Novel Kefir Exopolysaccharides (KEPS) Mitigate Lipopolysaccharide (LPS)-Induced Systemic Inflammation in Luciferase Transgenic Mice through Inhibition of the NF-κB Pathway
Abstract
:1. Introduction
2. Materials and Methods
2.1. Obtaining a Novel KEPS Extract from Kefir Grain Fermentation
2.2. Microorganism, Fermentation Conditions and Extraction of KE
2.3. Molecular Weight Measurement of KEPS and KE
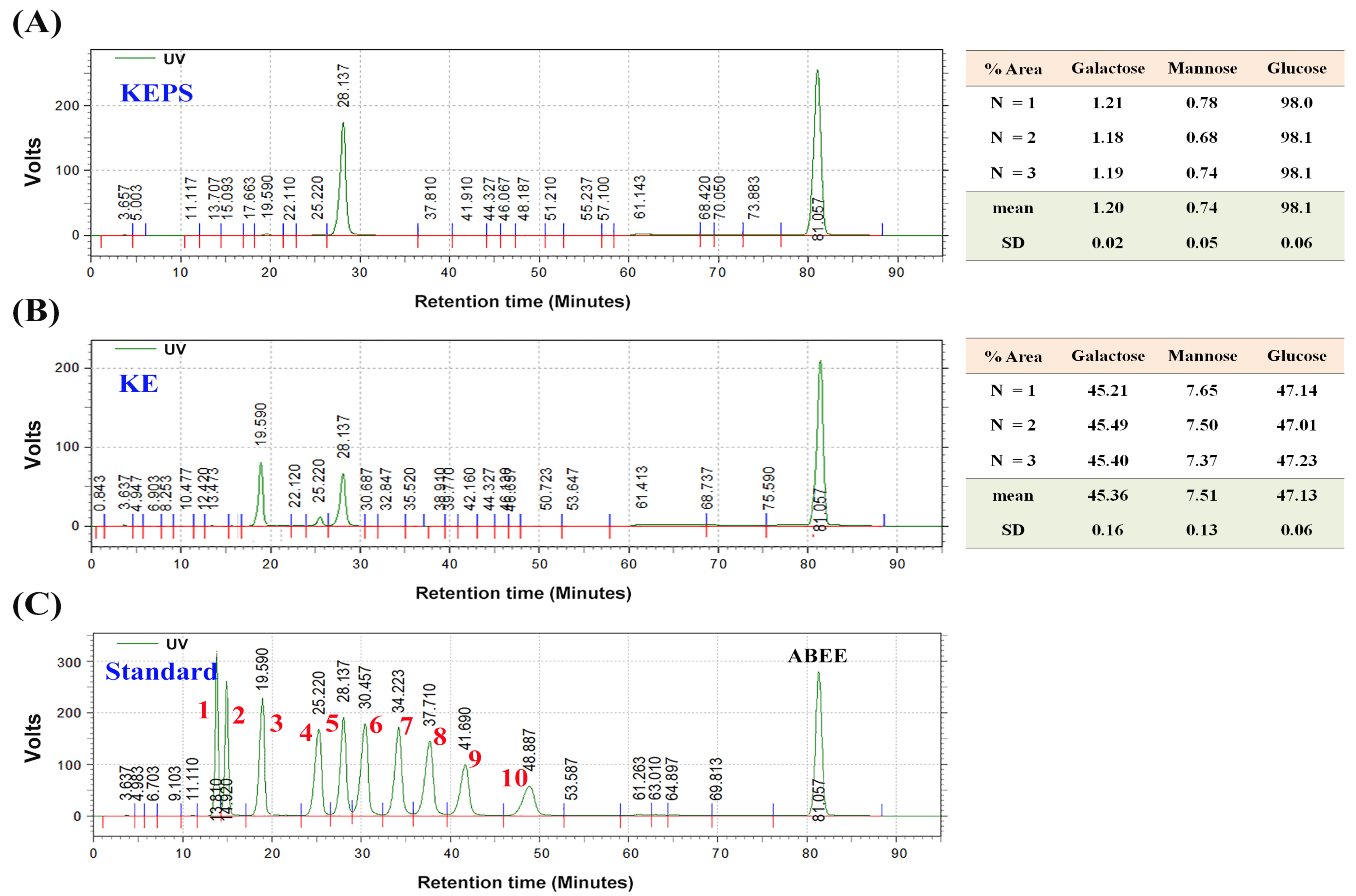
2.4. Monosaccharide Composition in KEPS and KE
2.5. Cell Culture and MTT Assay
2.6. Anti-Inflammation Assessments of KEPS and KE In Vitro
2.7. Animals
2.8. Bioluminescence Imaging
2.9. Western Blot Analysis
2.10. Statistical Analysis
3. Results
3.1. Molecular Weight and Monosaccharide Composition of KEPS and KE
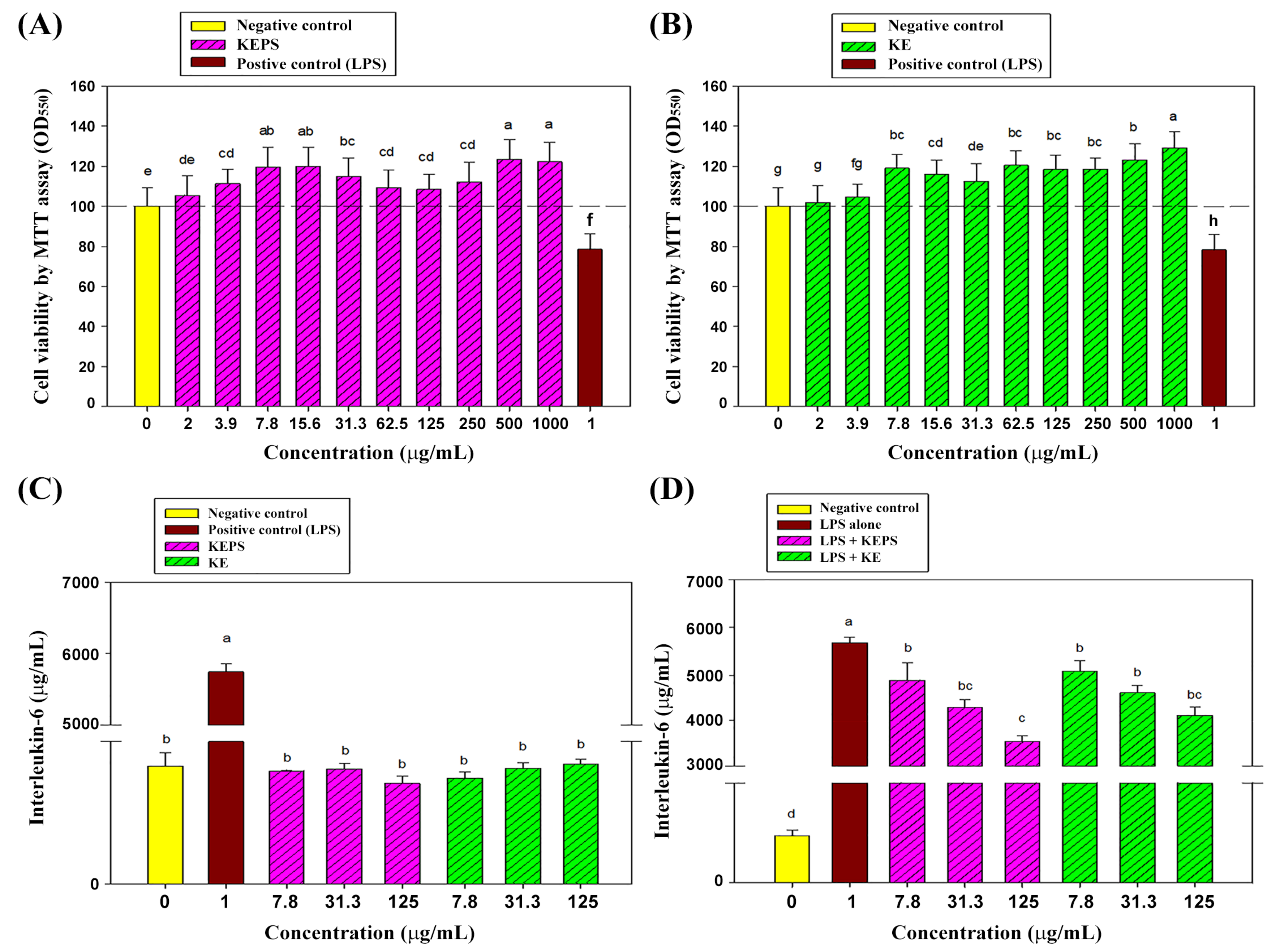
3.2. Effect of the KEPS and KE Treatment on RAW264.7 Cells Viability
3.3. KEPS and KE Decreased the Pro-Inflammatory Cytokine Secretion in LPS-Induced RAW264.7 Cells
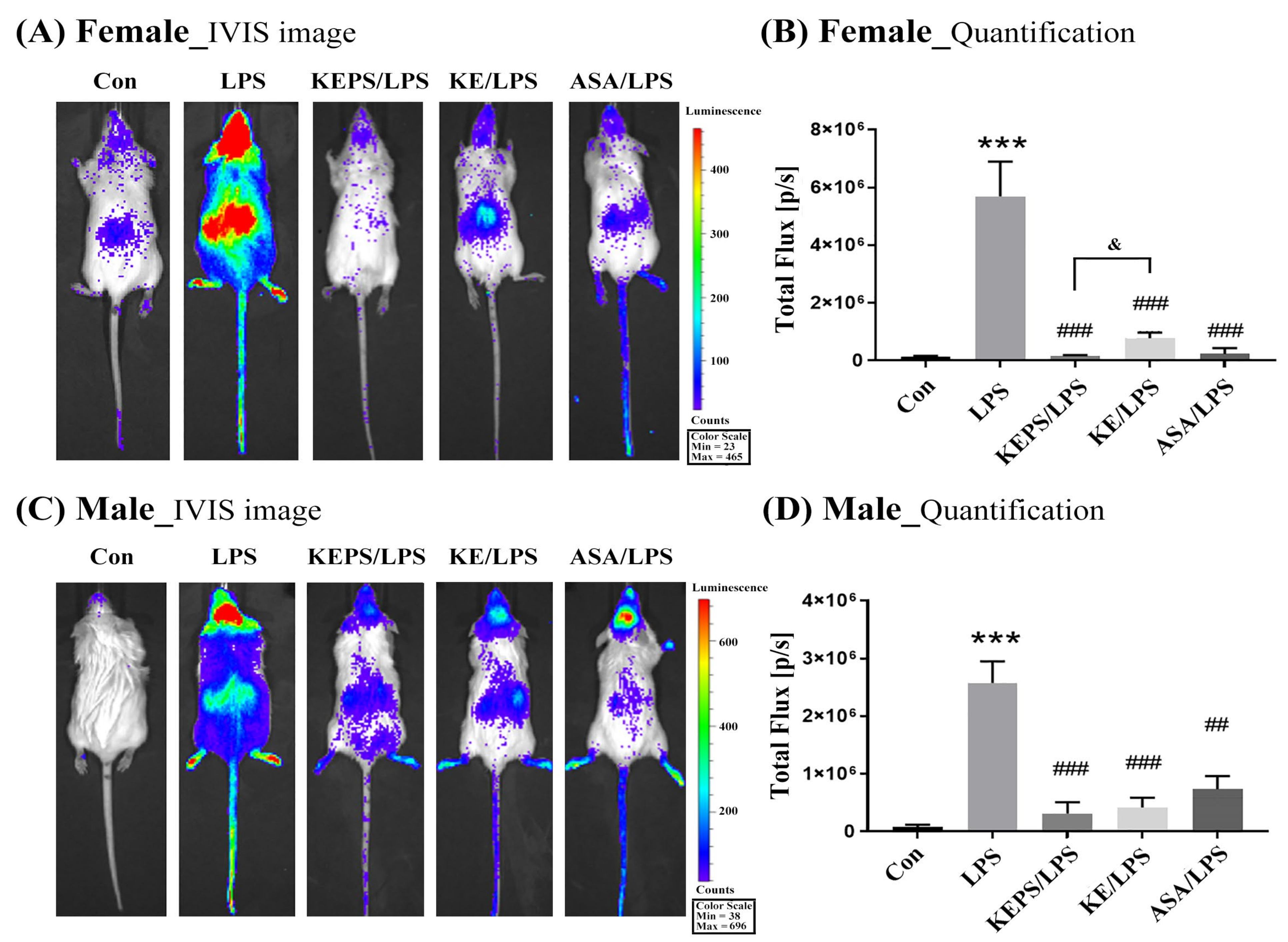
3.4. KEPS and KE Inhibited the LPS-Induced NF-κB Activation in NF-κB-luciferase+/+ Transgenic Mice
3.5. KEPS and KE Decreased the Inflammatory Mediator p-NF-κB Activation in LPS-Induced NF-κB-luciferase+/+ Transgenic Mice
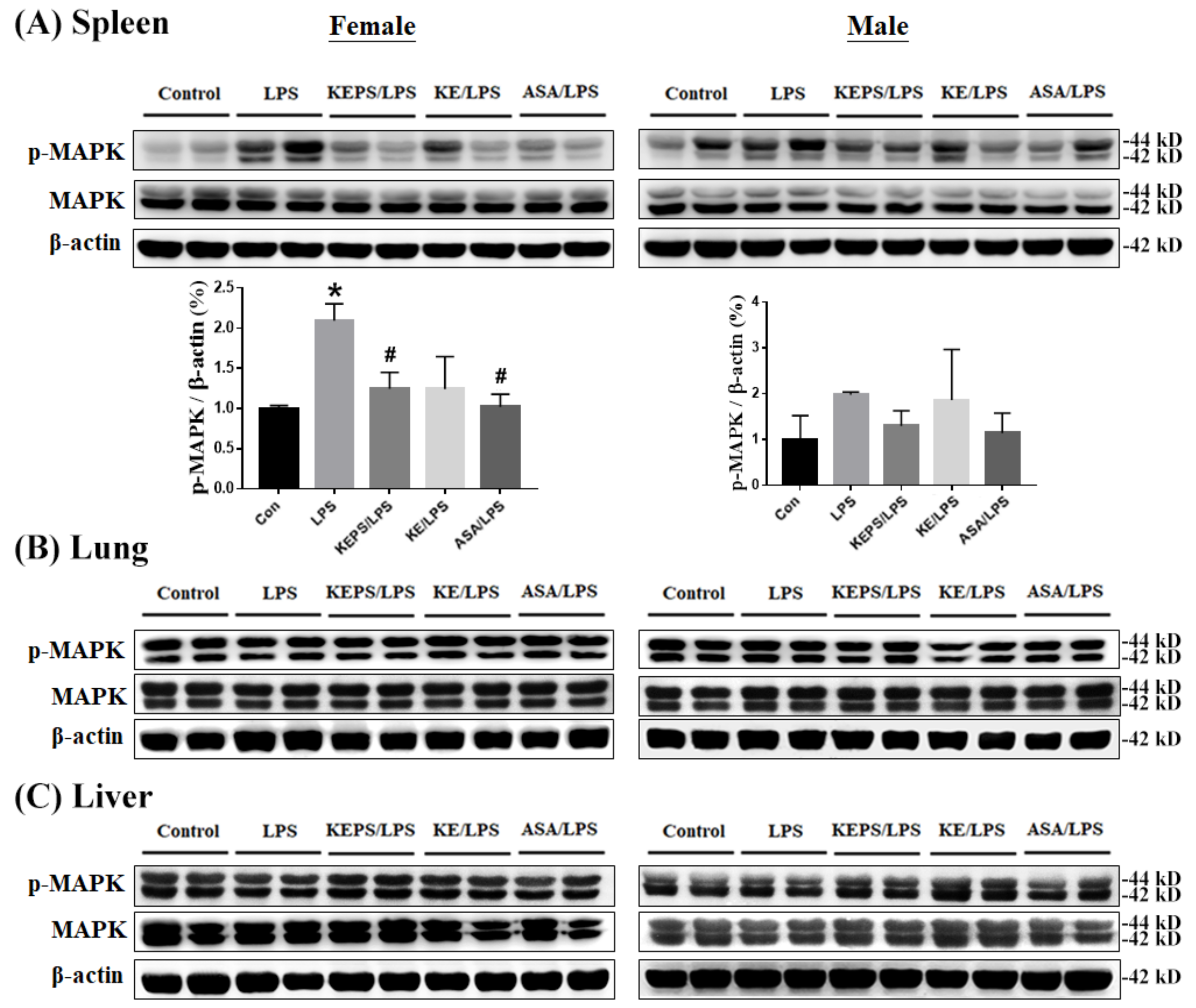
3.6. KEPS and KE Decreased the Inflammatory Mediator p-MAPK Expression in LPS-Induced NF-κB-luciferase+/+ Transgenic Mice
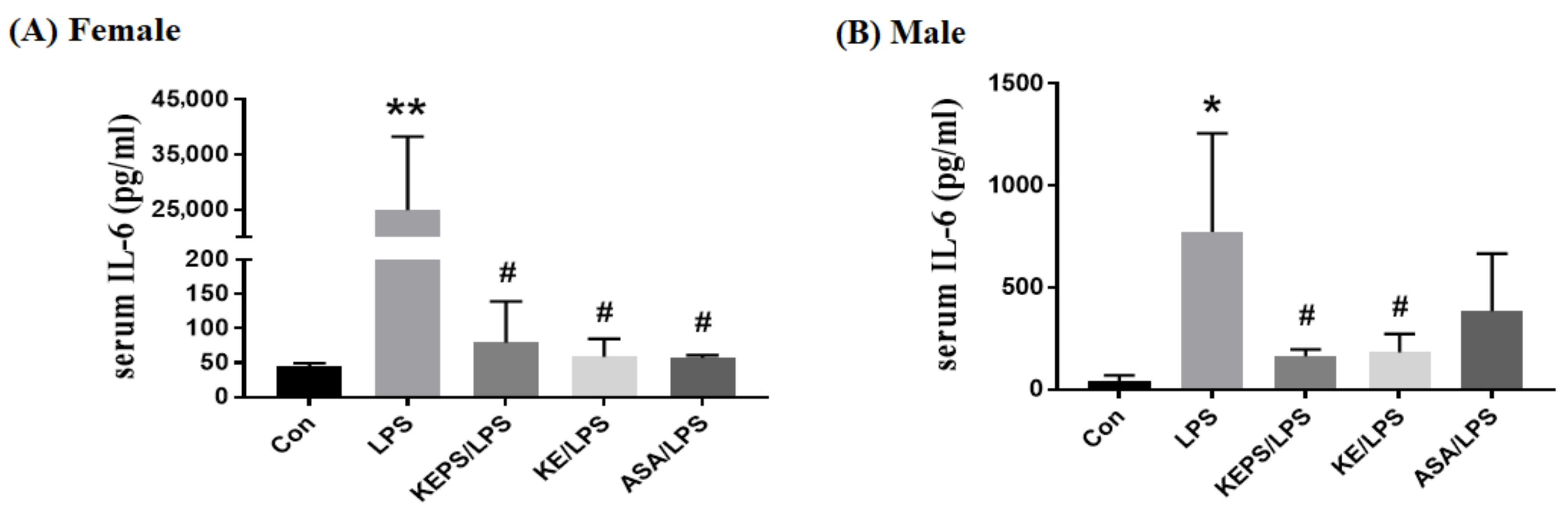
3.7. KEPS and KE Decreased the Serum Pro-Inflammatory Cytokine IL-6 Content in LPS-Induced NF-κB-luciferase+/+ Transgenic Mice
4. Discussion
5. Conclusions
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, Y.; Pischetsrieder, M. Identification and relative quantification of bioactive peptides sequentially released during simulated gastrointestinal digestion of commercial kefir. J. Agric. Food Chem. 2017, 65, 1865–1873. [Google Scholar] [CrossRef]
- De Lima, M.D.S.F.; da Silva, R.A.; da Silva, M.F.; da Silva, P.A.B.; Costa, R.M.P.B.; Teixeira, J.A.C.; Porto, A.L.F.; Cavalcanti, M.T.H. Brazilian kefir-fermented sheep’s milk, a source of antimicrobial and antioxidant peptides. Probiotics Antimicrob. Proteins 2018, 10, 446–455. [Google Scholar] [CrossRef] [PubMed]
- Tung, Y.T.; Chen, H.L.; Wu, H.S.; Ho, M.H.; Chong, K.Y.; Chen, C.M. Kefir peptides prevent hyperlipidemia and obesity in high-fat-diet-induced obese rats via lipid metabolism modulation. Mol. Nutri. Food Res. 2018, 62, 1700505. [Google Scholar] [CrossRef]
- Ghasemlou, M.; Khodaiyan, F.; Jahanbin, K.; Gharibzahedi, S.M.; Taheri, S. Structural investigation and response surface optimisation for improvement of kefiran production yield from a low-cost culture medium. Food Chem. 2012, 133, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Radhouani, H.; Goncalves, C.; Maia, F.R.; Oliveira, J.M.; Reis, R.L. Biological performance of a promising kefiran-biopolymer with potential in regenerative medicine applications: A comparative study with hyaluronic acid. J. Mater. Sci. Mater. Med. 2018, 29, 124. [Google Scholar] [CrossRef]
- Rodrigues, K.L.; Carvalho, J.C.; Schneedorf, J.M. Anti-inflammatory properties of kefir and its polysaccharide extract. Inflammopharmacology 2005, 13, 485–492. [Google Scholar] [CrossRef]
- Tan, K.X.; Chamundeswari, V.N.; Loo, S.C.J. Prospects of kefiran as a food-derived biopolymer for agri-food and biomedical applications. RSC Adv. 2020, 10, 25339–25351. [Google Scholar] [CrossRef] [PubMed]
- Correia, S.; Gonçalves, C.; Oliveira, J.M.; Radhouani, H.; Reis, R.L. Impact of kefiran exopolysaccharide extraction on its applicability for tissue engineering and regenerative medicine. Pharmaceutics 2022, 14, 1713. [Google Scholar] [CrossRef]
- Furman, D.; Campisi, J.; Verdin, E.; Carrera-Bastos, P.; Targ, S.; Franceschi, C.; Ferrucci, L.; Gilroy, D.W.; Fasano, A.; Miller, G.W.; et al. Chronic inflammation in the etiology of disease across the life span. Nat. Med. 2019, 25, 1822–1832. [Google Scholar] [CrossRef]
- Jang, W.Y.; Hwang, J.Y.; Cho, J.Y. Ginsenosides from Panax ginseng as key modulators of NF-κB signaling are powerful anti-inflammatory and anticancer agents. Int. J. Mol. Sci. 2023, 24, 6119. [Google Scholar] [CrossRef]
- Hassan, N.H.; Mehanna, S.; Hussien, A.M.; Ibrahim, M.A.; Hassanen, E.I. The potential mechanism underlying the hepatorenal toxicity induced by hymexazol in rats and the role of NF-κB signaling pathway. J. Biochem. Mol. Toxicol. 2023, 37, e23304. [Google Scholar] [CrossRef]
- Xin, Y.; Tian, M.; Deng, S.; Li, J.; Yang, M.; Gao, J.; Pei, X.; Wang, Y.; Tan, J.; Zhao, F.; et al. The key drivers of brain injury by systemic inflammatory responses after sepsis: Microglia and neuroinflammation. Mol. Neurobiol. 2023, 60, 1369–1390. [Google Scholar] [CrossRef]
- Chuang, K.C.; Lai, Y.W.; Ko, C.H.; Yen, C.C.; Chen, H.L.; Lan, Y.W.; Chen, C.F.; Chen, W.; Chen, C.M. Therapeutic effects of kefir peptides on adjuvant-induced arthritis in rats through anti-inflammation and downregulation of matrix metalloproteinases. Life Sci. 2023, 317, 121411. [Google Scholar] [CrossRef] [PubMed]
- Rogler, G.; Singh, A.; Kavanaugh, A.; Rubin, D.T. Extraintestinal manifestations of inflammatory bowel disease: Current concepts, treatment, and implications for disease management. Gastroenterology 2021, 161, 1118–1132. [Google Scholar] [CrossRef]
- Tung, M.C.; Lan, Y.W.; Li, H.H.; Chen, H.L.; Chen, S.Y.; Chen, Y.H.; Lin, C.C.; Tu, M.Y.; Chen, C.M. Kefir peptides alleviate high-fat diet-induced atherosclerosis by attenuating macrophage accumulation and oxidative stress in ApoE knockout mice. Sci. Rep. 2020, 10, 8802. [Google Scholar] [CrossRef]
- Gao, Z.; Wu, C.; Wu, J.; Zhu, L.; Gao, M.; Wang, Z.; Li, Z.; Zhan, X. Antioxidant and anti-inflammatory properties of an aminoglycan-rich exopolysaccharide from the submerged fermentation of Bacillus thuringiensis. Int. J. Biol. Macromol. 2022, 220, 1010–1020. [Google Scholar] [CrossRef]
- Chang, G.R.; Lin, W.Y.; Fan, H.C.; Tu, M.Y.; Liu, Y.H.; Yen, C.C.; Cidem, A.; Chen, W.; Chen, C.M. Kefir peptides ameliorate osteoporosis in AKR1A1 knockout mice with vitamin C deficiency by promoting osteoblastogenesis and inhibiting osteoclastogenesis. Biomed. Pharmacother. 2022, 156, 113859. [Google Scholar] [CrossRef] [PubMed]
- Tu, M.Y.; Chen, H.L.; Tung, Y.T.; Kao, C.C.; Hu, F.C.; Chen, C.M. Short-term effects of kefir-fermented milk consumption on bone mineral density and bone metabolism in a randomized clinical trial of osteoporotic patients. PLoS ONE 2015, 10, e0144231. [Google Scholar] [CrossRef]
- Prado, M.R.; Boller, C.; Zibetti, R.G.; de Souza, D.; Pedroso, L.L.; Soccol, C.R. Anti-inflammatory and angiogenic activity of polysaccharide extract obtained from Tibetan kefir. Microvasc. Res. 2016, 108, 29–33. [Google Scholar] [CrossRef] [PubMed]
- Dailin, D.J.; Elsayed, E.A.; Othman, N.Z.; Malek, R.; Phin, H.S.; Aziz, R.; Wadaan, M.; El Enshasy, H.A. Bioprocess development for kefiran production by Lactobacillus kefiranofaciens in semi industrial scale bioreactor. Saudi J. Biol. Sci. 2016, 23, 495–502. [Google Scholar] [CrossRef]
- Cheirsilp, B.; Shimizu, H.; Shioya, S. Enhanced kefiran production by mixed culture of Lactobacillus kefiranofaciens and Saccharomyces cerevisiae. J. Biotechnol. 2003, 100, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Zi, W.; Pei, Z.; Liu, S. Characterization of polysaccharides and their antioxidant properties from Plumula nelumbinis. Saudi Pharm. J. 2018, 26, 656–664. [Google Scholar] [CrossRef]
- Yasuno, S.; Murata, T.; Kokubo, K.; Yamaguchi, T.; Kamei, M. Two-mode analysis by high-performance liquid chromatography of rho-aminobenzoic ethyl ester-derivatized monosaccharides. Biosci. Biotechnol. Biochem. 1997, 61, 1944–1946. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Ishida, M.; Takekuni, C.; Nishi, K.; Sugahara, T. p-Synephrine suppresses inflammatory responses in lipopolysaccharide-stimulated RAW264.7 cells and alleviates systemic inflammatory response syndrome in mice. Food Funct. 2022, 13, 5229–5239. [Google Scholar] [CrossRef]
- Chen, Y.H.; Chen, H.L.; Fan, H.C.; Tung, Y.T.; Kuo, C.W.; Tu, M.Y.; Chen, C.M. Anti-inflammatory, antioxidant, and antifibrotic effects of kefir peptides on salt-induced renal vascular damage and dysfunction in aged stroke-prone spontaneously hypertensive rats. Antioxidants 2020, 9, 790. [Google Scholar] [CrossRef] [PubMed]
- Park, M.Y.; Ha, S.E.; Kim, H.H.; Bhosale, P.B.; Abusaliya, A.; Jeong, S.H.; Park, J.S.; Heo, J.D.; Kim, G.S. Scutellarein inhibits LPS-induced inflammation through NF-κB/MAPKs signaling pathway in RAW264.7 cells. Molecules 2022, 27, 3782. [Google Scholar] [CrossRef]
- Yen, C.C.; Chang, W.H.; Tung, M.C.; Chen, H.L.; Liu, H.C.; Liao, C.H.; Lan, Y.W.; Chong, K.Y.; Yang, S.H.; Chen, C.M. Lactoferrin protects hyperoxia-induced lung and kidney systemic inflammation in an in vivo imaging model of NF-κB/luciferase transgenic mice. Mol. Imaging Biol. 2020, 22, 526–538. [Google Scholar] [CrossRef] [PubMed]
- Kuo, W.Y.; Hwu, L.; Wu, C.Y.; Lee, J.S.; Chang, C.W.; Liu, R.S. STAT3/NF-κB-regulated lentiviral TK/GCV suicide gene therapy for cisplatin-resistant triple-negative breast cancer. Theranostics 2017, 7, 647–663. [Google Scholar] [CrossRef]
- Fukuchi, M.; Mitazaki, S.; Saito-Moriya, R.; Kitada, N.; Maki, S.A.; Izumi, H.; Mori, H. Bioluminescence imaging using d-luciferin and its analogs for visualizing Bdnf expression in living mice; different patterns of bioluminescence signals using distinct luciferase substrates. J. Biochem. 2022, 172, 321–327. [Google Scholar] [CrossRef]
- Lan, Y.W.; Yang, J.C.; Yen, C.C.; Huang, T.T.; Chen, Y.C.; Chen, H.L.; Chong, K.Y.; Chen, C.M. Predifferentiated amniotic fluid mesenchymal stem cells enhance lung alveolar epithelium regeneration and reverse elastase-induced pulmonary emphysema. Stem. Cell Res. Ther. 2019, 10, 163. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Yang, T.; Chen, X.; Song, Y. Changes of lipopolysaccharide-induced acute kidney and liver injuries in rats based on metabolomics analysis. J. Inflamm. Res. 2021, 14, 1807–1825. [Google Scholar] [CrossRef]
- Baranova, I.N.; Souza, A.C.; Bocharov, A.V.; Vishnyakova, T.G.; Hu, X.; Vaisman, B.L.; Amar, M.J.; Chen, Z.; Kost, Y.; Remaley, A.T.; et al. Human SR-BI and SR-BII potentiate lipopolysaccharide-induced inflammation and acute liver and kidney injury in mice. J. Immunol. 2016, 196, 3135–3147. [Google Scholar] [CrossRef]
- Kany, S.; Vollrath, J.T.; Relja, B. Cytokines in inflammatory disease. Int. J. Mol. Sci. 2019, 20, 6008. [Google Scholar] [CrossRef]
- Dou, X.; Yan, D.; Liu, S.; Gao, L.; Shan, A. Thymol alleviates LPS-induced liver inflammation and apoptosis by inhibiting NLRP3 inflammasome activation and the AMPK-mTOR-autophagy pathway. Nutrients 2022, 14, 2809. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.; Wang, Q.; Zhang, H.; Tai, S.; Liu, H.; Zhang, D. A synthetic peptide AWRK6 alleviates lipopolysaccharide-induced liver injury. Int. J. Mol. Sci. 2018, 19, 2661. [Google Scholar] [CrossRef] [PubMed]
- Rittirsch, D.; Flierl, M.A.; Day, D.E.; Nadeau, B.A.; McGuire, S.R.; Hoesel, L.M.; Ipaktchi, K.; Zetoune, F.S.; Sarma, J.V.; Leng, L.; et al. Acute lung injury induced by lipopolysaccharide is independent of complement activation. J. Immunol. 2008, 180, 7664–7672. [Google Scholar] [CrossRef]
- Reutershan, J.; Basit, A.; Galkina, E.V.; Ley, K. Sequential recruitment of neutrophils into lung and bronchoalveolar lavage fluid in LPS-induced acute lung injury. Am. J. Physiol. Lung Cell Mol. Physiol. 2005, 289, L807–L815. [Google Scholar] [CrossRef] [PubMed]
- Ortona, E.; Pierdominici, M.; Rider, V. Editorial: Sex hormones and gender differences in immune responses. Front. Immunol. 2019, 10, 1076. [Google Scholar] [CrossRef]
- Taneja, V. Sex hormones determine immune response. Front. Immunol. 2018, 9, 1931. [Google Scholar] [CrossRef] [PubMed]
- Kosyreva, A.M.; Dzhalilova, D.S.; Makarova, O.V.; Tsvetkov, I.S.; Zolotova, N.A.; Diatroptova, M.A.; Ponomarenko, E.A.; Mkhitarov, V.A.; Khochanskiy, D.N.; Mikhailova, L.P. Sex differences of inflammatory and immune response in pups of Wistar rats with SIRS. Sci. Rep. 2020, 10, 15884. [Google Scholar] [CrossRef] [PubMed]
- Spolarics, Z. The X-files of inflammation: Cellular mosaicism of X-linked polymorphic genes and the female advantage in the host response to injury and infection. Shock 2007, 6, 597–604. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Kraus, V.B. Does lipopolysaccharide-mediated inflammation have a role in OA? Nat. Rev. Rheumatol. 2016, 12, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Noailles, A.; Maneu, V.; Campello, L.; Lax, P.; Cuenca, N. Systemic inflammation induced by lipopolysaccharide aggravates inherited retinal dystrophy. Cell Death Dis. 2018, 9, 350. [Google Scholar] [CrossRef] [PubMed]
- Heinrich, P.C.; Behrmann, I.; Haan, S.; Hermanns, H.M.; Müller-Newen, G.; Schaper, F. Principles of interleukin (IL)-6-type cytokine signalling and its regulation. Biochem. J. 2003, 374, 1–20. [Google Scholar] [CrossRef]
- Luna, J.M.; Moon, Y.P.; Liu, K.M.; Spitalnik, S.; Paik, M.C.; Cheung, K.; Sacco, R.L.; Elkind, M.S.V. High-sensitivity C-reactive protein and interleukin-6-dominant inflammation and ischemic stroke risk: The northern Manhattan study. Stroke 2014, 45, 979–987. [Google Scholar] [CrossRef] [PubMed]
- Bronte, V.; Pittet, M.J. The spleen in local and systemic regulation of immunity. Immunity 2013, 39, 806–818. [Google Scholar] [CrossRef]
- Poole, J.A.; Gaurav, R.; Schwab, A.; Nelson, A.J.; Gleason, A.; Romberger, D.J.; Wyatt, T.A. Post-endotoxin exposure-induced lung inflammation and resolution consequences beneficially impacted by lung-delivered IL-10 therapy. Sci. Rep. 2022, 12, 17338. [Google Scholar] [CrossRef]
- Jirillo, E.; Caccavo, D.; Magrone, T.; Piccigallo, E.; Amati, L.; Lembo, A.; Kalis, C.; Gumenscheimer, M. The role of the liver in the response to LPS: Experimental and clinical findings. J. Endotoxin. Res. 2002, 8, 319–327. [Google Scholar] [CrossRef]
- Angelin, J.; Kavitha, M. Exopolysaccharides from probiotic bacteria and their health potential. Int. J. Biol. Macromol. 2020, 162, 853–865. [Google Scholar] [CrossRef]
- Ciszek-Lenda, M.; Nowak, B.; Sróttek, M.; Gamian, A.; Marcinkiewicz, J. Immunoregulatory potential of exopolysaccharide from Lactobacillus rhamnosus KL37: Effects on the production of inflammatory mediators by mouse macrophages. Int. J. Exp. Pathol. 2011, 92, 382–391. [Google Scholar] [CrossRef] [PubMed]
- Kwon, M.; Lee, J.; Park, S.; Kwon, O.H.; Seo, J.; Roh, S. Exopolysaccharide isolated from Lactobacillus plantarum L-14 has anti-inflammatory effects via the Toll-like receptor 4 pathway in LPS-induced RAW 264.7 cells. Int. J. Mol. Sci. 2020, 21, 9283. [Google Scholar] [CrossRef] [PubMed]
- Sung, W.W.; Lin, Y.Y.; Huang, S.D.; Cheng, H.L. Exopolysaccharides of Bacillus amyloliquefaciens Amy-1 mitigate inflammation by inhibiting ERK1/2 and NF-κB pathways and activating p38/Nrf2 pathway. Int. J. Mol. Sci. 2022, 23, 10237. [Google Scholar] [CrossRef] [PubMed]
- Luang-In, V.; Saengha, W.; Deeseenthum, S. Characterization and bioactivities of a novel exopolysaccharide produced from lactose by Bacillus tequilensis PS21 isolated from Thai milk kefir. Microbiol. Biotechnol. Lett. 2018, 46, 9–17. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, X.; Jiang, Y.; Zhao, W.; Guo, T.; Cao, Y.; Teng, J.; Hao, X.; Zhao, J.; Yang, Z. Antioxidant status and gut microbiota change in an aging mouse model as influenced by exopolysaccharide produced by Lactobacillus plantarum YW11 isolated from Tibetan kefir. J. Dairy Sci. 2017, 100, 6025–6041. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Shi, J.; Yang, X.; Nan, B.; Liu, Y.; Wang, Z. Chemical and physical characteristics and antioxidant activities of the exopolysaccharide produced by Tibetan kefir grains during milk fermentation. Int. Dairy J. 2014, 43, 15–21. [Google Scholar] [CrossRef]
- Werning, M.L.; Hernández-Alcántara, A.M.; Ruiz, M.J.; Soto, L.P.; Dueñas, M.T.; López, P.; Frizzo, L.S. Biological functions of exopolysaccharides from lactic acid bacteria and their potential benefits for humans and farmed animals. Foods 2022, 11, 1284. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liao, C.-H.; Yen, C.-C.; Chen, H.-L.; Liu, Y.-H.; Chen, Y.-H.; Lan, Y.-W.; Chen, K.-R.; Chen, W.; Chen, C.-M. Novel Kefir Exopolysaccharides (KEPS) Mitigate Lipopolysaccharide (LPS)-Induced Systemic Inflammation in Luciferase Transgenic Mice through Inhibition of the NF-κB Pathway. Antioxidants 2023, 12, 1724. https://doi.org/10.3390/antiox12091724
Liao C-H, Yen C-C, Chen H-L, Liu Y-H, Chen Y-H, Lan Y-W, Chen K-R, Chen W, Chen C-M. Novel Kefir Exopolysaccharides (KEPS) Mitigate Lipopolysaccharide (LPS)-Induced Systemic Inflammation in Luciferase Transgenic Mice through Inhibition of the NF-κB Pathway. Antioxidants. 2023; 12(9):1724. https://doi.org/10.3390/antiox12091724
Chicago/Turabian StyleLiao, Chun-Huei, Chih-Ching Yen, Hsiao-Ling Chen, Yu-Hsien Liu, Yu-Hsuan Chen, Ying-Wei Lan, Ke-Rong Chen, Wei Chen, and Chuan-Mu Chen. 2023. "Novel Kefir Exopolysaccharides (KEPS) Mitigate Lipopolysaccharide (LPS)-Induced Systemic Inflammation in Luciferase Transgenic Mice through Inhibition of the NF-κB Pathway" Antioxidants 12, no. 9: 1724. https://doi.org/10.3390/antiox12091724
APA StyleLiao, C.-H., Yen, C.-C., Chen, H.-L., Liu, Y.-H., Chen, Y.-H., Lan, Y.-W., Chen, K.-R., Chen, W., & Chen, C.-M. (2023). Novel Kefir Exopolysaccharides (KEPS) Mitigate Lipopolysaccharide (LPS)-Induced Systemic Inflammation in Luciferase Transgenic Mice through Inhibition of the NF-κB Pathway. Antioxidants, 12(9), 1724. https://doi.org/10.3390/antiox12091724







