Metabolome and Metagenome Integration Unveiled Synthesis Pathways of Novel Antioxidant Peptides in Fermented Lignocellulosic Biomass of Palm Kernel Meal
Abstract
1. Introduction
2. Materials and Methods
2.1. Enzymes and Microbial Strains
2.2. Palm Kernel Meal Hydrolysis and Lactobacilli Fermentation
2.3. Metabolome Analysis
2.4. Metagenome Analysis
2.5. Antioxidant Capacity of PKM and Polyphenols and Flavonoids Content
2.6. Statistical Analysis
3. Results
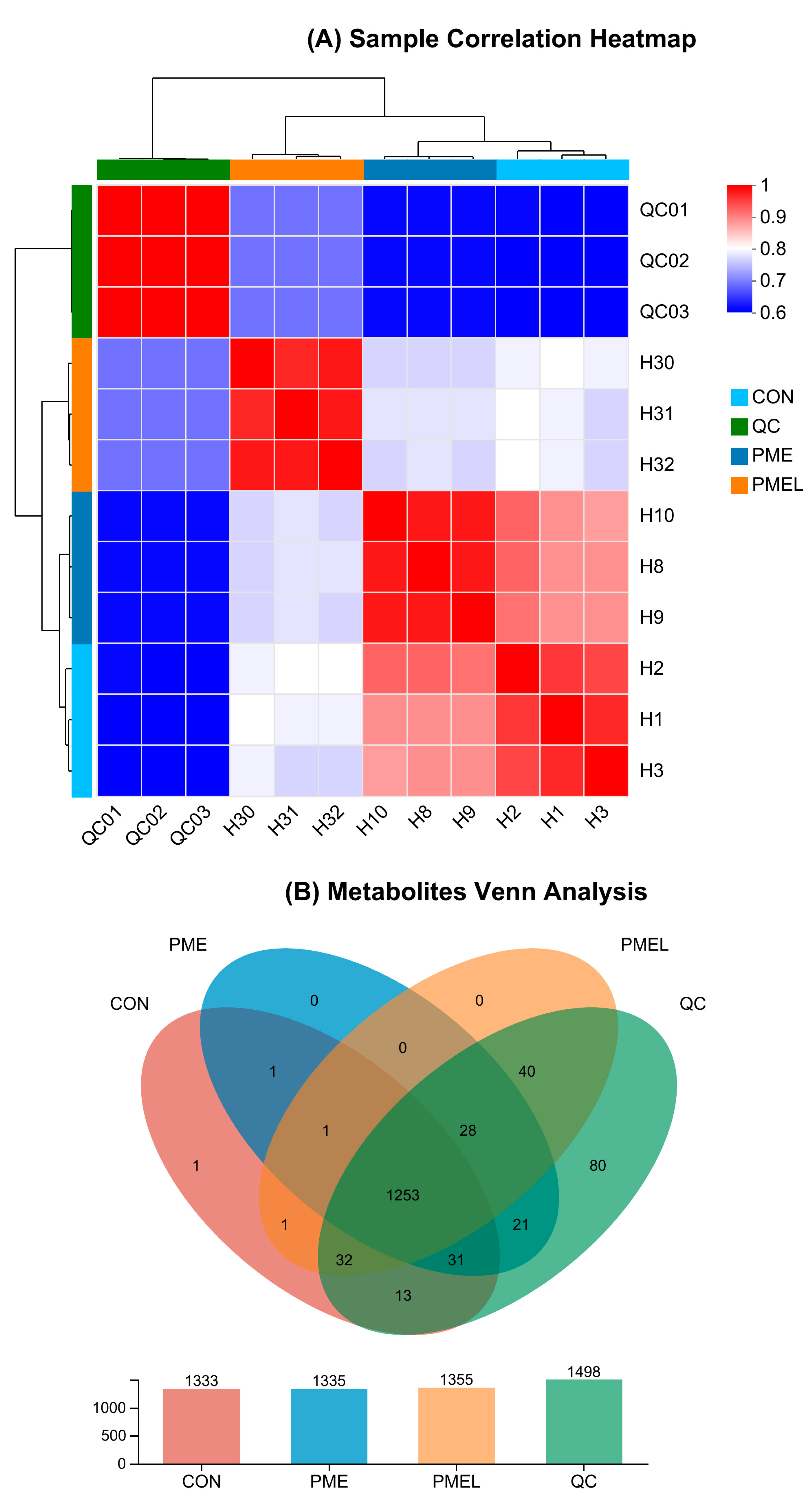
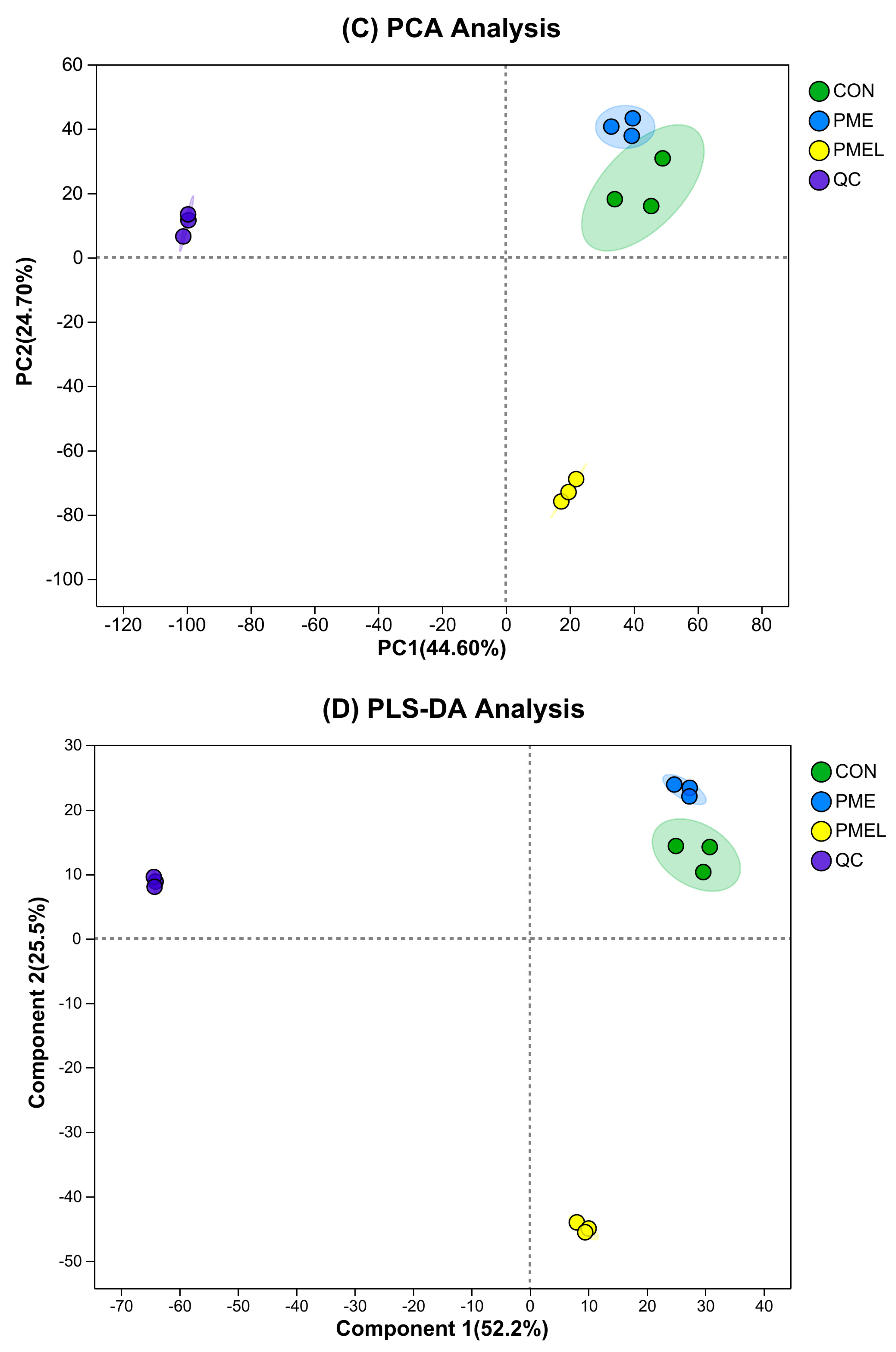
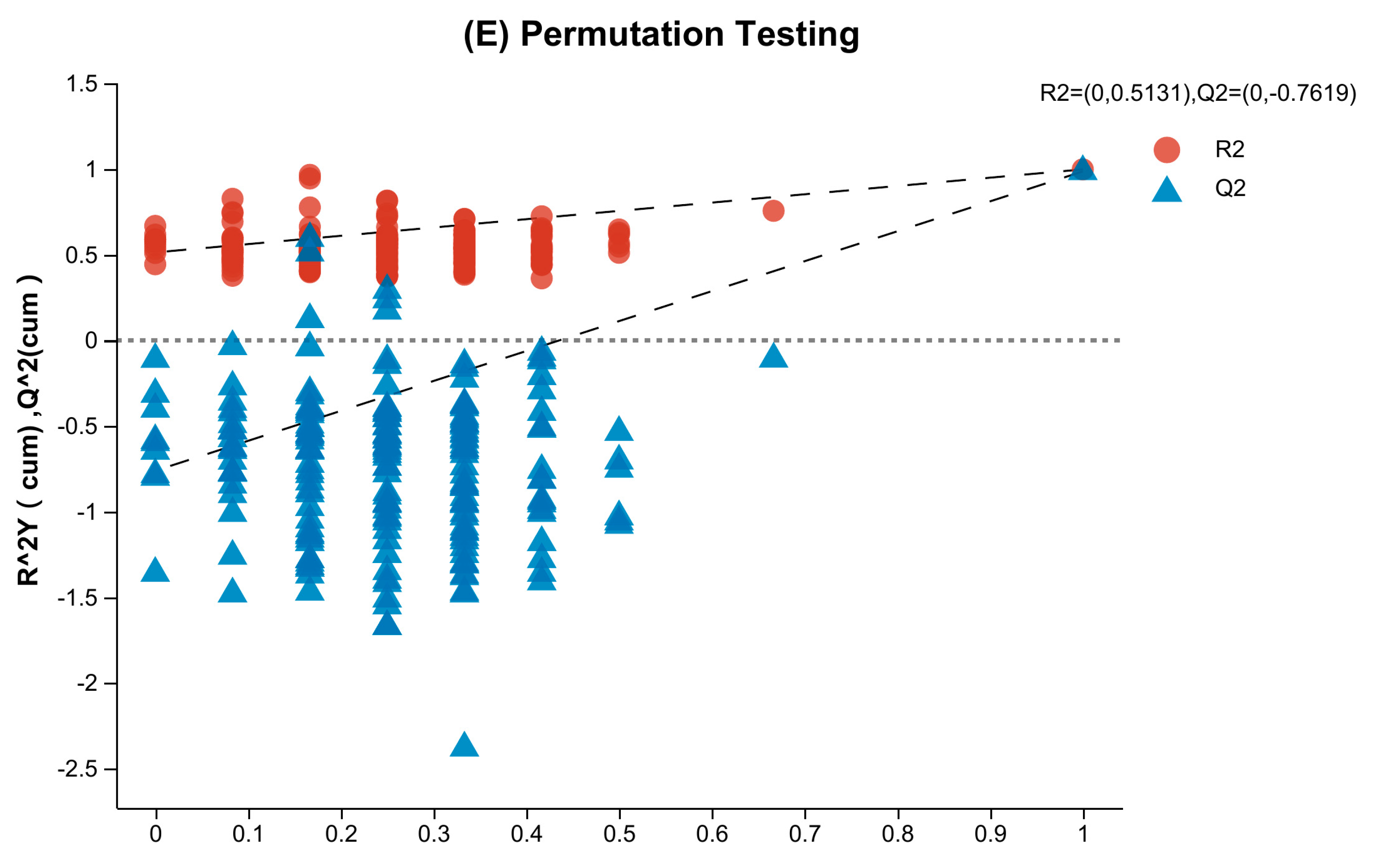
3.1. Multi-Statistical Analysis
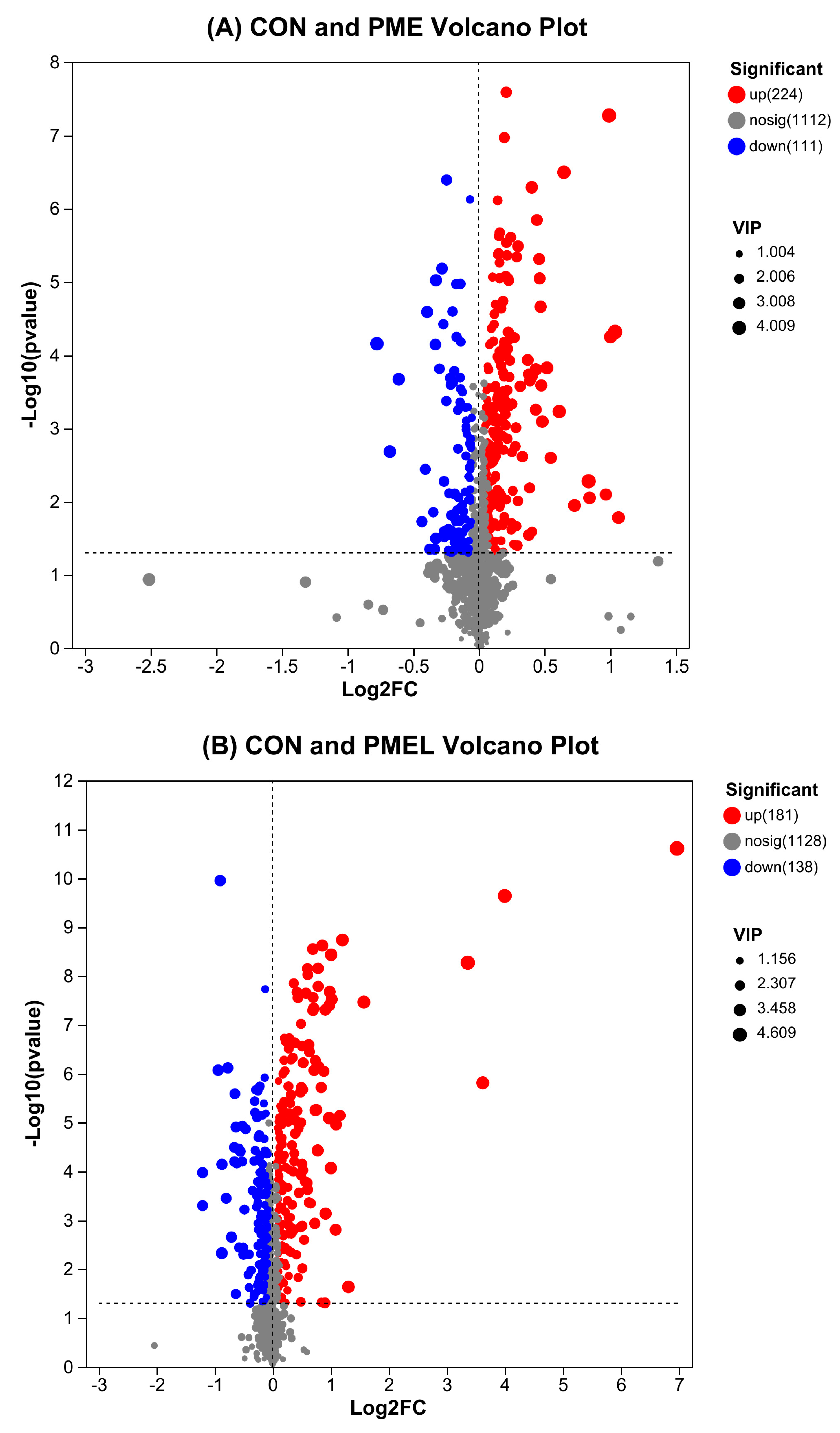
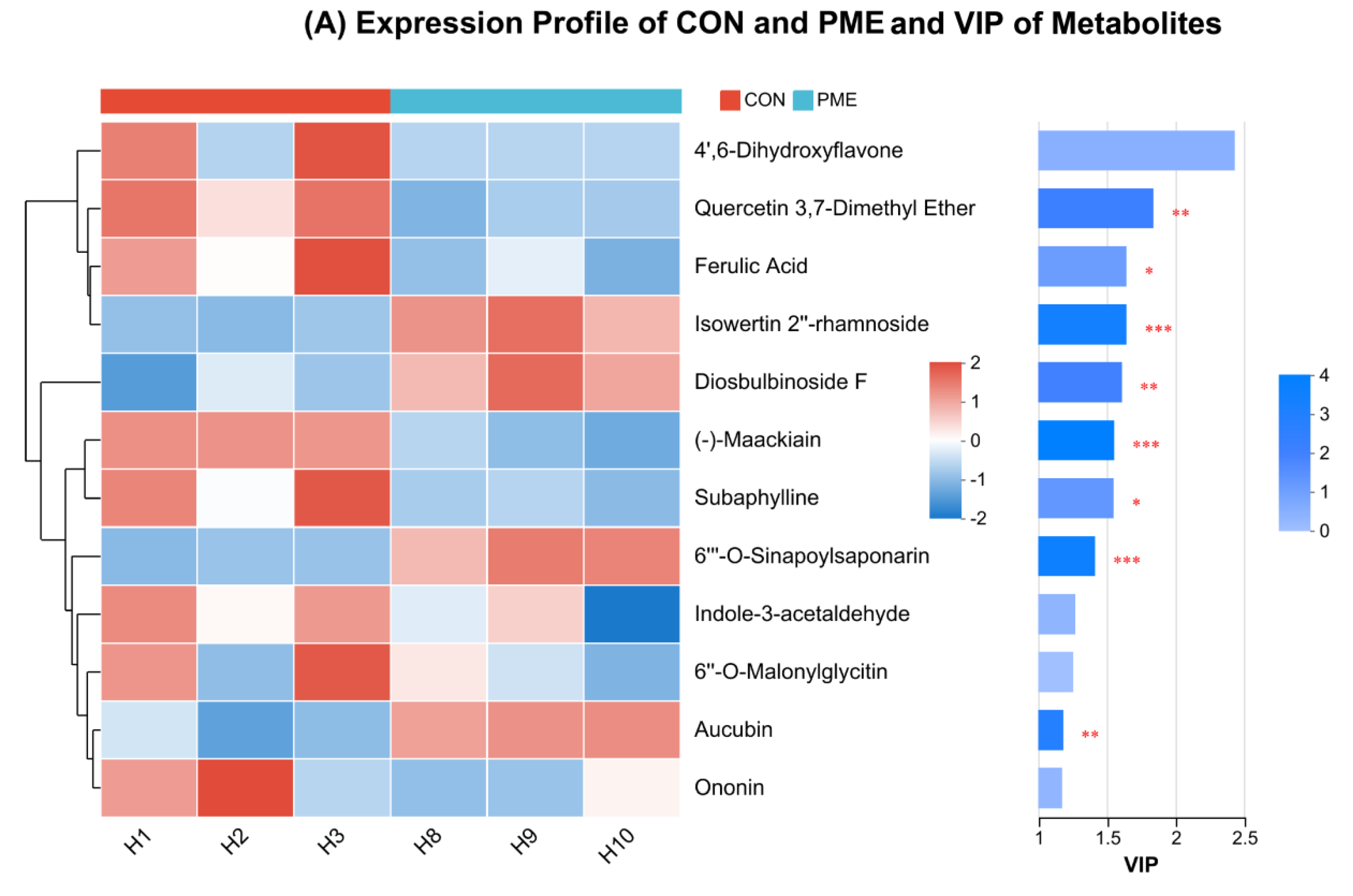
3.2. Expression of Distinct Metabolites
3.3. Compound Categorization in Accordance with KEGG and HMDB Databases
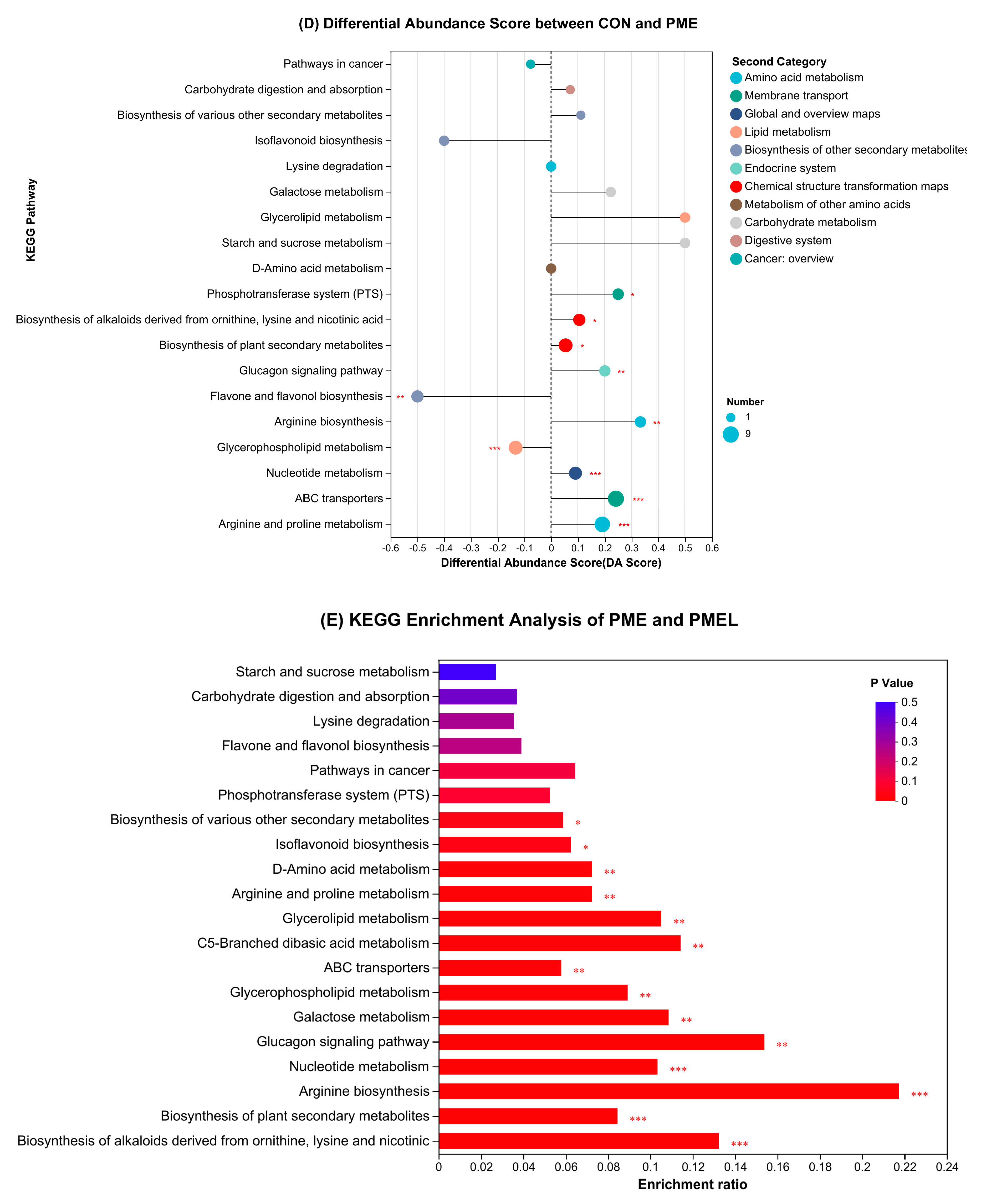
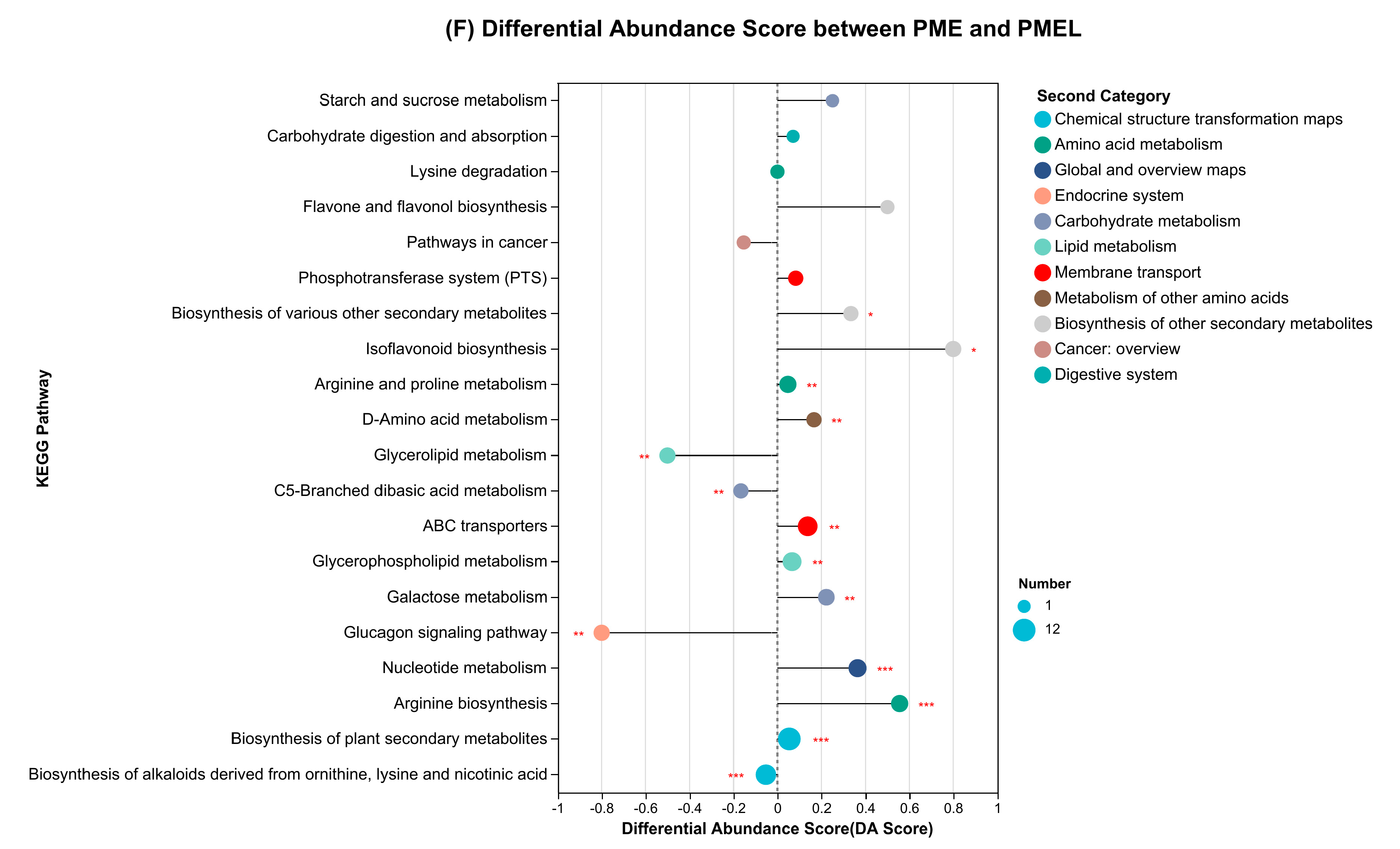
3.4. Enrichment Analysis of KEGG Pathway and Differential Abundance Score
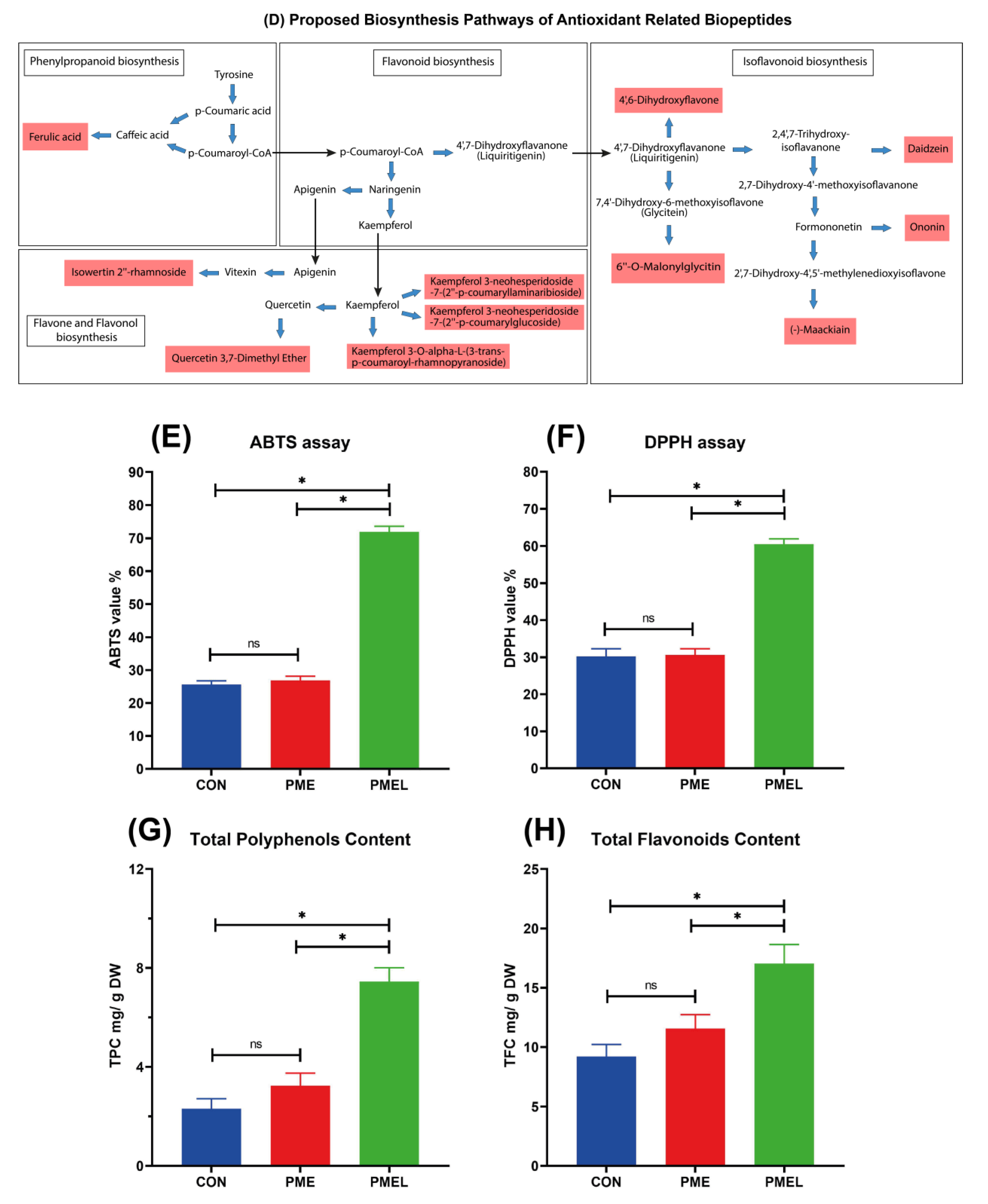
3.5. Metabolites Possessing Antioxidant Potential and Their VIP Assessment, Total Flavonoids and Polyphenols Content, and Antioxidant Capability
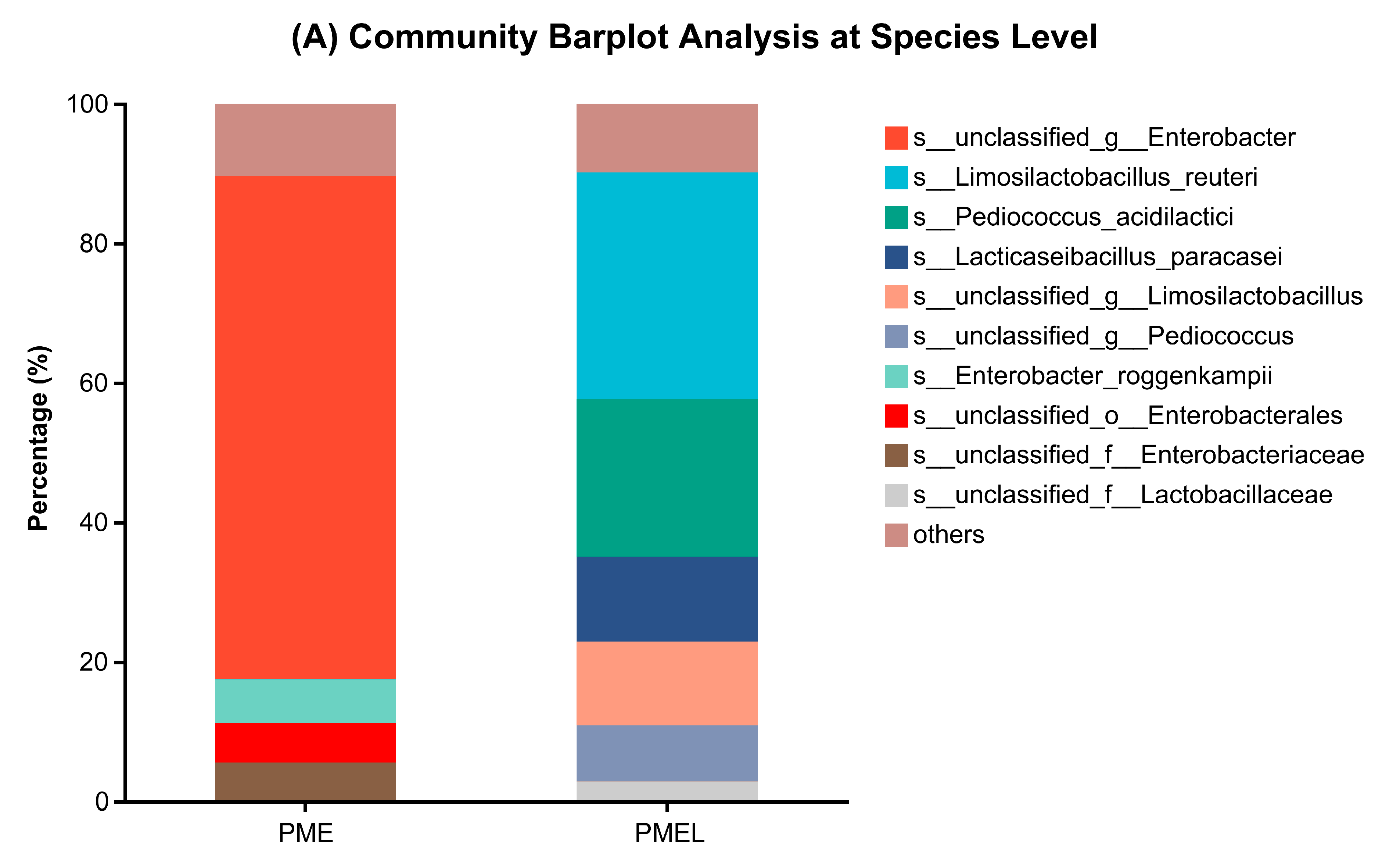
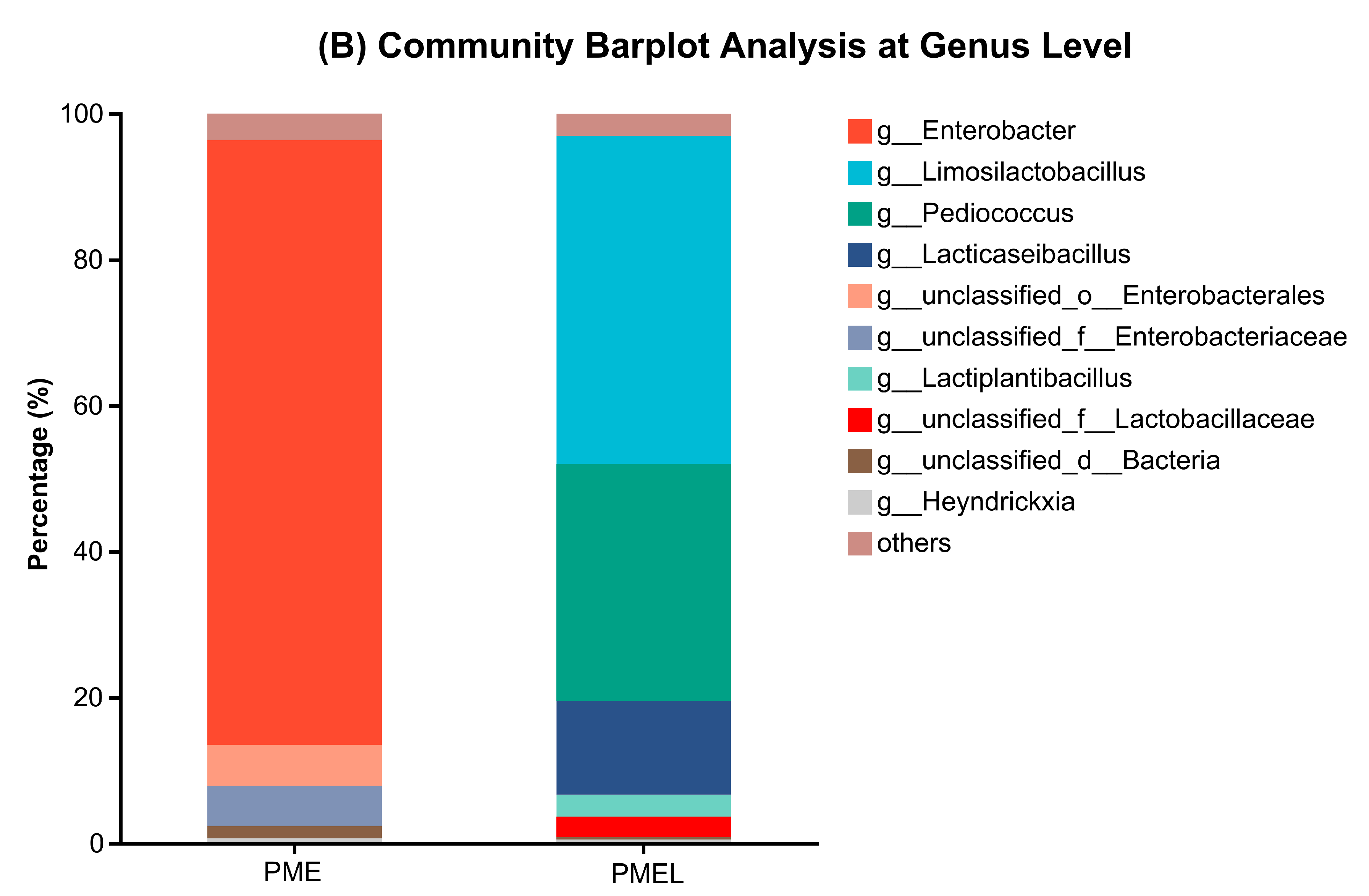
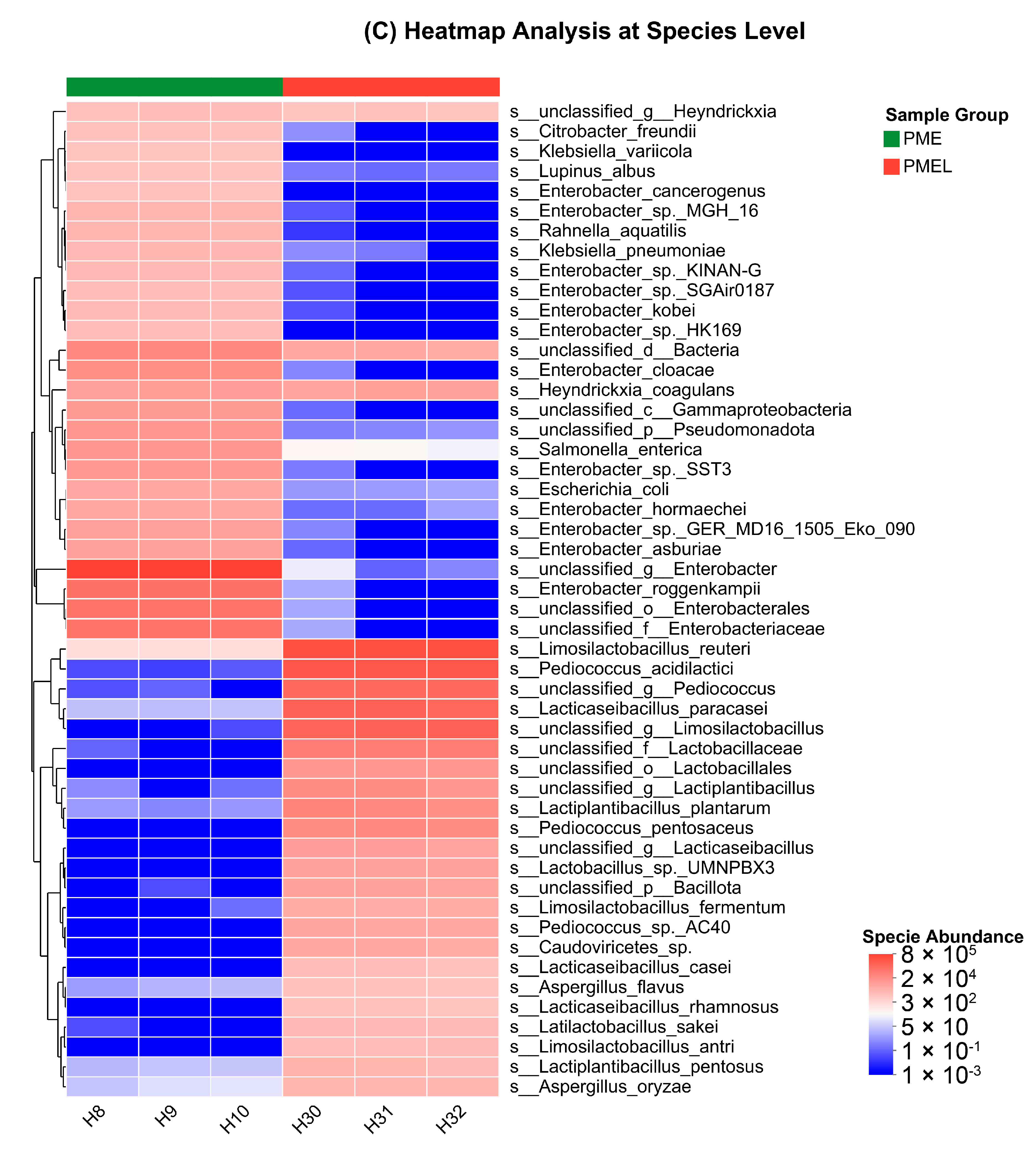
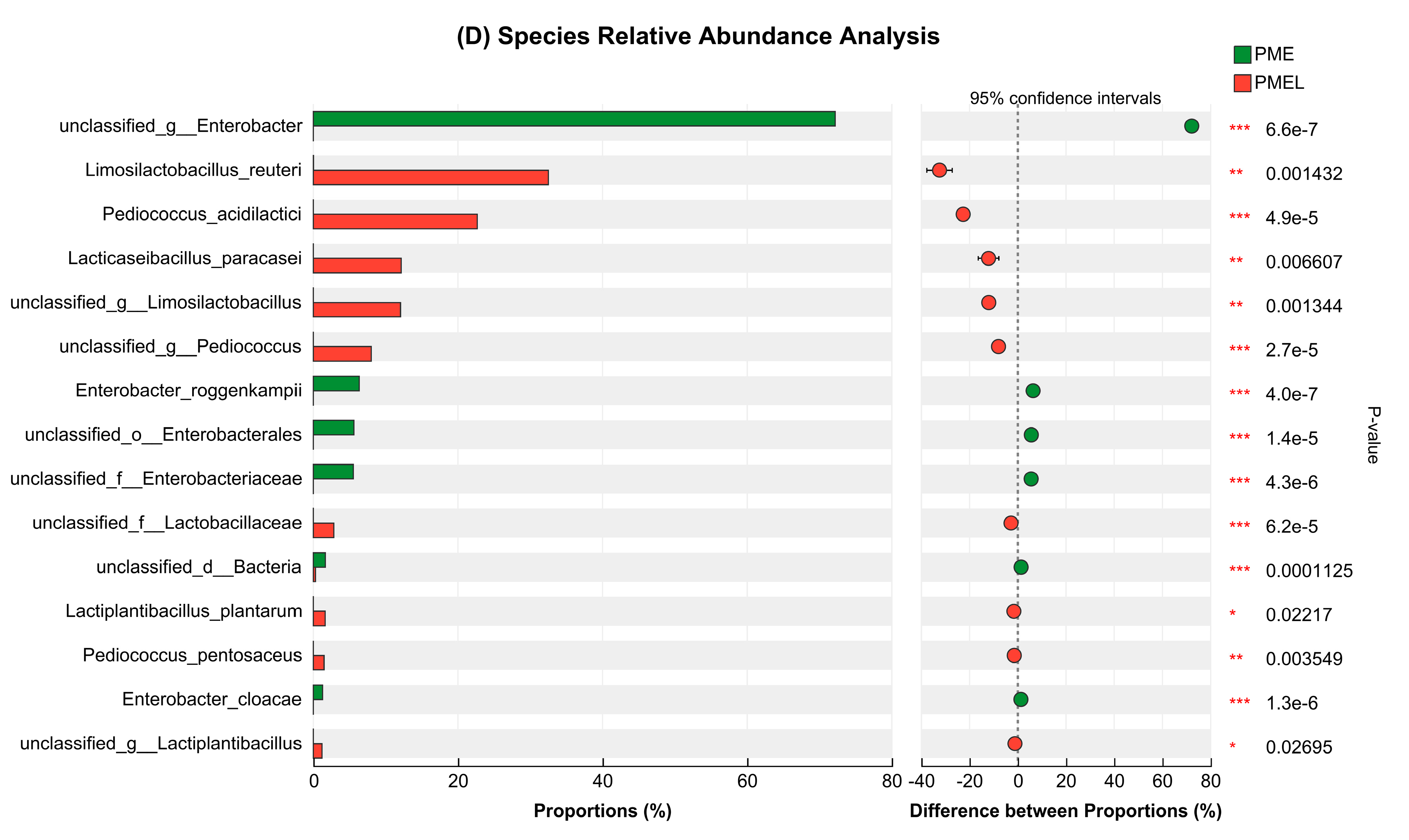
3.6. Metagenome of Bacterial Microbial Community
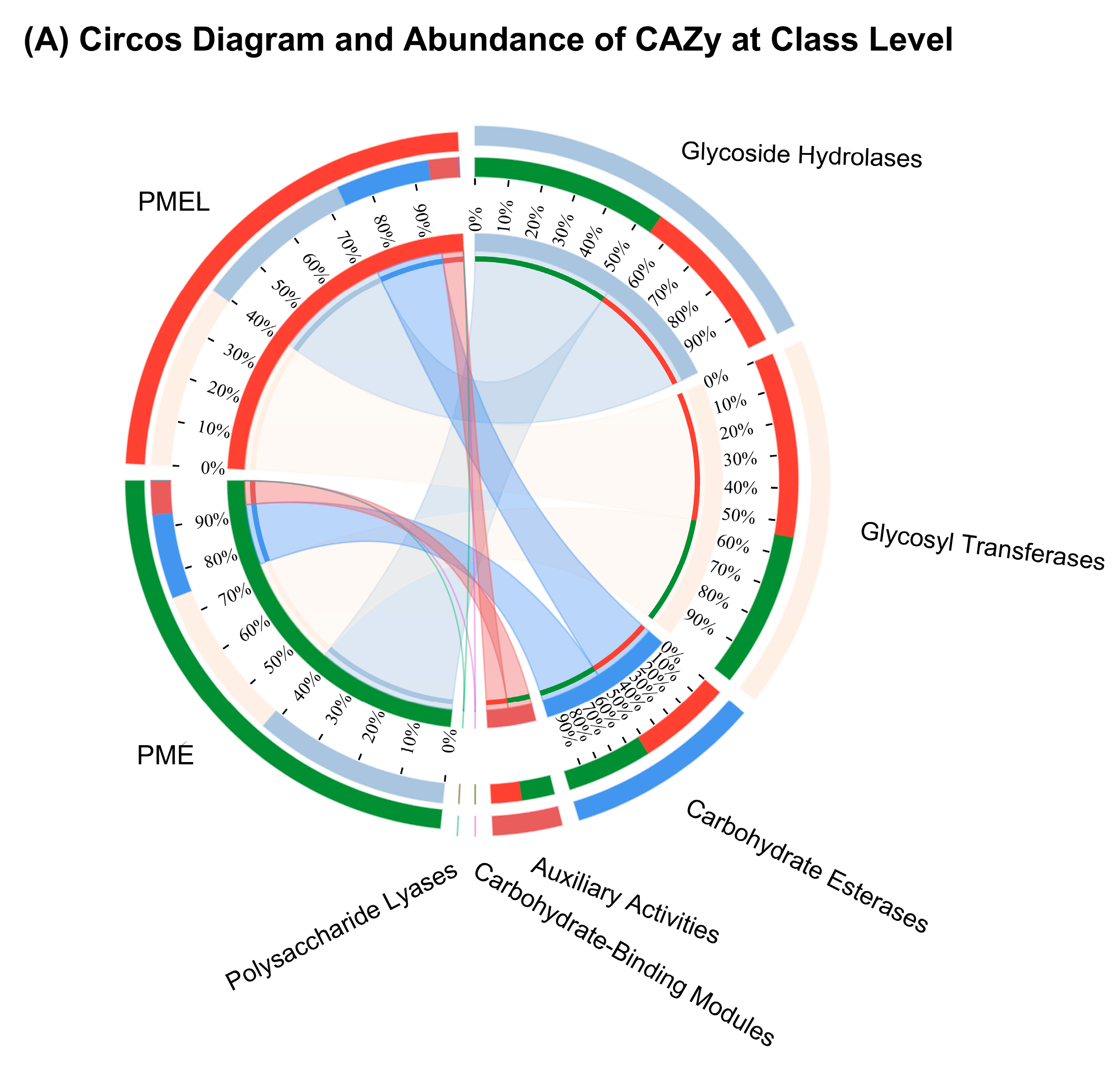
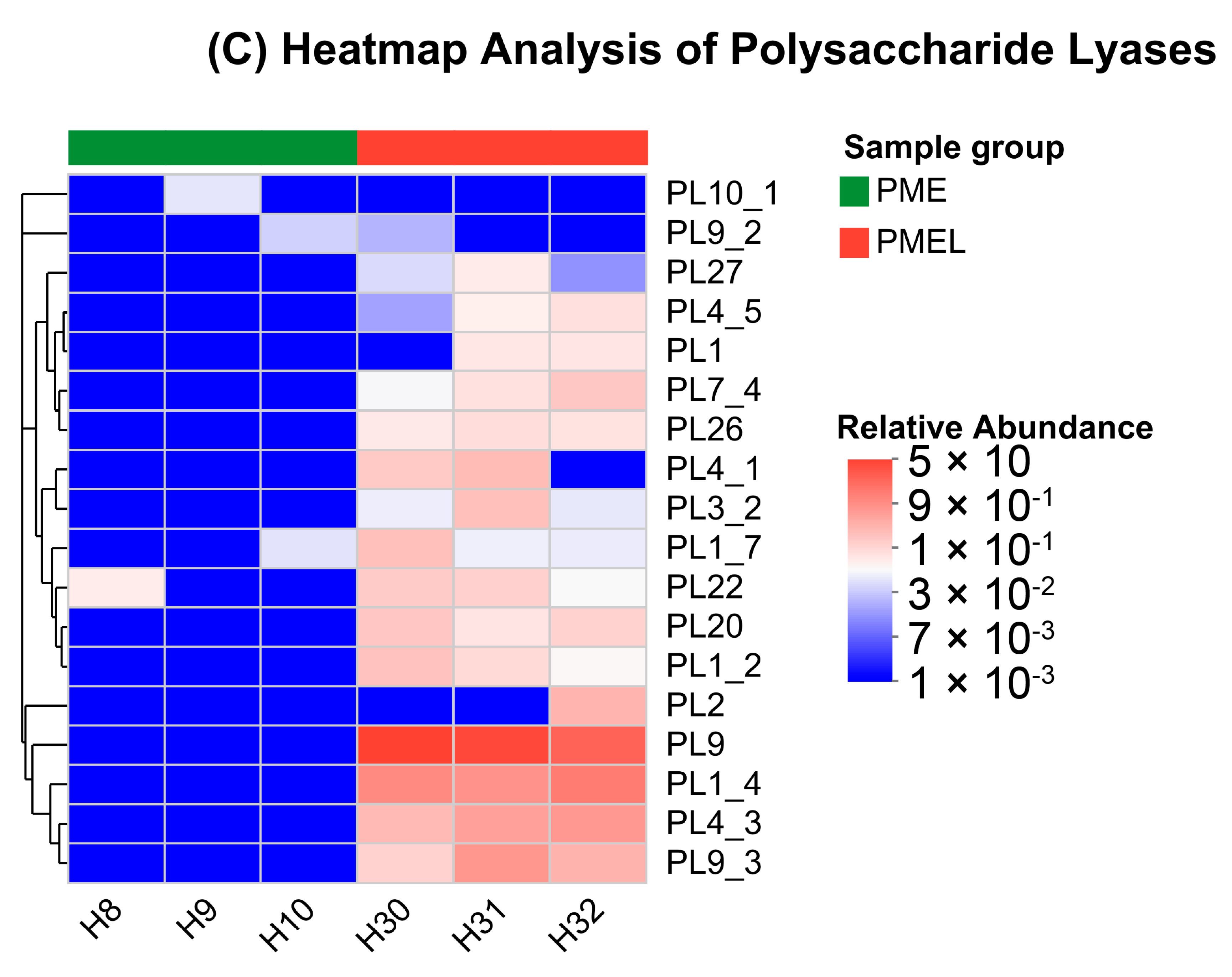
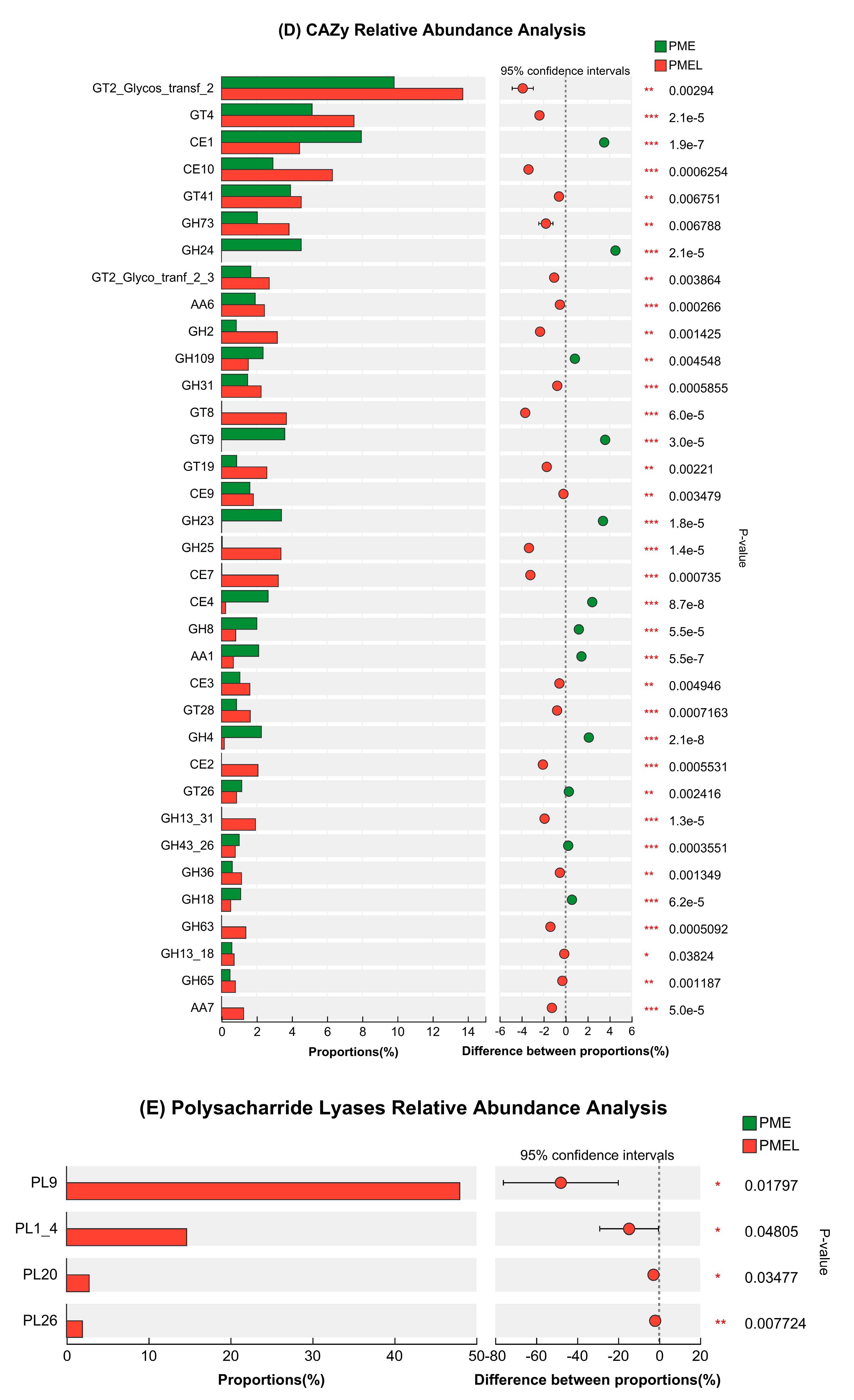
3.7. Carbohydrates Active Enzymes (CAZy) Analysis
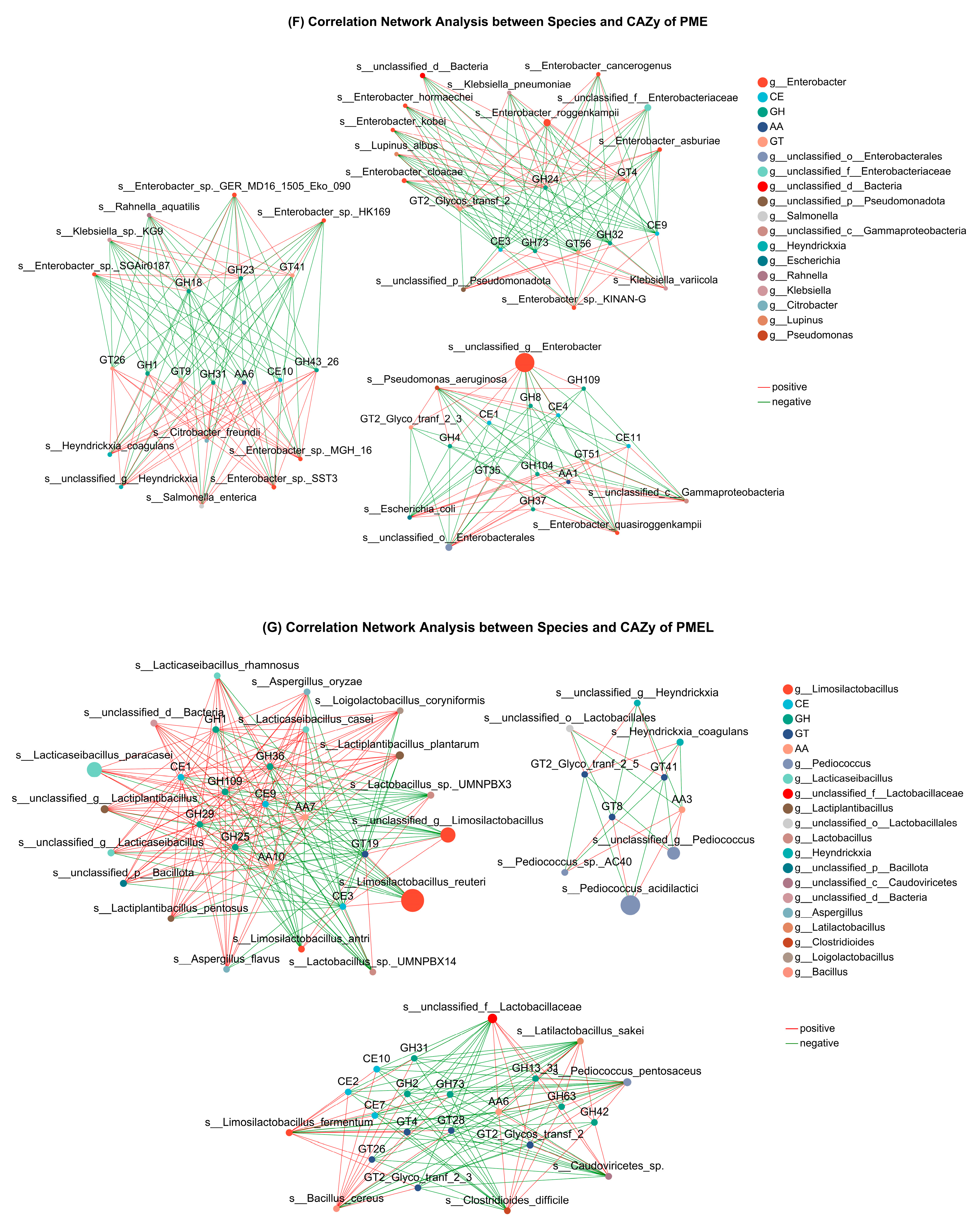
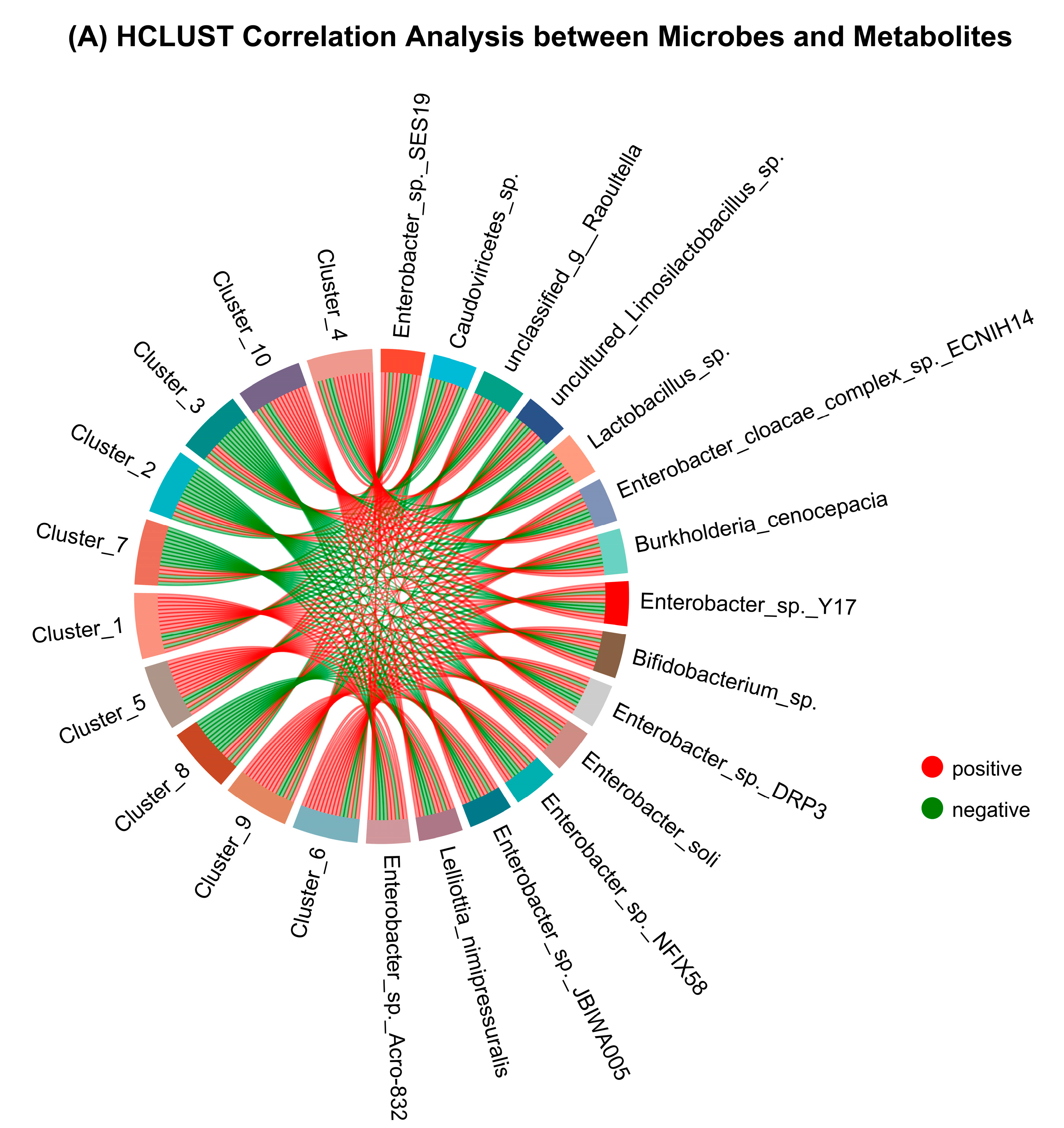
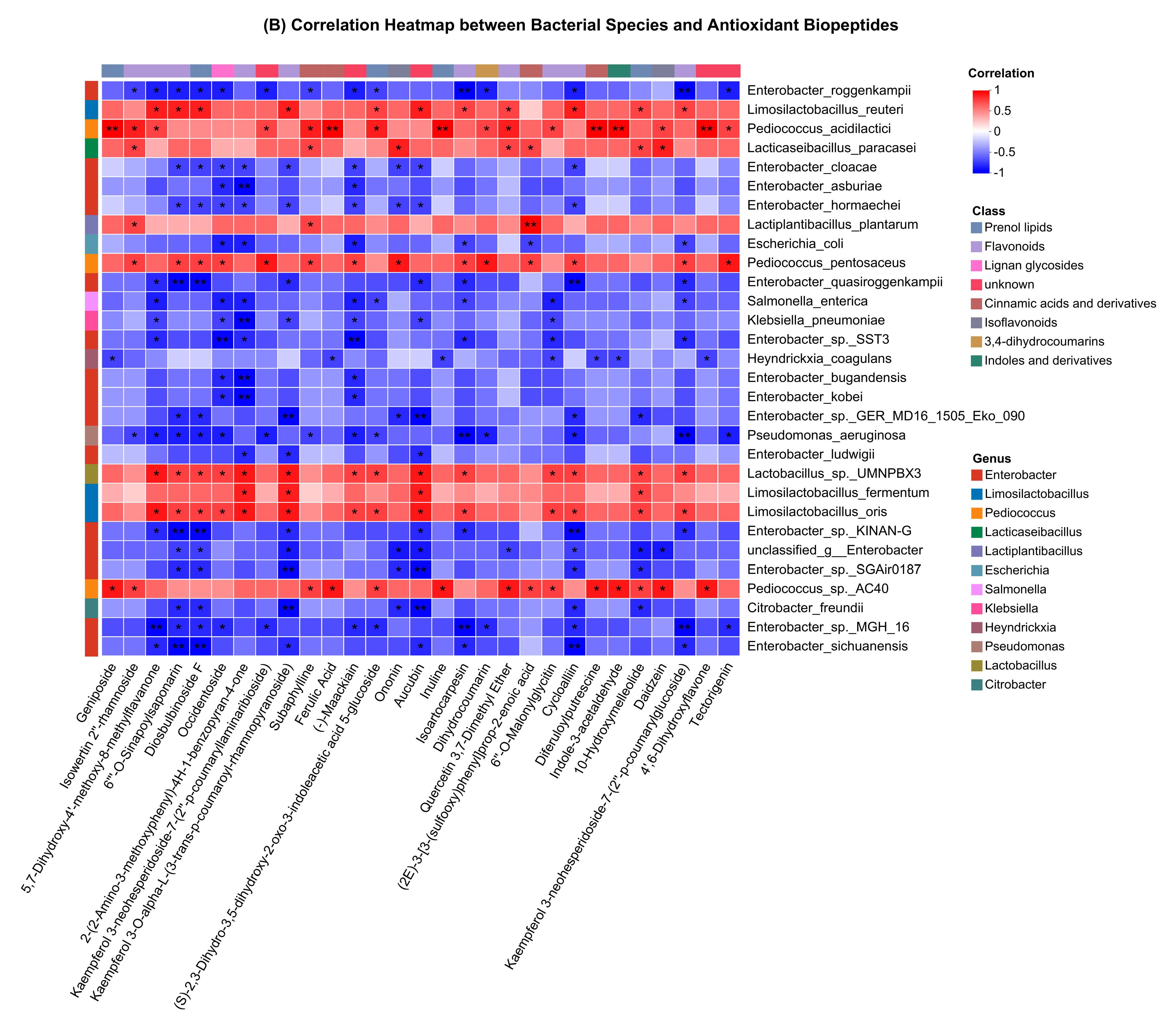
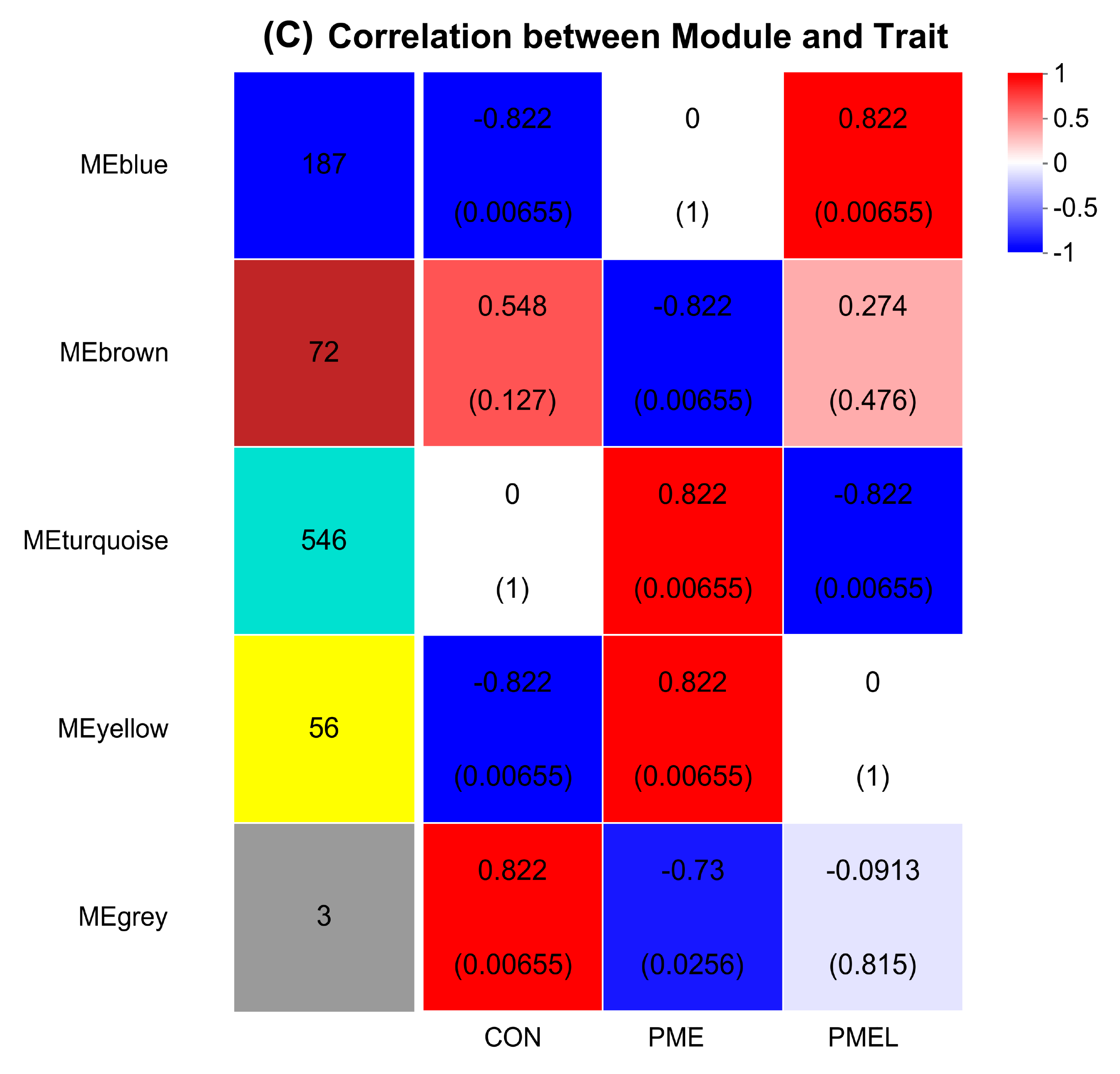
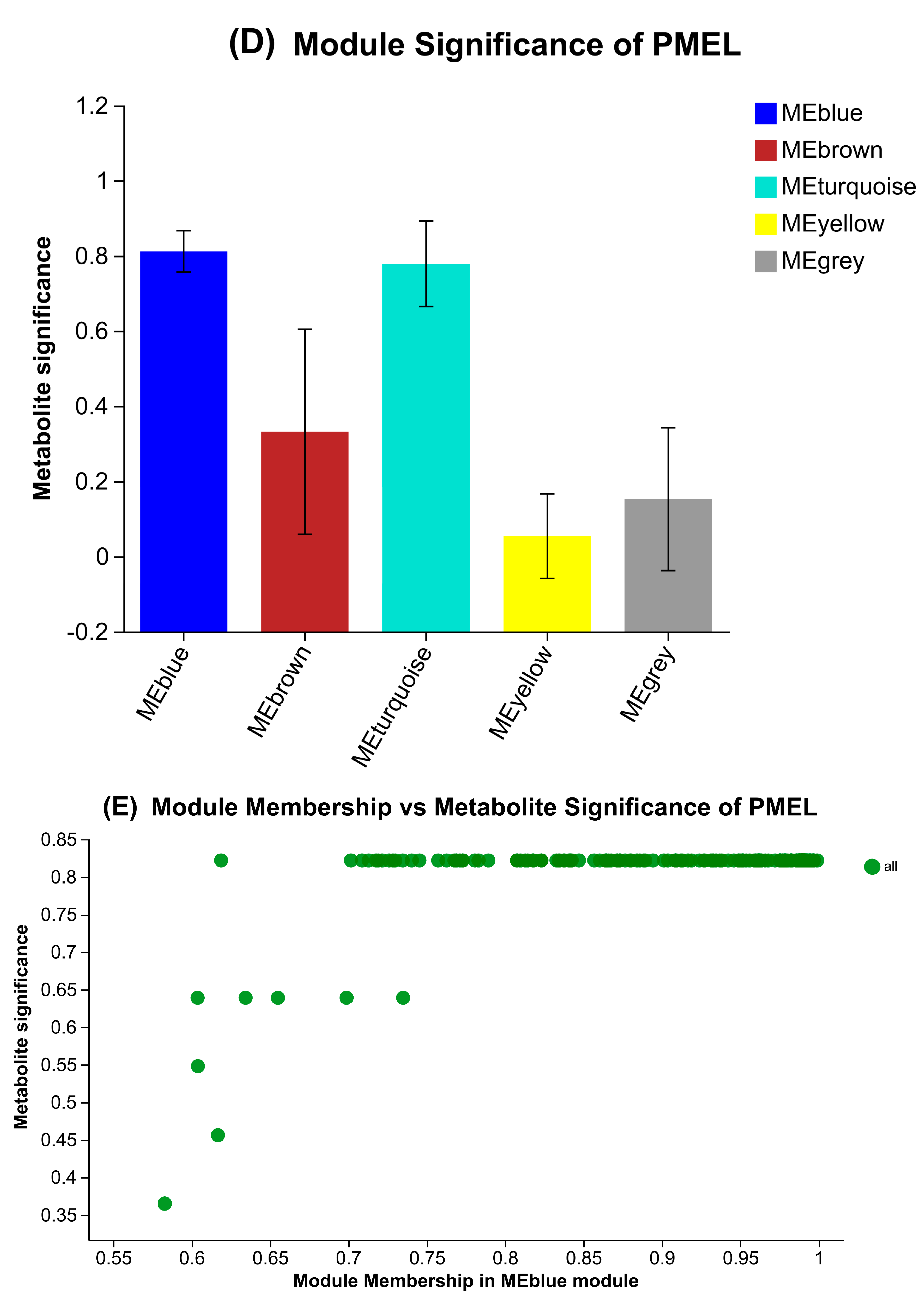
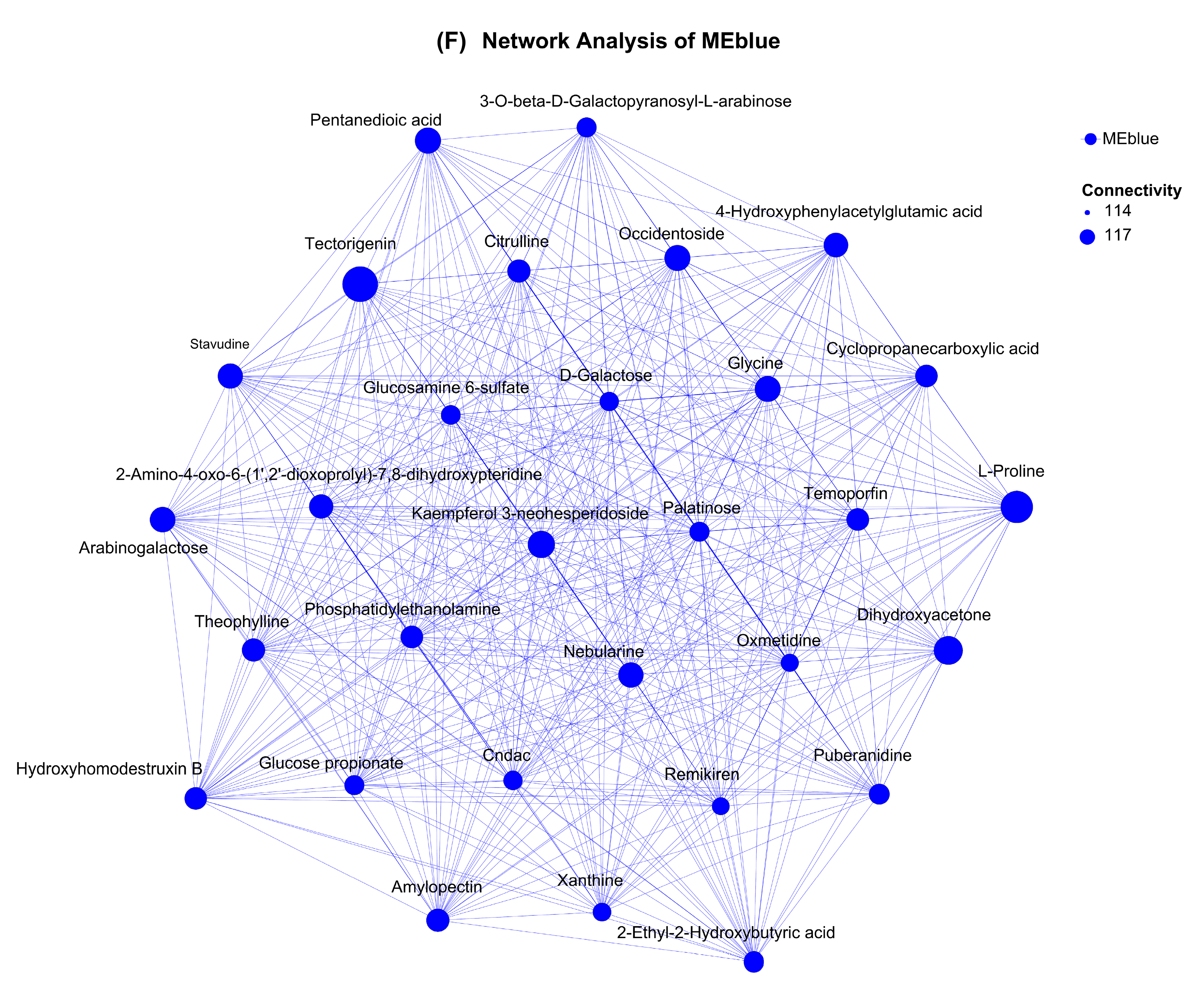
3.8. Species and Metabolites Correlation Analysis and WGCNA
4. Discussion
4.1. Fermented Palm Kernel Meal, Metabolomic, and Metagenomic Profile
4.2. Correlation Evaluation of Microbial Population with Antioxidant Biopeptides and CAZy
4.3. Polyphenolic and Flavonoid Content and Antioxidant Capability
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gustavsson, J.; Cederberg, C.; Sonesson, U.; Van Otterdijk, R.; Meybeck, A. Global Food Losses and Food Waste; Save Food Congress. The Swedish Institute for Food and Biotechnology: Düsseldorf, Germany, 2011. [Google Scholar]
- Teixeira, G.L.; Ibañez, E.; Block, J.M. Emerging Lipids from Arecaceae Palm Fruits in Brazil. Molecules 2022, 27, 4188. [Google Scholar] [CrossRef]
- USDA. Global Market Analysis, Foreign Agricultural Service, United States Department of Agriculture. Oilseeds: World Markets and Trade. Available online: https://apps.fas.usda.gov/psdonline/circulars/oilseeds.pdf (accessed on 4 September 2024).
- Safi, C.; Humblet, N.-P.; Geerdink, P.; Theunissen, M.; Beelen, B.; Voogt, J.; Mulder, W. Valorisation of proteins from palm kernel meal. Bioresour. Technol. Rep. 2022, 18, 101050. [Google Scholar] [CrossRef]
- Galanakis, C.M. Proteins: Sustainable Source, Processing and Applications; Elsevier Science: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Amaral-Júnior, J.M.d.; Morais, E.d.; Lima, A.C.S.; Martorano, L.G.; Nahúm, B.d.S.; Sousa, L.F.; Lourenço-Júnior, J.d.B.; Rodrigues, T.C.G.d.C.; Silva, J.A.R.d.; Silva, A.L.d.C.; et al. Effect of Palm Kernel Cake Supplementation on Voluntary Feed Intake, In Situ Rumen Degradability and Performance in Buffaloes in the Eastern Amazon. Animals 2023, 13, 934. [Google Scholar] [CrossRef]
- Mohamad Asri, N.; Muhialdin, B.J.; Zarei, M.; Saari, N. Low molecular weight peptides generated from palm kernel cake via solid state lacto-fermentation extend the shelf life of bread. LWT 2020, 134, 110206. [Google Scholar] [CrossRef]
- Sulaiman, F.; Abdullah, N.; Gerhauser, H.; Shariff, A. An outlook of Malaysian energy, oil palm industry and its utilization of wastes as useful resources. Biomass Bioenergy 2011, 35, 3775–3786. [Google Scholar] [CrossRef]
- Pelaez, R.D.R.; Oliveira, M.E.C.; Miller, R.N.G.; de Almeida, J.R.M.; de Siqueira, F.G. Biotechnological valorization of lignocellulosic residues from the oil palm industry: Status and perspectives. Biomass Convers. Biorefinery 2024, 14, 3077–3099. [Google Scholar] [CrossRef]
- Vakili, M.; Rafatullah, M.; Ibrahim, M.H.; Salamatinia, B.; Gholami, Z.; Zwain, H.M. A review on composting of oil palm biomass. Environ. Dev. Sustainability 2015, 17, 691–709. [Google Scholar] [CrossRef]
- Sundu, B.; Kumar, A.; Dingle, J. Palm kernel meal in broiler diets: Effect on chicken performance and health. Worlds Poult. Sci. J. 2006, 62, 316–325. [Google Scholar] [CrossRef]
- Cheah, W.Y.; Siti-Dina, R.P.; Leng, S.T.K.; Er, A.C.; Show, P.L. Circular bioeconomy in palm oil industry: Current practices and future perspectives. Environ. Technol. Innov. 2023, 30, 103050. [Google Scholar] [CrossRef]
- Eggleston, H.; Buendia, L.; Miwa, K.; Ngara, T.; Tanabe, K. 2006 IPCC Guidelines for National Greenhouse Gas Inventories; Institute for Global Environmental Strategies (IGES): Hayama, Japan, 2006. [Google Scholar]
- Sathitkowitchai, W.; Ayimbila, F.; Nitisinprasert, S.; Keawsompong, S. Selection of pretreatment method and mannanase enzyme to improve the functionality of palm kernel cake. J. Biosci. Bioeng. 2022, 134, 301–306. [Google Scholar] [CrossRef]
- Stein, H.H.; Casas, G.A.; Abelilla, J.J.; Liu, Y.; Sulabo, R.C. Nutritional value of high fiber co-products from the copra, palm kernel, and rice industries in diets fed to pigs. J. Anim. Sci. Biotechnol. 2015, 6, 56. [Google Scholar] [CrossRef]
- Sindhu, R.; Binod, P.; Pandey, A. Biological pretreatment of lignocellulosic biomass–An overview. Bioresour. Technol. 2016, 199, 76–82. [Google Scholar] [CrossRef]
- Hou, Y.; Wu, Z.; Dai, Z.; Wang, G.; Wu, G. Protein hydrolysates in animal nutrition: Industrial production, bioactive peptides, and functional significance. Bioact. Pept. Food 2022, 209–232. [Google Scholar] [CrossRef]
- Shahidi, F.; Kamil, Y.J. Enzymes from fish and aquatic invertebrates and their application in the food industry. Trends Food Sci. Technol. 2001, 12, 435–464. [Google Scholar] [CrossRef]
- Khubber, S.; Marti-Quijal, F.J.; Tomasevic, I.; Remize, F.; Barba, F.J. Lactic acid fermentation as a useful strategy to recover antimicrobial and antioxidant compounds from food and by-products. Curr. Opin. Food Sci. 2022, 43, 189–198. [Google Scholar] [CrossRef]
- Feng, T.; Wang, J. Oxidative stress tolerance and antioxidant capacity of lactic acid bacteria as probiotic: A systematic review. Gut Microbes 2020, 12, 1801944. [Google Scholar] [CrossRef]
- Olukomaiya, O.O.; Fernando, W.C.; Mereddy, R.; Li, X.; Sultanbawa, Y. Solid-state fermentation of canola meal with Aspergillus sojae, Aspergillus ficuum and their co-cultures: Effects on physicochemical, microbiological and functional properties. LWT 2020, 127, 109362. [Google Scholar] [CrossRef]
- Gänzle, M.G. Lactic metabolism revisited: Metabolism of lactic acid bacteria in food fermentations and food spoilage. Curr. Opin. Food Sci. 2015, 2, 106–117. [Google Scholar] [CrossRef]
- Hakim, A.H.; Zulkifli, I.; Farjam, A.S.; Awad, E.A. Feeding fermented palm kernel cake with higher levels of dietary fat improved gut bacterial population and blood lipid concentration but not the growth performance in broiler chickens. Ital. J. Anim. Sci. 2021, 20, 1671–1680. [Google Scholar] [CrossRef]
- Hammes, W.P.; Hertel, C. The genera lactobacillus and carnobacterium. Prokaryotes 2006, 4, 320–403. [Google Scholar]
- Kumar, V.; Ahluwalia, V.; Saran, S.; Kumar, J.; Patel, A.K.; Singhania, R.R. Recent developments on solid-state fermentation for production of microbial secondary metabolites: Challenges and solutions. Bioresour. Technol. 2021, 323, 124566. [Google Scholar] [CrossRef]
- Xu, D.; Ding, W.; Ke, W.; Li, F.; Zhang, P.; Guo, X. Modulation of metabolome and bacterial community in whole crop corn silage by inoculating homofermentative Lactobacillus plantarum and heterofermentative Lactobacillus buchneri. Front. Microbiol. 2019, 9, 3299. [Google Scholar] [CrossRef]
- Yu, M.; Li, Z.; Rong, T.; Tian, Z.; Deng, D.; Lu, H.; Zhang, R.; Ma, X. Integrated metagenomics-metabolomics analysis reveals the cecal microbial composition, function, and metabolites of pigs fed diets with different starch sources. Food Res. Int. 2022, 154, 110951. [Google Scholar] [CrossRef]
- Cui, Y.; Wang, X.; Yue, Y.; Du, G.; Chen, H.; Ning, M.; Yuan, Y.; Yue, T. Metagenomic features of Tibetan kefir grains and its metabolomics analysis during fermentation. LWT 2023, 175, 114502. [Google Scholar] [CrossRef]
- Tavares, E.Q.; De Souza, A.P.; Buckeridge, M.S. How endogenous plant cell-wall degradation mechanisms can help achieve higher efficiency in saccharification of biomass. J. Exp. Bot. 2015, 66, 4133–4143. [Google Scholar] [CrossRef]
- Kuhad, R.C.; Gupta, R.; Singh, A. Microbial cellulases and their industrial applications. Enzym. Res. 2011, 2011, 280696. [Google Scholar] [CrossRef]
- Sawatdeenarunat, C.; Surendra, K.C.; Takara, D.; Oechsner, H.; Khanal, S.K. Anaerobic digestion of lignocellulosic biomass: Challenges and opportunities. Bioresour. Technol. 2015, 178, 178–186. [Google Scholar] [CrossRef]
- Singh, S.B. Enzyme catalysis and its role in food processing industries. In Enzymes in Food Technology: Improvements and Innovations; Springer: Berlin/Heidelberg, Germany, 2018; pp. 143–165. [Google Scholar]
- Agrawal, R.; Kumari, P.; Sivagurunathan, P.; Satlewal, A.; Kumar, R.; Gupta, R.P.; Puri, S.K. Pretreatment process and its effect on enzymatic hydrolysis of biomass. In Current Status and Future Scope of Microbial Cellulases; Elsevier: Amsterdam, The Netherlands, 2021; pp. 145–169. [Google Scholar]
- Xue, Y.; Guo, C.; Hu, F.; Liu, J.; Mao, S. Hepatic metabolic profile reveals the adaptive mechanisms of ewes to severe undernutrition during late gestation. Metabolites 2018, 8, 85. [Google Scholar] [CrossRef]
- Li, M.; Liu, M.; Wang, B.; Shi, L. Metabonomics Analysis of Stem Extracts from Dalbergia sissoo. Molecules 2022, 27, 1982. [Google Scholar] [CrossRef]
- Jia, X.; Chen, H.; Wang, X.; Nie, X.; Xiang, L.; Liu, D.; Zhao, Z. Effects of Fermentation Period on the Non-Volatile Metabolites of Chinese Ultra-Long-Term Solid Fermented Kohlrabi Based on Non-Targeted Metabolomic Analysis. Fermentation 2023, 9, 753. [Google Scholar] [CrossRef]
- Qamar, H.; Li, Y.; He, R.; Waqas, M.; Song, M.; Deng, D.; Cui, Y.; Yang, P.; Liu, Z.; Qammar, B.; et al. Integrated Metabolomics and Metagenomics Unveiled Biomarkers of Antioxidant Potential in Fermented Brewer’s Grains. Antioxidants 2024, 13, 872. [Google Scholar] [CrossRef]
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. fastp: An ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [CrossRef]
- Li, D.; Liu, C.-M.; Luo, R.; Sadakane, K.; Lam, T.-W. MEGAHIT: An ultra-fast single-node solution for large and complex metagenomics assembly via succinct de Bruijn graph. Bioinformatics 2015, 31, 1674–1676. [Google Scholar] [CrossRef]
- Hyatt, D.; Chen, G.-L.; LoCascio, P.F.; Land, M.L.; Larimer, F.W.; Hauser, L.J. Prodigal: Prokaryotic gene recognition and translation initiation site identification. BMC Bioinform. 2010, 11, 119. [Google Scholar] [CrossRef]
- Fu, L.; Niu, B.; Zhu, Z.; Wu, S.; Li, W. CD-HIT: Accelerated for clustering the next-generation sequencing data. Bioinformatics 2012, 28, 3150–3152. [Google Scholar] [CrossRef]
- Li, R.; Li, Y.; Kristiansen, K.; Wang, J. SOAP: Short oligonucleotide alignment program. Bioinformatics 2008, 24, 713–714. [Google Scholar] [CrossRef]
- Buchfink, B.; Xie, C.; Huson, D.H. Fast and sensitive protein alignment using DIAMOND. Nat. Methods 2015, 12, 59–60. [Google Scholar] [CrossRef]
- Zhu, J.; Wang, R.; Zhang, Y.; Lu, Y.; Cai, S.; Xiong, Q. Metabolomics Reveals Antioxidant Metabolites in Colored Rice Grains. Metabolites 2024, 14, 120. [Google Scholar] [CrossRef]
- Xiong, Q.; Zhang, J.; Sun, C.; Wang, R.; Wei, H.; He, H.; Zhou, D.; Zhang, H.; Zhu, J. Metabolomics revealed metabolite biomarkers of antioxidant properties and flavonoid metabolite accumulation in purple rice after grain filling. Food Chem. X 2023, 18, 100720. [Google Scholar] [CrossRef]
- Qamar, H.; Waqas, M.; Li, A.; Iqbal, M.; Mehmood, K.; Li, J. Plastrum Testudinis Extract Mitigates Thiram Toxicity in Broilers via Regulating PI3K/AKT Signaling. Biomolecules 2019, 9, 784. [Google Scholar] [CrossRef]
- Waqas, M.; Qamar, H.; Zhang, J.; Yao, W.; Li, A.; Wang, Y.; Iqbal, M.; Mehmood, K.; Jiang, X.; Li, J. Puerarin enhance vascular proliferation and halt apoptosis in thiram-induced avian tibial dyschondroplasia by regulating HIF-1α, TIMP-3 and BCL-2 expressions. Ecotoxicol. Environ. Saf. 2020, 190, 110126. [Google Scholar] [CrossRef]
- Seddik, H.A.; Bendali, F.; Gancel, F.; Fliss, I.; Spano, G.; Drider, D. Lactobacillus plantarum and its probiotic and food potentialities. Probiotics Antimicrob. Proteins 2017, 9, 111–122. [Google Scholar] [CrossRef]
- Liu, D.-M.; Huang, Y.-Y.; Liang, M.-H. Analysis of the probiotic characteristics and adaptability of Lactiplantibacillus plantarum DMDL 9010 to gastrointestinal environment by complete genome sequencing and corresponding phenotypes. LWT 2022, 158, 113129. [Google Scholar] [CrossRef]
- Yang, S.-J.; Lee, J.-E.; Lim, S.-M.; Kim, Y.-J.; Lee, N.-K.; Paik, H.-D. Antioxidant and immune-enhancing effects of probiotic Lactobacillus plantarum 200655 isolated from kimchi. Food Sci. Biotechnol. 2019, 28, 491–499. [Google Scholar] [CrossRef]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative stress: Harms and benefits for human health. Oxid. Med. Cell Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef]
- Gulcin, İ. Antioxidants and antioxidant methods: An updated overview. Arch. Toxicol. 2020, 94, 651–715. [Google Scholar] [CrossRef]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef]
- Karak, P. Biological activities of flavonoids: An overview. Int. J. Pharm. Sci. Res 2019, 10, 1567–1574. [Google Scholar]
- Calderon-Montano, J.M.; Burgos-Morón, E.; Pérez-Guerrero, C.; López-Lázaro, M. A review on the dietary flavonoid kaempferol. Mini Rev. Med. Chem. 2011, 11, 298–344. [Google Scholar] [CrossRef]
- Chen, J.; Chua, K.-W.; Chua, C.C.; Yu, H.; Pei, A.; Chua, B.H.; Hamdy, R.C.; Xu, X.; Liu, C.-F. Antioxidant activity of 7, 8-dihydroxyflavone provides neuroprotection against glutamate-induced toxicity. Neurosci. Lett. 2011, 499, 181–185. [Google Scholar] [CrossRef]
- Tsuji, P.A.; Stephenson, K.K.; Wade, K.L.; Liu, H.; Fahey, J.W. Structure-activity analysis of flavonoids: Direct and indirect antioxidant, and antiinflammatory potencies and toxicities. Nutr. Cancer 2013, 65, 1014–1025. [Google Scholar] [CrossRef]
- Costantino, L.; Rastelli, G.; Gamberini, M.C.; Vinson, J.A.; Bose, P.; Iannone, A.; Staffieri, M.; Antolini, L.; Del Corso, A.; Mura, U. 1-Benzopyran-4-one antioxidants as aldose reductase inhibitors. J. Med. Chem. 1999, 42, 1881–1893. [Google Scholar] [CrossRef]
- Gupta, A.; Marquess, A.R.; Pandey, A.K.; Bishayee, A. Jackfruit (Artocarpus heterophyllus Lam.) in health and disease: A critical review. Crit. Rev. Food Sci. Nutr. 2023, 63, 6344–6378. [Google Scholar] [CrossRef]
- Wang, J.; Fang, X.; Ge, L.; Cao, F.; Zhao, L.; Wang, Z.; Xiao, W. Antitumor, antioxidant and anti-inflammatory activities of kaempferol and its corresponding glycosides and the enzymatic preparation of kaempferol. PLoS ONE 2018, 13, e0197563. [Google Scholar] [CrossRef]
- Rüfer, C.E.; Kulling, S.E. Antioxidant activity of isoflavones and their major metabolites using different in vitro assays. J. Agric. Food Chem. 2006, 54, 2926–2931. [Google Scholar] [CrossRef]
- Heim, K.E.; Tagliaferro, A.R.; Bobilya, D.J. Flavonoid antioxidants: Chemistry, metabolism and structure-activity relationships. J. Nutr. Biochem. 2002, 13, 572–584. [Google Scholar] [CrossRef]
- Miadoková, E. Isoflavonoids—An overview of their biological activities and potential health benefits. Interdiscip. Toxicol. 2009, 2, 211–218. [Google Scholar] [CrossRef]
- Rong, J.; Fu, F.; Han, C.; Wu, Y.; Xia, Q.; Du, D. Tectorigenin: A review of its sources, pharmacology, toxicity, and pharmacokinetics. Molecules 2023, 28, 5904. [Google Scholar] [CrossRef]
- Guru, A.; Sudhakaran, G.; Velayutham, M.; Murugan, R.; Pachaiappan, R.; Mothana, R.A.; Noman, O.M.; Juliet, A.; Arockiaraj, J. Daidzein normalized gentamicin-induced nephrotoxicity and associated pro-inflammatory cytokines in MDCK and zebrafish: Possible mechanism of nephroprotection. Comp. Biochem. Physiol. Part C: Toxicol. Pharmacol. 2022, 258, 109364. [Google Scholar] [CrossRef]
- Bhuia, M.S.; Aktar, M.A.; Chowdhury, R.; Ferdous, J.; Rahman, M.A.; Al Hasan, M.S.; Islam, M.T. Therapeutic potentials of ononin with mechanistic insights: A comprehensive review. Food Biosci. 2023, 56, 103302. [Google Scholar] [CrossRef]
- Merenkova, S.; Zinina, O.; Yu, K. Microbial Fermentation of Grain Raw Materials. Prospects for Food Technology: An Analytical Review. Вестник Южнo-Уральскoгo гoсударственнoгo университета. Серия: Пищевые и биoтехнoлoгии 2021, 9, 5–15. [Google Scholar]
- Verni, M.; Verardo, V.; Rizzello, C.G. How fermentation affects the antioxidant properties of cereals and legumes. Foods 2019, 8, 362. [Google Scholar] [CrossRef]
- Yang, F.; Chen, C.; Ni, D.; Yang, Y.; Tian, J.; Li, Y.; Chen, S.; Ye, X.; Wang, L. Effects of Fermentation on Bioactivity and the Composition of Polyphenols Contained in Polyphenol-Rich Foods: A Review. Foods 2023, 12, 3315. [Google Scholar] [CrossRef]
- Gao, Y.; Zhou, H.; Wang, Y.; Nussio, L.G.; Yang, F.; Ni, K. Insights into fermentation with lactic acid bacteria on the flavonoids biotransformation of alfalfa silage. Chem. Biol. Technol. Agric. 2024, 11, 73. [Google Scholar] [CrossRef]
- Salvetti, E.; Fondi, M.; Fani, R.; Torriani, S.; Felis, G.E. Evolution of lactic acid bacteria in the order Lactobacillales as depicted by analysis of glycolysis and pentose phosphate pathways. Syst. Appl. Microbiol. 2013, 36, 291–305. [Google Scholar] [CrossRef]
- LeBlanc, J.G.; Chain, F.; Martín, R.; Bermúdez-Humarán, L.G.; Courau, S.; Langella, P. Beneficial effects on host energy metabolism of short-chain fatty acids and vitamins produced by commensal and probiotic bacteria. Microb. Cell Factories 2017, 16, 79. [Google Scholar] [CrossRef]
- Outeiriño, D.; Costa-Trigo, I.; Ochogavias, A.; Pinheiro de Souza Oliveira, R.; Pérez Guerra, N.; Salgado, J.M.; Domínguez, J.M. Biorefinery of brewery spent grain to obtain bioproducts with high value-added in the market. N. Biotechnol. 2024, 79, 111–119. [Google Scholar] [CrossRef]
- Linares, D.M.; Martín, M.; Ladero, V.; Alvarez, M.A.; Fernández, M. Biogenic Amines in Dairy Products. Crit. Rev. Food Sci. Nutr. 2011, 51, 691–703. [Google Scholar] [CrossRef]
- Paul Ross, R.; Morgan, S.; Hill, C. Preservation and fermentation: Past, present and future. Int. J. Food Microbiol. 2002, 79, 3–16. [Google Scholar] [CrossRef]
- Chai, K.F.; Voo, A.Y.H.; Chen, W.N. Bioactive peptides from food fermentation: A comprehensive review of their sources, bioactivities, applications, and future development. Compr. Rev. Food Sci. Food Saf. 2020, 19, 3825–3885. [Google Scholar] [CrossRef]
- Soriano, A.P.; Mamuad, L.L.; Kim, S.-H.; Choi, Y.J.; Jeong, C.D.; Bae, G.S.; Chang, M.B.; Lee, S.S. Effect of Lactobacillus mucosae on In vitro Rumen Fermentation Characteristics of Dried Brewers Grain, Methane Production and Bacterial Diversity. Asian-Australas J. Anim. Sci. 2014, 27, 1562–1570. [Google Scholar] [CrossRef]
- Mamuad, L.; Kim, S.; Choi, Y.; Soriano, A.; Cho, K.; Lee, K.; Bae, G.; Lee, S. Increased propionate concentration in Lactobacillus mucosae–fermented wet brewers grains and during in vitro rumen fermentation. J. Appl. Microbiol. 2017, 123, 29–40. [Google Scholar] [CrossRef]
- Hugenholtz, J. Citrate metabolism in lactic acid bacteria. FEMS Microbiol. Rev. 1993, 12, 165–178. [Google Scholar] [CrossRef]
- Makarova, K.; Slesarev, A.; Wolf, Y.; Sorokin, A.; Mirkin, B.; Koonin, E.; Pavlov, A.; Pavlova, N.; Karamychev, V.; Polouchine, N. Comparative genomics of the lactic acid bacteria. Proc. Natl. Acad. Sci. 2006, 103, 15611–15616. [Google Scholar] [CrossRef]
- Šušković, J.; Kos, B.; Beganović, J.; Leboš Pavunc, A.; Habjanič, K.; Matošić, S. Antimicrobial activity–the most important property of probiotic and starter lactic acid bacteria. Food Technol. Biotechnol. 2010, 48, 296–307. [Google Scholar]
- Bairagi, A.; Ghosh, K.; Sen, S.; Ray, A. Evaluation of the nutritive value of Leucaena leucocephala leaf meal, inoculated with fish intestinal bacteria Bacillus subtilis and Bacillus circulans in formulated diets for rohu, Labeo rohita (Hamilton) fingerlings. Aquac. Res. 2004, 35, 436–446. [Google Scholar] [CrossRef]
- Abdeshahian, P.; Samat, N.; Hamid, A.A.; Yusoff, W.M. Utilization of palm kernel cake for production of beta-mannanase by Aspergillus niger FTCC 5003 in solid substrate fermentation using an aerated column bioreactor. J. Ind. Microbiol. Biotechnol. 2010, 37, 103–109. [Google Scholar] [CrossRef]
- Wang, L.; Zhou, H.; He, R.; Xu, W.; He, G. Effects of soybean meal fermentation by Lactobacillus plantarum P8 on growth, immune responses, and intestinal morphology in juvenile turbot (Scophthalmus maximus L.). Aquaculture 2016, 464, 87–94. [Google Scholar] [CrossRef]
- Gupta, S.; Abu-Ghannam, N. Probiotic fermentation of plant based products: Possibilities and opportunities. Crit. Rev. Food Sci. Nutr. 2012, 52, 183–199. [Google Scholar] [CrossRef]
- Meng, F.-B.; Lei, Y.-T.; Li, Q.-Z.; Li, Y.-C.; Deng, Y.; Liu, D.-Y. Effect of Lactobacillus plantarum and Lactobacillus acidophilus fermentation on antioxidant activity and metabolomic profiles of loquat juice. LWT 2022, 171, 114104. [Google Scholar] [CrossRef]
- Xu, H.; Sun, J.; Zhao, Z.; Ma, X.; Li, C.; Liu, L.; Zhang, G. Lactobacillus plantarum ZLC-18 fermentation improve tyrosinase inhibition activity and antioxidant capacity in soybean hulls. Int. J. Food Sci. Technol. 2022, 57, 4518–4527. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, R.; Zhang, Y.; Yang, Y.; Sun, X.; Zhang, Q.; Yang, N. Biotransformation of phenolics and metabolites and the change in antioxidant activity in kiwifruit induced by Lactobacillus plantarum fermentation. J. Sci. Food Agric. 2020, 100, 3283–3290. [Google Scholar] [CrossRef]
- Scalbert, A.; Manach, C.; Morand, C.; Rémésy, C.; Jiménez, L. Dietary polyphenols and the prevention of diseases. Crit. Rev. Food Sci. Nutr. 2005, 45, 287–306. [Google Scholar] [CrossRef]
- Rodríguez, H.; Landete, J.M.; de las Rivas, B.; Muñoz, R. Metabolism of food phenolic acids by Lactobacillus plantarum CECT 748T. Food Chem. 2008, 107, 1393–1398. [Google Scholar] [CrossRef]
- Michlmayr, H.; Kneifel, W. β-Glucosidase activities of lactic acid bacteria: Mechanisms, impact on fermented food and human health. FEMS Microbiol. Lett. 2014, 352, 1–10. [Google Scholar] [CrossRef]
- Liang, T.; Jiang, T.; Liang, Z.; Zhang, N.; Dong, B.; Wu, Q.; Gu, B. Carbohydrate-active enzyme profiles of Lactiplantibacillus plantarum strain 84-3 contribute to flavor formation in fermented dairy and vegetable products. Food Chem. X 2023, 20, 101036. [Google Scholar] [CrossRef]
- Liu, X.; Wu, Z.; Hu, T.; Lin, X.; Liang, H.; Li, W.; Jin, X.; Xiao, L.; Fang, X.; Zou, Y. Comparative genomic analysis reveals niche adaption of Lactobacillus acidophilus. J. Appl. Microbiol. 2023, 134, lxad287. [Google Scholar] [CrossRef]
- Li, Z.; Li, Y.; Xiao, C.; Yan, Z.; Pan, R.; Gao, Y.; Li, B.; Wei, J.; Qiu, Y.; Liu, K. Genomic and metabolic features of the Lactobacillus sakei JD10 revealed potential probiotic traits. Microbiol. Res. 2022, 256, 126954. [Google Scholar] [CrossRef]
- Kostrzynska, M.; Bachand, A. Use of microbial antagonism to reduce pathogen levels on produce and meat products: A review. Can. J. Microbiol. 2006, 52, 1017–1026. [Google Scholar] [CrossRef]
- Kaoutari, A.E.; Armougom, F.; Gordon, J.I.; Raoult, D.; Henrissat, B. The abundance and variety of carbohydrate-active enzymes in the human gut microbiota. Nat. Rev. Microbiol. 2013, 11, 497–504. [Google Scholar] [CrossRef]
- Rodríguez, H.; Curiel, J.A.; Landete, J.M.; de las Rivas, B.; de Felipe, F.L.; Gómez-Cordovés, C.; Mancheño, J.M.; Muñoz, R. Food phenolics and lactic acid bacteria. Int. J. Food Microbiol. 2009, 132, 79–90. [Google Scholar] [CrossRef]
- Akbari, B.; Baghaei-Yazdi, N.; Bahmaie, M.; Mahdavi Abhari, F. The role of plant-derived natural antioxidants in reduction of oxidative stress. Biofactors 2022, 48, 611–633. [Google Scholar] [CrossRef]
- Ryu, J.Y.; Kang, H.R.; Cho, S.K. Changes over the fermentation period in phenolic compounds and antioxidant and anticancer activities of blueberries fermented by Lactobacillus plantarum. J. Food Sci. 2019, 84, 2347–2356. [Google Scholar] [CrossRef]
- Verni, M.; Pontonio, E.; Krona, A.; Jacob, S.; Pinto, D.; Rinaldi, F.; Verardo, V.; Díaz-de-Cerio, E.; Coda, R.; Rizzello, C.G. Bioprocessing of brewers’ spent grain enhances its antioxidant activity: Characterization of phenolic compounds and bioactive peptides. Front. Microbiol. 2020, 11, 1831. [Google Scholar] [CrossRef]
- Mahajan, G.; Sharma, V.; Ray, R.C.; Gupta, R. 10—Lactic acid bacteria as potential sources of enzymes: From genes to industrial applications. In Lactic Acid Bacteria as Cell Factories; Montet, D., Ray, R.C., De Carvalho Azevedo, V.A., Paramithiotis, S., Eds.; Woodhead Publishing: Amsterdam, The Netherlands, 2023; pp. 183–197. [Google Scholar]
- Filannino, P.; Di Cagno, R.; Gobbetti, M. Metabolic and functional paths of lactic acid bacteria in plant foods: Get out of the labyrinth. Curr. Opin. Biotechnol. 2018, 49, 64–72. [Google Scholar] [CrossRef]
- Ezejiofor, T.I.N.; Enebaku, U.E.; Ogueke, C. Waste to wealth-value recovery from agro-food processing wastes using biotechnology: A review. Br. Biotechnol. J. 2014, 4, 418–481. [Google Scholar] [CrossRef]
- Hammes, W.P.; Brandt, M.J.; Francis, K.L.; Rosenheim, J.; Seitter, M.F.; Vogelmann, S.A. Microbial ecology of cereal fermentations. Trends Food Sci. Technol. 2005, 16, 4–11. [Google Scholar] [CrossRef]
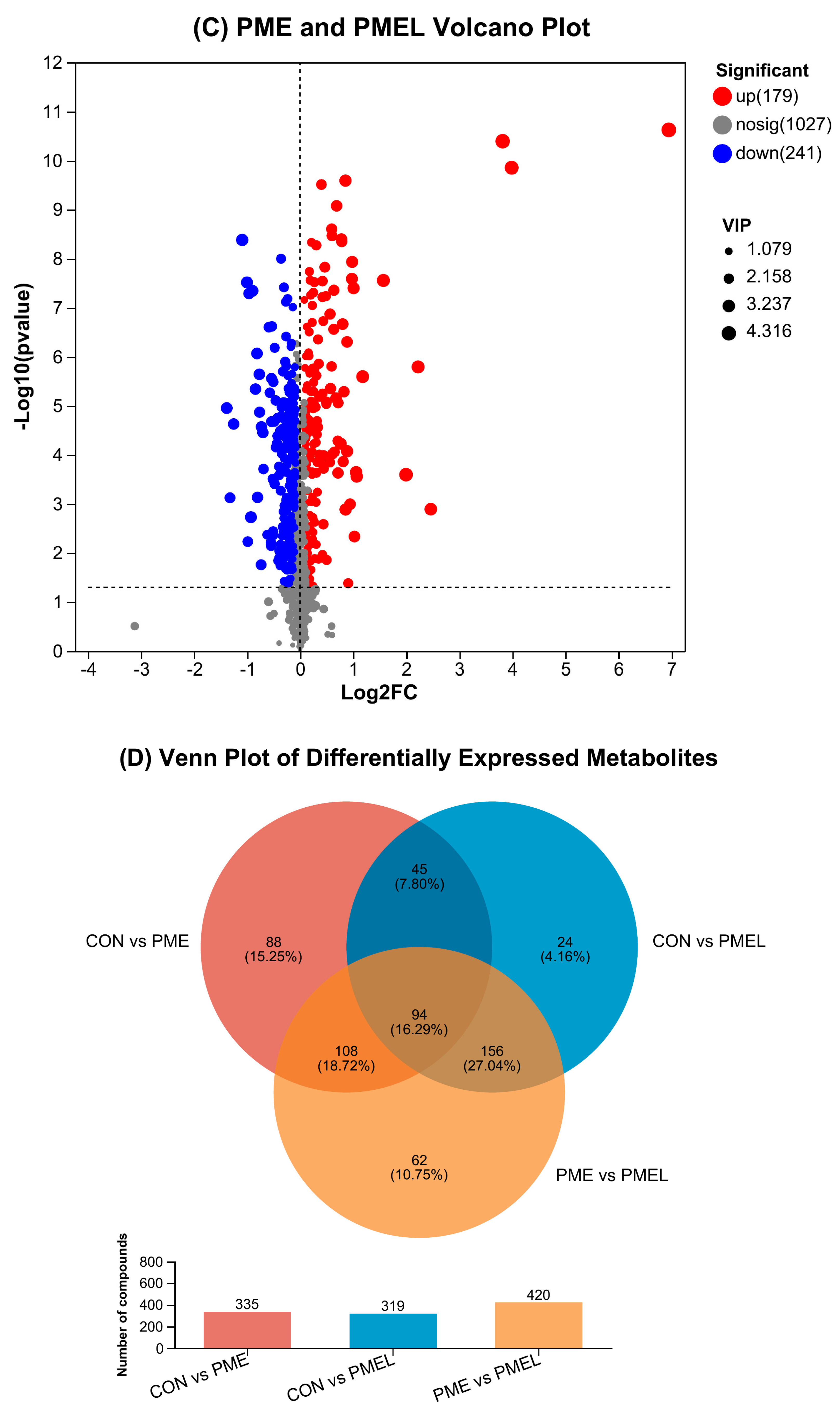
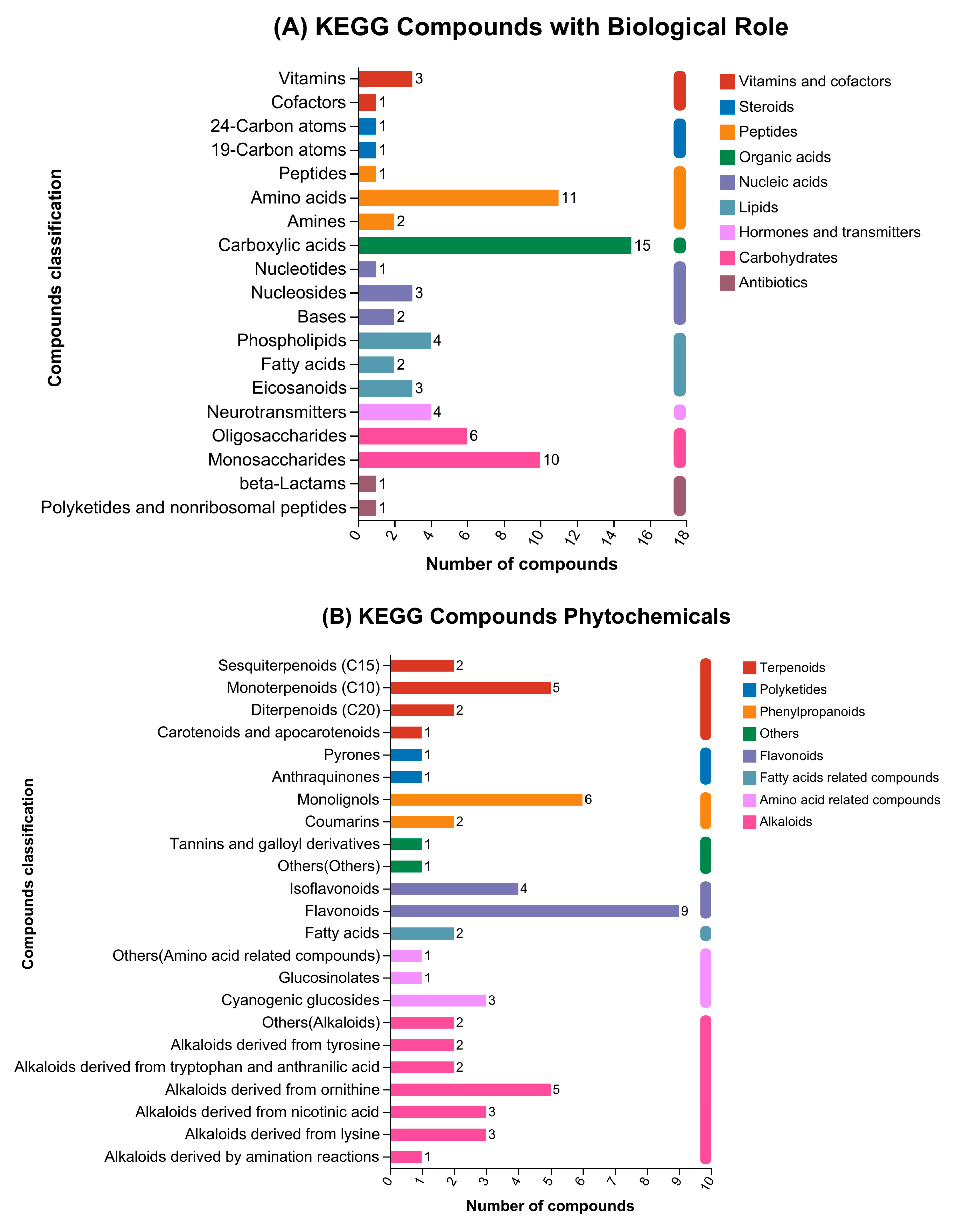


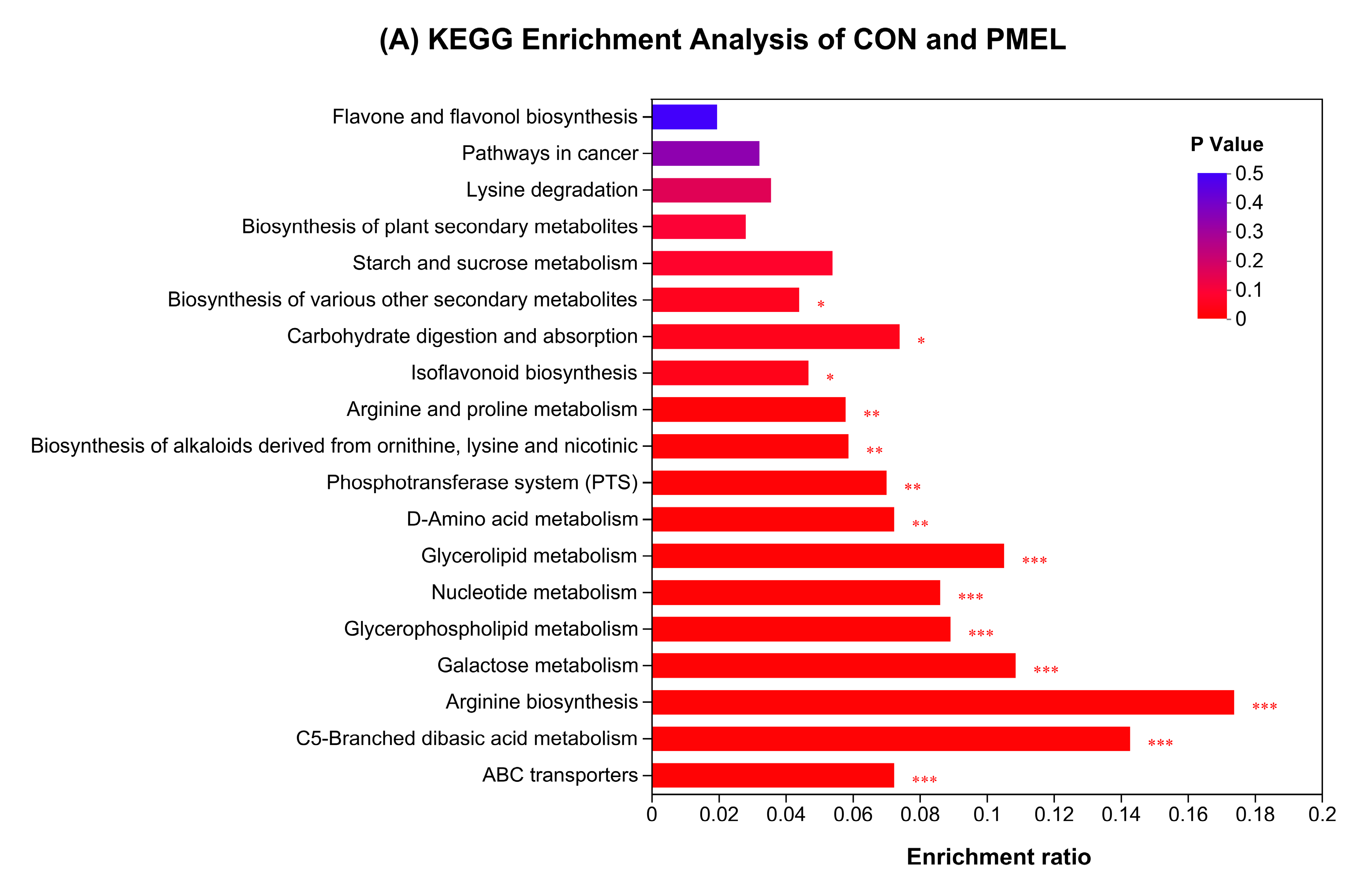
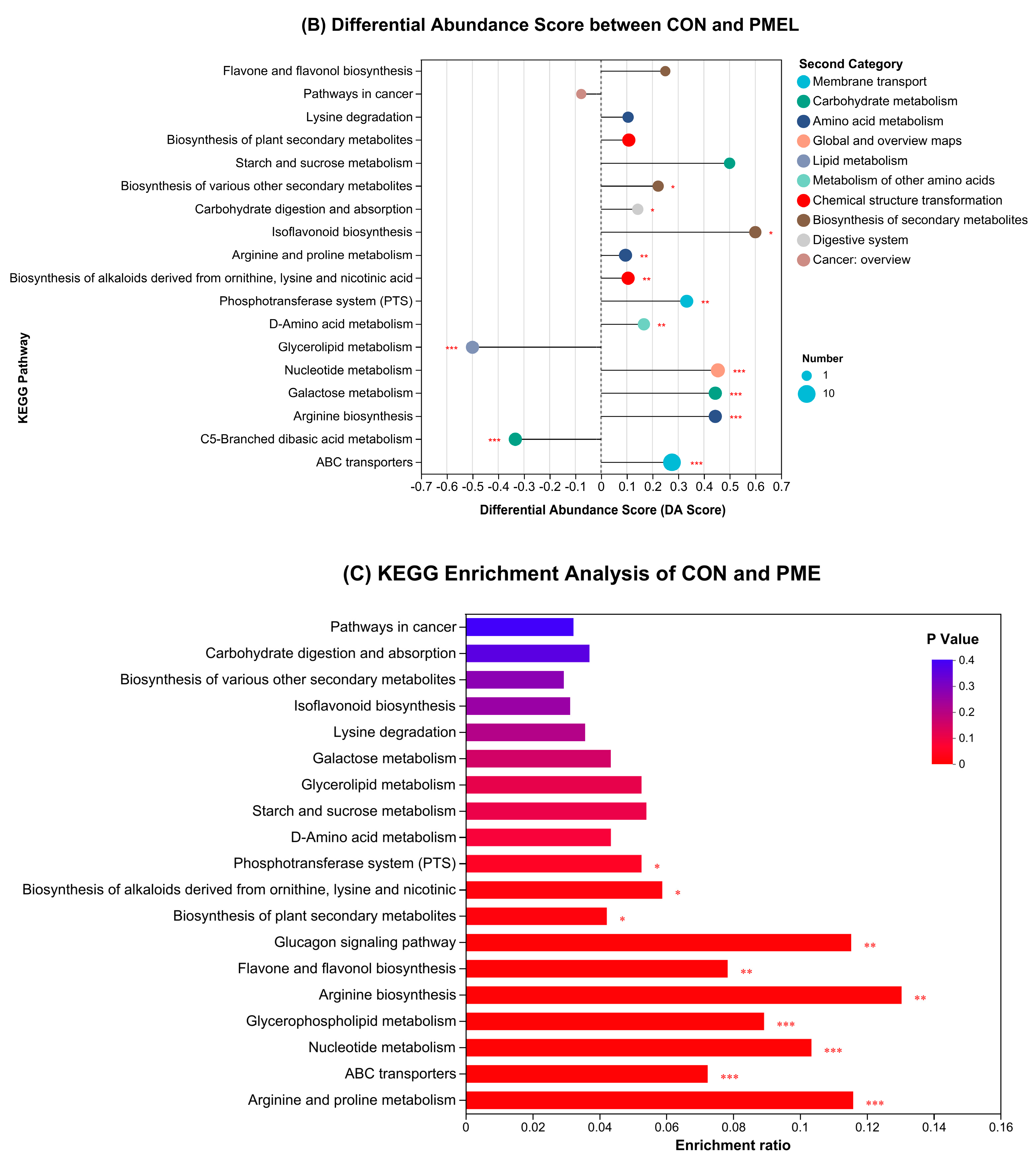


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qamar, H.; He, R.; Li, Y.; Song, M.; Deng, D.; Cui, Y.; Yu, M.; Ma, X. Metabolome and Metagenome Integration Unveiled Synthesis Pathways of Novel Antioxidant Peptides in Fermented Lignocellulosic Biomass of Palm Kernel Meal. Antioxidants 2024, 13, 1253. https://doi.org/10.3390/antiox13101253
Qamar H, He R, Li Y, Song M, Deng D, Cui Y, Yu M, Ma X. Metabolome and Metagenome Integration Unveiled Synthesis Pathways of Novel Antioxidant Peptides in Fermented Lignocellulosic Biomass of Palm Kernel Meal. Antioxidants. 2024; 13(10):1253. https://doi.org/10.3390/antiox13101253
Chicago/Turabian StyleQamar, Hammad, Rong He, Yuanfei Li, Min Song, Dun Deng, Yiyan Cui, Miao Yu, and Xianyong Ma. 2024. "Metabolome and Metagenome Integration Unveiled Synthesis Pathways of Novel Antioxidant Peptides in Fermented Lignocellulosic Biomass of Palm Kernel Meal" Antioxidants 13, no. 10: 1253. https://doi.org/10.3390/antiox13101253
APA StyleQamar, H., He, R., Li, Y., Song, M., Deng, D., Cui, Y., Yu, M., & Ma, X. (2024). Metabolome and Metagenome Integration Unveiled Synthesis Pathways of Novel Antioxidant Peptides in Fermented Lignocellulosic Biomass of Palm Kernel Meal. Antioxidants, 13(10), 1253. https://doi.org/10.3390/antiox13101253






