Valorization of Olive Leaf Polyphenols by Green Extraction and Selective Adsorption on Biochar Derived from Grapevine Pruning Residues
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. BC Production
2.3. The Extraction of Polyphenolic Compounds
2.4. Analysis of Polyphenolic Compounds in Olive Leaf Extracts
2.5. Antioxidant Capacity of Olive Leaf Extracts
2.6. Adsorption Capacity of BC Derived from Grapevine Pruning Residues
2.7. Statistical Analysis
3. Results
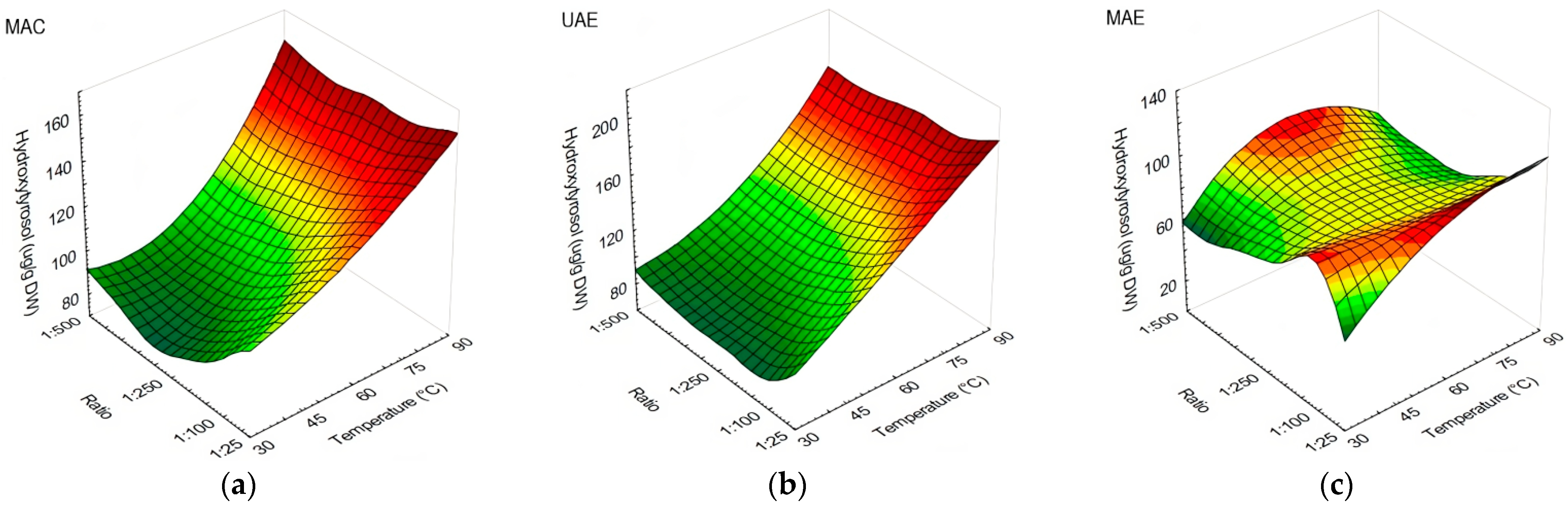
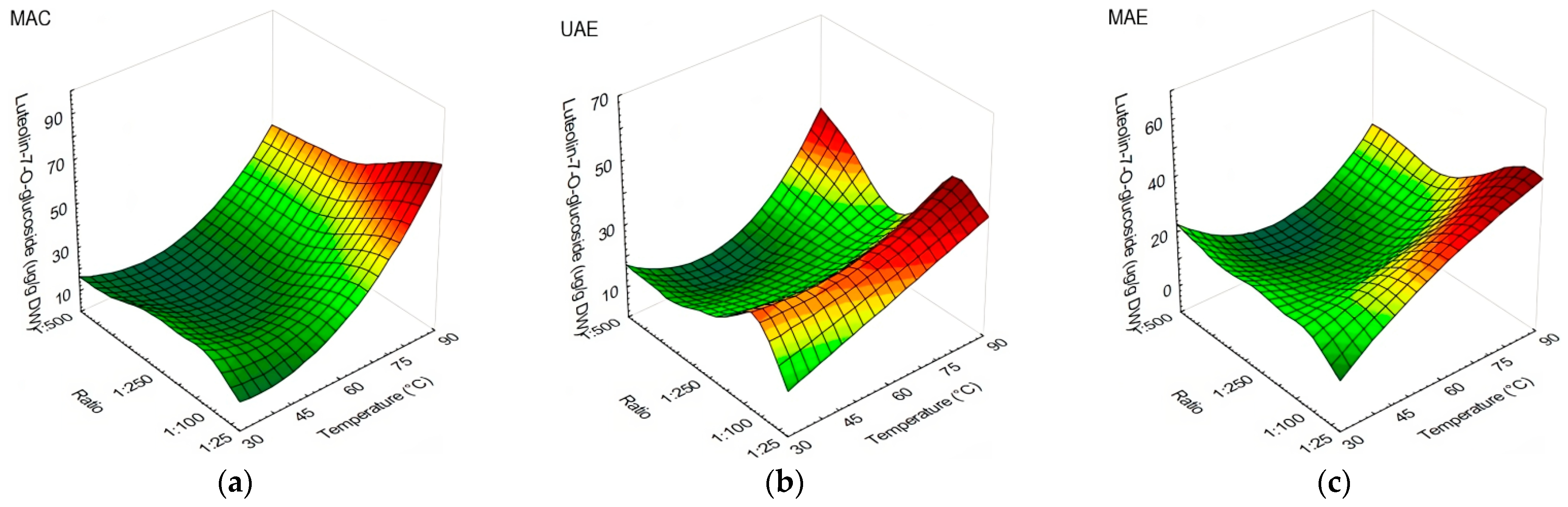
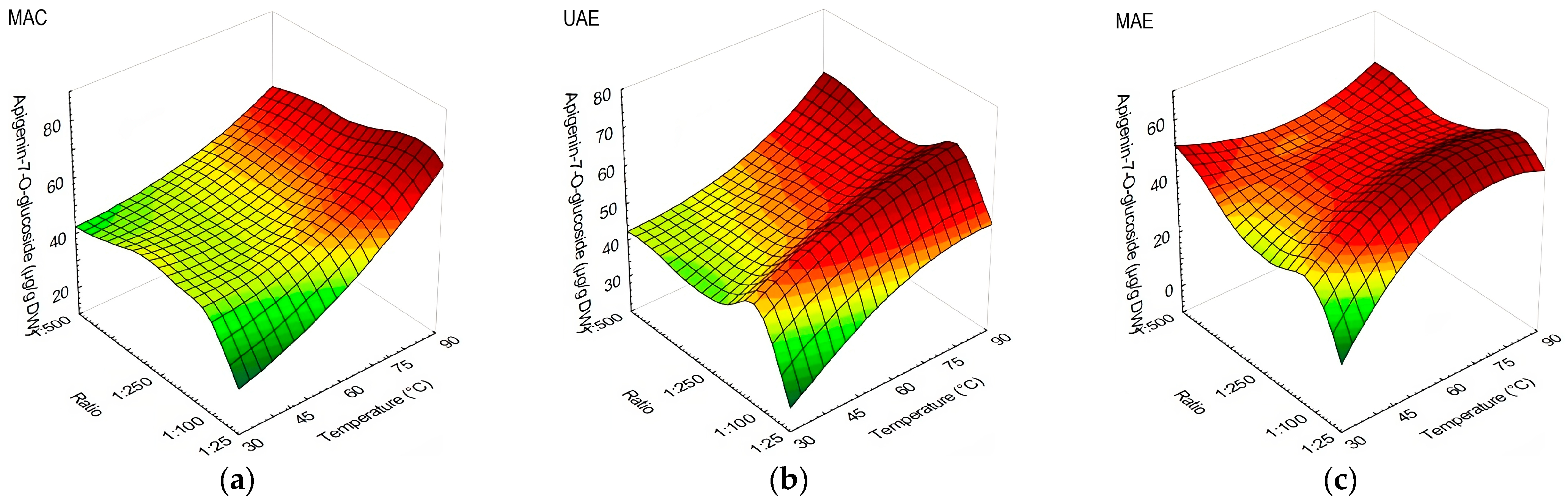
3.1. The Influences of Extraction Method, Temperature, and Solid-to-Liquid Ratio on Olive Leaves’ Polyphenolic Compound Content and Antioxidant Capacity
3.2. Adsorption Capacity
3.2.1. Langmuir and Freundlich Isotherms
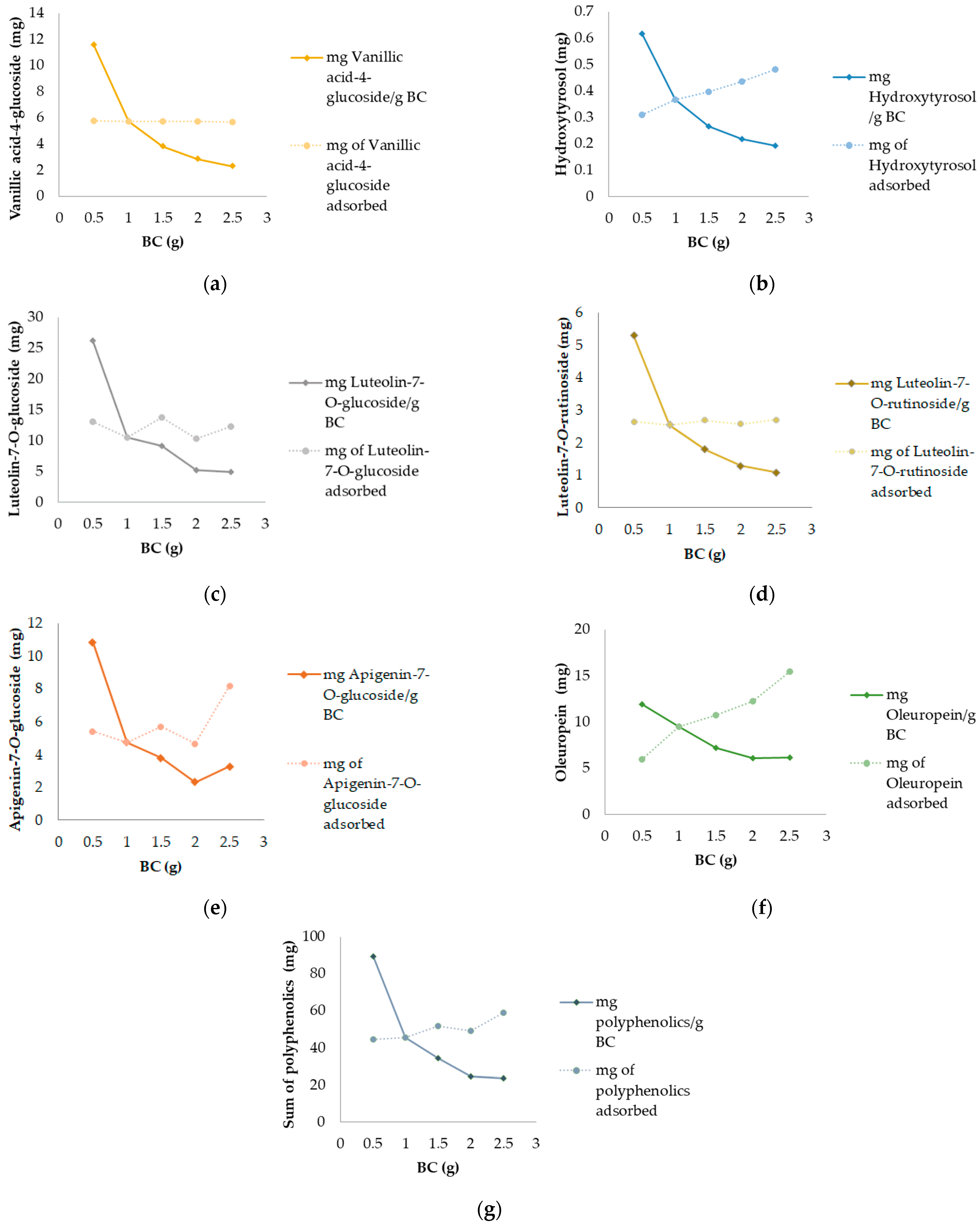
3.2.2. Effects of BC Dosages in Polyphenolic Adsorption
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lorenz, K.; Lal, R. Environmental Impact of Organic Agriculture. In Advances in Agronomy; Donald, L.S., Ed.; Academic Press Inc.: London, UK, 2016; Volume 139, pp. 99–152. ISBN 9780128047736. [Google Scholar]
- Chen, Y.-H.; Wen, X.-W.; Wang, B.; Nie, P.-Y. Agricultural Pollution and Regulation: How to Subsidize Agriculture? J. Clean. Prod. 2017, 164, 258–264. [Google Scholar] [CrossRef]
- Ghosh, A.; Misra, S.; Bhattacharyya, R.; Sarkar, A.; Singh, A.K.; Tyagi, V.C.; Kumar, R.V.; Meena, V.S. Agriculture, Dairy and Fishery Farming Practices and Greenhouse Gas Emission Footprint: A Strategic Appraisal for Mitigation. Environ. Sci. Pollut. Res. 2020, 27, 10160–10184. [Google Scholar] [CrossRef] [PubMed]
- Baque, M.A.; Moh, S.H.; Lee, E.J.; Zhong, J.J.; Paek, K.Y. Production of Biomass and Useful Compounds from Adventitious Roots of High-Value Added Medicinal Plants Using Bioreactor. Biotechnol. Adv. 2012, 30, 1255–1267. [Google Scholar] [CrossRef] [PubMed]
- Yen, H.W.; Yang, S.C.; Chen, C.H.; Jesisca; Chang, J.S. Supercritical Fluid Extraction of Valuable Compounds from Microalgal Biomass. Bioresour. Technol. 2015, 184, 291–296. [Google Scholar] [CrossRef] [PubMed]
- EU Eurostat. Available online: https://ec.europa.eu/eurostat (accessed on 13 September 2023).
- Ahmad, S.; Ahmad Chatha, Z.; Nasir, M.A.; Aziz, A.; Ahmad Virk, N.; Rehman Khan, A. Effect of Pruning on the Yield and Quality of Kinnow Fruit. J. Agric. Soc. Sci. 2006, 2, 51–53. [Google Scholar]
- Albert, T.; Karp, K.; Starast, M.; Paal, T. The Effect of Mulching and Pruning on the Vegetative Growth and Yield of the Half-High Blueberry. Agron. Res. 2010, 8, 759–769. [Google Scholar]
- Ruiz, E.; Romero-García, J.M.; Romero, I.; Manzanares, P.; Negro, M.J.; Castro, E. Olive-Derived Biomass as a Source of Energy and Chemicals. Biofuels Bioprod. Biorefining 2017, 11, 1077–1094. [Google Scholar] [CrossRef]
- Molina-Alcaide, E.; Yáñez-Ruiz, D.R. Potential Use of Olive By-Products in Ruminant Feeding: A Review. Anim. Feed Sci. Technol. 2008, 147, 247–264. [Google Scholar] [CrossRef]
- Araújo, Y.R.V.; de Góis, M.L.; Junior, L.M.C.; Carvalho, M. Carbon Footprint Associated with Four Disposal Scenarios for Urban Pruning Waste. Environ. Sci. Pollut. Res. 2018, 25, 1863–1868. [Google Scholar] [CrossRef]
- da Rosa, G.S.; Martiny, T.R.; Dotto, G.L.; Vanga, S.K.; Parrine, D.; Gariepy, Y.; Lefsrud, M.; Raghavan, V. Eco-Friendly Extraction for the Recovery of Bioactive Compounds from Brazilian Olive Leaves. Sustain. Mater. Technol. 2021, 28, e00276. [Google Scholar] [CrossRef]
- De Marco, E.; Savarese, M.; Paduano, A.; Sacchi, R. Characterization and Fractionation of Phenolic Compounds Extracted from Olive Oil Mill Wastewaters. Food Chem. 2007, 104, 858–867. [Google Scholar] [CrossRef]
- Benincasa, C.; Santoro, I.; Nardi, M.; Cassano, A.; Sindona, G. Eco-Friendly Extraction and Characterisation of Nutraceuticals from Olive Leaves. Molecules 2019, 24, 3481. [Google Scholar] [CrossRef]
- Martín-García, B.; Pimentel-Moral, S.; Gómez-Caravaca, A.M.; Arráez-Román, D.; Segura-Carretero, A. Box-Behnken Experimental Design for a Green Extraction Method of Phenolic Compounds from Olive Leaves. Ind. Crops Prod. 2020, 154, 112741. [Google Scholar] [CrossRef]
- Cruz, R.M.S.; Brito, R.; Smirniotis, P.; Nikolaidou, Z.; Vieira, M.C. Extraction of Bioactive Compounds from Olive Leaves Using Emerging Technologies. In Ingredients Extraction by Physicochemical Methods in Food; Grumezescu, A.M., Holban, A.M., Eds.; Academic Press Inc.: London, UK, 2017; Volume 4, pp. 441–461. ISBN 9780128112021. [Google Scholar]
- Mourtzinos, I.; Anastasopoulou, E.; Petrou, A.; Grigorakis, S.; Makris, D.; Biliaderis, C.G. Optimization of a Green Extraction Method for the Recovery of Polyphenols from Olive Leaf Using Cyclodextrins and Glycerin as Co-Solvents. J. Food Sci. Technol. 2016, 53, 3939–3947. [Google Scholar] [CrossRef]
- Lama-Muñoz, A.; Contreras, M.d.M.; Espínola, F.; Moya, M.; Romero, I.; Castro, E. Content of Phenolic Compounds and Mannitol in Olive Leaves Extracts from Six Spanish Cultivars: Extraction with the Soxhlet Method and Pressurized Liquids. Food Chem. 2020, 320, 126626. [Google Scholar] [CrossRef]
- Irakli, M.; Chatzopoulou, P.; Ekateriniadou, L. Optimization of Ultrasound-Assisted Extraction of Phenolic Compounds: Oleuropein, Phenolic Acids, Phenolic Alcohols and Flavonoids from Olive Leaves and Evaluation of Its Antioxidant Activities. Ind. Crops Prod. 2018, 124, 382–388. [Google Scholar] [CrossRef]
- European Commission. The EU Chemical Strategy for Sustainability towards a Toxic-Free Environment. Chim. Oggi Chem. Today 2020, 39, 40–41. [Google Scholar]
- Maraulo, G.E.; dos Santos Ferreira, C.; Mazzobre, M.F. β-Cyclodextrin Enhanced Ultrasound-Assisted Extraction as a Green Method to Recover Olive Pomace Bioactive Compounds. J. Food Process. Preserv. 2021, 45, e15194. [Google Scholar] [CrossRef]
- Fernández, M.d.L.; Espino, M.; Gomez, F.J.; Silva, M.F. Novel Approaches Mediated by Tailor-Made Green Solvents for the Extraction of Phenolic Compounds from Agro-Food Industrial by-Products. Food Chem. 2018, 239, 671–678. [Google Scholar] [CrossRef]
- Abd-Elsalam, H.A.H.; Gamal, M.; Naguib, I.A.; Al-Ghobashy, M.A.; Zaazaa, H.E.; Abdelkawy, M. Development of Green and Efficient Extraction Methods of Quercetin from Red Onion Scales Wastes Using Factorial Design for Method Optimization: A Comparative Study. Separations 2021, 8, 137. [Google Scholar] [CrossRef]
- Santillán, D.; Sotés, V.; Iglesias, A.; Garrote, L. Adapting Viticulture to Climate Change in the Mediterranean Region: Evaluations Accounting for Spatial Differences in the Producers-Climate Interactions. BIO Web Conf. 2019, 12, 01001. [Google Scholar] [CrossRef]
- Spinelli, R.; Nati, C.; Pari, L.; Mescalchin, E.; Magagnotti, N. Production and Quality of Biomass Fuels from Mechanized Collection and Processing of Vineyard Pruning Residues. Appl. Energy 2012, 89, 374–379. [Google Scholar] [CrossRef]
- Liang, L.; Xi, F.; Tan, W.; Meng, X.; Hu, B.; Wang, X. Review of Organic and Inorganic Pollutants Removal by Biochar and Biochar-Based Composites. Biochar 2021, 3, 255–281. [Google Scholar] [CrossRef]
- Zhao, L.; Xiao, D.; Liu, Y.; Xu, H.; Nan, H.; Li, D.; Kan, Y.; Cao, X. Biochar as Simultaneous Shelter, Adsorbent, PH Buffer, and Substrate of Pseudomonas Citronellolis to Promote Biodegradation of High Concentrations of Phenol in Wastewater. Water Res. 2020, 172, 115494. [Google Scholar] [CrossRef] [PubMed]
- Komnitsas, K.A.; Zaharaki, D. Morphology of Modified Biochar and Its Potential for Phenol Removal from Aqueous Solutions. Front. Environ. Sci. 2016, 4, 26. [Google Scholar] [CrossRef]
- Prelac, M.; Palcic, I.; Cvitan, D.; Andelini, D.; Repaji’c, M.; Curko, J.; Kovaˇcevi´c, T.K.; Goreta Ban, S.; Užila, Z.; Ban, D.; et al. Biochar from Grapevine Pruning Residues as an Efficient Adsorbent of Polyphenolic Compounds. Materials 2023, 16, 4716. [Google Scholar] [CrossRef]
- Wang, J.; Wang, S. Preparation, Modification and Environmental Application of Biochar: A Review. J. Clean. Prod. 2019, 227, 1002–1022. [Google Scholar] [CrossRef]
- Han, Y.; Boateng, A.A.; Qi, P.X.; Lima, I.M.; Chang, J. Heavy Metal and Phenol Adsorptive Properties of Biochars from Pyrolyzed Switchgrass and Woody Biomass in Correlation with Surface Properties. J. Environ. Manag. 2013, 118, 196–204. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT—Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Ou, B.; Hampsch-Woodill, M.; Prior, R.L. Development and Validation of an Improved Oxygen Radical Absorbance Capacity Assay Using Fluorescein as the Fluorescent Probe. J. Agric. Food Chem. 2001, 49, 4619–4626. [Google Scholar] [CrossRef] [PubMed]
- Langmuir, I. The Adsorption of Gases on Plane Surfaces of Mica. J. Am. Chem. Soc. 1938, 40, 1361–1403. [Google Scholar] [CrossRef]
- Markhali, F.S.; Teixeira, J.A.; Rocha, C.M.R. Olive Tree Leaves—A Source of Valuable Active Compounds. Processes 2020, 8, 1177. [Google Scholar] [CrossRef]
- Rahmanian, N.; Jafari, S.M.; Wani, T.A. Bioactive Profile, Dehydration, Extraction and Application of the Bioactive Components of Olive Leaves; Elsevier: Amsterdam, The Netherlands, 2015; Volume 42, ISBN 9190864174. [Google Scholar]
- Castro-Puyana, M.; Marina, M.L.; Plaza, M. Water as Green Extraction Solvent: Principles and Reasons for Its Use. Curr. Opin. Green Sustain. Chem. 2017, 5, 31–36. [Google Scholar] [CrossRef]
- Prelac, M.; Palčić, I.; Cvitan, D.; Anđelini, D.; Repajić, M.; Ćurko, J.; Kovačević, T.K.; Ban Goreta, S.; Užila, Z.; Ban, D.; et al. From Waste to Green: Water-Based Extraction of Polyphenols from Onion Peel and Their Adsorption on Biochar from Grapevine Pruning Residues. Antioxidants 2023, 12, 1697. [Google Scholar] [CrossRef]
- Dobrinčić, A.; Repajić, M.; Elez Garofulić, I.; Tuden, L.; Dragović-Uzelac, V.; Levaj, B. Comparison of Different Extraction Methods for the Recovery of Olive Leaves Polyphenols. Processes 2020, 8, 1008. [Google Scholar] [CrossRef]
- Khemakhem, I.; Ahmad-Qasem, M.H.; Catalán, E.B.; Micol, V.; García-Pérez, J.V.; Ayadi, M.A.; Bouaziz, M. Kinetic Improvement of Olive Leaves’ Bioactive Compounds Extraction by Using Power Ultrasound in a Wide Temperature Range. Ultrason. Sonochem. 2017, 34, 466–473. [Google Scholar] [CrossRef]
- Goldsmith, C.D.; Vuong, Q.V.; Stathopoulos, C.E.; Roach, P.D.; Scarlett, C.J. Optimization of the Aqueous Extraction of Phenolic Compounds from Olive Leaves. Antioxidants 2014, 3, 700–712. [Google Scholar] [CrossRef] [PubMed]
- Lukić, I.; Pasković, I.; Žurga, P.; Germek, V.M.; Brkljača, M.; Marcelić, Š.; Ban, D.; Grozić, K.; Lukić, M.; Užila, Z.; et al. Determination of the Variability of Biophenols and Mineral Nutrients in Olive Leaves with Respect to Cultivar, Collection Period and Geographical Location for Their Targeted and Well-Timed Exploitation. Plants 2020, 9, 1667. [Google Scholar] [CrossRef] [PubMed]
- Pasković, I.; Lukić, I.; Žurga, P.; Germek, V.M.; Brkljača, M.; Koprivnjak, O.; Major, N.; Grozić, K.; Franić, M.; Ban, D.; et al. Temporal Variation of Phenolic and Mineral Composition in Olive Leaves Is Cultivar Dependent. Plants 2020, 9, 1099. [Google Scholar] [CrossRef]
- Polić Pasković, M.; Vidović, N.; Lukić, I.; Žurga, P.; Majetić Germek, V.; Goreta Ban, S.; Kos, T.; Čoga, L.; Tomljanović, T.; Simonić-Kocijan, S.; et al. Phenolic Potential of Olive Leaves from Different Istrian Cultivars in Croatia. Horticulturae 2023, 9, 594. [Google Scholar] [CrossRef]
- Zakraoui, M.; Hannachi, H.; Pasković, I.; Vidović, N.; Polić Pasković, M.; Palčić, I.; Major, N.; Goreta Ban, S.; Hamrouni, L. Effect of Geographical Location on the Phenolic and Mineral Composition of Chetoui Olive Leaves. Foods 2023, 12, 2565. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Qu, J.; Feng, S.; Chen, T.; Yuan, M.; Huang, Y.; Liao, J. Liangshan Olive Leaves and Their Antioxidant and Their Antioxidant and Anticancer Activities. Foods 2019, 8, 657. [Google Scholar] [CrossRef] [PubMed]
- Palmeri, R.; Siracusa, L.; Carrubba, M.; Parafati, L.; Proetto, I.; Pesce, F.; Fallico, B. Olive Leaves, a Promising Byproduct of Olive Oil Industry : Assessment of Metabolic Profiles and Antioxidant Capacity as a Function of Cultivar and Seasonal Change. Agronomy 2022, 12, 2007. [Google Scholar] [CrossRef]
- Şahin, S.; Malik, N.S.A.; Perez, J.L.; Brockington, J.E. Seasonal Changes of Individual Phenolic Compounds in Leaves of Twenty Olive Cultivars Grown in Texas. J. Agric. Sci. Technol. 2012, 2, 242. [Google Scholar]
- Talhaoui, N.; Gómez-Caravaca, A.M.; Roldán, C.; León, L.; De la Rosa, R.; Fernández-Gutiérrez, A.; Segura-Carretero, A. Chemometric Analysis for the Evaluation of Phenolic Patterns in Olive Leaves from Six Cultivars at Different Growth Stages. J. Agric. Food Chem. 2015, 63, 1722–1729. [Google Scholar] [CrossRef] [PubMed]
- Ranalli, A.; Contento, S.; Lucera, L.; Di Febo, M.; Marchegiani, D.; Di Fonzo, V. Factors Affecting the Contents of Iridoid Oleuropein in Olive Leaves (Olea europaea L.). J. Agric. Food Chem. 2006, 54, 434–440. [Google Scholar] [CrossRef] [PubMed]
- Clodoveo, M.L.; Crupi, P.; Annunziato, A.; Corbo, F. Innovative Extraction Technologies for Development of Functional Ingredients Based on Polyphenols from Olive Leaves. Foods 2022, 11, 103. [Google Scholar] [CrossRef]
- Xie, P.; Huang, L.; Zhang, C.; You, F. Reduced Pressure Extraction of Oleuropein from Olive Leaves (Olea europaea L.) with Ultrasound. Food Bioprod. Process. 2013, 93, 29–38. [Google Scholar] [CrossRef]
- da Rosa, G.S.; Vanga, S.K.; Gariepy, Y.; Raghavan, V. Comparison of Microwave, Ultrasonic and Conventional Techniques for Extraction of Bioactive Compounds from Olive Leaves (Olea europaea L.). Innov. Food Sci. Emerg. Technol. 2019, 58, 102234. [Google Scholar] [CrossRef]
- Rafiee, Z.; Jafari, S.M.; Alami, M.; Khomeiri, M. Microwave-Assisted Extraction of Phenolic Compounds from Olive Leaves; A Comparison with Maceration. J. Anim. Plant Sci. 2011, 21, 738–745. [Google Scholar]
- Valinger, D.; Kušen, M.; Benković, M.; Jurina, T.; Panić, M.; Redovniković, I.R.; Kljusurić, J.G.; Tušek, A.J. Enhancement of the Green Extraction of Bioactive Molecules from Olea Europaea Leaves. Separations 2022, 9, 33. [Google Scholar] [CrossRef]
- Vizzarri, V.; Ienco, A.; Benincasa, C.; Perri, E.; Pucci, N.; Cesari, E.; Novellis, C.; Rizzo, P.; Pellegrino, M.; Zaffina, F.; et al. Phenolic Extract from Olive Leaves as a Promising Endotherapeutic Treatment against Xylella Fastidiosa in Naturally Infected Olea Europaea (Var. europaea) Trees. Biology 2023, 12, 1141. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Gutiérrez, M.; Bascón-Villegas, I.; Rodríguez, A.; Pérez-Rodríguez, F.; Fernández-Prior, Á.; Rosal, A.; Carrasco, E. Valorisation of Olea europaea L. Olive Leaves through the Evaluation of Their Extracts: Antioxidant and Antimicrobial Activity. Foods 2021, 10, 966. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Wang, J. Comparison of Linearization Methods for Modeling the Langmuir Adsorption Isotherm. J. Mol. Liq. 2019, 296, 111850. [Google Scholar] [CrossRef]
- Al-Ghouti, M.A.; Da’ana, D.A. Guidelines for the Use and Interpretation of Adsorption Isotherm Models: A Review. J. Hazard. Mater. 2020, 393, 122383. [Google Scholar] [CrossRef] [PubMed]
- Aliakbarian, B.; Casazza, A.A.; Perego, P. Kinetic and Isotherm Modelling of the Adsorption of Phenolic Compounds from Olive Mill Wastewater onto Activated Carbon. Food Technol. Biotechnol. 2015, 53, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Perwitasari, D.S.; Ardian, Y.; Pracesa, Y.; Pangestu, M.A.; Sampe Tola, P. Langmuir and Freundlich Isotherm Approximation on Adsorption Mechanism of Chrome Waste by Using Tofu Dregs. Nusant. Sci. Technol. Proc. 2021, 2021, 106–112. [Google Scholar]
- Abodif, A.M.; Abodif, A.M.; Meng, L.; Ma, S.; Ahmed, A.S.A.; Belvett, N.; Wei, Z.Z.; Ning, D. Mechanisms and Models of Adsorption: TiO2-Supported Biochar for Removal of 3,4-Dimethylaniline. ACS Omega 2020, 5, 13630–13640. [Google Scholar] [CrossRef]
- Abid, N.; Masmoudi, M.A.; Megdiche, M.; Barakat, A.; Ellouze, M.; Chamkha, M.; Ksibi, M.; Sayadi, S. Biochar from Olive Mill Solid Waste as an Eco-Friendly Adsorbent for the Removal of Polyphenols from Olive Mill Wastewater. Chem. Eng. Res. Des. 2022, 181, 384–398. [Google Scholar] [CrossRef]
- Goktepeli, G.; Yildiz, S.; Yel, E. Phenol Adsorption on Magnetic Biochar Derived from Olive Pomace: Equilibrium, Kinetic and Thermodynamics. Hacet. J. Biol. Chem. 2021, 49, 133–145. [Google Scholar] [CrossRef]
- Freundlich, H.M.F. Over the Adsorption in Solution. J. Phys. Chem 1906, 57, 1100–1107. [Google Scholar]
- Ismail, M.G.B.H.; Weng, C.N.; Rahman, H.A.; Zakaria, N.A. Freundlich Isotherm Equilibrium Equastions in Determining Effectiveness a Low Cost Absorbent to Heavy Metal Removal in Wastewater (Leachate) At Teluk Kitang Landfill, Pengkalan Chepa, Kelantan, Malaysia. J. Geogr. Earth Sci. 2013, 1, 55477126. [Google Scholar]
- Anah, L.; Astrini, N. Isotherm Adsorption Studies of Ni(II) Ion Removal from Aqueous Solutions by Modified Carboxymethyl Cellulose Hydrogel. IOP Conf. Ser. Earth Environ. Sci. 2018, 160, 012017. [Google Scholar] [CrossRef]
- Hadrich, F.; Geißen, S.U.; Chamkha, M.; Sayadi, S. Optimizing the Extraction Conditions of Hydroxytyrosol from Olive Leaves Using a Modified Spherical Activated Carbon: A New Experimental Design. Biomed. Res. Int. 2022, 2022, 6199627. [Google Scholar] [CrossRef] [PubMed]
- Senol, A.; Hasdemir, İ.M.; Hasdemir, B.; Kurdaş, İ. Adsorptive Removal of Biophenols from Olive Mill Wastewaters (OMW) by Activated Carbon: Mass Transfer, Equilibrium and Kinetic Studies. Asia-Pac. J. Chem. Eng. 2017, 12, 128–146. [Google Scholar] [CrossRef]
- Fseha, Y.H.; Shaheen, J.; Sizirici, B. Phenol Contaminated Municipal Wastewater Treatment Using Date Palm Frond Biochar: Optimization Using Response Surface Methodology. Emerg. Contam. 2023, 9, 100202. [Google Scholar] [CrossRef]
- Da̧browski, A.; Podkościelny, P.; Hubicki, Z.; Barczak, M. Adsorption of Phenolic Compounds by Activated Carbon—A Critical Review. Chemosphere 2005, 58, 1049–1070. [Google Scholar] [CrossRef]


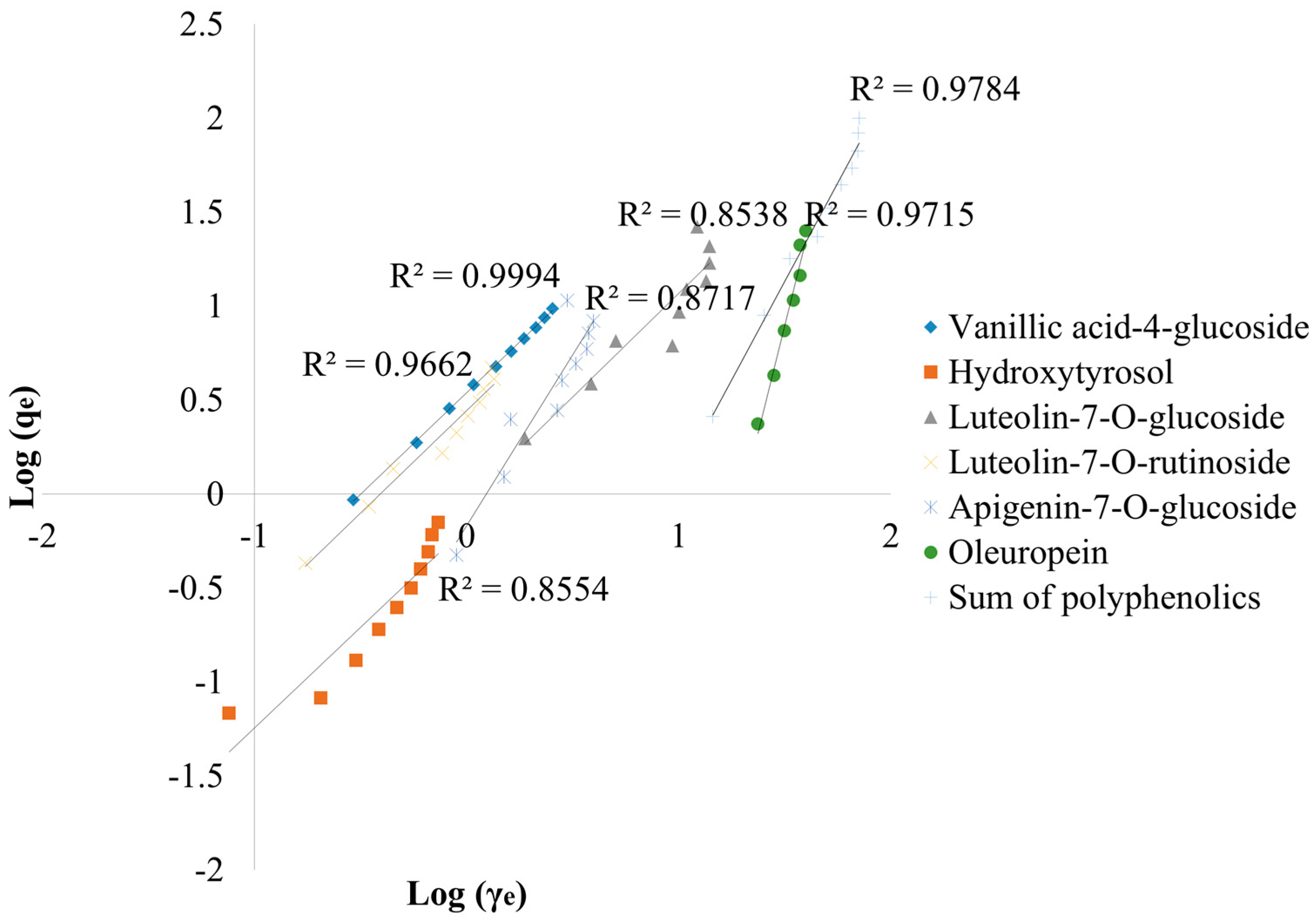
| Parameters | VA-4-g | Hyty | L-7-g | L-7-r | A-7-g | Olp | Sum | |
|---|---|---|---|---|---|---|---|---|
| Langmuir isotherm | qmax (mg/g) | 1.54 | 3.08 | 1.21 | 2.44 | 0.47 | 0.05 | 6.83 |
| KL (L/mg) | −0.28 | 0.38 | 102 | −10.1 | −4.17 | −41.20 | 9.64 | |
| RL | (−2.62)–(−0.01) | 0.05–0.35 | 0.00–0.91 | (−105)–(−0.02) | (−0.66)–(−0.05) | 0.00–1.32 | 0.02–0.50 | |
| R2 | 0.999 | 0.999 | 0.549 | 0.990 | 0.918 | 0.980 | 0.945 | |
| Freundlich isotherm | Kf (mg/g) × (L/g)n | 0.32 | 1.20 | 1.44 | 0.40 | 1.35 | 20.4 | 9.49 |
| 1/n | 0.93 | 0.80 | 0.78 | 0.89 | 0.48 | 0.21 | 0.47 | |
| R2 | 0.999 | 0.855 | 0.854 | 0.966 | 0.872 | 0.972 | 0.978 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prelac, M.; Major, N.; Cvitan, D.; Anđelini, D.; Repajić, M.; Ćurko, J.; Kovačević, T.K.; Goreta Ban, S.; Užila, Z.; Ban, D.; et al. Valorization of Olive Leaf Polyphenols by Green Extraction and Selective Adsorption on Biochar Derived from Grapevine Pruning Residues. Antioxidants 2024, 13, 1. https://doi.org/10.3390/antiox13010001
Prelac M, Major N, Cvitan D, Anđelini D, Repajić M, Ćurko J, Kovačević TK, Goreta Ban S, Užila Z, Ban D, et al. Valorization of Olive Leaf Polyphenols by Green Extraction and Selective Adsorption on Biochar Derived from Grapevine Pruning Residues. Antioxidants. 2024; 13(1):1. https://doi.org/10.3390/antiox13010001
Chicago/Turabian StylePrelac, Melissa, Nikola Major, Danko Cvitan, Dominik Anđelini, Maja Repajić, Josip Ćurko, Tvrtko Karlo Kovačević, Smiljana Goreta Ban, Zoran Užila, Dean Ban, and et al. 2024. "Valorization of Olive Leaf Polyphenols by Green Extraction and Selective Adsorption on Biochar Derived from Grapevine Pruning Residues" Antioxidants 13, no. 1: 1. https://doi.org/10.3390/antiox13010001
APA StylePrelac, M., Major, N., Cvitan, D., Anđelini, D., Repajić, M., Ćurko, J., Kovačević, T. K., Goreta Ban, S., Užila, Z., Ban, D., & Palčić, I. (2024). Valorization of Olive Leaf Polyphenols by Green Extraction and Selective Adsorption on Biochar Derived from Grapevine Pruning Residues. Antioxidants, 13(1), 1. https://doi.org/10.3390/antiox13010001











