long-term exposure to air pollution in fertile men: A cross-sectional study. Environ. Pollut. 2022, 300, 118994. [Google Scholar] [CrossRef] [PubMed]
- Wyck, S.; Herrera, C.; Requena, C.E.; Bittner, L.; Hajkova, P.; Bollwein, H.; Santoro, R. Oxidative stress in sperm affects the epigenetic reprogramming in early embryonic development. Epigenetics Chromatin 2018, 11, 60. [Google Scholar] [CrossRef] [PubMed]
- Chabory, E.; Damon, C.; Lenoir, A.; Kauselmann, G.; Kern, H.; Zevnik, B.; Garrel, C.; Saez, F.; Cadet, R.; Henry-Berger, J.; et al. Epididymis seleno-independent glutathione peroxidase 5 maintains sperm DNA integrity in mice. J. Clin. Investig. 2009, 119, 2074–2085. [Google Scholar] [CrossRef] [PubMed]
- Noblanc, A.; Peltier, M.; Damon-Soubeyrand, C.; Kerchkove, N.; Chabory, E.; Vernet, P.; Saez, F.; Cadet, R.; Janny, L.; Pons-Rejraji, H.; et al. Epididymis response partly compensates for spermatozoa oxidative defects in snGPx4 and GPx5 double mutant mice. PLoS ONE 2012, 7, e38565. [Google Scholar] [CrossRef]
- Gharagozloo, P.; Gutierrez-Adan, A.; Champroux, A.; Noblanc, A.; Kocer, A.; Calle, A.; Perez-Cerezales, S.; Pericuesta, E.; Polhemus, A.; Moazamian, A.; et al. A novel antioxidant formulation designed to treat male infertility associated with oxidative stress: Promising preclinical evidence from animal models. Hum. Reprod. 2016, 31, 252–262. [Google Scholar] [CrossRef]
- Vaisvila, R.; Ponnaluri, V.K.C.; Sun, Z.; Langhorst, B.W.; Saleh, L.; Guan, S.; Dai, N.; Campbell, M.A.; Sexton, B.S.; Marks, K.; et al. Enzymatic methyl sequencing detects DNA methylation at single-base resolution from picograms of DNA. Genome Res. 2021, 31, 1280–1289. [Google Scholar] [CrossRef]
- Kocer, A.; Henry-Berger, J.; Noblanc, A.; Champroux, A.; Pogorelcnik, R.; Guiton, R.; Janny, L.; Pons-Rejraji, H.; Saez, F.; Johnson, G.D.; et al. Oxidative DNA damage in mouse sperm chromosomes: Size matters. Free Radic. Biol. Med. 2015, 89, 993–1002. [Google Scholar] [CrossRef]
- Chan, D.; Shao, X.; Dumargne, M.C.; Aarabi, M.; Simon, M.M.; Kwan, T.; Bailey, J.L.; Robaire, B.; Kimmins, S.; San Gabriel, M.C.; et al. Customized MethylC-Capture Sequencing to Evaluate Variation in the Human Sperm DNA Methylome Representative of Altered Folate Metabolism. Environ. Health Perspect. 2019, 127, 87002. [Google Scholar] [CrossRef]
- Spiegel, J.; Adhikari, S.; Balasubramanian, S. The Structure and Function of DNA G-Quadruplexes. Trends Chem. 2020, 2, 123–136. [Google Scholar] [CrossRef]
- Fleming, A.M.; Burrows, C.J. 8-Oxo-7,8-dihydroguanine, friend and foe: Epigenetic-like regulator versus initiator of mu-tagenesis. DNA Repair 2017, 56, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Moazamian, A.; Gharagozloo, P.; Aitken, R.J.; Drevet, J.R. Oxidative stress and reproductive function: Sperm telomeres, oxidative stress, and infertility. Reproduction 2022, 164, F125–F133. [Google Scholar] [CrossRef] [PubMed]
- Obeid, R.; Holzgreve, W.; Pietrzik, K. Is 5-methyltetrahydrofolate an alternative to folic acid for the prevention of neural tube defects? J. Perinat Med. 2013, 41, 469–483. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Xu, J.; Lin, Y.L.; Cabrera, R.M.; Chen, Q.; Zhang, C.; Steele, J.W.; Han, X.; Gross, S.S.; Wlodarczyk, B.J.; et al. Excess folic acid intake increases DNA de novo point mutations. Cell Discov. 2023, 9, 22. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Safarinejad, M.R.; Shafiei, N.; Safarinejad, S. Relationship between Genetic Polymorphisms of Methylenetetrahydrofolate Reductase (C677t, A1298c, and G1793a) as Risk Factors for Idiopathic Male Infertility. Reprod Sci. 2011, 18, 304–315. [Google Scholar] [CrossRef]
- Menezo, Y.; Elder, K.; Clement, A.; Clement, P. Folic Acid, Folinic Acid, 5 Methyl Tetrahydrofolate Supplementation for Mutations That Affect Epigenesis through the Folate and One-Carbon Cycles. Biomolecules 2022, 12, 197. [Google Scholar] [CrossRef]
- Jenkins, T.; Aston, K.; Carrell, D.; DeVilbiss, E.; Sjaarda, L.; Perkins, N.; Mills, J.L.; Chen, Z.; Sparks, A.; Clemons, T.; et al. The Impact of Zinc and Folic Acid Supplementation on Sperm DNA Methylation: Results from the Folic Acid and Zinc Supplementation Randomized Clinical Trial (Fazst). Fertil. Steril. 2022, 117, 75–85. [Google Scholar] [CrossRef]
- Chen, H.; Scott-Boyer, M.P.; Droit, A.; Robert, C.; Belleannee, C. Sperm Heterogeneity Accounts for Sperm DNA Methylation Variations Observed in the Caput Epididymis, Independently From DNMT/TET Activities. Front. Cell Dev. Biol. 2022, 10, 834519. [Google Scholar] [CrossRef]
- Wu, Y.H.; Huang, Y.F.; Wu, P.Y.; Chang, T.H.; Huang, S.C.; Chou, C.Y. The downregulation of miR-509-3p expression by collagen type XI alpha 1-regulated hypermethylation facilitates cancer progression and chemoresistance via the DNA methyltransferase 1/Small ubiquitin-like modifier-3 axis in ovarian cancer cells. J. Ovarian Res. 2023, 16, 124. [Google Scholar] [CrossRef]
- Zamora-Fuentes, J.M.; Hernandez-Lemus, E.; Espinal-Enriquez, J. Methylation-related genes involved in renal carcinoma progression. Front. Genet. 2023, 14, 1225158. [Google Scholar] [CrossRef]
- Song, B.; Chen, Y.; Wang, C.; Li, G.; Wei, Z.; He, X.; Cao, Y. Poor semen parameters are associated with abnormal methylation of imprinted genes in sperm DNA. Reprod. Biol. Endocrinol. 2022, 20, 155. [Google Scholar] [CrossRef] [PubMed]
- Aitken, R.J.; Drevet, J.R. The Importance of Oxidative Stress in Determining the Functionality of Mammalian Spermatozoa: A Two-Edged Sword. Antioxidants 2020, 9, 111. [Google Scholar] [CrossRef] [PubMed]
- Drevet, J.R.; Aitken, R.J. Oxidation of Sperm Nucleus in Mammals: A Physiological Necessity to Some Extent with Adverse Impacts on Oocyte and Offspring. Antioxidants 2020, 9, 95. [Google Scholar] [CrossRef] [PubMed]
- Damon-Soubeyrand, C.; Bongiovanni, A.; Chorfa, A.; Goubely, C.; Pirot, N.; Pardanaud, L.; Piboin-Fragner, L.; Vachias, C.; Bravard, S.; Guiton, R.; et al. Three-dimensional imaging of vascular development in the mouse epididymis. Elife 2023, 12, e82748. [Google Scholar] [CrossRef] [PubMed]
- Sharma, U.; Conine, C.C.; Shea, J.M.; Boskovic, A.; Derr, A.G.; Bing, X.Y.; Belleannee, C.; Kucukural, A.; Serra, R.W.; Sun, F.; et al. Biogenesis and function of tRNA fragments during sperm maturation and fertilization in mammals. Science 2016, 351, 391–396. [Google Scholar] [CrossRef]
- Deshpande, S.S.; Nemani, H.; Arumugam, G.; Ravichandran, A.; Balasinor, N.H. High-fat diet-induced and genetically inherited obesity differentially alters DNA methylation profile in the germline of adult male rats. Clin. Epigenetics 2020, 12, 179. [Google Scholar] [CrossRef]
- Xue, J.; Gharaibeh, R.Z.; Pietryk, E.W.; Brouwer, C.; Tarantino, L.M.; Valdar, W.; Ideraabdullah, F.Y. Impact of vitamin D depletion during development on mouse sperm DNA methylation. Epigenetics 2018, 13, 959–974. [Google Scholar] [CrossRef]
- Hug, E.; Villeneuve, P.; Bravard, S.; Chorfa, A.; Damon-Soubeyrand, C.; Somkuti, S.G.; Moazamian, A.; Aitken, R.J.; Gharagozloo, P.; Drevet, J.R.; et al. Loss of Nuclear/DNA Integrity in Mouse Epididymal Spermatozoa after Short-Term Exposure to Low Doses of Dibutyl Phthalate or Bisphenol AF and Its Mitigation by Oral Antioxidant Supplementation. Antioxidants 2023, 12, 1046. [Google Scholar] [CrossRef]
- Miao, M.; Zhou, X.; Li, Y.; Zhang, O.; Zhou, Z.; Li, T.; Yuan, W.; Li, R.; Li, D.K. LINE-1 hypomethylation in spermatozoa is associated with Bisphenol A exposure. Andrology 2014, 2, 138–144. [Google Scholar] [CrossRef]
- Oluwayiose, O.A.; Marcho, C.; Wu, H.; Houle, E.; Krawetz, S.A.; Suvorov, A.; Mager, J.; Richard Pilsner, J. Paternal preconception phthalate exposure alters sperm methylome and embryonic programming. Environ. Int. 2021, 155, 106693. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hug, E.; Renaud, Y.; Guiton, R.; Ben Sassi, M.; Marcaillou, C.; Moazamian, A.; Gharagozloo, P.; Drevet, J.R.; Saez, F. Exploring the Epigenetic Landscape of Spermatozoa: Impact of Oxidative Stress and Antioxidant Supplementation on DNA Methylation and Hydroxymethylation. Antioxidants 2024, 13, 1520. https://doi.org/10.3390/antiox13121520
Hug E, Renaud Y, Guiton R, Ben Sassi M, Marcaillou C, Moazamian A, Gharagozloo P, Drevet JR, Saez F. Exploring the Epigenetic Landscape of Spermatozoa: Impact of Oxidative Stress and Antioxidant Supplementation on DNA Methylation and Hydroxymethylation. Antioxidants. 2024; 13(12):1520. https://doi.org/10.3390/antiox13121520
Chicago/Turabian StyleHug, Elisa, Yoan Renaud, Rachel Guiton, Mehdi Ben Sassi, Charles Marcaillou, Aron Moazamian, Parviz Gharagozloo, Joël R. Drevet, and Fabrice Saez. 2024. "Exploring the Epigenetic Landscape of Spermatozoa: Impact of Oxidative Stress and Antioxidant Supplementation on DNA Methylation and Hydroxymethylation" Antioxidants 13, no. 12: 1520. https://doi.org/10.3390/antiox13121520
APA StyleHug, E., Renaud, Y., Guiton, R., Ben Sassi, M., Marcaillou, C., Moazamian, A., Gharagozloo, P., Drevet, J. R., & Saez, F. (2024). Exploring the Epigenetic Landscape of Spermatozoa: Impact of Oxidative Stress and Antioxidant Supplementation on DNA Methylation and Hydroxymethylation. Antioxidants, 13(12), 1520. https://doi.org/10.3390/antiox13121520







