The Influence of Caramel Carbon Quantum Dots and Caramel on Platelet Aggregation, Protein Glycation and Lipid Peroxidation
Abstract
:1. Introduction
2. Experimental Methods
2.1. Chemicals and Reagents
2.2. Preparation of Caramel and Carbon Quantum Dots (CQDs)
2.3. Tested Solutions
- Dilutions of the base CQD solution—three-fold (Q1), ten-fold (Q2), thirty-fold (Q3) and one-hundred-fold (Q4);
- Dilutions of the base caramel solution—three-fold (C1), ten-fold (C2), thirty-fold (C3) and one-hundred-fold (C4);
- 500 mM sucrose (S500);
- Glucose—500 mM (G 500), 50 mM (G 50);
- Fructose—500 mM (F 500), 50 mM (F50);
- 250 mM glucose + 250 mM fructose (G + F);
- Erythritol—500 mM (E 500), 50 mM (E 50), 5 mM (E 5);
- Xylitol—500 mM (X 500), 50 mM (X 50), 5 mM (X 5).
2.4. Whole Blood Aggregation
2.5. Impact on Platelet Viability (Cytotoxicity Test)
2.6. In Vitro Protein Glycation
2.6.1. Preparation of Glycated BSA Samples
2.6.2. Advanced Glycation End Products Formation
2.6.3. Fructosamine Content
2.7. Oxidative Stress in Adipose Tissue
2.7.1. Isolation of Adipose Tissue and Incubation with Tested Solutions
2.7.2. Lipid Peroxidation Assay
2.7.3. Total Reducing Power of Adipose Tissue
2.8. Ferric Reducing Antioxidant Power (FRAP)
2.9. In Vitro Integration of the Red Blood Cell Membrane
2.9.1. Preparation of Blood Sample for Red Blood Cells Lysis Assay
2.9.2. Red Blood Cells Lysis Assay
2.10. Statistical Analysis
3. Results
3.1. Carbon Quantum Dots and Caramel
3.2. Effect of Carbon Quantum Dots and Caramel on Platelet Aggregation
3.3. Effect of Carbon Quantum Dots and Caramel on Platelet Viability
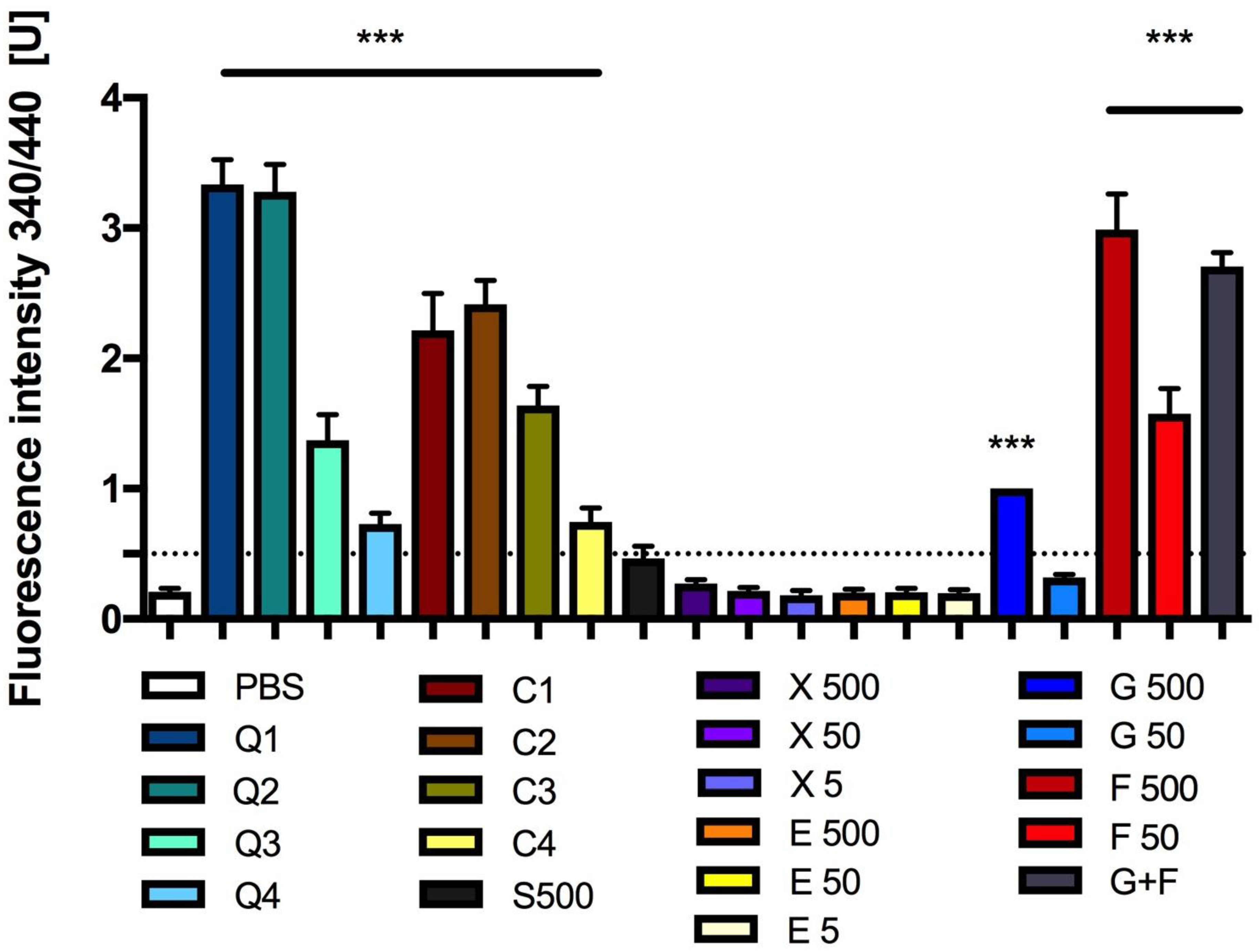
3.4. Effect of Carbon Quantum Dots and Caramel on Protein Glycation
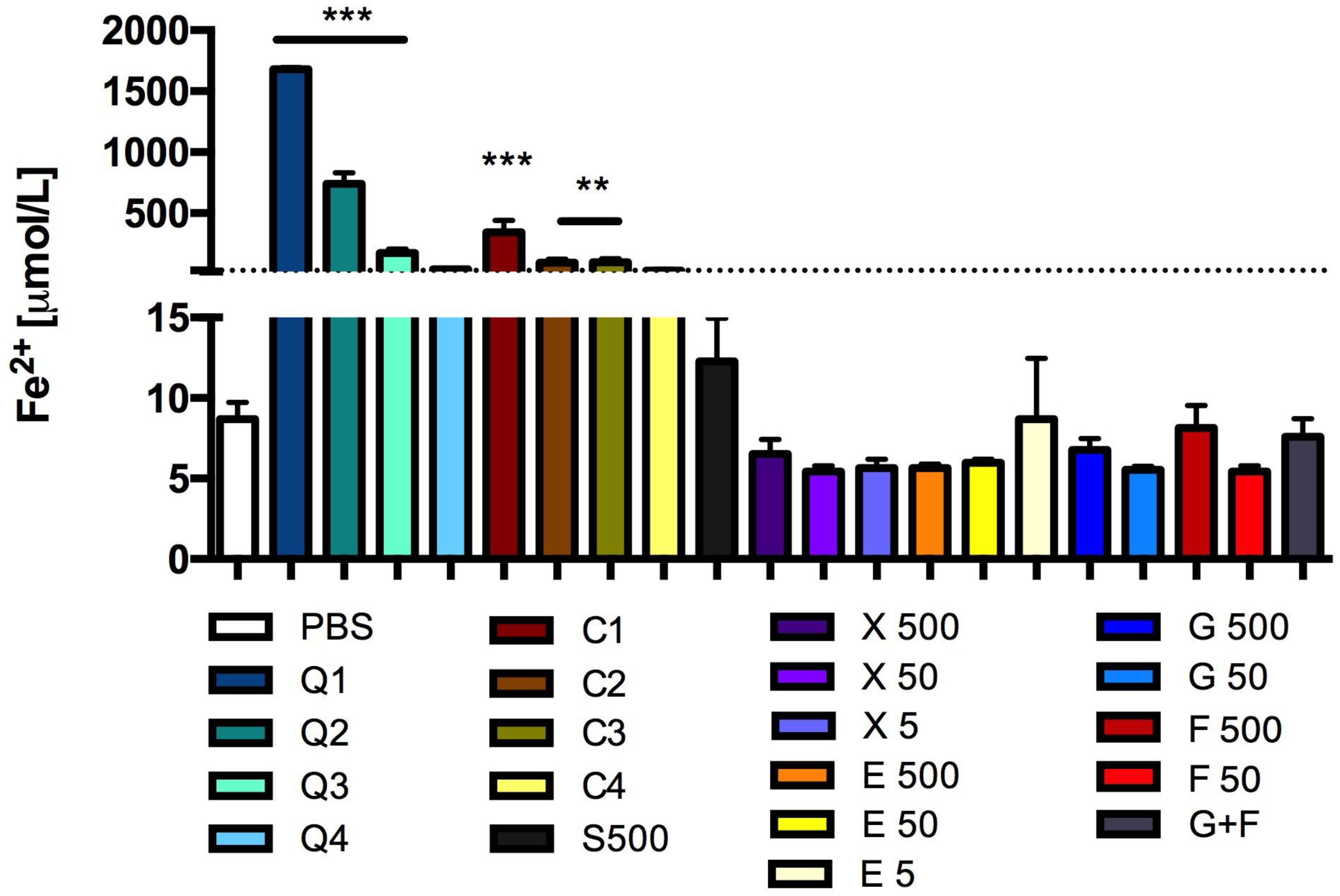
3.5. Effect of Carbon Quantum Dots and Caramel on Lipid Peroxidation
3.6. In Vitro Reducing Properties of Carbon Quantum Dots and Caramel
3.7. Effect of Carbon Quantum Dots and Caramel on Integrity of the Red Blood Cell Membrane
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Saraiva, A.; Carrascosa, C.; Raheem, D.; Ramos, F.; Raposo, A. Natural Sweeteners: The Relevance of Food Naturalness for Consumers, Food Security Aspects, Sustainability and Health Impacts. Int. J. Environ. Res. Public. Health. 2020, 17, 6285. [Google Scholar] [CrossRef] [PubMed]
- Witkowski, M.; Nemet, I.; Alamri, H.; Wilcox, J.; Gupta, N.; Nimer, N.; Haghikia, A.; Li, X.S.; Wu, Y.; Saha, P.P.; et al. The artificial sweetener erythritol and cardiovascular event risk. Nat. Med. 2023, 29, 710–718. [Google Scholar] [CrossRef] [PubMed]
- Grembecka, M. Natural sweeteners in a human diet. Rocz. Państwowego Zakładu Hig. 2015, 66, 195–202. [Google Scholar]
- Sengar, G.; Sharma, H.K. Food caramels: A review. J. Food Sci. Technol. 2014, 51, 1686–1696. [Google Scholar] [CrossRef] [PubMed]
- Vollmuth, T.A. Caramel color safety—An update. Food Chem. Toxicol. 2018, 111, 578–596. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.C.; Wang, M.; Yong, A.M.; Wong, S.Y.; Zhang, X.H.; Tan, H.; Chang, A.Y.; Li, X.; Wang, J. Intrinsically Fluorescent Carbon Dots with Tunable Emission Derived from Hydrothermal Treatment of Glucose in the Presence of Monopotassium Phosphate. Chem. Commun. 2011, 47, 11615−11617. [Google Scholar] [CrossRef] [PubMed]
- Wojnicki, M.; Hessel, V. Quantum materials made in microfluidics—Critical review and perspective. Chem. Eng. J. 2022, 438, 135616. [Google Scholar] [CrossRef]
- Pho, H.Q.; Jiao, Y.; Jiao, Y.; Sun, W.; Tansu, N.; Wojnicki, M.; Shabbir, H.; Rogóż, K.; Tran, N.N.; Lewis, D.; et al. Quantum Materials; Wiley: Hoboken, NJ, USA, 2023. [Google Scholar] [CrossRef]
- Shabbir, H.; Wojnicki, M. Recent Progress of Non-Cadmium and Organic Quantum Dots for Optoelectronic Applications with a Focus on Photodetector Devices. Electronics 2023, 12, 1327. [Google Scholar] [CrossRef]
- de Mello Rocha, A.P.; Alayo, M.I.; da Silva, D.M. Synthesis of Nitrogen-Doped Graphene Quantum Dots from Sucrose Carbonization. Appl. Sci. 2022, 12, 8686. [Google Scholar] [CrossRef]
- Yang, H.-L.; Bai, L.-F.; Geng, A.-R.; Chen, H.; Xu, L.-T.; Xie, X.-C.; Wang, D.-J.; Gu, H.-W.; Wang, X.-M. Carbon quantum dots: Preparation, optical properties, and biomedical applications. Mater. Today Adv. 2023, 18, 100369. [Google Scholar] [CrossRef]
- Cui, L.; Ren, X.; Sun, M.; Liu, H.; Xia, L. Carbon Dots: Synthesis, Properties and Applications. Nanomaterials 2021, 11, 3419. [Google Scholar] [CrossRef] [PubMed]
- Wölnerhanssen, B.K.; Meyer-Gerspach, A.C.; Beglinger, C.; Islam, M.S. Metabolic effects of the natural sweeteners xylitol and erythritol: A comprehensive review. Crit. Rev. Food Sci. Nutr. 2020, 60, 1986–1998. [Google Scholar] [CrossRef] [PubMed]
- Mazi, T.A.; Stanhope, K.L. Erythritol: An in-depth discussion of its potential to be a beneficial dietary component. Nutrients 2023, 15, 204. [Google Scholar] [CrossRef] [PubMed]
- Msomi, N.Z.; Erukainure, O.L.; Salau, V.F.; Olofinsan, K.A.; Islam, M.S. Comparative effects of xylitol and erythritol on modulating blood glucose; inducing insulin secretion; reducing dyslipidemia and redox imbalance in a type 2 diabetes rat model. Food Sci. Human Well 2023, 12, 2052–2060. [Google Scholar] [CrossRef]
- Ruanpeng, D.; Thongprayoon, C.; Cheungpasitporn, W.; Harindhanavudhi, T. Sugar and artificially sweetened beverages linked to obesity: A systematic review and meta-analysis. QJM 2017, 110, 513–520. [Google Scholar] [CrossRef] [PubMed]
- Romo-Romo, A.; Aguilar-Salinas, C.A.; Brito-Córdova, G.X.; Gómez-Díaz, R.A.; Almeda-Valdes, P. Sucralose decreases insulin sensitivity in healthy subjects: A randomized controlled trial. Am. J. Clin. Nutr. 2018, 108, 485–491. [Google Scholar] [CrossRef] [PubMed]
- Imamura, F.; O’Connor, L.; Ye, Z.; Mursu, J.; Hayashino, Y.; Bhupathiraju, S.N.; Forouhi, N.G. Consumption of sugar sweetened beverages, artificially sweetened beverages and fruit juice and incidence of type 2 diabetes: Systematic review, meta-analysis and estimation of population attributable fraction. Br. Med. J. 2015, 351, h3576. [Google Scholar] [CrossRef]
- Mossavar-Rahmani, Y.; Kamensky, V.; Manson, J.E.; Silver, B.; Rapp, S.R.; Haring, B.; Beresford, S.A.A.; Snetselaar, L.; Wassertheil-Smoller, S. Artificially sweetened beverages and stroke, coronary heart disease and all-cause mortality in the Women’s Health Initiative. Stroke 2019, 50, 555–562. [Google Scholar] [CrossRef]
- Malik, V.S.; Li, Y.; Pan, A.; De Koning, L.; Schernhammer, E.; Willett, W.C.; Hu, F.B. Long-term consumption of sugar-sweetened and artificially sweetened beverages and risk of mortality in US adults. Circulation 2019, 139, 2113–2125. [Google Scholar] [CrossRef]
- Mullee, A.; Romaguera, D.; Pearson-Stuttard, J.; Viallon, V.; Stepien, M.; Freisling, H.; Fagherazzi, G.; Mancini, F.R.; Boutron-Ruault, M.-C.; Kühn, T.; et al. Association between soft drink consumption and mortality in ten European countries. JAMA Intern. Med. 2019, 179, 1479–1490. [Google Scholar] [CrossRef]
- Nammahachak, N.; Aup-Ngoen, K.K.; Asanithic, P.; Horpratumd, M.; Chuangchote, S.; Ratanaphan, S.; Surareungchai, W. Hydrothermal synthesis of carbon quantum dots with size tunability via heterogeneous nucleation. RSC Adv. 2022, 12, 31729–31733. [Google Scholar] [CrossRef] [PubMed]
- Bhatwadekar, A.D.; Ghole, V.S. Rapid method for the preparation of an AGE-BSA standard calibrator using thermal glycation. J. Clin. Lab. Anal. 2005, 19, 11–15. [Google Scholar] [CrossRef] [PubMed]
- Chukwuma, C.I.; Shahidul, I. Effects of xylitol on carbohydrate digesting enzymes activity, intestinal glucose absorbcion and muscle glucose uptake: A multi-mode study. Food Funct. 2015, 6, 955–962. [Google Scholar] [CrossRef] [PubMed]
- Erukainure, O.L.; Matsabisa, M.G.; Salau, V.F.; Olofinsan, K.A.; Oyedemi, S.O.; Chukwuma, C.I.; Nde, A.L.; Islam, M.S. Cannabidiol improves glucose utilization and modulates glucose-induced dysmetabolic activities in isolated rats’ peripheral adipose tissues. Biomed. Pharmacother. 2022, 149, 112863. [Google Scholar] [CrossRef] [PubMed]
- Mazzara, E.; Carletti, R.; Petrelli, R.; Mustafa, A.M.; Caprioli, G.; Fiorini, D.; Scortichini, S.; Dall’Acqua, S.; Sut, S.; Nunez, S.; et al. Greem ectraction of hemp (Cannabis sativa L.) using microwave method for recovery of three valuable fractions (essential oil, phenolic compounds and cannabinoids): A central composite design optimization study. J. Sci. Food Agric. 2022, 102, 6220–6235. [Google Scholar] [CrossRef]
- Grzegorczyk-Karolak, I.; Gołąb, K.; Gburek, J.; Wysokińska, H.; Matkowska, A. Inhibition of Advanced Glycation End-Product Formation and Antioxidant Activity by Extracts and Polyphenols from Scutellaria alpina L. and S. altissima L. Molecules 2016, 21, 739. [Google Scholar] [CrossRef]
- Adisakwattana, S.; Sompong, W.; Meeprom, A.; Sathaporn, N.; Yibchok-anun, S. Cinnamic acid and its derivatives inhibit fructose-mediated protein glycation. Int. J. Mol. Sci. 2012, 13, 1778–1789. [Google Scholar] [CrossRef]
- Kubacka, M.; Kazek, G.; Kotańska, M.; Filipek, B.; Waszkielewicz, A.M.; Mogilski, S. Anti-aggregation effect of aroxyalkyl derivatives of 2-methoxyphenylpiperazine is due to their 5-HT2A and α2-adrenoceptor antagonistic properties. A comparison with ketanserin, sarpogrelate, prazosin, yohimbine and ARC239. Eur. J. Pharmacol. 2018, 818, 263–270. [Google Scholar] [CrossRef]
- Kubacka, M.; Mogilski, S.; Bednarski, M.; Pociecha, K.; Świerczek, A.; Nicosia, N.; Schabikowski, J.; Załuski, M.; Chłoń-Rzepa, G.; Hockemeyer, J.; et al. Antiplatelet Effects of Selected Xanthine-Based Adenosine A2A and A2B Receptor Antagonists Determined in Rat Blood. Int. J. Mol. Sci. 2023, 24, 13378. [Google Scholar] [CrossRef]
- Trichler, S.A.; Bulla, S.C.; Thomason, J.; Lunsford, K.V.; Bulla, C. Ultra-pure platelet isolation from canine whole blood. BMC Vet. Res. 2013, 9, 144. [Google Scholar] [CrossRef]
- Vinson, J.A.; Howard, T.B.I.I.I. Inhibition of protein glycation and advanced glycation end products by ascorbic acid and other vitamins and nutrients. J. Nutr. Biochem. 1996, 7, 659–663. [Google Scholar] [CrossRef]
- Ansi, V.A.; Renuka, N.K. Sucrose derived luminescent carbon dots as a promising bio-medical agent. Mater. Today Procesing. 2019, 18, 1724–1728. [Google Scholar] [CrossRef]
- Shabbir, H.; Csapó, E.; Wojnicki, M. Carbon quantum dots: The role of surface functional groups and proposed mechanisms for metal ion sensing. Inorganics 2023, 11, 262. [Google Scholar] [CrossRef]
- Sarkar, S.; Banerjee, D.; Ghorai, U.K.; Chattopadhyay, K.K. Size dependent photoluminescence property of hydrothermally synthesised crystalline carbon quantum dots. J. Luminesc. 2016, 178, 314–323. [Google Scholar] [CrossRef]
- Johnson, R.N.; Metcalf, P.A.; Baker, J.R. Fructosamine: A new approach to the estimation of serum glycosylprotein. An index of diabetic control. Clin. Chim. Acta 1983, 127, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Deo, P.; Chern, C.; Peake, B.; Tan, S.-Y. Non-nutritive sweeteners are in concomitant with the formation of endogenous and exogenous advanced glycation end-products. Int. J. Food Sci. Nutr. 2020, 71, 706–714. [Google Scholar] [CrossRef] [PubMed]
- Kowalczyk-Pachel, D.; Iciek, M.; Wydra, K.; Nowak, E.; Górny, M.; Filip, M.; Włodek, L.; Lorenc-Koci, E. Cysteine Metabolism and Oxidative Processes in the Rat Liver and Kidney after Acute and Repeated Cocaine Treatment. PLoS ONE 2016, 11, e0147238. [Google Scholar] [CrossRef]
- Ohkawa, H.; Ohshi, N.; Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef]
- Benzie, I.; Strain, J. Direct measure of total antioxidant activity of biological fluids and modified version for simultaneous measurement of total antioxidant power and ascorbic. Enzymology 1999, 299, 15–27. [Google Scholar]
- Shabbir, H.; Wojtaszek, K.; Rutkowski, B.; Csapó, E.; Bednarski, M.; Adamiec, A.; Głuch-Lutwin, M.; Mordyl, B.; Druciarek, J.; Kotańska, M.; et al. Milk-Derived Carbon Quantum Dots: Study of Biological and Chemical Properties Provides Evidence of Toxicity. Molecules 2022, 27, 8728. [Google Scholar] [CrossRef]
- Kotańska, M.; Mika, K.; Szafarz, M.; Dziubina, A.; Bednarski, M.; Müller, C.E.; Sapa, J.; Kieć-Kononowicz, K. PSB 603—A known selective adenosine A2B receptor antagonist–has anti-inflammatory activity in mice. Biomed. Pharmacother. 2021, 135, 111164. [Google Scholar] [CrossRef] [PubMed]
- Kotańska, M.; Kubacka, M.; Bednarski, M.; Nicosia, N.; Szafarz, M.; Jawień, W.; Müller, C.E.; Kieć-Kononowicz, K. The GPR18 Agonist PSB-KD-107 Exerts Endothelium-Dependent Vasorelaxant Effects. Pharmaceuticals 2021, 14, 799. [Google Scholar] [CrossRef] [PubMed]
- Parvin, M.S.; Das, N.; Jahan, N.; Akhter, M.A.; Nahar, L.; Islam, M.E. Evaluation of in vitro anti-inflammatory and antibacterial potential of Crescentia cujete leaves and stem bark. BMC Res. Notes 2015, 8, 412–418. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Guo, Z.; Zhang, Y.; Xue, W.; Liu, Z. Blood compatibility evaluations of fluorescent carbon dots. ACS Appl. Mater. Interfaces 2015, 7, 19153–19162. [Google Scholar] [CrossRef]
- Fournet, M.; Bonte, F.; Desmouliere, A. Glycation Damage: A Possible Hub for Major Pathophysiological Disorders and Aging. Aging Dis. 2018, 9, 880–900. [Google Scholar] [CrossRef]
- Sudic, D.; Razmara, M.; Forslund, M.; Ji, Q.; Hjemdahl, P.; Li, N. High glucose levels enhance platelet activation: Involvement of multiple mechanisms. Br. J. Heamatol. 2006, 133, 315–322. [Google Scholar] [CrossRef]
- Tang, W.H.; Stitham, J.; Gleim, S.; Di Febbo, C.; Porreca, E.; Fava, C.; Tacconelli, S.; Capone, M.; Evangelista, V.; Levantesi, G.; et al. Glucose and collagen regulate human platelet activity through aldose reductase induction of thromboxane. J. Clin. Investig. 2011, 121, 4462–4476. [Google Scholar] [CrossRef]
- Yngen, M.; Östenson, C.G.; Hu, H.; Li, N.; Hjemdahl, P.; Wallén, N.H. Enhanced P-selectin expression and increased soluble CD40 Ligand in patients with Type 1 diabetes mellitus and microangiopathy: Evidence for platelet hyperactivity and chronic inflammation. Diabetologia 2004, 47, 537–540. [Google Scholar] [CrossRef]
- De la Cruz, J.P.; Maximo, M.A.; Blanco, E.; Moreno, A.; Sánchez de la Cuesta, F. Effect of erythrocytes and prostacyclin production in the effect of fructose and sorbitol on platelet activation in human whole blood in vitro. Thromb. Res. 1997, 86, 515–524. [Google Scholar] [CrossRef]
- Hootman, K.C.; Trezzi, J.-P.; Kraemer, L.; Burwell, L.S.; Dong, X.; Guertin, K.A.; Jaeger, C.; Stover, P.J.; Hiller, K.; Cassano, P.A.; et al. Erythritol is a pentose-phosphate pathway metabolite and associated with adiposity gain in young adults. Proc. Natl. Acad. Sci. USA 2017, 114, E4233–E4240. [Google Scholar] [CrossRef]
- Lee, T.-Y.; Jayakumar, T.; Thanasekaran, P.; Lin, K.-C.; Chen, H.-M.; Veerakumar, P.; Sheu, J.-R. Carbon dot nanoparticles exert inhibitory effects on human platelets and reduce mortality in mice with acute pulmonary thromboembolism. Nanomaterials 2020, 10, 1254. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Zhao, Y.; Luo, J.; Xiong, W.; Liu, X.; Cheng, J.; Wang, Y.; Zhang, M.; Qu, H. Hemostatic bioactivity of novel Pollen Typhae Carbonisata-derived carbon quantum dots. J. Nanobiotechnol. 2017, 15, 60. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhang, Y.; Liu, X.; Kong, H.; Wang, Y.; Qin, G.; Cao, P.; Song, X.; Yan, X.; Wang, Q.; et al. Novel carbon quantum dots from egg yolk oil and their haemostatic effects. Sci. Rep. 2017, 7, 4452. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wang, Y.; Yan, X.; Zhang, M.; Zhang, Y.; Cheng, J.; Lu, F.; Qu, H.; Wang, Q.; Zhao, Y. Novel phellodendri cortex (huang bo)-derived carbon dots and their hemostatic effect. Nanomedicine 2018, 13, 391–405. [Google Scholar] [CrossRef] [PubMed]
- Massucco, P.; Mattiello, L.; Russo, I.; Traversa, M.; Doronzo, G.; Anfossi, G.; Trovati, M. High glucose rapidly activates the nitric oxide/cyclic nucleotide pathway in human platelets via an osmotic mechanism. Thromb. Haemost. 2005, 93, 517–526. [Google Scholar] [CrossRef] [PubMed]
- Assert, R.; Scherk, G.; Bumbure, A.; Pirags, V.; Schatz, H.; Pfeiffer, A.F. Regulation of protein kinase C by short term hyperglycaemia in human platelets in vivo and in vitro. Diabetologia 2001, 44, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Bains, Y.; Gugliucci, A. Ilex paraguariensis and its main component chlorogenic acid inhibit fructose formation of advanced glycation endproducts with amino acids at conditions compatible with those in the digestive system. Fitoterapia 2017, 117, 6e10. [Google Scholar] [CrossRef]
- Zhao, W.; Devamanoharan, P.S.; Varma, S.D. Fructose induced deactivation of glucose-6-phosphate dehydrogenase activity and its prevention by pyruvate: Implications in cataract prevention. Free Rad. Res. 1998, 29, 315–320. [Google Scholar] [CrossRef]
- Brownlee, M.; Cerami, A.; Vlassara, H. Advanced glycosylation end products in tissue and the biochemical basis of diabetic complications. N. Engl. J. Med. 1988, 318, 1315–1321. [Google Scholar]
- Thorpe, S.R.; Baynes, J.W. Maillard reaction products in tissue proteins: New products and new perspectives. Amino Acids. 2003, 25, 275–281. [Google Scholar] [CrossRef]
- Deo, P.; Glenn, J.V.; Powell, L.A.; Stitt, A.W.; Ames, J.M. Upregulation of oxidative stress markers in human microvascular endothelial cells by complexes of serum albumin and digestion products of glycated casein. J. Biochem. Mol. Toxicol. 2009, 23, 364–372. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, N. Advanced glycation end products-role in pathology of diabetic complications. Diabetes Res. Clin. Pract. 2005, 67, 3–21. [Google Scholar] [CrossRef] [PubMed]
- Aragno, M.; Mastrocola, R. Dietary sugars and endogenous formation of advanced glycation end products: Emerging mechanisms of disease. Nutrients 2017, 9, 385. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, A.M.; Hori, O.; Cao, R.; Yan, S.D.; Brett, J.; Wautier, J.L.; Ogawa, S.; Kuwabara, K.; Matsumoto, M.; Stern, D. RAGE: A novel cellular receptor for advanced glycation end products. Diabetes 1996, 45 (Suppl. S3), S77–S80. [Google Scholar] [CrossRef]
- Vlassara, H. Advanced glycation end-products and atherosclerosis. Ann. Med. 1996, 28, 419–426. [Google Scholar] [CrossRef]
- Kellow, N.J.; Savige, G.S. Dietary advanced glycation end-product restriction for the attenuation of insulin resistance, oxidative stress and endothelial dysfunction: A systematic review. Eur. J. Clin. Nutr. 2013, 67, 239–248. [Google Scholar] [CrossRef]
- Van Puyvelde, K.; Mets, T.; Njemini, R.; Beyer, I.; Bautmans, I. Effect of advanced glycation end product intake on inflammation and aging: A systematic review. Nutr. Rev. 2014, 72, 638–650. [Google Scholar] [CrossRef]
- Faist, V.; Erbersdobler, H.F. Metabolic transit and in vivo effects of melanoidins and precursor compounds deriving from the Maillard reaction. Ann. Nutr. Metab. 2001, 45, 1–12. [Google Scholar] [CrossRef]
- Baynes, J.W. Role of oxidative stress in development of complications in diabetes. Diabetes 1991, 40, 405–412. [Google Scholar] [CrossRef]
- Koya, D.; King, G.L. Protein kinase C activation and the development of diabetic complications. Diabetes 1998, 47, 859–866. [Google Scholar] [CrossRef]
- Kennedy, A.L.; Lyons, T.J. Glycation, oxidation, and lipoxidation in the development of diabetic complications. Metabolism 1997, 46, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Baynes, J.W.; Thorpe, S.R. Glycoxidation and lipoxidation in atherogenesis. Free Radic. Biol. Med. 2000, 28, 1708–1716. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zhao, L.; Du, F.; Wu, Y.; Cai, R.; Xu, L.; Jin, H.; Zou, S.; Gong, A.; Du, F. Facile synthesis of cerium-doped carbon quantum dots as a highly efficient antioxidant for free radical scavenging. Nanotechnology 2019, 30, 325101. [Google Scholar] [CrossRef] [PubMed]
- Šafranko, S.; Stanković, A.; Hajra, S.; Kim, H.J.; Strelec, I.; Dutour-Sikirić, M.; Weber, I.; Bosnar, M.H.; Grbčić, P.; Pavelić, S.K.; et al. Preparation of Multifunctional N-Doped Carbon Quantum Dots from Citrus clementina Peel: Investigating Targeted Pharmacological Activities and the Potential Application for Fe3+ Sensing. Pharmaceuticals 2021, 14, 857. [Google Scholar] [CrossRef]
- Shahid, S.; Mohiyuddin, S.; Packirisamy, G. Synthesis of Multi-Color Fluorescent Carbon Dots From Mint Leaves: A Robust Bioimaging Agent with Potential Antioxidant Activity. J. Nanosci. Nanotechnol. 2020, 20, 6305–6316. [Google Scholar] [CrossRef]
- Chen, M.; Leng, Y.; He, C.; Li, X.; Zhao, L.; Qu, Y.; Wu, Y. Red blood cells: A potential delivery system. J. Nanobiotechnol. 2023, 21, 288. [Google Scholar] [CrossRef]
- Raval, J.S.; Fontes, J.; Banerjee, U.; Yazer, M.H.; Mank, E.; Palmer, A.F. Ascorbic acid improves membrane fragility and decreases haemolysis during red blood cell storage. Transfus. Med. 2013, 23, 87–93. [Google Scholar] [CrossRef]
- Foster, D.W. Diabetes mellitus. In Harrison’s Principles of Internal Medicine; Willson, J.D., Braunwald, E., Isselbacher, K.J., Petersdorf, R.G., Martin, J.B., Fauci, A.S., Root, R.K., Eds.; McGraw-Hill, Inc.: New York, NY, USA, 1991; Volume 2, pp. 1739–1759. [Google Scholar]








Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kotańska, M.; Wojtaszek, K.; Kubacka, M.; Bednarski, M.; Nicosia, N.; Wojnicki, M. The Influence of Caramel Carbon Quantum Dots and Caramel on Platelet Aggregation, Protein Glycation and Lipid Peroxidation. Antioxidants 2024, 13, 13. https://doi.org/10.3390/antiox13010013
Kotańska M, Wojtaszek K, Kubacka M, Bednarski M, Nicosia N, Wojnicki M. The Influence of Caramel Carbon Quantum Dots and Caramel on Platelet Aggregation, Protein Glycation and Lipid Peroxidation. Antioxidants. 2024; 13(1):13. https://doi.org/10.3390/antiox13010013
Chicago/Turabian StyleKotańska, Magdalena, Konrad Wojtaszek, Monika Kubacka, Marek Bednarski, Noemi Nicosia, and Marek Wojnicki. 2024. "The Influence of Caramel Carbon Quantum Dots and Caramel on Platelet Aggregation, Protein Glycation and Lipid Peroxidation" Antioxidants 13, no. 1: 13. https://doi.org/10.3390/antiox13010013
APA StyleKotańska, M., Wojtaszek, K., Kubacka, M., Bednarski, M., Nicosia, N., & Wojnicki, M. (2024). The Influence of Caramel Carbon Quantum Dots and Caramel on Platelet Aggregation, Protein Glycation and Lipid Peroxidation. Antioxidants, 13(1), 13. https://doi.org/10.3390/antiox13010013






