Carbon Monoxide-Loaded Red Blood Cell Prevents the Onset of Cisplatin-Induced Acute Kidney Injury
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture and Treatment
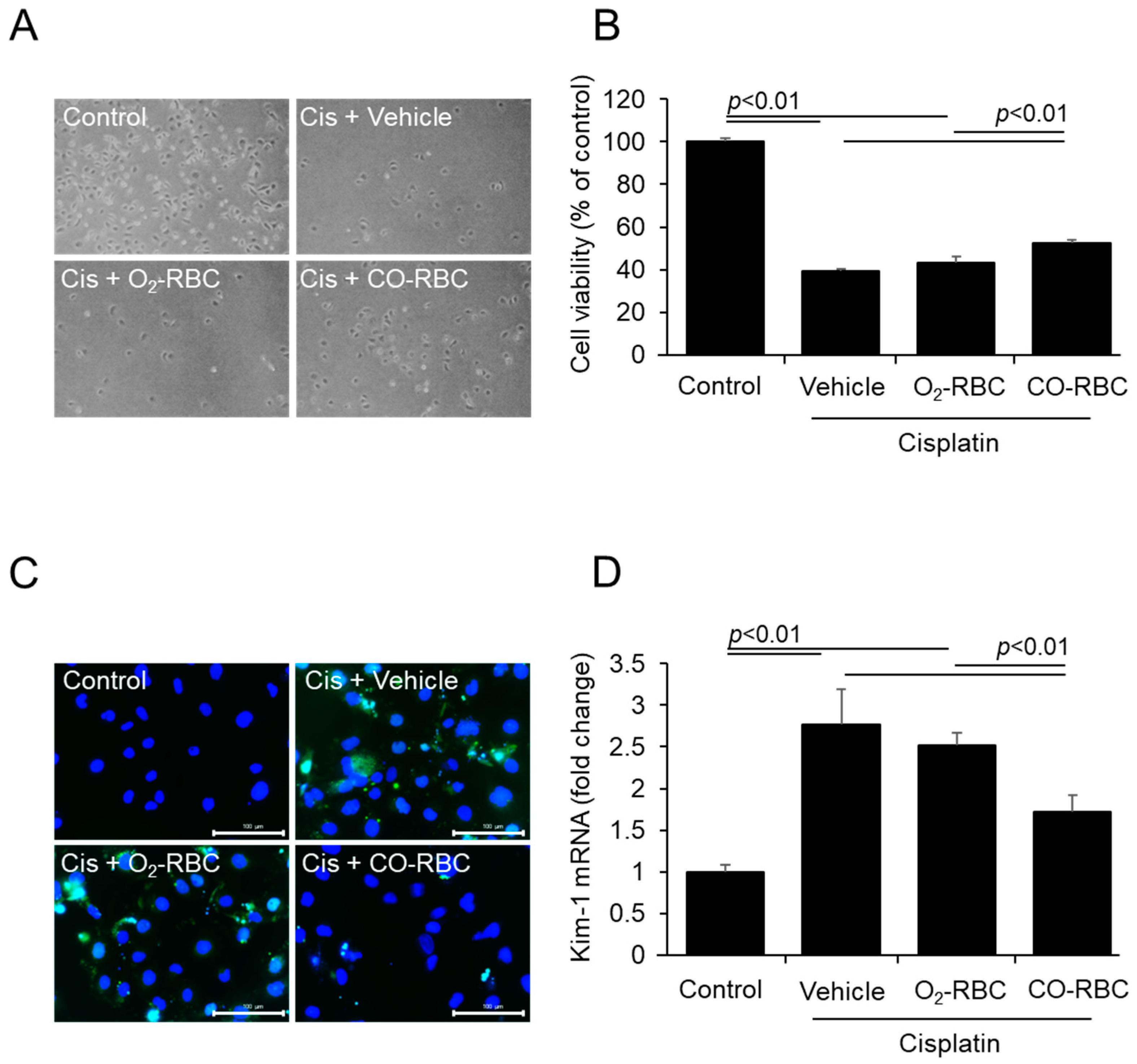
2.2. WST-8 Assay
2.3. TUNEL Staining
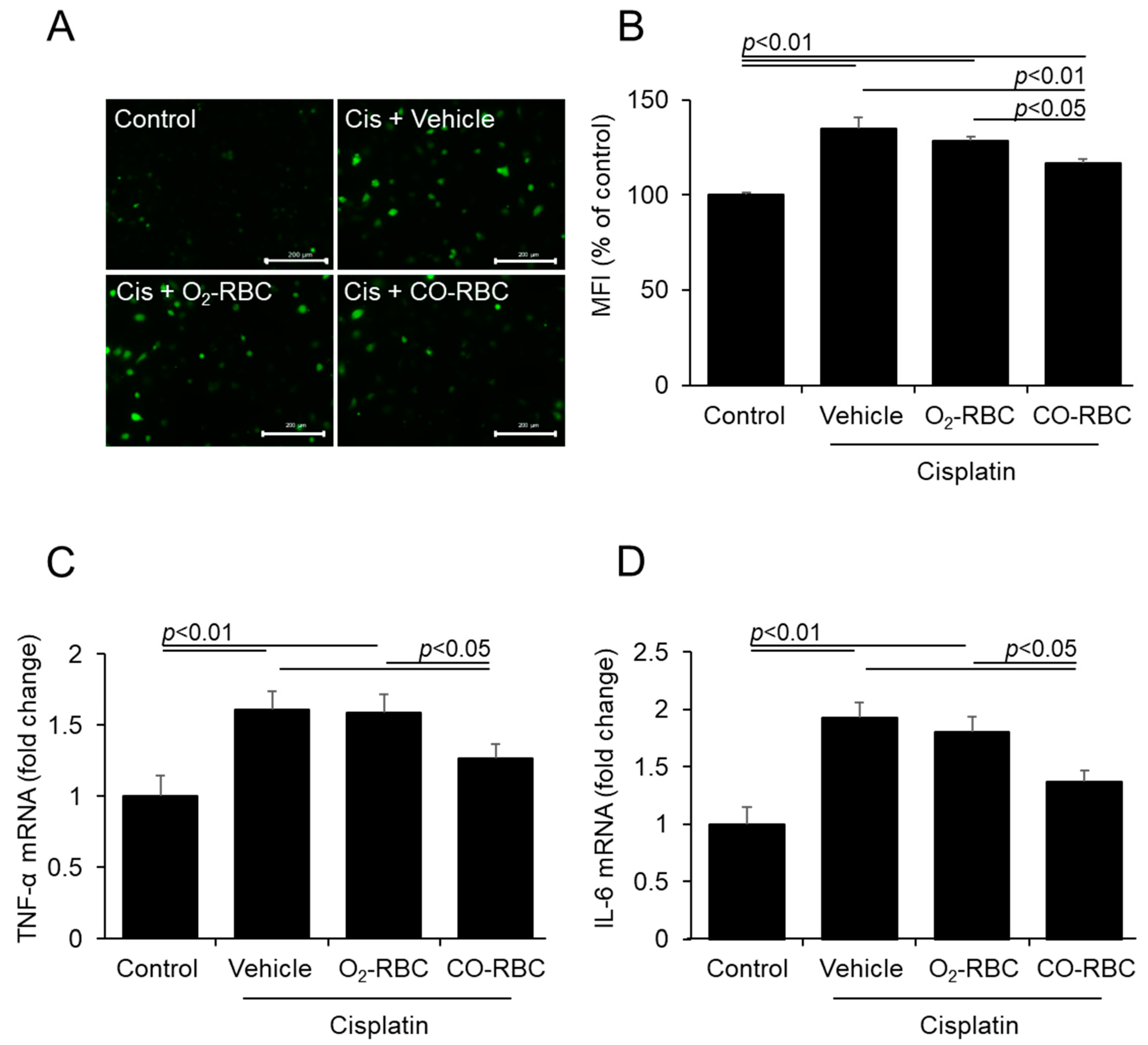
2.4. Measurement of ROS Levels
2.5. Immunostaining Analysis
2.6. Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR) Analysis
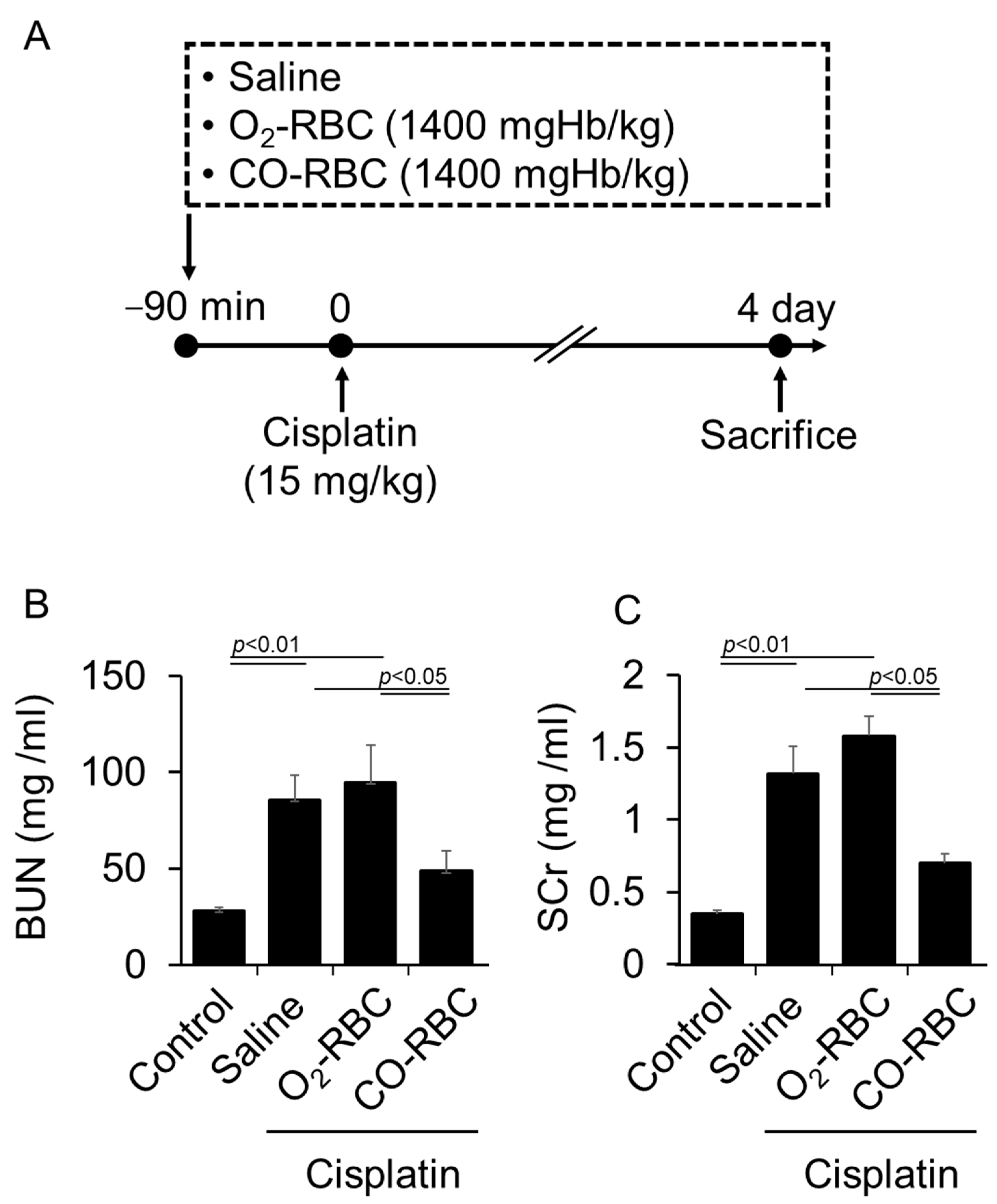
2.7. Cisplatin-Induced AKI Mice Model
2.8. Preparation of O2-RBC and CO-RBC
2.9. Analysis of Renal Function
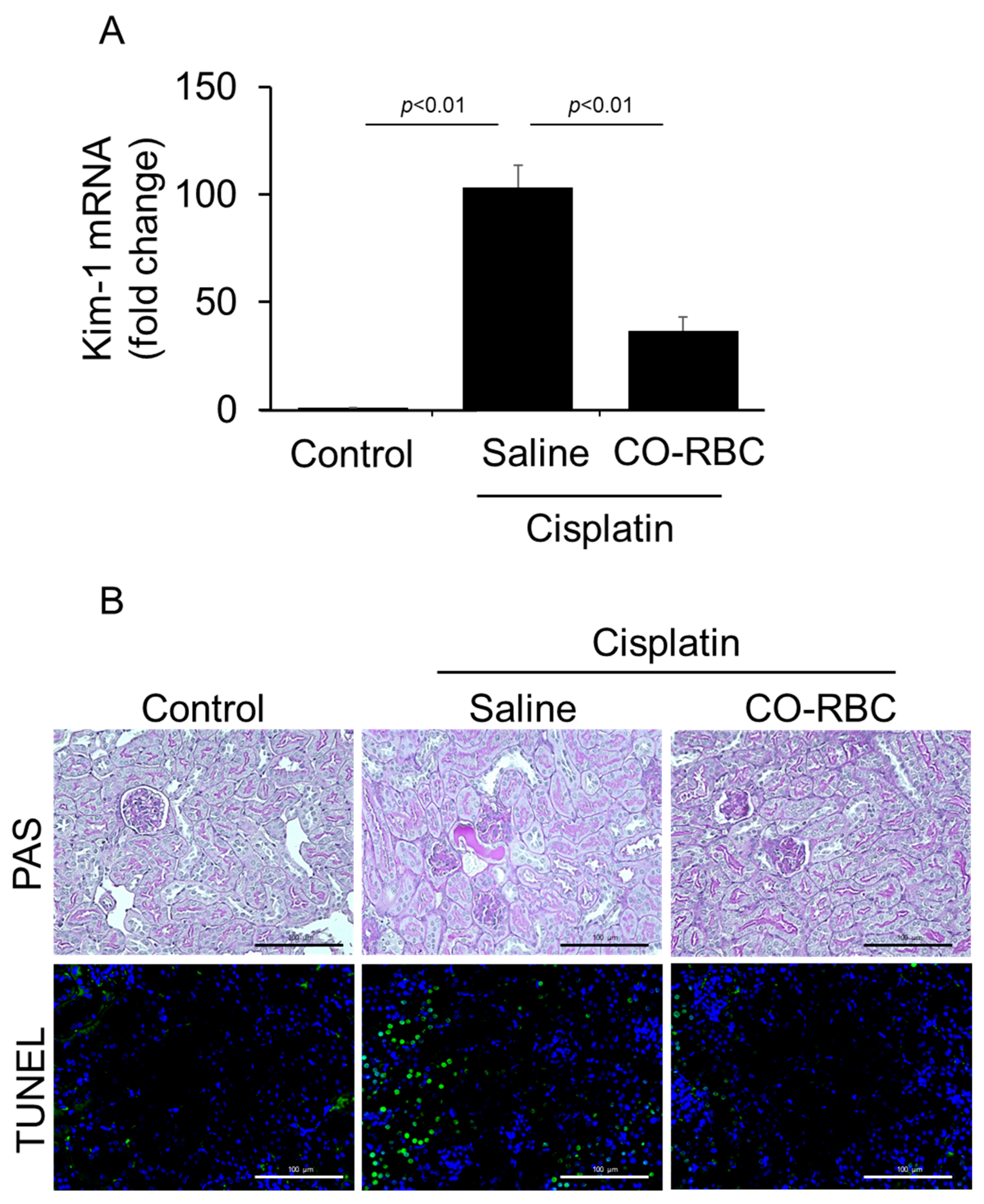
2.10. Histologic Examination of Kidney Tissue
2.11. Diacron-Reactive Oxygen Metabolites (d-ROMs) Test
2.12. Quantification of CO Levels in Kidney and Brain
2.13. Statistics
3. Results
3.1. CO-RBC Alleviates Cisplatin-Induced Cytotoxicity in HK-2 Cells
3.2. CO-RBC Exerts Antioxidant and Anti-Inflammatory Effects on Cisplatin-Treated HK-2 Cells
3.3. CO-RBC Exerts a Renoprotective Effect on Cisplatin-Induced AKI Mice
3.4. CO-RBC Protects the Kidney from Cisplatin-Induced Damage
3.5. CO-RBC Exerts Antioxidant Effects on Cisplatin-Induced AKI Mice
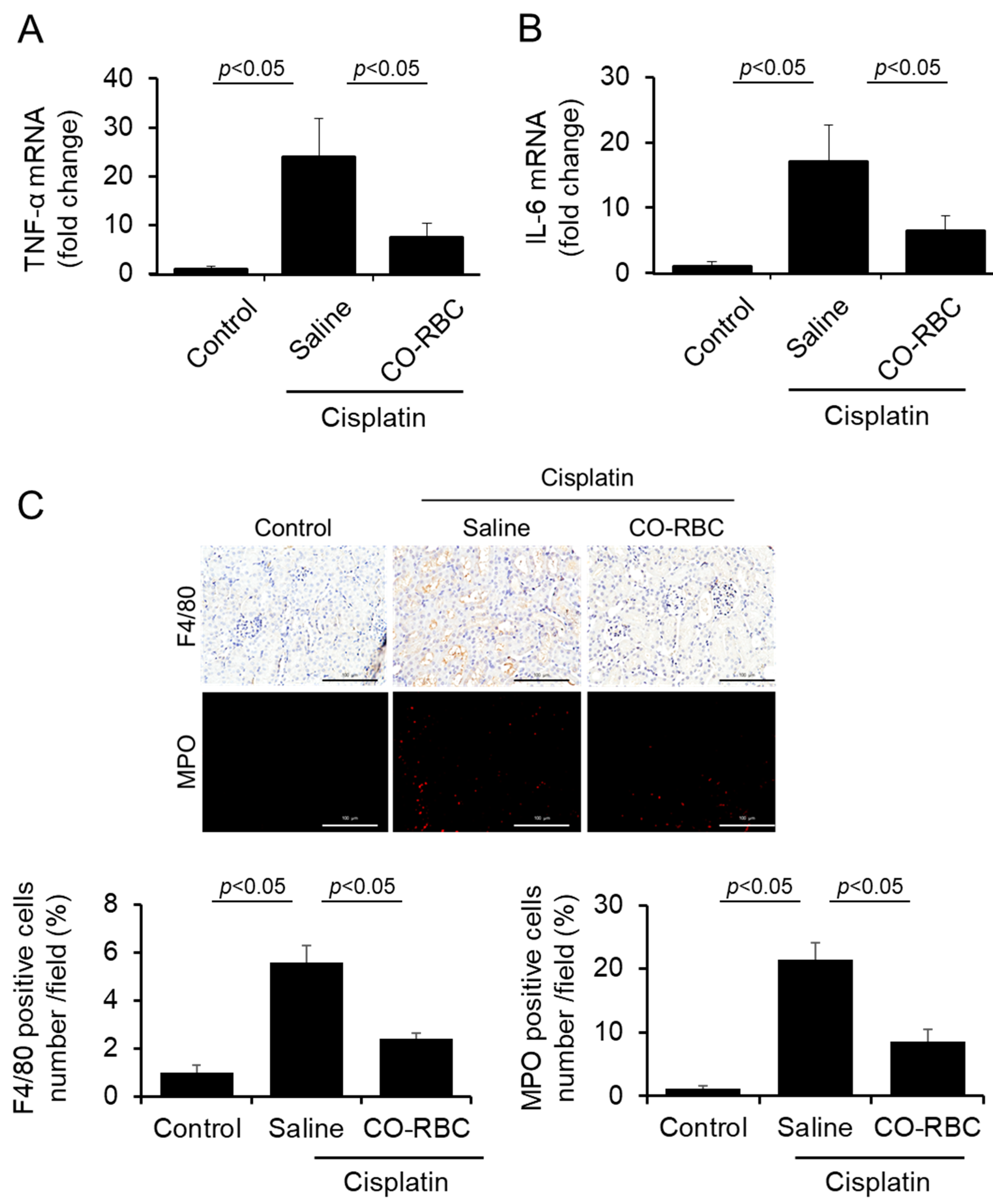
3.6. CO-RBC Exerts Anti-Inflammatory Effects on Cisplatin-Induced AKI Mice
3.7. CO-RBC Does Not Weaken the Anti-Tumor Effect of Cisplatin in B16-F10 Melanoma Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dasari, S.; Tchounwou, P.B. Cisplatin in cancer therapy: Molecular mechanisms of action. Eur. J. Pharmacol. 2014, 740, 364–378. [Google Scholar] [CrossRef]
- Choy, H.; Park, C.; Yao, M. Current status and future prospects for satraplatin, an oral platinum analogue. Clin. Cancer Res. 2008, 14, 1633–1638. [Google Scholar] [CrossRef]
- Fisher, D.E. Apoptosis in cancer therapy: Crossing the threshold. Cell 1994, 78, 539–542. [Google Scholar] [CrossRef]
- Price, P.M.; Yu, F.; Kaldis, P.; Aleem, E.; Nowak, G.; Safirstein, R.L.; Megyesi, J. Dependence of cisplatin-induced cell death in vitro and in vivo on cyclin-dependent kinase 2. J. Am. Soc. Nephrol. 2006, 17, 2434–2442. [Google Scholar] [CrossRef] [PubMed]
- Marullo, R.; Werner, E.; Degtyareva, N.; Moore, B.; Altavilla, G.; Ramalingam, S.S.; Doetsch, P.W. Cisplatin induces a mitochondrial-ROS response that contributes to cytotoxicity depending on mitochondrial redox status and bioenergetic functions. PLoS ONE 2013, 8, e81162. [Google Scholar] [CrossRef] [PubMed]
- Oun, R.; Moussa, Y.E.; Wheate, N.J. The side effects of platinum-based chemotherapy drugs: A review for chemists. Dalton Trans. 2018, 47, 6645–6653. [Google Scholar] [CrossRef]
- Lebwohl, D.; Canetta, R. Clinical development of platinum complexes in cancer therapy: An historical perspective and an update. Eur. J. Cancer 1998, 34, 1522–1534. [Google Scholar] [CrossRef]
- Shiraishi, F.; Curtis, L.M.; Truong, L.; Poss, K.; Visner, G.A.; Madsen, K.; Nick, H.S.; Agarwal, A. Heme oxygenase-1 gene ablation or expression modulates cisplatin-induced renal tubular apoptosis. Am. J. Physiol. Ren. Physiol. 2000, 278, F726–F736. [Google Scholar] [CrossRef]
- Ozkok, A.; Edelstein, C.L. Pathophysiology of cisplatin-induced acute kidney injury. BioMed Res. Int. 2014, 2014, 967826. [Google Scholar] [CrossRef] [PubMed]
- Srisawat, N.; Sileanu, F.E.; Murugan, R.; Bellomod, R.; Calzavacca, P.; Cartin-Ceba, R.; Cruz, D.; Finn, J.; Hoste, E.E.; Kashani, K.; et al. Variation in risk and mortality of acute kidney injury in critically ill patients: A multicenter study. Am. J. Nephrol. 2015, 41, 81–88. [Google Scholar] [CrossRef]
- Rachman, A.; Wafa, S.; Nugroho, P.; Koesnoe, S. The effect of mannitol addition to hydration on acute kidney injury event after high dose cisplatin chemotherapy: An ambispective cohort study. BMC Cancer 2022, 22, 395. [Google Scholar] [CrossRef] [PubMed]
- Dickenmann, M.; Oettl, T.; Mihatsch, M.J. Osmotic nephrosis: Acute kidney injury with accumulation of proximal tubular lysosomes due to administration of exogenous solutes. Am. J. Kidney Dis. 2008, 51, 491–503. [Google Scholar] [CrossRef] [PubMed]
- Oh, G.S.; Kim, H.J.; Shen, A.; Lee, S.B.; Khadka, D.; Pandit, A.; So, H.S. Cisplatin-induced Kidney Dysfunction and Perspectives on Improving Treatment Strategies. Electrolyte Blood Press 2014, 12, 55–65. [Google Scholar] [CrossRef]
- Kawai, Y.; Nakao, T.; Kunimura, N.; Kohda, Y.; Gemba, M. Relationship of intracellular calcium and oxygen radicals to Cisplatin-related renal cell injury. J. Pharmacol. Sci. 2006, 100, 65–72. [Google Scholar] [CrossRef]
- Ramesh, G.; Reeves, W.B. p38 MAP kinase inhibition ameliorates cisplatin nephrotoxicity in mice. Am. J. Physiol. Ren. Physiol. 2005, 289, F166–F174. [Google Scholar] [CrossRef]
- Huang, S.J.; Huang, J.; Yan, Y.B.; Qiu, J.; Tan, R.Q.; Liu, Y.; Tian, Q.; Guan, L.; Niu, S.S.; Zhang, Y.; et al. The renoprotective effect of curcumin against cisplatin-induced acute kidney injury in mice: Involvement of miR-181a/PTEN axis. Ren. Fail. 2020, 42, 350–357. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Wei, W.; Huang, J.; Liu, X.; Ci, X. Isoorientin Attenuates Cisplatin-Induced Nephrotoxicity Through the Inhibition of Oxidative Stress and Apoptosis via Activating the SIRT1/SIRT6/Nrf-2 Pathway. Front. Pharmacol. 2020, 11, 264. [Google Scholar] [CrossRef]
- Tan, R.Z.; Wang, C.; Deng, C.; Zhong, X.; Yan, Y.; Luo, Y.; Lan, H.Y.; He, T.; Wang, L. Quercetin protects against cisplatin-induced acute kidney injury by inhibiting Mincle/Syk/NF-κB signaling maintained macrophage inflammation. Phytother. Res. 2020, 34, 139–152. [Google Scholar] [CrossRef]
- Yuan, L.; Yang, J.; Li, Y.; Liu, F.; Yuan, Y.; Tang, X. Matrine alleviates cisplatin-induced acute kidney injury by inhibiting mitochondrial dysfunction and inflammation via SIRT3/OPA1 pathway. J. Cell. Mol. Med. 2022, 26, 3702–3715. [Google Scholar] [CrossRef]
- Zhang, C.; Wenger, T.; Mattern, J.; Ilea, S.; Frey, C.; Gutwein, P.; Altevogt, P.; Bodenmüller, W.; Gassler, N.; Schnabel, P.A.; et al. Clinical and mechanistic aspects of glucocorticoid-induced chemotherapy resistance in the majority of solid tumors. Cancer Biol. Ther. 2007, 6, 278–287. [Google Scholar] [CrossRef]
- Manohar, S.; Leung, N. Cisplatin nephrotoxicity: A review of the literature. J. Nephrol. 2018, 31, 15–25. [Google Scholar] [CrossRef]
- Wu, L.; Wang, R. Carbon monoxide: Endogenous production, physiological functions, and pharmacological applications. Pharmacol. Rev. 2005, 57, 585–630. [Google Scholar] [CrossRef]
- Adach, W.; Olas, B. A comparison of multifunctional donors of carbon monoxide: Their anticoagulant, antioxidant, anti-aggregatory and cytotoxicity activities in an in vitro model. Nitric Oxide 2020, 97, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Watabe, Y.; Taguchi, K.; Sakai, H.; Enoki, Y.; Maruyama, T.; Otagiri, M.; Kohno, M.; Matsumoto, K. Bioinspired carbon monoxide delivery using artificial blood attenuates the progression of obliterative bronchiolitis via suppression of macrophage activation by IL-17A. Eur. J. Pharm. Biopharm. 2022, 170, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Kang, I.S.; Kim, R.I.; Kim, C. Carbon Monoxide Regulates Macrophage Differentiation and Polarization toward the M2 Phenotype through Upregulation of Heme Oxygenase 1. Cells 2021, 10, 3444. [Google Scholar] [CrossRef]
- Bathoorn, E.; Slebos, D.J.; Postma, D.S.; Koeter, G.H.; van Oosterhout, A.J.; van der Toorn, M.; Boezen, H.M.; Kerstjens, H.A. Anti-inflammatory effects of inhaled carbon monoxide in patients with COPD: A pilot study. Eur. Respir. J. 2007, 30, 1131–1137. [Google Scholar] [CrossRef]
- Yoon, Y.E.; Lee, K.S.; Lee, Y.J.; Lee, H.H.; Han, W.K. Renoprotective Effects of Carbon Monoxide-Releasing Molecule 3 in Ischemia-Reperfusion Injury and Cisplatin-Induced Toxicity. Transplant. Proc. 2017, 49, 1175–1182. [Google Scholar] [CrossRef] [PubMed]
- Opoku-Damoah, Y.; Zhang, R.; Ta, H.T.; Xu, Z.P. Vitamin E-facilitated carbon monoxide pro-drug nanomedicine for efficient light-responsive combination cancer therapy. Biomater. Sci. 2021, 9, 6086–6097. [Google Scholar] [CrossRef]
- Kawahara, B.; Faull, K.F.; Janzen, C.; Mascharak, P.K. Carbon Monoxide Inhibits Cytochrome P450 Enzymes CYP3A4/2C8 in Human Breast Cancer Cells, Increasing Sensitivity to Paclitaxel. J. Med. Chem. 2021, 64, 8437–8446. [Google Scholar] [CrossRef]
- Wang, S.B.; Zhang, C.; Chen, Z.X.; Ye, J.J.; Peng, S.Y.; Rong, L.; Liu, C.J.; Zhang, X.Z. A Versatile Carbon Monoxide Nanogenerator for Enhanced Tumor Therapy and Anti-Inflammation. ACS Nano 2019, 13, 5523–5532. [Google Scholar] [CrossRef]
- Kawahara, B.; Ramadoss, S.; Chaudhuri, G.; Janzen, C.; Sen, S.; Mascharak, P.K. Carbon monoxide sensitizes cisplatin-resistant ovarian cancer cell lines toward cisplatin via attenuation of levels of glutathione and nuclear metallothionein. J. Inorg. Biochem. 2019, 191, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Motterlini, R.; Mann, B.E.; Foresti, R. Therapeutic applications of carbon monoxide-releasing molecules. Expert Opin. Investig. Drugs 2005, 14, 1305–1318. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.W.; Irvine, D.J.; Discher, D.E.; Mitragotri, S. Bio-inspired, bioengineered and biomimetic drug delivery carriers. Nat. Rev. Drug Discov. 2011, 10, 521–535. [Google Scholar] [CrossRef]
- Taguchi, K.; Maruyama, T.; Otagiri, M. Use of Hemoglobin for Delivering Exogenous Carbon Monoxide in Medicinal Applications. Curr. Med. Chem. 2020, 27, 2949–2963. [Google Scholar] [CrossRef]
- Ogaki, S.; Taguchi, K.; Watanabe, H.; Otagiri, M.; Maruyama, T. Carbon monoxide-bound red blood cells protect red blood cell transfusion-induced hepatic cytochrome P450 impairment in hemorrhagic-shock rats. Drug Metab. Dispos. 2013, 41, 141–148. [Google Scholar] [CrossRef]
- Ogaki, S.; Taguchi, K.; Watanabe, H.; Ishima, Y.; Otagiri, M.; Maruyama, T. Carbon monoxide-bound red blood cell resuscitation ameliorates hepatic injury induced by massive hemorrhage and red blood cell resuscitation via hepatic cytochrome P450 protection in hemorrhagic shock rats. J. Pharm. Sci. 2014, 103, 2199–2206. [Google Scholar] [CrossRef]
- Ogaki, S.; Taguchi, K.; Maeda, H.; Watanabe, H.; Ishima, Y.; Otagiri, M.; Maruyama, T. Kupffer cell inactivation by carbon monoxide bound to red blood cells preserves hepatic cytochrome P450 via anti-oxidant and anti-inflammatory effects exerted through the HMGB1/TLR-4 pathway during resuscitation from hemorrhagic shock. Biochem. Pharmacol. 2015, 97, 310–319. [Google Scholar] [CrossRef]
- Taguchi, K.; Ogaki, S.; Nagasaki, T.; Yanagisawa, H.; Nishida, K.; Maeda, H.; Enoki, Y.; Matsumoto, K.; Sekijima, H.; Ooi, K.; et al. Carbon Monoxide Rescues the Developmental Lethality of Experimental Rat Models of Rhabdomyolysis-Induced Acute Kidney Injury. J. Pharmacol. Exp. Ther. 2020, 372, 355–365. [Google Scholar] [CrossRef] [PubMed]
- Nagasaki, T.; Maeda, H.; Taguchi, K.; Yanagisawa, H.; Nishida, K.; Kobayashi, K.; Wada, N.; Noguchi, I.; Murata, R.; Sakai, H.; et al. A bioinspired carbon monoxide delivery system prevents acute kidney injury and the progression to chronic kidney disease. Redox Biol. 2022, 54, 102371. [Google Scholar] [CrossRef]
- Widdop, B. Analysis of carbon monoxide. Ann. Clin. Biochem. 2002, 39, 378–391. [Google Scholar] [CrossRef] [PubMed]
- Parks, J.; Worth, H.G. Carboxyhemoglobin determination by second-derivative spectroscopy. Clin. Chem. 1985, 31, 279–281. [Google Scholar] [CrossRef] [PubMed]
- Kwon, S.Y.; Bae, O.N.; Noh, J.Y.; Kim, K.; Kang, S.; Shin, Y.J.; Lim, K.M.; Chung, J.H. Erythrophagocytosis of lead-exposed erythrocytes by renal tubular cells: Possible role in lead-induced nephrotoxicity. Environ. Health Perspect. 2015, 123, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Taguchi, K.; Urata, Y.; Anraku, M.; Maruyama, T.; Watanabe, H.; Sakai, H.; Horinouchi, H.; Kobayashi, K.; Tsuchida, E.; Kai, T.; et al. Pharmacokinetic study of enclosed hemoglobin and outer lipid component after the administration of hemoglobin vesicles as an artificial oxygen carrier. Drug Metab. Dispos. 2009, 37, 1456–1463. [Google Scholar] [CrossRef] [PubMed]
- Taguchi, K.; Nagao, S.; Yamasaki, K.; Sakai, H.; Seo, H.; Maruyama, T.; Otagiri, M. Biological Responsiveness and Metabolic Performance of Liposome-Encapsulated Hemoglobin (Hemoglobin-Vesicles) in Apolipoprotein E-Deficient Mice after Massive Intravenous Injection. Biol. Pharm. Bull. 2015, 38, 1606–1616. [Google Scholar] [CrossRef]
- Nair, A.B.; Jacob, S. A simple practice guide for dose conversion between animals and human. J. Basic Clin. Pharm. 2016, 7, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.M.; Kim, H.K.; Shim, W.; Anwar, M.A.; Kwon, J.W.; Kwon, H.K.; Kim, H.J.; Jeong, H.; Kim, H.M.; Hwang, D.; et al. Mechanism of Cisplatin-Induced Cytotoxicity Is Correlated to Impaired Metabolism Due to Mitochondrial ROS Generation. PLoS ONE 2015, 10, e0135083. [Google Scholar] [CrossRef] [PubMed]
- Han, W.K.; Bailly, V.; Abichandani, R.; Thadhani, R.; Bonventre, J.V. Kidney Injury Molecule-1 (KIM-1): A novel biomarker for human renal proximal tubule injury. Kidney Int. 2002, 62, 237–244. [Google Scholar] [CrossRef]
- Kodama, A.; Watanabe, H.; Tanaka, R.; Kondo, M.; Chuang, V.T.; Wu, Q.; Endo, M.; Ishima, Y.; Fukagawa, M.; Otagiri, M.; et al. Albumin fusion renders thioredoxin an effective anti-oxidative and anti-inflammatory agent for preventing cisplatin-induced nephrotoxicity. Biochim. Biophys. Acta 2014, 1840, 1152–1162. [Google Scholar] [CrossRef]
- Yao, X.; Panichpisal, K.; Kurtzman, N.; Nugent, K. Cisplatin nephrotoxicity: A review. Am. J. Med. Sci. 2007, 334, 115–124. [Google Scholar] [CrossRef]
- Shah, A.; Xia, L.; Masson, E.A.; Gui, C.; Momen, A.; Shikatani, E.A.; Husain, M.; Quaggin, S.; John, R.; Fantus, I.G. Thioredoxin-Interacting Protein Deficiency Protects against Diabetic Nephropathy. J. Am. Soc. Nephrol. 2015, 26, 2963–2977. [Google Scholar] [CrossRef]
- Zhang, M.Z.; Wang, X.; Yang, H.; Fogo, A.B.; Murphy, B.J.; Kaltenbach, R.; Cheng, P.; Zinker, B.; Harris, R.C. Lysophosphatidic Acid Receptor Antagonism Protects against Diabetic Nephropathy in a Type 2 Diabetic Model. J. Am. Soc. Nephrol. 2017, 28, 3300–3311. [Google Scholar] [CrossRef] [PubMed]
- Igarashi, G.; Iino, K.; Watanabe, H.; Ito, H. Remote ischemic pre-conditioning alleviates contrast-induced acute kidney injury in patients with moderate chronic kidney disease. Circ. J. 2013, 77, 3037–3044. [Google Scholar] [CrossRef] [PubMed]
- Faubel, S.; Lewis, E.C.; Reznikov, L.; Ljubanovic, D.; Hoke, T.S.; Somerset, H.; Oh, D.J.; Lu, L.; Klein, C.L.; Dinarello, C.A.; et al. Cisplatin-induced acute renal failure is associated with an increase in the cytokines interleukin (IL)-1beta, IL-18, IL-6, and neutrophil infiltration in the kidney. J. Pharmacol. Exp. Ther. 2007, 322, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.I.; Zeb, A.; Kim, M.S.; Rana, I.; Khan, N.; Qureshi, O.S.; Lim, C.W.; Park, J.S.; Gao, Z.; Maeng, H.J.; et al. Controlled therapeutic delivery of CO from carbon monoxide-releasing molecules (CORMs). J. Control. Release 2022, 350, 652–667. [Google Scholar] [CrossRef]
- Tayem, Y.; Johnson, T.R.; Mann, B.E.; Green, C.J.; Motterlini, R. Protection against cisplatin-induced nephrotoxicity by a carbon monoxide-releasing molecule. Am. J. Physiol. Ren. Physiol. 2006, 290, F789–F794. [Google Scholar] [CrossRef]
- Taguchi, K.; Suzuki, Y.; Tsutsuura, M.; Hiraoka, K.; Watabe, Y.; Enoki, Y.; Otagiri, M.; Sakai, H.; Matsumoto, K. Liposomal Artificial Red Blood Cell-Based Carbon Monoxide Donor Is a Potent Renoprotectant against Cisplatin-Induced Acute Kidney Injury. Pharmaceutics 2021, 14, 57. [Google Scholar] [CrossRef]
- Boehning, D.; Snyder, S.H. Circadian rhythms. Carbon monoxide and clocks. Science 2002, 298, 2339–2340. [Google Scholar] [CrossRef]
- Campbell, N.K.; Fitzgerald, H.K.; Dunne, A. Regulation of inflammation by the antioxidant haem oxygenase 1. Nat. Rev. Immunol. 2021, 21, 411–425. [Google Scholar] [CrossRef]
- Almeida, A.S.; Figueiredo-Pereira, C.; Vieira, H.L. Carbon monoxide and mitochondria-modulation of cell metabolism, redox response and cell death. Front. Physiol. 2015, 6, 33. [Google Scholar] [CrossRef]
- Lavitrano, M.; Smolenski, R.T.; Musumeci, A.; Maccherini, M.; Slominska, E.; Di Florio, E.; Bracco, A.; Mancini, A.; Stassi, G.; Patti, M.; et al. Carbon monoxide improves cardiac energetics and safeguards the heart during reperfusion after cardiopulmonary bypass in pigs. FASEB J. 2004, 18, 1093–1095. [Google Scholar] [CrossRef]
- Kaczara, P.; Motterlini, R.; Kus, K.; Zakrzewska, A.; Abramov, A.Y.; Chlopicki, S. Carbon monoxide shifts energetic metabolism from glycolysis to oxidative phosphorylation in endothelial cells. FEBS Lett. 2016, 590, 3469–3480. [Google Scholar] [CrossRef] [PubMed]
- Yan, W.; Xu, Y.; Yuan, Y.; Tian, L.; Wang, Q.; Xie, Y.; Shao, X.; Zhang, M.; Ni, Z.; Mou, S. Renoprotective mechanisms of Astragaloside IV in cisplatin-induced acute kidney injury. Free Radic. Res. 2017, 51, 669–683. [Google Scholar] [CrossRef] [PubMed]
- McSweeney, K.R.; Gadanec, L.K.; Qaradakhi, T.; Ali, B.A.; Zulli, A.; Apostolopoulos, V. Mechanisms of Cisplatin-Induced Acute Kidney Injury: Pathological Mechanisms, Pharmacological Interventions, and Genetic Mitigations. Cancers 2021, 13, 1572. [Google Scholar] [CrossRef] [PubMed]
- Privratsky, J.R.; Ide, S.; Chen, Y.; Kitai, H.; Ren, J.; Fradin, H.; Lu, X.; Souma, T.; Crowley, S.D. A macrophage-endothelial immunoregulatory axis ameliorates septic acute kidney injury. Kidney Int. 2023, 103, 514–528. [Google Scholar] [CrossRef]
- Ruan, Y.; Wang, L.; Zhao, Y.; Yao, Y.; Chen, S.; Li, J.; Guo, H.; Ming, C.; Gong, F.; Chen, G. Carbon monoxide potently prevents ischemia-induced high-mobility group box 1 translocation and release and protects against lethal renal ischemia-reperfusion injury. Kidney Int. 2014, 86, 525–537. [Google Scholar] [CrossRef]
- Taguchi, K.; Nagao, S.; Maeda, H.; Yanagisawa, H.; Sakai, H.; Yamasaki, K.; Wakayama, T.; Watanabe, H.; Otagiri, M.; Maruyama, T. Biomimetic carbon monoxide delivery based on hemoglobin vesicles ameliorates acute pancreatitis in mice via the regulation of macrophage and neutrophil activity. Drug Deliv. 2018, 25, 1266–1274. [Google Scholar] [CrossRef]
- Ciarimboli, G.; Ludwig, T.; Lang, D.; Pavenstädt, H.; Koepsell, H.; Piechota, H.J.; Haier, J.; Jaehde, U.; Zisowsky, J.; Schlatter, E. Cisplatin nephrotoxicity is critically mediated via the human organic cation transporter 2. Am. J. Pathol. 2005, 167, 1477–1484. [Google Scholar] [CrossRef]
- Filipski, K.K.; Mathijssen, R.H.; Mikkelsen, T.S.; Schinkel, A.H.; Sparreboom, A. Contribution of organic cation transporter 2 (OCT2) to cisplatin-induced nephrotoxicity. Clin. Pharmacol. Ther. 2009, 86, 396–402. [Google Scholar] [CrossRef]
- Iwata, K.; Aizawa, K.; Kamitsu, S.; Jingami, S.; Fukunaga, E.; Yoshida, M.; Yoshimura, M.; Hamada, A.; Saito, H. Effects of genetic variants in SLC22A2 organic cation transporter 2 and SLC47A1 multidrug and toxin extrusion 1 transporter on cisplatin-induced adverse events. Clin. Exp. Nephrol. 2012, 16, 843–851. [Google Scholar] [CrossRef]
- Zhang, J.; Zhou, W. Ameliorative effects of SLC22A2 gene polymorphism 808 G/T and cimetidine on cisplatin-induced nephrotoxicity in Chinese cancer patients. Food Chem. Toxicol. 2012, 50, 2289–2293. [Google Scholar] [CrossRef]
- Franke, R.M.; Kosloske, A.M.; Lancaster, C.S.; Filipski, K.K.; Hu, C.; Zolk, O.; Mathijssen, R.H.; Sparreboom, A. Influence of Oct1/Oct2-deficiency on cisplatin-induced changes in urinary N-acetyl-beta-D-glucosaminidase. Clin. Cancer Res. 2010, 16, 4198–4206. [Google Scholar] [CrossRef] [PubMed]
- Katsuda, H.; Yamashita, M.; Katsura, H.; Yu, J.; Waki, Y.; Nagata, N.; Sai, Y.; Miyamoto, K. Protecting cisplatin-induced nephrotoxicity with cimetidine does not affect antitumor activity. Biol. Pharm. Bull. 2010, 33, 1867–1871. [Google Scholar] [CrossRef] [PubMed]
- Sprowl, J.A.; van Doorn, L.; Hu, S.; van Gerven, L.; de Bruijn, P.; Li, L.; Gibson, A.A.; Mathijssen, R.H.; Sparreboom, A. Conjunctive therapy of cisplatin with the OCT2 inhibitor cimetidine: Influence on antitumor efficacy and systemic clearance. Clin. Pharmacol. Ther. 2013, 94, 585–592. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Park, D.J.; Kim, J.H.; Jeong, E.Y.; Jung, M.H.; Kim, T.H.; Yang, J.I.; Lee, G.W.; Chung, H.J.; Chang, S.H. Glutamine protects against cisplatin-induced nephrotoxicity by decreasing cisplatin accumulation. J. Pharmacol. Sci. 2015, 127, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Gong, W.; Lu, L.; Zhou, Y.; Liu, J.; Ma, H.; Fu, L.; Huang, S.; Zhang, Y.; Zhang, A.; Jia, Z. The novel STING antagonist H151 ameliorates cisplatin-induced acute kidney injury and mitochondrial dysfunction. Am. J. Physiol. Ren. Physiol. 2021, 320, F608–F616. [Google Scholar] [CrossRef]
- Guo, Y.; Wang, M.; Mou, J.; Zhao, Z.; Yang, J.; Zhu, F.; Pei, G.; Zhu, H.; Wang, Y.; Xu, G.; et al. Pretreatment of Huaiqihuang extractum protects against cisplatin-induced nephrotoxicity. Sci. Rep. 2018, 8, 7333. [Google Scholar] [CrossRef]
- Ramkumar, V.; Mukherjea, D.; Dhukhwa, A.; Rybak, L.P. Oxidative Stress and Inflammation Caused by Cisplatin Ototoxicity. Antioxidants 2021, 10, 1919. [Google Scholar] [CrossRef]
- Behrouzi, A.; Xia, H.; Thompson, E.L.; Kelley, M.R.; Fehrenbacher, J.C. Oxidative DNA Damage and Cisplatin Neurotoxicity Is Exacerbated by Inhibition of OGG1 Glycosylase Activity and APE1 Endonuclease Activity in Sensory Neurons. Int. J. Mol. Sci. 2022, 23, 1909. [Google Scholar] [CrossRef]
- Pardridge, W.M. Drug transport across the blood-brain barrier. J. Cereb. Blood Flow Metab. 2012, 32, 1959–1972. [Google Scholar] [CrossRef]
- Nyberg, S.; Abbott, N.J.; Shi, X.; Steyger, P.S.; Dabdoub, A. Delivery of therapeutics to the inner ear: The challenge of the blood-labyrinth barrier. Sci. Transl. Med. 2019, 11, eaao0935. [Google Scholar] [CrossRef]
- Moody, B.F.; Calvert, J.W. Emergent role of gasotransmitters in ischemia-reperfusion injury. Med. Gas Res. 2011, 1, 3. [Google Scholar] [CrossRef]
- López-Lázaro, M. Two preclinical tests to evaluate anticancer activity and to help validate drug candidates for clinical trials. Oncoscience 2015, 2, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Cui, Q.; Liang, X.L.; Wang, J.Q.; Zhang, J.Y.; Chen, Z.S. Therapeutic implication of carbon monoxide in drug resistant cancers. Biochem. Pharmacol. 2022, 201, 115061. [Google Scholar] [CrossRef] [PubMed]
- Bamias, A.; Tzannis, K.; Harshman, L.C.; Crabb, S.J.; Wong, Y.N.; Kumar Pal, S.; De Giorgi, U.; Ladoire, S.; Agarwal, N.; Yu, E.Y.; et al. Impact of contemporary patterns of chemotherapy utilization on survival in patients with advanced cancer of the urinary tract: A Retrospective International Study of Invasive/Advanced Cancer of the Urothelium (RISC). Ann. Oncol. 2019, 30, 1841. [Google Scholar] [CrossRef] [PubMed]
- Corsi, M.P.; Shea, K.; Knoebel, R.W. Impact of transitioning inpatient chemotherapy regimens to the outpatient setting. J. Oncol. Pharm. Pract. 2020, 26, 1324–1330. [Google Scholar] [CrossRef]
- Biagiotti, S.; Paoletti, M.F.; Fraternale, A.; Rossi, L.; Magnani, M. Drug delivery by red blood cells. IUBMB Life 2011, 63, 621–631. [Google Scholar] [CrossRef]
- Wang, C.; Huang, J.; Zhang, Y.; Jia, H.; Chen, B. Construction and evaluation of red blood cells-based drug delivery system for chemo-photothermal therapy. Colloids Surf. B Biointerfaces 2021, 204, 111789. [Google Scholar] [CrossRef]
- Koleva, L.; Bovt, E.; Ataullakhanov, F.; Sinauridze, E. Erythrocytes as Carriers: From Drug Delivery to Biosensors. Pharmaceutics 2020, 12, 276. [Google Scholar] [CrossRef]
- Villa, C.H.; Anselmo, A.C.; Mitragotri, S.; Muzykantov, V. Red blood cells: Supercarriers for drugs, biologicals, and nanoparticles and inspiration for advanced delivery systems. Adv. Drug Deliv. Rev. 2016, 106, 88–103. [Google Scholar] [CrossRef]
- Lucas, A.; Lam, D.; Cabrales, P. Doxorubicin-loaded red blood cells reduced cardiac toxicity and preserved anticancer activity. Drug Deliv. 2019, 26, 433–442. [Google Scholar] [CrossRef]
- Cabrales, P.; Tsai, A.G.; Intaglietta, M. Modulation of perfusion and oxygenation by red blood cell oxygen affinity during acute anemia. Am. J. Respir. Cell Mol. Biol. 2008, 38, 354–361. [Google Scholar] [CrossRef] [PubMed]
- Villela, N.R.; Cabrales, P.; Tsai, A.G.; Intaglietta, M. Microcirculatory effects of changing blood hemoglobin oxygen affinity during hemorrhagic shock resuscitation in an experimental model. Shock 2009, 31, 645–652. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nagasaki, T.; Maeda, H.; Yanagisawa, H.; Nishida, K.; Kobayashi, K.; Wada, N.; Noguchi, I.; Iwakiri, R.; Taguchi, K.; Sakai, H.; et al. Carbon Monoxide-Loaded Red Blood Cell Prevents the Onset of Cisplatin-Induced Acute Kidney Injury. Antioxidants 2023, 12, 1705. https://doi.org/10.3390/antiox12091705
Nagasaki T, Maeda H, Yanagisawa H, Nishida K, Kobayashi K, Wada N, Noguchi I, Iwakiri R, Taguchi K, Sakai H, et al. Carbon Monoxide-Loaded Red Blood Cell Prevents the Onset of Cisplatin-Induced Acute Kidney Injury. Antioxidants. 2023; 12(9):1705. https://doi.org/10.3390/antiox12091705
Chicago/Turabian StyleNagasaki, Taisei, Hitoshi Maeda, Hiroki Yanagisawa, Kento Nishida, Kazuki Kobayashi, Naoki Wada, Isamu Noguchi, Ryotaro Iwakiri, Kazuaki Taguchi, Hiromi Sakai, and et al. 2023. "Carbon Monoxide-Loaded Red Blood Cell Prevents the Onset of Cisplatin-Induced Acute Kidney Injury" Antioxidants 12, no. 9: 1705. https://doi.org/10.3390/antiox12091705
APA StyleNagasaki, T., Maeda, H., Yanagisawa, H., Nishida, K., Kobayashi, K., Wada, N., Noguchi, I., Iwakiri, R., Taguchi, K., Sakai, H., Saruwatari, J., Watanabe, H., Otagiri, M., & Maruyama, T. (2023). Carbon Monoxide-Loaded Red Blood Cell Prevents the Onset of Cisplatin-Induced Acute Kidney Injury. Antioxidants, 12(9), 1705. https://doi.org/10.3390/antiox12091705







