Verbascoside-Rich Plant Extracts in Animal Nutrition
Abstract
:1. Introduction
2. Biological Effects of Verbascoside
2.1. Antioxidant Activity
2.2. Anti-inflammatory Activity
2.3. Antibacterial Activity
2.4. Other Biological Activities
3. Verbascoside-Rich Plant Extracts in Animal Nutrition
3.1. Monogastric Animals
3.1.1. Pig
3.1.2. Hares
3.1.3. Rabbit
| Supplement | Dose mg/kg Feed | Animal | Effect on Antioxidant Parameters | Treatment vs. Control, % | Ref. |
|---|---|---|---|---|---|
| Verbenaceae extract (Lippia Citriodora) | 5 | New Zealand White × Californian rabbit 80 d | Improved of blood | [80] | |
| TBARS | −41.04 ** | ||||
| ROMs | −31.71 ** | ||||
| Vitamin A | +65.98 ** | ||||
| Vitamin E | +92.18 ** | ||||
| Verbenaceae extract (Lippia Citriodora) | 5 10 | New Zealand White rabbit 80 d | Improved of muscle | [81] | |
| TBARS (d 7) | −37 ** | ||||
| −34 ** | |||||
| Vitamin E | +21.42 | ||||
| +71.42 | |||||
| (p = 0.07) | |||||
| Verbenaceae extract (Lippia Citriodora) | 5 | New Zealand White × Californian rabbit 80 d | Improved of muscle | [83] | |
| TBARS (72 h) | −47.73 ** | ||||
| Vitamin A | +76.74 * | ||||
| Vitamin E | +30,332 * |
3.1.4. Equidae
3.1.5. Poultry
3.1.6. Fish
3.2. Ruminants
Small Ruminants
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Achilonu, M.; Shale, K.; Arthur, G.; Naidoo, K.; Mbatha, M. Phytochemical Benefits of Agroresidues as Alternative Nutritive Dietary Resource for Pig and Poultry Farming. J. Chem. 2018, 2018, 1035071. [Google Scholar] [CrossRef]
- Badyal, S.; Singh, H.; Yadav, A.K.; Sharma, S.; Bhushan, I. Plant secondary metabolites and their uses. Plant Archiv. 2020, 20, 3336–3340. [Google Scholar]
- Velu, G.; Palanichamy, V.; Rajan, A.P. Phytochemical and Pharmacological Importance of Plant Secondary Metabolites in Modern Medicine. In Bioorganic Phase in Natural Food: An Overview; Roopan, S.M., Madhumitha, G., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 135–156. [Google Scholar]
- Mahfuz, S.; Shang, Q.; Piao, X. Phenolic compounds as natural feed additives in poultry and swine diets: A review. J. Anim. Sci. Biotechnol. 2021, 12, 48. [Google Scholar] [CrossRef] [PubMed]
- Ignat, I.; Volf, I.; Popa, V.I. A critical review of methods for characterisation of polyphenolic compounds in fruits and vegetables. Food Chem. 2011, 126, 1821–1835. [Google Scholar] [CrossRef]
- Lipiński, K.; Mazur, M.; Antoszkiewicz, Z.; Purwin, C. Polyphenols in monogastric nutrition—A review. Ann. Anim. Sci. 2017, 17, 41–58. [Google Scholar] [CrossRef]
- Scarpati, M.L.; Monache, D. Isolation from Verbascum sinuatum of two new glucosides, verbascoside and isoverbascoside. Ann. Chim. 1963, 53, 356–367. [Google Scholar]
- Birkofer, L.; Kaiser, C.; Thomas, U. Acteosid und Neoacteosid; Zuckerester aus Syringa vulgaris (L.). Z. Für Naturforschung 1968, 23b, 1051–1058. [Google Scholar] [CrossRef]
- Alipieva, K.I.; Orhan, I.E.; Cankaya, I.I.; Kostadinova, E.P.; Georgiev, M.I. Treasure from garden: Chemical profiling, pharmacology and biotechnology ofmulleins. Phytochem. Rev. 2014, 13, 417–444. [Google Scholar] [CrossRef]
- Schlauer, J.; Budzianowski, J.; Kukulczanka, K.; Ratajczak, L. Acteoside and related phenylethanoid glycosides in Byblis liniflora Salisb. plants propagated in vitro and its systematic significance. Acta Soc. Bot. Pol. 2004, 73, 9–15. [Google Scholar] [CrossRef]
- Taskova, R.M.; Gotfredsen, C.H.; Jensen, S.R. Chemotaxonomic markers in Digitalideae (Plantaginaceae). Phytochemistry 2005, 66, 1440–1447. [Google Scholar] [CrossRef]
- Bied, H.K.; Bedgood, D.R., Jr.; Prenzler, P.D.; Robards, K. Bioscreening of Australian olive mill waste extracts: Biophenol content, antioxidant, antimicrobial and molluscicidal activities. Food Chem. Toxicol. 2007, 45, 1238–1248. [Google Scholar]
- De Marco, E.; Savarese, M.; Paduano, A.; Sacchi, R. Characterization and fractionation of phenolic compounds extracted from olive oil mill wastewaters. Food Chem. 2007, 104, 858–867. [Google Scholar] [CrossRef]
- Reid, A.M.; Juvonen, R.; Huuskonen, P.; Lehtonen, M.; Pasanen, M.; Lall, N. In Vitro Human Metabolism and Inhibition Potency of Verbascoside for CYP Enzymes. Molecules 2019, 24, 2191. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Ren, Q.; Wu, L. The pharmacokinetic property and pharmacological activity of acteoside: A review. Biomed. Pharmacother. 2022, 153, 113296. [Google Scholar] [CrossRef]
- Zhang, W.; Huo, S.X.; Wen, Y.L.; Xing, H.; Chen, X.J. Pharmacokinetics of acteoside following single dose intragastric and intravenous administrations in dogs. Chin. J. Nat. Med. 2015, 13, 634–640. [Google Scholar] [CrossRef]
- Xue, Z.; Yang, B. Phenylethanoid Glycosides: Research Advances in Their Phytochemistry, Pharmacological Activity and Pharmacokinetics. Molecules 2016, 21, 991. [Google Scholar] [CrossRef]
- Cui, Q.; Pan, Y.; Xu, X.; Zhang, W.; Wu, X.; Qu, S.; Liu, X. The metabolic profile of acteoside produced by human or rat intestinal bacteria or intestinal enzyme in vitro employed UPLC-Q-TOF-MS. Fitoterapia 2016, 109, 67–74. [Google Scholar] [CrossRef]
- Qi, M.; Xiong, A.; Li, P.; Yang, Q.; Yang, L.; Wang, Z. Identification of acteoside and its major metabolites in rat urine by ultra-performance liquid chromatography combined with electrospray ionization quadrupole time-of-flight tandem mass spectrometry. J. Chromatogr. B 2013, 940, 77–85. [Google Scholar] [CrossRef]
- Su, D.; Li, W.; Xu, Q.M.; Liu, Y.L.; Song, Y.G.; Feng, Y.L. New metabolites of acteoside identified by ultra-performance liquid chromatography/quadrupole-time-of-flight MSE in rat plasma, urine, and feces. Fitoterapia 2016, 112, 45–55. [Google Scholar] [CrossRef]
- Kallingal, A.; Thachan Kundil, V.; Ayyolath, A.; Karlapudi, A.P.; Muringayil Joseph, T.E.; Jayadevi, V. Molecular modeling study of tectoquinone and acteoside from Tectona grandis linn: A new SARS-CoV-2 main protease inhibitor against COVID-19. J. Biomol. Struct. Dyn. 2020, 40, 1764–1775. [Google Scholar] [CrossRef]
- Etemad, L.; Zafari, R.; Vahdati-Mashhadian, N.; Adel Moallem, S.; Shirvan, Z.O.; Hosseinzadeh, H. Acute, sub-acute and cell toxicity of verbascoside. Iran. J. Pharm. Res. 2016, 15, 521–525. [Google Scholar] [PubMed]
- Etemad, L.; Shirvan, Z.O.; Vahdati-Mashhadian, N.; Moallem, S.A.; Zafari, R.; Hosseinzadeh, H. Acute, subacute, and cell toxicity of the aqueous extract of Lippia citriodora. Jundishapur. J. Nat. Pharm. Prod. 2016, 11, e32546. [Google Scholar] [CrossRef]
- Cheimonidi, C.; Samara, P.; Polychronopoulos, P.; Tsakiri, E.N.; Nikou, T.; Myrianthopoulos, V.; Sakellaropoulos, T.; Zoumpourlis, V.; Mikros, E.; Papassideri, I.; et al. Selective cytotoxicity of the herbal substance acteoside against tumor cells and its mechanistic insights. Redox Biol. 2018, 16, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Gutteridge, J.M. Free radicals in disease processes: A compilation of cause and consequence. Free Radic. Res. Commun. 1993, 19, 141–158. [Google Scholar] [CrossRef] [PubMed]
- Alipieva, K.; Korkina, L.; Orhan, I.E.; Georgiev, M.I. Verbascoside—A review of its occurrence, (bio)synthesis and pharmacological significance. Biotechnol. Adv. 2014, 32, 1065–1076. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.H.; Shiou, L.Y.; Chien, M.Y.; Hou, W.; Hu, M. Antioxidant and antihypertensive activities of acteoside and its analogs. Bot. Stud. 2012, 53, 421–429. [Google Scholar]
- Burgos, C.; Muñoz-Mingarro, D.; Navarro, I.; Martín-Cordero, C.; Acero, N. Neuroprotective Potential of Verbascoside Isolated from Acanthus mollis L. Leaves through Its Enzymatic Inhibition and Free Radical Scavenging Ability. Antioxidants 2020, 9, 1207. [Google Scholar] [CrossRef]
- Potapovich, A.I.; Lulli, D.; Fidanza, P.; Kostyuk, V.A.; De Luca, C.; Pastore, S.; Korkina, L.G. Plant polyphenols differentially modulate inflammatory responses of human keratinocytes by interfering with activation of transcription factors NFκB and AhR and EGFR-ERK pathway. Toxicol. Appl. Pharmacol. 2011, 255, 138–149. [Google Scholar] [CrossRef]
- Pastore, S.; Lulli, D.; Fidanza, P.; Potapovich, A.I.; Kostyuk, V.A.; De Luca, C. Plant polyphenols regulate chemokine expression and tissue repair in human keratinocytes through interaction with cytoplasmic and nuclear components of epidermal growth factor receptor system. Antioxid. Redox Signal. 2012, 16, 314–328. [Google Scholar] [CrossRef]
- Anfuso, C.D.; Giurdanella, G.; Longo, A.; Cosentino, A.; Agafonova, A.; Rusciano, D.; Lupo, G. Antioxidant Activity of Cyanidin-3-O-Glucoside and Verbascoside in an in Vitro Model of Diabetic Retinopathy. Front. Biosci. 2022, 27, 308. [Google Scholar] [CrossRef]
- Kwiecień, I.; Miceli, N.; D’Arrigo, M.; Marino, A.; Ekiert, H. Antioxidant Potential and Enhancement of Bioactive Metabolite Production in In Vitro Cultures of Scutellaria lateriflora L. by Biotechnological Methods. Molecules 2022, 27, 1140. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Wang, S.; Pan, J.; Ma, K. Verbascoside: A neuroprotective phenylethanoid glycosides with anti-depressive properties. Phytomedicine 2023, 120, 155027. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.Y.; Jiang, J.G.; Yang, L.; Wang, D.W.; Zhu, W. Anti-ageing active ingredients from herbs and nutraceuticals used in traditional Chinese medicine: Pharmacological mechanisms and implications for drug discovery. Br. J. Pharm. 2017, 174, 1395–1425. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Qin, S.; Zhao, S. Osteoarthritis is Prevented in Rats by Verbascoside via Nuclear Factor kappa B (NF-κB) Pathway Downregulation. Med. Sci. Monit. 2020, 6, e921276. [Google Scholar] [CrossRef] [PubMed]
- Moon, E.Y.; Lee, J.H.; Lee, J.W.; Song, J.H.; Pyo, S. ROS/Epac1- mediated Rap1/NF-kappaB Activation Is Required for the Expression of BAFF in Raw264.7 Murine Macrophages. Cell Signal. 2011, 23, 1479–1488. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.H.; Chuang, H.C.; Hsiao, G.; Hou, T.Y.; Wang, C.C.; Huang, S.C.; Li, B.Y.; Lee, Y.L. Acteoside exerts immunomodulatory effects on dendritic cells via aryl hydrocarbon receptor activation and ameliorates Th2-mediated allergic asthma by inducing Foxp3+ regulatory T cells. Int. Immunopharmacol. 2022, 106, 108603. [Google Scholar] [CrossRef] [PubMed]
- Song, H.S.; Choi, M.Y.; Ko, M.S.; Jeong, J.M.; Kim, Y.H.; Jang, B.H.; Sung, J.H.; Kim, M.G.; Whang, W.K.; Sim, S.S. Competitive inhibition of cytosolic Ca2+- dependent phospholipase A2 by acteoside in RBL-2H3 cells. Arch. Pharm. Res. 2012, 35, 905–910. [Google Scholar] [CrossRef]
- Childs, C.E.; Calder, P.C.; Miles, E.A. Diet and Immune Function. Nutrients 2019, 11, 1933. [Google Scholar] [CrossRef]
- Hagihara, M.; Crandon, J.L.; Nicolau, D.P. The efficacy and safety of antibiotic combination therapy for infections caused by Gram-positive and Gram-negative organisms. Expert Opin. Drug Saf. 2012, 11, 221–233. [Google Scholar] [CrossRef]
- Álvarez-Martínez, F.J.; Barrajón-Catalán, E.; Micol, V. Tackling Antibiotic Resistance with Compounds of Natural Origin: A Comprehensive Review. Biomedicines 2020, 8, 405. [Google Scholar] [CrossRef]
- Ghaemi, E.O.; Khorshidi, D.; Moradi, A.; Seifi, A.; Mazendrani, M.; Bazouri, M.; Mansourian, A.R. The efficacy of ethanolic extract of Lemon verbena on the skin infection due to Staphylococcus aureus in an animal model. Pak. J. Biol. Sci. 2007, 10, 4132–4135. [Google Scholar] [CrossRef] [PubMed]
- Agampodi, V.A.; Katavic, P.; Collet, C.; Collet, T. Antibacterial and Anti-inflammatory Activity of Extracts and Major Constituents Derived from Stachytarpheta indica Linn. Leaves and Their Potential Implications for Wound Healing. Appl. Biochem. Biotechnol. 2022, 194, 6213–6254. [Google Scholar] [CrossRef] [PubMed]
- Friščić, M.; Petlevski, R.; Kosalec, I.; Madunić, J.; Matulić, M.; Bucar, F.; Hazler Pilepić, K.; Maleš, Ž. Globularia alypum L. and Related Species: LC-MS Profiles and Antidiabetic, Antioxidant, Anti-Inflammatory, Antibacterial and Anticancer Potential. Pharmaceuticals 2022, 15, 506. [Google Scholar] [CrossRef] [PubMed]
- Fazly Bazzaz, B.S.; Khameneh, B.; Zahedian Ostad, M.R.; Hosseinzadeh, H. In vitro evaluation of antibacterial activity of verbascoside, Lemon verbena extract and caffeine in combination with gentamicin against drug-resistant Staphylococcus aureus and Escherichia coli clinical isolates. Avicenna J. Phytomed. 2018, 8, 246–253. [Google Scholar] [PubMed]
- Shi, C.; Ma, Y.; Tian, L.; Li, J.; Qiao, G.; Liu, C.; Cao, W.; Liang, C. Verbascoside: An Efficient and Safe Natural Antibacterial Adjuvant for Preventing Bacterial Contamination of Fresh Meat. Molecules 2022, 27, 4943. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.Q.; Zhou, W.Y.; Feng, Y.; Li, Y.; Liu, K.; Liu, L.Z.; Lin, D.X.; He, Z.D.; Wu, X.L. Acteoside and acyl-migrated acteoside, compounds in Chinese Kudingcha tea, inhibit alpha-amylase in vitro. J. Med. Food 2017, 20, 577–585. [Google Scholar] [CrossRef] [PubMed]
- Shimada, H.; Urabe, Y.; Okamoto, Y.; Li, Z.; Kawase, A.; Morikawa, T.; Tu, P.; Muraoka, O.; Iwaki, M. Major constituents of Cistanche tubulosa, echinacoside and acteoside, inhibit sodium-dependent glucose cotransporter 1-mediated glucose uptake by intestinal epithelial cells. J. Funct. Foods 2017, 39, 91–95. [Google Scholar] [CrossRef]
- Khan, R.A.; Hossain, R.; Roy, P.; Jain, D.; Mohammad Saikat, A.S.; Roy Shuvo, A.P.; Akram, M.; Elbossaty, W.F.; Khan, I.N.; Painuli, S.; et al. Anticancer effects of acteoside: Mechanistic insights and therapeutic status. Eur. J. Pharm. 2022, 916, 174699. [Google Scholar] [CrossRef]
- Chen, S.; Liu, H.; Wang, S.; Jiang, H.; Gao, L.; Wang, L.; Teng, L.; Wang, C.; Wang, D. The Neuroprotection of Verbascoside in Alzheimer’s Disease Mediated through Mitigation of Neuroinflammation via Blocking NF-κB-p65 Signaling. Nutrients 2022, 14, 1417. [Google Scholar] [CrossRef]
- Wang, C.; Cai, X.; Wang, R.; Zhai, S.; Zhang, Y.; Hu, W.; Zhang, Y.; Wang, D. Neuroprotective effects of verbascoside against Alzheimer’s disease via the relief of endoplasmic reticulum stress in A beta-exposed U251 cells and APP/PS1 mice. J. Neuroinflamm. 2020, 17, 309. [Google Scholar] [CrossRef]
- Jaramillo-Morales, O.A.; Díaz-Cervantes, E.; Via, L.D.; Garcia-Argaez, A.N.; Espinosa-Juárez, J.V.; Ovando-Zambrano, J.C.; Muñoz-Pérez, V.M.; Valadez-Vega, C.; Bautista, M. Hepatoprotective Activity, In Silico Analysis, and Molecular Docking Study of Verbascoside from Leucophyllum frutescens in Rats with Post-Necrotic Liver Damage. Sci. Pharm. 2023, 91, 40. [Google Scholar] [CrossRef]
- Christaki, E.; Giannenas, I.; Bonos, E.; Bonos, E.; Florou-Paneri, P. Innovative uses of aromatic plants as natural supplements in nutrition. In Feed Additives: Aromatic Plants and Herbs in Animal Nutrition and Health; Florou-Paneri, P., Christaki, E., Giannenas, I., Eds.; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Puppel, K.; Kapusta, A.; Kuczyńska, B. The etiology of oxidative stress in the various species of animals, a review. J. Sci. Food Agric. 2015, 95, 2179–2184. [Google Scholar] [CrossRef] [PubMed]
- Lykkesfeldt, J.; Svendsen, O. Oxidants and antioxidants in disease: Oxidative stress in farm animals. Vet. J. 2007, 173, 502–511. [Google Scholar] [CrossRef] [PubMed]
- Buchet, A.; Belloc, C.; Leblanc-Maridor, M.; Merlot, E. Effects of age and weaning conditions on blood indicators of oxidative status in pigs. PLoS ONE 2017, 12, e0178487. [Google Scholar] [CrossRef] [PubMed]
- Tufarelli, V.; Colonna, M.A.; Losacco, C.; Puvača, N. Biological Health Markers Associated with Oxidative Stress in Dairy Cows during Lactation Period. Metabolites 2023, 13, 405. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Xing, M.; Gu, X. Research Progress on Oxidative Stress and Its Nutritional Regulation Strategies in Pigs. Animals 2021, 11, 1384. [Google Scholar] [CrossRef] [PubMed]
- Sejian, V.; Bhatta, R.; Gaughan, J.B.; Dunshea, F.R.; Lacetera, N. Review: Adaptation of animals to heat stress. Animal 2018, 12, S431–S444. [Google Scholar] [CrossRef]
- Gonzalez-Rivas, P.A.; Chauhan, S.S.; Ha, M.; Fegan, N.; Dunshea, F.R.; Warner, R.D. Effects of heat stress on animal physiology, metabolism, and meat quality: A review. Meat Sci. 2020, 162, 108025. [Google Scholar] [CrossRef]
- Manuelian, C.L.; Pitino, R.; Simoni, M.; Mavrommatis, A.; De Marchi, M.; Righi, F.; Tsiplakou, E. Plant Feed Additives as Natural Alternatives to the Use of Synthetic Antioxidant Vitamins on Livestock Mammals’ Performances, Health, and Oxidative Status: A Review of the Literature in the Last 20 Years. Antioxidants 2021, 10, 1461. [Google Scholar] [CrossRef]
- Di Giancamillo, A.; Rossi, R.; Pastorelli, G.; Deponti, D.; Carollo, V.; Casamassima, D.; Domeneghini, C.; Corino, C. The effects of dietary verbascoside on blood and liver oxidative stress status induced by a high n-6 polyunsaturated fatty acids diet in piglets. J. Anim. Sci. 2015, 93, 2849–2859. [Google Scholar] [CrossRef]
- Campbell, J.M.; Crenshaw, J.D.; Polo, J. The biological stress of early weaned piglets. J. Anim. Sci. Biotechnol. 2013, 4, 19. [Google Scholar] [CrossRef] [PubMed]
- Maina, S.; Misinzo, G.; Bakari, G.; Kim, H.-Y. Human, Animal and Plant Health Benefits of Glucosinolates and Strategies for Enhanced Bioactivity: A Systematic Review. Molecules 2020, 25, 3682. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Espinosa, C.D.; Abelilla, J.J.; Casas, G.A.; Lagos, L.V.; Lee, S.A.; Kwon, W.B.; Mathai, J.K.; Navarro, D.M.D.L.; Jaworski, N.W.; et al. Non-antibiotic feed additives in diets for pigs: A review. Anim. Nutr. 2018, 4, 113–125. [Google Scholar] [CrossRef] [PubMed]
- Pastorelli, G.; Rossi, R.; Corino, C. Influence of Lippia citriodora verbascoside on growth performance, antioxidant status, and serum immunoglobulins content in piglets. Czech J. Anim. Sci. 2012, 57, 312–322. [Google Scholar] [CrossRef]
- Rossi, R.; Pastorelli, G.; Corino, C. Application of KRL test to assess total antioxidant activity in pigs: Sensitivity to dietary antioxidants. Res. Vet. Sci. 2013, 94, 372–377. [Google Scholar] [CrossRef] [PubMed]
- Rossi, R.; Corino, C.; Modina, S.; Di Giancamillo, A. Dietary verbascoside influences gut morphology and the expression of α-Transducin and α-Gustducin in the small intestine of weaned piglets exposed to n-6 polyunsaturated fatty acids-induced oxidative stress. Animals 2019, 9, 20. [Google Scholar] [CrossRef] [PubMed]
- Di Giancamillo, A.; Rossi, R.; Vitari, F.; Carollo, V.; Deponti, D.; Corino, C.; Domeneghini, C. Changes in nitrosative stress biomarkers in swine intestine following dietary intervention with verbascoside. Histol. Histopathol. 2013, 28, 715–723. [Google Scholar]
- Zheng, R.; Bowei, J.; Lin, C.; Qiang, H.; Limei, W.; Ruoyu, G.; Yucheng, L.; Junping, H.; Jianhua, Y. Microbiome-metabolomics analysis reveals the potential effect of verbascoside in alleviating cognitive impairment in db/db mice. Food Funct. 2023, 14, 3488–3508. [Google Scholar]
- Rossi, R.; Pastorelli, G.; Cannata, S.; Tavaniello, S.; Maiorano, G.; Corino, C. Effect of long term dietary supplementation with plant extract on carcass characteristics meat quality and oxidative stability in pork. Meat Sci. 2013, 95, 542–548. [Google Scholar] [CrossRef]
- Rossi, R.; Ratti, S.; Pastorelli, G.; Crotti, A.; Corino, C. The effect of dietary vitamin E and verbascoside on meat quality and oxidative stability of muscle in medium-heavy pigs. Food Res. Int. 2014, 65, 88–94. [Google Scholar] [CrossRef]
- Rossi, R.; Stella, S.; Ratti, S.; Maghin, F.; Tirloni, E.; Corino, C. Effects of antioxidant mixtures in the diet of finishing pigs on the oxidative status and shelf life of Longissimus dorsi muscle packaged under modified atmosphere. J. Anim. Sci. 2017, 11, 4986–4997. [Google Scholar] [CrossRef] [PubMed]
- Pastorelli, G.; Rossi, R.; Ratti, S.; Corino, C. Plant extracts in heavy pig feeding: Effects on quality of meat and Cremona salami. Anim. Prod. Sci. 2016, 56, 1199–1207. [Google Scholar] [CrossRef]
- Rossi, R.; Ratti, S.; Moretti, V.M.; Vasconi, M.; Corino, C. Sensory characteristics and volatile compounds of dry cured ham Speck are affected by pig dietary supplementation with antioxidant mixture. J. Sci. Food Agric. 2021, 101, 1134–1142. [Google Scholar] [CrossRef] [PubMed]
- Serra, V.; Salvatori, G.; Pastorelli, G. Dietary Polyphenol Supplementation in Food Producing Animals: Effects on the Quality of Derived Products. Animals 2021, 11, 401. [Google Scholar] [CrossRef] [PubMed]
- Casamassima, D.; Palazzo, M.; Vizzarri, F.; Cinone, M.; Corino, C. Effect of dietary phenylpropanoid glycoside-based natural extracts on blood parameters and productive performance in intensively-reared young hares. Czech J. Anim. Sci. 2013, 58, 270–278. [Google Scholar] [CrossRef]
- Vizzarri, F.; Nardoia, M.; Palazzo, M. Effect of dietary Lippia citriodora extract on productive performance and meat quality parameters in hares (Lepus europaeus Pall. ) Archiv. Anim. Breed. 2014, 57, 1–7. [Google Scholar] [CrossRef]
- Liu, M.J.; Li, J.X.; Guo, H.Z.; Lee, K.M.; Qin, L.; Chan, K.M. The effects of verbascoside on plasma lipid peroxidation level and erythrocyte membrane fluidity during immobilization in rabbits: A time course study. Life Sci. 2003, 73, 883–892. [Google Scholar] [CrossRef]
- Casamassima, D.; Palazzo, M.; Vizzarri, F.; Costagliola, C.; Corino, C.; Di Costanzo, A. Dietary effects of plant extracts, based on verbascoside, lycopene and horseradish on several blood variables and plasma oxidative status in growing rabbits. Liv. Sci. 2017, 206, 148–153. [Google Scholar] [CrossRef]
- Palazzo, M.; Vizzarri, F.; Nardoia, M.; Ratti, S.; Pastorelli, G.; Casamassima, D. Dietary Lippia citriodora extract in rabbit feeding: Effects on quality of carcass and meat. Archiv. Anim. Breed. 2015, 58, 355–364. [Google Scholar] [CrossRef]
- Lo Fiego, D.P.; Santoro, P.; Macchioni, P.; Mazzoni, D.; Piattoni, F.; Tassone, F.; Leonibus, E. The effect of dietary supplementation of vitamins C and E on the a-tocopherol content of muscles, liver and kidney, on the stability of lipids, and on certain meat quality parameters of the longissimus dorsi of rabbits. Meat Sci. 2004, 67, 319–327. [Google Scholar] [CrossRef]
- Vizzarri, F.; Palazzo, M.; D’Alessandro, A.G.; Casamassima, D. Productive performance and meat quality traits in growing rabbit following the dietary supplementation of Lippia citriodora, Raphanus sativus and Solanum lycopersicum extracts. Livest. Sci. 2017, 200, 53–59. [Google Scholar] [CrossRef]
- Palazzo, M.; Vizzarri, F.; Cinone, M.; D’Alessandro, A.; Martemucci, G.; Casamassima, D. Dietary effect of Lemon verbena extract on selected blood parameters and on plasma oxidative profile in Avelignese horses. Anim. Sci. J. 2019, 90, 222–228. [Google Scholar] [CrossRef] [PubMed]
- D’Alessandro, A.G.; Vizzarri, F.; Palazzo, M.; Martemucci, G. Dietary verbascoside supplementation in donkeys: Effects on milk fatty acid profile during lactation, and serum biochemical parameters and oxidative markers. Animal 2017, 11, 1505–1512. [Google Scholar] [CrossRef] [PubMed]
- Rossi, R.; Ratti, S.; Pastorelli, G.; Maghin, F.; Martemucci, G.; Casamassima, D.; D’Alessandro, A.G.; Corino, C. Effect of dietary plant extract on meat quality and sensory parameters of meat from Equidae. J. Sci. Food Agric. 2017, 97, 4690–4696. [Google Scholar] [CrossRef] [PubMed]
- Lorenzo, J.M.; Sarriés, M.V.; Tateo, A.; Polidori, P.; Franco, D.; Lanza, M. Carcass characteristics, meat quality and nutritional value of horsemeat: A review. Meat Sci. 2014, 96, 1478–1488. [Google Scholar] [CrossRef] [PubMed]
- Polidori, P.; Cavallucci, C.; Beghelli, D.; Vincenzetti, S. Physical and chemical characteristics of donkey meat from Martina Franca breed. Meat Sci. 2009, 82, 469–471. [Google Scholar] [CrossRef] [PubMed]
- De Marco, M.; Lopez Salcedo, W.; Pastorelli, G.; Rossi, R.; Corino, C.; Bergagna, S.; Mellia, E.; Gennero, M.S.; Biasibetti, E.; Capucchio, M.T.; et al. Effects of verbascoside supplemented diets on growth performance, blood traits, meat quality, lipid oxidation and histological features in broiler chickens. Ital. J. Anim. Sci. 2015, 14, 3712. [Google Scholar] [CrossRef]
- Mehrparvar, M.; Mazhari, M.; Esmaeilipour, O.; Sami, M. Effect of Lippia citridora leaves powder on growth performance, carcass traits, blood metabolites and meat quality of broilers. Iran. J. Vet. Med. 2016, 10, 307–317. [Google Scholar]
- Rafiee, F.; Mazhari, M.; Ghoreishi, M.; Esmaeilipour, O. Effect of Lemon verbena powder and vitamin C on performance and immunity of heat-stressed broilers. J. Anim. Physiol. Anim. Nutr. 2016, 100, 807–812. [Google Scholar] [CrossRef]
- Matshogo, T.B.; Mlambo, V.; Marume, U.; Sebola, N. Growth performance, blood parameters, carcass characteristics and meat quality traits in Potchefstroom Koekoek chickens fed Lippia javanica leaf meal. Tropic. Anim. Health Prod. 2018, 50, 1787–1795. [Google Scholar] [CrossRef]
- Mpofu, D.A.; Marume, U.; Mlambo, V.; Hugo, A. The effects of Lippia javanica dietary inclusion on growth performance, carcass characteristics and fatty acid profiles of broiler chickens. Anim. Nutr. 2016, 2, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Mnisi, C.M.; Matshogo, T.B.; van Niekerk, R.; Mlambo, V. Growth performance, haemo-biochemical parameters and meat quality characteristics of male Japanese quails fed a Lippia javanica-based diet. S. Afr. J. Anim. Sci. 2017, 47, 5. [Google Scholar] [CrossRef]
- Olivier, D.K.; Shikanga, E.A.; Combrinck, S.; Krause, R.W.M.; Regnier, T.; Dlamini, T.P. Phenylethanoid glycosides from Lippia javanica. S. Afr. J. Bot. 2010, 76, 58–63. [Google Scholar] [CrossRef]
- Hong, S.; Oh, G.W.; Kang, W.G.; Kim, O. Anticoccidial effects of the Plantago asiatica extract on experimental Eimeria tenella infection. Lab. Anim. Res. 2016, 32, 1053870. [Google Scholar] [CrossRef] [PubMed]
- Reverter, M.; Sarter, S.; Caruso, D.; Avarre, J.C.; Combe, M.; Pepey, E. Aquaculture at the crossroads of global warming and antimicrobial resistance. Nat. Comm. 2020, 11, 1870. [Google Scholar] [CrossRef] [PubMed]
- Reverter, M.; Tapissier-Bontemps, N.; Sarter, S.; Sasal, P.; Caruso, D. Moving towards more sustainable aquaculture practices: A meta-analysis on the potential of plant-enriched diets to improve fish growth, immunity and disease resistance. Rev. Aquac. 2020, 13, 537–555. [Google Scholar] [CrossRef]
- Hoseinifar, S.H.; Shakouri, M.; Van Doan, H.; Shafiei, S.; Yousefi, M.; Raeisi, M.; Yousefi, S.; Harikrishnan, R.; Reverter, M. Dietary supplementation of Lemon verbena (Aloysia citrodora) improved immunity, immune-related genes expression and antioxidant enzymes in rainbow trout (Oncorrhyncus mykiss). Fish Shellfish. Immunol. 2020, 99, 379–385. [Google Scholar] [CrossRef]
- Di Leo Lira, P.; van Baren, C.M.; López, S.; Molina, A.; Heit, C.; Viturro, C.; de Lampasona, M.P.; Catalán, C.A.; Bandoni, A. Northwestern Argentina: A Center of Genetic Diversity of Lemon Verbena (Aloysia citriodora Paláu, Verbenaceae). Chem. Biodivers. 2013, 10, 251–261. [Google Scholar] [CrossRef]
- Salomón, R.; Firmino, J.P.; Reyes-López, F.E.; Andre, K.B.; González-Silvera, D.; Esteband, M.A.; Tort, L.; Quintela, J.C.; Pinilla-Rosas, J.M.; Vallejos-Vidal, E. The growth promoting and immunomodulatory effects of a medicinal plant leaf extract obtained from Salvia officinalis and Lippia citriodora in Gilthead seabream (Sparus aurata). Aquaculture 2020, 524, 735291. [Google Scholar] [CrossRef]
- Salomón, R.; Reyes-Lopez, F.E.; Tort, L.; Firmino, J.P.; Sarasquete, C.; Ortiz-Delgado, J.B.; Quintela, J.C.; Pinilla-Rosas, J.M.; Vallejos-Vidal, E.; Gisbert, E. Medicinal plant leaf extract from sage and Lemon verbena promotes intestinal immunity and barrier function in Gilthead seabream (Sparus aurata). Front. Immunol. 2021, 12, 670279. [Google Scholar] [CrossRef]
- Salomón, R.; Dolors, F.M.; Reyes-López, F.E.; Tort, L.; Firmino, J.P.; Quintela, J.C.; Pinilla-Rosas, J.M.; Vallejos-Vidal, E.; Gisbert, E. Phytogenics from sage and Lemon verbena promote growth, systemic immunity and disease resistance in atlantic salmon (Salmo salar). Front Mar. Sci. 2022, 9, 828497. [Google Scholar] [CrossRef]
- Navarrete, S.; Kemp, P.D.; Pain, S.J.; Back, P.J. Bioactive compounds, aucubin and acteoside, in plantain (Plantago lanceolata L.) and their effect on in vitro rumen fermentation. Anim. Feed Sci. Technol. 2016, 222, 158–167. [Google Scholar] [CrossRef]
- Casamassima, D.; Nardoia, M.; Palazzo, M.; Vizzarri, F.; Corino, C. Effect of dietary extruded linseed, verbascoside and vitamin E supplements on selected serum biochemical parameters and plasma oxidative status in Lacaune ewes. Slov. Vet. Res. 2014, 51, 89–100. [Google Scholar]
- Casamassima, D.; Palazzo, M.; D’alessandro, A.G.; Colella, G.E.; Vizzarri, F.; Corino, C. The effect of Lemon verbena (Lippia citriodora) verbascoside on the productive performance, plasma oxidative status, and some blood metabolites in suckling lambs. J. Anim. Feed Sci. 2013, 22, 204–212. [Google Scholar] [CrossRef]
- Da Silva, N.I.S.; de Moura, J.P.P.; de Lucena Nascimento, M.E.; Gomes Machado, F.C.; Pinto Costa, T.G.; de Araújo Filho, J.M.; Cordão, M.A.; Edvan, R.L.; Oliveira, R.L.; Bezerra, L.R. Effect of Lippia alba hay as phytogenic feed additive on the lactation performance, milk composition, and rumen and blood parameters of Alpine goats. Small Rumin. Res. 2022, 215, 106767. [Google Scholar] [CrossRef]
- Ferraz Gomes, A.; Prates Almeida, M.; Freire Leite, M.; Schwaiger, S.; Stuppner, H.; Halabalaki, M.; Amaral, J.G.; David, M. Seasonal variation in the chemical composition of two chemotypes of Lippia alba. Food Chem. 2019, 273, 186–193. [Google Scholar] [CrossRef]
- Aguiar, S.; Costa, M.C.C.D.; Nacimento, S.C.; Sena, K.C.F.R. Antimicrobial activity of Lippia alba (Mill.) N. E. Brown (Verbenaceae). Rev. Bras. Farmacogn. 2008, 18, 436–440. [Google Scholar] [CrossRef]
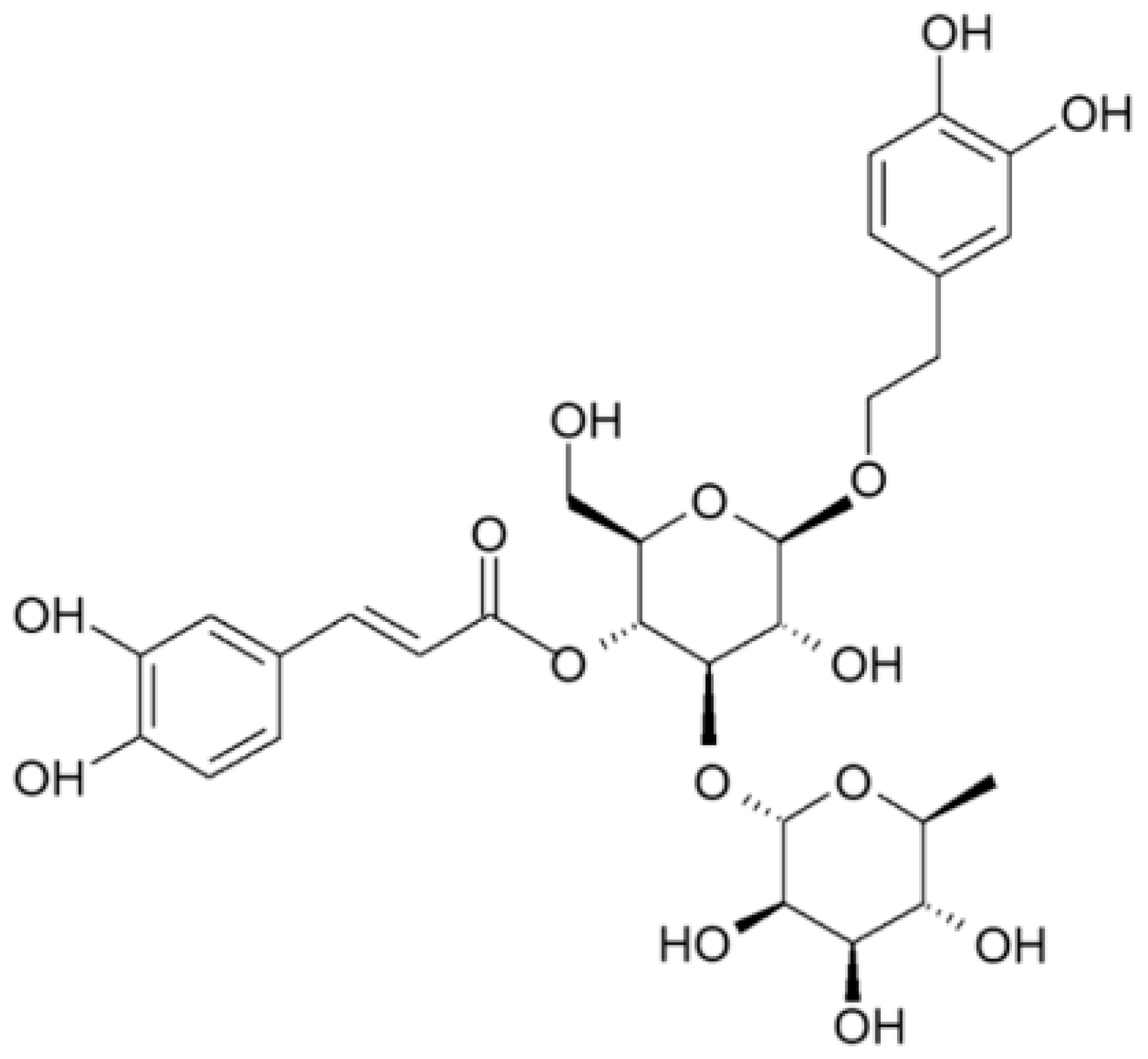

| Compounds | g/kg Extract |
|---|---|
| Gallic acid | 1.755 ± 0.07 |
| 3.4-dihydroxybenzoic acid | 0.450 ± 0.04 |
| Methyl gallate | 1.955 ± 0.09 |
| Isoverbascoside | 0.455 ± 0.04 |
| Verbascoside | 4.470 ± 0.08 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rossi, R.; Mainardi, E.; Vizzarri, F.; Corino, C. Verbascoside-Rich Plant Extracts in Animal Nutrition. Antioxidants 2024, 13, 39. https://doi.org/10.3390/antiox13010039
Rossi R, Mainardi E, Vizzarri F, Corino C. Verbascoside-Rich Plant Extracts in Animal Nutrition. Antioxidants. 2024; 13(1):39. https://doi.org/10.3390/antiox13010039
Chicago/Turabian StyleRossi, Raffaella, Edda Mainardi, Francesco Vizzarri, and Carlo Corino. 2024. "Verbascoside-Rich Plant Extracts in Animal Nutrition" Antioxidants 13, no. 1: 39. https://doi.org/10.3390/antiox13010039
APA StyleRossi, R., Mainardi, E., Vizzarri, F., & Corino, C. (2024). Verbascoside-Rich Plant Extracts in Animal Nutrition. Antioxidants, 13(1), 39. https://doi.org/10.3390/antiox13010039






