Antioxidant and Cytotoxic Potential of Carlina vulgaris Extract and Bioactivity-Guided Isolation of Cytotoxic Components
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Standards
2.2. Plant Material
2.3. Extraction, Fractionation, and Subfraction
2.4. Chromatographic Analysis (UHPLC–HR/QTOF/MS–PDA)
2.5. Isolation of the Active Compounds
2.6. Nuclear Magnetic Resonance (NMR) Spectroscopy
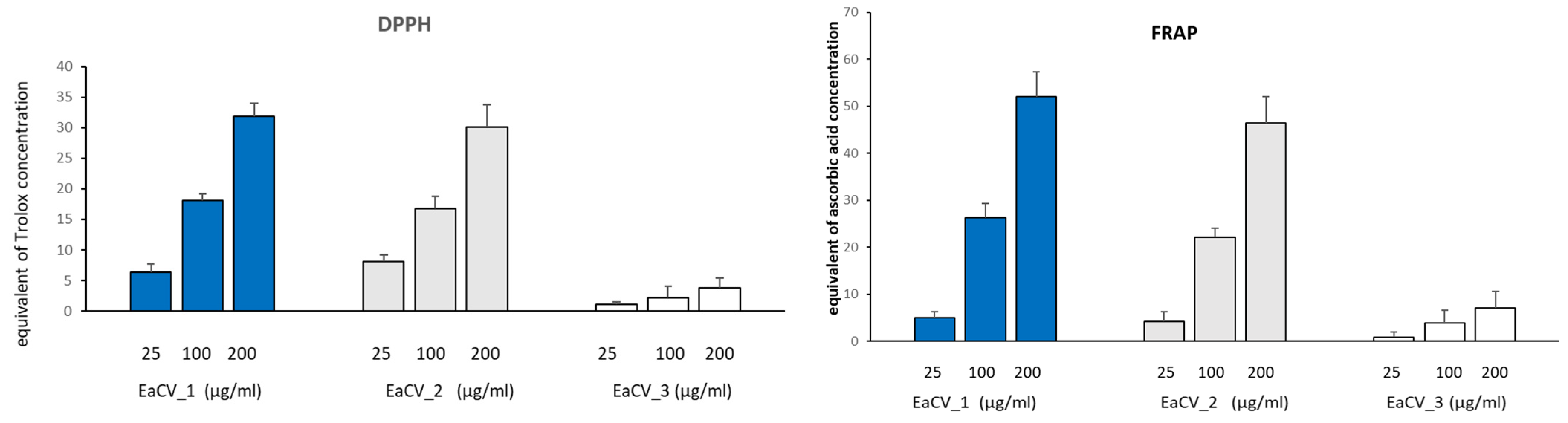
2.7. Antioxidant Activity
2.7.1. DPPH Radical Scavenging Assay
2.7.2. Ferric Ion Reducing Antioxidant Power (FRAP Assay)
2.8. Cell Culture
2.8.1. MTT Assay
2.8.2. Neutral Red Uptake Assay
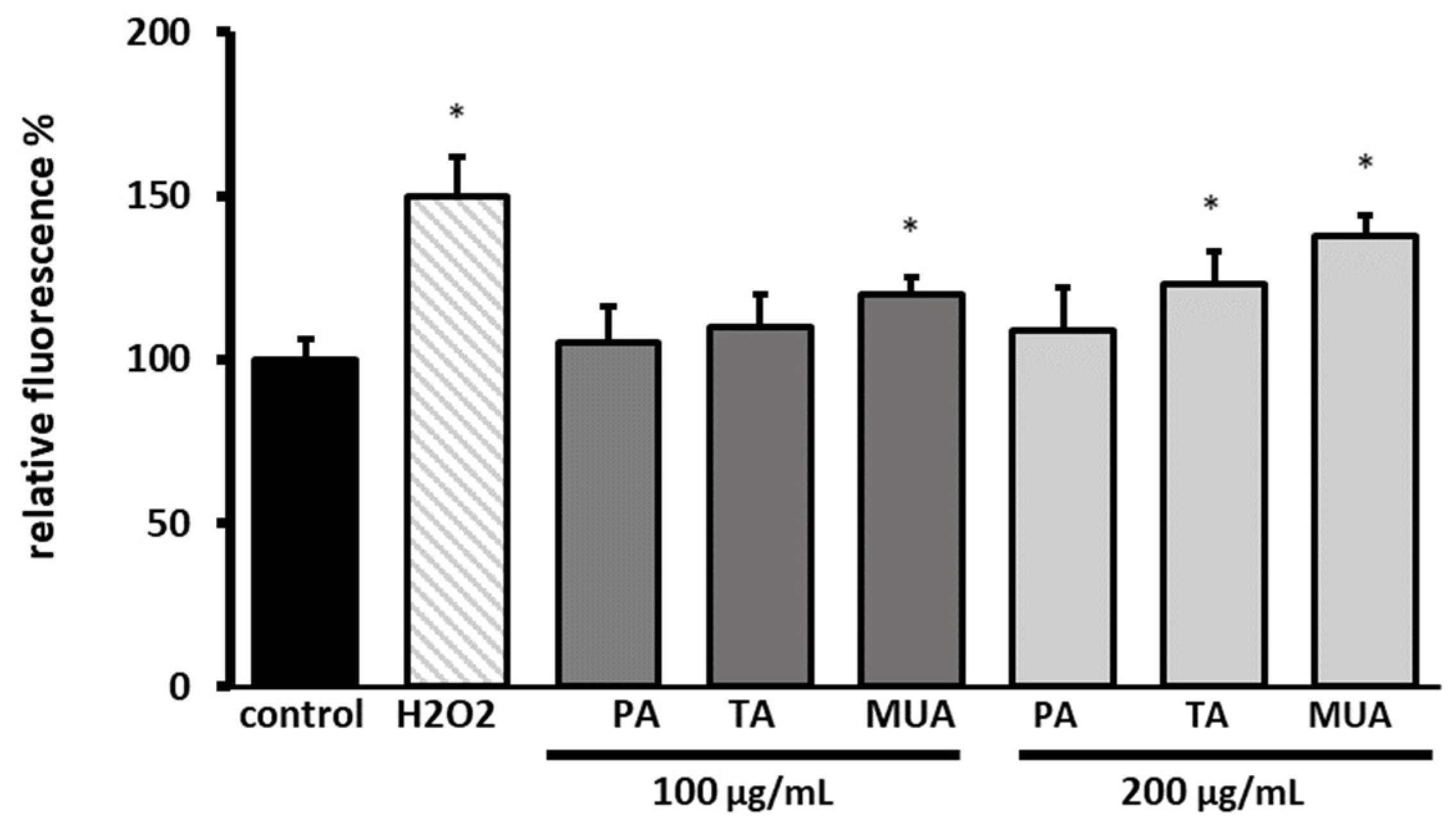
2.8.3. Detection of Intracellular Levels of Reactive Oxygen Species (ROS)
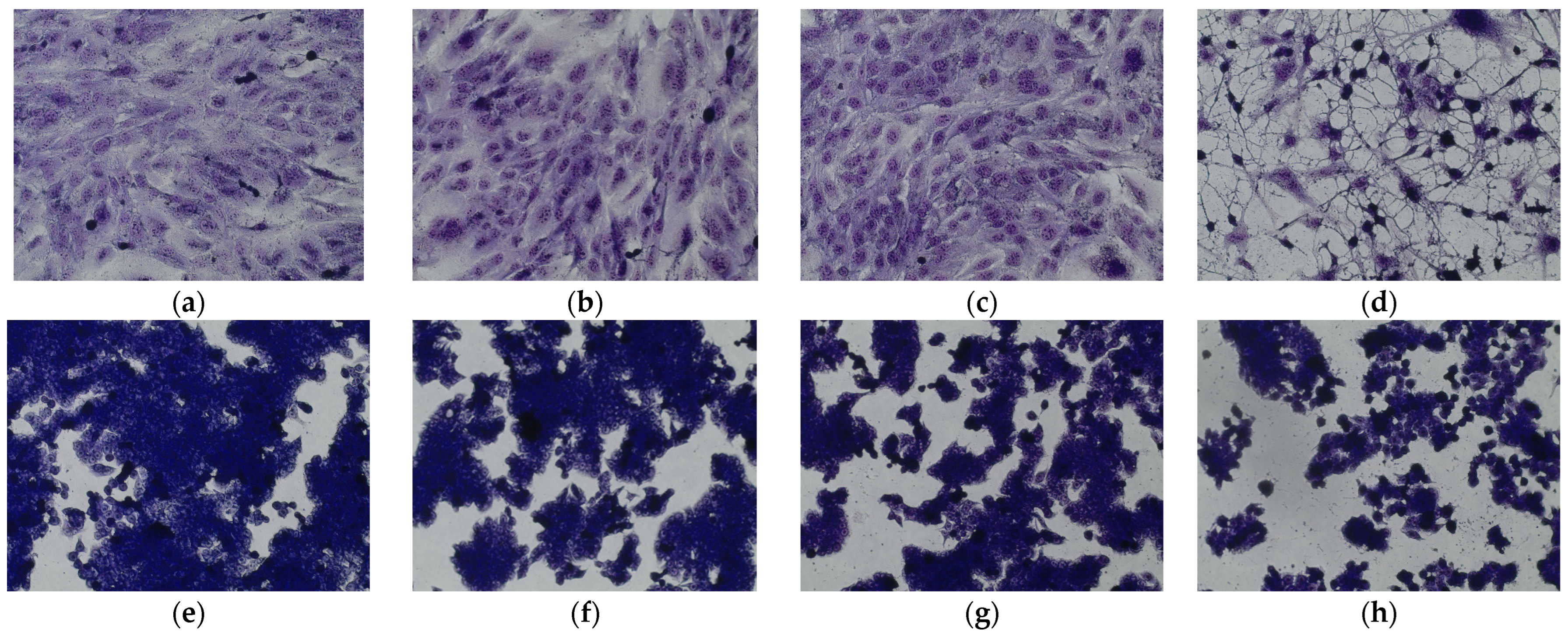
2.8.4. Cellular Morphology Analysis, May–Grünwald–Giemsa (MGG) Staining
2.9. Statistical Analysis
3. Results
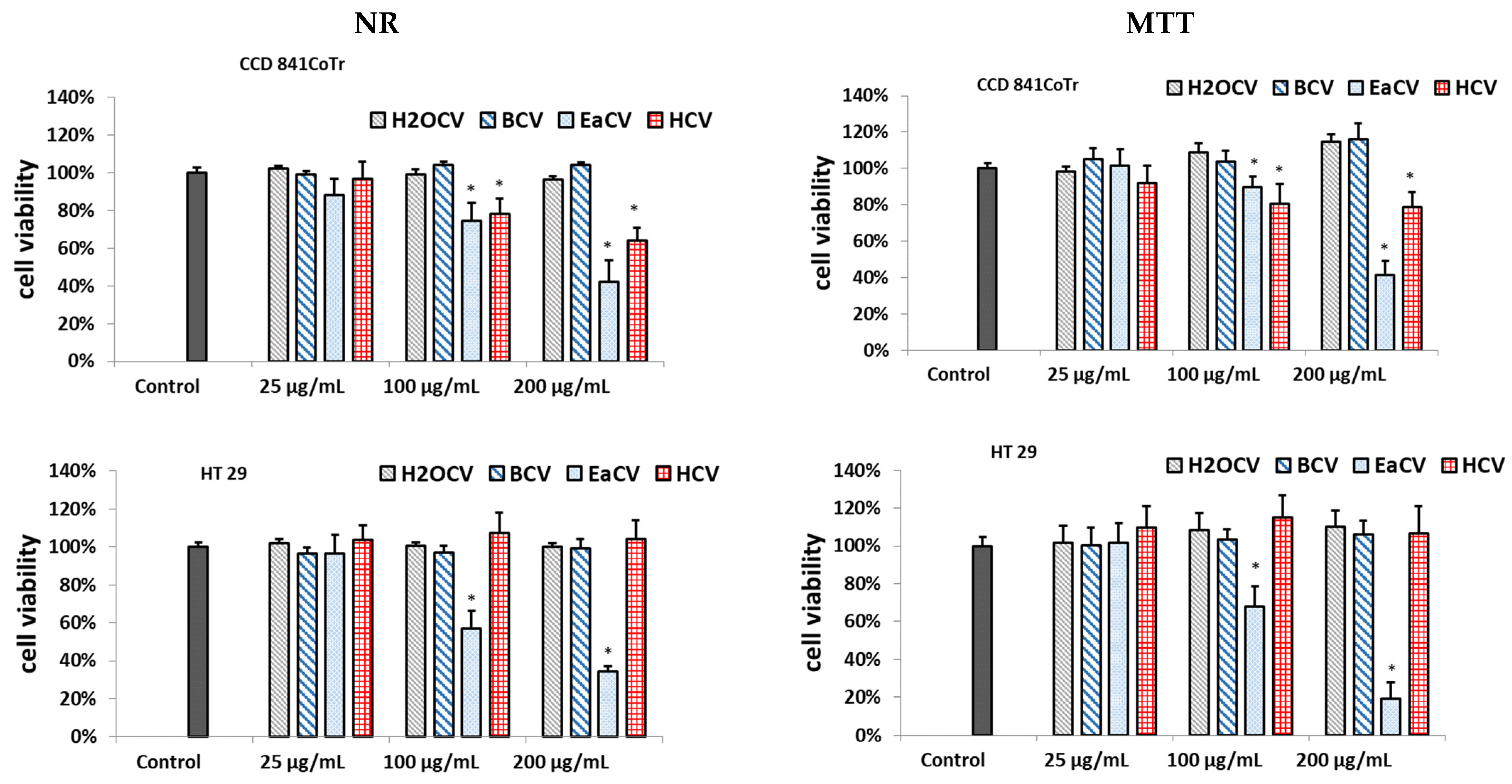
3.1. Cytotoxicity Assessment of C. vulgaris Fractions
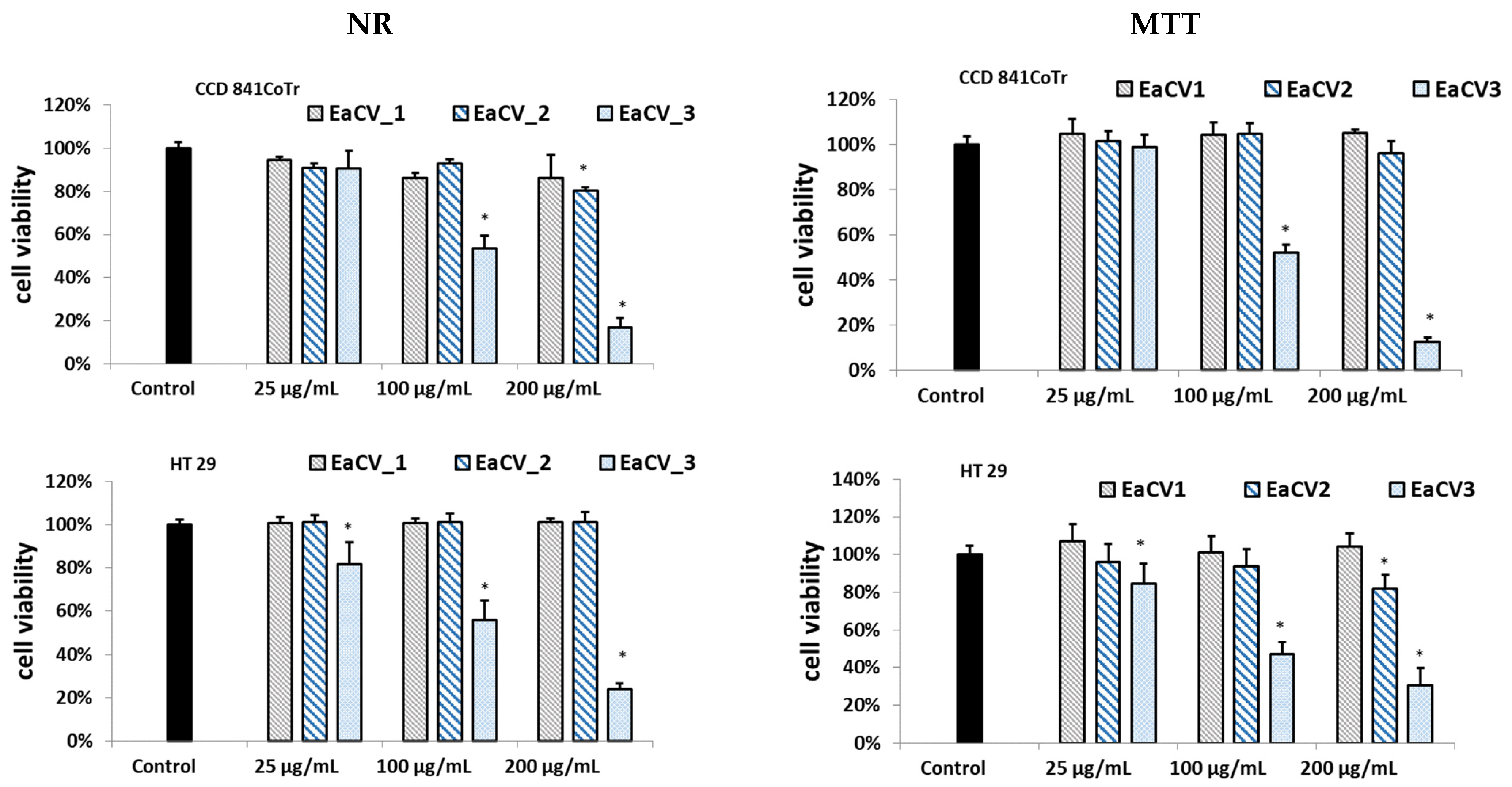
3.2. Subfractions
3.2.1. Biological Assay
3.2.2. Phytochemical Characterization of the Subfractions
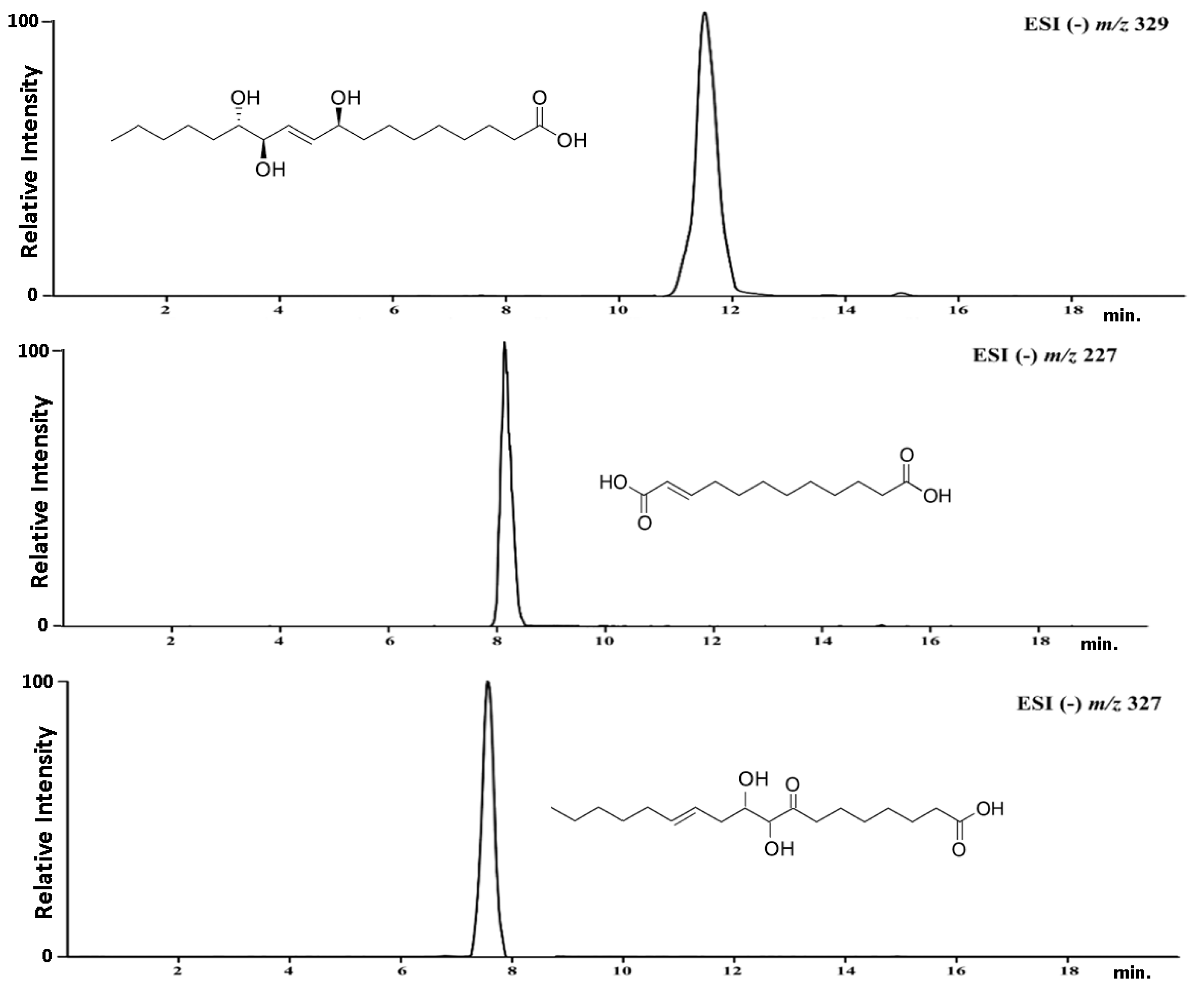
3.3. Isolation of the Target Compounds and Structure Elucidation
- Traumatic acid:HR MS/MS: C12H20O4; m/z –H 227.1285 (M-H); 183.7652; 165.53621H NMR (500 MHz, MeOH–4D): δ (ppm) 7.11 (1H, dd, J = 8.1; 1.8 Hz H–3); 6.00 (1H, d, J = 8.1 Hz H–2), 2.30 (2H, t, J = 7.2 Hz H–11); 2.18 (2H, dd, J = 7.1; 1.8 Hz H–4); 1.52 (2H, dd, J = 7.1 Hz H–10); 1.29 (10H; s H–5; H–6; H–7; H–8; H–9)13C NMR (125 MHz, MeOH–4D): δ (ppm) 178.4 (C–12); 171.5 (C–1); 153.6 (C–3); 120.4 (C–2); 34.0 (C–11); 33.1 (C–4); 29.6 (C–5; C–6; C–7; C–8; C–9); 24.7 (C–10).
- Pinellic acid:HR MS/MS: C18H34O5; m/z-H 329.2337 (M-H); 229.3276; 211.3278; 171.76821H NMR (500 MHz, MeOH–4D): δ (ppm) 5.72 (1H; dd; J = 15.6; 5.1; Hz; H–10); 5.65 (1H; J = 15.6; 5.2 Hz; H–11); 4.05 (1H; m; H–12); 3.91 (1H; dd J = 5.5; 5.0 Hz; H–9); 3.41 (1H; m; H–13); 2.27 (2H; t; J = 7.6 Hz; H–2); 1.52 (2H; m; H–3); 1.48 (2H; m; H–8); 1.44 (2H, m, H–14); 1.31 (2H; m; H–17); 1.29 (4H; m; H–4; H–5); 1.25 (8H; m; H–6; 7; 15; 16), 0.89 (3H; t, J = 6.3 Hz; H–18).13C NMR (125 MHz, MeOH–4D): δ (ppm) 177.6 (C–1); 136.6 (C–10); 131.2 (C–11); 76.4 (C–13); 75.8 (C–12); 73.0 (C–9); 38.3 (C–8); 35.1 (C–2); 33.6 (C–14); 33.2 (C–16); 30.5 (C–6); 30.4 (C–5); 30.1 (C–4); 26.6 (C–15); 26.5 (C–7); 26.1 (C–3); 23.7 (C–17); 13.9 (C–18).
- 9,10-dihydroxy-8-oxsooctadec-12-enic acid:HR MS/MS: C18H32O5; m/z-H 327.2180; 211.3278; 171.76821H NMR (500 MHz, MeOH–4D): δ (ppm) 5.72 (1H; dd; J = 15.6; 5.1; Hz; H–12); 5.65 (1H; J = 15.6; 5.2; H–13); 4.05 (1H; m; H–10); 3.91 (1H; dd J = 5.5; 5.0 Hz; H–9); 2.45 (2H; t; J = 7.1 Hz; H–7); 2.30 (2H; m; H–2); 2.23 (1H; m; H–11); 2.18 (1H; m; H–14), 1.98 (1H; m; H–11); 1.57 (4H, m; H–3; 6), 1.31 (2H; m; H–17); 1.29 (8H; m; H–4; 5; 15; 16); 0.90 (3H; t; J = 6.3 Hz; H–18)13C NMR (125 MHz, MeOH–4D): δ (ppm) 204.2 (C–8);178.4 (C–1); 133.6 (C–13); 124.2 (C–12); 83.4 (C–9); 64.7 (C–10); 43.8 (C–7); 34.3 (C–2); 33.7 (C–14); 31.9 (C–16); 30.4 (C–11); 29.6 (C–15); 29.0 (C–6); 28.8 (C–5); 27.6 (C–4); 24.7 (C–3); 22.8 (C–17); 14.1 (C–18).
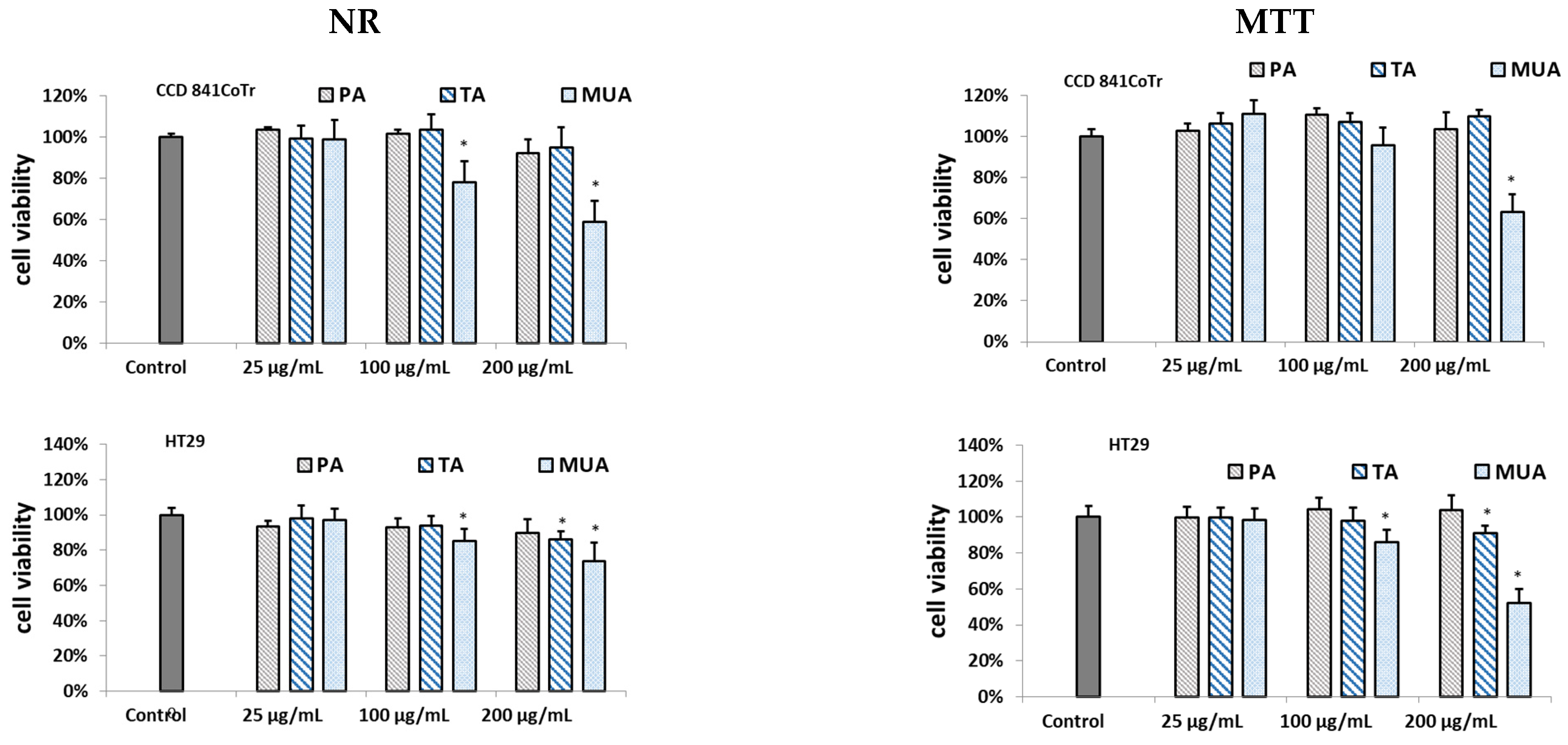
3.4. Biological Assay of the Isolated Compounds
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Elshafie, H.S.; Camele, I.; Mohamed, A.A. A Comprehensive Review on the Biological, Agricultural and Pharmaceutical Properties of Secondary Metabolites Based-Plant Origin. Int. J. Mol. Sci. 2023, 24, 3266. [Google Scholar] [CrossRef]
- Mukherjee, P.; Venkatesh, P.; Ponnusankar, S. Ethnopharmacology and Integrative Medicine—Let the History Tell the Future. J. Ayurveda Integr. Med. 2010, 1, 100. [Google Scholar] [CrossRef]
- Strzemski, M.; Wójciak-Kosior, M.; Sowa, I.; Załuski, D.; Verpoorte, R. Historical and Traditional Medical Applications of Carlina acaulis L. A Critical Ethnopharmacological Review. J. Ethnopharmacol. 2019, 239, 111842. [Google Scholar] [CrossRef] [PubMed]
- Dordević, S.; Tadić, V.; Petrović, S.; Kukić-Marković, J.; Dobrić, S.; Milenković, M.; Hadžifejzović, N. Bioactivity Assays on Carlina acaulis and C. Acanthifolia Root and Herb Extracts. Dig. J. Nanomater. Biostruct. 2012, 7, 1213–1222. [Google Scholar]
- Spinozzi, E.; Ferrati, M.; Cappellacci, L.; Caselli, A.; Perinelli, D.R.; Bonacucina, G.; Maggi, F.; Strzemski, M.; Petrelli, R.; Pavela, R.; et al. (Asteraceae): Biology, Phytochemistry, and Application as a Promising Source of Effective Green Insecticides and Acaricides. Ind. Crops Prod. 2023, 192, 116076. [Google Scholar] [CrossRef]
- Kavallieratos, N.G.; Nika, E.P.; Skourti, A.; Spinozzi, E.; Ferrati, M.; Petrelli, R.; Maggi, F.; Benelli, G. Carlina acaulis Essential Oil: A Candidate Product for Agrochemical Industry Due to Its Pesticidal Capacity. Ind. Crops Prod. 2022, 188, 115572. [Google Scholar] [CrossRef]
- Rosato, A.; Barbarossa, A.; Mustafa, A.M.; Bonacucina, G.; Perinelli, D.R.; Petrelli, R.; Maggi, F.; Spinozzi, E. Comprehensive Evaluation of the Antibacterial and Antifungal Activities of Carlina acaulis L. Essential Oil and Its Nanoemulsion. Antibiotics 2021, 10, 1451. [Google Scholar] [CrossRef] [PubMed]
- Link, P.; Roth, K.; Sporer, F.; Wink, M. Carlina acaulis Exhibits Antioxidant Activity and Counteracts Aβ Toxicity in Caenorhabditis Elegans. Molecules 2016, 21, 871. [Google Scholar] [CrossRef]
- Lunz, K.; Stappen, I. Back to the Roots—An Overview of the Chemical Composition and Bioactivity of Selected Root-Essential Oils. Molecules 2021, 26, 3155. [Google Scholar] [CrossRef] [PubMed]
- Strzemski, M.; Wojnicki, K.; Sowa, I.; Wojas-Krawczyk, K.; Krawczyk, P.; Kocjan, R.; Such, J.; Latalski, M.; Wnorowski, A.; Wójciak-Kosior, M. In Vitro Antiproliferative Activity of Extracts of Carlina acaulis Subsp. Caulescens and Carlina Acanthifolia Subsp. Utzka. Front. Pharmacol. 2017, 8, 371. [Google Scholar] [CrossRef]
- Wnorowski, A.; Wnorowska, S.; Wojas-Krawczyk, K.; Grenda, A.; Staniak, M.; Michalak, A.; Woźniak, S.; Matosiuk, D.; Biała, G.; Wójciak, M.; et al. Toxicity of Carlina Oxide—A Natural Polyacetylene from the Carlina acaulis Roots—In Vitro and in Vivo Study. Toxins 2020, 12, 239. [Google Scholar] [CrossRef]
- Sowa, I.; Mołdoch, J.; Paduch, R.; Strzemski, M.; Szkutnik, J.; Tyszczuk-Rotko, K.; Dresler, S.; Szczepanek, D.; Wójciak, M. Polyphenolic Composition of Carlina acaulis L. Extract and Cytotoxic Potential against Colorectal Adenocarcinoma and Cervical Cancer Cells. Molecules 2023, 28, 6148. [Google Scholar] [CrossRef]
- Strzemski, M.; Wójciak-Kosior, M.; Sowa, I.; Rutkowska, E.; Szwerc, W.; Kocjan, R.; Latalski, M. Carlina Species as a New Source of Bioactive Pentacyclic Triterpenes. Ind. Crops Prod. 2016, 94, 498–504. [Google Scholar] [CrossRef]
- Strzemski, M.; Wójciak-Kosior, M.; Sowa, I.; Załuski, D.; Szwerc, W.; Sawicki, J.; Kocjan, R.; Feldo, M.; Dresler, S. Carlina vulgaris L. as a Source of Phytochemicals with Antioxidant Activity. Oxidative Med. Cell. Longev. 2017, 2017, 1891849. [Google Scholar] [CrossRef]
- Sowa, I.; Mołdoch, J.; Dresler, S.; Kubrak, T.; Soluch, A.; Szczepanek, D.; Strzemski, M.; Paduch, R.; Wójciak, M. Phytochemical Profiling, Antioxidant Activity, and Protective Effect against H2O2-Induced Oxidative Stress of Carlina Vulgaris Extract. Molecules 2023, 28, 5422. [Google Scholar] [CrossRef]
- Belabbes, R.; Mami, I.R.; Dib, M.E.A.; Mejdoub, K.; Tabti, B.; Costa, J.; Muselli, A. Chemical Composition and Biological Activities of Essential Oils of Echinops Spinosus and Carlina Vulgaris Rich in Polyacetylene Compounds. Curr. Nutr. Food Sci. 2020, 16, 563–570. [Google Scholar] [CrossRef]
- Sowa, I.; Paduch, R.; Strzemski, M.; Zielińska, S.; Rydzik-Strzemska, E.; Sawicki, J.; Kocjan, R.; Polkowski, J.; Matkowski, A.; Latalski, M.; et al. Proliferative and Antioxidant Activity of Symphytum Officinale Root Extract. Nat. Prod. Res. 2018, 32, 605–609. [Google Scholar] [CrossRef]
- Ziemlewska, A.; Nizioł-Łukaszewska, Z.; Bujak, T.; Zagórska-Dziok, M.; Wójciak, M.; Sowa, I. Effect of Fermentation Time on the Content of Bioactive Compounds with Cosmetic and Dermatological Properties in Kombucha Yerba Mate Extracts. Sci. Rep. 2021, 11, 18792. [Google Scholar] [CrossRef] [PubMed]
- Dzobo, K. The Role of Natural Products as Sources of Therapeutic Agents for Innovative Drug Discovery. In Comprehensive Pharmacology; Elsevier: Amsterdam, The Netherlands, 2022. [Google Scholar] [CrossRef]
- Savchenko, T.; Degtyaryov, E.; Radzyukevich, Y.; Buryak, V. Therapeutic Potential of Plant Oxylipins. Int. J. Mol. Sci. 2022, 23, 14627. [Google Scholar] [CrossRef] [PubMed]
- Knieper, M.; Viehhauser, A.; Dietz, K.-J. Oxylipins and Reactive Carbonyls as Regulators of the Plant Redox and Reactive Oxygen Species Network under Stress. Antioxidants 2023, 12, 814. [Google Scholar] [CrossRef]
- Cambiaggi, L.; Chakravarty, A.; Noureddine, N.; Hersberger, M. The Role of α-Linolenic Acid and Its Oxylipins in Human Cardiovascular Diseases. Int. J. Mol. Sci. 2023, 24, 6110. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.Y.; Park, K.J.; Subedi, L.; Lee, G.S.; Lee, J.-H.; Lee, W.-M.; Choi, S.U.; Hong, S.-M.; Kim, S.Y.; Kim, C.S. Anti-Inflammatory, Neurotrophic, and Cytotoxic Oxylipins Isolated from Chaenomeles Sinensis Twigs. Antioxidants 2023, 12, 284. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Ma, D. The Role of N-3 Polyunsaturated Fatty Acids in the Prevention and Treatment of Breast Cancer. Nutrients 2014, 6, 5184–5223. [Google Scholar] [CrossRef] [PubMed]
- Montecillo-Aguado, M.; Tirado-Rodriguez, B.; Antonio-Andres, G.; Morales-Martinez, M.; Tong, Z.; Yang, J.; Hammock, B.D.; Hernandez-Pando, R.; Huerta-Yepez, S. Omega-6 Polyunsaturated Fatty Acids Enhance Tumor Aggressiveness in Experimental Lung Cancer Model: Important Role of Oxylipins. Int. J. Mol. Sci. 2022, 23, 6179. [Google Scholar] [CrossRef]
- Panigrahy, D.; Edin, M.L.; Lee, C.R.; Huang, S.; Bielenberg, D.R.; Butterfield, C.E.; Barnés, C.M.; Mammoto, A.; Mammoto, T.; Luria, A.; et al. Epoxyeicosanoids Stimulate Multiorgan Metastasis and Tumor Dormancy Escape in Mice. J. Clin. Investig. 2012, 122, 178–191. [Google Scholar] [CrossRef]
- Zhang, G.; Panigrahy, D.; Mahakian, L.M.; Yang, J.; Liu, J.-Y.; Stephen Lee, K.S.; Wettersten, H.I.; Ulu, A.; Hu, X.; Tam, S.; et al. Epoxy Metabolites of Docosahexaenoic Acid (DHA) Inhibit Angiogenesis, Tumor Growth, and Metastasis. Proc. Natl. Acad. Sci. USA 2013, 110, 6530–6535. [Google Scholar] [CrossRef]
- Khan, R.S.; Senthi, M.; Rao, P.C.; Basha, A.; Alvala, M.; Tummuri, D.; Masubuti, H.; Fujimoto, Y.; Begum, A.S. Cytotoxic Constituents of Abutilon Indicum Leaves against U87MG Human Glioblastoma Cells. Nat. Prod. Res. 2015, 29, 1069–1073. [Google Scholar] [CrossRef]
- Samra, R.M.; Soliman, A.F.; Zaki, A.A.; Ashour, A.; Al-Karmalawy, A.A.; Hassan, M.A.; Zaghloul, A.M. Bioassay-Guided Isolation of a New Cytotoxic Ceramide from Cyperus rotundus L. S. Afr. J. Bot. 2021, 139, 210–216. [Google Scholar] [CrossRef]
- Jabłońska-Trypuć, A.; Krętowski, R.; Wołejko, E.; Wydro, U.; Butarewicz, A. Traumatic Acid Toxicity Mechanisms in Human Breast Cancer MCF-7 Cells. Regul. Toxicol. Pharmacol. 2019, 106, 137–146. [Google Scholar] [CrossRef]
- Fauser, J.K.; Matthews, G.M.; Cummins, A.G.; Howarth, G.S. Induction of Apoptosis by the Medium-Chain Length Fatty Acid Lauric Acid in Colon Cancer Cells Due to Induction of Oxidative Stress. Chemotherapy 2013, 59, 214–224. [Google Scholar] [CrossRef]
- Fauser, J.K.; Prisciandaro, L.D.; Cummins, A.G.; Howarth, G.S. Fatty Acids as Potential Adjunctive Colorectal Chemotherapeutic Agents. Cancer Biol. Ther. 2011, 11, 724–731. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Kim, H.S.; Seo, Y.R. Understanding of ROS-Inducing Strategy in Anticancer Therapy. Oxidative Med. Cell. Longev. 2019, 2019, 5381692. [Google Scholar] [CrossRef] [PubMed]
- Nizami, Z.N.; Aburawi, H.E.; Semlali, A.; Muhammad, K.; Iratni, R. Oxidative Stress Inducers in Cancer Therapy: Preclinical and Clinical Evidence. Antioxidants 2023, 12, 1159. [Google Scholar] [CrossRef] [PubMed]
| No. | Compound | Fraction Amount (mg/g d.m. of Fraction) | ||
|---|---|---|---|---|
| EaCV_1 | EaCV_2 | EaCV_3 | ||
| 1 | 3-caffeoquinic acid | 23.20 ± 0.13 | ND | ND |
| 2 | 5-caffeoquinic acid | 157.69 ± 0.13 | ND | ND |
| 3 | densifloside | 113.73 ± 0.28 | ND | ND |
| 4 | carlinoside | ND | 167.27 ± 0.66 | ND |
| 5 | schaftoside | ND | 84.25 ± 0.07 | ND |
| 6 | isoschaftoside I | ND | 207.09 ± 0.05 | ND |
| 7 | vitexin | ND | 214.98 ± 0.39 | ND |
| 8 | apigenin di-C arabinoside | ND | 2.68 ± 0.05 | ND |
| 9 | taxifolin | ND | 3.38 ± 0.30 | ND |
| 10 | rutin | ND | 69.20 ± 0.32 | ND |
| 11 | nicotiflorin | ND | 52.66 ± 0.31 | ND |
| 12 | traumatic acid | ND | ND | 137.04 ± 5.77 |
| 13 | 9,10-dihydroxy-8-oxsooctadec-12-enic acid | ND | ND | 124.99 ± 0.07 |
| 14 | pinellic acid | ND | ND | 140.71 ± 0.42 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sowa, I.; Paduch, R.; Mołdoch, J.; Szczepanek, D.; Szkutnik, J.; Sowa, P.; Tyszczuk-Rotko, K.; Blicharski, T.; Wójciak, M. Antioxidant and Cytotoxic Potential of Carlina vulgaris Extract and Bioactivity-Guided Isolation of Cytotoxic Components. Antioxidants 2023, 12, 1704. https://doi.org/10.3390/antiox12091704
Sowa I, Paduch R, Mołdoch J, Szczepanek D, Szkutnik J, Sowa P, Tyszczuk-Rotko K, Blicharski T, Wójciak M. Antioxidant and Cytotoxic Potential of Carlina vulgaris Extract and Bioactivity-Guided Isolation of Cytotoxic Components. Antioxidants. 2023; 12(9):1704. https://doi.org/10.3390/antiox12091704
Chicago/Turabian StyleSowa, Ireneusz, Roman Paduch, Jarosław Mołdoch, Dariusz Szczepanek, Jacek Szkutnik, Paweł Sowa, Katarzyna Tyszczuk-Rotko, Tomasz Blicharski, and Magdalena Wójciak. 2023. "Antioxidant and Cytotoxic Potential of Carlina vulgaris Extract and Bioactivity-Guided Isolation of Cytotoxic Components" Antioxidants 12, no. 9: 1704. https://doi.org/10.3390/antiox12091704
APA StyleSowa, I., Paduch, R., Mołdoch, J., Szczepanek, D., Szkutnik, J., Sowa, P., Tyszczuk-Rotko, K., Blicharski, T., & Wójciak, M. (2023). Antioxidant and Cytotoxic Potential of Carlina vulgaris Extract and Bioactivity-Guided Isolation of Cytotoxic Components. Antioxidants, 12(9), 1704. https://doi.org/10.3390/antiox12091704








