Abstract
This study aimed to evaluate the antioxidant and antiaging effects of Indian almond (Terminalia catappa Linn.) leaf extract (TCE) on high-glucose (GLU)-induced obese Caenorhabditis elegans. Since TCE contains high contents of flavonoids and phenolics, strong radical scavenging activity was confirmed in vitro. The stress-resistance effect of TCE was confirmed under thermal and oxidative stress conditions at nontoxic tested concentrations (6.25, 12.5, and 25 μg/mL). GLU at 2% caused lipid and reactive oxygen species (ROS) accumulation in C. elegans, and TCE inhibited lipid and ROS accumulation under both normal and 2% GLU conditions in a concentration-dependent manner. In addition, TCE proved to be effective in prolonging the lifespan of C. elegans under normal and 2% GLU conditions. The ROS reduction effect of TCE was abolished in mutants deficient in daf-16/FOXO and skn-1/Nrf-2. In addition, the lifespan-extending effect of TCE in these two mutants disappeared. The lifespan-extending effect was abolished even in atgl-1/ATGL-deficiency mutants. The TCE effect was reduced in aak-1/AMPK-deficient mutants and completely abolished under 2% GLU conditions. Therefore, the effect of prolonging lifespan by inhibiting lipid and ROS accumulation under the high GLU conditions of TCE is considered to be the result of atgl-1, daf-16, and skn-1 being downregulated by aak-1. These results suggest that the physiological potential of TCE contributes to antiaging under metabolic disorders.
1. Introduction
Obesity has the potential to progress into a more severe metabolic syndrome (MS), and also causes other MSs, such as arteriosclerosis, hyperglycemia, insulin resistance, and diabetes [1,2]. In particular, obesity resulting from high sugar intake is recognized as a fatal risk factor that increases mortality by developing into serious chronic diseases, such as cancer, cardiovascular disease, osteoarthritis, kidney disease, and liver disease [2,3,4].
Caenorhabditis elegans consumes food through the mouth. The ingested food ingredients are absorbed by the intestinal cells. Excess calorie intake induces fat accumulation in the intestine. The shape and size of lipid droplets produced can be observed under a microscope because the torso of C. elegans is transparent [5,6,7]. Therefore, C. elegans that have exposed to compounds that cause or inhibit obesity are suitable models for identifying whether a compound is involved in obesity [8,9]. Since C. elegans genes are highly homologous with mammalian genes for energy metabolism, studies using gene-deficient mutants provide insight into understanding the signaling pathway [10]. In particular, since C. elegans have short lifespans of less than four weeks, they can be beneficial when used to study lifespans related to various health conditions and nutrient intake [10].
AMP-activated protein kinase (AMPK) plays a central role in energy homeostasis by regulating the GLU and lipid metabolism [11]. Therefore, AMPK is an important factor when studying the relationship between various nutritional metabolic stresses and aging [12]. C. elegans is an important model system for studying AMPK action. In C. elegans, AMPK consists of two catalytic α subunits: aak-1 and aak-2 [13]. Excessive reactive oxygen species (ROS) are generated during metabolic stress, and AMPK mediates an antioxidant system that reduces ROS through the regulation of the transcription factor FOXO and Nrf2 [14]. In particular, C. elegans is employed to gain insight into the intervention of daf-16/FOXO in AMPK signaling pathways related to the antioxidant system [15,16,17]. Furthermore, skn-1/Nrf2 is a transcription regulator involved in the detoxification of ROS, which plays an important preservation role in stress resistance by downregulating a series of target genes involved in antioxidant enzyme activity [14]. Recent reports have shown that activated AMPK leads to a boost in Nrf2 [18]. Thus, daf-16 and skn-1 play key roles in improving oxidative stress and regulating downstream genes to extend the lifespan of C. elegans [19]. On the other hand, phosphorylated AMPK induces the nuclear localization of FOXO1, which binds to the promoter region to increase the expression of adipose triglyceride lipase (ATGL). ATGL, a key lipase, hydrolyzes triglycerides to form diglycerides and fatty acids [20]. This series of processes helps to extend the lifespan by regulating lipid metabolism in C. elegans.
To find novel health-functional material for improving the risks of MSs, we tested the benefits of Indian almond (Terminalia catappa Linn.) leaf extract (TCE). T. catappa is a large tropical tree that is native to Southeast Asia, Australia, and the Pacific. TCE contains gallic acid, corilagin, ellagic acid, rutin, kaempferol, chebulagic acid, punicalagin, punicalin, and quercetin [21,22]. In addition, TCE is known to have various medicinal effects, such as possessing anti-inflammatory, analgesic, antioxidant, and antibacterial properties, as well as liver protection and skin protection [23,24,25,26,27,28].
Since the antioxidant-activity-based cell-protective effect of TCE has been reported in various research, the in vivo lifespan extension and antiaging effects of TCE had been expected. Therefore, we induced metabolic stress in C. elegans through high-GLU feeding and investigated whether TCE exhibits positive physiological activity in metabolic disease models and ultimately has a longevity effect.
2. Materials and Methods
2.1. Sample Preparation
Indian almond (Terminalia catappa) leaves were collected in Myanmar in 2020 and their extract was received from the pharmaceutical research department (Yangon, Myanmar). The collected leaves were dried in the shade for more than 4 days until they were completely dry. All dry leaves were reflux-extracted for 16 h, using 70% (v/v) alcohol at 70 °C. The solvent was removed from the extract using a rotary evaporator (EYELA, Tokyo Rikakikai Co., Tokyo, Japan) at 60 °C. Terminalia catappa leaf extract (TCE) was dissolved in 10 mg/mL of dimethyl sulfoxide (DMSO), prepared as a stock solution, and stored at −80 °C until analyzed.
All reagents used in our study were HPLC- or molecular-biology-grade. Unless stated otherwise, all the materials were purchased from Sigma Chemical Co. (St. Louis, MO, USA).
2.2. LC-MS/MS Analysis
To identify phenolic compounds in TCE, we used UPLC-QTOF-MS. The analytical conditions are shown in Table 1. Solvents used for analysis were of HPLC grade and purchased from Millipore (Bedford, MA, USA).

Table 1.
UPLC-QTOF-MS analysis condition of phenolic compounds in TCE.
2.3. Total Phenol Contents (TPC) and Total Flavonoid Contents (TFC)
The total phenolic contents (TPC) in the TCE were determined with the Folin–Ciocalteu (FC) method. Briefly, 700 μL sample (mixed with FC in 1:1 ratio) and 700 μL sodium carbonate were mixed and placed in the dark for 1 h. An absorbance reading was performed at 720 nm. The total phenolic contents of the sample were calculated using the equation for the gallic acid standard curve. The results were expressed as gallic acid equivalents (GAE)/g.
To determine the total flavonoid contents (TFC), 500 μL of the sample, 1.5 mL of sodium nitrite (5%), 10 μL of aluminum chloride (10%), and 1 mL of sodium hydroxide (1 N) were mixed. The absorbance was measured at 415 nm [29]. The total flavonoid contents of the samples were calculated using the equation for the quercetin standard curve. The results were expressed as quercetin equivalents (QE)/g.
2.4. Radical Scavenging Capacity
To measure the scavenging capacity against 2,2-azino-bis-3-ethylbenzothiazoline-6-sulphonic acid (ABTS) radical, we modified and used the method from Gullon et al. [30]. To produce ABTS cations, the mixture containing 7.0 mM ABTS (in 20 mM sodium acetate buffer, pH 4.5) and 2.45 mM potassium persulfate was kept in the dark overnight. The ABTS solution was diluted to an absorbance of 0.7 ± 0.01 at 734 nm. An amount of 50 µL of samples reacted with 950 µL of ABTS solution and then was placed for 10 min at room temperature. An absorbance at 734 nm was recorded.
A 2,2-diphenyl-1-picrylhydrazyl (DPPH) assay was modified by Li et al. [31]. Simply, a sample of the same volume and a DPPH solution (0.2 mM) were mixed and left at room temperature for 30 min. The reaction mixture was recorded at 517 nm using a microplate reader (microplate reader SYNERGY HTX, Biotek, CA, USA).
ABTS and DPPH radical-scavenging activity was calculated using the following formula: radical scavenging activity (%) = [1 − (sample O.D./blank O.D.)] × 100
Furthermore, we presented the value of the antioxidant capacity, which was calculated using an ascorbic acid (AA) calibration curve and expressed in micrograms of AA per gram of sample dry weight (DW).
2.5. Worm Study
2.5.1. Cultivation
For experimental use of C. elegans strain N2 (wild-type) and its derivative mutant strains, skn-1 (zu67) IV and daf-16::GFP (zls356) were obtained from the Caenorhabditis Genetics Center (CGC) in the U.S.A. Strains daf-16 (tm5030), atgl-1 (tm12352), and aak-1 (tm1944) were obtained from the National BioResource Project (NBRP) of Japan.
All strains were kept on nematode growth medium (NGM) plates that were spread with E. coli OP50. They were maintained at a temperature of 20 °C throughout the entire experiment. Age synchronization of nematodes was achieved by separating the eggs from gravid adults using a solution containing 6% sodium hypochlorite and 5 M NaOH [32].
2.5.2. Acute Toxicity
Synchronized L4 was washed twice with M9 buffer and suspended in M9 buffer containing cholesterol. One milliliter of the suspension was transferred to each well of a 24-well plate (20–30 worms/well) and mixed with 10 μL of various concentrations of TCE. Then, the plates were left at 20 °C for 24 h. Acute toxicity results were expressed as percent survival after counting the live worms.
2.5.3. Oil Red O Staining
To determine lipid contents, oil red O staining was partially modified and performed [33]. Simply, cultivated worms were fixed in 4% formaldehyde for 24 h, then dehydrated with 60% isopropanol at −70 °C for 15 min. The dehydrated worms were washed three times with M9 buffer and dyed with an oil red O solution for 2 h. The stained worms were washed with M9 buffer, then observed under a microscope (Nikon, Seoul, Republic of Korea). The relative strength of the stained lipid droplets in the worms was quantified using Image J 1.8.0. software.
2.5.4. Triglyceride (TG) Assay
The cultured L4 worms were resuspended in 500 μL PBS containing 0.05% Tween-20 solution. They were homogenized on ice using a glass homogenizer (ALLSHENG, regional, national) for 5 min to collect the pellet and then centrifuged at 1000× g for 5 min. The obtained supernatant was analyzed for TG content. TG content was measured using the absorbance at 570 nm with a TG kit (Biomax, Guri, Republic of Korea).
2.5.5. Determination of Stress Resistance
In the case of thermal and oxidative stress analysis, 50 synchronized N2 L1 larvae were adjusted as follows. OP50 and TCE were mixed and seeded on NGM plates.
To evaluate the effect on thermal stress, the worm plate was maintained at 20 °C for 72 h, incubated at 35 °C for 4 h, and then transferred back to 20 °C. In addition, to test the effect on oxidative stress, pretreated worms were then transferred to a 24-well plate containing a concentration of 80 μM juglone (5-hydroxy-1,4-naphthoquinone, Sigma-Aldrich, St. Louis, MI, USA). The plate was incubated at 20 °C.
After incubation, the living and the dead worms were counted and subsequently scored, as described in the study conducted by Rathor et al. [34].
2.5.6. DCF–DA Assay
L1 worms were treated with different concentrations of extracts for 64 h and then incubated with 100 μM H2DCF-DA for 1 h in the dark. After that, the nematodes were mounted in NAN3 (2%) onto microscope slides. The slides were viewed using a Nikon ECLIPS Ci (Nikon, Seoul, Republic of Korea) fluorescence microscope. The fluorescence intensities were examined using Image J software. An average of 15 worms per group was selected for quantification.
2.5.7. Daf-16 Nuclear Localization
Daf-16 (zIs356) nematode is daf-16::GFP mutant, which is a transgenic mutant nematode fused with green fluorescent protein and daf-16. After daf-16 mutants in the L1 stage were treated with extracts for 64 h, they were immobilized on microscope slides with 2% NaN3. The patterns of daf-16::GFP expression were evaluated as “cytosolic”, “intermediate”, and “nuclear”, which counted the percentage of each treatment group.
2.5.8. Lifespan Assay
Young adult nematodes were transferred to M9 buffer with cholesterol. Then, different concentrations of extracts were added, including 120 μM of 5-Fluoro-2’-deoxyuridine (FUDR, 98%) (Alfa Aesar, Seoul, Republic of Korea), to prevent spawning, and carbenicillin (50 μg/mL), to prevent bacterial infection. The transfer day was designated as day 1, the old medium was removed and fresh medium containing the extract was added every other day until all worms died. The results were expressed as a percentage of the survival rate (% survival rate).
2.5.9. Statistical Analysis
Results are presented as the means ± standard deviations (SD) of three independent replicates. The significance of intergroup differences was determined using one-way analysis of variance (ANOVA), followed by Tukey’s multiple range test. SPSS 27.0 was used for all statistical analyses except lifespan. p-values < 0.05 were considered to be significant. The results of the lifespan and the mean lifespan were analyzed with the Kaplan–Meier method using the OASIS application (https://sbi.postech.ac.kr/oasis/, accessed on 21 July 2023). p-values of survival differences were determined with the log-rank test.
3. Results
3.1. TCE Components Profile
As a result of the UPLC-QTOF-MS analysis, seven compounds were identified as bio-phenolic components of TCE (Figure 1), including phenolic acids such as gallic acid and ellagic acid, flavonoids such as orientin, vitexin, and terpenes or terpenoids such as arjungenin, arjunolic acid, and betulinic acid. The molecular weight of each peak is presented in Figure S1.
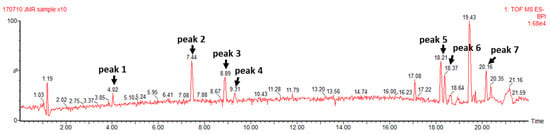
Figure 1.
UPLC-QTOF-MS chromatogram of TCE. Peak 1: gallic acid, peak 2: orientin, peak 3: vitexin, peak 4: ellagic acid, peak 5: arjungenin, peak 6: arjunolic acid, and peak 7: betulinic acid.
3.2. Total Phenol and Total Flavonoid Contents and Antioxidant Activity of TCE
The total phenol content (TPC) and the total flavonoid content (TFC) of TCE were 693.65 ± 60.3 GAE/g and 268.39 ± 10 QE/g, respectively. The results were expressed as 50% inhibition concentration (IC50) and trolox equivalents (TE) (Table 2). The DPPH scavenging capacity and IC50 of TCE were 84.33 ± 0.23 μTE/g and 18.82 ± 0.04 μg/mL, respectively. The ABTS scavenging capacity and IC50 of TCE were 2.23 ± 0.09 μTE/g and 125.77 ± 0.47 μg/mL, respectively (Table 2).

Table 2.
Total phenolic content (TPC), total flavonoid content (TFC), and the antioxidant activity evaluated using the DPPH and ABTS assays of TCE.
3.3. Safety of TCE
To evaluate the safety of TCE, acute toxicity tests were performed with different doses of TCE (3.125–400 μg/mL). No toxicity was observed at 3.125–25 μg/mL, but worm survival was reduced in the concentration range of 50–400 μg/mL of TCE, which was determined to be a toxic concentration. Therefore, safety concentrations (6.25 μg/mL, 12.5 μg/mL, 25 μg/mL) of TCE were treated in further experiments (Figure 2).
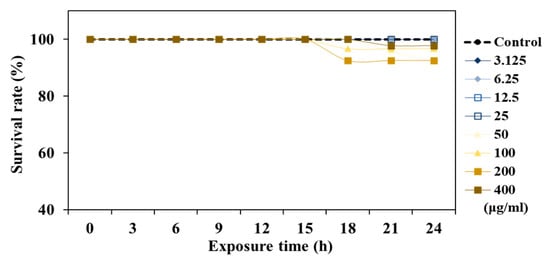
Figure 2.
Safety of TCE at different concentrations (3.125–400 μg/mL).
3.4. Inhibitory Effect on Lipid Accumulation of TCE
To understand the work of TCE on lipid metabolism, we investigated the effect of TCE on fat accumulation in C. elegans under normal or 2% GLU conditions. The TCE group exhibited a concentration-dependent decrease in total fat accumulation in normal and 2% GLU conditions compared to the control group (Figure 3A,B). Consistently, the TCE-treated worms had reduced triglyceride (TG) content compared to the control group under both normal and 2% GLU-treated groups (Figure 3C).
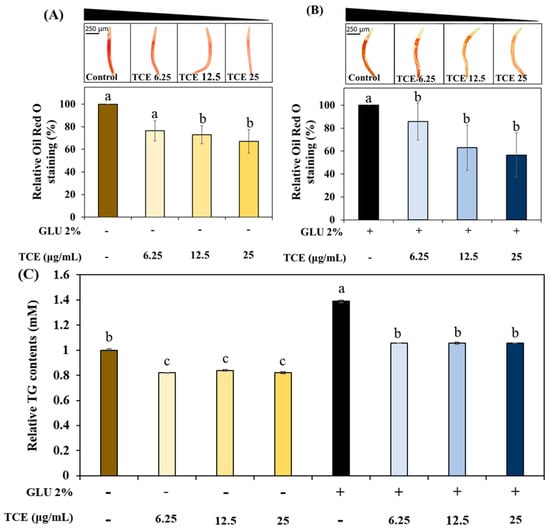
Figure 3.
Inhibition of TCE on lipid accumulation. Total fat contents under (A) normal and (B) 2% GLU (glucose) conditions. (C) TG content in the worms (more than 2000 worms were used in each independent experiment). Bars represent the means ± SDs (n = 3 plates and 10 worms for oil red O staining assay). One thousand worms were used in each independent group for the TG assay. Statistical analysis was performed using one-way ANOVA, followed by Tukey’s post hoc test. Different letters above bars mean statistically significant differences (p < 0.05).
3.5. Evaluation of TCE Stress Resistance under Various Stress Conditions
To evaluate the stress resistance of TCE, we analyzed its ability to improve resistance to thermal and oxidative stress. TCE showed an increase in survival compared to the control group under thermal stress (Figure 4A) and oxidative stress (Figure 4B) conditions. However, there was no statistically significant difference.
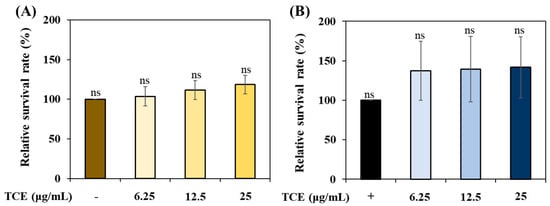
Figure 4.
The stress resistance of TCE under (A) oxidative stress condition and (B) thermal stress condition. Bars represent the means ± SDs (n = 3 plates and 10 worms). Statistical analysis was performed using one-way ANOVA, followed by Tukey’s post hoc test (p < 0.05). ns: no significance.
3.6. Inhibitory Effects on Glucose-Induced ROS Accumulation of TCE
We investigated whether GLU can induce oxidative stress. Treatment of GLU at concentrations of 1%, 2%, and 5% revealed that GLU induces oxidative stress in a concentration-dependent manner, which is similar to 80 μM juglone (Figure 5A). TCE-treated N2 worms resulted in a reduction in oxidative stress compared to the control group under normal (Figure 5B,D) and even GLU (Figure 5B,D) conditions.
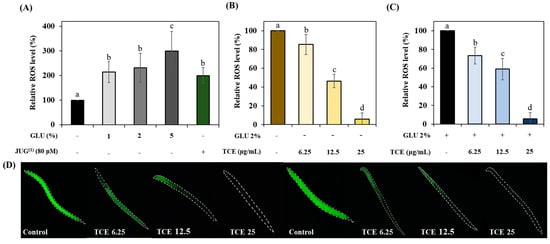
Figure 5.
The inhibitory effect on ROS accumulation of TCE. (A) GLU (glucose)-induced oxidative stress and ROS content under (B) normal and (C) 2% GLU conditions. (D) Images of green-fluorescent-stained ROS under normal and 2% GLU conditions. Bars represent the means ± SDs (n = 3 plates and 10 worms). Statistical analysis was performed using one-way ANOVA, followed by Tukey’s post hoc test. Different lowercase letters means statistically significant differences (p < 0.05). (1) Juglone.
3.7. TCE Effect on Lifespan Prolonging
To evaluate the effect of TCE on the lifespan of C. elegans, tested worms were incubated until all worms died while preventing progeny production at 20 °C. The results indicated that TCE treatment can extend the lifespan of cultured C. elegans under normal conditions by a maximum of 16 days (Figure 6A). Even in GLU conditions, the TCE treatment extended the lifespan of C. elegans by a maximum of 20 days (Figure 6B).
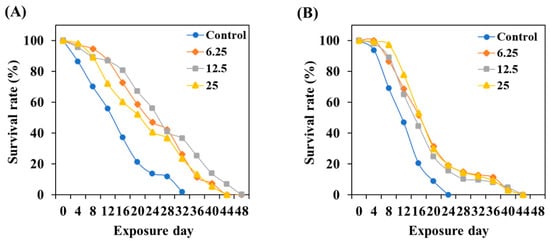
Figure 6.
Effect of TCE on lifespan in wild-type worms under (A) normal and (B) 2% GLU (AD) conditions. Fifty worms were used in each independent group.
3.8. Role of Daf-16 in TCE Effect
To investigate localization to the nucleus of daf-16 due to the TCE treatment, L1 larvae of daf-16::GFP were treated with TCE for 64 h. In TCE-treated worms, the intensity of the daf-16 nuclear replacement increased compared to the control group in a concentration-dependent manner (Figure 7A). Similarly, it was observed that the nuclear expression of daf-16 increased compared to the control group in TCE-treated worms that were also in the GLU condition (Figure 7B).
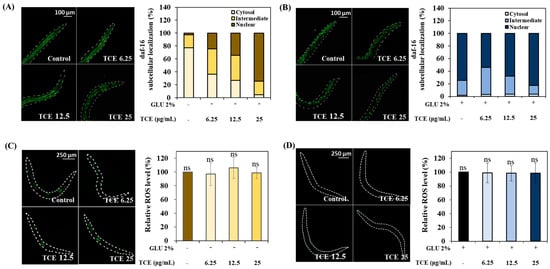
Figure 7.
Daf-16 nuclear localization and determination of ROS level in daf-16 null mutants. Daf-16 nuclear localization under (A) normal and (B) 2% GLU (glucose) conditions. ROS levels under (C) normal and (D) 2% GLU (glucose) conditions. Bars represent the means ± SDs (n = 3 plates and 10 worms). Statistical analysis was performed using one-way ANOVA, followed by Tukey’s post hoc test (p < 0.05). ns: no significance.
To precisely identify the role of daf-16 in TCE action, the ROS intensity was analyzed using daf-16 null worms. Daf-16 null worms were treated with TCE; there was no significant difference observed in ROS expression under both normal and GLU conditions (Figure 7C,D). As a result, the ROS-reducing effect of TCE in the wild type was abolished, suggesting that daf-16 was involved in the TCE effect.
3.9. Role of Skn-1 in TCE Effect
Besides identifying the relationship between the action of TCE and the role of daf-16, we also investigated the impact of TCE on skn-1 transcription factors by utilizing skn-1 knockdown worms.
Similar to the daf-16 result, there was no significant difference in the ROS expression between the control and the TCE-treatment group in both normal and GLU conditions in skn-1 null worms (Figure 8A,B). These results suggest that the expression of skn-1, along with daf-16, affects the TCE effect.
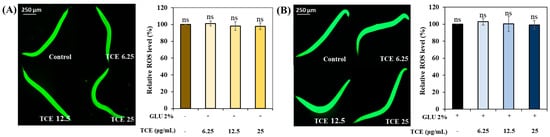
Figure 8.
Determination of ROS level in skn-1 knockdown mutants. (A) ROS level under normal condition. (B) ROS level under GLU condition. Bars represent the means ± SDs (n = 3 plates and 10 worms). Statistical analysis was performed using one-way ANOVA, followed by Tukey’s post hoc test (p < 0.05). ns: no significance.
3.10. The Role of Various Metabolic Stress Regulators in the Lifespan-Extending Effect of TCE
To gain insight into how TCE interacts with the various genes that regulate energy metabolism, we used daf-16, skn-1, atgl-1, and aak-1 knockdown mutant worms to evaluate the effect of TCE on longevity.
Daf-16, skn-1, atgl-1, and aak-1 gene knockdown mutants were treated with different concentrations of TCE, respectively. In earlier experiments, the lifespan of the wild-type, N2 was extended depending on the concentration of the treated TCE (Figure 5). However, the lifespan of the four mutants ended similarly, with no statistical difference according to the TCE concentration (Figure 8, Table S1). This result means that the lifespan-extending effect of TCE is related to the daf-16, skn-1, atgl-1, and aak-1 genetic factors.
4. Discussion
Recent research suggests that obesity is not simply attributable to caloric imbalance but is influenced by systemic factors associated with insulin metabolism [35,36,37]. Therefore, we induced metabolic disorders using an excessive GLU intake model and evaluated the level of accumulated fat, a representative phenotype of obesity. Our results showed that the treatment of TCE in high-GLU-ingested worms reduces fat and TG accumulation (Figure 3). These results suggest that TCE can prevent high-sugar-induced obesity by primarily suppressing body fat accumulation.
Excessive secretion of insulin due to a high-sugar diet can trigger systemic oxidative stress, inflammation, and aging. These metabolic stresses contribute to the pathogenesis of obesity and diabetes, which can subsequently lead to various complications [38,39,40]. Our study found that 2% GLU caused ROS accumulation of as much as 80 μM JUG. Numerous studies have verified hypotheses suggesting a link between GLU-diet-derived ROS overproduction and lifespan, and they support our results [41,42]. However, TCE exhibited a protective effect against GLU-induced oxidative damage (Figure 5).
Normally, transcription factors daf-16/FOXO and skn-1/Nrf2 are located primarily in the cytoplasm, but when exposed to internal or external stress, these factors translocate into the nucleus [43,44]. Nuclear expression of these factors initiates a series of stress controls, such as regulating the activity of antioxidant enzymes to prevent ROS overproduction [45]. The inhibitory effect of TCE, as well as the promotional effect of GLU on ROS production in daf-16 and skn-1 knockdown mutations, has been abolished (Figure 8). Furthermore, the lifespan-extending effect of TCE has been abolished in daf-16 and skn-1 mutants (Figure 9). Therefore, we clearly confirmed that the in vivo antioxidant effect of TCE on GLU-induced oxidative toxicity is related to daf-16 and skn-1, and we conclude that the longevity effect of TCE is based on the antioxidant defense system. Daf-16/FOXO and skn-1/Nrf2 are downstream transcription factors expressed by the activation of aak-1/AMPK [46,47]. In our lifespan result of the aak-1 knockdown mutant, The TCE effect shown in N2 was abolished (Figure 9). Taken together, it is believed that the inhibitory effect of TCE against oxidative stress ultimately contributed to extending the lifespan of C. elegans (Figure 6). The in vitro antioxidant effect of TCE has been verified through numerous studies, and the preventive effects on skin aging and osteoporosis related to antioxidant properties have been reported [28,48]. However, the effect of TCE on lifespan extension has not been reported. In particular, this is the first discovery that the antioxidant-based metabolic disease improvement and longevity effect of TCE depends on the AMPK pathway.
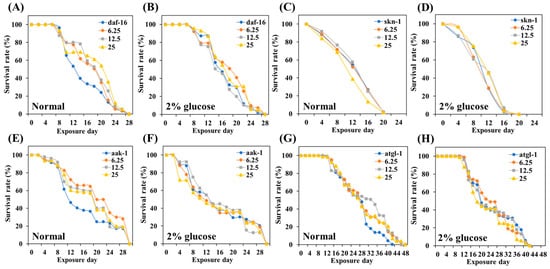
Figure 9.
Effect of TCE on lifespan in various mutants. (A,B) survival curve of daf-16 under normal and GLU conditions. (C,D) Survival curve of skn-1 under normal and GLU conditions. (E,F) Survival curve of aak-1 under normal and GLU conditions. (G,H) Survival curve of atgl-1 under normal and GLU conditions. Fifty worms were used in each independent group.
Meanwhile, AMPK is a key regulator in energy metabolism and is particularly well known to control GLU and lipid metabolism [11,48]. In metabolic regulation, because AMPK activation induces health-beneficial physiological effects, AMPK is considered an important therapeutic target for controlling human diseases, including MS [49]. We found that the accumulated fat was reduced due to GLU feeding in TCE-treated worms. Thus, further investigation of the genetic factors related to lipid metabolism controlled by AMPK was needed. Fat accumulation is controlled by the balance between fat synthesis (lipogenesis) and fat degradation (lipolysis and beta-oxidation) [49]. Daf-16 is a factor that mediates the antioxidant system to respond to stress and is also involved in lipogenesis-related factors in lipid accumulation [50]. Therefore, increased daf-16 expression through TCE treatment also means that TCE intervenes in daf-16 expression to regulate fat accumulation. In addition, AMPK downregulates atgl-1/ATGL, which is a major lipase in lipolysis [51]. The TCE effect on the lifespan extension was abolished in the atgl-1 knockdown mutant, and this result implies that the effect of TCE is due to the action of atgl-1. A number of studies focusing on lipid metabolism related to AMPK activation have demonstrated that the intervention of daf-16 and atgl-1 attenuates lipid levels and prolongs their lifespan [20,46,51].
In conclusion, TCE prolonged the lifespan by improving antioxidant activity and lipid metabolism in nematode models of high-GLU-diet-induced metabolic disorders. It was confirmed that the TCE effect is influenced by aak-1/AMPK, which is responsible for energy metabolism. The activation of aak-1/AMPK regulates oxidative stress by inducing the nuclear transition of skn-1 and daf-16. In addition, AMPK downregulates daf-16 and atgl-1, which are in charge of lipogenesis and lipolysis, respectively. TCE intervened in the action of those genetic factors related to energy metabolism. In other words, it is believed that the effect of TCE depends on skn-1, daf-16, and atgl-1 belonging to the aak-1/AMPK pathway. Therefore, it has been proven that Indian almond leaves have the potential to prevent and improve metabolic diseases by regulating genetic factors through the AMPK pathway. This study is the first to experimentally demonstrate that TCE has a longevity effect by modulating AMPK to relieve metabolic stress.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/antiox13010014/s1, Figure S1: Mass spectra corresponding to individual peaks obtained from UPLC-QTOF-MS. Table S1: Influence of TCE on lifespan under normal and 2% GLU (glucose) conditions in various knockdown mutants.
Author Contributions
Y.K., S.-b.L., M.C. and S.C. performed the experiments; Y.K. analyzed the data and contributed to the writing; M.J. designed the experiment and wrote the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the National Research Foundation of Korea (NRF) and was funded by the Korean government (MSIP) (Grant no. 2021R1G1A1092967).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Pi-Sunyer, X. The medical risks of obesity. Postgrad. Med. 2009, 121, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Francisqueti, F.V.; Nascimento, A.F.; Minatel, I.O.; Dias, M.C.; Luvizotto, R.d.A.M.; Berchieri-Ronchi, C.; Ferreira, A.L.A.; Corrêa, C.R. Metabolic syndrome and inflammation in adipose tissue occur at different times in animals submitted to a high-sugar/fat diet. J. Nutr. Sci. 2017, 6, e41. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, D.T.d.; Fernandes, I.d.C.; Sousa, G.G.d.; Santos, T.A.P.d.; Paiva, N.C.N.d.; Carneiro, C.M.; Evangelista, E.A.; Barboza, N.R.; Guerra-Sá, R. High-sugar diet leads to obesity and metabolic diseases in ad libitum-fed rats irrespective of caloric intake. Arch. Endocrinol. Metab. 2020, 64, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R.J.; Segal, M.S.; Sautin, Y.; Nakagawa, T.; Feig, D.I.; Kang, D.-H.; Gersch, M.S.; Benner, S.; Sánchez-Lozada, L.G. Potential role of sugar (fructose) in the epidemic of hypertension, obesity and the metabolic syndrome, diabetes, kidney disease, and cardiovascular disease. Am. J. Clin. Nutr. 2007, 86, 899–906. [Google Scholar] [PubMed]
- Wormbook. Available online: http://www.wormbook.org/chapters/www_feeding/feeding.html (accessed on 21 May 2012).
- McKay, R.M.; McKay, J.P.; Avery, L.; Graff, J.M. C. elegans: A model for exploring the genetics of fat storage. Dev. Cell 2003, 4, 131–142. [Google Scholar] [CrossRef]
- Corsi, A.K.; Wightman, B.; Chalfie, M. A transparent window into biology: A primer on Caenorhabditis elegans. Genetics 2015, 200, 387–407. [Google Scholar] [CrossRef]
- Dwyer, D.S.; Donohoe, D.; Lu, X.H.; Aamodt, E.J. Mechanistic connections between glucose/lipid disturbances and weight gain induced by antipsychotic drugs. Int. Rev. Neurobiol. 2005, 65, 211–247. [Google Scholar]
- Zheng, J.; Enright, F.; Keenan, M.; Finley, J.; Zhou, J.; Ye, J.; Greenway, F.; Senevirathne, R.N.; Gissendanner, C.R.; Manaois, R. Resistant starch, fermented resistant starch, and short-chain fatty acids reduce intestinal fat deposition in Caenorhabditis elegans. J. Agric. Food Chem. 2010, 58, 4744–4748. [Google Scholar] [CrossRef]
- Murphy, C.T.; McCarroll, S.A.; Bargmann, C.I.; Fraser, A.; Kamath, R.S.; Ahringer, J.; Li, H.; Kenyon, C. Genes that act downstream of DAF-16 to influence the lifespan of Caenorhabditis elegans. Nature 2003, 424, 277–283. [Google Scholar] [CrossRef]
- Bai, J.; Zhu, Y.; He, L.; Zhang, J.; Li, J.; Pan, R.; Zhang, J.; Zhao, Y.; Cui, L.; Lu, H. Saponins from bitter melon reduce lipid accumulation via induction of autophagy in C. elegans and HepG2 cell line. Curr. Res. Food Sci. 2022, 5, 1167–1175. [Google Scholar] [CrossRef]
- Salminen, A.; Kaarniranta, K. AMP-activated protein kinase (AMPK) controls the aging process via an integrated signaling network. Ageing Res. Rev. 2012, 11, 230–241. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, M.; Roy, R. 5′-AMP-Activated Protein Kinase Signaling in Caenorhabditis elegans. AMP-Act. Protein Kinase 2016, 107, 375–388. [Google Scholar]
- Greer, E.L.; Dowlatshahi, D.; Banko, M.R.; Villen, J.; Hoang, K.; Blanchard, D.; Gygi, S.P.; Brunet, A. An AMPK-FOXO pathway mediates longevity induced by a novel method of dietary restriction in C. elegans. Curr. Biol. 2007, 17, 1646–1656. [Google Scholar] [CrossRef] [PubMed]
- Ankeny, R.A. The natural history of Caenorhabditis elegans research. Nat. Rev. Genet. 2001, 2, 474–479. [Google Scholar] [CrossRef] [PubMed]
- Fatt, H.V.; Dougherty, E.C. Genetic control of differential heat tolerance in two strains of the nematode Caenorhabditis elegans. Science 1963, 141, 266–267. [Google Scholar] [CrossRef] [PubMed]
- Nigon, V.; Dougherty, E.C. Reproductive patterns and attempts at reciprocal crossing of Rhabditis elegans Maupas, 1900, and Rhabditis briggsae Dougherty and Nigon, 1949 (Nematoda: Rhabditidae). J. Exp. Zool. 1949, 112, 485–503. [Google Scholar] [CrossRef]
- Kosztelnik, M.; Kurucz, A.; Papp, D.; Jones, E.; Sigmond, T.; Barna, J.; Traka, M.H.; Lorincz, T.; Szarka, A.; Banhegyi, G. Suppression of AMPK/aak-2 by NRF2/SKN-1 down-regulates autophagy during prolonged oxidative stress. FASEB J. 2019, 33, 2372. [Google Scholar] [CrossRef]
- Zhang, H.; Davies, K.J.; Forman, H.J. Oxidative stress response and Nrf2 signaling in aging. Free Radic. Biol. Med. 2015, 88, 314–336. [Google Scholar] [CrossRef]
- Chen, W.-L.; Chen, Y.-L.; Chiang, Y.-M.; Wang, S.-G.; Lee, H.-M. Fenofibrate lowers lipid accumulation in myotubes by modulating the PPARα/AMPK/FoxO1/ATGL pathway. Biochem. Pharmacol. 2012, 84, 522–531. [Google Scholar] [CrossRef]
- Anand, A.; Divya, N.; Kotti, P. An updated review of Terminalia catappa. Pharmacogn. Rev. 2015, 9, 93. [Google Scholar] [CrossRef]
- Abiodun, O.O.; Rodríguez-Nogales, A.; Algieri, F.; Gomez-Caravaca, A.M.; Segura-Carretero, A.; Utrilla, M.P.; Rodriguez-Cabezas, M.E.; Galvez, J. Antiinflammatory and immunomodulatory activity of an ethanolic extract from the stem bark of Terminalia catappa L. (Combretaceae): In vitro and in vivo evidences. J. Ethnopharmacol. 2016, 192, 309–319. [Google Scholar] [CrossRef]
- Fan, Y.; Xu, L.; Gao, J.; Wang, Y.; Tang, X.; Zhao, X.; Zhang, Z. Phytochemical and antiinflammatory studies on Terminalia catappa. Fitoterapia 2004, 75, 253–260. [Google Scholar] [CrossRef]
- Ratnasooriya, W.; Dharmasiri, M.; Rajapakse, R.; De Silva, M.; Jayawardena, S.; Fernando, P.; De Silva, W.; Nawela, A.; Warusawithana, R.; Jayakody, J. Tender leaf extract of Terminalia catappa antinociceptive activity in rats. Pharm. Biol. 2002, 40, 60–66. [Google Scholar] [CrossRef]
- Liu, T.-Y.; Ho, L.-K.; Tsai, Y.-C.; Chiang, S.-H.; Chao, T.-W.; Li, J.-H.; Chi, C.-W. Modification of mitomycin C-induced clastogenicity by Terminalia catappa L. in vitro and in vivo. Cancer Lett. 1996, 105, 113–118. [Google Scholar] [CrossRef]
- Nair, R.; Chanda, S. Antimicrobial activity of Terminalia catappa, Manilkara zapota and Piper betel leaf extract. Indian J. Pharm. Sci. 2008, 70, 390. [Google Scholar]
- Yang, S.-F.; Chen, M.-K.; Hsieh, Y.-S.; Yang, J.-S.; Zavras, A.-I.; Hsieh, Y.-H.; Su, S.-C.; Kao, T.-Y.; Chen, P.-N.; Chu, S.-C. Antimetastatic effects of Terminalia catappa L. on oral cancer via a down-regulation of metastasis-associated proteases. Food Chem. Toxicol. 2010, 48, 1052–1058. [Google Scholar] [CrossRef]
- Wen, K.-C.; Shih, I.; Hu, J.-C.; Liao, S.-T.; Su, T.-W.; Chiang, H.-M. Inhibitory effects of Terminalia catappa on UVB-induced photodamage in fibroblast cell line. Evid.-Based Complement. Altern. Med. 2010, 2011, 904532. [Google Scholar]
- Jang, M.; Choi, H.Y.; Kim, G.H. Phenolic components rich ethyl acetate fraction of Orostachys japonicus inhibits lipid accumulation by regulating reactive oxygen species generation in adipogenesis. J. Food Biochem. 2019, 43, e12939. [Google Scholar] [CrossRef]
- Gullon, B.; Pintado, M.E.; Fernández-López, J.; Pérez-Álvarez, J.A.; Viuda-Martos, M. In vitro gastrointestinal digestion of pomegranate peel (Punica granatum) flour obtained from co-products: Changes in the antioxidant potential and bioactive compounds stability. J. Funct. Foods 2015, 19, 617–628. [Google Scholar] [CrossRef]
- Li, Z.; Lan, Y.; Miao, J.; Chen, X.; Chen, B.; Liu, G.; Wu, X.; Zhu, X.; Cao, Y. Phytochemicals, antioxidant capacity and cytoprotective effects of jackfruit (Artocarpus heterophyllus Lam.) axis extracts on HepG2 cells. Food Biosci. 2021, 41, 100933. [Google Scholar] [CrossRef]
- Pandey, S.; Tiwari, S.; Kumar, A.; Niranjan, A.; Chand, J.; Lehri, A.; Chauhan, P.S. Antioxidant and anti-aging potential of Juniper berry (Juniperus communis L.) essential oil in Caenorhabditis elegans model system. Ind. Crops Prod. 2018, 120, 113–122. [Google Scholar] [CrossRef]
- Bai, J.; Farias-Pereira, R.; Jang, M.; Zhang, Y.; Lee, S.M.; Kim, Y.-S.; Park, Y.; Ahn, J.B.; Kim, G.-H.; Kim, K.-H. Azelaic acid promotes caenorhabditis elegans longevity at low temperature via an increase in fatty acid desaturation. Pharm. Res. 2021, 38, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Rathor, L.; Pant, A.; Nagar, A.; Tandon, S.; Trivedi, S.; Pandey, R. Trachyspermum ammi L. (Carom) oil induces alterations in SOD-3, GST-4 expression and prolongs lifespan in Caenorhabditis elegans. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2017, 87, 1355–1362. [Google Scholar] [CrossRef]
- Steinberg, H.O.; Chaker, H.; Leaming, R.; Johnson, A.; Brechtel, G.; Baron, A.D. Obesity/insulin resistance is associated with endothelial dysfunction. Implications for the syndrome of insulin resistance. J. Clin. Investig. 1996, 97, 2601–2610. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Ballantyne, C.M. Metabolic inflammation and insulin resistance in obesity. Circ. Res. 2020, 126, 1549–1564. [Google Scholar] [CrossRef] [PubMed]
- AsghariHanjani, N.; Vafa, M. The role of IGF-1 in obesity, cardiovascular disease, and cancer. Med. J. Islam. Repub. Iran 2019, 33, 56. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Sánchez, A.; Madrigal-Santillán, E.; Bautista, M.; Esquivel-Soto, J.; Morales-González, Á.; Esquivel-Chirino, C.; Durante-Montiel, I.; Sánchez-Rivera, G.; Valadez-Vega, C.; Morales-González, J.A. Inflammation, oxidative stress, and obesity. Int. J. Mol. Sci. 2011, 12, 3117–3132. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, R.; Azevedo, I. Chronic inflammation in obesity and the metabolic syndrome. Mediat. Inflamm. 2010, 2010, 289645. [Google Scholar] [CrossRef]
- Koliaki, C.; Liatis, S.; Kokkinos, A. Obesity and cardiovascular disease: Revisiting an old relationship. Metabolism 2019, 92, 98–107. [Google Scholar] [CrossRef]
- Zhang, H.; Qin, J.; Lan, X.; Zeng, W.; Zhou, J.; Huang, T.-E.; Xiao, W.-L.; Wang, Q.-Q.; Sun, S.; Su, W. Handelin extends lifespan and healthspan of Caenorhabditis elegans by reducing ROS generation and improving motor function. Biogerontology 2022, 23, 115–128. [Google Scholar] [CrossRef]
- Balaban, R.S.; Nemoto, S.; Finkel, T. Mitochondria, oxidants, and aging. Cell 2005, 120, 483–495. [Google Scholar] [CrossRef] [PubMed]
- Newsholme, P.; Cruzat, V.F.; Keane, K.N.; Carlessi, R.; de Bittencourt, P.I.H., Jr. Molecular mechanisms of ROS production and oxidative stress in diabetes. Biochem. J. 2016, 473, 4527–4550. [Google Scholar] [CrossRef] [PubMed]
- Xu, A.; Zhang, Z.; Ko, S.H.; Fisher, A.L.; Liu, Z.; Chen, L. Microtubule regulators act in the nervous system to modulate fat metabolism and longevity through DAF-16 in C. elegans. Aging Cell 2019, 18, e12884. [Google Scholar] [CrossRef] [PubMed]
- Blackwell, T.K.; Steinbaugh, M.J.; Hourihan, J.M.; Ewald, C.Y.; Isik, M. SKN-1/Nrf, stress responses, and aging in Caenorhabditis elegans. Free Radic. Biol. Med. 2015, 88, 290–301. [Google Scholar] [CrossRef] [PubMed]
- Wan, Q.-L.; Zheng, S.-Q.; Wu, G.-S.; Luo, H.-R. Aspirin extends the lifespan of Caenorhabditis elegans via AMPK and DAF-16/FOXO in dietary restriction pathway. Exp. Gerontol. 2013, 48, 499–506. [Google Scholar] [CrossRef]
- Wang, S.; Yi, X.; Wu, Z.; Guo, S.; Dai, W.; Wang, H.; Shi, Q.; Zeng, K.; Guo, W.; Li, C. CAMKK2 defines ferroptosis sensitivity of melanoma cells by regulating AMPK—NRF2 pathway. J. Investig. Dermatol. 2022, 142, 189–200.e188. [Google Scholar] [CrossRef]
- Koyama, T.; Nakajima, C.; Nighimoto, S.; Takami, M.; Woo, J.-T.; Yazawa, K. Suppressive effects of the leaf of Terminalia catappa L. on osteoclast differentiation in vitro and bone weight loss in vivo. J. Nutr. Sci. Vitaminol. 2012, 58, 129–135. [Google Scholar] [CrossRef][Green Version]
- Jang, M.; Choi, S.I. Schisandrin C isolated from Schisandra chinensis fruits inhibits lipid accumulation by regulating adipogenesis and lipolysis through AMPK signaling in 3T3-L1 adipocytes. J. Food Biochem. 2022, 46, e14454. [Google Scholar] [CrossRef]
- Wang, K.; Chen, S.; Zhang, C.; Huang, J.; Wu, J.; Zhou, H.; Jin, L.; Qian, X.; Jin, J.; Lyu, J. Enhanced ROS production leads to excessive fat accumulation through DAF-16 in Caenorhabditis elegans. Exp. Gerontol. 2018, 112, 20–29. [Google Scholar] [CrossRef]
- Bai, J.; Farias-Pereira, R.; Zhang, Y.; Jang, M.; Park, Y.; Kim, K.H. C. elegans ACAT regulates lipolysis and its related lifespan in fasting through modulation of the genes in lipolysis and insulin/IGF-1 signaling. BioFactors 2020, 46, 754–765. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).